Abstract
The International Agency for Research on Cancer (IARC) and the US National Cancer Institute (NCI) have initiated a series of cancer-focused seminars [Scelo G, Hofmann JN, Banks RE et al. International cancer seminars: a focus on kidney cancer. Ann Oncol 2016; 27(8): 1382–1385]. In this, the second seminar, IARC and NCI convened a workshop in order to examine the state of the current science on esophageal squamous cell carcinoma etiology, genetics, early detection, treatment, and palliation, was reviewed to identify the most critical open research questions. The results of these discussions were summarized by formulating a series of ‘difficult questions’, which should inform and prioritize future research efforts.
Keywords: esophageal squamous cell carcinoma (ESCC), epidemiology, genomics, early detection, clinical research
Introduction
Esophageal cancer is the eighth most common cancer worldwide, and the sixth most common cause of cancer death [1,2]. It has two dominant histologic types: esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC). In 2012, there were an estimated 398 000 new ESCC cases worldwide, representing 87% of all esophageal cancer [3].
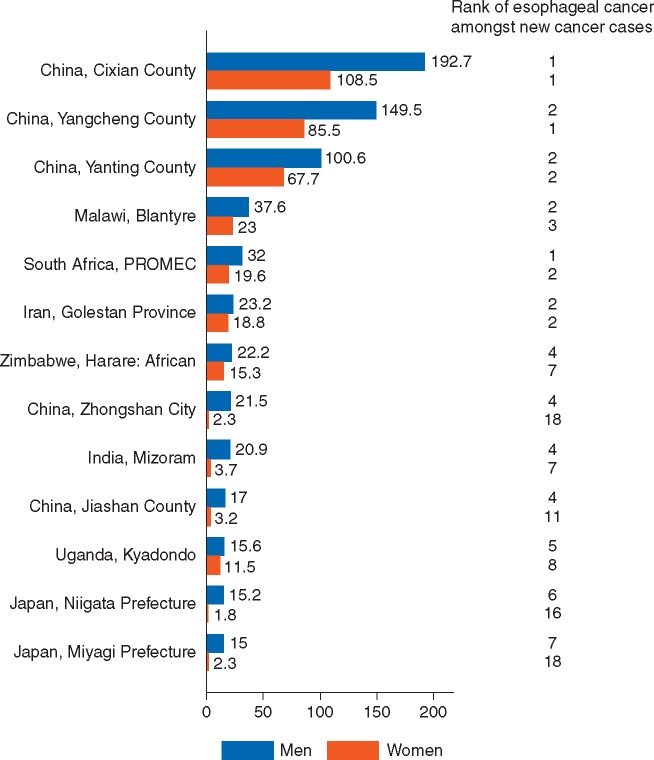
The geographic distribution of ESCC is one of the most uneven of all cancers, with high incidence regions stretching across Asia from China to Iran, from eastern to southern Africa, and in parts of southern South America (Figure 1) [3]. Recognized ESCC hotspots, past and present, exist within sharply defined regions including Linxian China, Golestan Province in Iran, Western Kenya south to Malawi, the Eastern Cape province of South Africa, Calvados in France, Southern Brazil and Uruguay [3]. The burden of ESCC is particularly striking in these high incidence areas: in Cinxian county, China, esophageal cancer is the most common cancer in both men and women, in South Africa it is the most common cancer in men, second most common in women (Figure 2) [4].
Figure 1.
Incidence of esophageal squamous cell carcinoma (age standardized rates, per 100 000) for females (A) and males (B); GLOBOCAN 2012.
Figure 2.
Cancer incidence in five continents, Vol. X: Registries with age-standardized incidence in either gender of at least 15 per 100 000.
ESCC is also marked by striking etiologic heterogeneity. In low and medium incidence populations ESCC is largely attributable to smoking and alcohol and incidence rates are three to four times higher in men than in women. This was also true of historically highly affected areas such as Calvados and France [5]. In the USA, population attributable risks as high as 89% have been reported for ESCC, based on smoking habits and consumption of alcohol, fruit, and vegetables [6], with similar attributable risks reported for esophageal cancer death from the UK (89%) [7], Nordic countries (89%) [8], and France (74%) [9]. In contrast, tobacco use and alcohol consumption are less common and less intensely practiced and play a lesser role in the etiology of ESCC in high incidence areas in Asia [10]. Similar studies in China have estimated that 46% of esophageal cancer deaths are attributable to the combined effects of smoking [11, 12], drinking alcohol, and low fruit and vegetable intake [13]. Most high incidence regions also have male : female ratios that approach 1 : 1 [14].
ESCC research is made particularly compelling because of the rapidly fatal course of the cancer, the late stage presentation juxtaposed with the important contribution of modifiable risk factors. Five-year survival rates for esophageal cancer are 20% in the USA [15], 21% in China [16], 12% in Europe [17], and <5% in the lower resource settings [18, 19] suggesting that primary and secondary prevention are key to reducing mortality from this disease.
