Abstract
Background
Conventional criteria for tumor progression may not fully reflect the clinical benefit of immunotherapy or appropriately guide treatment decisions. The phase II IMvigor210 study demonstrated the efficacy and safety of atezolizumab, a programmed death-ligand 1-directed antibody, in patients with platinum-treated locally advanced or metastatic urothelial carcinoma. Patients could continue atezolizumab beyond Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 progression at the investigator’s discretion: this analysis assessed post-progression outcomes in these patients.
Patients and methods
Patients were treated with atezolizumab 1200 mg i.v. every 3 weeks until loss of clinical benefit. Efficacy and safety outcomes in patients who experienced RECIST v1.1 progression and did, or did not, continue atezolizumab were analyzed descriptively.
Results
In total, 220 patients who experienced progression from the overall cohort (n = 310) were analyzed: 137 continued atezolizumab for ≥ 1 dose after progression, 19 received other systemic therapy, and 64 received no further systemic therapy. Compared with those who discontinued, patients continuing atezolizumab beyond progression were more likely to have had a baseline Eastern Cooperative Oncology Group performance status of 0 (43.1% versus 31.3%), less likely to have had baseline liver metastases (27.0% versus 41.0%), and more likely to have had an initial response to atezolizumab (responses in 11.7% versus 1.2%). Five patients (3.6%) continuing atezolizumab after progression had subsequent responses compared with baseline measurements. Median post-progression overall survival was 8.6 months in patients continuing atezolizumab, 6.8 months in those receiving another treatment, and 1.2 months in those receiving no further treatment. Atezolizumab exposure-adjusted adverse event frequencies were generally similar before and following progression.
Conclusion
In this single-arm study, patients who continued atezolizumab beyond RECIST v1.1 progression derived prolonged clinical benefit without additional safety signals. Identification of patients most likely to benefit from atezolizumab beyond progression remains an important challenge in the management of metastatic urothelial carcinoma.
ClinicalTrials.gov ID
Keywords: atezolizumab, immunotherapy, post-progression outcomes, programmed death-ligand 1, PD-L1, urothelial cancer
Introduction
More than 165 000 patients are estimated to die annually from urothelial cancer worldwide [1]. First-line platinum-based chemotherapy is associated with overall survival (OS) of approximately 9–15 months [2–4]; however, there is no global standard for patients who progress after platinum therapy and the median OS is approximately 7–9 months [5]. However, cancer immunotherapies are addressing this high unmet need in locally advanced or metastatic urothelial cancer (mUC).
Immune checkpoints, including programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1), can inhibit development of active immune responses [6], and PD-L1 or PD-1 inhibition has produced promising results in a variety of solid tumors [7, 8]. Atezolizumab (anti-PD-L1) selectively targets PD-L1 to block interactions with receptors PD-1 and B7.1 to reinvigorate and stimulate anticancer immunity, while leaving the PD-L2/PD-1 interaction intact [6, 9]. Atezolizumab demonstrated durable responses and safety across multiple cancers, including mUC [7, 9–12]. In the United States, Europe, and elsewhere, atezolizumab is approved for previously treated metastatic non-small-cell lung cancer, untreated cisplatin-ineligible mUC, and mUC that has progressed after platinum-based chemotherapy. In addition, other PD-L1- or PD-1-targeting antibodies are approved for platinum-treated mUC [13], and pembrolizumab is also approved for cisplatin-ineligible patients.
Commonly used criteria for assessing progression, Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) [14], were formulated for conventional anticancer agents—largely based on radiographic criteria—before the advent of immune checkpoint inhibitors. Responses to such agents, including atezolizumab, can manifest as delayed or nonclassical responses [15, 16]; for instance, infiltration of the tumor microenvironment can transiently increase tumor size, leading to apparent progressive disease (PD) per RECIST v1.1. Such criteria may therefore be inadequate to fully assess clinical benefit or guide treatment [17, 18]. Patients in the platinum-treated cohort of IMvigor210 who developed PD per RECIST v1.1 were permitted to continue atezolizumab until investigator-deemed loss of clinical benefit. Initial results from this study reported on the overall cohort of patients, which had a median follow-up of 11.7 months [11]. To better understand the efficacy and safety of atezolizumab beyond RECIST v1.1 PD, in the current updated analysis, we descriptively evaluated post-progression outcomes in patients from IMvigor210.
