Abstract
Background
In the 2007 World Cancer Research Fund/American Institute for Cancer Research Second Expert Report, the expert panel judged that there was strong evidence that alcoholic drinks and body fatness increased esophageal cancer risk, whereas fruits and vegetables probably decreased its risk. The judgments were mainly based on case–control studies. As part of the Continuous Update Project, we updated the scientific evidence accumulated from cohort studies in this topic.
Methods
We updated the Continuous Update Project database up to 10 January 2017 by searching in PubMed and conducted dose–response meta-analyses to estimate summary relative risks (RRs) and 95% confidence intervals (CIs) using random effects model.
Results
A total of 57 cohort studies were included in 13 meta-analyses. Esophageal adenocarcinoma risk was inversely related to vegetable intake (RR per 100 g/day: 0.89, 95% CI: 0.80–0.99, n = 3) and directly associated with body mass index (RR per 5 kg/m2: 1.47, 95% CI: 1.34–1.61, n = 9). For esophageal squamous cell carcinoma, inverse associations were observed with fruit intake (RR for 100 g/day increment: 0.84, 95% CI: 0.75–0.94, n = 3) and body mass index (RR for 5 kg/m2 increment: 0.64, 95% CI: 0.56–0.73, n = 8), and direct associations with intakes of processed meats (RR for 50 g/day increment: 1.59, 95% CI: 1.11–2.28, n = 3), processed and red meats (RR for 100 g/day increment: 1.37, 95% CI: 1.04–1.82, n = 3) and alcohol (RR for 10 g/day increment: 1.25, 95% CI: 1.12–1.41, n = 6).
Conclusions
Evidence from cohort studies suggested a protective role of vegetables and body weight control in esophageal adenocarcinomas development. For squamous cell carcinomas, higher intakes of red and processed meats and alcohol may increase the risk, whereas fruits intake may play a protective role.
Keywords: esophageal cancer, diet, anthropometry, dose–response, meta-analysis
Key Message
This is a dose–response meta-analysis of prospective studies investigating associations between dietary, anthropometric factors and the risk of esophageal cancer. The results suggested protective effect of vegetables in esophageal adenocarcinoma (AC), and fruits in squamous cell carcinoma (SCC); as well as adverse effect of body fatness in AC, and red and processed meat and alcohol in SCC.
Introduction
Esophageal cancer is the eighth most common cancer and the eighth leading cause of cancer death in the world, with 456 000 new cases and 400 000 deaths in 2012, respectively [1]. At the time of diagnosis, only ∼25% have a localized disease [2]. The 5-year survival rate is only 15%–20% in western countries [3]. Globally, there is a significant variation in esophageal cancer incidence. Esophageal squamous cell carcinoma (SCC) remains the most prevalent histologic type, particularly in ‘esophageal belt’ countries (e.g. Kazakhstan, Mongolia, Northern parts of China, Iran) [4]. In developed countries (e.g. UK [2], United States, Australia [5]), the incidence of esophageal adenocarcinoma (AC) has been increasing rapidly, exceeding the incidence of SCC.
Smoking and alcohol are the major risk factors for esophageal SCC [6]. In 2007, the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AIRC) Second Expert Report [7] concluded that there was strong evidence that alcohol intake is a cause of esophageal cancer, and intake of vegetables, fruits, and foods containing β-carotene and vitamin C probably reduce its risk; body fatness is a cause of esophageal AC, and maté probably increases the risk of esophageal SCC. For other foods and dietary components, the association with esophageal cancer was judged as ‘limited’. The majority of evidence was from case–control studies and not many studies reported results specifically on esophageal SCC and AC.
Since the 2007 WCRF/AICR report, at least 70 relevant publications from prospective studies have been identified. Many of which investigated the associations by histologic types of esophageal cancer, as the etiology of the diseases is different. Modifiable risk factors are important because there are potential public health implications. We conducted an updated systematic review and meta-analysis of dietary and anthropometric factors and the risk of esophageal cancer, SCC and AC, as part of the work for the WCRF CUP [8].
Methods
Search strategy
This is an update of the 2007 WCRF/AICR report for which the search was conducted through December 2005 in eight electronic databases [7]. All cohort studies included in the report were indexed in PubMed, therefore, we updated the search in PubMed up to 10 January 2017. In addition, reference lists of relevant publications were screened for any further publications. The protocol of the CUP including the search strategy can be accessed at http://www.wcrf.org/sites/default/files/protocol_oesophageal_cancer.pdf [9].
Study selection
Included studies met the following criteria: (i) were designed as cohort, nested case–control or case–cohort study; (ii) reported relative risk (RR) estimates (hazard ratio, odds ratio, or risk ratio) with confidence intervals (CIs); (iii) reported quantifiable measures of dietary intakes or anthropometric factors. When several publications from the same study were identified, the most recent publication with the largest number of cases was selected for the review.
According to the protocol, the dose–response meta-analysis is updated in the CUP when at least two new studies had been published after the 2007 WCRF/AICR report and the total number of studies with sufficient data for meta-analysis is five or more. Additional analyses of related exposures were conducted even though the numbers did not meet the criteria.
Data extraction
The following data were extracted from each study: study characteristics (first author, publication year, country, study name, follow-up period, and losses); study sample characteristics (sex, ethnicity, number of cases and non-cases, study size); exposure details (assessment method, exposure level); outcome details (type of esophageal cancer, case ascertainment method); RR estimates and corresponding 95% CIs; and adjustment variables.
Statistical analysis
We combined the dose–response RR estimates in the studies using a random-effects model, which considers both within-study and between-study variation [10]. When the dose–response RR estimates were not available in the studies, we used Greenland and Longnecker method to derive the linear dose–response trend from the natural logarithm of the risk estimates across the exposure categories [11]. For this method, at least three categories are required, each presenting the number of person-years, cases, RRs and CIs for that category. Where this information was not provided explicitly, it was derived as specified in the protocol [9]. In brief, the following rules were applied: person-years or cases per quantile were estimated by dividing the total number of cases or person-years by the number of quantiles; missing person-years per category were estimated proportionally if the number of cases per category, RRs, and total person-years were available. When food or drinks consumption was reported in servings or times, these were converted to grams/day using standard serving sizes as stated in the protocol [9]. Where intakes were reported per 1000 kcal/day, the mean energy intake of the study population was used to estimate total intake per day. Summary RRs were estimated for men, women, and both combined. When reported separately, the RRs for men and women were first combined using a fixed effect meta-analysis before pooling with other studies. Summary RRs were calculated for any type of esophageal cancer and for SCC and AC separately. When the RRs were available by cancer types only, the combined RR for esophageal cancer was estimated using Hamling’s method [12]. Hamling’s method was also used to change the reference category if it did not correspond to the lowest exposure category. Floating CIs were converted to conventional 95% CIs using the method described by Orsini et al. [13]. When the study reported observed and calibrated risk estimates, the calibrated one was used for the dose–response meta-analysis.
