Abstract
The rapid development of immunomodulatory cancer therapies has led to a concurrent increase in the application of informatics techniques to the analysis of tumors, the tumor microenvironment, and measures of systemic immunity. In this review, the use of tumors to gather genetic and expression data will first be explored. Next, techniques to assess tumor immunity are reviewed, including HLA status, predicted neoantigens, immune microenvironment deconvolution, and T-cell receptor sequencing. Attempts to integrate these data are in early stages of development and are discussed in this review. Finally, we review the application of these informatics strategies to therapy development, with a focus on vaccines, adoptive cell transfer, and checkpoint blockade therapies.
Keywords: computational biology, bioinformatics, immunotherapy, neoantigens, checkpoint blockade, adoptive cell transfer
Introduction
The quest to understand and improve how the immune system identifies and eradicates cancer can make use of data from dozens of molecular, cellular, and tissue profiling technologies. The primary focus of this review will be bioinformatic analyses of data generated by high-throughput DNA and RNA sequencing of bulk normal and tumor cell populations.
First, computational tools that consider cancer and the immune system separately will be reviewed, followed by a discussion of how to better understand the interaction of the immune system and cancer. Finally, methods to dissect therapeutic responses to interventions such as vaccination, checkpoint blockade, and adoptive cell transfer (ACT) will be reviewed (Figure 1).
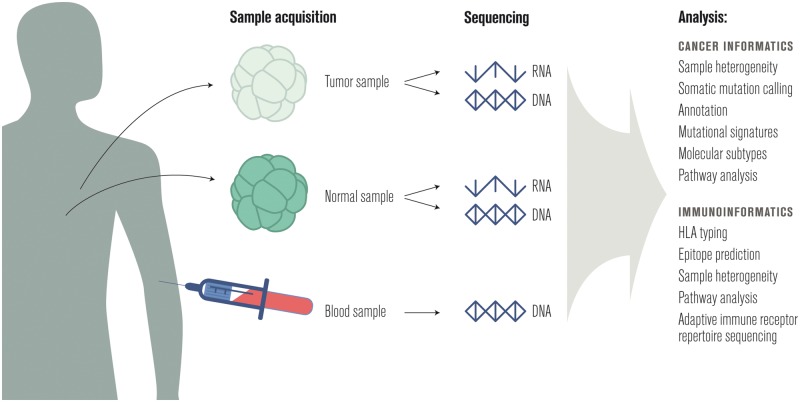
Figure 1.
A sample workflow for informatics in cancer immunotherapy.
Cancer informatics
Cancer is a disease of the genome [1], so it is common to profile the mutations and transcripts present in both the patient’s peripheral blood or nearby normal tissue and one or more tumor samples [2]. A summary of common uses of this data in cancer research follows; for a more detailed review, see Ding et al. [3].
Sample heterogeneity
The proportion of tumor cells in a cancer tissue sample is known as the ‘cellularity’ or ‘purity’ of the sample. Cellularity is an important metric for any type of downstream analysis since it is directly associated with the intensity of the tumor signal in genomic data. Inferring cellularity is complicated in some cancers because of the challenges involved with pathologically excising normal tissue; in addition, nearby cells that appear normal microscopically often harbor somatic mutations similar to those found in cancer cells [4]. ABSOLUTE [5] and ESTIMATE [6] are two commonly used tools to compute cellularity from sequencing data; recent work compared these tools to each other and to pathology-based estimates of cellularity and found low, but positive, correlation between sequence- and pathology-based estimates [7]. While ESTIMATE uses gene expression as input, ABSOLUTE uses somatic copy number to quantify cellularity and hence is sensitive to errors in copy number estimates.
Within the population of tumor cells there is heterogeneity [8, 9]. Tumor cells can be partitioned into clonal families, and it is sometimes possible to reconstruct the phylogeny of tumor cells to better understand the spatial and temporal evolution of tumor heterogeneity [10–14]. Tools like THetA [15], PyClone [16], SciClone [17], PhyloWGS [18], and QuantumClone [19] can partition tumors into subclones, Canopy [20] can infer the evolutionary relationship between these clones, and visualization tools like BubbleTree [21] and fishplot [22] can be useful to better comprehend tumor evolution [23]. These tools operate on genomic data obtained from either a single tumor sample (THetA, PyClone, SciClone, Canopy, bubbletree) or multiple samples (QuantumClone, fishplot) that are spatially or temporally distinct from each other.
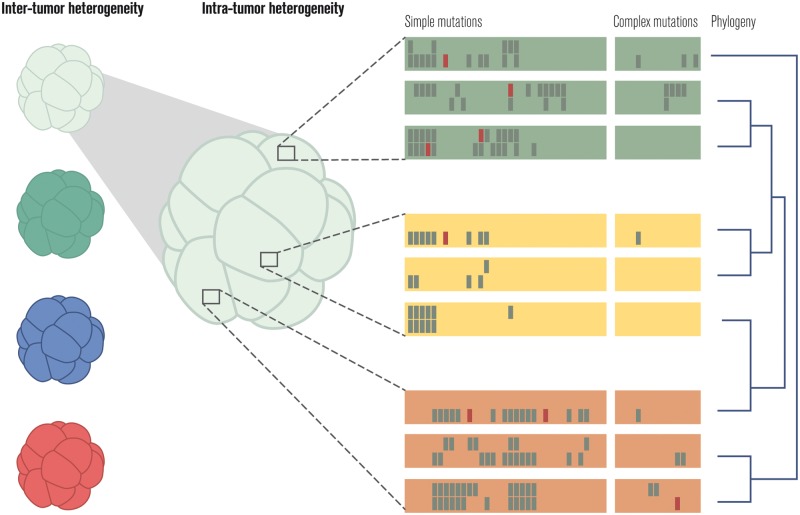
Single-cell sequencing technologies have been employed to improve sample heterogeneity estimates [24–26]; these approaches are reviewed in Kuipers et al. [27] (Figure 2).
Figure 2.
Quantifying heterogeneity across and within tumors. Simple mutations correspond to single-nucleotide variants and short (e.g. 30 nucleotides) insertions and deletions (i.e. indels); complex mutations include all other genome alterations.
Somatic mutation calling
The Genomic Data Commons [28] makes available single-nucleotide variant and short insertion and deletion (indel) calls from MuTect2, SomaticSniper [29], VarScan2 [30], and MuSE [31]. A recent ICGC benchmarking exercise [32] made use of two additional callers, MuTect [33] and Strelka [34]. In practice, it is common to combine the results of several somatic mutation callers to produce a consensus list of high-confidence calls [35, 36].
While simple mutations are the most frequent form of genomic alteration in cancer cells, more complex mutations are almost always present and are often oncogenic drivers. Complex mutations can be organized into categories [37], and it is common to use a different tool for each category of complex mutation.
Software for structural variant detection includes DELLY [38], Meerkat [39], and novoBreak [40]. It is helpful to have whole-genome DNA-seq data when using these tools, since these variations often affect large chromosomal regions, and are difficult to detect with whole-exome DNA-seq data.
Two recent benchmarks [41, 42] of gene fusion detection software found that EricScript [43], SOAPfuse [44], FusionCatcher [45], and JAFFA [46] executed well. These tools make use of RNA-seq data as input.
Somatic copy number alterations (SCNAs) for the TCGA project were estimated with ABSOLUTE [47]. Recent benchmarks [48, 49] of software for SCNA calling from sequencing data highlighted ADTEx [50] and EXCAVATOR [51] as top performers. Tools not included in those benchmarks that may be useful include seqCNA [52], Sequenza [53], hapLOHseq [54], and CNVkit [55].
Challenges to the field of bioinformatics include the need for continuous updates to these programs, and the conflict between repeated benchmarking to delineate the best program versus consistent use of the same program to compare results between studies.
The somatic mutation callers discussed above make assumptions about sequencing protocols and read preprocessing software and methods. For example, many simple mutation detection methods expect whole-exome data, and the choice of the exome capture kit can alter the somatic mutations identified. Sequencing depth is a critical consideration for detecting somatic mutations, as higher depth can better resolve sample heterogeneity issues. Usually one normal and one tumor genome are sequenced; however, some somatic mutation callers try to infer somatic mutations without a normal sample [56], whereas others can make use of RNA-seq data [57, 58] or data from DNA-seq of multiple samples. Biopsy and tissue preservation methods can also impact somatic mutations detected [59, 60].
Standard read preprocessing includes alignment to the human reference genome, base quality score recalibration, duplicate removal, and realignment of indels [61]. It is also important to profile the quality of the sequencing data and the alignments [62]. Significant coordination of many software tools is required to implement quality control, alignment, post-alignment processing, and both simple and complex mutation calling [63]. Most laboratories still manually examine somatic mutation calls with tools like IGV [64] or pileup.js [65] to confirm their accuracy, as in Alioto et al. [32]. Whole pipelines have been published that can serve as a model for investigators setting up their own computational programs [66–68]. As of 2017, there is no consensus on the most sensitive and specific pipeline.
In fact, despite years of work on somatic mutation calling, it is still common for two laboratories to produce highly discordant results on the same input material, even for simple mutations called from targeted panels [69] or whole-exome sequencing [70]. Attempts to benchmark somatic mutation calling pipelines often consider only concordance comparisons [71] or validate on simulated data [72], and the most commonly used validation technique, Sanger sequencing, is time-consuming and not always accurate [73]. In an effort to address this quandary and provide model data, high-coverage whole-genome, whole-exome, and targeted panel data have been made available for a single acute myeloid leukemia (AML) case [74]. Although this high coverage data will be useful, it still does not capture the significant impact of variation in sequencing protocols across sites [75].
Sample heterogeneity further complicates the validation process. Ultimately, a standards body should provide isogenic cell lines that can allow for benchmarking of a site’s somatic mutation calling process, similar to Genome in a Bottle for germline mutation calling [76].
Annotation
After enumerating high-confidence somatic mutations in a tumor sample, the next step is to annotate each mutation with its expected impact. Basic annotations include whether the mutation occurs within a gene, whether it alters the protein produced from that gene, and the specific amino acid alterations expected. Cancer-related annotations include whether the mutation occurs within a ‘driver’ gene [57] that is known to contribute to the progression of cancer and whether a drug exists that can target cells carrying the mutation.
Common tools for the annotation of somatic mutations include VEP [77], SnpEff [78], ANNOVAR [79, 80], and Oncotator [81]. These tools make use of databases such as ENSEMBL [82, 83], RefSeq [84], and the UCSC Known Genes database [85] for basic annotations, and COSMIC [86], CIViC [87], and PMKB [88] for cancer-related annotations. The calling and annotation of somatic mutations is reviewed in Van Allen et al. [89]. It is important to note that there are differences among the annotation databases that can impact downstream analyses [90–92].
Mutational signatures
Researchers have disaggregated the biological processes generating somatic mutations in cancer by examining the trinucleotide context of somatic mutations in thousands of tumor samples [93]. These mutagenic processes include disruptions to the DNA damage repair pathway [94], environmental insults such as smoking [95] and UV radiation, and chemotherapy [96]. Tools like deconstructSigs [97] facilitate the determination of the mutagenic processes present in a single tumor sample. Lessons from several years of mutational signature detection have been distilled in a review [98].
Molecular subtypes
In addition to the intra-tumor heterogeneity discussed above, tumors also vary across individuals [99] and can be categorized in a variety of ways, including clinically (e.g. by organ and stage) and pathologically (e.g. by grade and cell morphology). DNA-seq and RNA-seq data, the focus of this review, can also be used to categorize cancers into subtypes [100]. It is common to start with a single cancer, as categorized by organ or tissue of origin, and identify molecular subtypes using DNA-seq, RNA-seq, and other forms of sequencing data. Many of the TCGA research network publications attempt to identify molecular subtypes of a specific cancer in this way, for example, in the cases of head and neck squamous cell [101] and clear cell renal cell [102] carcinomas. Another approach is to look for molecular subtypes across a variety of cancers [103].
Pathway analysis
Systems biologists have mapped the hallmarks of cancer [104] and other cell behaviors on to the underlying protein–protein interaction and transcriptional regulatory networks that implement the behaviors [105–107]. These gene sets or pathways are collected in databases like KEGG [108, 109], GeneSetDB [110], MSigDB [111], and Reactome [112].
Grouping altered genes according to these gene sets and pathways increases the statistical power of computational analyses and also permits a higher-level view of altered cellular mechanisms based on our prior information on genes’ roles. Tools like Gene Set Enrichment Analysis (GSEA) [113, 114] and Ensemble of Gene Set Enrichment Analyses (EGSEA) [115] leverage gene sets for a better comparison of two groups of samples. This type of analysis is commonly used when comparing normal samples to tumors, tumors to a panel of normal samples (when the matched normal sample is not available) [116], or two sets of tumor samples that have different phenotypes (such as responders and non-responders). When a more granular understanding of the altered pathways for individual samples is needed, it is more common to use tools like single-sample GSEA (ssGSEA) [117], gene set variation analysis (GSVA) [118], and moGSA [119] that perform single-sample gene set or pathway enrichment analysis.
