Abstract
Background
Distant metastasis accounts for 90% of deaths from colorectal cancer (CRC). Genomic heterogeneity has been reported in various solid malignancies, but remains largely under-explored in metastatic CRC tumors, especially in primary to metastatic tumor evolution.
Patients and methods
We conducted high-depth whole-exome sequencing in multiple regions of matched primary and metastatic CRC tumors. Using a total of 28 tumor, normal, and lymph node tissues, we analyzed inter- and intra-individual heterogeneity, inferred the tumor subclonal architectures, and depicted the subclonal evolutionary routes from primary to metastatic tumors.
Results
CRC has significant inter-individual but relatively limited intra-individual heterogeneity. Genomic landscapes were more similar within primary, metastatic, or lymph node tumors than across these types. Metastatic tumors exhibited less intratumor heterogeneity than primary tumors, indicating that single-region sequencing may be adequate to identify important metastasis mutations to guide treatment. Remarkably, all metastatic tumors inherited multiple genetically distinct subclones from primary tumors, supporting a possible polyclonal seeding mechanism for metastasis. Analysis of one patient with the trio samples of primary, metastatic, and lymph node tumors supported a mechanism of synchronous parallel dissemination from the primary to metastatic tumors that was not mediated through lymph nodes.
Conclusions
In CRC, metastatic tumors have different but less heterogeneous genomic landscapes than primary tumors. It is possible that CRC metastasis is, at least partly, mediated through a polyclonal seeding mechanism. These findings demonstrated the rationale and feasibility for identifying and targeting primary tumor-derived metastasis-potent subclones for the prediction, prevention, and treatment of CRC metastasis.
Keywords: colorectal cancer, metastasis, whole-exome sequencing, heterogeneity
Introduction
Colorectal cancer (CRC) has high prevalence and mortality worldwide [1]. Distant metastasis accounts for ∼90% of CRC deaths. Once metastasized, 5-year survival drops to 13.1%, compared with 90.1% for non-metastatic CRC patients [2]. To enable the early assessment of metastasis potential and its effective prevention and treatment, it is crucial to discover and exploit the principles that govern the initiation and growth of CRC metastasis.
Large-scale efforts [3] have revealed comprehensive genomic mutation profiles in primary tumors of CRC patients; however, genomic landscapes of metastatic tumors remain under-explored. Recent studies revealed extensive intratumor heterogeneity (ITH), in which distinct cell populations, called subclones, carry distinct mutations. Knowledge of subclones profoundly impacts our understanding of tumor progression, drug resistance, and metastasis [4, 5]. It is increasingly clear that cancer genomes develop and evolve in a complex fashion [6–9], and without considering ITH, genome-informed precision medicine may not be fruitful due to insufficient understanding of the dynamic processes leading to metastasis.
Multiregion whole-exome sequencing (WES) or whole-genome sequencing have demonstrated ITH in several cancer types to date; however, most previous studies analyzed only primary tumors but not metastatic tumors [10]. Therefore, the subclonal architecture of metastatic tumors remains under-explored, and little is known about how their subclonal architecture relate to that of the primary tumors from which they derive.
In the current study, we sought to explore the effect of ITH on CRC metastasis. We conducted high-depth (>150×) multiregion WES in 28 tissues from four CRC patients with matched primary and metastatic tumors to characterize ITH and subclone architecture and revealed the clonal and subclonal evolutionary routes from primary to metastatic tumors of the same patients.
Patients and methods
Patient description
This study included 28 samples from four CRC patients (supplementary Table S1, available at Annals of Oncology online). There were 23 multiregion tumor samples (supplementary Table S2, available at Annals of Oncology online), including 10 primary tumors, 3 positive lymph nodes, and 10 metastatic tumors, along with 5 normal samples (one adjacent normal tissue for each patient and one additional normal lymph node for patient A02). For all patients, we arbitrarily labeled tumor regions as Pri-1, Pri-2, etc. for primary tumors, Met-1, Met-2, etc. for metastatic tumors, and Lym-1, Lym-2, etc. for positive lymph nodes. All metastatic tumors and positive lymph nodes were resected 1–3 years after the removal of the corresponding primary tumors.
