Abstract
Background
The absence of a survival benefit for whole brain radiotherapy (WBRT) among randomized trials has been attributed to a competing risk of death from extracranial disease. We re-analyzed EORTC 22952 to assess the impact of WBRT on survival for patients with controlled extracranial disease or favorable prognoses.
Patients and methods
We utilized Cox regression, landmark analysis, and the Kaplan–Meier method to evaluate the impact of WBRT on survival accounting for (i) extracranial progression as a time-dependent covariate in all patients and (ii) diagnosis-specific graded prognostic assessment (GPA) score in patients with primary non-small-cell lung cancer (NSCLC).
Results
A total of 329 patients treated per-protocol were included for analysis with a median follow up of 26 months. One hundred and fifteen (35%) patients had no extracranial progression; 70 (21%) patients had progression <90 days, 65 (20%) between 90 and 180 days, and 79 (24%) patients >180 days from randomization. There was no difference in the model-based risk of death in the WBRT group before [hazard ratio (HR) (95% CI)=0.70 (0.45–1.11), P = 0.133), or after [HR (95% CI)=1.20 (0.89–1.61), P = 0.214] extracranial progression. Among 177 patients with NSCLC, 175 had data available for GPA calculation. There was no significant survival benefit to WBRT among NSCLC patients with favorable GPA scores [HR (95% CI)=1.10 (0.68–1.79)] or unfavorable GPA scores [HR (95% CI)=1.11 (0.71–1.76)].
Conclusions
Among patients with limited extracranial disease and one to three brain metastases at enrollment, we found no significant survival benefit to WBRT among NSCLC patients with favorable GPA scores or patients with any histology and controlled extracranial disease status. This exploratory analysis of phase III data supports the practice of omitting WBRT for patients with limited brain metastases undergoing SRS and close surveillance.
Clinical Trials Number
Keywords: stereotactic radiosurgery, SRS, whole brain radiotherapy, WBRT, brain metastases
Introduction
Whole brain radiotherapy (WBRT) administered in addition to neurosurgery or stereotactic radiosurgery (SRS) consistently led to improved intracranial control of metastatic brain disease in randomized controlled trials but without an associated overall survival (OS) benefit [1–5]. Both the efficacy of salvage therapy and the competing risk of death from extracranial disease have been put forward to explain this observation [5, 6].
The relative importance of these two factors has significant clinical implications. If there were perfect salvage therapy, upfront WBRT would never be indicated in addition to SRS or surgery for patients with limited metastases. If, however, competing risk from systemic disease is the prime factor that decouples improved intracranial control and OS, then there may be a patient population with limited competing systemic risk that would benefit from WBRT. In fact, re-analysis of a prior randomized trial of SRS with or without WBRT suggested such an OS benefit from WBRT for patients with good prognostic grading [6].
The European Organization for Research and Treatment of Cancer (EORTC) 22952-26001 study is the largest prospective trial of SRS or neurosurgery with or without WBRT in the setting of limited brain metastases, finding no impact of WBRT on OS [4]. Furthermore, EORTC 22952 was unique in requiring stable extracranial disease or brain only metastases at enrollment, and is thus enriched for patients with a potentially reduced risk of death from non-CNS causes at enrollment [4]. While this population with seemingly low competing risk from systemic disease had no benefit in the overall population, we re-analyzed EORTC 22952 to determine whether WBRT improved OS for patients with controlled extracranial disease following treatment and for patients with favorable prognostic factors.
Methods–patients
Study design
This is an unplanned secondary analysis of the phase III EORTC 22952-26001 trial which randomized patients with one to three brain metastases with stable systemic disease or asymptomatic primary tumors with brain only metastasis to adjuvant WBRT or observation after SRS or surgical resection [4]. Eligibility criteria included patients aged 18 years or older, WHO performance status ≤2 at baseline, one to three brain metastases, and stable systemic cancer for 3 months or asymptomatic synchronous primary tumor without metastases outside the CNS. Maximum tumor sizes were 3.5 cm for single and 2.5 cm for multiple metastases. The original protocol was approved by ethics committees at participating institutions and all patients provided written informed consent. This secondary analysis was approved by the EORTC and the Fox Chase Cancer Center institutional review board.
