Abstract
Glycine plays several roles in human metabolism, e.g. as one-carbon donor, in purine synthesis, and as a component of glutathione. Glycine is decarboxylated via the glycine cleavage system (GCS) that yields concurrent generation of a one-carbon unit as 5,10-methylenetetrahydrofolate (methyleneTHF). Serine hydroxymethyltransferase (SHMT) catalyzes the interconversion of glycine and serine, another one-carbon donor. The quantitative role of glycine in human one-carbon metabolism has received little attention. To quantify whole body glycine flux, glycine to serine flux, and rate of glycine cleavage in humans. A primed, constant infusion with 9.26 μmol/(kg·h) [1,2-13C2]glycine and 1.87 μmol/(kg·h) [2H3]leucine was used to quantify the kinetic behavior of glycine in young, healthy volunteers (n=5) in fed state. The isotopic enrichment of infused tracers and metabolic products in plasma, as well as breath 13CO2 enrichment, were determined for use in kinetic analysis. Serine synthesis by direct conversion from glycine via SHMT occurred at 193 ± 28 μmol/(kg·h) (mean ± SEM), which comprised 41% of the 463 ± 55 μmol/(kg·h) total glycine flux. Nearly half (46%) of the glycine-to-serine conversion occurred using GCS-derived methyleneTHF one-carbon units. Based on breath 13CO2 measurement, glycine decarboxylation (190 ± 41 μmol/(kg·h)) accounted for 39 ± 6% of whole body glycine flux. This study is the first to quantify human glycine cleavage and glycine-to-serine SHMT kinetics. GCS is responsible for a substantial proportion of whole body glycine flux and constitutes a major route for the generation of one-carbon units.
INTRODUCTION
Glycine, a non-essential amino acid, has multiple roles in human metabolism, including as a one-carbon donor, as a substrate in purine and protein synthesis, and as a precursor of glutathione. In the central nervous system, glycine is an inhibitory neurotransmitter (1). In one-carbon metabolism, glycine serves both as a donor and acceptor of one-carbon units. Abnormal one-carbon metabolism – as in cases of enzymatic defects, low vitamin B-12 and/or folate status – may yield anemia, DNA hypomethylation, neural tube defects, vascular diseases, and neurological disorders (2). The quantitative role of glycine in one-carbon metabolism has not been fully elucidated.
Through the mitochondrial glycine cleavage system (GCS), a four-protein enzymatic complex, glycine is catabolized to CO2, ammonia, and a one-carbon unit in form of 5,10-methylenetetrahydrofolate (methyleneTHF) (3). Mutations affecting genes encoding the components of the GCS can cause reduced catalytic activity of the enzyme complex (4). Such loss-of-function mutations cause accumulation of glycine to pathological levels responsible for the conditions of nonketotic hyperglycinemia or glycine encephalopathy (1, 4). Typical assays for GCS activity in vitro involve incubation with [carboxyl-14C]glycine and measurement of the released 14CO2 (5).
Glycine is an important precursor of serine. Serine hydroxymethyltransferase (SHMT) catalyzes the reversible formation of serine from glycine and a one-carbon unit donated by 5,10-methylenetetrahydrofolate (methyleneTHF). Because GCS forms methyleneTHF, GCS and SHMT are able to synthesize serine in a concerted manner (6, 7). Both amino acids, glycine and serine, are gluconeogeneic via pyruvate (1) and serve as one-carbon donors. Serine was shown to be the main source of one-carbon units for regeneration of methionine from homocysteine (8) and, thus, S-adenosylmethionine, the primary agent used in biological methylation reactions.
Tracers labeled with stable isotopes facilitate the study of in vivo kinetics in metabolism. [15N]glycine was the first amino acid tracer used in kinetic research to investigate whole body protein turnover (9, 10). [15N]glycine remains the most widely used tracer for this purpose (9, 11, 12). Human tracer protocols using [15N]glycine have been designed to investigate glycine nitrogen metabolism (13), de novo glycine synthesis (14), dietary effects on glycine metabolism (15, 16), and lipoprotein metabolism (17). With the use of [13C1]glycine, Cetin et al. (18) investigated placental glycine transport.
We describe here a new steady state tracer protocol to quantify whole body glycine flux and glycine to serine flux after primed, constant infusion of [1,2-13C2]glycine and [2H3]leucine in healthy male and female volunteers. [2H3]Leucine was included to determine any nutritional effects on protein turnover when this protocol is applied to studies with nutritional interventions (8, 19). In addition to analysis of plasma from serial blood samples collected during the infusion, we also measured breath 13CO2 enrichment (20) to quantify the flux of glycine decarboxylation through the GCS and estimated the concurrent glycine-based generation of one-carbon units. Through the use of this protocol, we examined GCS flux, the rate of glycine-to-serine conversion and the fraction of this conversion specifically using a glycine-derived one-carbon unit in healthy young adults. Overall, these findings demonstrate the utility of this protocol and provide new insight into quantitative aspects of human glycine and one-carbon metabolism.
