Abstract
Glioblastoma (WHO grade IV astrocytoma) is the most frequent primary brain tumor in adults, representing a highly heterogeneous group of neoplasms that are among the most aggressive and challenging cancers to treat. An improved understanding of the molecular pathways that drive malignancy in glioblastoma has led to the development of various biomarkers and the evaluation of several agents specifically targeting tumor cells and the tumor microenvironment. A number of rational approaches are being investigated, including therapies targeting tumor growth factor receptors and downstream pathways, cell cycle and epigenetic regulation, angiogenesis and antitumor immune response. Moreover, recent identification and validation of prognostic and predictive biomarkers have allowed implementation of modern trial designs based on matching molecular features of tumors to targeted therapeutics. However, while occasional targeted therapy responses have been documented in patients, to date no targeted therapy has been formally validated as effective in clinical trials. The lack of knowledge about relevant molecular drivers in vivo combined with a lack of highly bioactive and brain penetrant-targeted therapies remain significant challenges. In this article, we review the most promising biological insights that have opened the way for the development of targeted therapies in glioblastoma, and examine recent data from clinical trials evaluating targeted therapies and immunotherapies. We discuss challenges and opportunities for the development of these agents in glioblastoma.
Keywords: glioma, cancer genomics, targeted therapies, precision medicine, personalized medicine, biomarkers
Introduction
Glioblastoma is the most frequent and aggressive primary malignant brain tumor in adults [1], with a median overall survival (OS) between 10 and 20 months [2–4]. Standard of care is maximal safe surgery followed by concomitant radio-chemotherapy and adjuvant chemotherapy with temozolomide, which can be combined with intermediate-frequency alternating electric fields [2, 4, 5]. Once recurrence occurs, therapeutic options are limited, including bevacizumab and nitrosoureas, although bevacizumab is not approved in Europe. Unlike in most other cancers, this lack of progress has been sustained despite growing insight into the biology of the disease [6–11]. Fortunately, these significant advances have continued to stimulate the development and re-purposing of numerous targeted therapies in clinical trials.
Genomic landscape of glioblastoma
Glioblastomas constitute a highly heterogeneous group of invasive malignant brain tumors [12]. It was the first tumor to undergo comprehensive molecular characterization [6–10, 13–15]. Briefly, these studies showed that most tumors harbor recurrent molecular alterations disrupting core pathways involved in regulation of growth (receptor tyrosine kinase [RTK], mitogen-activated protein kinase [MAPK] and phosphoinositide 3-kinase [PI3K] signaling pathways), cell cycle, DNA repair and apoptosis (Retinoblastoma/E2F and p53 tumor suppressor pathways) as well as control of chromatin state and telomere length (Table 1). Frequently, these alterations derive from copy number aberrations (CNAs). The most common amplification events involve chromosomes 7 (EGFR/MET/CDK6), 12 (CDK4 and MDM2) and 4 (PDGFRA), while recurrent homozygous deletions are found in chromosomes 9 (CDKN2A/B) and 10 (PTEN). In addition, genome-wide sequencing highlighted single nucleotide variants (SNVs) and short insertions and deletions, resulting in recurrent mutations in the TERT promoter, PTEN, TP53, EGFR, PIK3CA, PIK3R1, NF1 and RB1 [10].
Table 1.
Genomic alterations and example targeted therapies in glioblastoma
| Gene | Alteration or target | Target frequency in glioblastomaa (%) | Candidate therapy (drug example) |
|---|---|---|---|
| Growth factor receptors | |||
| EGFR | Deletion (EGFRvIII), mutation, translocation and/or amplification | 55 | EGFR vaccine or antibody-drug conjugate (rindopepimut, ABT-414) |
| KIT | Amplification, mutation | 10 | KIT inhibitor (imatinib) |
| PDGFRA | Amplification | 15 | PDGFR inhibitor (dasatinib) |
| FGFR1, FGFR3 | Translocation (e.g. FGFR3-TACC3) | 3 | FGFR1/3 inhibitor (JNJ-42756493) |
| MET | Amplification, translocation | 3 | MET inhibitor (cabozantinib) |
| MAPK and PI3K/mTOR signaling pathways | |||
| PTEN | Deletion, mutation | 40 | AKT inhibitor, mTOR inhibitor (voxtalisib) |
| PIK3CA | Amplification, mutation | 10 | mTOR inhibitor, PI3K inhibitor (buparlisib) |
| NF1 | Deletion, mutation | 14 | MEK inhibitor (trametinib) |
| BRAF | Mutation (BRAF V600E) | 2 | BRAF inhibitor (vemurafenib), MEK inhibitor (trametinib) |
| Cell cycle pathways | |||
| MDM2 | Amplification | 10 | MDM2 inhibitor (AMG232) |
| TP53 | Wild-type (no mutations) | 60 | MDM2 inhibitor (AMG232) |
| CDK4/6 | Amplification | 20 | CDK4/6 inhibitor (ribociclib) |
| RB1 | Wild-type (no mutations) | 90 | CDK4/6 inhibitor (ribociclib) |
| Others | |||
| IDH1 | Mutation | 6 | IDH1 inhibitor (AG120) |
| MYC, MYCN | Amplification | 5 | Bromodomain inhibitor (OTX-015) |
Source: cbioportal.org (glioblastoma TCGA dataset, n = 281 tumor samples with sequencing and CNA data) [10].
