Abstract
The previously unexplored metal-catalyzed [5 + 2] cycloadditions of vinylcyclopropanes (VCPs) and electron-rich alkynes (ynol ethers) have been found to provide a highly efficient, direct route to dioxygenated seven-membered rings, a common feature of numerous natural and non-natural targets and building blocks for synthesis. The reactions proceed in high yield at room temperature and tolerate a broad range of functionalities. Substituted VCPs were found to react with high regioselectivity.
Graphical abstract
New reactions, reagents, and catalysts change how we think about bond construction, thereby enabling new strategic choices for step economical and greener, if not ideal, syntheses.1 As part of our studies on new cycloaddition reactions,2,3 we previously reported a route to seven-membered rings involving the metal-catalyzed [5 + 2] cycloaddition of vinylcyclopropanes (VCPs) and π-components.4 Rhodium catalysts have proven to be the most general for this CC bond activation process, working thus far intramolecularly with alkynes, alkenes and allenes and intermolecularly with alkynes and activated allenes as 2C components.2,3,5
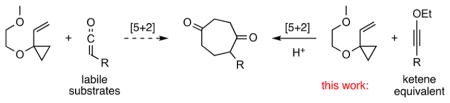
To extend the reach of these [5 + 2] cycloaddition reactions and more generally other [m + n] processes, we have been exploring the use of π-component equivalents of otherwise inaccessible, difficult to use, or unsafe π-systems including allene6 and buta-1,2,3-triene4c equivalents of gaseous allenes and cumulenes as well as tetramethyleneethane7 (TME) equivalents of the unstable and difficult to access TME. Here we report the use of ynol ethers (Scheme 1, left) as ketene (Scheme 1, right) equivalents in [5 + 2] cycloadditions with VCPs.8
Scheme 1.
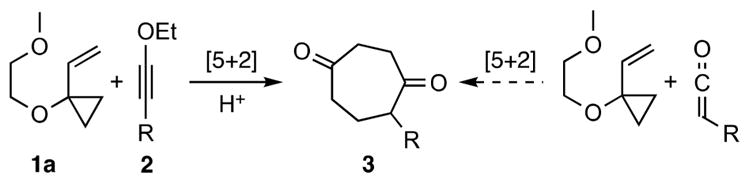
Use of Ynol Ethers as Ketene Equivalents
While ketenes can be used as π-components in some metal-catalyzed cycloadditions,9 their electron-poor nature, propensity to dimerize, and incompatibility with a range of functionalities limits their utility.10 In contrast, ynol ethers are electron-rich and easily prepared by alkylation of the parent metal alkoxyacetylide.11 However, their use in metal-catalyzed cycloadditions is largely unexplored and potentially problematic due to their reported “instability” in the presence of cationic rhodium complexes.12,13 Beyond their mechanistic interest, the study of ynol ethers as 2C components in [5 + 2] cycloadditions is further motivated by the potential use of such a process in accessing diverse targets.2,14 Numerous natural (estimated at >3000)15 and non-natural products, including many of research and therapeutic importance,16 incorporate functionalities derivable from cycloheptan-1,4-diones (CHDs).17,18 Yet few methods exist for the direct construction of such systems.19 We have now found that the metal-catalyzed cycloaddition of ynol ethers and VCPs provides a solution to this problem.
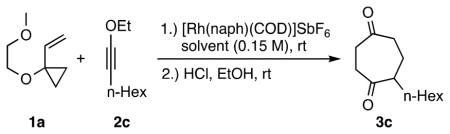
To determine the suitability of ynol ethers as substrates20 in [5 + 2] cycloadditions, 1-ethoxy-1-octyne (2c, R = n-hexyl) was chosen as a test reactant in an initial catalyst screening (for substrate syntheses, see Supporting Information (SI)).
We first tested [RhCl(CO)2]2 as a catalyst in the reaction of 2c at 25 °C with commercially available VCP 1a. Cycloadduct 3c did not form. Upon heating at 90 °C, the reaction gave 3c albeit in only 52% yield. A recently introduced cationic Rh(I) catalyst ([Rh(dnCOT)(MeCN)2]SbF6)4a,b provided only complex mixtures. In contrast, [Rh(naph)(COD)]SbF6, another cationic Rh catalyst,21 gave promising initial results (Table 1, entry 1:60% of 3c), working even at 25 °C in 2,2,2-trifluorethanol (TFE), and was thus selected for further study.