Heterogeneity was evaluated by Cochran Q test and I2 statistics [14]. The sources of heterogeneity were explored in stratified analysis by sex, cancer type (AC, SCC), geographic location, exposure assessment method, length of follow-up, number of cases, year of publication, and adjustment factors when possible. Sensitivity of the summary risk estimate was investigated in influence-analyses by excluding each study in turn.
Publication or small study bias was assessed by Egger’s test and visual inspection of the funnel plot when at least five studies were included in the analysis [15]. Statistical tests with a P value of <0.05 were considered significant. Analyses were conducted using Stata version 12 software (Stata Corp, College Station, TX).
Results
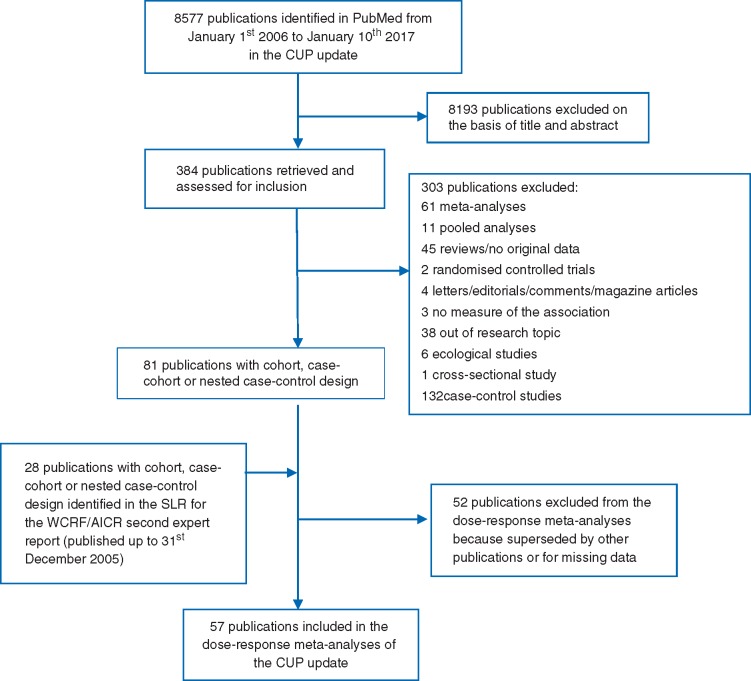
The study search flowchart is available in Figure 1. Of the 109 publications, 57 publications from 34 different cohort studies were included in the dose–response meta-analyses of dietary and anthropometric factors and the risk of esophageal cancer, and SCC and AC. Fifteen studies were conducted in Asia, 11 in Europe, and 8 in the USA. Cases were ascertained through cancer, death or health registries, medical records, death certificates, active follow-up, or a combination of the methods. No studies were based on self-reported, unverified cases. Validated dietary questionnaire was used in all but one Chinese study [16–18]. Weight and height data were measured in eight studies [16, 17, 19–26], self-reported in eight [27–34] and taken from medical records in one [35]. Most studies adjusted for multiple main confounding factors such as alcohol, smoking, education, and body mass index (BMI). Main characteristics of each included publication are provided in supplementary Table S1, available at Annals of Oncology online. The excluded publications and reasons for exclusions are in supplementary Table S2, available at Annals of Oncology online.
Figure 1.
Flow chart of the study selection.
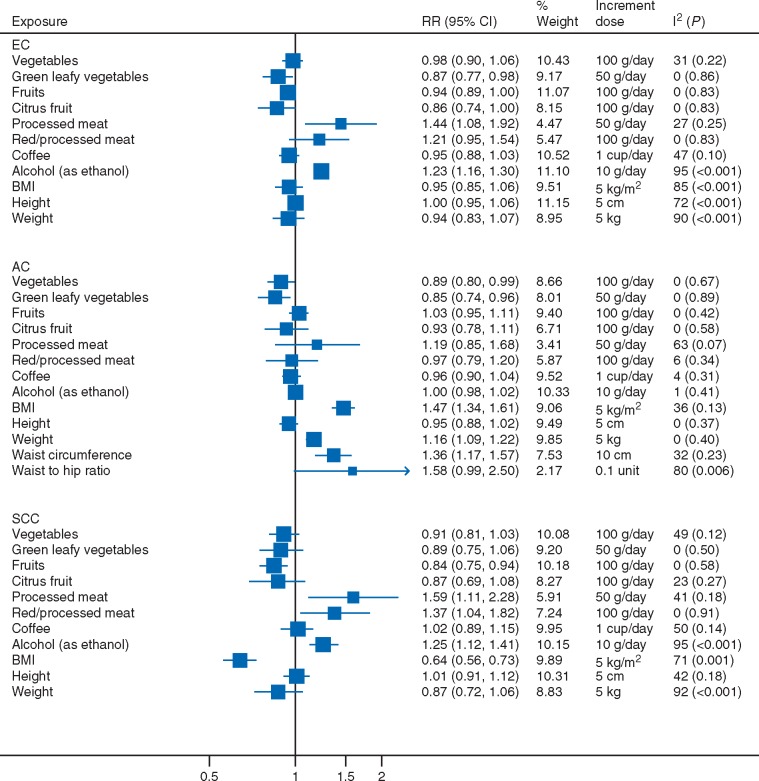
A summary of the dose–response relationships examined in the present study is shown in Figure 2.
Figure 2.
Summary of the dose–response relationships for the dietary and anthropometric factors and risk of esophageal cancer, adenocarcinoma and squamous cell carcinoma. EC, esophageal cancer; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Fruits and vegetables
Meta-analyses were conducted for vegetables, green leafy vegetables, fruits and citrus fruits. Only two studies reported on total fruit and vegetable intake [36, 37], and found significant inverse associations with SCC [36, 37] and no association with AC [36]. There was not enough information for other vegetables or fruits [17, 38–40] (results not tabulated).
