Abstract
For the majority of patients with newly diagnosed follicular lymphoma (FL), current treatments, while not curative, allow for long remission durations. However, several important needs remain unaddressed. Studies have consistently shown that ∼20% of patients with FL experience disease progression within 2 years of first-line treatment, and consequently have a 50% risk of death in 5 years. Better characterization of this group of patients at diagnosis may provide insight into those in need of alternate or intensive therapies, facilitate a precision approach to inform clinical trials, and allow for improved patient counseling. Prognostic methods to date have employed clinical parameters, genomic methods, and a wide assortment of biological and biochemical markers, but none so far has been able to adequately identify this high-risk population. Advances in the first-line treatment of FL with chemoimmunotherapy have led to a median progression-free survival (PFS) of approximately 7 years; creating a challenge in the development of clinical trials where PFS is a primary end point. A surrogate end point that accurately predicts PFS would allow for new treatments to reach patients with FL sooner, or lessen toxicity, time, and expense to those patients requiring little to no therapy. Quality of response to treatment may predict PFS and overall survival in FL; as such complete response rates, either alone or in conjunction with PET imaging or minimal residual disease negativity, are being studied as surrogates, with complete response at 30 months after induction providing the strongest surrogacy evidence to date. A better understanding of how to optimize quality of life in the context of this chronic illness is another important focus deserving of further study. Ongoing efforts to address these important unmet needs are herein discussed.
Keywords: follicular lymphoma, indolent lymphoma, progression-free survival, surrogate end point
Introduction
Follicular lymphoma (FL), the second most common non-Hodgkin lymphoma (NHL) in Western countries, accounts for ∼20% of NHL cases globally [1]. Although indolent in nature and often responding well to initial therapy, advanced-stage FL is an incurable malignancy characterized by frequent relapses and often increasingly aggressive disease, with risk of transformation to a more aggressive histology [2].
The natural history of FL has changed over the past several years, largely due to the incorporation of novel therapies and the monoclonal antibody rituximab. Overall survival (OS) has significantly improved and now approaches two decades [3, 4]. However, considerable clinical heterogeneity to the disease exists. Early disease recurrence in particular has significant clinical implications, and relapse within 24 months of first line treatment is estimated to occur consistently in as many as 20% of patients [5–8]. Progression within 24 months of initial chemotherapy (PFS 24) has recently been established as a robust predictor of survival in FL and associated with inferior outcomes, with only 34%–50% of patients being alive at 5 years [9]. With findings validated in multiple independent cohorts of patients, the reproducible frequency of early relapse is indicative of a uniquely high-risk subset of FL patients with heterogeneous disease biology.
Currently available clinical prognostic markers cannot adequately identify patients who will experience early relapsing or chemorefractory disease. Gene expression profiling (GEP) studies established the importance of the tumor microenvironment (TME) and sentinel mutations in FL prognosis [10–13]. However, there are currently no accurate markers associated with, or predictive of, early progressive disease. A recent clinical-genetic risk model (m7-FLIPI) including the mutation status of seven genes, the FLIPI, and the Eastern Cooperative Oncology Group (ECOG) performance status, was found to improve the risk stratification for survival in high FLIPI risk FL patients receiving first-line treatment but had limited use in predicting PFS 24 [14]. However, it was not able to accurately predict all patients with subsequent early relapse. Similarly, there is no standard treatment of early relapsed FL. The National Clinical Trials Network of the National Cancer Institute recently convened a lymphoma clinical trials planning meeting to determine priorities for lymphoma clinical trials research. Consensus from this meeting established that the top priority in FL was to impact this defined group of high-risk patients [15]. Increased knowledge about biologic determinants of early relapsing FL will facilitate the development of innovative, precision approaches tailored to individual patients. This review examines strides made in developing new prognostic tools and surrogate FL clinical trial end points that may help improve outcomes for these high-risk patients.
Front line treatment—overview
For patients with asymptomatic, low tumor burden, advanced stage FL, the timing of first-line treatment initiation is of important consideration. The watch-and-wait approach is generally implemented until the occurrence of symptoms or signs of advancing lymphoma (e.g. B symptoms, organ involvement, ascites or pleural effusion, rapid progression, or bone marrow infiltration) [16, 17]. Most studies have found no significant difference in survival between watchful waiting versus induction chemotherapy [18] or observation versus treatment with chemoimmunotherapy or rituximab monotherapy [19], suggesting that watchful waiting is a viable option to defer toxicities associated with active treatments. Given the long natural history of FL, consideration of quality of life (QoL) in selecting therapy is paramount. Although the issue remains controversial, at least one recent study reported improved time to next treatment, progression-free survival (PFS), and QOL, but not OS in asymptomatic patients treated with rituximab induction and maintenance versus observation [20].
The landmark trials adding rituximab to first-line chemotherapy improved outcomes for patients requiring treatment. In randomized studies, rituximab added to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) [21] and cyclophosphamide, vincristine, and prednisone (CVP) [22] significantly improved response and survival versus chemotherapy alone. The FOLL05 study identified superior 3-year time to treatment failure and PFS for R-CHOP and rituximab, fludarabine and mitoxantrone (R-FM) induction therapy versus R-CVP, but with fewer grade 3/4 neutropenia for R-CVP/R-CHOP than R-FM [23]. Similarly, the prospective, multicenter, US observational National LymphoCare Study (NLCS) of patients with newly diagnosed FL showed lower 5-year PFS (49% versus 58% versus 64%; P = 0.029) and 5-year OS (76% versus 86% versus 86%; P = 0.021) rates with R-CVP versus R-CHOP versus R-fludarabine-based regimens, respectively [24]. First-line bendamustine with rituximab (BR) had non-inferior responses versus R-CHOP/R-CVP in indolent NHL (iNHL) or mantle cell lymphoma (MCL) in the BRIGHT study [25]. In another randomized study in iNHL and MCL, BR showed significantly longer median PFS than R-CHOP (69.5 versus 31.2 months, P < 0.0001) and better tolerability [6].
Studies including ECOG1496 (CVP ± rituximab maintenance) and PRIMA (CVP, CHOP, or FCM + rituximab maintenance versus observation) found significant benefit in PFS (but not OS) with rituximab maintenance following first-line FL treatment [5, 26, 27], although a short (8 month) course of rituximab maintenance did not significantly improve 2-year PFS versus observation (81% versus 69%, HR = 0.74, P = 0.226) in older patients whose disease had responded to rituximab plus fludarabine, mitoxantrone, and dexamethasone (R-FND) [28]. For patients with low tumor burden FL, rituximab maintenance versus retreatment provided similar disease control and health-related QOL (HRQOL) in the ECOG E4402 (RESORT) study [29].
As these studies highlight, most newly diagnosed FL patients treated per current paradigms enjoy long PFS and OS despite an incurable illness. However, large randomized studies have consistently shown that irrespective of treatment choice, at least 20% of patients with newly diagnosed FL experience disease progression within 2 years of first-line therapy [5, 6, 8, 23]. Moreover, these patients have poorer OS compared with those patients who did not relapse within 2 years [9].