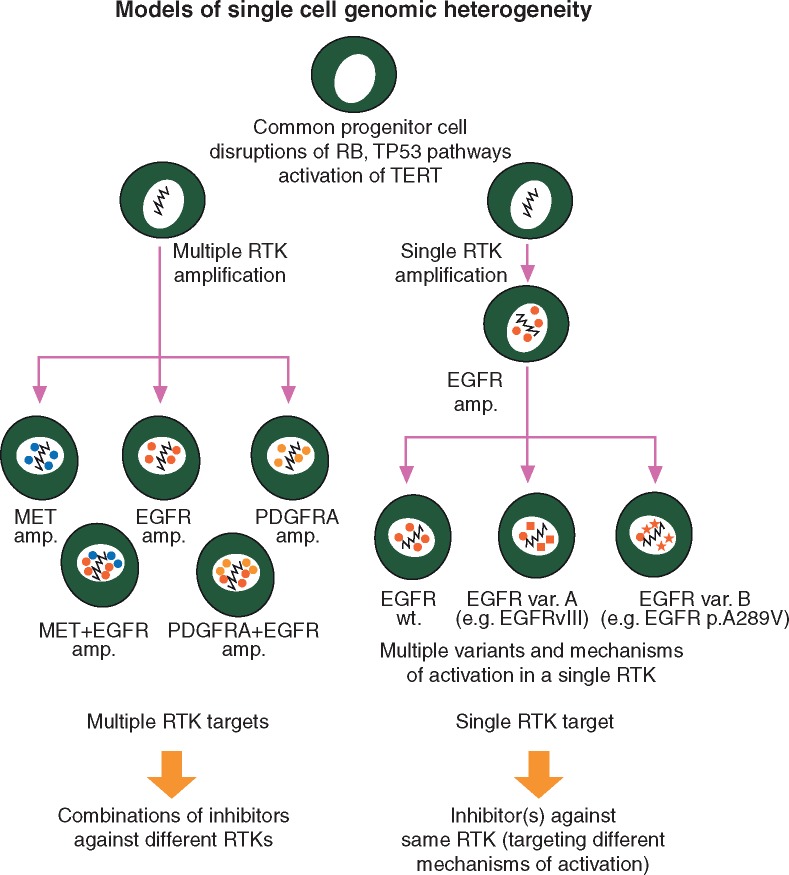
Most of these recurrent and biologically relevant genomic variants continue to be attractive targets for drug development [16–23] (Table 1). However, none of the recurrent genomic variants in glioblastoma has been strongly associated with clear prognostic and predictive value so far. This challenges the assumption that these variants are necessarily obligate cancer drivers in glioblastoma and suggests that strong cancer cell plasticity and redundancy among alterations that drive tumor growth may contribute to therapy failure more than previously assumed (Figure 1). Increasingly, it is being recognized that glioblastomas are characterized by significant inter- and intra-tumor genomic heterogeneity, which can exist as temporal or spatial [10, 24–33]. This represents challenges for appropriate driver identification due to glioblastoma being inherently limited in the amount and locations that one can sample, as well as the limited opportunities for reoperation [34–36]. The evidence that such heterogeneity might be relevant comes from multisector genome-wide sequencing of primary and post-treatment tumors, which revealed substantial divergence in the landscape of driver alterations between primary and recurrent tumors [30, 32, 33, 37, 38]. Moreover, heterogeneity at the single cell level can exist as multiple genomic alterations within redundant pathways (e.g. mosaic amplifications of EGFR, MET and PDFGRA) [10, 24, 29, 39, 40], or multiple unique variants of a single gene (e.g. multiple EGFR oncogenic variants in a single cell) [29, 30, 40], which overall results in heterogeneity in drug sensitivity within individual tumor cells [41] (Figure 1).
Figure 1.
Cellular heterogeneity of RTK aberrations in glioblastoma: implications for appropriate drug targeting (adapted from Francis et al. [30]). Dynamics of the glioblastoma genome may generate or select for subclonal populations of tumor cells that are highly resistant to treatment, overall suggesting that comprehensive characterization of tumor heterogeneity is a prerequisite for the success of pharmacological inhibition of RTK alterations. Left, multiple amplifications of distinct RTK genes can be observed in non-overlapping subclonal populations from individual tumors, or within individual tumor cells. In other cases (right), tumor heterogeneity may exist as multiple alterations within a single RTK gene.
Targeting growth factor receptors and their downstream signaling pathways
Drugs directed against alterations that lead to constitutive activation of growth factor RTKs are the most common type of targeted therapy in all types of cancer with successful responses seen in many cancers. These drugs have also been of great interest in glioblastoma because direct alterations in RTKs and/or downstream MAPK/PI3K signaling pathways represent a hallmark of this tumor (Table 1) [10].
EGFR-targeted therapies
EGFR amplification, rearrangement or point mutations are found in approximately half of glioblastomas and multiple aberrations in EGFR often co-exist within an individual tumor [10, 30, 42–44]. Nearly 20% of glioblastomas harbor deletion of exons 2–7 of EGFR, resulting in EGFRvIII, a constitutively active oncogenic variant frequently associated with EGFR amplification. Preclinical studies have demonstrated that EGFRvIII-driven tumors are only weakly sensitive to first generation EGFR tyrosine kinase inhibitors (TKI) erlotinib and gefitinb [45, 46]. Indeed, EGFRvIII—as most other EGFR SNVs found in glioblastoma—alters the extracellular domain of EGFR in glioblastoma, while in contrast lung adenocarcinomas typically harbor direct activating mutations in the kinase domain [45].