Table 1.
Optimization Studies
| |||||
|---|---|---|---|---|---|
| entry | solvent | catalyst loading [mol %] | ynol ether [equiv] | t [h] | yield [%] |
| 1 | TFE | 3 | 1.1 | 8 | 60 |
| 2 | TFE | 3 | 3.0 | 12 | 35 |
| 3 | TFE | 3 | 1.1 | 4.5 | 0a,b |
| 4 | TFE | 5 | 1.1 | 4 | 74 |
| 5 | TFE | 5 | 1.1c | 2 | 91 |
| 6 | TFE | 0 | 1.1 | 4 | 0d |
[Rh] and 2c were stirred in TFE for 2 h prior to the reaction.
1a was recovered.
2c was added dropwise over the course of 2 h.
Reaction was carried out at 80 °C.
Interestingly, when excess ynol ether 2c (3.0 equiv) was used to increase the yield, cycloadduct 3c was obtained but in only 35% yield, suggesting that the ynol ether inhibits catalysis (Table 1, entry 2). To test this point, the catalyst was stirred with ynol ether 2c for 2 h after which VCP 1a was added (entry 3). No cycloadduct was formed and only starting materials were isolated. To overcome this substrate inhibition problem, the catalyst loading was increased (5 mol %) and the amount of the ynol ether was decreased (1.1 equiv, entry 4). An improved yield (74%) was obtained. Finally, to further minimize the inhibitory effect of the ynol ether, 2c was added dropwise over 2 h. Under these conditions, the cycloadduct was formed in excellent yield (91%, entry 5). No reaction was observed in the absence of catalyst, even when the reaction was heated for 4 h (entry 6).
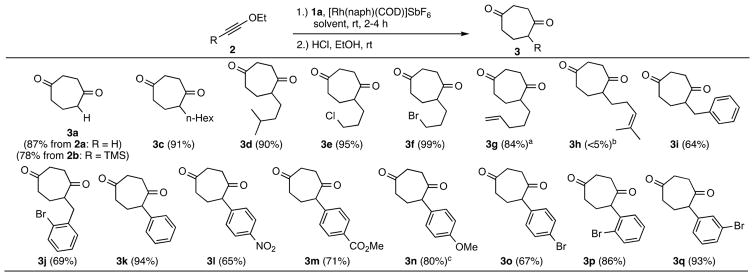
Using the above conditions, a broad range of ynol ethers yielded CHDs in good to excellent yields (Scheme 2). Terminal alkyne 2a (EtOCCH) and TMS-analogue 2b (EtOCCTMS), equivalents of ketene itself, reacted efficiently, both giving dione 3a after workup. Alkyl-substituted (2c–2g, 2i–2j) and aryl-substituted ynol ethers (2k–2q) were also effective substrates. Halogen containing substrates (2e, 2f) reacted efficiently along with terminal alkene 2g (84% yield). Of mechanistic interest, trisubstituted alkene 2h gave a complex mixture, potentially due to catalyst deactivation by chelative coordination. Supporting this hypothesis, the otherwise efficient reaction of 2d with VCP 1a, in the presence of 2h, yielded no cycloadduct 3d. Benzyl substituted ynol ethers (2i and 2j) also worked moderately well. For aryl-containing ynol ethers, a solvent mixture of 1,2-dichloroethane (DCE) and TFE (1:1) was used.6 Phenyl derivative 2k gave cycloadduct 3k in 94% isolated yield and 65–80% yields were obtained for both electron-rich and electron-poor aryl derivatives. The electron-rich anisole 2n required slower addition (4 h) to overcome its hypothesized coordinative deactivation of the catalyst. Supporting this idea, slower addition of the ynol ether produced cycloadduct 3n in 80% yield (see SI, Table S1). Nitro-groups (3l), esters (3m), additional ethers (3n) and aryl-bromides (3j, 3o–3q) were also well tolerated. Bromide substitution was accommodated at all aryl positions, providing versatile handles for subsequent diversification.
