Abstract
Background
Copanlisib is a pan-class I phosphatidylinositol 3-kinase inhibitor with predominant activity against the α- and δ-isoforms.
Patients and methods
This phase II study evaluated the response rate of copanlisib administered intravenously on days 1, 8, and 15 of a 28-day cycle, in patients with indolent or aggressive malignant lymphoma. Archival tumor tissues were used for immunohistochemistry, gene-expression profiling, and mutation analysis.
Results
Thirty-three patients with indolent lymphoma and 51 with aggressive lymphoma received copanlisib. Follicular lymphoma (48.5%) and peripheral T-cell lymphoma (33.3%) were the most common histologic subtypes. Most patients (78.6%) had received prior rituximab and 54.8% were rituximab-refractory. Median duration of treatment was 23 and 8 weeks in the indolent and aggressive cohorts, respectively (overall range 2–138). Eighty patients were evaluated for efficacy. The objective response rate was 43.7% (14/32) in the indolent cohort and 27.1% (13/48) in the aggressive cohort; median progression-free survival was 294 days (range 0–874) and 70 days (range 0–897), respectively; median duration of response was 390 days (range 0–825) and 166 days (range 0–786), respectively. Common adverse events included hyperglycemia (57.1%; grade ≥3, 23.8%), hypertension (54.8%; grade ≥3, 40.5%), and diarrhea (40.5%; grade ≥3, 4.8%), all generally manageable. Neutropenia occurred in 28.6% of patients (grade 4, 11.9%). Molecular analyses showed enhanced antitumor activity in tumors with upregulated phosphatidylinositol 3-kinase pathway gene expression.
Conclusion
Intravenous copanlisib demonstrated promising efficacy and manageable toxicity in heavily pretreated patients with various subtypes of indolent and aggressive malignant lymphoma. Subtype-specific studies of copanlisib in patients with follicular, peripheral T-cell, and mantle cell lymphomas are ongoing.
This trial is registered with ClinicalTrials.gov number NCT01660451 (Part A).
Keywords: copanlisib, treatment, malignant lymphoma, PI3K inhibitor
Introduction
Non-Hodgkin’s lymphoma comprises a heterogeneous group of malignant lymphomas, with both indolent and aggressive subtypes [1]. The B-cell receptor (BCR) signaling pathway is critical for the development, proliferation, and survival of malignant B-cells. Drugs targeting BCR pathway kinases, including the Bruton’s tyrosine kinase inhibitor ibrutinib [2] and the phosphatidylinositol 3-kinase (PI3K)-δ isoform inhibitor idelalisib [3], have proved to be effective treatment options in patients with refractory follicular or relapsed mantle cell lymphoma.
However, fatal and/or serious toxicities have been associated with idelalisib use [3, 4] and, recently, frequent serious adverse events, including hepatic and gastrointestinal toxicity, colitis, opportunistic infections, autoimmune toxicities, and pneumonitis, have raised safety concerns around idelalisib in combination with standard therapies [5–7]. Therefore, new approaches, such as inhibitors of multiple PI3K isoforms, have been developed to both mitigate toxicity issues and improve efficacy [8–10].
Copanlisib (BAY 80-6946; Bayer AG, Berlin, Germany) is an intravenous pan-class I PI3K inhibitor with predominant activity against the PI3K-α and PI3K-δ isoforms [11]. A first-in-human phase I study established the maximum tolerated dose of copanlisib as 0.8 mg/kg administered on days 1, 8, and 15 of a 28-day cycle [12]. In an expansion cohort including non-Hodgkin’s lymphoma patients, severe toxicities were low, and there were early signs of efficacy, including complete response (CR) or partial response (PR) in all six patients with relapsed or refractory follicular lymphoma (FL), and one of three patients with diffuse large B-cell lymphoma (DLBCL) [12].
This open-label, uncontrolled, phase II study evaluated the efficacy and safety of intravenous copanlisib administered intermittently in heavily pretreated patients with relapsed or refractory, indolent or aggressive malignant lymphoma (ClinicalTrials.gov identifier: NCT01660451; Part A). Biomarker analyses were carried out to identify a possible gene signature profile that may associate with response.
Patients and methods
Study design and patient eligibility
The study population comprised two cohorts of 30 patients each with either indolent lymphoma or chronic lymphocytic leukemia (CLL), or aggressive malignant lymphoma, relapsed or refractory to two or more prior lines of therapy. Following early signs of clinical activity, the protocol was amended to enroll additional patients with aggressive lymphoma [mantle cell lymphoma (MCL) and T-cell lymphoma]. Separate extension studies for relapsed or refractory, indolent lymphoma (NCT01660451, Part B) and DLBCL (NCT02391116) are ongoing. The study protocol and amendments were approved by all relevant institutional review boards and ethics committees. All patients gave written, informed consent.
The primary efficacy variable was objective response rate (ORR), defined as the proportion of patients who achieved a CR, an unconfirmed CR (uCR), or a PR [13], or CR or PR for patients with CLL [14]. The database cut-off date was 4 November 2013 for the primary analysis set, and 1 October 2015 including expansion patients. Secondary variables included progression-free survival, overall survival, and duration of response. Additional variables included time to response, lesion size, biomarkers, and safety.
The indolent cohort consisted of histologically confirmed grade 1, 2, or 3a FL, marginal zone lymphoma, lymphoplasmacytic lymphoma/Waldenström macroglobulinemia, or CLL. Aggressive lymphomas included histologically confirmed grade 3b FL, transformed indolent lymphoma, DLBCL, mediastinal large B-cell lymphoma, MCL, unspecified peripheral T-cell lymphoma (PTCL), anaplastic large-cell lymphoma primary systemic type, or angio-immunoblastic T-cell lymphoma. Additional inclusion criteria and exclusion criteria are described in the supplementary materials, available at Annals of Oncology online.