Methods
The design and primary outcomes from the single-arm phase II study of atezolizumab in mUC (IMvigor210) were reported previously [11]. This post hoc analysis reports on previously platinum-treated patients (cohort 2) who experienced PD per RECIST v1.1 as determined by a central independent review facility (IRF; BioClinica, Princeton, NJ): 220 of 310 patients from the overall cohort. Further details on eligibility, dosing, endpoints, assessments, and statistical considerations are provided in the supplementary material, available at Annals of Oncology online. Patients could continue treatment with atezolizumab beyond IRF-assessed RECIST v1.1 PD provided the following conditions were met: evidence of stabilization or improvement of disease-related symptoms, no unequivocal signs of progression (e.g. worsened laboratory parameters), no decline in Eastern Cooperative Oncology Group performance status (ECOG PS) attributed to progression, and no signs of progression unmanageable by protocol-allowed interventions. Tumor assessments (supplementary material, available at Annals of Oncology online) for patients treated beyond PD occurred at either the next scheduled assessment or at 6 (±2) weeks as an unscheduled assessment if the scan frequency was every 12 weeks (or earlier if clinically indicated). Outcomes were evaluated descriptively, both in those who continued atezolizumab (≥ 1 dose after PD) and those who received any other systemic treatment or no treatment after progression. Confirmed IRF-assessed objective response rates (ORRs) per RECIST v1.1 were reported with respect to study baseline, and changes in the sum of target lesion longest diameters (SLDs) were calculated relative to either study baseline or a reset baseline based on measurements at the time of PD. All post-PD scans were included in the post-PD best overall response evaluations for this analysis. Details on immune-modified RECIST (imRECIST) assessments, radiographic criteria designed specifically for responses to cancer immunotherapies, are in the supplementary material, available at Annals of Oncology online. OS was defined as the time from first dose of atezolizumab to death (from any cause), and post-progression OS was defined from IRF-assessed PD to death. Adverse event (AE) frequencies and grades were recorded overall and as adjusted incidence rates based on safety follow-up duration per 100 patient-years.
Results
Patients and treatment
From the overall cohort of 310 patients, 220 experienced IRF-assessed RECIST v1.1 PD and were included in this analysis, 137 of whom received atezolizumab beyond PD. Of the other 83 patients, 19 received another systemic treatment after PD (mainly chemotherapy; n = 16; supplementary Table S1, available at Annals of Oncology online), and 64 had received no other systemic treatment at the time of analysis (4 July 2016). Baseline patient and disease characteristics were largely comparable between groups although several adverse prognostic factors at baseline (e.g. liver metastases, ECOG PS 1, hemoglobin < 10 g/dl) [19] were more common in patients who did not continue atezolizumab (Table 1). A numerically lower rate of palliative radiotherapy use occurred for patients who received no systemic therapy beyond progression (supplementary Table S2, available at Annals of Oncology online). Patients who received atezolizumab beyond progression were also more likely to have had high baseline PD-L1 expression on tumor-infiltrating immune cells. At time of PD, 24 patients who continued atezolizumab (17.6%), 12 who received other therapy (63.2%), and 36 (66.7%) with no subsequent systemic therapy experienced worsening of ECOG PS from baseline; further, 69 (50.4%), 9 (47.4%), and 39 (60.9%) patients, respectively, experienced new lesions at PD.
Table 1.
