Abstract
Aims
Single-chamber (VR-ICD) and subcutaneous (S-ICD) implantable cardioverter-defibrillators are effective to protect patients against sudden death but expose them to higher risk of inappropriate shock (IS). We sought to quantify the annual rate and influencing factors of ISs in VR- and S-ICDs from the literature.
Methods and results
PubMed, Embase, and Cochrane Library were searched for full text articles with IS rates. Poisson distribution estimated proportion of patients with ISs; rates were annualized based on follow-up duration. Random effects meta-analysis accounted for study-to-study variation. Out of 3264 articles, 16 qualified for the meta-analysis. Across studies, 6.4% [95% confidence interval (CI) 5.1–7.9%] of patients received an IS per year. Meta-regression analyses demonstrated that IS rates were lower in more recent studies [rate ratio (RR) per year: 0.93, 95% CI: 0.87–0.98; P = 0.01] and trended lower in studies with longer follow-up (RR per year: 0.78, 95% CI: 0.60–1.01; P = 0.06). Use of S-ICDs (RR: 1.81, 95% CI: 0.86–3.81; P = 0.12) and ventricular tachycardia zone programmed on (RR: 1.13, 95% CI: 0.65–1.97; P = 0.66) were not associated with a significantly increased change in risk. The IS rate observed in one of the more recent studies was significantly lower than predicted after accounting for covariates (RR: 0.29, 95% CI: 0.14–0.60; P < 0.001).
Conclusions
A comprehensive review of the literature shows that 6.4% of patients with ICDs experienced their first IS annually. One of the 16 studies was better than predicted with the lowest reported rate (1.9%) and could not be explained by timing of the study or other covariates.
Keywords: Implantable cardioverter-defibrillator, Subcutaneous ICD, Sudden cardiac death, Inappropriate shocks, Meta-analysis
What’s new?
Our study showed a relatively constant annual appropriate shock rate of 5.8% and an annual inappropriate shock rate of 6.4% which later progressively reduced over time, and significantly dropped to 1.9% in one of the more recent studies.
Meta-regression analyses demonstrated that inappropriate shock rates were lower in more recent studies and trended lower in studies with longer follow-up.
Use of subcutaneous implantable cardioverter-defibrillators and ventricular tachycardia zone programmed on were not associated with a significantly increased change in risk.
These observations call for further investigation in the contemporary outcomes of heart failure patients and in those indicated for an implantable cardioverter-defibrillator.
Introduction
The pivotal trials demonstrating effective prevention of sudden cardiac death with implantable cardioverter-defibrillators (ICDs) for both secondary and primary prevention were established primarily by devices without atrial leads.1–5 ICDs with right atrial leads (i.e. dual-chamber) were developed to enhance rhythm diagnosis (ventriculo-atrial association) with the intent to reduce the inappropriate shock (IS) rate for atrial fibrillation (AF) and supraventricular tachycardias as well as to provide atrial rate and atrioventricular conduction support, when clinically indicated.
A recent retrospective cohort study of admission in the National Cardiovascular Data Registry’s ICD registry from 2006 to 2009 found that among patients receiving an ICD for primary prevention without a pacing indication, the use of a dual-chamber device compared with a single-chamber device was associated with a higher risk of device-related complications and similar 1 year mortality and hospitalization outcomes.6 Additionally, a recent systematic assessment of ISs comparing dual-chamber ICDs with single-chamber devices revealed no clear superiority of dual-chamber ICDs vs. single-chamber in reducing ISs.7 Thus, an atrial lead is not recommended for a substantial proportion of ICD-indicated patients.8
Since the publication of the ICD landmark trials,1–4 significant technological advances have occurred and device programming has substantially changed which may have influenced the IS rate. However, it is unknown whether the IS rate in single-chamber ICDs (VR-ICDs) has changed over time. In light of all of the above considerations, we sought to quantify the annualized frequency of first ISs among VR-ICDs and subcutaneous ICDs (S-ICDs) and to determine influencing factors associated with their occurrence through a systematic review and meta-analysis. Furthermore, we assessed whether the annualized rate of appropriate shocks in these patients changed over time.
Methods
Search strategy
The systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.9 A systematic search of PubMed (Medline), Embase, and Cochrane Library was performed to identify articles with VR-ICD or S-ICD IS rates published through July 2015. The following Boolean search terms were utilized: ‘implantable defibrillator OR ICD’ AND ‘shock OR shocks OR therapy’ AND inappropriate.