METHODS
Materials
[1,2-13C2]Glycine and L-[5,5,5-2H3]leucine were purchased from Cambridge Isotopes Laboratories (Andover, MA). Isotope solutions were prepared in isotonic saline, filter sterilized and analyzed to assure lack of pyrogenicity and microbial contamination.
Human subjects
Healthy adult male and non-pregnant female subjects (20–40y) were recruited and met the following inclusion criteria: no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; and a body mass index of < 28kg/m2. Medical history, dietary habits, and demographic data were assessed by a questionnaire. Adequate nutritional status for folate, vitamins B-12 and B-6 was defined as serum folate >7nmol/L, serum vitamin B-12 >200pmol/L and plasma pyridoxal 5′-phosphate (PLP) >30nmol/L, respectively, and plasma total homocysteine concentration <12μmol/L. For determination of general health, subjects were screened by standard clinical measures for normal hematological pattern, blood chemistry, and thyroid status, and underwent a physical examination. All subjects gave written informed consent. The University of Florida Institutional Review Board and the General Clinical Research Center (GCRC) Scientific Advisory Committee approved this protocol.
Dietary treatment
All meals were prepared by the Bionutrition Unit of the GCRC. Prior to the infusion day subjects consumed nutritionally adequate meals with standardized composition for 2 d to minimize dietary variation immediately prior to the study.
Analytical methods
Screening measurements
Serum folate and vitamin B-12 were analyzed with the use of a commercial chemiluminescence-based assay (Elecsys, Roche Diagnostics, Indianapolis, IN). Plasma PLP concentration was measured as the semicarbazone-derivative by reverse-phase HPLC with fluorescence detection (21). Plasma total homocysteine concentration was measured as the ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivative by reverse-phase HPLC with fluorescence detection (22).
Gas chromatography-mass spectrometry (GC/MS) analysis of amino acid isotopic enrichment
Plasma free amino acids were isolated, derivatized, and analyzed as previously described (8). The N-heptafluorobutyramide-n-propyl ester derivatives were dried, dissolved in ethyl acetate and stored at −20°C until analysis. Isotopic enrichment was determined by negative chemical ionization-GC/MS with the use of a Thermo-Finnigan DSQ GC-MS and a 30m poly (5% diphenyl and 95% dimethylsiloxane) fused silica capillary column (Equity 5, Supelco, Bellefonte, PA). The relative abundance of specific ions was determined by selected-ion monitoring at the following mass/charge (m/z) ratios: glycine (293-295); serine (519-520); leucine (349-352); and methionine (367-372). Isotopic enrichments are expressed as molar ratios (mol % excess) of labeled to nonlabeled isotopomers after correction for the natural abundance of stable isotopes essentially as performed by Storch et al. (23). Natural abundance measured for glycine M+2, leucine M+3, serine M+1, and serine M+2 was 1.2, 0.2, 17, and 2.6 mol % excess, respectively.
Breath CO2
For determination of the isotopic enrichment of breath 13CO2, samples were collected in Exetainer tubes provided by Metabolic Solutions, Inc. (Nashua, NH) and shipped to Metabolic Solutions for isotope ratio – mass spectrometry analysis. Total CO2 production rate, i.e. VCO2, was determined with the use of a metabolic cart (TrueMax 2400; ParvoMedics, Sandy, UT). Measurements were taken at 30-s intervals for ~5 min until 4 consecutive time points differed by no more than ± 0.01L/min.
Infusion protocol
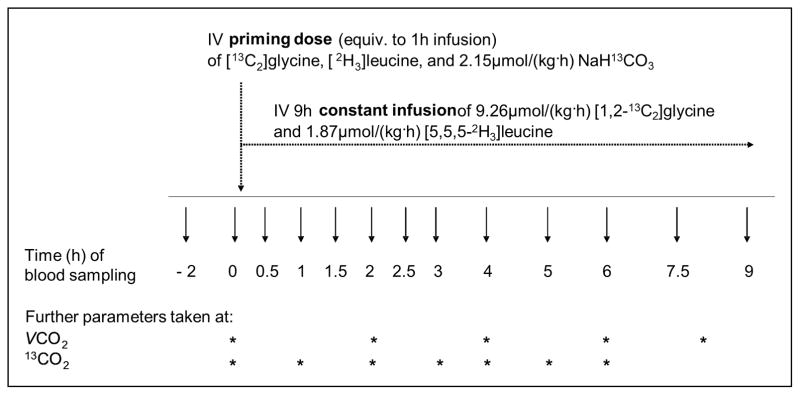
The infusion protocol is illustrated in Figure 2. Subjects were admitted to the GCRC on the evening before the infusion protocol and consumed no food and drinks, except water, between 2100 and initiation of the infusion. On the morning of the infusion, an angiocatheter was inserted in the antecubital vein of each arm; one for the tracer infusion and one for blood collection. Fasting blood samples were taken 2 h before infusion (at ~0700) for measurement of plasma PLP and total homocysteine concentrations, as well as for measurement of background isotopic enrichment of amino acids. Infusions were initiated at ~0900 with a 5 min, ~20mL priming dose that delivered 9.26 μmol/kg [1,2-13C2]glycine and 1.87 μmol/kg [5,5,5-2H3]leucine, respectively, along with a simultaneous priming dose of 2.15 μmol/kg of NaH13C03. The 9-h constant infusion followed immediately after the priming dose and delivered ~20mL infusion solution/h that contained 9.26 μmol/kg [1,2-13C2]glycine and 1.87 μmol/kg [5,5,5-2H3]leucine.
