Abstract
The genomic-plasticity of the immune system creates a broad immune repertoire engaged to tackle cancer cells. Promising clinical activity has been observed with several immune therapy strategies in solid tumors including melanoma, lung, kidney, and bladder cancers, albeit as yet immunotherapy-based treatment approaches in pancreatic ductal adenocarcinoma (PDAC) remain to have proven value. While translational and early clinical studies have demonstrated activation of antitumor immunity, most recent late-phase clinical trials have not confirmed the early promise in PDAC except in MSI-High PDAC patients. These results may in part be explained by multiple factors, including the poorly immunogenic nature of PDAC along with immune privilege, the complex tumor microenvironment, and the genetic plasticity of PDAC cells. These challenges have led to disappointments in the field, nonetheless they have also advanced our understanding that may tailor the future steps for immunotherapy for PDAC. Therefore, there is significant hope that progress is on the horizon.
Keywords: immunotherapy, immune evasion, PD-1, PD-L1, pancreatic cancer, mismatch repair
Introduction
pancreatic ductal adenocarcinoma (PDAC) is one of the most challenging cancers for patients, physicians, and scientists, due to its complex molecular characteristics, tumor microenvironment, and immune privilege. Surgical removal of the primary tumor is currently the only curative treatment, applicable to 10%–15% of patients diagnosed with this disease, albeit most patients who do undergo surgery ultimately recur and die of their disease. For the majority of patients with advanced pancreatic adenocarcinoma, systemic therapies are the mainstay of disease control. Although cytotoxic regimens such as FOLFIRINOX [1], gemcitabine, and nab-paclitaxel [2] have improved clinical outcomes, there has not been significant progress with targeted treatments. This is believed to be due to multiple compensatory pathways that provide redundant signaling to escape the inhibitory effects of targeted therapy [3]. Recently, more attention has been paid to immunotherapy to abolish these rebound and bypass mechanisms. Herein, we review immunotherapy in PDAC from early interaction between cancer cells and the immune system to developing strategies of immunotherapy to counteract this disease.
Immune surveillance, immunoediting, and immune privilege
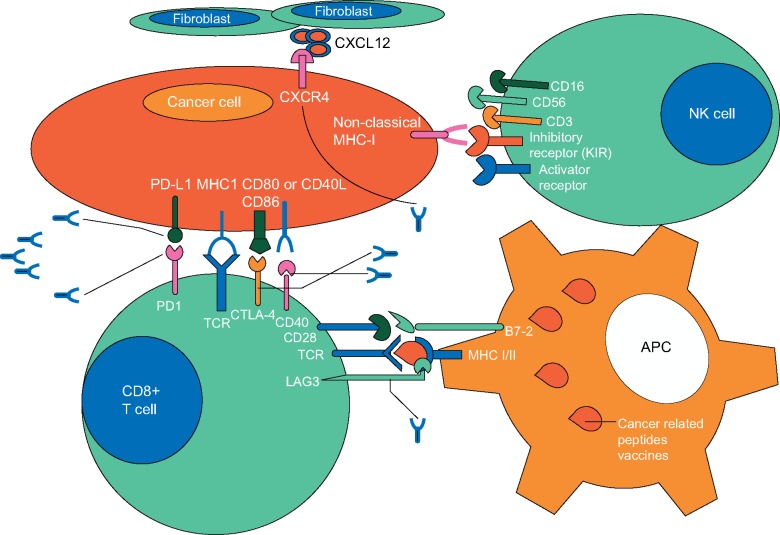
The immune response against cancers cell has been investigated over decades and it has been identified that the immune system actively patrols the body [4]. Once transformed, cancer cells are recognized by immune effector cells such as natural killer cells (NK) and cytotoxic T cells and are destroyed before development of clinical disease [5]. However, throughout this malignant transformation process, cancer cells may gain multiple mechanisms to evade immune response and be rendered ‘invisible’. This dynamic process between the immune system and the cancer cell is called ‘immunoediting’. For example, abundant expression of B7-H1, better known as programmed death ligand 1 (PD-L1), induces T-cell apoptosis and evades immune reaction in many tumors including PDAC [6]. Interestingly expression of PD-L1 is augmented with interferon gamma which is also a mediator of immune activation, suggesting that cancer cells are able to counteract via compensatory pathways to minimize immune response [6]. Cancer cells are also capable of altering their metabolism in the tumor microenvironment to evade the immune system. For example, increased expression of indoleamine-2,3-dioxygenase (IDO) depletes tryptophan, which is an important amino acid for routine functioning of immune system cells including NK and cytotoxic T cells in PDAC [7]. Moreover, PDAC cells secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) which induces the infiltration of myeloid derived cells to the tumor microenvironment and suppresses antigen specific T-cell response [8]. Myeloid cell-inflamed PDAC [8], unlike tumor infiltrating T-cell inflammation [9], creates a safe haven for PDAC cells and is associated with more aggressive behavior. T regulatory cells (Tregs), negative regulators of tumor-specific cytotoxic T cells, also infiltrate the tumor microenvironment of PDAC and suppress antitumor immunity [10]. Tregs are recruited by various tumor cells throughout an epithelial-to-mesenchymal (EMT) process via releasing mediators such as C-C motif chemokine ligand 2 (CCL2) [11]. Moreover, cancer cells abolish cell-mediated immunity by downregulating expression of antigen presenting molecules including major histocompatibility antigen (MHC) class I [12].
PDAC cells also generate immune tolerance by directly interacting with activated tumor antigen-specific T cells; a process called ‘immune privilege’. Many pathways have been shown to be involved in this process. For example, PDAC cells are capable of downregulating Fas receptor signaling to evade immune attack and intensify Fas ligand expression which induces apoptosis of activated antitumor cytotoxic T cells [13–15]. Foxp3 (forkhead box P3), a transcription regulator, highly expressed on both Tregs and PDAC cells, may also mediate immune privilege by suppressing proliferation of activated cytotoxic T cells [16]. Collectively, these mechanisms facilitate escape of cancer cells from immune system recognition but also underpin opportunities in cancer immunotherapy which are further detailed below (Figure 1).
Figure 1.
Interaction between immune and cancer cells and their receptors (PD-1, PD-L1, and CTLA4), which are targeted by monoclonal antibodies.
Cancer vaccines
Cancer cells express various antigens that are potentially immunogenic due to mutations creating neoepitopes or simply due to aberrant expression of certain proteins. Utility of these antigens as cancer vaccines has been interrogated in translational and clinical studies in different models using peptide-based and whole cell cancer vaccines. In peptide-based vaccines, anticancer immune activation is generated via specific predetermined antigens that are potentially immunogenic, while whole cell cancer vaccines sensitize T cells against cell to cell recognition which is further discussed below.