Etiologic risk factors
ESCC in Europe and in the US are predominantly driven by alcohol and tobacco, which have a strong synergistic effect on ESCC risk [5]. The Calvados region of Northern France exhibited exceptionally high rates in the past, although these are now moderate. The cause for the past high incidence rates is largely due to the heavy consumption of a particular type of brandy, that was often distilled at home and drunk hot [20]. The existence of high incidence regions in Asia and Africa [21] are more difficult to explain, since many suspected risk factors are also prevalent elsewhere, suggesting the presence of locally operating risk factors yet to be identified and/or the likely co-occurrence of multiple interacting factors. Nonetheless, a number of risk factors consistently associated with ESCC in high incidence regions have been identified, many of which are often present in the lower socioeconomic groups most affected by the disease.
In addition to tobacco use and alcohol consumption, the International Agency for Research on Cancer (IARC) monograph series have identified several exposures as causally linked to ESCC including acetaldehyde from alcoholic beverages [22], betel quid with or without tobacco [23], and X- and gamma-radiation [23].
The consumption of hot foods and beverages has been suggested to be an etiologic factor for ESCC across diverse populations in China, Iran, Brazil, and Tanzania [24–27]. Hot beverages were recently classified as probably carcinogenic to the esophagus in the IARC Monograph Series [28]. Maté is a herbal infusion, that is popular in southern Brazil, Uruguay, Paraguay, and Argentina, and traditionally it is drunk very hot (>65 °C). Maté consumed ‘hot’ or ‘very hot’ [26] has been associated with an increased risk of ESCC [29]. Elevated risk of ESCC has also been linked to the consumption of very hot tea and other beverages [24, 25, 27, 28], however, a paucity of published data led the IARC Working Group to conclude that there is limited evidence in humans for the carcinogenicity of drinking very hot beverages, and inadequate evidence in humans to evaluate the carcinogenicity of drinking maté that is not very hot.
Poor diet has been suggested to play a role in ESCC risk, and studies have investigated the effects of specific foods, nutrients, or dietary patterns [30–32]. The excess risk associated with poor diet may stem from specific nutritional deficiencies, rather than poor nutrition in general. One micronutrient which has been studied with respect to ESCC is selenium. Regions with both selenium deficient soil or crops and high incidence of ESCC have been identified across the world, including the Taihang mountains in China and parts of east Africa [33]. Several intervention and observational studies have shown that selenium deficiency may be a risk factor for upper gastrointestinal cancers in Linxian, in the Taihang mountains [34–36], but this has not been formally tested in other regions of the world with high incidence of ESCC.
Another apparent risk factor for ESCC is polycyclic aromatic hydrocarbon (PAH) exposure. PAHs are environmental carcinogens produced during incomplete combustion of organic material, including tobacco, coal, and wood. High PAH exposure has been documented in urine samples from healthy subjects in Linxian, China [37], Golestan, Iran [38], and Rio Grande do Sul, Brazil [39], as well as in non-tumor esophageal tissue from ESCC cases in Golestan, Iran [40]. High PAH content has also been detected in staple foods in Linxian [41] and commercially processed maté leaves and beverages from Rio Grande do Sul [42, 43]. Such findings form the basis of a compelling hypothesis that PAHs from both tobacco and non-tobacco sources contribute to the pathogenesis of ESCC. Although exposure to PAHs in these regions is high and these chemicals are known to be carcinogenic, definitive association studies for ESCC have not been completed.
We note that several recent studies [37, 44–48] strongly suggest that Human Papilloma Virus plays little if any role in ESCC etiology, so that anti-HPV vaccines will not play a role in ESCC prevention.
Other less studied but possible risk factors in high incidence populations include poor oral hygiene/tooth loss [49–53], prolonged close contact with ruminant animals [54–56], and the oral microbiome [57, 58].
Challenges outstanding
The next generation of etiologic studies
ESCC risk factors are likely acting in combination with each other, or with some as yet unidentified factor(s). Existing cohorts in Linxian, China [10] and Golestan, Iran [59] provide important resources for studying ESCC in distinct populations. However, the concentration of ESCC in low-resource areas, such as eastern and southern Africa, precludes the rapid implementation of multiple large cohort studies. In the absence of cohorts, multiple case–control studies of ESCC etiology are needed in understudied populations, particularly in east Africa. These studies must ensure high quality data collection and should use harmonized, but locally tailored, data and biospecimen collection protocols. Putative risk factors that have suggestive, but not conclusive, evidence of their ESCC risk include: hot beverage/food consumption, animal exposure, oral health, opium [60], pickled foods [61], salt tea [62], fermented milk [63], and nitrosamines. Further studies to identify exposure sources and routes are needed, to enable translation to primary prevention of ESCC in high incidence regions. Definitive prospective (cohort) studies using biomarkers of exposure (such as PAH metabolites in urine) are needed. Lastly, comparing and contrasting the population attributable risks for identified risk factors in high and low incidence areas would be a worthy addition to the ESCC literature.
