Abstract
Background
Activating events along the PI3K/mTOR pathway are common in head and neck squamous cell carcinomas (HNSCC), and preclinical studies suggest additive or synergistic effects when combining mTORC1 inhibitors with carboplatin and paclitaxel chemotherapy.
Patients and methods
In this single-institution phase II study, the combination of temsirolimus 25 mg, carboplatin AUC 1.5, and paclitaxel 80 mg/m2 administered on days 1 and 8 of a 21-day cycle was evaluated in 36 patients with recurrent and/or metastatic (R/M) HNSCC. The primary end point was objective response rate after two cycles of treatment. Secondary end points include the safety and tolerability profile and overall survival. Correlative studies with exome mutational analysis were performed in pre-treatment biopsy samples from 21 patients.
Results
Fifteen (41.7%) patients had an objective response, which were all partial responses, and 19 (52.3%) patients had stable disease as best response. The two patients who were designated as ‘non-responders’ were removed from study prior to two cycles of treatment, but are included in the efficacy and safety analyses. The median duration on study was 5.3 months and the median progression-free survival and overall survival were 5.9 months (95% confidence interval, 4.8–7.1) and 12.8 months (95% confidence interval, 9.8–15.8), respectively. The most common grade 3 and 4 adverse events were hematologic toxicities. Three (3.8%) patients developed neutropenic fever on study. Three of four patients with PIK3CA mutations experienced tumor regressions, and responses were also seen in patients with other genetic alterations in the PI3K/mTOR pathway.
Conclusion
The combination of temsirolimus with low-dose weekly carboplatin and paclitaxel appears to have meaningful clinical efficacy in the treatment of R/M HNSCC. This regimen has a relatively high response rate compared to other treatments evaluated in R/M HNSCC, and potential associations with genetic alterations in the PI3K/mTOR pathway should be further explored.
Keywords: temsirolimus, mTOR inhibition, head and neck cancer, squamous cell carcinoma
Introduction
For patients with recurrent and/or metastatic (R/M) squamous cell carcinoma of the head and neck (HNSCC), first-line therapy with cetuximab in combination with platinum-based chemotherapy yields an overall response rate (ORR) of 36%, and a median progression-free survival (mPFS) and overall survival (mOS) of 5.6 and 10.1 months, respectively [1]. Grade 3 or 4 adverse events occurred in 82% of patients, including a 22% incidence of neutropenia. There remains a critical need for systemic therapy with improved tolerability and efficacy for R/M HNSCC.
Alterations in the PI3K/mTOR (phospoinositide-3-kinase/mammalian target of rapamycin) pathway are common oncogenic events in HNSCC [2]. mTORC1 inhibitors, rapamycin and temsirolimus, have achieved growth inhibition in HNSCC xenografts [3, 4]. Further pre-clinical data suggests that mTORC1 inhibition, with everolimus and temsirolimus, can sensitize cancer cell lines to platinum and/or taxane-based chemotherapy [5, 6]. Specifically, in HNSCC lines, rapamycin has been shown to provide synergistic efficacy when combined with carboplatin and paclitaxel [5].
We conducted the phase I study that established the safety and tolerability of temsirolimus 25 mg flat dose in combination with low-dose carboplatin (AUC 1.5) and paclitaxel (80 mg/m2), all given on days 1 and 8 of a 21-day cycle [7]. Among the six patients with R/M HNSCC treated at this dose level in the phase I study, confirmed objective radiographic responses were observed in four patients.
Here we report the results of a single-arm phase II study of this regimen of temsirolimus 25 mg flat dose in combination with low-dose carboplatin and paclitaxel in patients with R/M HNSCC.
Patients and methods
This was a single-center, non-randomized phase II study. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC), and subjects provided written informed consent.
Patient population
Study subjects were 18 years or older, and had R/M, non-nasopharynx HNSCC that was pathologically confirmed. Patients had adequate function of bone marrow (absolute neutrophil count ≥ 1.5×109/l, platelets ≥ 100×109/l, and hemoglobin ≥ 9 g/dl), kidneys (serum creatinine ≤ 1.3 mg/dl, or creatinine clearance ≥ 45 ml/min per Cockroft and Gault formula), and liver (total bilirubin ≤ 1.0 mg/dl; alkaline phosphatase, aspartate transaminase and alanine transaminase ≤ 1.5× upper limit of normal). Measurable disease by RECIST v1 criteria [8] and Karnofsky Performance Status (KPS) of at least 70% were required. Patients were excluded if they had received more than two prior cytotoxic regimens for R/M disease. Other key exclusions were prior exposure to mTOR inhibitors, history of any brain metastasis, active interstitial pneumonitis, or any serious underlying medical condition that would impair the patient’s ability to receive protocol treatment.
