Abstract
Background
Homologous recombination defects in BRCA1/2-mutated tumors result in sensitivity to poly(ADP-ribose) polymerase inhibitors, which interfere with DNA damage repair. Veliparib, a potent poly(ADP-ribose) polymerase inhibitor, enhanced the antitumor activity of platinum agents and temozolomide in early phase clinical trials. This phase II study examined the safety and efficacy of intermittent veliparib with carboplatin/paclitaxel (VCP) or temozolomide (VT) in patients with BRCA1/2-mutated breast cancer.
Patients and methods
Eligible patients ≥18 years with locally recurrent or metastatic breast cancer and a deleterious BRCA1/2 germline mutation were randomized 1 : 1 : 1 to VCP, VT, or placebo plus carboplatin/paclitaxel (PCP). Primary end point was progression-free survival (PFS); secondary end points included overall survival (OS) and overall response rate (ORR).
Results
Of 290 randomized patients, 284 were BRCA+, confirmed by central laboratory. For VCP versus PCP, median PFS was 14.1 and 12.3 months, respectively [hazard ratio (HR) 0.789; 95% CI 0.536–1.162; P = 0.227], interim median OS 28.3 and 25.9 months (HR 0.750; 95% CI 0.503–1.117; P = 0.156), and ORR 77.8% and 61.3% (P = 0.027). For VT (versus PCP), median PFS was 7.4 months (HR 1.858; 95% CI 1.278–2.702; P = 0.001), interim median OS 19.1 months (HR 1.483; 95% CI 1.032–2.131; P = 0.032), and ORR 28.6% (P < 0.001). Safety profile was comparable between carboplatin/paclitaxel arms. Adverse events (all grades) of neutropenia, anemia, alopecia, and neuropathy were less frequent with VT versus PCP.
Conclusion
Numerical but not statistically significant increases in both PFS and OS were observed in patients with BRCA1/2-mutated recurrent/metastatic breast cancer receiving VCP compared with PCP. The addition of veliparib to carboplatin/paclitaxel significantly improved ORR. There was no clinically meaningful increase in toxicity with VCP versus PCP. VT was inferior to PCP. An ongoing phase III trial is evaluating VCP versus PCP, with optional continuation single-agent therapy with veliparib/placebo if chemotherapy is discontinued without progression, in this patient population.
Clinical trial information
Keywords: PARP inhibitors, metastatic breast cancer, platinum, temozolomide
Introduction
Mutations in BRCA1/2 genes are the most common inherited mutations associated with breast cancer [1]. The homologous recombination DNA damage repair pathway is defective in BRCA+ tumor cells. Poly(ADP-ribose) polymerase (PARP)-1 and PARP-2 function in detecting and repairing DNA damage [2], and PARP inhibitors are synthetic lethal in cells with DNA damage defects [3, 4]. Monotherapy with PARP inhibitors has antitumor activity in patients with BRCA+ cancers; PARP inhibitors have gained regulatory approval in patients with ovarian cancer [5–7].
Veliparib (ABT-888) is a potent, orally bioavailable, selective PARP-1/2 inhibitor that crosses the blood–brain barrier [8]. Veliparib monotherapy has demonstrated antitumor activity in BRCA+ patients [5] and has shown promising results in combination with DNA-damaging therapies in early phase clinical trials. In a phase I dose-escalation study of veliparib plus carboplatin/paclitaxel, the objective response rate (ORR) was 57% (8/14) among patients with metastatic breast cancer receiving the maximum administered dose of veliparib [9]. Tolerability of veliparib with carboplatin/paclitaxel was comparable with that expected for carboplatin/paclitaxel alone [9]. These results, along with recent data indicating platinum sensitivity among BRCA+ patients [10, 11], suggest veliparib plus platinum may be an effective therapy for patients with BRCA+ breast cancer.
In a phase II study examining veliparib plus temozolomide in patients with metastatic breast cancer, response rate was 25% (7/28) and clinical benefit rate (CBR) 50% among BRCA+ patients [12, 13]. While single-agent temozolomide has limited activity in breast cancer [14], these data suggest the combination may provide patients with BRCA-mutated metastatic breast cancer a novel all-oral regimen with reduced hematologic toxicities and alopecia compared with other combination regimens.
Here, we report primary results of the BROCADE study evaluating safety and efficacy of intermittent veliparib with temozolomide (VT) or veliparib with carboplatin/paclitaxel (VCP) compared with placebo plus carboplatin/paclitaxel (PCP) in the biomarker-selected population of BRCA+ patients with locally recurrent or metastatic breast cancer.
