Abstract
Background
T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive disease. In this study, we report our experience from 119 patients with T-PLL.
Patients and methods
We reviewed the clinico-pathologic records of 119 consecutive patients with T-PLL, who presented to our institution between 1990 and 2016.
Results
One hundred and nineteen patients with T-PLL were analysed. Complex karyotype and aberrations in chromosome 14 were seen in 65% and 52% patients, respectively. Seventy-five patients (63%) were previously untreated and 43 (37%) were initially treated outside our institution. Sixty-three previously untreated patients (84%) received frontline therapies. Overall, 95 patients (80%) have died. Median overall survival (OS) from diagnosis was 19 months [95% confidence interval (CI) 16–26 months]. Using recursive partitioning (RP), we found that patients with hemoglobin < 9.3 g/dl, lactate dehydrogenase (LDH) ≥ 1668 IU/l, white blood cell ≥ 208 K/l and β2M ≥ 8 mg/l had significantly inferior OS and patients with hemoglobin < 9.3 g/dl had inferior progression-free survival (PFS). In multivariate analysis, we identified that presence of pleural effusion [hazard ratio (HR) 2.08 (95% CI 1.11–3.9); P = 0.02], high LDH (≥ 1668 IU/l) [HR 2.5 (95% CI 1.20–4.24); P < 0.001)], and low hemoglobin (< 9.3 g/dl) [HR 0.33 (95% CI 0.14–0.75); P = 0.008] were associated with shorter OS. Fifty-five previously untreated patients received treatment with an alemtuzumab-based regimen (42 monotherapy and 13 combination with pentostatin). Overall response rate, complete remission rate (CR) for single-agent alemtuzumab and alemtuzumab combined with pentostatin were 83%, 66% and 82%, 73% respectively. In patients who achieved initial CR, stem cell transplantation was not associated with longer PFS and OS.
Conclusion
Outcomes in T-PLL remain poor. Multicenter collaborative effort is required to conduct prospective studies.
Keywords: T-PLL, prolymphocytic leukemia, prolymphocytes
Introduction
T-cell prolymphocytic leukemia (T-PLL) is an uncommon, mature lymphoid leukemia that is typically associated with a poor prognosis. T-PLL is diagnosed based on characteristic clinical, morphologic, immunophenotypic [1], cytogenetic and molecular features which may explain its aggressive clinical course [2]. Cytogenetic features [3] include chromosome 14 aberrations involving gene rearrangements of proto-oncogenes TCL1 (T-cell leukemia 1) or MTCP1 (mature T-cell proliferation 1; on chromosome X), aberration in chromosomes 8 (especially isochromosome 8 potentially leading to overexpression of MYC), deletion chromosome 11q22.3 (ATM gene deletion), or high prevalence of complex karyotype. Approximately 70%–80% of patients with T-PLL show an overexpression of TCL-1 by immunohistochemistry (IHC) or by chromosomal analysis using FISH and/or karyotyping. TCL1 belongs to beta barrel family of proteins localized in the cytoplasm. TCL1 and MTCP1 oncoprotein are structurally similar with 40% identical amino acid residues [4]. TCL1 is shown to promote kinase activity and transphophorylation of AKT and promotes the nuclear transport of AKT [5, 6]. Furthermore, overexpression of TCL-1 has been shown to modulate and amplify the AKT activation mediated by stimulation of T-cell receptor (TCR) in cells from T-PLL patients and induce hyperproliferation [7]. A recent study [8] has demonstrated gain-of-function mutations of IL-2RG, JAK-1/JAK-3 and STAT5B using whole-genome and whole-exome sequencing in T-PLL [9, 10]. Specifically, shorter survival was noted in patients with JAK-3 p.M11I mutations. Inhibition of STAT5B promoted apoptosis of T-PLL cells.
