Introduction: treatment strategies in advanced breast cancer
The treatment landscape for advanced breast cancer has changed radically over the last 30–40 years with the refinement of surgical and radiographic techniques, approval of a range of chemotherapy regimens in the adjuvant and metastatic settings, and improvements in screening procedures. A deeper understanding of the molecular pathways underpinning tumor growth has led to the identification and development of a number of targeted therapies with particularly high activity in breast cancer. As an example, the prognosis of women with human epidermal growth factor receptor 2-positive (HER2+) breast cancer has improved dramatically since the introduction of HER2-targeted therapies [1]. Meanwhile, in hormone receptor-positive (HR+) breast cancer, the mammalian target of rapamycin (mTOR) inhibitor everolimus has been shown to be effective in combination with exemestane [2].
Overall, these innovations have led to the earlier detection of breast cancers and better treatments, resulting in significant improvements in patient survival and quality of life. Finding future treatments for breast cancer will depend on gaining more insights into the pathways driving tumor growth and the mechanisms of acquired and de novo resistance. Investigational targeted agents currently in clinical development include the phosphatidylinositol 3-kinase (PI3K) inhibitor alpelisib (BYL719) and the cyclin-dependent kinase (CDK) 4/6 inhibitor ribociclib (LEE011).
Translating basic research into novel therapies in breast cancer
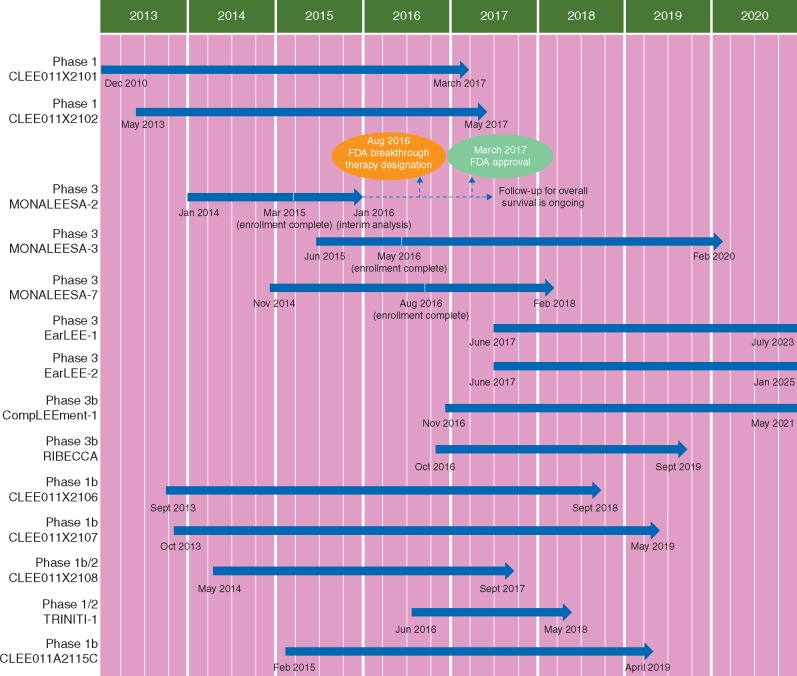
A thorough understanding of disease biology and patient needs lies at the heart of developing novel, effective therapies. Novartis has a long-standing heritage in developing treatment options for patients with breast cancer, targeting a range of oncogenic pathways—including the non-steroidal aromatase inhibitor letrozole and everolimus. Activation of the cyclin D–CDK4/6–inhibitor of CDK4 (INK4)–retinoblastoma (Rb) pathway is common in breast cancer, and the efficacy of combined CDK4/6 inhibition with endocrine therapy has been demonstrated in the first- and second-line treatment of advanced breast cancer [3, 4]. These findings encouraged Novartis to expedite the development of ribociclib (LEE011), an orally bioavailable, selective, ATP-competitive inhibitor of CDK4/6 (Figure 1) [5]. In preclinical studies, ribociclib demonstrated high levels of growth inhibition in HR+ breast cancer cell lines [6]. Additionally, in xenograft models of HR+ breast cancer, ribociclib demonstrated synergistic antitumor activity in doublet combinations with endocrine therapies including fulvestrant and letrozole [6]. In the first-in-human Phase 1 trial of adult patients with advanced solid tumors or lymphoma, single-agent ribociclib displayed promising signs of clinical activity; of 31 patients, 9 experienced stable disease and 1 patient had a partial response [5]. Ribociclib clinical development rapidly progressed from early-phase single-agent and dose-finding doublet combination trials to the recent Phase 3 MONALEESA-2 trial based on Phase 1 preliminary data indicating the absence of a drug–drug interaction between ribociclib and letrozole in the CLEE011X2107 trial [7]; frequent Data Monitoring Committee meetings mitigated any potential risk. The efficacy and safety of ribociclib is now being investigated in a number of other Phase 3 trials in different patient populations and therapy