Abstract
Aims
A recommendation for a subcutaneous-implantable cardioverter-defibrillator (S-ICD) has been added to recent European Society of Cardiology Guidelines. However, the S-ICD is not ideally suitable for patients who need pacing. The aim of this survey was to analyse the current practice of ICD implantation and to evaluate the actual suitability of S-ICD.
Methods and results
The survey ‘S-ICD Why Not?’ was an independent initiative taken by the Italian Heart Rhythm Society (AIAC). Clinical characteristics, selection criteria, and factors guiding the choice of ICD type were collected in consecutive patients who underwent ICD implantation in 33 Italian centres from September to December 2015. A cardiac resynchronization therapy (CRT) device was implanted in 39% (369 of 947) of patients undergoing de novo ICD implantation. An S-ICD was implanted in 12% of patients with no CRT indication (62 of 510 with available data). S-ICD patients were younger than patients who received transvenous ICD, more often had channelopathies, and more frequently received their device for secondary prevention of sudden death. More frequently, the clinical reason for preferring a transvenous ICD over an S-ICD was the need for pacing (45%) or for antitachycardia pacing (36%). Nonetheless, only 7% of patients fulfilled conditions for recommending permanent pacing, and 4% of patients had a history of monomorphic ventricular tachycardia that might have been treatable with antitachycardia pacing.
Conclusion
The vast majority of patients needing ICD therapy are suitable candidates for S-ICD implantation. Nevertheless, it currently seems to be preferentially adopted for secondary prevention of sudden death in young patients with channelopathies.
Keywords: Implantable cardioverter-defibrillator, Subcutaneous, Survey, Indication, Pacing, Ventricular arrhythmias, Sudden death
Introduction
What’s new?
This nation-wide survey provided original data on the current practice of implantable cardioverter-defibrillator (ICD) implantation, and specifically on the adoption of subcutaneous-ICD (S-ICD) therapy.
The typical profile of an S-ICD recipient is different from that of the overall ICD population, in that an S-ICD seems to be preferred in young patients with channelopathies, mainly in the context of secondary prevention.
The most common reasons for preferring a transvenous ICD over an S-ICD are not supported by specific conditions, such as the need for permanent pacing or antitachycardia pacing.
Implantable cardioverter-defibrillators (ICDs) are an established therapy for the prevention of sudden cardiac death (SCD).1 Conventional ICDs rely on transvenous leads to deliver defibrillation shocks [transvenous-ICD (T-ICD)] and, if necessary, to provide cardiac pacing. Implantable cardioverter-defibrillator therapy is not free from procedural complications; these are mainly associated with the insertion of transvenous leads, e.g. pneumothorax, cardiac tamponade, and vascular damage.2 Moreover, the long-term risks of device-related complications are of great concern, especially in view of the improved survival of ICD recipients.3 In order to avoid the risks involved in accessing the heart via the vascular system and to overcome recurring problems with transvenous leads, a subcutaneous ICD (S-ICD) has recently been developed in which the electrode system is placed entirely subcutaneously, outside the chest. The available data suggest that S-ICDs are effective in terminating life-threatening ventricular arrhythmias (VAs).4–6 Consequently, a Class IIa recommendation for S-ICD has been added to the most recent European Society of Cardiology (ESC) Guidelines for patients with VAs.1 However, S-ICDs are not suitable for patients who require pacing for bradycardia or cardiac resynchronization therapy (CRT), nor for those who suffer from VAs that can easily be terminated by antitachycardia pacing (ATP).
The Italian survey ‘S-ICD Why Not?’ was an independent initiative taken by the Italian Heart Rhythm Society (Associazione Italiana Aritmologia e Cardiostimolazione—AIAC). The primary aim of this survey was to provide information, including clinical characteristics, selection criteria, and factors guiding the choice of ICD type, in a representative sample of consecutive patients who underwent ICD implantation in Italian clinical practice. Data were analysed to measure the actual suitability for S-ICD and to evaluate the adoption of this novel therapy.