Figure 2.
[13C2]Glycine tracer infusion protocol.
Blood samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7.5, and 9 h of the infusion. These samples were placed immediately on ice and were centrifuged within 15 min after the blood draw (1500g, 4°C, 10 min). Plasma was stored in micro-centrifuge tubes at −80°C. Red cells were washed, lysed, snap frozen in liquid nitrogen, and stored at −80°C for measurement of glutathione concentration and enrichment. These data will be presented in a separate manuscript.
To measure 13CO2 production, breath samples were collected into Exetainer tubes at times 0, 1, 2, 3, 4, 5, and 6 h of infusion. Measurements of the total CO2 production rate (VCO2) were conducted at 0, 2, 4, 6, and 8 h of infusion. The subjects received hourly a nutritive formula to maintain a fed state (23). This formula provided an intake of energy and a balanced pattern of amino acids at a rate based on requirements of 0.8 g protein·kg−1·d−1, 126 and 130 kJ·kg−1·d−1(30 and 31 kcal·kg−1·d−1) for women and men, respectively), which equals an hourly protein dose of ~0.03 g/kg with 5.23 and 5.44 kJ·kg−1·d−1 (1.25 and 1.30 kcal·kg−1·d−1) for women and men, respectively.
Kinetic principles and analysis
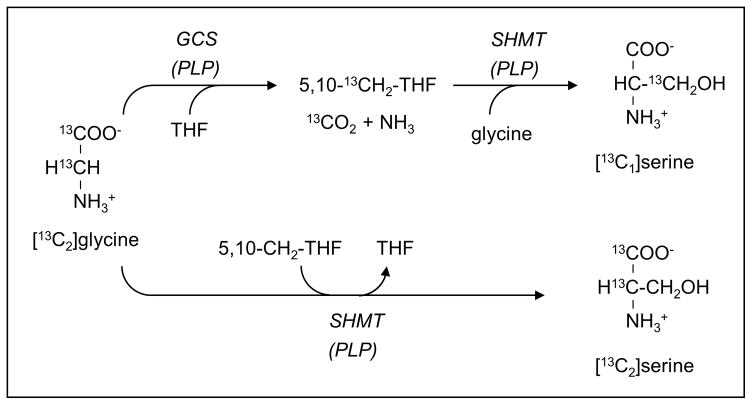
The tracer model is based on the general principle as our previous studies of the role of serine in one-carbon metabolism (8, 19, 24), analogous to that of Schalinske and Steele (25). The combined use of [1,2-13C2]glycine and [2H3]leucine permitted the determination of the kinetics of whole body glycine turnover, the conversion of glycine to serine, the rate of glycine decarboxylation, and its role as a source of one-carbon units in one-carbon metabolism. [2H3]Leucine is included to evaluate any nutritional effects on protein turnover in applications involving dietary interventions (8, 19). As the glycine tracer is decarboxylated and catabolized via the GCS, the original glycine 2-carbon 13C-labeled carbon is transferred to tetrahydrofolate (THF) to yield methyleneTHF. The major focus of this protocol was the determination of the quantitative aspects of glycine decarboxylation, glycine-to-serine interconversion by SHMT (as monitored by the formation of [13C2]serine, and the contribution of glycine-derived one-carbon units (as methyleneTHF) in serine formation via SHMT (as indicated by the formation of [13C1]serine) (Figure 1). We also examined in this protocol the use of glycine-derived one-carbon units for homocysteine remethylation. In this process, the one-carbon unit of methyleneTHF is reduced by 5,10-methylenetetrahydrofolate reductase to yield 5-methylTHF, then transferred to homocysteine via methionine synthase to generate methionine (or [methyl-13C1]methionine if derived in labeled form from the glycine tracer) (8, 24). The rate of remethylation of homocysteine through methionine synthase using a one-carbon unit derived from the glycine tracer (via GCS) provides a measure of the in vivo rate of this pathway.
Figure 1.
Schematic of carbon flow from infused [13C2]glycine to give singly or doubly labeled serine. This is the basis of differentiating whether labeled serine is generated directly from glycine via SHMT (lower pathway yielding [13C2]serine) or via SHMT using a glycine-derived methyleneTHF (upper pathway yielding [13C1]serine).
Plateau enrichments (Ep) for all infused amino acid tracers were calculated as the mean of the isotopic enrichments for the ~1.5-h to 9-h time points for the infused [13C2]glycine and [2H3]leucine tracers. Plateau enrichments of all labeled metabolic products were determined by fitting enrichment data to single exponential curves defined by the equation
| (1) |
In this equation, E is the enrichment at time t (h), while Ef and k are the enrichment at infinity (i.e., plateau enrichment) and rate constant (h−1) from the fitted curve, respectively (26). Data were fit to a single exponential regression equation using the “exponential rise to maximum” function of SigmaPlot 2002 (Version 8.02; SPSS, Inc., Chicago, IL).