Although pathway-level analyses can provide valuable insights about differences among groups or individual samples by making use of biologically meaningful groups of genes, researchers need to carefully consider whether a group- or individual-level enrichment is feasible for their sample set, which pathway databases would be relevant for their field, and the false discovery rate they will use to adjust the results. Given that a majority of gene sets we use for these analyses are curated from previous observations, there is a significant overlap and redundancy across databases, and there is no clear consensus on many of the core biological pathways; the results of these pathway analyses can easily be overinterpreted or biased because of a poor understanding of the methodology being used.
Immunoinformatics
As immune modulatory agents join the armamentarium of anticancer therapies, bioinformaticians involved in translational studies are increasingly using tools that profile the state of the immune system, both local and systemic.
HLA typing
Although the immune system has many cell types, the primary effector cells of tumor immunity are T cells [120]. T cells are activated in part by major histocompatibility complex (MHC) proteins presenting peptides for inspection on the surface of target cells [121]. In humans, the MHC genes are known as human leukocyte antigens (HLA), and they vary widely across individuals. There are three class I and three class II HLA genes, with the former class presenting antigen to CD8+ T cells and the latter to CD4+ T cells [122]. Enumerating the 12 alleles of these genes present in an individual is known as HLA typing. Most HLA typing methods make use of a database of known HLA alleles that is maintained by International ImMunoGeneTics (IMGT) [123].
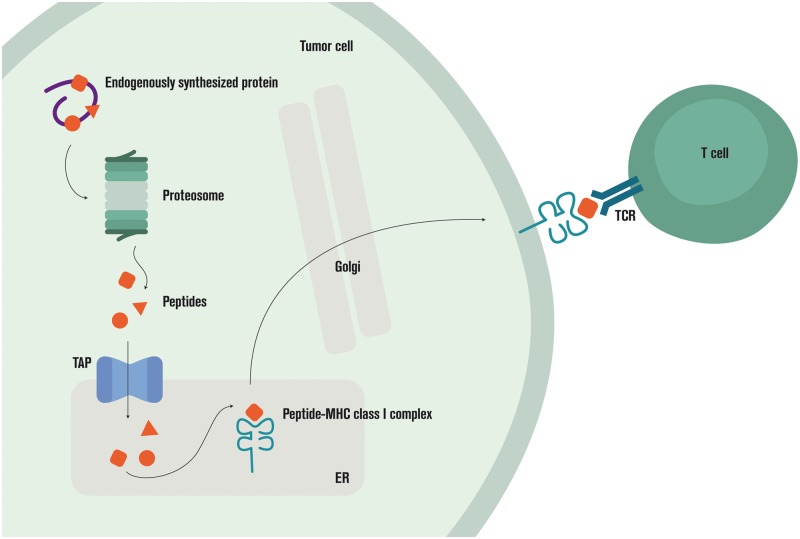
High-resolution HLA typing can now be carried out with DNA or RNA sequencing rather than serology [124]. Software for HLA typing includes ATHLATES [125] and POLYSOLVER [126], which take whole-exome DNA-seq data; seq2HLA [127], which works with RNA-seq data; and OptiType [128], which works with either DNA-seq or RNA-seq data. A recent benchmark of seven algorithms found OptiType to be the most accurate on whole-exome DNA-seq data for class I HLA typing [129]. Promising new directions for HLA typing include using long read DNA-seq [130–132] and representing the polymorphic HLA region as a graph (Figure 3) [133].
Figure 3.
Antigen processing and presentation.
Epitope prediction
The antigen-processing machinery (APM) of a cell is responsible for sampling proteins and peptides from the cell interior for display to T cells [134]. If a presented peptide generates a T-cell response, it is referred to as an epitope and said to be immunogenic.
There are many stages in the class I APM pathway that can be predicted, including proteasomal cleavage, MHC binding, and presentation. The Immune Epitope Database (IEDB) [135] has curated measurements of each stage. Using IEDB data as input, researchers have built comprehensive predictors of how the class I APM pathway will process a protein [136], although informatic prediction of processing remains to be validated. The most critical value to predict as a proxy for immunogenicity appears to be the binding affinity of peptide to the MHC protein [137]. Predictors for p/MHC binding affinity have been progressively improving for decades, led by the NetMHC family of predictors, accounting for complexities such as HLA alleles with little or no training data [138], variable peptide lengths [139], and variable binding affinity distributions across HLA alleles [140]. Other top performing predictors on the IEDB MHC class I benchmark [141] include SMMPMBEC [142], MHCflurry [143], and the IEDB Consensus predictor [144].
Class II epitope prediction is currently far more difficult than class I because the class II APM pathway is complex [145], and class II epitopes have more variable lengths and binding positions. For a recent review of class I and class II epitope prediction software, see Lund et al. [146].
Advances in mass spectrometry have allowed direct and high-throughput measurement of presented peptides [147–150], so it is likely that presentation will soon replace MHC binding affinity as the most common outcome to predict as a proxy for immunogenicity. Beyond presentation, high-throughput measurements of TCR interactions with pMHC complexes are in development [151, 152]. It may one day be possible to predict pMHC/TCR interactions rather than p/MHC binding or pMHC presentation.
Predicting epitopes is complicated by our incomplete understanding of the origin of the peptides presented by a cell [153]. Some have suggested that defective ribosomal products account for a significant fraction of presented peptides [154]; others claim that proteasome-generated spliced peptides are important [155–157]. There is even evidence that pMHC complexes can be transferred between cells [158].
Also complicating epitope prediction are posttranslational modifications [159, 160] and epitopes found on nonclassical MHC proteins like HLA-E [161–163].
Sample heterogeneity
With DNA and/or RNA sequencing data from bulk tissue, it is sometimes possible to estimate the relative proportion of immune cell types present in the sample. This computation is referred to as ‘deconvolution’ [164, 165] and usually relies on the identification of ‘signature’ genes whose expression levels can be used to distinguish between cell types [166]. Software tools for immune infiltrate deconvolution include CIBERSORT [167], TIMER [168], and MCP-counter [169].
These tools are often trained on expression data from isolated immune cell subtypes, such as those generated by the Immunological Genome Project (ImmGen) [170], and differ in the number and kind of cell subtypes estimated: TIMER estimates 6 immune cell subtypes, MCP-counter estimates 8 immune cell subtypes and 2 stromal cell subtypes, and CIBERSORT estimates 22 immune cell subtypes. CIBERSORT and TIMER estimate relative frequencies of immune cell subtypes, whereas MCP-counter can estimate absolute abundances. Users should take care when using CIBERSORT on RNA-seq data, as the model is trained on expression microarray data.
Pathway analysis
Tools for gene set and pathway analysis can be used on populations of immune cells just as they can be on cancer cells. Although gene sets and pathways for immune cell behaviors such as antigen processing, interferon response, and cytolysis are available from many of the same databases that hold data on cancer pathways, there are also immune-specific databases like DC-ATLAS [171] and InnateDB [172].
Adaptive immune receptor repertoire sequencing
New library preparation, sequencing, and analysis technologies have enabled the high-throughput sequencing of B- and T-cell receptor repertoires [173–175]. The original protocols could only sequence unpaired single chains of these receptors; recent protocols make it possible to sequence paired chains [176, 177] to give a complete picture of the receptor repertoire. New algorithms allow repertoire sequences to be extracted from DNA-seq or RNA-seq data from bulk tissue [178–180] or single cells [181], although the output of these approaches relative to dedicated receptor sequencing remains to be thoroughly explored.
Public databases of repertoire sequences [182] allow individual samples to be placed into a larger context, and the community is actively working to standardize file formats and processing pipelines [183, 184].
Repertoire sequence populations can be summarized and compared across conditions to understand vaccination [185], aging [186], and more [187, 188]. Receptor diversity is an often-computed summary metric [189], and individual receptors are frequently grouped into clonal families as an intermediate step in these analyses [190]. The Repertoire Dissimilarity Index is another proposed measure that can be used to compare two samples [191].
Cancer–immune interactions
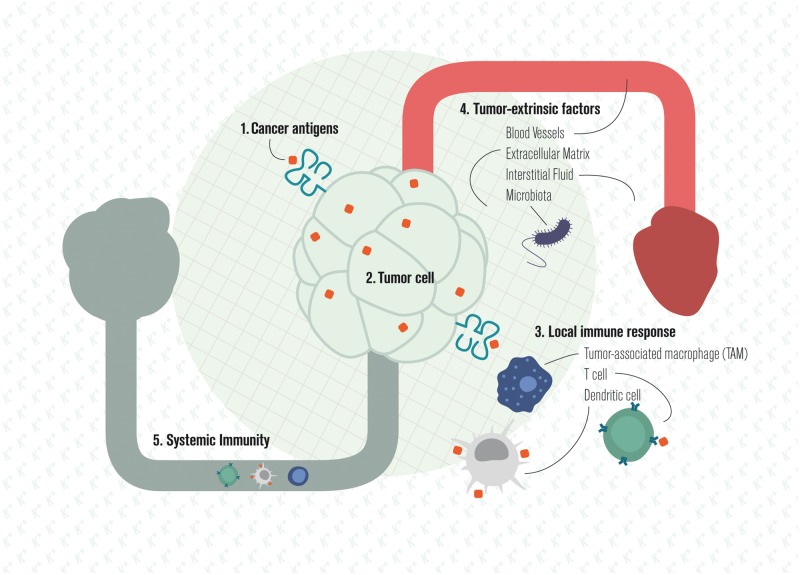
Cancer and the immune system are both complex entities that interact and evolve over time. Milestones in the analysis of their interactions include work on cancer immunosurveillance [192–194], immunoediting [195–198], immune escape [199], and the cancer–immunity cycle (Figure 4) [200].
Figure 4.
Local and systemic cancer–immune interactions.
Cancer antigens
What are the specific targets of the T-cell response to cancer [201]? Many are self-antigens that are aberrantly expressed or posttranslationally modified [202], whereas others are non-self viral [203] or somatic mutation-derived tumor antigens [204]. Somatic mutation-derived tumor antigens are also called ‘neoantigens’ and are important targets for tumor rejection [205–210].
The feasibility of identifying neoantigens with whole-exome DNA-seq data was demonstrated in 2008 [211] and has since been used to interrogate the neoantigen-directed response in many cancers [212]. Open source software for computing neoantigens from sequencing data includes Epidisco [213], ProTECT [214], and pVAC-Seq [215]. The TRON Cell Line Portal (TCLP) catalogs predicted neoantigens for common cancer cell lines [216]. The Cancer Immunome Atlas (TCIA) does the same for 20 solid tumor types from TCGA [217]. A recent analysis of predicted neoantigens for over 60 000 patients found very few shared neoantigens [218].
It has become possible to directly identify peptides presented by cancer cells biopsied from human patients using mass spectrometry [219] and to screen for T cells specific for >1000 peptide targets in a single tumor sample with high-throughput MHC tetramer assays [220]. Data from these assays are expected to improve the quality of cancer neoantigen prediction.
Although the neoantigen burden of a tumor associates with patient survival [221], this effect cannot be separated from the impact of mutation burden [222]. The field is currently working to determine whether specific mutagenic processes like chemotherapy are more likely to create neoantigens than others [96] and if certain kinds of neoantigens are more predictive of response than others. It is common to use RNA-seq data to lower the ranking of neoepitopes with no evidence of expression and to exclude neoepitopes determined to be similar to self-peptides. HLA binding affinity of validated neoepitopes and their corresponding unmutated wild-type peptides have been closely examined with mixed results [223, 224]. Clonal neoantigens appear to be better than subclonal ones at eliciting a productive antitumor immune response [225].
Tumor-intrinsic immune escape
As tumors grow, their constituent clones evolve to evade the immune system [226–229]. Some direct mechanisms of evasion include resistance to T-cell lysis [230] or elimination of T-cell targets through down-regulation of the APM or editing out cancer antigens [134, 231]. Cancer cells also up-regulate inhibitory checkpoint ligands such as PD-L1 through a variety of mechanisms [232–236]. Although the impact of interferon on cancer cells is complex and still being elucidated, there is strong evidence that the disruption of interferon response pathways plays an important role in immune evasion [237–239].
Sometimes, pathways whose primary function upon activation is to equip the tumor with a hallmark of cancer behavior have immune suppression as a secondary function, such as the MAPK [240, 241] and WNT [242] pathways. Recent evidence indicates that large chromosomal disruptions, common in cancer cells, may aid immune evasion [243].
Local immune response
Software for the deconvolution of immune cells in a tissue sample can be used to measure the local immune response to a tumor. Research in colorectal cancer demonstrated that the ‘immune contexture’ of the tumor could be used to predict patient survival [244–247]. Tumors heavily infiltrated by anticancer immune cells are said to have ‘hot’ tumor microenvironments (TMEs); tumors that are devoid of tumor infiltrating immune populations are considered ‘cold’ [248]. Researchers have associated positive patient outcomes to effector T cells and mature dendritic cells [249] and poor outcomes to regulatory T cells [250, 251], myeloid-derived suppressor cells [252], and tumor-associated macrophages [253, 254]. The poor outcomes may be related to immune infiltrate promoting metastasis [255].
