Abstract
Background
Programmed cell death protein-1 (PD-1) blockade therapies have demonstrated durable responses and prolonged survival in a variety of malignancies. Treatment is generally well tolerated although immune-related adverse events (irAEs) can occur. Autoimmune thyroid dysfunction is among the most common irAE, but an assessment of the clinical, mechanistic, and immunologic features has not been previously described.
Patient and methods
Patients with advanced non-small-cell lung cancer (NSCLC) treated with pembrolizumab at Memorial Sloan Kettering Cancer Center (n = 51) as part of KEYNOTE-001 (NCT01295827) were included. Thyroid function test and anti-thyroid antibodies were assessed prospectively at each study visit, beginning before the first treatment. Frequency of development of thyroid dysfunction, association with anti-thyroid antibodies, clinical course, and relationship with progression-free survival and overall survival to treatment with pembrolizumab was evaluated.
Results
Of 51 patients treated, 3 were hypothyroid and 48 were not at baseline. Ten of 48 [21%, 95% confidence interval (CI) 10% to 35%] patients developed thyroid dysfunction requiring thyroid replacement. Anti-thyroid antibodies were present in 8 of 10 patients who developed thyroid dysfunction, compared with 3 of 38 who did not (80% versus 8%, P < 0.0001). Thyroid dysfunction occurred early (median, 42 days) in the pembrolizumab course, and a majority (6 of 10 patients) experienced brief, transient hyperthyroidism preceding the onset of hypothyroidism; no persistent hyperthyroidism occurred. Both hyperthyroidism and hypothyroidism were largely asymptomatic. Overall survival with pembrolizumab was significantly longer in subjects who developed thyroid dysfunction (hazard ratio, 0.29; 95% CI 0.09–0.94; P = 0.04).
Conclusions
Thyroid dysfunction during pembrolizumab treatment of NSCLC is common and is characterized by early-onset, frequently preceded by transient hyperthyroidism, closely associated with anti-thyroid antibodies, and may be associated with improved outcomes. The presence of antibody-mediated toxicity in T-cell-directed therapy suggests an under-recognized impact of PD-1 biology in modulating humoral immunity.
Keywords: PD-1, non-small cell lung cancer, thyroid dysfunction, hypothyroidism, pembrolizumab
Introduction
Therapies targeting the programmed cell death protein-1 (PD-1) pathway have profoundly improved outcomes for patients with a variety of malignancies [1, 2]. PD-1 is an inhibitory co-receptor primarily expressed on the surface of activated T cells which, upon binding to PD-L1 or PD-L2 ligands, modulates T-cell effector function, including proliferation [3], cytokine production [4], and survival [5]. Interruption of PD-1/PD-L1 signaling by monoclonal antibodies can re-invigorate T-cell-mediated anti-tumor immunity, producing durable anti-cancer responses in a subset of patients.
Despite the broad enhancement in the host immune response, PD-1/PD-L1 blockade therapy is usually well tolerated. Side-effects associated with PD-1/PD-L1 blockade have been characterized by a distinct range of toxic effects, termed immune-related adverse events (irAE). Thyroid dysfunction is one of the most common irAEs, with incidence up to 10% in patients treated with PD-1/PD-L1 blockade [2]. Given this frequency, it is critical to examine the clinical manifestations and management of PD-1/PD-L1 blockade-mediated thyroid dysfunction.
The underlying etiology of thyroid dysfunction associated with PD-1/PD-L1 blockade remains uncertain. Thyroid conditions such as Graves’ disease or Hashimoto’s thyroiditis are often associated with anti-thyroid antibodies. It is uncertain whether PD-1/PD-L1 blockade—typically conceived as enhancing T-cell activity—similarly produces thyroid dysfunction as a result of a B-cell-mediated response [6–8]. In this context, we hypothesized that a systematic assessment of immune-related thyroid dysfunction may provide important insight not only into the mechanisms that can drive irAEs but also into the fundamental biological impact of PD-1/PD-L1 blockade. To explore this hypothesis, we examined the clinical course of thyroid abnormalities, their association with thyroid antibodies, and their relationship with clinical outcomes in a cohort of patients with advanced non-small-cell lung cancer (NSCLC) treated with pembrolizumab.