Steady state kinetics of amino acid tracers were calculated using standard equations (12), including correction for overestimation of intracellular enrichment from plasma enrichment data (12, 23, 26), as discussed below. The flux of an amino acid is the rate of appearance of that amino acid from endogenous production (de novo synthesis and protein breakdown), absorption, and the tracer infusion, and is calculated from the plateau enrichment of the corresponding amino acid tracer. Specifically, the flux of leucine (QLeu) in the plasma pool is calculated as:
| (2) |
where: ILeu is the [2H3]leucine infusion rate, ELeu is the enrichment of the [2H3]leucine tracer and EpLeu is the plateau enrichment of [2H3]leucine in plasma. The plateau enrichment of plasma leucine was not corrected for overestimation of intracellular enrichment, consistent with previous studies using leucine flux as a relative indicator of protein turnover (19, 24, 27).
Glycine flux (QGly) was calculated from plasma [13C2]glycine enrichment after correcting for the overestimation of the intracellular [13C2]glycine enrichment that occurs when the plasma plateau enrichment of the glycine tracer is used. This prediction of intracellular [13C2]glycine enrichment (Ep′Gly) was accomplished by multiplying the observed plasma [13C2]glycine enrichment by a correction factor of 0.4 derived from previous glycine tracer infusion studies in humans (17, 28).
| (3) |
Flux values for labeled metabolic products, i.e., serine M+1 and serine M+2, are estimated assuming a serine flux of QSer = 271 μmol/(kg·h) (8).
| (4) |
The synthesis rates of metabolic products derived from the infused tracers were calculated from the flux and the plateau enrichment of the infused tracer after correction for intracellular isotopic dilution (8). The rate of serine M+1 synthesis, which was indicative of serine synthesis using a glycine-derived one-carbon unit, was thus calculated as:
| (5) |
In analogous fashion, the rate of serine M+2 synthesis, reflective of direct conversion of glycine to serine, was:
| (6) |
The rate of production of 13CO2 provided a direct measurement of the whole body flux of the decarboxylation of the glycine tracer. This was measured in standard fashion as in amino acid oxidation studies (14, 23). In this procedure, the rate of 13CO2 release (V13CO2, in units of μmol·h−1·kg−1 body weight) and the rate of glycine catabolism via decarboxylation (CGly, μmol·h−1·kg−1 body weight), were calculated as follows:
| (7) |
where: E13CO2 is breath CO2 enrichment plateau, VCO2 is the rate of total CO2 production, and 0.81 is the assumed fraction of CO2 release from the body pool of bicarbonate and W is body weight (14).
| (8) |
where: Ep′Gly is the plateau enrichment of plasma [13C2]glycine corrected for intracellular overestimation and EiGly is the enrichment of the infused glycine tracer. The fraction of glycine flux occurring via glycine decarboxylation was calculated as:
| (9) |
All data are presented as mean ± standard error of the mean (SEM).
RESULTS
The subjects (3 male, 2 female, age 21–28 y) had a body mass index below 25 kg/m2. Their serum folate, vitamin B-12, and plasma PLP concentrations were in the normal range and their plasma total homocysteine (<12 μmol/L) indicated normal one-carbon metabolism.
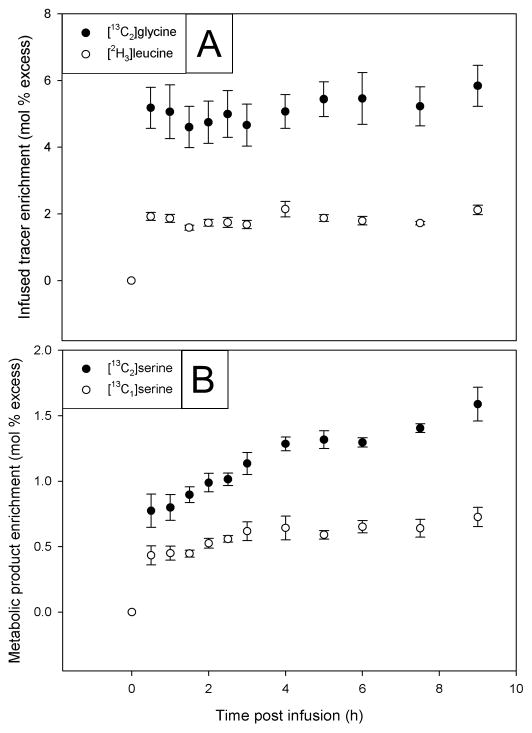
After primed, constant infusion with 9.26 μmol/(kg·h) [13C2]glycine and 1.87 μmol/(kg·h) [2H3]leucine, plasma enrichments of the infused stable isotope labeled amino acids were measured in all subjects (see Figure 3). The plateau enrichment and flux values derived from the infused glycine and leucine tracers and the glycine-derived metabolic products (Figure 1) are shown in Table 1. Using the data of [13C2]serine enrichment, the mean (± SEM) rate of serine synthesis directly from [13C2]glycine via SHMT was 193 ± 28μmol/(kg·h) comprising 41% of the 463 ± 55 μmol/(kg·h) total glycine flux. The rate of serine synthesis via GCS and SHMT using a glycine cleavage-derived one-carbon unit via SHMT (a labeled one-carbon unit coupled to an unlabeled glycine to yield [13C1]serine, i.e., serine M+1) was 88 ± 13 μmol/(kg·h) and thus contributed to 46% of the glycine-to-serine conversion. The enrichment of M+3 serine from coupling of a glycine-derived 13C one-carbon unit with the infused [13C2]glycine was below detection limits (data not shown). Under the conditions of this protocol, the enrichment of [13C1]methionine (i.e., M+1) was typically <0.2 mol % excess, which was below the limit of precise measurement..