Multiregion WES, data processing, and analysis
We employed an empirically optimized protocol to extract DNA from formalin-fixed, paraffin-embedded samples and used NimbleGen SeqCap EZ Exome V2.0 kit for library construction and Illumina HiSeq 2000 for paired-end sequencing. After best-practice protocol for sequencing quality control (QC) and alignment, we used MuTect [11] to call mutations in tumors and obtained a high-confidence mutation set after rigorous filtering. We inferred phylogenetic trees of tumor blocks based on the mutation patters in each of the patients using PHYLIP [12]. Finally, we inferred subclones using PyClone [13], and constructed subclonal architectures and evolutionary routes based on the mutation patterns in the subclones. Details of data processing, analysis, and QC are described in supplementary materials, available at Annals of Oncology online, including supplementary Tables S3 and S4 and Figures S1–S3, available at Annals of Oncology online.
Results
Inter- and intra-individual genomic heterogeneity revealed by mutation sharing analysis
The average sequencing depth of cleaned WES data after removing duplicates was ∼150× (ranging from 123 to 209×), and the average insert size of paired-end reads was 100 bp (ranging from 91 to 113 bp). Supplementary Tables S5–S8, available at Annals of Oncology online list the complete mutations for each patient. The mutations in all patients exhibited signatures characterized predominantly by C > T transition, with four substitution patterns, ACG > ATG, CCG > CTG, GCG > GTG, and TCG > TTG, at the neighboring trinucleotides (supplementary Figure S4, available at Annals of Oncology online), consistent with those reported for CRC [9, 14]. Moreover, we identified known driver genes that are frequently reported in previous studies, such as APC, KRAS, and TP53 (supplementary Tables S5–S8, available at Annals of Oncology online). Recurrent mutations were rare between different patients, indicating the significant inter-individual heterogeneity. Three non-synonymous mutations were shared by two patients, including mutations in MUC2 (between patients A01 and A04), KRT8 (between A01 and A03), and MAML2 (between A01 and A02). The percentage of trunk mutations shared by all tumor regions versus all types of trunk mutations was 76%, 46%, 71%, and 96% in patient A01, A02, A03, and A04, respectively, indicating the varying ITH levels among patients.
Primary and metastatic tumors differ significantly based on analysis of mutation CCFs
We further explored ITH based on mutation CCFs, which provided a finer scale than mutation sharing analysis. We obtained ∼20 000 accurately called germline heterozygous variants in exonic and intronic regions (supplementary Table S4, available at Annals of Oncology online), and incorporated the inferred CNAs (supplementary Figure S5 and Tables S9–S12, available at Annals of Oncology online) to estimate CCFs of mutations. Supplementary Figure S6, available at Annals of Oncology online shows the pair-wise relationships of CCFs of all mutations across all tumor regions in each patient. For all patients, the CCFs were generally consistent within either all primary tumor regions or all metastatic tumor regions, but drastically different between primary and metastatic tumor regions. Supplementary Figure S7, available at Annals of Oncology online shows the estimated clusters of mutations and their CCFs in each tumor region in A01. All regions contained several clusters with varying CCFs, and two mutations in SMARCE1 gene and one in PADI1 gene formed a cluster with CCF close to 1.0 across all tumor regions, representing clonal mutations. Mutations with low CCFs were abundant in all tumor regions, both primary and metastatic tumors included, indicating the pervasiveness of ITH. Clustering of other patients is in supplementary Figure S8, available at Annals of Oncology online.
Metastatic tumors exhibit less ITH than primary tumors
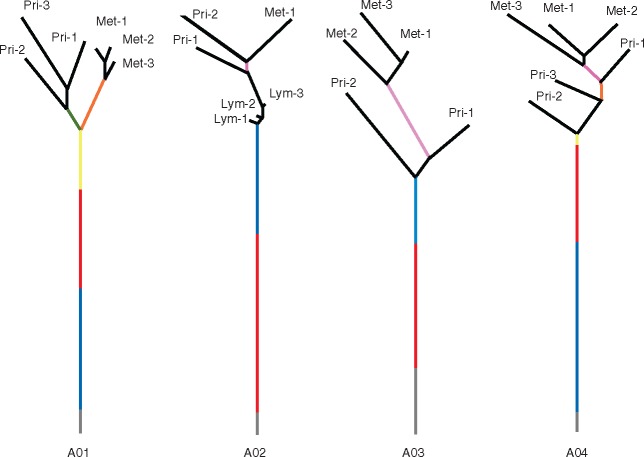
We constructed phylogenetic trees (Figure 1) of all tumor regions in each patient based on identified mutations [12]. We divided trunk mutations into different clones or subclones according to their estimated CCFs: the mutations in the root of the trunk were clonal mutations and others were subclonal mutations with decreasing CCFs along the trunk of the tree upwards. The clones or subclones were represented by different colors, and the numbers of mutations in them were proportional to the lengths. For all patients, a large number of mutations were shared by all tumor regions, as represented by the lengths of tree trunks (Figure 1). Importantly, genomic diversity appeared to be reduced within metastatic tumors compared with primary tumors, as evidenced by the shorter distances among metastatic tumor regions than those among primary tumor regions (Figure 1, with an exception for patient A02 who had only one metastatic tumor block analyzed).