Identifying patients at risk of short survival
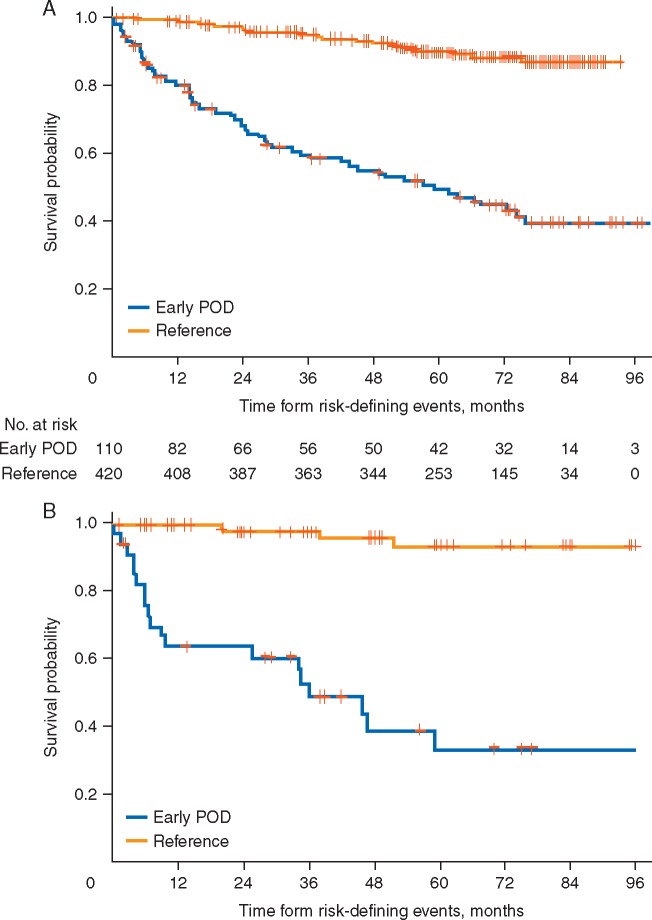
Validated prognostic methods that identify patients at the time of diagnosis who are at risk of shortened survival could inform therapy selection and clinical trial enrollment, improve stratification and data interpretation, and enhance patient counseling. An analysis of NLCS data from 588 patients with stage II–IV FL receiving first-line R-CHOP showed that consistent with other studies, 19% patients relapsed within 2 years of diagnosis [9]. Patients with early relapsing disease were significantly more likely than patients without early progression (>2 years) to have high-risk Follicular Lymphoma International Prognostic Index (FLIPI) scores (P = 0.007). Importantly, OS was markedly reduced in the early progression group, with a 5-year survival rate of 50% from the 2-year risk-defining event (i.e. progression or death) compared with 90% for patients without early progression (Figure 1). The OS hazard ratio for early versus non-early progressors receiving first-line R-CHOP was 6.44 after adjusting for FLIPI score. Exploratory analyses of NLCS patients treated with first-line R-CVP and R-fludarabine yielded similar results. Validation of these data in an independent cohort of FL patients found similar rates of poor (34%) 5-year OS in early relapsing disease. Given the marked worse prognosis associated with early relapse after first-line chemoimmunotherapy, this event may provide an informative trial selection criterion.
Figure 1.
Overall survival from risk-defining event after diagnosis in patients treated with R-CHOP in (A) the National LymphoCare Study Group, and (B) a validation set of patients with first-line FL treated with R-CHOP. Early progression of disease (POD) was defined as progression within 24 months of diagnosis. Reprinted with permission. ©2015 American Society of Clinical Oncology. All rights reserved. Casulo, C et al: J Clin Oncol 33(23), 2015: 2516–2522 [9].
There exist several previously reported prognostic methods that may be useful in identifying at diagnosis the patients who relapse within 2 years. In patients with newly diagnosed FL, FLIPI is the most widely used clinical prognostic index (Table 1) [30]. For FL patients, FLIPI score incorporates better predictive factors for estimating survival than the International Prognostic Index [30], and although originally based on data from the pre-rituximab era, studies employing various treatment regimens have confirmed its prognostic abilities [31, 32]. FLIPI score has also been reported to predict for the risk of histological transformation in grade 1 and 2 FL [33], and was prognostic for 5-year survival after first-line progression [34].
Table 1.
International prognostic indices in first-line FL
| Index | Risk parameters | Risk categories | Prognostic for |
|---|---|---|---|
| FLIPI | Age >60 years | 0–1: low | OS [30] |
| Stage III/IV | 2: intermediate | TTF from diagnosis [29] | |
| Hemoglobin <12 g/dl | 3–5: poor | Risk of transformation [31] | |
| LDH >ULN | 5-year survival from first progression [32] | ||
| >4 Nodal areas | |||
| FLIPI2 | Age >60 years | 0: low | 3-year PFS from diagnosis [33] |
| Hemoglobin <12 g/dl | 1–2: intermediate | 3-year OS from diagnosis [33] | |
| Serum β2M >ULN | 3–5: poor | 5-year PFS from diagnosis [34, 35] | |
| Bone marrow involvement | 5-year OS from diagnosis [34, 35] | ||
| Lymph node diameter >6 cm | |||
| m7-FLIPI | FLIPI score >2 | <0.8: low | 5-year FFS from treatment |
| ECOG PS > 1 | ≥0.8: high | initiation [14] | |
| Non-silent mutation in | 5-year OS from treatment initiation [14] | ||
| EZH2 | |||
| ARID1A | |||
| MEF2B | |||
| EP300 | |||
| FOX01 | |||
| CREBBP | |||
| CARD11 |
ARID1A, AT-rich interactive domain-containing protein 1A; β2M, beta-2 microglobulin; CARD11, caspase recruitment domain family, member 11; CREBBP, cyclic adenosine monophosphate (cAMP) response element-binding protein; ECOG PS, Eastern Cooperative Oncology Group performance status; EP300, E1A binding protein 300; EZH2, enhancer of zeste homolog 2; FFS, failure-free survival; FL, follicular lymphoma; FLIPI, follicular lymphoma International Prognostic Index; FOX01, forkhead box protein 01; LDH, lactate dehydrogenase; MEF2B, myocyte enhancer factor 2B; OS, overall survival; PFS, progression-free survival; TTF, time to treatment failure; ULN, upper limit of normal.
The International Follicular Lymphoma Prognostic Factor Project subsequently considered FL prognostic indices in the rituximab era using more recently reported clinical parameters (e.g. β2-microglobulin) and PFS as end points [35]. The resulting index, FLIPI2 (Table 1), stratifies patients into low-, intermediate-, and high-risk groups, which show 3-year PFS rates of 91%, 69%, and 51%, respectively (P < 0.00001) [35]. In addition, FLIPI2 does not require the assignment of lymph node groups, which with FLIPI may result in inconsistent scoring [36]. In comparison studies, FLIPI2 has superior prognostic ability, although FLIPI is still more commonly used [36, 37].