Intervention
WBRT was administered to 30 Gy in 10 fractions. SRS was prescribed to 25 Gy at the center of the metastases, with a minimum dose of 20 Gy to the surface of the planning target volume (1–2 mm margin in addition to gross tumor volume). Patients undergoing SRS completed a short steroid taper around the time of their procedure. Surgical patients underwent complete resection with the extent of resection assessed similarly for all patients of a single institution. CT/MRI were strongly recommended.
Statistical analysis
Baseline characteristics of patients were compared across treatment arms using Chi-squared tests. The primary analysis population was defined as ‘as treated per-protocol’, i.e. patients who actually received/did not receive WBRT in accordance with their randomly assigned treatment (supplementary Figure S1, available at Annals of Oncology online). In secondary analyses, we also considered ‘as treated’ and ‘intent to treat’ populations; analyses and definitions of populations are listed in the supplementary data, available at Annals of Oncology online. We used Kaplan–Meier curves to estimate survival functions in patient groups defined by receipt of WBRT, and compared survival between groups using log-rank tests. We used Cox proportional hazards regression to adjust for important patient factors. We included time to extracranial progression as a time-dependent covariate, and estimated hazard ratios (HRs) for WBRT both before and after extracranial progression. For context, we also created cumulative incidence curves for extracranial progression in a competing risks framework. We further carried out a landmark analysis, examining survival in the subset of patients who survived without extracranial progression to at least 6 months. Diagnosis-specific GPA values were calculated for patients with non-small-cell lung cancer (NSCLC) and dichotomized as favorable (GPA 2.5–4.0) or unfavorable (GPA 0.5–2.0) prognoses to analyze the relationship of prognosis and WBRT and to compare results with prior studies [6]. For the GPA calculation, we considered the following performance status conversion: ECOG/WHO 0 = KPS 90%–100%, ECOG/WHO 1 = KPS 70–80, and ECOG/WHO 2 = KPS < 70 [7]. Statistical significance was defined as P < 0.05. Analyses were carried out using SAS (version 9.4) and Stata (version 12.1) statistical software.
Results
Overall, a total of 329 patients were included in the analysis (supplementary Figure S1, available at Annals of Oncology online). The median follow-up among living patients was 26 months. Baseline patient characteristics reflected the original publication (Table 1) [4]. A total of 115 (35%) patients had no extracranial progression during study follow-up, while 70 (21%) patients had extracranial progression <90 days, 65 (20%) between 90 and 180 days, and 79 (24%) patients >180 days.
Table 1.
Baseline patient characteristics according to per-protocol receipt of whole brain radiotherapy (WBRT)
| No WBRT | WBRT | P | |
|---|---|---|---|
| Age (median) | 60.0 | 60.6 | 0.30 |
| Local therapy | |||
| Complete surgery | 78 | 77 | 0.95 |
| Radiosurgery | 87 | 87 | |
| ECOG performance status | |||
| 0 | 77 | 65 | 0.44 |
| 1 | 71 | 80 | |
| 2 | 17 | 19 | |
| Primary site | |||
| Breast | 16 | 17 | 0.94 |
| Colo-rectum | 15 | 14 | |
| Kidney | 13 | 16 | |
| Lung | 88 | 89 | |
| Melanoma | 7 | 8 | |
| Other/unknown | 26 | 20 | |
| Number of metastases | |||
| 1 | 131 | 133 | 0.93 |
| 2 | 25 | 23 | |
| 3 | 9 | 8 | |
| Macroscopic tumor outside brain | |||
| Absent | 80 | 75 | 0.87 |
| Present | 78 | 81 | |
| Unknown | 7 | 8 | |
| Extracranial progression | |||
| No | 60 | 55 | 0.59 |
| Yes | 105 | 109 | |
A total of 175 NSCLC patients had data available for GPA calculation; 101 (58%) had favorable diagnosis-specific GPA scores. The distribution of GPA among NSCLC patients was 3.5–4.0 (13), 2.5–3.0 (88), 1.5–2.0 (65), and 0.5–1.0 (9). Baseline patient characteristics for NSCLC patients stratified by GPA are listed in supplementary Table S1, available at Annals of Oncology online.