Figure 1.
Phylogenetic trees showing the relationships of tumor samples in each patient based on mutation sharing and CCFs. The gray line represents trunk mutations that are clonal in all samples. The black line represents leaf mutations that are subclonal and private to the corresponding tumor samples. All other colored lines represent branch mutations that are subclonal and shared by at least two tumor samples. The lengths of the trunk and the branches of the tree are proportional to the number of corresponding mutations.
Subclonal architecture suggests a possible polyclonal seeding in CRC metastasis
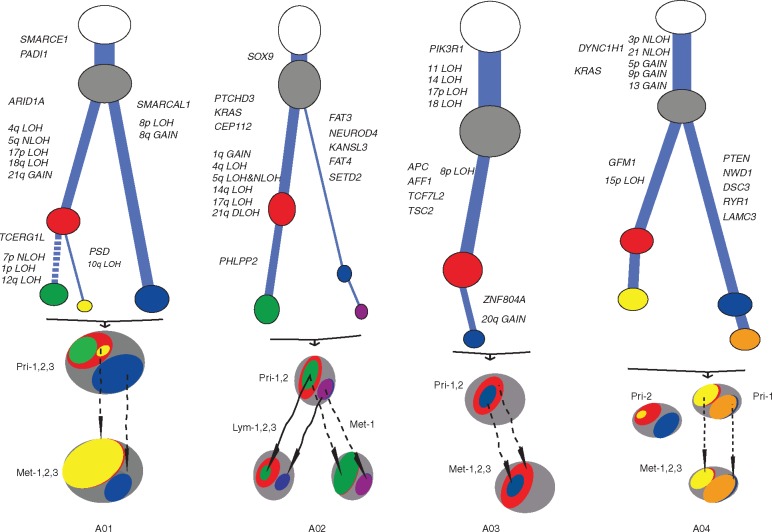
We next analyzed the subclonal architectures using mutation CCFs. In Figure 2, we used different colors of ovals to label the clones or subclones, with the size representing their corresponding CCFs. In patient A01 (Figure 2), we observed four subclones (2, 3, 4, and 5) with varying CCFs in each of the primary tumor regions, and three of them (2, 3, and 5) were also in the metastatic tumor. By comparison, cluster 6 contained subclonal mutations in each of the metastatic tumor regions but not in any primary tumor. Mutations in cluster 5 had low CCFs in the primary tumor but a much higher CCF in metastatic tumor, suggesting that this subclone might contain important drug-resistant and/or metastasis-promoting mutations. Cluster 6 was not observed in primary tumor regions and thus likely either contained de novo mutations during the growth of the metastasis or were not sampled in the primary tumor blocks. For each patient, the linear and/or branching relationships of all subclones were deduced and depicted based on mutation CCFs, ignoring the leaf mutations (the last cluster in each patient) due to the lack of information to infer their relationships (Figure 2, left panels). For example, in the primary tumor of patient A01, both the yellow and the green subclones derived in the same parental red subclonal cells (i.e. they were both linear in relationship to the red subclone) but they occupied different subpopulations of cells (i.e. they were branches in relationship to each other within the red subclone). Clearly, both the yellow and the blue subclones were inherited in the metastatic tumors, indicating a possible polyclonal metastasis seeding mechanism in this patient.
Figure 2.
Subclonal architecture for all patients. Each panel represents a patient and each row in one panel represents a tumor sample from the corresponding patient. The area of the ovals is proportional to the estimated CCF. For each patient, the most left panel shows the constructed subclonal architecture. Cluster 1 shows the clonal trunk mutations in all tumor regions, the last cluster shows the subclonal leaf mutations observed in only one tumor region, and clusters in between shows subclonal trunk and branch mutations that are shared by two or more tumor regions.