Rindopepimut is an EGFRvIII peptide vaccine that demonstrated signs of activity in preclinical models of glioblastoma and early phase trials [16, 47, 48]. The recently completed randomized phase II study ReACT evaluated the association of rindopepimut plus bevacizumab in EGFRvIII-positive recurrent glioblastoma. Advantage to rindopepimut therapy was reported across multiple endpoints including 2-year OS rate and progression-free survival (PFS), although the trial failed to meet its primary endpoint [49] (Table 2). Preliminary analyses from the phase III randomized study of rindopepimut in newly diagnosed EGFRvIII-positive glioblastoma indicated that its benefit on OS will not reach statistical significance (23 months from diagnosis in both arms), resulting in the closure of the trial [50]. Subgroup analyses suggested that rindopepimut might have failed due to reduced amount of EGFRvIII antigen burden in patients that underwent gross total resection (2-year survival rate of 30% in patients with non-minimal residual disease versus 19% in patients with minimal residual disease), although these results will need confirmation after longer follow-up. Further development of rindopepimut is uncertain.
Table 2.
Recently reported trials of targeted therapies in recurrent glioblastoma
| Design | Drug regimen | Target(s) | Population, number of patients (n) | Median OS (months) | Median PFS (months) | PFS-6 | Reference |
|---|---|---|---|---|---|---|---|
| Growth factor receptors, MAPK/PI3K signaling pathways | |||||||
| Phase II, randomized | Onartuzumab + bevacizumab (ona/beva) or bevacizumab + placebo (beva/p) | MET (onartuzumab), VEGF (bevacizumab) | First recurrence, n = 64 (ona/beva), n = 65 (beva/p) | 8.8 (ona/beva), 12.6 (beva/p) | 3.9 (ona/beva), 2.9 (beva/p) | 33.9% (ona/beva), 29% (beva/p) | [97] |
| Phase II, double-blind, randomized | Bevacizumab + rindopepimut (beva/rindo) or bevacizumab + placebo (beva/p) | VEGF (bevacizumab), EGFRvIII (rindopepimut) | First or second recurrence, bevacizumab-naïve, EGFRvIII- positive, n = 73 | 11.6 (beva/rindo), 9.3 (beva/p) | NA | 28% (beva/rindo), 16% (beva/p) | [49] |
| Phase II, randomized | Temozolomide (TMZ) or afatinib (afa) or afatinib + temozolomide (afa/TMZ) | EGFR (Afatinib) | First recurrence, n = 39 (TMZ), n = 41 (afa), n = 39 (afa/TMZ) |
|
|
|
[62] |
| Phase I, single-agent | ABT-414 | EGFR, EGFRvIII | Any recurrence, n = 60 | 9.0 | NA | 28.3% | [52] |
| Phase II, single-agent | PX-866 | PI3K | First recurrence, n = 33 | NA | NA | 17% | [101] |
| Phase II, single-arm | Buparlisib + bevacizumab | PI3K (buparlisib), VEGF (bevacizumab) | First recurrence, n = 68 | 10.8 | 5.3 | NA | [102] |
| Phase II, single-arm | Erlotinib + sorafenib | EGFR (erlotinib), VEGFR, PDGFR, Raf kinases (sorafenib) | NA, n = 56 | 5.7 | 2.5 | 14% | [71] |
| DNA repair and other epigenetic modifiers | |||||||
| Phase II, randomized | Bevacizumab (beva) + vorinostat (beva/vor) or bevacizumab (beva) | Histone deacetylases (vorinostat), VEGF (bevacizumab) | First recurrence, n = 49 (beva/vor), n = 41 (beva) | 8.3 (beva/vor), 7.0 (beva) | 4.2 (beva/vor), 3.6 (beva) | NA | [154] |
| Phase II, randomized | Sequential temozolomide + veliparib | PARP (veliparib) | NA, n = 141 | 10.3 (beva-naïve), 4.7 (beva-resistant) | 2.0 (beva-naïve), 2.0 (beva-resistant) | 17.0% (beva-naïve), 4.4% (beva-resistant) | [129] |
| Phase II, single-arm | Panobinostat + bevacizumab | Histone deacetylases (panobinostat), VEGF (bevacizumab) | First or second recurrence, n = 24 | 9.0 | 5.0 | 30.4% | [143] |
| Antiangiogenics | |||||||
| Phase III, double-blind, randomized | Lomustine + bevacizumab (lom/beva) vs. lomustine (lom) | VEGF (beva) | First recurrence, n = 288 (lom/beva), n = 149 (lom) | 9.1 (lom/beva), 8.6 (lom) | 4.2 (lom/beva), 1.5 (lom) | NA | [169] |
| Phase III, double-blind, randomized | CCNU + placebo (CCNU) or cediranib (ced) or cediranib + CCNU (CCNU/ced) | VEGFR1-3 and PDGFR (ced) | First recurrence, n = 65 (CCNU), n = 131 (ced), n = 129 (CCNU/ced) | 9.8 (CCNU), 8.0 (ced), 9.4 (CCNU/ced) | 2.7 (CCNU), 3.1 (ced), 4.2 (CCNU/ced) | 24.5% (CCNU), 16% (ced), 34.5% (CCNU/ced) | [155] |
| Phase II, randomized | Axitinib (axi) or physician’s choice (lomustine or bevacizumab) | First recurrence, n = 22 (axi), n = 22 (control) | 6.3 (axi), 3.7 (control) | 34% (axi), 28% (control) | [165] | ||
| Phase II, randomized | Bevacizumab + placebo (beva/p) vs. bevacizumab + dasatinib (beva/dasa) | SRC, c-KIT, EPHA2, PDGFR (dasa), VEGF (beva) | First or second recurrence, n = 38 (beva/p), n = 83 (beva/dasa) | 7.9 (beva/p), 7.2 (beva/dasa) | NA | 18.4% (beva/p), 27.2% (beva/dasa) | [164] |
| Phase II, single-agent | Dasatinib | SRC, c-KIT, EPHA2, PDGFR | First recurrence, overexpression of at least 2 putative dasatinib targets, n = 50 | 7.9 | 1.7 | 6% | [84] |