Despite an improved understanding into the molecular biology of T-PLL, the disease continues to present a therapeutic challenge for clinicians. In a recent analysis of SEER database [11], the median survival was 21 months and better outcomes were noted after the introduction of alemtuzumab (after 2000) for the therapy of T-PLL. None of the currently available therapies are very effective in producing durable remissions in patients with T-PLL [12]. Overall response rates (ORR) with alemtuzumab-based regimens vary from 50% to 90% and complete remission rates (CR) vary from 60% to 80% in different studies, with short duration of response [13–16]. Other chemotherapies such as nelarabine, single-agent bendamustine [17], combination of pentostatin with alemtuzumab [18], fludarabine, mitoxantrone cyclophosphamide followed by alemtuzumab consolidation [19] have been used with limited success. Allogenic stem cell transplantation (SCT) may induce longer relapse-free survival in few patients [20, 21]. Patients who received total body irradiation and shorter time from diagnosis to SCT had better relapse-free survival; however, treatment-related mortality and relapses remain common [22–24]. Rarity of this disease precludes the development of large-scale prospective clinical trials. Here, in this analysis, we are reporting the clinical experience of the largest series of patients with T-PLL from a single institution.
Methods
We reviewed the clinico-pathologic records of 119 consecutive patients with T-PLL, at our institution. Survival distributions was estimated by Kaplan–Meier method. Cox proportional hazard model for survival was analysed. We also carried out classification and regression tree analysis of various parameters to identify the optimal cut off points for predicting survival. Details are available in supplementary, available at Annals of Oncology online.
Results
Patient characteristics
We analysed the characteristics, prognostic factors, outcomes and treatments of 119 patients with T-PLL. Supplementary Table S1, available at Annals of Oncology online, provides the summary of the clinical and laboratory characteristics of the patients according to the treatment status. At the initial presentation, 20 patients (17%) presented with performance status 2 or higher. Lymphadenopathy, splenomegaly, skin lesions, pleural effusion and hepatomegaly were seen in 58%, 38%, 28%, 12% and 11%, of patients, respectively. Small-cell variant of T-PLL was detected in 21/106 patients (20%) and 57% patients with small-cell variant co-expressed CD4 and CD8. Overall, 108 patients had karyotype data available for analysis. Complex karyotype and aberrations in chromosome 14 were seen in 70 (65%) and 56 (52%) patients, respectively.
Treatments and survival outcomes
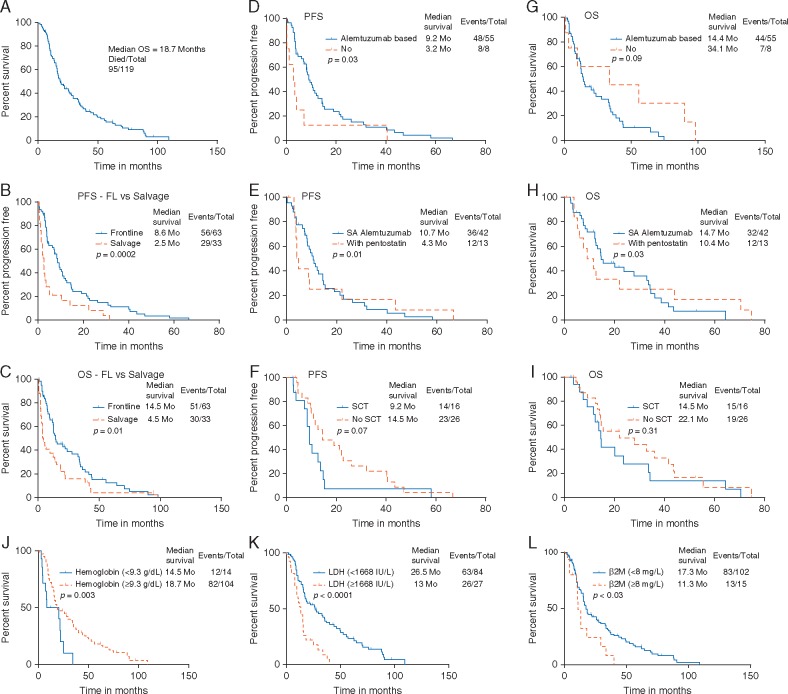
Overall median survival from initial diagnosis was 19 months [95% confidence interval (CI), 16–26 months] (Figure 1A). At initial presentation to our center, 75 patients (63%) were previously untreated and 43 patients (36%) were previously treated. Treatment and follow-up information were available in 67/75 (89%) previously untreated patients.