Eligible patients received 0.8 mg/kg copanlisib intravenously over a 1-h infusion on days 1, 8, and 15 of a 28-day cycle until disease progression, worsening of Eastern Cooperative Oncology Group performance status of ≥3, or unacceptable toxicity. Details of plasma glucose requirements pre-infusion, management of post-infusion hyperglycemia, and permitted dose reductions are described in the supplementary materials, available at Annals of Oncology online.
Dose reductions to 0.6 and 0.4 mg/kg were permitted if clinically significant toxicities were observed; re-escalation was not permitted. Treatment was discontinued if the 0.4 mg/kg dose was not tolerated.
Assessments
Tumor assessments were carried out at screening and every two cycles thereafter during year 1, every three cycles during year 2, and every six cycles during year 3. Radiologic evaluation of efficacy was carried out by central blinded independent review; in patients with CLL, treatment response was determined by investigator assessment. Patients were followed off-study for overall survival at 3-month intervals for up to 3 years. Safety was evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Measurements of glycated hemoglobin, plasma glucose, and blood pressure are described in the supplementary materials, available at Annals of Oncology online. Archival formalin-fixed paraffin-embedded tumor tissues were evaluated for biomarkers, for which detailed methods are described in the supplementary materials, available at Annals of Oncology online.
Statistical methods
Pre-specified primary evaluation was to reject the null hypothesis of a true ORR of ≤5% in the indolent or aggressive lymphoma cohorts, separately. ORR was evaluated using a one-sided exact binomial test (significance level of 5%) and was designed to have 95% power per cohort, if the true ORR was 30%, resulting in a requirement of 30 assessable patients per cohort. Exact binomial Clopper–Pearson confidence intervals (CI) with confidence level of 90% were provided for ORR. Time-to-event variables were analyzed using Kaplan–Meier methodology. In order to study promising lymphoma histologies in more detail, the aggressive cohort was expanded with an additional 17 patients, beyond those recruited for the primary evaluation. Data from these patients were included in a descriptive analysis of the study. Detailed methods are described in the supplementary materials, available at Annals of Oncology online.
Results
Patients
Eighty-four patients received copanlisib: 33 in the indolent cohort and 34 in the aggressive cohort for the primary analysis, and 17 additional patients with aggressive lymphoma (four patients with MCL and 13 with PTCL) enrolled into an expansion cohort (supplementary Figure S1, available at Annals of Oncology online). The majority had advanced-stage disease at study entry (Ann Arbor stage III/IV, 73.7% indolent, 88.2% aggressive) and had received a median of three lines of systemic anticancer therapy (Table 1).
Table 1.
Baseline demographics and disease characteristics
| Primary analysis set |
||||
|---|---|---|---|---|
| Indolent cohort | Aggressive cohort | Aggressive cohort, all | Totala | |
| (n=33) | (n=34) | (n=51) | (N=84) | |
| Sex, n (%) | ||||
| Male | 15 (45.5) | 17 (50.0) | 29 (56.9) | 44 (52.4) |
| Female | 18 (54.5) | 17 (50.0) | 22 (43.1) | 40 (47.6) |
| Median age, years (range) | 68.0 (46–89) | 68.0 (22–90) | 63.0 (22–90) | 66.5 (22–90) |
| Median time from initial diagnosis to start of study treatment, months (range) | 119.8 (20–244) | 29.7 (6–212) | 24.0 (6–281) | 47.6 (6–281) |
| Median time since first progression, months (range) | 68.5 (10–177) | 11.5 (0–100) | 11.2 (0–100) | 19.7 (0–177) |
| Median time since most recent progression to start of study treatment, months (range) | 5.1 (1–31) | 3.9 (0–18) | 4.1 (0–21) | 4.1 (0–31) |
| Most recent histology of tumor, n (%) | ||||
| Indolent lymphoma or CLL | 33 (100) | 0 | 0 | 33 (39.3) |
| CLL | 13 (39.4) | 0 | 0 | 13 (15.5) |
| FL | 16 (48.5) | 0 | 0 | 16 (19.0) |
| Grade 1, 2, or 3ab | 15 (45.5) | 0 | 0 | 15 (17.9) |
| MZL | 3 (9.1) | 0 | 0 | 3 (3.6) |
| SLL | 1 (3.0) | 0 | 0 | 1 (1.2) |
| Aggressive lymphoma | 0 | 34 (100) | 51 (100) | 51 (60.7) |
| DLBCL | 0 | 15 (44.1) | 15 (29.4) | 15 (17.9) |
| FL, grade 3b | 0 | 1 (2.9) | 1 (2.0) | 1 (1.2) |
| MCL | 0 | 7 (20.6) | 11 (21.6) | 11 (13.1) |
| Mediastinal large B-cell lymphoma | 0 | 1 (2.9) | 1 (2.0) | 1 (1.2) |
| PTCL | 0 | 4 (11.8) | 17 (33.3) | 17 (20.2) |
| Anaplastic large-cell lymphoma | 0 | 0 | 3 (5.9) | 3 (3.6) |
| Angio-immunoblastic TCL | 0 | 1 (2.9) | 4 (7.8) | 4 (4.8) |
| PTCLc | 0 | 3 (8.8) | 10 (19.6) | 10 (11.9) |
| Transformed indolent FL | 0 | 6 (17.6) | 6 (11.8) | 6 (7.1) |
| Stage at study entry, n (%)d | ||||
| I | 1 (3.0) | 0 | 1 (2.0) | 2 (2.9) |
| II | 4 (12.1) | 3 (8.8) | 5 (9.8) | 9 (10.7) |
| III | 5 (15.2) | 7 (20.6) | 12 (23.5) | 17 (20.2) |
| IV | 9 (42.4) | 24 (70.6) | 33 (64.7) | 42 (50.0) |
| Prior systemic anticancer therapy lines, median (range) | 4 (2–10) | 3 (2–9) | 3 (1–9) | 3 (1–10) |
Total includes all patients from the ‘Indolent’ and ‘Aggressive, all’ cohorts
Data missing for one patient.