Scheme 2. Substrate Scoped.
aAdditional 12% of double bond migration byproduct were isolated. bComplex product mixture was formed. c2n was added over 4 h. dReaction conditions: 5 mol % catalyst, 1.0 equiv VCP, 1.1 equiv ynol ether added dropwise over 2 h. Solvent: TFE (3a–3h), TFE/DCE 1:1 (3i–3q). For aryl substituted ynol ether (3k–3q), the reaction mixture was stirred for additional 2 h.
While many alkyl-substituted ynol ethers can be made in pure form,20 their purification over silica results in substantial decomposition. The use of crude ynol ethers was therefore tested as an alternative. Two substrates (2c and 2d), purified and unpurified (see SI, Table S2), gave identical yields. The aryl substrates were more robust and were purified using triethylamine neutralized silica.
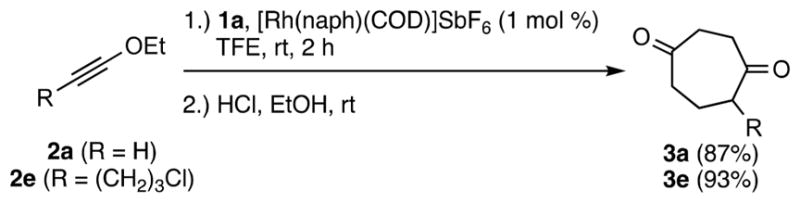
Next, catalyst loading and reaction scale were investigated (see SI, Table S3). With 5 mol % catalyst, ynol ether, 2a gave cycloadduct 3a in 87% isolated yield (Scheme 2). Significantly, a near equivalent yield (86%) was obtained with 1 mol % of catalyst. When tested on a 1 mmol scale at room temperature using 1 mol % of catalyst, 3a was obtained in 87% yield (Scheme 3). To check substrate generality, 2e was also tested, giving 3e in 93% isolated yield (Scheme 3).
Scheme 3.
Cycloaddition with Reduced Catalyst Loading at 1 mmol Scale
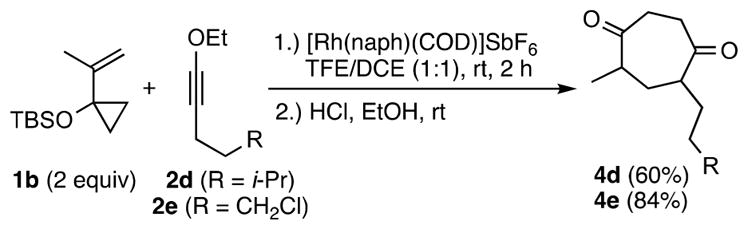
To explore regioselectivity, the reactivity of VCP 1b was examined. In this case, 2 equiv of VCP 1b provided improved yields. Significantly, only the 5,7-dialkyl substituted cycloadducts 4d and 4e were isolated to indicate a 1:1 mixture of diastereomers (Scheme 4).
Scheme 4.
Regioselective Access to 5,7-Disubstituted Cyclohepta-1,4-diones
Two regioisomers are possible depending on the ynol ether orientation during insertion. Previous studies have shown that alkyl-substituted terminal alkynes exhibit moderate regioselectivity (up to 7:1) using VCP 1b.22 Internal ynol ethers have not been tested previously. Providing the first experimental data on this issue of more general mechanistic and synthetic importance, ynol ethers 2d and 2e were found to react with excellent regioselectivity (>20:1).
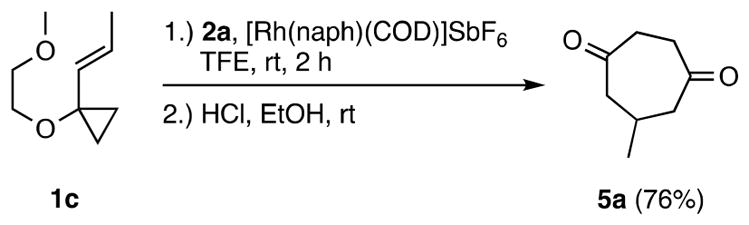
To determine whether access to 6-substituted CHDs could also be achieved, the reaction of VCP 1c was examined. As observed with ynol ethers 2d and 2e (Scheme 4), the cycloaddition of VCP 1c and ynol ether 2a proceeded with excellent regioselectivity to give only one regioisomer, CHD 5a, in 76% yield (Scheme 5).
