Abstract
Background
Hypermethylation of promoter CpG islands [CpG island methylator phenotype (CIMP)] represents a unique pathway for the development of colorectal cancer (CRC), characterized by lack of chromosomal instability and a low rate of adenomatous polyposis coli (APC) mutations, which have both been correlated with taxane resistance. Similarly, small bowel adenocarcinoma (SBA), a rare tumor, also has a low rate of APC mutations. This phase II study evaluated taxane sensitivity in SBA and CIMP-high CRC.
Patients and methods
The primary objective was Response Evaluation Criteria in Solid Tumors version 1.1 response rate. Eligibility included Eastern Cooperative Oncology Group performance status 0/1, refractory disease, and SBA or CIMP-high metastatic CRC. Nab-paclitaxel was initially administered at a dose of 260 mg/m2 every 3 weeks but was reduced to 220 mg/m2 owing to toxicity.
Results
A total of 21 patients with CIMP-high CRC and 13 with SBA were enrolled from November 2012 to October 2014. The efficacy-assessable population (patients who received at least three doses of the treatment) comprised 15 CIMP-high CRC patients and 10 SBA patients. Common grade 3 or 4 toxicities were fatigue (12%), neutropenia (9%), febrile neutropenia (9%), dehydration (6%), and thrombocytopenia (6%). No responses were seen in the CIMP-high CRC cohort and two partial responses were seen in the SBA cohort. Median progression-free survival was significantly greater in the SBA cohort than in the CIMP-high CRC cohort (3.2 months compared with 2.1 months, P = 0.03). Neither APC mutation status nor CHFR methylation status correlated with efficacy in the CIMP-high CRC cohort. In vivo testing of paclitaxel in an SBA patient-derived xenograft validated the activity of taxanes in this disease type.
Conclusion
Although preclinical studies suggested taxane sensitivity was associated with chromosomal stability and wild-type APC, we found that nab-paclitaxel was inactive in CIMP-high metastatic CRC. Nab-paclitaxel may represent a novel therapeutic option for SBA.
Keywords: nab-paclitaxel, CIMP, colorectal cancer, small bowel adenosscarcinoma
Introduction
Colorectal cancer (CRC) is a heterogeneous disease with two major pathways of development. Approximately 80% of CRCs show chromosomal instability (CIN) and are characterized by widespread chromosomal gains and losses. Errors in chromosomal segregation during mitosis are caused by errors in the spindle checkpoint. The adenomatous polyposis coli (APC) protein represents a critical component of the spindle complex because it is involved in mitotic spindle attachment to chromosomes. A number of studies have demonstrated that inactivating mutations in APC result in the development of CIN [1, 2]. The second major pathway of CRC carcinogenesis, occurring in ∼20% of CRCs, is the transcriptional inactivation of tumor suppressor genes by methylation of cytosine nucleotides at promoter CpG islands, termed CpG island methylator phenotype (CIMP-high) [3]. As expected, CIMP-high and CIN have been shown to be inversely correlated with near mutual exclusivity [4, 5].
Taxanes generate antitumor activity through direct binding to tubulin, with resultant suppression of microtubule dynamics. This results in the inability of cells to proceed through mitosis and results in cell cycle arrest and subsequent cell death. Although taxanes demonstrate a wide spectrum of antitumor activity and are active in upper gastrointestinal cancers, taxanes are not currently utilized in the treatment of lower gastrointestinal tract adenocarcinomas such as CRC. Initial phase II clinical trials investigating taxanes—single-agent paclitaxel in 14 patients and single-agent docetaxel in 19 patients—demonstrated no partial responses in unselected CRC. However, in a larger phase II study of the novel oral taxane tesetaxel in 39 unselected CRC patients, confirmed responses were seen in 4 patients (10%) and stable disease was seen in 14 patients (36%) [6].
Preclinical work has demonstrated a strong correlation between taxane sensitivity and CIN [7, 8]. In vitro studies in CRC cell lines have also supported the link between APC mutation status and taxane resistance [9, 10]. In fact, the CRC cell line HCT-116, which is CIMP-high and has wild-type APC, demonstrated sensitivity to paclitaxel, while the HCT-29 cell line, which is non-CIMP and has mutant APC, demonstrated resistance [9]. In addition, resistance to the microtubular toxin nocodazole was induced in the highly sensitive HCT-116 CRC cell line by the introduction of an APC-mutant construct [10]. Moreover, an analysis of 352 cell lines from the Sanger Institute identified APC mutations as the strongest genetic event that correlated with paclitaxel resistance (supplementary Figure S1, available at Annals of Oncology online). In parallel, aberrant methylation of the Checkpoint with Forkhead and Ring finger domains (CHFR) has been reported to be associated with sensitivity to taxanes in multiple histologies, including CRC [11]. CHFR is a mitotic checkpoint protein that is activated in the face of microtubular stress. Absence of the protein through methylation results in catastrophic cellular stress with microtubular injury, resulting in cell death, and provides a biological rationale for increased sensitivity to taxanes in tumors with CHFR methylation.