Figure 1.
Survival outcomes in patients with T-PLL—overall and according to the treatment status at the time of initial presentation to MDACC—frontline versus salvage treatment. (A) All patients from the time of initial diagnosis, median OS was 19 months. (B) PFS is significantly longer in patients treated in frontline setting (P = 0.0002). (C) OS is significantly longer in patients treated in frontline setting (P = 0.01). Survival outcomes according to the type of frontline therapy. (D and G) PFS and OS in patients who received therapy with alemtuzumab (single-agent alemtuzumab or in combination with pentostatin) versus other therapies (P = 0.03 for PFS and P = 0.09 for OS). (E and H) PFS and OS in patients who received alemtuzumab (single agent alemtuzumab versus alemtuzumab with pentostatin (P = 0.01 for PFS and P = 0.03 for OS). Survival outcomes according to treatment with SCT in patients who achieved complete remission after frontline therapy. (F) PFS was not significantly different in patients with/without SCT (P = 0.07). (I) OS was not significantly different in patients with/without SCT (P = 0.31). Survival outcomes in patients with T-PLL, according to the prognostic factors identified by recursive partitioning (RP) analysis. (J–L) OS was significantly shorter in patients with low hemoglobin (<9.3 g/dl), high serum LDH (≥1668 IU/l) and high β2M (≥8 mg/l) level.
Frontline patients
Among these 67 previously untreated patients, 4 patients remained in observation and 63 received treatment (55 with alemtuzumab-based regimens and 8 with non-alemtuzumab-based therapy). The median time from initial diagnosis to first treatment of these 63 patients was 2.3 months (range, 0.1–52 months). Fourteen patients were considered to have an indolent disease, 10 with treatment requirement after ≥ 19 months after initial diagnosis and 4 patients remained under observation. For the 55 patients treated with alemtuzumab-based therapy, 42 (76%) were treated with single-agent alemtuzumab and 13 (24%) with a combination of alemtuzumab and pentostatin. ORR and CR in patients who received frontline single-agent alemtuzumab (n = 42) and alemtuzumab combined with pentostatin (n = 13) were 81% and 61% in single-agent alemtuzumab cohort while 82% and 73% in alemtuzumab combined with pentostatin cohort, respectively. Non-alemtuzumab-based therapies are described in supplementary, available at Annals of Oncology online.
Patients with relapsed or refractory disease
We next analysed the response to salvage treatments in previously treated patients (before presentation at our center). Information for salvage treatments was available in 29 patients. Relapses occurred in 25/29 patients with a median time to relapse of 2.5 months (0.3–31 months). The ORR with salvage combination alemtuzumab with pentostatin (n = 5) was 75% compared with 46% with single-agent alemtuzumab (n = 15), summarized in Table 1. There was no difference in PFS or OS according to the type of salvage therapy (single-agent alemtuzumab versus alemtuzumab with pentostatin versus single-agent nelarabine) (supplementary Figure S1A and B, available at Annals of Oncology online).
Table 1.
Summary of the type of treatments, treatment responses and survival outcomes in patients with T-PLL (frontline and salvage setting)
| Alemtuzumab monotherapy |
Alemtuzumab + pentostatin |
Nelarabine | |||
|---|---|---|---|---|---|
| First line | Salvage | First line | Salvage | All | |
| n = 42 | n = 15 | n = 13 | n = 5 | n = 5 (one frontline and four salvage) | |
| Overall response (%) | 81 | 46 | 82 | 75 | 20 |
| Complete response (%) | 61 | 46 | 73 | 50 | 0 |
| Median OS (months)* | 15 | 15 | 10.4 | 2.6 | 2 |
| Median PFS (months)* | 11 | 3 | 4.3 | 2.6 | 2 |
| Median number of prior regimen (range) | – | 1 (1–4) | – | 3 (1–3) | 2 (1–3) |
Censored at stem cell transplant, total of 29 patients were treated with salvage therapy and shown above is information on 24 patients, 5 patients were treated with miscellaneous treatments (Fordosine, HyperCVAD, fludarabine-based regimen).