Methods
Study design
All Italian centres with experience in S-ICD implantation were invited to participate. Centres were asked to enrol consecutive patients at the time of de novo implantation of a new single- or dual-chamber T-ICD or an S-ICD, in a 3 month row, between 1 September 2015 and 31 December 2015. At the time of implantation, all patients provided written informed consent for data storage and analysis. Data were collected by means of online internet entry. An electronic case report form was created to capture demographics and clinical characteristics, selection criteria assessed prior to implantation, and factors guiding the choice of ICD type. The contents of the form are detailed in Appendix. All centres were also asked to report the total number of implantation procedures performed during the observation period, i.e. de novo, replacement and upgrade implantations of single-or dual-chamber T-ICD, ICD for CRT (CRT-D), and S-ICD. Secondary prevention of SCD was defined as the ICD implantation in patients with documented ventricular fibrillation, haemodynamically not tolerated or recurrent sustained ventricular tachycardia in the absence of reversible causes.1 The prevalence of conditions for a recommendation for pacing according to ESC Guidelines8 was measured to quantify the actual need for permanent pacing.
Statistical analysis
Descriptive statistics are reported as means ± SD for normally distributed continuous variables or medians with 25th–75th percentiles in the case of skewed distribution. Categorical variables are reported as percentages. Differences between mean data were compared by means of a t-test for Gaussian variables, and by the Mann–Whitney non-parametric test for non-Gaussian variables. Differences in proportions were compared by means of χ2 analysis or Fisher’s exact test, as appropriate. A P-value <0.05 was considered significant for all tests. All statistical analyses were performed by means of STATISTICA software, version 7.1 (StatSoft, Inc., Tulsa, OK, USA).
Results
Participating centres and study population
The participating centres numbered 33 (the complete list is reported in Appendix), 29 (88%) of which belonged to the fourth quartile of the ICD volume distribution (>50 ICDs per year), according to the Italian ICD Registry of AIAC.
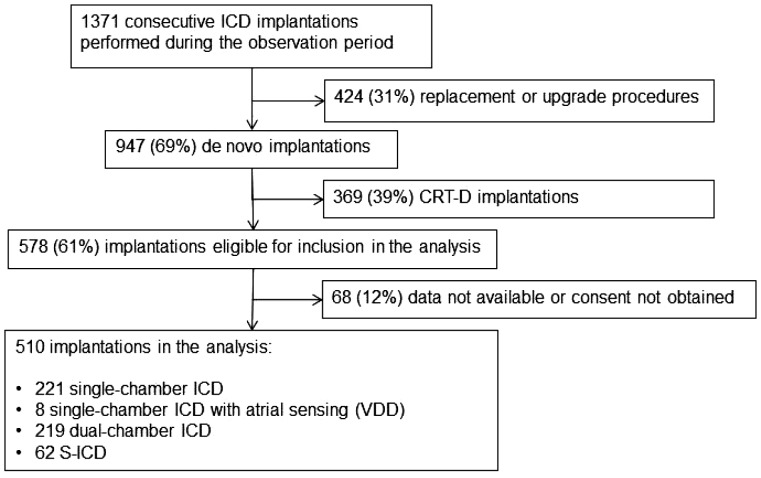
A total of 1371 consecutive ICD procedures were performed during the 3 month observation period. The procedure was a replacement or an upgrade of a previous ICD system in 424 (31%) patients and a de novo implantation in 947 (69%) patients. Of the latter, 369 (39%) were CRT-Ds; the remaining 578 (61%) cases were eligible for inclusion, i.e. single- or dual-chamber T-ICDs and S-ICDs (Figure 1). In 68 (12%) of these cases, data were not available or consent was not obtained.
Figure 1.
Diagram of the study: number of cases in analysis.