Remarkably, similar to patient A01, data for all of the other patients also revealed the existence of multiple subclones in metastatic tumors (Figure 2). For patient A03, the subclones inherited in the metastatic tumor were linear rather than branching as in other patients. However, it was clear that the seeding was also polyclonal, as the CCFs of the mutations in the red and blue subclones differed in the metastatic tumor, inconsistent with the single-clonal seeding mechanism in which the CCFs in the two subclones should be identical in the metastatic tumor.
Subclonal evolution further suggests polyclonal seeding in CRC metastasis
We next analyzed the evolutionary routes of subclones in primary tumors to reveal their progressions to metastatic tumors, with a particular goal to trace the metastatic subclones back to their ancestors in the primary tumors (Figure 3). We assigned those potentially important mutated genes that were either reported as CRC driver genes [15] or in the Catalogue of Somatic Mutations in Cancer (COSMIC) database (version 70) and CNAs to each subclone to provide insights into the timing of the mutations. The sizes of subclones correspond to mutation CCFs, and the same colors were used as for those in Figure 2. For patient A01, multiple subclones were present during the evolutionary route, including the cells carrying the yellow subclonal genomic aberrations (PSD, 10q LOH) and the cells carrying the mutations in the blue subclone (SMARCAL1, 8p LOH, 8q Gain). The green subclone could have derived from either the red or the blue subclone, but we were not able to unambiguously resolve the linear relationship based on the available mutation data, and therefore arbitrarily put it under the red subclone (an alternative was to put it under the blue subclone). For patient A03, the two subclones (blue and red) disseminated to the metastatic tumor in a linear relationship, a pattern different from other patients. The two subclones in the metastatic tumor maintained similar relative CCFs to those of the primary tumor during the metastasis progression. For patient A04, the subclonal architectures in the two primary tumor regions were largely different from each other and thus depicted separately. Based on the subclonal relationships, it was likely that the metastatic tumor originated from cancer cells with a similar subclonal composition to that of the Pri-1 but not Pri-2 region. The two subclones that disseminated to the metastatic tumor were either minor (yellow) or nondetectable (orange) in the Pri-2 region. Overall, data from all patients in our study suggested a polyclonal seeding mechanism for CRC metastasis.
Figure 3.
Subclonal evolutionary routes from primary to metastatic/lymph node tumors for all patients. The color scheme is the same as that in Figure 2. The length of the branches in the tree represents the number of somatic mutations in the corresponding subclone, and the thickness of the branches represents the proportion of the tumor cells occupied by the corresponding subclone. The dashed link between subclones indicates that the relationship is not definitive. Mutated genes and CNAs derived in each subclone are labeled along the branches.
Parallel metastatic dissemination to distant organs independent of lymph nodes
Patient A02 was the only patient with lymph nodes analyzed. The three positive lymph nodes were tightly clustered together, indicating their high genetic similarity (Figure 1). The lung metastasis, which was removed 3 years later after the removal of the primary tumor, had a drastically different genetic landscape from those of the positive lymph nodes (Figure 1), suggesting that genetically distinct cancer cells from the primary tumor might have migrated to lung and formed the metastasis, likely independent from lymph node invasion. These findings were also supported by the clustering analysis, which demonstrated the higher consistency of mutation CCFs among the three positive lymph nodes than other tumor regions (supplementary Figure S6, available at Annals of Oncology online). There were extensive but shared subclonal mutations in all positive lymph nodes, suggesting that these subclones likely existed in the same primary tumor and synchronously disseminated to the positive lymph nodes. A02 in Figure 3 supported that the subclones from primary tumors disseminated in parallel to both the positive lymph nodes and the metastatic tumor, suggesting that the metastatic tumor might have derived directly from the primary tumor rather than from positive lymph nodes.