Scheme 5.
Regioselective Access to 6-Substituted Cyclohepta-1,4-dione
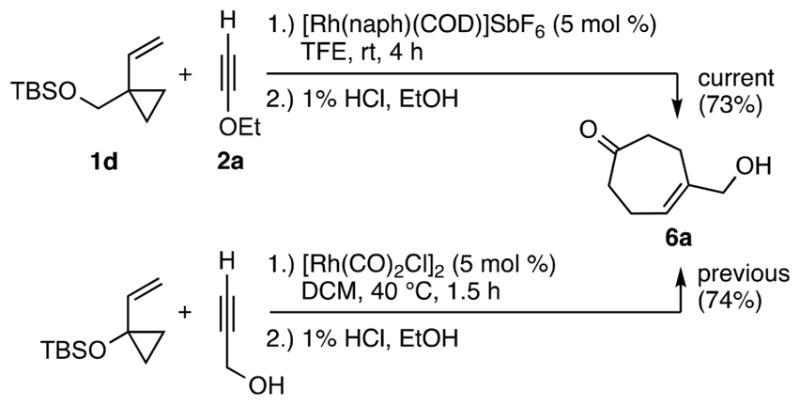
Significantly, this method is not limited to oxygen substituted VCPs. Alkyl substituted VCPs also work well, as shown by the reaction of VCP 1d with ynol ether 2a, which gave cycloheptenone 6a in 73% (Scheme 6, top). This method provides a strategically complementary route to cycloheptenones, as one can choose the more accessible VCPs and alkynes to produce a common product.5a
Scheme 6.
Application of an Alkyl-Substituted VCP
To further test functional group tolerance, the reaction of VCP 1a with ynol ether 2k was conducted in the combined presence of acetone, ethyl acetate, diethyl ether, triethyl amine, cyclohexene and maleic anhydride (0.3 equiv of each). Using the conditions given in Scheme 2, cycloadduct 3k was isolated in 86% yield, indicating broad functional group tolerance. Prompted by these results and the previously reported preference for DCE and TFE as solvents,21b the cycloaddition was conducted in acetone. Significantly, excellent yields were obtained in a room temperature reaction that was complete in 30 min (Scheme 7). Slow addition was not required. Acetone is thus a superb non-halogenated solvent option for both aryl- and alkyl-ynol ether substrates.
Scheme 7.
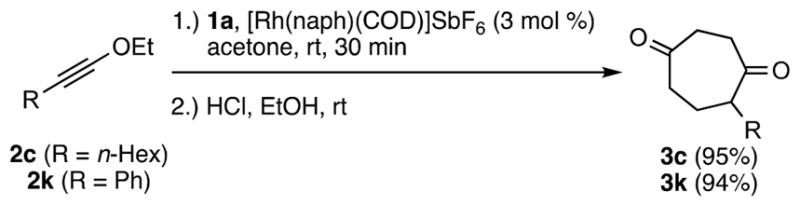
Use of Acetone As a Solvent
In summary, we report the first use of ynol ethers as ketene equivalents in the rhodium-catalyzed intermolecular [5 + 2] cycloaddition reaction with VCPs and the first study of reaction regioselectivity. The cycloaddition proceeds at room temperature within minutes to hours and provides substituted cyclohepta-1,4-diones in good to excellent yields. The reaction tolerates a wide range of functionality commonly encountered in synthesis and can be run in various solvents (DCE, TFE, acetone). Substituted VCPs can also be used and react with unprecedentedly high regioselectivity. For cost, safety and time considerations, these exploratory experiments were conducted on a small scale but are not affected by a 10-fold scale increase and can be done with a catalyst loading of 1 mol %. The use of these substituted CHDs in synthesis and as scaffolds in designed libraries will be reported in due course.