The remaining 510 patients constituted the population in analysis. The device implanted was a single-chamber T-ICD in 221 (43%) patients, a single-lead T-ICD with atrial sensing (VDD) in 8 (2%) patients, a dual-chamber ICD in 219 (43%) patients, and an S-ICD in 62 (12%) patients.
Clinical characteristics
Table 1 shows the baseline clinical variables in the overall population and in patients who underwent implantation of T-ICD and S-ICD. The two groups differed greatly, in that S-ICD patients were younger, showed better systolic function and functional status, less frequently presented with structural cardiomyopathy and more often had inherited channelopathies. Patients who received an S-ICD also had less coronary artery disease and fewer comorbidities, and more frequently received their device for secondary prevention of SCD.
Table 1.
Demographics and baseline clinical parameters
| Parameter | All patients (n=510) | Transvenous ICD (n=448) | Subcutaneous ICD (n=62) | P-values |
|---|---|---|---|---|
| Male gender, n (%) | 399 (78) | 354 (79) | 45 (73) | 0.250 |
| Age, years | 65 ±13 | 67 ±11 | 47 ±13 | <0.001 |
| BMI classification | 0.001 | |||
| Underweight, n (%) | 10 (2) | 10 (2) | 0 (0) | |
| Normal weight, n (%) | 189 (37) | 154 (34) | 35 (56) | |
| Overweight and obese, n (%) | 311 (61) | 286 (64) | 25 (40) | |
| LV ejection fraction, % | 36 ±11 | 34 ±10 | 49 ±14 | <0.001 |
| New York Heart Association | <0.001 | |||
| Class I, n (%) | 107 (21) | 66 (15) | 41 (66) | |
| Class II, n (%) | 270 (53) | 256 (57) | 14 (23) | |
| Class III, n (%) | 128 (25) | 121 (27) | 7 (11) | |
| Class IV, n (%) | 5 (1) | 5 (1) | 0 (0) | |
| Secondary prevention of SCD, n (%) | 123 (24) | 91 (20) | 32 (52) | <0.001 |
| Cardiomyopathy | ||||
| Ischaemic, n (%) | 286 (56) | 268 (60) | 18 (29) | <0.001 |
| Dilated, n (%) | 97 (19) | 94 (22) | 3 (5) | 0.002 |
| Hypertrophic, n (%) | 25 (5) | 16 (4) | 9 (15) | <0.001 |
| Hypertensive, n (%) | 16 (3) | 15 (3) | 1 (2) | 0.706 |
| Valvular, n (%) | 20 (4) | 18 (4) | 2 (3) | 1.000 |
| ARVD, n (%) | 10 (2) | 7 (2) | 3 (5) | 0.110 |
| Congenital, n (%) | 5 (1) | 4 (1) | 1 (2) | 0.478 |
| Other, n (%) | 5 (1) | 3 (1) | 2 (3) | 0.114 |
| Channelopathies/Other | ||||
| Idiopathic VF, n (%) | 20 (4) | 13 (3) | 7 (11) | 0.001 |
| Brugada, n (%) | 15 (3) | 2 (0.4) | 13 (21) | <0.001 |
| Long QT syndrome, n (%) | 5 (1) | 3 (1) | 2 (3) | 0.114 |
| Other, n (%) | 6 (1) | 5 (1) | 1 (2) | 0.542 |
| Coronary artery disease, n (%) | 293 (57) | 275 (61) | 18 (29) | <0.001 |
| Myocardial infarction, n (%) | 269 (53) | 252 (56) | 17 (27) | <0.001 |
| Coronary artery bypass graft, n (%) | 97 (19) | 92 (21) | 5 (8) | 0.019 |
| PTCA, n (%) | 194 (38) | 181 (40) | 13 (21) | 0.003 |
| Chronic kidney disease, n (%) | 87 (17) | 83 (19) | 4 (6) | 0.018 |
| Diabetes, n (%) | 134 (27) | 127 (28) | 7 (11) | 0.004 |
| COPD, n (%) | 82 (16) | 80 (18) | 2 (3) | 0.001 |
BMI, body mass index; LV, left ventricular; SCD, sudden cardiac death; ARVD, arrhythmogenic right ventricular dysplasia; VF, ventricular fibrillation; PTCA, percutaneous transluminal coronary angioplasty; COPD, chronic obstructive pulmonary disease.
