Abstract
Background
We hypothesized that increased baseline BMI and BMI change would negatively impact clinical outcomes with adjuvant breast cancer systemic therapy.
Methods
Data from chemotherapy trials MA.5 and MA.21; endocrine therapy MA.12, MA.14 and MA.27; and trastuzumab HERA/MA.24 were analyzed. The primary objective was to examine the effect of BMI change on breast cancer-free interval (BCFI) landmarked at 5 years; secondary objectives included BMI changes at 1 and 3 years; BMI changes on disease-specific survival (DSS) and overall survival (OS); and effects of baseline BMI. Stratified analyses included trial therapy and composite trial stratification factors.
Results
In pre-/peri-/early post-menopausal chemotherapy trials (N = 2793), baseline BMI did not impact any endpoint and increased BMI from baseline did not significantly affect BCFI (P = 0.85) after 5 years although it was associated with worse BCFI (P = 0.03) and DSS (P = 0.07) after 1 year. BMI increase by 3 and 5 years was associated with better DSS (P = 0.01; 0.01) and OS (P = 0.003; 0.05). In pre-menopausal endocrine therapy trial MA.12 (N = 672), patients with higher baseline BMI had worse BCFI (P = 0.02) after 1 year, worse DSS (P = 0.05; 0.004) after 1 and 5 years and worse OS (P = 0.01) after 5 years. Increased BMI did not impact BCFI (P = 0.90) after 5 years, although it was associated with worse BCFI (P = 0.01) after 1 year. In post-menopausal endocrine therapy trials MA.14 and MA.27 (N = 8236), baseline BMI did not significantly impact outcome for any endpoint. BMI change did not impact BCFI or DSS after 1 or 3 years, although a mean increased BMI of 0.3 was associated with better OS (P = 0.02) after 1 year. With the administration of trastuzumab (N = 1395) baseline BMI and BMI change did not significantly impact outcomes.
Conclusions
Higher baseline BMI and BMI increases negatively affected outcomes only in pre-/peri-/early post-menopausal trial patients. Otherwise, BMI increases similar to those expected in healthy women either did not impact outcome or were associated with better outcomes.
Clinical Trials numbers
CAN-NCIC-MA5; National Cancer Institute (NCI)-V90-0027; MA.12-NCT00002542; MA.14-NCT00002864; MA.21-NCT00014222; HERA, NCT00045032;CAN-NCIC-MA24; MA-27-NCT00066573.
Keywords: BMI, disease specific survival, triple negative, weight change, overall survival
Introduction
Reports indicating an association between obesity and incidence of breast cancer and outcome led to lifestyle recommendations about diet, weight loss, and regular exercise in early breast cancer guidelines [1–5].
Obesity has been implicated as a significant risk factor in developing breast cancer. Weight gain occurs in the majority of women following early breast cancer systemic therapy [6–9]. Women rarely return to their pre-diagnosis weight [10, 11]. In the Nurse's Health Study, weight at diagnosis and weight gain by 12 months were significantly associated with increased breast cancer rates; weight gain increased recurrence risk to 1.35–1.64 [12]. Smaller and older studies generally reported that weight gain after breast cancer diagnosis affected prognosis [13–15]; however, this was not always seen [16].
We examined here whether baseline BMI or weight change impacted outcome in more contemporary phase III adjuvant trials of chemotherapy, endocrine therapy, and trastuzumab.
Methods
Study design
Trials were approved by Health Regulatory authorities and centers’ Institutional Review Boards, and patients provided informed consent. We utilized prospectively gathered individual patient data, with trials classified by menopausal status and type of trial therapy.
Pre-, peri-, and early post-menopausal trials
Adjuvant chemotherapy
MA.5 randomized women to cyclophosphamide, epirubicin, and fluorouracil (CEF), or significantly inferior cyclophosphamide, methotrexate, and fluorouracil (CMF) [17]. We utilized 10-year follow-up data [18]. MA.21 randomized women to CEF, or dose dense epirubicin and cyclophosphamide followed by paclitaxel (EC/T), or significantly inferior doxorubicin and cyclophosphamide followed by paclitaxel (AC/T) [19]. We utilized final analysis median 8-year follow-up data [20].
Adjuvant endocrine therapy
MA.12 was a placebo-controlled trial of tamoxifen in women who completed CMF, CEF, or AC. Tamoxifen was directionally better at median 9.7 years follow-up [21].
