Abstract
Background
Cixutumumab is a fully human IgG1 monoclonal antibody to the insulin-like growth factor type I receptor that can potentially reverse resistance and enhance the efficacy of chemotherapy.
Methods
Bevacizumab-eligible patients with stage IV or recurrent non-squamous, non-small-cell lung cancer and good performance status were randomized to receive standard doses of paclitaxel, carboplatin, and bevacizumab to a maximum of six cycles followed by bevacizumab maintenance (CPB) until progression (arm A) or CPB plus cixutumumab 6 mg/kg i.v. weekly (arm B).
Results
Of 175 patients randomized, 153 were eligible and treated (78 in arm A; 75 in arm B). The median progression-free survival was 5.8 months (95% CI 5.4–7.1) in arm A versus 7 months (95% CI 5.7–7.6) in arm B (P = 0.33); hazard ratio 0.92 (95% CI 0.65–1.31). Objective response was 46.2% versus 58.7% in arm A versus arm B (P = 0.15). The median overall survival was 16.2 months in arm A versus 16.1 months in arm B (P = 0.95). Grade 3/4 neutropenia and febrile neutropenia, thrombocytopenia, fatigue, and hyperglycemia were increased with cixutumumab.
Conclusions
The addition of cixutumumab to CPB increased toxicity without improving efficacy and is not recommended for further development in non-small-cell lung cancer. Both treatment groups had longer OS than historical controls which may be attributed to several factors, and emphasizes the value of a comparator arm in phase II trials.
ClinicalTrials.gov Identifier
Keywords: cixutumumab, non-small-cell lung cancer, bevacizumab, paclitaxel, carboplatin
Introduction
Lung cancer is a common and lethal malignancy that accounts for most cancer-related deaths in the United States and worldwide [1, 2]. Patients typically present at advanced disease stages when treatment is rarely curative. In the 1990s, several new chemotherapeutic agents were shown to have significant activity in non-small-cell lung cancer (NSCLC). These agents include the taxanes (paclitaxel and docetaxel), gemcitabine, irinotecan, and vinorelbine. Phase II trials have demonstrated that all of these agents have reported single agent activity of 20%–30%. Having demonstrated activity as single agents, these new drugs were then evaluated in combination. A previous study by our group (E1594) demonstrated that the efficacy of platinum doublets, including carboplatin/paclitaxel, cisplatin/paclitaxel, cisplatin/gemcitabine, and cisplatin/docetaxel, is comparable in the treatment of advanced NSCLC [3]. However, the addition of a third cytotoxic agent has not improved survival despite higher response rates [4]. With standard contemporary platinum-based chemotherapy regimens, median survival of patients with advanced NSCLC is 8–10 months and 1-year survival ∼40% [5]. The incorporation of a third novel, molecular targeted agent to a platinum doublet has been the subject of multiple phase III randomized studies. In a phase III trial (E4599) that enrolled a total of 878 patients with advanced non-squamous NSCLC, the median progression-free survival (PFS) was 6.4 versus 4.5 months (P < 0.0001) and the overall survival (OS) 12.5 versus 10.2 months (P = 0.007) in the bevacizumab arm versus the carboplatin/paclitaxel alone arm [6]. A higher incidence of grade 4 neutropenia (24% versus 16%, P = 0.006), grade 3–4 hypertension (6% versus 0.7%, P < 0.001), and grade 3–4 bleeding (5% versus 0.7%, P < 0.001) was observed in the bevacizumab arm. The identification of novel agents that can further enhance the efficacy of platinum doublets plus bevacizumab in patients who are candidates for bevacizumab remains a major focus of clinical investigation in NSCLC.