Electrocardiogram on implantation and clinical indications for pacing
The findings of the baseline electrocardiogram (ECG) and the arrhythmic history of the patients are presented in Table 2. Twenty-eight (5%) patients presented with sick sinus syndrome, and 8 (2%) with second-degree Mobitz II or third-degree atrioventricular block. Overall, conditions for a Class I recommendation for permanent pacing were present in 36 (7%) patients. An additional 10 (2%) patients had conditions for a Class IIa (should be considered) recommendation and 3 (1%) patients for a Class IIb (may be considered) recommendation.
Table 2.
Electrocardiogram on implantation and arrhythmic history
| Parameter | All patients (n=510) | Transvenous ICD (n=448) | Subcutaneous ICD (n=62) | P-values |
|---|---|---|---|---|
| Atrial fibrillation, n (%) | 64 (13) | 64 (14) | 0 (0) | <0.001 |
| Sick sinus syndrome, n (%) | 28 (5) | 28 (6) | 0 (0) | 0.037 |
| Chronotropic incompetence, n (%) | 32 (6) | 30 (7) | 2 (3) | 0.407 |
| PR interval duration, ms | 174 ±37 | 177 ±36 | 156 ±32 | <0.001 |
| Atrioventricular block | ||||
| First-degree (PR interval >200ms), n (%) | 61 (12) | 57 (13) | 4 (6) | 0.209 |
| Second-degree Mobitz I, n (%) | 5 (1) | 5 (1) | 0 (0) | 1.000 |
| Second-degree Mobitz II, n (%) | 3 (1) | 3 (1) | 0 (0) | 1.000 |
| Third-degree, n (%) | 5 (1) | 5 (1) | 0 (0) | 1.000 |
| QRS duration, ms | 105 ±20 | 107 ±20 | 96 ±12 | <0.001 |
| QRS duration > 120 ms, n (%) | 62 (12) | 61 (14) | 1 (2) | 0.003 |
| Left bundle branch block, n (%) | 28 (5) | 27 (6) | 1 (2) | 0.233 |
| Right bundle branch block, n (%) | 22 (4) | 20 (4) | 2 (3) | 1.000 |
| Left anterior fascicular block, n (%) | 33 (6) | 32 (7) | 1 (2) | 0.162 |
| Intraventricular conduction delay, n (%) | 12 (2) | 11 (2) | 1 (2) | 1.000 |
| History of | ||||
| Ventricular fibrillation, n (%) | 75 (15) | 57 (13) | 18 (30) | <0.001 |
| Polymorphic ventricular tachycardia, n (%) | 27 (5) | 19 (4) | 8 (13) | 0.004 |
| Monomorphic ventricular tachycardia, n (%) | 68 (13) | 59 (13) | 7 (12) | 0.679 |
| With syncope, n (%) | 19 (4) | 16 (4) | 3 (5) | 0.717 |
Analysis of the arrhythmic history revealed the occurrence of monomorphic ventricular tachycardia (MVT) with syncope in 19 (4%) patients, i.e. patients potentially indicated for receiving ATP therapy.