Triple negative disease
Exploratory investigations used centrally-reviewed ER, PR, and HER2 for MA.5 and MA.12, and locally assessed for MA.21.
Post-menopausal trials
Adjuvant chemotherapy
No phase III adjuvant breast cancer chemotherapy trials were available.
Adjuvant endocrine therapy
MA.14 was a trial of tamoxifen ± Octreotide LAR with no significant treatment differences at final analysis median 7.9 years follow-up [22]. In MA.27, the aromatase inhibitors exemestane and anastrozole did not differ significantly at final analysis median 4.1-years follow-up [23].
Pre- and post-menopausal trials
Adjuvant herceptin
Exploratory adjuvant herceptin examinations arose from three sources: (1) 1-year herceptin therapy arm of HERceptin Adjuvant (HERA) trial at median 4-year follow-up [24]; (2) MA.27, when trastuzumab was a stratification factor [23]; and (3) MA.21, when trastuzumab was permitted after June 2005 [20].
Baseline patient characteristics
For cross-trial comparisons, baseline patient characteristics/stratification factors were categorized in a common way: age (≤49; ≥50 years); menopausal status (pre/peri/uncertain;post/missing); race (Caucasian/white;other); ECOG performance status (0;other); nodal status (N−;N+;unknown); T status (T1/in situ; ≥T2;unknown); hormone receptor status (positive;negative;unknown); adjuvant chemotherapy (yes;no); adjuvant anthracyclines (yes;no).
The manuscript was written by the authors, with all authors reviewing the final paper.
Patient population
CCTG trials patients who received trial treatment were included, categorized by treatment received. MA.21 patients completed trial therapy before the DSMC-mandated release of interim analysis. HERA patients randomized to the 1-year herceptin arm were included.
Study endpoints
BMI change from baseline was defined as [(BMI at timepoint)–(baseline BMI)]. We examined whether BMI change affected future clinical outcome in those without an outcome event, by 1, 3, or 5 years [25]. The primary objective was the effect of BMI change by 5 years on following breast cancer-free interval (BCFI), where BCFI was defined as time from randomization until first invasive or DCIS recurrence, contralateral disease, or breast cancer death; censoring was at non-breast second primary invasive cancer, non-breast cancer death, or longest follow-up. Secondary objectives included effects of BMI change by 1 and 3 years; effects of BMI change on disease-specific survival (DSS) and on OS; and, effects of baseline BMI. For CCTG trials, DSS was defined as time from randomization to death with or from breast cancer; for HERA, DSS was defined as death following recurrence without a cardiac event. OS was defined as time from randomization to death. Patient change in BMI was censored at BCFI event prior to BMI assessment: for 1 year examinations (11, ≤18) months; for 3-year (35, ≤42) months; for 5-year (59, ≤66) months. HERA trial examinations were restricted to 1-year BMI assessments in the period (330, ≤400) days.
Statistical analysis
Weight was routinely collected at baseline and follow-up for CCTG led trials; height was uniformly collected at baseline. Patients were predominantly excluded in landmarked analyses following 1, 3, and 5 years BMI due to prior clinical outcome; therefore, patients included after timepoints are characterized by their baseline BMI [25]. HERA patients routinely had only baseline weight so patient characteristics were compared with exact Fisher tests by whether BMI was available at baseline and 1-year.
BMI change and baseline BMI effects were assessed separately by menopausal status and type of adjuvant systemic therapy. Effects were assessed with stratified Cox models due to substantive evidence against the Cox assumption of proportional hazards; stratification was by treatment and baseline patient characteristics. Graphical depiction of log-linear BMI effect on hazard by outcome was displayed with Forest plots, indicating the number of patients assessed after 1, 3, and 5 years, mean (change in) patient BMI with standard error, effect on outcome with hazard ratio (HR), 95% HR confidence interval, and Wald statistic with nominal significance a two-sided P-value of 0.05, unadjusted for multiplicity.
