Landscapes affect natural dengue vector control through food webs in both terrestrial and aquatic environments.
Abstract
The epidemiology of vector-borne diseases is governed by a structured array of correlative and causative factors, including landscape (for example, rural versus urban), abiotic (for example, weather), and biotic (for example, food web) factors. Studies of mosquito-borne diseases rarely address these multiple factors at large spatial scales, which limits insights into how human alterations of landscapes and food webs alter mosquito abundance. We used structural equation modeling to identify the relative magnitude and direction of landscape, abiotic, and food web factors on Aedes larvae and adults across 70 sites in northern Thailand. Food web factors were modeled as mosquito-predator trophic cascades. Landscape context affected mosquito-predator communities in aquatic and terrestrial environments via cascading food web interactions. Several mosquito predators within these food webs showed potential as biocontrol agents in mosquito population control, but their potentials for control were landscape-dependent. In terrestrial food webs, the habitat-sensitive tokay gecko structured mosquito-predator communities, indicating that a conservation approach to vector control could be a useful addition to existing control efforts.
INTRODUCTION
Pollution, habitat destruction, and other forms of environmental degradation have often been associated with health risks (1), and as a result, environmental management is now applied in many ways to mitigate diseases, such as cholera and malaria. Nevertheless, many aspects remain poorly explored, including the role of ecological interactions in disease control.
Dengue fever, a mosquito-borne disease transmitted by several species in the genus Aedes Meigen 1818, has been linked to anthropogenic alterations of the environment, such as deforestation, urbanization, and climate change (2–5). Although mosquito control efforts have increased tremendously over the past few decades, dengue fever is still rising (6), with a global estimate of 390 million dengue infections annually (7). One reason for failing control efforts is the lack of a comprehensive understanding of Aedes mosquitoes and their role in the broader ecological context of the food webs, habitats, and landscapes in which they are embedded (8). To develop more effective mosquito control strategies, we need to answer questions such as how do other species interact with Aedes mosquitoes and what environmental factors affect these interactions? The answers will allow us to start to determine how we might control Aedes populations using an environmental management approach (9).
In the context of mosquito control, predator-prey interactions play a particularly important role because they drive community dynamics. Small-scale experimental work shows that numerous predators of Aedes spp. differ in their predation rates and potential impacts on mosquito population density. For instance, we showed that, although both dragonfly and damselfly larvae prey on Aedes aegypti (L. 1762) larvae, their predation rates differed and were dependent on predator body size (10). Further, the few field studies conducted in this system suggest that local habitat conditions and landscape context affect the dynamics of these predator-prey interactions. We showed, for example, that mosquito larval densities in container-like habitats were strongly affected by predator colonization rates, which, in turn, were highly dependent on habitat type and landscape context (11). In general, predation of only the aquatic larval life stages of mosquitoes has been considered; however, recent work shows that terrestrial predators, such as spiders and lizards, can achieve high predation rates on adult mosquitoes (12–16). These adult mosquitoes, occurring largely in urban areas that are densely populated by people, are responsible for disease prevalence and spread.
Here, we aimed to determine the role of predation in Aedes population dynamics by investigating food web trophic cascades in both aquatic and terrestrial environments. We recorded population densities of mosquitoes and their predators near buildings within different habitats and seasons and then modeled the effects of these factors on the aquatic and terrestrial food webs. The terrestrial food web included adult Aedes mosquitoes, house geckos (Hemidactylus spp.), tokay geckos (Gecko gekko, L. 1758), spiders, and domestic cats. The aquatic food web included Aedes larvae and a suite of predators that occur in water-filled, container-like habitats, such as water storage containers, discarded tires, tree holes, and small pools. We used a comprehensive approach to modeling dengue vector mosquitoes by including many predator-prey interactions from both aquatic and terrestrial habitats based on our empirical data set. Before this study, we conducted a series of predation rate experiments and behavioral studies that provide a strong theoretical basis for the predator-prey interactions in our models (table S1) (10–13, 17–19). We used structural equation models to estimate the strength of possible direct and indirect effects within aquatic and terrestrial Aedes-predator food webs while controlling for external factors such as landscape context, seasonality, and habitat characteristics across 70 sites in west Thailand (Fig. 1).
Fig. 1. Path diagrams for the terrestrial and aquatic SEMs.
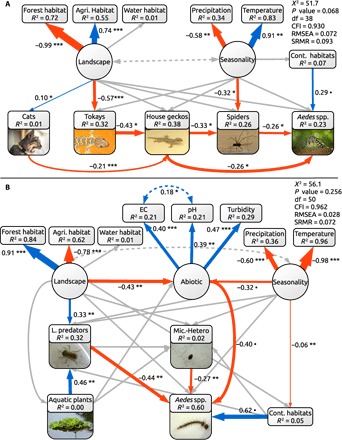
The terrestrial model (n = 70) used a robust maximum likelihood estimator (A). In the aquatic model (n = 156), turbidity (clear/murky), aquatic plants (presence/absence), and Aedes spp. larvae (presence/absence, due to zero inflation) were modeled as binomial variables, using a weighted least squares estimation with robust standard errors (B). R2 values represent variance explained. Model fit was assessed using χ2 statistics, comparative fit index (CFI), root mean square errors of approximation (RMSEA), and standardized root mean square residual (SMRS). Solid arrows display paths from independent to dependent variables, dotted arrows display covariances, circles are latent variables, and rectangles are measured variables. Blue arrows, positive effects; red arrows, negative effects; gray arrows, nonsignificant effects. Effects represent correlations based on the best fitting covariance structure for each model and might be causative, but are not so by definition. Path coefficients (standardized effect sizes) are near each arrow. ***P < 0.001, **P < 0.01, *P < 0.05, and •P < 0.1. “Agri. habitat,” “Water habitat,” and “Forest habitat” are total area of agricultural, surface water, and forested areas in the landscape, which was a 250-m-radius area centered on the focal building. “Precipitation” represents the total monthly precipitation, and “Temperature” represents the monthly (aquatic model) and two-monthly (terrestrial model) mean temperature before sampling. “Cont. habitats” represents the number of container-like habitats in the landscape. EC is electrical conductivity, and pH is the acidity of container habitat water. L. predators, large predators; Mic.-Hetero, micro-Heteroptera.