Includes eight patients with PTCL, not otherwise specified.
Data missing for 14 patients with indolent lymphoma.
CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; PTCL, peripheral T-cell lymphoma; SLL, small lymphocytic lymphoma; TCL, T-cell lymphoma.
The majority of patients had received chemotherapy with or without immunotherapy or immunotherapy monotherapy (supplementary Table S1, available at Annals of Oncology online). Twenty-three patients (69.7%) in the indolent cohort were refractory to one or more regimens with rituximab and 15 (45.5%) were refractory to one or more regimens with bendamustine.
Copanlisib treatment
Median treatment duration was 13.9 weeks overall (range 2.0–137.9): 22.7 weeks (5.7 cycles) in the indolent cohort and 8.0 weeks (2.0 cycles) in the aggressive cohort. Patients with indolent or aggressive lymphoma received a median number of 15 (range 1–101) and six (range 1–95) copanlisib infusions, respectively; patients received a median of 90.5% of the planned dose overall. Fifty patients (59.5%) had dose interruptions or delays because of adverse events, and 11 (13.1%) had dose reduction because of adverse events (supplementary Results, available at Annals of Oncology online); interruptions or delays had a median duration of 1 week (range 0.1–1.7).
Efficacy
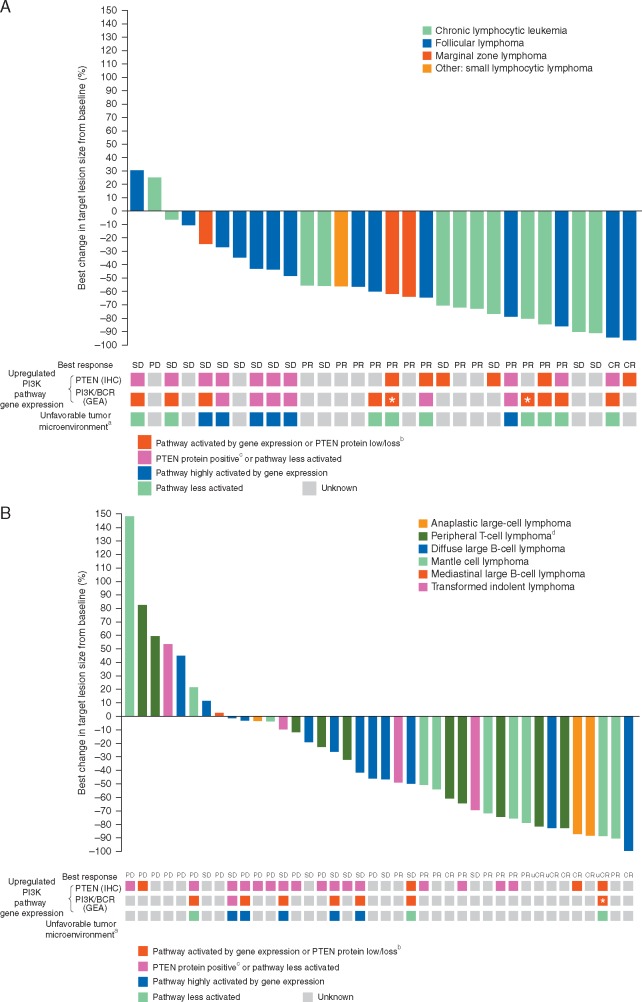
Sixty-six patients were included in the per protocol analysis set for the primary efficacy analysis. The ORR was 43.8% (90% CI 28.7–59.7) in the indolent cohort and 29.4% (90% CI 16.9–44.8) in the aggressive cohort, statistically confirming the hypothesis of an ORR >5% (P < 0.0001 in each cohort) (Table 2). A waterfall plot of best change in target lesion size from baseline per investigator assessment indicated that 66.7% of patients (20/30) in the indolent cohort (Figure 1A) and 42.5% (17/40) in the aggressive cohort (Figure 1B) had ≥50% reduction in lesion size.
Table 2.
Response evaluation by independent assessment (per protocol set)
| n (%) | Indolent cohort | Aggressive cohort, primary analysis set | Aggressive cohort, all |
|---|---|---|---|
| (n=32)a | (n=34) | (n=48)b | |
| Best response | |||
| Complete response | 2 (6.3) | 0 | 2 (4.2) |
| Unconfirmed complete response | 1 (3.1) | 4 (11.8) | 2 (4.2) |
| Partial response | 11 (34.4) | 6 (17.7) | 9 (18.8) |
| Stable disease | 15 (46.9) | 6 (17.7) | 11 (22.9) |
| Progressive disease | 1 (3.1) | 10 (29.4) | 16 (33.3) |
| Not available/not evaluablec | 2 (6.3) | 8 (23.5) | 8 (16.7) |
| Objective response rate | 14 (43.8) | 10 (29.4) | 13 (27.1) |
| Disease control rated | 29 (90.6) | 16 (47.1) | 24 (50.0) |
One patient was excluded as they did not have any measurable lesion as per Cheson criteria at baseline.