Results
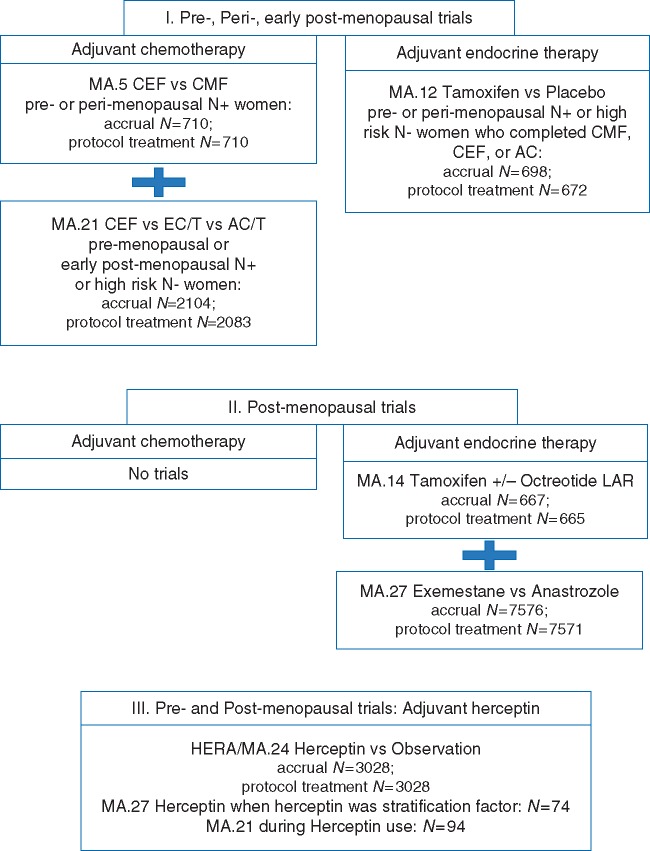
A CONSORT diagram (Figure 1) indicates patients included by trial- and tumor-type. Table 1 provides trial-level comparative baseline characteristics.
Figure 1.
CONSORT diagram: patients in phase III adjuvant trials, classified by menopausal status and type of trial systemic therapy. CEF is cyclophosphamide, epirubicin, fluorouracil; CMF is cyclophosphamide, methotrexate, fluorouracil; AC is doxorubicin, cyclophosphamide; EC/T is epirubicin, cyclophosphamide followed by paclitaxel; AC/T is doxorubicin, cyclophosphamide followed by paclitaxel.
Table 1.
Baseline patient characteristics
| Factors | MA.5 | MA.12 | MA.14 | MA.21 | MA.27 | HERA |
|---|---|---|---|---|---|---|
| Total N | 710 | 672 | 665 | 2083 | 7571 | 3028 |
| Age < 50 | 84% | 82% | 3% | 61% | 3% | 51% |
| Menopausal status: Pre/uncertain | 100% | 100% | 0% | 68% | 0% | 54% |
| Race White | n/a | n/a | 97% | 89% | 95% | 83% |
| ECOG performance 0 | 81% | 65% | 78% | 84% | 82% | 91% |
| Node positive | 100% | 75% | 47% | 72% | 28% | 64% |
| T1/in situ | 37% | 43% | 58% | 35% | 72% | 35% |
| Hormone receptor positive | 68% | 75% | 91% | 61% | 100% | 51% |
| Chemotherapy | 100% | 100% | 31% | 100% | 31% | 100% |
| Anthracyclines | 49% | 55% | 25% | 100% | 28% | 93% |
Pre-, peri-, and early post-menopausal trials
Adjuvant chemotherapy
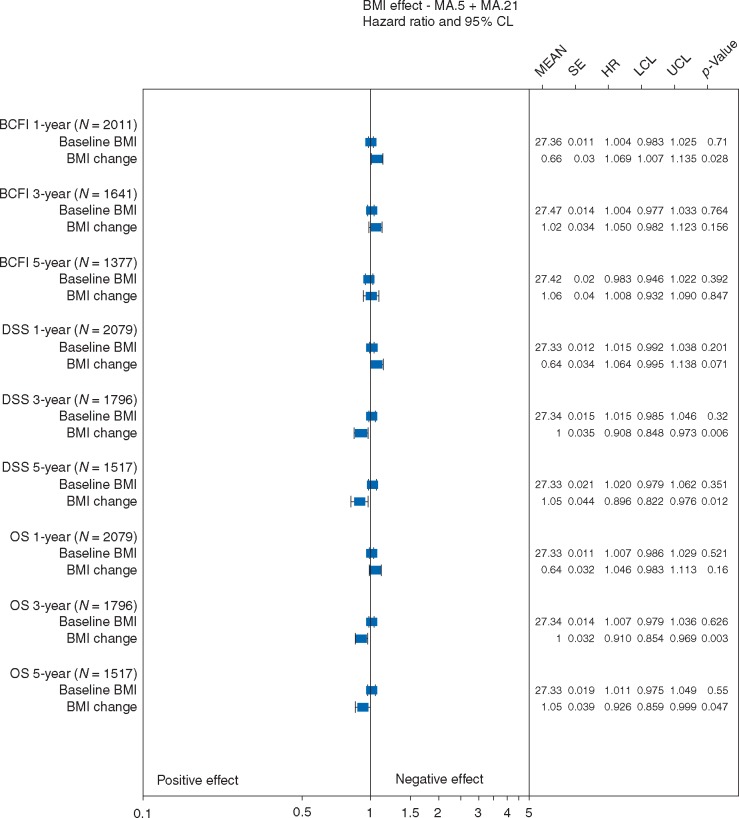
