Abstract
Background
Ramucirumab, the human immunoglobulin G1 monoclonal antibody receptor antagonist of vascular endothelial growth factor receptor 2, has been approved for treating gastric/gastroesophageal junction, non-small-cell lung, and metastatic colorectal cancers. With the completion of six global, randomized, double-blind, placebo-controlled, phase III trials across multiple tumor types, an opportunity now exists to further establish the safety parameters of ramucirumab across a large patient population.
Materials and methods
An individual patient meta-analysis across the six completed phase III trials was conducted and the relative risk (RR) and associated 95% confidence intervals (CIs) were derived using fixed-effects or mixed-effects models for all-grade and high-grade adverse events (AEs) possibly related to vascular endothelial growth factor pathway inhibition. The number needed to harm was also calculable due to the placebo-controlled nature of all six registration standard trials.
Results
A total of 4996 treated patients (N = 2748 in the ramucirumab arm and N = 2248 in the control, placebo arm) were included in this meta-analysis. Arterial thromboembolic events [ATE; all-grade, RR: 0.8, 95% CI 0.5–1.3; high-grade (grade ≥3), RR: 0.9, 95% CI 0.5–1.7], venous thromboembolic events (VTE; all-grade, RR: 0.7, 95% CI 0.5–1.1; high-grade, RR: 0.7, 95% CI 0.4–1.2), high-grade bleeding (RR: 1.1, 95% CI 0.8–1.5), and high-grade gastrointestinal (GI) bleeding (RR: 1.1, 95% CI 0.7–1.7) did not demonstrate a definite increased risk with ramucirumab. A higher percentage of hypertension, proteinuria, low-grade (grade 1–2) bleeding, GI perforation, infusion-related reaction, and wound-healing complications were observed in the ramucirumab arm compared with the control arm.
Conclusions
Ramucirumab may be distinct among antiangiogenic agents in terms of ATE, VTE, high-grade bleeding, or high-grade GI bleeding by showing no clear evidence for an increased risk of these AEs in this meta-analysis of a large and diverse patient population. Ramucirumab is consistent with other angiogenic inhibitors in the risk of developing certain AEs.
Clinical Trial Numbers: NCT00917384 (REGARD), NCT01170663 (RAINBOW), NCT01168973 (REVEL), NCT01183780 (RAISE), NCT01140347 (REACH), and NCT00703326 (ROSE).
Keywords: VEGF, VEGFR, ramucirumab, antiangiogenic, adverse events, meta-analysis
Introduction
Ramucirumab (CYRAMZA®, Eli Lilly and Company, Indianapolis, IN, USA) is a fully human immunoglobulin G1 monoclonal antibody with high affinity binding to the vascular endothelial growth factor receptor 2 (VEGFR-2) extracellular domain, blocking binding of multiple VEGF ligands and receptor activation. Ramucirumab has received approval for second-line therapy in gastric, lung, and colorectal cancers [1, 2].
Risk–benefit assessment is an important component of physician and patient decision making in selecting cancer treatments. The need to minimize treatment-related toxicity while maximizing efficacy is paramount. To date, the results of six global, randomized, double-blind, placebo-controlled, phase III clinical trials with different tumor types have been published to present the efficacy and safety profile of ramucirumab [3–9] (Table 1).
Table 1.
Ramucirumab double-blind randomized controlled phase III clinical trials
| Trial | Indication | Treatment arms | Patients randomized per arma | Date first patient enrolled | NCI CTCAE | Trial registry number |
|---|---|---|---|---|---|---|
| REGARDb [3] | Advanced gastric or GEJ adenocarcinoma | Ramucirumab 8 mg/kg i.v. Q2W plus BSC or placebo plus BSC | RAM: n = 238 | 6 October 2009 | v4.02 | NCT00917384 |
| I4T-IE-JVBD | Control: n = 117 | |||||
| RAINBOWb [4] I4T-IE-JVBE | Advanced gastric or GEJ adenocarcinoma | Ramucirumab 8 mg/kg i.v. on days 1 and 15, plus paclitaxel 80 mg/m2 i.v. on days 1, 8, and 15 of a 28-day cycle or placebo i.v. plus paclitaxel 80 mg/m2 i.v. on days 1, 8, and 15 of a 28-day cycle | RAM: n = 330 Control: n = 335 | 23 December 2010 | v4.02 | NCT01170663 |
| REVELb [5] | Stage IV NSCLC | Ramucirumab 10 mg/kg i.v. plus docetaxel 75 mg/m2 on day 1 of a 21-day cycle or placebo plus docetaxel 75 mg/m2 on day 1 of a 21-day cycle | RAM: n = 628 | 03 December 2010 | v4.0 | NCT01168973 |
| I4T-MC-JVBA | Control: n = 625 | |||||
| RAISEb [6] | Metastatic CRC | Ramucirumab 8 mg/kg i.v. plus FOLFIRI Q2W or placebo plus FOLFIRI Q2W | RAM: n = 536 | 13 December 2010 | v4.02 | NCT01183780 |
| I4T-MC-JVBB | Control: n = 536 | |||||
| REACHb [7] | Advanced HCC | Ramucirumab 8 mg/kg i.v. Q2W plus BSC or placebo Q2W plus BSC | RAM: n = 283 | 04 November 2010 | v4.0 | NCT01140347 |
| I4T-IE-JVBF | Control: n = 282 | |||||
| ROSEc [8] | Metastatic breast cancer | Ramucirumab 10 mg/kg i.v. plus docetaxel 75 mg/m2 Q3W or docetaxel 75 mg/m2 plus placebo Q3W | RAM: n = 759 | 11 August 2008 | v3.0 | NCT00703326 |
| I4T-IE-JVBC | Control: n = 385 |
Intent-to-treat population.
The primary end point for these studies was overall survival.
The primary end point for this study was progression-free survival.
BSC, best supportive care; CRC, colorectal carcinoma; GEJ, gastroesophageal junction; FOLFIRI, leucovorin (folinic acid), fluorouracil, and irinotecan; HCC, hepatocellular carcinoma; i.v., intravenous; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; NSCLC, non-small-cell lung cancer; Q2W, every two weeks; Q3W, every 3 weeks; RAM, ramucirumab; v, version.
The purpose of this report is to (i) examine the incidence of adverse events possibly attributed to VEGF pathway inhibition based on data from six phase III clinical trials, (ii) determine specific patient- and treatment-related factors that may be associated with an increased adverse event risk, and (iii) explore how specific observed adverse events may be managed in the clinical setting.
Pooled data from these trials provide an opportunity to evaluate relatively infrequent adverse events at the individual patient level. While conditions such as thrombosis and bowel perforation may occur as part of the natural history of advanced cancers, using only registration standard placebo-controlled trials in evaluating reported adverse events permits an unbiased estimate of the number needed to trigger one additional adverse event compared with the control arm [the number needed to harm (NNH)], whereas uncontrolled trials coalesce causation and natural history.