The median overall survival (OS) and progression-free survival (PFS) of patients are summarized in Table 1. Patients who received frontline treatment had a significantly longer PFS and OS compared with those who were treated with the first salvage (P = 0.0002 and 0.01, respectively; Figure 1B and C. Furthermore, patients who received frontline therapy with alemtuzumab-based regimens (monotherapy or combined with chemotherapy) had significantly longer PFS when compared with patients treated with non-alemtuzumab-based therapies (P = 0.03 for PFS and 0.09 for OS; Figure 1D and E. Moreover, patients who received single-agent alemtuzumab had significantly longer PFS and OS when compared with patients treated with pentostatin and alemtuzumab (P = 0.01 and 0.03, respectively; Figure 1F and G.
Sixteen patients underwent allogeneic SCT in first CR, 73% of 16 patients received an unrelated donor SCT. None of the patients received autologous SCT. We did not observe any significant difference in PFS or OS (Figure 1H and I) among patients who did or did not undergo SCT after achieving initial CR with any frontline therapies. We have summarized the details of SCT in supplementary Table S2, available at Annals of Oncology online. Eight patients (50%) relapsed after SCT.
For the total cohort, 43 patients were treated before initial presentation to our center. Details of treatment responses, follow ups outside our center was not available for review; however, the median number of prior treatments was 2 (range, 1–6); 46% of prior therapies were fludarabine based, 12% with single-agent chlorambucil, 14% with single-agent pentostatin, 16% with single-agent alemtuzumab and 12% with miscellaneous regimen.
Prognostic factors and survival outcomes
Using recursive partitioning (RP), we have identified an optimal cut off value for certain baseline parameters which significantly correlated with PFS and OS: hemoglobin, serum lactate dehydrogenase (LDH), and β2M levels. Patients with low hemoglobin (<9.3 g/dl), high serum LDH (≥1668 IU/l), and high serum β2M (≥8 mg/l) had significantly inferior PFS (supplementary Figure S2A and C, available at Annals of Oncology online) and OS Figure 1J–L. Furthermore, we detected that OS was significantly inferior in patients showing TCL-1 rearrangement by cytogenetics; however, a similar prognostic impact was not observed in patients with positive TCL-1 expression by IHC. PFS was not significantly different in patients with or without TCL-1 expression (supplementary Figure S3A–D, available at Annals of Oncology online). Survival was not significantly different in patients with or without small-cell variant of T-PLL.
Finally, we carried out univariate and multivariate analyses to identify the factors predictive for OS. All patients in this analysis were previously untreated and were analysed from the time of initial diagnosis (supplementary Table S3, available at Annals of Oncology online). In multivariate analysis, factors independently predictive for significantly increased risk of death were presence of pleural effusion [hazard ratio (HR) 2.08 (95% CI 1.11–3.90); P = 0.02], low hemoglobin (<9.3 g/dL) 0.33 (0.14–0.75; P = 0.008), high LDH level (≥ 1668 IU/l) 2.52 (1.2–4.24; P <0.001), and high white blood cell count [white blood cell (WBC) ≥ 208 K/l] 3.35 (0.98–11.49; P = 0.05). For PFS, we identified that patients with non-Caucasian ethnicity, absence of small-cell variant of T-PLL, and high β2M (≥8 mg/l) had significantly higher risk of disease progression after initial therapy (supplementary Table S4, available at Annals of Oncology online).
Discussion
In this study, we have analysed, to our knowledge, the largest series of patients with T-PLL from a single institution. We described the patient and disease characteristics, responses to treatment, long-term outcomes, and factors that may be useful in determining prognosis.