Translating etiologic data into public health policy
Tobacco on its own, or in combination with alcohol, continue to have an important role in esophageal cancer in many regions, and while they cannot explain the high incidence rates that are observed in Asia, they do present opportunities for prevention. Currently, many parts of Africa have low tobacco use rates and limiting the tobacco epidemic in this region will continue to be a public health priority. Evidence for the consumption of hot beverages increasing risk of ESCC is mounting and, in high risk areas, public health campaigns targeting the consumption of very hot beverages should be considered.
Genetics and somatic studies
There is strong evidence for the role of genetics in the etiology of ESCC. Numerous previous studies have consistently found strong increased risk of ESCC in persons who reported a positive family history of ESCC [10, 64–68]. Estimates of cancer heritability based on genome-wide association studies (GWAS) showed that ESCC had the highest heritability of all 13 cancers evaluated [68]. In addition, a familial esophageal cancer syndrome, tylosis, inherited as an autosomal dominant trait, has been reported and associated with mutations in RHBDF2 [69].
Polymorphisms in ADH1B, ADH7, and ALDH2 are known to alter ethanol metabolism, and have been linked to altered risk of ESCC among alcohol consumers in both Asian and European populations [70, 71]. In other hypothesis-driven single nucleotide polymorphism (SNP) studies, a rare BRCA2 variant was shown to have a large association (between threefold and sixfold) in separate European and Iranian populations [72, 73]. A number of genome wide association studies (GWAS) of ESCC have been undertaken to date in China [74, 75] and Europe [76]. A comprehensive joint analysis of the Chinese studies, which included 5337 ESCC cases and 5787 controls, with a replication study which included 9654 cases and 10 058 controls [77] confirmed a number of loci, identified two new susceptibility loci, and found a new locus in the HLA class II region that was limited to the population in the high-incidence Taihang Mountain region in China. Cumulatively, ESCC GWAS in Asians have identified a total of 16 risk loci to date, including 14 with main effects and two others evident only because of an interaction with alcohol use. Five of these GWAS loci have also been tested for ESCC association in African populations, and RUNX1 and PLCE1 showed some limited evidence of association [78].
Many of the early somatic mutations studies of ESCC focused on TP53 [79, 80]. The distinct etiologic heterogeneity of ESCC may be reflected in the TP53 mutation patterns observed in tumors from different high and low ESCC incidence regions, though this does not always appear predictable; that is, the proportion of tumors containing TP53 mutations is not necessarily highest in high ESCC incidence areas [81–84]. With the advent of Next-Generation Sequencing, a more complete characterization of common mutations is already underway [85–92].
Genomic studies of ESCC tumors have also reported promising candidate DNA methylation markers for early detection, and tumor prognosis [93], however, studies have been notably limited in their size [94–96]. Whole exome studies of ESCC have identified mutation signatures similar to those seen in other squamous cancers and distinct from esophageal adenocarcinomas [48, 89, 97]. A recent report from Malawi, the first of its kind from Africa, identified a subset of tumors where TP53 mutations were rare, and a subset of tumors with an unknown mutation signature, consistent with an unidentified environmental exposure [48], but the data remain too sparse to draw strong conclusions.
Challenges outstanding
Ethnically diverse and adequately powered GWAS and tumor sequencing studies
Large scale GWAS of ESCC beyond subjects of Chinese ethnicity are needed, particularly from eastern and south-eastern Africa, north-eastern Iran, South America and central Asia. Large scale tumor sequencing studies could also help to highlight mutation signatures [98] linked to underlying causes, and may help to identify to what extent risk factors are common across high risk areas. Both of these projects will require large scale, high quality biorepositories that combine lifestyle data, biologic sample collection, appropriate ethical and consent oversight, and biorepository infrastructure support. Cancer Research UK recently funded a large mutation signature study that aims to include 1000 ESCC from low and high incidence populations, which will shed new light on the potential similarities and differences in etiologic agents across the globe.
Molecular data from Africa are underrepresented in the literature
Particular focus and support should be given to genetic and genomic studies originating in Africa, which have been notably limited to date and have often been small in size and underpowered considering the lower linkage disequilibrium patterns observed in African populations. The significantly younger age profile of African ESCC cases may make genetic and genomic studies there particularly relevant.
Early detection, treatment, and care
The pathogenesis of ESCC has been well characterized, including identification of a recognizable precursor lesion, esophageal squamous dysplasia (ESD), which affords the possibility of intervention to detect and manage precursor lesions and prevent tumor development. In one region of exceptionally high ESCC incidence in China, endoscopic screening of asymptomatic adults with use of Lugol’s iodine to detect ESD, and endoscopic mucosal resection and/or ablation to treat high-grade ESD has proven effective in reducing both ESCC incidence and mortality [99].