| Phase II, single-arm | Pazopanib + lapatinib | VEGFR1-3, c-Kit, PDGFR (pazopanib), EGFR (lapatinib) | NA, n = 41 | NA | 1.9 | 7.5% | [65] |
| Phase II, single-agent | Sunitinib | VEGFR1-2, c-Kit, PDGFR, FLT3, CSF-1R, RET | NA, n = 63 | 9.4 (beva-naïve), 4.4 (beva-resistant) | 1 (beva-naïve), 1 (beva-resistant) |
|
[152] |
| Phase II, single-agent | Nintedanib | VEGFR1-3, FGFR1-3, PDGFR | First or second recurrence, n = 25 | 6 | 1 | NA | [157] |
| Immune checkpoint inhibitors | |||||||
| Phase II/III, randomized | Nivolumab (3 mg/kg, nivo1) or nivolumab (1 mg/kg) + ipilimumab (3 mg/kg, nivo1/ipi3) or nivolumab (3 mg/kg) + ipilimumab (1 mg/kg, nivo3/ipi1) | PD1 (nivolumab), CTLA-4 (ipilimumab) | First recurrence, n = 10 (nivo3), n = 10 (nivo1/ipi3), n = 20 (nivo3/ipi1) | 10.5 (nivo3), 9.3 (nivo1/ipi3), 7.3 (nivo3/ipi1) | 1.9 (nivo3), 2.1 (nivo1/ipi3), 2.4 (nivo3/ipi1) | NA | [183] |
| Phase II, single-agent | Durvalumab | PD-L1 | First or second recurrence, bevacizumab-naïve, n = 30 | 6.7 | 3.2 | 20% | [185] |
| Phase Ib, single-agent | Pembrolizumab | PD1 | Any recurrence, n = 26 | 14.0 | 3.0 | 44% | [184] |
Abbreviations: NA, data not available.
Other EGFRvIII-targeted therapies are being evaluated. ABT-414 is an antibody drug conjugate (ADC) consisting of an anti-EGFR MAb, conjugated to the tubulin inhibitor monomethylauristatin F. ABT-414 demonstrated cytotoxicity against glioblastoma patient-derived xenograft models expressing either wild-type EGFR or EGFRvIII [51]. Preliminary data from a phase I trial of ABT-414 monotherapy in EGFR-amplified recurrent glioblastoma showed a 6 months PFS rate of 28.3% [52] (Table 2). OS from trial entry was 9 months, which was considered encouraging, as 56% of patients had already undergone two to three prior therapies. No dose-limiting toxicity was reported, although specific ocular toxicities were frequently observed (mostly reversible blurred vision, with some patients presenting with keratitis or corneal epithelial microcysts). The clinical development of ABT-414 is ongoing with randomized phase II/III trials (Table 3).
Table 3.
Ongoing randomized phase 3 trials evaluating investigational agents in glioblastoma
| ClinicalTrials.gov Identifier | Population | Treatment arms | Primary endpoint | Statusa | Sponsor |
|---|---|---|---|---|---|
| Newly diagnosed glioblastoma | |||||
| NCT00045968 | Sufficient tumor lysate after surgery | Experimental: RT/TMZ followed by TMZ + DCVax-L (dendritic cells vaccine) | PFS | Accrual suspended | Northwest Biotherapeutics |
| Comparator: RT/TMZ followed by TMZ + placebo | |||||
| NCT02617589 | Unmethylated MGMT promoter | Experimental: RT + nivolumab (anti-PD1 MAb) | OS | Recruiting | Bristol-Myers Squibb |
| Comparator: RT/TMZ followed by TMZ | |||||
| NCT02546102 | HLA-A2 positive patients | Experimental: RT/TMZ followed by TMZ + ICT-107 (dendritic cells vaccine) | OS | Recruiting | ImmunoCellular Therapeutics |
| Comparator: RT/TMZ followed by TMZ + placebo | |||||
| NCT02573324 | EGFR-amplified | Experimental: RT/TMZ/ABT-414 followed by TMZ + ABT-414 (anti-EGFR ADC) | OS | Recruiting | AbbVie |
| Comparator: RT/TMZ/placebo followed by TMZ + placebo | |||||
| NCT02152982 | Unmethylated MGMT promoter | Experimental: RT/TMZ + followed by TMZ + veliparib (PARP inhibitor) | OS | Not yet recruiting | National Cancer Institute |
| Comparator: RT/TMZ followed by TMZ + placebo | |||||
| Recurrent glioblastoma | |||||
| NCT0201771 | First progression | Experimental: nivolumab (anti-PD1 MAb) | OS | Accrual completed | Bristol-Myers Squibb |
| Comparator: bevacizumab | |||||
| NCT02511405 | First or second progression | Experimental: bevacizumab + VB-111 (viral toxin) | OS | Recruiting | Vascular Biogenics |
| Comparator: bevacizumab | |||||
| NCT02414165 | First or second progression, candidate for resection | Experimental: TOCA-511 (viral gene therapy injected in tumor resection cavity) + TOCA-FC (5-fluorocytosine) | OS | Recruiting | Tocagen |
| Comparator: Investigator's choice (single agent lomustine or temozolomide or bevacizumab) | |||||
Source: clinicaltrials.gov (November 2016).
In addition, several trials have evaluated more broadly effective EGFR-targeted therapies (Table 2). A variety of first and second generation EGFR/HER2 TKI or anti-EGFR monoclonal antibodies (Mab) have been evaluated as monotherapy [53–62] or in association with various agents or radiation therapy [63–79]. The results of these trials have been comprehensively reviewed elsewhere [18, 80]. Overall, disappointing results were reported despite some anectodotal response observed, suggesting the lack of efficacy of the currently available agents. Further studies evaluating novel agents or combinations are warranted to re-evaluate the value of EGFR inhibition in molecularly selected populations.