Baseline patient and disease characteristicsa
| Characteristic | Post-progression atezolizumab treatment | No post-progression atezolizumab treatment | Post-progression treatment in the 83 patients not receiving post-progression atezolizumab |
|
|---|---|---|---|---|
| Other systemic treatment | No systemic treatment | |||
| (n = 137) | (n = 83) | (n = 19) | (n = 64) | |
| Median (range) age, years | 66.0 (36–84) | 67.0 (32–91) | 65.0 (45–91) | 67.5 (32–86) |
| Male sex, n (%) | 110 (80.3) | 59 (71.1) | 12 (63.2) | 47 (73.4) |
| Primary site bladder, n (%) | 102 (74.5) | 62 (74.7) | 15 (78.9) | 47 (73.4) |
| ECOG PS, n (%) | ||||
| 0 | 59 (43.1) | 26 (31.3) | 8 (42.1) | 18 (28.1) |
| 1 | 78 (56.9) | 57 (68.7) | 11 (57.9) | 46 (71.9) |
| Metastatic site, n (%) | ||||
| Visceralb | 112 (81.8) | 72 (86.7) | 16 (84.2) | 56 (87.5) |
| Liver | 37 (27.0) | 34 (41.0) | 8 (42.1) | 26 (40.6) |
| Bone | 20 (14.6) | 22 (26.5) | 7 (36.8) | 15 (23.4) |
| Lymph node only | 18 (13.1) | 10 (12.0) | 3 (15.8) | 7 (10.9) |
| Hemoglobin < 10 g/dl, n (%) | 22 (16.1) | 24 (28.9) | 3 (15.8) | 21 (32.8) |
| No of Bellmunt risk factors, n (%)c | ||||
| 0 | 49 (35.8) | 11 (13.3) | 4 (21.1) | 7 (10.9) |
| 1 | 47 (34.3) | 37 (44.6) | 8 (42.1) | 29 (45.3) |
| 2 | 33 (24.1) | 27 (32.5) | 7 (36.8) | 20 (31.3) |
| 3 | 8 (5.8) | 8 (9.6) | 0 | 8 (12.5) |
| Glomerular filtration rate | 50 (36.5) | 37 (44.6) | 7 (36.8) | 30 (46.9) |
| < 60 ml/min, n (%) | ||||
| Prior systemic regimens in metastatic setting, n (%) | ||||
| 0 | 29 (21.2) | 11 (13.3) | 1 (5.3) | 10 (15.6) |
| 1 | 55 (40.1) | 34 (41.0) | 13 (68.4) | 21 (32.8) |
| 2 | 22 (16.1) | 23 (27.7) | 2 (10.5) | 21 (32.8) |
| 3 | 16 (11.7) | 11 (13.3) | 3 (15.8) | 8 (12.5) |
| ≥ 4 | 15 (10.9) | 4 (4.8) | 0 | 4 (6.3) |
| Prior platinum-based chemotherapy, n (%) | ||||
| Cisplatin | 104 (75.9) | 60 (72.3) | 11 (57.9) | 49 (76.6) |
| Carboplatin onlyd | 33 (24.1) | 22 (26.5) | 8 (42.1) | 14 (21.9) |
| PD-L1 status, n (%) | ||||
| IC2/3e | 49 (35.8) | 15 (18.1) | 4 (21.1) | 11 (17.2) |
| IC1f | 43 (31.4) | 36 (43.4) | 9 (47.4) | 27 (42.2) |
| IC0g | 45 (32.8) | 32 (38.6) | 6 (31.6) | 26 (40.6) |
Refers to characteristics at study baseline for patients with indicated treatment patterns following first independent review facility–assessed Response Evaluation Criteria In Solid Tumors version 1.1 progressive disease.
Visceral metastasis defined as liver, lung, bone, or any nonlymph node or soft tissue metastasis.
Defined by baseline ECOG PS > 0, hemoglobin level < 10 g/dl, and/or liver metastases.