Vegetable intake was not related to the risk of esophageal cancer (all types combined) (RR per 100 g/day = 0.98, 95% CI: 0.90–1.06, I2 = 31%, 5 studies) [16, 37, 38, 40, 41] and SCC (RR = 0.91, 95% CI: 0.81–1.03, I2 = 49%, 4 studies) [16, 36, 37, 40], but was statistically significantly inversely associated with AC (RR = 0.89, 95% CI: 0.80–0.99, I2 = 0%, 3 studies) [36, 38, 40], with one study contributing 82% weight in the analysis [36] (supplementary Figure S1, available at Annals of Oncology online). All three studies reported non-significant inverse associations in the analyses adjusted for multiple main confounders; of which two additionally adjusted for fruit intake [36, 40]. Green leafy vegetable intake was significantly inversely associated with the risk of esophageal cancer (RR per 50 g/day = 0.87, 95% CI: 0.77–0.98, I2 = 0%, 6 studies) [17, 36–40] and AC (RR = 0.85, 95% CI: 0.74–0.96, I2 = 0%, 3 studies) [36, 38, 40], but not SCC (RR = 0.89, 95% CI: 0.75–1.06, I2 = 0%, 4 studies) [17, 36, 37, 40] (supplementary Figure S2, available at Annals of Oncology online). Only one study additionally adjusted for intake of fruits and other vegetables [40].
Fruit intake was not related to all esophageal cancer (RR per 100 g/day = 0.94, 95% CI: 0.89–1.00, I2 = 0%, 4 studies) [37, 38, 40, 41] and AC (RR = 1.03, 95% CI: 0.95–1.11, I2 = 0%, 3 studies) [36, 38, 40], but was inversely associated with SCC (RR = 0.84, 95% CI: 0.75–0.94, I2 = 0%, 3 studies) [36, 37, 40] (supplementary Figure S3, available at Annals of Oncology online). Studies on SCC adjusted for all major confounders and two studies additionally adjusted for vegetable intake [36, 40]. For citrus fruits, no significant associations were observed (supplementary Figure S4, available at Annals of Oncology online). The summary RRs per 100 g/day were 0.86 (95% CI: 0.74–1.00, I2 = 0%) for all esophageal cancer (6 studies) [36–40, 42], 0.93 (95% CI: 0.78–1.11, I2 = 0%) for AC (3 studies) [36, 38, 40], and 0.87 (95% CI: 0.69–1.08, I2 = 23%) for SCC (3 studies) [36, 37, 40].
In influence analyses, the results remained the same except when the only study on mortality was excluded [39], citrus fruit became significantly inversely associated with all esophageal cancer risk (RR = 0.85, 95% CI: 0.73–0.99).
Red and processed meats
Meta-analyses were conducted for intakes of processed meat as well as processed and red meat combined. Meta-analyses were not conducted for total meat [38, 43–45], specific red meats [39, 46, 47], poultry [38, 39, 48, 49], and fish [39, 43, 45, 48, 50, 51] because of insufficient information. Reported results were mostly non-significant (results not tabulated).
Processed meat intake was associated with increased risk of all esophageal cancer (RR per 50 g/day = 1.44, 95% CI: 1.08–1.92, I2 = 27%, 4 studies) [39, 49, 52–54] (supplementary Figure S5, available at Annals of Oncology online). No significant association was observed for AC (RR = 1.19, 95% CI: 0.85–1.68, I2 = 63%, 3 studies) [49, 52, 53] but a direct association was observed for SCC (RR = 1.59, 95% CI: 1.11–2.28, I2 = 41%, 3 studies) [52–54].
For processed and red meat intake combined, the associations were not significant for all esophageal cancer (RR per 100 g/day = 1.21, 95% CI: 0.95–1.54, I2 = 0%, 3 studies) [49, 52–54] and AC (RR = 0.97, 95% CI: 0.79–1.20, I2 = 6%, 3 studies) [49, 52, 53], but was significant with SCC (RR = 1.37, 95% CI: 1.04–1.82, I2 = 0%, 3 studies) [52–54] (supplementary Figure S6, available at Annals of Oncology online).
Hot drinks
Six [39, 55–59], two [57, 59], and three studies [57–59] were included in the meta-analyses of coffee and all esophageal cancer, AC and SCC risk, respectively. There were no significant associations (supplementary Figure S7, available at Annals of Oncology online). The summary RRs per 1 cup/day were 0.95 (95% CI: 0.88–1.03, I2 = 47%) for all esophageal cancer, 0.96 (95% CI: 0.90–1.04, I2 = 4%) for AC, and 1.02 (95% CI: 0.89–1.15, I2 = 50%) for SCC.
Meta-analysis was not conducted for high-temperature drinks [16, 50, 57], tea [39, 50, 57, 59], green tea [39, 60], and black tea [39] due to low number of studies with sufficient data (results not tabulated). Drinking hot liquid and hot tea were non-significantly inversely associated with SCC [16, 57] and AC [57] risk in two studies and significantly directly associated with esophageal cancer mortality in one study [50], comparing drinking hot versus not hot tea.
Alcoholic drinks
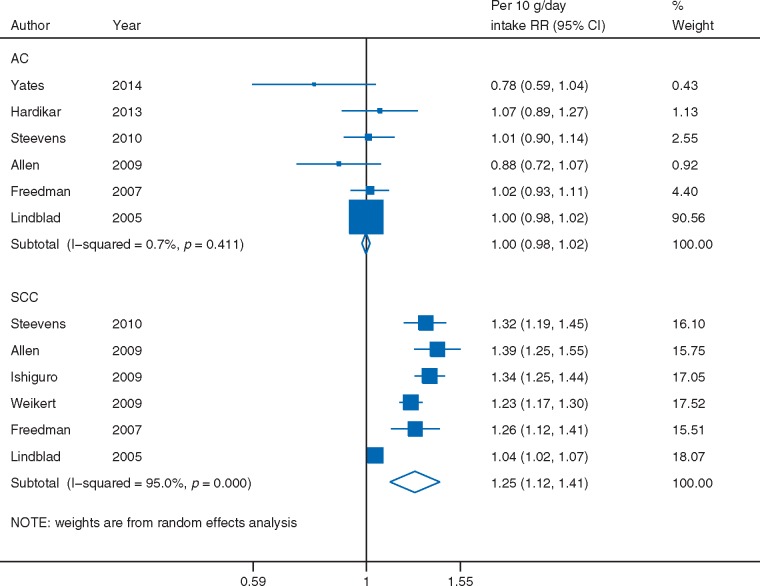
Alcohol intake, as measured in grams of ethanol, was significantly directly associated with all esophageal cancer risk (RR per 10 g/day = 1.23, 95% CI: 1.16–1.30, I2 = 95%, 18 studies) [21, 35, 43, 50, 61–74] (supplementary Figure S8, available at Annals of Oncology online). The association was restricted to SCC (RR = 1.25, 95% CI: 1.12–1.41, I2 = 95%, 6 studies) [35, 61, 63, 64, 69, 70] and not observed with AC (RR = 1.00, 95% CI: 0.98–1.02, I2 = 1%, 6 studies) [21, 35, 61, 63, 69, 75] (Figure 3).