In this study, we hypothesized that nab-paclitaxel would demonstrate antitumor activity in patients with CIMP-high CRC because this subset of patients would be enriched for wild-type APC and chromosomal stability. In addition, because one of the most unique molecular findings of small bowel adenocarcinoma (SBA) is the low rate of APC mutations, we hypothesized that nab-paclitaxel would also demonstrate antitumor activity in this rare and understudied tumor type [12].
Methods
Study design and treatment
This is an open-label, single-institution, phase II study of nab-paclitaxel for histologically confirmed metastatic SBA or CIMP-high CRC.Nab-paclitaxel was given as a 260 mg/m2 intravenous infusion every 21 days. However, following the enrollment of four patients, two serious adverse events (grade 3 neutropenic fever and grade 4 sepsis) occurred and the starting dose was amended to 220 mg/m2. Restaging was conducted every three cycles.
Patients
For CRC patients, enrollment was restricted to those with MD Anderson conducted Clinical Laboratory Improvement Amendment certified CIMP-high CRC, which was defined as hypermethylation in at least two of six methylation-specific PCR markers (MLH1, P16, P14, MINT1, MINT2, and MINT31). Eligibility criteria were: Eastern Cooperative Oncology Group performance status of ≤1, adequate organ function, Common Terminology Criteria for Adverse Events 4.0 peripheral neuropathy ≤grade 1, no prior treatment with taxanes, and refractory disease (defined for SBA as prior treatment with fluoropyrimidine and oxaliplatin or for CRC as prior treatment with fluoropyrimidine, oxaliplatin, irinotecan, and anti-EGFR therapy for those with wild-type KRAS). The study was conducted with approval by the MD Anderson Institutional Review Board, and all participants provided written informed consent (NCT01730586).
Tumor mutational analysis and CHFR methylation analysis
Pretreatment paraffin-embedded tumor tissue was collected for mutational analysis including full APC gene sequencing, microsatellite stability status by immunohistochemistry, and CHFR methylation by standard methylation-specific PCR and DREAMing (Discrimination of Rare EpiAlleles by Melt) (supplementary Methods, available at Annals of Oncology online) [13].
Blood samples were collected at baseline in 20 patients with CIMP-high CRC. For circulating cell-free CHFR methylation analysis, all genomic DNA was bisulfite treated based upon the ‘Methylation-on-Beads’ bisulfite conversion technique as previously published (supplementary Methods, available at Annals of Oncology online).
SBA and CIMP-high patient-derived xenograft experiment
A fresh tumor sample from a patient with a distal duodenal APC wild-type microsatellite stable adenocarcinoma who was not enrolled in the clinical trial and a CIMP-high BRAF V600E microsatellite stable CRC were implanted into the subcutaneous flanks of female NOD-SCID-gamma mice. Upon successful uptake of the tumor, 10 mice per patient-derived xenograft (PDX) model were randomized to receive a phosphate-buffered saline control or 12.5 mg/kg paclitaxel administered intraperitoneally twice weekly for eight doses. Tumor measurements were recorded twice weekly.
Statistical analysis
The primary end points were response rate (as per RECIST version 1.1) in the efficacy-assessable population (defined as those who had received at least three cycles of nab-paclitaxel) for the CIMP-high CRC cohort and the SBA cohort. Secondary end points included safety, progression-free survival (PFS), and overall survival for both disease cohorts as well as an exploratory end point evaluating efficacy by APC mutation status.
Assuming a null hypothesis of ≤1% response rate, a sample size of 15 patients with CIMP-high CRC would be required to demonstrate a response rate of 20% using a binomial one-sample test with a two-sided α of 0.025 and power of 91%. For the SBA cohort, a sample size of 10 patients would be required to demonstrate a response rate of 20% using a binomial one-sample test with a two-sided α of 0.025 and power of 85%. Descriptive statistics included Fisher’s exact test, Wilcoxon rank-sum test, two-sided log-rank test, and Kaplan–Meier method. All P values presented are two-sided and statistical significance is P-value ≤0.05.