Factors guiding the choice of implantable cardioverter-defibrillator type
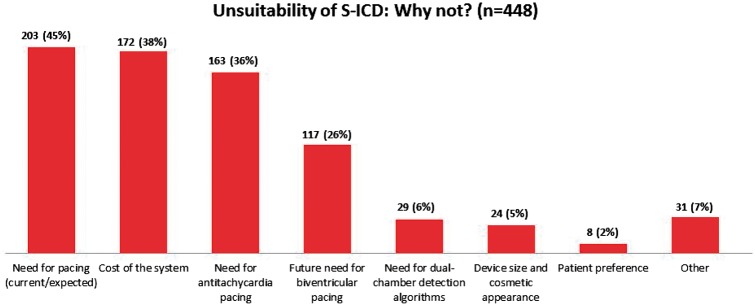
The main reasons for choosing a T-ICD over an S-ICD (n = 448) are reported in Figure 2. Among clinical factors, the current or expected need for pacing was reported in 203 (45%) patients. Of these, only 28 had conditions for a Class I recommendation for permanent pacing on implantation. In 163 (36%) patients, a T-ICD was preferred owing to the potential need for ATP therapy. Of these, only nine had a history of MVT with syncope. The possible development of CRT indications during follow-up, to be managed in future by device upgrade, was reported as the reason for preferring a T-ICD in 117 (26%) patients. In this group, only seven patients had a left bundle branch block, and 25 patients had a QRS duration >120 ms.
Figure 2.
Factors for preferring a transvenous ICD over an S-ICD (n = 448). Multiple factors were reported per patient.
The cost of the system was reported as the reason for preferring a T-ICD in 172 (38%) patients.
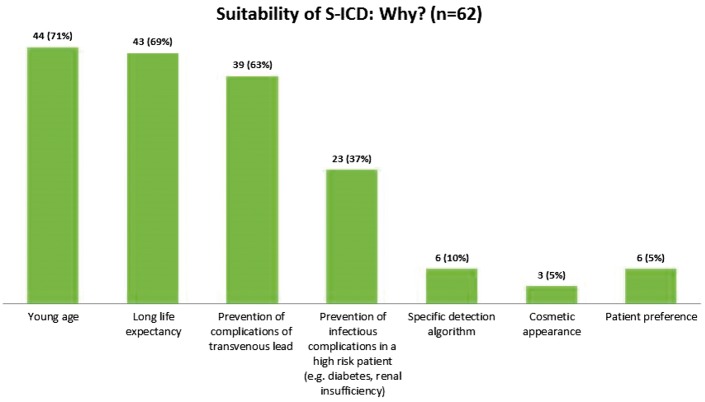
The drivers for S-ICD implantation (n = 62) are reported in Figure 3. The most frequent were young age, long life expectancy, and the possibility of avoiding complications.
Figure 3.
Factors for preferring an S-ICD over a transvenous ICD (n = 62). Multiple factors were reported per patient.
Discussion
The results of this survey demonstrate that, in current Italian clinical practice, the typical profile of an S-ICD recipient is different from that of the overall T-ICD population, in that an S-ICD seems to be preferred in young patients with channelopathies, mainly in the context of secondary SCD prevention. We also found that a high proportion of patients necessitating therapy for the prevention of SCD might be suitable candidates for S-ICD implantation. Actually, the most common reasons for preferring a T-ICD over an S-ICD are not supported by specific conditions, such as the need for permanent pacing or ATP therapy.
As the body of evidence on the safety and efficacy of S-ICD continues to grow,4–7 a Class IIa recommendation for S-ICD has been added to the most recent ESC Guidelines for patients with VAs.1 However, as the S-ICD does not provide pacing, it is not ideally suitable for patients who need pacing therapy for bradycardia support, CRT, or ATP therapy.
This nation-wide survey analysed the practice of ICD implantation in a large number of centres. The participating centres constituted a representative sample (about 40%) of the Italian ICD centres belonging to the fourth quartile of the ICD volume distribution (>50 ICDs per year), according to the Italian ICD Registry of AIAC.9 Similarly, the devices in analysis constituted a sample of about 40% of all ICD implantation procedures performed in Italy during the observation period.