Study selection and data extraction
Search criteria and methodology were approved by all authors. Titles and abstracts retrieved in the search were reviewed, and observational and comparative studies reporting IS rates in ICDs were selected. Studies were included if results reported VR-ICD or S-ICD IS rates, were in the English language, and included human subjects ≥18 years of age. Case reports, review articles, abstracts, and editorials were excluded, as were studies with <100 patients with single chamber/subcutaneous devices and studies with <6 months of follow-up. In the event that there were multiple publications from the same study, the latest study with the most complete data available was selected, and the other publications were not used in order to avoid overlapping cohorts. Final determination of article eligibility was assessed by two authors (J.H.H. and D.H.F.). For included studies, only data on VR-ICD/S-ICD patients were extracted. Extracted data included: number of patients, follow-up duration, IS rate, appropriate shock rate, proportion with AF at baseline, use of a slow VT zone, and prolonged detection. Data were extracted by one author (D.H.F.) and were reviewed by additional authors (T.R., J.H.H., and A.A.).
Meta-analysis
Meta-analysis methods provided a structured, model-based way to combine information from multiple studies. To estimate the annual first IS rate among patients implanted with a VR-ICD or S-ICD, we used random-effects Poisson regression. This method models the number of patients with a first IS via a Poisson distribution, where the expected number of patients with an IS is a function of the IS rate and the total number of years of patient follow-up observed in the study. Modeling patient counts based on the total years of follow-up captures the fact that a study with more patients or with longer average follow-up per patient is expected to observe more patients with an IS than a shorter or smaller study with the same annual rate. We modelled study-to-study variation in the number of patients with an IS that exceeds the degree of variation expected with the Poisson distribution with normally distributed random effects. These methods were also used to estimate the rate of first appropriate shocks.
Meta-regression was used to examine how study-level factors contribute to differences in IS rates. Average duration of follow-up and study timing were assessed for their relationships to IS rate. When the study was conducted served as a proxy for a collection of treatment changes over time (e.g. improved ICD technology, programming strategies, patient selection) and was measured by the middle year of study enrolment. We further examined how IS rates differ by the study’s ICD programming (with vs. without a VT zone) and by device type (VR-ICD vs. S-ICD). To assess whether these factors were sufficient to explain each study’s IS rate, we tested whether a study’s observed rate was in agreement with the model-estimated expected IS rates based on all other studies’ data given the study’s mean follow-up, middle enrolment year, ICD programming, and device type.
Statistics
The Poisson meta-analysis with normal random effects was performed with the metafor package for R statistical software.10 Heterogeneity was assessed with the I2 index,11 expressing the proportion of variation that is not explained by Poisson sampling variability. The IS rate was modelled on the log scale as a linear combination of the regression factors. P-values <0.05 were considered statistically significant.
Results
A total of 3264 articles were retrieved after excluding duplicates. After excluding 3095 articles for not meeting inclusion/exclusion criteria, 169 articles remained to be assessed for eligibility. Following assessment of the full-text articles, 153 were excluded for reasons such as: shocks were not separated by device type, the device type was not specified, or rates of inappropriate therapy (rather than just shocks) were given, and two studies were excluded due to reporting IS rates for SVT only.12,13 This left us with 16 studies to be included in the analysis (Figure 1, Table 1).
Figure 1.
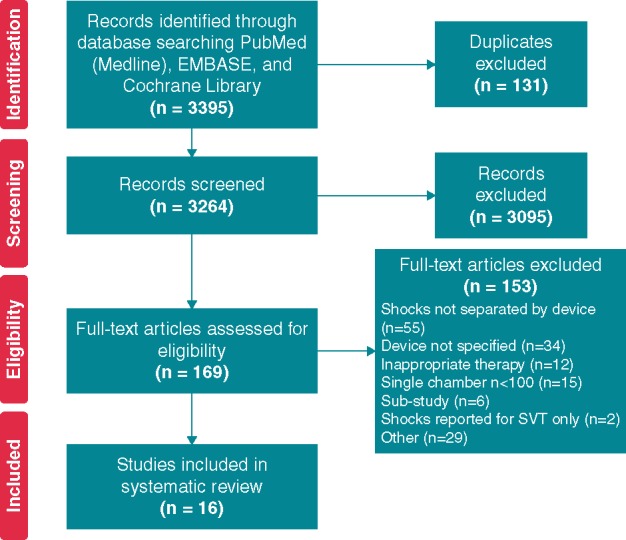
PRISMA flow diagram. Diagram depicting systematic review process, following the PRISMA approach.