Figure 3.
Plasma enrichment of infused amino acids (A) and metabolic products (B) in healthy men and women during primed, constant infusion with 9.26 μmol/(kg·h) [13C2]glycine and 1.87 μmol/(kg·h) [2H3]leucine. Values are means ± SEM, n=5.
Table 1.
Plateau enrichments of stable isotope-labeled amino acids and their metabolic products and the corresponding flux values in healthy men and women 1
| Plateau enrichment (Ep) [mol % excess] | Whole Body Flux (Q) [μmol/(kg·h)] | |
|---|---|---|
| Infused amino acids (AA) | Qinfused AA | |
| [2H3]leucine | 1.82 ± 0.13 | 102 ± 7 |
| [13C2]glycine | 5.10 ± 0.72 | 463 ± 55 |
|
| ||
| Metabolic products | ||
| QGly→Ser M+x | ||
| [13C1]serine (Ser M+1) 2 | 0.63 ± 0.05 | 88 ± 13 |
| [13C2]serine (Ser M+2) 3 | 1.39 ± 0.07 | 193 ± 28 |
| QGly→CO2 | ||
| [13CO2] | 0.037 ± 0.012 | 190 ± 41 |
Values are means ± SEM, n=5.
Serine synthesis via SHMT using a glycine-derived one-carbon unit.
Serine synthesis from glycine via SHMT.
The plateau enrichment of breath 13CO2 after primed, constant glycine infusion rate of 9.26 μmol/(kg·h) was 0.037 ± 0.012 mol % excess (mean ± SEM). This corresponded to a rate of 13CO2 release of 3.6 ± 0.6 μmol/(kg·h), and a rate of glycine catabolism via decarboxylation of 190 ± 41 μmol/(kg·h). This indicates that glycine catabolism generating CO2, which we assumed to be primarily via the GCS, accounted for 39 ± 6% of whole body glycine flux.
The leucine flux (102 ± 7 μmol/(kg·h)) was ~20% higher than that observed in previous studies from this laboratory (e.g., 8, 23), which reflected greater intake of dietary amino acids in the nutritive formula used in this protocol.
DISCUSSION
Sources of 13CO2
This is the first study to quantify human GCS. Using 13CO2 enrichment to enable the calculation of glycine decarboxylation, glycine cleavage accounted for 39% of the whole body glycine flux in these healthy men and women as determined by primed, constant infusion with [13C2]glycine. Although the greatest portion of CO2 release from glycine occurs from glycine decarboxylase activity of the GCS, we acknowledge that CO2 also might be generated by several other processes. That both carbon atoms of glycine ultimately can yield CO2 was demonstrated in studies of patients with inherited enzymatic defects of GCS. In hepatocytes of patients with hyperglycinemia, CO2 formation could be detected after incubation with labeled glycine even though these patients have little or no enzyme activity of GCS (3).
In healthy individuals, CO2 can be formed from glycine through pathways of folate or pyruvate metabolism in addition to decarboxylation in the GCS. One-carbon units derived from the 2-carbon of glycine are transferred to THF forming methyleneTHF which can be enzymatically oxidized to 10-formyl-THF by 5,10-methylenetetrahydrofolate dehydrogenase. 10-formyltetrahydrofolate dehydrogenase converts 10-formylTHF to THF and CO2, which appears to be a mechanism of achieving a regeneration of THF and as a means of regulating the 10-formylTHF pool (29). Recent mathematical modeling predicts that 10-formylTHF dehydrogenase would contribute to CO2 generation at a rate of ~30% of the rate of GCS in hepatic metabolism (30). Thus, the reaction catalyzed by 10-formyl-THF dehydrogenase might contribute partially to 13CO2 formation after [13C2]glycine infusion. However, as glycine is the direct substrate for GCS, we proposed that CO2 formation through the GCS is likely to be substantially faster and quantitatively greater than that from 10-formyl-THF dehydrogenase.
Glycine and serine are interconvertible through SHMT, and serine dehydratase transforms serine to pyruvate which enters the tricarboxylic acid (TCA) cycle either as oxaloacetate or acetyl-CoA. The decarboxylation of pyruvate by pyruvate dehydrogenase to acetyl-CoA and CO2 also could contribute to 13CO2 formation in this protocol. However, the activity of serine dehydratase is lower in humans than other mammalian species (31) and, thus, would contribute minimally extent to CO2 formation from glycine. The report that pyruvate oxidation rate was only ~10 μmol/(kg·min) in healthy subjects (32) is consistent with our assumption that generation of CO2 from glycine by pyruvate oxidation is far less than that from the GCS. An alternative glycine tracer study is planned to quantify the CO2 production rate while distinguishing between its formation by GCS and 10-formylTHF dehydrogenase reactions.