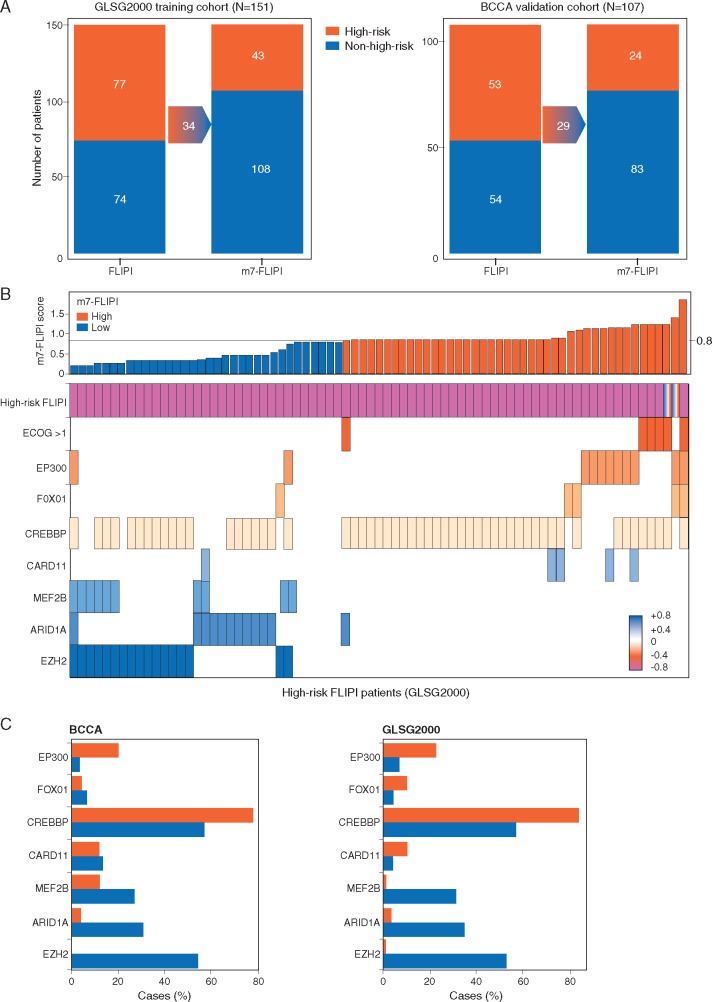
These two FLIPI measures cannot predict response to specific treatments, particularly with newer targeted agents [38]. To address this issue and improve prognostic accuracy, the German Low-Grade Lymphoma Study Group (GLSG) explored the incorporation of gene mutational status into FLIPI scoring by multivariable analysis for recurrent mutations in complete sequences of 74 genes from biopsies of 151 patients with previously untreated, symptomatic, advanced stage FL within 1 year of receiving R-CHOP and interferon maintenance [14]. Non-silent mutations from seven genes were incorporated into a model that included FLIPI and Eastern Cooperative Oncology Group performance status (ECOG PS) to yield the m7-FLIPI (Table 1). In the validation cohort, m7-FLIPI-defined high- versus low-risk groups had 5-year failure-free survival (FFS) rates of 25% versus 68% (HR = 3.58, P < 0.0001), respectively. Risk stratification by m7-FLIPI outperformed stratification methods evaluated by FLIPI and FLIPI combined with ECOG PS. Notably, not all gene mutations were associated with higher risk. Approximately half of the patients defined as high-risk by FLIPI were downgraded to low-risk by m7-FLIPI and had outcomes consistent with the low-risk group (Figure 2) [14]. In most cases, the difference in classification was due to a mutation of the transcriptional repressor EZH2 that lowered the 5-year FFS risk among high-risk FLIPI patients. However, there are limitations on the ability of m7-FLIPI to prospectively identify patients who progress within 2 years of diagnosis (i.e. early progressors). In the same GLSG patient group plus 107 patients from a Canadian population-based registry who received R-CVP plus rituximab maintenance [i.e. British Columbia Cancer Agency (BCCA)], high-risk m7-FLIPI patients were shown to be highly enriched among early progressors (within the first 2 years), but it was also evident that a significant proportion of the early progressors had been classified as low-risk by m7-FLIPI [39].
Figure 2.
Reclassification of risk category by m7-FLIPI. (A) Migration plot showing reclassification of patients by m7-FLIPI in both cohorts. (B) m7-FLIPI score for all high-risk FLIPI patients from the GLSG2000 cohort, along with the ECOG PS and molecular predictors. Boxes indicate high-risk FLIPI, an ECOG performance status of more than 1, or a mutation in the indicated gene, and the color code indicates the coefficient of the individual m7-FLIPI predictor. (C) Relative frequencies of molecular predictors by m7-FLIPI category in high-risk FLIPI patients from the BCCA and GLSG2000 cohorts are shown. Reprinted from The Lancet Oncology, 16/9, Pastore et al., Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry, 1111–1122, ©2015, with permission from Elsevier [14].
Other genomic methods have been explored for their possible prognostic utility in FL. GEP of 191 untreated FL biopsy specimens has shown 2 gene signatures based on molecular features of immune cells present at diagnosis that helped predict median survival within 4 quartiles [10]. A separate study identified a select genetic profile of 81 genes that could accurately distinguish low-grade from high-grade FL [40]. A study of differential gene expression in samples from FL patients treated with CHOP, identified 14 genes, including cyclin B1 (CCNB1), with high expression in a complete response (CR) group and low expression in a progressive disease (PD) group [41] (Table 2). High CCNB1 expression was independently prognostic in favor of improved OS when evaluated with FLIPI by multivariate analysis. A genome-wide comparative hybridization study reported copy number variants in 16 regions that were independent predictors from the International Prognostic Index (IPI) for OS in a multivariate model [42]. Subsequent work by the same group associated a mutation in the gene for tumor necrosis factor receptor superfamily member 14 (TNFRSF14) with significantly inferior OS and disease-specific survival [43]. Genome-wide copy number analysis from 198 FL and 79 transformed FL patient samples identified abnormalities in chromosomes X and 6 that predicted for shorter OS [44]. In a GEP analysis of 128 FL tumor specimens, chromosomal deletions affecting the cyclin-dependent kinase inhibitor 2A/B gene were significantly associated with inferior survival [45]. In addition to these genomic aberrations, age (>60 years), extranodal involvement, and high LDH were also significant predictors for shorter OS by multivariate analysis. Large genome wide profiling studies have established that epigenetic modification is especially important in FL; for example, MLL2 mutations are found in 89% of FL and are thought to be drivers of lymphomagenesis [46, 47]. Many other markers have also been evaluated for their association with outcomes (Table 2) [41, 48–68].
Table 2.
Potential prognostic markers in first-line FL at diagnosis
| Marker at diagnosis | N | Frequency | Risk increased | P-value | Comment |
|---|---|---|---|---|---|
| CCNB1 [41] | 57 | High CCNB1 expression led to better OS | 0.010 | FL responders to CHOP CCNB1 was independently prognostic for OS along with FLIPI | |
| BCL2 mutated [48] | 128a | 12% | With versus without BCL2 mutation | Grade 1 or 2 FL patients | |
| Transformation: HR 3.6 | <0.0001 | FLIPI-independent risk factor | |||
| Death from lymphoma: median 9.5 versus 20.4 years | 0.012 | Based on pre-rituximab era data | |||
| BM BCL2/IgH+ cells >1/102 [49] | 76 | 23% high BM BCL2+ | High versus low/intermediate BM BCL2+ | 0.007 | All patients treated with CHOP followed by R in responders who remained BCL2+ |
| CR: 26% versus 61% intermediate versus 71% low | 0.02 | ||||
| 5-year EFS: 32% versus 59% | |||||
| TP53 mutated [50] | 185 | 6% | Mutated versus wt TP53 (adjusted for IPI) | Also correlates with low expression of IR1 (P=0.016), but not IR2 (P=0.53), gene signature [10] | |
| Shorter PFS: HR 3.6 | <0.001 | ||||
| Shorter OS: HR 2.7 | 0.009 | ||||
| No association with transformation | |||||
| TP53 mutated [51] | 29 | 28% | No difference between TP53 mutated versus wtTP53 in OS from diagnosis or transformation | 0.19 | Samples from FL pre- and post-transformation to DLBCL |
| 0.45 | |||||
| PB ALC <1×109/l [52] | 228 | 28% | ALC <1×109/l versus ≥1×109/l | FLIPI-independent risk factor | |
| Median OS: 73 versus 175 months | <0.0001 | Based on pre-rituximab era data | |||
| PB ALC <0.89×109/l [53] | 79 | 49% | ALC <0.89×109/l versus ≥0.89×109/l | ALC measured pre-rituximab treatment | |
| Median TTP: 8.2 versus 36.5 months | <0.0009 | FLIPI-independent risk factor | |||
| CR: 13% versus 58% | <0.0001 | ||||
| PB AMC >0.63×109/l [54] | 428 | 25% | AMC ≤0.63×109/l versus >0.63×109/l | AMC was FLIPI-independent risk factor | |
| 5-year PFS: 61% versus 44% | 0.001 | ALC ≤1×109/l was not significantly associated with CR or 5-year PFS | |||
| CR: 77% versus 54% | <0.001 | ||||
| PB AMC ≥0.57×109/l [55] | 355 | 37% | AMC <0.57×109/l versus ≥0.57×109/l | FLIPI-independent risk factor | |
| Median OS: Not reached versus 10.2 years (HR 2.6) | <0.0001 | ||||