Time to extracranial progression
There was no significant difference in time to extracranial progression according to the receipt of WBRT (supplementary Figure S2, available at Annals of Oncology online). OS according to receipt of WBRT before extracranial progression yielded similar estimates of survival in the two groups (Figure 1A). In the model-based risk of death, WBRT was not statistically significantly associated with mortality either before extracranial progression [HR = 0.70, 95% CI = (0.45, 1.11)] or following extracranial progression [HR = 1.20, 95% CI = (0.89, 1.61)] (Table 2). Baseline patient characteristics of the intention to treat and as treated analyses are listed in supplementary Tables S2 and S5, available at Annals of Oncology online, respectively. Unadjusted OS in the intention to treat and as treated analyses produced findings similar to the per-protocol analysis (supplementary Figures S3 and S5, available at Annals of Oncology online). Model-based risk of death for WBRT did not differ significantly either before extracranial progression or following extracranial progression in intention to treat (supplementary Table S3, available at Annals of Oncology online) or as treated (supplementary Table S6, available at Annals of Oncology online) populations.
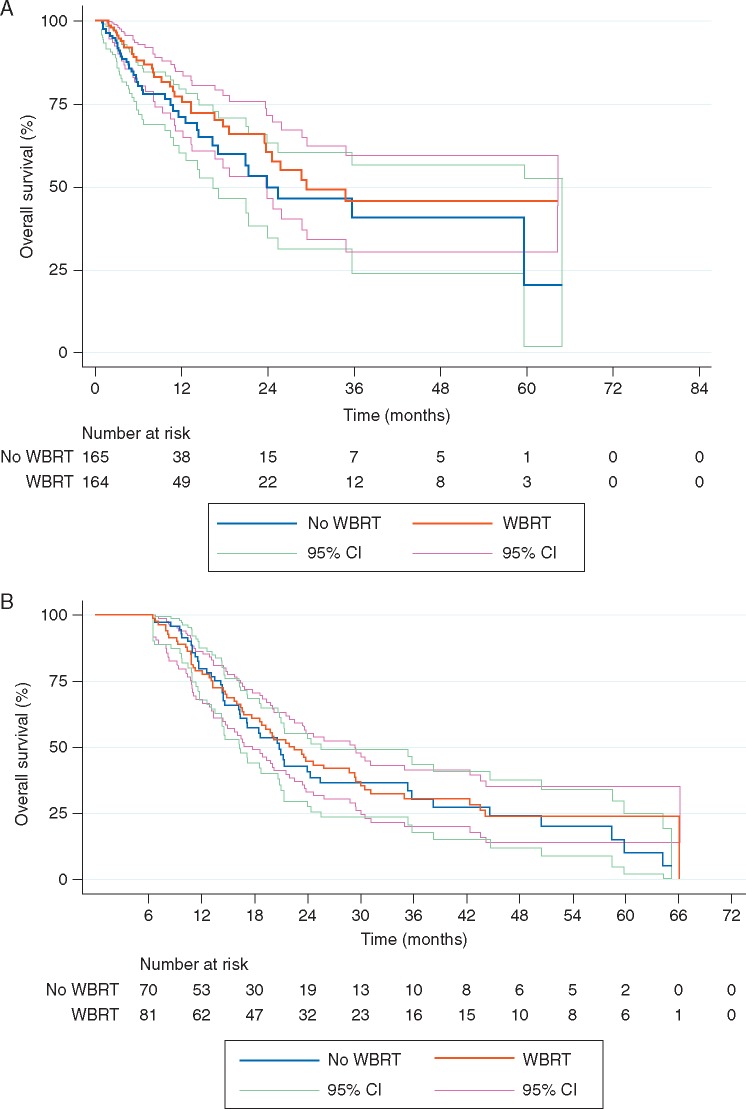
Figure 1.
