Abstract
Desmoid-type fibromatosis is a rare and locally aggressive monoclonal, fibroblastic proliferation characterized by a variable and often unpredictable clinical course. Currently, there is no established or evidence-based treatment approach available for this disease. Therefore, in 2015 the European Desmoid Working Group published a position paper giving recommendations on the treatment of this intriguing disease. Here, we present an update of this consensus approach based on professionals’ AND patients’ expertise following a round table meeting bringing together sarcoma experts from the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group with patients and patient advocates from Sarcoma PAtients EuroNet. In this paper, we focus on new findings regarding the prognostic value of mutational analysis in desmoid-type fibromatosis patients and new systemic treatment options.
Keywords: desmoid, aggressive fibromatosis, EORTC/STBSG, patient advocacy groups, SPAEN, treatment algorithm
Key Message
This is a consensus approach to sporadic desmoid-type fibromatosis from European countries. An initial watchful waiting approach is useful to document actual tumor progression. Active therapies need to be individualized on a multidisciplinary basis for patients with clearly progressing disease.
Introduction
General issues and epidemiology
Desmoid-type fibromatosis (DF) is a rare monoclonal, fibroblastic proliferation characterized by a variable and often unpredictable clinical course. In the International Classification of Diseases, it is classified as D48.1. According to the World Health Organization (WHO), DF is a ‘clonal fibroblastic proliferation that arises in the deep soft tissues and is characterized by infiltrative growth and a tendency toward local recurrence but an inability to metastasize’, even though they may be multifocal in the same limb or body part [1]. DF is a distinct rare entity (incidence five to six cases per 1 million of the population per annum [2]) with a peak age of 30–40 years [2, 3]. Approximately 5%–10% arises in the context of familial adenomatous polyposis (FAP); however, this will not be discussed in this paper.
Level of evidence
Considering the variable clinical presentations, anatomic locations and biologic behaviors, a highly individualized treatment approach by expert teams is required. Due to the rarity of the disease, the level of evidence available for common types of cancer is unlikely ever to be available for DF. There is no published phase III randomized clinical study; only few phase II trials and mainly retrospective analyses are available. As for rare cancers and diseases, a higher level of uncertainty needs to be accepted in DF both for regulatory and for clinical decision-making.
Methodology
This position paper adheres to the European Organization for Research and Treatment of Cancer (EORTC) Policy 19 on ‘Guidelines, Expert Opinions, and the use of EORTC Results in Promotional Material on Cancer Care’ (http://www.eortc.org/app/uploads//03/POL019.pdf) and has formal EORTC Board approval. The level of evidence available and the grade of recommendation are classified according to the ESMO guidelines. In 2015, the European Desmoid Working Group published a first position paper giving recommendations on the treatment of DF [4]. Here, we present an update of this consensus approach based on professionals’ and patients’ expertise following a 2nd Round Table Meeting on 23 February 2017 bringing together soft tissue tumor experts from the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with patients and patient advocates from Sarcoma PAtients EuroNet (SPAEN). In this paper, we focus on new findings regarding the prognostic value of the mutational analysis in DF patients and an update on systemic treatment options.
Pathology/molecular biology
Biopsy
The histopathologic confirmation of DF is mandatory before initiating treatment. A diagnosis of DF can be readily established on core biopsies using 14G or 16G needles, while neither incisional nor excisional biopsy is recommended as the initial diagnostic modality. According to the rarity of DF and manifold potential histologic mimics, some reference centers have reported rates of misdiagnosed cases as high as 30%–40% during initial work-up [2, 5]. Noteworthy, nuclear accumulation of β-catenin on immunostaining has been observed in non-DF soft tissue neoplasms as well, while activating mutations in CTNNB1 (the gene encoding β-catenin) were confined to DF in the differential diagnostic setting compared with other soft tissue neoplasms [6]. Accordingly, we strongly recommend that DF diagnosis should be confirmed by an expert soft tissue pathologist and ideally mutational analysis should be strongly considered in diagnostically equivocal or uncertain cases [7].
Resection specimen
Although the macroscopic appearance of DF is typically nodular with a bulky mass appearance (Figure 1A), tentacle-like spiculated extensions with infiltrative growth are regularly found (Figure 1B). Accordingly, resection margins should be evaluated carefully by extensive sampling [8]. Intra-operative frozen section evaluation of resection margins is not regularly recommended. The macroscopic and microscopic aspects of DF have been described in detail in the first consensus paper [4].
Figure 1.

(A) Macroscopic picture of DF. Note finger-like extensions (arrow) into muscle (M). (B) Microscopic picture of DF arising from deep fascia (F). Note the infiltrative growth into skeletal muscle (arrows). (C) Screen-shot of next-generation sequencing analysis of β-catenin T41A mutation, with missense mutation A > G in only a subset of the reads.
Molecular biology
Approximately 85%–90% of DF harbors mutations in the β-catenin gene, leading to nuclear accumulation of β-catenin protein (Figure 2). β-Catenin mutations and APC mutations appear to be mutually exclusive in DF, thus, detection of a somatic β-catenin mutation may help to exclude a syndromal condition [9]. Vice versa, β-catenin wildtype status in DF should raise suspicion for FAP, with more extensive diagnostic clinical work-up (e.g. colonoscopy). Mutation analysis of β-catenin has been proposed as a specific diagnostic tool for establishing DF diagnosis, particularly in challenging or diagnostically ambiguous cases [10]. In some cases (e.g. with low tumor cell content), application of next-generation sequencing is slightly more sensitive compared with classical Sanger sequencing as it detects cases with low mutational allelic fractions, with reported frequencies of 93%–95% [11] (Figure 1C). Mutations of β-catenin in DF cluster in the N-terminal region comprising codons 32–45 encoded by exon 3. Although T41A and S45F are by far the most common mutations in DF accounting for roughly 50% and 25%, respectively, S45P is the third most common mutation at around 9% and very rare missense mutations and deletions affecting codons 32–49 have been observed as well [11]. Thus, all codons 32–49 should be included in a mutation analysis, and the sensitivity of the assay should be adequate to the estimated tumor cell content.