Methods
Patients
Patients with stage IV NSCLC treated with pembrolizumab at Memorial Sloan Kettering Cancer Center (n = 51) as part of KEYNOTE-001 (NCT01295827) were included. All patients received at least one dose of pembrolizumab as previously described [2]. Treatment was given until progression of the disease [determined by immune-related response criteria (irRC) assessed every 9 weeks], unacceptable toxicity, or withdrawal of consent. All patients were followed up for survival until death, loss-to-follow-up, or withdrawal of consent.
Assessments
Thyroid function tests (TFTs), including thyroid-stimulating hormone (TSH), free T4, and T3, were measured at each study visit (every 2 or 3 weeks), beginning before the first treatment. Anti-thyroid antibodies (anti-microsomal and anti-thyroglobulin) were measured at pre-dose of cycle 1 and cycle 2 and then at every other cycle following cycle 2 (details in supplementary material, available at Annals of Oncology online).
At baseline, patients were divided into two groups: (i) hypothyroid for those lacking native thyroid function and/or receiving thyroid hormone-replacement therapy or (ii) non-hypothyroid for those with native thyroid function and no thyroid hormone replacement therapy. Those patients who were hypothyroid at baseline (n = 3) were considered separately. In non-hypothyroid patients (n = 48), thyroid outcomes during treatment with pembrolizumab were characterized as either (i) no thyroid dysfunction: normal TSH levels throughout or transient alterations in TSH (fewer than two consecutive laboratories) not requiring treatment or (ii) thyroid dysfunction: at least two consecutive abnormal TSH levels, ultimately requiring treatment. The clinical severity of those with thyroid dysfunction was graded using CTCAE 4.0 criteria (details in supplementary material and supplementary table S1, available at Annals of Oncology online).
Progression-free survival (PFS) and overall survival (OS) to treatment with pembrolizumab were determined using investigator-determined irRC. Those without progression or death were censored as of the last on-study imaging assessment before the datalock on 7 March 2016.
Statistical analyses
Baseline hypothyroid subjects were excluded from primary analyses (n = 3). Among non-hypothyroid patients, incidence of thyroid dysfunction and anti-thyroid antibodies was described, along with exact 95% confidence intervals.
Median levels of TSH and anti-thyroid antibodies levels were characterized over time. Associations between the development of thyroid dysfunction, anti-thyroid antibodies, other (non-thyroid) irAEs, and clinical features in response to pembrolizumab were examined using Fisher’s exact test. PFS and OS among the two main groups were depicted using Kaplan–Meier method and compared using univariate Cox proportional hazards model with score tests. All statistical tests were two sided and P < 0.05 was set as the level of significance. Statistical analyses were carried out using R (version 3.1.1; R Development Core Team).
Systematic review and meta-analysis
A systematic review was carried out of published studies of PD-1, PD-L1, and/or CTLA-4 blockade therapy in patients with advanced melanoma, NSCLC, renal cell carcinoma (RCC), as of 1 May 2016 (supplementary material, available at Annals of Oncology online). Trials in combination with cytotoxic chemotherapy were excluded. Sample size and reported incidence of hypothyroidism, hyperthyroidism, and/or thyroiditis were collected from each trial and summed as separate events within groups of individual diseases treated with PD-1/PD-L1 blockade, CTLA-4 blockade, PD-1/PD-L1 plus CTLA-4 blockade, respectively. Meta-analysis to describe the incidence of hypothyroidism was conducted using logistic regression. Heterogeneity among studies was tested by the DerSimonian–Laird method. If there was significant evidence of heterogeneity, a random intercept was added to the model. Comparisons of incidence of hypothyroidism in melanoma patients treated with PD-1 blockade versus CTLA-4 blockade versus PD-1/PD-L1 plus CTLA-4 blockade, as well as incidence in PD-1-treated melanoma versus NSCLC versus RCC, were conducted by including the treatment as a covariate in the regression model. R package meta and metafor were used for the analyses.