Potential functional relevance of the identified mutated genes
Finally, we conducted a literature review and summarized the potential functional relevance of the mutated genes. Given the large number of identified mutations/genes (supplementary Tables S5–S8, available at Annals of Oncology online), we focused on those more robust genes with nonsynonymous trunk mutations (present in all primary, metastatic, lymph nodes, or all tumor regions of the same patients). There were 29, 58, 30 and 67 such genes for patient A01, A02, A03 and A04, respectively (supplementary Table S13, available at Annals of Oncology online). Based on the COSMIC and Kyoto Encyclopedia of Genes and Genomes databases and on the literature review, we assigned each gene to one of the following categories: category 1 (implicated in CRC or cancer metastasis), category 2 (implicated in cancers other than CRC and not in metastasis), and category 3 (not implicated in cancer or metastasis). For patient A01, A02, A03, and A04, the percentage of genes is 44.8%, 29.3%, 43.3%, and 35.8%, respectively, in category 1, and 27.6%, 25.9%, 20.0%, 28.4%, respectively, in category 2. The large number of category 1 and 2 genes further substantiated the reliability and physiological relevance of the genes identified in our study. Because many mutations in COSMIC may not have functional impact, we further curated a highly conservative list of mutations that are either reported with functional evidence in previous studies or stop-gain mutations in tumor suppressor genes (supplementary Table S14, available at Annals of Oncology online). The most prominent functional mutation identified is KRAS G12D mutation that has been extensively reported in previous studies with compelling functional impacts on CRC tumorigenesis and treatment response [16–18].
Discussion
Using multiregion high-depth WES of paired primary and metastatic CRC tumors, we reported here the varying levels of inter- and intra-individual genomic heterogeneity, the subclonal architecture, and the evolutionary relationship of subclones. Although ITH is pervasive, the subclones are in general consistent across tumor blocks, suggesting that single-region sequencing of primary or metastatic tumor may be adequate to identify important mutations to guide treatment, although for some patients (e.g. A04) a single biopsy in primary tumor may not be adequate due to elevated mutational differences in primary versus metastatic tumors. For the patient with the trio of primary and metastatic tumors along with positive lymph nodes, we observed that the metastatic tumor and positive lymph nodes originated from independent parental cancer cells in the primary tumors. In-depth evaluations of identified mutations and genes substantiated their physiological relevance and, at the same time, revealed many novel genes that could serve as promising targets for the prediction, prevention, and treatment of CRC metastasis.
The prevailing single-cell origin theory for metastasis [19, 20] has been seriously challenged recently and emerging evidence supports polyclonal seeding. Findings from our current study lend further support to the polyclonal seeding theory in CRC, which, if further proven to be a major metastasis seeding mechanism in large-scale studies, will have profound implications in the prevention and treatment of CRC metastasis. In such scenarios, it is warranted to systematically identify subclones and subclone-specific genetic mutations and their functions to identify subclonal driver genes for better understanding of the metastasis mechanism.
Many genes with non-synonymous trunk mutations identified in this study had been previously associated with CRC treatment resistance, including KRAS [21], TTN [22], ABCA13 [23], SOX9 [24], APC [25], ATM [26], MMP16 [27], MUC2 [28], and IL24 [29] (supplementary Table S13, available at Annals of Oncology online). We also identified genes that were implicated in treatment response in malignancies other than CRC, such as KRT8 [30], SMARCE1 [31], TCF4 [32, 33], and TPO [34]. The presence of demonstrated functional non-synonymous mutations and stop-gain mutations in tumor suppressor genes (supplementary Table S14, available at Annals of Oncology online) further substantiated the robustness of our analyses. It should be noted that the large numbers of mutations that do not have available functional evidence may also include genuine functional mutations that have not been functionally characterized in current literature. Additional future studies are needed to investigate their functional roles. However, we only focused on genes with non-synonymous trunk mutations and might have missed important genes with branch or even leaf mutations.
Overall, our study is one of the first attempts to use multiregion high-depth WES of paired primary and metastatic tumors to tackle the mechanism of subclonal dissemination from primary to metastatic tumors or lymph nodes in CRC. Although the sample size of the current study is relatively small, our data painted a comprehensive picture on subclonal architecture and evolutionary history that warrants further well-powered and in-depth explorations.
Funding
National Cancer Institute (CA162201); National Human Genome Research Institute (HG006857); American Cancer Society (123741-RSG-13-003-01-CCE); Pennsylvania Department of Health (HRFF 09F RFA-08-07-06).
