Abstract
Aims
Cardiac resynchronization therapy with implantable defibrillator backup (CRT-D) improves outcomes, but predictors and markers of response remain limited. Physical activity information collected by CRT devices may provide insights to CRT response and the relationship between activity changes and survival.
Methods and results
Patients entered into the LATITUDE remote monitoring system from 2008 to 2012 after receipt of a new CRT-D were eligible. Mean daily activity was calculated from LATITUDE uploads at baseline (first 3–10 days following implant) and 6 months (180–210 days). Pairwise differences for baseline—6-month activity were calculated, and survival according to quintiles of 6-month activity change was assessed. Cox regression was used to examine the adjusted association between survival and baseline–6-month activity change. A total of 26 509 patients were followed for a median of 2.3 years (mean age 70.2 ± 11.0 years, 70.7% male). Mean baseline activity was 66.2 ± 47.7 min/day, with mean paired increase at 6 months of 37.1 ± 48.2 min/day [95% CI (confidence interval), 36.5–37.6, P < 0.0001], though 15.5% of patients did not improve or worsened at 6 months. Survival at 3 years was significantly higher in the largest baseline—6-month activity change quintile vs. the lowest quintile (88.9% vs. 62.1%, log-rank P-value < 0.001). Adjusted for age and gender, higher 6-month activity change was associated with a lower risk of death (adjusted hazard ratios 0.65 per 30 min increase in activity, 95% CI, 0.63–0.67).
Conclusions
Change in physical activity between baseline and 6 months following CRT implantation is strongly associated with survival.
Keywords: Cardiac resynchronization therapy, Heart failure, Outcomes research, Remote monitoring, Exercise
What's new?
This study evaluated physical activity information collected automatically by patients' devices to describe activity patterns following cardiac resynchronization therapy (CRT) implantation.
A total of 26 509 patients were followed for a median of 2.3 years with mean paired increase in activity at 6 months of 37.1 ± 48.2 min/day (95% CI, 36.5–37.6, P < 0.0001), though 15.5% of patients did not improve or worsened.
Survival at 3 years was significantly higher in the largest baseline–6-month activity change quintile vs. the lowest quintile (88.9% vs. 62.1%, log-rank P-value < 0.001).
After adjustment for age and gender, higher 6-month activity change was associated with a lower risk of death (adjusted HR 0.65 per 30 min increase in activity, 95% CI, 0.63–0.67).
Thus, device-detected activity patterns may improve characterization of CRT response.
Introduction
Cardiac resynchronization therapy (CRT) improves survival, reduces heart failure hospitalizations, and improves quality of life for patients with persistent systolic heart failure and electromechanical dyssynchrony in spite of optimal medical therapy.1 Yet despite the clinical trial and real-world success of CRT, it is commonly reported that a substantial proportion of CRT recipients do not respond.2 Recent studies evaluating CRT in patients with milder heart failure, in whom adverse events are less frequent, included echocardiographic parameters, heart failure scores, and events such as diuretic escalation alongside traditional measures such as hospitalizations and mortality.3,4 This range of endpoints challenges attempts to find a consistent definition of ‘response’ and characterization of clinical trajectories after device implantation. Thus, an objective, quantifiable, and robust measure of CRT response applicable across the spectrum of heart failure severity may provide new insights into patients' response to treatment.
Many cardiac implantable electrical devices automatically collect patient physical activity information through analysis of embedded accelerometer data.5 Prior analyses in implantable cardioverter-defibrillator (ICD) patients, including many with CRT devices (CRT-D), have identified an inverse relationship between device-adjudicated activity and mortality.6,7 Other studies evaluating activity alongside other device-based diagnostics have shown promise in predicting events in CRT patients, but with relatively small cohorts that provided only limited descriptions of activity patterns.8 Activity quantification might provide a robust, continuous measure of CRT response closely aligned with the treatment goal of relieving heart failure symptoms and improving or preserving patients' functional status.
The goals of this study were to characterize patient activity patterns from CRT devices and their relationship to survival following implantation. Specifically, we hypothesized that changes in physical activity between the time of the implant procedure and 6 months post-implant would be associated with subsequent survival.