Investigations utilized 2793 patients: 710 node positive (N+) MA.5 patients; 2083 MA.21 patients, 72% of whom were N+, 28% high risk node negative (Table 1). Mean baseline BMI did not significantly impact BCFI, DSS, or OS (Figure 2) after any timepoint. Mean BMI increased at 1-year by 0.7; increased BMI was associated with worse BCFI (P = 0.03) and DSS (P = 0.07) after 1-year. The mean BMI change at 5 years was 1.1, which had no significant effect on BCFI (P = 0.85) after 5 years. However, mean BMI increase of 1 at 3 years and 1.1 at 5 years was associated with better DSS (P = 0.01; 0.01) and better OS (P = 0.003; 0.05) after 3 and 5 years.
Figure 2.
Effects of baseline BMI and BMI change on breast cancer free interval (BCFI), disease specific survival (DSS), overall survival (OS): Pre-, peri-, and early post-menopausal adjuvant chemotherapy trials. SE is standard error; HR is hazard ratio; LCL is the lower 95% confidence limit; UCL is the upper 95% confidence limit; P-value is based on a two-sided Wald statistic from a stratified Cox model.
Adjuvant endocrine therapy
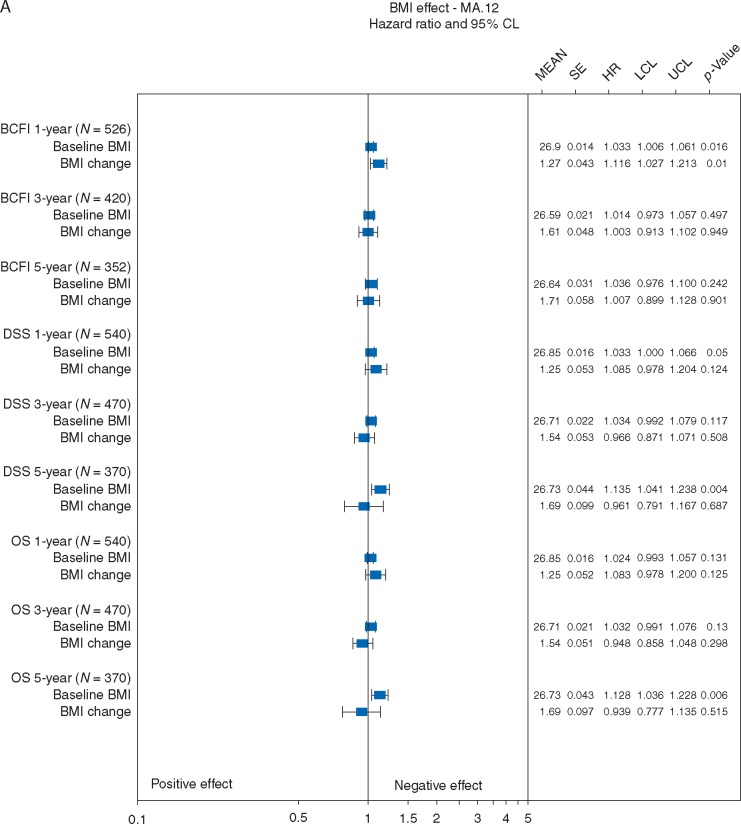
BMI effects were assessed in 672 MA.12 patients, 75% of whom were N+, all of whom received adjuvant chemotherapy. Patients with higher baseline BMI had worse BCFI (P = 0.02; Figure 3A) after 1-year, worse DSS (P = 0.05; 0.004) after 1 and 5 years and worse OS (P = 0.01) after 5 years. Mean BMI change at 1-year was 1.3; increased BMI was associated with worse BCFI (P = 0.01) after 1-year. Mean BMI change at 5 years was 1.7, and did not significantly impact BCFI (P = 0.90). Mean BMI changes of 1.3–1.7 did not significantly impact DSS or OS after any timepoint.