(A) Overall survival (OS) according to receipt of whole brain radiotherapy before extracranial progression (per-protocol analysis). Patients were censored at the time of follow up or extracranial progression. Thus, this plot displays the survival effect of WBRT among patients without evidence of extracranial progression (estimated from all patients, using their extracranial progression free interval). (B) Landmark analysis of OS according to the receipt of whole brain radiotherapy (WBRT). The landmark represented the absence of extracranial progression at 6 months. Patients with events before 6 months were excluded from analysis.
Table 2.
Cox regression analysis of overall survival according to per-protocol receipt of whole brain radiotherapy
| Parameter | Hazard ratio | [95% CI] | P |
|---|---|---|---|
| Whole brain radiotherapya | |||
| Before extracranial progression | 0.70 | [0.45–1.11] | 0.133 |
| After extracranial progression | 1.20 | [0.89–1.61] | 0.214 |
| Age (continuous) | 1.02 | [1.01–1.04] | <0.001 |
| Extracranial progression | 4.51 | [3.03–6.72] | <0.001 |
| Local therapy | |||
| Radiosurgery | 1.00 | Referent | – |
| Surgery | 0.86 | [0.66–1.14] | 0.293 |
| ECOG performance status | |||
| 0 | 1.00 | Referent | – |
| 1 | 1.08 | [0.83–1.40] | 0.571 |
| 2 | 1.70 | [1.13–2.55] | 0.011 |
| Primary site lung (versus other) | 1.32 | [1.02–1.70] | 0.033 |
| Number of metastases (2–3 versus 1) | 1.09 | [0.79–1.49] | 0.609 |
| Macroscopic tumor present outside brain | |||
| Absent | 1.00 | Referent | – |
| Present | 1.10 | [0.84–1.44] | 0.490 |
| Unknown | 1.64 | [0.83–3.24] | 0.154 |
Extracranial progression was included as a time-dependent covariate.
Reference group = arms randomized to no whole brain radiotherapy.
Landmark analysis
The time to systemic progression with death as a competing risk (i.e. the cumulative incidence of systemic progression over time) is shown in supplementary Figure S2, available at Annals of Oncology online. The impact of WBRT on OS for patients with controlled systemic disease at 6 months (landmark) following randomization is shown in Figure 1B. Patients who died within 6 months following randomization were excluded to limit bias. There was no impact of WBRT on survival among patients with controlled disease at 6 months (P = 0.51).
NSCLC diagnosis-specific GPA
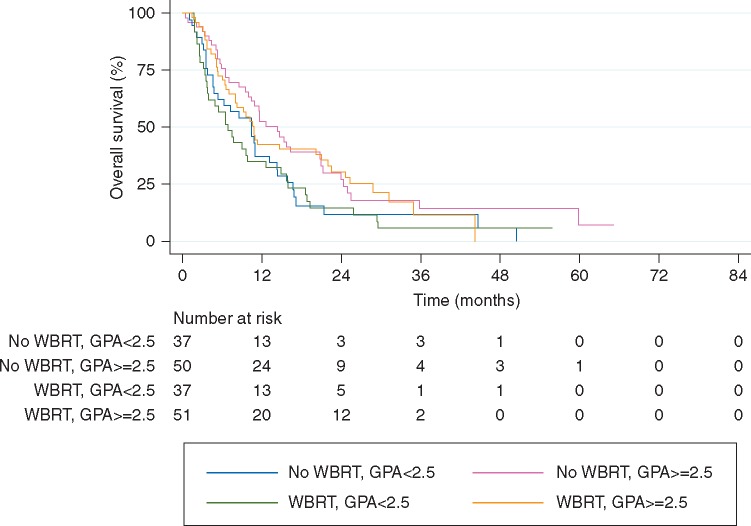
OS did not vary according to the use of WBRT in NSCLC patients with either favorable or unfavorable GPA scores (Table 3; Figure 2) in the per-protocol analysis. Analysis according to intention to treat (supplementary Table S4 and Figure S4, available at Annals of Oncology online) and as treated (supplementary Table S7 and Figure S6, available at Annals of Oncology online) showed similar findings.