Methods
Data source
This study analysed data from the ALTITUDE registry, the details of which have been published previously.7 In brief, the ALTITUDE registry was created in 2008 with the goal of prospectively analysing data drawn from CRT and ICD devices enrolled in the LATITUDE clinical remote monitoring system (Boston Scientific Corp., Marlboro, MA, USA). All new Boston Scientific ICD and CRT-D devices have been eligible for LATITUDE enrolment since 2006. The remote system consists of a base station capable of device interrogation and transmission that is placed in the patient's home, with data collection performed either on a patient-initiated basis or automatically via wireless telemetry, depending on the specific device model. Enrolment in the remote follow-up system is made at the discretion of the implanting physician at the time of device implantation or at routine post-implantation follow-up clinic visits.
ALTITUDE studies are conducted using de-identified data from the LATITUDE network. Investigator-initiated proposals to ALTITUDE are reviewed by an independent physician leadership panel, and projects with sufficient scientific merit are supported. Several prior studies have successfully queried the ALTITUDE database to assess arrhythmic events and survival.7,9
This study was reviewed by the institutional review board at the Hebrew SeniorLife Institute for Aging Research.
Study population
Patients receiving new CRT-D devices from 1 January 2008 to 31 December 2012 who were entered into the LATITUDE network were eligible for inclusion. This window was selected to accord with introduction of an ICD software platform that supported daily recording and upload of patient activity. Study follow-up ended on 1 March 2013 to allow collection of baseline activity information for all included subjects, while also providing sufficient lag time for reporting of deaths into the National Death Index. Patients without a compatible CRT platform, missing demographic information (including social security numbers), <6 months of follow-up, or without usable patient activity data were excluded from analysis (Figure 1). Thus, patients who died prior to reaching 6 months of follow-up were excluded. Most exclusions for unusable activity data arose from cases with infrequent transmissions to LATITUDE and truncated device memory, leading to loss of historical activity information.
Figure 1.
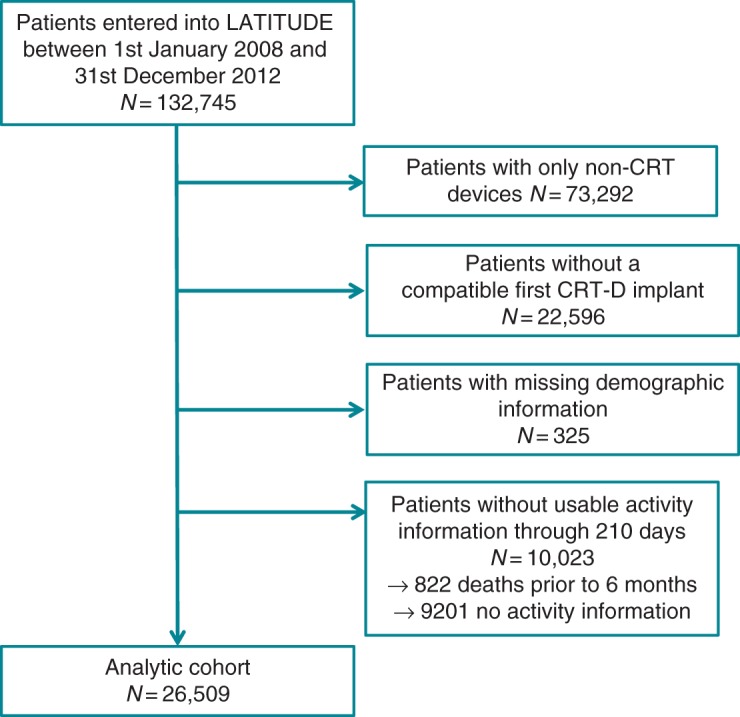
Derivation of study cohort.
Patient activity
Patient activity in Boston Scientific devices is measured through an integrated circuit accelerometer embedded in the pulse generator itself, which in applicable patients can also be used for rate responsive pacing. The accelerometer detects both the frequency and the amplitude of patient motion, and translates this into a proportional electrical signal. A proprietary algorithm interprets this signal and specifies whether the sensor exceeds a threshold of 25 milligravities, corresponding to an approximate walking speed of 2 miles/h, in order to determine a state of ‘active’ or ‘not active’ for a given minute. The sensor maintains a log of the percent of time a patient is considered active or not active daily for each 24 h period. The device models marketed during the study period are capable of storing up to one year of daily patient activity data. At each LATITUDE upload, all available activity data are uploaded. Thus, for our analysis, complete patient activity data were available for each patient with at least one upload per 12-month rolling period.