However, mass endoscopic screening to detect and remove ESD would present a substantial challenge even in a high-resource environment and is not tenable in most high incidence regions. A robust risk stratification system might substantially increase efficiency, but efforts to date have not been promising [100]. Previous studies have explored the use of esophageal balloon, or sponge cytology, as a less invasive primary screening test for detecting dysplasia and carcinoma among asymptomatic individuals [101, 102], but the accuracy of detection using routine cytologic readings was too low to be clinically useful. Recently sponge cytology in combination with molecular markers has proven effective for detecting Barrett’s esophagus [103, 104] and this may prove useful for primary detection of ESD as well.
Early detection of ESCC is critical because disease prognosis and management are determined by the stage at which ESCC is detected. The advent of endoscopic treatment (radiofrequency ablation, cryotherapy, endoscopic mucosal resection, submucosal dissection) for high-grade ESD, or early ESCC in China, Iran and Kenya offers tangible potential for early detection and cancer prevention in high incidence regions. For palliative care, the availability of self-expanding metal stents could dramatically improve the quality of life for patients presenting with late stage tumors. Lack of fluoroscopy devices in many areas has necessitated the development of stenting techniques that do not require fluoroscopy and are safe and reproducible [19].
Challenges outstanding
Optimizing population-based screening strategies
In China, where population-based endoscopic screening is already underway in high ESCC incidence areas, there is a need to optimize the screening strategy (which age groups to include, optimal screening intervals, appropriate follow-up methods, etc.).
Development of a primary non-endoscopic screening test and risk stratification models for ESD
Development of accurate, low cost methods for primary screening for high-grade ESD should be prioritized. Accurate and comprehensive knowledge of local risk factors could also help build locally effective risk prediction models.
Priorities for the development of early detection markers
Future field studies of ESCC should incorporate collecting appropriate tissue samples and blood. Developing biorepositories of tumor, adjacent normal tissue, precursor lesions (low- and high-grade ESD), and blood with linked questionnaire/demographic data, will provide a resource for development of early detection markers. Molecular profiling of ESCC tumors has shown promise in identifying markers for early detection and should be continued and expanded. Comparative studies of molecular profiles from tumors and dysplastic lesions originating in different low- and high-incidence areas may be particularly insightful.
Knowledge transfer for physicians in high incidence regions
The success of large scale screening trials in China also creates a valuable training resource for endoscopists and surgeons all over the world. Short-term training programs in high-incidence areas of China could enable physicians from other high risk regions to expand their capacity, whether in the use of Lugol’s iodine for lesion detection, endoscopic therapy of high risk lesions, esophagectomy, for operable invasive tumors, and/or in esophageal stent placement for palliation.
Access to palliative care in Africa
In many places nearly all patients with ESCC present with advanced obstructing lesions that are only amenable to palliation, which in low-resource settings is generally limited to the placement of stents. Producing affordable stents and providing resources and training to allow physicians to offer stent palliation to ESCC patients in low resource settings must be prioritized given the clear evidence that stent palliation can be very valuable to patients [19].
Conclusion
Decades of study in ESCC high incidence regions of China [10, 34, 36, 49, 50, 102, 105] and a growing body of evidence from Iran [25, 38, 40, 46, 51, 55, 59, 100, 106–108] and recent studies in Africa [21, 27, 48, 109–111] have provided insights on the epidemiology, etiology, early detection, and management of ESCC. Programs in high incidence regions in China and Iran are currently informing new field studies in Africa and elsewhere, and offer opportunities for powerful pooled epidemiologic studies, real-time data sharing, and knowledge transfer. Definitive ESCC risk factors should be tested in primary prevention studies. Future studies should work to describe unknown triggers working with highly prevalent regional exposures. Additional genetic and genomic studies are needed, particularly in Africa, which is not well represented in the current genomic literature. Such studies could provide data for risk stratification and contribute to our understanding of etiologic heterogeneity. Development of a clinically useful non-endoscopic primary screening test should be a high priority, because it could dramatically impact the ESCC burden in high incidence regions. Increasing the availability of affordable stents could significantly improve the quality of life of those patients with late-stage tumors.
Progress to date means there is now great promise for ESCC cancer prevention, early detection, and treatment.
Difficult research questions that should be prioritized
What explains the distinctive geographic pattern of high ESCC incidence in regions of Asia, Africa, and South America. Is there a common underlying cause or combination of causes?
Tobacco and alcohol are important in some areas (e.g. Europe and the USA), but appear to play a lesser role in Asia. What is the role of these risk factors, which are amenable to preventive interventions, in the high risk regions of Africa?
Can we produce definitive evidence that high temperature foods and beverages modulate ESCC risk in different populations, and should we design public health interventions to reduce risk from this exposure?