| PB ALC/AMC <4.7 [56] | 99 | 77% | ALC/AMC <4.7 versus ≥4.7 5-year PFS: 46% versus 77% | 0.022 | FLIPI-independent risk factor |
| Superior PFS also shown for cut-offs | |||||
| ALC ≥1.1×109/l and AMC <0.32×109/l | |||||
| Elevated serum IL-2R, IL-1RA, and CXCL9 [57] | 264 | N/A | Elevated cytokines correlated with shorter EFS | 0.013 (IL-2R) | Adjusted for IPI and initial therapy |
| HR 2.05 (IL-2R); 1.57 (IL-1RA); 1.96 (CXCL9) | 0.042 (IL-1RA) | ||||
| 0.0012 (CXCL9) | |||||
| Serum 25(OH)D | 183 | 15% | 25(OH)D <20 versus ≥20 ng/mL | IPI-independent risk | |
| <20 ng/mL (SWOG) [58] | 5-year PFS: 42% versus 65% | 0.011 | factor | ||
| 5-year OS: 82% versus 92% | 0.003 | ||||
| Serum 25(OH)D | 240 | 25% | 25(OH)D <10 versus ≥10 ng/mL | IPI-independent risk factor | |
| <10 ng/mL (PRIMA) [58] | 5-year PFS: 48% versus 61% | 0.013 | |||
| 5-year OS: 88% versus 94% | 0.14 | ||||
| High CD163+ macrophages [59] | 76a (BCCA) | 9% | High versus low CD163 + 5-year PFS (BCCA): 29% versus 61% | 0.004 | BCCA patients were treated with R-CVP |
| 144a (PRIMA) | 12% | 5-year PFS (PRIMA): 55% versus 37% | 0.030 | PRIMA patients were treated with R-CHOP followed by R maintenance or observation | |
| CD68+ macrophages >15/hpf [60] | 99 | 12% | CD68+ macrophages: <15 versus >15/hpf | IPI-independent risk factor | |
| Median PFS: 7.1 versus 1.7 years | 0.001 | Based on pre-rituximab era data | |||
| Median OS: 16.3 versus 5.0 years | <0.001 | ||||
| TAMs content <67% [61] | 96 | 33% | TAM <67% versus >67% | FLIPI- and R-FLIPI-independent risk factor | |
| 5-year PFS: 38% versus 67% | 0.006 | All patients received R-CHOP | |||
| 5-year OS: 90% versus 97% | 0.116 | ||||
| High lymph node CD8+ T-cell levels >8.6% [62] | 122 | 25% | DSS: HR 0.18 | 0.002 | High CD8+ cell levels correlated with 5× lower risk of death |
| OS: HR 0.19 | 0.001 | FLIPI-independent risk factor | |||
| No significance with survival identified based per levels of CD19+, CD3+, CD4+, CD4+/CD3+, or CD8+/CD3+ | |||||
| CD4 + >PD-1low >26% or CD8+ PD-1low >45% T-cells [63] | 32 | ∼50% | Shorter OS | 0.007 (CD4+) | Low levels of PD-1 (but not high PD-1) in CD4+ or CD8+ T-cells had significantly shorter OS |
| 0.026 (CD8+) | |||||
| Follicular FOXP3+ T cell pattern [64] | 102 | 36% | Follicular versus diffuse pattern of expression | IPI-independent risk factor | |
| Median PFS: 2.2 versus 8.8 years | 0.001 | Based on pre-rituximab era data | |||
| Median OS: 7.1 years versus NR | <0.001 | ||||
| Median RT: 13.3 years versus NR | 0.004 | ||||
| PD-1+ cells ≤5%, 6%–33%, or > 33% [65] | 100 | 25%, 50%, 25% | 5-year PFS: 20%, 46%, 48%, respectively | 0.038 | FLIPI-independent risk factor |
| 5-year OS: 50%, 77%, 95%, respectively | 0.004 | ||||
| 5-year RT: 29 (≤5% PD-1+) versus 7% (>5% PD-1+) | 0.05 | ||||
| Lymph node MVD ≤51 [66] | 46 | 55% | MVD ≤51 versus >51 | IPI-independent risk factor | |
| Median PFS: 13 versus 47 months | 0.02 | Based on pre-rituximab era data | |||
| Median OS: 59 versus >94 months | 0.03 | ||||
| Presence of tumor sclerosis in lymph nodes [67] | 157 | 14% | Shorter OS | 0.0034 | FLIPI-independent risk factor |
| Based on pre-rituximab era data | |||||
| Low skeletal muscle density (SMD)b [69] | 145 | 41% | SMD lowb versus high | FLIPI-independent risk factor | |
| Median PFS: 69.6 versus 106.7 months | 0.01 | ||||
| Median OS: 92.7 months versus NR | 0.0002 | ||||
| ORR: 83% versus 96% | 0.01 | ||||
Data were adjusted for multivariate analysis where available.
In the validation cohort.
Defined as <36.6 and <33.1 Hounsfield units for non-overweight (BMI ≤25 kg/m2) and overweight (BMI ≤25 and >25 kg/m2) patients, respectively.
25(OH)D, 25-hydroxyvitamin D; ALC, absolute lymphocyte count; AMC, absolute monocyte count; BCCA, British Columbia Cancer Agency; BCL2, B-cell lymphoma 2; BM, bone marrow; CCNB1, cyclin B1; CD, cluster of differentiation (cell surface marker); CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; CXCL, chemokine (CXC motif) ligand; DLBCL, diffuse large B-cell lymphoma; DSS, disease-specific survival; EFS, event-free survival; FL, follicular lymphoma; FLIPI, follicular lymphoma International Prognostic Index; FOXP3+, forkhead box P3-positive; hpf, high powered field; HU, Hounsfield units; HR, hazard ratio; IL, interleukin; IPI, International Prognostic Index; IR1 or IR2, immune response 1 or 2; MVD, microvessel density; N/A, not available; NR, not reached; ORR, overall response rate; OS, overall survival; PB, peripheral blood; PD-1, programmed cell death protein 1; PFS, progression-free survival; PRIMA, Primary Rituximab and Maintenance; R, rituximab; RT, risk of transformation; SMD, skeletal muscle density; SWOG, Southwest Oncology Group; TAM, tumor-associated macrophage; TP53, tumor protein p53; TTP, time to progression; wt, wild type
Low serum levels of vitamin D at diagnosis were associated with significantly shorter 5-year PFS and OS for 183 patients receiving CHOP plus rituximab or iodine-131 tositumomab in multiple SWOG studies (S9800, S9911, or S0016) and significantly shorter 5-year PFS, but not OS in 240 patients receiving R-chemotherapy plus rituximab maintenance or observation (PRIMA) [58]. Further studies are required to determine whether serum vitamin D levels or supplementation may represent a modifiable lifestyle factor in FL.
Existing prognostic methods for identifying patients with newly diagnosed FL who are at risk of early progression and short survival have many limitations and have not yet been fully explored to determine whether these prognostic methods are able to identify at diagnosis the patients relapsing within 2 years. FLIPI and its modified versions, FLIPI2 and m7-FLIPI, have some utility in risk stratification, but do not fully define this population. Other methods have been investigated less widely. Although proposed prognostic factors have been tested in validation cohorts after training cohorts, independent confirmation of prognostic power in multiple large trials is lacking. The ability to successfully identify the 20% of patients who are at risk of early progression and shorter survival from the time of diagnosis will be a major milestone on the road to improving outcomes in FL and will foster clinical trials in this population.
Predictive models for FL are needed to match patients with particular management strategies and to risk stratify and predict outcomes for patients with first-line treatment strategies (e.g. watchful waiting, chemoimmunotherapy, chemotherapy-free immunotherapy). Currently, clinic-pathologic and genomic prognostic markers are not being used in every day practice but clinical trials in development will be evaluating the feasibility and reproducibility of using novel pathologic biomarkers to risk stratify patients. The upcoming Southwest Oncology Group (SWOG) 1608 study will be prospectively evaluating The M7-FLIPI, where patients with early relapsing FL will be randomized to receive novel treatment strategies including a PI3kinase inhibitor, lenalidomide, or CHOP, all with anti CD20 antibody obinutuzumab.