Refers to carboplatin with no other prior platinum. One patient in the no post-progression atezolizumab and no systemic treatment categories who received another platinum agent is not included.
≥ 5% PD-L1 expression on IC.
< 5% and ≥ 1% PD-L1 expression on IC.
< 1% PD-L1 expression on IC.
ECOG PS, Eastern Cooperative Oncology Group performance status; IC, tumor-infiltrating immune cell; PD-L1, programmed death-ligand 1.
Atezolizumab exposure is shown in supplementary Table S3, available at Annals of Oncology online. Median time from PD to the first post-PD dose of atezolizumab was 3 days, and the 137 patients receiving post-PD atezolizumab were treated for a median of 1.6 months (3 doses); > 80% received ≥ 2 doses. More than one-third of patients (n = 53) were treated for > 3 months beyond progression, and 11.7% (n = 16) were treated for > 1 year.
Efficacy
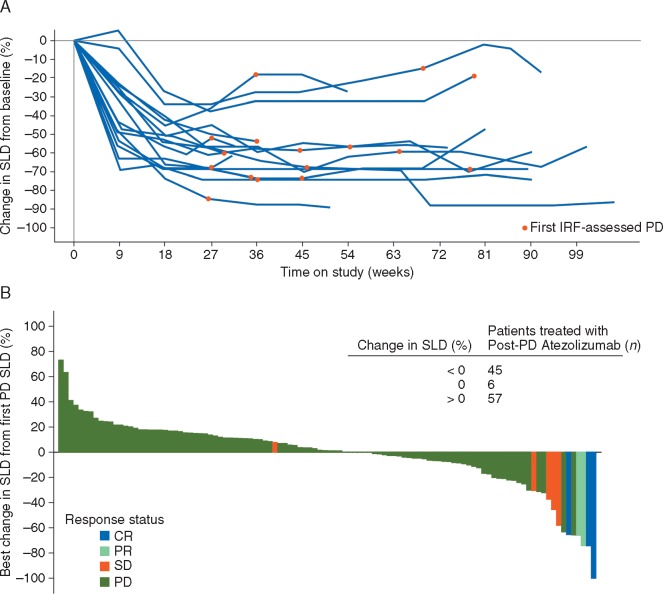
Sixteen of the 137 patients treated with atezolizumab beyond PD (11.7%) had an objective RECIST v1.1 response to atezolizumab before PD, compared with one patient (1.2%) who did not continue atezolizumab after PD (supplementary Table S4, available at Annals of Oncology online). Changes in tumor burden from baseline in pre-progression responders to atezolizumab are plotted in Figure 1A. None of these initial responders to atezolizumab subsequently achieved a post-PD response with respect to study baseline although the magnitude of SLD reduction appeared to be maintained on study. Regardless of initial response status, the time to PD was similar among all subgroups evaluated (supplementary Table S5, available at Annals of Oncology online).
Figure 1.
Changes in sum of target lesion longest diameters (SLDs) on study. (A) Changes in SLDs from the start of treatment in patients treated with atezolizumab, after Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) progression, who achieved a confirmed objective response before RECIST v1.1 progression [per independent review facility assessment (IRF)] (n = 16) The first occurrence of IRF-assessed PD is indicated by red circles. (B) Best post-progressive disease (PD) change in SLDs (from time of PD) for all patients treated with atezolizumab post-PD and who had a post-PD evaluation (n = 108). Response status refers to best overall post-PD response (per IRF) relative to study baseline. CR, complete response; PR, partial response; SD, stable disease.