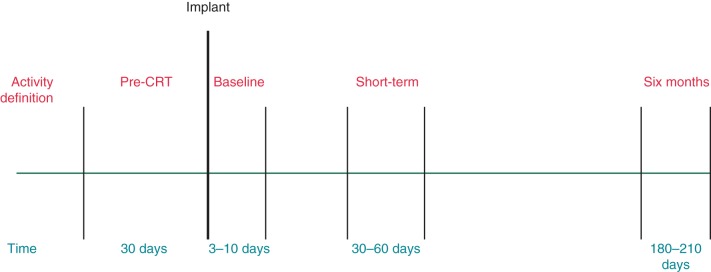
For our analysis, we defined ‘baseline’ patient activity as the mean minutes per day considered active in the first 3–10 days following implant (Figure 2). This was selected to allow procedural recovery (including possible lead revision procedures) balanced against the analytic focus on possible increases in activity from improved heart failure symptoms from the CRT implantation itself. In addition, we defined ‘short-term’ activity as the mean minutes per day in the 30- to 60-day period following implantation, and ‘6-month’ activity as the mean minutes per day in the 180- to 210-day period following implantation (Figure 2).
Figure 2.
Definitions of activity ‘windows’ according to timing relative to implantation of CRT device.
Finally, for a pre-specified subgroup analysis, we identified those patients with a prior non-CRT ICD with usable activity information who received a new CRT device in the study period. For these patients, we characterized their ‘pre-CRT’ activity as the mean minutes per day in the 30 days prior to their CRT implantation.
Survival
Vital status and, when applicable, date of death were drawn from the Boston Scientific Data Tracking database, which monitors patient's clinical status through two complementary means. First, the Tracking database is linked to the National Death Index, which allows for rolling updates of patient's vital status. For our study, we selected 1 March 2013 as our last follow-up date to allow for a potential lag in reporting. In addition, deaths reported to Boston Scientific as part of routine patient care are included on the Boston Scientific Tracking database records. Patient follow-up was censored at the date of death, the last LATITUDE transmission, or end of study.
Additional variables
Demographic variables captured in the ALTITUDE registry include age at device implant and sex. Device-specific details included the date of implant and the presence of a prior implant.
Statistical analysis
For the main analysis, all baseline demographic data, clinical information, and procedural variables were described using frequencies for categorical variables and means/medians with SDs/interquartile ranges for continuous variables. For baseline (3–10 days), short-term (30–60 days), and 6-month (180–210 days) patient activity, we identified the mean ± SD minutes per day active. Next, we calculated the pairwise differences between baseline and short-term activity, and baseline and 6-month activity. We then divided the cohort into quintiles according to the baseline—6-month activity difference and summarized activity levels as well as baseline characteristics for each quintile. Patient characteristics across these quintiles were compared using ANOVA for continuous variables or χ2 test for categorical variables.
Unadjusted survival for the overall cohort and each baseline—6-month activity difference quintile was evaluated with the Kaplan–Meier method, with calculation of hazard ratios (HR) and 95% confidence intervals (CIs) using the log-rank test for association between quintile and survival with 4 df. To further evaluate the relationship between change in patient activity and survival, we used Cox regression and considered change in activity from baseline to 6 months as a continuous variable with an incremental change of 30 min/day considered the unit of analysis. This model included age, gender, prior ICD implant, and baseline (days 3–10) minutes per day of activity, yielding adjusted hazard ratios (AHR) with 95% CIs.
For our pre-specified subgroup analysis of patients upgraded to CRT, we identified their baseline demographic and clinical characteristics similarly to the overall cohort. We also calculated the mean ± SD of ‘pre-CRT’ activity from the 30 days prior to the CRT implant and performed pairwise comparisons between pre-CRT activity and activity at 6 months. In this upgrade group, we again evaluated the relationship between activity changes (pre-CRT to 6 months) and survival using Cox regression with adjustment for age, gender, and pre-CRT activity level.
Results
Baseline characteristics
Of 132 745 patients enrolled in the ALTITUDE database during the study period, 26 509 CRT-D recipients were eligible for analysis (Figure 1). The median follow-up time for this cohort was 2.3 years (25–75% IQR, 1.4–3.4 years). Table 1 illustrates the characteristics of the overall cohort, which had a mean age of 70.2 ± 11.0 years, was 70.7% male and included 10.7% with a previous ICD implanted. Baseline (days 3–10) mean physical activity for the entire cohort was 66.2 ± 47.7 min/day.