Table 3.
Cox regression analysis of overall survival according to the per-protocol receipt of whole brain radiotherapy and diagnosis-specific graded prognostic assessment score for lung cancer patients
| Parameter | Hazard ratio | [95% CI] | P |
|---|---|---|---|
| Whole brain radiotherapya | |||
| GPA <2.5 | 1.10 | [0.68–1.79] | 0.690 |
| GPA ≥2.5 | 1.11 | [0.71–1.76] | 0.641 |
| Local therapy | |||
| Radiosurgery | 1.00 | Referent | – |
| Surgery | 0.96 | [0.69–1.35] | 0.828 |
| GPA ≥2.5 | 0.64 | [0.40–1.03] | 0.067 |
Reference group = arms randomized to no whole brain radiotherapy and treated per-protocol.
Figure 2.
Overall survival among non-small-cell lung cancer (NSCLC) patients according to receipt of whole brain radiotherapy stratified by diagnosis-specific Graded Prognostic Assessment (GPA) score (per-protocol). NSCLC patients (n = 175) were stratified according to GPA scores of <2.5 (unfavorable) and ≥2.5 (favorable).
Discussion
No phase III randomized trial has demonstrated that the improved intracranial control resulting from the addition of WBRT to SRS or surgery for patients with limited brain metastases translates into a survival benefit. One potential reason for this disconnect may be the competing risk of death from systemic disease, which may dilute or eliminate any benefit from WBRT [5, 6]. If progressive systemic disease leads to death before intracranial progression, improving intracranial control would not have any effect on survival. Even in cases where intracranial progression would have been the proximal cause of death, if systemic disease is progressive on a similar time scale, improved intracranial control might merely trade one cause of death for another or provide only marginal benefit that might be diluted in a population. Furthermore, salvage therapy need not be perfect; rather it only requires extending the time line of intracranial progression beyond that of systemic disease in order to have no demonstrated benefit from improved initial CNS control or quality of life. WBRT treats the possibility of microscopic disease, marginally improves control of known disease in addition to local therapy, and may not address future metastatic disease to the brain. Thus the risk of death from systemic progression must be relatively low compared with that of microscopic CNS disease in order to derive a benefit from WBRT. Though the diagnosis of brain metastasis is frequently perceived as the portent of a grim prognosis, systemic disease status and its response to therapy is typically the driver of mortality [8, 9]. Retrospective analyses have shown that the risk of extracranial disease progression is a major prognostic factor [10, 11]. In fact, the Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) prognostic index, derived from pooled RTOG trial data in brain metastases, had two of four prognostic factors (controlled primary and brain only metastases) related to risk from systemic progressive disease [12].
While several other randomized studies showed no survival benefit from the addition of WBRT to ablative local therapy in oligometastatic disease, the EORTC 22952 was unique in allowing only patients with no evidence of progressive extracranial disease on study. While this eligibility criterion may have constituted an ideal population from which to isolate a survival signal from WBRT given the hypothetically decreased risk from systemic disease, no improved survival was seen with increased intracranial control [4]. In fact, in spite of the restrictive eligibility criterion, ∼40% of patients experienced extracranial progression by 6 months. We hypothesized that if the patients that were truly at low risk for disease progression could be identified, perhaps a benefit from WBRT would be discovered. In order to answer this question, we conducted a landmark analysis for patients that had not progressed by 6 months, intending to identify factors that predicted systemic stability should there have been a benefit to WBRT in that group. But instead we found no improved survival for patients that had not progressed systemically. There was no difference in risk of death related to WBRT before extracranial progression nor was WBRT associated with survival after progression of ECD (HR = 0.70, P = 0.133; HR = 1.20, P = 0.214, respectively).