Treatment
On days 1 and 8 of each 21-day cycle, patients received paclitaxel (P) 80 mg/m2, carboplatin (C) AUC 1.5, and temsirolimus (T) 25 mg flat dose intravenously. The sequence of administration was P→C→T. Premedications and anti-emetics were provided according to institutional guidelines. Total carboplatin dose (mg) was calculated as target AUC x [glomerular filtration rate (GFR) +25], and was capped based on a maximum GFR estimate of 125 ml/min. As such, the maximum allowable dosage was 225 mg for target AUC 1.5.
On day 1 of each cycle, patients provided routine bloodwork (complete blood cell count, basic metabolic panel with magnesium, liver function tests, lipid panel) and underwent physical examination. On day 8 of each cycle, a complete blood cell count was obtained. At any time after six cycles of study treatment, patients who maintained stable disease (or continued response) had the option of remaining on treatment with temsirolimus alone.
Adverse event assessments were performed according to Common Terminology Criteria for Adverse Events version 3.0 (CTCAE). Radiologic response assessments according to RECIST v1 criteria [8] were required after every two cycles for the first six cycles, and after every third cycle thereafter.
Study end points and statistical considerations
The primary end point of the study was the ORR (CR or PR) after two cycles of treatment. Six patients from phase I who were treated at the phase II recommended dose [7] were included in the efficacy analysis for response rate as stipulated by the protocol. As such, 30 patients were planned for this phase II study to achieve the sample size of 36 patients evaluable for the primary end point. No more than 20 of 36 patients could have received prior chemotherapy for R/M disease. The ORR for cisplatin and paclitaxel in the first line setting for R/M disease was 26%, per historic control [9], with an ORR of 15% for chemotherapy in the second or third line [10, 11]. As such, the estimated ORR was p0 = (0.26×0.5) + (0.15×0.5) = 0.21. Therefore, a 21% response rate would be considered not promising, while a 41% response rate would be considered promising, with Type I and II error rates set to 0.05 and 0.2, respectively.
Upon conclusion of the study, if 12 or more patients had a response out of a total of 36 patients enrolled, the regimen would be considered worthy of further investigation. Secondary end points were safety and tolerability of the regimen and the mOS using Kaplan–Meier methodology, beginning at the start of the treatment.
Targeted exome next-generation DNA sequencing
For patients with adequate pre-treatment formalin-fixed paraffin-embedded tumor tissue for correlative studies, targeted exome mutational analysis was performed using MSKCC-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets). The assay involves massively parallel sequencing, coupled with solution-phase exon capture. Exon capture was performed on barcoded pools of sequence libraries by hybridization (Nimblegen SeqCap Target Enrichment) using custom oligonucleotides to capture all exons and select introns of 341 cancer genes [12].
Results
Patient characteristics
Thirty-nine patients were enrolled between March 2011 and May 2013 and all patients had been followed until time of death. Two patients were found to be ineligible following enrollment and were never treated. One patient was found to be ineligible after cycle 1, due to a primary diagnosis of nasopharynx cancer. These three patients were replaced and therefore, 36 patients were eligible for evaluation.
Table 1 shows the characteristics of all eligible patients who received treatment on study. Of the 36 patients, 27 (75%) were men. The median age was 57 years (range 30–85 years) and the median KPS was 80% (range 70%–90%). The primary tumor sites included: oral cavity (28%), oropharynx (OPSCC) (31%), hypopharynx (3%), larynx (31%), paranasal sinus (3%), and unknown primary (6%). Twenty-five (69%) patients had at least a 10 pack-year smoking history. Twenty-four (67%) patients received no prior systemic treatment in the R/M setting, while 11 received one line of prior treatment and 1 received two lines of prior treatment. Four patients each received prior treatment with a platinum agent or a taxane. One patient received prior treatment with both carboplatin and paclitaxel.
Table 1.