Insulin-like growth factor type I receptor (IGF-IR) is a member of the insulin receptor family that plays critical roles in epithelial cancer cell development, proliferation, motility, and survival [7]. IGF-IR shares extensive homology with the insulin receptor. IGF-IR is expressed on the cell surface as preformed dimmers and is activated through the binding of two high affinity binding ligands, insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF-II) [8]. A large number of preclinical and clinical studies have implicated the IGF-IR and its ligands, IGF-I and IGF-II, in the development, maintenance, and progression of cancer. Immunohistochemical analysis of human tumor samples has indicated that a majority of tumor sections across many tissue types stained positive for IGF-IR [9]. IGFs have been shown to be strong mitogens for a variety of solid tumors [7–10], including lung cancers; this effect is mediated through the IGF-IR. The principal pathways for transduction of the IGF signal are the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways.
IGF-IR signaling has also been shown to protect tumor cells from the cytotoxic effects of chemotherapy and radiation and may be an important factor in tumor cell drug resistance [11, 12]. Inhibition of IGF-IR signaling concurrent with chemotherapy treatment enhances tumor cell apoptosis in several models, including cisplatin-treated ovarian cell lines [13], gemcitabine-treated pancreatic cancer xenografts [14], and vinorelbine-treated breast and non-small-cell lung xenografts [15]. IGF-I and IGF-II may also contribute to tumor angiogenesis; these factors are capable of inducing VEGF synthesis in tumor cells [16, 17].
Cixutuxumab or IMC-A12 (Imclone, Inc.) is a fully human IgG1 monoclonal antibody to the IGF-IR that possesses high affinity for IGF-IR and acts as an antagonist of IGF-I and IGF-II ligand binding and signaling [18, 19]. Cixutumumab does not bind to or recognize the human insulin receptor [20]. Cixutumumab inhibited ligand-induced growth of numerous human tumor cell lines in vitro, including that for breast, prostate, pancreatic, lung, and colon [18, 19]. In vivo tumor growth inhibition has also been demonstrated against in xenograft models including human colon, breast, lung, pancreatic, prostate, and renal carcinoma [18]. Down-regulation of IGF-1 R phosphorylation in endothelial cells was also observed with in vivo treatment of cixutumumab [22]. Cixutumumab plus chemotherapy has been tested in xenograft models, and enhanced tumor control was observed in combination with a number of agents including paclitaxel, 5-fluorouracil, cisplatin, and irinotecan [19, 21].
Two phase I studies evaluated weekly (3–15 mg/kg) or every-2-weeks (6–15 mg/kg) dosing of cixutumumab in patients with advanced solid tumors [22]. A total of 24 and 16 patients were enrolled in the weekly and every-2-week dosing studies, respectively. Severe adverse events (AE) were infrequent; one serious AE (grade 3 electrocardiogram QT prolongation) was deemed possibly cixutumumab-related (10 mg/kg every-2-weeks). Hyperglycemia was a common AE. A maximum tolerated dose was not identified; pre-determined target serum minimum concentrations (60 μg/ml) were achieved with ≥6 mg/kg weekly and ≥10 mg/kg every-2-week dosing. Overall, stable disease was achieved in 25% of all patients.
Cixutumumab is well tolerated and has the potential of reversing resistance to chemotherapy and thus enhancing the efficacy of platinum-based chemotherapy for advanced NSCLC. Our aim was to study the combination of an IGF-IR inhibitor with chemotherapy in patients with bevacizumab-eligible advanced NSCLC, i.e. predominantly of adenocarcinoma histology.