Patients and methods
BROCADE is a randomized, partially blinded phase II trial (NCT01506609) at 86 sites in 20 countries. The study was approved by an independent ethics committee/independent review board at each site and carried out in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Patients
Eligible patients were ≥18 years of age with histologically or cytologically confirmed locally recurrent or metastatic breast cancer with a deleterious BRCA1/2 germline mutation. Human epidermal growth factor receptor 2 mutation-positive (HER2+) patients were enrolled if they were ineligible for or progressed on prior HER2-directed therapy. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, radiologically evaluable disease, and adequate hematologic, renal, and hepatic function. Exclusion criteria included >2 prior lines of cytotoxic therapy for metastatic disease, prior breast cancer treatment with temozolomide, platinum, or PARP inhibitor, prior taxane for metastatic breast cancer (unless patient received ≤1 cycle or therapy was >1 year before enrollment), pre-existing neuropathy grade >1, and history of brain metastases or uncontrolled seizure disorder.
Treatment
Patients were randomized 1 : 1 : 1 to PCP, VCP, or VT. Randomization was stratified by hormone receptor status [estrogen receptor (ER) and/or progesterone receptor (PgR) positive, ER and/or PgR negative], prior cytotoxic therapy (yes, no), and ECOG performance status (0–1, 2). The carboplatin/paclitaxel arms were double-blinded; treatment was open-label for the VT arm. Placebo/veliparib dose was 120 mg BID orally on days 1–7 (21-day cycle) in the carboplatin/paclitaxel arms. Carboplatin (area under the curve 6 mg/ml/min) and paclitaxel (175 mg/m2) were administered intravenously on day 3. In the VT arm, veliparib dose was 40 mg BID orally on days 1–7. Temozolomide started at 150 mg/m2 QD orally on days 1–5 (28-day cycle), and was escalated to 200 mg/m2 at cycle 2 if well-tolerated during the first cycle. Treatment was until disease progression or unmanageable toxicity. Veliparib/placebo could be continued if either carboplatin or paclitaxel was discontinued for toxicity, but veliparib/placebo was discontinued if both carboplatin and paclitaxel were discontinued. If either veliparib or temozolomide was discontinued because of toxicity, both were discontinued.
On-study evaluation
Tumor response was assessed by computed tomography scan utilizing Response Evaluation Criteria in Solid Tumors v1.1 at screening, 9-week intervals thereafter, and final visit. Post-treatment and survival information was collected monthly after discontinuation. Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Statistical methodology and end point analysis
Primary efficacy end point was progression-free survival (PFS) by independent central review. Secondary end points included overall survival (OS), ORR, and CBR (progression-free rate at week 18).
The study enrolled 290 patients to accrue 159 total PFS events (112 on carboplatin/paclitaxel arms) and 194 total OS events (136 on carboplatin/paclitaxel arms) to provide ≥80% power at two-sided α = 0.05 [assuming a hazard ratio (HR) of 0.58 and 0.61 for PFS and OS, respectively] to detect a statistically significant treatment effect for VCP versus PCP or for VT versus PCP.
Results of the primary analysis are reported, with interim OS (164 deaths). Efficacy analyses included all randomized patients with deleterious germline BRCA1/2 mutations confirmed by central laboratory (Myriad). Safety analyses included patients receiving ≥1 dose of study drug. The log-rank test, stratified by ER/PgR status and prior cytotoxic therapy, compared PFS and OS between each veliparib-containing arm and the placebo arm. CBR was estimated and compared using Kaplan–Meier estimates. ORR was estimated and compared using the Cochran–Mantel–Haenszel test stratified by ER/PgR status and prior cytotoxic therapy. Comparisons of safety data used Fisher’s exact test. Statistical significance was determined by a two-sided P value ≤ 0.05. Analyses were carried out using SAS v9.2 or higher (SAS Institute, Inc., Cary, NC) under the UNIX operating system.
Additional details are available in Supplementary Methods at Annals of Oncology online.
Results
Patient characteristics
Two hundred ninety-four patients were randomized between January 2012 and April 2015 (supplementary Figure S1, available at Annals of Oncology online). Four patients in the carboplatin/paclitaxel arms received veliparib/placebo at 80 mg BID and were excluded from efficacy analyses per protocol. Of the 290 patients randomized after this amendment (VCP, N = 97; PCP, N = 99; VT, N = 94), 282 received ≥1 dose of study drug, and 284 had a centrally confirmed deleterious BRCA1/2 mutation. Baseline patient characteristics are in Table 1.
Table 1.