combinations (Table 1). In March 2017, ribociclib in combination with an aromatase inhibitor as an initial endocrine-based therapy for the treatment of postmenopausal women with HR+, HER2− advanced or metastatic breast cancer was approved by the US Food and Drug Administration (FDA), based on positive results from the planned interim analysis of MONALEESA-2 (discussed below) [8]. In addition, based on preclinical data with ribociclib in combination with endocrine therapies and PI3K inhibitors, a series of ongoing Phase 1 and 2 trials are investigating triplet therapy combinations in HR+ breast cancer [9–11].
Figure 1.
Ribociclib (LEE011) development program timeline.
Table 1.
Key trials in the ribociclib (LEE011) development program
| Study | Phase | Combination partner | Patient population | Setting and prior therapy requirements | N | Estimated completion |
|---|---|---|---|---|---|---|
| CLEE011X2101 (NCT01237236) | 1 | Single agent | Patients with Rb+ advanced solid tumors or lymphomas | ≥1L: Any number of prior therapies | 132 | March 2017 |
| CLEE011X2102 (NCT01747876) | 1 | Single agent | Pediatric patients with MRT or NB (other tumors with CDK4/6 pathway abnormalities permitted in the dose escalation portion) | ≥1L: Any number of prior therapies | 32 | May 2017 |
| MONALEESA-2 (NCT01958021) | 3 | Letrozole | Postmenopausal women with HR+/HER2− ABC | 1L: No prior treatment for ABC | 668 | Completed (positive interim analysis Jan 2016) |
| MONALEESA-3 (NCT02422615) | 3 | Fulvestrant | Men and postmenopausal women with HR+/HER2− ABC | 1L and 2L: No prior therapy or progressed on prior ET | 725 | Feb 2020 |
| MONALEESA-7 (NCT02278120) | 3 | Goserelin+tamoxifen or NSAI | Pre- or perimenopausal women with HR+/HER2− ABC | 1L: No prior ET for ABC | 672 | Feb 2018 |
| EarLEE-1 (NCT03078751) | 3 | Tamoxifen or letrozole or anastrozole or exemestane | Men and pre- or postmenopausal women with HR+/HER2− high-risk EBC | Adjuvant | ≈2000 | July 2023 |
| EarLEE-2 (NCT03081234) | 3 | Tamoxifen or letrozole or anastrozole or exemestane | Men and pre- or postmenopausal women with HR+/HER2− intermediate-risk EBC | Adjuvant | ≈4000 | Jan 2025 |
| CompLEEment-1 (NCT02941926) | 3b | Letrozole+goserelina | Men and pre- or postmenopausal women with HR+/HER2− ABC | 1L and 2L: No prior systemic ET and ≤1 prior line of chemotherapy for ABC | ≈3000 | May 2021 |
| RIBECCA (NCT03096847) | 3b | Letrozole+goserelina | Men and pre- or postmenopausal women with HR+/HER2− ABC | 1L, 2L, 3L, and 4L: ≤1 prior line of chemotherapy and ≤2 prior lines of ET for ABC | ≈500 | Sept 2019 |
| CLEE011X2106 (NCT01857193) | 1b | Exemestane±everolimus | Postmenopausal women with HR+/HER2− ABC | ≥1L: Progressed on prior NSAI therapy | ≈142 | Sept 2018 |
| CLEE011X2107 (NCT01872260) | 1b | Letrozole±alpelisib | Postmenopausal women with HR+/HER2− ABC | 1L (expansion phase): No prior systemic treatment for ABC | ≈225 | May 2019 |
| CLEE011X2108 (NCT02088684) | 1b/2 | Fulvestrant±alpelisib | Postmenopausal women with HR+/HER2− ABC | ≥1L: Progressed on prior AI therapy | ≈70 | Sept 2017 |
| TRINITI-1 (NCT02732119) | 1/2 | Everolimus+exemestane | Men and postmenopausal women with HR+/HER2− ABC | ≥1L (Phase 2): Progressed on prior ET and a CDK4/6i | ≈51 | May 2018 |
| CLEE011A2115C (NCT02333370) | 1b | Letrozole or fulvestrant or tamoxifen+goserelinb | Pre- or postmenopausal Asian women with HR+/HER2− ABC | 1L: No prior treatment for ABC | ≈86 | April 2019 |
In men and premenopausal women only.