Results
Characteristics of thyroid dysfunction in patients treated with pembrolizumab
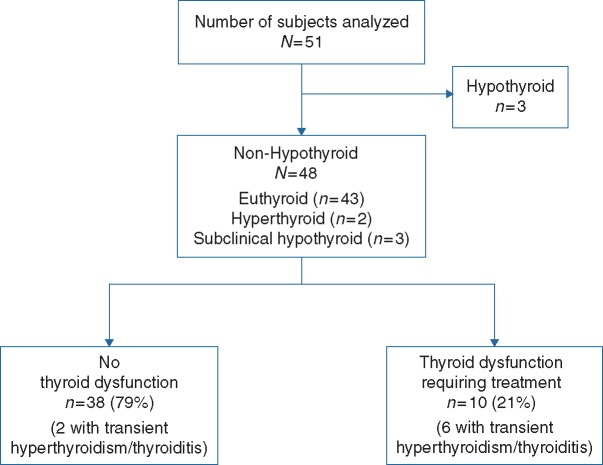
Fifty-one patients with advanced NSCLC treated with pembrolizumab were analyzed: three were hypothyroid at baseline (two had primary hypothyroidism and one had surgically resected thyroid) and were considered separately. Of non-hypothyroid patients, 10 of 48 (21%, 95% CI 10% to 35%) developed thyroid dysfunction and ultimately required thyroid hormone replacement (Figure 1). The severity of hypothyroidism was initially grade 1 in 5 (50%) and grade 2 in 4 (40%) of the 10 subjects; one patient presented grade 3 symptoms (supplementary Table S2, available at Annals of Oncology online). In the five patients with asymptomatic, initially grade 1 hypothyroidism, the median TSH level at the time of initiation of treatment was 42.13 mIU/L (range 5.8–128.92, one patient with levels <10 mIU/L). In symptomatic (i.e. initially grade 2–3) hypothyroidism the median TSH was 88.15 mIU/L (range 34.77–118.01). Long-term thyroid hormone replacement was delivered to all patients with thyroid dysfunction; no evident recovery of thyroid function was observed although discontinuation of hormone replacement was not attempted.
Figure 1.
Consort diagram: subjects treated with pembrolizumab. At baseline, patients were classified according to their thyroid status as hypothyroid and non-hypothyroid. Patients were started on pembrolizumab and thyroid abnormalities were followed up while they remained on treatment.
Six of 10 patients who developed thyroid dysfunction experienced a preceding period of asymptomatic transient hyperthyroidism (Figure 1). No patients required β-blocker, corticosteroids, or methimazole therapy. No patients with TFT abnormalities required delay or discontinuation of pembrolizumab due to clinical impact of the thyroid disorders.
TSH kinetics and association of thyroid dysfunction with anti-thyroid antibodies
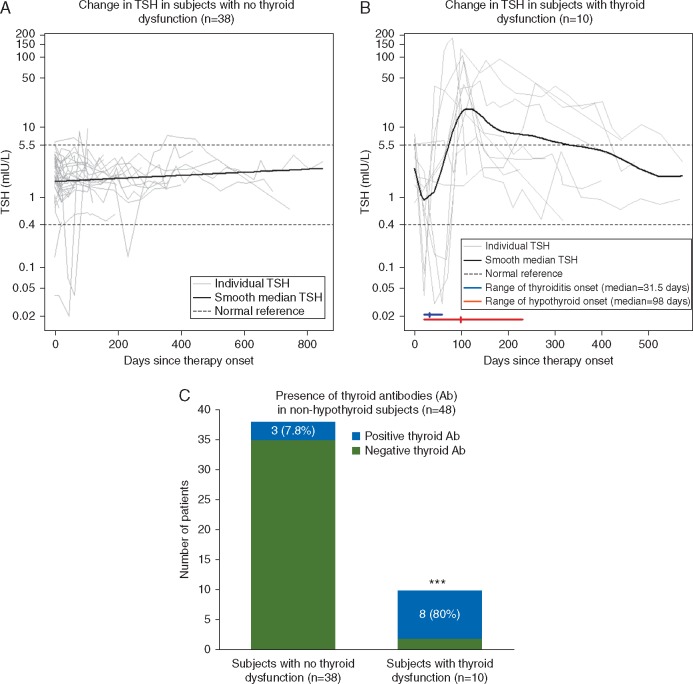
In patients who did not develop thyroid dysfunction, TSH levels remained in the normal range or fluctuated briefly and mildly outside the normal range but returned back to normal (Figure 2A). In patients who developed thyroid dysfunction (Figure 2B), six of 10 initially presented with transient hyperthyroidism (deep decrease in TSH and increased T3 and free T4), occurring shortly after initiation of pembrolizumab. The median onset of transient hyperthyroidism was 32 days (range 21–59 days) with a median duration of 27 days (range 21–60 days). The onset of hypothyroidism presented later (median time of onset 98 days, range 20–231 days).