Targeting other receptor tyrosine kinases
Oncogenic FGFR–TACC fusions are found in nearly 3% of glioblastomas, with promising evidence of actionability provided by preclinical studies [21, 81]. Encouraging evidence of activity was recently reported in a phase I study evaluating JNJ-42756493—a highly selective pan-FGFR TKI—in three patients harboring FGFR3–TACC3-positive glioblastomas [21, 82]. Phase II clinical trials evaluating other selective FGFR inhibitors (e.g. BGJ398 and AZD4547) are currently ongoing [83].
PDGFRA amplification is found in nearly 15% of glioblastomas [10]. This receptor is highly active in all glioma types and represents one of the more underexplored targets for therapy. A recently reported phase II trial evaluated the efficacy of dasatinib, a multikinase inhibitor targeting PDGFR, c-KIT, SRC and EPHA2 [84] (Table 2). Despite the fact that patients were selected on the basis of overexpression of at least 2 putative dasatinib targets, no response was reported. Additional trials evaluated other multikinase inhibitors without showing any consistent clinical activity in glioblastoma [85–89].
Finally, preclinical evidences indicated an oncogenic role for c-MET signaling pathway activation in glioblastoma, notably by promoting tumor growth and invasiveness as well as drug resistance [90–94]. Rare responses have been documented in patients receiving crizotinib, a c-MET/ALK inhibitor and represent some of the first evidence of targeted therapy success [95, 96]. MET amplification or mutation as well as overexpression of c-MET or its ligand, the hepatocyte growth factor (HGF), have been proposed as predictive biomarkers, although efficacy and its molecular determinants remain unclear to date. A recently reported randomized phase II trial investigated the safety and efficacy of bevacizumab plus onartuzumab—a MAb against MET—versus bevacizumab plus placebo in recurrent glioblastoma (Table 2) [97]. Overall, there was no evidence of clinical benefit with bevacizumab plus onartuzumab compared with bevacizumab plus placebo, although exploratory biomarker analyses suggested benefit in patients with umethylated O6-methylguanine–DNA methyltransferase (MGMT) or high HGF expression in tumor tissue. Further understanding of the role of these RTKs in the progression of glioblastoma, as well as evaluation of highly brain penetrant and potent inhibitors is warranted.
Targeting PI3K/AKT/mTOR and MAPK signaling pathways
In light of the disappointing activity observed with existing RTK inhibitors, agents designed to interfere with downstream molecules remain attractive. The PI3K/AKT/mTOR signaling pathway is dysregulated in the vast majority of glioblastomas through various molecular alterations (Table 1). mTOR inhibitors such as temsirolimus and everolimus have been FDA-approved to treat various solid cancers including subependymal giant cell astrocytoma, a low grade brain tumor arising in patients with tuberous sclerosis complex, with good response in this special type of astrocytoma. However, when evaluated in glioblastoma as monotherapy, or in combination with either EGFR TKIs, bevacizumab or temozolomide and radiation therapy, these agents have not demonstrated significant clinical activity [66–70, 98–100].
Nonetheless, it has been hypothesized that a subset of patients may benefit from PI3K/AKT/mTOR signaling inhibition, and novel agents with a broader range of activity are currently being evaluated. PX-866 is an oral PI3K inhibitor recently tested in a phase II trial [101]. While the study was negative, durable stabilization was observed in 21% of patients. No association between outcome and PTEN, PIK3CA or PIK3R1 status was observed. The dual PI3K/mTOR inhibitor voxtalisib and the pan-class I PI3K inhibitor buparlisib have been evaluated in other trials. Preliminary results from phase II trials evaluating buparlisib indicated activity in association with bevacizumab [102], while limited efficacy was observed in patients receiving buparlisib as monotherapy, even in the presence of PIK3CA, PIK3R1 or PTEN molecular alterations (Table 2).
Targeting of MAPK pathway signaling, activated in all glioblastoma, is also a rational approach. A small subset of patients (3%), especially those with giant cell or epithelioid morphology (11%), harbors the BRAF V600E mutation [103], a well-known targetable oncogene. The BRAF inhibitor vemurafenib has shown promising efficacy in individual patients with BRAF-mutant (V600E) high-grade gliomas of non-glioblastoma types [104, 105]. The RAF multikinase inhibitor sorafenib has been evaluated in several small phase I/II studies as monotherapy or in combination with bevacizumab, temozolomide, temsirolimus [106–110] or radiation therapy and temozolomide [111]. Unfortunately, limited efficacy was observed and has not supported further development of sorafenib in glioblastoma (Table 2). Future preclinical studies and trials should focus on combined inhibition of MAPK and other pathways, as well as identifying predictive biomarkers. The presence of responses in other glioma types with BRAF alterations suggests these agents may be some of the most promising for future success in targeted therapies.
Targeting DNA repair and cell cycle control pathways
Disruption of p53 and Retinoblastoma/E2F tumor suppressor pathways is found in more than 80% of glioblastomas [10]. TP53 encodes the tumor suppressor protein p53 that causes cell-cycle arrest and promotes apoptosis upon DNA damage [112]. TP53 mutation/deletion results in growth advantage and clonal expansion of glioma cells, as well as impairment of DNA repair, promoting overall genetic instability and transformation [113, 114]. Besides direct gene mutation or deletion, p53 inactivation may be caused by MDM2 or MDM4 amplification (20% of patient overall) [10, 115]. The first therapeutic strategies targeting p53 were centered on attempting reactivation of the pathway using gene therapy or pharmacological approaches, although these have failed to demonstrate clinical efficacy [116]. A key disadvantage of the original nutlin-based drugs was the low potency and poor blood–brain barrier (BBB) penetration. However, MDM2 inhibition has recently re-emerged as an attractive strategy to restore p53 function with advances in the chemical properties of nutlin-based agents (RG7112, RG7388), as well as other classes of agents recently developed (HDM201, AMG232). Preclinical studies have demonstrated striking antitumor efficacy in MDM2-amplified glioblastoma models [117, 118]. Most importantly, TP53-wild-type models also showed marked response to these agents and blood–tumor and blood–brain penetration of the more novel agents has been in a range as feasible for clinical trials. Given that about 50% of glioblastoma patients have TP53-wild-type tumors this represents an attractive strategy for the majority of patients.