Patients with T-PLL continue to have poor outcomes [2] and there is only a minority of patients who do not require treatment after the initial diagnosis. Despite the recent advances in our understanding of the molecular biology of T-PLL, the discovery of newer, more effective therapies in this uncommon and aggressive leukemia remains an unmet need [12]. None of the currently available treatment modalities (including SCT) have significantly improved the outcomes in T-PLL [23]. Our data suggest that single-agent alemtuzumab as an initial therapy is associated with high response rates and is the best available treatment of T-PLL. However, durable remissions are still uncommon and relapsed disease accounts for the majority of failures. Furthermore, in this study, attempts to prolong remission with allogenic SCT after initial CR did not improve survival. We identified that hemoglobin <9.3 g/dl, serum LDH level ≥ 1668 IU/l, high WBC count, and presence of a pleural effusion at initial diagnosis significantly predicted for poor OS. These factors were not previously recognized to have distinct prognostic impact in T-PLL. We also identified that non-Caucasian ethnicity, absence of small-cell variant of T-PLL and high β2M (≥8 mg/l) had significantly higher risk of disease progression after initial therapy. Precise reasons to explain these findings are unclear, as to date, no study has reported on the clinico-pathologic characterization of small-cell variant or impact of race in T-PLL. Additionally, it is possible that similar to other lymphoproliferative disorders, serum LDH and β2 microglobin may reflect disease burden in T-PLL. Importantly, the characteristics of the previously treated patients (e.g. elevated β2 microglobulin, more lymphadenopathy and organomegaly) (Table 1) were suggestive of bulky disease.
Previously published retrospective studies were limited in the number of patients (range, 10–86), and data were obtained from multiple centers and/or different countries. One prior study reported data from 86 patients with T-PLL [7] and identified that overexpression of TCL-1 oncoprotein was associated with aggressive disease, short lymphocyte doubling time, enhanced TCR and AKT signaling, and rapid cell proliferation. Herling et al [7] reported that aged >65 years and higher WBC count (>40 K/μl) was associated with an increased risk of death. However, these cut offs were arbitrary and were not derived by statistical analysis. In our study, we based the cut off derived from RP analysis of different baseline variables and identified an optimal, clinically applicable cut-off value, which was not reported in previously published studies. We further applied these variables into the final cox regression model for survival outcomes and identified their prognostic relevance.
The role of SCT after initial CR in patients with T-PLL remains controversial, largely due to high rate of early relapses, and high transplant related mortality [21]; only a small fraction of patients have 3-year survival of 5%–20% [20, 24]. Two multi-institutional studies have reported that patients with T-PLL who have undergone SCT (auto or allogeneic) have better PFS and OS compared with patients who did not undergo SCT [21, 22]. In contrast, our data show that the median survival in 16 patients who underwent allogeneic SCT in first CR was 14.5 months compared with 22 months (P= not significant) in 26 patients in first CR after different frontline therapies who did not receive SCT. The explanation for this lack of benefit is not clear, but could be related to differences in conditioning regimens, the relative resistance to cytotoxic chemotherapy in T-PLL, and the lack of any robust graft versus leukemia effect in T-PLL [22, 25, 26]. Nonetheless, effective post remission therapy is a critical need for improving outcomes in these patients. Longer term consolidation or maintenance strategies, immunotherapeutic approaches, or targeted therapies should be investigated to maintain remissions achieved after effective frontline therapy.
Currently, data are very sparse on the cytokine milieu of T-PLL. However, the initial clinical presentation with B-symptoms, lymphadenopathy, organomegaly, pleural and pericardial effusions are suggestive of a cytokine-mediated syndrome. Indeed, about 76% patients with T-PLL have activating somatic mutations in IL2RG/JAK1/JAK3/STAT5B [8, 10] identified through whole-exome sequencing or by next-generation sequencing [8, 27, 28]. The net effect of these mutations is constitutive activation of JAK-STAT signaling and up regulation of STAT5B target genes [8]. It is possible that a pan-JAK inhibitor or STAT5 inhibitor may have a therapeutic effect in T-PLL.