Supplementary Material
Acknowledgments
Fellowship support was graciously provided by the Swiss National Science Foundation (C.E.), the National Science Foundation Graduate Research Fellowship (B.F., DGE-114747), the Japan Society for the Promotion of Science (F.I.), and the German Academic Exchange Service (B.S.). Grant support provided by the National Science Foundation (CHE848280) and National Institute of Health (R37 CA031845) is gratefully acknowledged.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b02765.
Experimental procedures and characterization data for all reactions and products (PDF)
References
- 1.(a) Wender PA, Miller BL. Nature. 2009;460:197. doi: 10.1038/460197a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wender PA. Nat Prod Rep. 2014;31:433. doi: 10.1039/c4np00013g. [DOI] [PubMed] [Google Scholar]; (c) Roschangar F, Sheldon RA, Senanayake CH. Green Chem. 2015;17:752. [Google Scholar]; (d) Sheldon RA. Green Chem. 2017;19:18. [Google Scholar]
- 2.Wender PA, Croatt MP, Deschamps NM. In: Comprehensive Organometallic Chemistry III. Ojima I, editor. Vol. 10. Elsevier Ltd; Amsterdam: 2007. pp. 603–648. [Google Scholar]
- 3.For recent examples, see: Wender PA, Gamber GG, Williams TJ. Rhodium(I)-Catalyzed [5 + 2], [6 + 2], and [5 + 2+1] Cycloadditions: New Reactions for Organic Synthesis. In: Evans PA, editor. Modern Rhodium-Catalyzed Organic Reactions. Wiley; Weinheim: 2005. pp. 263–299.Trost BM, Hu Y, Horne DB. J Am Chem Soc. 2007;129:1178. doi: 10.1021/ja073272b.Liu CH, Yu ZX. Angew Chem. 2017;129:8793. doi: 10.1002/anie.201702288.Zuo G, Louie J. J Am Chem Soc. 2005;127:5798. doi: 10.1021/ja043253r.Fürstner A, Majima K, Martin R, Krause H, Kattnig E, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:1992. doi: 10.1021/ja0777180.Ashfeld BL, Martin SF. Org Lett. 2005;7:4535. doi: 10.1021/ol051945u.Lee SI, Park SY, Park JH, Jung IG, Choi SY, Chung YK. J Org Chem. 2006;71:91. doi: 10.1021/jo051685u.Straker RN, Peng Q, Mekareeya A, Paton RS, Anderson EA. Nat Commun. 2016;7:10109. doi: 10.1038/ncomms10109.Inagaki F, Sugikubo K, Miyashita Y, Mukai C. Angew Chem, Int Ed. 2010;49:2206. doi: 10.1002/anie.200906994.Wegner HA, de Meijere A, Wender PA. J Am Chem Soc. 2005;127:6530. doi: 10.1021/ja043671w.Hong X, Stevens MC, Liu P, Wender PA, Houk KN. J Am Chem Soc. 2014;136:17273. doi: 10.1021/ja5098308.Melcher MC, von Wachenfeldt H, Sundin A, Strand D. Chem - Eur J. 2015;21:531. doi: 10.1002/chem.201405729.Murakami M, Itami K, Ito Y. Angew Chem, Int Ed Engl. 1995;34:2691.Murakami M, Itami K, Ubukata M, Tsuji I, Ito Y. J Org Chem. 1998;63:4. doi: 10.1021/jo9718859.
- 4.For recent examples, see: Wender PA, Lesser AB, Sirois LE. Org Synth. 2011;88:109.Wender PA, Lesser AB, Sirois LE. Angew Chem, Int Ed. 2012;51:2736. doi: 10.1002/anie.201108270.Wender PA, Fournogerakis DN, Jeffreys MS, Quiroz RV, Inagaki F, Pfaffenbach M. Nat Chem. 2014;6:448. doi: 10.1038/nchem.1917.Wender PA, Axtman AD, Golden JE, Kee JM, Sirois LE, Quiroz RV, Stevens MC. Org Chem Front. 2014;1:1166. doi: 10.1039/c4qo00228h.Mustard TJL, Wender PA, Cheong PHY. ACS Catal. 2015;5:1758. doi: 10.1021/cs501828e.
