Abstract
Background
RAS assessment is mandatory for therapy decision in metastatic colorectal cancer (mCRC) patients. This determination is based on tumor tissue, however, genotyping of circulating tumor (ct)DNA offers clear advantages as a minimally invasive method that represents tumor heterogeneity. Our study aims to evaluate the use of ctDNA as an alternative for determining baseline RAS status and subsequent monitoring of RAS mutations during therapy as a component of routine clinical practice.
Patients and methods
RAS mutational status in plasma was evaluated in mCRC patients by OncoBEAM™ RAS CRC assay. Concordance of results in plasma and tissue was retrospectively evaluated. RAS mutations were also prospectively monitored in longitudinal plasma samples from selected patients.
Results
Analysis of RAS in tissue and plasma samples from 115 mCRC patients showed a 93% overall agreement. Plasma/tissue RAS discrepancies were mainly explained by spatial and temporal tumor heterogeneity. Analysis of clinico-pathological features showed that the site of metastasis (i.e. peritoneal, lung), the histology of the tumor (i.e. mucinous) and administration of treatment previous to blood collection negatively impacted the detection of RAS in ctDNA. In patients with baseline mutant RAS tumors treated with chemotherapy/antiangiogenic, longitudinal analysis of RAS ctDNA mirrored response to treatment, being an early predictor of response. In patients RAS wt, longitudinal monitoring of RAS ctDNA revealed that OncoBEAM was useful to detect emergence of RAS mutations during anti-EGFR treatment.
Conclusion
The high overall agreement in RAS mutational assessment between plasma and tissue supports blood-based testing with OncoBEAM™ as a viable alternative for genotyping RAS of mCRC patients in routine clinical practice. Our study describes practical clinico-pathological specifications to optimize RAS ctDNA determination. Moreover, OncoBEAM™ is useful to monitor RAS in patients undergoing systemic therapy to detect resistance and evaluate the efficacy of particular treatments.
Keywords: ctDNA, RAS mutations, colorectal cancer, liquid biopsy, tumor dynamics, heterogeneity
Introduction
Monoclonal antibodies (moAb) directed against EGFR—cetuximab and panitumumab—are standard components of treatment regimens for metastatic colorectal cancer (mCRC) patients, either alone or in combination with chemotherapy. The current standard of care is to determine mutations in RAS in all mCRC tumors before initiating treatment, as critical biomarkers of innate resistance to anti-EGFR [1]. Moreover, all mCRC patients that initially respond to anti-EGFR therapy eventually develop resistance, which in ∼50% of cases is due to the emergence of RAS mutations [2–5]. Currently, RAS mutation determination is carried out in formalin fixed paraffin-embedded samples from tumor tissue.
Circulating DNA fragments carrying tumor specific sequence alterations (circulating tumor DNA, ctDNA) are found in the cell-free fraction of blood, representing a variable and generally small fraction of the total circulating cell-free DNA (cfDNA). Tumor genotyping using ctDNA offers potential advantages particularly in the metastatic setting as a safe minimally invasive alternative to tissue [3].
Prior studies have demonstrated a high degree of concordance between somatic mutations detected in tumor tissue and those determined in ctDNA of patients with advanced tumors [6, 7]. The use of ctDNA has also demonstrated utility to predict treatment response to chemotherapy. Previous ctDNA studies utilized massively parallel (direct) sequencing of tumor tissue in order to identify somatic alterations specific to individual patients, which were subsequently incorporated into the development of a personalized gene panel to detect these mutations in blood samples. Although useful in a research setting, a personalized NGS panel approach is currently not amenable to routine clinical practice in that it requires significant dedicated resources in highly qualified research laboratories. Alternatively, blood-based tests that encompass a panel of the most frequently occurring mutations for a given tumor type and which can be used to interrogate the plasma of patients with high sensitivity present a practical approach for routine clinical care. The first and only test thus far for the determination of RAS mutations in ctDNA with European Conformity (CE-marked) in vitro diagnostic (CE-IVD) is the OncoBEAM RAS CRC assay, which detects 34 mutations in exons 2, 3, and 4 in the KRAS and NRAS genes as recommended by current clinical practice treatment guidelines (NCCN, ESMO, EMA).