Figure 3.
Alcohol intake and esophageal cancer risk by histologic type, dose–response meta-analysis. AC, adenocarcinoma; SCC, squamous cell carcinoma.
The substantial proportion of between-study heterogeneity in the analysis on SCC was largely due to one study [35]; when omitted in sensitivity analysis, the relationship remained similar (RR = 1.30, 95% CI: 1.24–1.36) but heterogeneity dropped from 95% to 39%. In this study, the reference category of alcohol consumption was relatively high (≤2 units per day), most of the study participants were in the lowest two categories, and 42% was of unknown alcohol consumption [35].
Anthropometric measurements
For BMI, 17 studies were included in the meta-analyses of esophageal cancer [16, 19–24, 26–35], nine in AC [19–21, 23, 25, 27, 33–35] and eight in SCC [16, 19, 20, 23, 26, 33–35].
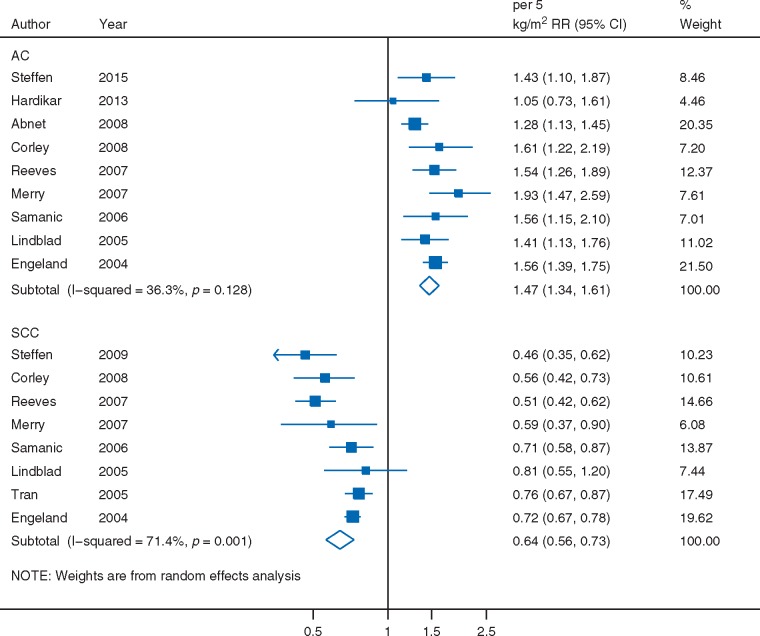
BMI was not associated with all esophageal cancer risk (RR per 5 kg/m2 = 0.95, 95% CI: 0.85–1.06, I2 = 85%) (supplementary Figure S9, available at Annals of Oncology online). Significant associations, that were directly related to AC (RR = 1.47, 95% CI: 1.34–1.61) and inversely to SCC (RR = 0.64, 95% CI: 0.56–0.73) were observed (Figure 4).
Figure 4.
BMI and esophageal cancer risk by histologic type, dose–response meta-analysis. AC, adenocarcinoma; SCC, squamous cell carcinoma.
The moderate (I2 = 36%) and high (I2 = 71%) proportions of between-study heterogeneity in the respective analyses on AC and SCC were not explained by the exposure assessment method, adjustment for smoking, geographic location, and sex; where the associations remained significant in the subgroup analyses (supplementary Table S3, available at Annals of Oncology online).
Meta-analyses were also conducted for weight and height; and for waist circumference and waist-to-hip ratio, where only two [25, 76] and three studies [21, 25, 76] had reported results on the risk of AC, respectively.
The associations of weight with all esophageal cancer [16, 26, 30, 76, 77], AC [25, 76] and SCC risk [16, 26] followed those observed for BMI (supplementary Figure S10, available at Annals of Oncology online). Waist circumference and waist-to-hip ratio were directly associated with AC risk (supplementary Figure S11, available at Annals of Oncology online). The summary RRs were 1.36 (95% CI: 1.17–1.57, I2 = 32%) for each 10 cm increase of waist circumference and 1.58 (95% CI: 0.99–2.50, I2 = 80%) for each 0.1 unit increase of waist-to-hip ratio.
Height was not associated with esophageal cancer (RR per 5 cm = 1.00, 95% CI: 0.95–1.06, I2 = 72%, 9 studies) [16, 20, 26, 30, 33, 76, 78–80], AC (RR = 0.95, 95% CI: 0.88–1.02, I2 = 0%, 3 studies) [25, 33, 76] or SCC (RR = 1.01, 95% CI: 0.91–1.12, I2 = 42%, 3 studies) [16, 26, 33] (supplementary Figure S12, available at Annals of Oncology online).
Test of publication or small study bias
Egger’s test indicated significant evidence of small study bias for alcohol and esophageal cancer (all) (P < 0.01) and SCC (P = 0.01). The asymmetry in the funnel plot of SCC was driven by a study that reported a weaker association [35].
Visual inspection of the funnel plot suggested small studies reported stronger inverse associations between vegetable intake and esophageal cancer (all) risk than expected, but the Egger’s test was not statistically significant (P = 0.15). All other P-values for Egger’s test >0.18.
Interactions with smoking: fruits and vegetables, processed and red meat, alcohol, and BMI
Studies did not observe statistically significant interactions between smoking and intakes of fruit and vegetable [36, 40], processed and red meat [52], and alcohol [44, 64, 66, 69] in relation to esophageal cancer [36, 44, 66], AC [40, 52], or SCC [37, 40, 52, 64] risks and mortality [66].
