Abstract
Background
Sarcomas are rare but aggressive diseases. Specialized multidisciplinary management is not implemented for all patients in most countries. We investigated the impact of a multidisciplinary tumor board (MDTB) presentation before treatment in a nationwide study over 5 years.
Patients and methods
NETSARC (netsarc.org) is a network of 26 reference sarcoma centers with specialized MDTB, funded by the French National Cancer Institute to improve the outcome of sarcoma patients. Since 2010, presentation to an MDTB and second pathological review are mandatory for sarcoma patients in France. Patients’ characteristics and follow-up are collected in a database regularly monitored and updated. The management and survival of patients presented to these MDTB before versus after initial treatment were analyzed.
Results
Out of the 12 528 patients aged ≥15 years, with a first diagnosis of soft tissue and visceral sarcoma obtained between 1 January 2010 and 31 December 2014, 5281 (42.2%) and 7247 (57.8%) were presented to the MDTB before and after the initiation of treatment, respectively. The former group had generally worse prognostic characteristics. Presentation to a MDTB before treatment was associated with a better compliance to clinical practice guidelines, for example, biopsy before surgery, imaging, quality of initial surgery, and less reoperations (all P < 0.001). Local relapse-free survival and relapse-free survival were significantly better in patients presented to a MDTB before initiation of treatment, both in univariate and multivariate analysis.
Conclusion
The compliance to clinical practice guidelines and relapse-free survival of sarcoma patients are significantly better when the initial treatment is guided by a pre-therapeutic specialized MDTB.
Keywords: sarcoma, multidisciplinarity, tumor board, clinical practice guidelines, relapse, survival
Introduction
Soft tissue and visceral sarcomas (STS) constitute a heterogeneous group of rare connective tissue cancers, in terms of histology, molecular biology, and clinical presentations, and an estimated incidence of 5.6–5.9/100 000 per year [1–4]. Because of their rarity, STS are often initially misrecognized, misdiagnosed, and as a consequence not treated according to clinical practice guidelines (CPG) [5–7]. Inadequate diagnostic procedures and treatment, for instance, enucleation of the tumor as initial surgery without initial imaging or biopsy, are observed in a large fraction of patients and often qualified as ‘whoops’ procedures [4–8].
However, optimal surgical removal of sarcoma, with en bloc resection, is the mainstay of the curative treatment of localized STS [4–8]. The quality of initial surgery is a major prognostic factor for recurrence-free survival and overall survival in all series [9–12]. In all CPGs, it is recommended that the management of sarcoma patients should be carried out by a dedicated multidisciplinary team, including expert pathologists, radiologist, surgeons, radiation oncologist and medical oncologists treating a large number of patients. In most countries, the diagnosis and treatment of patients with sarcoma can be carried out in any oncology facility. Conversely, in some countries (e.g. Scandinavian countries, UK), the management of sarcoma patients must be carried out in dedicated reference centers [6–8, 13]. Most CPG recommended that patients with a suspected diagnosis of STS should be referred at a sarcoma center before any treatment [6–8].
The French National Cancer Institute (INCa), validated and supported the creation of a clinical network for sarcoma (NetSarc) in 2009, which was missioned to improve the management and outcome of sarcoma patients. Twenty-six reference centers were identified in this clinical network. Netsarc is linked to a sarcoma pathological reference network (RRePS ‘Network for expert pathology diagnosis in sarcoma’), of 23 reference centers for sarcoma pathology in charge of the second histological review of each suspected case of sarcoma. A common database (netsarc.org) gathering all cases of histologically reviewed sarcoma presented in multidisciplinary tumor boards (MDTB) was created and implemented, describing the diagnostic, therapeutic management, and the clinical outcome in terms of relapse and survival.
As of 8 December 2016, this database included 37 833 sarcoma patients, with 12 528 patients aged >15 years enrolled between 1 January 2010 and 31 December 2014. The aim of the present study is to evaluate the impact of the presentation of the patients with soft tissue or visceral sarcoma to a specialized MDTB before the initial treatment.
Patients and methods
The network
Each Netsarc center proposes a MDTB board gathering at minimum sarcoma specialized, pathologist(s), radiologist(s), surgeon(s), radiation oncologist(s), medical oncologist(s), and often molecular biologist(s), orthopedist(s), pediatrician(s). All sarcoma patient cases, discussed in MDTB within NetSarc centers were recorded in the database, by a dedicated team of Clinical research assistant (CRAs), supervised by three Coordinating centers (Centre Leon Berard, Gustave Roussy, Institut Bergonié). Patient files may be presented by the primary care physician at any step: before any diagnostic procedure, before initial biopsy, before primary surgery, after primary surgery, at relapse, and/or in case of a possible inclusion in a clinical trial. Patients and treatment data were prospectively included and regularly updated by the dedicated study coordinators. Monitoring of the centers is carried out by the three coordinating centers on a regular basis.