Table 1.
Studies included in meta-analysis
| Study | Publication year | Device type | Randomization | No. patients | Follow-up (months) | % AF | VT rate cutoff (lowest) | Slow VT Rx |
|---|---|---|---|---|---|---|---|---|
| MADIT II4 | 2002 | VR-ICD | – | 405 | 20 | NR | 160 bpma | Yes |
| SCD HeFT2 | 2005 | VR-ICD | – | 808 | 45.5 | NR | NR | No |
| Sacher et al.14 | 2006 | VR-ICD | – | 196 | 21 | NR | 170 bpma | Yes |
| DATAS15 | 2008 | VR-ICD | – | 111 | 15.6 | NR | 167 bpm | Yes |
| Kleemann et al.16 | 2011 | VR-ICD | – | 596 | 62.5 | NR | 167 bpm | Yes |
| González-Enríquez et al.17 | 2012 | VR-ICD | – | 332 | 12 | NR | NR | No |
| Yang et al.18 | 2012 | VR-ICD | – | 136 | 29 | NR | 160 bpm | Yes |
| RIGHT19 | 2012 | VR-ICD | VITALITY 2 | 507 | 18.3 | NR | 150 bpm | Yes |
| Medtronic | 504 | 18.3 | NR | 150 bpm | Yes | |||
| Olde Nordkamp et al.20 | 2012 | S-ICD | – | 118 | 18 | 11 | NR | No |
| Deyell et al.21 | 2013 | VR-ICD | – | 354 | 30 | NR | NR | Yes |
| ECOST22 | 2013 | VR-ICD | Active | 161 | 24.2 | NR | 150 bpm | Yes |
| Conventional | 141 | 24.2 | NR | 150 bpm | Yes | |||
| S-ICD IDE23 | 2013 | S-ICD | – | 314 | 11 | 15 | NR | No |
| OPTION24 | 2014 | VR-ICD | – | 223 | 23.4 | 11 | 170 bpm | Yes |
| DECREASE25 | 2015 | VR-ICD | Conventional | 112 | 12 | NR | 171 bpm | Yes |
| Progressive | 120 | 12 | NR | 187 bpm | No | |||
| EFFORTLESS26 | 2015 | S-ICD | – | 581 | 21.4 | 17 | NR | No |
| PainFree SST27 | 2015 | VR-ICD | – | 751 | 24 | 22 | 167 bpm | Yes |
NR, not reported.
Assumed rate, lowest rate reported in manuscript.
The final population for the meta-analysis included 6470 patients with 14 696 patient-years cumulative follow-up (mean 919 patient-years). Middle year of enrolment for the studies ranged from 1999 to 2011, with the average middle year of enrolment being mid-way through 2005. Of the 16 studies included in the meta-analysis, 13 were VR-ICD studies and three were S-ICD studies. Thirteen studies were prospective and three studies were retrospective. Five of 16 studies reported percentage of VR-ICD or S-ICD patients with AF. A total of 926 patients received ISs across the 16 studies.