RESULTS
Aedes were exposed to different predators in the terrestrial and aquatic environments (Fig. 2). In the terrestrial environment, five gecko species were observed on buildings; of these five species, three were very common: Hemidactylus frenatus (Schlegel, 1836), Hemidactylus platyurus (Schneider, 1792), and Gecko gekko. Spider assemblages were dominated by Pholcidae (cellar spiders), mainly Crossopriza lyoni (Blackwall, 1867). Other common spider families included Salticidae (jumping spiders) and Araneidae (orb-weaver spiders). Hersiliidae (tree trunk spiders) were often observed in forest landscapes but were rare in urban and agricultural landscapes. Gecko and spider diversity and richness, as well as spider and tokay abundance, were higher in forest landscapes than in urban and agricultural landscapes (Figs. 3A and 4). In the aquatic environment, many different mosquito larval predators were encountered in water-filled, container-like habitats including predatory mosquito larvae belonging to the genera Toxorhynchites (elephant mosquito) and Lutzia. The most common groups of mosquito larvae (or egg) predators were Anura (tadpoles), Corixidae (water boatmen), Notonectidae (backswimmers), Pleidae (pygmy backswimmers), Toxorhynchites sp., and Veliidae (smaller water striders); within these groups, there was also a large variation in species composition. Fish, usually guppies introduced by humans, were uncommon. Of the six aquatic predator groups, tadpoles, water boatmen, pygmy backswimmers, and Toxorhynchites occurred more frequently in forest landscapes than in urban and agricultural landscapes (Fig. 3B). Aquatic predators were more species-rich in forest landscapes than in urban, but not agricultural, landscapes. Adult Aedes mosquitoes were more common in urban and forest landscapes than in agricultural landscapes (Fig. 3A). Aedes larvae were present in 32% of the container-like habitats; this number was slightly lower in forest landscapes than in agricultural and urban landscapes (Fig. 3B).
Fig. 2. A simplified food web that includes Aedes and its predators in the aquatic and terrestrial environment.
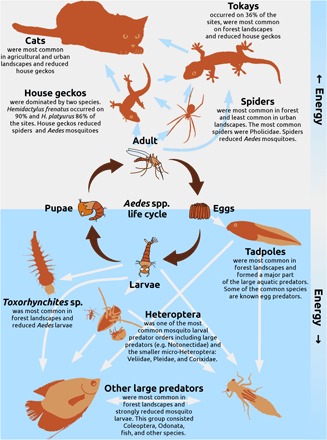
Blue and white arrows show the direction of energy flow from prey to predator. In the center of the figure, the life cycle (dark brown arrows) of Aedes shows that adult female mosquitoes lay eggs, which develop into larvae that will pupate, after which adult mosquitoes will emerge. In these different life cycle stages, Aedes spp. are exposed to different predators in the terrestrial (upper gray area) and aquatic (lower blue area) environments.
Fig. 3. Mean abundance ± SE and % presence of terrestrial and aquatic taxa in or near buildings in three landscape classes.
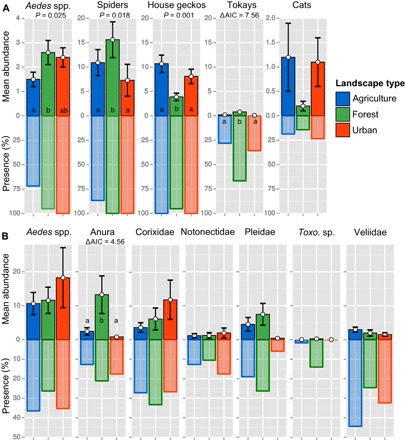
Green bars display forest landscapes, which were those areas in which forest (canopy cover > 30%) constituted 30% or more of the study area. Red bars are urban landscapes (urban areas constitute 25% or more of the landscape), and all other areas are considered agricultural landscapes (blue bars). The sample size (n) for the terrestrial species (A) in agriculture, forest, and urban landscapes was 32, 21, and 17, respectively; for aquatic species (B), this was 63, 57, and 34. Mean abundance of Aedes, house geckos, and spiders was compared using linear models (significant P values are given in the figure) and Tukey’s post hoc test (groups are noted with letters). For other taxa, we used zero-inflated negative binomial models. When AIC values were more than two points higher than the null model, the habitat effect was considered significant and Tukey’s post hoc test was then used on the count and zero-inflated models. Aedes spp. abundance is given as trapped mosquitoes per 24 hours, for spiders, geckos, and tokays; this is the number of individuals per 100-m2 wall, and for cats, this is the number per household. Abundance of aquatic taxa is given as individuals per water-filled container-like habitats.
Fig. 4. Predator taxon diversity and richness for the three main predator groups in different landscape types.