- 5.For seminal intramolecular [5 + 2] cycloaddition with alkynes and activated allenes, see: Wender PA, Rieck H, Fuji M. J Am Chem Soc. 1998;120:10976.Wegner HA, de Meijere A, Wender PA. J Am Chem Soc. 2005;127:6530. doi: 10.1021/ja043671w.. For recent studies and lead references on other metal-catalyzed [5 + 2] cycloadditions, see: Shu XZ, Scheinebeck CM, Li X, Zhou X, Song W, Chen L, Guzei IA, Tang W. Org Lett. 2015;17:5128. doi: 10.1021/acs.orglett.5b02665.Ylijoki KEO, Kirk AD, Böcklein S, Witherell RD, Stryker JM. Organometallics. 2015;34:3335. For key contributions from other groups see references 2, 3, and 14.
- 6.Wender PA, Inagaki F, Pfaffenbach M, Stevens M-C. Org Lett. 2014;16:2923. doi: 10.1021/ol501114q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wender PA, Jeffreys MS, Raub AG. J Am Chem Soc. 2015;137:9088. doi: 10.1021/jacs.5b04091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a review on ketenes, see: Tidwell TT. Eur J Org Chem. 2006;2006:563.Tidwell TT. Angew Chem, Int Ed. 2005;44:5778. doi: 10.1002/anie.200500098.Tidwell TT. Ketenes. 2. John Wiley & Sons; Hoboken, NJ: 1995. 2005.Temperley C, Ketenes M. Their Cumulene Analogues, and Their S, Se, and Te Analogues. In: Katritzky AR, Taylor RJK, editors. Comprehensive Organic Functional Group Transformations II. Vol. 3. Elsevier Science; Amsterdam: 2005. pp. 573–603.Hyatt A, Raynolds PW. Org React. 1994;45:159.
- 9.For an example of Rh-catalyzed ketene cycloadditions, see: Kondo T, Niimi M, Yoshida Y, Wada K, Mitsudo T, Kimura Y, Toshimitsu A. Molecules. 2010;15:4189. doi: 10.3390/molecules15064189.Kim I, Roh SW, Lee DG, Lee C. Org Lett. 2014;16:2482. doi: 10.1021/ol500856z.
- 10.Hanford WE, Sauer JC. Organic Reactions. John Wiley & Sons; Hoboken, NJ: 2014. Preparation of Ketenes and Ketene Dimers; pp. 109–126. [Google Scholar]
- 11.For the general preparation of ynol ethers, see: Gray VJ, Wilden JD. Org Biomol Chem. 2016;14:9695. doi: 10.1039/c6ob01776b.Shindo M, Sato Y, Shishido K. Tetrahedron. 1998;54:2411.Kowalski CJ, Fields KW. J Am Chem Soc. 1982;104:321.Akai S, Kitagaki S, Naka T, Yamamoto K, Tsuzuki Y, Matsumoto K, Kita Y. J Chem Soc, Perkin Trans. 1996;1:1705.Schöllkopf U, Hoppe I. Angew Chem, Int Ed Engl. 1975;14:765.
- 12.Alkyl ethynyl ethers could not be employed in the presence of Lewis acidic cationic Rh(I) complex due to their instability. For an alternative use of aryloxy ethers in [2 + 2+2] cycloadditions, see: Miyauchi Y, Noguchi K, Tanaka K. Org Lett. 2012;14:5856. doi: 10.1021/ol3027158.For an example of an ynol ether cycloaddition in total synthesis, see: Alayrac C, Schollmeyer D, Witulski B. Chem Commun. 2009:1464. doi: 10.1039/b820291e.
- 13.For a review on ynolate chemistry, see: Shindo M. Tetrahedron. 2007;63:10. doi: 10.1016/j.tet.2007.07.033.For reviews on the chemistry of ynol ethers, see: Minehan TG. Acc Chem Res. 2016;49:1168. doi: 10.1021/acs.accounts.6b00107.