The aim of the present study was to evaluate the clinical applications of the OncoBEAM RAS CRC assay in routine clinical practice for the diagnosis, assessment of response to chemotherapy/antiangiogenic treatment and monitoring of acquired resistance to anti-EGFR therapy in mCRC patients.
Materials and methods
Study design and sample collection
A retrospective-prospective study was carried out in two Spanish Institutions. Patients with histologically confirmed metastatic colorectal cancer and anti-EGFR treatment naïve were eligible for the study. Blood samples were collected in all patients before the administration of anti-EGFR treatment. For those patients undergoing monitoring, serial blood samples were collected every 4 weeks coinciding with the treatment visit and at the moment of progressive disease. See full inclusion criteria and regulatory aspects in supplementary material, available at Annals of Oncology online.
OncoBEAM™ RAS CRC assay was used to detect RAS mutations in plasma, and RAS mutation detection in tissue samples were carried out according to standard-of-care (SoC) procedures validated by each hospital (details in supplementary material and Table S4, available at Annals of Oncology online).
Statistical analysis
Variables were described using median and interquartile range (IQR) when continuous, and percentage when categorical. For mutant allele fraction (MAF) levels comparisons between different groups regarding clinical variables, we carried out Mann–Whitney U test for dichotomic variables and Kruskal–Wallis test for polycothomic variables. Tests were carried out under SPSS v.22 with a significance level of P < 0.05. Graphics were built using R 3.3.1.
Results
Patient characteristics and concordance of extended RAS determination in plasma versus tissue
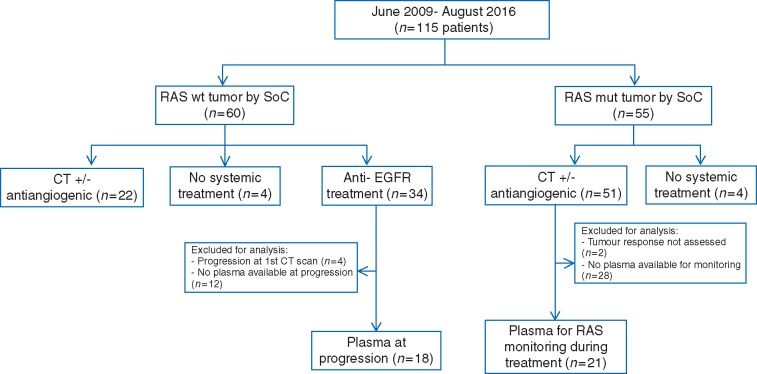
From June 2009 to August 2016, 115 patients with mCRC were included, all of them had at least one baseline blood draw. Study flowchart is presented in Figure 1. Clinico-pathological characteristics of the patients are described in supplementary Table S1, available at Annals of Oncology online. At the time of basal ctDNA collection, all patients were naïve to anti-EGFR treatment and 82 patients (71%) had not received any therapy in the metastatic setting. The median time from tumor tissue specimen collection to ctDNA collection was 47.5 days (range 0–1783 days) in therapy-naïve patients.
Figure 1.
Study flowchart. Number of patients included in each of the analysis endpoints and reasons for exclusion are depicted.