The NETSARC database
The objectives of NETSARC are as follows: (i) to obtain an exhaustive description of the incident and prevalent population of sarcoma patients in France, by cross comparison of the pathological review database (rreps.org) and of the clinical database (netsarc.org), (ii) to monitor the diagnostic and initial treatment procedures, and (iii) to monitor patient outcome in particular survival and relapse. The database includes a limited set of data, on purpose, describing patients and tumor characteristics, surgery, relapse and survival. The center which carried out the first resection is documented. The surgical resection system (R) from the Union International Centre le cancer (UICC) was chosen to define the quality of surgery. This system distinguishes the quality of resection (R), using the surgical and pathological report: R0 = macroscopically complete en bloc resection with in sano resection margins, R1 = same, but with tumor cells visible on resection margins, and R2 = macroscopic residual disease. All data presented here were extracted from the netsarc.org database accessible online.
Statistical analyses
The categorical data were summarized by the frequencies and percentages, and the continuous covariates have been summarized with median, range and numbers of non-missing observations. The statistical test used for comparison was a chi-square (or a Fisher’s exact) test for categorical covariates. A Kruskal–Wallis test was used for covariates with more than two ordered categories or for continuous variables. The diagnostic date is the date of histological diagnosis (biopsy or first surgery). Survival is calculated from the date of diagnosis to the date of last follow-up or death. Local relapse-free survival (LRFS) is computed from the diagnostic date to the date of the last follow-up or the date of the first local recurrence. Relapse-free survival (RFS) is computed from the date of diagnosis to the date of the last follow-up or the date of the first metastasis. Survival curves were plotted using a Kaplan–Meier method. Survival was compared using the log-rank test. Univariate and multivariate analyses for LRFS and RFS included the following: (i) classical prognostic factors for sarcoma, and (ii) variables differing across the two groups (MDTB before versus after treatment) which had a prognostic impact on LRFS, RFS, or OS. These included gender, age, grade [14], sarcoma size, site, depth, presentation at the MDTB before versus after initiation of treatment. Cox proportional hazard model was used for the multivariate analysis, introducing parameters significant in univariate analysis. Factors included in the multivariate model were identified by a backward selection procedure which entails including all the covariates in the model and removing those whose P value is >0.05 one at a time. At each step of the model, all included variables were tested and removed if they were no longer associated with the outcome considering a 5% type one error (P ≥0.05). All statistical tests were two-sided. All statistical analyses were carried out using SPSS (version 19.0).
Results
Patient population
As of 8 December 2016, 37 833 patients were prospectively included in the Netsarc database. The patient population studied here is that of patients aged 15 years and above, with an histologically reviewed and confirmed soft tissue or visceral sarcoma, diagnosed in France from 1 January 2010 to 31 December 2014, in any anatomic site. Bone sarcomas and desmoid tumors were not included. About 12 528 patients match these criteria and are included in this analysis (Table 1).
Table 1.
Description of the patient population
| Characteristics | Incident patients N = 12 528 |
|---|---|
| Gender | |
| Male | 6207 (49.6%) |
| Female | 6321 (51.1%) |
| Age at first diagnosis | |
| Median (min–max) | 61 (18–101) |
| Type of tumor | |
| Soft tissue | 9604 (76.7%) |
| Visceral | 2924 (23.3%) |
| Site | |
| Head and neck | 860 (6.9%) |
| Trunk | 6791 (54.2%) |
| Retroperitoneum | 1225 (9.8%) |
| Trunk wall | 1396 (11.1%) |
| Upper limb | 1126 (8.9%) |
| Lower limb | 3751 (29.9%) |
| Size of the tumor | |
| Median (min–max) | 96 (1–940) |
| Histology | |
| Leiomyosarcoma | 1617 (12.9%) |
| GIST | 1103 (8.8%) |
| Dediff liposarcoma (LPS) | 987 (7.9%) |
| Well diff. LPS | 906 (7.2%) |
| Myxoid LPS | 347 (2.8) |
| UPS | 890 (7.1%) |
| Myxofibrosarcoma | 561 (4.5%) |
| Other > 100 cases | 4576 (36.5%) |
| Other < 100 cases | 1541 (12.3%) |
| Grade | |
| 1 | 1802 (14.5%) |
| 2 | 2544 (20.3%) |
| 3 | 3546 (28.3%) |
| Unknown | 2945 (23.5%) |
| Non-applicable | 1691 (13.5%) |
| Metastases at diagnosis | |
| Yes | 1594 (12.7%) |
| No | 9646 (77.0%) |
| Unknown | 1288 (10.2%) |
| No of pts managed by NETSARC center | |
| Median (min–max) | 234 (44–1351) |
Patient characteristics
We compared the characteristics of patients presented in a Netsarc MDTB prior (N = 5281, 42.2%) or after (N = 7247, 57.8%) the primary treatment (Table 2). The proportion of patients presented to 1 of the 26 Netsarc MDTB before initiation of treatment increased from 38.9% to 45.7% (P < 0.001) between 2010 and 2014. Overall, the characteristics of patients presented to a NETSARC MDTB before treatment were less favorable, with a higher proportion of patients with metastasis at diagnosis and with deep seated sarcomas, larger tumors, of worse grade (all P < 0.001, see Table 2). Locations were also significantly different, with less visceral sarcomas in patients referred to MDTB before the initiation of first treatment, more retroperitoneal sarcomas, but less head and neck sarcomas (P < 0.001 for all sites, Table 2)
Table 2.