- 14.For reviews on seven membered ring synthesis, see: Nguyen TV, Hartmann JM, Enders D. Synthesis. 2013;45:845.Pellissier H. Adv Synth Catal. 2011;353:189.Ylijoki KEO, Stryker JM. Chem Rev. 2013;113:2244. doi: 10.1021/cr300087g.Clavier H, Pellissier H. Methods and Applications of Cycloaddition Reactions in Organic Syntheses. John Wiley & Sons; Hoboken, NJ: 2014. Recent Developments in The [5 + 2] Cycloaddition; pp. 631–654.Kulinkovich OG. Cyclopropanes in Organic Synthesis. John Wiley & Sons, Inc; Hoboken, NJ: 2015. Cycloheptanes; pp. 285–332.Buono G, Clavier H, Giodano L, Tenaglia A. Stereoselective Multiple Bond-Forming Transformations in Organic Synthesis. John Wiley & Sons; Hoboken, NJ: 2015. Seven- and Eight-Membered Carbocycles; pp. 211–240.Butenschön H. Angew Chem, Int Ed. 2008;47:5287. doi: 10.1002/anie.200801738.Harmata M. Chem Commun. 2010;46:8904. doi: 10.1039/c0cc03621h.Harmata M. Chem Commun. 2010;46:8886. doi: 10.1039/c0cc03620j.
- 15.On the basis of The Dictionary of Natural Productshttp://dnp.chemnetbase.com, using a 1,4-cycloheptadiol substructure. Isomers are considered unique scaffolds.
- 16.(a) Wender PA, Lee HY, Wilhelm RS, Williams PD. J Am Chem Soc. 1989;111:8954. [Google Scholar]; (b) Wender PA, Kee JM, Warrington JM. Science. 2008;320:649. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wender PA, Buschmann N, Cardin NB, Jones LR, Kan C, Kee JM, Kowalski JA, Longcore KE. Nat Chem. 2011;3:615. doi: 10.1038/nchem.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jørgensen L, McKerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. Science. 2013;341:878. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]
- 17.For examples in synthesis, see: Mehta G, Krishnamurthy N, Karra SR. J Chem Soc, Chem Commun. 1989:1299.Audenaert F, De Keukeleire D, Vandewalle M. Tetrahedron. 1987;43:5593.
- 18.For an example in non-natural clinical candidate scaffold, see: Leahy DK, Fan Y, Desai LV, Chan C, Zhu J, Luo G, Chen L, Hanson RL, Sugiyama M, Rosner T, Cuniere N, Guo Z, Hsiao Y, Gao Q. Org Lett. 2012;14:4938. doi: 10.1021/ol302262q.
- 19.For syntheses of CHDs, see: Bondon D, Pietrasanta Y, Pucci B. Tetrahedron. 1976;32:2401.(b) Ref 9a. Schulz SR, Blechert S. Angew Chem, Int Ed. 2007;46:3966. doi: 10.1002/anie.200604553.Schick H, Roatsch B, Schwarz H, Hauser A, Schwarz S. Liebigs Ann Chem. 1992;1992:419.Cope AC, Scheiner P, Youngquist MJ. J Org Chem. 1963;28:518.Seebach D, Jones NR, Corey EJ. J Org Chem. 1968;33:300.Frontier AJ, Danishefsky SJ, Koppel GA, Meng D. Tetrahedron. 1998;54:12721.
- 20.For syntheses of alkyl-substituted ynol ethers, see: Sakamoto T, Yasuhara A, Kondo Y, Yamanaka H. Chem Pharm Bull. 1994;42:2032.Löffler A, Himbert G. Synthesis. 1992;1992:495.Jouvin K, Bayle A, Legrand F, Evano G. Org Lett. 2012;14:1652. doi: 10.1021/ol300491d.
- 21.For preparation and recent examples of the use of [Rh(naph) (COD)]SbF6, see: Wender PA, Williams TJ. Angew Chem, Int Ed. 2002;41:4550. doi: 10.1002/1521-3773(20021202)41:23<4550::AID-ANIE4550>3.0.CO;2-D.Wender PA, Sirois LE, Stemmler R, Williams TJ. Org Lett. 2010;12:1604. doi: 10.1021/ol100337m.
- 22.Liu P, Sirois LE, Cheong PHY, Yu ZX, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127. doi: 10.1021/ja103253d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.