Figure 2.
Comparison of thyroid-stimulating hormone (TSH) kinetics in non-hypothyroid subjects treated with pembrolizumab and association with thyroid antibodies. TSH levels were measured over time in subjects treated with pembrolizumab, bold line represents the median TSH level among (A) subjects who did not develop thyroid dysfunction and (B) subjects who developed thyroid dysfunction. Grey lines represent each of the subjects. Dashed lines represent normal TSH ranges (0.4–5.5 mIU/L). Median time (hash) and range (horizontal line) of onset of transient hyperthyroidism/thyroiditis (blue) and hypothyroidism (red) were also described. (C) Incidence of anti-thyroid antibodies was estimated in non-hypothyroid at baseline treated with pembrolizumab. A patient was considered to have positive antibodies if either anti-thyroglobulin or anti-microsomal antibodies were present at any point during the treatment. ***P < 0.0001 two-tailed Fisher’s exact test.
Eleven of forty-eight (23%, 95% CI 12% to 37%) patients had positive anti-thyroglobulin and/or anti-microsomal antibodies, 4 of whom were present at baseline and 7 developed during pembrolizumab treatment. Anti-thyroid antibodies were present in 8 of 10 patients who developed thyroid dysfunction, compared with 3 of 38 who did not (80% versus 8%, P < 0.0001) (Figure 2C and supplementary Figure S1, available at Annals of Oncology online). Six of the seven patients in whom anti-thyroid antibodies developed, antibody onset coincided with onset of transient hyperthyroidism and preceded hypothyroidism (supplementary Figure S2, available at Annals of Oncology online).
There was no difference in the incidence of other (non-thyroid) irAE in subjects who developed thyroid dysfunction compared with those who did not (supplementary Figure S3 and Table S3, available at Annals of Oncology online).
Association of immune-related thyroid dysfunction and clinical outcomes
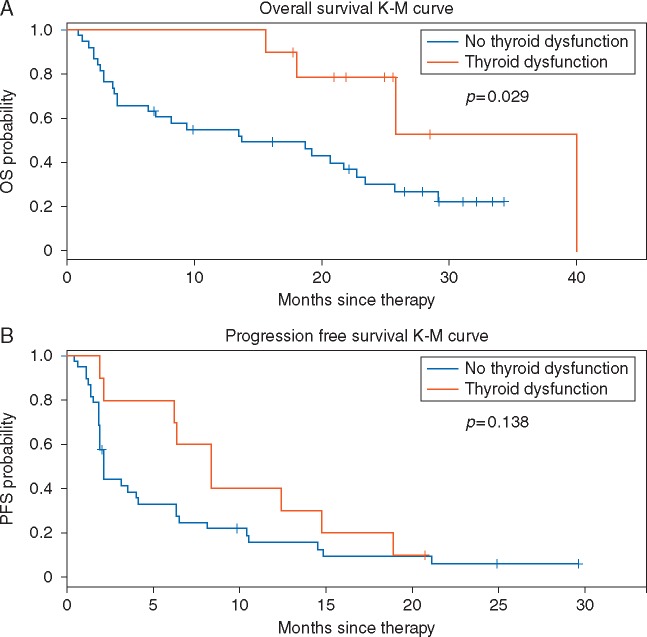
There were no evident differences in the baseline clinical characteristics of patients with or without thyroid dysfunction (supplementary Table S4, available at Annals of Oncology online). In patients who developed thyroid dysfunction, the median OS on pembrolizumab was significantly longer than those without thyroid dysfunction (median 40 versus 14 months, HR 0.29; 95% CI 0.09–0.94, P = 0.029, Figure 3A). PFS was numerically but not statistically significantly longer (median 8 versus 2 months, HR 0.58; 95% CI 0.27–1.21, P = 0.14, Figure 3B).