Results
Patient characteristics
A total of 21 patients with CIMP-high CRC and 13 with SBA were enrolled from November 2012 to October 2014. Baseline characteristics are shown in Table 1. The median number of prior treatments was three for CRC and two for SBA. Peritoneal metastases were common in SBA (n = 11, 85%), and liver metastases were common in CIMP-high CRC (n = 11, 52%). The median number of cycles administered was 3 for both the CIMP-high CRC cohort (range 1–6) and the SBA cohort (range 1–17).
Table 1.
Baseline characteristics of patients with CpG island methylator phenotype (CIMP-high) colorectal cancer (CRC; n = 21) and patients with small bowel adenocarcinoma (n = 13)
| Characteristic | No. (%) |
|
|---|---|---|
| CIMP-high CRC | SBA | |
| Median age (range) | 57 years (33–76 years) | 58 years (40–76 years) |
| Female | 7 (33) | 7 (54) |
| Race/ethnicity | ||
| White | 18 (86) | 7 (54) |
| African American | 1 (5) | 4 (31) |
| Asian | 2 (10) | 2 (15) |
| Histologic grade | ||
| Moderate | 17 (81) | 9 (69) |
| Poor | 4 (19) | 4 (31) |
| Mucinous histological characteristics | 5 (24) | 3 (23) |
| Primary lesion | ||
| Duodenum | 4 (31) | |
| Jejunum/ileum | 9 (69) | |
| Right colon | 6 (29) | |
| Left colon | 15 (71) | |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 3 (14) | 6 (46) |
| 1 | 18 (86) | 7 (54) |
| Metastatic sites | ||
| Liver | 11 (52) | 3 (23) |
| Lung | 10 (48) | 1 (8) |
| Peritoneum | 8 (38) | 11 (85) |
| Median number of prior lines of therapy (range) | 3 (2–6) | 2 (1–7) |
| CIMP-high | 21 (100) | N/A |
N/A, not applicable.
The molecular characteristics for the efficacy-assessable population are shown in supplementary Table S1, available at Annals of Oncology online. A KRAS mutation was present in six patients with CIMP-high CRC (40%) and eight patients with SBA (80%). A wild-type APC gene was present in four patients with CIMP-high CRC (27%) and eight patients with SBA (80%).
Toxicity
All adverse events occurring in >10% of patients are shown in supplementary Table S2, available at Annals of Oncology online. No grade 5 adverse events were seen. The most common grade 3 or 4 adverse events were fatigue (n = 4, 12%), neutropenia (n = 3, 9%), dehydration (n = 2, 6%), and thrombocytopenia (n = 2, 6%). Grade 3 febrile neutropenia occurred in three patients (9%).
Efficacy
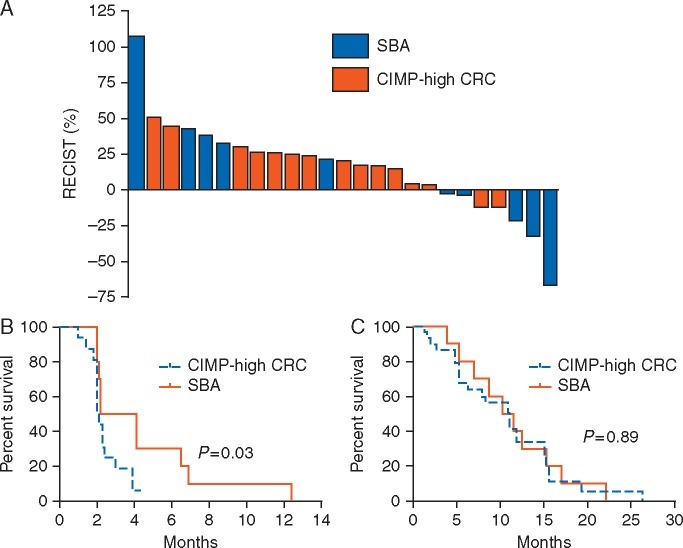
A total of 15 patients with CIMP-high CRC and 10 with SBA were assessable for response assessment, Figure 1A and supplementary Table S3, available at Annals of Oncology online. No responses were seen in the CIMP-high CRC cohort, and two responses where seen in the SBA cohort (jejunal primary in both cases), supplementary Figure S2, available at Annals of Oncology online. Stable disease was seen in three (20%) CIMP-high CRC and three (30%) SBA patients (ileal primary in two patients and duodenal primary in one patient). PFS and overall survival for both tumor cohorts are shown in Figure 1B and C. In an unplanned exploratory analysis, the SBA cohort showed a statistically significant improvement in PFS compared with the CIMP-high CRC cohort (median PFS = 3.2 months compared with 2.1 months, P-value = 0.03). Similar findings were seen when the analysis was conducted on an intent-to-treat basis (supplementary Table S4 and Figure S3, available at Annals of Oncology online).