Baseline characteristics of eligible patients (n = 36)
| Characteristic | Number of patients |
|---|---|
| Sex | |
| Men | 27 (75%) |
| Women | 9 (25%) |
| Median age (years) | 57 (range: 30–85) |
| Karnofsky Performance Status | |
| 90% | 9 (25%) |
| 80% | 22 (61%) |
| 70% | 5 (14%) |
| Subsite of primary tumor | |
| Oral cavity | 10 (28%) |
| Oropharynx | 11 (31%) (p16-positive 7, p16-negative 2, p16 status unknown 2) |
| Hypopharynx | 1 (3%) |
| Larynx | 11 (31%) |
| Paranasal sinus | 1 (3%) |
| Unknown Primary with neck node involvement | 2 (6%) |
| Smoking history | |
| Never or negligible | 7 (19%) |
| <10 pack-years | 4 (11%) |
| 10–20 pack-years | 8 (22%) |
| 20–40 pack-years | 9 (25%) |
| >40 pack-years | 8 (22%) |
| Extent of Recurrent/Distant Metastatic (DM) disease | |
| Locoregional recurrence alone | 6 (17%) |
| DM recurrence alone | 14 (39%) |
| Locoregional and DM recurrence | 12 (33%) |
| Locoregional with DM disease de novo | 4 (11%) |
| Sites of DM disease | |
| Lung | 24 (67%) |
| Lung Only | 17 (47%) |
| Liver | 3 (8%) |
| Bone | 4 (11%) |
| Prior radiation therapya | 32 (89%) |
| Prior systemic therapy for R/M disease | |
| No prior chemotherapy | 24 (67%) |
| 1 line of prior systemic therapy | 11 (31%) |
| 2 lines of prior systemic therapy | 1 (3%) |
Thirty-two patients received definitive radiation therapy to the head and neck. Two of these patients received radiation to a previous primary head and neck cancer and not to a presumed second head and neck cancer that was treated on study. An additional two patients received re-irradiation or a second definitive course of radiation to locoregional recurrent disease.
Efficacy
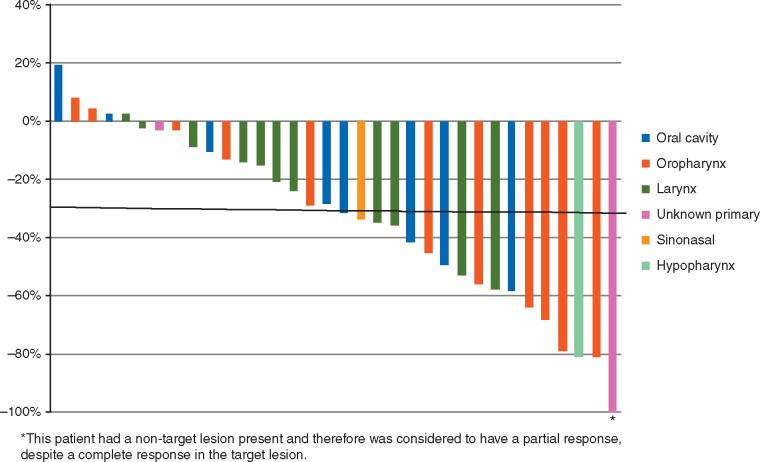
Thirty-four patients were evaluable for an objective response (OR) after two cycles of treatment (Figure 1). Fifteen patients had an OR. There were no complete responses. All 15 ORs were partial responses (PR). Stable disease (SD) was present as best response in 19 patients. Two patients were not evaluable for OR due to adverse events that occurred during cycle 1 that lead to removal from study and were considered non-responders. Therefore, 15 of 36 or 41.7% [95% confidence interval (CI), 27.9–60.3] of patients were considered to have had an OR.
Figure 1.
Waterfall plot of best response per RECIST v1 (n = 34).
Of the four patients with p16-positive OPSCC, three had a PR and one had SD as best overall response, while of the three patients with p16-negative OPSCC, two had a PR and one had SD as best overall response. Among the 12 patients who had received prior treatment in the R/M setting, two patients achieved a PR. One patient with an OR had received cisplatin-based therapy in the first-line setting.
The median duration on study for all 36 patients on study was 5.3 months. Excluding the two patients removed from study during cycle 1, the median duration and cycles of treatment on study were 5.7 months (range 1.1–17.7) and 8 (range 2–22), respectively. Twenty (59%) patients received >6 cycles of treatment. Eleven patients received temsirolimus monotherapy after cycle 6. An additional four patients received temsirolimus monotherapy beginning after cycles 7–14. Following discontinuation of carboplatin and paclitaxel, patients remained on temsirolimus for a median of 3 cycles prior to POD.