In premenopausal women only.
1L, first-line; 2L, second-line; 3L, third-line; 4L, fourth-line; ABC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin-dependent kinase; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; EBC, early breast cancer; ET, endocrine therapy; HER2−, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; MRT, malignant rhabdoid tumor; NB, neuroblastoma; NSAI, non-steroidal aromatase inhibitor; Rb+, retinoblastoma-positive.
Community approach to clinical trials: MONALEESA-2 case study
Novartis’ strong collaboration with clinicians at trial sites across the world supports fast, efficient, and appropriate patient recruitment onto clinical trials. This was exemplified by the rapid completion of the MONALEESA-2 trial. In MONALEESA-2, the first patient was randomized in January 2014 with enrollment completed after ∼14 months (March 2015); the planned interim analysis was performed after ∼24 months (January 2016). Positive interim results showed that the trial met its primary endpoint, leading to the recommendation by the Independent Data Monitoring Committee to stop the trial early (May 2016). This was followed by the US FDA granting Breakthrough Therapy designation after around 31 months (August 2016), granting Priority Review three months later (November 2016), followed by approval for first-line ribociclib in combination with aromatase inhibitor therapy 5 months later (March 2017). This represents a rapid development timeline from first patient enrollment to trial read-out, considering the median duration of Phase 3 oncology trials in 2014 was ∼50 months [12].
Completion of MONALEESA-2 was supported by several key trial design elements. First, a large study steering committee was consulted on the design and conduct of the study; the globally distributed steering committee aided in the consideration of local regulatory requirements and fostered effective inter-investigator communication, resulting in efficient trial conduct with high regional participation. In total, 668 patients were enrolled at 223 sites, across 29 countries worldwide. Second, recruitment was facilitated by permitting patients who had received ≤14 days of prior letrozole or anastrozole onto the trial, increasing the potential pool of eligible patients. Finally, the interim analysis for MONALEESA-2 was planned after 211 PFS events (70% of the planned 302 events), providing an early but mature look at the trial data. The chosen timing of interim analysis and the pre-specified efficacy criteria were guided by early discussions with health authorities, and supported by previous experience in the BOLERO-2 trial [2], enabling a stable and robust assessment of treatment effect prior to final analysis. In MONALEESA-2, a stringent Haybittle–Peto type boundary ensured that early trial declaration of superiority would only occur if the HR was ≤0.56 with P < 0.0000129 [8]. Rigorous statistical testing procedures are also in place for the secondary endpoint of overall survival.
Similar testing strategies are being employed throughout the ribociclib development program; by implementing robust statistical criteria for interim analyses, data can potentially be analyzed early for superior efficacy, allowing expedited regulatory filings and, if approved, for patients to benefit from novel therapies at the earliest possible opportunity.
Ribociclib in other patient populations
Novartis is also taking an innovative approach to investigating ribociclib in under-served indications that have not been previously investigated with other CDK4/6 inhibitors. Key trials include the following:
MONALEESA-3 (NCT02422615), which is investigating ribociclib in combination with fulvestrant in men (as well as postmenopausal women) with HR+/HER2− advanced breast cancer in the first- and second-line settings. This trial was designed in part to anticipate the results from the Phase 3 FALCON study investigating fulvestrant vs anastrozole for the first-line treatment of HR+ advanced breast cancer. The first patient was enrolled onto MONALEESA-3 in June 2015, and enrollment was completed in May 2016.