A validated surrogate end point for first-line FL trials
For most patients, advances in the treatment of FL have led to considerable improvements in survival. Four-year OS and PFS of 91% and 61%, respectively, were reported with first-line CHOP plus anti-CD20 antibodies in a combined analysis of multiple SWOG studies from 1974 to 2004, which represented a significant improvement over chemotherapy-based regimens [69]. A single-institution study of patients with previously untreated grade 1 or 2 FL treated at Stanford University from 1960 to 2003 found improvements in median OS [but not event-free survival (EFS)] from the pre-anthracycline (1960–1975) and anthracycline (1976–1986) eras to the aggressive chemotherapy/purine analog (1987–1996) and rituximab (1997–2003) eras [4]. Ten-year OS rates were 54%, 54%, 68%, and 73% in those eras, respectively, with improvements attributed to better treatment options and supportive care rather than general life expectancy gains. In the current chemoimmunotherapy era, median survival is likely approaching 20 years.
Although OS remains the ultimate standard by which cancer therapies are judged, PFS represents a more practical end point for clinical trials in FL. Unlike OS, treatment effects measured by PFS are not diluted by the effects of subsequent therapy [70]. Median PFS with first-line chemoimmunotherapy induction and rituximab maintenance is now >7 years [71]. The use of PFS as a primary end point for regulatory approval of new FL therapies still necessitates lengthy trials to reach the primary end point for most patients. Phase III studies enrolling in excess of 1000 patients require more than 8 years to complete [72]. For example, the ongoing RELEVANCE trial (NCT01650701) of rituximab plus lenalidomide versus rituximab plus chemotherapy followed by rituximab, has enrolled >1000 patients and is expected to require >12 years to complete [73]. During the ensuing time lag between the start of registration studies and commercial availability, perhaps 15 years later, newer treatments might be developed or existing therapies consolidated, with potential loss of therapeutic and clinical relevance for the ongoing study [74]. Clearly, expediting the development of novel therapies in first-line FL to appropriately risk-stratify patients at diagnosis is desirable from many points of view.
A surrogate end point able to reliably predict treatment effects on PFS earlier could shorten the length of time to reach a primary end point, thus allowing effective new treatments to reach patients with FL sooner. Requirements for surrogate end points have been mathematically defined [75] and precedents exist for their use in other forms of cancer [76, 77], where they have at times served as efficacy end points for FDA approval [78].
Overall response rate (ORR) is considered an acceptable end point for FDA accelerated approval of oncology drugs, although it seldom provides a true measure of clinical benefit, particularly in the context of indolent disease with generally long survival outcomes [78]. Several studies suggest that quality of response, as determined by CR, may predict survival in FL. In the pre-rituximab era, the GELF86 study showed significantly longer OS in patients who achieved CR rather than PR (adjusted HR = 0.53, P < 0.001) [79]. Another study of low-grade lymphomas also found a significant (P < 0.0001) association of CR with survival [80]. A compilation of trial-level data from 20 clinical trials (published 1978–2005) in 5128 patients with indolent lymphoma identified a significant correlation between CR and the 3-year EFS/PFS ratio (P = 0.0007), but not with individual 3-year EFS, PFS, or OS end points [81]. A separate meta-analysis of first-line induction and/or consolidation (but not rituximab maintenance) trials (published 2001–2006) in 2421 patients with indolent lymphoma found a significant correlation between higher CR and lower risk of disease progression (P < 0.001) [82].
More recently, the Follicular Lymphoma Analysis of Surrogacy Hypothesis group analyzed trial-level and individual patient-level data from 13 randomized, first-line, induction and/or maintenance studies in FL (N = 3837) published on/after 1990 for which sufficient data on CR at 30 months after induction (CR30) were available [71]. CR30 correlated with PFS using both linear regression () and copula bivariate () models, and CR30 met all pre-specified requirements for surrogacy (, ). CR at the earlier 24-month time point (CR24) met the linear regression criteria, but did not achieve the correlation using the copula bivariate criteria. Thus, the authors concluded that first-line FL chemoimmunotherapy effects on CR30 and PFS were highly consistent on both a trial- and individual patient-level, validating its use as a surrogate end point in this setting. Whether or not CR30 will maintain its prognostic significance in an era of novel agents remains yet to be determined and we anticipate will require further prospective validation in clinical trials.
Positron emission tomography (PET) has been investigated alongside malignant lymphoma response criteria [83] and its role is increasingly being investigated in FL clinical trials. Recent identification of baseline total metabolic tumor volume correlated with PET imaging represents an early predictor of high-risk patients and assists with risk-adapted approaches to treatment [84]. Several studies have posited a correlation between post-induction PET negativity and favorable outcomes [85–90]. An analysis of end-of-induction PET scans in the PRIMA study found that PET+ patients had significantly inferior 42-month PFS (33% versus 71% PET−, P < 0.001), and a significantly higher risk of death (HR = 7.0, P = 0.0011) [88]. A prospective study from GELA/GOELAMS examined PET scan status for 121 patients with previously untreated FL [85]. Patients received six cycles of R-CHOP followed by two additional rituximab infusions and were evaluated by PET after four cycles of R-CHOP (interim) and at the end of treatment. Interim scans demonstrated that PET negativity was associated with significantly higher 2-year PFS rates (86% versus 61% PET+, P = 0.0046). End-of-treatment PET scans confirmed a strong association between PET− status and improved 2-year PFS (87% PET− versus 51% PET+, P < 0.001), as well as significantly improved 2-year OS (100% versus 88%, respectively; P = 0.0128). These results support a potential role for treatment evaluation based on PET status during or after induction. A third large study examined PET scans taken within 3 months of the end of chemoimmunotherapy induction in the FOLL05 trial, arriving at a similar conclusion [87]. Here the 3-year PFS rates favored the PET− group (66% versus 35% PET+, P < 0.001) and post-induction PET status was independent of conventional response, FLIPI score, and treatment arm. A pooled analysis of the PRIMA, PET-Folliculaire, and FOLL05 studies confirmed that PET status was a significant predictor of survival and that response assessments by PET (rather than conventional CT) were better correlated with long-term survival [90]. Further evaluation of a subset of the FOLL05 patients showed that although PET and minimal residual disease (MRD) status were not strongly correlated with each other, they could be used as complementary evaluations at the end of therapy [91].
MRD negativity as determined by the absence of detectable BCL2/IgH tumor cells in bone marrow and peripheral blood by polymerase chain reaction (PCR) assays may also predict improved outcomes in FL. An analysis of patients with detectable BCL2/IgH at screening (53%) in the FOLL05 trial associated MRD-negative status with significantly improved 3-year PFS: 66% MRD− versus 41% MRD+ (P = 0.015) at 12 months and 84% MRD− versus 50% MRD+ at 24 months (P = 0.014) [92]. Significance was seen in patients with PR and CR. Analysis of the phase III ML17638 trial of the R-FND regimen followed by rituximab consolidation or observation in first-line FL reported similar results [93]. The BCL2/IgH marker was found in 51% of 227 patients screened at diagnosis. Among these patients, end-of-therapy MRD negativity predicted improved 3-year PFS in both arms (combined, 72% MRD− versus 39% MRD+, P < 0.007).
The role of end-of-treatment MRD in predicting outcomes, however, remains somewhat controversial. As noted above, ∼50% of patients do not have detectable levels of a molecular marker at screening, and results may depend on the specific PCR method employed or evaluation time point. Not all studies have associated MRD status with prognosis, as shown in the EORTC 20981 study of CHOP or R-CHOP followed by rituximab maintenance or observation in relapsed/resistant FL, where no significant prognostic value was identified based on BCL2/IgH levels for response or PFS from second randomization [94].
Thus, although promising candidates for surrogate end points to speed clinical trials in first-line FL continue to be explored, none have been fully confirmed as primary end points in large, prospective studies. A streamlined process resulting in reproducible, feasible and cost-effective assessments of MRD could facilitate its routine incorporation into the daily practice of treating FL. Furthermore this will facilitate the discovery and use of other novel technologies such as next generation sequencing.