Table 1.
Demographic and clinical characteristics of the entire study population and stratified by quintile of activity change from baseline and at 6 months of follow-up
| Characteristic (n) | Entire study (26 509) | Quintile 1 (5301) | Quintile 2 (5302) | Quintile 3 (5303) | Quintile 4 (5301) | Quintile 5 (5302) | P-value* |
|---|---|---|---|---|---|---|---|
| Age | 70.2 ± 11.0 | 72.0 ± 10.8 | 72.8 ± 9.9 | 71.2 ± 10.4 | 69.3 ± 10.7 | 65.6 ± 11.6 | <0.0001 |
| Male (%) | 70.7 | 77.7 | 70.7 | 67.9 | 67.4 | 69.8 | <0.0001 |
| Prior ICD implant (%) | 10.7 | 13.5 | 12.6 | 10.2 | 9.0 | 7.9 | <0.0001 |
| Mean activity (min/day) at baseline | 66.2 ± 47.7 | 79.2 ± 64.0 | 55.9 ± 40.6 | 60.3 ± 39.8 | 64.4 ± 40.3 | 71.0 ± 45.5 | <0.0001 |
| Change in activity baseline → 6 months mean min/day (range) | 37.1 ± 48.2 (−770 to 1006) | −13.8 ± 26.1 (−770 to 4.1) | 12.3 ± 4.7 (4.1–20.4) | 29.1 ± 5.3 (20.4–38.7) | 51.0 ± 7.9 (38.7–65.9) | 106.8 ± 50.0 (66.0–1006) | <0.0001 |
ICD, implantable cardioverter-defibrillator; CRT, cardiac resynchronization therapy.
*P-value for comparison across quintiles by ANOVA.
Activity patterns following cardiac resynchronization therapy implantation
Figure 3 demonstrates the distribution of 6-month activity changes across the entire cohort. Short-term (days 30–60) mean physical activity for the entire cohort was 99.7 ± 60.9 min/day, and activity at 6 months (days 180–210) further increased to 103.2 ± 65.6 min/day. Mean paired differences between baseline and 6-month activity was 37.1 ± 48.2 min/day (95% CI, 36.5–37.6, P < 0.0001). In contrast, mean paired differences between 30 and 60 days and 6 months was only 3.5 min/day (95% CI, 3.1–3.9, P < 0.0001), indicating that the majority of change seen by 6 months had already manifested by 60 days. Overall, 15.5% of patients did not improve or worsened at 6 months.
Figure 3.
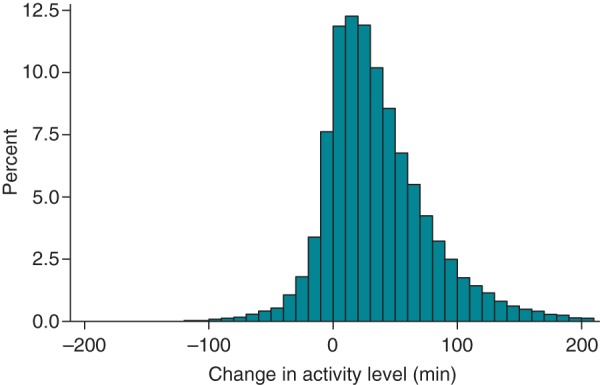
Activity changes for entire cohort, measured as the difference in activity between baseline (average minutes per day over days 3–10) and 6 months (average minutes per day over days 180–210).
Quintiles of baseline–6-month activity change and associated patient characteristics are shown in Table 1. Paired activity changes varied from a decrease in mean activity of 13.8 ± 26.1 min/day (range: −770 to 4.1 min/day) in the lowest quintile to an increase of 106.8 + 50.0 min/day (range: 66–1006 min/day) in the top quintile (P < 0.0001 for trend across quintiles). Patients in the highest vs. lowest quintiles of 6-month activity change tended to be younger (65.6 ± 11.6 vs. 72.0 ± 10.8 years, P < 0.0001) and were less likely to have a prior ICD (7.9% vs. 13.5%, P < 0.0001).