About 50%–60% of patients exhibited complex karyotype and most abnormalities were observed in chromosome 14, in addition to the previously described del 11q, del 17p and chromosome 8 aberrations. Chromosome 14 abnormalities are a hallmark of T-PLL and involve the TCL-1 oncogene. Presence of complex karyotype [29, 30] and high incidence of ATM mutations [31] is known to be associated with refractory disease and poor prognosis in lymphoid malignancies. Two thirds of patients with T-PLL have ATM mutations [32, 33] and patients with ataxia telangiectasia may have a higher risk of transformation to T-PLL [34]. Inactivating mutations in ATM or loss of material at chromosome 11q23 (at the ATM gene locus) lead to deficient signaling of double-stranded DNA repair and cell cycle arrest after DNA damage. It is possible that in T-PLL [35, 36], these genetic aberrations may explain the molecular complexity and refractoriness to chemotherapy. Exploiting double-stranded DNA repair defects may be another important strategy to selectively target T-PLL cells. At present, this study lacks complete information on the molecular characteristics of these patients; therefore, it is difficult to provide a mechanistic link between cytogenetic finding and clinical course.
We further noted that although the response rate to alemtuzumab with pentostatin is marginally better then single agent alemtuzumab (73 versus 61%), the PFS and OS was significantly longer in patients treated with single-agent alemtuzumab. Since this is a retrospective study, it is possible that selection bias for either regimen may have affected the outcomes i.e. patients with bulky, more aggressive disease may have received the combination regimen and therefore had inferior outcomes. The PFS was better with alemtuzumab-based regimens compared with non-alemtuzumab-based therapies; for OS, the trend of increased OS with non-alemtuzumab-based therapies could be misleading, since virtually all of these patients received salvage therapy with alemtuzumab. Few patients who were treated with nelarabine and fordosine did not respond well.
There are several limitations to our study. This is a retrospective analysis that allows selection bias and uncontrolled analysis. Treatment and complete follow-up information was not available for all the patients, and third of patients were previously treated before coming to our center. However, when analyzing for outcomes, responses and prognostic factors, care was taken to make sure complete and original source data were available for all analysed patients. There was no uniform pattern of follow-up and management was according to individual physician’s choice, but the treatment modalities were examined and presented individually. While cytogenetic, morphology and flow cytometric studies were available for the majority of patients, our study is limited by the lack of complete molecular genetic data on all patients. Collaborations are now underway to retrospectively examine available banked samples and create protocols to prospectively study newly diagnosed patients.
In summary, T-PLL continues to pose a therapeutic challenge and newer modalities are required to treat this leukemia. Rarity of this disease limits the conduct of large-scale clinical trials, and multicenter collaborative effort is required to conduct prospective studies. Studies are underway to further define the genomic imbalances in patients with T-PLL and identify newer therapeutic targets and recognize the signaling pathways which can provide drug resistance in T-PLL.
Key Message
T-PLL remains a therapeutic challenge. Outcomes are poor in spite of stem cell transplant. We have reported the outcomes and prognostic factors from the largest single centre series of patients with T-PLL. We identified that hemoglobin < 9.3 g/dL, serum LDH level ≥ 1668 IU/l and presence of pleural effusion in patients with T-PLL were predictive for increased risk of death.
Funding
Our funding was from the following sources - Supported in part by the MD Anderson Cancer Center Support grant CA016672 (PI: Dr Ronald DePinho) and award number P01 CA049639 (PI: Dr Richard Champlin) from the National Cancer Institute. None of the authors are employed by the National Institutes of Health. JC is recipient of grant from the National Cancer Institute (PI of Project 1 of P01 CA049639).