The local immune response can be characterized not just by the cell types present but also by the states of those cells and their specific targets in the tumor. A proposed ‘cytolytic signature’ attempts to capture cancer cell–killing activity of immune cells in the TME [256]. Other work has used MHC multimer staining [257, 258] and TCR-seq of tumor-infiltrating lymphocytes (TILs) [259–261] to enumerate the targets and magnitude of the local T-cell response to cancer.
Although the immune contexture can be deconvolved from RNA-seq of a bulk tumor sample, other measurements may provide more insight. Recent work has shown that the epigenetic profile of a cell, as measured by ATAC-seq, is more stable than RNA-seq of cells of the same type or in the same state [262]. Deconvolution algorithms that take ATAC-seq data as input will likely be more accurate than those that use RNA-seq. It is also likely that high-dimensional single-cell profiling technologies such as mass cytometry or single-cell RNA-seq will provide a more detailed understanding of the immune contexture.
Immunohistochemistry (IHC) images capture the spatial distribution of immune cells, and new multiplex protocols allow staining of up to a few dozen antigens on a single slide [263, 264]. Beyond IHC, new techniques like histo-cytometry [265] and multiplexed ion beam imaging (MIBI) [266] make high dimensional quantification of cells possible while capturing their spatial distribution.
Tumor-extrinsic immune escape
The TME is more than just tumor and immune cells: stromal cells, blood and lymphatic vessels, extracellular matrix, interstitial fluid, and microbiota all contribute to the immunogenicity of a tumor [267, 268]. The tumor vasculature is usually studied through the lens of preventing angiogenesis to starve the tumor of nutrients [269]. Blood vessels, however, also permit T-cell trafficking to tumors and can be altered in cancer to facilitate immune escape [270, 271]. Tumors can also develop local lymphatics whose role in the immune response to cancer is still being elucidated [272].
Stromal cells modulate the immune contexture of the TME in multiple ways, including altering the contents of the tumor interstitial fluid or remodeling the extracellular matrix to exclude immune cells [273, 274]. They are also an important source of inhibitory checkpoint ligands [275]. Features of the extracellular environment that alter the immune contexture of the TME include ion [276], cytokine [277], and oxygen [278] concentrations, and the tumor microbiome [279, 280]. A particular area of recent focus is the competition for metabolites between clonally expanding tumor and T cells [281, 282]. Although DNA-seq and RNA-seq data from tumor biopsies can be used to look for pathways known to modulate the TME, investigation of tumor-extrinsic immune escape is aided significantly by metabolomic data [283].
Systemic immune response
Measurement of the tumor draining or sentinel lymph node [284] as well as profiling of the peripheral blood [285] can provide additional information about the immune response to cancer [286, 287]. In addition, the diversity of the peripheral adaptive immune receptor repertoire can be predictive of disease progression [288, 289], and probing the antigen specificity of the antibody [290] or T-cell [291] repertoire can reveal an immune response to tumor antigens. PhIP-seq is a new technology that allows for low-cost, high-throughput profiling of the antigen specificity of the antibody repertoire [292, 293].
Finally, it is well known that cancer incidence increases with age. Measures of aging in the immune system [294] could potentially be used to predict tumor/immune interactions and guide treatment decisions [295].
Summary measures
It has been suggested that the various measures of tumor/immune interactions should be integrated into a single ‘immunogram’ [296, 297] or ‘immunophenotype’ [217, 298, 299]. These summary measures and visualizations can be useful for identifying immune molecular subtypes, prognosis, and clinical decision support, or suggesting new combination therapies [300].
Therapeutic interventions
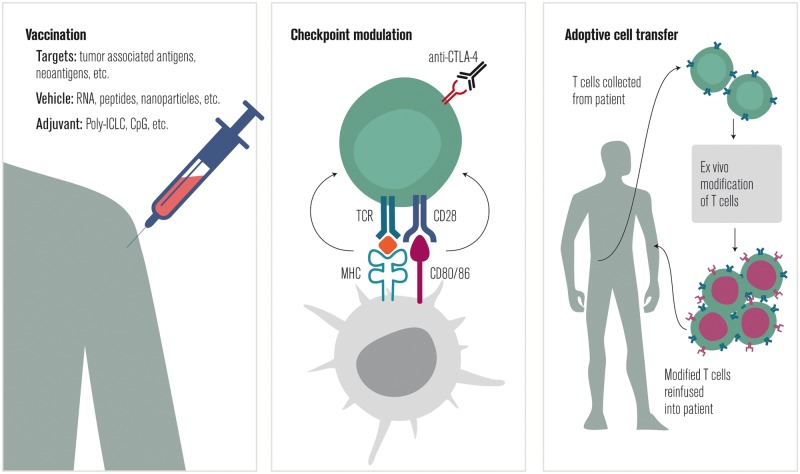
The understanding gained from studying the interaction of the immune system and cancer can be used to design interventions that improve the immune response to cancer. Although conventional cancer therapies have immunological effects [301, 302], here, three approaches to cancer immunotherapy that utilize bioinformatics and neoantigen prediction will be discussed: therapeutic vaccination, checkpoint modulation, and ACT (Figure 5).
Figure 5.
Categories of therapeutic interventions in cancer immunotherapy.
Vaccination
In 2010, the FDA approved sipuleucel-T, a dendritic cell vaccine targeting the tumor antigen prostatic acid phosphatase, for use in prostate cancer [303]. Since 2011, CIMAvax-EGF, a vaccine targeting the growth factor receptor ligand EGF in lung cancer [304, 305], has been available in Cuba and is currently in clinical trials in the United States.
These approvals came after decades of development of many approaches to vaccination against established cancer [306–308] and inspired dozens of additional clinical trials [309–311].
Therapeutic cancer vaccines differ by target, delivery method, and adjuvants [312–315]. The major challenge in therapeutic cancer vaccination is not generating a tumor-directed immune response but rather overcoming immune evasion [316] and generating T-cell memory [317]. Several approaches to the personalization of therapeutic cancer vaccines have been proposed [318], including loading dendritic cells with material obtained from the tumor directly [319] or specifically targeting neoantigens [320–322].
The efficacy of neoantigen vaccines was demonstrated in mouse models in 2014 [258], and the first publication of results in human trials was in 2015 [323]. The neoantigens targeted by these vaccines are often identified by neoepitope prediction from sequencing or mass spectrometry data [324], although ranking factors may need to be tuned to this task: what makes a good endogenous neoantigen may not be identical to what makes a good target for neoantigen vaccination.
One surprising finding from the analysis of the first neoantigen vaccine responses was that class II epitopes are important for vaccine effectiveness even when vaccine epitopes are predicted based on MHC I prediction algorithms [325, 326]. An important concern with neoantigen vaccines is that some tumors may not have enough neoantigen-generating mutations to target [327].
In addition to target selection, bioinformatics can be used to optimize vaccine delivery and adjuvant composition. Prediction of how the breadth, intensity, and duration of the immune response to vaccination relate to antigen load, schedule, and injection site is an active area of research [328–331].
In two small phase I studies published in Nature [332, 333], two independent groups treated patients with stage IIIC or IV melanoma with RNA- and peptide-based neoantigen vaccines, respectively. The vaccines exhibited a favorable safety profile, and clinical outcomes were better than would be expected in the setting of IIIC or metastatic disease, including some documented tumor regressions. Furthermore, both studies suggested activation of naive T-cell populations. Interestingly, although the study by Ott et al. [333] prioritized HLA class I neoepitope prediction, the CD4 positive T-cell responses predominated, although CD8 T cells were also found to be activated.
Checkpoint modulation
The relatively recent success of checkpoint blockade therapies in melanoma [334], followed by other malignancies [335–338], has motivated a resurgence of research into immune checkpoint receptors and their ligands [339, 340]. The FDA has approved antibodies targeting CTLA-4 and PD-1/PD-L1, and new immune checkpoint targets are the subject of active investigation [341–345].
Because pembrolizumab was approved for non-small-cell lung cancer in conjunction with a companion diagnostic, the IHC 22C3 pharmDx test [346, 347]; there has been an intense focus on the association of PD-L1 level with response to blockade of the PD-1/PD-L1 axis [348]. Using PD-L1 level as a biomarker is difficult: PD-L1 is expressed by many cell types, its expression in those cells varies over time [275], its function varies depending on the cell type on which it is expressed [349], there are multiple causes of its presence or absence [350, 351], and it appears to interact with other biomarkers [352]. Nevertheless, work continues in order to understand the role of PD-L1 expression in response to other therapies [353] and in other indications [354]. The Blueprint PD-L1 IHC Assay Comparison Project has reported its phase 1 results comparing four assays for measuring PD-L1 [355].
Beyond immune checkpoint receptor or ligand expression, commonly proposed biomarkers for response to checkpoint blockade include mutation burden [356–358], mismatch-repair status [359], and loss of the interferon response pathway [360–363]. The composition of the gut microbiome [364, 365] and overall immune system state (as inferred from peripheral blood samples) [366–368] have also been implicated.
A major challenge for the field is to formulate a mechanistic model of checkpoint blockade that will allow predictive biomarkers that associate with response to intervention to be separated from prognostic biomarkers that associate with disease progression unrelated to intervention [369–371]. Lessons about which cells respond to checkpoint blockade from chronic infection [262, 372] may be important in the construction of the mechanistic models.
Additional challenges include finding biomarkers that predict adverse events [373–375], developing on-treatment biomarkers that predict response to therapy early in the course of treatment [376, 377], and the interpretation of predictive models to elucidate mechanisms of resistance [378] and suggest new combination therapies [379–381].
Adoptive cell transfer
In 1988, it was reported that TILs from melanoma, when expanded in vitro and reinfused into the host, could cause tumor regression [382]. In 2002, host lymphodepletion before ACT was used to improve response rates [383]. For many tumors, however, TILs are not available, and T cells specific for tumor antigens are created in vitro through genome editing. Autologous lymphocytes from the peripheral blood edited to express either a cancer antigen-specific TCR [384] or a cell type-specific chimeric antigen receptor [385] has been used to treat cancer in humans. For more detailed reviews of ACT, see Rosenberg and Restifo [386] and Fesnak et al. [387].
One primary use of bioinformatics in ACT is to identify new targets for cell therapy [388–390]. Similar techniques to those used to discover targets for vaccination are employed in this domain. Bioinformatics can also examine how the targets evolve in response to ACT [391, 392].
To determine the optimal cell product, detailed measurements are carried out on cells before and after transfer. An increase in the number of T-cell targets [393] and the persistence of antitumor T cells [394] are post-transfer features associated with positive outcomes. Evolving technologies enable more detailed tracking of the fate of adoptively transferred cells in vivo [395], including with PET [396–398].
Another use of bioinformatics in ACT is to discover the expansion and enrichment protocol that generates the optimal cell product for reinfusion. Cytokines [399], metabolites [400], and small molecules [401] have all been shown to impact therapeutic efficacy. Artificial antigen-presenting cells can also be used [402].
Finally, informatics can help determine how to best prepare the patient for ACT. Total body irradiation and chemotherapy are commonly used to deplete lymphocytes in the host before ACT; it is not yet clear whether intensive myeloablative lymphodepletion, which requires hematopoietic stem cell transplantation to reconstitute the host immune system, is more effective than transient, nonmyeloablative lymphodepletion [403–405].
Discussion
Conclusion
Informatics techniques to analyze tumors, the TME, and systemic immunity have evolved alongside the increased use of immunomodulatory therapies in cancer. The rate of progress has been dizzying: the first published use of NGS to predict neoantigens involved spreadsheets and manual curation [211]. Just 4 years later, the Schreiber laboratory and collaborators had streamlined the process into a pipeline [406]. Immune deconvolution techniques have enriched the types of data that can be extracted from existing RNA-seq or microarray data, giving a more complete picture of the tumor and its immune microenvironment. This deepened understanding is being brought to the next level by techniques such as single-cell RNA-seq and MIBI.
However, challenges abound. First, the determination of sample quality and tumor proportion (in the case of human tumor samples) is uncertain and serves as an unstable foundation for the intricate and often elegant work of translational bioinformatics. Second, as demonstrated by the array of software packages that can be used at each stage of the analytic process, there is no single accepted or superior method with which to perform each step. Consequently, research groups working with distinct methods may generate substantially different results and conclusions from the same dataset. Third, the application of statistical methods to the ‘multi-omics’ data being generated poses a substantial challenge, especially given that many translational studies feature small sample sizes and many studied variables: a perfect storm for false discovery, a risk of which the field must be critically aware. Finally, as preclinical data have demonstrated, metabolomics likely play an important role in tumor immunity, but quality data are difficult to collect when using human samples.
As bioinformatics techniques continue to improve, they are likely to play an ever-growing role in the triage of patients to off-the-shelf therapies, as in the case of mutation load with checkpoint blockade agents, or be used to determine what therapy is given, as in the case of vaccination or ACT.
Acknowledgement
The authors would like to thank Arman Aksoy for his input to this review.
Funding
Parker Institute for Cancer Immunotherapy (no grant number applies) (JH) and the National Cancer Institute at the National Institutes of Health Cancer Center Support Grant (2P30CA008748-48 to AS).
This supplement was sponsored by F. Hoffmann-La Roche.
Disclosure
JH is the principal investigator on a sponsored research agreement with Neon Therapeutics. AS is the Translational Medicine Lead for Adaptive Biotechnologies.