Baseline patient demographics and clinical characteristics
| Characteristic, n (%) | PCP | VCP | VT |
|---|---|---|---|
| (n = 99) | (n = 97) | (n = 94) | |
| Age (years) | |||
| Median | 46 | 44 | 46 |
| Range | 24–66 | 25–65 | 22–70 |
| Sex | |||
| Female | 97 (98.0) | 95 (97.9) | 92 (97.9) |
| Male | 2 (2.0) | 2 (2.1) | 2 (2.1) |
| ECOG status | |||
| 0–1 | 93 (93.9) | 92 (94.8) | 91 (96.8) |
| 2 | 6 (6.1) | 5 (5.2) | 3 (3.2) |
| Prior cytotoxic therapy in the neoadjuvant/adjuvant setting | |||
| Yes | 72 (72.7) | 76 (78.4) | 69 (73.4) |
| No | 27 (27.3) | 21 (21.6) | 25 (26.6) |
| No. of prior cytotoxic regimens for recurrent/metastatic diseasea | |||
| 0 | 62 (62.6) | 74 (76.3) | 66 (70.2) |
| 1 | 30 (30.3) | 15 (15.5) | 18 (19.1) |
| 2 | 5 (5.1) | 7 (7.2) | 9 (9.6) |
| >2 | 2 (2.0) | 1 (1.0) | 1 (1.1) |
| Time since initial breast cancer diagnosis (years) | |||
| Median | 3.0 | 3.2 | 2.9 |
| Range | 0.1–22.0 | 0.1–25.4 | 0.0–27.8 |
| Measurable disease at baselineb | |||
| Yes | 81 (83.5) | 73 (77.7) | 72 (76.6) |
| No | 16 (16.5) | 21 (22.3) | 22 (23.4) |
| Receptor status | |||
| ER+ and/or PgR+ | 56 (56.6) | 57 (58.8) | 54 (57.4) |
| HER2 positivec | 7 (7.1) | 3 (3.1) | 5 (5.3) |
| Triple negative (ER–/PgR–/HER2–) | 42 (42.4) | 40 (41.2) | 38 (40.4) |
| BRCA1 mutation positived | 53 (53.5) | 51 (52.6) | 49 (52.1) |
| BRCA2 mutation positived | 46 (46.5) | 44 (45.4) | 43 (45.7) |
| Number of metastasis sites | |||
| No metastasis | 5 (5.1) | 4 (4.1) | 10 (10.6) |
| 1 | 38 (38.4) | 39 (40.2) | 38 (40.4) |
| 2 | 28 (28.3) | 30 (30.9) | 29 (30.9) |
| 3 | 18 (18.2) | 13 (13.4) | 13 (13.8) |
| ≥4 | 10 (10.1) | 11 (11.3) | 4 (4.3) |
| Liver or lung metastasis | |||
| Yes | 58 (58.6) | 58 (59.8) | 48 (51.1) |
| No | 41 (41.4) | 39 (40.2) | 46 (48.9) |
P = NS for all comparisons.
Chemotherapy regimens reported as neoadjuvant/adjuvant that began >1 year after first chemotherapy regimen were considered recurrent/metastatic.
Based on central imaging; five patients with status missing (PCP, 2; VCP, 3).
Positive in either primary or metastasis.
Based on core laboratory.
ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PCP, placebo plus carboplatin/paclitaxel; PgR, progesterone receptor; TNBC, triple-negative breast cancer; VCP, veliparib plus carboplatin/paclitaxel; VT, veliparib plus temozolomide.
Efficacy
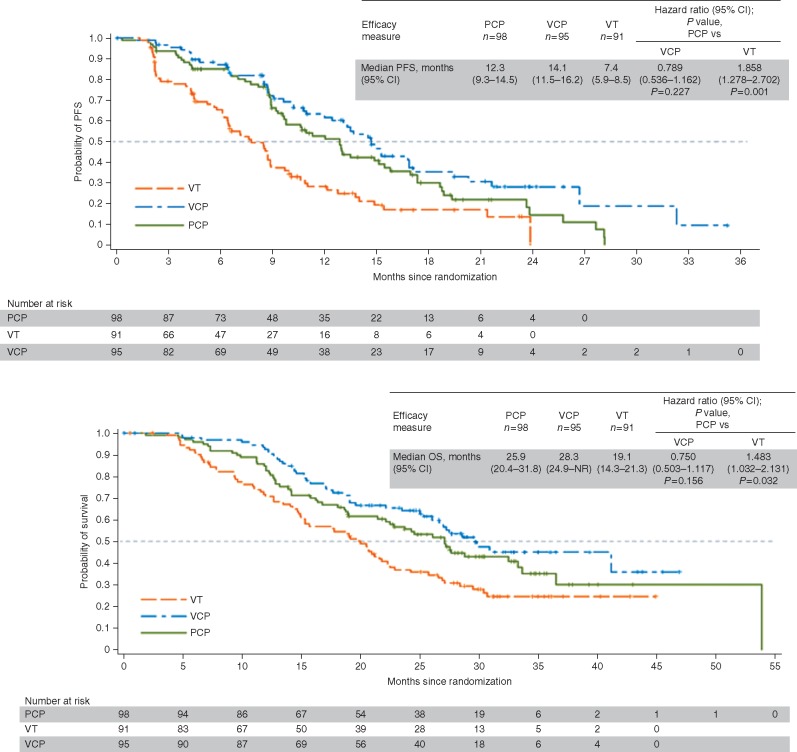
Median PFS for VCP and PCP was 14.1 months (95% CI 11.5–16.2) and 12.3 months (95% CI 9.3–14.5) [HR 0.789 (95% CI 0.536–1.162); P = 0.227] (Figure 1A). Interim median OS for VCP and PCP was 28.3 months (95% CI 24.9–not reached) and 25.9 months (95% CI 20.4–31.8) [HR 0.750 (95% CI 0.503–1.117); P = 0.156] (Figure 1B). ORR was 78% (56/72) and 61.3% (49/80) for VCP and PCP, respectively (P = 0.027) (Table 2). Among patients with PFS events, 15/68 on PCP and 10/60 on VCP had new central nervous system metastases. Across subgroups defined by baseline characteristics, including triple-negative breast cancer (TNBC) versus non-TNBC and BRCA1 versus BRCA2 mutation, PFS outcomes generally favored VCP versus PCP (supplementary Figure S2, available at Annals of Oncology online).