Figure 3A.
Effects of baseline BMI and BMI change on breast cancer free interval (BCFI), disease specific survival (DSS), overall survival (OS): Pre- and peri-menopausal adjuvant endocrine therapy. SE is standard error; HR is hazard ratio; LCL is the lower 95% confidence limit; UCL is the upper 95% confidence limit; P-value is based on a two-sided Wald statistic from a stratified Cox model.
Triple negative disease
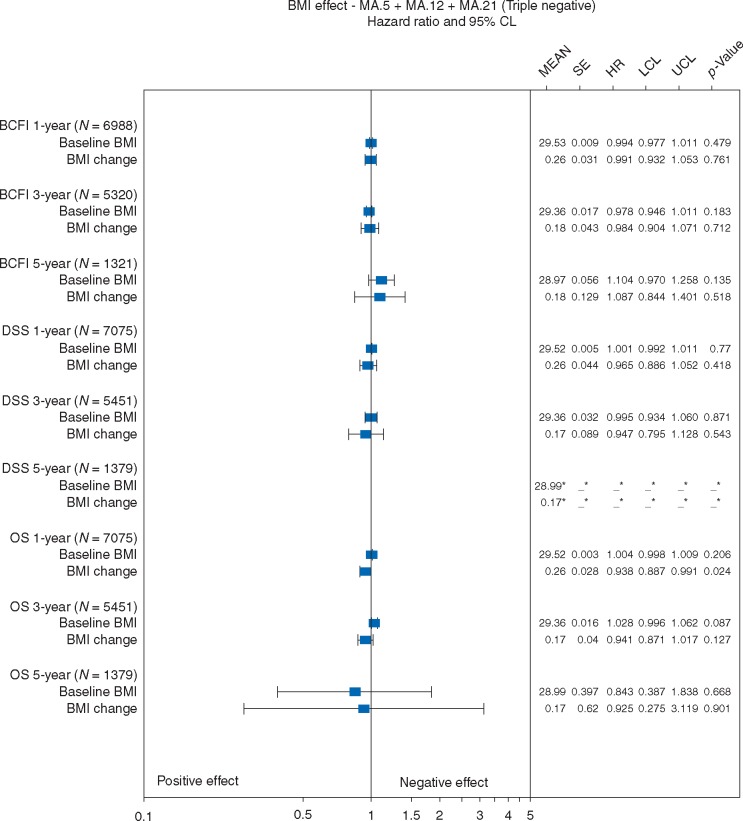
BMI effects on BCFI were assessable for N = 1250, 948, and 739 triple negative patients after 1, 3, and 5 years. Baseline BMI did not significantly impact BCFI, DSS, or OS (Figure 3B). Mean BMI increases of 1.1–1.6 did not significantly impact BCFI after any timepoint; nor, DSS or OS after 1 or 5 years. Increased mean BMI of 1.4 at 3 years was associated afterwards with better DSS and OS (P = 0.05; 0.01).
Figure 3B.
Effects of baseline BMI and BMI change on breast cancer free interval (BCFI), disease specific survival (DSS), and overall survival (OS): Triple negative patients. SE is standard error; HR is hazard ratio; LCL is the lower 95% confidence limit; UCL is the upper 95% confidence limit; P-value is based on a two-sided Wald statistic from a stratified Cox model.