Post-PD tumor assessments were evaluable for 108 of the 137 patients who continued atezolizumab beyond PD, and best changes in tumor burden with respect to time of PD are shown in Figure 1B for these patients. Forty-five patients (32.8% of 137) experienced a reduction in SLD of any magnitude with respect to measurements at PD. Five RECIST v1.1 objective responses (3.6%) were observed with respect to baseline in patients continuing atezolizumab after PD (best post-PD response of partial response in two patients and complete response in three patients); none of these patients previously experienced a response (supplementary Table S4, available at Annals of Oncology online). Similar results were observed using imRECIST criteria: five patients (5.1%) who continued atezolizumab following imRECIST PD (n = 99) achieved a post-PD imRECIST objective response (one complete and four partial responses). Post-progression scans were unavailable for 18 of 19 patients who received other systemic therapies and for 62 of 64 patients who received no subsequent systemic therapy. No RECIST v1.1 responses were seen in the three patients who did have scans although one patient (1.6% of 64) who received no further treatment had a reduction in SLD.
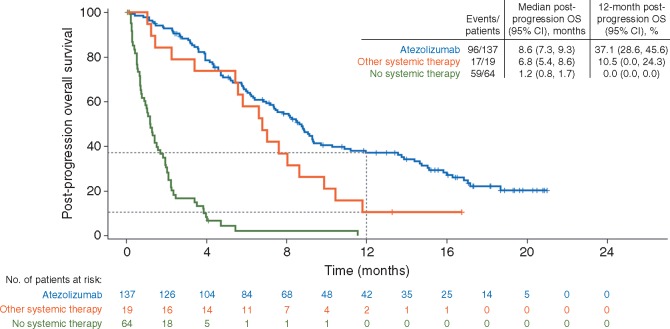
With a median follow-up of 21.2 months from start of treatment (range, 0.6+ to 4.5), median OS was 12.8 months in patients who continued atezolizumab, 8.8 months in patients who received only other systemic therapy, and 2.9 months in patients who received no systemic therapy (supplementary Table S6, available at Annals of Oncology online). With a median follow-up of 17.1 months from time of first progression (range, 0–21.0 months), median post-PD OS was 8.6 months in patients who continued atezolizumab, 6.8 months in patients who received other systemic treatment, and 1.2 months in patients who received no treatment beyond progression (Figure 2). OS at 12 months beyond progression was 37.1%, 10.5%, and 0% in these subgroups, respectively (Figure 2).
Figure 2.
Overall survival (OS) from time of progression. Kaplan–Meier plot of OS by treatment status beyond progression. Inset displays median post-progression OS and 12-month OS rates. Median post-progression survival follow-up durations were 17.3 months (range, 0.2+ to 21.0 months) in patients who received atezolizumab beyond progression 15.0 months (range, 1.0+ to 16.7 months) in patients who received other systemic therapy, and not estimable (range, 0-11.5+ months) in patients who received no systemic therapy beyond progression.
Compared with all patients who received post-PD atezolizumab, post-PD OS was numerically longer in those with a baseline ECOG PS 0, no visceral metastases, or only lymph node disease (median post-PD OS: 14.4, 14.7, and 14.7 months, respectively, for each subgroup). Median post-PD OS in patients continuing atezolizumab was 7.3 months in those with a baseline ECOG PS 1 and 8.2 months in those with visceral metastases. Patients with liver metastases at baseline who continued atezolizumab had a median post-PD OS of 6.0 months (9.0 months in those without liver metastases). In the 16 patients who had initially responded and continued treatment beyond progression, median post-PD OS was 10.3 months, whereas median post-PD OS was 8.4 months in the 121 patients who had not responded and continued treatment beyond progression. Post-PD OS by baseline and clinical characteristics for patients who did or did not continue atezolizumab is shown in supplementary Table S7, available at Annals of Oncology online.