Figure 2.

Immunohistochemistry of a DF with characteristic β-catenin staining.
Prognostic relevance of β-catenin mutations
A significant correlation between β-catenin S45F mutation and an increased risk of recurrence after resection was observed in four independent studies [12–15], and S45F mutations were overrepresented in a clinical trial of DF patients with RECIST progressive disease [16]. Notably, in that trial DF with S45F mutation showed the highest progression arrest rate of 85% when treated with 2 years of imatinib 800 mg/day, compared with only 43% progression arrest rate in DF with β-catenin wild-type status. Taken together, these findings strongly encourage mutation analysis of β-catenin in DF to identify patients with a probably more aggressive course, and to estimate response to imatinib therapy. However, to date there are no prospective data on the prognostic value of β-catenin (CTNNB1) mutation status at the time of first diagnosis, but studies addressing this point are ongoing.
Imaging
Diagnosis
MRI is the mainstay of imaging in DF and can be used for diagnosis, local staging and follow-up [17, 18]. Once the diagnosis is established, follow-up MRI is often carried out without intravenous contrast, minimizing risk for the patient [19], and the key diagnostic feature of hypointense bands is identifiable on T2W images [20]. An association has been shown between lesion growth and high T2W signal intensity [21], but prediction of behavior has been challenging [22]. An increase in collagen deposition and decrease in extracellular space results in a decrease in T2W signal intensity [23, 24]; also in lesions responding to treatment [25, 26]. Lesions are frequently intermuscular, infiltrating along facial planes [27, 28] and can be multifocal although usually in the same body part.
Follow-up and response assessment
The dimension-based RECIST is currently employed within clinical trials [29]. The lack of radiation exposure makes targeted MRI ideal for follow-up. MRI surveillance has been used to assess response to treatment with a decrease in T2W signal and lesion size [30] and FDG PET/CT may give an early indication of response in patients treated with imatinib [31]. However, future applications should be selected so that the benefit of imaging outweighs the risk of radiation exposure particularly where multiple assessments for non-malignant pathology are carried out in young patients.
Indication for treatment
Immediate surgery is no more the standard treatment of DF. Retrospective series have shown progression-free survival rates of 50% at 5 years for asymptomatic patients managed with a front-line conservative ‘watchful waiting’ approach [32–35]. These patients remained under close observation, such that no patient was lost to follow-up and treatment plans could be altered if tumors progressed. No significant prognostic factors identified patients who do not need treatment from those who need active therapy at diagnosis. This is further complicated by the fact that tumor growth but also tumor site and size may be decision factors, as same sized tumors may remain asymptomatic in some sites and be life-threatening in others. Spontaneous regressions are observed in as many as 20%–30% of cases (Figure 3) [36]. There may be sites where regression is more common (i.e. abdominal wall [37]), however, regression has been observed at all sites [38]. It is reasonable to consider watchful waiting as an initial step when asymptomatic tumors are located at critical sites (i.e. mesentery) before undertaking subsequent treatments (IV, B); the same is valid for intra-abdominal DF [39].
Figure 3.
Examples of spontaneous regression of DF at different sites. (A) Intra-abdominal DF. (B) Scapular girdle DF.
Surgery
Before 2000, the management of sporadic DF mirrored that of soft tissue sarcoma with surgery as the standard of care. Multiple retrospective single institution case series have reported local control rates after complete surgical resection to be ∼80% at 5 years. Tumor location was found to be a risk factor for recurrence, with abdominal wall DF portending a better prognosis, followed by intra-abdominal DF, trunk DF and extremity DF portending a worse outcome. Recurrent disease was found to be a risk factor for further recurrence. Surgical margins, however, do not consistently correlate with recurrence [40], while β-catenin mutational status does (Tables 1 and 2). A recently published nomogram incorporates tumor site, size and patient age in estimating the risk of local recurrence; however, surgical margins are not included [41]. This observation led to a reassessment of the overall management, and preservation of function became a priority. Therefore, many investigators proposed to further limit morbidity by considering an initial observation period in all patients, especially when surgery would involve loss of function [32–35]. When the surveillance approach fails, surgery is still a valid option (IV, A). In case of progression medical treatment or radiotherapy should also be considered factoring localization and age. When carried out, surgical resection should be aimed at obtaining microscopic negative margins, although function preservation—especially for tumors located in the extremities and girdles—should always be an important goal and other alternatives, including radiation therapy, can be considered when appropriate. Furthermore, a large sporadic mesenteric/retroperitoneal DF may be treated by surgical resection due to tumor size and possible related symptoms.
Table 1.
Prognostic factors in DF: surgical margins and clinical outcome in sporadic DF
| Period | No. of patient | Primary/ Recurrent | Median FU (months) | 5-year DFS | 5-year DFS (M+/M−) | 10-year DFS | 10-year DFS (M+/M−) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Merchant et al. [76] | 1982–1997 | 105 | All primary | 49 | 75% | 76%/74% | N/R | N/R | 0.51 |
| Gronchi et al. [77] | 1966–2001 | 203 | 128 Primary | 130 | 81% | 79%/82% | 76% | 74%/77% | 0.5 |
| 75 Recurrence | 153 | 59% | 47%/65% | 59% | 47%/65% | 0.19 | |||
| Lev et al. [78] | 1995–2005 | 189 | 140/49 | 68 | 80% | 80%/80% | 79% | 79%/79% | |
| Bonvalot et al. [33] | 1988–2003 | 89 | All primary | 76 | 44% | 35%/60% | N/R | N/R | 0.09 |
| Huang et al. [79] | 1987–2007 | 151 | 113 Primary | 102 | 87% | 64%/92% | 85% | 64%/92% | 0.0001 |
| 38 Recurrence | 102 | 56% | 35%/71% | 56% | 35%/71% | 0.09 | |||
| Salas et al. [40] | 1965–2008 | 370 | All primary | 53 | 60% | 60%/60% | 50% | 50%/50% | |
| Mullen et al. [80] | 1970–2009 | 177 | 133/44 | 40 | 61% | 52%/82% | 60% | 52%/77% | 0.008 |
| Crago et al. [41] | 1982–2011 | 495 | 382/113 | 60 | 69% | 69%/69% | 65% | 65%/65% | |
| Van Broekhoven et al. [81] | 1989–2011 | 132 | All primary | 38 | 82.4% | 80%/85% | N/R | N/R | 0.7 |
| Cates et al. [8] | 1983–2011 | 92 | All primary | 38 | N/R | 58%/87% | N/R | 50%/87% | 0.02 |
Background in light blue: studies showing an association of quality of surgical margins and risk of local relapse. Background in dark blue: studies NOT showing any association of quality of surgical margins and risk of local relapse.