One exception was for the inverse associations of BMI with SCC risk that was only significant among smokers and not among non-smokers in the European Prospective Investigation into Cancer and Nutrition study (EPIC) (P interaction <0.01) [26]. The pooled study, Me-Can, reported similar findings but the P-values for interaction were 0.41 for current smokers and 0.59 for former smokers versus never smokers [81].
Discussion
The summary data from cohort studies indicate that esophageal AC risk is inversely associated with vegetable intake and directly associated with higher body adiposity (as assessed by BMI and waist circumference), and that the risk of esophageal SCC is inversely related to fruit intake and directly associated with intakes of red and processed meats and alcohol.
Fruits and vegetables
The estimated risk reductions were 11% for AC per 100 g/day increment of vegetable and 16% for SCC per 100 g/day increment of fruit intake. Whether vegetables and fruits have an independent role in esophageal cancer risk awaits further confirmation as not all studies included in the analyses provided mutually adjusted results. Our findings are in agreement with another meta-analysis on fruit intake [82] and SCC risk but not with a published meta-analysis that reported no association between vegetable intake and risk of AC [83]. The latter included the same prospective studies as in our meta-analysis but compared highest versus lowest intakes and did not conduct dose–response meta-analysis.
Fruits and vegetables are good sources of vitamin C and phytochemicals such as β-carotene that are tumor inhibiting, as shown in recent experimental studies [84, 85]. Risk reductions of esophageal and gastric cancers were reported in the Shandong Intervention Trial of vitamin C, E and selenium supplementation [86]; and in observational studies, inverse associations between esophageal cancer risk and dietary vitamin C intake [87], and carotenoids [88].
Red and processed meats
The summary results of cohort studies show that SCC risk increases 59% for an increment of 50 g/day of processed meats and 37% for an increment of 100 g/day of processed and red meats. A direct but not significant association of processed meat with AC was observed.
Previously published meta-analyses reported direct but not significant associations between processed meat intake and all types of esophageal cancer [89, 90] and SCC [91] risk. The present meta-analysis includes more recent publications [49, 54] with more cases. A published meta-analysis [92] reported a significant direct association with AC that was mainly influenced by case–control studies. Plausible mechanisms explaining the associations include heme iron, polycyclic aromatic hydrocarbons, dietary N-nitroso compounds (NOC) and endogenous nitrosation, that are potentially carcinogenic to humans [49, 53, 92, 93]. Direct associations between heme iron intake and risk of AC [49, 52], and SCC [93]; heterocyclic amines formed in high temperature cooked meat and AC risk [52]; dietary nitrite and risk of esophageal cancer [94] and SCC [93]; endogenous NOC index and esophageal cancer risk [94]; and intake of N-nitrosodimethylamine (NDMA) and risk of esophageal cancer [94], and SCC [93] were observed.
Hot drinks
Coffee intake was not associated with esophageal cancer risk which is in agreement with a previous meta-analysis of prospective cohort studies that compared the highest versus the lowest intakes [95]. Our meta-analysis has one additional cohort [55].
Drinking very hot beverages, traditionally at ∼70 °C in some areas of China, Iran, Turkey, and South America, was recently classified as probably carcinogenic to humans by the World Health Organization [96]. The epidemiologic evidence comes mainly from case–control studies, in which the direct associations were stronger than those observed in the few cohort studies that had reported results [16, 39, 50, 57, 59]. The weaker association could partly be due to the fact that these cohort studies were mostly from geographical areas where hot beverages are not normally consumed at very high temperatures. Further confirmation from prospective studies is needed.
Alcohol
Our meta-analysis showed that alcohol intake increased the risk of SCC. The results are in concordance with published meta-analyses [97, 98]. The main mechanisms implicated in the carcinogenesis are related to alcohol metabolite acetaldehyde [97] and chronic alcohol consumption induced enzymes generating reactive oxygen species [99]. The specific mechanism of SCC carcinogenesis is related to alcohol metabolism in saliva where acetaldehyde concentration is 10–100 times higher than in the blood [99]. In smokers, the conversion of acetaldehyde to non-toxic acetate in saliva is inhibited.
Anthropometry
An increment of 5 kg/m2 of BMI was associated with 47% increased risk of AC. Weight and waist circumference were also associated with increased risk of esophageal AC. In contrast, BMI increment of 5 kg/m2 was associated with 36% reduced risk of SCC. Our results are consistent with previously published meta-analyses on AC [100, 101] and SCC [101]. The between-study heterogeneity observed in BMI and SCC remained unexplained, but the heterogeneity was due to the magnitude rather than the direction of the associations in the studies.
The observed inverse association of BMI with SCC risk is similar to what is observed in other smoking-related cancers, as smokers have higher risk of SCC and tend to be slimmer. However, this is not supported by all studies [26]. Increased levels of estrogens associated with higher BMI may have a protective role in SCC development [26].
On the contrary, the EPIC study reported direct associations between waist circumference, and waist-to-hip ratio and the risks of SCC in smokers and non-smokers [26]. The analyses were adjusted for BMI. In agreement with this study, Lin, 2015 reported direct but not significant association for waist circumference in a multivariate-adjusted model including BMI and smoking [79]. Whether waist circumference and waist-to-hip ratio are less confounded by smoking than BMI in the association with SCC risk, independent or not of BMI, needs to be investigated further as current evidence is lacking.
Major proposed mechanisms to explain the association between body fatness and risk of cancer involve insulin, insulin-like growth factor, adipokines, inflammation, and immune responses [102]. Pressure from excess body fat in abdominal area may cause gastroesophageal reflux disease (GERD) which in turn may lead to premalignant condition of Barrett’s esophagus, a precursor of esophageal AC [24].
Strengths and limitations
This meta-analysis has several strengths. In the previous WCRF/IARC systematic literature review, the evidence was mainly from case–control studies [7]. In this review, we included studies of a prospective design, preventing recall bias and providing stronger level of evidence.
We reviewed all dietary and anthropometric exposures on esophageal cancer for which dose–response meta-analyses were conducted during the Continuous Update Project (CUP), presenting an overview of available evidence on thirteen exposures. When the number of studies was sufficient, sensitivity, and stratified analyses were conducted to test the robustness of findings and explore possible sources of heterogeneity. Evidence of heterogeneity was not detected or was low for many analyses. This review particularly focuses on esophageal AC and SCC, which are more frequent in Western and Asian countries, respectively. Publications from studies based in these areas allow the examination of the differences in cancer etiology by histologic type. In terms of study quality, most studies in our review assessed dietary intake using validated food frequency questionnaires and the esophageal cancer diagnosis was documented.