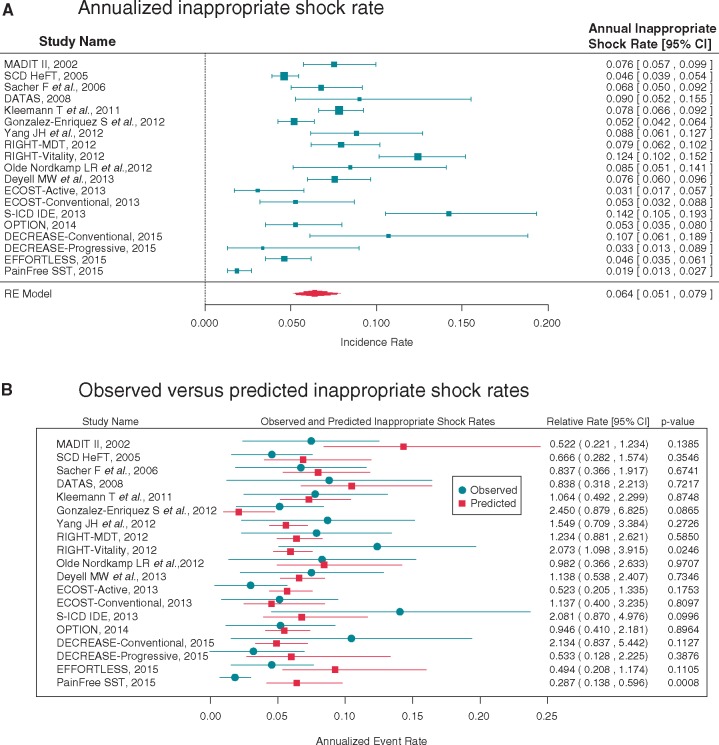
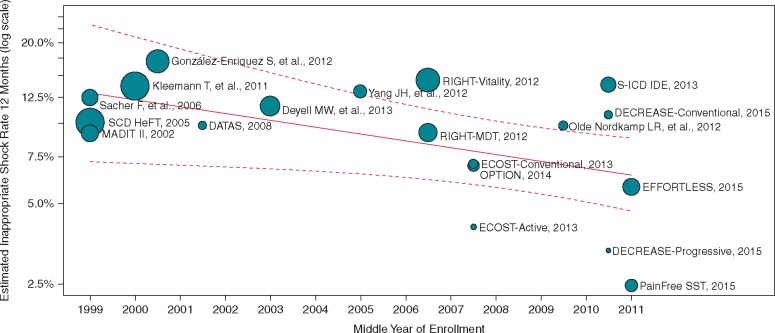
A random effects Poisson meta-analysis estimated an annualized IS rate of 6.4% (95% CI 5.1–7.9) with substantial variability between studies (Figure 2A). The I2 statistic was 90.1%. A meta-regression analysis adjusting for mean follow-up time and the middle year of enrolment explained a statistically significant degree of variability (P = 0.04), but the heterogeneity remained high (I2 = 85.5%). This meta-regression model found that studies with longer mean follow-up had lower annualized IS rates. The annualized rate was estimated to decrease by a factor of 0.76 (95% CI 0.60, 0.95, P = 0.02) for each additional year of mean follow-up. Each additional year in a study’s middle year of enrolment was associated with a reduction in the IS rate by a factor of 0.94 (95% CI 0.89, 1.0, P = 0.04), with studies conducted more recently having lower IS rates than those conducted farther in the past (Figure 3). A more detailed meta-regression model included device type and slow VT zone programming, study factors that were reported across all studies (Table 2). AF prevalence was not reported in all studies and prolonged detection was only used in a single study. Therefore these variables were not included in the model. Subcutaneous ICD studies were estimated to have an IS rate 1.81 (95% CI 0.86, 3.80, P = 0.12) times the transvenous shock rate, but the difference was not statistically significant. Use of a slow VT zone was estimated to result in an IS rate 1.13 (95% CI 0.65, 1.97, P = 0.66) times higher, which was also not statistically significant. The addition of these variables did not significantly reduce the unexplained heterogeneity (I2 = 82.4%). Using this model, the expected IS rate for a 1-year study of transvenous single-chamber ICDs with a slow VT zone with enrolment at the same time as the most recent studies in this analysis is 5.3% (95% prediction interval from 2.5% to 11.4%).
Figure 2.
(A) Annualized inappropriate shock rate. Random effects Poisson meta-analysis depicting annualized inappropriate shock rate. In the model, the expected number of patients with an inappropriate shock is a function of the inappropriate shock rate and the total number of years of patient follow-up observed in the study. (B) Observed vs. predicted inappropriate shock rates. A meta-regression analysis adjusting for mean follow-up time, the middle year of enrolment, device type, and slow VT zone programming was used to calculate predicted inappropriate shock rates. Each individual study’s observed (annualized) inappropriate shock rate was compared with the predicted rate. Results are shown as relative rates.
Figure 3.
Estimated inappropriate shock rate by middle year of patient enrolment into each given study. A meta-regression analysis of the inappropriate shock rate by study at 12 months after adjusting for calendar year and mean follow-up duration. Each bubble shows a study and the size of bubble is proportional to the inverse of the variance of the log-risk ratio.
Table 2.