The preliminary studies on the therapy with S-ICD mostly included patients that were considered more suitable, e.g. patients with difficult venous access, young patients facing a lifetime of device therapy, or those at particular risk of bacteraemia. Similarly to our S-ICD population, the mean age at implantation in the pooled analysis of two large prospective studies [IDE (S-ICD System IDE Clinical Investigation) and EFFORTLESS (Post-Market S-ICD Registry)]6 was as low as 50 years. Interestingly, compared with published studies we observed an even higher proportion of secondary prevention indications (52% vs. 30%) and a higher mean value of ejection fraction (49% vs. 39%). This demonstrates that the patients currently receiving S-ICD in Italian clinical practice represent a very selected group out of the general population currently indicated for ICD and thus potentially suitable for S-ICD, according to the most recent guidelines.1
In order to quantify the actual need for permanent pacing in the study population, we prospectively looked for the prevalence of conditions for a Class I recommendation for pacing on implantation; we found a proportion of 7%, whereas criteria for a weaker recommendation were met by an additional 3% of patients. Considering the possibility of developing the need for pacing after implantation, de Bie et al.10 performed a single-centre retrospective study on patients without a pre-existing indication for pacing. Among predictors of the unsuitability for an S-ICD, they found a prolonged QRS duration, which was present in 12% of patients in our population. Similarly, in a post hoc analysis of the MADIT-II study,11 it was shown that the need for pacing or CRT was very low (<2% per year), during follow-up in patients with an ICD indication, and even lower in those with normal PR interval (PR >200 ms was present in 12% of patients in our survey). Nonetheless, the relationship between right ventricular pacing and adverse outcomes in patients with ICD was previously shown,12 thus providing the rationale for implementing strategies and pacing modalities that minimize ventricular pacing.13 Similarly, it has been suggested to reduce atrial pacing, as an increasing risk relationship was shown between its cumulative percentage and the severity of atrioventricular decoupling in ICD patients.14
In this study, the proportion of patients with a CRT indication was 39% of all consecutive patients undergoing de novo ICD implantations. However, the possible development of CRT indications after implantation, i.e. need for device upgrade to a CRT-D system, was previously shown to be very rare (0.3% at 1-year follow-up, and <6% of the cases of S-ICD unsuitability) and not associated with additional risks.10 Nonetheless, the best strategy for ICD candidates considered at risk of the future development of CRT-D indications is still unclear, mainly in the light of the well-known complications associated with transvenous device replacement and upgrade.15 Indeed, de novo CRT-D implantation after removal of an S-ICD could be safer than upgrading a single- or dual-chamber T-ICD to a CRT-D system.
The need for ATP therapy constitutes an additional potential barrier to the adoption of S-ICD. However, the role of ATP therapy has recently been questioned. ATP utilization has increased significantly in the last decade without notable effects on shock incidence and survival.16 In the delayed-therapy arm of the Multicenter Automatic Defibrillator Implantation Trial—Reduce Inappropriate Therapy (MADIT-RIT), a primary prevention trial, the 1-year incidence of pacing therapies was only 4%, with low rates of appropriate shocks and unnecessary ATP therapies.17 Similarly, the PainFree SST secondary prevention trial18 showed recently a 1-year rate of ATP therapies equal to 5.4%, adopting a prolonged detection programming strategy. Current guidelines provide recommendations for customized ATP programming in cases of previous MVT.19 In our study, the prevalence of unstable MVT was 4%. This kind of patient is suitable for different approaches: early implantation of a T-ICD, a hybrid approach by combining S-ICD implantation and catheter ablation,20 or even S-ICD followed by possible additional implantation of the upcoming leadless system capable of delivering ATP therapy.21 Additional investigations are warranted in order to compare strategies and measure outcomes.
Overall, our results on S-ICD suitability seem to be in agreement with preliminary S-ICD experiences. In a recent analysis of 882 patients who received an S-ICD, extraction of the S-ICD because of the need for pacing occurred in four patients (0.4%) over 22 months.6 Specifically, one patient developed a new bradycardia indication, one device was extracted in order to upgrade to a CRT, one patient needed ATP, and one patient with arrhythmic storms underwent replacement with a T-ICD in an attempt to suppress arrhythmias by means of overdrive pacing.