Perhaps extracranial disease is not the only factor that may interact with a potential benefit from WBRT and patients with other good prognostic factors might benefit. Aoyama et al. showed in a post hoc analysis of the JROSG 99-1 trial that there was longer survival associated with WBRT for patients with NSCLC and a favorable graded prognostic assessment (GPA) score (2.5–4.0) at diagnosis but not for those with a poor GPA. The GPA is a more contemporary prognostic index that reflects the heterogeneity in survival between and within primary cancers better than RPA [13]. In addition to extracranial disease status, age, performance status, and number of brain metastases factor in to the NSCLC GPA used in re-analysis.
Contrary to the JROSG re-analysis, however, we found no survival associations with WBRT for NSCLC cancer patients with a favorable GPA at time of randomization (Table 3; Figure 2). Again, replicating the analysis in the intention to treat and as treated populations yielded similar estimates (supplementary Tables S4 and S7, available at Annals of Oncology online, respectively).
There are several possible reasons for these discordant findings. The original EORTC 22952 trial was not powered to detect an OS difference. Sample size was small in both our unplanned analysis (n = 101) and the JROSG secondary analysis (n = 47). We considered the possibility that the current analysis may lack power and constitute a type II error. Because post hoc power calculations are flawed [14], we directly compared the estimates of benefit for the two analyses. The JROSG analysis reported an increased risk of death for patients with DS-GPA >2.5 who did not receive WBRT, with an HR of 1.92, which is equivalent to an HR of 0.52 for receipt of WBRT. This magnitude of benefit from WBRT was outside of the lower bound 95% confidence interval of our estimate [HR (95% CI) = 1.11 (0.71–1.76)] among the comparable favorable DS-GPA patient group. In fact the HR for death in our population was slightly over 1, a non-significant trend towards a detriment with WBRT. Therefore, while a false negative error certainly remains a possibility in our analysis, a large benefit with a similar magnitude to that seen in the JROSG study is unlikely.
Differences in the populations studied and the biology of disease may also play a role in the discordant findings. JROSG 99-1 included patients from a consortium of 11 Japanese centers. The biologic heterogeneity of NSCLC cancer between Asian and non-Asian populations has been well documented, specifically in the incidence of epidermal growth factor receptor (EGFR) tyrosine kinase mutations [15]. It is conceivable that the ability to detect a survival benefit from WBRT among patients with NSCLC and a favorable GPA in JROSG-99-1 reflects an enriched population of EFGR-mutated cancers with different patterns of failure and responses to systemic therapy, which in turn permit superior upfront detection of patients who will benefit from WBRT. That is, biologic differences may account for an actual survival benefit seen for WBRT in the Japanese analysis but not in the European one. Perhaps the transition to a molecularly driven lung-specific GPA (Lung-molGPA) [16, 17] would have helped normalize the differences between the JROSG and our study. While our model-based analysis using progression of extracranial disease as a time-dependent covariate and landmark analysis may mitigate some differences between the populations in terms of risk of systemic progression, the availability of and response to systemic therapy after progression for EGFR mutant tumors is not accounted for in this analysis.
Finally, the results of either or both analyses may be a product of the limitations of secondary analyses. In short, they are exploratory and not designed to definitively answer the hypothesis in question. Further investigation into populations that may benefit from the upfront delivery of WBRT might include tumor molecular factors as well as other clinical factors such as the risk for leptomeningeal disease [18–22].