Relationship between change in activity and survival
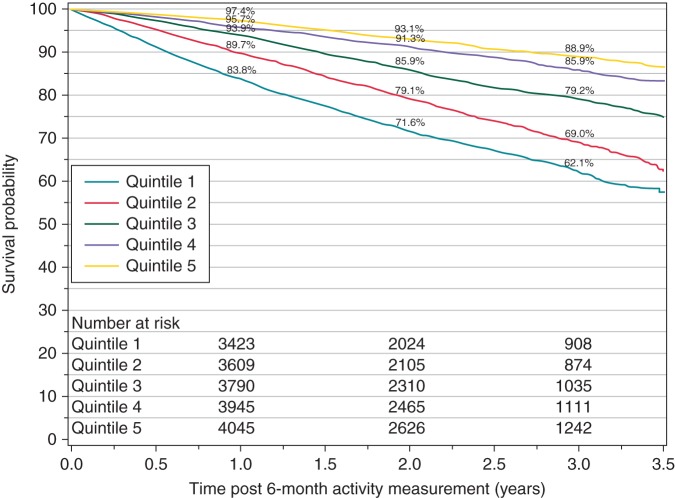
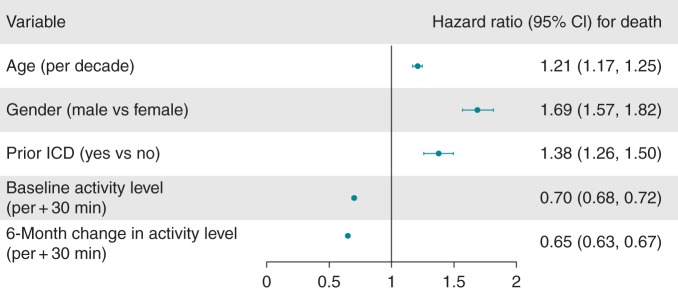
Unadjusted survival from 6-month post-CRT-D implant in the highest vs. lowest quintile of activity change (baseline—6 months) was 97.4% vs. 83.8% at 1 year, and 88.9% vs. 62.1% at 3 years (log-rank P < 0.0001 for overall survival across quintiles, Figure 4). After adjustment for age, gender, and baseline activity, 6-month activity change remained associated with a lower risk for death (AHR 0.65 per 30 min increase in activity, 95% CI, 0.63–0.67; Figure 5).
Figure 4.
Survival and number of patients at risk for study population stratified by quintile of activity change between baseline (average min/day over days 3–10) and 6 months (average min/day over days 180–210).
Figure 5.
Multivariate analysis of patient characteristics and survival following CRT-defibrillator implantation. Baseline and 6-month change in activity level HR describe hazard for death assumed with every 30 min increase in activity, adjusted for other factors.
Subgroup analysis of patients upgraded to cardiac resynchronization therapy
The pre-specific subgroup of patients upgraded to CRT whose prior ICD had been entered in ALTITUDE included 932 patients (mean age 70.0 ± 10, 80% male). The mean physical activity pre-CRT (30–60 days prior to CRT implant) for this group was 100.0 ± 60.1 min/day. Mean paired differences between pre-CRT and 6-month activity showed a decrease of 8.1 min/day and ranged from a decrease in 67.6 ± 49.7 in the lowest quintile to an increase of 42.7 ± 29.5 in the highest quintile. After adjustment for age and gender, mortality was lower for every 30 min increase in both pre-CRT activity (HR 0.68, 95% CI, 0.6–0.77, P < 0.0001) and 6-month activity change (HR 0.73, 95% CI, 0.62–0.86, P = 0.0001).
Discussion
This report provides a large, nationwide assessment of activity patterns following CRT implantation. Overall, physical activity following CRT implantation improved by an average of >30 min/day, but varied widely, with 15.5% of patients showing diminished activity at 6 months. We identified a strong association between activity changes at 6 months following CRT implantation and survival, with a 26% absolute difference in survival after only 3 years (88.9% vs. 62.1%) between quintiles with the highest and lowest improvement in activity. This relationship remained significant after adjustment for age and gender, and extended to our subgroup analysis of patients for whom their CRT implantation procedure was an upgrade from a single- or dual-chamber system.