Patients’ characteristics, procedures, and NETSARC MDTB
| Patient characteristics | NETSARC MDTB before treatment |
P value | |
|---|---|---|---|
| Yes (N = 5281) | No (N = 7247) | ||
| Gender | |||
| Female | 2507 (47.5%) | 3814 (52.6%) | |
| Male | 2774 (52.5%) | 3433 (47.4%) | |
| Tumor size (mean, SE) | 109.6 (80.6) | 85.6 (75.4) | <0.001 |
| Site | |||
| Soft tissue | 4492 (85.1%) | 5112 (70.5) | <0.001 |
| Visceral | 789 (14.9%) | 2135 (29.5%) | |
| Deep seated | |||
| No | 671 (12.7%) | 1574 (21.7%) | <0.001 |
| Yes | 4449 (84.2%) | 5377 (74.2%) | |
| Unknown | 161 (3.0%) | 314 (4.1%) | |
| Grade | |||
| 1 | 716 (13.6%) | 1086 (15.0%) | <0.001 |
| 2 | 1058 (20.0%) | 1486 (20.5%) | |
| 3 | 1463 (27.7%) | 2083 (28.7%) | |
| Unknown | 1345 (25.4%) | 1600 (22.1%) | |
| NA | 699 (13.2%) | 992 (13.6%) | |
| Metastasis at diagnosis | |||
| Yes | 832 (15.8%) | 762 (10.5%) | <0.001 |
| No | 3904 (73.9%) | 5742 (79.2%) | |
| Unknown | 545 (10.3%) | 743 (10.3%) | |
| Diagnostic procedures | |||
| Imaging of the primary tumor | |||
| Yes | 4642 (87.9%) | 4375 (60.4%) | <0.001 |
| No | 171 (3.2%) | 623 (8.6%) | |
| Unknown | 468 (8.9%) | 2249 (31.0%) | |
| Diagnostic biopsy | |||
| Yes | 4633 (87.7%) | 3036 (41.9%) | <0.001 |
| No | 481 (9.1%) | 3315 (45.7%) | |
| Unknown | 167 (3.2%) | 896 (12.4%) | |
| Therapeutic procedurea | |||
| Quality of first surgeryb | |||
| R0 | 1436 (52.6%) | 1968 (32.2%) | <0.001 |
| R1 | 845 (30.9%) | 1965 (32.1%) | |
| R2 | 204 (7.1%) | 1148 (18.8%) | |
| NE | 246 (9.1%) | 1032 (16.9%) | |
| Reexcision after first surgery | |||
| Yes | 165 (6.0%) | 1065 (17.4%) | <0.001 |
| No | 2320 (85.0%) | 4916 (65.7%) | |
| NE | 246 (9.1%) | 1032 (16.9%) | |
| Quality of final surgeryb | |||
| R0 | 1571 (57.5%) | 2845 (46.5%) | <0.001 |
| R1 | 773 (28.3%) | 1529 (25.0%) | |
| R2 | 141 (5.1%) | 707 (11.5%) | |
| NE | 246 (9.1%) | 1032 (16.9%) | |
Missing data in 3684 patients who had not been operated at the time of the analysis. Only non-metastatic patients in whom surgery was documented are described; these are 8844 patients, in whom MDTB was before treatment (N = 2731), or after treatment (N = 6113).
Quality of resection (R) is classified as: R0 = macroscopically complete en bloc resection with in sano resection margins, R1 = same, but with tumor cells visible on resection margins, R2 = macroscopic residual disease.