Figure 1.
Kaplan–Meier curve of (A) progression-free survival and (B) overall survival. NR, not reached; OS, overall survival; PCP, placebo plus carboplatin/paclitaxel; PFS, progression-free survival; VCP, veliparib plus carboplatin/paclitaxel; VT, veliparib plus temozolomide.
Table 2.
Tumor response
| Variable | PCP | VCP | VT |
|---|---|---|---|
| (n = 98) | (n = 95) | (n = 91) | |
| ORR (CR+PR), n/N (%), (95% CI) | 49/80 (61.3) | 56/72 (77.8)* | 20/70 28.6*** |
| (49.7–71.9) | (66.4–86.7) | (18.4–40.6) | |
| Complete response, n/N (%) | 3/80 (3.8) | 4/72 (5.6) | 1/70 (1.4) |
| Partial response, n/N (%) | 46/80 (57.5) | 52/72 (72.2) | 19/70 (27.1) |
| Median duration of overall response, months (95% CI) | 11.1 | 11.7 | 6.3 |
| (9.5–15.7) | (8.5–14.1) | (4.2–12.4) | |
| Clinical benefit rate | 87.0 | 90.7 | 73.0* |
| (week 18 progression-free rate), % (95% CI) | (78.3–92.4) | (82.2–95.2) | (62.2–81.2) |
For ORR, CR, and PR, proportions of patients with confirmed responses are shown; these analyses included all patients with measurable disease at baseline. Duration of overall response included all patients with an objective response. Assessment was per independent radiology reviewer.
*, ***P value <0.05 or 0.001 level for comparison with PCP.
CR, complete response; ORR, objective response rate; PCP, placebo plus carboplatin/paclitaxel; PR, partial response; VCP, veliparib plus carboplatin/paclitaxel; VT, veliparib plus temozolomide.
In the VT arm, median PFS [7.4 months (95% CI 5.9–8.5)], median OS [19.1 months (14.3–21.3)], and ORR (28.6%, 20/70) were inferior to PCP (Figure 1 and Table 2). Duration of overall response among patients receiving VT was 6.3 months (95% CI 4.2–12.4). Among 76 patients with PFS events, 3 had new central nervous system metastases.
Treatment exposure and tolerability
Median (range) veliparib/placebo exposure for PCP and VCP arms was 10 (1–33) cycles and 12 (1–48) cycles, respectively. For PCP and VCP, median (range) carboplatin exposure was 9 (1–31) and 9 (1–42) cycles, and paclitaxel exposure was 9 (1–33) and 9 (1–48) cycles. For VT, median (range) exposure was 6 (1–26) cycles for veliparib and temozolomide.
The most common any grade treatment-emergent adverse events with PCP versus VCP were neutropenia (74.0% and 74.2%, respectively), thrombocytopenia (69.8% and 71.0%), and nausea (58.3% and 71.0%) [P = not significant (NS) for each comparison] (Table 3). Grade 3/4 adverse events in ≥30% of patients treated with PCP or VCP were neutropenia (55.2% and 55.9%) and thrombocytopenia (26.0% and 31.2%) (P = NS for each comparison). Rates of veliparib/placebo, carboplatin, and paclitaxel interruption, dose reduction, and discontinuation due to adverse events were comparable between the two carboplatin/paclitaxel-containing arms (P = NS) (supplementary Table S1, available at Annals of Oncology online). Fifty-two patients (52.5%) in the PCP arm and 51 (52.6%) in the VCP arm received colony-stimulating factors at some point. The serious adverse event rate was 27.1% for PCP and 34.4% for VCP (P = NS).