Cell cycle progression is frequently deregulated through various recurrent molecular alterations including inactivation of CDKN2A/CDKN2B and RB1 as well as amplification of CDK4 and CDK6 (Table 1) [10, 119]. Novel agents designed to inhibit CDK4 and CDK6 have demonstrated strong antitumor efficacy in RB1-wild-type glioblastoma models [120–123], and have been subsequently evaluated in phase II. Results from this study as well as other trials evaluating newer compounds (NCT02345824) should shed light on the value of CDK inhibitors in glioblastoma, and the biomarker profile of the patients that may respond.
Finally, synthetic lethal approaches have been developed as novel strategies to target tumors harboring alterations disrupting DNA repair and tumor suppressor pathways. WEE1—a nuclear serine/threonine kinase—acts as a gatekeeper against mitotic catastrophe in glioblastoma. Recent preclinical works demonstrated that small-molecule inhibition of WEE1 sensitized glioblastoma to DNA damaging agents including radiation therapy [124–126]. Combination of the WEE1 inhibitor AZD1775 with radiation therapy and temozolomide is currently being evaluated (NCT01849146). Other promising strategies exploiting synthetic lethal interactions include association of DNA repair inhibitors (e.g. the PARP inhibitors veliparib and olaparib) with radiation therapy and/or temozolomide, which have demonstrated antitumor efficacy in animal models [127–128], and are currently evaluated in randomized trials (Table 3) [129].
Targeting epigenetic deregulation and tumor metabolism
Targeting isocitrate dehydrogenase
IDH1 mutations are found in 6% of primary glioblastomas [7, 130–132]. These mutations confer a gain-of-function, resulting in the production of d-2-hydroxyglutarate (D2HG), which interferes with cellular metabolism and epigenetic regulation [132, 133]. Small-molecule inhibitors of mutant IDH enzymes have demonstrated activity in preclinical models [17], and are being evaluated in phase I/II trials (NCT02073994, NCT02481154). Preliminary reports indicated favorable safety profile and signs of activity, mainly in patients with lower grade tumors [134]. IDH1 peptide vaccines represent an alternative approach that has demonstrated activity in preclinical models [135, 136], and are being evaluated in clinical trials (NCT02454634, NCT02193347).
Targeting histone deacetylase and other epigenetic modifiers
Histone deacetylase (HDAC) inhibitors represent an emerging class of therapeutics that has shown activity in hematologic malignancies. Despite encouraging efficacy in preclinical models including histone H3-mutant pediatric glioblastoma [137–139], only modest activity has been observed in clinical trials evaluating HDAC inhibitors as a single agent, or in combination with temozolomide, bortezomib or bevacizumab [140–143] (Table 2). Beyond HDAC inhibitors, other epigenetic modifiers have recently gained interest for the treatment of glial tumors. These include BET bromodomain proteins inhibitors and EZH2 inhibitors, which have recently entered in clinical trials (NCT01897571, NCT02711137), and have both demonstrated antitumor activity in preclinical models [144–147].
Targeting tumor angiogenesis
A multitude of anti-angiogenic targeted therapies have been evaluated in clinical trials of glioblastoma as monotherapy or in combinations with various agents, all with no significant survival benefit to patients [63, 84, 106, 148–168] (Table 2). In 2009, bevacizumab received provisional FDA-approval for the treatment of recurrent glioblastoma on the basis of radiographic response rates ranging from 28% to 59% reported in two single-arm trials [148, 149]. However, subsequent trials failed to demonstrate superiority of bevacizumab alone or combined with lomustine in terms of OS [161, 169]. In newly diagnosed glioblastoma, two recently reported placebo-controlled randomized trials evaluating the benefit from the addition of bevacizumab to standard of care showed no difference in OS, while significant improvement in PFS was demonstrated in both trials (extension of median PFS of 3.4 and 4.6 months) [162, 163].
Given the encouraging preclinical data, what went wrong? The lack of the target being expressed in tumor cells is something that became clearer with time. The level of dependency of the tumor ecosystem on the vasculature now appears to be low. Despite the lack of clear survival benefit of antiangiogenic agents in glioblastoma, prolonged PFS with long-lasting tumor response or stabilization has been proposed to be present in a subset of patients receiving bevacizumab. The identification of biomarkers to predict response of antiangiogenics agents may therefore be warranted. One possibility for this comes from post-hoc analysis from the AVAglio randomized phase III trial [170], which reported significant OS advantage of adding bevacizumab to standard of care in patients with proneural IDH1 wild-type tumors, albeit this needs to be validated further in an independent trial.
Immunotherapies
Immunotherapy for glioblastoma has gained considerable interest over the past years. The concept of the central nervous system (CNS) as an ‘immune privileged site’ has been recently challenged by the discovery of the CNS lymphatic system, which is connected to the deep cervical lymph nodes [171–174]. Therapeutic targeting of immune checkpoint programmed cell death 1 (PD1)/programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated molecule-4 (CTLA-4) using MAbs has been associated with significant clinical benefit in several human malignancies [175, 176]. These treatments aim at enhancing antitumor immune responses, by blocking negative regulatory pathways in T-cell activation. In glioblastoma, PD-L1 is expressed in some patients [177, 178], and preclinical studies have provided rationale for the evaluation of immune checkpoint blockers (ICBs) [179–182].