Three patients were excluded because: baseline computed tomography or magnetic resonance imaging of all suspected disease sites and tumor evaluations were not taken within 28 days before starting study treatment (one patient); no measurable lesion was observed (one patient); and no post-baseline tumor assessment was available and discontinuation was not caused by a drug-related toxicity, death, or progression by clinical judgment before disease was re-evaluated (one patient).
Includes patients without post-baseline tumor assessment.
Disease control rate was defined as the proportion of patients with a complete response, an unconfirmed complete response, a partial response, or stable disease.
Figure 1.
Percent best change in target lesion size from baseline (investigator assessment) in the indolent (A) and aggressive (B) cohorts with corresponding status of baseline upregulation of PI3K/PTEN pathway gene expression and tumor microenvironment (full analysis set). * indicates NOTCH1 mutation by next-generation sequencing. aUnfavorable tumor microenvironment gene-expression signature was defined as high (greater than the median value) weighted gene-expression scores combining genes expressed in stromal, inflammatory, and immune response pathways. bUpregulation of PI3K/PTEN pathway gene expression was defined as PTEN protein loss (0% of tumor cells stained positive for PTEN by IHC) or low PTEN protein expression (1%–4% of tumor cells stained positive), and/or a high PI3K/BCR gene-expression signature (defined by a weighted gene-expression score greater than the median value). cPositive PTEN protein expression was defined as ≥5% of cells staining positive for PTEN by IHC. dIncludes peripheral T-cell lymphoma, peripheral T-cell lymphoma not otherwise specified, and angio-immunoblastic T-cell lymphoma. BCR, B-cell receptor; CR, complete response; GEA, gene-expression analysis; IHC, immunohistochemistry; PD, progressive disease; PR, partial response; SD, stable disease; uCR, unconfirmed complete response.
CRs/uCRs were observed in three of 15 FL patients (20.0%) and PRs in a further three patients (20.0%); the ORR was 40% (supplementary Table S2, available at Annals of Oncology online). PRs were observed in five of the 13 patients with CLL (ORR 38.5%), two of the three patients with marginal zone lymphoma (ORR 66.7%), and one patient with small lymphocytic lymphoma (ORR 100%). In the initial set of patients with aggressive lymphoma, five of seven with MCL and two of four with PTCL had CR/uCR or PR, prompting additional enrollment, expanding the response analysis to 48 patients. The ORR for the aggressive lymphoma expansion cohort was 27.1% (90% CI 16.8–39.6). Objective responses were achieved in patients with DLBCL (one PR; ORR 6.7%), PTCL (two CRs, one PR; ORR 21.4%), MCL (two uCRs, five PRs; ORR 63.6%), and transformed FL (both PRs; ORR 33.3%) (supplementary Table S2, available at Annals of Oncology online).
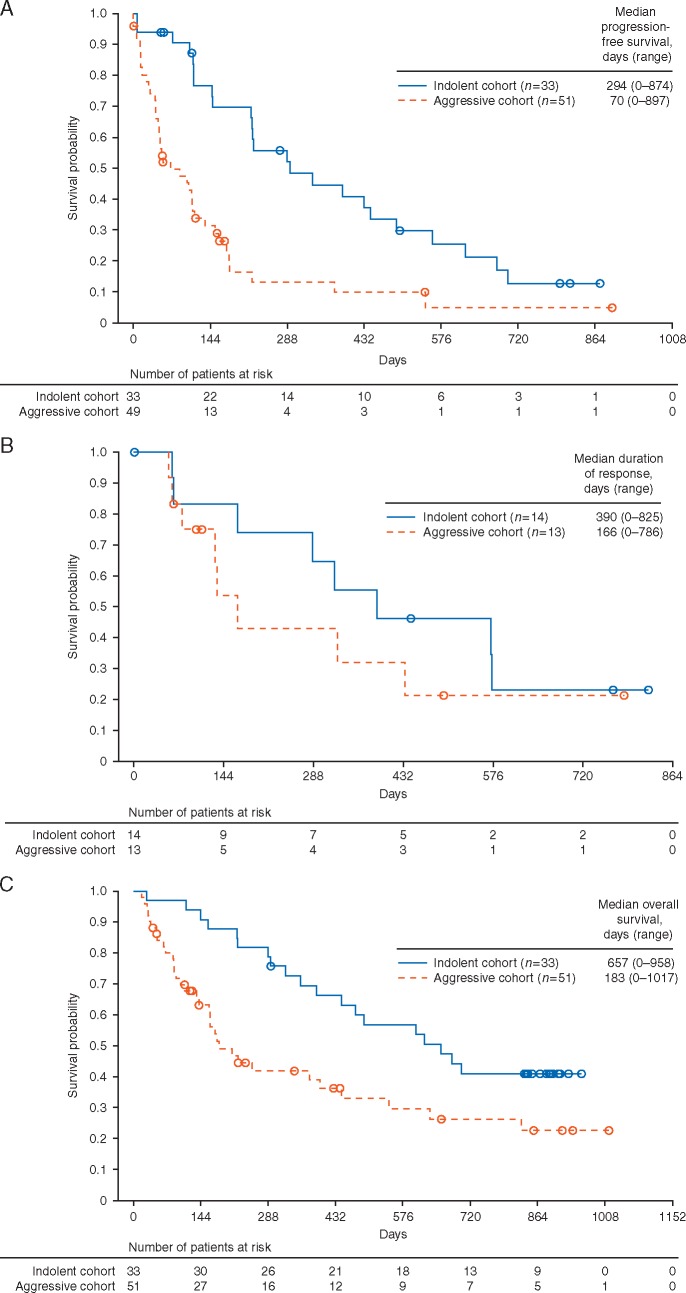
Median time to response was 52 days (range 0–109) in the indolent cohort and 51 days (range 0–117) in the aggressive cohort, and was generally observed at the first response assessment (supplementary Figure S2, available at Annals of Oncology online). Median progression-free survival was 294 days (range 0–874) in the indolent cohort and 70 days (range 0–897) in the aggressive cohort (Figure 2A). At 12 months, progression-free survival was 45% and 13% in the indolent and aggressive cohorts, respectively. The median duration of response was 390 days (range 0–825) and 166 days (range 0–786 days) in the indolent and aggressive cohorts, respectively (Figure 2B). Median overall survival was 657 days in the indolent cohort (range 0–958) and 183 days in the aggressive cohort (range 0–1017) (Figure 2C). At 12 months, overall survival was 69% and 42% in patients with indolent and aggressive lymphoma, respectively.