Methods of analysis
A meta-analysis was conducted to review reported adverse events across the six completed phase III ramucirumab trials. An overview of the trials and all randomized patients (intent-to-treat population) is provided in Table 1, with the data based on the primary database lock for each trial. As all studies were placebo-controlled, the term ‘control arm’ is used herein to pool studies with placebo and those with chemotherapy plus placebo. Adverse events possibly attributed to VEGF inhibition, based on literature review [10], were evaluated in patients receiving at least one dose of study drug (safety population). Consolidated adverse event terms are defined in the supplementary Appendix, available at Annals of Oncology online. Although only arterial thromboembolic events (ATE) are considered associated with the antiangiogenic class [10], venous thromboembolic events (VTE) are also reported along with ATE, but the association between antiangiogenic agents and VTE remains unclear [11–14]. Grading of the adverse events was based on Common Terminology Criteria for Adverse Events, versions 3.0–4.02.
The relative risk (RR) and the associated 95% confidence interval (CI) were calculated for all-grade and severe/high-grade (grade ≥3) adverse events. The overall RR and 95% CI were derived using fixed-effects or mixed-effects models. In addition, for rare, severe, and fatal events, a simple pooled result or absolute risk difference without adjustment is presented. To determine consistency among studies, the meta-analyses included a statistical test of heterogeneity to determine whether any differences in RR of an adverse event were due to chance or actual differences in study results. The assumption of homogeneity was considered rejected for P < 0.10 from Cochran’s Q test. RRs were derived using a random-effects model only if the significant heterogeneity was identified among studies. Otherwise, a fixed-effects model based on the inverse variance weighting of the selected studies was used to pool the RR. The NNH and NNH leading to discontinuation were derived by calculating the inverse of the attributable risk: specifically, 1/(experimental rate − control rate). When the calculated NNH numerical value in a given section is a negative number, due to the incidence being lower in ramucirumab than in the control arm, such values are reported in data tables and not in the Results section. The statistical analysis was carried out in R3.1.1 [R Core Team (2016)] [15].
Results
A total of 4996 randomized patients received at least one dose of the study drug (safety population of 2748 received ramucirumab and 2248 received placebo). Patient characteristics and demographics are presented in Table 2, with the ramucirumab exposure being presented in the supplementary Table S1, available at Annals of Oncology online. Major inclusion criteria for study enrollment, such as adequate hematologic, hepatic, coagulation, and renal function at baseline, were similar across all trials.
Table 2.
Patient characteristics in the intent-to-treat population of ramucirumab double-blind randomized controlled phase III clinical trials
| REGARD |
RAINBOW |
REVEL |
RAISE |
REACH |
ROSE |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAM n = 238 | Control n = 117 | RAM n = 330 | Control n = 335 | RAM n = 628 | Control n = 625 | RAM n = 536 | Control n = 536 | RAM n = 283 | Control n = 282 | RAM n = 759 | Control n = 385 | |
| Median age, years (range) | 60 (30–86) | 60 (24–87) | 61 (25–83) | 61 (24–84) | 62 (21–85) | 61 (25–86) | 62 (21–83) | 62 (33–87) | 64 (28–87) | 62 (25–85) | 54 (24–82) | 54 (29–81) |
| <65, n (%) | 156 (66) | 71 (61) | 204 (62) | 212 (63) | 391 (62) | 407 (65) | 324 (60) | 321 (60) | 150 (53) | 162 (57) | 629 (83) | 325 (84) |
| ≥65, n (%) | 82 (34) | 46 (39) | 126 (38) | 123 (37) | 237 (38) | 218 (35) | 212 (40) | 215 (40) | 133 (47) | 120 (43) | 130 (17) | 60 (16) |
| Gender, n (%) | ||||||||||||
| Male | 169 (71) | 79 (68) | 229 (69) | 243 (73) | 419 (67) | 415 (66) | 289 (54) | 326 (61) | 236 (83) | 242 (86) | 0 (0) | 0 (0) |
| Female | 69 (29) | 38 (32) | 101 (31) | 92 (27) | 209 (33) | 210 (34) | 247 (46) | 210 (39) | 47 (17) | 40 (14) | 759 (100) | 385 (100) |
| Race, n (%) | ||||||||||||
| White | 181 (76) | 91 (78) | 208 (63) | 199 (59) | 526 (84) | 503 (80) | 405 (76) | 410 (76) | 139 (49) | 137 (49) | 676 (89) | 341 (89) |
| Asian | 39 (16) | 17 (15) | 110 (33) | 121 (36) | 74 (12) | 86 (14) | 111 (21) | 103 (19) | 131 (46) | 135 (48) | 31 (4) | 20 (5) |
| Black | 4 (2) | 2 (2) | 6 (2) | 6 (2) | 17 (3) | 16 (3) | 14 (3) | 16 (3) | 5 (2) | 3 (1) | 27 (4) | 14 (4) |
| Other | 14 (6) | 7 (6) | 6 (2) | 9 (3) | 10 (2) | 20 (3) | 4 (1) | 2 (<1) | 8 (3) | 7 (3) | 25 (3) | 10 (3) |
| Not reported/ missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) | 2 (<1) | 5 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ECOG PS, n (%) | ||||||||||||
| 0 | 67 (28) | 31 (26) | 117 (35) | 144 (43) | 207 (33) | 199 (32) | 263 (49) | 259 (48) | 159 (56) | 153 (54) | 439 (58) | 240 (62) |
| 1 | 171 (72) | 85 (73) | 213 (65) | 191 (57) | 420 (67) | 425 (68) | 268 (50) | 273 (51) | 124 (44) | 129 (46) | 318 (42) | 143 (37) |
| 2 or 3 | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 2 (<1) | 0 (0) | 0 (0) | 2 (<1) | 2 (<1) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) | 4 (1) | 2 (<1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; RAM, ramucirumab.
The following sections focus on some specific adverse events reported in these six randomized trials.
Hypertension
Arterial hypertension was defined as either new onset or a worsening grade of pre-existing hypertension during the trial. As shown in Table 3, there were 585 (21.3%) and 167 (7.4%) patients with all-grade hypertension in the ramucirumab and control arms, respectively. The corresponding RR was 2.7 (95% CI 2.3–3.2). A total of 246 (9.0%) patients in the ramucirumab arm experienced grade ≥3 hypertension compared with 57 (2.5%) in the control arm (RR: 3.7, 95% CI 2.8–4.9). This 6.5% increase for grade ≥3 hypertension in the ramucirumab arm represents an NNH of 1 in 16 patients (Table 4). There were only two (0.07%) reported instances of grade 4 hypertensive crisis in the ramucirumab arm (zero in control) among these trials. There were no deaths due to hypertension. In most cases, hypertension was controlled using standard antihypertensive treatment, while patients continued to receive ramucirumab therapy. Only 0.3% of patients (8/2748) discontinued ramucirumab treatment due to hypertension, or 1 in 344 patients who started ramucirumab [16].
Table 3.