Peptide-based cancer vaccines
Mesothelin, an overexpressed peptide present in various cancers including PDAC, has been extensively evaluated as a target antigen. In an animal model, activation of cytotoxic T cells via virus-like particles containing human mesothelin induced substantial tumor suppression [17]. Mesothelin has also been used for in vivo cross priming of T cells that are implemented in whole cell cancer vaccine studies [18]. Mucin1 (MUC1), a cell surface associated glycoprotein expressed in PDAC, has also been investigated. In a phase I/II study, 12 patients who underwent surgical resection received MUC1-pulsed autologous dendritic cells (DCs) as an adjuvant therapy. About 4 out of 12 (25%) had a 4-year disease-free survival [19]. The patients received a total of four doses over 6 months. Fluctuations in circulating CD4+ and CD8+ cells were observed before and after vaccinations suggesting a real-time negative regulation of activated T cells. To avoid self-tolerance and enhance an immune response, MUC1 has been engineered to express more antigenic epitopes and murine models have shown improved outcomes though this approach has yet to be explored in clinical studies [20] (Table 1).
Table 1.
Selected completed trials assessing vaccine-based immunotherapeutic approaches in PDAC
| Identification | Trial and strategy | Mechanism of action | Study design | Results |
|---|---|---|---|---|
| Lepisto et al. [19] | Phase 1/2 study of mucin-1 loaded dendritic cells in adjuvant setting | Activation of T cells against Mucin-1 expressing PDAC cells | 1 × 106 Dendritic cells loaded with mucin-1 peptide were administered in the adjuvant setting at week 1, week 3, week 6 and 6 months to 12 months | Well tolerated with no toxicity. N=4 of 12 patients had 4-year disease-free survival |
| Bernhardt et al. [21] | Phase 1/2 study of telomerase as a cancer vaccine in combination with GM-CSF in advanced stage PDAC patients | Induction of T-cell clones reactive to telomerase |
|
Well tolerated. Median OS 8.6 months in intermediate group and was significantly better compared with low- and high-dose groups (P=0.006 and P=0.05, respectively) |
| Middleton et al. [22] | Phase III study of telomerase as a cancer vaccine in combination with gemcitabine and capecitabine in advanced stage PDAC patients | Induction of T-cell clones reactive to telomerase and enhancement of clinical response to chemotherapy |
|
Neither concurrent or sequential telomerase vaccine therapy improved the clinical outcomes (P=0.6) |
| Jaffee et al. [28] | Phase I study of GVAX alone in stage 1, 2, and 3 PDAC patients | Activation of CTLs with GM-CSF secreting PDAC cells |
|
|
| Laheru et al. [29] | Phase I study of GVAX in combination with low-dose cyclophosphamide (Cy/GVAX) in advanced PDAC | Activation of CTLs with GM-CSF secreting PDAC cells and inhibition of regulatory T cells | Arm A: six doses of GVAX with 21-day intervals.Arm B: cyclophosphamide 1 day prior to GVAX initiation followed by 6 doses of GVAX | Both approaches were well tolerated with minimal toxicity. GVAX in combination with cyclophosphamide demonstrated a tendency toward better clinical outcomes (4.3 versus 2.3 months) |
| Le et al. [30] | Phase II trial of Cy/GVAX in combination with CRS-207, as compared with GVAX alone in advanced stage PDAC patients | Activation of CTLs with GM-CSF secreting PDAC cells, mesothelin and inhibition of T regulatory cells |
|
OS was 6.1 months versus 3.9 months in arm A and arm B, respectively (P=0.02). Enhanced mesothelin specific T-cell clones were associated with a better prognosis |
| ECLIPSE trial [31] | Phase IIb trial of Cy/GVAX in combination with CRS-207 versus GVAX alone versus physician choice chemotherapy in advanced stage PDAC patients | Activation of cytotoxic T cells with GM-CSF secreting PDAC cells and inhibition of T regulatory cells |
|
No evidence of benefit. OS was 3.8 months, 5.4 months and 4.6 months in GVAX and CRS-207, CSR-207 alone and chemotherapy alone, respectively |
| Hardacre et al. [33] | Phase II trial of Algenpantucel-L in combination with chemotherapy in resectable PDAC | Induction of cytotoxic T cells against PDAC cells with hyperacute rejection (hypothetical) | Single arm: 100 million vaccine cells injected intradermally for up to 14 vaccinations over 8 months in combination with chemo in adjuvant setting | A trend toward improved clinical outcomes. Twelve-month disease-free survival and 12-month overall survival were 62% and 86%, respectively |
| IMPRESS trial [34] | Phase III trial of Algenpantucel-L in combination with chemotherapy versus chemotherapy alone in resected PDAC patients | Induction of cytotoxic T cells against PDAC cells with hyperacute rejection (hypothetical) |
|
No evidence for benefit. Three- and four-year survival were 41.4% versus 42.1% and 32.6% versus 32.7% for the control and study groups, respectively. The median OS was 30.4 and 27.3 months for the control and study group, respectively |
PDAC, pancreatic adenocarcinoma; CTL, cytotoxic T cells; DCs, dendritic cells; OS, overall survival; DLT, dose-limiting toxicity.
Telomerase, an immortality-related ribounucleo-protein, is frequently overexpressed in transformed malignant cells rendering it a potential target antigen for immunotherapy. Telomerase in combination with GM-CSF was identified to be safe and early signals were promising for antitumor immunity [21]. However, a recent phase III trial of a combination of telomerase peptide with chemotherapy did not show any improvement in survival outcomes [22]. Currently, human telomerase reverse transcriptase (hTERT), a subunit of the telomerase enzyme, is being evaluated both as a single agent and in combination with IL-12 DNA in a phase I trial for solid tumors including PDAC (Table 2; NCT02960594). Survivin, a well-known tumor-related antigen, has also been investigated as a cancer vaccine. A gemcitabine-resistant patient treated with a survivin-based vaccination strategy had a complete remission although disease progression occurred once the vaccination was discontinued [23]. Wilms’ tumor 1 (WT1), a mutated peptide expressed in various cancers, including PDAC, has been used to sensitize effector T cells to PDAC [24]. DCs were designed to present WT1 via either MHC class I, II, or I/II (combined model) and the best clinical response was observed with MHC class I/II combined model which was associated with an increased delayed hypersensitivity reaction. Administration of biweekly MHC-restricted WT1 vaccine, when combined with gemcitabine, also appears to be a safe approach in advanced stage PDAC patients [25]. More recently, to overcome progressive self-tolerance to cancer related antigens, exploration of personalized peptides known as cancer peptides with an ability to activate pre-existing host immunity in a HLA specific manner has been conducted [26, 27]. Early phase studies of these peptides have shown a tolerable safety profile and signals of clinical benefit in both chemotherapy-naive and chemotherapy-resistant patients with advanced stage PDAC [26, 27].