Patient and methods
Eligible patients were adults with histologically or cytologically confirmed with non-squamous, NSCLC with advanced disease defined as either recurrent disease after prior radiation or surgery or stage IV (M1a or M1b) based on the TNM staging system (American Joint Committee on Cancer 7th edition, 2009). All patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1, adequate hematologic and biochemical laboratory parameters, including fasting blood glucose within normal range (fasting < 120 mg/dl or below institutional upper limit of normal); and measurable disease (< 4 weeks before randomization) as defined by the revised Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) [23] and were previously untreated with chemotherapy or biologic/molecular targeted therapy for advanced NSCLC. Prior chemotherapy and/or biological/molecular targeted therapy as part of initial potentially curative therapy (one regimen of induction and/or adjuvant and/or concurrent chemoradiotherapy) was allowed provided it had been completed 1 year or more before randomization. Prior treatment with cixutumumab or another IGF-IR inhibitor was not allowed. Patients with poorly controlled diabetes mellitus, neuropathy > Common Terminology Criteria for Adverse Events (CTCAE) grade 1 at baseline, concurrent therapeutic anti-coagulation and history of thrombotic or hemorrhagic disorders were excluded. All patients met commonly used criteria for bevacizumab eligibility, including a urine dipstick ≤ 0–1+ or a urine protein creatinine ratio < 1.0, no history of bleeding diathesis or coagulopathy; no ≥ grade 2 bleeding or any bleeding requiring intervention within 4 weeks before randomization. Hypertension must have been well-controlled (≤ 150/90) on a stable regimen of anti-hypertensive therapy. History of gross hemoptysis (defined as ≥ 1/2 teaspoon of bright red blood) was an exclusion factor. Patients with the following medical conditions within 6 months before randomization were excluded: abdominal fistula, gastrointestinal perforation, intra-abdominal abscess, previous myocardial infarction, history of any central nervous system, cerebrovascular ischemia or active heart or vascular disease. Serious non-healing wound, ulcer, bone fracture, major surgical procedure within 4 weeks or minor surgery within 7 days before randomization, therapy with daily aspirin (>325 mg/day) or non-steroidal anti-inflammatory agents or other agents known to inhibit platelet function, were exclusion factors.
Patient evaluation and treatment plan
Patients were evaluated with laboratory tests and baseline scans, including a head computed tomography or magnetic resonance imaging, within 4 weeks before randomization. Cixutumumab (IMC-A12; Imclone Systems Inc.) was supplied under a contract with the NCI. For both arms, paclitaxel 200 mg/m2 was administered intravenously (i.v.) over 3 h, followed by carboplatin area under the curve (AUC) of 6.0 (with the maximum carboplatin dose limited to 900 mg starting from December 2010) intravenously over 30 min, then followed by bevacizumab 15 mg/kg intravenously >30–90 min, all on day 1 of each cycle (21-day cycle). For patients in arm B, cixutumumab 6 mg/kg i.v. over 1 h was additionally given on days 1, 8, and 15 of each cycle (immediately following bevacizumab when applicable). Each regimen was repeated every 21 days up to a total of six cycles until documented disease progression or intolerable toxicity. For cycles 7 and beyond, bevacizumab 15 mg/kg i.v. was administered over 30 min on day 1 of each cycle to patients on both arms. For patients in arm B, cixutumumab 6 mg/kg i.v. over 1 h was additionally given on days 1, 8, and 15 of each cycle (immediately following bevacizumab when applicable).
Statistical considerations
This was an open label, phase II randomized trial with progression-free survival (PFS) as primary end point. It was assumed that the addition of cixutumumab would increase the median PFS from 6 months (based on E4599 results) to 9 months. Assuming the survival distribution to be exponential, the above hypothesis corresponds to a 33% reduction in the PFS failure hazard rate. To detect such a reduction in the failure hazard rate with 85% power while maintaining a one-sided significance level of 10%, the log-rank test indicated the study would require 162 eligible patients to give full information of 133 PFS events with a 9-month accrual period (with the accrual rate expected to be 18 patients per month) and a 14-month follow-up period. Assuming a 10% ineligibility rate, a total of 180 patients (90 on each arm) would be accrued in order to obtain 162 eligible patients. Treatments were randomly assigned using permuted blocks within strata. The stratification factor used was ECOG PS (0 versus 1). Institutions obtained treatment assignments from a web registration program.
For safety considerations, a two-stage design was also used for each arm in this study. The target toxicities considered for this study were ≥ grade 3 bleeding, ≥ grade 3 febrile neutropenia, and any grade infection with grade 3–4 neutrophils. The above combined toxicity rate was expected to be ∼10%. A true combined toxicity rate of 0.25 with clearly attributable target toxicities would be considered unacceptable for the treatment regimen. The first stage was to accrue 18 patients on each arm.