Diagnostic before treatment
Clinical practice guidelines recommend that imaging and biopsy should be carried out before any therapeutic approach in sarcoma [6–8]. A higher number of patients presented to a Netsarc MDTB before treatment had adequate imaging of the tumor before treatment (Table 3). Similarly, a higher proportion of patients presented to a MDTB before surgery had biopsy before surgery: only 41% of the patients treated before presentation to a NetSARC MDTB had a biopsy versus 85.7% in patients presented to a MDT (Table 2).
Table 3.
Multivariate analysis of prognostic factors for survival
| Beta | E.S. | HR | P value | |
|---|---|---|---|---|
| Local relapse-free survival | ||||
| MDTB after treatment | 0.590 | 0.071 | 1.804 | 0.000 |
| Grade 3 | 0.272 | 0.064 | 1.312 | 0.000 |
| Age | 0.007 | 0.002 | 1.007 | 0.000 |
| Tumor size | 0.002 | 0.000 | 1.002 | 0.000 |
| Internal trunk | −0.353 | 0.081 | 0.703 | 0.000 |
| Limb site | 0.343 | 0.079 | 0.710 | 0.000 |
| Grade NA | −0.340 | 0.114 | 0.712 | 0.003 |
| Grade 1 | 0.377 | 0.096 | 0.686 | 0.000 |
| Relapse-free survival | ||||
| Grade 3 | 0.634 | 0.072 | 1.886 | 0.000 |
| MDT after treatment | 0.234 | 0.052 | 1.263 | 0.000 |
| Age | 0.005 | 0.001 | 1.005 | 0.001 |
| Tumor size | 0.002 | 0.000 | 1.002 | 0.000 |
| Limb site | −0.289 | 0.065 | 0.749 | 0.000 |
| Grade NA | −0.317 | 0.106 | 0.728 | 0.003 |
| Internal trunk | −0.151 | 0.068 | 0.860 | 0.025 |
| Grade 1 | −0.482 | 0.096 | 0.618 | 0.000 |
Cox model was carried out including all significant variables in univariate analysis and using a backward selection procedure which entails including all the covariates in the model and removing those whose P value is >0.05 one at a time. At each step of the model, all included variables were tested and removed if they were no longer associated with the outcome considering a 5% type one error (P ≥0.05).
Grade NA, not applicable.
Quality of surgical resection
We then focused on the 8844 patients with a documented absence of metastasis and in whom surgery was mentioned in the database. As expected, a higher proportion of patients presented to MDTB after treatment had reported surgery, since this was a selection criteria (‘treatment before’) for this group. The quality of the primary surgery carried out after versus before presentation to the Netsarc MDTB was superior with significantly more R0 resections, less R2 resections, and less resections non-evaluable for this parameter (Table 2) (P < 0.001). About 1065 (17.4%) patients had secondary resection when treatment was carried out before NetSarc MDTB versus 165 (6.0%) in those where treatment was given after MDTB (P < 0.001). After this secondary resection, the quality of the final surgery remained better for patients presented to a MDTB before versus after surgery with significantly more R0 resections and less R2 resections. Re-operation do not compensate for the inadequate initial patient management (P < 0.001) (Table 2).
Survival
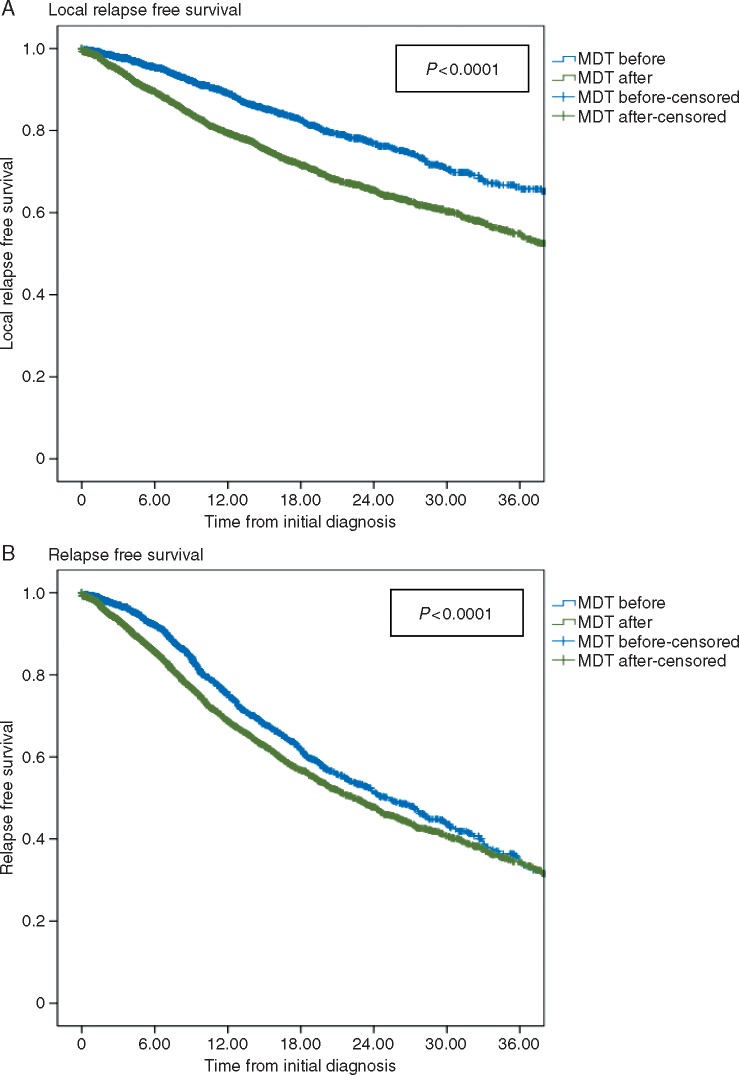
We analyzed here the population of 9646 patients without diagnosed metastasis at initial diagnosis. With a median follow-up of 26 months, the LRFS of patients treated before presentation to a MDTB was significantly worse than that of the remaining patients, with a 2-year LRFS of 65.4%, versus 76.9% in these two groups, respectively (Figure 1A, P < 0.001). RFS was also significantly worse for patients treated before presentation to a MDTB than for those presented before, with a 2-year LRFS of 46.6%, versus 51.7% in each groups (Figure 1B, P < 0.001). Multivariate analysis in non-metastatic patients included the parameters differing between the two groups (MDTB before versus after treatment), with prognostic value for LRFS and RFS (gender, age, size, tumor site, grade, depth, presentation to a MDTB before treatment). The latter was associated with the highest risk ratio for LRFS and was also a strong independent negative prognostic factor for RFS (Table 3). Overall survival was too early to assess given the median follow-up (not shown).