Figure 3.
Association between immune-related thyroid dysfunction and clinical outcomes. Kaplan–Meier (K–M) plots of (A) overall survival (OS) and (B) progression-free survival (PFS) in subjects who developed thyroid dysfunction (n = 10) versus subjects who did not (n = 38).
Systematic review and meta-analysis of incidence of thyroid dysfunction in subjects treated with checkpoint inhibitors
Thirty-five published trials of checkpoint inhibitors were identified in melanoma (n = 2969 with PD-1/PD-L1; n = 1877 with CTLA-4; n = 460 with PD-1 plus CTLA-4 blockade), NSCLC (n = 1712 with PD-1/PD-L1; n = 99 with PD-1/PD-L1 plus CTLA-4 blockade) and RCC (n = 201 with PD-1 blockade). There was no reported incidence of thyroid dysfunction in trials in monotherapy with PD-L1 inhibitors (supplementary Table S5, available at Annals of Oncology online). Among patients with melanoma, the rate of hypothyroidism was 3.6% with CTLA-4 blockade compared with 7.5% with PD-1 blockade (P = 0.012) and up to 14.6% in those treated with combination therapy (P = 0.034 compared with PD-1 monotherapy) (Figure 4). Rates of hypothyroidism in patients treated with PD-1 monotherapy were consistent across diseases (7.5% in melanoma, 7.1% in NSCLC, and 6.5% in RCC, P = 0.79).
Figure 4.
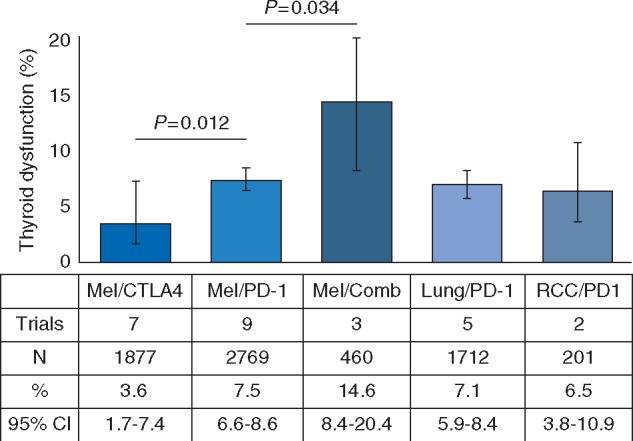
Incidence of thyroid dysfunction in patients with melanoma (Mel), lung cancer, renal cell carcinoma (RCC), and bladder cancer treated with checkpoint inhibitors. The percent incidence of hypothyroidism is depicted (with associated 95% confidence intervals) within each disease and treatment cohort. P values of individual comparisons are reported. Comb, Combination therapy.
Discussion
This is the first study to describe the clinical and immunologic features of thyroid dysfunction in patients treated with PD-1 blockade. In this cohort, 21% of patients with NSCLC treated with pembrolizumab developed thyroid dysfunction, which was strongly associated with anti-thyroid antibodies. Nearly all cases occurred early in the course of PD-1 therapy, with relatively uniform timing and pattern of onset. Many patients who ultimately developed hypothyroidism were preceded by a transient and asymptomatic period of hyperthyroidism. Hypothyroidism presented with no or mild symptoms and did not preclude the continuation of immunotherapy. Of note, two patients with pre-existing hypothyroidism required no adjustment in thyroid replacement while one required an increased dose. Overall, this experience generally supports the safe use of PD-1 blockade in patients with prior, ongoing, or new autoimmune thyroid conditions.
The incidence of thyroid dysfunction in this series is higher than in reports of larger anti-PD-1/PD-L1 clinical trials, where rates range from 2% to 10% (supplementary Table S5, available at Annals of Oncology online). Routine surveillance of thyroid function studies at each study visit increases identification of these disorders, as symptoms of thyroid dysfunction are often absent or vague. We note that there is possible under-reporting of thyroid dysfunction in larger clinical trials as CTCAE grading depends on TSH changes, clinical symptoms, and/or the need for treatment rather than using TSH levels alone. We suggest that given the subtle clinical presentation of thyroid function, routine TSH levels should be obtained before and during anti-PD-1 treatment, especially during the initial months of treatment. Additionally, as there are not specific guidelines about management of hypothyroidism in asymptomatic patients treated with PD-1 inhibitors, we propose using clinical guidelines based on other clinical scenarios in which patients with subclinical hypothyroidism and TSH levels ≥10 mIU/L should receive thyroid-replacement therapy [9, 10].