Figure 1.
Efficacy outcomes stratified by tumor type for the efficacy-assessable population of patients with CpG island methylator phenotype (CIMP-high) colorectal cancer (CRC; n = 15) and patients with small bowel adenocarcinoma (SBA; n = 10). (A) Best radiographic response according to Response Evaluation Criteria in Solid Tumors (RECIST). (B) Progression-free survival curves for each cohort. (C) Overall survival curves for each cohort.
In a preplanned exploratory analysis, efficacy outcomes were evaluated in relation to the presence or the absence of an APC mutation. In the CIMP-high CRC cohort, all four patients with a wild-type APC gene demonstrated progressive disease as best response. In the SBA cohort, the two partial responses were both in patients with a wild-type APC gene. The one SBA patient with an APC mutation demonstrated progressive disease as best response. The one CRC patient with high microsatellite instability demonstrated progressive disease and the three patients with MLH1 methylation demonstrated progressive disease.
CHFR methylation analysis
Five patients had archived tumor available for CHFR methylation analysis. Two samples showed CHFR methylation and three samples were CHFR unmethylated. Of the two patients with methylated CHFR, one patient had stable disease for 5 months and the other was unevaluable as the subject was removed from study due to toxicity.
DREAMing analysis of baseline plasma CHFR methylation status was conducted in 20 CIMP-high CRC patients. A total of six patients had methylated CHFR >10 heavily methylated copies per milliliters of plasma, supplementary Figure S4, available at Annals of Oncology online, including two of the three stable disease patients. No correlation with the rate of stable disease or PFS was present with regard to CHFR methylation status, though the number of patients was very small.
SBA patient-derived xenograft
A patient-derived xenograft (PDX) was established from a patient with SBA not enrolled in the clinical trial. This patient underwent a primary resection of a moderately differentiated distal duodenal adenocarcinoma that had microsatellite stability and wild-type APC. Treatment with intraperitoneal paclitaxel resulted in a statistically significant reduction in tumor growth (P-value = 0.007) in the duodenal cancer, whereas no significant difference in the CIMP-high CRC cancer was seen (P-value =0.5), supplementary Figure S5, available at Annals of Oncology online.
Discussion
The current study reports for the first time the effects of a taxane in SBA and CIMP-high CRC. The results did not demonstrate that nab-paclitaxel had clinical activity in CIMP-high metastatic CRC, but activity was observed in SBA. Because there are limited treatment options for SBA and taxanes are not utilized in the treatment of this disease, these preliminary clinical findings, further replicated in a separate PDX model, support further exploration of taxane therapy in SBA.
The rationale for the exploration of taxane therapy in CIMP-high CRC was based upon the strong preclinical and clinical correlation between CIN and taxane sensitivity [9, 10, 14, 15]. This was initially determined by Swanton et al., who carried out a large RNA interference screen in colon, breast, and non-small-cell lung cancer cell lines to identify genes influencing paclitaxel sensitivity [7, 8, 14]. Most genes discovered through this screen involved the spindle complex, and silencing of these genes resulted in not only paclitaxel resistance but also the development of CIN [8]. Further efforts identified a CIN gene expression profile that correlated with taxane efficacy in NCI-60 colorectal and breast cancer cell lines and patient samples from an ovarian cancer clinical trial [7].
The CIN and antitubulin response assessment (CINATRA) clinical trial was initiated to investigate the microtubule targeting agent patupilone in CRC according to CIN status. That study was halted after the enrollment of 29 patients owing to excessive toxicity, and no correlation with CIN was conducted [16]. Although our results are limited by sample size and the use of a surrogate biomarker, the lack of clinical activity in our trial does not support the correlation between chromosomal stability and taxane sensitivity.