Meta-regression analysis
| Estimate (95% CI) | P-value | |
|---|---|---|
| Intercepta | 0.047 (0.024, 0.090) | <0.001 |
| Middle enrolment year (per year) | 0.93 (0.87, 0.98) | 0.01 |
| Mean follow-up (per year) | 0.78 (0.60, 1.01) | 0.06 |
| S-ICD vs. Transvenous | 1.81 (0.86, 3.80) | 0.12 |
| VT zone programmed (Yes vs. No) | 1.13 (0.65, 1.97) | 0.66 |
Intercept for 2011 Middle Enrolment Year, 1 Year Mean Follow-up, Transvenous ICD, with no VT Zone programmed.
Each individual study’s observed IS rate was compared with the expected rate based on this more detailed model estimated using the results of all other studies. The observed and expected IS rates are shown in Figure 2B along with their relative rate (observed/expected). Two studies had rates that were statistically different from the expected rate after accounting for device type, VT zone programming, mean follow-up duration, and middle enrolment year. The PainFree SST study had a lower IS rate than expected (relative rate 0.287; 95% CI 0.138, 0.596, P = 0.0008). The Vitality arm of the RIGHT study had a higher IS rate than expected (relative rate 2.073; 95% CI 1.098, 3.915, P = 0.025).
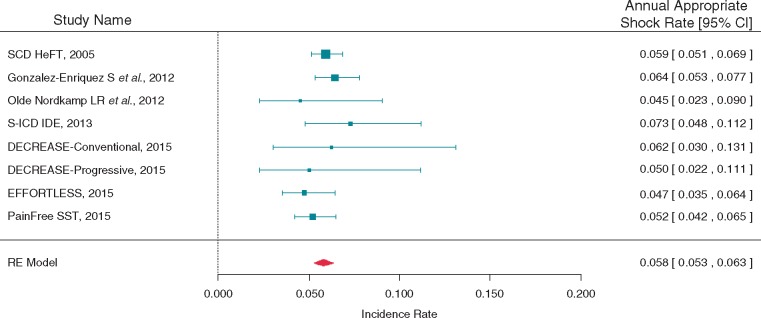
To understand the appropriate shock rates for VR-ICD's and S-ICDs, a meta-analysis was also performed within a subgroup of the 16 studies that reported appropriate shocks. Seven studies with 3136 patients followed for 6631 patient-years were included. Three of the studies were of S-ICDs. The annualized appropriate shock rate was estimated at 5.8% (95% CI 5.3, 6.3) (Figure 4). The observed heterogeneity in appropriate shock rates was in line with that expected by a Poisson distribution (I2 = 0.0%).
Figure 4.
Appropriate shock meta-analysis. Meta-analysis depicting annualized appropriate shock rate within a subgroup of the 16 studies that reported appropriate shocks.
Discussion
The objective of this study was to systematically assess the annualized rate of inappropriate and appropriate shocks in VR-ICDs. Our analysis covered approximately 15 years of clinical trials in which a VR-ICD was used and the rate of IS was reported. To our knowledge, this is the most comprehensive assessment of appropriate and inappropriate ICD shock rates to date. Our study showed a relatively constant annual appropriate shock rate of 5.8% and an annual IS rate of 6.4% which later progressively reduced over time, and significantly dropped to 1.9% in one of the more recent studies. This study was not designed nor had the intention of demonstrating that transvenous technology is superior to subcutaneous technology, nor that one ICD brand is better than another one. Rather, our analysis demonstrates that the rate of ISs, independently of the implantation approach used and manufacturer, has dramatically changed over time and is continuously reducing. This is important, and in our opinion, reassuring information for patients and families, general practitioners, cardiologists, scientific societies, and regulators. Furthermore, one may consider the single-chamber ICD an effective choice to protect patients against sudden death without compromising safety at an excellent cost-effectiveness ratio.