Table 3.
Most common (≥20% of patients) and grade 3–4 (≥5% of patients) treatment-emergent adverse events
| Treatment-emergent adverse events, n (%) | PCP | VCP | VT | |||
|---|---|---|---|---|---|---|
| (n = 96) |
(n = 93) |
(n = 93) |
||||
| All grades | Grade 3–4 | All Grades | Grade 3–4 | All Grades | Grade 3–4 | |
| Any adverse event | 94 (97.9) | 80 (83.3) | 93 (100) | 73 (78.5) | 92 (98.9) | 67 (72.0) |
| Hematologic | ||||||
| Anemia | 49 (51.0) | 17 (17.7) | 53 (57.0) | 16 (17.2) | 26 (28.0)** | 7 (7.5)* |
| Febrile neutropenia | 3 (3.1) | 3 (3.1) | 8 (8.6) | 8 (8.6) | 1 (1.1) | 1 (1.1) |
| Leukopenia | 27 (28.1) | 11 (11.5) | 28 (30.1) | 15 (16.1) | 16 (17.2) | 11 (11.8) |
| Neutropenia | 71 (74.0) | 53 (55.2) | 69 (74.2) | 52 (55.9) | 46 (49.5)*** | 34 (36.6)* |
| Thrombocytopenia | 67 (69.8) | 25 (26.0) | 66 (71.0) | 29 (31.2) | 73 (78.5) | 45 (48.4)** |
| Non-hematologic | ||||||
| Abdominal pain | 15 (15.6) | 0 | 22 (23.7) | 1 (1.1) | 15 (16.1) | 1 (1.1) |
| Alopecia | 55 (57.3) | 1 (1.0) | 62 (66.7) | 1 (1.1) | 10 (10.8)*** | 0 |
| Arthralgia | 31 (32.3) | 0 | 34 (36.6) | 0 | 14 (15.1)** | 0 |
| Asthenia | 16 (16.7) | 3 (3.1) | 23 (24.7) | 3 (3.2) | 17 (18.3) | 2 (2.2) |
| Back pain | 22 (22.9) | 2 (2.1) | 28 (30.1) | 1 (1.1) | 24 (25.8) | 2 (2.2) |
| Bone pain | 12 (12.5) | 1 (1.0) | 22 (23.7) | 0 | 7 (7.5) | 1 (1.1) |
| Constipation | 28 (29.2) | 0 | 36 (38.7) | 0 | 38 (40.9) | 1 (1.1) |
| Cough | 15 (15.6) | 1 (1.0) | 19 (20.4) | 0 | 12 (12.9) | 0 |
| Decreased appetite | 20 (20.8) | 1 (1.0) | 22 (23.7) | 2 (2.2) | 20 (21.5) | 1 (1.1) |
| Diarrhea | 27 (28.1) | 7 (7.3) | 36 (38.7) | 4 (4.3) | 19 (20.4) | 2 (2.2) |
| Dizziness | 17 (17.7) | 0 | 23 (24.7) | 0 | 7 (7.5)* | 0 |
| Drug hypersensitivity | 17 (17.7) | 0 | 19 (20.4) | 5 (5.4)*,a | 0*** | 0 |
| Dyspnea | 23 (24.0) | 3 (3.1) | 14 (15.1) | 0 | 8 (8.6)** | 0 |
| Fatigue | 57 (59.4) | 8 (8.3) | 47 (50.5) | 5 (5.4) | 44 (47.3) | 3 (3.2) |
| Headache | 31 (32.3) | 0 | 33 (35.5) | 1 (1.1) | 27 (29.0) | 1 (1.1) |
| Insomnia | 22 (22.9) | 0 | 14 (15.1) | 0 | 20 (21.5) | 0 |
| Myalgia | 21 (21.9) | 0 | 32 (34.4) | 0 | 8 (8.6)* | 1 (1.1) |
| Nausea | 56 (58.3) | 2 (2.1) | 66 (71.0) | 1 (1.1) | 70 (75.3)* | 2 (2.2) |
| Pain in extremity | 23 (24.0) | 2 (2.1) | 16 (17.2) | 2 (2.2) | 13 (14.0) | 0 |
| Peripheral neuropathyb | 57 (59.4) | 5 (5.2) | 64 (68.8) | 7 (7.5) | 15 (16.1)*** | 0 |
| Pyrexia | 20 (20.8) | 0 | 19 (20.4) | 0 | 9 (9.7)* | 0 |
| Upper respiratory tract infection | 10 (10.4) | 0 | 20 (21.5)* | 0 | 14 (15.1) | 0 |
| Vomiting | 23 (24.0) | 1 (1.0) | 26 (28.0) | 1 (1.1) | 40 (43.0)** | 3 (3.2) |
*, **, ***Statistically significant compared with PCP at the 0.05, 0.01, or 0.001 level, respectively.