Several clinical trials evaluating ICBs are ongoing (Tables 2 and 3), including randomized phase III trials of the anti-PD1 nivolumab. Preliminary data on efficacy and safety of ICBs as monotherapy or in combination were recently reported [183–186] (Table 2). Overall, the response rates observed with these agents in recurrent disease were low; however, the observation of a relative increase in 6-month PFS and OS suggested a possible benefit in a subset of patients. Recent studies in non-CNS cancers have indicated that patients whose tumors bear high neoantigen and/or mutation load may derive enhanced clinical benefit from immune checkpoint inhibitors [187–190]. Partial responses to nivolumab were recently reported in two pediatric patients that developed hypermutant glioblastoma in the context of biallelic mismatch repair deficiency [191], suggesting that this subset of patients may be responsive to this strategy.
Beyond targeting of immune checkpoints, other approaches taking advantage of the immune system and the tumor microenvironment are being explored. Dendritic cell and peptide vaccines have entered clinical trials, with promising signs of activity reported in preclinical studies and early phase trials [16, 47, 48, 135, 136, 192–197]. These encouraging results need further confirmation in the ongoing larger randomized trials. Other immune-cells based approaches include engineered chimeric antigen receptor (CAR T)/NK cells re-directed to specific tumor antigens (e.g. EGFRvIII), which have demonstrated promising antitumor efficacy in animal models [198, 199], and are currently evaluated in several phase I/II trials (NCT01109095, NCT02442297, NCT02664363, NCT01454596). However, these novel approached will require further standardization and optimization efforts, and costs and technical issues associated with cell-based therapy will likely limit its widespread application.
Development of targeted therapies in glioblastoma: current state of the art and future directions
Lessons learned from the clinical development of targeted therapies
Unlike the experience in some other human malignancies harboring activating oncogenic alterations (e.g. EGFR or ALK in lung adenocarcinoma), efforts in the field of precision medicine have not yet demonstrated consistent clinical activity in glioblastoma. Several factors may explain such disappointing results. A central element of precision medicine is the matching of a selective drug and its mechanism of action using a robust biomarker (e.g. a molecular assay defining a specific biologic subgroup) to select patients that are expected to benefit from the drug (‘selecting the right drug for the right patient’). Before evaluation in large trials, scientists and investigators should provide: (i) strong evidence of antitumor activity in disease-relevant models [200] and (ii) proof-of-concept (i.e. demonstration of the feasibility) as well as evidence of effective target modulation in early phase trials. In glioblastoma, few if any trials that evaluated targeted therapies have met these preliminary requirements.
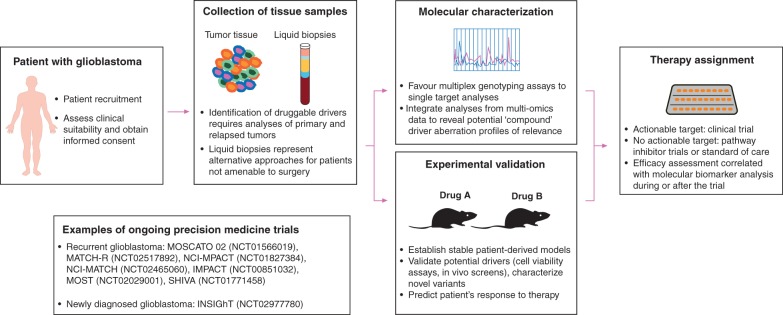
As far as target relevance and selection are concerned, most of the trials had not implemented molecular enrichment for patient selection. It is likely that most patients have received investigational agents in the absence of the relevant target in their tumor. Defining relevant targets is often challenging. Although early studies suggested that EGFR and PTEN status could predict response to EGFR-targeted therapies [201, 202], outcome was not correlated with the presence of EGFR amplification, EGFRvIII, PTEN loss or other molecular alterations in subsequent studies, and molecular predictors for the efficacy of EGFR targeted therapies remain undetermined. Future precision medicine studies should more largely implement systematic molecular characterization, including assessment of non-invasive biomarkers [203, 204], which will theoretically enable physicians to identify the most relevant targets for each patient, and allow further correlation of molecular profile with outcome (Figure 2).
Figure 2.
Current implementation of precision medicine in glioblastoma. Practical implications of implementing precision medicine approaches in glioblastoma are depicted in this figure. Appropriate molecular profiling requires analysis of tumor tissue from the relapsed tumor. Further steps include target identification and selection, and treatment selection. Main limitations include difficulties in obtaining tumor tissue from relapse, target prioritization, and availability of optimal drugs in the context of CNS disease and related molecular alterations. This figure features pictures from ‘Servier medical art’ by Servier, used under Creative Commons Attribution 3.0 France.