Glycine and Human One-Carbon Metabolism
During glycine decarboxylation, one molecule of methyleneTHF is formed per molecule of CO2 in the GCS. In spite of the potential overestimation of GCS flux in this study, the glycine cleavage rate of 190 μmol/(kg·h) observed in this study implies a high rate of methyleneTHF formation from glycine. MethyleneTHF is used for serine synthesis via SHMT, formation of 10-formylTHF or 5-methylTHF, and the formation of various metabolic products derived from these folates (serine, thymidylate, methionine and purines). In the current protocol, the rate of serine synthesis using a glycine derived methyleneTHF was 88 μmol/(kg·h). Previous studies in this laboratory and others have shown that homocysteine remethylation flux is in the range of only 2–8 μmol/(kg·h) (24). Overall, these data suggest a very substantial flow of carbon units from glycine into other aspects of one-carbon metabolism such as purine and thymidylate synthesis.
The use of [13C2]glycine in this protocol has allowed independent assessment of serine synthesis by SHMT and the fraction of that synthesis specifically using a glycine-derived methyleneTHF. Under the conditions of this protocol, serine formation by SHMT accounted for 41% of whole body glycine flux. Kalhan et al. (33) showed that serine contributes to 15–20% of plasma glycine pool in pregnant and non-pregnant women. A similar ratio of glycine to serine and serine to glycine formation was observed in fetal lamb hepatocytes (6). The net flux through cytosolic SHMT (cSHMT) mainly occurs in the glycine to serine direction (29, 34, 35). However, increases in cellular glycine concentration cause a reversal of the mitochondrial SHMT (mSHMT) from its steady state direction of serine to glycine formation (30, 35) and yield net formation of serine. Serine synthesis is important because of its role as a substrate in the synthesis of glucose via pyruvate, phosphatidylserine, cystathionine, and neuromodulators (36). Serine also serves as the main one-carbon donor for remethylation of homocysteine to methionine (8), although remethylation accounts for a small part in the whole body serine flux.
Aside from providing novel experimental data describing novel quantitative aspects of several phases of human one-carbon metabolism, this and our previous studies (8, 24) also yield experimental confirmation of predictions of mathematical modeling (30). These include: (a) glycine is an important source of one-carbon units to support a high rate of serine synthesis; and (b) homocysteine remethylation from 5-methylTHF via methionine synthase constitutes a small fraction of human one-carbon metabolic flux, as mentioned above. Our experimental study also suggests that the GCS is an important source of glycine-derived one-carbon units needed to support the high steady state fluxes of thymidylate and purine synthesis predicted in mathematical modeling (30).
A single-pool model using plasma amino acid enrichment for calculation of whole body amino acid kinetics often overestimates the whole body amino acid pool. To compensate for such overestimation in the case of plasma glycine enrichment, we employed a correction factor of 0.4 (12, 17, 28, 37). This is based on consensus data from studies using metabolically produced surrogate indicators of intracellular glycine, including urinary hippurate (12) and plasma apolipoprotein B-100 in VLDL (17, 28, 37), both of which reflect the enrichment of hepatic free glycine pools.
The whole body glycine flux in our healthy male and female volunteers was 463 μmol/(kg·h), which is consistent with a report of 458 μmol/(kg·h), also determined at fed state, by Gersovitz et al. (15). Because the flux of an amino acid is the rate of appearance of that amino acid from endogenous production (de novo synthesis and protein breakdown), the tracer infusion, and absorption (12), the intake of amino acids during a tracer infusion increases the amino acid flux. Thus, glycine flux determined at fasting state was only about half of the flux at fed state, 240μmol/(kg·h), as reported by Robert et al. (14). Measurements of glycine metabolism have been shown to be independent of the route of administration (38). Intracellular dilution of glycine enrichment results from glyoxylate metabolism (39), de novo synthesis, protein degradation, and interconversion from serine. Thirty-five percent of systemic flux of glycine occurs from endogenous synthesis (40). Pathways removing glycine from its pool are formation of serine and glutathione, transamination, protein synthesis, and gluconeogenesis.
Summary
This infusion protocol provides new quantitative information about human glycine and one-carbon metabolism. The glycine decarboxylation rate accounted for over a third of whole body glycine flux. This finding of a high rate of glycine degradation via GCS is supported by the symptoms of hyperglycinemia in patients with enzymatic defects in the glycine decarboxylase (1, 4). Nearly half of the methyleneTHF formed by GCS was used for serine formation, with a substantial supply of glycine-derived methyleneTHF available to support other requirements in human one-carbon metabolism. This protocol is being applied in a protocol with additional subjects to investigate glycine kinetics, the sensitivity of glycine metabolism to gender, marginal deficiency of vitamin B-6, and the kinetics of nucleotide and glutathione synthesis.