Table 2.
Selected ongoing clinical trials evaluating immunotherapeutic approaches in PDAC
| Identifier | Trial and strategy | Mechanism of action | Design of clinical trial | Primary and secondary end points |
|---|---|---|---|---|
| NCT00727441 | Phase II study of GVAX vaccine±cyclophosphamide in resectable PDAC | Induction of effector immune cells and inhibition of T regulatory cells via whole cell cancer vaccine |
|
PE: safety, feasibility, and immune response SE: OS and PFS |
| NCT02451982 | Phase 1/2 study of neoadjuvant/adjuvant GVAX vaccine±nivolumab (anti PD-1) | Induction of effector immune cells with whole cell cancer vaccine±removal of negative regulatory signals |
|
PE: median IL17A expression in vaccine-induced lymphoid aggregates SE: OS and DFS |
| NCT01896869 | Phase II study of Ipilimumab (anti-CTLA4) and GVAX vaccine in metastatic PDAC | Induction of effector immune cells with whole cell cancer vaccine±removal of negative regulatory signals |
|
|
| NCT02548169 | Phase I study of antigen-loaded Dendritic cell in combination with chemotherapy | Induction of effector immune cells |
|
|
| NCT02243371 | Phase II study of GVAX vaccine and CRS-207±nivolumab in metastatic PDAC patients | Induction of effector immune cells with whole cell cancer vaccine±removal of negative regulatory signals |
|
|
| NCT01473940 | Phase I study of ipilimumab with gemcitabine in advanced stage PDAC | Induction of effector immune cells by removing negative regulatory signals |
|
|
| NCT02558894 | Phase II study of durvalumab (anti-PD-L1)±tremelimumab (anti-CTLA4) in metastatic PDAC | Induction of effector immune cells by removing negative regulatory signals with immune check point inhibitors |
|
|
| NCT02268825 | Phase I, IIA study of pembrolizumab (anti-PD-1) in combination with mFOLFOX in advanced stage GI cancers | Induction of effector immune cells by removing negative regulatory signals with immune check point inhibitor | Single arm: pembrolizumab day 1 of each cycle of FOLFOX (total 14 days) | PE: safety and tolerability in combination with mFOLFOX |
| NCT02309177 | Phase I study of Nivolumab (anti-PD-1) with Nab-paclitaxel±gemcitabine in advanced stage PDAC | Induction of effector immune cells by removing negative regulatory signals with immune check point inhibitor |
|
|
| NCT02303990 (RADVAX trial) | Phase I study of Pembrolizumab in combination with hypofractionated radiation therapy | Induction of effector immune cells by immune check point inhibitor and sensitization of T cells by radiotherapy | Single arm: pembrolizumab along with radiation treatment | PE: safety and dose limiting toxicities |
| NCT02077881 | Phase 1/2 study of Indoximod (IDO inhibitor) in combination with gemcitabine and nab-paclitaxel in metastatic PDAC | Induction of effector immune cells by modifying tumor metabolism | Single arm: indoximod twice daily×28 days each cycle. Gemcitabine, Nab-paclitaxel days 1, 8, and 15 28 days |
|
Sx, surgery; CTX, cyclophosphamide; PE, primary end point; SE, secondary end points; OS, overall survival; PFS, progression-free survival; RR, response rate; DFS, disease-free survival; TTP, time-to-progression.
Whole cell cancer vaccines
In the early 1990s, tumor cells were genetically engineered to secrete GM-CSF (GVAX) and foster immune activation. A phase I study of this approach in PDAC showed a favorable safety profile and suggested signals for enhanced antitumor immunity [28]. However, one of the most common challenges observed in vaccine-based clinical trials is compensatory infiltration of Tregs into the tumor microenvironment leading to non-durable immune responses. In an open-label phase I safety study, a combination of GVAX with low-dose cyclophosphamide (Cy) (250 mg/m2), the latter used to deplete Tregs, relatively improved survival outcomes were observed compared with GVAX alone [29] (2.3 versus 4.3 months). This approach was further developed in a clinical trial with the addition of mesothelin-expressing listeria monocytogenes (CRS-207) to boost immune response [30]. In a phase II trial, previously treated advanced PDAC patients were enrolled in two arms to receive two doses Cy/GVAX followed by four doses of CRS-207 (A) versus six doses of Cy/GVAX alone (B) [30]. The authors reported a better overall survival (OS) in arm A (6.1 versus 3.9 months P = 0.02). The presence of a mesothelin-specific CD8+ T-cell response was found to be associated with an improved course in both groups. In a phase IIB trial (ECLIPSE) of this approach, previously treated advanced stage PDAC patients were enrolled in three arms to receive Cy/GVAX and CRS-207 versus CRS-207 alone versus physician’s choice of single agent chemotherapy. The results of this study was disappointing with evidence of lack of efficacy for the combination of CRS-207 and Cy/GVAX compared with chemotherapy although there was a signal from the CRS-207 alone arm compared with chemotherapy alone (5.4 versus 4.6 months, respectively) [31].
The potential benefits of GVAX in PDAC have also been investigated in different clinical settings. For example, a single arm phase II clinical trial assessed GVAX in combination with chemotherapy in an adjuvant setting [32]. Individuals received 5×108 GVAX after 8–10 weeks of surgery, followed by 5-FU based chemoradiation [32]. In this uncontrolled single-arm study, the incorporation of immunotherapy with adjuvant chemoradiation was reported to be safe and potential signals for better outcomes were observed compared with historical controls. Currently, the rationale of using Cy/GVAX±CSR-207 in combination with chemotherapy and checkpoint inhibitors is being examined in neoadjuvant settings (Table 2; NCT00727441, NCT02451982). The clinical utility of Cy/GVAX in locally advanced disease is currently being studied in a phase II trial in combination with SBRT (Stereotactic body radiation therapy) and pembrolizumab (NCT02648282).
Algenpantucel-L, a whole cell cancer vaccine composed of irradiated cancer cells expressing α-GT(alpha-1,3-galactosyltransferase), has been investigated in PDAC. In a single arm phase II trial, safety was demonstrated in combination with chemotherapy and chemoradiotherapy and promise identified in an adjuvant setting [33]. However, a phase III trial of algenpantucel-L (IMPRESS) did not reach to its primary end point [34]. A press-release in 2016 announced the results which showed that overall survival in control and study groups were 30.4 versus 27.3 months, respectively [34].