Definition of resection margins is not consistent in all studies: definition of positive/negative varies from <1 mm/≥1 mm to 0 mm/>0 mm. The sampling protocol of the surgical specimen (number of sections to evaluate surgical margins) is not reported in any of the series, but one where the critical number of sections looked to be 7 [8].
Table 2.
Prognostic factors in DF: β-catenin (CTNNB1) mutational status and clinical outcome in sporadic DF
| Period | No. of patients | Primary/ recurrent | Median FU (months) | 5-year DFS | 5-year DFS WT/ T41A/S45F | 10-year DFS | 10-year DFS (M+/M−) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Lazar et al. [12] | 1985–2005 | 138 | 89/39 | N/R | 49% | 65%/57%/23% | N/R | N/R | 0.0001 |
| Dômont et al. [13] | 1987–2007 | 101 | 57/44 | 62 | 49% | 75%/43%a | N/R | N/R | 0.02 |
| Colombo et al. [14] | 1998–2011 | 179 | All primary | 50 | 70% | 91%/66%/45% | N/R | N/R | 0.05 |
| Mullen et al. [82] | 1984–2009 | 115 | 95/20 | N/R | 71% | 74%/55%/60% | N/R | N/R | 0.28 |
| Van Broekhoven et al. [15] | 1989–2013 | 101 | All primary | 41 | 77% | 87%/88%/46% | N/R | N/R | 0.001 |
Background in light blue: studies NOT showing any association of ß-catenin mutational status and risk of local relapse. Background in dark blue: Studies showing an association of β-catenin mutational status and risk of local relapse.
Comments: Of note, S45F mutated tumors are more common in extremity DF in all series. In Colombo et al. [14], the largest series so far, the administration of RT seemed to offset the negative prognostic impact of S45F.
All mutated tumors were considered together. When the 3 different mutated tumors were considered separately, only a trend for a worse outcome of S45F could be observed.
FU, follow-up; DFS, disease-free survival; M+, positive margins; M−, negative margins; N/R, not reported.
Therefore, watchful waiting is a reasonable approach to minimize overtreatment and unnecessary morbidity in a subset of patients (IV, B). Prospective observational studies are presently underway to validate these results and possibly shed more light on the biologic background of this intriguing disease (NCT01801176, NCT02547831 and NTR4714) [42]. Spontaneous regressions of DF may have to do with the immunologic environment of the host. Studies are ongoing to better understand the role of immunity in the course of the disease. However no studies with immunomodulators have been run or planned so far. In distinct clinical situations such as complications (occlusion, perforation etc. with or without systematic resection of all the mass) or major cosmetic issues patients can be operated upfront. On the other hand, pain and pregnancy should not be considered per se as unequivocal indication for surgery. As a matter of fact, while the progression risk during pregnancy is as high as 40%–50%, this can be safely managed. An active treatment is required in less than half of the patients and only a minority requires an operation. Moreover, DF does not increase the obstetric risk and it should not be a contraindication to future pregnancy. There are presently no data to recommend a specific delay between the onset of a watchful waiting approach and pregnancy, although it is reasonable to wait at least a year or two in order to understand whether the disease is stable or progressing and no active therapies are in fact needed [43].
Isolated limb perfusion and cryoablation
In patients with progressive, locally advanced extremity DF, where resection would result in important functional sacrifice, isolated limb perfusion (ILP) with tumor necrosis factor alpha and melphalan seems to be a very effective treatment option [44, 45]. With a median follow-up of 7 years, 90% of 25 patients had disease control; of these, 40% developed disease progression after a median of 2 years [45]. This modality can be followed by substantial side effects, although the use of low dose TNF (1 mg) and moderate temperature (never above 39 °C) have made this procedure safer than in the past. Therefore, it can be considered an option even in this condition, as long as it is delivered as above.
Cryoablation has been reported in case series to be an effective alternative treatment of small and moderately sized extra-abdominal DF. It is of limited use in patients with larger tumors that can only be partially treated due to the involvement of vital structures. Continuing research is necessary [46, 47] and a non-randomized phase II study in France is ongoing (NCT02476305).
Of note, both approaches are not available in every center and do require particular expertise.
Radiotherapy
There is no change regarding previously made recommendations for asymptomatic patients, operable symptomatic and/or progressive patients and inoperable symptomatic and/or progressive patients [48–51]. Radiotherapy to a dose of 56 Gy in 28 once-daily fractions of 2 Gy has been shown to provide adequate local control in the majority of progressive patients (III, A) [50].
Radiotherapy techniques
Regardless of the indication, radiotherapy should be delivered by the best available techniques such as Intensity Modulated Radiotherapy (IMRT) and Image Guided Radiotherapy (IGRT). Coregistration with (contrast enhanced) MRI sequences, preferably in treatment position, is imperative. Whether the chosen dose should be applied by conventional, linear accelerator based photons or proton beam therapy is an issue of debate and future research [52].
Given the proximity of radiation sensitive organs in the abdominal cavity, radiotherapy to the abdominal wall per se is not contraindicated, but should be regarded as a challenge and only to be applied with great caution applying modern techniques such as IMRT and IGRT, taking respiratory motion into account.