Safety
Safety was evaluated in patients treated with atezolizumab beyond progression (Table 2). Treatment-related AEs were reported before PD in 91 patients (66.4%) and following PD in 73 patients (53.3%). The safety profile was similar to that in the overall study population [11]; fatigue was the most common treatment-related AE of any grade (in 32.1% before PD and 12.4% post-PD), followed by nausea and pruritus (11.7% each before PD and 4.4% an 8.0%, respectively, after PD) in this analysis. Grade 3 treatment-related AEs were reported in 10 patients (7.3%) before PD and in 12 patients (8.8%) after PD. Grade 4 treatment-related AEs occurred in two patients (1.5%) before PD and in one patient (0.7%) after PD. No treatment-related deaths were reported. Pre- and post-PD AE incidence rates adjusted for safety follow-up duration are shown in Table 2. Treatment-related AE incidence rates per 100 patient-years appeared similar, albeit numerically lower in the post-PD setting, regardless of grade. Three patients (2.2%) had AEs regardless of attribution that led to atezolizumab withdrawal post-PD.
Table 2.
Adverse event incidence in patients treated with post-progression atezolizumab
| Patients treated with post-progression atezolizumab (n = 137) |
||
|---|---|---|
| Before or at progression | After progression | |
| All-grade treatment-related AEs, n (%) | 91 (66.4) | 73 (53.3) |
| Total patient-years at risk | 17.8 | 31.2 |
| Exposure-adjusted AE incidence rate per 100 patient-years | 512.1 | 234.3 |
| Grade 3-4 treatment-related AEs, n (%)a | 12 (8.8) | 13 (9.5) |
| Total patient-years at risk | 42.7 | 60.0 |
| Exposure-adjusted AE incidence rate per 100 patient-years | 28.1 | 21.7 |
| AEs leading to withdrawal, n (%)b | 1 (0.7) | 3 (2.2) |
| Total patient-years at risk | 46.3 | 60.7 |
| Exposure-adjusted AE incidence rate per 100 patient-years | 2.2 | 4.9 |
No treatment-related grade 5 AEs were observed.
Regardless of attribution, including grade 2 fatigue (before or at progression), grade 3 retroperitoneal infection and acute kidney injury (n = 1 each), and grade 5 cerebral hemorrhage (n = 1).
AE, adverse event.
Discussion
Cancer immunotherapies are shifting the treatment landscape and outlook for patients with mUC. However, use of these agents requires reevaluation of assessments of clinical benefit to appropriately guide treatment duration and characterize outcomes [17, 18]. Patients from the platinum-treated cohort of this phase II study could receive atezolizumab until loss of clinical benefit, and primary outcomes from the overall cohort reported a tolerable safety profile coupled with durable objective responses [11]. In this post hoc analysis, we report that many patients who continued atezolizumab beyond progression derived prolonged clinical benefit, including new responses with respect to baseline and reductions in SLD. These observations suggest that further to response durability and OS seen in the overall cohort [11], atezolizumab treatment beyond progression may result in non-classical responses and durable tumor burden reductions, which may contribute to prolonged OS seen in these patients (post-PD median 8.6 months).
Prolonged post-PD OS (median > 14 months) was observed in some patient subgroups with favorable baseline prognostic characteristics, particularly ECOG PS 0, lymph node-only disease, or no visceral metastases. Furthermore, the median post-PD OS of 8.4 months in patients who continued beyond progression without prior RECIST v1.1 response to atezolizumab (10.3 months for responders) suggests that achieving an initial response is not the sole indicator of survival benefit. However, although < 50% of patients treated beyond PD were known to have received subsequent non-protocol therapy (supplementary Table S1, available at Annals of Oncology online), potential confounding effects from such therapies cannot be eliminated. Yet, these OS data—based on a study with long-term follow-up—generally compare well with historic observations for platinum-treated mUC [5]. The safety profile of atezolizumab before and after progression was generally similar and agrees with that seen in the overall study cohort [11]. When adjusted for safety follow-up, treatment-related AE rates before or after progression were comparable, but numerically lower after progression. These data suggest prolonged tolerability in this population that included heavily pre-treated patients, consistent with observations from the overall phase I study cohort of previously treated patients with mUC, wherein most treatment-related AEs occurred earlier on study [20].