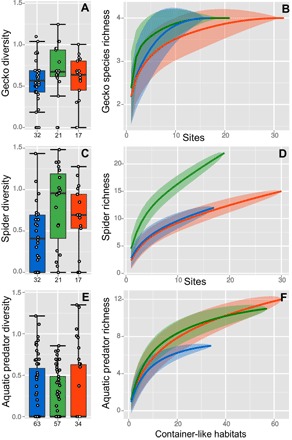
In the boxplots, the horizontal bar gives the median, the box gives the interquartile ranges, the whiskers give the largest and lowest values with a maximum extent of 1.5 times the interquartile range, and the values on the x axis show the sample size (n). Diversity is given as Hill numbers (the exponential of Shannon’s entropy index). Predator richness is given as rarefied curves, which show the increase in recorded taxa with increasing number of observed individuals. Confidence intervals were calculated following Chao’s bootstrap method (30). Curves that are leveling off indicate that predator richness was estimated well by the sampling. When curves do not level off, it is expected that more taxa would have been detected with greater sample sizes. (A) Gecko species diversity on buildings in agricultural (yellow), forest (green), and urban (red) landscapes. (B) Rarefied gecko species richness curves, (C) spider family diversity, (D) rarefied spider family richness curves, (E) aquatic predator diversity (including invertebrate families, Anura, and fish), and (F) rarefied aquatic predator richness curves.
Terrestrial food web model
The terrestrial food web model shows that the species comprising the Aedes food webs near buildings interact indirectly through trophic cascades. Tokay geckos and cats are at the top of this food web, and their abundance was significantly correlated with the abundances of other species through direct and indirect interactions (Fig. 1A and table S2). Both house geckos and spiders were identified as important predators of Aedes mosquitoes; however, geckos prey on spiders and thus have both direct, negative and indirect, positive effects on mosquito abundance. Landscape type (urban, forest, and agricultural) affected the nature of these interactions. Tokay geckos were more abundant in forest landscapes than in urban and agricultural landscapes, which were negatively and nonlinearly related to house gecko densities and therefore were indirectly correlated with an increase in spider densities and total predator diversity, resulting in fewer Aedes mosquitoes in forest landscapes (Fig. 5). In contrast, this keystone predator was mostly absent in urban landscapes, which resulted in higher house gecko abundance and indirectly in lower spider densities, causing a net increase of Aedes mosquitoes (Fig. 5). This variation in predator communities among landscape types was also reflected in mean site predator diversity and predator species richness, where forest landscapes had more diverse and species-rich predator communities than urban and agricultural landscapes (Fig. 4). Seasonality also affected Aedes populations; after periods of increased precipitation and reduced temperature (rainy season), Aedes populations were generally higher (Fig. 1). Seasonal effects were partially mitigated by an increase in spiders, whose abundances also increased under these circumstances.
Fig. 5. Predicted values based on structural equation models.
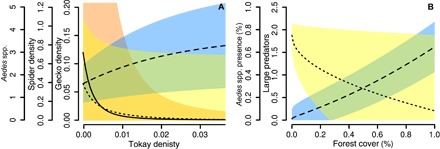
In (A), the change in species abundance as an effect of changes in tokay density is given (individuals per 100 m2). Aedes spp. abundance (trapped individuals per 24 hours) is shown in yellow with a small dashed line, spider density (individuals per 100 m2) is shown in blue with a large dashed line, and gecko density (individuals per 100 m2) is shown in red with a solid line. In (B), the change in species abundance is shown as an effect of change in forest cover. The probability of Aedes spp. presence is shown in yellow with a small dashed line, and the total abundance of large predators is given in blue with a large dashed line. Solid colors represent 95% confidence intervals.
Aquatic food web model
Our aquatic food web model shows that Aedes presence was negatively correlated with the abundance of both large predators, such as tadpoles and backswimmers, and micro-Heteroptera, which included Corixidae (water boatmen), Pleidae (pygmy backswimmers), and Veliidae (smaller water striders). The effect of large predators on Aedes presence was twice that of micro-Heteroptera on Aedes. The effects of landscape context on Aedes presence were indirectly mediated by food web interactions through a trophic cascade involving large aquatic predators (Fig. 1B and table S3). Landscapes that contained a greater area of forest and less agriculture had higher predator abundances, which negatively affected Aedes presence (Fig. 5). Landscape context directly explained variation in abundance of large predators, but not in micro-Heteroptera abundance or Aedes larvae presence. No direct or indirect effects explained a significant amount of variation in micro-Heteropteran abundance. The presence of aquatic plants was positively correlated with large predator abundance, which, in turn, was indirectly negatively correlated with Aedes presence. The number of container-like habitats in the landscape had a relatively large, albeit nonsignificant, effect on Aedes presence.
DISCUSSION
Our findings suggest that Aedes populations are controlled by the interaction between landscape, abiotic, and biotic factors in terrestrial and aquatic habitats. In particular, trophic interactions indirectly mediate complex relationships between dengue vectors and both local- and landscape-scale conditions. These findings are important validation for the few existing small-scale, experimental studies (12, 14–16), showing that mosquito predators in the terrestrial environment are of major importance for the large-scale natural control of mosquitoes. These findings have implications for current, integrated vector management strategies, especially regarding biological control (20), because current practice is focused on the release of aquatic predators in breeding habitats while ignoring the potential of naturally occurring aquatic and terrestrial predators.
Landscape context affects microclimatic conditions at sites and, therefore, the availability of Aedes breeding habitat. Breeding habitat availability and microclimatic conditions are considered major underlying factors of a landscape-Aedes relationship (2, 21). However, our study suggests that the effects of food web processes exceed these factors. Landscape context was related to Aedes populations within both the terrestrial and aquatic food webs, but only via indirect effects mediated through cascading interactions. Landscape context and habitat type have often been reported to be strongly related to Aedes presence, but the underlying mechanism is often simplified to the number of available breeding habitats and their microclimatic conditions (22, 23). Our study suggests that food web context may explain these relationships. We observed, for example, that the abundance of top predators, which were more common in forest landscapes, was related to an increase in total predator richness, which consequently diversified the food web and was further related to lower Aedes populations in both the terrestrial and aquatic systems. Our data did not show an effect of breeding habitats on Aedes in the terrestrial system; however, this effect may have been captured by the inclusion of seasonality in precipitation and temperature because container habitats are largely ephemeral and usually only fill with water during periods of rain as well as by abiotic factors that differ substantially in new breeding habitats, where predators are more likely to be absent.