Summary of the incidence and relative risk of adverse events across the six completed phase III ramucirumab clinical trials
| HTN, n (%) |
Proteinuria, n (%) |
Bleeding, n (%) |
GI bleeding, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Safety population | All grade | Grade ≥ 3 | All grade | Grade ≥ 3 | All grade | Grade ≥ 3 | All grade | Grade ≥ 3 | ||
| RAM | N = 2748 | 585 (21.3) | 246 (9.0) | 259 (9.4) | 31 (1.1) | 1031 (37.5) | 74 (2.7) | 186 (6.8) | 45 (1.6) | |
| Control | N = 2248 | 167 (7.4) | 57 (2.5) | 70 (3.1) | 1 (0.04)a | 426 (19.0) | 62 (2.8) | 103 (4.6) | 36 (1.6) | |
| Relative risk (95% CI) | 2.7 (2.3–3.2) | 3.7 (2.8–4.9) | 3.4 (2.6–4.3) | 8.3 (2.9–24.1) | 2.0 (1.8–2.2) | 1.1 (0.8–1.5) | 1.6 (1.3–2.0) | 1.1 (0.7–1.7) | ||
| GI perforation, n (%) | ATE, n (%) | VTEb, n (%) | IRR, n (%) | Wound-healing complications, n (%) | ||||||
| All grade | Grade ≥ 3 | All grade | Grade ≥ 3 | All grade | Grade ≥ 3 | All gradeb | Grade ≥ 3 | All grade | Grade ≥ 3 | |
| RAM | 30 (1.1) | 28 (1.0) | 38 (1.4) | 21 (0.8) | 106 (3.9) | 56 (2.0) | 180 (6.6) | 28 (1.0) | 14 (0.5) | 5 (0.2) |
| Control | 7 (0.3) | 6 (0.3) | 40 (1.8) | 19 (0.8) | 116 (5.2) | 61 (2.7) | 104 (4.6) | 13 (0.6) | 4 (0.2) | 0 (0)a |
| Relative risk (95% CI) | 3.2 (1.5–7.0) | 3.2 (1.4–7.3) | 0.8 (0.5–1.3) | 0.9 (0.5–1.7) | 0.7 (0.5–1.1) | 0.7 (0.4–1.2) | 1.4 (0.8–2.3) | 1.5 (0.8–2.7) | 2.0 (0.8–5.1) | 1.9 (0.5–7.5) |
For rare events (events that were not observed in at least one treatment arm in any study), the relative risk might not be reliable due to large variability.
Random-effects analysis model utilized due to significant identified heterogeneity.
ATE, arterial thromboembolic events; CI, confidence interval; GI, gastrointestinal; HTN, hypertension; IRR, infusion-related reactions; RAM, ramucirumab; VTE, venous thromboembolic events.
Table 4.
The NNH in each adverse event from the six completed phase III ramucirumab clinical trials
| SUMMARY |
REGARD |
RAINBOW |
REVEL |
RAISE |
REACH |
ROSE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Safety | RAM | Control | RAM | Control | RAM | Control | RAM | Control | RAM | Control | RAM | Control | RAM | Control |
| population | N = 2748 | N = 2248 | n = 236 | n = 115 | n = 327 | n = 329 | n = 627 | n = 618 | n = 529 | n = 528 | n = 277 | n = 276 | n = 752 | n = 382 |
| NNH |
NNH |
NNH |
NNH |
NNH |
NNH |
NNH |
||||||||
| All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | |
| Hypertension | 7 | 16 | 12 | 20 | 5 | 8 | 17 | 29 | 6 | 12 | 8 | 11 | 6 | 20 |
| Proteinuria | 16 | 92 | 280 | 236 | 9 | 82 | 39 | 627 | 8 | 35 | 8 | 46 | 27 | 251 |
| Bleeding | 5 | −1535 | 71 | 128 | 4 | 54 | 7 | 788 | 5 | 133 | 8 | −68 | 4 | −111 |
| GI bleeding | 46 | 2768 | 372 | 280 | 25 | 47 | 91 | 318 | 18 | 133 | 145 | −46 | 44 | −153 |
| GI perforation | 128 | 133 | −4523 | −4523 | 109 | 82 | 158 | 211 | 88 | 88 | N/C | N/C | 84 | 94 |
| ATE | −252 | −1235 | 59 | 79 | 317 | 17 931 | −197 | −296 | −105 | −263 | −138 | −276 | −408 | 248 |
| VTE | −77 | −148 | −32 | −33 | −67 | −111 | −31 | −86 | 53 | 48 | 140 | −138 | −56 | −55 |
| IRR | 52 | 227 | −76 | N/C | 46 | 164 | −116 | 666 | 35 | 265 | 15 | 92 | −1217 | 3420 |
| Wound healing | 302 | 550 | N/C | N/C | N/C | N/C | −309 | N/C | 106 | 529 | −276 | N/C | 94 | 188 |
NNH calculated via the following formula: 1/(ramucirumab rate − control rate). Negative values indicate that the incidence of the given adverse event was higher in the control than in the ramucirumab arm.
ATE, arterial thromboembolic events; GI, gastrointestinal; IRR, infusion-related reactions; N/C, not calculable; NNH, number needed to harm; RAM, ramucirumab; VTE, venous thromboembolic events.
Similar proportions of patients in both treatment arms received antihypertensive agents as concurrent therapy (Table 5), except in RAINBOW where the ramucirumab arm was 17% higher than the control arm, possibly due to a higher rate of hypertension in this trial.
Table 5.
Antihypertensive agents used in the completed phase III ramucirumab clinical trials
| Patients receiving concurrent antihypertensive therapiesa,b |
||
|---|---|---|
| RAM, n (%) | Control, n (%) | |
| REGARD | 101 (42.8) | 46 (40.0) |
| RAINBOW | 180 (55.0) | 124 (37.7) |
| REVEL | 159 (25.4) | 109 (17.6) |
| RAISE | 329 (62.2) | 286 (54.2) |
| REACH | 223 (80.5) | 201 (72.8) |
| ROSE | 379 (50.4) | 167 (43.7) |
Patient is only counted once for each category.
Concurrent antihypertensive therapy includes any antihypertensive therapy received between treatment start date and 30 days after treatment end, including therapies that may have started before treatment start date.
Antihypertensive therapies included diuretics, peripheral vasodilators, beta-blocking agents, calcium channel antagonists, renin angiotensin agents, and other antihypertensive therapy.
RAM, ramucirumab.
Proteinuria
There were 259 (9.4%) patients experiencing any-grade proteinuria in the ramucirumab arm and 70 (3.1%) patients in the control arm (RR: 3.4, 95% CI 2.6–4.3) (Table 3). Thirty-one (1.1%) patients in the ramucirumab arm experienced grade ≥3 proteinuria (including only one grade 4 and no grade 5 events) versus one (0.04%) patient in the control arm. The NNH for grade ≥3 proteinuria was 1 in 92 patients (Table 4). Twenty-seven (1.0%) patients in the ramucirumab arm and one (0.04%) patient in the control arm discontinued investigational drug due to proteinuria. The NNH for proteinuria leading to discontinuation was 1 in 107 patients. Three patients (0.1%) with nephrotic syndrome, exclusively from the RAISE trial, were identified in the ramucirumab arm, all of whom were Asian patients with pre-existing hypertension treated with at least two antihypertensive medications.