Post-menopausal trials
Adjuvant endocrine therapy
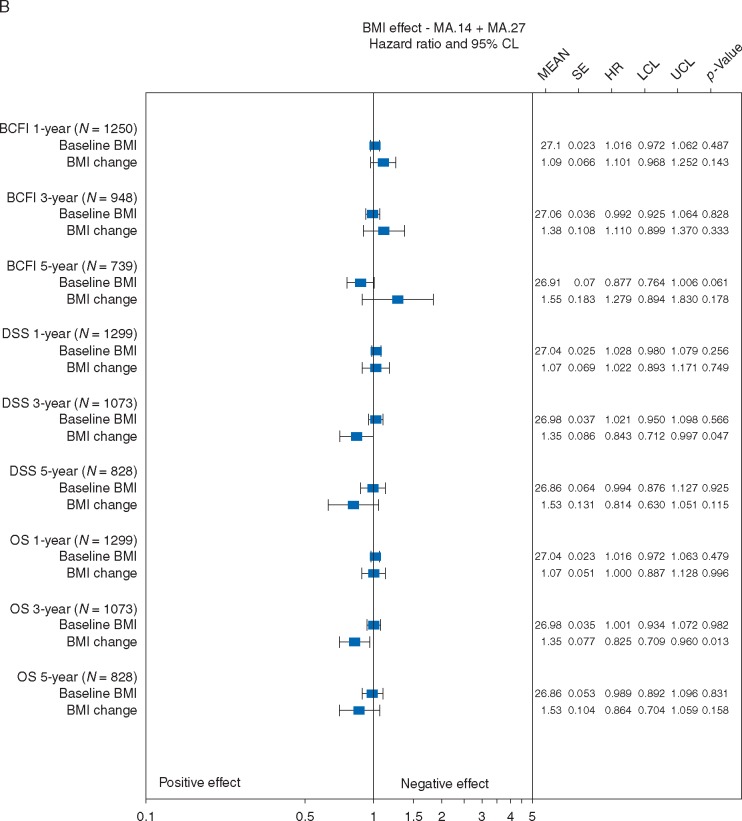
BMI effects were assessed in 8236 patients: 665 MA.14 patients, 47% of whom were N+, with 31% receiving chemotherapy; and 7571 MA.27 patients who were 28% N+, with 31% receiving chemotherapy (Table 1). Baseline BMI did not significantly impact outcome for any endpoint, at any timepoint (Figure 4), Mean BMI change of 0.2–0.3 did not significantly impact BCFI at any timepoint, or DSS after 1 or 3 years. Mean increased BMI of 0.3 at 1 year was associated with better OS (P = 0.02) after 1 year.
Figure 4.
Effects of baseline BMI and BMI change on breast cancer free interval (BCFI), disease specific survival (DSS), overall survival (OS): Post-menopausal adjuvant endocrine therapy. SE is standard error; HR is hazard ratio; LCL is the lower 95% confidence limit; UCL is the upper 95% confidence limit; P-value is based on a two-sided Wald statistic from a stratified Cox model. *indicates Cox model not fit due to lack of events.
Pre- and post-menopausal trials
Adjuvant herceptin
Trastuzumab therapy was received by 1491 HERA patients, 1249 of whom (supplementary Table S1, available at Annals of Oncology online), were assessable for BMI changes by 1-year; trastuzumab patients assessable at 1-year were not significantly different from those who were not (P = 0.14–0.78). Mean baseline BMI and mean BMI change of 0.4 did not significantly impact later BCFI, DSS, or OS for trastuzumab-treated patients (supplementary Figure S1, available at Annals of Oncology online).
Discussion
A priori, we expected higher baseline BMI and increased BMI to negatively impact outcome [1–16]. We examined the impact of baseline BMI and change in BMI in six contemporary phase III trials. Obesity, particularly abdominal obesity, determines systemic endocrine environment, encourages breast cancer development, and elevated circulating insulin, that is mitogenic, anti-apoptotic and pro-angiogenic [26, 27]. Several adipokines produced by adipose tissue are related to hyperinsulinemia and angiogenesis promotion, contributing to aggressive breast cancer [28]. Potentially, obesity increases estradiol levels, the mitotic rate of breast epithelial cells [29], disease recurrence [30], and chronic low-grade inflammation linked to tumor progression [31]. Generally, this hypothesis was not supported in our study.
Caan et al reported with 12 915 cases diagnosed 1990–2006, that weight gain did not impact DSS, although it negatively impacted OS [32]. Bradshaw et al. found for 1033 cases diagnosed 1996–1997 that weight gains > 10%, or losses > 5% after diagnosis led to worse OS and DSS [33]. Which leads to whether to target weight gain, or changes in dietary fat.
Consensus is that higher baseline BMI is a risk factor for breast cancer development and in earlier treatment-eras, may have impacted outcome. Cases included in a meta-analysis were diagnosed between 1963 and 2005 and reported that obese patients had elevated risk of relapse and poor outcomes [34]. In a study follow-up with 213 075 survivors diagnosed between 1957 and 2009, baseline BMI significantly impacted DSS; the association was weaker in clinical trials than observational studies [35]. Cecchini et al. [36] reported in four randomized trials that baseline BMI was associated with lower survival with ER positive tumors.