Although cancer vaccines are clearly able to activate antitumor immunity, lack of significant clinical benefit and durable immunity has led to investigation of combination approaches of vaccine and immune modulatory agents [35]. Ipilimumab combined with GVAX appears to have favorable survival outcomes and further sustained antitumor immunity compared with ipilimumab alone in a small phase I study in advanced PDAC (OS; 5.7 versus 3.6 months, respectively) [35]. Clinical trials are currently investigating the role of this combination and a vaccine only approach in late stage PDAC patients (NCT01896869, NCT02548169). Combination of Cy/GVAX and CRS-207 with or without nivolumab is also currently being investigated in both metastatic PDAC and neoadjuvant/adjuvant settings (Table 2; NCT02243371, NCT02451982).
Checkpoint inhibitors and other immune modulatory strategies
Recent discoveries have revealed many immunomodulatory receptors involved in immune evasion [36, 37] (Figure 1). Monoclonal antibodies targeting these inhibitory signals, also called immune checkpoint inhibitors, can induce antitumor immunity [38, 39]. Promising outcomes observed in other solid tumors have led to investigation of checkpoint inhibitors in PDAC. Ipilimumab, an anti-CTLA4 antibody, in a phase II study as a single agent treatment was found to be safe, though did not demonstrate significant activity [40]. In this study, 1 of 27 patients, had a delayed response after initial progression (Table 3). Tremelimumab, another anti-CTLA4 antibody, was assessed and combined with gemcitabine in the treatment of metastatic PDAC patients [41]. In this single arm, phase I study, no dose-limiting toxicity was observed and the median OS was 7.4 months with two patients achieving a partial response. A phase I study of another immune checkpoint inhibitor, BMS-936559 (an anti-PD-L1 antibody), showed no responses in 14 patients who had been previously treated for advanced PDAC although significant tumor regressions were observed in other solid tumors such as melanoma and lung cancer [42]. Due to the lack of signal for immune response with the use of single agent checkpoint inhibitors, combination durvalumab and tremelimumab (anti-PD-L1, anti-CTLA4 antibodies) has been assessed and results are awaited (NCT02558894). Pembrolizumab and nivolumab, anti-PD-1 antibodies, other immune checkpoint inhibitors, are currently being evaluated in combination with FOLFOX, and gemcitabine and-Nab-paclitaxel with/without gemcitabine, respectively, in early phase studies for safety and efficacy (NCT02268825, NCT02309177). An abscopal effect induced by radiotherapy at a distant site to which the radiation is being administered, may also enhance the effect of immunotherapy and several trials are currently investigating this approach, e.g. pembrolizumab in combination with hypofractionated radiotherapy in advanced PDAC (RADVAX trial, NCT02303990).
Table 3.
Selected trials assessing novel immunomodulatory agents in PDAC
| Identification | Trial and strategy | Mechanism of action | Study design | Results |
|---|---|---|---|---|
| Royal et al. [40] | Phase II study of single agent ipilimumab (CTLA4 inhibitor) in locally advanced and metastatic PDAC patients | Check point inhibitor to overcome T-cell exhaustion | Single arm: patients received Ipilimumab intravenously (3.0 mg/kg every 3 weeks; 4 doses/course) for a maximum of 2 courses | Three patients had grade >3 adverse effects. N = 1 of 27 had a delayed objective response, but had confirmed POD initially |
| Aglietta et al. [41] | Phase I study of single agent tremelimumab in combination with gemcitabine in chemotherapy naive PDAC patients | Check point inhibitor (CTLA4 inhibitor) to overcome T-cell exhaustion | Single arm: 34 patients received tremelimumab on the first day of the 84-day cycle and standard gemcitabine dosing (1000 mg/m2 on days 1, 8, and 15 of each 28-day cycles) | Well tolerated. Grade 3/4 asthenia and nausea. No DLT. Two patients out of 34 had a partial response all of whom received complete course of tremelimumab |
| Brahmer et al. [42] | Phase I study of BMS-936559 (an anti-PD-L1 antibody) as a single agent in advanced stage PDAC patients | Check point inhibitor (CTLA4 inhibitor) to overcome T-cell exhaustion | Single arm: multiple solid tumor patients included. Fourteen PDAC patients received BMS-936559 with a dose of 0.3–10 mg/kg on days 1, 15, and 29 | Well tolerated. No objective response was demonstrated in PDAC patients |
| Beatty at al. [43] | Phase I study of anti-CD40 antibody in combination with gemcitabine in advanced stage PDAC patients | CTL activation with CD40 provocation | Single arm: CP-870,893 (anti-40 antibody) at 0.1 or 0.2 mg/kg were infused on day 3 of each 28-day cycle along with standard gemcitabine treatment | One dose limiting event (CVC). Grade 1/2 cytokine release syndrome. Mixed response in metastatic lesions with decrease FDG uptake |
| Nywening et al. [48] | Phase Ib trial of CCR2 inhibitors in combination with FOLFIRINOX in borderline resectable and locally advanced PDAC patients | Inhibition of tumor associated macrophages to remove the negative/inhibitory feedback on cytotoxic T cells |
|
One patient had dose limiting event. Otherwise well tolerated. Objective response in FOLFIRINOX alone and experimental arm were 49% versus 0%, respectively. Disease control was achieved in 97% of experiment group and 80% with FOLFIRINOX alone |
| Kondo et al. [54] | Generation of adoptive immunotherapy against Mucin-1 by using DCs in unresectable and recurrent PDAC patients | CTLs reactive to mucin-1 were generated by DCs presenting mucin-1 to create antitumor immunity | Single arm: N = 20 patients received expanded clone CTLs which were generated ex vivo environment | Well tolerated. N = 5 had stable disease. One patient had complete response. Median OS was 9.8 months |
| Hecht et al. [73] | Phase 1/2 trial of intratumoral endoscopic ultrasound injection of ONYX-015 in unresectable PDAC | Proliferation of genetically modified adenoviruses viruses in p53-mutant pancreatic cancer cells to induce oncolysis | Single arm; N = 21 received 2 x 1010 (n = 3) or 2 x 1011 (n = 18) viral copies/treatment which were injected directly into pancreatic tumor. Patients also received standard dose of gemcitabine | Feasible approach. Generally, well tolerated. N = 10 either had partial response or stable disease and N = 11 patients had progressive disease |
PDAC, pancreatic adenocarcinoma; CTL, cytotoxic T cells; DCs, dendritic cells; OS, overall survival; DLT, dose-limiting toxicity.