This study expands the current understanding of the contemporary outcome in terms of shock rate in patients receiving VR-ICDs. In the early randomized clinical trials, incidence of inappropriate ICD therapy widely ranged from 9% in the AVID trial1 and 11.5% in the MADIT-II trial4, both reported during 2 year follow-up. This high incidence has progressively decreased and currently ranges from a 1.9% to 4.6% annualized rate as shown in the more recent prospective studies27 and large observational studies.26 Several factors have contributed to this impressive reduction in IS rate, including major technical improvements in lead manufacturing and lead performance monitoring, prolongation of arrhythmia detection times, improved discrimination algorithms, and greater adoption of remote monitoring for patient management. Although our systematic review by design was not able to identify the factor(s) most likely contributing to this remarkable reduction in annualized IS rate, the striking low IS rate of the PainFree SST study provides some insights. PainFree SST patients received a device in which a novel suite of detection algorithms in conjunction with routine implementation of a proven programming strategy was activated as default at the time of device implantation. A recent historical comparison of a prospective database found that the use of SST algorithms in new generation CRT-Ds significantly reduced ISs compared with standard CRT-Ds without the algorithms.28 This combination of algorithms and programming resulted in an annualized IS rate of 1.9% for single-chamber ICDs in the PainFree SST study, which was by far lower than the 5% probability and the 3% probability of inappropriate therapy in the MADIT-RIT29 and ADVANCE III studies30, respectively. Notably, the MADIT-RIT study included only dual-chamber or triple-chamber ICDs indicated for primary prevention of sudden death, and the patients had a lower prevalence of AF than the PainFree SST study.
The progressive reduction in IS rates has not been paralleled by a similar remarkable reduction in the annualized appropriate shock rate. This indicates that, despite major advancements in heart failure therapy and patient management over the last decade, the risk of repeated life-threatening arrhythmias in VR-ICD patients has mostly remained unchanged. Conversely, technology and device programming have effectively helped in maximizing the benefit of ICDs. Repeated or multiple shocks are painful, psychologically devastating, possible sources of aggravation of cardiac function, and costly due to hospitalization subsequent to ICD firing.6,31–33 Additionally, a significant association between ICD shock and mortality has been demonstrated,32 although the level of association seems to be stronger for appropriate shocks.33
By reducing the burden of ISs, one may expect a substantial reduction in health care resource utilization and in the cost-effectiveness ratio, as well as increased patient quality-of-life31 and longevity.33 Although these important benefits are common to single-, dual-, and triple-chamber ICDs, there are additional benefits specific to single-chamber ICDs. Dual-chamber devices are more costly for initial implant, are associated with an increased risk of device-related in-hospital complications,6 require a slightly longer follow-up visit,34 and have a greater risk of generator depletion,35 all of which are associated with increased costs compared with VR-ICDs. Although further benefit may be expected by the use of an S-ICD, in our study this type of device was estimated to have a non-statistically significant IS rate 1.81 times the transvenous shock rate. However, a recently presented EFFORTLESS analysis showing an annual IS rate of 3.7% of 985 subjects with S-ICDs followed to 3.1 years suggests that this rate may be improving.36 Also, upcoming modifications to S-ICD devices that include a new T-wave oversensing algorithm and/or a 9 Hz filter are expected to help reduce the level of ISs.37,38 Finally, the SMART study was a head-to-head comparison of discrimination algorithms of various device manufactures and demonstrates that both subcutaneous and transvenous ICDs accurately detect the presence of ventricular tachyarrhythmias with sensitivity approaching 100%.39 However, there were marked differences in the specificity of devices to inhibit therapy for atrial arrhythmias. In addition, the use of dual-chamber discrimination algorithms incorporating both atrial and ventricular intracardiac signals did not significantly improve specificity for atrial arrhythmias compared with single-chamber discrimination.
The annualized rate of ISs was estimated to decrease by a factor of 0.76 for each additional year of mean follow-up. A likely factor here is that some patients are at higher risk of ISs than others. Those at higher risk tend to have events early in follow-up, leaving a lower risk group at the later follow-up times. As the model is only looking at the first event, studies with longer follow-up have lower rates.
Our model-estimated analyses show that most studies observed IS rates that are in agreement with their expected rates—the rate based on mean follow-up, when the study was conducted, whether the device used a subcutaneous or transvenous lead, and whether a VT zone was programmed. The only study that had a significantly lower IS rate than expected based on all other study results was PainFree SST. The low rate of ISs in that study may be attributable to one or more factors that could not be modelled due to being unique to a single study or to incomplete reporting across studies. Factors that may account for the low rate include the specific discrimination algorithms the device employs, programming for delayed-detection, patient-management techniques, or patient risk factors. Recently, Biton et al.40 reported age as an inverse risk factor for inappropriate therapy from the MADIT-RIT trial.