Our study included randomized trials with enrollment during 1989–2008 with most patients enrolling after 2000 to contemporary treatments. We incorporated the effects of prognostic factors, type of treatment (adjuvant chemotherapy, endocrine, or trastuzumab) and specific subtype (triple negative). Higher baseline BMI only negatively impacted outcomes in the pre-menopausal endocrine trial MA.12. Increases in BMI by 1-year only negatively affected later outcomes in the pre-/peri-/early post-menopausal trials (MA.12; MA.5/MA.21). Otherwise, BMI increases, similar to those expected in healthy women, either did not impact outcome or were associated with better outcomes. To the best of our knowledge, this is the first study to examine the impact of BMI change in a phase III adjuvant trastuzumab trial; our results are supported by a previous finding that trastuzumab impact had no association with baseline BMI [37].
Baseline BMI increased over time from 26.5 in the oldest trial, MA.5, to 29.6 in MA.27, opening in 2003. We postulate that modest gains in higher unhealthy baseline BMI may not add much risk, or that new regimens and treatments mask baseline BMI effect with effective contemporary treatments superseding BMI effects.
The strength of this study is its large size with >5-year follow-up for all trials except MA.27 and HERA/MA.24, and contemporary treatments. Patient weight was measured at multiple timepoints, 1, 3, and 5 years after accrual, and the effects of baseline BMI and BMI change were adjusted for baseline risk factors.
The limitations of the study include that height was consistently measured only at baseline and menopausal status was that at trial enrollment. Some of our treatments are no longer commonly used. Compliance of oral medications is not incorporated. HERA was the predominant source of trastuzumab treatment data; however, weight was only required at baseline. Hormone receptor status was predominantly locally determined. Clinical trials may not represent clinical practice experience.
Other studies have also reported the impact of weight gain in pre-menopausal patients receiving third generation adjuvant treatments [38–40]. The Nurses' Health Study also found associations with weight were stronger in pre-menopausal women [12].
In summary, we found higher baseline BMI only negatively impacted outcomes in the pre-menopausal endocrine trial MA.12. Further, only increased BMI by 1-year negatively affected outcomes in the pre-/peri-/early post-menopausal MA.12 endocrine trial and (MA.5, MA.21) chemotherapy trials. Otherwise, BMI increases, similar to those expected in healthy women, and either did not impact outcome or were associated with better outcomes. With improved survival the focus should concentrate on good nutrition, weight control and exercise for our patients, although we have not shown that these consistently improve breast cancer outcomes [41].
Supplementary Material
Acknowledgements
The authors thank the HERA study team for the kind provision of the HERA data for this work.
Funding
This work was supported by grants to the CCTG from the Canadian Cancer Society Research Institute (grant #021039) and the US National Institutes of Health (CA77202).
Disclosure
Authors have declared no conflicts of interest.
References
- 1. Ligibel JA, Alfano CM, Kerry S. et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J Clin Oncol 2014; 31: 3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rock CL, Doyle C, Demark-Wahnefried W. et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012; 62: 243–274. [DOI] [PubMed] [Google Scholar]
- 3. Kushi LH, Doyle C, McCullough M. et al. American cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012; 62: 30–67. [DOI] [PubMed] [Google Scholar]
- 4. Schmitz KH, Courneya KS, Matthews C. et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010; 42: 1409–1426. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network: NCCN guidelines for supportive care, survivorship. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive (27 February 2015, date last accessed).