Figure 2.
Progression-free survival (full analysis set) (A), duration of response (per protocol set) (B), and overall survival (full analysis set) (C) in patients in the indolent or aggressive cohorts receiving copanlisib.
Biomarkers
Loss of or low tumor PTEN protein expression was detected in six of 16 patients in the indolent cohort (including 2/10 with FL) and four of 26 patients in the aggressive cohort (supplementary Table S3, available at Annals of Oncology online). High expression of PI3K and/or BCR pathway genes occurred in nine of 15 patients in the indolent cohort (including 4/10 with FL) and seven of nine patients in the aggressive cohort.
In the indolent cohort, 13 of 18 patients (including 6/11 with FL) had upregulated PI3K pathway gene expression (supplementary Table S3, available at Annals of Oncology online). Upregulation of PI3K pathway gene expression was observed in 10 of 11 patients with either a CR or a PR, or ≥50% best decrease in target lesion size from baseline, but only three of seven patients with <50% best decrease in target lesion size (Figure 1A). In the aggressive cohort, nine of 26 patients had upregulation of PI3K pathway gene expression (supplementary Table S3, available at Annals of Oncology online), including three of seven patients with ≥50% best reduction in target lesion size (of whom two had CR/uCR) and six of 13 patients with <50% best decrease in target lesion size (Figure 1B).
Results from DNA next-generation sequencing are described in the supplementary materials, available at Annals of Oncology online. An unfavorable tumor microenvironment gene signature was more frequently expressed in patients with indolent or aggressive lymphoma who were less responsive to copanlisib, with <50% best decrease in target lesion size (Figure 1A and B).
Safety
Treatment-emergent adverse events (TEAEs) are shown in Table 3. Drug-related TEAEs are shown in supplementary Table S4, available at Annals of Oncology online. The most common TEAEs were hyperglycemia (59.5%), hypertension (54.8%), fatigue (48.8%), and diarrhea (40.5%). In most cases, grade 3 was the worst grade of TEAE (overall 60.7%; 51/84). Serious grade 3, 4, and 5 TEAEs were reported in 31.0% (26/84), 4.8% (4/84), and 11.9% (10/84) of patients, respectively. Serious grade 3 or 4 TEAEs occurring in two or more patients included: grade 3 lung infection (10.7%; 9/84); grade 3 diarrhea and grade 3 febrile neutropenia (3.6% each; 3/84); and grade 4 decreased neutrophil count, grade 3 hyperglycemia, grade 3 pneumonitis (one infectious and one possibly infectious), grade 3 pancreatitis, grade 3 cardiac disorders—other, and grade 3 infection/infestations—other (2.4% each; 2/84). There was one case of serious grade 3 hypertension and one case of serious grade 3 acute coronary syndrome. Serious drug-related TEAEs were recorded in 32.1% of patients (supplementary Table S5, available at Annals of Oncology online).
Table 3.