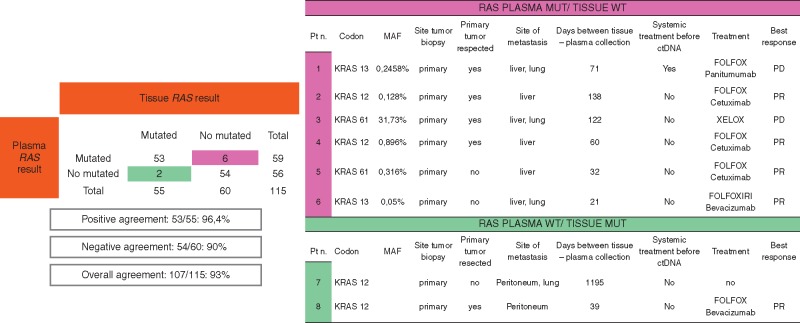
Of the 115 patients included in the study, 55 (47.8%) and 59 (51.3%) were shown to have RAS mutations in their tumor samples as detected by SoC RAS tissue testing and as detected in ctDNA by RAS OncoBEAM, respectively (supplementary Figure S1, available at Annals of Oncology online). The overall concordance of RAS results between ctDNA RAS OncoBEAM assay and standard techniques for tissue analysis was 93% (107/115 patients), kappa index 0.844 (95% CI 0.746–0.941) (Figure 2).
Figure 2.
Comparison of RAS mutations detected in tissue versus plasma and analysis of discrepancies. Overall concordance analysis between RAS mutations detected in tumor by SoC and BEAMing plasma. Positive agreement (patients RAS mutated in plasma and tissue analysis) and negative agreement (patients wild-type in tissue and plasma). Clinico-pathological and treatment characteristics from eight discordant cases.
Characterization of RAS tissue versus RAS plasma discordant cases
Among 55 patients in whom a RAS mutation was detected in tissue, 53 also had a RAS mutation in plasma (positive percentage agreement, PPA of 96.4%) (Figure 2). There were only two cases with RAS mutated tissue in whom no RAS mutation was detected in ctDNA. Both had localized tumors at the time of diagnosis that were initially removed. At relapse, when ctDNA extraction was carried out, both patients had minimal tumor burden: one with only peritoneum metastasis and the other had one infra-centimetric lung metastasis and one single implant in the peritoneum.
Among 60 patients determined to be RAS wt in tissue, no RAS mutations were observed in ctDNA in 54 cases (negative percentage agreement, NPA of 90%). In the remaining 6 patients, plasma RAS BEAMing detected a RAS mutation that SoC tissue testing had not revealed. In all of these cases, the primary tumor served as the source for RAS mutational analysis and had been removed before ctDNA sampling. Notably, all six patients had, at least, liver metastasis when blood was drawn for ctDNA analysis.
Interestingly, in five out of the six RAS tissue-/plasma+ discordant cases, the RAS MAF detected in ctDNA was under 1%. We compared the MAF of concordant and discordant cases. As shown in Figure 3A, there was a trend towards lower RAS plasma MAF in discordant cases compared with patients with concordant RAS in tissue and plasma (median RAS MAF 0.281% and 2.317%, respectively; P=0.193).
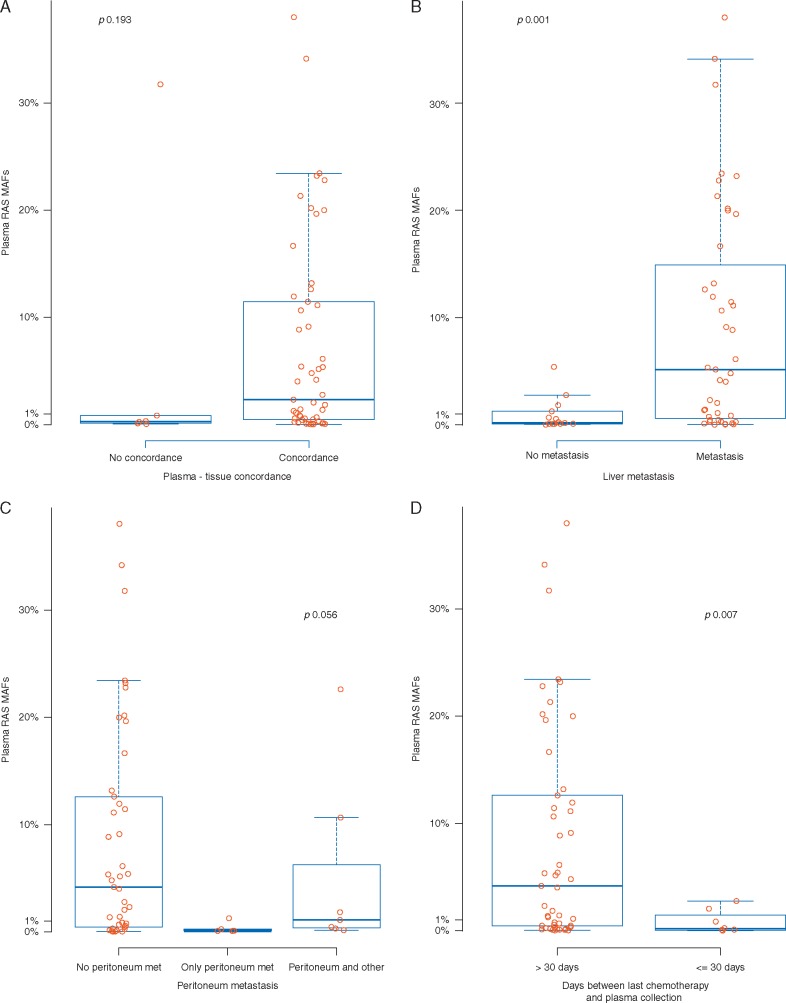
Figure 3.