References
- 1. Stratton MR, Campbell PJ, Futreal PA.. The cancer genome. Nature 2009; 458(7239): 719–724.http://dx.doi.org/10.1038/nature07943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mardis ER. Genome sequencing and cancer. Curr Opin Genet Dev 2012; 22(3): 245–250.http://dx.doi.org/10.1016/j.gde.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding L, Wendl MC, McMichael JF, Raphael BJ.. Expanding the computational toolbox for mining cancer genomes. Nat Rev Genet 2014; 15(8): 556–570.http://dx.doi.org/10.1038/nrg3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martincorena I, Roshan A, Gerstung M. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015; 348(6237): 880–886.http://dx.doi.org/10.1126/science.aaa6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter SL, Cibulskis K, Helman E. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol 2012; 30(5): 413–421.http://dx.doi.org/10.1038/nbt.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshihara K, Shahmoradgoli M, Martínez E. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013; 4: 2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aran D, Sirota M, Butte AJ.. Systematic pan-cancer analysis of tumour purity. Nat Commun 2015; 6: 8971.http://dx.doi.org/10.1038/ncomms9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nowell PC. The clonal evolution of tumor cell populations. Science 1976; 194(4260): 23–28.http://dx.doi.org/10.1126/science.959840 [DOI] [PubMed] [Google Scholar]
- 9. Gerlinger M, Rowan AJ, Horswell S. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366(10): 883–892.http://dx.doi.org/10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding L, Raphael BJ, Chen F, Wendl MC.. Advances for studying clonal evolution in cancer. Cancer Lett 2013; 340(2): 212–219.http://dx.doi.org/10.1016/j.canlet.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGranahan N, Swanton C.. Biological and Therapeutic Impact of Intratumor Heterogeneity in Cancer Evolution. Cancer Cell 2015; 27(1): 15–26.http://dx.doi.org/10.1016/j.ccell.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 12. McGranahan N, Swanton C.. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017; 168(4): 613–628.http://dx.doi.org/10.1016/j.cell.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 13. Scott J, Marusyk A.. Somatic clonal evolution: a selection-centric perspective. Biochim Biophys Acta 2017; 1867(2): 139–150. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz R, Schäffer AA.. The evolution of tumour phylogenetics: principles and practice. Nat Rev Genet 2017; 18(4): 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oesper L, Mahmoody A, Raphael BJ.. THetA: inferring intra-tumor heterogeneity from high-throughput DNA sequencing data. Genome Biol 2013; 14(7): R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roth A, Khattra J, Yap D. et al. PyClone: statistical inference of clonal population structure in cancer. Nat Methods 2014; 11(4): 396–398.http://dx.doi.org/10.1038/nmeth.2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller CA, White BS, Dees ND. et al. SciClone: inferring clonal architecture and tracking the spatial and temporal patterns of tumor evolution. PLoS Comput Biol 2014; 10(8): e1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deshwar AG, Vembu S, Yung CK. et al. PhyloWGS: reconstructing subclonal composition and evolution from whole-genome sequencing of tumors. Genome Biol 2015; 16(1): 35.http://dx.doi.org/10.1186/s13059-015-0602-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deveau P, Daage LC, Oldridge D. et al. Clonal assessment of functional mutations in cancer based on a genotype-aware method for clonal reconstruction. bioRxiv 2016; 054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang Y, Qiu Y, Minn AJ, Zhang NR.. Assessing intratumor heterogeneity and tracking longitudinal and spatial clonal evolutionary history by next-generation sequencing. Proc Natl Acad Sci USA 2016; 113(37): E5528–E5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu W, Kuziora M, Creasy T. et al. BubbleTree: an intuitive visualization to elucidate tumoral aneuploidy and clonality using next generation sequencing data. Nucleic Acids Res 2016; 44(4): e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller CA, McMichael J, Dang HX. et al. Visualizing tumor evolution with the fishplot package for R. BMC Genomics 2016; 17(1): 880.http://dx.doi.org/10.1186/s12864-016-3195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krzywinski M. Visualizing clonal evolution in cancer. Mol Cell 2016; 62(5): 652–656.http://dx.doi.org/10.1016/j.molcel.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 24. Subramanian A, Schwartz R.. Reference-free inference of tumor phylogenies from single-cell sequencing data. BMC Genomics 2015; 16(Suppl 11): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jahn K, Kuipers J, Beerenwinkel N.. Tree inference for single-cell data. Genome Biol 2016; 17: 86.http://dx.doi.org/10.1186/s13059-016-0936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zafar H, Tzen A, Navin N. et al. SiFit: a method for inferring tumor trees from single-cell sequencing data under finite-site models. bioRxiv 2016; 091595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuipers J, Jahn K, Beerenwinkel N.. Advances in understanding tumour evolution through single-cell sequencing. Biochim Biophys Acta 2017; 1867(2): 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grossman RL, Heath AP, Ferretti V. et al. Toward a shared vision for cancer genomic data. N Engl J Med 2016; 375(12): 1109–1112.http://dx.doi.org/10.1056/NEJMp1607591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larson DE, Harris CC, Chen K. et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 2012; 28(3): 311–317.http://dx.doi.org/10.1093/bioinformatics/btr665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koboldt DC, Zhang Q, Larson DE. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 2012; 22(3): 568–576.http://dx.doi.org/10.1101/gr.129684.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan Y, Xi L, Hughes DST. et al. MuSE: accounting for tumor heterogeneity using a sample-specific error model improves sensitivity and specificity in mutation calling from sequencing data. Genome Biol 2016; 17(1): 178.http://dx.doi.org/10.1186/s13059-016-1029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alioto TS, Buchhalter I, Derdak S. et al. A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat Commun 2015; 6: 10001.http://dx.doi.org/10.1038/ncomms10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cibulskis K, Lawrence MS, Carter SL. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013; 31(3): 213–219.http://dx.doi.org/10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saunders CT, Wong WSW, Swamy S. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 2012; 28(14): 1811–1817.http://dx.doi.org/10.1093/bioinformatics/bts271 [DOI] [PubMed] [Google Scholar]
- 35. Fang LT, Afshar PT, Chhibber A. et al. An ensemble approach to accurately detect somatic mutations using SomaticSeq. Genome Biol 2015; 16(1): 197.http://dx.doi.org/10.1186/s13059-015-0758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SY, Jacob L, Speed TP.. Combining calls from multiple somatic mutation-callers. BMC Bioinformatics 2014; 15(1): 154.http://dx.doi.org/10.1186/1471-2105-15-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenman CD, Pleasance ED, Newman S. et al. Estimation of rearrangement phylogeny for cancer genomes. Genome Res 2012; 22(2): 346–361.http://dx.doi.org/10.1101/gr.118414.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rausch T, Zichner T, Schlattl A. et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 2012; 28(18): i333–i339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang L, Luquette LJ, Gehlenborg N. et al. Diverse mechanisms of somatic structural variations in human cancer genomes. Cell 2013; 153(4): 919–929.http://dx.doi.org/10.1016/j.cell.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chong Z, Ruan J, Gao M. et al. novoBreak: local assembly for breakpoint detection in cancer genomes. Nat Methods 2017; 14(1): 65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu S, Tsai W-H, Ding Y. et al. Comprehensive evaluation of fusion transcript detection algorithms and a meta-caller to combine top performing methods in paired-end RNA-seq data. Nucleic Acids Res 2016; 44(5): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar S, Vo AD, Qin F, Li H.. Comparative assessment of methods for the fusion transcripts detection from RNA-Seq data. Sci Rep 2016; 6: 21597.http://dx.doi.org/10.1038/srep21597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benelli M, Pescucci C, Marseglia G. et al. Discovering chimeric transcripts in paired-end RNA-seq data by using EricScript. Bioinformatics 2012; 28(24): 3232–3239.http://dx.doi.org/10.1093/bioinformatics/bts617 [DOI] [PubMed] [Google Scholar]
- 44. Jia W, Qiu K, He M. et al. SOAPfuse: an algorithm for identifying fusion transcripts from paired-end RNA-Seq data. Genome Biol 2013; 14(2): R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicorici D, Satalan M, Edgren H. et al. FusionCatcher—a tool for finding somatic fusion genes in paired-end RNA-sequencing data. bioRxiv 2014; 011650. [Google Scholar]
- 46. Davidson NM, Majewski IJ, Oshlack A.. JAFFA: high sensitivity transcriptome-focused fusion gene detection. Genome Med 2015; 7(1): 43.http://dx.doi.org/10.1186/s13073-015-0167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zack TI, Schumacher SE, Carter SL. et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet 2013; 45(10): 1134–1140.http://dx.doi.org/10.1038/ng.2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao M, Wang Q, Wang Q. et al. Computational tools for copy number variation (CNV) detection using next-generation sequencing data: features and perspectives. BMC Bioinformatics 2013; 14(Suppl 11): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nam J-Y, Kim NKD, Kim SC. et al. Evaluation of somatic copy number estimation tools for whole-exome sequencing data. Brief Bioinformatics 2016; 17(2): 185–192.http://dx.doi.org/10.1093/bib/bbv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amarasinghe KC, Li J, Hunter SM. et al. Inferring copy number and genotype in tumour exome data. BMC Genomics 2014; 15: 732.http://dx.doi.org/10.1186/1471-2164-15-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magi A, Tattini L, Cifola I. et al. EXCAVATOR: detecting copy number variants from whole-exome sequencing data. Genome Biol 2013; 14(10): R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mosen-Ansorena D, Telleria N, Veganzones S. et al. seqCNA: an R package for DNA copy number analysis in cancer using high-throughput sequencing. BMC Genomics 2014; 15(1): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Favero F, Joshi T, Marquard AM. et al. Sequenza: allele-specific copy number and mutation profiles from tumor sequencing data. Ann Oncol 2015; 26(1): 64–70.http://dx.doi.org/10.1093/annonc/mdu479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. San Lucas FA, Sivakumar S, Vattathil S. et al. Rapid and powerful detection of subtle allelic imbalance from exome sequencing data with hapLOHseq. Bioinformatics 2016; 32(19): 3015–3017.http://dx.doi.org/10.1093/bioinformatics/btw340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Talevich E, Shain AH, Botton T, Bastian BC.. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol 2016; 12(4): e1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hiltemann S, Jenster G, Trapman J. et al. Discriminating somatic and germline mutations in tumour DNA samples without matching normals. Genome Res 2015; 25(9): 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilkerson MD, Cabanski CR, Sun W. et al. Integrated RNA and DNA sequencing improves mutation detection in low purity tumors. Nucleic Acids Res 2014; 42(13): e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Radenbaugh AJ, Ma S, Ewing A. et al. RADIA: RNA and DNA integrated analysis for somatic mutation detection. PLoS One 2014; 9(11): e111516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kokkat TJ, Patel MS, McGarvey D. et al. Archived formalin-fixed paraffin-embedded (FFPE) blocks: a valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv Biobank 2013; 11(2): 101–106.http://dx.doi.org/10.1089/bio.2012.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hedegaard J, Thorsen K, Lund MK. et al. Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS One 2014; 9(5): e98187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bao R, Huang L, Andrade J. et al. Review of current methods, applications, and data management for the bioinformatics analysis of whole exome sequencing. Cancer Inform 2014; 13(Suppl 2): 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ewels P, Magnusson M, Lundin S, Käller M.. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016; 32(19): 3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Doig K, Papenfuss AT, Fox S.. Clinical cancer genomic analysis: data engineering required. Lancet Oncol 2015; 16(9): 1015–1017.http://dx.doi.org/10.1016/S1470-2045(15)00195-3 [DOI] [PubMed] [Google Scholar]