Among factors for preferring a transvenous ICD over an S-ICD, the need for dual-chamber detection algorithms was reported in 6% of patients, suggesting that this may not be a relevant concern. Plausibly, this can be explained by the inconclusive literature on the superiority of dual-chamber ICD in reducing inappropriate shocks. However, it should be considered that in our population a dual-chamber ICD was adopted in 43% of patients, in the majority of cases in the absence of an indication for pacing. This is an additional finding confirming that patient characteristics play a small role in the decision to place a specific ICD type.
In addition to clinical considerations, in this survey the cost of the system proved to be an additional barrier to the adoption of S-ICD in current clinical practice. However, this may be specific to the Italian situation and not applicable to other healthcare systems.
In summary, our results seem to suggest that a treatment gap exists between the guidelines and the clinical care of patients. Ongoing studies on the S-ICD will serve to confirm the efficacy of the therapy22 and to build confidence in its usefulness. The addition of S-ICD to the tools for treating patients at risk of SCD has been a significant advance. The application of strategies to facilitate the implementation of guidelines (e.g. clinical decision support and reminder systems) could enhance the use of a novel recommended therapy, such as the S-ICD, thereby improving outcomes.
Limitations
Our findings might be affected by a bias, in that the participation in present survey was limited to centres with experience in S-ICD implantation. In particular, data about the therapy adoption and the factors guiding the choice of device could have been different if a larger sample of implanting centres was considered. Moreover, our results may not be applicable to other populations with different underlying demographics or to other healthcare systems. In addition, although the importance of consecutive inclusion was emphasized repeatedly to all participants, we cannot confirm that all patients were included consecutively. Similarly, the accuracy of the data was not audited. In addition, it should be mentioned that some patients suitable for S-ICD according to the absence of a pacing indication or previous MVT may be ineligible according to the manufacturer’s surface ECG screening template. A previous study that explored this issue found a proportion of 15% of patients who did not satisfy ECG criteria.23
Conclusions
The present nation-wide survey revealed that an S-ICD was implanted in 12% of patients with no CRT indication, and was adopted preferentially for secondary prevention of SCD in young patients with channelopathies. Moreover, although the most common reasons for preferring a T-ICD over an S-ICD were the need for permanent pacing or ATP therapy, at the time of ICD implantation, only 7% of patients fulfilled conditions for Class I recommendation for permanent pacing. An additional 4% of patients presented with a history of unstable MVT that might have been treatable with ATP. The vast majority of patients needing therapy for SCD prevention might therefore be suitable candidates for S-ICD implantation.
Conflict of interest: M.L. and S.V. are employees of Boston Scientific. The other authors report no conflicts.
Funding
This was an independent study. No external funding was received for this project.