Correlation of circulating RAS mutations and clinico-pathological characteristics. (A) Differences in RAS ctDNA mutant allele fraction (MAF) in patients with concordant RAS plasma and tissue determination compared with discordant cases (RAS wild-type in plasma and RAS mutated in tissue or RAS mutated in plasma and RAS wild-type in tissue). (B) Differences in RAS ctDNA MAF according to patients with liver metastasis versus patients without liver involvement. (C) Differences in RAS ctDNA MAF according to patients without peritoneum involvement, patients with only peritoneum metastasis and patients with peritoneum plus another metastatic site. (D) Differences in RAS ctDNA MAF according to treatment naïve patients versus patients with previous systemic treatment received within a month prior ctDNA blood extraction.
Differences in RAS ctDNA MAF according to clinico-pathological characteristics
The global median RAS plasma MAF was 1.84% (IQR: 0.284–11.290) (supplementary Table S1, available at Annals of Oncology online). No differences in MAFs were observed in relation to age, gender, initial stage at diagnosis or primary site of disease (right versus left).
While no differences in RAS MAF were seen in relation to the number of metastatic sites, differences were observed depending on the site of metastasis location (Figure 3B and C). Patients with liver involvement had higher RAS ctDNA compared with those without liver metastases (4.806% versus 0.203%; P=0.001). In contrast, MAF from patients having only peritoneum metastases was lower (0.1%) than patients without peritoneum involvement (4.026%), than patients with peritoneum metastasis in addition to at least one other metastatic site (1.109%; P =0.056). MAF was lower in patients with only lung metastatic involvement (0.033%). Of note, in two patients that presented with tumors having mucinous histology MAF was below the median (0.451% and 0.161%, respectively; P < 0.05).
We then sought to analyze the impact of treatment on RAS mutation detection in plasma. No differences were observed in RAS plasma MAF between patients in whom the primary tumor had been removed before basal ctDNA extraction (4.026%) and patients in whom ctDNA was extracted from blood drawn at the time primary tumors had not been resected (1.558%; P=0.584). Regarding the relation between systemic treatment and plasma RAS mutation detection, 8 of 59 RAS mutant patients (13.6%) had received previous treatment with chemotherapy (comprising 5FU, oxaliplatin and/or irinotecan) ± anti-VEGF within a month prior ctDNA blood extraction. In all of these patients, lower RAS plasma MAFs were observed (0.173%; range 0.074–1.156) when compared with treatment-naïve patients (4.178%; range 0.451–12.620; P=0.007) (Figure 3D), emphasizing the relevance of blood draw timing for an accurate RAS determination.