Of the five cases of drug hypersensitivity in the VCP arm, four were assessed as probably related to carboplatin and one as probably related to veliparib.
Includes reported events of neuralgia, peripheral neuropathy, peripheral sensory neuropathy, polyneuropathy, and sensory disturbance.
PCP, placebo plus carboplatin/paclitaxel; VCP, veliparib plus carboplatin/paclitaxel; VT, veliparib plus temozolomide.
In the VT arm, the most common any grade treatment-emergent adverse events were neutropenia (49.5%), thrombocytopenia (78.5%), and nausea (75.3%). Nausea occurred more frequently with VT than PCP (P = 0.014), while anemia (P < 0.01), alopecia (P < 0.001), neutropenia (P < 0.001), and neuropathy (P < 0.001) occurred less frequently. Nineteen (20.2%) patients in the VT arm received colony-stimulating factors. Temozolomide was escalated from 150 to 200 mg/kg2 at cycle 2 in 36% of patients. The serious adverse event rate was 17.2% for VT, comparable with the rate for PCP (P = NS).
Discussion
BROCADE evaluated veliparib combined with two different chemotherapy backbones in patients with BRCA+ locally recurrent/metastatic breast cancer. Numerical increases in PFS and OS were observed with VCP compared with carboplatin/paclitaxel alone, although these did not meet statistical significance. The addition of veliparib to carboplatin/paclitaxel significantly improved ORR from 61.3% to 77.8% (P = 0.027).
Carboplatin/paclitaxel was highly active, supporting emerging data indicating sensitivity of BRCA+ breast cancer to platinum [10, 11]. In a study examining carboplatin or docetaxel as first-line metastatic therapy in patients with TNBC or BRCA+ breast cancer, PFS was 6.8 months for carboplatin and 4.8 months for docetaxel in BRCA+ patients [11]. A phase III trial explored olaparib monotherapy in patients with BRCA+ metastatic breast cancer who had ≤2 previous chemotherapy regimens for metastatic disease and prior anthracycline/taxane treatment; median PFS was 7.0 months and median OS was 19.3 months [15]. Median PFS was 12.3 months for PCP and 14.1 months for VCP and median OS was 25.9 months for PCP and 28.3 months for VCP in our study of predominantly first-line metastatic patients. Although patient populations and treatment durations were not standard across these three trials, these extended PFS times suggest durable disease control is achievable with PCP and VCP combination regimens. This response needs to be balanced with tolerability and quality of life, and it will be important to consider quality of life and patient-reported outcomes in future analyses. Patients in this study remained on study treatment of a median 10 cycles of PCP and 12 cycles of VCP, with ranges up to 48 cycles.
The VCP safety profile was comparable with that of PCP. Patients receiving VCP did not experience more veliparib/placebo, carboplatin, or paclitaxel dose reductions, discontinuations, or interruptions than patients receiving placebo. While a previous report evaluating carboplatin/paclitaxel with or without the PARP inhibitor olaparib indicated an increase in any-grade neutropenia among patients receiving olaparib [16], the incidence of neutropenia was essentially unchanged by the addition of veliparib to carboplatin/paclitaxel in the current study. Duration of exposure to veliparib/placebo, carboplatin, and paclitaxel was comparable whether patients were randomized to veliparib or placebo.
PFS, OS, and ORR were each inferior with VT compared with PCP. However, 73.0% of patients experienced clinical benefit, and 28.6% had an objective response. Although VT was not compared with a single-agent control, these responses are notable because of the reported limited activity of single-agent temozolomide in patients with breast cancer (although BRCA status of patients in this previous trial is unknown) [14]. In the VT arm, veliparib was dosed intermittently at 40 mg BID (based on phase I/II trial experience with this combination). This is a potentially low dose for combinatorial efficacy; 400 mg BID is the recommended phase II monotherapy dose [12, 17, 18]. The VT regimen provided tolerability advantages over PCP, with less frequent neutropenia, anemia, alopecia, and neuropathy. Given the tolerability profile, an all-oral regimen like VT may be suitable for selected patients unable or unwilling to be treated with intravenous platinum-based combination chemotherapy regimens.
Emerging data indicate that PARP inhibitors function through both inhibition of PARP activity and ‘trapping’ of PARP at sites of DNA damage. The relative contribution of these mechanisms to PARP inhibitor function may be context dependent. Trapping has been reported in cells treated with PARP inhibitors and alkylating agents [19]. However, the same has not been observed with PARP inhibitors and platinum agents [19, 20]. These data suggest the lower trapping efficiency of veliparib [20, 21], may not be impactful for its efficacy as part of the VCP regimen. Data have also suggested that trapping efficiency and tolerability are inversely related [22], which may limit the tolerability of higher efficiency trapping agents in similar regimens [23].