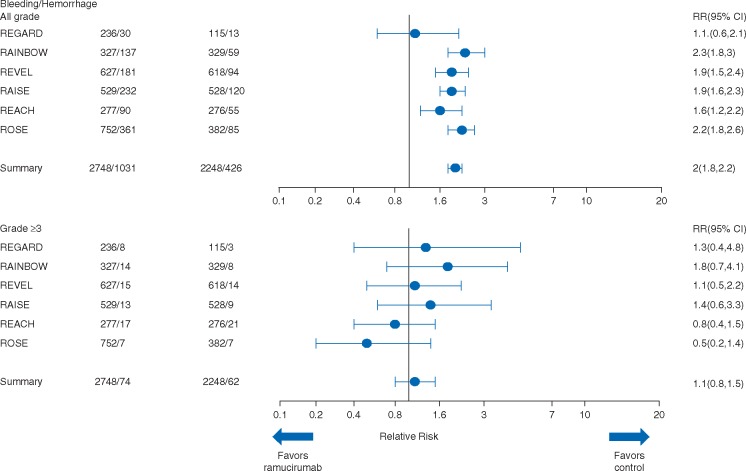
Hemorrhage/bleeding
Bleeding (all grades) was reported in 1031 (37.5%) and 426 (19.0%) patients in the ramucirumab and control arms, respectively (Table 3, Figure 1) (RR: 2.0, 95% CI 1.8–2.2). Low-grade (grade 1–2) epistaxis was the most frequently reported bleeding event in the ramucirumab arm [ranging from 5% (REGARD) to 40% (ROSE)], with the exception of three grade 3 events. Grade ≥3 bleeding was reported in 74 (2.7%) patients in the ramucirumab arm and 62 (2.8%) patients in the control arm (RR: 1.1, 95% CI 0.8–1.5).
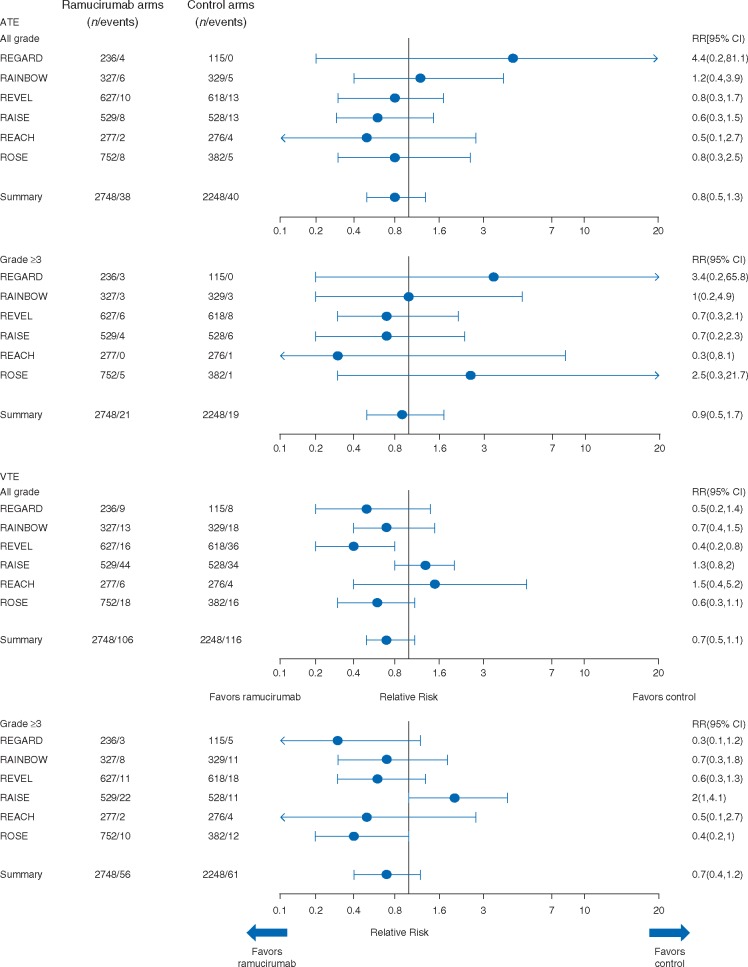
Figure 1.
Forest plots of the incidence and relative risk of ATE, VTE, and bleeding adverse events in completed phase III ramucirumab clinical trials. ATE, arterial thromboembolic events; CI, confidence interval; VTE, venous thromboembolic events; RR, relative risk.
Gastrointestinal (GI) bleeding of any grade was reported in 186 (6.8%) patients in the ramucirumab arm versus 103 (4.6%) in the control arm (RR: 1.6, 95% CI 1.3–2.0) (Table 3). Grade ≥3 events were infrequent and occurred at the same rate (1.6%) in both treatment groups (RR: 1.1, 95% CI 0.7–1.7). Thus, no difference was observed for high-grade (grade ≥3) bleeding/GI bleeding between the ramucirumab and control arms.
There were six (0.2%) patients in the ramucirumab arm who experienced grade 4 bleeding events and four of them were GI bleeding events. Ten (0.4%) patients in the control arm experienced grade 4 bleeding events, and four of them were GI bleeding events. Fatal hemorrhage/bleeding events were reported in 17 (0.6%) patients in the ramucirumab arm: GI bleeding (n = 9), pulmonary hemorrhage (n = 6), hepatic hemorrhage (n = 1), and hemorrhagic shock (n = 1). There were 14 (0.6%) fatal hemorrhage/bleeding events in the control arm: GI bleeding (n = 5), pulmonary hemorrhage (n = 6), intracranial hemorrhage (n = 2), and aortic aneurysm rupture (n = 1). Twenty-five patients (0.9%) discontinued ramucirumab treatment due to bleeding/hemorrhage [GI bleeding (n = 15), intracranial hemorrhage (n = 3), epistaxis (n = 3), hepatic hemorrhage (n = 2), hematuria (n = 1), and hemoptysis (n = 1)] compared with 17 patients (0.8%) discontinuing placebo treatment [GI bleeding (n = 14), hepatic hemorrhage (n = 1), menorrhagia (n = 1), and hemorrhage in unknown location (n = 1)]. The discontinuation rate was low and similar between the two arms.
As pulmonary hemorrhage has been a concern for non-small-cell lung cancer (NSCLC) patients treated with antiangiogenic agents [17], we compared the incidence of all-grade pulmonary hemorrhage in both squamous and nonsquamous NSCLC histologies in the REVEL trial and showed it to be similar between the treatment arms (squamous: ramucirumab arm all-grade = 9.6%, high-grade = 1.9%; control arm all-grade = 12.4%, high-grade = 2.4%; nonsquamous: ramucirumab arm all-grade = 7.3%, high-grade = 1.1%; control arm all-grade = 5.7%, high-grade = 0.9%) [5].