The mPFS was 5.9 months (95% CI, 4.8–7.1) (Figure 2 in Supplementary Material S1). The mOS was 12.8 months (95% CI, 9.8–15.8).
Reasons for removal from study and toxicity
The majority of patients were removed from study due to progression of disease (POD) (81%) with 25 (69%) patients removed for radiologic POD per RECIST v1 criteria and 4 (11%) patients removed for clinical POD (Table 2 in Supplementary Material S2). Three patients were removed due to excessive toxicity. One was removed for grade 3 elevation in alkaline phosphatase during cycle 3. The other two patients were removed during cycle 1 for severe infections requiring hospitalization. One patient developed neutropenic fever with underlying osteomyelitis and the other patient developed sepsis and acute respiratory failure due to pneumonia. The latter was considered a severe adverse event. One patient was removed for a hypersensitivity reaction to paclitaxel during cycle 9. Two patients were removed due to withdrawal of consent. One patient with recurrent locoregional base of tongue cancer withdrew consent after receiving a major response to receive re-irradiation. Another patient withdrew to pursue other treatment in the setting of slow disease growth that did not meet POD per RECIST v1 criteria. One patient, a 45-year-old man with oral cavity cancer, died while on study of unclear cause during cycle 4.
The most common toxicities of any grade (S3), regardless of attribution, experienced by two-thirds the patients include: anemia (94%), hyperglycemia (94%), fatigue (89%), leukopenia (81%), hypercholesterolemia (78%), hypoalbuminemia (75%), and thrombocytopenia (75%). The most common grade 3 adverse events, regardless of attribution, include: lymphopenia (61%), leukopenia (31%), dysphagia (25%), neutropenia (22%), and anemia (19%). Dysphagia was not attributed to the treatment on study, but rather to recurrent disease or sequelae from radiation treatment. Three (8%) patients developed neutropenic fever on study. There were four grade 4 adverse events that include: leukopenia, anemia, pneumonia, and hypercalcemia, all reported in one patient. As noted, there was one grade 5 toxicity or sudden death on study of unknown attribution.
Hyperglycemia and hypercholesterolemia are common toxicities associated with mTOR inhibitors. Of the 34 patients with hyperglycemia of all grades, 16 had pre-existing hyperglycemia (as defined by fasting glucose > 160 mg/dl). Two patients had grade 3 hyperglycemia and both had pre-existing diabetes mellitus. Twenty-eight patients had grade 1 or 2 hypercholesterolemia with only two patients having elevated cholesterol at baseline.
Fourteen patients required a dose reduction in temsirolimus. Eleven patients required one dose level reduction to 20 mg flat dose and three patients required two dose level reductions to 15 mg flat dose. Among these 14 patients, two patients also required a dose reduction in paclitaxel to 60 mg/m2 and one patient also required dose reductions in both carboplatin to AUC 1 and paclitaxel to 60 mg/m2. Dose reductions were due to myelosuppression (five patients), mucositis (four patients), fatigue (three patients), and liver function abnormalities (one patient).
Correlative molecular analysis
Targeted exome mutational analysis was performed using MSKCC-IMPACT on biopsy specimens from 21 patients on study. Four (19%) patients had a mutation in PIK3CA. Missence mutations in PIK3CAE545K and PIK3CA E542K were identified in three patients and one patient, respectively. Among patients with a PIK3CA mutation, one had a PR with a CR in the target lesion and three had SD as best response. The patient with the PR, in addition to harboring a mutation in PIK3CAE545K, also had a mutation in TP53, and mutations of unknown significance in AXIN2, KDM6A, BRCA1, and ERCC4. Of the three patients with PIK3CA mutant tumors with SD, two had tumor regression. The median duration on treatment for these patients was 4.4 months (range 2.3–15.0). One of the four patients with a PIK3CAE545K mutation also had mutations in AKT3S472F and MTORD1123H and D1108H. This patient had SD for 5.1 months. An additional patient on study had an AKT3W330C missence mutation and had a PR to study treatment. No other patient had an MTOR mutation. One patient had a PIK3CA amplification and had SD on study. Another patient had a PTENR130Q mutation and achieved a PR.
Both patients with mutations in TSC2, one with a TSC2R751 truncating mutation and one with a TSC2R485Q missence mutation, had a PR. One of two patients with a mutation in TSC1, specifically TSC1 G305R missense mutation, had a PR and the other patient with TSC1K820E missense mutation had 24% regression in a target lesion. Given that hyperactivation of mTORC1 is the primary alteration driving the growth of TSC mutant tumors [13], a TSC1/2 mutation should be predictive of response to temsirolimus.