QoL as end points in FL
For most patients, a diagnosis of FL introduces challenges extending over many years. In this context, success is measured not only in terms of response and survival, but also in how the disease affects a patient’s ability to perform daily activities, their treatment-free time, overall outlook, and finances. HRQOL as determined by patient-reported outcomes (PROs) has become an increasingly important factor in making treatment decisions. The instruments most often used to quantify patient HRQOL in cancer are the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [95] and the Functional Assessment of Cancer Therapy—General (FACT-G) [96]. Others such as the EuroQol Group EQ-5D [97] and lymphoma-specific questionnaires such as Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) [98] are also in use.
Differences in HRQOL between arms were seen in the BRIGHT trial of BR versus R-CHOP or R-CVP in first-line iNHL and MCL [25]. As a secondary end point, HRQOL as assessed by the EORTC QLQ-C30 typically incorporates five functional scales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, nausea/vomiting, and pain), 6 single-item scales, and global health status [96]. Patients in the BR arm of the BRIGHT study reported similar or improved scores versus R-CHOP/R-CVP for cognitive, physical, social, emotional, and global health scales, as well as reductions in dyspnea, constipation, and fatigue [99]. Although not all improvements were clinically or statistically significant, the results provided further support for the use of BR in these patients. In other trials, including PRIMA, which used the EORTC QLQ-C30 and the FACT-G questionnaire to assess HRQOL, a lack of differences between treatment arms was observed [5], which is also useful in informing treatment decisions. For patients in the ‘low risk’ group without early progression, these parameters are increasingly important.
The use of PROs to assess specific issues such as illness-related anxiety was exemplified in the randomized phase III E4402 (RESORT) trial, which compared time with treatment failure and disease-related outcomes in patients with previously untreated iNHL who were randomized to maintenance rituximab versus rituximab retreatment [100]. Several specifically chosen instruments, including FACT-G, were used to assess anxiety and characterize patient coping styles as ‘active’ (patients reporting that medical visits and ongoing treatment reduced anxiety) and ‘avoidant’ (patients reporting a preference to avoid medical visits due to increased anxiety). In the study, rituximab retreatment was not associated with increased anxiety relative to rituximab maintenance regardless of coping style, but avoidant coping was associated with higher anxiety and worse HRQOL.
HRQOL questionnaires can also be used to assess financial difficulties, often a major consideration for patients and a factor that can influence treatment decisions, sometimes making combination regimens with new agents economically untenable. Cost-effectiveness has emerged as a factor to be routinely considered alongside efficacy and safety, as shown in the case of rituximab [101–104].
Conclusions
In recent decades, advances in the treatment of FL have greatly improved survival for most patients with this malignancy, but have yet to prove curative. Heterogeneity in outcomes, likely reflecting underlying pathobiological differences among patients with FL, is evident in the ∼20% of patients who progress within 2 years of diagnosis with best current treatments [5, 6, 8, 23]. The ability to prospectively identify these patients represents the first step in improving outcomes in this high-risk group. Prognostic methods (e.g. FLIPI and related clinical indices, GEP, TME markers) have so far not adequately defined this population.
With median PFS now approximately 7 years (including maintenance), clinical trials of new agents using PFS as an end point may require more than a decade to complete, impairing timely availability of newer treatments for patients. Surrogate end points (e.g. ORR, CR, PET− CR, and MRD− CR) have been explored, but have not been adequately validated for use as primary end points. An additional consequence of long survival in FL is the increasing importance of QOL. Most large first-line FL trials now include HRQOL assessment as a secondary end point.
Despite the considerable progress that has been made in the treatment of newly diagnosed FL, these and other challenges still remain. Future goals of therapy will strive for treatments that are shorter in duration or free of systemic chemotherapy, well tolerated and biologically rational. To achieve these goals, we still need to better understand what are the biologic determinants of poor risk disease? Who will benefit from an aggressive or more conservative treatment approach at diagnosis? Who will require maintenance and for what duration? What is the optimal way to optimize the use of PET scanning and MRD analysis? The answers to these questions will provide a rich resource of information with which we will be well poised to optimize treatment of all patients with FL.
Acknowledgements
Editorial support for this manuscript was provided by Robert Rydzewski MS, CMPP and Julie Kern, PhD, CMPP of Bio Connections LLC, funded by Celgene Corporation.
Funding
None declared.
Disclosure
CC has been in an advisory board role for Infinity; and her research institution has received research support from Celgene. LN has been in an advisory/consulting role for AbbVie, Gilead, Infinity, Pharmacyclics, and TG Therapeutics; and her research institution has received research support from AbbVie, Janssen, and TG Therapeutics. NHF has been in an advisory/consulting role for AbbVie, Celgene, Infinity, and Roche; and his research institution has received research support from AbbVie, Celgene, and Roche. JWF has been in an advisory/consulting role for Bayer and his research institution has received research funding from Seattle Genetics. CRF has served as a consultant for Genentech/Roche (unpaid), Gilead, AbbVie, Celgene, Seattle Genetics, Prescription Solutions, Clinical Care Options, American Society of Hematology, American Society of Clinical Oncology; and his research institution has received research funding from AbbVie, Acerta, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceutical, Millennium/Takeda, Spectrum, Onyx Pharmaceuticals, Eastern Cooperative Oncology Group, Southwest Oncology Group, Mayo Clinic, and National Cancer Institute/National Institute of Health.
References
- 1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997; 89: 3909–3918. [PubMed] [Google Scholar]
- 2. Johnson PW, Rohatiner AZ, Whelan JS. et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol 1995; 13: 140–147. [DOI] [PubMed] [Google Scholar]
- 3. Swenson WT, Wooldridge JE, Lynch CF. et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol 2005; 23: 5019–5026. [DOI] [PubMed] [Google Scholar]
- 4. Tan D, Horning SJ, Hoppe RT. et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 2013; 122: 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salles G, Seymour JF, Offner F. et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011; 377: 42–51. [DOI] [PubMed] [Google Scholar]
- 6. Rummel MJ, Niederle N, Maschmeyer G. et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013; 381: 1203–1210. [DOI] [PubMed] [Google Scholar]
- 7. Maurer MJ, Bachy E, Ghesquières H. et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol 2016; 91(11): 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Press OW, Unger JM, Rimsza LM. et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol 2013; 31: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casulo C, Byrtek M, Dawson KL. et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol 2015; 33: 2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dave SS, Wright G, Tan B. et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004; 351: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 11. Ame-Thomas P, Hoeller S, Artchounin C. et al. CD10 delineates a subset of human IL-4 producing follicular helper T cells involved in the survival of follicular lymphoma B cells. Blood 2015; 125: 2381–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green MR, Vicente-Duenas C, Romero-Camarero I. et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat Commun 2014; 5: 3904.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green MR, Gentles AJ, Nair RV. et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013; 121: 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pastore A, Jurinovic V, Kridel R. et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol 2015; 16: 1111–1122. [DOI] [PubMed] [Google Scholar]
- 15. Maddocks K, Barr PM, Cheson BD. et al. Recommendations for clinical trial development in follicular lymphoma. JNCI J Natl Cancer Inst 2016; 109(3). pii: djw255. doi: 10.1093/jnci/djw255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiddemann W, Cheson BD.. How we manage follicular lymphoma. Leukemia 2014; 28: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 17. Ghielmini M, Vitolo U, Kimby E. et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol 2013; 24: 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brice P, Bastion Y, Lepage E. et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 1997; 15: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 19. Nastoupil LJ, Sinha R, Byrtek M. et al. Outcomes following watchful waiting for stage II-IV follicular lymphoma patients in the modern era. Br J Haematol 2016; 172: 724–734. [DOI] [PubMed] [Google Scholar]
- 20. Ardeshna KM, Qian W, Smith P. et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol 2014; 15: 424–435. [DOI] [PubMed] [Google Scholar]
- 21. Hiddemann W, Kneba M, Dreyling M. et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005; 106: 3725–3732. [DOI] [PubMed] [Google Scholar]
- 22. Marcus R, Imrie K, Solal-Celigny P. et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008; 26: 4579–4586. [DOI] [PubMed] [Google Scholar]