Combining radiotherapy and surgery
Post-operative radiation has not demonstrated a conclusive benefit after first surgery regardless of resection margins. However, adjuvant radiotherapy may reduce the risk of recurrence after incomplete surgical resection, particularly in patients with recurrent tumors [53]; comparable conclusions have been drawn by different meta-analyses [54–56]. Therefore, careful consideration on the morbidity of salvage surgery in case of local recurrence after surgery only compared with late morbidity of adjuvant radiotherapy is mandatory in every individual case.
Medical therapy
Systemic treatment options comprise antihormonal therapies with no histologic support from the presence of ER-/PR-positivity but from availability and reimbursement, non-steroidal anti-inflammatory drugs (NSAIDs), low-dose chemotherapy, tyrosine kinase inhibitors, and full-dose chemotherapy including liposomal doxorubicin [57, 58]. Recently, new treatment strategies have emerged such as targeting the Notch signaling pathway [59].
Anti-hormonal agents such as tamoxifen may be used—alone [60] or in combination with NSAIDs [61]—as first medical treatment, mainly because of their limited toxicity, rare adverse events and low costs [62] (III, B). However, response rates have been found to be low and no clear relationship between symptom changes, size or MRI signal changes could be demonstrated [63]. Therefore, a general recommendation for its use cannot be given.
When the relevant issue is critical anatomic site, in the case of hormonal therapy failure or for aggressively growing, symptomatic or even life-threatening DF, chemotherapy is advisable using either a ‘low dose’ regimen with methotrexate and/or vinblastine/vinorelbine [64–67] (III, B). Conventional dose chemotherapy using anthracycline-based regimes is another option if more rapid response is desired (e.g. for intra-abdominal or head and neck DF) [65]. It is usually administered for six to eight cycles, i.e. until the maximum tolerated dose of anthracycline is reached; however, using lower dosages and more cycles may be possible. Pegylated liposomal doxorubicin has been reported in uncontrolled patient series to have significant activity with acceptable toxicity and, importantly in this young patient population, less cardiac toxicity than conventional doxorubicin [68, 69].
There is prospective, uncontrolled evidence for the activity of the tyrosine kinase inhibitor (TKI) imatinib in progressive DF patients with high rates of stabilization (60%–80%) despite rather low response rates (6%–16%) with a well-known toxicity profile [70–72] (III, B). In the most recent publication of the German Interdisciplinary Sarcoma Group (GISG) imatinib induced sustained progression arrest in RECIST progressive DF patients. In addition, nilotinib had the potential to stabilize DF growth even after imatinib failure [73]. In a retrospective cohort, the use of sorafenib revealed a higher response rate with 25% and a disease stabilization rate of 70% [25]; however, the updated analysis revealed a response rate of 18% which is in the same range as described for imatinib [74]; no prospective data are available yet. Currently, sorafenib is being evaluated in a phase III, placebo-controlled setting (NCT02066181), presently closed to patient entry. In a cohort of eight patients treated with pazopanib, partial responses were reported in three and disease stabilization in five patients without any radiologic disease progression [75].
Notch signaling is a new systemic treatment strategy. Gamma-secretase cleaves intracellular Notch resulting in Notch signaling. PF-03084014 is an oral, reversible gamma-secretase inhibitor. A phase II study of PF-03084014 has been conducted in 17 DF patients (in contrast to ∼150 patients prospectively treated with imatinib) who had progressed following at least one line of therapy. Five partial responses (29%) were shown and 12 out of the 17 patients demonstrated stable disease; there were no disease progressions [59]. Unfortunately, the drug is not available at present and no trial is currently underway.
Ongoing European studies
A randomized phase II trial (DESMOPAZ) evaluating pazopanib versus methotrexate plus vinblastine in 94 patients is ongoing in France (NCT01876082). In Italy, a phase II study evaluating toremifene in DF is recruiting (NCT02353429). In Spain, there is an ongoing study with nab-paclitaxel in DF and Ewing sarcomas. Another trial is evaluating the mTOR inhibitor sirolimus in children and young adults with desmoid-type fibromatosis (NCT01265030).
In general, it is reasonable to employ the less toxic before the more toxic therapies in a stepwise fashion. Due to the lack of randomized data, we are still not in the situation to propose a definitive sequence of the existing systemic treatment options. Out of the variety of possible systemic treatment options, one can be chosen taking into account the dynamic growth of the tumor and the urgency of treatment, the expected response rate, the planned treatment duration and the toxicity of the administered drug. Note, that often long-term treatment periods are necessary with some TKIs to achieve tumor shrinkage and control tumor growth. Comparative and randomized studies are highly encouraged in the medical treatment setting to gain more evidence-based data which could help to guide us through the treatment plan. Many drugs described above are not licensed for DF and, therefore, not available or reimbursed in most European countries. Efforts are needed to make tyrosine kinase or gamma-secretase inhibitors accessible and involving patient advocacy groups such as SPAEN is essential in pushing that forward.
Main challenges for DF patients—the patients’ perspective
DF diagnosis is often hampered by misdiagnosis resulting in a long timeframe from first symptoms until correct diagnosis. Patients are often relieved to get the diagnosis of a ‘benign disease’ underestimating the possible aggressive course. Uncertainty in diagnosis, treatment and possible recurrence often requires psychologic support. Considering the peak age of ∼35 years, patients often feel they are losing their independence just at the time they are starting to gain it.
Comprehensive programs especially for adolescents are needed including physical, psychologic and social support. Follow-up does not follow patterns of more common cancer types, being highly individualized according to physical, psychologic and social aspects. There is room for a symptom-driven follow-up strategy and a strict recommendation on follow-up procedures cannot be given. After 1 year of follow-up DF patients should not be discouraged to become pregnant. There may be a risk of tumor development during or after pregnancy. However, if the tumor has been stable before pregnancy, it is most likely to regress again afterwards [43].
Experts may recommend getting in touch with other patients to relieve the feeling of isolation and to help to restore a sense of normality. National and international patient advocacy groups such as SPAEN can be of substantial support here (http://www.sarcoma-patients.eu/en/).