- 6. Goodwin PJ, Ennis M, Pritchard KI. et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol 1999; 17: 120–129. [DOI] [PubMed] [Google Scholar]
- 7. Makari-Judson G, Braun B, Jerry DJ. et al. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol. 2014; 5: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saquib N, Flatt SW, Natarajan L. et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women's healthy eating and living (WHEL) study. Breast Cancer Res Treat 2007; 105: 177–186. [DOI] [PubMed] [Google Scholar]
- 9. Gu K, Chen X, Zheng Y. et al. Weight change patterns among breast cancer survivors: results from the shanghai breast cancer survival study. Cancer Causes Control 2010; 21: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makari-Judson G, Judson CH, Mertens WC.. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J 2007; 13: 258–265. [DOI] [PubMed] [Google Scholar]
- 11. Chlebowski RT, Aiello E, McTiernan A.. Weight loss in breast cancer patient management. J Clin Oncol 2002; 20: 1128–1143. [DOI] [PubMed] [Google Scholar]
- 12. Kroenke CH, Chen WY, Rosner B. et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 2005; 23: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 13. Chlebowski RT, Weiner JM, Reynolds R. et al. Long-term survival following relapse after 5-FU but not CMF adjuvant breast cancer therapy. Breast Cancer Res Treat 1986; 7: 23–30. [DOI] [PubMed] [Google Scholar]
- 14. Camoriano JK, Loprinzi CL, Ingle JN. et al. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol 1990; 8: 1327–1334. [DOI] [PubMed] [Google Scholar]
- 15. Levine EG, Raczynski JM, Carpenter JT.. Weight gain with breast cancer adjuvant treatment. Cancer 1991; 67: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 16. Caan BJ, Kwan ML, Hartzell G. et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 2008; 19: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine MN, Bramwell VH, Pritchard KI. et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998; 16: 2651–2658. [DOI] [PubMed] [Google Scholar]
- 18. Cheang MC, Voduc KD, Tu D. et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res 2012; 18: 2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burnell M, Levine MN, Chapman JA. et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol 2010; 28: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burnell M, Shepherd L, Gelmon K. et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of the final relapse free survival analysis. Cancer Res 2012; 72(24 Suppl): 194s–195s. (Abstract). [Google Scholar]
- 21. Bramwell VH, Pritchard KI, Tu D. et al. A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada-Clinical Trials Group trial, MA.12). Ann Oncol 2010; 21: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pritchard KI, Shepherd LE, Chapman JA. et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011; 29: 3869–3876. [DOI] [PubMed] [Google Scholar]
- 23. Goss PE, Ingle JN, Pritchard KI. et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27–a randomized controlled phase III trial. J Clin Oncol. 2013; 31: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piccart-Gebhart MJ, Procter M, Leyland-Jones B. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–1672. [DOI] [PubMed] [Google Scholar]
- 25. Anderson JR, Cain KC, Gelber RD.. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 2008; 26: 3913–3915. [DOI] [PubMed] [Google Scholar]
- 26. Teitelman G, Alpert S, Hanahan D.. Proliferation, senescence, and neoplastic progression of beta cells in hyperplasic pancreatic islets. Cell 1988; 52: 97–105. [DOI] [PubMed] [Google Scholar]
- 27. Goodwin PJ, Ennis M, Pritchard KI. et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 2002; 20: 42–51. [DOI] [PubMed] [Google Scholar]
- 28. Rose DP, Komninou D, Stephenson GD.. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 2004; 5: 153–165. [DOI] [PubMed] [Google Scholar]
- 29. Key TJ. Serum oestradiol and breast cancer risk. Endocr Relat Cancer 1999; 6: 175–180. [DOI] [PubMed] [Google Scholar]
- 30. Rock CL, Flatt SW, Laughlin GA. et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev 2008; 17: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Kruijsdijk RC, van der Wall E, Visseren FL.. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 2009; 18: 2569–2578. [DOI] [PubMed] [Google Scholar]
- 32. Caan BJ, Kwan ML, Shu XO. et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012; 21: 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bradshaw PT, Ibrahim JG, Stevens J. et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 2012; 23: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Protani M, Coory M, Martin JH.. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010; 123: 627–635. [DOI] [PubMed] [Google Scholar]
- 35. Chan DS, Vieira AR, Aune D. et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014; 25: 1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cecchini RS, Swain SM, Costantino JP. et al. Body mass index at diagnosis and breast cancer survival prognosis in clinical trial populations from NRG oncology/NSABP B-30, B-31, B-34, and B-38. Cancer Epidemiol Biomarkers Prev 2016; 25(1): 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crozier JA, Moreno-Aspitia A, Ballman KV. et al. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer 2013; 119: 2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaudhray LN, Wen S, Xioa J. et al. Weight change associated with third-generation adjuvant chemotherapy in breast cancer patients. J Community Support Oncol 2014; 12: 355–360. [DOI] [PubMed] [Google Scholar]
- 39. Pfeiler G, Konigsberg R, Fesl C. et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol 2011; 29: 2653–2659. [DOI] [PubMed] [Google Scholar]
- 40. Mamounas EP, Jeong JH, Wickerham DL. et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the national surgical adjuvant breast and bowel project B-33 trial. J Clin Oncol. 2008; 26: 1965–1971. [DOI] [PubMed] [Google Scholar]
- 41. Chapman JA, Meng D, Shepherd L. et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst 2008; 100: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.