The primary analyses (except toxicity analysis) were carried out based on all eligible patients who started protocol treatment. PFS was defined as the time from randomization to progression (evaluated using the RECIST 1.1 criteria) or to death without documentation of progression. OS was measured from randomization to death from any cause, censored at the date of last contact. Time-to-event was estimated using the Kaplan–Meier method and compared by the log-rank test [24], stratified by ECOG PS. Cox proportional hazards models [25], stratified on ECOG PS, were used to estimate hazard ratios and test for significance for PFS and OS. Objective response was evaluated using the RECIST 1.1 criteria. Response rate was defined as the proportion of patients with complete or partial response among all eligible and treated patients. Toxicities were evaluated using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. All patients who started protocol treatment (regardless of eligibility status) were included in the toxicity analysis. The Fisher’s exact test and the Wilcoxon rank sum test were used for comparison between groups when appropriate. For the primary end point (PFS), a one-sided P value was reported with a P value < 0.10 considered statistically significant. All other P values were two-sided with a P value < 0.05 considered statistically significant. Confidence intervals (CI) were at the 95% level.
Results
A total of 175 patients were enrolled (88 in arm A and 87 in arm B). In June 2013, the clinical development program for cixutumumab was terminated by the manufacturer that prompted earlier closure than planned (planned accrual was 180). Among the 175 patients, 14 were ineligible (including 2 who never started therapy); 2 were with questionable eligibility status due to no protocol therapy received and no forms submitted; additional 6 patients never started protocol therapy, leading to 153 eligible patients who started protocol treatment (78 in arm A and 75 in arm B, refer to supplementary Figure S1, available at Annals of Oncology online, for details). All efficacy analyses were based on eligible and treated patients and toxicity analysis on all treated patients regardless of eligibility status (83 in arm A and 82 in arm B).
Patient and disease characteristics
Supplementary Table S1, available at Annals of Oncology online, displays patient demographics and disease characteristics at the time of enrollment (N = 153). For any variable listed in supplementary Table S1, available at Annals of Oncology online, no statistically significant difference was observed between the two arms.
Treatment delivery and discontinuation
The median number of cycles received was 6.5 cycles for arm A (range: 1–42) and 6 cycles for arm B (range 1–63), respectively. For either arm, the main reason for treatment discontinuation was disease progression [50.7% (arm A) versus 54.7% (arm B), P = 0.63], followed by toxicity/complication [27.3% (arm A) versus 29.3% (arm B), P = 0.86].
Efficacy
Progression-free survival
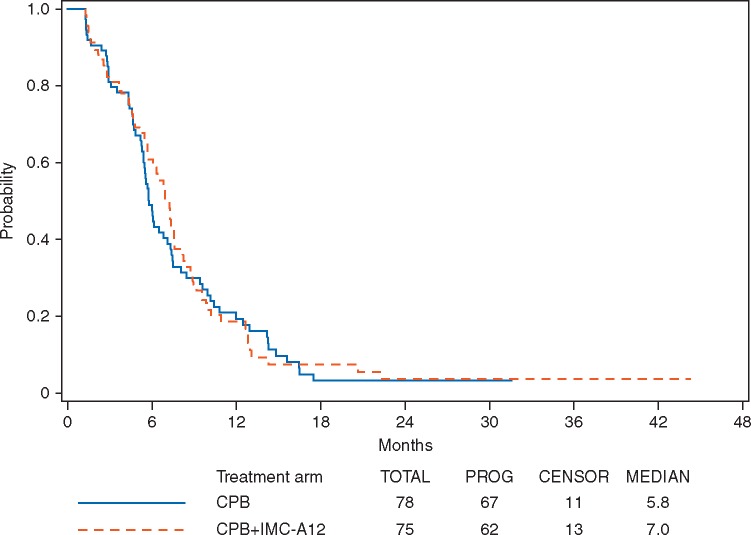
Among the 153 eligible and treated patients, 118 patients (77%) had documented disease progression (61 and 57 patients in arm A and arm B, respectively). Figure 1 shows PFS curves by treatment arm. The hazard ratio (CPB + cixutumumab/CPB) for progression was 0.92 with 95% CI of (0.65–1.31) (Wald P = 0.33, one-sided) with a median PFS of 5.8 months (95% CI 5.4–7.1 months) and 7.0 months (95% CI 5.7–7.6 months) for the CPB arm and the CPB + cixutumumab arm, respectively. A stratified log-rank test shows that there was no statistically significant difference in PFS between the two arms (P = 0.33, one-sided).