PAH exposure is common to each of the high incidence populations in Asia, Africa, and South America, but can we complete definitive association studies using biomarkers for this exposure?
Are dietary/mineral deficiencies a risk factor for ESCC in the high risk regions of China and elsewhere?
Is it possible to develop better animal models of ESD, to improve experimental testing of proposed ESCC etiologic mechanisms?
Is it possible to develop non-endoscopic screening tools, using one or more molecular markers, to identify high grade dysplasia and risk stratify asymptomatic adults in high incidence populations?
Can we identify the appropriate ages, and follow-up intervals, for ongoing endoscopic screening programs?
What is the role of endoscopic therapy in the treatment of precursor and early invasive ESCC lesions, and which can be carried out safely and effectively outside of specialized centers?
Are stents that best choice for palliative care and how can reasonably priced palliative care be delivered to patients in low-resource areas such as Africa?
Funding
This seminar was funded jointly by the International Agency for Research on Cancer (IARC) and the Division of Cancer Epidemiology and Genetics (DCEG) of the US National Cancer Institute Intramural Research Program (no grant number applicable).
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Scelo G, Hofmann JN, Banks RE. et al. International cancer seminars: a focus on kidney cancer. Ann Oncol 2016; 27(8): 1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay JSI, Ervik M, Dikshit R. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013.
- 3. Arnold M, Soerjomataram I, Ferlay J. et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015; 64(3): 381–387. [DOI] [PubMed] [Google Scholar]
- 4. Forman D, Bray F, Brewster DH. et al. Lyon, France: IARC Scientific Publication No. 164.
- 5. Tuyns AJ. Oesophageal cancer in non-smoking drinkers and in non-drinking smokers. Int J Cancer 1983; 32(4): 443–444. [DOI] [PubMed] [Google Scholar]
- 6. Engel LS, Chow WH, Vaughan TL. et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003; 95(18): 1404–1413. [DOI] [PubMed] [Google Scholar]
- 7. Parkin DM, Boyd L, Walker LC.. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 2011; 105(Suppl 2): S77–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olsen JH, Andersen A, Dreyer L. et al. Summary of avoidable cancers in the Nordic countries. APMIS Suppl 1997; 76: 141–146. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer (IARC) (2007) Attributable Causes of Cancer in France in the Year 2000. IARC Working Group Reports 3. IARC: Lyon.
- 10. Tran GD, Sun XD, Abnet CC. et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005; 113(3): 456–463. [DOI] [PubMed] [Google Scholar]
- 11. Liu BQ, Peto R, Chen ZM. et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. Br Med J 1998; 317(7170): 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu D, Kelly TN, Wu X. et al. Mortality attributable to smoking in China. N Engl J Med 2009; 360(2): 150–159. [DOI] [PubMed] [Google Scholar]
- 13. Wang JB, Fan JH, Liang H. et al. Attributable causes of esophageal cancer incidence and mortality in China. PLoS ONE 2012; 7(8): e42281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamangar F, Chow WH, Abnet CC. et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009; 38(1): 27–57, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 16. Zeng H, Zheng R, Guo Y. et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer 2015; 136(8): 1921–1930. [DOI] [PubMed] [Google Scholar]
- 17. De Angelis R, Sant M, Coleman MP. et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5 – a population-based study. Lancet Oncol 2014; 15(1): 23–34. [DOI] [PubMed] [Google Scholar]
- 18. Dawsey SP, Tonui S, Parker RK. et al. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS ONE 2010; 5(11): e14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White RE, Parker RK, Fitzwater JW. et al. Stents as sole therapy for oesophageal cancer: a prospective analysis of outcomes after placement. Lancet Oncol 2009; 10(3): 240–246. [DOI] [PubMed] [Google Scholar]
- 20. Launoy G, Milan C, Day NE. et al. Oesophageal cancer in France: potential importance of hot alcoholic drinks. Int J Cancer 1997; 71(6): 917–923. [DOI] [PubMed] [Google Scholar]
- 21. McCormack VA, Menya D, Munishi MO. et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int J Cancer 2017 Jan 15; 140(2): 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. IARC. Acetaldehyde. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer (IARC) 1999; 319–335.
- 23. IARC. A Review of Human Carcinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer (IARC) 2011.
- 24. Gao Y, Hu N, Han XY. et al. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case–control study. Cancer Epidemiol 2011; 35(6): e91–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Islami F, Pourshams A, Nasrollahzadeh D. et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case–control study. Br Med J 2009; 338: b929.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lubin JH, De Stefani E, Abnet CC. et al. Mate drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case–control studies. Cancer Epidemiol Biomarkers Prev 2013; 23(1): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munishi MO, Hanisch R, Mapunda O. et al. Africa's oesophageal cancer corridor: do hot beverages contribute? Cancer Causes Control 2015; 26(10): 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loomis D, Guyton KZ, Grosse Y. et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016; 17(7): 877–878. [DOI] [PubMed] [Google Scholar]
- 29. Lubin JH, De Stefani E, Abnet CC. et al. Mate drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case–control studies. Cancer Epidemiol Biomarkers Prev 2014; 23(1): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Oesophageal Cancer 2016.