- 64. Robinson JT, Thorvaldsdóttir H, Winckler W. et al. Integrative genomics viewer. Nat Biotechnol 2011; 29(1): 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vanderkam D, Aksoy BA, Hodes I. et al. pileup.js: a JavaScript library for interactive and in-browser visualization of genomic data. Bioinformatics 2016; 32(15): 2378–2379.http://dx.doi.org/10.1093/bioinformatics/btw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rashid M, Robles-Espinoza CD, Rust AG, Adams DJ.. Cake: a bioinformatics pipeline for the integrated analysis of somatic variants in cancer genomes. Bioinformatics 2013; 29(17): 2208–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bao R, Hernandez K, Huang L. et al. ExScalibur: a high-performance cloud-enabled suite for whole exome germline and somatic mutation identification. PLoS One 2015; 10(8): e0135800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. do Valle ÍF, Giampieri E, Simonetti G. et al. Optimized pipeline of MuTect and GATK tools to improve the detection of somatic single nucleotide polymorphisms in whole-exome sequencing data. BMC Bioinformatics 2016; 17(12): 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuderer NM, Burton KA, Blau S. et al. Comparison of 2 commercially available next-generation sequencing platforms in oncology. JAMA Oncol 2017; 3(7): 996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qiu P, Pang L, Arreaza G. et al. Data interoperability of whole exome sequencing (WES) based mutational burden estimates from different laboratories. Int J Mol Sci 2016; 17(5): 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim SY, Speed TP.. Comparing somatic mutation-callers: beyond Venn diagrams. BMC Bioinformatics 2013; 14: 189.http://dx.doi.org/10.1186/1471-2105-14-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ewing AD, Houlahan KE, Hu Y. et al. Combining tumor genome simulation with crowdsourcing to benchmark somatic single-nucleotide-variant detection. Nat Methods 2015; 12(7): 623–630.http://dx.doi.org/10.1038/nmeth.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Beck TF, Mullikin JC NISC Comparative Sequencing Program Biesecker LG.. Systematic evaluation of sanger validation of next-generation sequencing variants. Clin Chem 2016; 62(4): 647–654.http://dx.doi.org/10.1373/clinchem.2015.249623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Griffith M, Miller CA, Griffith OL. et al. Optimizing cancer genome sequencing and analysis. Cell Syst 2015; 1(3): 210–223.http://dx.doi.org/10.1016/j.cels.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buchhalter I, Hutter B, Alioto TS. et al. A comprehensive multicenter comparison of whole genome sequencing pipelines using a uniform tumor-normal sample pair. bioRxiv 2014; 013177. [Google Scholar]
- 76. Zook JM, Chapman B, Wang J. et al. Integrating human sequence data sets provides a resource of benchmark SNP and indel genotype calls. Nat Biotechnol 2014; 32(3): 246–251.http://dx.doi.org/10.1038/nbt.2835 [DOI] [PubMed] [Google Scholar]
- 77. McLaren W, Gil L, Hunt SE. et al. The Ensembl variant effect predictor. Genome Biol 2016; 17(1): 122.http://dx.doi.org/10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cingolani P, Platts A, Wang LL. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012; 6(2): 80–92.http://dx.doi.org/10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang H, Wang K.. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc 2015; 10(10): 1556–1566.http://dx.doi.org/10.1038/nprot.2015.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang K, Li M, Hakonarson H.. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38(16): e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ramos AH, Lichtenstein L, Gupta M. et al. Oncotator: cancer variant annotation tool. Hum Mutat 2015; 36(4): E2423–E2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Flicek P, Amode MR, Barrell D. et al. Ensembl 2014. Nucleic Acids Res 2014; 42(Database issue): D749–D755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aken BL, Ayling S, Barrell D. et al. The Ensembl gene annotation system. Database 2016; 2016: baw093. doi: 10.1093/database/baw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pruitt KD, Tatusova T, Maglott DR.. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 2007; 35(Database): D61–D65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hsu F, Kent WJ, Clawson H. et al. The UCSC known genes. Bioinformatics 2006; 22(9): 1036–1046.http://dx.doi.org/10.1093/bioinformatics/btl048 [DOI] [PubMed] [Google Scholar]
- 86. Forbes SA, Beare D, Gunasekaran P. et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res 2015; 43(Database issue): D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Griffith M, Spies NC, Krysiak K. et al. CIViC: a knowledgebase for expert-crowdsourcing the clinical interpretation of variants in cancer. bioRxiv 2016; 072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang L, Fernandes H, Zia H. et al. The Precision Medicine Knowledge Base: an online application for collaborative editing, maintenance and sharing of structured clinical-grade cancer mutations interpretations. bioRxiv 2016; 059824. [Google Scholar]
- 89. Van Allen EM, Wagle N, Levy MA.. Clinical analysis and interpretation of cancer genome data. J Clin Oncol 2013; 31(15): 1825–1833.http://dx.doi.org/10.1200/JCO.2013.48.7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao S, Zhang B.. A comprehensive evaluation of Ensembl, RefSeq, and UCSC annotations in the context of RNA-seq read mapping and gene quantification. BMC Genomics 2015; 16(1): 97.http://dx.doi.org/10.1186/s12864-015-1308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frankish A, Uszczynska B, Ritchie GRS. et al. Comparison of GENCODE and RefSeq gene annotation and the impact of reference geneset on variant effect prediction. BMC Genomics 2015; 16(Suppl 8): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McCarthy DJ, Humburg P, Kanapin A. et al. Choice of transcripts and software has a large effect on variant annotation. Genome Med 2014; 6(3): 26.http://dx.doi.org/10.1186/gm543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alexandrov LB, Nik-Zainal S, Wedge DC. et al. Signatures of mutational processes in human cancer. Nature 2013; 500(7463): 415–421.http://dx.doi.org/10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jeggo PA, Pearl LH, Carr AM.. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer Cancer 201516(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 95. Alexandrov LB, Ju YS, Haase K. et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016; 354(6312): 618–622.http://dx.doi.org/10.1126/science.aag0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O’Donnell T, Christie EL, Buros J. et al. Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer. bioRxiv 2016; 090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rosenthal R, McGranahan N, Herrero J. et al. deconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol 2016; 17(1): 31.http://dx.doi.org/10.1186/s13059-016-0893-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Petljak M, Alexandrov LB.. Understanding mutagenesis through delineation of mutational signatures in human cancer. Carcinogenesis 2016; 37(6): 531–540.http://dx.doi.org/10.1093/carcin/bgw055 [DOI] [PubMed] [Google Scholar]
- 99. De Sousa E Melo F, Vermeulen L, Fessler E, Medema JP.. Cancer heterogeneity—a multifaceted view. EMBO Rep 2013; 14(8): 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Song Q, Merajver SD, Li JZ.. Cancer classification in the genomic era: five contemporary problems. Hum Genomics 2015; 9: 27.http://dx.doi.org/10.1186/s40246-015-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517(7536): 576–582.http://dx.doi.org/10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013; 499(7456): 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoadley KA, Yau C, Wolf DM. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014; 158(4): 929–944.http://dx.doi.org/10.1016/j.cell.2014.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011; 144(5): 646–674.http://dx.doi.org/10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 105. Khatri P, Sirota M, Butte AJ.. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol 2012; 8(2): e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vogelstein B, Papadopoulos N, Velculescu VE. et al. Cancer genome landscapes. Science 2013; 339(6127): 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Consequences TM. Pathway Analysis working group of the International Cancer Genome Consortium. Pathway and network analysis of cancer genomes. Nat Methods 2015; 12(7): 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kanehisa M, Goto S.. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 2000; 28(1): 27–30.http://dx.doi.org/10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kanehisa M, Sato Y, Kawashima M. et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 2016; 44(D1): D457–D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Araki H, Knapp C, Tsai P, Print C.. GeneSetDB: a comprehensive meta-database, statistical and visualisation framework for gene set analysis. FEBS Open Bio 2012; 2: 76–82.http://dx.doi.org/10.1016/j.fob.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liberzon A, Birger C, Thorvaldsdóttir H. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015; 1(6): 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fabregat A, Sidiropoulos K, Garapati P. et al. The reactome pathway Knowledgebase. Nucleic Acids Res 2016; 44(D1): D481–D487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mootha VK, Lindgren CM, Eriksson K-F. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 114. Subramanian A, Tamayo P, Mootha VK. et al. Gene Set Enrichment Analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102(43): 15545–15550.http://dx.doi.org/10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alhamdoosh M, Ng M, Wilson NJ. et al. Combining multiple tools outperforms individual methods in gene set enrichment analyses. Bioinformatics 2017; 33(3): 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ahn T, Lee E, Huh N, Park T.. Personalized identification of altered pathways in cancer using accumulated normal tissue data. Bioinformatics 2014; 30(17): i422–i429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barbie DA, Tamayo P, Boehm JS. et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 2009; 462(7269): 108–112.http://dx.doi.org/10.1038/nature08460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hänzelmann S, Castelo R, Guinney J.. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013; 14: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Meng C, Kuster B, Peters B. et al. moGSA: integrative single sample gene-set analysis of multiple omics data. bioRxiv 2016; 046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gajewski TF, Schreiber H, Fu Y-X.. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14(10): 1014–1022.http://dx.doi.org/10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Smith-Garvin JE, Koretzky GA, Jordan MS.. T cell activation. Annu Rev Immunol 2009; 27: 591–619.http://dx.doi.org/10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Swain SL. T cell subsets and the recognition of MHC class. Immunol Rev 1983; 74: 129–142.http://dx.doi.org/10.1111/j.1600-065X.1983.tb01087.x [DOI] [PubMed] [Google Scholar]
- 123. Lefranc M-P, Giudicelli V, Ginestoux C. et al. IMGT®, the international ImMunoGeneTics information system®. Nucleic Acids Res 2009; 37(Database): D1006–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Erlich H. HLA DNA typing: past, present, and future. Tissue Antigens 2012; 80(1): 1–11.http://dx.doi.org/10.1111/j.1399-0039.2012.01881.x [DOI] [PubMed] [Google Scholar]
- 125. Liu C, Yang X, Duffy B. et al. ATHLATES: accurate typing of human leukocyte antigen through exome sequencing. Nucleic Acids Res 2013; 41(14): e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shukla SA, Rooney MS, Rajasagi M. et al. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol 2015; 33(11): 1152–1158.http://dx.doi.org/10.1038/nbt.3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Boegel S, Löwer M, Schäfer M. et al. HLA typing from RNA-Seq sequence reads. Genome Med 2012; 4(12): 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Szolek A, Schubert B, Mohr C. et al. OptiType: precision HLA typing from next-generation sequencing data. Bioinformatics 2014; 30(23): 3310–3316.http://dx.doi.org/10.1093/bioinformatics/btu548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kiyotani K, Mai TH, Nakamura Y.. Comparison of exome-based HLA class I genotyping tools: identification of platform-specific genotyping errors. J Hum Genet 2017; 62(3): 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chang C-J, Chen P-L, Yang W-S, Chao K-M.. A fault-tolerant method for HLA typing with PacBio data. BMC Bioinformatics 2014; 15: 296.http://dx.doi.org/10.1186/1471-2105-15-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ammar R, Paton TA, Torti D. et al. Long read nanopore sequencing for detection of HLA and CYP2D6 variants and haplotypes. F1000Res 2015; 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cereb N, Kim HR, Ryu J, Yang SY.. Advances in DNA sequencing technologies for high resolution HLA typing. Hum Immunol 2015; 76(12): 923–927.http://dx.doi.org/10.1016/j.humimm.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 133. Dilthey AT, Gourraud P-A, Mentzer AJ. et al. High-accuracy HLA type inference from whole-genome sequencing data using population reference graphs. PLoS Comput Biol 2016; 12(10): e1005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Leone P, Shin E-C, Perosa F. et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst 2013; 105(16): 1172–1187.http://dx.doi.org/10.1093/jnci/djt184 [DOI] [PubMed] [Google Scholar]
- 135. Vita R, Overton JA, Greenbaum JA. et al. The Immune Epitope Database (IEDB) 3.0. Nucleic Acids Res 2015; 43(Database issue): D405–D412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tenzer S, Peters B, Bulik S. et al. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell Mol Life Sci 2005; 62(9): 1025–1037.http://dx.doi.org/10.1007/s00018-005-4528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sette A, Vitiello A, Reherman B. et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol 1994; 153(12): 5586–5592. [PubMed] [Google Scholar]
- 138. Nielsen M, Lundegaard C, Blicher T. et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One 2007; 2(8): e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lundegaard C, Lund O, Nielsen M.. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics 2008; 24(11): 1397–1398.http://dx.doi.org/10.1093/bioinformatics/btn128 [DOI] [PubMed] [Google Scholar]