GI perforation
There were 30 (1.1%) patients who experienced all-grade GI perforation in the ramucirumab arm and 7 (0.3%) patients in the control arm (RR: 3.2, 95% CI 1.5–7.0) (Table 3). Grade ≥3 GI perforation was reported in 28 (1.0%) patients in the ramucirumab arm versus 6 (0.3%) patients in the control arm (RR: 3.2, 95% CI 1.4–7.3). Ten (0.4%) patients in the ramucirumab arm experienced grade 4 GI perforation compared with three (0.1%) patients in the control arm. Seven (0.3%) patients in the ramucirumab arm and one (0.04%) patient in the control arm experienced grade 5 GI perforation. Thus, one additional grade ≥3 GI perforation event would occur in every 133 patients treated with ramucirumab (Table 4). The fatal GI perforation rate in the ramucirumab arm was 1 in 393 treated patients, compared with 1 in 2248 in the control arm. This implies an increased absolute risk of fatal GI perforation events of approximately 1 in 476 patients treated with ramucirumab.
Arterial thromboembolic events
Overall, there were 38 (1.4%) and 40 (1.8%) cases of all-grade ATE in the ramucirumab and control arms, respectively (RR: 0.8, 95% CI 0.5–1.3) (Table 3, Figure 1). A total of 21 (0.8%) patients in the ramucirumab arm experienced grade ≥3 ATE versus 19 (0.8%) patients in the control arm (RR: 0.9, 95% CI 0.5–1.7).
Across all trials, seven (0.3%) grade 4 events were reported in the ramucirumab arm, with four of these events not recovered or resolved, whereas four grade 4 events were reported in the control arm. Seven grade 5 ATE events were reported in the ramucirumab arm (six myocardial events and one cerebrovascular event) and 10 grade 5 ATE events were reported in the control arm (seven myocardial events and three cerebrovascular events). Overall, the mortality rate for ATE was low and similar between the ramucirumab (0.3%) and control arms (0.4%).
Venous thromboembolic events
VTEs presented here include both symptomatic and non-symptomatic VTE events. Overall, 106 (3.9%) patients with all-grade VTEs were reported in the ramucirumab arm versus 116 (5.2%) in the control arm (RR: 0.7, 95% CI 0.5–1.1) (Table 3, Figure 1). Fifty-six (2.0%) patients with grade ≥3 VTEs were reported in the ramucirumab arm and 61 (2.7%) patients in the control arm (RR: 0.7, 95% CI 0.4–1.2).
Across all trials, 11 (0.4%) grade 4 VTEs were reported in the ramucirumab arm and 11 (0.5%) grade 4 VTEs were reported in the control arm. There were three grade 5 VTEs in the ramucirumab arm and six in the control arm; all grade 5 VTEs in both arms were pulmonary embolism (PE). The fatal VTE rate was low and similar in both the ramucirumab (0.1%) and control arms (0.3%). Ten (0.4%) patients discontinued ramucirumab treatment due to VTEs and 15 (0.7%) patients discontinued treatment in the control arm, primarily due to deep vein thrombosis or PE.
Infusion-related reactions
Any-grade infusion-related reactions (IRR) was reported in 180 (6.6%) patients in the ramucirumab arm compared with 104 (4.6%) patients in the control arm (RR: 1.4, 95% CI 0.8–2.3) (Table 3). The NNH for all-grade IRR was 1 in 52 and for grade ≥3 was 1 in 227 patients (Table 4). A total of 28 (1.0%) patients in the ramucirumab arm experienced grade ≥3 IRR versus 13 (0.6%) in the control arm (RR: 1.5, 95% CI 0.8–2.7). Four (0.1%) grade 4 events were reported in the ramucirumab arm and two (0.1%) in the control arm, with the outcome in the ramucirumab arm being either recovered or resolved. There were no grade 5 IRR reported in these trials. Twelve patients (0.4%) in the ramucirumab arm discontinued ramucirumab, whereas three (0.1%) patients in the control arm discontinued placebo due to an IRR. The NNH for IRR leading to discontinuation was 1 in 330 patients treated with ramucirumab.
Reversible posterior leukoencephalopathy syndrome
Reversible posterior leukoencephalopathy syndrome (RPLS) was reported in two (0.04%) patients in these trials, one grade 2 RPLS in ramucirumab arm and one grade 2 RPLS in control arm, both in the RAISE trial. Both patients’ RPLS status was confirmed with magnetic resonance imaging. Therefore, the incidence was approximately 1 in 2700 ramucirumab-treated patients.
Wound-healing complications
All-grade wound-healing complications were reported in 14 (0.5%) patients in the ramucirumab arm, occurring only in the RAISE (n = 6) and ROSE (n = 8) trials (Table 3 and supplementary Table S2, available at Annals of Oncology online), and in 4 (0.2%) patients in the control arm (RR: 2.0, 95% CI 0.8–5.1). Five (0.2%) patients in the ramucirumab arm experienced grade ≥3 wound-healing complications with zero patients in the control arm. The NNH for all-grade and grade ≥3 wound-healing complication events was 1 in 302 and 1 in 550 patients in the ramucirumab and control arms, respectively (Table 4).
Discussion and clinical implications
This meta-analysis represents one of the largest individual patient meta-analyses of an antiangiogenic drug and has some notable findings. First, similar to the published safety data for other antiangiogenics as a ‘class effect’, we observed a higher percentage of low-grade bleeding, GI perforation, wound-healing complications, hypertension, and proteinuria in the ramucirumab arm compared with the control arm. The rates and severity of these events are consistent with those seen in other antiangiogenic trials [10, 18–20]. In addition, the safety profile described here is consistent with the ramucirumab labels [1, 2].
Ramucirumab may differ from other antiangiogenics in relation to bleeding and thromboembolism, as no evidence for increased risk of ATE, VTE, high-grade bleeding, or high-grade GI bleeding was found in this meta-analysis. The lack of an increased risk of ATE, VTE, or high-grade bleeding differs from published studies with other antiangiogenics [11, 12, 21–25]. The mechanisms underlying these differences remain unclear, although it cannot be ruled out that differences in the incidence of these adverse events between ramucirumab and other antiangiogenics may be related to differences in the patient populations.
Arterial hypertension is recognized as a common adverse event associated with antiangiogenic therapies [26, 27]. The mechanisms of hypertension associated with VEGF inhibition are thought to include decreased production of nitric oxide in the wall of arterioles and other resistance vessels [28], increased activation of the endothelin-1 system [30], and/or capillary rarefaction [29]. Once detected, hypertension is readily managed with antihypertensives and rarely delays or stops cancer treatment. Preexisting hypertension should be controlled before starting ramucirumab treatment, and monitoring of blood pressure is recommended during therapy [1]. Although hypertension was common and required treatment, only 1 out of 344 ramucirumab patients in these trials discontinued therapy due to hypertension.