- 31. Li WQ, Park Y, Wu JW. et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol 2013; 11(9): 1130–1136 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sewram V, Sitas F, O'Connell D. et al. Diet and esophageal cancer risk in the Eastern Cape Province of South Africa. Nutr Cancer 2014; 66(5): 791–799. [DOI] [PubMed] [Google Scholar]
- 33. Schaafsma T, Wakefield J, Hanisch R. et al. Africa's oesophageal cancer Corridor: geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS ONE 2015; 10(10): e0140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiao YL, Dawsey SM, Kamangar F. et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst 2009; 101(7): 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Limburg PJ, Wei W, Ahnen DJ. et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology 2005; 129(3): 863–873. [DOI] [PubMed] [Google Scholar]
- 36. Mark SD, Qiao YL, Dawsey SM. et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000; 92(21): 1753–1763. [DOI] [PubMed] [Google Scholar]
- 37. Roth MJ, Qiao Y-L, Rothman N. et al. High urine 1-hydroxypyrene glucuronide concentrations in Linxian, China, an area of high risk for squamous oesophageal cancer. Biomarkers 2001; 6(5): 381–386. [DOI] [PubMed] [Google Scholar]
- 38. Kamangar F, Strickland PT, Pourshams A. et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res 2005; 25(1B): 425–428. [PubMed] [Google Scholar]
- 39. Fagundes RB, Abnet CC, Strickland PT. et al. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking mate in healthy subjects from Rio Grande do Sul, Brazil. BMC Cancer 2006; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abedi-Ardekani B, Kamangar F, Hewitt SM. et al. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut 2010; 59(9): 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roth MJ, Strickland KL, Wang GQ. et al. High levels of carcinogenic polycyclic aromatic hydrocarbons present within food from Linxian, China may contribute to that region's high incidence of oesophageal cancer. Eur J Cancer 1998; 34(5): 757–758. [DOI] [PubMed] [Google Scholar]
- 42. Golozar A, Fagundes RB, Etemadi A. et al. Significant variation in the concentration of carcinogenic polycyclic aromatic hydrocarbons in yerba mate samples by brand, batch, and processing method. Environ Sci Technol 2012; 46(24): 13488–13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamangar F, Schantz MM, Abnet CC. et al. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev 2008; 17(5): 1262–1268. [DOI] [PubMed] [Google Scholar]
- 44. Koshiol J, Wei WQ, Kreimer AR. et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer 2010; 127(1): 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sitas F, Egger S, Urban MI. et al. InterSCOPE study: associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. J Natl Cancer Inst 2012; 104(2): 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halec G, Schmitt M, Egger S. et al. Mucosal alpha-papillomaviruses are not associated with esophageal squamous cell carcinomas: Lack of mechanistic evidence from South Africa, China and Iran and from a world-wide meta-analysis. Int J Cancer 2016; 139(1): 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Herbster S, Ferraro CT, Koff NK. et al. HPV infection in Brazilian patients with esophageal squamous cell carcinoma: interpopulational differences, lack of correlation with surrogate markers and clinicopathological parameters. Cancer Lett 2012; 326(1): 52–58. [DOI] [PubMed] [Google Scholar]
- 48. Liu W, Snell JM, Jeck WR. et al. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight 2016; 1(16): e88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abnet CC, Qiao YL, Mark SD. et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001; 12(9): 847–854. [DOI] [PubMed] [Google Scholar]
- 50. Abnet CC, Qiao YL, Dawsey SM. et al. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol 2005; 34(2): 467–474. [DOI] [PubMed] [Google Scholar]
- 51. Abnet CC, Kamangar F, Islami F. et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008; 17(11): 3062–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dar NA, Islami F, Bhat GA. et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer 2013; 109(5): 1367–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guha N, Boffetta P, Wunsch Filho V. et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case–control studies. Am J Epidemiol. 2007; 166(10): 1159–1173. [DOI] [PubMed] [Google Scholar]
- 54. Plowright W. Malignant neoplasia of the oesophagus and rumen of cattle in Kenya. J Comp Pathol 1955; 65(2): 108–114. [DOI] [PubMed] [Google Scholar]
- 55. Nasrollahzadeh D, Ye W, Shakeri R. et al. Contact with ruminants is associated with esophageal squamous cell carcinoma risk. Int J Cancer 2015; 136(6): 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dar NA, Islami F, Bhat GA. et al. Contact with animals and risk of oesophageal squamous cell carcinoma: outcome of a case–control study from Kashmir, a high-risk region. Occup Environ Med 2014; 71(3): 208–214. [DOI] [PubMed] [Google Scholar]