Aquatic predators are preferentially considered as biocontrol agents for mosquitoes (24) because it is relatively easy to add an aquatic predator to a small, confined container-like habitat. However, we demonstrate here that terrestrial predators are also important for the control of mosquitoes around houses. Geckos and spiders have previously been proposed as potential biological control agents (15, 16), but these suggestions have received little to no attention. The use of terrestrial predators in biological control should be reconsidered because these predators (geckos and spiders) also occur inside buildings, where most of the blood-fed Aedes mosquitoes reside. Aedes mosquitoes that have consumed a blood meal have a higher chance of carrying a dengue virus (blood feeding is the main source of pathogen transmission to the mosquito) and are more likely to reproduce (25). By reducing mosquitoes in these residential environments, where humans are most vulnerable and exposed, the effects of multiple forms of control can be maximized. However, in western-designed buildings, which are sealed for air conditioning, these predators are less common and tend to be unwanted in western cultures due to the risk of salmonella infection, fecal droppings, and poisoning, in the case of certain spider species (25, 26). Hence, the application of biological control using the species in the terrestrial system modeled here may be restricted to the developing world where spiders and geckos are more likely to be present in homes. Where appropriate, a combined terrestrial and aquatic conservation vector control approach would increase the colonization of predators in these habitats and is, therefore, most likely to be successful in the long term. We have previously shown that landscape characteristics, such as land use and habitat isolation, affect the colonization rates of aquatic predators (11). Manipulating the local environment by increasing urban green space, such as gardens and parks, could facilitate colonization by aquatic predators and potentially also terrestrial predators.
Conservation biological control may be one important step toward sustainable and effective management of disease vectors. Through the conservation of natural enemies, vector populations can be reduced by readily available species and cost-effective interventions. For agricultural pests, the idea of conservation-based control methods has been well studied (26) and, in several cases, has already been successfully implemented (27–29). We believe that, within mosquito-predator systems, a similar approach is also likely to be successful. Our data suggest that in these systems (i) mosquito populations are landscape-dependent, with these effects mediated through predators, (ii) predator diversity is important due to the different life stages of mosquitoes, and (iii) colonization rates play a crucial role in the establishment of predator-mosquito communities (11). Although source reduction is one of the most effective, direct measures to reduce mosquito abundance, it is often impossible to completely reduce dengue vectors using source reduction only, especially in developing countries where discarded containers are common. Here, a combination of control measures may lead to a more efficient and effective control of mosquitoes. Our models suggest that Aedes mosquitoes are part of complex food webs that are affected by landscape context through various trophic cascades in both terrestrial and aquatic systems, which are strong indicators that landscape management and conservation biological control have a great deal of potential in the control of Aedes mosquitoes.
MATERIALS AND METHODS
Study area
Data were collected at 70 sites in the Kamphaeng Phet province, northwest Thailand, between February 2014 and October 2014. This period included all three seasons: the end of the cool season (February), the hot season (March to June), and the rainy season (July to October). All sites were centered on one-story buildings with stone walls. All species data were collected from all the walls of each focal building or in close proximity to it (<1 m) during a 3-day period.
Species data
Mosquitoes were collected using baited BG-Sentinel traps (Biogents) (31). These traps were deployed on three different sides of each focal building for 24 hours spread over a 2-day period, resulting in a total of 72 trapping hours per site. Every 24 hours, traps were emptied and mosquitoes and other insects were transported to the laboratory. In some cases, traps were compromised by rain or wind. These values were removed from the data, after which the total number of Aedes mosquitoes per site (building) was averaged over a 24-hour period. Because of physical damage, it was not possible to identify all mosquitoes to species level. Therefore, all mosquitoes belonging to the genus Aedes were used for analysis. For specimens that could be identified to species (n = 255 of 415 individuals), 91% belonged to either A. aegypti (67%) or Aedes albopictus (25%), which was considered sufficient to justify this generalization to the family level. A. aegypti and A. albopictus differ in some aspects of their ecology, such as habitat preferences and larval competition (25), and so, because we did not differentiate these species, any separate effects of these species within the food web will be confounded. However, they are both diurnal species, select similar breeding habitats, feed mostly on human hosts, generally only fly short distances, and are the main vectors of dengue fever (25); therefore, the effect on the overall conclusions is likely to be minimal.
All spiders that were present on all the outer walls and overhangs were counted during a single survey and identified to species where possible. Afterward, the walls and overhangs were measured to calculate the total surface area, which was used to calculate the spider density and allowed comparison of densities among different buildings. Geckos were counted half an hour after dusk. House geckos are attracted to artificial lights; therefore, we placed a 12-V light near a wall of the focal building, lit 30 min before counting, to standardize the light among buildings. All geckos were identified to species level. Geckos were grouped into two categories: house geckos (mainly Hemidactylus species) and the larger tokays (Gekko gecko). Species counts were divided by the total sampling surface to calculate density. The number of cats that were residents of the focal building was determined by a short inquiry with residents/landlords.
Bats (Chiroptera) were also included as a predator in the terrestrial system. We used an ultrasound bat detector (Batcon) with digital recorder to record echolocation calls of bats. We recorded 5 min of echolocation calls at 7 to 15 random locations (depending on weather) around the focal building and repeated this for three nights. Recordings were later analyzed, and, where possible, the species was identified using an existing databank for local species. A preliminary analysis of the first 30 sites showed no signs of a relationship between bat feeding activity and Aedes abundance; therefore, sampling was discontinued and bats were not included in the study further.