- 23. Federico M, Luminari S, Dondi A. et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 2013; 31: 1506–1513. [DOI] [PubMed] [Google Scholar]
- 24. Nastoupil LJ, Sinha R, Byrtek M. et al. Comparison of the effectiveness of frontline chemoimmunotherapy regimens for follicular lymphoma used in the United States. Leuk Lymphoma 2015; 56: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 25. Flinn IW, van der Jagt R, Kahl BS. et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014; 123: 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hochster H, Weller E, Gascoyne RD. et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol 2009; 27: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seymour JF, Feugier P, Offner F. et al. Updated 6 year follow-up of the PRIMA study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. Blood (ASH Abstract Meeting Abstract) 2013; 122: Abstract 509. [Google Scholar]
- 28. Vitolo U, Ladetto M, Boccomini C. et al. Rituximab maintenance compared with observation after brief first-line R-FND chemoimmunotherapy with rituximab consolidation in patients age older than 60 years with advanced follicular lymphoma: a phase III randomized study by the Fondazione Italiana Linfomi. J Clin Oncol 2013; 31: 3351–3359. [DOI] [PubMed] [Google Scholar]
- 29. Kahl BS, Hong F, Williams ME. et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol E4402. J Clin Oncol 2014; 32: 3096–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solal-Celigny P, Roy P, Colombat P. et al. Follicular lymphoma international prognostic index. Blood 2004; 104: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 31. Buske C, Hoster E, Dreyling M. et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 2006; 108: 1504–1508. [DOI] [PubMed] [Google Scholar]
- 32. Arcaini L, Colombo N, Passamonti F. et al. Correlation of the FLIPI score for follicular lymphoma with period of diagnosis and type of treatment. Leuk Res 2006; 30: 277–282. [DOI] [PubMed] [Google Scholar]
- 33. Gine E, Montoto S, Bosch F. et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol 2006; 17: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 34. Montoto S, Lopez-Guillermo A, Altes A. et al. Predictive value of follicular lymphoma international prognostic index (FLIPI) in patients with follicular lymphoma at first progression. Ann Oncol 2004; 15: 1484–1489. [DOI] [PubMed] [Google Scholar]
- 35. Federico M, Bellei M, Marcheselli L. et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009; 27: 4555–4562. [DOI] [PubMed] [Google Scholar]
- 36. Press OW, Unger JM, Rimsza LM. et al. A comparative analysis of prognostic factor models for follicular lymphoma based on a phase III trial of CHOP-rituximab versus CHOP + 131iodine–tositumomab. Clin Cancer Res 2013; 19: 6624–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arcaini L, Merli M, Passamonti F. et al. Validation of follicular lymphoma international prognostic index 2 (FLIPI2) score in an independent series of follicular lymphoma patients. Br J Haematol 2010; 149: 455–457. [DOI] [PubMed] [Google Scholar]
- 38. Lopez-Guillermo A. A novel clinicogenetic prognostic score for follicular lymphoma. Lancet Oncol 2015; 16: 1011–1012. [DOI] [PubMed] [Google Scholar]
- 39. Jurinovic V, Kridel R, Staiger AM. et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood. 2016; 128: 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glas AM, Kersten MJ, Delahaye LJ. et al. Gene expression profiling in follicular lymphoma to assess clinical aggressiveness and to guide the choice of treatment. Blood 2005; 105: 301–307. [DOI] [PubMed] [Google Scholar]
- 41. Bjorck E, Ek S, Landgren O. et al. High expression of cyclin B1 predicts a favorable outcome in patients with follicular lymphoma. Blood 2005; 105: 2908–2915. [DOI] [PubMed] [Google Scholar]
- 42. Cheung KJ, Shah SP, Steidl C. et al. Genome-wide profiling of follicular lymphoma by array comparative genomic hybridization reveals prognostically significant DNA copy number imbalances. Blood 2009; 113: 137–148. [DOI] [PubMed] [Google Scholar]
- 43. Cheung KJ, Johnson NA, Affleck JG. et al. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 2010; 70: 9166–9174. [DOI] [PubMed] [Google Scholar]
- 44. Bouska A, McKeithan TW, Deffenbacher KE. et al. Genome-wide copy-number analyses reveal genomic abnormalities involved in transformation of follicular lymphoma. Blood 2014; 123: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwaenen C, Viardot A, Berger H. et al. Microarray-based genomic profiling reveals novel genomic aberrations in follicular lymphoma which associate with patient survival and gene expression status. Genes Chromosom Cancer 2009; 48: 39–54. [DOI] [PubMed] [Google Scholar]
- 46. Pasqualucci L, Trifonov V, Fabbri G. et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 2011; 43: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morin RD, Mendez-Lago M, Mungall AJ. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011; 476: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Correia C, Schneider PA, Dai H. et al. BCL2 mutations are associated with increased risk of transformation and shortened survival in follicular lymphoma. Blood 2015; 125: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rambaldi A, Carlotti E, Oldani E. et al. Quantitative PCR of bone marrow BCL2/IgH+ cells at diagnosis predicts treatment response and long-term outcome in follicular non-Hodgkin lymphoma. Blood 2005; 105: 3428–3433. [DOI] [PubMed] [Google Scholar]
- 50. O'Shea D, O'Riain C, Taylor C. et al. The presence of TP53 mutation at diagnosis of follicular lymphoma identifies a high-risk group of patients with shortened time to disease progression and poorer overall survival. Blood 2008; 112: 3126–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davies AJ, Lee AM, Taylor C. et al. A limited role for TP53 mutation in the transformation of follicular lymphoma to diffuse large B-cell lymphoma. Leukemia 2005; 19: 1459–1465. [DOI] [PubMed] [Google Scholar]
- 52. Siddiqui M, Ristow K, Markovic SN. et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol 2006; 134: 596–601. [DOI] [PubMed] [Google Scholar]
- 53. Behl D, Ristow K, Markovic SN. et al. Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. Br J Haematol 2007; 137: 409–415. [DOI] [PubMed] [Google Scholar]
- 54. Marcheselli L, Bari A, Anastasia A. et al. Prognostic roles of absolute monocyte and absolute lymphocyte counts in patients with advanced-stage follicular lymphoma in the rituximab era: an analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Br J Haematol 2015; 169: 544–551. [DOI] [PubMed] [Google Scholar]
- 55. Wilcox RA, Ristow K, Habermann TM. et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma 2012; 53: 575–580. [DOI] [PubMed] [Google Scholar]
- 56. Kumagai S, Tashima M, Fujikawa J. et al. Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol 2014; 99: 737–742. [DOI] [PubMed] [Google Scholar]
- 57. Mir MA, Maurer MJ, Ziesmer SC. et al. Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood 2015; 125: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kelly JL, Salles G, Goldman B. et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J Clin Oncol 2015; 33: 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kridel R, Xerri L, Gelas-Dore B. et al. The prognostic impact of CD163-positive macrophages in follicular lymphoma: a study from the BC Cancer Agency and the Lymphoma Study Association. Clin Cancer Res 2015; 21: 3428–3435. [DOI] [PubMed] [Google Scholar]
- 60. Farinha P, Masoudi H, Skinnider BF. et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 2005; 106: 2169–2174. [DOI] [PubMed] [Google Scholar]
- 61. Taskinen M, Karjalainen-Lindsberg ML, Nyman H. et al. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res 2007; 13: 5784–5789. [DOI] [PubMed] [Google Scholar]
- 62. Wahlin BE, Sander B, Christensson B, Kimby E.. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res 2007; 13: 388–397. [DOI] [PubMed] [Google Scholar]
- 63. Yang ZZ, Grote DM, Ziesmer SC. et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 2015; 5: e281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farinha P, Al-Tourah A, Gill K. et al. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood 2010; 115: 289–295. [DOI] [PubMed] [Google Scholar]
- 65. Carreras J, Lopez-Guillermo A, Roncador G. et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 2009; 27: 1470–1476. [DOI] [PubMed] [Google Scholar]
- 66. Koster A, van Krieken JH, Mackenzie MA. et al. Increased vascularization predicts favorable outcome in follicular lymphoma. Clin Cancer Res 2005; 11: 154–161. [PubMed] [Google Scholar]
- 67. Klapper W, Hoster E, Rolver L. et al. Tumor sclerosis but not cell proliferation or malignancy grade is a prognostic marker in advanced-stage follicular lymphoma: the German Low Grade Lymphoma Study Group. J Clin Oncol 2007; 25: 3330–3336. [DOI] [PubMed] [Google Scholar]
- 68. Chu MP, Lieffers J, Ghosh S. et al. Skeletal muscle radio-density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PLoS ONE 2015; 10: e0127589.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fisher RI, LeBlanc M, Press OW. et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol 2005; 23: 8447–8452. [DOI] [PubMed] [Google Scholar]
- 70. Broglio KR, Berry DA.. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009; 101: 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shi Q, Flowers CR, Hiddemann W. et al. Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: an individual patient-level analysis of multiple randomized trials. J Clin Oncol 2017; 35: 552–560. [DOI] [PubMed] [Google Scholar]
- 72. Fine G, Horning S.. Perspective of clinical research in follicular NHL: interaction between science and industry. Best Pract Res Clin Haematol 2011; 24: 313–321. [DOI] [PubMed] [Google Scholar]
- 73. Morschhauser F, Salles G, Flinn I, et al. The “RELEVANCE” trial: A phase 3 open label randomized study to compare the efficacy and safety of rituximab plus lenalidomide (CC-5013) versus rituximab plus chemotherapy followed by rituximab in subjects with previously untreated follicular lymphoma ICML Abstracts 2013:136.