References
- 1. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. et al. Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 2. Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P. et al. ; Investigators of the Ontario ICD Database. Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol 2010;55:774–82. [DOI] [PubMed] [Google Scholar]
- 3. Ranasinghe I, Parzynski CS, Freeman JV, Dreyer RP, Ross JS, Akar JG. et al . Long-Term Risk for Device-Related Complications and Reoperations After Implantable Cardioverter-Defibrillator Implantation: An Observational Cohort Study. Ann Intern Med 2016. May 3. doi: 10.7326/M15-2732. [DOI] [PubMed] [Google Scholar]
- 4. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M. et al . Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–53. [DOI] [PubMed] [Google Scholar]
- 5. Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB. et al. ; EFFORTLESS Investigators. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J 2014;35:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DA, Boersma LV. et al . Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol 2015;65:1605–15. [DOI] [PubMed] [Google Scholar]
- 7. Boveda S, Lenarczyk R, Haugaa K, Fumagalli S, Madrid AH, Defaye P. et al. Implantation of subcutaneous implantable cardioverter defibrillators in Europe: results of the European Heart Rhythm Association survey. Europace 2016;18:1434–9. [DOI] [PubMed] [Google Scholar]
- 8. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 9. Proclemer A, Zecchin M, D'Onofrio A, Botto GL, Facchin D, Rebellato L. et al. [ The Pacemaker and Implantable Cardioverter-Defibrillator Registry of the Italian Association of Arrhythmology and Cardiac Pacing–Annual report 2014.] G Ital Cardiol (Rome) 2016;17:95–107. [DOI] [PubMed] [Google Scholar]
- 10. de Bie MK, Thijssen J, van Rees JB, Putter H, van der Velde ET, Schalij MJ. et al . Suitability for subcutaneous defibrillator implantation: results based on data from routine clinical practice. Heart 2013;99:1018–23. [DOI] [PubMed] [Google Scholar]
- 11. Kutyifa V, Zareba W, Rosero S, Mcnitt S, Polonsky B, Moss AJ.. The need for pacing in patients who qualify for an ICD: clinical implications. Eur Heart J 2014;35(Suppl. 1):513–850. [Google Scholar]
- 12. Sharma AD, Rizo-Patron C, Hallstrom AP, O'Neill GP, Rothbart S, Martins JB. et al .; DAVID Investigators. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005;2:830–4. [DOI] [PubMed] [Google Scholar]
- 13. Olshansky B, Day JB, Moore S, Gering L, Rosenbaum M, McGuire M. et al. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation 2007;115:9–16. [DOI] [PubMed] [Google Scholar]
- 14. Sweeney MO, Ellenbogen KA, Tang AS, Johnson J, Belk P, Sheldon T.. Severe atrioventricular decoupling, uncoupling, and ventriculoatrial coupling during enhanced atrial pacing: incidence, mechanisms, and implications for minimizing right ventricular pacing in ICD patients. J Cardiovasc Electrophysiol 2008;19:1175–80. [DOI] [PubMed] [Google Scholar]
- 15. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R. et al. ; REPLACE Registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010;122:1553–61. [DOI] [PubMed] [Google Scholar]
- 16. Saxon L, Varma N, Lindenfeld J, Wold N, Jones PW, Hayes DL. et al . ICD programming trends and relationship to survival-the altitude dataset. Heart Rhythm 2013;10:1420–4. [Google Scholar]
- 17. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP. et al. ; MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 18. Sterns LD, Meine M, Kurita T, Meijer A, Auricchio A, Ando K. et al . Extended detection time to reduce shocks is safe in secondary prevention patients: The secondary prevention substudy of PainFree SST. Heart Rhythm 2016;13:1489–96. [DOI] [PubMed] [Google Scholar]
- 19. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J. et al . 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2016;18:159–83. [DOI] [PubMed] [Google Scholar]
- 20. Wissner E, Stevenson WG, Kuck KH.. Catheter ablation of ventricular tachycardia in ischaemic and non-ischaemic cardiomyopathy: where are we today? A clinical review. Eur Heart J 2012;33:1440–50. [DOI] [PubMed] [Google Scholar]
- 21. Tjong FV, Brouwer TF, Kooiman KM, Smeding L, Koop B, Soltis B. et al . Communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol 2016;67:1865–6. [DOI] [PubMed] [Google Scholar]
- 22. Olde Nordkamp LR, Knops RE, Bardy GH, Blaauw Y, Boersma LV, Bos JS. et al . Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J 2012;163:753–60. [DOI] [PubMed] [Google Scholar]
- 23. Randles DA, Hawkins NM, Shaw M, Patwala AY, Pettit SJ, Wright DJ.. How many patients fulfil the surface electrocardiogram criteria for subcutaneous implantable cardioverter-defibrillator implantation? Europace 2014;16:1015–21. [DOI] [PubMed] [Google Scholar]