To our knowledge, this is the first systematic review and meta-analysis of appropriate and inappropriate ICD shock rates. Thus, our findings suggest that a contemporary benchmark for future studies of ICD performance should be annualized rates of 5.8% for appropriate shocks and 6.4% for ISs; and one study included in our analysis (PainFree SST) suggests that a rate of 1 in 50 patients receiving an IS is achievable. Although the appropriate shock rates were highly uniform across studies, IS rates appear to be influenced by factors that include the technology configuration, device programming, and rhythm discrimination algorithms. We could not assess patient factors due to inadequate reporting across studies. Therefore, it is important that future reports uniformly include these parameters for comparison.
Limitations
Our study shares similar limitations with previous studies using a similar methodology. First, there was some heterogeneity in the studies with respect to inclusion criteria, patient population, and criteria for ICD implantation. Additionally, co-morbidities and other patient risk factors which may have influenced the use of a VR-ICD rather than a DR-ICD cannot be accounted for. However, the relative balance in the patient characteristics between the VR- and DR- cohort within each of the selected studies should mitigate this concern. Although we used a random-effects model to account for some of this variation, it should be acknowledged that we cannot account for heterogeneity completely through this method. In addition, several potential contributing factors to ISs (i.e. remote monitoring, AF) were not reported for all studies, which prevented estimating the degree of association. This may have contributed to the excess heterogeneity observed across studies in the IS rates.
Conclusions
A comprehensive review of the literature shows that 6.4% of patients with ICDs typically experienced a first IS annually, with a strong trend towards reduction in the most recent years. This is important information that should reassure patients and their families, general practitioners, cardiologists, scientific societies, and regulators. Notably, one of the 16 studies was better than predicted with the lowest reported rate (1.9%) and could not be explained by timing of the study or other covariates which emphasizes the need to continue investing in novel optimization algorithms and modern ICD programming.
Conflict of interest: A. A. is a consultant for Boston Scientific, Medtronic, LivaNova; and receives speaker fees from Medtronic, Boston Scientific, and LivaNova. J.H.H. is an employee of and receives compensation from Medtronic. E.J.S. is a consultant for Medtronic and Boston Scientific; and receives speaker fees from Medtronic. L.D.S. is a consultant for Medtronic, Boston Scientific, and St. Jude Medical. T.K. is a consultant for Medtronic, St. Jude Medical, Biotronik, and Boston Scientific; and receives speaker fees from Medtronic and St. Jude Medical. A.M. receives fellowship support from, and is a consultant for Medtronic. D.H.F. is an employee of and receives compensation from Medtronic. T.R. receives consulting fees from Medtronic.
References
- 1. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med 1997;337:1576–83. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H. et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med 1996;335:1933–40. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 5. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 6. Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP. et al. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA 2013;309:2025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goncalves J, Pereira T.. Inappropriate shocks in patients with ICDs: single chamber versus dual chamber. Arq Bras Cardiol 2013;101:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR. et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. J Am Coll Cardiol 2014;64:1143–77. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 11. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 12. Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W.. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol 2005;28:926–32. [DOI] [PubMed] [Google Scholar]
- 13. Friedman PA, McClelland RL, Bamlet WR, Acosta H, Kessler D, Munger TM. et al. Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation 2006;113:2871–9. [DOI] [PubMed] [Google Scholar]
- 14. Sacher F, Probst V, Iesaka Y, Jacon P, Laborderie J, Mizon-Gerard F. et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation 2006;114: 2317–24. [DOI] [PubMed] [Google Scholar]
- 15. Almendral J, Arribas F, Wolpert C, Ricci R, Adragao P, Cobo E. et al. Dual-chamber defibrillators reduce clinically significant adverse events compared with single-chamber devices: results from the DATAS (Dual chamber and Atrial Tachyarrhythmias Adverse events Study) trial. Europace 2008;10:528–35. [DOI] [PubMed] [Google Scholar]