Mosquito larvae and aquatic predators were sampled across a variety of container-like aquatic habitats within a 250-m-radius area centered on the focal buildings. A total of 154 container-like aquatic habitats were sampled within these areas by emptying the complete content into a sieve (400-μm mesh). All aquatic fauna was identified to order level with the exception of Heteroptera, which were identified to family, and Culicidae, which were identified to genus. For later analysis, aquatic predators were divided into two groups: large predators and micro-Heteroptera. Micro-Heteroptera contained all species from the families Corixidae, Pleidae, and Veliidae, and large predators included all other predators such as Coleoptera, Odonata, Diplonychus spp., fish, Gerridae, Anura, Notonectidae, Lutzia spp., Naucoridae, Ranatra spp., and Toxorhynchites spp. We created these two groups based on strong allometric effects within the order of Heteroptera with regard to mosquito predation (18). Mosquito larvae were identified using the Illustrated Keys to the Mosquitoes of Thailand I–VI. Acidity was measured using pH paper (Merck KGaA). EC and temperature were measured using an EC meter (Mettler Toledo FG3-I). Turbidity was visually assessed and categorized into two classes: clear or cloudy.
Seasonality
Daily precipitation and temperature data were retrieved from the Tropical Rainfall Measurement Mission (~25-km resolution) and Terra MODIS satellite (1-km resolution), respectively (online Data Pool, courtesy of the NASA Land Processes Distributed Active Archive Center). These data were used to calculate variables that represent weather conditions over a certain period before each sampling date (1-, 2-, 4-, and 8-week mean temperature and total precipitation). During the preliminary analysis, the 4-week mean temperature explained most variation in the species data for the aquatic food web, and the 8-week mean temperature explained most variation in the terrestrial food web and was therefore used in the respective models. For precipitation, this was the 4-week total precipitation for both food webs. For each site, the temperature and precipitation values were used that corresponded to the location of the centered building.
Analysis
Food webs are systems in which a set of species forms a complex network of feeding relationships (32). To assess the effects of habitat type on the predator-prey interactions of adult Aedes mosquitoes and multiple predators, it is important to evaluate the complete network. For example, certain predator species may reduce mosquito densities but simultaneously feed on other predator species. Hence, the indirect effect (predation of predators) mitigates the direct effect (mosquito predation). Therefore, the total effect of the predator on mosquito populations is a combination of its direct and indirect effect. Our models were focused on predator-prey relationships, and we therefore did not include any competitive interactions in the food webs. However, a Culex-Aedes competitive relationship could have played a role in the aquatic system because they often co-occurred (33, 34). Nevertheless, Aedes is a superior competitor (33, 34), and EC, which correlates with food availability (35), was not significantly related to Aedes presence. We therefore conclude that a competitive interaction would not have strongly affected our models.
Structural equation modeling (SEM) is a multivariate technique that allows modeling of both direct and indirect effects using observed, as well as unobserved or “latent,” variables (36, 37). The latent variables not only generally encompass unmeasurable parameters, such as seasonality or habitat, but also can quantify unmeasured interactions between species in food webs (36). SEM has already been applied on several occasions to food webs, including systems with mosquito larvae (38–40). Modeling these complete ecological networks in this manner results in a better understanding of system processes (36).
Before the analyses, a hypothetical framework was created containing all hypothesized relationships (Fig. 1) (36). Relationships were based on a series of predator-prey experiments that we conducted previously (2, 11–13, 17–19) and on existing literature (table S1). This framework was then tested using SEM. This method is referred to as “confirmatory factor analysis” (36). Changes in this framework should be minimized (36). SEM analyzes the network of relationships by implying a set of covariances with which it reproduces the observed covariance matrix by estimating model parameters (36). When the observed covariance matrix and estimated covariances are not significantly different following a χ2 test, the model is considered a good fit to the data.
We started modeling all individual paths in the SEM using linear models based on a priori hypothesized trophic interactions. In the terrestrial model, some relationships between predators and prey were nonlinear because of density dependency. We developed several equations using nonlinear least square regression to linearize such relationships. Of these equations, those of the form resulted in the best fit based on the lowest AIC (Akaike information criterion) score. The response variables in the linearized predator-prey relationships were square root–transformed to reduce heteroscedasticity in the nonlinear relationships.
A robust maximum likelihood estimator was used, where normality was not achieved (36). In the aquatic model (n = 156), turbidity (clear/murky), aquatic plants (presence/absence), and Aedes spp. larvae (presence/absence, due to zero inflation) were modeled as binomial variables, using a weighted least squares estimation with robust maximum likelihood estimator (41).
The terrestrial model included two latent variables: “landscape” and “seasonality.” The landscape (250-m-radius sampling areas) variable was based on several component factors, including the amount of forest habitat, agricultural habitat, and water habitat (lakes, rivers, and canals). The seasonality variable was based on two component variables: temperature and precipitation. The aquatic model also included a latent variable representing the abiotic characteristics of the habitat including EC, acidity, and turbidity. All analyses were conducted in RStudio version 0.96.331 built on R 3.0.2 using the “lavaan” package (42).