This work extends prior assessments of physical activity among CRT recipients in several ways. The PARTNERS-HF study evaluated 694 patients with CRT-D according to a diagnostic algorithm that incorporated low patient activity (<1 h/day) with several other device-detected arrhythmic and autonomic markers into a heart failure prediction model demonstrating a five-fold increase in risk for clinical events associated with pre-specified thresholds.8 However, activity alone and changes in activity over time were not described in this study, which was not powered to evaluate survival. Vegh et al. 10 also identified an association between physical activity and heart failure hospitalizations in a single-centre study of 164 CRT patients, adjusted for clinical factors and medication use. Our study builds on the prior by providing more detail regarding the time course of activity changes with sufficient power to evaluate survival as an outcome.
In addition, our study linking device-detected activity to outcomes aligns with other work evaluating the mechanisms of CRT response in terms of exercise physiology. One study identified decreased isovolumic time and increased filling time as predictors of improved exercise capacity following CRT.11 Another small crossover assessment of CRT's impact on exercise physiology suggested that CRT did not affect resting haemodynamics but improved exercise duration.12 In a sub-study of the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure study, CRT improved 6-min walk testing and quality of life at 6 months without changing V02 max.13 Our work expands on these physiologic assessments to suggest that physical activity following CRT implantation reflects both the underlying cardiomyopathy and response to treatment.
More broadly, our study builds on attempts to characterize, predict, and improve response to CRT,14 particularly following a shift in clinical investigation towards patients with less severe CHF symptoms, in which clinical composite scores are increasingly utilized15 despite their limitations. Indeed, the distribution of improvement and worsening in activity we identified roughly mirror clinical responses noted in earlier trials of CRT, further supporting the use of these data as a meaningful surrogate for more challenging endpoints such as V02 max testing.16 Prospective studies seeking echocardiographic determinants of CRT response have not been fruitful,17 but may similarly be limited based on an unnecessarily narrow view of positive outcomes from CRT implantation. For healthier patients, in particular, survival and prevention of heart failure hospitalizations, or substantial changes in health status, may be too uncommon for practical use in clinical studies. New York Heart Association Class is commonly utilized for initial eligibility for CRT and in describing response, but is notoriously unreliable. Physical activity measurement, however, provides a granular and continuous measure that closely aligns with one of the primary goals of CRT, specifically improvement or maintenance of functional status. Thus, the association we have identified between activity changes and survival may support future prospective validation of device-detected activity measurement as a CRT response metric. In addition, the timing of activity changes we identified—with most of the improvement manifest at 60 days—may inform strategies for optimization including A-V and V-V timing or even lead revision. The ideal time to intervene in patients for whom ‘non-response’ to CRT is suspected on more global clinical grounds remains elusive, and the timing of functional changes we identified accords with smaller scale studies assessing improvements in autonomic18 and neurohormonal19 measures.
Our study has several potential limitations. We limited our cohort to patients who survived to 6 months in order to provide analytic focus on this medium-term time period, while excluding those patients with severe life-limiting conditions, in accord with recommendations to defer device implantation in patients not expected to survive for at least a year or more with a good quality of life. External validation of device-detected activity measurement remains relatively limited, and our study cohort included only a single manufacturer's CRT devices. Our models evaluating the relationship between activity changes and survival included a small number of covariates, and did not include data such as QRS morphology or echocardiographic measurements, both of which influence outcomes.20–22 Prospective studies evaluating activity will need to account for CRT indications and clinical variables including QRS morphology and echocardiographic parameters.
In addition, causality cannot be inferred from our observed association, and thus whether activity primarily influences survival or is merely similarly related to other important factors such as heart failure severity cannot be determined from this analysis. Our observation that the lowest quantile of activity change at 6 months had the highest baseline activity levels and that the upgrade subgroup overall experienced a decrease in activity, may reflect regression to the mean. Similarly, though we observed a dose–response relationship between activity and survival, this relationship is not linear and may reflect either a physiologic ‘threshold’ effect or positive feedback cycle from CRT response—survival for the 4th and 5th quintiles was similar despite a marked difference in activity change. Though theoretically modifiable, actual interventions targeting activity have been limited, and thus the clinical application of this measurement remains uncertain. Importantly, we did not have a non-CRT control group for comparison or a mechanism to determine cause of death, which further highlights the need for further inquiry regarding heart failure patients' clinical trajectories and activity levels as a therapeutic target.
Conclusions
In sum, device-detected activity change following CRT implantation varies widely and predicts survival. While a causal mechanism cannot be determined from this study, we suggest that physical activity may serve as a useful marker of risk among heart failure patients and may have a future role in characterizing response to CRT.