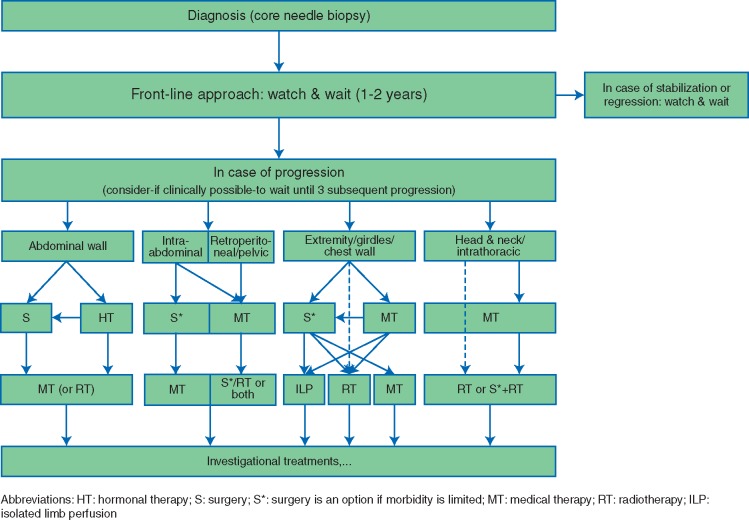
Consensus algorithm
A multidisciplinary discussion in soft tissue tumor boards is necessary to propose a personalized management; furthermore, a discussion with the patient is also necessary for tailoring this proposal to its objectives given the natural course of the disease. Patient advocacy groups are helpful to reinforce the explanations given by health professionals and avoid some misunderstanding especially about the wait and see policy. Second opinion by an expert pathologist as well as clinical management by an expert team is highly recommended (Figure 4).
Figure 4.
Consensus algorithm.
There is clear consensus that a conservative watch and wait strategy should be the front-line approach to newly diagnosed patients, irrespective of existing pain or other clinical symptoms, offering a way to understand the behavior of the disease and tailor next treatment steps. The time interval for a watch and wait approach could be 1–2 years and patients should be closely followed, preferably using contrast enhanced MRI. The first clinical and/or radiologic re-evaluation should be done within 8–12 weeks, then every 3 months in the first year, then 6 monthly up to the fifth year, and yearly thereafter. In the case of progression, alternative treatment options should be discussed. To define the cut-off for an active treatment, different factors have to be taken into account such as initial tumor size, growth rate, anatomical localization, risk to organs/nerves etc., compression and worsening of function. In most cases, the strategy is switched to a definitive treatment in the case of an objective tumor size progression in multiple (e.g. three) consecutive images and further steps should be tailored as described in the depicted algorithm (Figure 4).
In the case of a progressing DF localized at the abdominal wall, hormonal therapy might be an option. A more definitive strategy, of course, would be surgical resection or radiotherapy.
For intraabdominal DF, it was clearly agreed that surgery remains the main treatment in the case of progression, if the tumor is operable. For retroperitoneal or pelvic DF medical therapy should be the first therapeutic option. In the case of further progression or relapse, medical therapy, surgery or radiotherapy would be an option with a tendency toward surgery if the tumor is resectable with preservation of function.
For DF of the extremities, girdles or chest wall the decision for the type of the initial treatment should be guided by the expected postoperative functional impairment or morbidity. As this can be highly subjective, of course, postoperative consequences should be clearly discussed with the patient. If the lesion is not involving major vessels or nerves an observation strategy should be continued. If the lesion threatens to involve major vessels or nerves, surgical resection should not necessarily be considered the first option; the alternative would be medical therapy or radiotherapy alone. Other alternatives for a limb tumor include ILP which can be considered for tumors located in the extremities, especially advisable in multifocal disease and tumors of the hand or foot. No resection of the remnant tumor is usually proposed. In the case of further progression or relapse, definitive surgery could then be proposed. In the case of positive surgical margins and critical situations, adjuvant radiotherapy may be considered.
For critical anatomical localizations such as head and neck and intrathoracic sites medical therapy is generally considered the first line option. However, in selected conditions (elder age, patient intolerance/preference, comorbidities, lesion growing rapidly and threatening vital organs etc.) radiotherapy is a reasonable and effective first line alternative. In the case of further progression or relapse, radiotherapy should be discussed in these highly radiosensitive structures. If surgery is considered, additional radiotherapy should always be considered to minimize the risk of local relapse.
Disclaimer
These recommendations reflect the state of knowledge, current at the time of publication, on effective and appropriately validated data, as well as clinical consensus judgments when knowledge is lacking. The inevitable changes in the state of scientific information and technology mandate that periodic review, updating and revisions will be needed. Expert opinions users always are urged to seek out newer information that might impact the diagnostic and treatment recommendations contained within. These expert opinions do not apply to all patients, and must be adapted and tailored to each individual patient. Proper use, adaptation modifications or decisions to disregard these or other guidelines, in whole or in part, are entirely the responsibility of the clinician who uses the expert opinions. Ultimately, healthcare professionals must make their own treatment decisions about care on a case-by-case basis, after consultation with their patients, using their clinical judgment, knowledge and expertise. An expert opinion is not intended to take the place of physician or a researcher judgment in diagnosing and treatment of particular patients or in conducting specific research activities. Expert opinions may not be complete or accurate. The EORTC and members of their boards, officers and employees disclaim all liability for the accuracy or completeness of an expert opinion, and disclaim all warranties, express or implied to their incorrect use.