Summary of TEAEs irrespective of causality (safety analysis set)a
| n (%) | Grade 1 or 2 | Grade 3 or 4 | Total |
|---|---|---|---|
| (n=84) | (n=84) | (N=84) | |
| Patients with ≥1 TEAEs | 7 (8.3) | 67 (79.8) | 84 (100)b |
| Patients with any TEAE leading to permanent discontinuation of study drug | 3 (3.6) | 18 (21.4) | 21 (25.0)c |
| Patients with any TEAE leading to dose reduction | 3 (3.6) | 8 (9.5) | 11 (13.1) |
| Patients with any TEAE leading to dose interruption | 11 (13.1) | 39 (46.4) | 50 (59.5) |
| TEAEs occurring in ≥10% of patients overalld | |||
| Hyperglycemia | 29 (34.5) | 21 (25.0) | 50 (59.5) |
| Hypertension | 12 (14.3) | 34 (40.5) | 46 (54.8) |
| Fatigue | 31 (36.9) | 10 (11.9) | 41 (48.8) |
| Diarrhea | 30 (35.7) | 4 (4.8) | 34 (40.5) |
| Decreased neutrophil count | 4 (4.8) | 25 (29.8) | 29 (34.5) |
| Nausea | 26 (31.0) | 2 (2.4) | 28 (33.3) |
| Anemia | 12 (14.3) | 12 (14.3) | 24 (28.6) |
| Oral mucositis | 18 (21.4) | 1 (1.2) | 19 (22.6) |
| Lung infection | 5 (6.0) | 10 (11.9) | 17 (20.2) |
| Fever | 15 (17.9) | 1 (1.2) | 16 (19.0) |
| Decreased platelet count | 5 (6.0) | 10 (11.9) | 15 (17.9) |
| Headache | 15 (17.9) | 0 | 15 (17.9) |
| Urinary tract infection | 12 (14.3) | 2 (2.4) | 14 (16.7) |
| Dyspnea | 11 (13.1) | 3 (3.6) | 14 (16.7) |
| Constipation | 13 (15.5) | 0 | 13 (15.5) |
| Skin and subcutaneous disorders - other | 12 (14.3) | 0 | 12 (14.3) |
| Anorexia | 11 (13.1) | 1 (1.2) | 12 (14.3) |
| Vomiting | 10 (11.9) | 1 (1.2) | 11 (13.1) |
| Abdominal pain | 8 (9.5) | 2 (2.4) | 10 (11.9) |
| Musculoskeletal and connective tissue disorders - other | 10 (11.9) | 0 | 10 (11.9) |
| Cough | 10 (11.9) | 0 | 10 (11.9) |
| Infections and infestations - other | 7 (8.3) | 3 (3.6) | 10 (11.9) |
| Bronchial infection | 8 (9.5) | 1 (1.2) | 9 (10.7) |
| Upper respiratory infection | 8 (9.5) | 1 (1.2) | 9 (10.7) |
| Clinical laboratory | |||
| Increased alanine aminotransferasee | 19 (23.2) | 3 (3.7) | 22 (26.2) |
| Increased aspartate aminotransferasee | 21 (25.6) | 2 (2.4) | 23 (27.4) |
| Increased alkaline phosphatasee | 29 (35.4) | 1 (1.2) | 30 (35.7) |
| Adverse events of interest | |||
| Pneumonitis | 1 (1.2) | 2 (2.4) | 3 (3.6) |
October 2015 data set including all patients.
There were 10 deaths ≤30 days following the last infusion of the study drug, including infections and infestations in five patients (meningitis, pneumonia, lower respiratory tract infection, pyelonephritis, and septic shock), general disorders in two patients (deterioration in general physical health and multi-organ dysfunction syndrome), and acute respiratory failure, circulatory collapse, and progressive disease in one patient each.
Adverse events leading to permanent discontinuation of study drug included grade 3 fatigue, lung infection, pneumonitis, and maculo-papular rash in two patients each (2.4%), and grade 4 lipase increase, serum amylase increase, autoimmune disorder, and meningitis in one patient each (1.2%).
Common Terminology Criteria for Adverse Events version 4.0.
Two patients missing.
TEAE – treatment-emergent adverse event.
There were 10 deaths (Table 3), four of which were considered possibly drug-related (supplementary Table S5, available at Annals of Oncology online), including one case of meningitis caused by opportunistic infection with Cryptococcus neoformans in a patient with CLL occurring 8 days after one infusion of copanlisib.
Hyperglycemic and hypertension events were all grade ≤3 and transitory (supplementary Table S6, available at Annals of Oncology online). Seventeen patients received insulin to manage post-infusion hyperglycemia [grade 2, 35.3% (6/17); grade 3, 64.7% (11/17)], of whom nine had blood glucose ≤160 mg/dl. Increases in mean glycated hemoglobin from baseline to end of treatment were <0.5% in both indolent and aggressive cohorts (supplementary Table S6, available at Annals of Oncology online). Seventeen patients received post-dose antihypertensive treatment for grade 3 hypertension.
All-grade hematologic toxicities included decreased neutrophil count (34.5%; grade 3/4, 29.8%), anemia (28.6%; grade 3/4, 14.3%), and decreased platelet count (17.9%; grade 3/4, 11.9%), and infections and infestations were reported in 64.3% of patients (grade 1/2, 39.3%; drug-related all-grade, 29.8%). Infections of grade ≥3 occurring in two or more patients included lung infection (14.3%; 12/84) and skin infection, urinary tract infection, and other (2.4% each; 2/84). There were three reports of pneumonitis overall, including one grade 3 opportunistic infection with Pneumocystis jirovecii and one grade 1 non-infectious event. Diarrhea was mostly mild (grade 1, 22.6%; grade 2, 13.1%; grade ≥3, 4.8%) and was manageable with a standard symptomatic treatment such as loperamide. There were no reported cases of colitis or intestinal perforation.
TEAEs leading to permanent treatment discontinuation were reported in 25.0% of patients (Table 3). No patient discontinued because of hyperglycemia or hypertension.
Discussion
In this exploratory phase II study in heavily pretreated patients with relapsed or refractory, indolent or aggressive lymphoma, or CLL, copanlisib monotherapy was shown to be effective, confirming the early signal of activity reported in a lymphoma expansion cohort in the phase I study [12]. The ORR was 43.8% in the indolent cohort (CR/uCR 9.4%) and 27.1% in the aggressive cohort (CR/uCR 8.3%). A median duration of response of 12.8 months and median progression-free survival of 9.7 months were seen in the indolent cohort. The two largest subtypes of patients in the indolent cohort (FL and CLL) had ORRs of 40% (CR/uCR) and 38.5%, respectively. These results in the indolent cohort are similar to or slightly lower than the ORR and progression-free survival results reported by Flinn et al. [15] and Gopal et al. [16] with idelalisib in similar patient populations, although lower than those reported by Byrd et al. [17] for ibrutinib in patients with CLL and small lymphocytic lymphoma. Such cross-study comparisons are inconclusive due to the small numbers of patients, although the CR/uCR rate of 20% seen here in FL, as well as two CRs from six FL patients in the phase I study [12], are promising. A larger study in patients with indolent lymphoma is ongoing (NCT01660451, Part B).