The relatively uniform pattern of thyroid dysfunction during PD-1 blockade treatment—early-onset with initial period of transient hyperthyroidism followed by thyroid failure—is consistent with a mechanism of acute inflammation and destruction of the thyroid gland. We found that anti-thyroid antibodies are highly, and temporally, associated with nearly all cases of thyroid dysfunction. This is perhaps surprising given that PD-1 blockade is typically considered a T-cell-directed therapy. This finding highlights two provocative speculations that we believe may provide important insight to immunobiology of PD-1 and auto-immunity in general.
The first is that PD-1 (and PD-1 inhibitors) may, beyond the well-known impact on T-cell function, also importantly regulate the humoral immune response. PD-1 is highly expressed on activated B cells [11]. Also modulation of B cells via both T-cell-independent [12] and T-cell-dependent mechanisms [13, 14] have been described. Although some immune-related toxicity in PD-1 pre-clinical models may be antibody mediated [15, 16], this association appears to be under-studied in human trials. In addition, our meta-analysis highlights that occurrence of thyroid dysfunction may be more common with PD-1 blockade compared with CTLA-4 blockade. Recognition of the impact of PD-1 blockade, specifically, on humoral immunity could have important implications for understanding the etiology of irAEs and optimizing more precise management strategies. More importantly, it raises the question of whether some component of anti-tumor efficacy related to PD-1 blockade may similarly be antibody mediated.
The second is development of anti-thyroid antibodies shortly after initiation of pembrolizumab suggests that PD-1 inhibitors may be modulating auto-immune equilibrium and unmasking latent auto-immunity. Although the incidence of auto-immune thyroiditis is reported to be 4% in the general population, up to 11% of healthy subjects can present thyroid autoantibodies [17], suggesting that there are people characterized by sub-detectable levels of auto-immunity that, in absence of PD-1 blockade or other immune perturbations, remains quiescent [18]. Similar effects have been observed in patients treated with IFN-α for melanoma [19]. Although a breakdown of self-tolerance is suspected to lead to thyroid autoimmunity [20], the mechanisms of how PD-1 blockade may reveal such auto-immunity remain unclear.
Finally, it is notable that there is a correlation between the presence of thyroid dysfunction and increased survival with pembrolizumab. Although trends between irAEs and outcomes have been previously described in melanoma patients [21, 22], this is the first report in patients with NSCLC. We note that the early onset of thyroid dysfunction in this series suggests that this association is not simply related to patients who remain on therapy longer being at greater risk to toxicity. Whether there is a specific mechanistic link between anti-thyroid immunity and anti-tumor immunity is unknown and larger patient sample sizes are needed to validate this association.
In conclusion, thyroid dysfunction is common, occurs early, and is clinically mild in patients with NSCLC treated with PD-1 blockade. Importantly, this toxicity appears to be antibody mediated. We believe these data provide important clues toward a previously under-recognized role of PD-1 in modulating humoral immunity, which could be relevant, both for considering the mechanism of PD-1-mediated toxicity and anti-tumor efficacy. Given the rapid kinetics of anti-thyroid antibodies and toxicity, we propose these findings are suggestive of latent underlying auto-immunity, which may be more common than previously known and modifiable by PD-1 signaling. Although mechanistic studies are clearly needed to define the role of PD-1 in cancer and auto-immunity, our report suggests the immunologic consequences of pembrolizumab and other anti-PD-1 therapies extend far beyond their current conceptualization as T-cell checkpoint blockade therapy.
key message
In NSCLC treated with PD-1 blockade, thyroid dysfunction is common, characterized by early-onset, often preceded by transient hyperthyroidism, and can be associated with improved clinical outcomes. Thyroid toxicity appears to be antibody-mediated. These data provide insight to guide clinical management and highlight the under-recognized role of PD-1 in modulating humoral immunity.