Acknowledgements
Desmoid Working Group
S. Bauer, Sarcoma Center, West German Cancer Center, Essen, Germany; J. Y. Blay, Department of Medicine, Centre Léon Bérard, University Claude Bernard, Lyon, France; F. van Coevorden, Department of Surgical Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands; P. Dileo, Sarcoma Unit, University College Hospital, UCLH NHS Trust, London, UK; H. R. Dürr, Department of Orthopaedic Surgery, Campus Grosshadern, Ludwig-Maximilians-University Munich, Munich, Germany; M. Fiore, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; V. Grünwald, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Hannover, Germany; R. Jones, Medical Oncology, Royal Marsden Hospital London, London, UK; I. Judson, Medical Oncology, Royal Marsden Hospital London, London, UK; C. Kettelhack, Department of General Surgery, University Hospital Basel, Basel, Switzerland; K. Kopeckova, University Hospital Motol, Charles University, Prague, Czech Republic; A. Lazar, Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; L. H. Lindner, Department of Internal Medicine III, University Hospital Munich, Munich, Germany; J. Martin-Broto, MUsculoSkeletal Tumor Board of Excellence Sevilla (MUSTBE SEVILLA), Virgen del Rocío University Hospital, Sevilla, Spain; P. Rutkowski, Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland; S. Stacchiotti, Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; E. Stoeckle, Department of Surgery, Institut Bergonié, Bordeaux Cedex, France; C. Valverde, Oncology Department, Hospital Universitari Vall D'hebron, Barcelona, Spain; K. Verhoef, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, The Netherlands; E. Wardelmann, Gerhard-Domagk-Institute of Pathology, University Hospital Muenster, Muenster, Germany; M. Wartenberg, SPAEN Sarcoma PAtients EuroNet, Wölfersheim, Germany.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Contributor Information
Desmoid Working Group:
S Bauer, J Y Blay, F van Coevorden, P Dileo, H R Dürr, M Fiore, V Grünwald, R Jones, I Judson, C Kettelhack, K Kopeckova, A Lazar, L H Lindner, J Martin-Broto, P Rutkowski, S Stacchiotti, E Stoeckle, C Valverde, K Verhoef, E Wardelmann, and M Wartenberg
References
- 1. Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, WHO Classification of Tumours of Soft Tissue and Bone; 2013; 4th edition, Lyon: IARC.
- 2. Penel N, Coindre JM, Bonvalot S. et al. Management of desmoid tumours: a nationwide survey of labelled reference centre networks in France. Eur J Cancer 2016; 58: 90–96. [DOI] [PubMed] [Google Scholar]
- 3. Kasper B, Stroebel P, Hohenberger P.. Desmoid tumors - clinical features and treatment options for advanced disease. Oncologist 2011; 16: 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kasper B, Baumgarten C, Bonvalot S. et al. on behalf of the Desmoid Working Group. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise – a Sarcoma Patients EuroNet (SPAEN) and European Organisation For Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG) initiative. Eur J Cancer 2015; 51: 127–136. [DOI] [PubMed] [Google Scholar]
- 5. Huss S, Nehles J, Binot E. et al. β-catenin (CTNNB1) mutations and clinicopathological features of mesenteric desmoid-type fibromatosis. Histopathology 2013; 62: 294–304. [DOI] [PubMed] [Google Scholar]
- 6. Le Guellec S, Soubeyran I, Rochaix P. et al. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol 2012; 25: 1551–1558. [DOI] [PubMed] [Google Scholar]
- 7. Andritsch E, Beishon M, Bielack S. et al. ECCO essential requirements for quality cancer care: soft tissue sarcoma in adults and bone sarcoma. A critical review. Crit Rev Oncol Hematol 2017; 110: 94–105. [DOI] [PubMed] [Google Scholar]
- 8. Cates JM, Stricker TP.. Surgical resection margins in desmoid-type fibromatosis: a critical reassessment. Am J Surg Pathol 2014; 38: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 9. Wang WL, Nero C, Pappo A. et al. CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr. Dev Pathol.2012; 15: 361–367. [DOI] [PubMed] [Google Scholar]
- 10. Colombo C, Bolshakov S, Hajibashi S. et al. ′Difficult to diagnose′ desmoid tumours: a potential role for CTNNB1 mutational analysis. Histopathology 2011; 59: 336–340. [DOI] [PubMed] [Google Scholar]
- 11. Crago AM, Chmielecki J, Rosenberg M. et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer 2015; 54: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazar AJ, Tuvin D, Hajibashi S. et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008; 173: 1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dômont J, Salas S, Lacroix L. et al. High frequency of beta-catenin heterozygous mutations in extra-abdominal fibromatosis: a potential molecular tool for disease management. Br J Cancer 2010; 102: 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombo C, Miceli R, Lazar AJ. et al. CTNNB1 45F mutation is a molecular prognosticator of increased postoperative primary desmoid tumor recurrence: an independent, multicenter validation study. Cancer 2013; 119: 3696–3702. [DOI] [PubMed] [Google Scholar]
- 15. Van Broekhoven DL, Verhoef C, Grünhagen DJ. et al. Prognostic value of CTNNB1 gene mutation in primary sporadic aggressive fibromatosis. Ann Surg Oncol 2015; 22: 1464–1470. [DOI] [PubMed] [Google Scholar]
- 16. Kasper B, Gruenwald V, Reichardt P. et al. Correlation of CTNNB1 mutation status with progression arrest rate in RECIST progressive desmoid-type fibromatosis treated with imatinib: translational research results from a phase 2 Study of the German Interdisciplinary Sarcoma Group (GISG-01). Ann Surg Oncol 2016; 23: 1924–1927. [DOI] [PubMed] [Google Scholar]