The main limitation of this meta-analysis is the small number of studies on some exposures. For fruits, vegetables and red and processed meats, the small numbers in the stratified analyses reduced the possibility to detect a source of heterogeneity. Those results should be interpreted cautiously as replication is needed. For waist-to-hip ratio in relation to AC risk, sources of the significant between-study heterogeneity could not be explored as only three studies reported results; however, the direct association supported that of BMI and AC. For hot drinks, a meta-analysis was not possible as current data from prospective studies were limited.
The small number of studies reporting associations by smoking status limited a possibility to investigate confounding by smoking. Most studies included in this review were controlled for smoking, but residual confounding cannot be ruled out. For instance, no interaction with smoking was observed in North American [36] and European [40] studies on fruits and vegetables and esophageal cancers, but in a Japanese study [37], intake of fruits and vegetables was inversely related to SCC only in current smokers and consumers of >150 g/week of alcohol. It is also possible that the association is mainly observed in smokers because smokers have lower levels of serum vitamin C [103] and may benefit more from higher intakes.
Another limitation of this meta-analysis is the lack of information in individual studies on GERD symptoms which increase the risk of Barrett’s esophagus that may subsequently lead to AC. However, only ∼6%–12% of those with prolonged GERD symptoms will develop premalignant lesion of Barrett’s esophagus and only 0.5%–1% of these patients will be diagnosed with AC [6]. Therefore, adjusting for GERD symptoms is unlikely to significantly influence results, if not over adjusting the associations of interest for these intermediate factors.
Primary prevention of esophageal cancers may be a preferred option due to the aggressive nature of this disease and the lack of cost-effective screening. Body fatness, high alcohol and red and processed meat intakes are risk factors for other diseases and dietary patterns rich in plant foods may reduce the risk of other diseases including type 2 diabetes and CVD [104]. Therefore, existing primary prevention strategies for chronic diseases could also be used to reduce the burden of esophageal cancers.
Supplementary Material
Acknowledgements
TN is the principal investigator of the CUP at Imperial College London. CS managed the database for the CUP. SV and EP did the literature search and study selection. SV, DSMC, ARV, EP, LA, and DNR extracted the data. DSMC and SV carried out the statistical analyses. SV wrote the first draft of the original manuscript. SV and DSMC take responsibility for the integrity of the data and the accuracy of the data analysis. We thank the systematic literature review team at the Pennsylvania State University for their contributions to the esophageal cancer database.
Funding
This work was supported by the World Cancer Research Fund International as part of the CUP (grant number: 2007/SP01) (http://www.wcrf-uk.org/). The funder of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The views expressed in this review are the opinions of the authors. They may not represent the views of WCRF International/AICR and may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer risk.
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Rubenstein JH, Shaheen NJ.. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015; 149(2): 302–317.e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Napier KJ, Scheerer M, Misra S.. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6(5): 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eslick GD. Epidemiology of esophageal cancer. Gastroenterol Clin North Am 2009; 38(1): 17–25, vii. [DOI] [PubMed] [Google Scholar]
- 5. Thrift AP, Whiteman DC.. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012; 23(12): 3155–3162. [DOI] [PubMed] [Google Scholar]
- 6. Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A.. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21(26): 7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WCRF/AICR: Nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007.
- 8. WCRF International: Continuous Update Project (CUP), 2017. http://www.wcrf.org/int/research-we-fund/continuous-update-project-cup (2 February 2017, date last accessed).
- 9.CUP Team, Imperial College London: Protocol Version 2: Continuous Update and Systematic Literature Review of Randomised Controlled Trials and Prospective Studies on Food, Nutrition, Physical Activity and the Risk of Oesophageal Cancer. http://www.wcrf.org/sites/default/files/protocol_oesophageal_cancer.pdf (10 April 2017, date last accessed).
- 10. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 11. Greenland S, Longnecker MP.. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 1992; 135(11): 1301–1309. [DOI] [PubMed] [Google Scholar]
- 12. Hamling J, Lee P, Weitkunat R, Ambuhl M.. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27(7): 954–970. [DOI] [PubMed] [Google Scholar]
- 13. Orsini N. From floated to conventional confidence intervals for the relative risks based on published dose–response data. Comput Methods Programs Biomed 2010; 98(1): 90–93. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran GD, Sun XD, Abnet CC. et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005; 113(3): 456–463. [DOI] [PubMed] [Google Scholar]
- 17. Wang JB, Fan JH, Dawsey SM. et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep 2016; 6: 22619.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang SM, Fan JH, Jia MM. et al. Body mass index and long-term risk of death from esophageal squamous cell carcinoma in a Chinese population. Thoracic Cancer 2016; 7(4): 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corley DA, Kubo A, Zhao W.. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev 2008; 17(2): 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engeland A, Tretli S, Bjorge T.. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control 2004; 15(8): 837–843. [DOI] [PubMed] [Google Scholar]
- 21. Hardikar S, Onstad L, Blount PL. et al. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PLoS ONE 2013; 8(1): e52192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jee SH, Yun JE, Park EJ. et al. Body mass index and cancer risk in Korean men and women. Int J Cancer 2008; 123(8): 1892–1896. [DOI] [PubMed] [Google Scholar]
- 23. Samanic C, Chow WH, Gridley G. et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006; 17(7): 901–909. [DOI] [PubMed] [Google Scholar]
- 24. Smith M, Zhou M, Whitlock G. et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer 2008; 122(7): 1604–1610. [DOI] [PubMed] [Google Scholar]
- 25. Steffen A, Huerta JM, Weiderpass E. et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2015; 137(3): 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steffen A, Schulze MB, Pischon T. et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2009; 18(7): 2079–2089. [DOI] [PubMed] [Google Scholar]