Costimulatory molecules and chemokine pathways may also have important roles in recruitment of antitumor immunity. CD40, an APC receptor that upregulates T-cells function, targeted to boost antitumor immunity [43]. A trial evaluating a CD40 agonist combined with gemcitabine in 28 chemotherapy-naive advanced stage PDAC patients reported decreased FDG uptake in hepatic lesions of four patients [43]. Activation of CD40-CD40L receptor-ligand axis may enhance dendritic cell response and thus recruit CD8+ T cells to the tumor microenvironment [44]. Provocation of CD40 was also hypothesized to deplete the immunosuppressive tumor stroma which enhances cytotoxic T-cell activity against PDAC cells as observed in animal models [45]. Depletion of tumor-associated fibroblasts by targeting the CXCl12-CXCR4 axis may also enhance tumor specific T-cell infiltration when combined with an anti-PD-L1 antibody [46]. Ulocuplumab, an anti-CXCR4 antibody, has been investigated in combination with nivolumab in a phase 1/2 trial (NCT02472977) and results are awaited. Inhibition of PD-1 along with CXCR2, which is involved in recruitment of myeloid-derived suppressor cells, may also potentiate tumor immunity [47]. A recent phase Ib study evaluated PF-04136309, a CCR2(C-C chemokine receptor type2) inhibitor to suppress tumor associated macrophages, was evaluated in combination with FOLFIRINOX in borderline resectable and locally advanced PDAC [48]. The authors reported safe use of PF-04136309 in combination with FOLFIRINOX, and although only six patients were enrolled in the FOLFIRINOX alone arm (five evaluable for response), an improved objective tumor response was observed in the experimental arm compared with FOLFIRINOX alone (49% versus 0%) and improved disease control (97% versus 80%). However, the small size of the study precludes definitive conclusions and other reports of FOLFIRINOX in this setting have noted significantly higher response rates. Recently, a phase I study of this agent in combination with gemcitabine and nab-paclitaxel completed recruitment. (NCT02732938) and further decisions on development are pending.
Inhibition of the CCR5/CCL5 axis, which mediates homing of Tregs, inhibits tumor invasion and metastasis and enhances antitumor immune response in animal models [49]. Depletion of Tregs with or without a cancer vaccine reduces tumor burden in murine models [50]. Inhibited activity of IDO enhances tumor specific T-cell response and reduces conversion to Treg-like cells [51]. The combination of indoximod, an IDO inhibitor, with gemcitabine/nab-paclitaxel was evaluated in treatment-naive metastatic PDAC patients [52]. Interim results of this study showed an objective response in 11/30 (37%) patients including one complete response suggesting there may be additive role for use of IDO inhibitors in combination with chemotherapeutics [52].
Adoptive cell therapies
The repertoire of the adaptive immune system is one of the most important factors that determines the responsiveness of immunity against cancer vaccines. Lack of immune response and limited durability of induced immune activation via cancer vaccines and immunomodulatory agents have led to the evaluation of adoptive immune therapies in PDAC. In this approach, T-cell clones are expanded from tumor infiltrating lymphocytes (TILs), or genetically engineered T cells expressing either chimeric antigen receptors (CAR-T) or T-cell receptors (TCR).
Adoptive immune therapy strategies which have shown favorable responses in liquid tumors are also under investigation in PDAC. For example, MUC1-reactive cytotoxic T cells have been studied in PDAC patients [53]. Eight patients with unresectable and 20 patients with resectable PDAC, received an infusion of MUC1-reactive T cells that were expanded ex vivo. Early results showed reduced liver recurrence with adoptive T-cell treatment. The median survival time was similar to historical controls, and was 17.8 months in patients who underwent curative surgery with 1-, 2-, and 3-year survival rates of 83.3%, 32.4%, and 19.4%, respectively. A similar study demonstrated activity with infusion of anti-MUC1 cytotoxic T cells in which one patient had a complete response after adoptive T-cell infusion [54]. Natural-killer group-2 member D expressing NK and cytotoxic lymphocytes may also be effective once they are further activated with cytokines [55]. In a phase II study, exvivo expanded cytokine-induced killer cells in gemcitabine-refractory PDAC patients suggested encouraging clinical activity [56]. Cytokine-induced killer cells targeting CD133 via bispecific antibodies may also promise enhanced tumor immunity by selectively targeting more tumorigenic clones [57].
T cells genetically engineered to express chimeric antigen receptors (CAR-T cells) have been also explored as a therapy for PDAC. In one trial, MUC1-specific CAR-T cells were infused to mouse models and induced tumor suppression [58]. CAR-T cells with CD24 and Her2 receptors targeting PDAC stem cell markers were administered in animal models [59]. In this study, CAR-T cells inhibited the growth and metastasis of orthotopic tumor cells and extended the survival of the mice. In one early human study, mesothelin-specific CAR-T cells were infused safely with antitumor effect observed in one PDAC patient [60]. However, the stromal barrier in the tumor microenvironment may also pose challenges for genetically engineered T cells by inducing T-cell exhaustion [61]. For example, one study examined genetically engineered T cells expressing affinity-enhanced TCR against a mesothelin antigen and demonstrated progressive inhibition of T cells requiring multiple recurrent infusions to achieve a sustained antitumor response [62]. Although the trial suggested significant antitumor activity, it is important to note that cumulative inhibitory signals orchestrated by tumor stroma leading to tumor evasion may be a threat for the future of adoptive T-cell therapies. Nonetheless, adoptive T cells may surmount progressive exhaustion if their inhibitory signals are modified [63]. Enhanced infiltration of engineered T cells to the tumor microenvironment and diminished myeloid derived suppressor cells have been observed when the PD1-PD-L1 axis is genetically modified with adoptive T cells before infusion [63]. A recent report of a colorectal cancer patient with a KRAS G12D mutation reported significant tumor regression after infusion of expanded tumor infiltrating polyclonal CD8+ T cells reactive against the KRAS G12D antigen [64]. However, in the same study, the authors also observed a recurrent/new metastasis with loss of a subtype of class I MHC molecule indicating that cancer cells may mask antigenic peptides and evade immunity by modifying their antigen presenting molecules. Moreover, lack of common tumor infiltrating cytotoxic T cells in KRAS mutant PDAC patients suggests that cancer cells may selectively present peptides which induce T-cell anergy.
Aside from NK and cytotoxic T cells, recent studies indicate that tumor regression may also be achieved by tumor infiltrating CD4+ T cells that are reactive against specific tumor antigens [65]. This important discovery suggests that anticancer immunity may be further augmented with immune-orchestrating cells.