Post-PD OS was also assessed during follow-up in patients who discontinued atezolizumab in this exploratory analysis. Those treated with another systemic therapy had a median post-PD OS of 6.8 months, and those who received no further systemic treatment had a median post-PD OS of 1.2 months. These observations are in line with the lack of treatments and consensus in later palliative lines of therapy [5, 21]. However, because subsequent therapies (atezolizumab or non-protocol therapies) were not randomly allocated, subgroups were not controlled for imbalances, and efficacy was not evaluated by specific chemotherapy regimens, formal comparisons of outcomes between post-PD treatment subgroups are precluded. Treatment delays and patient heterogeneity could also underestimate clinical benefit in patients who discontinued atezolizumab. Furthermore, decisions regarding continuation of atezolizumab were based on investigator assessments of clinical benefit, and patients continuing atezolizumab would be expected to have more favorable prognostic factors, creating a protocol-specified selection bias in favor of atezolizumab. This latter factor might explain the apparent numerically lower rates of treatment-related AEs and longer OS in this study with respect to the overall cohort [11].
To our knowledge, this study was the first detailed analysis of post-progression outcomes in patients with mUC treated with a checkpoint inhibitor. Our data agree with similar reports from other tumor types [15, 22, 23] and limited data in mUC [24], which collectively demonstrate post-progression clinical benefit with immunotherapy; notably, in this study, patients who received any dose of atezolizumab were included for analyses (> 80% of patients received > 1 post-progression treatment dose), whereas other studies selected those treated > 6 weeks after progression [22, 23]; therefore, the scope for comparison is limited. Nevertheless, our study expands the knowledge on post-progression use of checkpoint inhibitors, and provides rationale for ongoing clinical studies employing pre-treatment with checkpoint inhibitors. A next critical step will be molecular-level characterization of the underlying biology of responses to anti-PD-L1 therapy after initial PD. Several molecular biomarkers of immunotherapy activity have already been reported from the platinum-treated IMvigor210 patients [11]. Unfortunately, very few patients from the cohort (n = 17) had evaluable biopsies at time of PD, and attempts to associate pre-treatment molecular data with post-progression outcomes are difficult. In this respect, our data emphasize the need for comprehensive, sequential collection of on-treatment tumor biopsies and warrant prospective evaluation in clinical trials.
The results of the current analysis suggest that previously platinum-treated patients with mUC can obtain prolonged clinical benefit from continuation of atezolizumab beyond RECIST v1.1 progression, with prolonged OS in some subgroups. However, further characterization of treatment patterns, post-progression outcomes, and biomarkers may require randomized controlled trials. Important future challenges include identifying patients most likely to derive benefit from atezolizumab, determining optimal treatment durations and sequencing, and developing treatment algorithms in mUC across lines of therapy.
Supplementary Material
Acknowledgements
We thank the patients, their families, and the clinical study site investigators and staff. Medical writing assistance for this manuscript was provided by John Carron, PhD, and Ashley J. Pratt, PhD, of Health Interactions and funded by F. Hoffmann-La Roche, Ltd.
Funding
F. Hoffmann-La Roche, Ltd. (no grant number applies); National Cancer Institute at the National Institutes of Health (P30 CA008748 to JER).