- 140. Karosiene E, Rasmussen M, Blicher T. et al. NetMHCIIpan-3.0, a common pan-specific MHC class II prediction method including all three human MHC class II isotypes, HLA-DR, HLA-DP and HLA-DQ. Immunogenetics 2013; 65(10): 711–724.http://dx.doi.org/10.1007/s00251-013-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Trolle T, Metushi IG, Greenbaum JA. et al. Automated benchmarking of peptide-MHC class I binding predictions. Bioinformatics 2015; 31(13): 2174–2181.http://dx.doi.org/10.1093/bioinformatics/btv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kim Y, Sidney J, Pinilla C. et al. Derivation of an amino acid similarity matrix for peptide: MHC binding and its application as a Bayesian prior. BMC Bioinformatics 2009; 10: 394.http://dx.doi.org/10.1186/1471-2105-10-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rubinsteyn A, O’Donnell T, Damaraju N, Hammerbacher J.. Predicting peptide-MHC binding affinities with imputed training data. bioRxiv 2016; 054775. [Google Scholar]
- 144. Moutaftsi M, Peters B, Pasquetto V. et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol 2006; 24(7): 817–819. [DOI] [PubMed] [Google Scholar]
- 145. Roche PA, Furuta K.. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015; 15(4): 203–216.http://dx.doi.org/10.1038/nri3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lund O, Karosiene E, Lundegaard C. et al. Bioinformatics identification of antigenic peptide: predicting the specificity of major MHC class I and II pathway players. Methods Mol Biol 2013; 960: 247–260. [DOI] [PubMed] [Google Scholar]
- 147. Caron E, Kowalewski DJ, Chiek Koh C. et al. Analysis of major histocompatibility complex (MHC) immunopeptidomes using mass spectrometry. Mol Cell Proteomics 2015; 14(12): 3105–3117.http://dx.doi.org/10.1074/mcp.O115.052431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M.. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteomics 2015; 14(3): 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pearson H, Daouda T, Granados DP. et al. MHC class I–associated peptides derive from selective regions of the human genome. J Clin Invest 2016; 126(12): 4690–4701.http://dx.doi.org/10.1172/JCI88590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Abelin JG, Keskin DB, Sarkizova S. et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity 2017; 46(2): 315–326.http://dx.doi.org/10.1016/j.immuni.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Birnbaum ME, Mendoza JL, Sethi DK. et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 2014; 157(5): 1073–1087.http://dx.doi.org/10.1016/j.cell.2014.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang S-Q, Parker P, Ma K-Y. et al. Direct measurement of T cell receptor affinity and sequence from naïve antiviral T cells. Sci Transl Med 2016; 8(341): 341ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Apcher S, Prado Martins R, Fåhraeus R.. The source of MHC class I presented peptides and its implications. Curr Opin Immunol 2016; 40: 117–122. [DOI] [PubMed] [Google Scholar]
- 154. Yewdell JW, Antón LC, Bennink JR.. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol 1996; 157(5): 1823–1826. [PubMed] [Google Scholar]
- 155. Berkers CR, de Jong A, Schuurman KG. et al. Peptide splicing in the proteasome creates a novel type of antigen with an isopeptide linkage. Ji 2015; 195(9): 4075–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liepe J, Marino F, Sidney J. et al. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science 2016; 354(6310): 354–358.http://dx.doi.org/10.1126/science.aaf4384 [DOI] [PubMed] [Google Scholar]
- 157. Delong T, Wiles TA, Baker RL. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 2016; 351(6274): 711–714.http://dx.doi.org/10.1126/science.aad2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dolan BP, Gibbs KD Jr, Ostrand-Rosenberg S.. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol 2006; 177(9): 6018–6024. [DOI] [PubMed] [Google Scholar]
- 159. Luban S, Li Z-G.. Citrullinated peptide and its relevance to rheumatoid arthritis: an update. Int J Rheum Dis 2010; 13(4): 284–287.http://dx.doi.org/10.1111/j.1756-185X.2010.01553.x [DOI] [PubMed] [Google Scholar]
- 160. Mohammed F, Cobbold M, Zarling AL. et al. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: a molecular basis for the presentation of transformed self. Nat Immunol 2008; 9(11): 1236–1243.http://dx.doi.org/10.1038/ni.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. de Kruijf EM, Sajet A, van Nes JGH. et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol 2010; 185(12): 7452–7459.http://dx.doi.org/10.4049/jimmunol.1002629 [DOI] [PubMed] [Google Scholar]
- 162. Zeestraten ECM, Reimers MS, Saadatmand S. et al. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer 2014; 110(2): 459–468.http://dx.doi.org/10.1038/bjc.2013.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hansen SG, Wu HL, Burwitz BJ. et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 2016; 351(6274): 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Abbas AR, Wolslegel K, Seshasayee D. et al. Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus. PLoS One 2009; 4(7): e6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shen-Orr SS, Gaujoux R.. Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Curr Opin Immunol 2013; 25(5): 571–578.http://dx.doi.org/10.1016/j.coi.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Abbas AR, Baldwin D, Ma Y. et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun 2005; 6(4): 319–331.http://dx.doi.org/10.1038/sj.gene.6364173 [DOI] [PubMed] [Google Scholar]
- 167. Newman AM, Liu CL, Green MR. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015; 12(5): 453–457.http://dx.doi.org/10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Li B, Severson E, Pignon J-C. et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol 2016; 17(1): 174.http://dx.doi.org/10.1186/s13059-016-1028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Becht E, Giraldo NA, Lacroix L. et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 2016; 17(1): 218.http://dx.doi.org/10.1186/s13059-016-1070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Heng TSP, Painter MW.. Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 2008; 9(10): 1091–1094. [DOI] [PubMed] [Google Scholar]
- 171. Cavalieri D, Rivero D, Beltrame L. et al. DC-ATLAS: a systems biology resource to dissect receptor specific signal transduction in dendritic cells. Immunome Res 2010; 6(1): 10.http://dx.doi.org/10.1186/1745-7580-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Breuer K, Foroushani AK, Laird MR. et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic Acids Res 2013; 41(D1): D1228–D1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Benichou J, Ben-Hamo R, Louzoun Y, Efroni S.. Rep-Seq: uncovering the immunological repertoire through next-generation sequencing. Immunology 2012; 135(3): 183–191.http://dx.doi.org/10.1111/j.1365-2567.2011.03527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Larimore K, McCormick MW, Robins HS, Greenberg PD.. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol 2012; 189(6): 3221–3230. [DOI] [PubMed] [Google Scholar]
- 175. Boyd SD, Marshall EL, Merker JD. et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med 2009; 1(12): 12ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Howie B, Sherwood AM, Berkebile AD. et al. High-throughput pairing of T cell receptor α and β sequences. Sci Transl Med 2015; 7(301): 301ra131. [DOI] [PubMed] [Google Scholar]
- 177. Lee ES, Thomas PG, Mold JE, Yates AJ.. Identifying T cell receptors from high-throughput sequencing: dealing with promiscuity in TCRα and TCRβ pairing. PLoS Comput Biol 2017; 13(1): e1005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Li B, Li T, Pignon J-C. et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet 2016; 48(7): 725–732.http://dx.doi.org/10.1038/ng.3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Mangul S, Mandric I, Yang HT. et al. Profiling adaptive immune repertoires across multiple human tissues by RNA Sequencing. bioRxiv 2016; 089235. [Google Scholar]
- 180. Brown SD, Raeburn LA, Holt RA.. Profiling tissue-resident T cell repertoires by RNA sequencing. Genome Med 2015; 7: 125.http://dx.doi.org/10.1186/s13073-015-0248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Eltahla AA, Rizzetto S, Pirozyan MR. et al. Linking the T cell receptor to the single cell transcriptome in antigen-specific human T cells. Immunol Cell Biol 2016; 94(6): 604–611.http://dx.doi.org/10.1038/icb.2016.16 [DOI] [PubMed] [Google Scholar]
- 182. DeWitt WS, Lindau P, Snyder TM. et al. A public database of memory and naive B-cell receptor sequences. PLoS One 2016; 11(8): e0160853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.About the Adaptive Immune Receptor Repertoire (AIRR) Community. http://airr.irmacs.sfu.ca (1 April 2017, date last accessed).
- 184. Zhang L, Cham J, Paciorek A. et al. 3D: diversity, dynamics, differential testing - a proposed pipeline for analysis of next-generation sequencing T cell repertoire data. BMC Bioinformatics 2017; 18(1): 129.http://dx.doi.org/10.1186/s12859-017-1544-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Laserson U, Vigneault F, Gadala-Maria D. et al. High-resolution antibody dynamics of vaccine-induced immune responses. Proc Natl Acad Sci USA 2014; 111(13): 4928–4933.http://dx.doi.org/10.1073/pnas.1323862111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. de Bourcy CFA, Angel CJL, Vollmers C. et al. Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc Natl Acad Sci USA 2017; 114(5): 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yaari G, Kleinstein SH.. Practical guidelines for B-cell receptor repertoire sequencing analysis. Genome Med 2015; 7: 121.http://dx.doi.org/10.1186/s13073-015-0243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lindau P, Robins HS.. Advances and applications of immune receptor sequencing in systems immunology. Current Opinion in Systems Biology 2017; 1: 62. [Google Scholar]
- 189. Greiff V, Bhat P, Cook SC. et al. A bioinformatic framework for immune repertoire diversity profiling enables detection of immunological status. Genome Med 2015; 7(1): 49.http://dx.doi.org/10.1186/s13073-015-0169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ralph DK, Matsen FA 4th. Likelihood-based inference of B cell clonal families. PLoS Comput Biol 2016; 12(10): e1005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bolen CR, Rubelt F, Vander Heiden JA, Davis MM.. The Repertoire Dissimilarity Index as a method to compare lymphocyte receptor repertoires. BMC Bioinformatics 2017; 18(1): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970; 13: 1–27. [DOI] [PubMed] [Google Scholar]
- 193. Burnet FM. Immunological surveillance in neoplasia. Immunol Rev 1971; 7: 3–25.http://dx.doi.org/10.1111/j.1600-065X.1971.tb00461.x [DOI] [PubMed] [Google Scholar]
- 194. Burnet FM. Implications of immunological surveillance for cancer therapy. Isr J Med Sci 1971; 7(1): 9–16. [PubMed] [Google Scholar]
- 195. Dunn GP, Bruce AT, Ikeda H. et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3(11): 991–998.http://dx.doi.org/10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 196. Dunn GP, Old LJ, Schreiber RD.. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22: 329–360.http://dx.doi.org/10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- 197. Dunn GP, Old LJ, Schreiber RD.. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21(2): 137–148.http://dx.doi.org/10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 198. Schreiber RD, Old LJ, Smyth MJ.. Cancer Immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331(6024): 1565–1570. [DOI] [PubMed] [Google Scholar]
- 199. Khong HT, Restifo NP.. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol 2002; 3(11): 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Chen DS, Mellman I.. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39(1): 1–10.http://dx.doi.org/10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 201. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T.. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14(2): 135–146.http://dx.doi.org/10.1038/nrc3670 [DOI] [PubMed] [Google Scholar]
- 202. Fu C, Zhao H, Wang Y. et al. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. Hladnikia 2016; 88(6): 275–286. [DOI] [PubMed] [Google Scholar]
- 203. Wang XG, Revskaya E, Bryan RA. et al. Treating cancer as an infectious disease—viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLoS One 2007; 2(10): e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Finnigan JP Jr, Rubinsteyn A, Hammerbacher J, Bhardwaj N.. Mutation-derived tumor antigens: novel targets in cancer immunotherapy. Oncology 2015; 29(12): 970–972, 974–975. [PubMed] [Google Scholar]
- 205. Lennerz V, Fatho M, Gentilini C. et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA 2005; 102(44): 16013–16018.http://dx.doi.org/10.1073/pnas.0500090102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Srivastava PK. Neoepitopes of cancers: looking back, looking ahead. Cancer Immunol Res 2015; 3(9): 969–977.http://dx.doi.org/10.1158/2326-6066.CIR-15-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Gubin MM, Artyomov MN, Mardis ER, Schreiber RD.. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest 2015; 125(9): 3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Schumacher TN, Schreiber RD.. Neoantigens in cancer immunotherapy. Science 2015; 348(6230): 69–74.http://dx.doi.org/10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 209. Bobisse S, Foukas PG, Coukos G, Harari A.. Neoantigen-based cancer immunotherapy. Ann Transl Med 2016; 4(14): 262.http://dx.doi.org/10.21037/atm.2016.06.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Yarchoan M, Johnson BA 3rd, Lutz ER. et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017; 17(4): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Segal NH, Parsons DW, Peggs KS. et al. Epitope landscape in breast and colorectal cancer. Cancer Res 2008; 68(3): 889–892.http://dx.doi.org/10.1158/0008-5472.CAN-07-3095 [DOI] [PubMed] [Google Scholar]
- 212. Rajasagi M, Shukla SA, Fritsch EF. et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood 2014; 124(3): 453–462.http://dx.doi.org/10.1182/blood-2014-04-567933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Vanderkam D, Aksoy BA, Hodes I. et al. pileup.js: a JavaScript library for interactive and in-browser visualization of genomic data. Bioinformatics 2016; 32(15): 2378–2379. https://github.com/hammerlab/epidisco (1 April 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.BD2KGenomics/protect. https://github.com/BD2KGenomics/protect (1 April 2017, date last accessed).