Funding
National Cancer Institute at the National Institutes of Health, Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (grant number: P30 CA008748), The Fiona and Stanley Druckenmiller Center for Lung Cancer Research (DCLCR), and KEYNOTE-001 (NCT01295827) was supported by Merck Sharp & Dohme, a subsidiary of Merck.
Disclosure
JEC reports honoraria from DAVAOncology; reports consulting or advisory role for Genentech/Roche, Clovis Oncology, AstraZeneca/MedImmune; received research funding from Lilly (MSKCC), Genentech/Roche (MSKCC), Bristol-Myers Squibb (MSKCC), AstraZeneca/MedImmune (MSKCC). JDW has stock or other ownership in Potenza Therapeutics and Vesuvius Pharmaceuticals; receives honoraria from Regeneron; reports consulting or advisory role for Bristol-Myers Squibb, Merck, MedImmune, ZIOPHARM Oncology, Polynoma, Polaris, Jounce Therapeutics, Genentech, F-Star, Beigene, Advaxis, Sellas, Lilly, Potenza Therapeutics, Tizona Therapeutics, Inc., Amgen, AstraZeneca, Chugai Pharma; received research funding from Bristol-Myers Squibb (MSKCC); receives patents, royalties, other intellectual property; co-inventor on an issued patent for DNA vaccines for treatment of cancer in companion animals; co-inventor on a patent for use of oncolytic Newcastle Disease virus; receives travel, accommodations, expenses from Bristol-Myers Squibb, Chugai Pharma, Roche, Janssen, Kadmon, Regeneron. Charles M. Rudin reports consulting or advisory role for Abbvie, AVEO, Boehringer Ingelheim, GlaxoSmithKline, Merck, Celgene, Novartis, Bristol-Myers Squibb; received research funding from Biomarin. MDH reports consulting or advisory role for Third Rock Ventures, Bristol-Myers Squibb, Merck, Genentech, Alexion Pharmaceuticals, Inovio Pharmaceuticals, AstraZeneca/MedImmune; received research funding from Bristol-Myers Squibb. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Ribas A, Hamid O, Daud A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016; 315: 1600–1609. [DOI] [PubMed] [Google Scholar]
- 2. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 3. Parry RV, Chemnitz JM, Frauwirth KA. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005; 25: 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett F, Luxenberg D, Ling V. et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol 2003; 170: 711–718. [DOI] [PubMed] [Google Scholar]
- 5. Ishida Y, Agata Y, Shibahara K, Honjo T.. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. J. J 1992; 11: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishimura H, Minato N, Nakano T, Honjo T.. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol 1998; 10: 1563–1572. [DOI] [PubMed] [Google Scholar]
- 7. Good-Jacobson KL, Szumilas CG, Chen L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 2010; 11: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol 2011; 187: 5183–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garber JR, Cobin RH, Gharib H. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18: 988–1028. [DOI] [PubMed] [Google Scholar]
- 10. Pearce SH, Brabant G, Duntas LH. et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J 2013; 2: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Velu V, Titanji K, Zhu B. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009; 458: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thibult ML, Mamessier E, Gertner-Dardenne J. et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol 2013; 25: 129–137. [DOI] [PubMed] [Google Scholar]
- 13. Kawamoto S, Tran TH, Maruya M. et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012; 336: 485–489. [DOI] [PubMed] [Google Scholar]
- 14. Sage PT, Francisco LM, Carman CV, Sharpe AH.. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013; 14: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishimura H, Nose M, Hiai H. et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 16. Okazaki T, Tanaka Y, Nishio R. et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003; 9: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 17. Hollowell JG, Staehling NW, Flanders WD. et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 489–499. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida H, Amino N, Yagawa K. et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab 1978; 46: 859–862. [DOI] [PubMed] [Google Scholar]
- 19. Gogas H, Ioannovich J, Dafni U. et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006; 354: 709–718. [DOI] [PubMed] [Google Scholar]
- 20. McLachlan SM, Rapoport B.. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev 2014; 35: 59–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Downey SG, Klapper JA, Smith FO. et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res 2007; 13: 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman-Keller M, Kim Y, Cronin H. et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016; 22: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.