In the aggressive cohort, the ORR ranged from 6.7% in DLBCL patients to 63.6% in MCL patients. Responses to ibrutinib have been reported to be higher in patients with activated B-cell (ABC) DLBCL compared with germinal center B-cell-like differentiation (GCB) DLBCL [18], but such subtyping of DLBCL was not available here. Based on expression of PI3K-α and PI3K-δ in DLBCL lymphoma tissues, together with heightened activity of copanlisib compared with selective PI3K-δ or PI3K-α inhibition in ABC DLBCL models [9, 10], exploration of copanlisib activity in ABC or GCB subtypes of DLBCL is ongoing (NCT02391116), and a rationale for combination with ibrutinib has been proposed [9, 10]. Objective responses have not been reported in studies with DLBCL patients treated with idelalisib.
The ORR of 63.6% (including two uCRs) in MCL patients was favorable compared with reports of idelalisib (ORR 40%) [19] or ibrutinib (ORR 67%) [20]. Copanlisib activity in MCL is consistent with expression of PI3K-α and PI3K-δ, with increased PI3K-α expression in later-stage disease [8]. In PTCL patients, the ORR was 21.4% and included two CRs, which was similar to that seen in a phase I study in PTCL patients with the PI3K-δ,γ inhibitor duvelisib [21]. Therefore, further study with copanlisib in relapsed or refractory MCL and PTCL may be warranted.
The safety profiles for PI3K inhibitors warrant careful scrutiny following the recent safety concerns for the oral agent idelalisib [5–7]. Hyperglycemia and hypertension were the most common TEAEs seen with copanlisib, were consistent with its target profile and route of administration, and were predicted [12]. Both were transient and manageable, and followed a similar pattern as previously reported [12], with blood pressure peaking 1–2 hours after the start of infusion and plasma glucose levels peaking 5–8 hours after the start of infusion, followed by a decline to baseline levels. No patients discontinued because of either adverse event. No hyperglycemic or hypertension events of grade ≥4 were reported, and serious events of grade 3 were reported in two patients and one patient, respectively, who all had baseline risk factors requiring planned hospitalization to adjust dosing. Cardiac events were infrequent and low, and glycated hemoglobin levels did not increase compared with baseline.
Infections are common for patients with hematologic malignancies such as CLL, and drugs inhibiting PI3K-δ or Bruton’s tyrosine kinase may exacerbate the rate and severity of infection [7, 22]. Copanlisib treatment decreased neutrophil count in 34.5% of patients (29.8% grade ≥3), yet serious febrile neutropenia was infrequent (three patients), as were opportunistic infections (two patients) and pneumonitis (three patients). Higher rates of pneumonitis have been reported with idelalisib in patients with relapsed lymphoma [11% (grade ≥3, 7%) and 12.5% (grade ≥3, 10%)] [16, 19]. Similarly, the incidence of pneumonitis here was low (3.6%; one grade 1 non-infectious, one grade 3 infectious, one grade 3 possibly infectious).
High rates of hepatic and gastrointestinal toxicity have been seen with idelalisib [22]. With idelalisib trials, the incidence of elevated aminotransferases ranged from 48% to 60% (all grade), with grade ≥3 ranging from 8% to 13% in phase II [15, 16, 19]. Here, elevated alanine aminotransferase and aspartate aminotransferase were incidental findings in 25.6% of patients, almost all of which were grade 1 (23.2% and 24.4%, respectively; grade 3, 3.7% and 2.4%, respectively). Diarrhea was the most common adverse event reported in a phase II study of idelalisib (all-grade, 43%; grade ≥3, 13%) [15, 16, 19]. Late-onset idelalisib-induced diarrhea has been reported, a possible symptom of autoimmune colitis [23]. In one study, 86% of patients (12/14) treated with idelalisib for ≥3 months with idelalisib-induced diarrhea had colitis with intra-epithelial lymphocytosis, crypt cell apoptosis, and neutrophilic infiltration of crypt epithelium [24]. Here, diarrhea was reported in 40.5% of patients (grade ≥3, 4.8%) and there were no reports of colitis. Only one case of colitis was reported with copanlisib in the phase I study [12]. This differentiation may reflect the intermittent administration of intravenous copanlisib versus oral agents dosed continuously.
Tumor gene expression and mutation analyses showed that consistent with the known low prevalence of PIK3CA mutations in lymphoma [25, 26], no mutations in PIK3CA, PIK3CB, PIK3CD, or PIK3CG, or PTEN, were detected. Upregulation of PI3K pathway gene expression was frequently observed in indolent and aggressive lymphoma types. Analysis of gene-expression data using response rate and progression-free survival (data not shown) as clinical outcomes demonstrated increased copanlisib antitumor activity in cases with activated PI3K/BCR signaling, which, together with low expression of unfavorable tumor microenvironment genes, is consistent with the proposed mechanism of action of copanlisib. These preliminary results are therefore currently under further evaluation in an extension cohort of patients with indolent lymphoma.
Overall, our data suggest that intravenous copanlisib may provide an effective therapeutic option for patients with relapsed or refractory, indolent or aggressive lymphoma whose disease has progressed after standard therapy. Moreover, the safety profile of intravenous intermittently dosed copanlisib is distinct and manageable, and potentially advantageous, with a lower incidence of fatal and/or severe hepatic and gastrointestinal toxicity compared with oral PI3K inhibitors. An extension study of copanlisib in patients with indolent lymphoma is ongoing, along with studies of copanlisib as monotherapy and in combination with standard chemotherapy in patients with indolent lymphoma (NCT02369016, NCT02367040, and NCT02626455) and aggressive lymphoma (NCT02391116).