- 27. Abnet CC, Freedman ND, Hollenbeck AR. et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008; 44(3): 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andreotti G, Hou L, Beane Freeman LE. et al. Body mass index, agricultural pesticide use, and cancer incidence in the Agricultural Health Study cohort. Cancer Causes Control 2010; 21(11): 1759–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ.. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348(17): 1625–1638. [DOI] [PubMed] [Google Scholar]
- 30. Fujino Y. Anthropometry, development history and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 2007; 8(Suppl): 105–112. [PubMed] [Google Scholar]
- 31. Hong JS, Yi SW, Yi JJ. et al. Body mass index and cancer mortality among Korean older middle-aged men: a prospective cohort study. Medicine (Baltimore) 2016; 95(21): e3684.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuriyama S, Tsubono Y, Hozawa A. et al. Obesity and risk of cancer in Japan. Int J Cancer 2005; 113(1): 148–157. [DOI] [PubMed] [Google Scholar]
- 33. Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA.. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 2007; 56(11): 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reeves GK, Pirie K, Beral V. et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Br Med J (Clin Res Ed) 2007; 335(7630): 1134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindblad M, Rodriguez LA, Lagergren J.. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case–control study. Cancer Causes Control 2005; 16(3): 285–294. [DOI] [PubMed] [Google Scholar]
- 36. Freedman ND, Park Y, Subar AF. et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer 2007; 121(12): 2753–2760. [DOI] [PubMed] [Google Scholar]
- 37. Yamaji T, Inoue M, Sasazuki S. et al. Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer 2008; 123(8): 1935–1940. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez CA, Pera G, Agudo A. et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Cancer 2006; 118(10): 2559–2566. [DOI] [PubMed] [Google Scholar]
- 39. Iso H, Kubota Y.. Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 2007; 8(Suppl): 35–80. [PubMed] [Google Scholar]
- 40. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA.. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011; 129(11): 2681–2693. [DOI] [PubMed] [Google Scholar]
- 41. George SM, Park Y, Leitzmann MF. et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr 2009; 89(1): 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li WQ, Kuriyama S, Li Q. et al. Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer 2010; 127(8): 1913–1922. [DOI] [PubMed] [Google Scholar]
- 43. Fan Y, Yuan JM, Wang R. et al. Alcohol, tobacco, and diet in relation to esophageal cancer: the Shanghai Cohort Study. Nutr Cancer 2008; 60(3): 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo W, Blot WJ, Li JY. et al. A nested case–control study of oesophageal and stomach cancers in the Linxian nutrition intervention trial. Int J Epidemiol 1994; 23(3): 444–450. [DOI] [PubMed] [Google Scholar]
- 45. Hirayama T. [A large scale cohort study on the effect of life styles on the risk of cancer by each site]. Gan No Rinsho 1990; Spec No: 233–242. [PubMed] [Google Scholar]
- 46. Kjaerheim K, Gaard M, Andersen A.. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: a prospective study of 10,900 Norwegian men. Cancer Causes Control 1998; 9(1): 99–108. [DOI] [PubMed] [Google Scholar]
- 47. Yu Y, Taylor PR, Li JY. et al. Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People's Republic of China. Cancer Causes Control 1993; 4(3): 195–202. [DOI] [PubMed] [Google Scholar]
- 48. Daniel CR, Cross AJ, Graubard BI. et al. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Philadelphia, Pa) 2011; 4(11): 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jakszyn P, Lujan-Barroso L, Agudo A. et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer 2013; 133(11): 2744–2750. [DOI] [PubMed] [Google Scholar]
- 50. Kinjo Y, Cui Y, Akiba S. et al. Mortality risks of oesophageal cancer associated with hot tea, alcohol, tobacco and diet in Japan. J Epidemiol 1998; 8(4): 235–243. [DOI] [PubMed] [Google Scholar]
- 51. Li WQ, Park Y, Wu JW. et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol 2013; 11(9): 1130–1136.e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cross AJ, Freedman ND, Ren J. et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol 2011; 106(3): 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA.. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann Oncol 2012; 23(9): 2319–2326. [DOI] [PubMed] [Google Scholar]
- 54. Steffen A, Bergmann MM, Sanchez MJ. et al. Meat and heme iron intake and risk of squamous cell carcinoma of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol Biomarkers Prev 2012; 21(12): 2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hashibe M, Galeone C, Buys SS. et al. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer 2015; 113(5): 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Naganuma T, Kuriyama S, Kakizaki M. et al. Coffee consumption and the risk of oral, pharyngeal, and esophageal cancers in Japan: the Miyagi Cohort Study. Am J Epidemiol 2008; 168(12): 1425–1432. [DOI] [PubMed] [Google Scholar]
- 57. Ren JS, Freedman ND, Kamangar F. et al. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur J Cancer 2010; 46(10): 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tverdal A, Hjellvik V, Selmer R.. Coffee intake and oral-oesophageal cancer: follow-up of 389,624 Norwegian men and women 40-45 years. Br J Cancer 2011; 105(1): 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zamora-Ros R, Lujan-Barroso L, Bueno-de-Mesquita HB. et al. Tea and coffee consumption and risk of esophageal cancer: the European prospective investigation into cancer and nutrition study. Int J Cancer 2014; 135(6): 1470–1479. [DOI] [PubMed] [Google Scholar]
- 60. Ishikawa A, Kuriyama S, Tsubono Y. et al. Smoking, alcohol drinking, green tea consumption and the risk of esophageal cancer in Japanese men. J Epidemiol 2006; 16(5): 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allen NE, Beral V, Casabonne D. et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 2009; 101(5): 296–305. [DOI] [PubMed] [Google Scholar]
- 62. Boffetta P, Garfinkel L.. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology (Cambridge, Mass) 1990; 1(5): 342–348. [DOI] [PubMed] [Google Scholar]
- 63. Freedman ND, Abnet CC, Leitzmann MF. et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007; 165(12): 1424–1433. [DOI] [PubMed] [Google Scholar]
- 64. Ishiguro S, Sasazuki S, Inoue M. et al. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study). Cancer Lett 2009; 275(2): 240–246. [DOI] [PubMed] [Google Scholar]
- 65. Kasum CM, Jacobs DR Jr, Nicodemus K, Folsom AR.. Dietary risk factors for upper aerodigestive tract cancers. Int J Cancer 2002; 99(2): 267–272. [DOI] [PubMed] [Google Scholar]