Disclosure
AN has consultancies with AstraZeneca, Bayer, Merck Sharp & Dohme, and Roche and has received research funding for his institution from Amgen, Astra Zeneca, and Merck Sharp & Dohme. RWJ has consultancies with Bristol-Myers Squibb, Eisai, Genoptix, and Nektar and has received research funding for his institution from Amgen, Bristol-Myers Squibb, Merck, Roche/Genentech, and X4P. YL has received grants from Sanofi and fees for consulting or advisory roles from Astellas, AstraZeneca, Janssen, Merck Sharp & Dohme, Roche, and Sanofi. JH-C had consultancies with Roche/Genentech. JLP-G has received grants or funding from Roche, had consultancies with Roche, and has received honoraria from Roche. DPP has ownership interest in Bellicum Pharmaceuticals and Tyme, Inc., has received honoraria from Astellas Pharma, Bayer, Bellicu Pharmaceuticals, Exelixis, Ferring, Genentech, Johnson and Johnson, Medivation, Merck Serono, Millennium, Pfizer, Progenics, and Sanofi, receives honoraria from Astellas Pharma, Bayer, Bellicum Pharmaceuticals, Exelixis, Ferring, Genentech, Johnson and Johnson, Medivation, Merck Serono, Millennium, Pfizer, Progenics, and Sanofi, and has consultancies with Bayer, Bellicum Pharmaceuticals, Dendreon, Exilixis, Ferring, Johnson & Johnson, Medivation, Millennium, Pfizer, and Sanofi. CLD, DT, BD, and CK are employees of Roche/Genentech and own Roche stock. QZ is a contractor of Roche/Genentech. JER has received grants or funding from Bristol-Myers Squibb and Roche and has had consultancies with Agensys, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, EMD-Serono, Merck, Roche, and Seattle Genetics.
References
- 1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. De Santis M, Bellmunt J, Mead G. et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von der Maase H, Sengelov L, Roberts JT. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–4608. [DOI] [PubMed] [Google Scholar]
- 4. Necchi A, Sonpavde G, Lo Vullo S. et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective international study of invasive/advanced cancer of the urothelium (RISC). Eur Urol 2017; 71: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raggi D, Miceli R, Sonpavde G. et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 2016; 27: 49–61. [DOI] [PubMed] [Google Scholar]
- 6. Chen DS, Mellman I.. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emens LA, Ascierto PA, Darcy PK. et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer 2017; 81: 116–129. [DOI] [PubMed] [Google Scholar]
- 9. Herbst RS, Soria JC, Kowanetz M. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDermott DF, Sosman JA, Sznol M. et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016; 34: 833–842. [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg JE, Hoffman-Censits J, Powles T. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balar AV, Galsky MD, Rosenberg JE. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farina MS, Lundgren KT, Bellmunt J.. Immunotherapy in urothelial cancer: recent results and future perspectives. Drugs 2017; 77: 1077–1089. [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 15. Gandara DR, von Pawel J, Sullivan R. et al. Atezolizumab treatment beyond disease progression in advanced NSCLC: results from the randomized Ph III OAK study. J Clin Oncol 2017; 35(Suppl): abstr 9001. [DOI] [PubMed] [Google Scholar]
- 16. Mazieres J, Fehrenbacher L, Rittmeyer A. et al. Non-classical response measured by immune-modified RECIST and post-progression treatment effects of atezolizumab in 2L/3L NSCLC: results from the randomized phase II study POPLAR. J Clin Oncol 2016; 34(Suppl): abstr 9032. [Google Scholar]
- 17. Seymour L, Bogaerts J, Perrone A. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodi FS, Hwu WJ, Kefford R. et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34: 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellmunt J, Choueiri TK, Fougeray R. et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28: 1850–1855. [DOI] [PubMed] [Google Scholar]
- 20. Petrylak PD, Powles T, Bellmunt J. et al. Atezolizumab (atezo) in patients with metastatic urothelial carcinoma (mUC): a 2-year clinical update from a phase Ia study. J Clin Oncol 2017; 15(Suppl 6): abstr 290. [Google Scholar]
- 21. Pal SK, Galsky MD, Lin S. et al. Second-line metastatic urothelial carcinoma treatment and survival in real-world patients in the United States. Ann Oncol 2016; 27: 266–295. [Google Scholar]
- 22. Long GV, Weber JS, Larkin J. et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol 2017; doi:10.1001/jamaoncol.2017.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. George S, Motzer RJ, Hammers HJ. et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol 2016; 2: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma P, Callahan MK, Bono P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016; 17: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.