MONALEESA-7 (NCT02278120), which is investigating ribociclib in combination with a non-steroidal aromatase inhibitor or tamoxifen plus goserelin in pre- and perimenopausal women with HR+/HER2− advanced breast cancer, in the first-line setting. The first patient was enrolled in November 2014, and enrollment was completed in August 2016.
TRINITI-1 (NCT02732119), which is investigating ribociclib in combination with everolimus and exemestane in men and postmenopausal women with HR+/HER2− advanced breast cancer who have progressed on a prior endocrine therapy and CDK4/6 inhibitor. Recruitment onto this trial is ongoing.
The ribociclib development program therefore includes a wide range of patients. As well as postmenopausal women, the MONALEESA trials include pre- and perimenopausal patient populations, in the first- and later-line settings (as well as specifically in the post-CDK4/6 inhibitor setting); and are also investigating men with advanced breast cancer, who remain a rare and under-served population.
The Novartis pipeline in breast cancer includes a number of promising compounds currently undergoing Phase 1–3 clinical trials, across the full range of breast cancer subtypes (HR+/HER2−, HER2+, and triple-negative breast cancer). In addition to ribociclib, other key Phase 3 compounds include PI3K/mTOR pathway inhibitors.
Conclusions
The development of ribociclib provides an example of rapid drug development, with successful transition directly from early-phase trials to a large-scale Phase 3 trial. The Phase 3 MONALEESA-2 trial was designed to quickly recruit patients and robustly analyze efficacy and safety as early as possible, leading to the combination of ribociclib and an aromatase inhibitor receiving FDA approval for the treatment of advanced breast cancer after the planned interim analysis, approximately 38 months after the first patient was enrolled. Ongoing clinical trials with ribociclib in a range of indications and treatment settings have the potential to increase treatment options for many more patients with advanced breast cancer and other indications in the future.
Acknowledgements
Ribociclib (LEE011) was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals. The authors thank Nirmal Jethwa, PhD, for medical editorial assistance with this manuscript. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Funding
Novartis Pharmaceuticals Corporation (no grant numbers apply).
Disclosure
All authors are employees of Novartis Pharmaceuticals Corporation.
References
- 1. Zurrida S, Veronesi U.. Milestones in breast cancer treatment. Breast J 2015; 21: 3–12. [DOI] [PubMed] [Google Scholar]
- 2. Baselga J, Campone M, Piccart M. et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med 2012; 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner NC, Ro J, André F. et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Martin M, Rugo HS. et al. PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2− advanced breast cancer (ABC). ASCO 2016: Abstr 507. [Google Scholar]
- 5. Infante JR, Cassier PA, Gerecitano JF. et al. A Phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 2016; 22: 5696–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Brien N, Di Tomaso E, Ayala R. et al. In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Am Assoc Cancer Res 2014: Abstr 4756. [Google Scholar]
- 7. Munster P, Hamilton E, Asano S. et al. Phase Ib study of LEE011 and BYL719 in combination with letrozole in estrogen receptor-positive, HER2-negative breast cancer (ER+, HER2− BC). ASCO 2014: Abstr 533. [Google Scholar]
- 8. Hortobagyi GN, Stemmer S, Burris H. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 9. Cortes J, Im S-A, Holgado E. et al. The next era of treatment for HR+/HER2– advanced breast cancer: clinical trials of triplet combinations. CoBrCa 2016: Abstr 179. [Google Scholar]
- 10. Oliveira M, Chavez-MacGregor M, Modi S. et al. Adding ribociclib to everolimus and exemestane in ER+/HER2– advanced breast cancer: feasibility and possible benefits. CoBrCa 2016: Abstr 141. [Google Scholar]
- 11. Juric D, Ismail-Khan R, Campone M. et al. Phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. San Antonio Breast Cancer Symp 2015: Abstr P3-14-01. [Google Scholar]
- 12. Alsumidaie M, Schiemann P. Why are cancer clinical trials increasing in duration? http://www.appliedclinicaltrialsonline.com/why-are-cancer-clinical-trials-increasing-duration (20 September 2016, date last accessed).