The current study was limited by the small sample size, based upon aggressive HR assumption of 0.58 for PFS and 0.61 for OS. The ongoing phase III study investigating veliparib added to carboplatin/paclitaxel in a larger BRCA+ breast cancer population will have greater power to detect a difference. Furthermore, there was no single-agent veliparib/placebo option for patients discontinuing chemotherapy. For several patients receiving PCP or VCP who had a CR or PR with a large tumor reduction at multiple assessments, reasons for treatment discontinuation included the very good response or to pursue less intense alternate therapy.
In a recently reported phase III trial in the neoadjuvant breast cancer setting, veliparib did not improve the efficacy of platinum-based chemotherapy in patients with TNBC [24]. Compared with the phase III neoadjuvant TNBC trial, the current trial and ongoing phase III trial of veliparib in advanced breast cancer enroll a more specific, biomarker-selected population of patients with germline BRCA1/BRCA2 mutations. This population has demonstrated sensitivity to PARP inhibitor therapy [15]. Furthermore, the phase III neoadjuvant TNBC study and the metastatic BRCA+ breast cancer veliparib trials use different doses of veliparib (50 mg BID continuous for 12 weeks versus 120 mg BID days 1–7 until progression, based on doses evaluated for each breast cancer type in earlier phase trials).
Patients with BRCA mutations are uniquely sensitive to both PARP inhibitors and platinum, suggesting VCP may be particularly active in this population. Platinum and PARP inhibitors share mechanisms of resistance, supporting their use in combination rather than sequentially [25]. In this phase II study, numerical trends in PFS and OS were observed with VCP over PCP, with a significant increase in ORR. The ongoing phase III trial in BRCA+ locally advanced/metastatic breast cancer (BROCADE3, NCT02163694) is powered to evaluate the efficacy of veliparib with carboplatin and weekly paclitaxel (N = 500). In contrast to the phase II study, patients in the phase III trial who discontinue carboplatin/paclitaxel due to toxicity or clinical benefit can continue veliparib/placebo at the monotherapy dose (300–400 mg BID).
Supplementary Material
Acknowledgements
The authors would like to thank the BROCADE investigators, patients and their families, study coordinators, and support staff. Medical writing support was provided by Delyth Eickermann, PhD, TRM Oncology, Atlanta, GA, funded by AbbVie Inc.
Funding
AbbVie Inc. provided financial support for this study (no grant number applies) and participated in the design, study conduct, analysis, and interpretation of the data, as well as the writing, review, and approval of this manuscript.
Disclosure
HSH has received research funding to institution from AbbVie, Corcept, Incyte, Karyopharm, Merrimack, Pfizer, Prescient, SeattleGenetics, and TapImmune. VD has received honoraria from AB Roche Genentech, Novartis, Pfizer, and AbbVie; and participated in symposia for Roche Genentech, Novartis, and Pfizer. MR has consulted for AstraZeneca and has received research support (institution) from AbbVie, BioMarin, Medivation, AstraZeneca, and Myriad. MC has received honoraria from Novartis, AstraZeneca, Pfizer, Sanofi, Roche, and Menarini; has had a consulting/advisory role with Novartis, AstraZeneca, Pfizer, Roche, and Menarini; participated in speaker’s bureaus for Novartis, AstraZeneca, Pfizer, and Roche; and received travel, accommodations, expenses, from Novartis, AstraZeneca, Pfizer, and Roche. MLT has had advisory roles for AstraZeneca and Tesaro; research funding (institution) from AbbVie, Medivation, and Tesaro. SMD has consulted for AbbVie; research support (institution) from AbbVie, Clovis, AstraZeneca, and Pharmamar. MF has advisory boards and honoraria from AstraZeneca and Pfizer. JEG has had a consulting or advisory role with Biogen, GTx Pharmaceuticals (spouse), Helix, Novartis (spouse), Pfizer (spouse), Pfizer, Sequenom, SV Life Sciences (spouse); and research funding from Myriad Genetics, Novartis (spouse), Pfizer (spouse), and Ambry Genetics. EHJ is a Co‐investigator on several Novartis trials but has never obtained any financial support. WG received research grants from the National Institutes of Health and the Breast Cancer Research Foundation, and is a consultant for Pfizer and Eli Lilly. SJI has consulted for AbbVie and Myriad; research support from Genentech, Pharmamar, and AbbVie. SP has consulted for AbbVie, Celldex, Pfizer, Esai; research funding to institution from AbbVie, Covance-Bayer, Lilly, Incyte, Novartis, Pfizer, Genentech, AstraZeneca, BioMarin, and Puma. CR, CN, QQ, and JQ are AbbVie employees and stock owners. SPS is a previous employee of AbbVie. All remaining authors have declared no conflicts of interest.