Figure 1.
Progression-free survival by treatment arm (p = 0.33, one-sided stratified log-rank); hazard ratio 0.92 (95% CI 0.65–1.31).
Overall survival
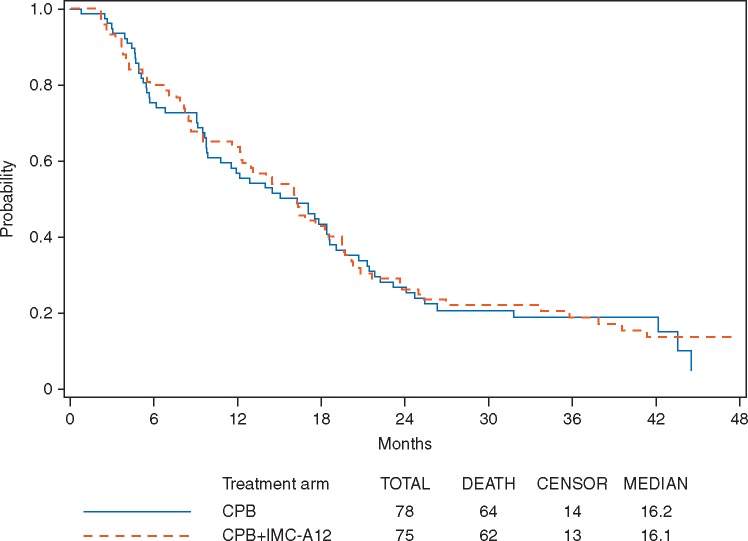
With a median follow-up of 36.6 months (range: 1.4–53.1 months), the median OS were 16.2 months (95% CI 9.9–18.6 months) and 16.1 months (95% CI 12.3–19.4 months) for arm A and arm B, respectively (Figure 2). A stratified log-rank test shows that there was no statistically significant difference in OS between the two arms (P = 0.95, two-sided). The hazard ratio (CPB + cixutumumab/CPB) for death was 0.99 with 95% CI of (0.69–1.41) (Wald P = 0.95).
Figure 2.
Overall survival by treatment arm (p = 0.95, two-sided stratified log-rank); hazard ratio 0.99 (95% CI 0.69–1.41).
Best overall response
The best overall response rate was 46.2% with CPB versus 58.7% with CPB plus cixutumumab, with no statistically significant difference between the two arms (P = 0.15) (supplementary Table S2, available at Annals of Oncology online).
Adverse events
Table 1 summarizes toxicity rate of grade 3 and higher (possibly, probably, or definitively related to protocol treatment). Common treatment-related grade 3/4 (%) events in arm A versus arm B were: febrile neutropenia, 0/0 versus 9/0 (P = 0.002); neutropenia, 16/17 versus 17/38 (P = 0.0006); thrombocytopenia, 5/5 versus 17/9 (P = 0.003); anemia, 7/0 versus 12/0 (P = 0.23); leukopenia, 5/0 versus 16/4 (P = 0.001); fatigue, 6/0 versus 20/1 (P = 0.002); nausea, 5/0 versus 11/0 (P = 0.12); hyperglycemia, 0/0 versus 10/1 (P = 0.0003); peripheral sensory neuropathy, 5/0 versus 5/0 (P = 1.00); hypertension, 14/0 versus 21/0 (P = 0.19); thromboembolic event, 7/1 versus 6/0 (P = 0.78). Among 165 patients receiving protocol treatment, 2 patients (intracranial hemorrhage in arm A and bronchopulmonary hemorrhage in arm B) were reported with treatment-related grade 5 AEs.