Supplementary Material
Acknowledgments
We would like to thank B. Case, M. Lau, A. Ellison, J. Tylianakis, J. Pannell, A. Paterson, T. Curran, M. Kovalenko, and four anonymous reviewers for feedback on the structural equation models and/or the manuscript; S. Kulthing and S. Wiengdao (Department of National Park, Wildlife and Plant Conservation) for allowing access to Khong Lan and Mae Wong National Park; P. Kaennark (head of the Kamphaeng Phet Silvicultural Research Station) for granting permission to conduct research on their premises; the Armed Forces Research Institute of Medical Sciences Kamphaeng Phet for training in mosquito identification; and C. Surin, C. Kongmuang, S. Koenraadt, F. P. Groothuis, M. Lehmann, G. Tkaczenko, T. Kaennark, P. Weterings, and A. Fischer for any additional help. Funding: This study was jointly funded through the Faculty of Natural Resources and Environment, Naresuan University and the Cat Drop Foundation. Author contributions: R.W., C.U., and H.L.B. designed the study. R.W. conducted the fieldwork. R.W. analyzed the data. H.L.B. reviewed the analysis. R.W. wrote the manuscript. C.U. and H.L.B. reviewed drafts of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper are archived on Figshare and can be found under DOI:10.6084/m9.figshare.5782428.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/eaap9534/DC1
table S1. Theoretical framework used to develop the hypothetical a priori models.
table S2. Parameter estimates (Est.) for all direct and indirect effects in the terrestrial structural equation model.
table S3. Parameter estimates (Est.) for all direct and indirect effects in the aquatic structural equation model.
REFERENCES AND NOTES
- 1.Patz J. A., Daszak P., Tabor G. M., Alonso Aguirre A., Pearl M., Epstein J., Wolfe N. D., Marm Kilpatrick A., Foufopoulos J., Molyneux D., Bradley D. J.; Members of the Working Group on Land Use Change Disease Emergence , Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 112, 1092–1098 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanwambeke S. O., Lambin E. F., Eichhorn M. P., Flasse S. P., Harbach R. E., Oskam L., Somboon P., van Beers S., van Benthem B. H. B., Walton C., Butlin R. K., Impact of Land-use change on dengue and malaria in northern Thailand. Ecohealth 4, 37–51 (2007). [Google Scholar]
- 3.Wu P. C., Lay J. G., Guo H. R., Lin C. Y., Lung S. C., Su H. J., Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 407, 2224–2233 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Tipayamongkholgul M., Lisakulruk S., Socio-geographical factors in vulnerability to dengue in Thai villages: A spatial regression analysis. Geospat. Health 5, 191–198 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Hales S., de Wet N., Maindonald J., Woodward A., Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 360, 830–834 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Murray N. E. A., Quam M. B., Wilder-Smith A., Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 5, 299–309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., Drake J. M., Brownstein J. S., Hoen A. G., Sankoh O., Myers M. F., George D. B., Jaenisch T., William Wint G. R., Simmons C. P., Scott T. W., Farrar J. J., Hay S. I., The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis B. R., Wilcox B. A., The ecological dimensions of vector-borne disease research and control. Cad. Saude Publica 25 (suppl. 1), S155–S167 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Moore S. M., Borer E. T., Hosseini P. R., Predators indirectly control vector-borne disease: Linking predator–prey and host–pathogen models. J. R. Soc. Interface 7, 161–176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weterings R., Umponstira C., Buckley H. L., Predation rates of mixed instar Odonata naiads feeding on Aedes aegypti and Armigeres moultoni (Diptera: Culicidae) larvae. J. Asia Pac. Entomol. 18, 1–8 (2015). [Google Scholar]
- 11.Weterings R., Umponstira C., Buckley H. L., Container-breeding mosquitoes and predator community dynamics along an urban-forest gradient: The effects of habitat type and isolation. Basic Appl. Ecol. 15, 486–495 (2014). [Google Scholar]
- 12.Weterings R., Umponstira C., Buckley H. L., Predation on mosquitoes by common Southeast Asian house-dwelling jumping spiders (Salticidae). Arachnology 16, 122–127 (2014). [Google Scholar]
- 13.Tkaczenko G. K., Fischer A. C., Weterings R., Prey preference of the Common House Geckos Hemidactylus frenatus and Hemidactylus platyurus. Herpetol. Notes 7, 483–488 (2014). [Google Scholar]
- 14.Reiskind M. H., Wund M. A., Experimental assessment of the impacts of northern long-eared bats on ovipositing Culex (Diptera : Culicidae) mosquitoes. J. Med. Entomol. 46, 1037–1044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canyon D. V., Hii J. L. K., The gecko: An environmentally friendly biological agent for mosquito control. Med. Vet. Entomol. 11, 319–323 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Strickman D., Sithiprasasna R., Southard D., Bionomics of the spider, Crossopriza lyoni (Araneae, Pholcidae), a predator of dengue vectors in Thailand. J. Arachnol. 25, 194–201 (1997). [Google Scholar]
- 17.Weterings R., Tadpoles of three common anuran species from Thailand do not prey on mosquito larvae. J. Vector Ecol. 40, 230–232 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Weterings R., Umponstira C., Buckley H. L., Density-dependent allometric functional response models. Ecol. Modell. 303, 12–18 (2015). [Google Scholar]