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Chen X, Cherian S.. Immunophenotypic characterization of T-cell prolymphocytic leukemia. Am J Clin Pathol 2013; 140: 727–735. [DOI] [PubMed] [Google Scholar]
- 2. Dearden C. Management of prolymphocytic leukemia. Hematol Am Soc Hematol Educ Program 2015; 2015: 361–367. [DOI] [PubMed] [Google Scholar]
- 3. Urbankova H, Holzerova M, Balcarkova J. et al. Array comparative genomic hybridization in the detection of chromosomal abnormalities in T-cell prolymphocytic leukemia. Cancer Genet Cytogenet 2010; 202: 58–62. [DOI] [PubMed] [Google Scholar]
- 4. Fu ZQ, Du Bois GC, Song SP. et al. Crystal structure of MTCP-1: implications for role of TCL-1 and MTCP-1 in T cell malignancies. Proc Natl Acad Sci USA 1998; 95: 3413–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auguin D, Barthe P, Royer C. et al. Structural basis for the co-activation of protein kinase B by T-cell leukemia-1 (TCL1) family proto-oncoproteins. J Biol Chem 2004; 279: 35890–35902. [DOI] [PubMed] [Google Scholar]
- 6. Pekarsky Y, Koval A, Hallas C. et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA 2000; 97: 3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herling M, Patel KA, Teitell MA. et al. High TCL1 expression and intact T-cell receptor signaling define a hyperproliferative subset of T-cell prolymphocytic leukemia. Blood 2008; 111: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiel MJ, Velusamy T, Rolland D. et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood 2014; 124: 1460–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellanger D, Jacquemin V, Chopin M. et al. Recurrent JAK1 and JAK3 somatic mutations in T-cell prolymphocytic leukemia. Leukemia 2014; 28: 417–419. [DOI] [PubMed] [Google Scholar]
- 10. Bergmann AK, Schneppenheim S, Seifert M. et al. Recurrent mutation of JAK3 in T-cell prolymphocytic leukemia. Genes Chromosomes Cancer 2014; 53: 309–316. [DOI] [PubMed] [Google Scholar]
- 11. Chandran R, Gardiner SK, Fenske TS, Spurgeon ES.. Survival trends in T cell prolymphocytic leukemia: A SEER database analysis. Leuk Lymphoma 2016; 57: 942–944. [DOI] [PubMed] [Google Scholar]
- 12. Dearden C. How I treat prolymphocytic leukemia. Blood 2012; 120: 538–551. [DOI] [PubMed] [Google Scholar]
- 13. Dearden CE, Matutes E, Cazin B. et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood 2001; 98: 1721–1726. [DOI] [PubMed] [Google Scholar]
- 14. Dearden CE, Khot A, Else M. et al. Alemtuzumab therapy in T-cell prolymphocytic leukemia: comparing efficacy in a series treated intravenously and a study piloting the subcutaneous route. Blood 2011; 118: 5799–5802. [DOI] [PubMed] [Google Scholar]
- 15. Keating MJ, Cazin B, Coutre S. et al. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. JCO 2002; 20: 205–213. [DOI] [PubMed] [Google Scholar]
- 16. Ravandi F, O'Brien S, Jones D. et al. T-cell prolymphocytic leukemia: a single-institution experience. Clin Lymphoma Myeloma. 2005; 6: 234–239. [DOI] [PubMed] [Google Scholar]
- 17. Herbaux C, Genet P, Bouabdallah K. et al. Bendamustine is effective in T-cell prolymphocytic leukaemia. Br J Haematol 2015; 168: 916–919. [DOI] [PubMed] [Google Scholar]
- 18. Ravandi F, Aribi A, O'Brien S. et al. Phase II study of alemtuzumab in combination with pentostatin in patients with T-cell neoplasms. J Clin Oncol 2009; 27: 5425–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopfinger G, Busch R, Pflug N. et al. Sequential chemoimmunotherapy of fludarabine, mitoxantrone, and cyclophosphamide induction followed by alemtuzumab consolidation is effective in T-cell prolymphocytic leukemia. Cancer 2013; 119: 2258–2267. [DOI] [PubMed] [Google Scholar]