Figure 1.
Local relapse-free survival and relapse-free survival in non-metastatic sarcoma patients according to the date of MDTB presentation. (A) Local relapse-free survival in patients presented to a MDTB before, versus after initiation of treatment. (B) Relapse-free survival in patients presented to a MDTB before, versus after initiation of treatment.
Discussion
Sarcomas are rare cancers for which multidisciplinary management in a reference center is recommended in all CPG [6–8]. Most sarcoma patients are however not managed in reference centers, worldwide. The impact of a management without the support of a specialized multidisciplinary team on relapse and survival of sarcoma patients is not precisely known.
The question addressed in this work was whether a multidisciplinary assessment conducted by a dedicated multidisciplinary sarcoma team, a ‘MDTB’ before the therapeutic procedure influences patient management and outcome. The general conclusion is that presentation of the patient to an MDTB before any treatment procedure is associated with a better compliance to the CPG, a better quality of surgery, a better RFS, with significantly less reoperation. Its impact on long-term outcome, survival, will need to be assessed with a longer follow-up.
Netsarc is a nationwide project aiming to improve the management and outcome of sarcoma patients. In order to be able to measure and report the outcome of sarcoma patients, the Netsarc database collected prospectively patient and tumor related information since 1 January 2010 from the 26 reference centers. This article reports an analysis of only incident patients with sarcoma aged ≥15 years with an initial diagnosis from 1 January 2010 to 31 December 2014. In view of the incidence of sarcomas, the expected number of incident case of sarcoma was close to 3900 per year in France; for soft tissue and visceral sarcoma of patients aged over 15 years (4.9/100 000 per year), it was close to 3070 per year. Overall, in this 5-year period, 15 350 incident cases of sarcomas were thus expected. The 12 528 patients matching these criteria identified in the Netsarc database represent 81.6% of this theoretical incidence, and therefore a large fraction of the incident population of sarcoma patient in this country.
This population of patients is a real life population: 12% of patients have metastasis; elderly patients >80 (n = 1474, 11.8%) and 90 (n = 107, 0.8%) also represent a significant proportion of this population usually not captured in clinical trials. Although the database aimed to be exhaustive, clinical and tumor parameters were not available for all patients; for example, unknown grade (23%) or unknown metastatic static status (10%). Information was lacking both in reference and non-reference centers, and this is an obvious objective of improvement for this project.
Patients whose files were discussed in a MDTB before treatment had worse prognosis features: more male patients, larger tumors, of higher grade, and more frequently metastatic. This suggests that primary care physicians may refer earlier sarcoma patient with bad prognostic features to the reference centers. Patients presented to a MDTB before treatment were managed more frequently in compliance with clinical practice guidelines. The difference was very significant for the imaging of the primary tumor, which was not done or unknown in 12% versus 40%, respectively for patients presented to MDTB before versus after treatment. In the former group, the percentage of patients without documented imaging remains too high (3% not done, and 8% unknown). But it must also be noted that not all patients presented in a MDTB before treatment were subsequently managed by the reference center: 17% of these patients were operated outside a reference center (not shown). This is also an important target for improvement.