Conclusion
Our secondary analysis of EORTC 22952-26001 found no association of WBRT with improved survival, regardless of the competing risk from systemic disease progression and regardless of favorable prognostic factors in NSCLC. These results are discordant with another secondary analysis; further investigations are warranted to elucidate possible subgroups that might benefit from aggressive intracranial control.
Supplementary Material
Acknowledgement
The authors would like to thank the European Organization for Research and Treatment of Cancer for collaboration and sharing of trial data to perform this analysis.
Funding
This publication was supported by grant number P30 CA006927 from the National Cancer Institute and National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The contributions of SC and LC (EORTC Headquarters) to this publication were supported by Fonds Cancer (FOCA) (no grant numbers apply) from Belgium.
Disclosure
The authors have declared no conflicts of interest relevant to this work. Disclosures outside of this work include: EH: research funding through Pfizer, paid to institution; AA: funding from Varian Medical Systems; BA: consulting relationship with Abbvie, BMS, Precision Health Economics, and Schlesinger Associates.
References
- 1. Patchell RA, Tibbs PA, Regine WF. et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998; 280(17): 1485–1489. [DOI] [PubMed] [Google Scholar]
- 2. Aoyama H, Shirato H, Tago M. et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006; 295(21): 2483–2491. [DOI] [PubMed] [Google Scholar]
- 3. Chang EL, Wefel JS, Hess KR. et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10(11): 1037–1044. [DOI] [PubMed] [Google Scholar]
- 4. Kocher M, Soffietti R, Abacioglu U. et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011; 29(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown PD, Jaeckle K, Ballman KV. et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. A Randomized Clinical Trial. JAMA 2016; 316(4): 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol 2015; 1(4): 457–464. [DOI] [PubMed] [Google Scholar]
- 7. ECOG Performance Status. http://ecog-acrin.org/resources/ecog-performance-status (20 March 2017, date last accessed).
- 8. Kondziolka D, Parry PV, Lunsford LD. et al. The accuracy of predicting survival in individual patients with cancer. J Neurosurg 2014; 120(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 9. Lin NU, Claus E, Sohl J. et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008; 113(10): 2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dyer MA, Kelly PJ, Chen Y-H. et al. Importance of extracranial disease status and tumor subtype for patients undergoing radiosurgery for breast cancer brain metastases. Int J Radiat Oncol Biol Phys 2012; 83(4): e479–e486. [DOI] [PubMed] [Google Scholar]
- 11. Dyer MA, Arvold ND, Chen Y-H. et al. The role of whole brain radiation therapy in the management of melanoma brain metastases. Radiat Oncol Lond Engl 2014; 9: 143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaspar L, Scott C, Rotman M. et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys 1997; 37(4): 745–751. [DOI] [PubMed] [Google Scholar]
- 13. Sperduto PW, Berkey B, Gaspar LE. et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008; 70(2): 510–514. [DOI] [PubMed] [Google Scholar]
- 14. Hoenig JM, Heisey DM.. The abuse of power. Am Stat 2001; 55(1): 19–24. [Google Scholar]
- 15. Midha A, Dearden S, McCormack R.. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015; 5(9): 2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 16. Sperduto PW, Yang TJ, Beal K. et al. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys 2016; 96(2): 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sperduto PW, Yang TJ, Beal K. et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 2017; 3(6): 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Ree TC, Dippel DW, Avezaat CJ. et al. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry 1999; 66(2): 225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahn JH, Lee SH, Kim S. et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 2012; 116(5): 984–993. [DOI] [PubMed] [Google Scholar]
- 20. Atalar B, Modlin LA, Choi CYH. et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. JAMA Oncol 2013; 87(4): 713–718. [DOI] [PubMed] [Google Scholar]
- 21. Brennan C, Yang TJ, Hilden P. et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. JAMA Oncol 2014; 88(1): 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trifiletti DM, Romano KD, Xu Z. et al. Leptomeningeal disease following stereotactic radiosurgery for brain metastases from breast cancer. J Neurooncol 2015; 124(3): 421–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.