- 20. Guillaume T, Beguin Y, Tabrizi R. et al. Allogeneic hematopoietic stem cell transplantation for T-prolymphocytic leukemia: a report from the French society for stem cell transplantation (SFGM-TC). Eur J Haematol 2015; 94: 265–269. [DOI] [PubMed] [Google Scholar]
- 21. Krishnan B, Else M, Tjonnfjord GE. et al. Stem cell transplantation after alemtuzumab in T-cell prolymphocytic leukaemia results in longer survival than after alemtuzumab alone: a multicentre retrospective study. Br J Haematol 2010; 149: 907–910. [DOI] [PubMed] [Google Scholar]
- 22. Wiktor-Jedrzejczak W, Dearden C, de Wreede L. et al. Hematopoietic stem cell transplantation in T-prolymphocytic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation and the Royal Marsden Consortium. Leukemia 2012; 26: 972–976. [DOI] [PubMed] [Google Scholar]
- 23. Herling M. Are we improving the outcome for patients with T-cell prolymphocytic leukemia by allogeneic stem cell transplantation? Eur J Haematol 2015; 94: 191–192. [DOI] [PubMed] [Google Scholar]
- 24. Matutes E, Polliack A.. T-cell prolymphocytic leukemia: survival improves with alemtuzemab, but stem cell transplant eligibility ′counts′ even more. Leuk Lymphoma 2016; 57: 746–747. [DOI] [PubMed] [Google Scholar]
- 25. de Lavallade H, Faucher C, Furst S. et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in a patient with T-cell prolymphocytic leukemia: graft-versus-tumor effect and long-term remission. Bone Marrow Transplant 2006; 37: 709–710. [DOI] [PubMed] [Google Scholar]
- 26. Sellner L, Bruggemann M, Schlitt M. et al. GvL effects in T-prolymphocytic leukemia: evidence from MRD kinetics and TCR repertoire analyses. Bone Marrow Transplant 2017; 52: 544–551. [DOI] [PubMed] [Google Scholar]
- 27. Stengel A, Kern W, Zenger M. et al. Genetic characterization of T-PLL reveals two major biologic subgroups and JAK3 mutations as prognostic marker. Genes Chromosomes Cancer 2016; 55: 82–94. [DOI] [PubMed] [Google Scholar]
- 28. Lopez C, Bergmann AK, Paul U. et al. Genes encoding members of the JAK-STAT pathway or epigenetic regulators are recurrently mutated in T-cell prolymphocytic leukaemia. Br J Haematol 2016; 173: 265–273. [DOI] [PubMed] [Google Scholar]
- 29. Herling CD, Klaumunzer M, Rocha CK. et al. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy. Blood 2016; 128: 395–404. [DOI] [PubMed] [Google Scholar]
- 30. Sarkozy C, Terre C, Jardin F. et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer 2014; 53: 106–116. [DOI] [PubMed] [Google Scholar]
- 31. Cuneo A, Bigoni R, Rigolin GM. et al. Acquired chromosome 11q deletion involving the ataxia teleangiectasia locus in B-cell non-Hodgkin's lymphoma: correlation with clinicobiologic features. JCO 2000; 18: 2607–2614. [DOI] [PubMed] [Google Scholar]
- 32. Stoppa-Lyonnet D, Soulier J, Lauge A. et al. Inactivation of the ATM gene in T-cell prolymphocytic leukemias. Blood 1998; 91: 3920–3926. [PubMed] [Google Scholar]
- 33. Yamaguchi M, Yamamoto K, Miki T. et al. T-cell prolymphocytic leukemia with der(11)t(1;11)(q21;q23) and ATM deficiency. Cancer Genet Cytogenet 2003; 146: 22–26. [DOI] [PubMed] [Google Scholar]
- 34. Narducci MG, Virgilio L, Isobe M. et al. TCL1 oncogene activation in preleukemic T cells from a case of ataxia-telangiectasia. Blood 1995; 86: 2358–2364. [PubMed] [Google Scholar]
- 35. Soulier J, Pierron G, Vecchione D. et al. A complex pattern of recurrent chromosomal losses and gains in T-cell prolymphocytic leukemia. Genes Chromosom Cancer 2001; 31: 248–254. [DOI] [PubMed] [Google Scholar]
- 36. Stilgenbauer S, Schaffner C, Litterst A. et al. Biallelic mutations in the ATM gene in T-prolymphocytic leukemia. Nat Med 1997; 3: 1155–1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.