Oncolytic viruses
Oncolytic viruses have a long history in cancer research as a therapeutic tool to manipulate T cells and to remove virus infected cancer cells. Cancer terminator virus (CTV) selectively infects cancer cells expressing progression elevated gene-3 (PEG3) by using its promoter region and leading to interferon gamma release [66]. Animal models suggest replication of this virus in PDAC cells may promote tumor suppression by fostering an immune response [66]. Herpes simplex virus (HSV) and reoviruses may also induce an antitumor effect in animal models of PDAC by enhancing cytotoxic T-cell infiltration [67–69]. Adenovirus selectively replicates in p53-mutant human cancer cells [70]. Animal models of PDAC cell lines with mutant p53 were treated with a mutated adenovirus (lacking 55-kDa E1B protein), known as ONYX-15, and the authors observed a marked reduction of tumor volume [71]. ONYX-15 has also been injected into primary PDAC site in a phase I trial with favorable safety although arguably a negative study as target viral replication was not achieved [72]. A phase I/II study investigated ONYX-15 in combination with gemcitabine and no response was observed until initiation of gemcitabine treatment suggesting limited activity in early phase of treatment [73]. Vaccina viruses have been engineered to express interleukin 10 and have been investigated in murine models which showed induction of CD8+ and CD4+ T cells and reduction of tumor implants [74]. Although oncolytic viruses can potentially promote apoptosis in cancer cells and lead to tumor regression, the transient nature of the responses, barriers to delivery of oncolytic viruses to cancer tissue, and the risk of infection are significant challenges for the future of oncologic virotherapy [75].
Discussion and future perspectives
The studies summarized herein have focused on concepts of immunotherapy in PDAC. The favorable responses seen in preclinical models unfortunately, have yet to be realized in clinical practice.
The absence of immune response to single agent checkpoint inhibitors such as CTLA4 or PD-1/PD-L1 antagonists is consistent with the known poorly immunogenic nature of PDAC. The evidence to date suggests that monotherapy with checkpoint inhibitors is not a promising strategy for the general PDAC population. However, checkpoint blockade may induce a significant clinical response in a subset of immunogenic, MSI (microsatellite instability)-High PDAC patients which have been reported with a frequency as low as 1% to as high as 22% of resected PDACs [76, 77]. Recent data suggest the frequency of MSI-High PDAC is closer to 1%–2%. In this subgroup, the malfunction of the mismatch repair machinery leads to microsatellite instability, which in turn leads to the generation of a higher volume of mutation-associated neoantigens that are targeted by the immune system [78]. Recently, the FDA approved the use of pembrolizumab in patients with MSI-H tumors including six PDAC patients based on the fast track review of promising clinical data obtained from five clinical studies that enrolled 149 patients with MSI-High tumors [79]. Results of an ongoing pembrolizumab trial in MSI-High non-colorectal gastrointestinal cancers including PDAC, has also demonstrated single agent activity to checkpoint inhibitors in a small subgroup of PDAC patients [80]. One emergent challenge is deciding which screening methods to use to appropriately identify this small subset of MSI-High PDAC patients responsive to checkpoint blockade. Checkpoint inhibitors may also boost antitumor immunity and achieve significant clinical response in T cell-inflamed PDAC which has been implicated to have a better prognosis perhaps due to cancer specific T-cell orchestrated antitumor response [9].
Genomic instability caused by mutations in DNA repair machinery genes such as BRCA and PALB2 [81] in a small subset of PDAC patients creates defective homologous repair (HR) which may lead to a significant mutation load and thus create varied antigens that are potentially immunogenic. This subgroup of PDAC along with a broader group of patients with defects in other genes involved in HR may also be vulnerable to immune check point inhibitors, a concept that is beginning to be explored. Moreover, PARP inhibitors which are promising agents in this subpopulation of PDAC, may further induce genomic instability in HR defective PDAC and create further antigens to facilitate response to immune checkpoint inhibitors. The combination of immune-modulating agents with PARP inhibitors and perhaps also platinum based chemotherapeutics, may uncover a potential synergy in PDAC with unstable genomes.
As aforementioned, most PDAC patients carry relatively less somatic mutations compared with other solid tumors [82] leading to observed limited immune response. Cancer vaccines, thus offer a strategy to circumvent this challenge. However, a phase IIB trial of GVAX and phase III trial algenpantucel-L have not shown any significant improvement in outcomes pointing to the fact that there are additional barriers for achievement of an effective antitumor immune response in PDAC. PDAC stroma bears one of most complicated tumor microenvironments that is inherently involved in downregulation of the immune reaction. Thus, cancer vaccines could be further investigated in combination with agents targeting stroma-facilitated inhibitory signals. Currently clinical trials are underway to investigate stroma modifying agents such as FAK inhibitors and recombinant hyaluronidase (PEGPH20) in combination with immune checkpoint inhibitors. Another important finding to be noted is that tumor-derived GM-CSF recruits myeloid-derived inflammatory cells and suppresses cytotoxic T-cell activity [8] although GM-CSF secreted by genetically engineered cancer cells in GVAX model is supposedly provoking an immune response [28]. However, the absence of durable immunity to GVAX and observed better responses to CRS-207 alone compared with combination of CRS-207 and GVAX in ECLIPSE trial [31] suggests that GM-CSF incorporated in GVAX may indeed recruit myeloid derived suppressor cells which could negatively impact GVAX vaccine and suppress cytotoxic T-cell infiltration. It is also important to note that evolving evidence suggests that myeloid cell-inflamed PDAC has a poorly immunogenic microenvironment in part due to continuous suppression of T cells by immature myeloid cells [8].
Although cytotoxic T cells are key components of cancer immunity, NK cells are also specially armed members of the immune system with unique features. NK cells eliminate targets including cancer cells without requiring MHC-restricted antigen recognition [83] a tool that may potentially overcome aforementioned obstacles. Loss of MHC class I expression, which is a strong signal for activation of NK cells, enables their cytotoxic effect on target cells. Progressive loss of MHC class I expression in PDAC [84] may also explain the absence of sustained immune response to MHC-restricted antigen presentation in vaccine treatment. Therefore, antitumor activity of NK cells should be further examined in poorly immunogenic tumors including PDAC.
Tumor heterogeneity remains another critical challenge in cancer treatment. Cancer stem cells are pluripotent cells that may form new clones with different properties and genetic signatures leading to tumor heterogeneity [85]. Cancer vaccines targeting certain antigens in cancer cells may induce destruction of clones expressing presumed target antigens however other clones lacking the target peptide may evade antitumor immunity. Thus, cancer vaccines targeting antigens universally present on pluripotent cancer stem cells may achieve sustained therapeutic efficacy. Knowledge regarding the role of cancer stem cells on immune suppression and their interaction with effector T cells may also broaden our horizons on immune evasion of cancer cells and may open new therapeutic paths for future cancer immunotherapy.