Response to anti-EGFR therapy and prognosis according to baseline RAS ctDNA determination
We then determined the impact of RAS detection in predicting response to anti-EGFR based therapy. Among 54 patients having RAS wt tumor tissue, 34 were treated with anti-EGFR monoclonal antibodies (31 with cetuximab and 3 with panitumumab-based regimens; 30 plus chemotherapy and 4 in monotherapy). Twenty-three achieved a complete or partial response (68%) and 7 patients (20%) had stable disease for more than 16 weeks. Among tissue RAS wt patients treated with anti-EGFR therapy, four were found to have a plasma RAS positive result (Figure 2, discordant patients #1, #2, #4, #5). Three of them achieved a partial response, whereas one showed progressive disease after administration of anti-EGFR treatment.
Despite the retrospective nature of our study, we determined the progression-free survival (PFS) according to RAS mutation determination in plasma and RAS mutation determination in tissue. PFS was 10.3 month (95% CI 7.7–25) for wt RAS tissue patients and 10.3 months (95% CI 7.7–19.8) for RAS wt plasma patients.
In addition, baseline high RAS ctDNA MAF have been associated with low survival [8]. We analyzed the prognosis impact of basal MAF levels in a cohort of 22 patients with at least 3 year of follow-up. Patients with MAF levels ≥1% had significant lower PFS and OS than those with basal levels <1% (supplementary Figure S2, available at Annals of Oncology online). These data suggest that ctDNA levels could also provide valuable information to predict the disease evolution in RAS mutant patients before treatment onset.
Longitudinal ctDNA RAS testing for assessing response to patients treated with systemic treatment
Because ctDNA analysis has the capacity to reflect tumor load [2], we examined the utility of OncoBEAM RAS CRC ctDNA testing to monitor the efficacy of response of patients to treatment.
RAS was longitudinally monitored in serial blood draws from 21 patients with baseline RAS mutations undergoing systemic therapy. Seven patients were treated with combination chemotherapy + antiangiogenic therapy, 12 received chemotherapy alone and 2 anti-EGFR-based treatment (supplementary Table S2, available at Annals of Oncology online). A first CT-scan was carried out concurrent with the ctDNA RAS monitoring to evaluate tumor response after 8–12 weeks of treatment. Analysis of RAS ctDNA at the time of this first CT-scan revealed a dramatic decrease in plasma RAS MAF in responding patients with a median of 100%. For patients with clinical response to treatment, no differences in MAFs were observed in relation to the type of response achieved (median MAF reduction in patients with SD 99% versus 100% in patients with PR; P = 0.21). However, MAF percentage of change was significantly lower in patients that progressed at first evaluation of response compared with patients with clinical benefit (PR + SD) (132% increase versus 99% reduction, respectively, P = 0.027)
In 10 out of 11 responding patients that subsequently progressed, RAS ctDNA MAF increased accordingly, though in most cases patients exhibited lower MAFs than at the time of diagnosis (Figure 4A; supplementary Table S2, available at Annals of Oncology online). Of note, in one patient having a basal KRAS mutation in codon 12 (basal RAS MAF 9.12%), a decrease in RAS MAF was initially observed that was quickly followed by an increase in RAS MAF at week 12 although the CT scan showed stable disease. This patient subsequently showed a rapid progression of disease and died 4 months later.