- 17. Otero S, Moskovic EC, Strauss DC. et al. Desmoid-type fibromatosis. Clin Radiol 2015; 70: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 18. Bashir U, Moskovic E, Strauss D. et al. Soft-tissue masses in the abdominal wall. Clin Radiol 2014; 69: e422–e431. [DOI] [PubMed] [Google Scholar]
- 19. Lee JC, Thomas JM, Phillips S. et al. Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol 2006; 186: 247–254. [DOI] [PubMed] [Google Scholar]
- 20. Hartman TE, Berquist TH, Fetsch JF.. MR imaging of extraabdominal desmoids: differentiation from other neoplasms. AJR Am J Roentgenol 1992; 158: 581–585. [DOI] [PubMed] [Google Scholar]
- 21. Healy JC, Reznek RH, Clark SK. et al. MR appearances of desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol 1997; 169: 465–472. [DOI] [PubMed] [Google Scholar]
- 22. Castellazzi G, Vanel D, Le Cesne A. et al. Can the MRI signal of aggressive fibromatosis be used to predict its behaviour? Eur J Radiol 2009; 69: 222–229. [DOI] [PubMed] [Google Scholar]
- 23. Walker EA, Petscavage JM, Brian PL. et al. Imaging features of superficial and deep fibromatoses in the adult population. Sarcoma 2012; 2012: 215810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sundaram M, McGuire MH, Schajowicz F.. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol 1987; 148: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 25. Gounder MM, Lefkowitz RA, Keohan ML. et al. Activity of sorafenib against desmoid tumor / deep fibromatosis. Clin Cancer Res 2011; 17: 4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maruzzo M, Martin-Liberal J, Messiou C. et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res 2015; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dinauer PA, Brixey CJ, Moncur JT. et al. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007; 27: 173–187. [DOI] [PubMed] [Google Scholar]
- 28. Rhim JH, Kim JH, Moon KC. et al. Desmoid-type fibromatosis in the head and neck: CT and MR imaging characteristics. Neuroradiology 2013; 55: 351–359. [DOI] [PubMed] [Google Scholar]
- 29. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumors: re-vised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 30. Murphey MD, Ruble CM, Tyszko SM. et al. From the archives of the AFIP: musculoskeletal fibromatoses-radiologic-pathologic correlation. Radiographics 2009; 29: 2143–2173. [DOI] [PubMed] [Google Scholar]
- 31. Kasper B, Dimitrakopoulou-Strauss A, Pilz LR. et al. Positron emission tomography as a surrogate marker for evaluation of treatment response in patients with desmoid tumors under therapy with imatinib. Biomed Res Int 2013; 2013: 389672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis JJ, Boland PJ, Leung DH. et al. The enigma of desmoid tumors. Ann Surg 1999; 229: 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonvalot S, Eldweny H, Haddad V. et al. Extra-abdominal primary fibromatosis: aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 2008; 34: 462–468. [DOI] [PubMed] [Google Scholar]
- 34. Fiore M, Rimareix F, Mariani L. et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 2009; 16: 2587–2593. [DOI] [PubMed] [Google Scholar]
- 35. Briand S, Barbier O, Biau D. et al. Wait-and-see policy as a first-line management for extra-abdominal desmoid tumors. J Bone Joint Surg Am 2014; 96: 631–638. [DOI] [PubMed] [Google Scholar]
- 36. Colombo C, Miceli R, Le Péchoux C. et al. Sporadic extra abdominal wall desmoid-type fibromatosis: surgical resection can be safely limited to a minority of patients. Eur J Cancer 2015; 51: 186–192. [DOI] [PubMed] [Google Scholar]
- 37. Bonvalot S, Ternes N, Fiore M. et al. Spontaneous regression of primary abdominal wall desmoids: more common than previously thought. Ann Surg Oncol 2013; 20: 4096–4102. [DOI] [PubMed] [Google Scholar]
- 38. Roussin S, Mazouni C, Rimareix F. et al. Toward a new strategy in desmoid of the breast? Eur J Surg Oncol 2015; 41: 571–576. [DOI] [PubMed] [Google Scholar]
- 39. Burtenshaw SM, Cannell AJ, McAlister ED. et al. Toward observation as first-line management in abdominal desmoid tumors. Ann Surg Oncol 2016; 23: 2212–2219. [DOI] [PubMed] [Google Scholar]
- 40. Salas S, Dufresne A, Bui B. et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 2011; 29: 3553–3558. [DOI] [PubMed] [Google Scholar]
- 41. Crago AM, Denton B, Salas S. et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg 2013; 258: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Broekhoven DL, Grünhagen DJ, van Dalen T. et al. Tailored beta-catenin mutational approach in extra-abdominal sporadic desmoid tumor patients without therapeutic intervention. BMC Cancer 2016; 16: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fiore M, Coppola S, Cannell AJ. et al. Desmoid-type fibromatosis and pregnancy: a multi-institutional analysis of recurrence and obstetric risk. Ann Surg 2014; 259: 973–978. [DOI] [PubMed] [Google Scholar]
- 44. Bonvalot S, Rimareix F, Causeret S. et al. Hyperthermic isolated limb perfusion in locally advanced soft tissue sarcoma and progressive desmoid-type fibromatosis with TNF 1 mg and melphalan (T1-M HILP) is safe and efficient. Ann Surg Oncol 2009; 16: 3350–3357. [DOI] [PubMed] [Google Scholar]
- 45. Van Broekhoven DL, Deroose JP, Bonvalot S. et al. Isolated limb perfusion using tumour necrosis factor α and melphalan in patients with advanced aggressive fibromatosis. Br J Surg 2014; 101: 1674–1680. [DOI] [PubMed] [Google Scholar]
- 46. Kujak JL, Liu PT, Johnson GB, Callstrom MR.. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol 2010; 39: 175–182. [DOI] [PubMed] [Google Scholar]
- 47. Schmitz JJ, Schmit GD, Atwell TD. et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10-year experience. AJR Am J Roentgenol 2016; 207: 190–195. [DOI] [PubMed] [Google Scholar]
- 48. http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf Version, 2.2017; (8 February 2017, date last accessed).