Overall, current evidence strongly suggests critical limiting challenges ahead for evolvement of immunotherapy in PDAC treatment, in particular, due to the poorly immunogenic nature of PDAC. It is important to note that, the innate aggressive behavior of PDAC may directly relate to its poor immunogenicity and lack of immune activation throughout malignant transformation and progression. However, the repertoire of the immune system is well-equipped with varied immune cells with different effector pathways including NK cells, cytotoxic T cells and T helper cells that lends a great potential for surmounting poor immunity in PDAC. Another important factor that immunotherapy based treatment trials should consider is identification of the right patient population that may show increased susceptibility to immune targeted strategies. Therefore, while progress to date has been limited in PDAC, there is optimism that based on emerging science and an abundance of targets and strategies, meaningful progress will be realized in PDAC.
Funding
David M. Rubenstein Center for Pancreatic Cancer; National Cancer Institute (P30 CA008748).
Disclosure
EMO has received research funding from Celgene, MabVax, Genentech, AstraZenica, and MedImmune. All remaining authors have declared no conflicts of interest.
References
- 1. Conroy T, Desseigne F, Ychou M. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364(19): 1817–1825. [DOI] [PubMed] [Google Scholar]
- 2. Von Hoff DD, Ervin T, Arena FP. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369(18): 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahin IH, Iacobuzio-Donahue CA, O’Reilly EM.. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert Opin Ther Targets 2016; 20(3): 341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swann JB, Smyth MJ.. Immune surveillance of tumors. J Clin Invest 2007; 117(5): 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunn GP, Bruce AT, Ikeda H. et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3(11): 991–998. [DOI] [PubMed] [Google Scholar]
- 6. Dong H, Strome SE, Salomao DR. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8): 793–800. [DOI] [PubMed] [Google Scholar]
- 7. Peng Y-P, Zhang J-J, Liang W-b. et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer 2014; 14(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayne LJ, Beatty GL, Jhala N. et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012; 21(6): 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukunaga A, Miyamoto M, Cho Y. et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28(1): e26–e31. [DOI] [PubMed] [Google Scholar]
- 10. Chellappa S, Hugenschmidt H, Hagness M. et al. Regulatory T cells that co-express RORγt and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. OncoImmunology 2016; 5(4): e1102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kudo-Saito C, Shirako H, Ohike M. et al. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis 2013; 30(4): 393–405. [DOI] [PubMed] [Google Scholar]
- 12. Pandha H, Rigg A, John J, Lemoine N.. Loss of expression of antigen‐presenting molecules in human pancreatic cancer and pancreatic cancer cell lines. Clin Exp Immunol 2007; 148(1): 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernstorff W, Spanjaard RA, Chan AK. et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery 1999; 125(1): 73–84. [DOI] [PubMed] [Google Scholar]
- 14. Whiteside TL, Tumor-induced death of immune cells: its mechanisms and consequences. Paper presented at: Seminars in Cancer Biology 2002. [DOI] [PubMed]
- 15. Joyce JA, Fearon DT.. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348(6230): 74–80. [DOI] [PubMed] [Google Scholar]
- 16. Hinz S, Pagerols-Raluy L, Oberg H-H. et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 2007; 67(17): 8344–8350. [DOI] [PubMed] [Google Scholar]
- 17. Li M, Bharadwaj U, Zhang R. et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther 2008; 7(2): 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas AM, Santarsiero LM, Lutz ER. et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004; 200(3): 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lepisto AJ, Moser AJ, Zeh H. et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 2008; 6(B): 955. [PMC free article] [PubMed] [Google Scholar]
- 20. Deguchi T, Tanemura M, Miyoshi E. et al. Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express α-Gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res 2010; 70(13): 5259–5269. [DOI] [PubMed] [Google Scholar]
- 21. Bernhardt S, Gjertsen M, Trachsel S. et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer 2006; 95(11): 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Middleton G, Silcocks P, Cox T. et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 2014; 15(8): 829–840. [DOI] [PubMed] [Google Scholar]
- 23. Wobser M, Keikavoussi P, Kunzmann V. et al. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 2006; 55(10): 1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koido S, Homma S, Okamoto M. et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)–specific MHC class I/II–restricted epitopes for pancreatic cancer. Clin Cancer Res 2014; 20(16): 4228–4239. [DOI] [PubMed] [Google Scholar]
- 25. Nishida S, Koido S, Takeda Y. et al. Wilms tumor gene (WT1) peptide–based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother 2014; 37(2): 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yutani S, Komatsu N, Yoshitomi M. et al. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep 2013; 30(3): 1094–1100. [DOI] [PubMed] [Google Scholar]
- 27. Yanagimoto H, Shiomi H, Satoi S. et al. A phase II study of personalized peptide vaccination combined with gemcitabine for non-resectable pancreatic cancer patients. Oncol Rep 2010; 24(3): 795. [DOI] [PubMed] [Google Scholar]
- 28. Jaffee EM, Hruban RH, Biedrzycki B. et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19(1): 145–156. [DOI] [PubMed] [Google Scholar]
- 29. Laheru D, Lutz E, Burke J. et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008; 14(5): 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le DT, Wang-Gillam A, Picozzi V. et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015; 33(12): 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biotech A. Aduro Biotech Announces Phase 2b ECLIPSE Trial Misses Primary Endpoint in Heavily Pretreated Metastatic Pancreatic Cancer Press release.2016; http://investors.aduro.com/phoenix.zhtml?c=242043&p=irol-newsArticle&ID=2168543 (12 September 2017, date last accessed).
- 32. Lutz E, Yeo CJ, Lillemoe KD. et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253(2): 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardacre JM, Mulcahy M, Small W. et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013; 17(1): 94–101. [DOI] [PubMed] [Google Scholar]
- 34. IMPRESS NGs. NewLink Genetics Announces Results from Phase 3 IMPRESS Trial of Algenpantucel-L for Patients with Resected Pancreatic Cancer Press release.2016; http://investors.linkp.com/releasedetail.cfm?releaseid=969978 (12 September 2017, date last accessed).