Abbreviations
- cSHMT
cytosolic serine hydroxymethyltransferase
- Ep
plateau enrichment
- GCS
glycine cleavage system
- I
infusion rate
- mSHMT
mitochondrial serine hydroxymethyltransferase
- Q
a measurement of flux (e.g., glycine flux=Qgly)
- PLP
pyridoxal 5′-phosphate
- SHMT
serine hydroxymethyltransferase
- THF
tetrahydrofolate
- VCO2
rate of CO2 production
- VLDL
very low density lipoproteins
Footnotes
This study was supported by NIH grant DK072398 and GCRC grant M01-RR00082.
Authors disclosures: YL was responsible for study coordination, subject recruitment and screening, data collection and analysis, and primary preparation of manuscript. JW was responsible for data collection and analysis. LRG was responsible for study coordination and subject recruitment and screening. PWS was a coinvestigator and was responsible for experimental design, clinical oversight, and manuscript preparation. JFG was the principal investigator and was responsible for experimental design, oversight of data collection, and manuscript preparation. None of the authors had a personal or financial interest in this research or a conflict of interest of any kind.
Literature cited
- 1.Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005;38:1051–8. doi: 10.1016/j.clinbiochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bailey LB, Gregory JF., 3rd Folate metabolism and requirements. J Nutr. 1999;129:779–82. doi: 10.1093/jn/129.4.779. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973;1:169–87. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- 4.Hiraga K, Kochi H, Hayasaka K, Kikuchi G, Nyhan WL. Defective glycine cleavage system in nonketotic hyperglycinemia. Occurrence of a less active glycine decarboxylase and an abnormal aminomethyl carrier protein. J Clin Invest. 1981;68:525–34. doi: 10.1172/JCI110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kure S, Narisawa K, Tada K. Enzymatic diagnosis of nonketotic hyperglycinemia with lymphoblasts. J Pediatr. 1992;120:95–8. doi: 10.1016/s0022-3476(05)80610-9. [DOI] [PubMed] [Google Scholar]
- 6.Narkewicz MR, Thureen PJ, Sauls SD, Tjoa S, Nikolayevsky N, Fennessey PV. Serine and glycine metabolism in hepatocytes from mid gestation fetal lambs. Pediatr Res. 1996;39:1085–90. doi: 10.1203/00006450-199606000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Thureen PJ, Narkewicz MR, Battaglia FC, Tjoa S, Fennessey PV. Pathways of serine and glycine metabolism in primary culture of ovine fetal hepatocytes. Pediatr Res. 1995;38:775–82. doi: 10.1203/00006450-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., 3rd Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–9. doi: 10.1152/ajpendo.00351.2003. (Erratum 2004;286:E674) [DOI] [PubMed] [Google Scholar]
- 9.Duggleby SL, Waterlow JC. The end-product method of measuring whole-body protein turnover: a review of published results and a comparison with those obtained by leucine infusion. Br J Nutr. 2005;94:141–53. doi: 10.1079/bjn20051460. [DOI] [PubMed] [Google Scholar]
- 10.Sprinson DB, Rittenberg D. The rate of interaction of the amino acids of the diet with the tissue proteins. J Biol Chem. 1949;180:715–26. [PubMed] [Google Scholar]
- 11.Jackson AA, Duggleby SL, Grove G. Whole body protein turnover can be measured non-invasively in women using the end product method with [15N]glycine to show changes with the menstrual cycle and pregnancy. Eur J Clin Nutr. 2000;54:329–36. doi: 10.1038/sj.ejcn.1600958. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine - Principles and Practice of Kinetic Analysis. New York: Wiley Liss; 1992. [Google Scholar]
- 13.Matthews DE, Conway JM, Young VR, Bier DM. Glycine nitrogen metabolism in man. Metabolism. 1981;30:886–93. doi: 10.1016/0026-0495(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 14.Robert JJ, Bier DM, Zhao XH, Matthews DE, Young VR. Glucose and insulin effects on the novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982;31:1210–8. doi: 10.1016/0026-0495(82)90006-3. [DOI] [PubMed] [Google Scholar]
- 15.Gersovitz M, Bier D, Matthews D, Udall J, Munro HN, Young VR. Dynamic aspects of whole body glycine metabolism: influence of protein intake in young adult and elderly males. Metabolism. 1980;29:1087–94. doi: 10.1016/0026-0495(80)90220-6. [DOI] [PubMed] [Google Scholar]
- 16.Yu YM, Yang RD, Matthews DE, Wen ZM, Burke JF, Bier DM, Young VR. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: effects of level of nitrogen and dispensable amino acid intake. J Nutr. 1985;115:399–410. doi: 10.1093/jn/115.3.399. [DOI] [PubMed] [Google Scholar]