- 215. Hundal J, Carreno BM, Petti AA. et al. pVAC-Seq: a genome-guided in silico approach to identifying tumor neoantigens. Genome Med 2016; 8(1): 11.http://dx.doi.org/10.1186/s13073-016-0264-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Scholtalbers J, Boegel S, Bukur T. et al. TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression. Genome Med 2015; 7(1): 118.http://dx.doi.org/10.1186/s13073-015-0240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Charoentong P, Finotello F, Angelova M. et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 2017; 18(1): 248–262.http://dx.doi.org/10.1016/j.celrep.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 218. Hartmaier RJ, Charo J, Fabrizio D. et al. Genomic analysis of 63, 220 tumors reveals insights into tumor uniqueness and targeted cancer immunotherapy strategies. Genome Med 2017; 9(1): 16.http://dx.doi.org/10.1186/s13073-017-0408-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bassani-Sternberg M, Bräunlein E, Klar R. et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun 2016; 7: 13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Bentzen AK, Marquard AM, Lyngaa R. et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat Biotechnol 2016; 34(10): 1037–1045.http://dx.doi.org/10.1038/nbt.3662 [DOI] [PubMed] [Google Scholar]
- 221. Brown SD, Warren RL, Gibb EA. et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res 2014; 24(5): 743–750.http://dx.doi.org/10.1101/gr.165985.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Nathanson T, Ahuja A, Rubinsteyn A. et al. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res 2017; 5(1): 84–91.http://dx.doi.org/10.1158/2326-6066.CIR-16-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Duan F, Duitama J, Al Seesi S. et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med 2014; 211(11): 2231–2248.http://dx.doi.org/10.1084/jem.20141308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Fritsch EF, Rajasagi M, Ott PA. et al. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res 2014; 2(6): 522–529.http://dx.doi.org/10.1158/2326-6066.CIR-13-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. McGranahan N, Furness AJS, Rosenthal R. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351(6280): 1463–1469.http://dx.doi.org/10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S.. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74: 181–273. [DOI] [PubMed] [Google Scholar]
- 227. Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov 2015; 5(9): 915–919.http://dx.doi.org/10.1158/2159-8290.CD-15-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Restifo NP, Smyth MJ, Snyder A.. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer 2016; 16(2): 121–126.http://dx.doi.org/10.1038/nrc.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A.. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168(4): 707–723.http://dx.doi.org/10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lee HM, Timme TL, Thompson TC.. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res 2000; 60(7): 1927–1933. [PubMed] [Google Scholar]
- 231. Restifo NP, Marincola FM, Kawakami Y. et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst 1996; 88(2): 100–108.http://dx.doi.org/10.1093/jnci/88.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Parsa AT, Waldron JS, Panner A. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13(1): 84–88.http://dx.doi.org/10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 233. Green MR, Monti S, Rodig SJ. et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116(17): 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Lastwika KJ, Wilson W 3rd, Li QK. et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 2016; 76(2): 227–238.http://dx.doi.org/10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- 235. Casey SC, Tong L, Li Y. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016; 352(6282): 227–231.http://dx.doi.org/10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lim S-O, Li C-W, Xia W. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell 2016; 30(6): 925–939.http://dx.doi.org/10.1016/j.ccell.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kaplan DH, Shankaran V, Dighe AS. et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 1998; 95(13): 7556–7561.http://dx.doi.org/10.1073/pnas.95.13.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Shankaran V, Ikeda H, Bruce AT. et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410(6832): 1107–1111.http://dx.doi.org/10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 239. Dunn GP, Bruce AT, Sheehan KCF. et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005; 6(7): 722–729.http://dx.doi.org/10.1038/ni1213 [DOI] [PubMed] [Google Scholar]
- 240. Liu C, Peng W, Xu C. et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013; 19(2): 393–403.http://dx.doi.org/10.1158/1078-0432.CCR-12-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Low HB, Zhang Y.. Regulatory roles of MAPK phosphatases in cancer. Immune Netw 2016; 16(2): 85–98.http://dx.doi.org/10.4110/in.2016.16.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Spranger S, Bao R, Gajewski TF.. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015; 523(7559): 231–235. [DOI] [PubMed] [Google Scholar]
- 243. Davoli T, Uno H, Wooten EC, Elledge SJ.. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017; 355(6322). doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Pagès F, Berger A, Camus M. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353(25): 2654–2666. [DOI] [PubMed] [Google Scholar]
- 245. Galon J, Fridman W-H, Pagès F.. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007; 67(5): 1883–1886. [DOI] [PubMed] [Google Scholar]
- 246. Fridman WH, Pagès F, Sautès-Fridman C, Galon J.. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12(4): 298–306. [DOI] [PubMed] [Google Scholar]
- 247. Angell H, Galon J.. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013; 25(2): 261–267.http://dx.doi.org/10.1016/j.coi.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 248. Wargo JA, Reddy SM, Reuben A, Sharma P.. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol 2016; 41: 23–31.http://dx.doi.org/10.1016/j.coi.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Roberts EW, Broz ML, Binnewies M. et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 2016; 30(2): 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther 2015; 4: 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Chaudhary B, Elkord E, Regulatory T.. Cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016; 4(3). doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kumar V, Patel S, Tcyganov E, Gabrilovich DI.. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 2016; 37(3): 208–220.http://dx.doi.org/10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Szebeni GJ, Vizler C, Nagy LI. et al. Pro-tumoral inflammatory myeloid cells as emerging therapeutic targets. Int J Mol Sci 2016; 17(11): 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Guo Q, Jin Z, Yuan Y. et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res 2016; 2016: 9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Kitamura T, Qian B-Z, Pollard JW.. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15(2): 73–86.http://dx.doi.org/10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Rooney MS, Shukla SA, Wu CJ. et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015; 160(1-2): 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. van Rooij N, van Buuren MM, Philips D. et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31(32): e439–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Gubin MM, Zhang X, Schuster H. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014; 515(7528): 577–581.http://dx.doi.org/10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Sainz-Perez A, Lim A, Lemercier B, Leclerc C.. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res 2012; 72(14): 3557–3569.http://dx.doi.org/10.1158/0008-5472.CAN-12-0277 [DOI] [PubMed] [Google Scholar]
- 260. Nakanishi K, Kukita Y, Segawa H. et al. Characterization of the T-cell receptor beta chain repertoire in tumor-infiltrating lymphocytes. Cancer Med 2016; 5(9): 2513–2521.http://dx.doi.org/10.1002/cam4.828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Levy E, Marty R, Gárate Calderón V. et al. Immune DNA signature of T-cell infiltration in breast tumor exomes. Sci Rep 2016; 6: 30064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Pauken KE, Sammons MA, Odorizzi PM. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016; 354(6316): 1160–1165.http://dx.doi.org/10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Dixon AR, Bathany C, Tsuei M. et al. Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev Mol Diagn 2015; 15(9): 1171–1186.http://dx.doi.org/10.1586/14737159.2015.1069182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Remark R, Merghoub T, Grabe N. et al. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol 2016; 1(1): aaf6925–aaf6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Gerner MY, Kastenmuller W, Ifrim I. et al. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 2012; 37(2): 364–376.http://dx.doi.org/10.1016/j.immuni.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Angelo M, Bendall SC, Finck R. et al. Multiplexed ion beam imaging of human breast tumors. Nat Med 2014; 20(4): 436–442.http://dx.doi.org/10.1038/nm.3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Chen F, Zhuang X, Lin L. et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med 2015; 13: 45.http://dx.doi.org/10.1186/s12916-015-0278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Smyth MJ, Ngiow SF, Ribas A, Teng MWL.. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016; 13(3): 143–158. [DOI] [PubMed] [Google Scholar]
- 269. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29(6 Suppl 16): 15–18. [DOI] [PubMed] [Google Scholar]
- 270. Hendry SA, Farnsworth RH, Solomon B. et al. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front Immunol 2016; 7: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Dieterich LC, Ikenberg K, Cetintas T. et al. Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-Cell activation. Front Immunol 2017; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Sautès-Fridman C, Lawand M, Giraldo NA. et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol 2016; 7: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Wallace MC, Friedman SL.. Hepatic fibrosis and the microenvironment: fertile soil for hepatocellular carcinoma development. Gene Expr 2014; 16(2): 77–84.http://dx.doi.org/10.3727/105221614X13919976902057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Joyce JA, Fearon DT.. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348(6230): 74–80.http://dx.doi.org/10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 275. Noguchi T, Ward JP, Gubin MM. et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 2017; 5(2): 106–117.http://dx.doi.org/10.1158/2326-6066.CIR-16-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Eil R, Vodnala SK, Clever D. et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016; 537(7621): 539–543.http://dx.doi.org/10.1038/nature19364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Atretkhany K-SN, Drutskaya MS, Nedospasov SA. et al. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther 2016; 168: 98–112. [DOI] [PubMed] [Google Scholar]
- 278. Kumar V, Gabrilovich DI.. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014; 143(4): 512–519.http://dx.doi.org/10.1111/imm.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Nakatsu G, Li X, Zhou H. et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun 2015; 6: 8727.http://dx.doi.org/10.1038/ncomms9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Alfano M, Canducci F, Nebuloni M. et al. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat Rev Urol 2016; 13(2): 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Chang C-H, Qiu J, O'Sullivan D. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015; 162(6): 1229–1241.http://dx.doi.org/10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Scharping NE, Delgoffe GM.. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccines (Basel) 2016; 4(4). doi: 10.3390/vaccines4040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Johnson CH, Spilker ME, Goetz L. et al. Metabolite and microbiome interplay in cancer immunotherapy. Cancer Res 2016; 76(21): 6146–6152.http://dx.doi.org/10.1158/0008-5472.CAN-16-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Rodolfo M, Castelli C, Rivoltini L.. Immune response markers in sentinel nodes may predict melanoma progression. Oncoimmunology 2014; 3(4): e28498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Gustafson MP, Lin Y, LaPlant B. et al. Immune monitoring using the predictive power of immune profiles. J Immunother Cancer 2013; 1(1): 7.http://dx.doi.org/10.1186/2051-1426-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zuckerman NS, Yu H, Simons DL. et al. Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. Int J Cancer 2013; 132(11): 2537–2547.http://dx.doi.org/10.1002/ijc.27933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Gutkin DW, Shurin MR.. Clinical evaluation of systemic and local immune responses in cancer: time for integration. Cancer Immunol Immunother 2014; 63(1): 45–57.http://dx.doi.org/10.1007/s00262-013-1480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Manuel M, Tredan O, Bachelot T. et al. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology 2012; 1(4): 432–440.http://dx.doi.org/10.4161/onci.19545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Gazzola A, Mannu C, Rossi M. et al. The evolution of clonality testing in the diagnosis and monitoring of hematological malignancies. Ther Adv Hematol 2014; 5(2): 35–47.http://dx.doi.org/10.1177/2040620713519729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Frietze KM, Roden RBS, Lee J-H. et al. Identification of anti-CA125 antibody responses in ovarian cancer patients by a novel deep sequence-coupled biopanning platform. Cancer Immunol Res 2016; 4(2): 157–164.http://dx.doi.org/10.1158/2326-6066.CIR-15-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Cohen CJ, Gartner JJ, Horovitz-Fried M. et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J Clin Invest 2015; 125(10): 3981–3991.http://dx.doi.org/10.1172/JCI82416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Larman HB, Laserson U, Querol L. et al. PhIP-Seq characterization of autoantibodies from patients with multiple sclerosis, type 1 diabetes and rheumatoid arthritis. J Autoimmun 2013; 43: 1–9.http://dx.doi.org/10.1016/j.jaut.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Xu GJ, Kula T, Xu Q. et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 2015; 348(6239): aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Simon AK, Hollander GA, McMichael A.. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015; 282(1821): 20143085.http://dx.doi.org/10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Hurez V, Padrón ÁS, Svatek RS, Curiel TJ.. Considerations for successful cancer immunotherapy in aged hosts. Clin Exp Immunol 2017; 187(1): 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Blank CU, Haanen JB, Ribas A, Schumacher TN.. CANCER IMMUNOLOGY. The “cancer immunogram”. Science 2016; 352(6286): 658–660. [DOI] [PubMed] [Google Scholar]
- 297. Karasaki T, Nagayama K, Kuwano H. et al. An immunogram for the cancer-immunity cycle: towards personalized immunotherapy of lung cancer. J Thorac Oncol 2017; 12(5): 791–803. [DOI] [PubMed] [Google Scholar]
- 298. Angelova M, Charoentong P, Hackl H. et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol 2015; 16(1): 64.http://dx.doi.org/10.1186/s13059-015-0620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Lizotte P, Ivanova E, Bittinger M. et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. Cancer Immunol Res 2016; 4(14): e89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Şenbabaoğlu Y, Gejman RS, Winer AG. et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 2016; 17(1): 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Galluzzi L, Buqué A, Kepp O. et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28(6): 690–714. [DOI] [PubMed] [Google Scholar]
- 302. Spiotto M, Fu Y-X, Weichselbaum RR.. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol 2016; doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363(5): 411–422.http://dx.doi.org/10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 304. Rodríguez PC, Rodríguez G, González G, Lage A.. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev 2010; 12(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 305. Rodriguez PC, Popa X, Martínez O. et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 2016; 22(15): 3782–3790. [DOI] [PubMed] [Google Scholar]
- 306. Finke LH, Wentworth K, Blumenstein B. et al. Lessons from randomized phase III studies with active cancer immunotherapies—outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 2007; 25: B97–B109. [DOI] [PubMed] [Google Scholar]
- 307. Klebanoff CA, Acquavella N, Yu Z, Restifo NP.. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011; 239(1): 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Guo C, Manjili MH, Subjeck JR. et al. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 2013; 119: 421–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Melero I, Gaudernack G, Gerritsen W. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014; 11(9): 509–524.http://dx.doi.org/10.1038/nrclinonc.2014.111 [DOI] [PubMed] [Google Scholar]