Table 1.
Treatment-related toxicities by treatment arm
| Toxicity | Treatment arm |
|||||
|---|---|---|---|---|---|---|
| A (n = 83) |
B (n = 82) |
|||||
| Grade |
Grade |
|||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Anemia | 7 | – | – | 12 | – | – |
| Febrile neutropenia | – | – | – | 9 | – | – |
| Fatigue | 6 | – | – | 20 | 1 | – |
| Fever | 1 | – | – | – | 2 | – |
| Infusion-related reaction | 1 | 1 | – | 1 | – | – |
| Pruritus | – | – | – | 1 | – | – |
| Anal fistula | – | – | – | 1 | – | – |
| Colitis | – | – | – | – | 1 | – |
| Colonic perforation | 1 | – | – | – | – | – |
| Dental caries | 1 | – | – | – | – | – |
| Diarrhea | 2 | – | – | 2 | – | – |
| Dysphagia | 1 | – | – | – | – | – |
| Mucositis oral | 1 | – | – | 1 | – | – |
| Nausea | 5 | – | – | 11 | – | – |
| Oral pain | – | – | – | 1 | – | – |
| Pancreatitis | – | – | – | 1 | – | – |
| Rectal pain | – | – | – | 1 | – | – |
| Rectal ulcer | – | – | – | 1 | – | – |
| Vomiting | 1 | – | – | 6 | 1 | – |
| Catheter-related infection | – | – | – | 1 | – | – |
| Enterocolitis infectious | 1 | – | – | – | – | – |
| Gum infection | – | – | – | 1 | – | – |
| Joint infection | – | – | – | 1 | – | – |
| Lung infection | – | – | – | 4 | – | – |
| Mucosal infection | – | – | – | 1 | – | – |
| Sepsis | – | – | – | – | 1 | – |
| Tooth infection | – | – | – | 1 | – | – |
| Urinary tract infection | 1 | – | – | 1 | – | – |
| Wound infection | – | – | – | 1 | – | – |
| Wound complication | – | – | – | 1 | – | – |
| Alanine aminotransferase increased | 1 | – | – | 2 | – | – |
| Aspartate aminotransferase increased | 1 | – | – | 1 | – | – |
| Lipase increased | – | – | – | 2 | – | – |
| Lymphocyte count decreased | 10 | 1 | – | 11 | – | – |
| Neutrophil count decreased | 16 | 17 | – | 17 | 38 | – |
| Platelet count decreased | 5 | 5 | – | 17 | 9 | – |
| Serum amylase increased | – | – | – | 1 | – | – |
| Weight gain | 1 | – | – | – | – | – |
| Weight loss | – | – | – | 1 | – | – |
| White blood cell decreased | 5 | – | – | 16 | 4 | – |
| Anorexia | 2 | – | – | 6 | 1 | – |
| Dehydration | 4 | 1 | – | 10 | – | – |
| Hyperglycemia | – | – | – | 10 | 1 | – |
| Hyperkalemia | – | – | – | 1 | – | – |
| Hypoalbuminemia | 1 | – | – | – | – | – |
| Hypocalcemia | – | 1 | – | – | – | – |
| Hypokalemia | – | 1 | – | 1 | – | – |
| Hypomagnesemia | 2 | 1 | – | – | – | – |
| Hyponatremia | 5 | 1 | – | 6 | 1 | – |
| Arthralgia | 1 | – | – | – | – | – |
| Back pain | 1 | – | – | 2 | – | – |
| Bone pain | 1 | – | – | 1 | – | – |
| Generalized muscle weakness | 1 | – | – | 2 | 1 | – |
| Muscle weakness lower limb | 1 | – | – | – | – | – |
| Myalgia | – | – | – | 2 | – | – |
| Musculoskeletal and connective—Other | – | – | – | 1 | – | – |
| Ataxia | 1 | – | – | – | – | – |
| Encephalopathy | – | – | – | – | 1 | – |
| Headache | – | – | – | 1 | – | – |
| Intracranial hemorrhage | – | – | 1 | – | – | – |
| Peripheral motor neuropathy | 1 | – | – | 1 | – | – |
| Peripheral sensory neuropathy | 5 | – | – | 5 | – | – |
| Reversible posterior leukoencephalopathy | – | – | – | 1 | – | – |