Proteinuria has been reported with antiangiogenic agents that block the effects of VEGF-A [31–33]; however, the underlying mechanism is not well understood. Inhibition of VEGF-dependent interactions between podocytes and glomerular endothelial cells lessens the integrity of the filtration barrier, leading to proteinuria [34–36]. Most patients enrolled in our trials had adequate renal function at baseline, and the incidence of high-grade proteinuria in the ramucirumab arm was 1.1% (RR: 8.3, 95% CI 2.9–24.1) (Table 3). During ramucirumab therapy, the clinician should monitor for the development or worsening of proteinuria [1]. In most cases, proteinuria was manageable during ramucirumab therapy. Only three (0.1%) patients in the ramucirumab arm reported nephrotic syndrome.
A meta-analysis of patients from 16 randomized trials with bevacizumab reported a proteinuria incidence of 2.2% and a significantly increased risk for high-grade proteinuria (RR: 4.79, 95% CI 2.71–8.46; P < 0.001) [32]. This same study also reported a 0.8% incidence of patients with nephrotic syndrome as well as an RR of 7.78 (95% CI 1.80–33.62; P = 0.006] [32].
The pathogenesis of antiangiogenic-associated bleeding is not well understood. Tumor-infiltrated vascular walls or injured mucosal membranes, which exhibit high VEGF dependence, may have an enhanced propensity to bleed [37]. Preclinical data demonstrate that capillaries with endothelial fenestrations are dependent on VEGF signaling [34, 38]. Thus, tumors with fenestrated capillaries, such as those arising in endocrine glands or the GI tract, may be particularly sensitive to anti-VEGF pathway agents, and more likely to develop capillary damage leading to hemorrhage.
Overall, the incidence rate of all-grade bleeding and GI bleeding events with ramucirumab was higher than the control arm. However, 72% of bleeding events (739/1031) in the ramucirumab arm were low-grade epistaxis requiring no intervention. Most importantly, the incidence rates of severe bleeding and GI bleeding (grade ≥ 3) were low and similar between the two treatment arms. Results from the REVEL NSCLC trial also demonstrated that, despite higher rates of all-grade pulmonary hemorrhage in both treatment arms in squamous histology patients (9.6% ramucirumab arm, 12.4% control arm) compared with nonsquamous histology (7.3% ramucirumab arm, 5.7% control arm), the incidence rate of pulmonary hemorrhage was similar between the two treatment arms within each histology [5].
GI perforation is a rare but serious adverse event and can be fatal due to severe peritonitis [19, 39]. Patients receiving ramucirumab in these studies had a low (1.0%) incidence of grade ≥3 GI perforation with an increased absolute risk of 0.7%. Despite this low incidence, patients and physicians should be aware of GI perforation and consider this in the differential diagnosis of patients with unexplained abdominal symptoms.
ATE s have been associated with some antiangiogenic therapeutic agents, particularly in the context of combination regimens including anti-VEGF antibodies and cytotoxic chemotherapy. Scappaticci et al. [12] reported that the risk of ATE was increased with chemotherapy plus bevacizumab versus chemotherapy alone (hazard ratio = 2.0, 95% CI 1.05–3.75, P = 0.031). Cancer patients have an intrinsically increased risk for thrombosis [23, 40]. The prevention and treatment of thromboembolic events is important, because they are the second most frequent cause of death in cancer patients [41]. The RRs from our results were 0.8 (95% CI 0.5–1.3) for all-grade ATEs and 0.9 (95% CI 0.5–1.7) for grade ≥3 ATEs. We cannot rule out whether the patient population included in our trials was better selected to reduce ATE risk factors due to prior knowledge of the potential risks for arterial embolic events. Since patients with any ATE (including myocardial infarction, unstable angina, cerebrovascular accident, or transient ischemic attack) within 6–12 months prior to randomization were excluded in all trials reported here except ROSE, the risk of developing ramucirumab-associated ATE for the patients with a recent history of ATE remains unclear. In aggregate, our data suggest that ramucirumab does not increase the risk of developing ATE.
Venous thrombosis is a common complication in patients with cancer [42, 43]. Compared with patients without cancer, thrombosis in patients with cancer is significantly more likely to be fatal [44]. Cancer patients with active malignancy have a four- to seven-fold higher incidence of symptomatic VTE than the general population [42, 45, 46]. VTEs have been reported with antiangiogenic therapies [11, 23–25, 47, 48], but this association is still controversial [11–14]. Our data demonstrated that ramucirumab did not increase the risk of VTE compared with the control arm (Table 3) (all-grade RR: 0.7, 95% CI 0.5–1.1; high-grade RR: 0.7, 95% CI 0.4–1.2).
Monoclonal antibodies may cause IRR [49, 50]. The incidence of all-grade and high-grade IRR was low for both treatment groups across these six trials (Tables 3 and 4), with the outcome of most reported IRR being either recovered or resolved, and no fatal IRR events were observed.
Despite previous reports of RPLS associated with antiangiogenic drug therapy [51, 52], in this meta-analysis there were single events in both treatment and control populations. Due to the extreme rarity of RPLS, a much larger patient population will be needed in order to further define this risk. Based on the evidence to date, it remains uncertain whether ramucirumab contributes to RPLS development.
VEGF mediates three effects for woundhealing: vasodilation to facilitate nutrient delivery and waste removal, increased vascular permeability for fibrinogen and plasminogen extravasation (providing a substrate for tissue growth), and angiogenesis for tissue formation and remodeling [53]. Clinical data on the effect of angiogenesis inhibitors on wound healing are limited. The risk of developing wound-healing complications with bevacizumab has been reported by Scappaticci et al. [54]. Patients experiencing major surgery concurrent with bevacizumab treatment demonstrated increased wound-healing complications, whereas no increased risk of wound-healing complications was observed in those given 5-fluorouracil/leucovorin-based chemotherapy plus bevacizumab 28–60 days post-surgical intervention versus chemotherapy-only treatment [54]. In the current study, the rate of the wound-healing complications was low overall in the ramucirumab arm (all-grade 0.5%).
A strength of this meta-analysis is that it represents one of the largest meta-analyses at the patient level of an antiangiogenic therapy, having evaluated 2748 ramucirumab-treated and 2248 placebo-treated patients. This permits the differentiation of study drug–related adverse events from toxicities of other anticancer therapy and the natural history of the disease.
One limitation of this meta-analysis is that despite a population of approximately 5000 patients, not all rare events may be observed. However, the 90% CI upper bound for the unobserved events rate in the current study population is less than 1/1000. Another (potential) limitation is that most trials reported here represented unique tumor types (with both REGARD and RAINBOW being gastric/gastroesophageal junction cancer), but supplementary Table S2, available at Annals of Oncology online, provides a summary of the incidence rate of a given AE in a given tumor type. Furthermore, although patients enrolled in these trials met study inclusion/exclusion criteria, had adequate organ function, and acceptable concurrent morbidities and medications, they may not reflect the general patient population receiving cancer treatment. Another concern in principle when combining data from multiple studies is the emergence of the so-called Simpson’s Paradox, in which a trend appears in different groups of data but disappears or reverses when these groups are combined [55, 56]. However, this is specifically a concern when studies are combined in which the randomization ratios differ substantially [57], which was not the case in the present meta-analysis: four out of the six studies had a common randomization of 1 : 1, and only the REGARD and ROSE trials, representing about 30% of the total analysis population, had a ratio of 2 : 1. Similarly, the presence/absence of chemotherapy or differences in the chemotherapy regime itself between studies is relevant on its own, particularly in the context of different proportions of patients receiving placebo in different studies.