The lack of compliance to the clinical practice guidelines was particularly impressive for the rate of pretreatment biopsy of the primary tumor in patients presented to the MDTB before versus after initiation of treatment (87% versus 41%). Optimal surgery of sarcoma can only be carried out with a proper knowledge of the diagnosis and extension and the tumor, as clearly stated in the CPG [6–12, 15]. This is lacking in over 50% of the patient not presented in a MDTB before treatment. As a consequence, the rate of optimal R0 surgery was very significantly lower in this group of patients (32% versus 52%), and conversely a very high rate of incomplete (R2 or unknown) surgery was observed in this group 36% versus 16%. It is well documented that R2 surgery exposes the patient to a major risk of local and distant relapse and death, while R0 surgery is associated with the best overall survival [16–18].
Significantly more patients had thus to be re-operated in the group not presented to a MDTB (17% versus 6%) exposing the patient to a higher cost and morbidity of the total treatment procedure [19, 20]. Of note, despite reoperation, the final results in terms of quality of surgery (R0, R1, and R2) remained significantly worse in the group of patient not presented to an MDTB before treatment. The 24-month overall survival of patients with final R2 or non-evaluable resection is 78.4% only, versus 92.4% for R0 and 88.5% for R1 resected patients (not shown) pointing to a major impact of these inadequate procedures on survival. Importantly also the outcome of this nationwide population of patients is worse than that reported in prospective series published from reference centers, probably because of less selections biases on age, performance status, or comorbidities, consistently with a previous smaller series [21, 22].
The impact on overall survival of the whole population is too early to be evaluated but lack of presentation to an MDTB will likely have a negative impact on overall survival. Obviously, this analysis will need to be reassessed with a longer follow-up. Finally, in multivariate analysis, presentation to a MDTB was identified as one of the strongest negative predictive factor for LRFS and RFS, in addition to the classical prognostic factors such as patients’ age, tumor size, grade, depth, and site, confirming the importance of this parameter for patient outcome.
Our series has several limitations. Obviously, this was not a prospective randomized study assessing the impact of MDTB, which would be technically difficult to organize in practice. In addition, an even more exhaustive coverage of the incident sarcoma patient population and of all parameters related to the tumor is required. The description of postoperative treatment is limited, while it may influence also survival in large series [23]. Importantly, an evaluation beyond a single nation will also be important to confirm these findings. This may become possible through the recently launched European Reference Network for Rare Cancers EURACAN (euracan.com), for sarcomas as well as other rare cancers.
Nevertheless, this nationwide series provides a tangible confirmation in a very large unselected nationwide population of the statements proposed in clinical practice guidelines for sarcomas. As such, it has relevance to the management of most rare cancers, which altogether represent 20% of all cancers and often share the same management issues [23–25]. The mechanisms of their worse prognosis are better understood in view of the present results. Given the magnitude of improvement of RFS observed when sarcoma patient files are discussed before treatment, these results strongly suggests that organizing patient management within reference centers offers the best option to improve the outcome of sarcoma patients.
In conclusion, these results show that the management of patients with sarcomas must be carried out under the supervision of a multidisciplinary team with experience as early as in the diagnostic phase, before any treatment is initiated. When this is not carried out, clinical practice guidelines are more often not followed, and risk of relapse and reoperation increase, with an increased rate of reoperation and cost for the patient.
Acknowledgements
The authors would like to thank the teams and leaders of the French National Cancer Institute (INCa) for the continuous support to the project. J-YB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
NetSARC (INCA) and RREPS (INCA) (no grant numbers apply), Association DAM’s (no grant number applies), Ensemble Contre Le GIST (no grant number applies), Eurosarc (FP7-278742), la Fondation ARC (no grant number applies), Infosarcome (no grant number applies), InterSARC (INCA) (no grant number applies), LabEx DEvweCAN (ANR-10-LABX-0061), Ligue de L’Ain contre le Cancer (no grant number applies), LYric (DGOS-INCa-4664), EURACAN (EU project 739521), and CHAFEA/European Commission FPA 739521 / SGA 769029.