- 19.Weterings R., Vetter K. C., Umponstira C., Factors influencing the predation rates of Anisops breddini (Hemiptera: Notonectidae) feeding on mosquito larvae. J. Entomol. Acarol. Res. 46, 107–111 (2014). [Google Scholar]
- 20.M. A. Rodríguez-pérez, A. F. V. Howard, F. Reyes-Villanueva, in Integrated Pest Management and Pest Control—Current and Future Tactics, M. L. Larramendy, S. Soloneski, Eds. (InTech, 2012), pp. 241–270. [Google Scholar]
- 21.Vallorani R., Angelini P., Bellini R., Carrieri M., Crisci A., Mascali Zeo S., Messeri G., Venturelli C., Temperature characterization of different urban microhabitats of Aedes albopictus (Diptera Culicidae) in central–northern Italy. Environ. Entomol. 44, 1182–1192 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Kamara F., Zhou G., Puthiyakunnon S., Li C., Liu Y., Zhou Y., Yao L., Yan G., Chen X.-G., Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLOS Negl. Trop. Dis. 8, e3301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delatte H., Toty C., Boyer S., Bouetard A., Bastien F., Fontenille D., Evidence of habitat structuring Aedes albopictus populations in Réunion Island. PLOS Negl. Trop. Dis. 7, e2111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaalan E. A.-S., D. V Canyon, Aquatic insect predators and mosquito control. Trop. Biomed. 26, 223–261 (2009). [PubMed] [Google Scholar]
- 25.A. N. Clements, The Biology of Mosquitoes: Volume 2, Sensory Reception and Behaviour (Chapman & Hall, ed. 1, 1999). [Google Scholar]
- 26.Tscharntke T., Bommarco R., Clough Y., Crist T. O., Kleijn D., Rand T. A., Tylianakis J. M., van Nouhuys S., Vidal S., Reprint of “Conservation biological control and enemy diversity on a landscape scale” [Biol. Control 43 (2007) 294–309]. Biol. Control 45, 238–253 (2008). [Google Scholar]
- 27.Aguilar-Fenollosa E., Pascual-Ruiz S., Hurtado M. A., Jacas J. A., Efficacy and economics of ground cover management as a conservation biological control strategy against Tetranychus urticae in clementine mandarin orchards. Crop Prot. 30, 1328–1333 (2011). [Google Scholar]
- 28.L. R. Baggen, G. M. Gurr, A. Meats, in Hymenoptera: Evolution, Biodiversity and Biological Control, A. D. Austin, M. Dowton, Eds. (CSIRO Publishing, 2000), pp. 388–395. [Google Scholar]
- 29.Van Alebeek F., Vijn M., Willemse J., Van Rijn P., A more beautiful landscape with less pests (Translated from Dutch). Ekoland 5, 26–27 (2012). [Google Scholar]
- 30.Chao A., Gotelli N. J., Hsieh T. C., Sander E. L., Ma K. H., Colwell R. K., Ellison A. M., Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014). [Google Scholar]
- 31.Williams C. R., Long S. A., Webb C. E., Bitzhenner M., Geier M., Russell R. C., Ritchie S. A., Aedes aegypti population sampling using BG-Sentinel traps in north Queensland Australia: Statistical considerations for trap deployment and sampling strategy. J. Med. Entomol. 44, 345–350 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Bascompte J., Disentangling the web of life. Science 325, 416–419 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Costanzo K. S., Muturi E. J., Lampman R. L., Alto B. W., The effects of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 48, 29–38 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Carrieri M., Bacchi M., Bellini R., Maini S., On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 32, 1313–1321 (2003). [Google Scholar]
- 35.Yadav P., Foster W. A., Mitsch W. J., Grewal P. S., Factors affecting mosquito populations in created wetlands in urban landscapes. Urban Ecosyst. 15, 499–511 (2012). [Google Scholar]
- 36.J. B. Grace, Structural Equation Modeling and Natural Systems (Cambridge Univ. Press, ed. 1, 2006). [Google Scholar]
- 37.B. McCune, J. B. Grace, Analysis of Ecological Communities (MjM Software Design, ed. 1, 2002). [Google Scholar]
- 38.Arhonditsis G. B., Stow C. A., Steinberg L. J., Kenney M. A., Lathrop R. C., McBride S. J., Reckhow K. H., Exploring ecological patterns with structural equation modeling and Bayesian analysis. Ecol. Model. 192, 385–409 (2006). [Google Scholar]
- 39.Gotelli N. J., Ellison A. M., Food-web models predict species abundances in response to habitat change. PLOS Biol. 4, e324 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baiser B., Ardeshiri R. S., Ellison A. M., Species richness and trophic diversity increase decomposition in a co-evolved food web. PLOS ONE 6, e20672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A. A. Beaujean, Latent Variable Modeling Using R: A Step-by-Step Guide (Routledge/Taylor & Francis, 2014). [Google Scholar]
- 42.Rosseel Y., lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012). [Google Scholar]
- 43.Aldstadt J., Koenraadt C. J. M., Fansiri T., Kijchalao U., Richardson J., Jones J. W., Scott T. W., Ecological modeling of Aedes aegypti (L.) pupal production in rural Kamphaeng Phet, Thailand. PLOS Negl. Trop. Dis. 5, e940 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chadee D. D., Aedes aegypti surveillance in Tobago, West Indies (1983-88). J. Am. Mosq. Control Assoc. 6, 148–150 (1990). [PubMed] [Google Scholar]
- 45.Scott T. W., Morrison A. C., Lorenz L. H., Clark G. G., Strickman D., Kittayapong P., Zhou H., Edman J. D., Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Population dynamics. J. Med. Entomol. 37, 77–88 (2000). [DOI] [PubMed] [Google Scholar]
- 46.G. W. Uetz, Habitat structure and spider foraging, in Habitat Structure: The Physical Arrangement of Objects in Space, S. S. Bell, E. D. McCoy, H. R. Mushinsky, Eds. (Springer, ed. 1, 1991), pp. 325–348. [Google Scholar]