- 35. Le DT, Lutz E, Uram JN. et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother (1997) 2013; 36(7): 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melero I, Berman DM, Aznar MA. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015; 15(8): 457–472. [DOI] [PubMed] [Google Scholar]
- 37. Laheru D, Jaffee EM.. Immunotherapy for pancreatic cancer – science driving clinical progress. Nat Rev Cancer 2005; 5(6): 459–467. [DOI] [PubMed] [Google Scholar]
- 38. Wolchok JD, Kluger H, Callahan MK. et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369(2): 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015; 373(2): 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Royal RE, Levy C, Turner K. et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33(8): 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aglietta M, Barone C, Sawyer M. et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014; 25(9): 1750–1755. [DOI] [PubMed] [Google Scholar]
- 42. Brahmer JR, Tykodi SS, Chow LQ. et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366(26): 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beatty GL, Torigian DA, Chiorean EG. et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013; 19(22): 6286–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serba S, Schmidt J, Wentzensen N. et al. Transfection with CD40L induces tumour suppression by dendritic cell activation in an orthotopic mouse model of pancreatic adenocarcinoma. Gut 2008; 57(3): 344–351. [DOI] [PubMed] [Google Scholar]
- 45. Beatty GL, Chiorean EG, Fishman MP. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331(6024): 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feig C, Jones JO, Kraman M. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci 2013; 110(50): 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steele CW, Karim SA, Leach JD. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 2016; 29(6): 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nywening TM, Wang-Gillam A, Sanford DE. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016; 17(5): 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan MC, Goedegebuure PS, Belt BA. et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 2009; 182(3): 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Viehl CT, Moore TT, Liyanage UK. et al. Depletion of CD4+ CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer–bearing mice. Ann Surg Oncol 2006; 13(9): 1252–1258. [DOI] [PubMed] [Google Scholar]
- 51. Liu X, Shin N, Koblish HK. et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010; 115(17): 3520–3530. [DOI] [PubMed] [Google Scholar]
- 52. Nathan B, Ignacio G-L, Pelin C. et al. Phase 2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: interim analysis. Paper presented at: ASCO Annual Meeting Proceedings 2016.
- 53. Kawaoka T, Oka M, Takashima M. et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep 2008; 20(1): 155. [PubMed] [Google Scholar]
- 54. Kondo H, Hazama S, Kawaoka T. et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res 2008; 28(1B): 379–387. [PubMed] [Google Scholar]
- 55. Morisaki T, Hirano T, Koya N. et al. NKG2D-directed cytokine-activated killer lymphocyte therapy combined with gemcitabine for patients with chemoresistant metastatic solid tumors. Anticancer Res 2014; 34(8): 4529–4538. [PubMed] [Google Scholar]
- 56. Chung MJ, Park JY, Bang S. et al. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother 2014; 63(9): 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang J, Li C, Wang Y. et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133 high cancer stem cells in vitro and in vivo. Clin Immunol 2013; 149(1): 156–168. [DOI] [PubMed] [Google Scholar]
- 58. Posey AD Jr, Schwab RD, Boesteanu AC. et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 2016; 44(6): 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maliar A, Servais C, Waks T. et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 2012; 143(5): 1375–1384. e1375. [DOI] [PubMed] [Google Scholar]
- 60. Beatty GL, Haas AR, Maus MV. et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014; 2(2): 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahmadzadeh M, Johnson LA, Heemskerk B. et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114(8): 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stromnes IM, Schmitt TM, Hulbert A. et al. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell 2015; 28(5): 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kobold S, Grassmann S, Chaloupka M. et al. Impact of a new fusion receptor on PD-1–mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 2015; 107(8): djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tran E, Robbins PF, Lu Y-C. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016; 375(23): 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tran E, Turcotte S, Gros A. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344(6184): 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sarkar D, Su Z-z, Vozhilla N. et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res 2005; 65(19): 9056–9063. [DOI] [PubMed] [Google Scholar]
- 67. Kasuya H, Nishiyama Y, Nomoto S. et al. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther 2007; 14(6): 533–542. [DOI] [PubMed] [Google Scholar]
- 68. Etoh T, Himeno Y, Matsumoto T. et al. Oncolytic viral therapy for human pancreatic cancer cells by reovirus. Clin Cancer Res 2003; 9(3): 1218–1223. [PubMed] [Google Scholar]
- 69. McAuliffe PF, Jarnagin WR, Johnson P. et al. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J Gastrointest Surg 2000; 4(6): 580–588. [DOI] [PubMed] [Google Scholar]
- 70. Bischoff JR, Kirn DH, Williams A, Heise C.. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996; 274(5286): 373. [DOI] [PubMed] [Google Scholar]
- 71. Motoi F, Sunamura M, Ding L. et al. Effective gene therapy for pancreatic cancer by cytokines mediated by restricted replication-competent adenovirus. Hum Gene Ther 2000; 11(2): 223–235. [DOI] [PubMed] [Google Scholar]
- 72. Mulvihill S, Warren R, Venook A. et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther 2001; 8(4): 308–315. [DOI] [PubMed] [Google Scholar]
- 73. Hecht JR, Bedford R, Abbruzzese JL. et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 2003; 9(2): 555–561. [PubMed] [Google Scholar]
- 74. Chard LS, Maniati E, Wang P. et al. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res 2015; 21(2): 405–416. [DOI] [PubMed] [Google Scholar]
- 75. Nemunaitis J, Khuri F, Ganly I. et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol 2001; 19(2): 289–298. [DOI] [PubMed] [Google Scholar]
- 76. Eatrides J, Coppola D, Wang H. et al. Microsatellite Instability in Pancreatic Cancer. J Clin Oncol 2016, doi: 10.1200/JCO.2016.34. [Google Scholar]
- 77. Nakata B, Wang YQ, Yashiro M. et al. Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin Cancer Res 2002; 8(8): 2536–2540. [PubMed] [Google Scholar]
- 78. Humphris JL, Patch AM, Nones K. et al. Hypermutation in pancreatic cancer. Gastroenterology 2017; 152(1): 68–74. e62. [DOI] [PubMed] [Google Scholar]
- 79. Release P. FDA Approves Pembrolizumab for Pancreatic Cancers with Mismatch Repair Deficiency. 2017; http://letswinpc.org/in-the-news/2017/05/24/fda-approves-pembrolizumab-for-pancreatic-cancers-with-mismatch-repair-deficiency/ (12 September 2017, date last accessed).
- 80. Le DT, Uram JN, Wang H. et al. PD-1 Blockade in Mismatch Repair Deficient Non-Colorectal Gastrointestinal Cancers. J Clin Oncol 2016, doi: 10.1056/NEJMoa1500596. [Google Scholar]
- 81. Sahin IH, Lowery MA, Stadler ZK. et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hepatol 2016; 10(8): 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alexandrov LB, Nik-Zainal S, Wedge DC. et al. Signatures of mutational processes in human cancer. Nature 2013; 500(7463): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Trinchieri G. Biology of natural killer cells. Adv Immunol 1989; 47: 187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Delp K, Momburg F, Hilmes C. et al. Functional deficiencies of components of the MHC class I antigen pathway in human tumors of epithelial origin. Bone Marrow Transplant 2000; 25: S88–S95. [DOI] [PubMed] [Google Scholar]
- 85. Visvader JE, Lindeman GJ.. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8(10): 755–768. [DOI] [PubMed] [Google Scholar]