- 57. Yu G, Gail MH, Shi J. et al. Association between upper digestive tract microbiota and cancer-predisposing states in the esophagus and stomach. Cancer Epidemiol Biomarkers Prev 2014; 23(5): 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nasrollahzadeh D, Malekzadeh R, Ploner A. et al. Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci Rep 2015; 5: 8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pourshams A, Khademi H, Malekshah AF. et al. Cohort Profile: The Golestan Cohort Study – a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol 2010; 39(1): 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kamangar F, Shakeri R, Malekzadeh R. et al. Opium use: an emerging risk factor for cancer? Lancet Oncol 2014; 15(2): e69–e77. [DOI] [PubMed] [Google Scholar]
- 61. Islami F, Ren JS, Taylor PR. et al. Pickled vegetables and the risk of oesophageal cancer: a meta-analysis. Br J Cancer 2009; 101(9): 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dar NA, Bhat GA, Shah IA. et al. Salt tea consumption and esophageal cancer: a possible role of alkaline beverages in esophageal carcinogenesis. Int J Cancer 2015; 136(6): E704–E710. [DOI] [PubMed] [Google Scholar]
- 63. Nieminen MT, Novak-Frazer L, Collins R. et al. Alcohol and acetaldehyde in African fermented milk mursik – a possible etiologic factor for high incidence of esophageal cancer in western Kenya. Cancer Epidemiol Biomarkers Prev 2013; 22(1): 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao Y, Hu N, Han X. et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer 2009; 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akbari MR, Malekzadeh R, Nasrollahzadeh D. et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer 2006; 119(5): 1047–1051. [DOI] [PubMed] [Google Scholar]
- 66. Hu N, Dawsey SM, Wu M. et al. Family history of oesophageal cancer in Shanxi Province, China. Eur J Cancer 1991; 27(10): 1336.. [DOI] [PubMed] [Google Scholar]
- 67. Li JY, Ershow AG, Chen ZJ. et al. A case–control study of cancer of the esophagus and gastric cardia in Linxian. Int J Cancer 1989; 43(5): 755–761. [DOI] [PubMed] [Google Scholar]
- 68. Sampson JN, Wheeler WA, Yeager M. et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J Natl Cancer Inst 2015; 107(12): djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blaydon DC, Etheridge SL, Risk JM. et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet 2012; 90(2): 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brooks PJ, Enoch MA, Goldman D. et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med 2009; 6(3): e50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hashibe M, McKay JD, Curado MP. et al. Multiple ADH genes are associated with upper aerodigestive cancers. Nat Genet 2008; 40(6): 707–709. [DOI] [PubMed] [Google Scholar]
- 72. Akbari MR, Malekzadeh R, Nasrollahzadeh D. et al. Germline BRCA2 mutations and the risk of esophageal squamous cell carcinoma. Oncogene 2008; 27(9): 1290–1296. [DOI] [PubMed] [Google Scholar]
- 73. Delahaye-Sourdeix M, Anantharaman D, Timofeeva MN. et al. A rare truncating BRCA2 variant and genetic susceptibility to upper aerodigestive tract cancer. J Natl Cancer Inst 2015; 107(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang LD, Zhou FY, Li XM. et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1. Nat Genet 2010; 42(9): 759–763. [DOI] [PubMed] [Google Scholar]
- 75. Abnet CC, Freedman ND, Hu N. et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet 2010; 42(9): 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McKay JD, Truong T, Gaborieau V. et al. A genome-wide association study of upper aerodigestive tract cancers conducted within the INHANCE consortium. PLoS Genet 2011; 7(3): e1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu C, Wang Z, Song X. et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet 2014; 46(9): 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bye H, Prescott NJ, Lewis CM. et al. Distinct genetic association at the PLCE1 locus with oesophageal squamous cell carcinoma in the South African population. Carcinogenesis 2012; 33(11): 2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Abedi-Ardekani B, Hainaut P.. Cancers of the upper gastro-intestinal tract: a review of somatic mutation distributions. Arch Iran Med 2014; 17(4): 286–292. [PubMed] [Google Scholar]
- 80. Bouaoun LSD, Ardin M, Hollstein M. et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat 2016; 37: 865–876. [DOI] [PubMed] [Google Scholar]
- 81. Gamieldien W, Victor TC, Mugwanya D. et al. p53 and p16/CDKN2 gene mutations in esophageal tumors from a high-incidence area in South Africa. Int J Cancer 1998; 78(5): 544–549. [DOI] [PubMed] [Google Scholar]
- 82. Tamura G, Maesawa C, Suzuki Y. et al. p53 gene mutations in esophageal cancer detected by polymerase chain reaction single-strand conformation polymorphism analysis. Jpn J Cancer Res 1992; 83(6): 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Putz A, Hartmann AA, Fontes PR. et al. TP53 mutation pattern of esophageal squamous cell carcinomas in a high risk area (Southern Brazil): role of life style factors. Int J Cancer 2002; 98(1): 99–105. [DOI] [PubMed] [Google Scholar]
- 84. Rossini A, de Almeida Simao T, Marques CB. et al. TP53 mutation profile of esophageal squamous cell carcinomas of patients from Southeastern Brazil. Mutat Res 2010; 696(1): 10–15. [DOI] [PubMed] [Google Scholar]