There was no apparent trend in the molecular signatures of patients with >50% regression in a target lesion or on treatment for >6 months. The patient who was on temsirolimus monotherapy for 12 cycles did not have mutational analysis performed.
Discussion
This phase II study demonstrates the efficacy of low-dose carboplatin and paclitaxel combined with temsirolimus on days 1 and 8 of a 21-day cycle in patients with R/M HNSCC. Fifteen ORs were seen, which exceeded the pre-specified threshold of 12 ORs for a positive study.
The toxicity profile of this regimen was acceptable and only 8.3% of patients were removed from study due to adverse events attributed to the study regimen. The addition of temsirolimus did not worsen the non-hematologic toxicity profile that is expected for low-dose weekly carboplatin and paclitaxel alone. The original design of the phase I study entailed a 28-day cycle with a high-dose bolus of carboplatin on day 1 [7]. However, this regimen was associated with an unacceptable risk of febrile neutropenia. The modified 21-day regimen evaluated in both the amended phase I study and this phase II study appears to be well tolerated, allowing for dose reductions of temsirolimus.
The study regimen may provide a research strategy to achieve a better balance of efficacy and toxicity in comparison with the current first-line, standard-of-care regimen of platinum-based chemotherapy (EXTREME) [1]. EXTREME exhibited a response rate, PFS, and OS of 36%, 5.6 months, and 10.1 months, respectively, while low-dose weekly carboplatin, paclitaxel, and temsirolimus had a response rate, PFS, and OS of 41.7%, 5.9 months, and 12.9 months, respectively. Approximately 20% of patients on the EXTREME regimen discontinued treatment due to any adverse event, compared to 13.9% of patients on this trial. However, cross-study comparisons cannot lead to formal conclusions. Given that this study had met its primary end point of an OR rate of at least 41%, this regimen of carboplatin, paclitaxel, and temsirolimus, is considered promising and worthy of further investigation. The response achieved in this study is superior to responses achieved with standard regimens in R/M HNSCC [1, 14], with the exception of weekly paclitaxel with cetuximab evaluated in a phase II study in the first-line [15]. The PFS appears compromised by allowing patients to continue on temsirolimus monotherapy following cycle 6. Many patients had progressive disease shortly after discontinuation of carboplatin and paclitaxel. Therefore, further development of this regimen would evaluate continued triplet therapy to achieve greater durability of disease control.
The enhanced efficacy of this study regimen appears to be due to synergistic activity between mTORC1 inhibition and cytotoxic chemotherapy. The relatively high response rate in this study, compared to historic studies [9, 16, 17] evaluating platinum-based therapy in combination with paclitaxel, is suggestive that the response is not solely attributed to these agents alone, and rather due to added efficacy with mTOR inhibition.
TEMHEAD is a phase II study that evaluated temsirolimus monotherapy in patients with HNSCC after progression on platinum chemotherapy and cetuximab [18]. There was no OR to temsirolimus, although tumor regressions were observed in 39.4% of this pre-treated population, suggestive of a signal of single-agent activity, but consistent with our findings that the benefit of mTOR inhibition is from additive activity.
Inhibition of the PI3K/mTOR pathway has recently been explored in the BERIL-1 trial that evaluated the addition of buparlisib, a pan-PI3K inhibitor, to weekly paclitaxel, in a randomized, placebo controlled, phase II trial [19]. Patients received treatment in the second-line following platinum-based chemotherapy. The ORR, mPFS, and mOS among patients who received buparlisib and paclitaxel were 39%, 4.6 months, and 10.4 months, respectively. Patients with HPV-positive and oropharynx primary tumors did not derive a benefit from the addition of buparlisib to paclitaxel. Further studies are necessary to learn how best to select patients for treatment with PI3K/mTOR pathway inhibitors, as well as to determine which line to incorporate PI3K/mTOR inhibition in the sequence of treatments for R/M disease, which also includes EGFR-directed therapy and immunotherapy. Similar to TEMHEAD and BERIL-1, the outcomes in our study were not dependent on identification of a PI3K/mTOR pathway alteration. Given the responses with our regimen in patients without a sensitizing mutation along the PI3K/mTOR pathway, mTOR activity is likely influenced by activating mutations in several other signaling pathways. An example of this is PTEN loss, which allows for activation of AKT signaling, and has been associated with response to mTOR inhibition [20]. One patient in this study exhibited a PTEN loss of function mutation and experienced an OR. Further pathways affecting mTOR activation should be explored in HNSCC to better appreciate response to therapeutic regimens that incorporate mTOR inhibition.