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 3. Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kreso A, O'Brien CA, van Galen P. et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 2013; 339: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tabassum DP, Polyak K.. Tumorigenesis: it takes a village. Nat Rev Cancer 2015; 15: 473–483. [DOI] [PubMed] [Google Scholar]
- 6. Neelakantan D, Drasin DJ, Ford HL.. Intratumoral heterogeneity: clonal cooperation in epithelial-to-mesenchymal transition and metastasis. Cell Adh Migr 2015; 9: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naxerova K, Jain RK.. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol 2015; 12: 258–272. [DOI] [PubMed] [Google Scholar]
- 8. Gundem G, Van Loo P, Kremeyer B. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015; 520: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim TM, Jung SH, An CH. et al. Subclonal genomic architectures of primary and metastatic colorectal cancer based on intratumoral genetic heterogeneity. Clin Cancer Res 2015; 21: 4461–4472. [DOI] [PubMed] [Google Scholar]
- 10. Andor N, Graham TA, Jansen M. et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med 2016; 22: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cibulskis K, Lawrence MS, Carter SL. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013; 31: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felsenstein J. PHYLIP – Phylogeny Inference Package (version 3.2). Cladistics 1989; 5: 164–166. [Google Scholar]
- 13. Roth A, Khattra J, Yap D. et al. PyClone: statistical inference of clonal population structure in cancer. Nat Methods 2014; 11: 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexandrov LB, Nik-Zainal S, Wedge DC. et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vogelstein B, Papadopoulos N, Velculescu VE. et al. Cancer genome landscapes. Science 2013; 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haigis KM, Kendall KR, Wang Y. et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet 2008; 40: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Nadauld L, Ootani A. et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med 2014; 20: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung KE, Maricevich MA, Richard LG. et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci USA 2010; 107: 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talmadge JE, Fidler IJ.. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010; 70: 5649–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fidler IJ. The pathogenesis of cancer metastasis: the ′seed and soil′ hypothesis revisited. Nat Rev Cancer 2003; 3: 453–458. [DOI] [PubMed] [Google Scholar]
- 21. Siravegna G, Mussolin B, Buscarino M. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015; 21: 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim R, Schell MJ, Teer JK. et al. Co-evolution of somatic variation in primary and metastatic colorectal cancer may expand biopsy indications in the molecular era. PLoS ONE 2015; 10: e0126670.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hlavata I, Mohelnikova-Duchonova B, Vaclavikova R. et al. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 2012; 27: 187–196. [DOI] [PubMed] [Google Scholar]
- 24. Espersen ML, Olsen J, Linnemann D. et al. Clinical implications of intestinal stem cell markers in colorectal cancer. Clin Colorectal Cancer 2015; 14: 63–71. [DOI] [PubMed] [Google Scholar]
- 25. Martino-Echarri E, Henderson BR, Brocardo MG.. Targeting the DNA replication checkpoint by pharmacologic inhibition of Chk1 kinase a strategy to sensitize APC mutant colon cancer cells to 5-fluorouracil chemotherapy. Oncotarget 2014; 5: 9889–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Y, Wan G, Spizzo R. et al. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol 2014; 8: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu XT, Xu Q, Tong JL. et al. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer 2012; 106: 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Carlo F, Witte TR, Hardman WE, Claudio PP.. Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS One.2013; 8: e69760.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Mo Y, Wang X. et al. Conditionally replicative adenovirus-based mda-7/IL-24 expression enhances sensitivity of colon cancer cells to 5-fluorouracil and doxorubicin. J Gastroenterol 2013; 48: 203–213. [DOI] [PubMed] [Google Scholar]
- 30. Zhou C, Nitschke AM, Xiong W. et al. Proteomic analysis of tumor necrosis factor-alpha resistant human breast cancer cells reveals a MEK5/Erk5-mediated epithelial-mesenchymal transition phenotype. Breast Cancer Res 2008; 10: R105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamaguchi T, Kurita T, Nishio K. et al. Expression of BAF57 in ovarian cancer cells and drug sensitivity. Cancer Sci 2015; 106: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovacs D, Migliano E, Muscardin L. et al. The role of Wnt/β-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: evidences from patients-derived cell lines. Oncotarget 2016; 7: 43295–43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi ZD, Qian XM, Liu CY. et al. Aspirin-/TMZ-coloaded microspheres exert synergistic antiglioma efficacy via inhibition of beta-catenin transactivation. CNS Neurosci Ther 2013; 19: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong-Feng Z, Ting L, Yong Z. et al. The TPO/c-MPL pathway in the bone marrow may protect leukemia cells from chemotherapy in AML Patients. Pathol Oncol Res 2014; 20: 309–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.