Supplementary Material
Acknowledgements
The authors wish to thank Liping Huang, David Martinez, Wei Shao, Jie Cheng, and Abraham Yeh for their assistance with statistical analysis.
Funding
This study was supported by research funding from Bayer HealthCare Pharmaceuticals, Inc. DC is funded by the National Institute for Health Research Biomedical Research Centre at the Royal Marsden and Institute of Cancer Research, London, UK. Tanja Torbica, PhD, of Complete HealthVizion, Manchester, UK, provided medical writing assistance in the development of the first draft, based on detailed discussion and feedback from all the authors, and was funded by Bayer HealthCare Pharmaceuticals, Inc. No grant number is applicable.
Disclosure
MD: Participated in advisory boards for and on a speaker bureau for Bayer. DC: Received research funding from Amgen, AstraZeneca, Bayer, Celgene, Merrimack, MedImmune, Merck Serono, and Sanofi. CT: Received honoraria from and participated on a board of directors or advisory board for AbbVie, Bayer HealthCare, Celgene, and Janssen; acted as a consultant for Janssen; and received research funding from Roche. FH, MG, and KK: Employees of Bayer AG. JG-V, IG, LL, CP, and BHC: Employees of Bayer HealthCare. MN: Employee of Bayer SA. PLZ: Participated in advisory boards for Roche, Janssen, Celgene, Gilead, Servier, Bayer, TG Pharmaceuticals, and Takeda. All remaining authors have declared no conflicts of interest.
References
- 1. Swerdlow SH, Campo E, Pileri SA. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Claro RA, McGinn KM, Verdun N. et al. FDA approval: ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res 2015; 21: 3586–3590. [DOI] [PubMed] [Google Scholar]
- 3. Miller BW, Przepiorka D, de Claro RA. et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res 2015; 21: 1525–1529. [DOI] [PubMed] [Google Scholar]
- 4. Center for Drug Evaluation and Research. Application number: 206545Orig1s000. Medical Review(s). Clinical Review. Zydelig® (Idelalisib). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206545Orig1s000MedR.pdf (20 March 2017, date last accessed).
- 5. Pongas G, Cheson BD.. PI3K signaling pathway in normal B cells and indolent B-cell malignancies. Semin Oncol 2016; 43: 647–654. [DOI] [PubMed] [Google Scholar]
- 6. Greenwell IB, Flowers CR, Blum KA, Cohen JB.. Clinical use of PI3K inhibitors in B-cell lymphoid malignancies: today and tomorrow. Expert Rev Anticancer Ther 2017; 17: 271–279. [DOI] [PubMed] [Google Scholar]
- 7. Zelenetz AD, Barrientos JC, Brown JR. et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2017; 18: 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iyengar S, Clear A, Bödör C. et al. P110α-mediated constitutive PI3K signaling limits the efficacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013; 121: 2274–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erdmann T, Klener P, Lynch JT. et al. Sensitivity to PI3K and AKT inhibitors is mediated by divergent molecular mechanisms in subtypes of DLBCL. Blood 2017;130: 310–322. [DOI] [PubMed] [Google Scholar]
- 10. Paul J, Soujon M, Wengner AM. et al. Simultaneous inhibition of PI3Kδ and PI3Kα induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-κB and AKT. Cancer Cell 2017; 31: 64–78. [DOI] [PubMed] [Google Scholar]
- 11. Liu N, Rowley BR, Bull CO. et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther 2013; 12: 2319–2330. [DOI] [PubMed] [Google Scholar]
- 12. Patnaik A, Appleman LJ, Tolcher AW. et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol 2016; 27: 1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheson BD, Horning SJ, Coiffier B. et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 1999; 17: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 14. Hallek M, Cheson BD, Catovsky D. et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flinn IW, Kahl BS, Leonard JP. et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 2014; 123: 3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gopal AK, Kahl BS, de Vos S. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014; 370: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrd JC, Furman RR, Coutre SE. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson WH, Young RM, Schmitz R. et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015; 21: 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kahl BS, Spurgeon SE, Furman RR. et al. A phase 1 study of the PI3Kδ inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood 2014; 123: 3398–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang ML, Blum KA, Martin P. et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015; 126: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horwitz SM, Porcu P, Flinn I. et al. Duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 2014; 124: 803. [Google Scholar]
- 22. U.S. Food & Drug Administration. FDA alerts healthcare professionals about clinical trials with Zydelig (idelalisib) in combination with other cancer medicines. http://www.fda.gov/Drugs/DrugSafety/ucm490618.htm (20 March 2017, date last accessed).
- 23. Coutré SE, Barrientos JC, Brown JR. et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma 2015; 56: 2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weidner AS, Panarelli NC, Geyer JT. et al. Idelalisib-associated colitis: histologic findings in 14 patients. Am J Surg Pathol 2015; 39: 1661–1667. [DOI] [PubMed] [Google Scholar]
- 25. Baohua Y, Xiaoyan Z, Tiecheng Z. et al. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol 2008; 17: 159–165. [DOI] [PubMed] [Google Scholar]
- 26. Marincevic M, Tobin G, Rosenquist R.. Infrequent occurrence of PIK3CA mutations in chronic lymphocytic leukemia. Leuk Lymphoma 2009; 50: 829–830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.