| Spasticity | – | – | – | 1 | – | – |
| Nervous system disorders—Other | 1 | – | – | – | – | – |
| Blurred vision | – | – | – | 1 | – | – |
| Confusion | – | – | – | 1 | – | – |
| Depression | – | – | – | – | 1 | – |
| Bronchopulmonary hemorrhage | – | – | – | 1 | – | 1 |
| Dyspnea | 1 | – | – | 2 | – | – |
| Pleuritic pain | 1 | – | – | – | – | – |
| Respiratory thoracic mediastinal—Other | 1 | – | – | – | – | – |
| Chronic kidney disease | – | – | – | 1 | – | – |
| Cystitis non-infective | – | – | – | 1 | – | – |
| Proteinuria | 2 | – | – | 5 | – | – |
| Irregular menstruation | 1 | – | – | – | – | – |
| Hypertension | 14 | – | – | 21 | – | – |
| Hypotension | 2 | – | – | 1 | – | – |
| Thromboembolic event | 7 | 1 | – | 6 | – | – |
| WORST DEGREE | 42 | 25 | 1 | 45 | 44 | 1 |
For safety considerations, a two-stage design was used for each arm in this study. At interim analysis, 37 patients (19 on arm A and 18 on arm B) were enrolled during stage 1 accrual with 16 cases started treatment in arm B. In this analysis, only two cases in arm B were observed with unacceptable toxicity, suggesting the regimen was potentially safe and accrual to its final accrual goal was then recommended.
Discussion
Our results demonstrate that the addition of cixutumumab to the combination of carboplatin, paclitaxel, and bevacizumab increased toxicity without improving efficacy. Therefore, this new regimen is not recommended for further development in NSCLC. Similarly, two other trials of cixutumumab in combination with first-line platinum doublet chemotherapy in patients with advanced NSCLC, including an ECOG-ACRIN trial in patients not eligible for bevacizumab, have shown disappointing results [26, 27].
Other agents in the class of IGF-IR inhibitors have been studied in NSCLC. A phase II study by Karp et al. [28] randomized patients with advanced NSCLC to receive carboplatin and paclitaxel with or without figitumumab, a monoclonal antibody against IGF-IR. The response rate was superior for patients who received the antibody in combination with chemotherapy (54% versus 41%). The progression-free survival was also longer in patients receiving figitumumab (P = 0.07). However, a subsequent phase III trial of this combination in patients with non-adenocarcinoma histology was negative [29].
It is important to note that patients in each arm of our study had longer OS than a historical control (E4599) with median OS that exceeded 16 months. It is very likely that this was due to patient selection and exclusion of poor prognosis patients. Another cixutumumab first-line trial for patients with advanced NSCLC not eligible for bevacizumab (E4508) was running simultaneously in the cooperative group. Other factors, such as advancement in second-line therapies and supportive care may have played a role. However, the fact of observed improved outcomes over historical controls due to reasons other than treatment effect emphasizes the importance of a contemporary comparator arm in phase II trials.