- 66. Kimm H, Kim S, Jee SH.. The independent effects of cigarette smoking, alcohol consumption, and serum aspartate aminotransferase on the alanine aminotransferase ratio in Korean men for the risk for esophageal cancer. Yonsei Med J. 2010; 51(3): 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nakaya N, Tsubono Y, Kuriyama S. et al. Alcohol consumption and the risk of cancer in Japanese men: the Miyagi cohort study. Eur J Cancer Prev 2005; 14(2): 169–174. [DOI] [PubMed] [Google Scholar]
- 68. Shen C, Schooling CM, Chan WM. et al. Alcohol intake and death from cancer in a prospective Chinese elderly cohort study in Hong Kong. J Epidemiol Community Health 2013; 67(10): 813–820. [DOI] [PubMed] [Google Scholar]
- 69. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA.. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut 2010; 59(1): 39–48. [DOI] [PubMed] [Google Scholar]
- 70. Weikert C, Dietrich T, Boeing H. et al. Lifetime and baseline alcohol intake and risk of cancer of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer 2009; 125(2): 406–412. [DOI] [PubMed] [Google Scholar]
- 71. Yaegashi Y, Onoda T, Morioka S. et al. Joint effects of smoking and alcohol drinking on esophageal cancer mortality in Japanese men: findings from the Japan collaborative cohort study. Asian Pac J Cancer Prev 2014; 15(2): 1023–1029. [DOI] [PubMed] [Google Scholar]
- 72. Yang L, Zhou M, Sherliker P. et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int J Epidemiol 2012; 41(4): 1101–1113. [DOI] [PubMed] [Google Scholar]
- 73. Yi SW, Hong JS, Yi JJ, Ohrr H.. Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: prospective cohort study. Medicine (Baltimore). 2016; 95(39): e4876.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yi SW, Sull JW, Linton JA. et al. Alcohol consumption and digestive cancer mortality in Koreans: the Kangwha Cohort Study. J Epidemiol 2010; 20(3): 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yates M, Cheong E, Luben R. et al. Body mass index, smoking, and alcohol and risks of Barrett's esophagus and esophageal adenocarcinoma: a UK prospective cohort study. Dig Dis Sci 2014; 59(7): 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. O'Doherty MG, Freedman ND, Hollenbeck AR. et al. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 2012; 61(9): 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tulinius H, Sigfusson N, Sigvaldason H. et al. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev 1997; 6(11): 863–873. [PubMed] [Google Scholar]
- 78. Batty GD, Shipley MJ, Langenberg C. et al. Adult height in relation to mortality from 14 cancer sites in men in London (UK): evidence from the original Whitehall study. Ann Oncol 2006; 17(1): 157–166. [DOI] [PubMed] [Google Scholar]
- 79. Green J, Cairns BJ, Casabonne D. et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol 2011; 12(8): 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sung J, Song YM, Lawlor DA. et al. Height and site-specific cancer risk: a cohort study of a Korean adult population. Am J Epidemiol 2009; 170(1): 53–64. [DOI] [PubMed] [Google Scholar]
- 81. Lindkvist B, Johansen D, Stocks T. et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 2014; 14: 103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu J, Wang J, Leng Y, Lv C.. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013; 133(2): 473–485. [DOI] [PubMed] [Google Scholar]
- 83. Li B, Jiang G, Zhang G. et al. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur J Nutr 2014; 53(7): 1511–1521. [DOI] [PubMed] [Google Scholar]
- 84. Abdel-Latif MM, Kelleher D, Reynolds JV.. Molecular mechanisms of constitutive and inducible NF-kappaB activation in oesophageal adenocarcinoma. Eur J Cancer 2015; 51(4): 464–472. [DOI] [PubMed] [Google Scholar]
- 85. Wang SK, Yang L, Wang TT. et al. Inhibition of proliferation and induction of apoptosis by the combination of beta-carotene and 1,25-dihydroxyvitamin D3 in human esophageal cancer EC9706 cells. Asian Pac J Cancer Prev 2012; 13(12): 6327–6332. [DOI] [PubMed] [Google Scholar]
- 86. Ma JL, Zhang L, Brown LM. et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012; 104(6): 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bo Y, Lu Y, Zhao Y. et al. Association between dietary vitamin C intake and risk of esophageal cancer: a dose–response meta-analysis. Int J Cancer 2016; 138(8): 1843–1850. [DOI] [PubMed] [Google Scholar]
- 88. Ge XX, Xing MY, Yu LF, Shen P.. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev 2013; 14(3): 1911–1918. [DOI] [PubMed] [Google Scholar]
- 89. Choi Y, Song S, Song Y, Lee JE.. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol 2013; 19(7): 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhu HC, Yang X, Xu LP. et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci 2014; 59(3): 664–673. [DOI] [PubMed] [Google Scholar]
- 91. Qu X, Ben Q, Jiang Y.. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol 2013; 23(12): 762–770.e761. [DOI] [PubMed] [Google Scholar]
- 92. Huang W, Han Y, Xu J. et al. Red and processed meat intake and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Cancer Causes Control 2013; 24(1): 193–201. [DOI] [PubMed] [Google Scholar]
- 93. Keszei AP, Goldbohm RA, Schouten LJ. et al. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr 2013; 97(1): 135–146. [DOI] [PubMed] [Google Scholar]
- 94. Loh YH, Jakszyn P, Luben RN. et al. N-Nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am J Clin Nutr 2011; 93(5): 1053–1061. [DOI] [PubMed] [Google Scholar]
- 95. Zheng JS, Yang J, Fu YQ. et al. Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutr Cancer 2013; 65(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 96. Loomis D, Guyton KZ, Grosse Y. et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016; 17(7): 877–878. [DOI] [PubMed] [Google Scholar]
- 97. Bagnardi V, Rota M, Botteri E. et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol 2013; 24(2): 301–308. [DOI] [PubMed] [Google Scholar]
- 98. Bagnardi V, Rota M, Botteri E. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer 2015; 112(3): 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Toh Y, Oki E, Ohgaki K. et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol 2010; 15(2): 135–144. [DOI] [PubMed] [Google Scholar]
- 100. Renehan AG, Tyson M, Egger M. et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371(9612): 569–578. [DOI] [PubMed] [Google Scholar]
- 101. Turati F, Tramacere I, La Vecchia C, Negri E.. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013; 24(3): 609–617. [DOI] [PubMed] [Google Scholar]
- 102. Aleman JO, Eusebi LH, Ricciardiello L. et al. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology 2014; 146(2): 357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schleicher RL, Carroll MD, Ford ES, Lacher DA.. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009; 90(5): 1252–1263. [DOI] [PubMed] [Google Scholar]
- 104. Medina-RemOn A, Kirwan R, Lamuela-Raventos RM, Estruch R.. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and mental health problems. Crit Rev Food Sci Nutr 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.