The strong in vitro correlation between APC mutational status and taxane resistance did support our efforts to further substratify our patients by APC mutational status. However, although our study consisted primarily of patients with CIMP-high CRC, only 27% of these patients had wild-type APC, and no efficacy correlation was seen. Despite selecting for CIMP-high CRC, the minimum threshold of two of the six methylated markers, which was present in 40% of the CIMP-high CRC cohort, may have resulted in the lower rate of BRAF V600E and MSI-high genetic alterations seen in this clinical trial. In addition based upon the correlation of CHFR methylation status and taxane sensitivity from laboratory analyses of CRC cell lines we also explored this correlation within this clinical trial [11]. Though we did not determine any correlation with CHFR methylation status, we did demonstrate the ability in clinical trial samples to potentially determine methylation status from circulating free DNA using a novel DREAM-ing assay. In line with prior estimates from tumor samples we demonstrated a rate of methylated CHFR in 6 of the 20 patients using this assay [17]. Two of the only three of patients with benefit, defined as stable disease, from this regimen had methylation by this exploratory assay (stable disease of 4.4 and 3.9 months, respectively). In part, the lack of correlations with clinical end points within this trial may reflect the overall lack of efficacy seen within the CIMP-high cohort and the limited numbers.
Our findings are similar to results from other clinical trials of taxanes in CRC. Although one study showed a 10% response rate in CRC treated with the oral taxane tesetaxel, other phase II studies have not demonstrated similar activity [6]. In addition, a recent clinical trial that was initiated after our clinical trial investigated weekly nab-paclitaxel in 41 patients with CRC; that trial demonstrated no responses and an overall disease control rate of 16% [18].
The more interesting finding from the current clinical trial relates to the efficacy seen in patients with SBA. Although the sample size of 10 patients is small, two patients demonstrated clear evidence of anticancer activity with partial responses that lasted 6.9 and 12.4 months. In addition, the PFS in the SBA cohort was significantly longer than the CIMP-high CRC, however, the comparison between these two cohorts is limited by differences in prognosis, trial eligibility, and molecular enrichment between the two cohorts.
Because the SBA cohort had a limited number of patients, we sought to provide convergent evidence of our findings by treating a SBA PDX model. Owing to the rarity of SBA, this model is, to the best of our knowledge, the only SBA PDX ever reported. Although only one model was available for this analysis, the findings of this analysis add support to the clinical trial findings.
At present, patients with SBA are treated on the basis of the paradigm for CRC and taxanes are not utilized. Recent molecular data have questioned this approach; a large-scale genomic comparison among 317 SBA patients, 6353 CRC patients, and 889 gastric carcinoma patients demonstrated unique genomic alterations in SBA compared with the neighboring cancers [12]. In particular, the rate of APC mutation was significantly different across all three cancers (P < 0.001), with a rate of 76% in CRC, 27% in SBA, and 8% in gastric cancers. Among the largest retrospective reviews of chemotherapy in SBA, ranging from 80 to 44 patients, the use of a taxane has not been reported [19, 20].
In conclusion, our clinical trial results demonstrate the safety of nab-paclitaxel at a dose of 220 mg/m2 every 3 weeks in intestinal cancers. The current clinical trial did not demonstrate clinical activity of nab-paclitaxel in CRC and did not validate the use of CIMP-high status as a predictive biomarker for nab-paclitaxel. However, trial findings and associated in vivo analysis of a novel SBA PDX do suggest that nab-paclitaxel may have activity in SBA. These results in combination with recent molecular findings demonstrate the unique differences between SBA and CRC, thus indicating that individual clinical trials are needed for rare tumor types.
Supplementary Material
Acknowledgements
We would like to acknowledge the patients and their families for participating in this study, Erica Goodoff from the Department of Scientific Publications for manuscript editing, and Dr Leslie Cope for his help in analyzing the CHFR methylation correlations on the study.
Funding
The clinical trial was supported by Celgene Corporation, Summit, NJ (no grant number applies). Research support was provided by the Kavanagh Family Foundation (no grant number applies), NIH Cancer Center Support Grant (CCSG) P30CA016672 and American Cancer Society (grant ID 127343-RSG-15-068-01-TBG; PI: NA).