- 85. Cao W, Wu W, Yan M. et al. Multiple region whole-exome sequencing reveals dramatically evolving intratumor genomic heterogeneity in esophageal squamous cell carcinoma. Oncogenesis 2015; 4: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hu N, Kadota M, Liu H. et al. Genomic landscape of somatic alterations in esophageal squamous cell carcinoma and gastric cancer. Cancer Res 2016; 76(7): 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang L, Zhou Y, Cheng C. et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet 2015; 96(4): 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lin DC, Hao JJ, Nagata Y. et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet 2014; 46(5): 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541(7636): 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sawada G, Niida A, Uchi R. et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology 2016; 150(5): 1171–1182. [DOI] [PubMed] [Google Scholar]
- 91. Qin HD, Liao XY, Chen YB. et al. Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. Am J Hum Genet 2016; 98(4): 709–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cheng C, Zhou Y, Li H. et al. Whole-genome sequencing reveals diverse models of structural variations in esophageal squamous cell carcinoma. Am J Hum Genet 2016; 98(2): 256–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ma K, Cao B, Guo M.. The detective, prognostic, and predictive value of DNA methylation in human esophageal squamous cell carcinoma. Clin Epigenetics 2016; 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lima SC, Hernandez-Vargas H, Simao T. et al. Identification of a DNA methylome signature of esophageal squamous cell carcinoma and potential epigenetic biomarkers. Epigenetics 2011; 6(10): 1217–1227. [DOI] [PubMed] [Google Scholar]
- 95. Li X, Zhou F, Jiang C. et al. Identification of a DNA methylome profile of esophageal squamous cell carcinoma and potential plasma epigenetic biomarkers for early diagnosis. PLoS One 2014; 9(7): e103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen C, Peng H, Huang X. et al. Genome-wide profiling of DNA methylation and gene expression in esophageal squamous cell carcinoma. Oncotarget 2016; 7(4): 4507–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Song Y, Li L, Ou Y. et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 2014; 509(7498): 91–95. [DOI] [PubMed] [Google Scholar]
- 98. Bignell GR, Greenman CD, Davies H. et al. Signatures of mutation and selection in the cancer genome. Nature 2010; 463(7283): 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wei WQ, Chen ZF, He YT. et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol 2015; 33(17): 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Etemadi A, Abnet CC, Golozar A. et al. Modeling the risk of esophageal squamous cell carcinoma and squamous dysplasia in a high risk area in Iran. Arch Iran Med 2012; 15(1): 18–21. [PMC free article] [PubMed] [Google Scholar]
- 101. Roth MJ, Liu SF, Dawsey SM. et al. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer 1997; 80(11): 2047–2059. [DOI] [PubMed] [Google Scholar]
- 102. Pan QJ, Roth MJ, Guo HQ. et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol 2008; 52(1): 14–23. [DOI] [PubMed] [Google Scholar]
- 103. Ross-Innes CS, Debiram-Beecham I, O'Donovan M. et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case–control study. PLoS Med 2015; 12(1): e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Iyer P, Johnson ML, Lansing R. et al. Discovery, validation and feasibility testing of highly discriminant DNA methylation markers for detection of Barrett's esophagus using a capsule sponge device. Gastroenterology 2016; 150(4 (S1)): S66–S67. [Google Scholar]
- 105. Wei WQ, Abnet CC, Qiao YL. et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr 2004; 79(1): 80–85. [DOI] [PubMed] [Google Scholar]
- 106. Islami F, Kamangar F, Aghcheli K. et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer 2004; 90(7): 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pourshams A, Saadatian-Elahi M, Nouraie M. et al. Golestan cohort study of oesophageal cancer: feasibility and first results. Br J Cancer 2005; 92(1): 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nasrollahzadeh D, Kamangar F, Aghcheli K. et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer 2008; 98(11): 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mwachiro MM, Burgert SL, Lando J. et al. Esophageal squamous dysplasia is common in asymptomatic Kenyans: a prospective, community-based, cross-sectional study. Am J Gastroenterol 2016; 111(4): 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. White RE, Chepkwony R, Mwachiro M. et al. Randomized trial of small-diameter versus large-diameter esophageal stents for palliation of malignant esophageal obstruction. J Clin Gastroenterol 2015; 49(8): 660–665. [DOI] [PubMed] [Google Scholar]
- 111. Cheng ML, Zhang L, Borok M. et al. The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cancer Epidemiol 2015; 39(2): 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]