Funding
D.B.K. is supported by a Paul B. Beeson Career Development Award in Aging Research (K23AG045963). S.L.M. is supported by NIH-NIA K24AG033640. The ALTITUDE study is supported by Boston Scientific.
Conflict of interest: T.R.: employment—North American Science Associates, Inc.; P.W.J.: employment—Boston Scientific; M.R.R.: Medtronic—consulting contract.
References
- 1. Boriani G, Nesti M, Ziacchi M, Padeletti L. Cardiac resynchronization therapy: an overview on guidelines. Card Electrophysiol Clin 2015;7:673–93. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol 2009;54:1837–46. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 5. Kadhiresan VA, Pastore J, Auricchio A, Sack S, Doelger A, Girouard S et al. A novel method—the activity log index—for monitoring physical activity of patients with heart failure. Am J Cardiol 2002;89:1435–7. [DOI] [PubMed] [Google Scholar]
- 6. Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M et al. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail 2014;7:279–87. [DOI] [PubMed] [Google Scholar]
- 7. Kramer DB, Mitchell SL, Monteiro J, Jones PW, Normand SL, Hayes DL et al. Patient activity and survival following implantable cardioverter-defibrillator implantation: the ALTITUDE activity study. J Am Heart Assoc 2015;4:e001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol 2010;55:1803–10. [DOI] [PubMed] [Google Scholar]
- 9. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–67. [DOI] [PubMed] [Google Scholar]
- 10. Vegh EM, Kandala J, Orencole M, Upadhyay GA, Sharma A, Miller A et al. Device-measured physical activity versus six-minute walk test as a predictor of reverse remodeling and outcome after cardiac resynchronization therapy for heart failure. Am J Cardiol 2014;113:1523–8. [DOI] [PubMed] [Google Scholar]
- 11. Jansen AH, Peels KH, Bracke F, van Dantzig JM, Meijer A, van der Voort PH et al. Relation of isovolumic times after cardiac resynchronization therapy to improvement in exercise capacity. Am J Cardiol 2007;99:75–8. [DOI] [PubMed] [Google Scholar]
- 12. Schlosshan D, Barker D, Lewis N, Pepper C, Tan LB. A mechanistic investigation into how long-term resynchronization therapy confers ongoing cardiac functional benefits and improved exercise capacity. Am J Cardiol 2009;103:701–8. [DOI] [PubMed] [Google Scholar]
- 13. De Marco T, Wolfel E, Feldman AM, Lowes B, Higginbotham MB, Ghali JK et al. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with QRS prolongation: COMPANION trial sub-study. J Card Fail 2008;14:9–18. [DOI] [PubMed] [Google Scholar]
- 14. Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol 2009;53:765–73. [DOI] [PubMed] [Google Scholar]
- 15. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–82. [DOI] [PubMed] [Google Scholar]
- 16. Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 2004;110:2864–8. [DOI] [PubMed] [Google Scholar]
- 17. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008;117:2608–16. [DOI] [PubMed] [Google Scholar]
- 18. Gilliam FR 3rd, Kaplan AJ, Black J, Chase KJ, Mullin CM. Changes in heart rate variability, quality of life, and activity in cardiac resynchronization therapy patients: results of the HF-HRV registry. Pacing Clin Electrophysiol 2007;30:56–64. [DOI] [PubMed] [Google Scholar]
- 19. Piepoli MF, Villani GQ, Corra U, Aschieri D, Rusticali G. Time course of effects of cardiac resynchronization therapy in chronic heart failure: benefits in patients with preserved exercise capacity. Pacing Clin Electrophysiol 2008;31:701–8. [DOI] [PubMed] [Google Scholar]
- 20. Bertini M, Hoke U, van Bommel RJ, Ng AC, Shanks M, Nucifora G et al. Impact of clinical and echocardiographic response to cardiac resynchronization therapy on long-term survival. Eur Heart J Cardiovasc Imaging 2013;14:774–81. [DOI] [PubMed] [Google Scholar]
- 21. Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005;112:1580–6. [DOI] [PubMed] [Google Scholar]
- 22. Hsing JM, Selzman KA, Leclercq C, Pires LA, McLaughlin MG, McRae SE et al. Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy: PROSPECT-ECG substudy. Circ Arrhythm Electrophysiol 2011;4:851–7. [DOI] [PubMed] [Google Scholar]