- 49. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(supp 3): iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 50. Keus RB, Nout RA, Blay JY. et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis—an EORTC STBSG and ROG study (EORTC 62991-22998). Ann Oncol 2013; 24: 2672–2676. [DOI] [PubMed] [Google Scholar]
- 51. Kriz J, Eich HT, Haverkamp U. et al. Radiotherapy is effective for desmoid tumors (aggressive fibromatosis)—long-term results of a German multicenter study. Oncol Res Treat 2014; 37: 255–260. [DOI] [PubMed] [Google Scholar]
- 52. DeLaney TF, Haas RLM.. Innovative radiotherapy of sarcoma: proton beam radiation. Eur J Cancer 2016; 62: 112–123. [DOI] [PubMed] [Google Scholar]
- 53. Janssen ML, van Broekhoven DL, Cates JM. et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg 2017; 104: 347–357. [DOI] [PubMed] [Google Scholar]
- 54. Wood TJ, Quinn KM, Farrokhyar F. et al. Local control of extra-abdominal desmoid tumors: systematic review and meta-analysis. Rare Tumors 2013; 5: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao X, Corbett T, Gupta AA. et al. A systematic review of active treatment options in patients with desmoid tumours. Curr Oncol 2014; 21: e613–e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nuyttens JJ, Rust PF, Thomas CR Jr, Turrisi AT III. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer 2000; 88: 1517–1523. [PubMed] [Google Scholar]
- 57. Janinis J, Patriki M, Vini L. et al. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol 2003; 14: 181–190. [DOI] [PubMed] [Google Scholar]
- 58. Al-Jazrawe M, Au M, Alman B.. Optimal therapy for desmoid tumors: current options and challenges for the future. Expert Rev Anticancer Ther 2015; 15: 1443–1458. [DOI] [PubMed] [Google Scholar]
- 59. Kummar S, O'Sullivan Coyne G, Do KT. et al. Clinical Activity of the γ-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis). J Clin Oncol. 2017; 35(14): 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fiore M, Colombo C, Radaelli S. et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer 2015; 51: 2800–2807. [DOI] [PubMed] [Google Scholar]
- 61. Quast DR, Schneider R, Burdzik E. et al. Long-term outcome of sporadic and FAP-associated desmoid tumors treated with high-dose selective estrogen receptor modulators and sulindac: a single-center long-term observational study in 134 patients. Fam Cancer 2016; 15: 31–40. [DOI] [PubMed] [Google Scholar]
- 62. Skapek SX, Anderson JR, Hill DA. et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a children’s oncology group (COG) phase II study. Pediatr Blood Cancer 2013; 60: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mitra I, Szucs Z, Libertini M. et al. Aggressive fibromatosis response to tamoxifen: MRI features with symptomatic correlation—the Royal Marsden experience. In CTOS Annual Meeting 2016, Lisbon (abstract #2549467) 2016.
- 64. Skapek SX, Ferguson WS, Granowetter L. et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a pediatric oncology group phase II trial. J Clin Oncol 2007; 25: 501–506. [DOI] [PubMed] [Google Scholar]
- 65. Garbay D, Le Cesne A, Penel N. et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG). Ann Oncol 2012; 23: 182–186. [DOI] [PubMed] [Google Scholar]
- 66. Mir O, Rahal C, Rimareix F. et al. Efficacy of oral vinorelbine in advanced/progressive desmoid tumours: an updated retrospective study in 50 patients. J Clin Oncol 2016; 34(suppl; abstr 11050). [Google Scholar]
- 67. Palassini E, Frezza AM, Mariani L. et al. Long-term efficacy of methotrexate plus vinblastine / vinorelbine in large series of patients affected by desmoid-type fibromatosis. Cancer J 2017; 23: 86–91. [DOI] [PubMed] [Google Scholar]
- 68. Constantinidou A, Jones RL, Scurr M. et al. Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 2009; 45: 2930–2934. [DOI] [PubMed] [Google Scholar]
- 69. Pang A, Macedo DVG, Carbini M, Maki RG.. Pegylated liposomal doxorubicin (PLD) as an active treatment option for desmoid tumor (DT) patients. J Clin Oncol 2016; 34(suppl; abstr 11032). [Google Scholar]
- 70. Heinrich MC, McArthur GA, Demetri GD. et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumour). J Clin Oncol 2006; 24: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 71. Penel N, Le Cesne A, Bui BN. et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol 2011; 22: 452–457. [DOI] [PubMed] [Google Scholar]
- 72. Kasper B, Grünwald V, Reichardt P. et al. Phase II study evaluating imatinib to induce progression arrest in RECIST progressive desmoid tumors not amenable to surgical resection with R0 intent or accompanied by unacceptable function loss—a study of the German Interdisciplinary Sarcoma Group (GISG). Ann Oncol 2014; 25(suppl 4): iv494. [Google Scholar]
- 73. Kasper B, Gruenwald V, Reichardt P. et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumors—final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer 2017; 76: 60–67. [DOI] [PubMed] [Google Scholar]
- 74. Munhoz RR, Lefkowitz RA, Kuk D. et al. Efficacy of sorafenib in patients with desmoid-type fibromatosis. J Clin Oncol 2016; 34(suppl; abstr 11065). [Google Scholar]
- 75. Szucs Z, Messiou C, Wong HH. et al. Pazopanib, a promising option in the landscape of treatment for aggressive fibromatosis. Anticancer Drugs 2017; 28: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Merchant NB, Lewis JJ, Woodruff JM. et al. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 1999; 86: 2045–2052. [PubMed] [Google Scholar]
- 77. Gronchi A, Casali PG, Mariani L. et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol 2003; 21: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 78. Lev D, Kotilingam D, Wei C. et al. Optimizing treatment of desmoid tumors. J Clin Oncol 2007; 25: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 79. Huang K, Fu H, Shi YQ. et al. Prognostic factors for extra-abdominal and abdominal wall desmoids: a 20-year experience at a single institution. J Surg Oncol 2009; 100: 563–569. [DOI] [PubMed] [Google Scholar]
- 80. Mullen JT, Delaney TF, Kobayashi WK. et al. Desmoid tumor: analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol 2012; 19: 4028–4035. [DOI] [PubMed] [Google Scholar]
- 81. Van Broekhoven DL, Verhoef C, Elias SG. et al. Local recurrence after surgery for primary extra-abdominal desmoid-type fibromatosis. Br J Surg 2013; 100: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 82. Mullen JT, DeLaney TF, Rosenberg AE. et al. β-Catenin mutation status and outcomes in sporadic desmoid tumors. Oncologist 2013; 18: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]