The safety signal for all therapeutics develops over time. Four additional phase III global, registrational, placebo-controlled trials with ramucirumab are under way (https://clinicaltrials.gov/). These ongoing controlled trials will add an additional 1500–2000 patients to the overall ramucirumab-treated population and will allow further analysis of ramucirumab safety at the patient level.
Conclusions
The importance of our results for clinical practice is that the risk of developing hypertension, proteinuria, all-grade bleeding, GI perforation, IRR, RPLS, and wound-healing delay are consistent with the reported adverse events associated with the angiogenesis inhibitor class. However, we do not observe an increased risk associated with ramucirumab for developing ATE, VTE, high-grade bleeding, or high-grade GI bleeding across these trials. Also, the majority of adverse events are low grade and manageable.
Supplementary Material
Acknowledgements
We thank the patients and their caregivers who participated in the trials, and the investigators and their support staff who conducted the trials comprising this analysis. Additional thanks to Jennifer Meyer Harris (Lilly USA, LLC, Indianapolis, IN, USA) for diligent contributions to the drafting, design, and critical revisions of this manuscript. Writing and editorial assistance, funded by Eli Lilly and Company, was provided by Matthew R. Distasi (Eli Lilly and Company, Indianapolis, IN, USA) and Melissa A. Wolferz (Eli Lilly and Company, Bridgewater, NJ, USA).
Funding
This work was supported by Eli Lilly and Company (no grant number is applicable).
Disclosure
DA reports honoraria/consultancy from Bayer, Biocompatibles, Boehringer Ingelheim, Eli Lilly and Company, Roche, Sanofi, and Servier; as well as honoraria presentations for Bayer, Eli Lilly and Company, Roche, and Servier. CSF has had consultant roles for Entrinsic Health, Genetech, Merck, Gilead Sciences, Sanofi, Dicerna, Five Prime Therapeutics, Merrimack, Bayer, Agios, Taiho, Kew, and Eli Lilly and Company. JT has had consultant/advisory roles for Amgen, Bayer, Boehringer Ingelheim, Celgene, Chugai, Imclone, Eli Lilly and Company, MSD, Merck Serono, Novartis, Roche, Sanofi, Symphogen, and Taiho. AO reports a grant from BMS. AXZ reports research support to his institution from Eli Lilly and Company. EBG reports grants to his institution from Eli Lilly and Company, AstraZeneca, Boehringer Ingelheim, BMS, Genentech, Merck, Mirati, Novartis, and Pfizer. JRM has had advisory roles for Eli Lilly and Company, Roche, and Pfizer, and holds shares in Pacylex Pharmaceuticals. LP-A has had advisory roles for Eli Lilly and Company, Roche, MSD, BMS, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Clovis, and Amgen. ADB declared no conflicts of interest. TO has had an editor role, research grant, and honoraria from Novartis; reports research grants and honoraria from Pfizer, Taiho, Bayer, Chugai, Eli Lilly and Company, Yakuruto Honsha, Ono Pharmaceutical, AstraZeneca, Merck Serono, Baxter, Nobelpharma Co.; and research grants from Nippon Boehringer Ingelheim, Dainippon Simitomo Pharma, Eisai Co., OncoTherapy Science Inc., Kyowa Hakko Kirin Co., Shizuoka Industry, Nano Carrier Co., Zeria Pharmaceutical Co., and Glaxo Smith Kline. TY reports grants from Boehringer Ingelheim GmbH and GlaxoSmithKline. HHY reports grants to his institution from Eli Lilly and Company. MD, DF, YZ, YL, PB, and AS are all employees and shareholders of Eli Lilly and Company. IC reports advisory board roles at Sanofi Oncology, Eli Lilly and Company, Bristol Meyers Squibb, MSD, Bayer, Roche, and Five Prime Therapeutics; honoraria from Taiho, Pfizer, Amgen, Eli Lilly and Company, and Gilead Science; research funding from Janssen-Cilag, Sanofi Oncology, Merck-Serono, and Novartis; and would like to acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research.
References
- 1. Cyramza [package insert US]. Eli Lilly and Company, Indianapolis, IN. April 2015; https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125477s011lbl.pdf (11 September 2017, date last accessed).
- 2. Cyramza [summary of product characteristics & package insert EMA]. Eli Lilly and Company, Indianapolis, IN. May 2017; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002829/WC500180724.pdf (11 September 2017, date last accessed).
- 3. Fuchs CS, Tomasek J, Yong CJ. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383 (9911): 31–39. [DOI] [PubMed] [Google Scholar]
- 4. Wilke H, Muro K, Van Cutsem E. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15 (11): 1224–1235. [DOI] [PubMed] [Google Scholar]
- 5. Garon EB, Ciuleanu TE, Arrieta O. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384 (9944): 665–673. [DOI] [PubMed] [Google Scholar]
- 6. Tabernero J, Yoshino T, Cohn AL. et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015; 16 (5): 499–508. [DOI] [PubMed] [Google Scholar]
- 7. Zhu AX, Park JO, Ryoo BY. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015; 16 (7): 859–870. [DOI] [PubMed] [Google Scholar]
- 8. Mackey JR, Ramos-Vazquez M, Lipatov O. et al. Primary results of ROSE/TRIO-12, a randomized placebo-controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. J Clin Oncol 2015; 33 (2): 141–148. [DOI] [PubMed] [Google Scholar]
- 9. Tabernero J, Takayuki Y, Cohn AL.. Correction to Lancet Oncol 2015; 16: 499–508. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015; 16 (6): e262. [DOI] [PubMed] [Google Scholar]
- 10. Chen HX, Cleck JN.. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 2009; 6 (8): 465–477. [DOI] [PubMed] [Google Scholar]
- 11. Nalluri SR, Chu D, Keresztes R. et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008; 300 (19): 2277–2285. [DOI] [PubMed] [Google Scholar]
- 12. Scappaticci FA, Skillings JR, Holden SN. et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst.2007; 99 (16): 1232–1239. [DOI] [PubMed] [Google Scholar]
- 13. Hurwitz HI, Saltz LB, Van Cutsem E. et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol 2011; 29 (13): 1757–1764. [DOI] [PubMed] [Google Scholar]
- 14. Economopoulou P, Kotsakis A, Kapiris I, Kentepozidis N.. Cancer therapy and cardiovascular risk: focus on bevacizumab. Cancer Manag Res 2015; 7: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (12 September 2017, date last accessed).