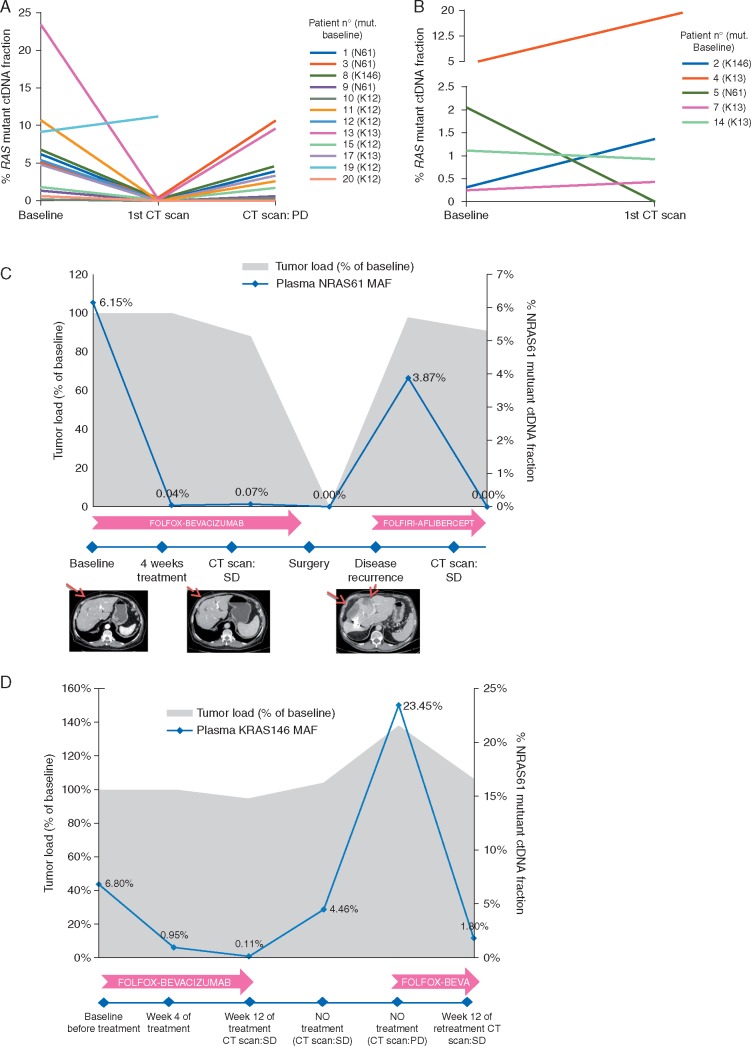
Figure 4.
Longitudinal analysis of plasma RAS ctDNA to evaluate response to treatment with chemotherapy ± antiangiogenic. (A) RAS ctDNA dynamics in nine patients with RAS mutated tumors treated with chemotherapy ± antiangiogenic that initially respond to treatment. Frequency of circulating RAS mutant alleles at baseline, at time of first CT-scan to evaluate treatment response and at disease progression. Decline and increase in circulating RAS MAF correlate with response and progression to treatment, respectively. (B) RAS ctDNA dynamics in 5 patients with RAS mutated tumors that progressed at first CTscan at 8–12 weeks from beginning of treatment. (C) Patient diagnosed with stage IV rectal cancer with liver metastasis. An NRAS codon 61 mutation was detected in tissue and plasma. After 4 weeks of treatment with FOLFOX + bevacizumab, plasma MAF dramatically decreased correlating with a stable disease observed in the CT-scan at week 12. The patient underwent surgery of the primary tumor and liver metastasis, and plasma RAS became undetectable. Eight months later, the patient relapsed and RAS MAF increased accordingly. Three months after initiating second line treatment with FOLFIRI + aflibercept, the patient achieved a stable disease by CT scan, and no plasma ctDNA RAS mutations were detected. (D) Monitoring ctDNA KRAS codon 146 mutation during treatment with FOLFOX-Bevacizumab in patient #3, diagnosed with a stage IV colon cancer with lung and liver metastasis. The colonoscopic biopsy analysis was RAS wt but plasma ctDNA showed a KRAS codon 146 mutation. Following removal of the primary tumor, re-analysis of RAS in the surgical sample confirmed the plasma result. The patient received FOLFOX + bevacizumab with an early decrease in RAS ctDNA that became undetectable at 12 weeks, alongside at the first CT scan. Treatment was discontinued and a subsequently increase in KRAS codon 146 MAF was observed, which then rapidly decreased when the chemotherapy was reintroduced. Gray area indicates tumor load. Blue line indicates changes in ctDNA KRAS146 frequency.