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Hadjihannas MV, Behrens J.. CIN by WNT: growth pathways, mitotic control and chromosomal instability in cancer. Cell Cycle 2006; 5(18): 2077–2081.http://dx.doi.org/10.4161/cc.5.18.3282 [DOI] [PubMed] [Google Scholar]
- 2. Kaplan KB, Burds AA, Swedlow JR. et al. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol 2001; 3(4): 429–432.http://dx.doi.org/10.1038/35070123 [DOI] [PubMed] [Google Scholar]
- 3. Simons CC, Hughes LA, Smits KM. et al. A novel classification of colorectal tumors based on microsatellite instability, the CpG island methylator phenotype and chromosomal instability: implications for prognosis. Ann Oncol 2013; 24(8): 2048–2056.http://dx.doi.org/10.1093/annonc/mdt076 [DOI] [PubMed] [Google Scholar]
- 4. Cheng YW, Pincas H, Bacolod MD. et al. CpG island methylator phenotype associates with low-degree chromosomal abnormalities in colorectal cancer. Clin Cancer Res 2008; 14(19): 6005–6013.http://dx.doi.org/10.1158/1078-0432.CCR-08-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goel A, Nagasaka T, Arnold CN. et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology 2007; 132(1): 127–138.http://dx.doi.org/10.1053/j.gastro.2006.09.018 [DOI] [PubMed] [Google Scholar]
- 6. Moore MR JC, Harker G, Lee F. et al. Phase II trial of DJ-927, an oral tubulin depolymerization inhibitor, in the treatment of metastatic colorectal cancer. J Clin Oncol 2006; 24(Suppl 18; Abstract 3591). [Google Scholar]
- 7. Swanton C, Nicke B, Schuett M. et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci USA 2009; 106(21): 8671–8676.http://dx.doi.org/10.1073/pnas.0811835106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swanton C, Marani M, Pardo O. et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007; 11(6): 498–512.http://dx.doi.org/10.1016/j.ccr.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 9. Tao W, South VJ, Zhang Y. et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell 2005; 8(1): 49–59.http://dx.doi.org/10.1016/j.ccr.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 10. Tighe A, Johnson VL, Taylor SS.. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci 2004; 117(26): 6339–6353.http://dx.doi.org/10.1242/jcs.01556 [DOI] [PubMed] [Google Scholar]
- 11. Pelosof L, Yerram SR, Ahuja N. et al. CHFR silencing or microsatellite instability is associated with increased antitumor activity of docetaxel or gemcitabine in colorectal cancer. Int J Cancer 2014; 134(3): 596–605.http://dx.doi.org/10.1002/ijc.28390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrock AB, Devoe CE, McWilliams R. et al. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol 2017; doi: 10.1001/jamaoncol.2017.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pisanic TR, Athamanolap P, Poh W. et al. DREAMing: a simple and ultrasensitive method for assessing intratumor epigenetic heterogeneity directly from liquid biopsies. Nucleic Acids Res 2015; 43(22): e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swanton C, Tomlinson I, Downward J.. Chromosomal instability, colorectal cancer and taxane resistance. Cell Cycle 2006; 5(8): 818–823.http://dx.doi.org/10.4161/cc.5.8.2682 [DOI] [PubMed] [Google Scholar]
- 15. Cahill DP, Lengauer C, Yu J. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 1998; 392(6673): 300–303.http://dx.doi.org/10.1038/32688 [DOI] [PubMed] [Google Scholar]
- 16. Moorcraft SY, Chau I, Peckitt C. et al. Patupilone in patients with pretreated metastatic/locally recurrent colorectal cancer: results of the phase II CINATRA trial. Invest New Drugs 2013; 31(5): 1339–1344.http://dx.doi.org/10.1007/s10637-013-9990-3 [DOI] [PubMed] [Google Scholar]
- 17. Brandes JC, van Engeland M, Wouters KA. et al. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis 2005; 26(6): 1152–1156.http://dx.doi.org/10.1093/carcin/bgi058 [DOI] [PubMed] [Google Scholar]
- 18. Ducreux M, Bennouna J, Adenis A. et al. Efficacy and safety of nab-paclitaxel in patients with previously treated metastatic colorectal cancer: a phase II COLO-001 trial. Cancer Chemother Pharmacol 2017; 79(1): 9–16.http://dx.doi.org/10.1007/s00280-016-3193-5 [DOI] [PubMed] [Google Scholar]
- 19. Overman MJ, Kopetz S, Wen S. et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer 2008; 113(8): 2038–2045.http://dx.doi.org/10.1002/cncr.23822 [DOI] [PubMed] [Google Scholar]
- 20. Zaanan A, Gauthier M, Malka D. et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer 2011; 117(7): 1422–1428.http://dx.doi.org/10.1002/cncr.25614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.