- 47.Lubin Y. D., Seasonal abundance and diversity of web-building spiders in relation to habitat structure on Barro Colorado Island, Panama. J. Arachnol. 6, 31–51 (1978). [Google Scholar]
- 48.Zug G. R., Vindum J. V., Koo M. S., Burmese Hemidactylus (Reptilia, Squamata, Gekkonidae): Taxonomic notes on tropical Asian Hemidactylus. Proc. Calif. Acad. Sci. 58, 387–405 (2007). [Google Scholar]
- 49.Newbery B., Dawson P., Jones D. N., Density of Asian house geckos Hemidactylus frenatus within suburban Brisbane. Queensl. Nat. 43, 8–13 (2005). [Google Scholar]
- 50.Aowphol A., Nabhitabhata J., Thirakhupt K., Voris H. K., Foraging ecology of the tokay gecko, Gekko gecko in a residential area in Thailand. Amphibia-Reptilia 27, 491–503 (2006). [Google Scholar]
- 51.Bucol A., Alcala A., Tokay gecko, Gekko gecko (Sauria: Gekkonidae) predation on juvenile house rats. Herpetol. Notes 6, 307–308 (2013). [Google Scholar]
- 52.Tang D.-Y., Liang Q., Liu S. L., Observation on the behaviour and habits of tokays (Gekko gecko Linnaeus). J. Sci. Med. Jinan Univ. 1985, 67–72 (1985). [Google Scholar]
- 53.Bobrov V. V., Spatial organization of a tropical lizard community in a forested area in northern Vietnam. Herpetozoa 6, 21–28 (1993). [Google Scholar]
- 54.Tang Y.-Z., Zhuang L.-Z., Wang Z.-W., Advertisement calls and their relation to reproductive cycles in Gekko gecko (Reptilia, Lacertilia). Copeia 2001, 248–253 (2001). [Google Scholar]
- 55.Gillies C., Clout M., The prey of domestic cats (Felis catus) in two suburbs of Auckland City, New Zealand. J. Zool. 259, 309–315 (2003). [Google Scholar]
- 56.Case T. J., Bolger D. T., Petren K., Invasions and competitive displacement among house geckos in the tropical Pacific. Ecology 75, 464–477 (1994). [Google Scholar]
- 57.Kirkpatrick R. D., Rauzon M. J., Foods of feral cats Felis catus on Jarvis and Howland Islands, Central Pacific Ocean. Biotropica 18, 72–75 (1986). [Google Scholar]
- 58.A. N. Clements, The Biology of Mosquitoes: Volume 1, Development, Nutrition and Reproduction (CABI, ed. 2, 2000). [Google Scholar]
- 59.Kulshrestha U. C., Kulshrestha M. J., Sekar R., Sastry G. S. R., Vairamani M., Chemical characteristics of rainwater at an urban site of south-central India. Atmos. Environ. 37, 3019–3026 (2003). [Google Scholar]
- 60.Fischer S., Pereyra D., Fernández L., Predation ability and non-consumptive effects of Notonecta sellata (Heteroptera: Notonectidae) on immature stages of Culex pipiens (Diptera: Culicidae). J. Vector Ecol. 37, 245–251 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Blaustein L., Influence of the predatory backswimmer, Notonecta maculata, on invertebrate community structure. Ecol. Entomol. 23, 246–252 (1998). [Google Scholar]
- 62.Saha N., Aditya G., Saha G. K., Habitat complexity reduces prey vulnerability: An experimental analysis using aquatic insect predators and immature dipteran prey. J. Asia Pac. Entomol. 12, 233–239 (2009). [Google Scholar]
- 63.Sih A., Foraging strategies and the avoidance of predation by an aquatic insect, aquatic insect, Notonecta hoffmanni. Ecology 63, 786–796 (1982). [Google Scholar]
- 64.Bowatte G., Perera P., Senevirathne G., Meegaskumbura S., Meegaskumbura M., Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol. Control. 67, 469–474 (2013). [Google Scholar]
- 65.Mokany A., Shine R., Biological warfare in the garden pond: Tadpoles suppress the growth of mosquito larvae. Ecol. Entomol. 28, 102–108 (2003). [Google Scholar]
- 66.Berry P. Y., The breeding patterns of seven species of Singapore Anura. J. Anim. Ecol. 33, 227–243 (1964). [Google Scholar]
- 67.Blaustein A. R., Wake D. B., Declining amphibian populations: A global phenomenon? Trends Ecol. Evol. 5, 203–204 (1990). [Google Scholar]
- 68.Ohba S.-y., Huynh T. T. T., Kawada H., Le L. L., Ngoc H. T., Hoang S. L., Higa Y., Takagi M., Heteropteran insects as mosquito predators in water jars in southern Vietnam. J. Vector Ecol. 36, 170–174 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Amrapala C., Sitthicharoenchai D., Thavara U., Tawatsin A., Chittihunsa T., Feeding ability of Micronecta grisea nymphal instars and adults on third instar Aedes aegypti larvae. Nat. Hist. J. Chulalongkorn Univ. 9, 189–200 (2009). [Google Scholar]
- 70.Miura T., Takahashi R., Predation of Microvelia pulchella (Hemiptera: Veliidae) on mosquito larvae. J. Am. Mosq. Control Assoc. 4, 91–93 (1988). [PubMed] [Google Scholar]
- 71.Andersen N. M., Yang C. M., Zettel H., Guide to the aquatic Heteroptera of Singapore and Peninsular Malaysia. -2. Veliidae. Raffles Bull. Zool. 50, 231–249 (2002). [Google Scholar]
- 72.Nieser N., Guide to aquatic Heteroptera of Singapore and peninsular Malaysia III. Pleidae and Notonectidae. Raffles Bull. Zool. 52, 79–96 (2004). [Google Scholar]
- 73.Focks D. A., Toxorhynchites as biocontrol agents. J. Am. Mosq. Control Assoc. 23, 118–127 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Campos R. E., Lounibos L. P., Life tables of Toxorhynchites rutilus (Diptera: Culicidae) in nature in southern Florida. J. Med. Entomol. 37, 385–392 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Steffan W. A., Evenhuis N. L., Biology of Toxorhynchites. Annu. Rev. Entomol. 26, 159–181 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/eaap9534/DC1
table S1. Theoretical framework used to develop the hypothetical a priori models.
table S2. Parameter estimates (Est.) for all direct and indirect effects in the terrestrial structural equation model.
table S3. Parameter estimates (Est.) for all direct and indirect effects in the aquatic structural equation model.