Representative time courses of ctDNA along with clinical and radiologic data on two subjects are provided (Figure 4C and D), showing the high accuracy of RAS plasma ctDNA dynamics as a surrogate marker of tumor load and a potential tool to evaluate early response to treatment.
ctDNA extended RAS for monitoring RAS mutations during and after withdrawal of anti-EGFR therapy
We and others previously reported that acquired resistance to anti-EGFR treatment is linked to the emergence of RAS mutations, that can be tracked in the blood of patients [2, 3, 8, 9]. In our study, we examined the value of OncoBEAM RAS CRC testing to detect the emergence of RAS mutations during anti-EGFR treatment. Plasma was available at the time of disease progression from 18 cases with acquired resistance to anti-EGFR therapy (i.e. disease progression after an initial complete response, partial response or stable disease for more than 16 weeks). Emergence of RAS mutations was detected in 7/18 patients (39%), 5 of them treated with chemotherapy + anti-EGFR and two with anti-EGFR monotherapy (supplementary Table S3, available at Annals of Oncology online). The most frequent mutations involved KRAS codon 12, KRAS codon 13 and NRAS codon 61. In three cases, different RAS mutations were concomitantly detected. Median RAS MAF detected at the time of anti-EGFR progression was 2.17% (range 0.024–24.957).
Discussion
Our study demonstrates that OncoBEAM RAS CRC assay is an efficient and accurate tool to be used in routine clinical practice with several applications in mCRC patients, including determination of baseline RAS at diagnosis to decide anti-EGFR therapy, assessment of efficacy to treatment and monitoring of the emergence of RAS mutations as a mechanism of resistance to anti-EGFR therapy.
The high overall agreement between baseline plasma and tissue RAS mutation status demonstrated in more than 100 patients evaluated in our study supports the use of blood-based testing with OncoBEAM™ RAS CRC as a viable alternative to tissue SoC for determining RAS mutation status in mCRC patients treated in routine clinical practice. Previous studies have shown that ctDNA can be detected in patients with mCRC by using personalized research panels with dPCR [7, 10, 11]. Recent publications have also shown a very high sensitivity with BEAMing to detect ctDNA mutations [12, 13]. However, to the best of our knowledge, this is the first study that explores the clinical use of plasma RAS determination by using a CE-marked assay in a daily clinical routine setting and in a large real world cohort of patients. Moreover, the minimal level of discordance (6%) between RAS tissue and plasma detection shown in our study is acceptable from a clinical point of view. In fact, it is far lower than the 5%–20% discrepancy found in RAS mutation detection when comparing two different tissue RAS testing SoC techniques [14, 15].
In an effort to explain plasma/tissue discrepancies as well as to better understand the biology of circulating tumor DNA, we identified several clinico-pathological features linked to low RAS ctDNA detection, including peritoneal/lung metastases or mucinous histology. In contrast, no correlation was found between the number of metastasis and RAS ctDNA mutations in our study. This data suggests that intrinsic biological characteristics of the tumor rather than tumor burden may impact ctDNA release. In order to appreciate the utility and further optimize the routine evaluation of RAS ctDNA determination in daily clinical practice, we also studied external factors that may influence the result of RAS ctDNA determination. We found that the administration of recent systemic treatment had a clear negative impact on the ability to detect RAS mutations in the blood of patients, emphasizing the importance of collecting plasma for basal RAS analysis before the initiation of any systemic treatment. On the contrary removal of the primary tumor before blood draw for RAS analysis did not impact the RAS mutation results. In global, our study shows that the pattern of genetic alterations in cancer patients is dynamic and is affected by intrinsic and extrinsic factors.