References
- 1. Easton DF. How many more breast cancer predisposition genes are there? Breast Cancer Res 1999; 1: 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ame JC, Spenlehauer C, de Murcia G.. The PARP superfamily. Bioessays 2004; 26: 882–893. [DOI] [PubMed] [Google Scholar]
- 3. Bryant HE, Schultz N, Thomas HD. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434: 913–917. [DOI] [PubMed] [Google Scholar]
- 4. Farmer H, McCabe N, Lord CJ. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921. [DOI] [PubMed] [Google Scholar]
- 5. Coleman RL, Sill MW, Bell-McGuinn K. et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation – an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015; 137: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman B, Shapira-Frommer R, Schmutzler RK. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandhu SK, Schelman WR, Wilding G. et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 2013; 14: 882–892. [DOI] [PubMed] [Google Scholar]
- 8. Donawho CK, Luo Y, Penning TD. et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007; 13: 2728–2737. [DOI] [PubMed] [Google Scholar]
- 9. Appleman LJ, Beumer JH, Jiang Y. et al. A phase I study of veliparib (ABT-888) in combination with carboplatin and paclitaxel in advanced solid malignancies. J Clin Oncol 2012; 30(Suppl): abst 3049. [Google Scholar]
- 10. Isakoff SJ, Mayer EL, He L. et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol 2015; 33: 1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tutt A, Ellis P, Kilburn L. et al. The TNT trial: a randomized phase III of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). In 37th Annual San Antonio Breast Cancer Symposium, Edition. San Antonio, TX 2014.
- 12. Isakoff SJ, Overmoyer B, Tung NM. et al. A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. J Clin Oncol 2010; 28: 15(Suppl): 1019. [Google Scholar]
- 13. Isakoff SJ, Overmoyer B, Tung NM. et al. A phase II trial expansion cohort of the PARP inhibitor veliparib (ABT888) and temozolomide in BRCA1/2 associated metastatic breast cancer. Cancer Res 2011; 71(Suppl 24): abstnr P3-16-05. [Google Scholar]
- 14. Trudeau ME, Crump M, Charpentier D. et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada – Clinical Trials Group (NCIC-CTG). Ann Oncol 2006; 17: 952–956. [DOI] [PubMed] [Google Scholar]
- 15. Robson M, Im SA, Senkus E. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 16. Clamp A, Jayson G.. PARP inhibitors in BRCA mutation-associated ovarian cancer. Lancet Oncol 2015; 16: 10–12. [DOI] [PubMed] [Google Scholar]
- 17. Molina J, Erlichman C, Northfelt D. et al. Ongoing phase 1 study of a novel PARP inhibitor, ABT-888 in combination with temozolamide; Pharmacokinetics, safety and anti-tumor activity. Cancer Res 2009; 69(Suppl 9): abst 3602. [Google Scholar]
- 18. Puhalla S, Beumer JH, Pahuja S. et al. Final results of a phase 1 study of single-agent veliparib (V) in patients (pts) with either BRCA1/2-mutated cancer (BRCA+), platinum-refractory ovarian, or basal-like breast cancer (BRCA-wt). J Clin Oncol 2014; 32(Suppl 15): abst 2570. [Google Scholar]
- 19. Murai J, Zhang Y, Morris J. et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther 2014; 349: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maag D, Solomon L, Hopkins T. et al. Rationale for the combination of veliparib with platinum-based chemotherapy. J Clin Oncol 2015; 33(Suppl 15): abst 2556. [Google Scholar]
- 21. Murai J, Huang SY, Das BB. et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012; 72: 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hopkins T, Solomon L, Shi Y. et al. PARP inhibitors trap PARP1 onto damaged DNA via catalytic inhibition and not by an allosteric mechanism. Eur J Cancer 2014; 50(Suppl 6): 82–83, abst 246. [Google Scholar]
- 23. Dhawan MS, Bartelink IH, Aggarwal RR. et al. A phase I study of carboplatin and talazoparib in patients with and without DNA repair mutations. J Clin Oncol 2017; 35(Suppl): abst 2527. [DOI] [PubMed] [Google Scholar]
- 24. Geyer CE, O’Shaughnessy J, Untch M. et al. Phase 3 study evaluating efficacy and safety of veliparib (V) plus carboplatin (Cb) or Cb in combination with standard neoadjuvant chemotherapy (NAC) in patients (pts) with early stage triple-negative breast cancer (TNBC). J Clin Oncol 2017; 35(Suppl): abst 520. [Google Scholar]
- 25. Fojo T, Bates S.. Mechanisms of resistance to PARP inhibitors–three and counting. Cancer Discov 2013; 3: 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.