Disclosure
PS, NP, EB, PA, SB, MR, AT, FB, PD-L, MT, DC, J-MC, FD, FD, AG, and AI have nothing to disclose. J-YB receives research support and honoraria from Eisai, Eli Lilly, GSK, Ignyta, Novartis, Pharmamar and Roche. CL received research grants, honoraria from Roche, BMS, MSD, GSK, Novartis, Amgen. CC received grants from Pfizer, Novartis, BMS. FG received research grants from Novartis and Takeda; IRC receives research support and honoraria from Eli Lilly, Pharmamar and Roche. ALC reports personal fees from Pharmamar, personal fees from Pfizer, personal fees from Lilly, personal fees from Novartis-GSK, personal fees from Amgen, outside the submitted work. All remaining authors have declared no conflicts of interest.
Appendix
Acknowledgement: Non-author Contributions to Data Collection, Analysis, or Writing/Editing Assistance (all individuals provided their approval to be quoted).
Physicians of the NetSarc network
BAY Jacques-Olivier MD, BOUCHÉ Olivier MD MD, BUI N’GUYEN Binh MD, CARRERE Sébastien MD, CAUSERET Sylvain MD, CHAIGNEAU Loïc MD, COLLARD Olivier MD, CORIAT Romain MD, CUPISSOL Didier MD, DELCAMBRE Corinne DI MARCO Antonio MD, DUJARDIN Franck MD, EYMARD Jean Christophe MD, FERRON Gwenael MD, FIORENZA Fabrice MD, GIMBERGUES Pierre MD, GOLDWASSER François MD, GUILLEMET Cécile MD, GUILLEMIN François; GUIRAMAND Jérôme MD, HONORE Charles MD, LE BRUN-LY Valérie MD, LE MAIGNAN Christine) MD, LINASSIER Claude MD, LOTZ Jean Pierre MD, MIR Olivier MD, PAUCHOT Julien MD, PERRIN Christophe MD, REVOL Marc MD, ROPARS Michaël MD, ROSSET Philippe MD, RUZIC Jean-Christophe MD, SOULIE Patrick MD, SPANO Jean-Philippe MD, VALLEE Antoine MD, VERHAEGHE Jean Luc MD.
Pathologists of the RRePS network
ADAM Julien MD, ANGOT Emilie MD, AUDARD Virginie MD, BATTISTTELA Maxime MD, BAZILLE Céline MD, BOUVIER Corinne MD, BURTIN Florence MD, CASSAGNEAU Elisabeth MD, CHARON-BARRA Céline MD, CHATEAU Marie-Christine MD, CHETAILLE Bruno MD, COLLIN Françoise MD, CROUE Anne MD, DE MURET Anne MD, DECOUVELAERE Anne-Valérie MD, DELFOUR Christophe MD, DEVOUASSOUX-SHISHEBORAN Mojgan MD, DOUCET Laurent MD, EMILE Jean François MD, FLEJOU Jean-François MD, GENESTIE Catherine MD, GHNASSIA Jean-Pierre MD, GOMEZ-BROUCHET Anne MD, GROS Philippe MD, GUINEBRETIERE Jean-Marc MD, KANTELIP Bernadette MD, KARANIAN Marie MD, LAE Marick MD, LAROUSSERIE Frédérique) MD, LE GUELLEC Sophie MD, LE LOARER François MD, LEROUX-BROUSSIER Agnès MD, LEROY Xavier MD, MARCELLIN Luc MD, MARIE Béatrice MD, MARTY Marion MD, MESCAM Lenaig MD, MICHELS Jean Jacques MD, MISHELLANY Florence MD, MITCOV Mona MD, MOREAU Anne MD, NEUVILLE Agnès MD, ORTONNE Nicolas MD, POMMEPUY Isabelle MD, PONELLE Tibor MD, PUGENS Gilles MD, RANCHERE-VINCE Dominique MD, ROBIN Yves-Marie MD, ROCHAIX Philippe MD, STOCK Nathalie MD, TERRIER Philippe MD, TRASSARD Martine MD, VACHER-LAVENU Marie-Cécile MD, VALO Isabelle MD, WEINGERTNER Noëlle MD, XERRI Luc, MD, PhD.
Study coordinators and clinical research assistants in charge of the data collection (both networks)
ALBERT Sabrina, ARNAUD Brigitte, BACONNIER Delphine, BARBEROUSSE Aurélia, BELCHEVA Sabina, BERCHOUD Juliane, BRIHMOUCHE KADOUCI Cherifa, CHELI Sandrine, CHEMIN-AIRIAU Claire, CHERRIER Grégory, COURREGES Jean-Baptiste, DEBAIGT Colin, DECOBECQ Valérie, DEURVEILHER Isabelle, DEVLIES Véronique, DION Adeline, FAUSTIN Jean-Baptiste, FLEITH Jérémy, GANELON Amandine, GARNIER Patricia, GUEN Laurence, HARMACHI Hajar, HOREAU Marie-Reine, IKONOMOVA Raina, ISSARTEL Nadine, JANY Bérangère, JEAN-DENIS Myriam, KADDOUR Nadira, LAURENT Carine, LEBLANC Noémie, MALCHIEN Isabelle, MARC Luz, MARQUIS Eric, MESLI Nouria, NGUYEN SIDINA Irina, PARTHONNEAU Jessica, PERVIEUX Lynda, PROVENT Stéphane, REVERDY Sandrine, RIEFFEL Laurent, ROSSET Sylvie, SCHOEN Hélène, SIDINA NGUYEN Irina, SMIS Pauline, TOURNIER Chantal, VANDERMEERSCH Sandy, ZERARKA Mehdi.