Funding
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA21115, CA23318, CA66636, CA180844, CA39229, CA189863, CA35267, CA17145, CA14548, CA180853, CA27525, CA180790, CA13650, CA15488, CA180864, CA180870, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Islami F, Torre LA, Jemal A.. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015; 4: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Jemal A.. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 3. Schiller JH, Harrington D, Belani CP. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92–98. [DOI] [PubMed] [Google Scholar]
- 4. Delbaldo C, Michiels S, Syz N. et al. Benefits of adding one drug to a single-agent or a two-drug combination in advanced non-small cell lung cancer (NSCLC). A meta-analysis of the literature. JAMA 2004;292:470–484. [DOI] [PubMed] [Google Scholar]
- 5. Argiris A, Schiller JH.. Can current treatments for advanced non-small-cell lung cancer be improved? JAMA 2004; 292: 499–500. [DOI] [PubMed] [Google Scholar]
- 6. Sandler A, Gray R, Perry MC. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 7. Pollak MN, Schernhammer ES, Hankinson SE.. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004; 4: 505–518. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Sun Y.. Insulin-like growth factor receptor-1 as an anti-cancer target: blocking transformation and inducing apoptosis. Curr Cancer Drug Targets 2002; 2: 191–207. [DOI] [PubMed] [Google Scholar]
- 9. Khandwala HM, McCutcheon IE, Flyvbjerg A. et al. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 2000; 21: 215–244. [DOI] [PubMed] [Google Scholar]
- 10. Le Roith D, Bondy C, Yakar S. et al. The somatomedin hypothesis: 2001. Endocr Rev 2001; 22: 53–74. [DOI] [PubMed] [Google Scholar]
- 11. Gooch JL, Van Den Berg CL, Yee D.. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death–proliferative and anti-apoptotic effects. Breast Cancer Res Treat 1999; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 12. Turner BC, Haffty BG, Narayanan L. et al. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res 1997; 57: 3079–3083. [PubMed] [Google Scholar]
- 13. Gotlieb WH, Bruchim I, Gu J. et al. Insulin-like growth factor receptor I targeting in epithelial ovarian cancer. Gynecol Oncol 2006; 100: 389–396. [DOI] [PubMed] [Google Scholar]
- 14. Maloney EK, McLaughlin JL, Dagdigian NE. et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res 2003; 63: 5073–5083. [PubMed] [Google Scholar]
- 15. Goetsch L, Gonzalez A, Leger O. et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer 2005; 113: 316–328. [DOI] [PubMed] [Google Scholar]
- 16. Akagi Y, Liu W, Zebrowski B. et al. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res 1998; 58: 4008–4014. [PubMed] [Google Scholar]
- 17. Warren RS, Yuan H, Matli MR. et al. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem 1996; 271: 29483–29488. [DOI] [PubMed] [Google Scholar]
- 18. Burtrum D, Zhu Z, Lu D. et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res 2003; 63: 8912–8921. [PubMed] [Google Scholar]
- 19. Wu JD, Odman A, Higgins LM. et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res 2005; 11: 3065–3074. [DOI] [PubMed] [Google Scholar]
- 20. Adams TE, Epa VC, Garrett TP. et al. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 2000; 57: 1050–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Chakravarty G, Kim S. et al. Growth-inhibitory effects of human anti-insulin-like growth factor-I receptor antibody (A12) in an orthotopic nude mouse model of anaplastic thyroid carcinoma. Clin Cancer Res 2006; 12: 4755–4765. [DOI] [PubMed] [Google Scholar]
- 22. Higano CS, Berlin J, Gordon M. et al. Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Invest New Drugs 2015; 33: 450–462. [DOI] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 24. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170. [PubMed] [Google Scholar]
- 25. Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972; 34: 187–220. [Google Scholar]
- 26. Hanna NH, Dahlberg SE, Kolesar JM. et al. Three-arm, randomized, phase 2 study of carboplatin and paclitaxel in combination with cetuximab, cixutumumab, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: an Eastern Cooperative Oncology Group (ECOG) study (E4508). Cancer 2015; 121: 2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novello S, Scagliotti G, de Castro G Jr. et al. An open-label, multicenter, randomized, phase II study of cisplatin and pemetrexed with or without cixutumumab (IMC-A12) as a first-line therapy in patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2017;12:383–389. [DOI] [PubMed] [Google Scholar]
- 28. Karp D, Paz-Ares L, Novello S. et al. High activity of the anti-IGF-IR antibody CP-751,871 in combination with paclitaxel and carboplatin in squamous NSCLC. J Clin Oncol 2008; 25: Abs #8015. [Google Scholar]
- 29. Langer CJ, Novello S, Park K. et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 2014; 32: 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.