Importantly, we also found a potential role of OncoBEAM RAS ctDNA assay in monitoring response and resistance during treatment. In patients with RAS mutant tumors, RAS plasma mirrored clinical and radiological response to treatment with chemotherapy drugs and was an early predictor of response. Likewise, Tie et al. [10] reported changes in ctDNA for mCRC patients treated with chemotherapy, although a more complex research-based approach was used. Moreover, we showed a potential use of RAS ctDNA in evaluating response to antiangiogenic drugs, which could be complementary to RECIST. On the other hand, in patients with RAS wt tumors treated with anti-EGFR, OncoBEAM RAS CRC was a valid tool to detect RAS mutations of resistance.
Despite the great value of the results presented, there are several limitations to our study. It is a retrospective analysis. Longitudinal blood extractions were only carried out in a limited number of patients. Additionally, given the low number of patients with specific clinico-pathological characteristics, our inferences from associations with P-values marginally <0.05% should be cautiously interpreted.
Overall, our data show that the OncoBEAM RAS CRC assay offers a minimally invasive and highly sensitive method for RAS assessment in plasma of mCRC patients which can be readily implemented into routine clinical practice to perform baseline diagnosis to select candidate patients to anti-EGR therapy. Moreover, we show a potential use of OncoBEAM RAS in assessing the dynamics of RAS to monitor response and resistance to treatment practice.
Supplementary Material
Acknowledgements
The authors wish to thank biobank of I.D.I.S.-C.H.U.S. (PT13/0010/0068) for providing part of the tissue samples from colorectal cancer patients including at Complexo Hospitalario Universitario de Santiago de Compostela. The authors want to thank Mario Martin for his valuable contribution in the statistical analysis and Cristina Blanco for data management support.
Funding
This work was supported by Instituto de Salud Carlos III grants PI15/00457, INT 16/00106 and DTS15/00048 (CM), PIE15/00008 and PI15/00146 (JA); 2014SGR740 (JA); Xarxa de Banc de Tumors de Catalunya and Fundació Cellex. This work was supported in part by Merck KGaA, Darmstadt, Germany.
Disclosure
FJ, DE and VS are employees of Sysmex-Inostics Inc. All remaining authors have declared no conflicts of interest.
References
- 1. Van Cutsem E, Cervantes A, Adam R.. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27(8): 1386–1422. [DOI] [PubMed] [Google Scholar]
- 2. MisaleYaeger SR, Hobor S.. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486(7404): 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diaz LA Jr, Williams RT, Wu J.. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486(7404): 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Emburgh BO, Arena S, Siravegna G.. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun 2016; 7: 13665.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montagut C, Dalmases A, Bellosillo B.. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012; 18(2): 221–223. [DOI] [PubMed] [Google Scholar]
- 6. Bettegowda C, Sausen M, Leary RJ.. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6(224): 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diehl F, Schmidt K, Choti MA.. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14(9): 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morelli MP, Overman MJ, Dasari A.. Characterizing the patterns of clonal selection in circulating tumor DNA from patients with colorectal cancer refractory to anti-EGFR treatment. Ann Oncol Off J Eur Soc Med Oncol/ESMO 2015; 26(4): 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arena S, Bellosillo B, Siravegna G.. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin Cancer Res 2015; 21(9): 2157–2166. [DOI] [PubMed] [Google Scholar]
- 10. Tie J, Kinde I, Wang Y.. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015; 26(8): 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siravegna G, Mussolin B, Buscarino M.. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015; 21(7): 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toledo R, Cubillo AA, Vega E.. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget 2016. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tabernero J, Lenz H-J, Siena S.. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol 2015; 16(8): 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azuara D, Santos C, Lopez-Doriga A.. Nanofluidic digital PCR and extended genotyping of RAS and BRAF for improved selection of metastatic colorectal cancer patients to anti-EGFR therapies. Mol Cancer Ther 2016; 15: 14–21. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez de Castro D, Angulo B, Gomez B.. A comparison of three methods for detecting KRAS mutations in formalin-fixed colorectal cancer specimens. Br J Cancer 2012; 107(2): 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.