- 16. Sashegyi A, Lin Y, Ferry D, Melemed A.. Comment on: Incidence and risk of hypertension with ramucirumab in cancer patients: a meta-analysis of published studies. Clin Drug Investig 2015; 35 (6): 405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson DH, Fehrenbacher L, Novotny WF. et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22 (11): 2184–2191. [DOI] [PubMed] [Google Scholar]
- 18. Gressett SM, Shah SR.. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother 2009; 43 (3): 490–501. [DOI] [PubMed] [Google Scholar]
- 19. Hapani S, Chu D, Wu S.. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009; 10 (6): 559–568. [DOI] [PubMed] [Google Scholar]
- 20. Faruque LI, Lin M, Battistella M. et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS One 2014; 9 (7): e101145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hang XF, Xu WS, Wang JX. et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2011; 67 (6): 613–623. [DOI] [PubMed] [Google Scholar]
- 22. Ahmadizar F, Onland-Moret NC, de Boer A. et al. Efficacy and safety assessment of the addition of bevacizumab to adjuvant therapy agents in cancer patients: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2015; 10 (9): e0136324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elice F, Rodeghiero F, Falanga A, Rickles FR.. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol 2009; 22 (1): 115–128. [DOI] [PubMed] [Google Scholar]
- 24. Elisei R, Schlumberger MJ, Muller SP. et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013; 31 (29): 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perkins SL, Cole SW.. Ziv-aflibercept (Zaltrap) for the treatment of metastatic colorectal cancer. Ann Pharmacother 2014; 48 (1): 93–98. [DOI] [PubMed] [Google Scholar]
- 26. Hamnvik OP, Choueiri TK, Turchin A. et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015; 121 (2): 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lankhorst S, Saleh L, Danser AJ, van den Meiracker AH.. Etiology of angiogenesis inhibition-related hypertension. Curr Opin Pharmacol 2015; 21: 7–13. [DOI] [PubMed] [Google Scholar]
- 28. Aparicio-Gallego G, Afonso-Afonso FJ, León-Mateos L. et al. Molecular basis of hypertension side effects induced by sunitinib. Anticancer Drugs 2011; 22 (1): 1–8. [DOI] [PubMed] [Google Scholar]
- 29. Kappers MH, van Esch JH, Sluiter W. et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010; 56 (4): 675–681. [DOI] [PubMed] [Google Scholar]
- 30. Des Guetz G, Mourad J-J, H D. et al. A mechanism of arterial hypertension induced by anti-VEGF therapy with bevacizumab: skin capillary rarefaction. Cancer Res 2007; 67 (9 Suppl): Abstract 1624–1624. [Google Scholar]
- 31. Lafayette RA, McCall B, Li N. et al. Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am J Nephrol 2014; 40 (1): 75–83. [DOI] [PubMed] [Google Scholar]
- 32. Wu S, Kim C, Baer L, Zhu X.. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 2010; 21 (8): 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Izzedine H, Massard C, Spano JP. et al. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer.2010; 46 (2): 439–448. [DOI] [PubMed] [Google Scholar]
- 34. Kamba T, McDonald DM.. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007; 96 (12): 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao N, Xu Q, Wang M. et al. Mechanism of kidney injury caused by bevacizumab in rats. Int J Clin Exp Pathol 2014; 7 (12): 8675–8683. [PMC free article] [PubMed] [Google Scholar]
- 36. Hayman SR, Calle JC, Jatoi A. et al. Urinary podocyte excretion and proteinuria in patients treated with antivascular endothelial growth factor therapy for solid tumor malignancies. Oncology 2014; 86 (5–6): 271–278. [DOI] [PubMed] [Google Scholar]
- 37. Sonpavde G, Bellmunt J, Schutz F, Choueiri TK.. The double edged sword of bleeding and clotting from VEGF inhibition in renal cancer patients. Curr Oncol Rep 2012; 14 (4): 295–306. [DOI] [PubMed] [Google Scholar]
- 38. Inai T, Mancuso M, Hashizume H. et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 2004; 165 (1): 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qi WX, Sun YJ, Tang LN. et al. Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol Hematol.2014; 89 (3): 394–403. [DOI] [PubMed] [Google Scholar]
- 40. Ay C, Dunkler D, Marosi C. et al. Prediction of venous thromboembolism in cancer patients. Blood 2010; 116 (24): 5377–5382. [DOI] [PubMed] [Google Scholar]
- 41. Hindi N, Cordero N, Espinosa E.. Thromboembolic disease in cancer patients. Support Care Cancer 2013; 21 (5): 1481–1486. [DOI] [PubMed] [Google Scholar]
- 42. Blom JW, Doggen CJ, Osanto S, Rosendaal FR.. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293 (6): 715–722. [DOI] [PubMed] [Google Scholar]
- 43. Agnelli G, George DJ, Kakkar AK. et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012; 366 (7): 601–609. [DOI] [PubMed] [Google Scholar]
- 44. Connolly GC, Phipps RP, Francis CW.. Platelets and cancer-associated thrombosis. Semin Oncol 2014; 41 (3): 302–310. [DOI] [PubMed] [Google Scholar]
- 45. Geerts WH, Bergqvist D, Pineo GF. et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (6 Suppl): 381S–453S. [DOI] [PubMed] [Google Scholar]
- 46. Heit JA, Silverstein MD, Mohr DN. et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160 (6): 809–815. [DOI] [PubMed] [Google Scholar]
- 47. Zangari M, Fink LM, Elice F. et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol 2009; 27 (29): 4865–4873. [DOI] [PubMed] [Google Scholar]
- 48. Khorana AA, Dalal M, Lin J, Connolly GC.. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013; 119 (3): 648–655. [DOI] [PubMed] [Google Scholar]
- 49. Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 2008; 13 (6): 725–732. [DOI] [PubMed] [Google Scholar]
- 50. Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007; 12 (5): 601–609. [DOI] [PubMed] [Google Scholar]
- 51. Tlemsani C, Mir O, Boudou-Rouquette P. et al. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Targ Oncol 2011; 6 (4): 253–258. [DOI] [PubMed] [Google Scholar]
- 52. Seet RC, Rabinstein AA.. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. QJM 2012; 105 (1): 69–75. [DOI] [PubMed] [Google Scholar]
- 53. Sharma K, Marcus JR.. Bevacizumab and wound-healing complications: mechanisms of action, clinical evidence, and management recommendations for the plastic surgeon. Ann Plast Surg 2013; 71 (4): 434–440. [DOI] [PubMed] [Google Scholar]
- 54. Scappaticci FA, Fehrenbacher L, Cartwright T. et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005; 91 (3): 173–180. [DOI] [PubMed] [Google Scholar]
- 55. Simpson EH. The interpretation of interaction in contingency tables. J R Stat Soc Series B Methodol 1951; 13 (2): 238–241. [Google Scholar]
- 56. El Naqa I. Perspectives on making big data analytics work for oncology. Methods 2016; 111: 32–44. [DOI] [PubMed] [Google Scholar]
- 57. Chuang-Stein C, Beltangady M.. Reporting cumulative proportion of subjects with an adverse event based on data from multiple studies. Pharmaceut Statist 2011; 10 (1): 3–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.