- 16. Kleemann T, Hochadel M, Strauss M, Skarlos A, Seidl K, Zahn R.. Comparison between atrial fibrillation-triggered implantable cardioverter-defibrillator (ICD) shocks and inappropriate shocks caused by lead failure: different impact on prognosis in clinical practice. J Cardiovasc Electrophysiol 2012;23:735–40. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Enriquez S, Rodriguez-Entem F, Exposito V, Castrillo-Bustamante C, Canteli A, Solloso A. et al. Single-chamber ICD, single-zone therapy in primary and secondary prevention patients: the simpler the better? J Interv Card Electrophysiol 2012;35:343–9. [DOI] [PubMed] [Google Scholar]
- 18. Yang JH, Byeon K, Yim HR, Park JW, Park SJ, Huh J. et al. Predictors and clinical impact of inappropriate implantable cardioverter-defibrillator shocks in Korean patients. J Korean Med Sci 2012;27:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gold MR, Ahmad S, Browne K, Berg KC, Thackeray L, Berger RD.. Prospective comparison of discrimination algorithms to prevent inappropriate ICD therapy: primary results of the Rhythm ID going head to head trial. Heart Rhythm 2012;9:370–7. [DOI] [PubMed] [Google Scholar]
- 20. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ. et al. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 2012;60:1933–9. [DOI] [PubMed] [Google Scholar]
- 21. Deyell MW, Qi A, Chakrabarti S, Yeung-Lai-Wah JA, Tung S, Khoo C. et al. Prognostic impact of inappropriate defibrillator shocks in a population cohort. Heart 2013;99:1250–5. [DOI] [PubMed] [Google Scholar]
- 22. Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS. et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M. et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–53. [DOI] [PubMed] [Google Scholar]
- 24. Kolb C, Sturmer M, Sick P, Reif S, Davy JM, Molon G. et al. Reduced risk for inappropriate implantable cardioverter-defibrillator shocks with dual-chamber therapy compared with single-chamber therapy: results of the randomized OPTION study. JACC Heart Fail 2014;2:611–9. [DOI] [PubMed] [Google Scholar]
- 25. Schwab JO, Bonnemeier H, Kleemann T, Brachmann J, Fischer S, Birkenhauer F. et al. Reduction of inappropriate ICD therapies in patients with primary prevention of sudden cardiac death: DECREASE study. Clin Res Cardiol 2015;104:1021–32. [DOI] [PubMed] [Google Scholar]
- 26. Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB. et al. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol 2015;195:126–33. [DOI] [PubMed] [Google Scholar]
- 27. Auricchio A, Schloss EJ, Kurita T, Meijer A, Gerritse B, Zweibel S. et al. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm 2015;12:926–36. [DOI] [PubMed] [Google Scholar]
- 28. Lunati M, Proclemer A, Boriani G, Landolina M, Locati E, Rordorf R. et al. Reduction of inappropriate anti-tachycardia pacing therapies and shocks by a novel suite of detection algorithms in heart failure patients with cardiac resynchronization therapy defibrillators: a historical comparison of a prospective database. Europace 2016;18:1391–8. [DOI] [PubMed] [Google Scholar]
- 29. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP. et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 30. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB. et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903–11. [DOI] [PubMed] [Google Scholar]
- 31. Mastenbroek MH, Pedersen SS, van der Tweel I, Doevendans PA, Meine M.. Results of ENHANCED Implantable Cardioverter Defibrillator Programming to Reduce Therapies and Improve Quality of Life (from the ENHANCED-ICD Study). Am J Cardiol 2016;117:596–604. [DOI] [PubMed] [Google Scholar]
- 32. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH. et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Proietti R, Labos C, Davis M, Thanassoulis G, Santangeli P, Russo V. et al. A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol 2015;31:270–7. [DOI] [PubMed] [Google Scholar]
- 34. Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J. et al. Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed joint survey. Europace 2011;13:1166–73. [DOI] [PubMed] [Google Scholar]
- 35. von Gunten S, Schaer BA, Yap SC, Szili-Torok T, Kuhne M, Sticherling C. et al . Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace 2015;18:710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boersma LV. Performance and outcomes in patients with the subcutaneous implantable cardiac defibrillator through mid term follow-up: The effortless Study. Presented at the Heart Rhythm Society's Annual Scientific Sessions San Francisco, CA; May 6, 2016.
- 37. Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam V. et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417–23. [DOI] [PubMed] [Google Scholar]
- 38. Theuns DA. Evaluation of a High Pass Filter Designed to Reduce Oversensing in the S-ICD. Presented at the Heart Rhythm Society's Annual Scientific Sessions San Francisco, C;A May 5, 2016.
- 39. Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA. et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol 2012;23:359–66. [DOI] [PubMed] [Google Scholar]
- 40. Biton Y, Huang DT, Goldenberg I, Rosero S, Moss AJ, Kutyifa V. et al. Relationship between age and inappropriate implantable cardioverter-defibrillator therapy in MADIT-RIT (Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy). Heart Rhythm 2016;13:888–93. [DOI] [PubMed] [Google Scholar]