In conclusion, the regimen of low-dose carboplatin and paclitaxel combined with temsirolimus appears to be efficacious, with an acceptable safety profile, in patients with R/M HNSCC. The clinically meaningful activity of mTOR inhibition plus low dose cytotoxic chemotherapy should be further explored in HNSCC, with continued attention to potential associations between efficacy and alterations in genes that encode components of the PI3K/mTOR pathway.
Funding
This study was approved and funded in part by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Pfizer, Inc. This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008747.
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Vermorken JB, Mesia R, Rivera F. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med 2008; 359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 2. Lui VWY, Hedberg ML, Li H. et al. Frequent Mutation of the PI3K Pathway in Head and Neck Cancer Defines Predictive Biomarkers. Cancer Discov 2013; 3: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amornphimoltham P, Patel V, Sodhi A. et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Research 2005; 65: 9953–9961. [DOI] [PubMed] [Google Scholar]
- 4. Nathan CO, Amirghahari N, Rong X. et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res 2007; 67: 2160–2168. [DOI] [PubMed] [Google Scholar]
- 5. Aissat N, Le Tourneau C, Ghoul A. et al. Antiproliferative effects of rapamycin as a single agent and in combination with carboplatin and paclitaxel in head and neck cancer cell lines. Cancer Chemother Pharmacol 2008; 62: 305–313. [DOI] [PubMed] [Google Scholar]
- 6. Mondesire WH, Jian W, Zhang H. et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res 2004; 10: 7031–7042. [DOI] [PubMed] [Google Scholar]
- 7. Fury MG, Sherman E, Ho A. et al. A phase I study of temsirolimus + carboplatin + paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother Pharmacol 2012; 70: 121–128. [DOI] [PubMed] [Google Scholar]
- 8. Therasse P, Arbuck SG, Eisenhauer EA. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 9. Gibson MK, Li Y, Murphy B. et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2005; 23: 3562–3567. [DOI] [PubMed] [Google Scholar]
- 10. Numico G, Merlano M.. Second-line treatment with docetaxel after failure of a platinum-based chemotherapy in squamous-cell head and neck cancer. Ann Oncol 2002; 13: 331–333. [DOI] [PubMed] [Google Scholar]
- 11. Merlano M, Conte PF, Tatarek R. et al. Ineffectiveness of 5-fluorouracil and cis-platin as second-line chemotherapy in head and neck cancer. Tumori 1984; 70: 267–269. [DOI] [PubMed] [Google Scholar]
- 12. Won HH, Scott SN, Brannon AR. et al. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp 2013; e50710: 1–9. PMID 24192750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moavero R, Romagnoli G, Graziola F, Curatolo P.. Mammalian Target of Rapamycin Inhibitors and Life-Threatening Conditions in Tuberous Sclerosis Complex. Semin Pediatr Neurol 2015; 22: 282–294. [DOI] [PubMed] [Google Scholar]
- 14. Forastiere AA, Metch B, Schuller DE. et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. Journal of Clinical Oncology 1992; 10: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 15. Hitt R, Irigoyen A, Cortes-Funes H. et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol 2012; 23: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 16. Adamo V, Ferraro G, Pergolizzi S. et al. Paclitaxel and cisplatin in patients with recurrent and metastatic head and neck squamous cell carcinoma. Oral Oncol 2004; 40: 525–531. [DOI] [PubMed] [Google Scholar]
- 17. Moosmann P, Egli F, Stahel RA, Jost L.. Weekly paclitaxel and carboplatin combination chemotherapy in patients with advanced squamous cell carcinoma of the head and neck. Onkologie 2003; 26: 568–572. [DOI] [PubMed] [Google Scholar]
- 18. Grunwald V, Keilholz U, Boehm A. et al. TEMHEAD: a single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO). Ann Oncol 2015; 26: 561–567. [DOI] [PubMed] [Google Scholar]
- 19. Soulieres D, Faivre S, Mesia R. et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol 2017; 18: 323–335. [DOI] [PubMed] [Google Scholar]
- 20. Holsinger FC, Piha-Paul SA, Janku F. et al. Biomarker-directed therapy of squamous carcinomas of the head and neck: targeting PI3K/PTEN/mTOR pathway. J Clin Oncol 2013; 31: e137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.