References
- 1. Ducimetière F, Lurkin A, Ranchère-Vince D. et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011; 6: e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mastrangelo G, Fadda E, Cegolon L. et al. A European project on incidence, treatment, and outcome of sarcoma. BMC Public Health 2010; 10: 188.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stiller CA, Trama A, Serraino D. et al. The RARECARE Working Group Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 2013; 49: 684–695. [DOI] [PubMed] [Google Scholar]
- 4. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL.. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res 2013; 33: 2597–2604. [PubMed] [Google Scholar]
- 5. Clark MA, Fisher C, Judson I, Thomas JM.. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353: 701–711. [DOI] [PubMed] [Google Scholar]
- 6. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3): iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 7. von Mehren M, Randall RL, Benjamin RS. et al. Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14: 758–786. [DOI] [PubMed] [Google Scholar]
- 8. Dangoor A, Seddon B, Gerrand C. et al. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016; 6: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandrasekar CR, Wafa H, Grimer RJ. et al. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90: 203–208. [DOI] [PubMed] [Google Scholar]
- 10. Siebenrock KA, Hertel R, Ganz R.. Unexpected resection of soft-tissue sarcoma. Arch Orthop Trauma Surg 2000; 120: 65–69. [PubMed] [Google Scholar]
- 11. Morii T, Aoyagi T, Tajima T. et al. Unplanned resection of a soft tissue sarcoma: clinical characteristics and impact on oncological and functional outcomes. J Orthop Sci off J Jpn Orthop Assoc 2015; 20: 373–379. [DOI] [PubMed] [Google Scholar]
- 12. Noria S, Davis A, Kandel R. et al. Residual disease following unplanned excision of a soft-tissue sarcoma of an extremity*. J Bone Joint Surg 1996; 78: 650–655. [DOI] [PubMed] [Google Scholar]
- 13. Alvegård T, Sundby Hall K, Bauer H, Rydholm A.. The Scandinavian Sarcoma Group: 30 years’ experience. Acta Orthop Suppl 2009; 80: 1–104. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F.. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: World Health Organization, IARC Press; 2013. [Google Scholar]
- 15. Charoenlap C, Imanishi J, Tanaka T. et al. Outcomes of unplanned sarcoma excision: impact of residual disease. Cancer Med 2016; 5: 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stojadinovic A, Leung DHY, Hoos A. et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002; 235: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gronchi A, Casali PG, Mariani L. et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol 2005; 23: 96–104. [DOI] [PubMed] [Google Scholar]
- 18. Stoeckle E, Gardet H, Coindre J-M. et al. Prospective evaluation of quality of surgery in soft tissue sarcoma. Eur J Surg Oncol 2006; 32: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 19. Perrier L, Kembou NS, Rascle P. et al. Economic impact of centralized histological reviews in patients with sarcoma, gist, and desmoid tumors. Value Health 2014; 17: A624.. [DOI] [PubMed] [Google Scholar]
- 20. Perrier L, Buja A, Mastrangelo G. et al. Clinicians’ adherence versus non adherence to practice guidelines in the management of patients with sarcoma: a cost-effectiveness assessment in two European regions. BMC Health Serv Res 2012; 12: 28–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derbel O, Heudel PE, Cropet C. et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS One 2017; 12(2): e0158406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagaria SP, Ashman JB, Daugherty LC. et al. Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol 2014; 109: 633–638. [DOI] [PubMed] [Google Scholar]
- 23. Gatta G, Capocaccia R, Trama A, Martínez-García C.. RARECARE Working Group. The burden of rare cancers in Europe. Adv Exp Med Biol 2010; 686: 285–303. [DOI] [PubMed] [Google Scholar]
- 24. Blay JY, Coindre JM, Ducimetière F, Ray-Coquard I.. The value of research collaborations and consortia in rare cancers. Lancet Oncol 2016; 17: e62–e69. [DOI] [PubMed] [Google Scholar]
- 25. Sandrucci S, Gatta G, Trama A. et al. Specialized teams or specialist networks for rare cancers? Eur J Surg Oncol 2015; 41: 1115–1117. [DOI] [PubMed] [Google Scholar]