- 310. Clifton GT, Kohrt HE, Peoples GE.. Critical issues in cancer vaccine trial design. Vaccine 2015; 33(51): 7386–7392.http://dx.doi.org/10.1016/j.vaccine.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 311. Romero P, Banchereau J, Bhardwaj N. et al. The Human Vaccines Project: a roadmap for cancer vaccine development. Sci Transl Med 2016; 8(334): 334ps9. [DOI] [PubMed] [Google Scholar]
- 312. Banday AH, Jeelani S, Hruby VJ.. Cancer vaccine adjuvants—recent clinical progress and future perspectives. Immunopharmacol Immunotoxicol 2015; 37(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 313. Ye Z, Li Z, Jin H, Qian Q.. Therapeutic cancer vaccines. Adv Exp Med Biol 2016; 909: 139–167. [DOI] [PubMed] [Google Scholar]
- 314. Thomas S, Prendergast GC.. Cancer vaccines: a brief overview. Methods Mol Biol 2016; 1403: 755–761. [DOI] [PubMed] [Google Scholar]
- 315. Khong H, Overwijk WW.. Adjuvants for peptide-based cancer vaccines. J Immunother Cancer 2016; 4(1): 56.http://dx.doi.org/10.1186/s40425-016-0160-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. van der Burg SH, Arens R, Ossendorp F. et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016; 16(4): 219–233.http://dx.doi.org/10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
- 317. van Duikeren S, Fransen MF, Redeker A. et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol 2012; 189(7): 3397–3403. [DOI] [PubMed] [Google Scholar]
- 318. Ophir E, Bobisse S, Coukos G. et al. Personalized approaches to active immunotherapy in cancer. Biochim Biophys Acta 2016; 1865(1): 72–82. [DOI] [PubMed] [Google Scholar]
- 319. Chiang CL-L, Coukos G, Kandalaft LE.. Whole tumor antigen vaccines: where are we? Vaccines (Basel) 2015; 3(2): 344–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Hacohen N, Fritsch EF, Carter TA. et al. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res 2013; 1(1): 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Fritsch EF, Hacohen N, Wu CJ.. Personal neoantigen cancer vaccines: the momentum builds. Oncoimmunology 2014; 3(6): e29311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Türeci Ö, Vormehr M, Diken M. et al. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin Cancer Res 2016; 22(8): 1885–1896. [DOI] [PubMed] [Google Scholar]
- 323. Carreno BM, Magrini V, Becker-Hapak M. et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348(6236): 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Rammensee H-G, Singh-Jasuja H.. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev Vaccines 2013; 12(10): 1211–1217.http://dx.doi.org/10.1586/14760584.2013.836911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Kreiter S, Vormehr M, van de Roemer N. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015; 520(7549): 692–696.http://dx.doi.org/10.1038/nature14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Ward JP, Gubin MM, Schreiber RD.. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv Immunol 2016; 130: 125–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Martin SD, Brown SD, Wick DA. et al. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS One 2016; 11(5): e0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. McLennan DN, Porter CJH, Charman SA.. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol 2005; 2(1): 89–96.http://dx.doi.org/10.1016/j.ddtec.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 329. Characiejus D, Jacobs JJL, Pašukonienė V. et al. Prediction of response in cancer immunotherapy. Anticancer Res 2011; 31(2): 639–647. [PubMed] [Google Scholar]
- 330. Henrickson SE, Perro M, Loughhead SM. et al. Antigen availability determines CD8+ T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity 2013; 39(3): 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Tscharke DC, Croft NP, Doherty PC, La Gruta NL.. Sizing up the key determinants of the CD8(+) T cell response. Nat Rev Immunol 2015; 15(11): 705–716. [DOI] [PubMed] [Google Scholar]
- 332. Sahin U, Derhovanessian E, Miller M. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017; 547(7662): 222–226.http://dx.doi.org/10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 333. Ott PA, Hu Z, Keskin DB. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017; 547(7662): 217–221.http://dx.doi.org/10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Hodi FS, O’Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8): 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813.http://dx.doi.org/10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Rosenberg JE, Hoffman-Censits J, Powles T. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387(10031): 1909–1920.http://dx.doi.org/10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Rizvi NA, Mazières J, Planchard D. et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16(3): 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ansell SM, Lesokhin AM, Borrello I. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372(4): 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Postow MA, Callahan MK, Wolchok JD.. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33(17): 1974–1982.http://dx.doi.org/10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Topalian SL, Drake CG, Pardoll DM.. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27(4): 450–461.http://dx.doi.org/10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Giuroiu I, Weber J.. Novel checkpoints and cosignaling molecules in cancer immunotherapy. Cancer J 2017; 23(1): 23–31.http://dx.doi.org/10.1097/PPO.0000000000000241 [DOI] [PubMed] [Google Scholar]
- 342. Janakiram M, Shah UA, Liu W. et al. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev 2017; 276(1): 26–39.http://dx.doi.org/10.1111/imr.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Allard B, Longhi MS, Robson SC, Stagg J.. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 2017; 276(1): 121–144.http://dx.doi.org/10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Dougall WC, Kurtulus S, Smyth MJ, Anderson AC.. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev 2017; 276(1): 112–120.http://dx.doi.org/10.1111/imr.12518 [DOI] [PubMed] [Google Scholar]
- 345. Ni L, Dong C.. New checkpoints in cancer immunotherapy. Immunol Rev 2017; 276(1): 52–65.http://dx.doi.org/10.1111/imr.12524 [DOI] [PubMed] [Google Scholar]
- 346. Roach C, Zhang N, Corigliano E. et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 2016; 24(6): 392–397.http://dx.doi.org/10.1097/PAI.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Jørgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev Mol Diagn 2016; 16(2): 131–133. [DOI] [PubMed] [Google Scholar]
- 348. Wang X, Teng F, Kong L, Yu J.. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016; 9: 5023–5039.http://dx.doi.org/10.2147/OTT.S105862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Lau J, Cheung J, Navarro A. et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 2017; 8: 14572.http://dx.doi.org/10.1038/ncomms14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Ribas A, Hu-Lieskovan S.. What does PD-L1 positive or negative mean? J Exp Med 2016; 213(13): 2835–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Scheel AH, Ansén S, Schultheis AM. et al. PD-L1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology 2016; 5(5): e1131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Liu X, Cho WC.. Precision medicine in immune checkpoint blockade therapy for non-small cell lung cancer. Clin Transl Med 2017; 6(1): 7.http://dx.doi.org/10.1186/s40169-017-0136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Novotny JF Jr, Cogswell J, Inzunza H. et al. Establishing a complementary diagnostic for anti-PD-1 immune checkpoint inhibitor therapy. Ann Oncol 2016; 27(10): 1966–1969.http://dx.doi.org/10.1093/annonc/mdw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Daud AI, Wolchok JD, Robert C. et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 2016; 34(34): 4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Hirsch FR, McElhinny A, Stanforth D. et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017; 12(2): 208–222.http://dx.doi.org/10.1016/j.jtho.2016.11.2228 [DOI] [PubMed] [Google Scholar]
- 356. Snyder A, Makarov V, Merghoub T. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371(23): 2189–2199.http://dx.doi.org/10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Rizvi NA, Hellmann MD, Snyder A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230): 124–128.http://dx.doi.org/10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Van Allen EM, Miao D, Schilling B. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015; 350(6257): 207–211.http://dx.doi.org/10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372(26): 2509–2520.http://dx.doi.org/10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Gao J, Shi LZ, Zhao H. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 2016; 167(2): 397–404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Benci JL, Xu B, Qiu Y. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016; 167(6): 1540–1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Zaretsky JM, Garcia-Diaz A, Shin DS. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375(9): 819–829.http://dx.doi.org/10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Shi LZ, Fu T, Guan B. et al. Interdependent IL-7 and IFN-γ signalling in T-cell controls tumour eradication by combined α-CTLA-4+α-PD-1 therapy. Nat Commun 2016; 7: 12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Vétizou M, Pitt JM, Daillère R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350(6264): 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Sivan A, Corrales L, Hubert N. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350(6264): 1084–1089.http://dx.doi.org/10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Snyder A, Nathanson T, Funt S. et al. Multi-omic analysis of urothelial cancer patients treated with PD-L1 blockade demonstrates the contribution of both systemic and somatic factors to the biology of response and resistance. bioRxiv 2016; 086843. [Google Scholar]
- 367. Wistuba-Hamprecht K, Martens A, Heubach F. et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. Eur J Cancer 2017; 73: 61–70.http://dx.doi.org/10.1016/j.ejca.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Spitzer MH, Carmi Y, Reticker-Flynn NE. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017; 168(3): 487–502.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Gosho M, Nagashima K, Sato Y.. Study designs and statistical analyses for biomarker research. Sensors 2012; 12(7): 8966–8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Topalian SL, Taube JM, Anders RA, Pardoll DM.. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16(5): 275–287.http://dx.doi.org/10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Gibney GT, Weiner LM, Atkins MB.. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17(12): e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Im SJ, Hashimoto M, Gerner MY. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016; 537(7620): 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Shahabi V, Berman D, Chasalow SD. et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013; 11: 75.http://dx.doi.org/10.1186/1479-5876-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Michot JM, Bigenwald C, Champiat S. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016; 54: 139–148.http://dx.doi.org/10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 375. Kourie HR, Paesmans M, Klastersky J.. Biomarkers for adverse events associated with immune checkpoint inhibitors. Biomark Med 2016; 10(10): 1029–1031.http://dx.doi.org/10.2217/bmm-2016-0211 [DOI] [PubMed] [Google Scholar]
- 376. Chen P-L, Roh W, Reuben A. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016; 6(8): 827–837.http://dx.doi.org/10.1158/2159-8290.CD-15-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Lesterhuis WJ, Bosco A, Millward MJ. et al. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat Rev Drug Discov 2017; 16(4): 264–272. [DOI] [PubMed] [Google Scholar]
- 378. O’Donnell JS, Long GV, Scolyer RA. et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017; 52: 71–81. [DOI] [PubMed] [Google Scholar]
- 379. Sharma P, Allison JP.. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161(2): 205–214.http://dx.doi.org/10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Swart M, Verbrugge I, Beltman JB.. Combination approaches with immune-checkpoint blockade in cancer therapy. Front Oncol 2016; 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Moynihan KD, Opel CF, Szeto GL. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 2016; 22(12): 1402–1410.http://dx.doi.org/10.1038/nm.4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Rosenberg SA, Packard BS, Aebersold PM. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988; 319(25): 1676–1680. [DOI] [PubMed] [Google Scholar]
- 383. Dudley ME, Wunderlich JR, Robbins PF. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298(5594): 850–854.http://dx.doi.org/10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Morgan RA, Dudley ME, Wunderlich JR. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314(5796): 126–129.http://dx.doi.org/10.1126/science.1129003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Kochenderfer JN, Wilson WH, Janik JE. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116(20): 4099–4102.http://dx.doi.org/10.1182/blood-2010-04-281931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Rosenberg SA, Restifo NP.. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348(6230): 62–68.http://dx.doi.org/10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Fesnak AD, June CH, Levine BL.. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016; 16(9): 566–581.http://dx.doi.org/10.1038/nrc.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Robbins PF, Lu Y-C, El-Gamil M. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19(6): 747–752.http://dx.doi.org/10.1038/nm.3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Linnemann C, Heemskerk B, Kvistborg P. et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 2013; 19(11): 1534–1541.http://dx.doi.org/10.1038/nm.3359 [DOI] [PubMed] [Google Scholar]
- 390. Orentas RJ, Nordlund J, He J. et al. Bioinformatic description of immunotherapy targets for pediatric T-cell leukemia and the impact of normal gene sets used for comparison. Front Oncol 2014; 4: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Ruella M, Maus MV.. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J 2016; 14: 357–362.http://dx.doi.org/10.1016/j.csbj.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Vyas M, Müller R, Pogge von Strandmann E.. Antigen loss variants: catching hold of escaping foes. Front Immunol 2017; 8(175): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Kvistborg P, Shu CJ, Heemskerk B. et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology 2012; 1(4): 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Rosenberg SA, Yang JC, Sherry RM. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17(13): 4550–4557.http://dx.doi.org/10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Chapuis AG, Desmarais C, Emerson R. et al. Tracking the fate and origin of clinically relevant adoptively transferred CD8+ T cells in vivo. Sci Immunol 2017; 2(8): eaal2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. McCracken MN, Vatakis DN, Dixit D. et al. Noninvasive detection of tumor-infiltrating T cells by PET reporter imaging. J Clin Invest 2015; 125(5): 1815–1826.http://dx.doi.org/10.1172/JCI77326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Tavaré R, Escuin-Ordinas H, Mok S. et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res 2016; 76(1): 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Mall S, Yusufi N, Wagner R. et al. Immuno-PET imaging of engineered human T cells in tumors. Cancer Res 2016; 76(14): 4113–4123.http://dx.doi.org/10.1158/0008-5472.CAN-15-2784 [DOI] [PubMed] [Google Scholar]
- 399. Hinrichs CS, Spolski R, Paulos CM. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008; 111(11): 5326–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Sukumar M, Liu J, Ji Y. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 2013; 123(10): 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Crompton JG, Sukumar M, Roychoudhuri R. et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res 2015; 75(2): 296–305.http://dx.doi.org/10.1158/0008-5472.CAN-14-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Eggermont LJ, Paulis LE, Tel J, Figdor CG.. Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends Biotechnol 2014; 32(9): 456–465.http://dx.doi.org/10.1016/j.tibtech.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Wrzesinski C, Paulos CM, Gattinoni L. et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest 2007; 117(2): 492–501.http://dx.doi.org/10.1172/JCI30414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Dudley ME, Yang JC, Sherry R. et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26(32): 5233–5239.http://dx.doi.org/10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Goff SL, Dudley ME, Citrin DE. et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol 2016; 34(20): 2389–2397.http://dx.doi.org/10.1200/JCO.2016.66.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Matsushita H, Vesely MD, Koboldt DC. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012; 482(7385): 400–404.http://dx.doi.org/10.1038/nature10755 [DOI] [PMC free article] [PubMed] [Google Scholar]