- 74. Illidge TM. Radioimmunotherapy of lymphoma: a treatment approach ahead of its time or past its sell-by date? J Clin Oncol 2010; 28: 2944–2946. [DOI] [PubMed] [Google Scholar]
- 75. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8: 431–440. [DOI] [PubMed] [Google Scholar]
- 76. Sargent DJ, Patiyil S, Yothers G. et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol 2007; 25: 4569–4574. [DOI] [PubMed] [Google Scholar]
- 77. Sargent DJ, Wieand HS, Haller DG. et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005; 23: 8664–8670. [DOI] [PubMed] [Google Scholar]
- 78. Johnson JR, Williams G, Pazdur R.. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 2003; 21: 1404–1411. [DOI] [PubMed] [Google Scholar]
- 79. Bachy E, Brice P, Delarue R. et al. Long-term follow-up of patients with newly diagnosed follicular lymphoma in the prerituximab era: effect of response quality on survival–a study from the groupe d'etude des lymphomes de l'adulte. J Clin Oncol 2010; 28: 822–829. [DOI] [PubMed] [Google Scholar]
- 80. Lopez-Guillermo A, Montserrat E, Bosch F. et al. Applicability of the International Index for aggressive lymphomas to patients with low-grade lymphoma. J Clin Oncol 1994; 12: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 81. Lee L, Wang L, Crump M.. Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points. Ann Oncol 2011; 22: 1392–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saville MW, Leonard JP, Hainsworth JD. et al. Role of different frontline regimens in achieving complete response in follicular lymphoma: a meta-analysis of CR rate and its relation to hazard rate for disease progression. Blood (ASH Annual Meeting Abstracts) 2006; 108: Abstract 2754. [Google Scholar]
- 83. Cheson BD, Pfistner B, Juweid ME. et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 84. Meignan M, Cottereau AS, Versari A. et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol 2016. [epub ahead of print], pii: JCO669440. [DOI] [PubMed] [Google Scholar]
- 85. Dupuis J, Berriolo-Riedinger A, Julian A. et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol 2012; 30: 4317–4322. [DOI] [PubMed] [Google Scholar]
- 86. Janikova A, Bolcak K, Pavlik T. et al. Value of [18F]fluorodeoxyglucose positron emission tomography in the management of follicular lymphoma: the end of a dilemma? Clin Lymphoma Myeloma 2008; 8: 287–293. [DOI] [PubMed] [Google Scholar]
- 87. Luminari S, Biasoli I, Versari A. et al. The prognostic role of post-induction FDG-PET in patients with follicular lymphoma: a subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi (FIL). Ann Oncol 2014; 25: 442–447. [DOI] [PubMed] [Google Scholar]
- 88. Trotman J, Fournier M, Lamy T. et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 2011; 29: 3194–3200. [DOI] [PubMed] [Google Scholar]
- 89. Zinzani PL, Musuraca G, Alinari L. et al. Predictive role of positron emission tomography in the outcome of patients with follicular lymphoma. Clin Lymphoma Myeloma 2007; 7: 291–295. [DOI] [PubMed] [Google Scholar]
- 90. Trotman J, Luminari S, Boussetta S. et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol 2014; 1: e17–e27. [DOI] [PubMed] [Google Scholar]
- 91. Luminari S, Galimberti S, Versari A. et al. Positron emission tomography response and minimal residual disease impact on progression-free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica 2016; 101: e66–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Galimberti S, Luminari S, Ciabatti E. et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res 2014; 20: 6398–6405. [DOI] [PubMed] [Google Scholar]
- 93. Ladetto M, Lobetti-Bodoni C, Mantoan B. et al. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood 2013; 122: 3759–3766. [DOI] [PubMed] [Google Scholar]
- 94. van Oers MH, Tonnissen E, Van Glabbeke M. et al. BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: results of a prospective randomized EORTC 20981 phase III intergroup study. J Clin Oncol 2010; 28: 2246–2252. [DOI] [PubMed] [Google Scholar]
- 95. Aaronson NK, Ahmedzai S, Bergman B. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 96. Yost KJ, Thompson CA, Eton DT. et al. The Functional Assessment of Cancer Therapy - General (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leuk Lymphoma 2013; 54: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Herdman M, Gudex C, Lloyd A. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cella D, Webster K, Cashy J. et al. Development of a measure of health-related quality of life for non-Hodgkin’s lymphoma clinical research: the Functional Assessment of Cancer Therapy - Lymphoma (FACT-Lym). Blood (ASH Annual Meeting Abstracts) 2005; 106: Abstract 750. [Google Scholar]
- 99. Burke JM, van der Jagt RH, Kahl BS. et al. Differences in quality of life between bendamustine-rituximab and R-CHOP/R-CVP in patients with previously untreated advanced indolent non-Hodgkin lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2016; 16: 182–190, e181. [DOI] [PubMed] [Google Scholar]
- 100. Wagner LI, Zhao F, Hong F. et al. Anxiety and health-related quality of life among patients with low-tumor burden non-Hodgkin lymphoma randomly assigned to two different rituximab dosing regimens: results from ECOG trial E4402 (RESORT). J Clin Oncol 2015; 33: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen Q, Ayer T, Nastoupil LJ. et al. Comparing the cost-effectiveness of rituximab maintenance and radioimmunotherapy consolidation versus observation following first-line therapy in patients with follicular lymphoma. Value Health 2015; 18: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Griffiths RI, Gleeson ML, Mikhael J, Danese MD.. Impact on medical cost, cumulative survival, and cost-effectiveness of adding rituximab to first-line chemotherapy for follicular lymphoma in elderly patients: an observational cohort study based on SEER-Medicare. J Cancer Epidemiol 2012; 2012: 978391.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Johnston KM, Bolbocean C, Connors J, Peacock S.. Cost-effectiveness of rituximab in follicular lymphoma. Expert Rev Pharmacoecon Outcomes Res 2012; 12: 569–577. [DOI] [PubMed] [Google Scholar]
- 104. Prica A, Chan K, Cheung M.. Frontline rituximab monotherapy induction versus a watch and wait approach for asymptomatic advanced-stage follicular lymphoma: a cost-effectiveness analysis. Cancer 2015; 121: 2637–2645. [DOI] [PubMed] [Google Scholar]