- 17.Arends J, Schafer G, Schauder P, Bircher J, Bier DM. Comparison of serine and hippurate as precursor equivalents during infusion of [15N]glycine for measurement of fractional synthetic rates of apolipoprotein B of very-low-density lipoprotein. Metabolism. 1995;44:1253–8. doi: 10.1016/0026-0495(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 18.Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, Battaglia FC. In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res. 1995;37:571–5. doi: 10.1203/00006450-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF., 3rd Deficiencies of folate and vitamin B(6) exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab. 2001;281:E1182–90. doi: 10.1152/ajpendo.2001.281.6.E1182. [DOI] [PubMed] [Google Scholar]
- 20.Parra MD, Martinez JA. Nutritional aspects of breath testing based on stable isotopes. Nutr Rev. 2006;64:338–47. doi: 10.1301/nr.2006.jul.338-347. [DOI] [PubMed] [Google Scholar]
- 21.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr. 1985;342:277–84. doi: 10.1016/s0378-4347(00)84518-1. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 23.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol. 1988;255:E322–31. doi: 10.1152/ajpendo.1988.255.3.E322. [DOI] [PubMed] [Google Scholar]
- 24.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF., 3rd Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–55. doi: 10.1093/ajcn/81.3.648. [DOI] [PubMed] [Google Scholar]
- 25.Schalinske KL, Steele RD. Quantitation of carbon flow through the hepatic folate-dependent one-carbon pool in rats. Arch Biochem Biophys. 1989;271:49–55. doi: 10.1016/0003-9861(89)90254-3. [DOI] [PubMed] [Google Scholar]
- 26.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–55. doi: 10.1152/ajpendo.2001.280.6.E947. [DOI] [PubMed] [Google Scholar]
- 27.Gregory JF, 3rd, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr. 2000;72:1535–41. doi: 10.1093/ajcn/72.6.1535. [DOI] [PubMed] [Google Scholar]
- 28.Cryer DR, Matsushima T, Marsh JB, Yudkoff M, Coates PM, Cortner JA. Direct measurement of apolipoprotein B synthesis in human very low density lipoprotein using stable isotopes and mass spectrometry. J Lipid Res. 1986;27:508–16. [PubMed] [Google Scholar]
- 29.Garcia-Martinez LF, Appling DR. Characterization of the folate-dependent mitochondrial oxidation of carbon 3 of serine. Biochemistry. 1993;32:4671–6. doi: 10.1021/bi00068a027. [DOI] [PubMed] [Google Scholar]
- 30.Nijhout HF, Reed MC, Lam SL, Shane B, Gregory JF, 3rd, Ulrich CM. In silico experimentation with a model of hepatic mitochondrial folate metabolism. Theor Biol Med Model. 2006;3:40. doi: 10.1186/1742-4682-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue HH, Sakaguchi T, Fujie M, Ogawa H, Ichiyama A. Flux of the L-serine metabolism in rabbit, human, and dog livers. Substantial contributions of both mitochondrial and peroxisomal serine:pyruvate/alanine:glyoxylate aminotransferase. J Biol Chem. 1999;274:16028–33. doi: 10.1074/jbc.274.23.16028. [DOI] [PubMed] [Google Scholar]
- 32.Shangraw RE, Jahoor F. Mechanism of dichloroacetate-induced hypolactatemia in humans with or without cirrhosis. Metabolism. 2004;53:1087–94. doi: 10.1016/j.metabol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Kalhan SC, Gruca LL, Parimi PS, O’Brien A, Dierker L, Burkett E. Serine metabolism in human pregnancy. Am J Physiol Endocrinol Metab. 2003;284:E733–40. doi: 10.1152/ajpendo.00167.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lin BF, Kim JS, Hsu JC, Osborne C, Lowe K, Garrow T, Shane B. Molecular biology in nutrition research: modeling of folate metabolism. Adv Food Nutr Res. 1996;40:95–106. doi: 10.1016/s1043-4526(08)60022-4. [DOI] [PubMed] [Google Scholar]
- 35.Kastanos EK, Woldman YY, Appling DR. Role of mitochondrial and cytoplasmic serine hydroxymethyltransferase isozymes in de novo purine synthesis in Saccharomyces cerevisiae. Biochemistry. 1997;36:14956–64. doi: 10.1021/bi971610n. [DOI] [PubMed] [Google Scholar]
- 36.de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. L-serine in disease and development. Biochem J. 2003;371:653–61. doi: 10.1042/BJ20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arends J, Chiu F, Bier DM. Analysis of plasma hippurate in humans using gas chromatography-mass spectrometry: concentration and incorporation of infused [15N]glycine. Anal Biochem. 1990;191:401–10. doi: 10.1016/0003-2697(90)90239-6. [DOI] [PubMed] [Google Scholar]
- 38.Stein TP, Settle RG, Albina JA, Dempsey DT, Melnick G. Metabolism of nonessential 15N-labeled amino acids and the measurement of human whole-body protein synthesis rates. J Nutr. 1986;116:1651–9. doi: 10.1093/jn/116.9.1651. [DOI] [PubMed] [Google Scholar]
- 39.Rowsell EV, Snell K, Carnie JA, Al-Tai AH. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969;115:1071–3. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeds PJ. Dispensable and indispensable amino acids for humans. J Nutr. 2000;130:1835S–40S. doi: 10.1093/jn/130.7.1835S. [DOI] [PubMed] [Google Scholar]