In trials that have failed despite molecular enrichment [50], other potential sources of failures have to be considered. As previously mentioned, the marked heterogeneity and plasticity of glioblastoma cells are likely major factors mediating the currently observed resistance to targeted therapies [31, 40, 205]. As an illustration, in a phase II trial, analyses of tissues from glioblastoma patients treated with gefitinib before debulking surgery revealed significant intratumoral accumulation of gefitinib associated with dephosphorylation of EGFR, while downstream canonical pathways were not significantly dephosphorylated when compared with untreated controls [31, 40, 206]. This indicated concomitant activation of redundant cell signaling pathways, a resistance mechanism observed in EGFR-driven glioma models [205]. This clearly implies that exploring combinations of targeted therapies to avoid emergence of resistant subclones is needed (Figure 1). Moreover, future studies should explore approaches that have the potential to more broadly inhibit tumor cell growth and survival [207, 208]. Agents that more broadly target pathways rather than single mutation variants have the potential to improve outcome in a much wider population of patients, even in the absence of actionable mutation targets identified by genomic profiling. As an illustration, novel MDM2 inhibitors have been reported to inhibit the growth of TP53-wild-type glioblastoma PDCLs, regardless of the tumor MDM2 amplification status [117, 118]. However, such approaches are expected to go along with more side effects. Other examples include synthetic lethal approaches and immunotherapy, which are investigated in large trials (Table 3).
Regarding drug relevance, most of the tested agents were neither primarily designed to inhibit alterations that are specific to glioblastoma, nor developed for targeting tumors located in the brain. Most currently available agents display inadequate pharmacokinetic properties due to poor crossing of the BBB [209–211]. The BBB is universally disrupted in glioblastomas but not necessarily within more infiltrative non-enhancing areas of the tumor. Given this mixed BBB setting, novel agents should be optimized for brain penetration. Other approaches include the use of tailored regimens (e.g. higher doses in pulsed schedules) and other strategies to actively break down the BBB (e.g. transient opening of the BBB by pulsed ultrasound) [212, 213], which may improve drug delivery and target inhibition using agents that are unlikely to adequately penetrate the tumor. In this context, having a molecular assay that confirms effective modulation of the target in the tumor is essential; otherwise conclusions on relevance of the target will remain elusive. Novel trial designs should more often incorporate tissue biomarkers collection during treatment, enabling evaluation of pharmacodynamics markers.
Novel biomarker-driven trial designs
Overall, considering the lack of clear demonstration of the benefit of targeted therapies in glioblastoma, proof-of concept in well molecularly characterized populations should be established in early phase and small-randomized phase II trials before further evaluation in registration trials. Academic groups and industry should collaborate in order to identify: (i) the best targets, drugs/combinations to be tested in clinical trials; (ii) the best population and (iii) the best biomarkers. Within the context of more precise and systematic molecular characterization of glioblastoma and increasing availability of novel targeted therapies, novel trial designs will be essential to more rapidly test agents. Practical implications for such precision medicine studies are represented in Figure 2.
A popular design is the ‘basket trial’ that involves screening of patients with cancer independent of tumor histology, for recruitment of a specific and often rare molecularly-defined population. A recently reported basket phase II trial evaluating vemurafenib in several BRAFV600-mutant non-melanoma tumors reported responses in high-grade glioma patients [105]. Similarly, crizotinib is currently investigated in MET-amplified glioblastomas (NCT02034981) as part of a larger trial with 23 molecularly defined cohorts. However, basket trials require robust preclinical studies to identify relevant biomarkers that will predict treatment response with high confidence, and well-established diagnostic assays available in real-time for patient selection [32, 214–220] (listed in supplementary Table S1, available at Annals of Oncology online). Moreover, such trial designs can present a major challenge when the molecular alterations in question are rare, requiring such trials to screen and reject a high number of patients who are then disappointed.
Other new approaches are multi-arm ‘master protocol’ and ‘umbrella’ trials, which most commonly involve screening for multiple targets [221], arms and agents, and yield added benefit that a higher proportion of patients may enter into the trial once screened. Such trials may include randomization between ‘standard’ and ‘molecularly tailored’ treatment arms, allowing assessing the utility of precision medicine approaches. These designs have been aided by translation of modern methods of high-throughput multiplex diagnostic assays, allowing to simultaneously measuring a host of targets using platforms such as targeted exomes or CGH/SNP arrays [208, 222]. These are now commonplace in an increasing number of centers and aid designing novel trials based on systematic molecular screening programs for treatment stratification (Figure 2). As an illustration, personalized medicine trials such as MOSCATO 02 (NCT01566019) and INSIGhT (NCT02977780) studies are currently evaluating the feasibility and the utility of genomic profiling to inform treatment decisions in patients with glioblastoma.
Conclusion
An improved understanding of the molecular pathways that drive malignancy in glioblastoma has led to the development of various biomarkers and several agents targeting specific molecular pathways in malignant cells. The concept of precision medicine driven by molecular stratification for the treatment of glioblastoma is appealing and scientifically sound; however, no evidence has yet demonstrated an improved patient outcome within the context of this disease, likely as a result of both scientific and logistical challenges that have hampered the success of clinical trials. The identification of relevant driver molecular events and highly bioactive and specific drugs remain the biggest challenges. With the recent incorporation in clinical practice of modern methods allowing molecular characterization and appropriate stratification of patients, there is hope that novel trials evaluating targeted therapies may be more effective. Identification of relevant targets, compounds and biomarkers for appropriate patient selection during early phase trials are essential for successful development of novel therapies.
Funding
MT receives research grants from Fondation pour la Recherche Médicale (FDM 41635). KLL receives funding from NIH (R01CA188288, R01 CA170592, P50 CA165962), The Ivy Foundation, The PLGA Foundation, and ABC2 Foundation.
Disclosure
MT and MS have declared no conflicts of interest. AI reports grants from Fondation ARC pour la recherche sur le Cancer, other from IntselChimos, personal fees from Novartis (janv 2014), other from Hoffmann-La Roche, other from Beta-Innov (Juillet 2014), personal fees from Lettre du Cancérologue, other from CArthera, personal fees from BMS (nov 2015), personal fees from Roche (dec 2015), personal fees from Cipla (dec 2015) (certified continuing education), outside the submitted work. DFCI receives funding for KLL's research with the following entities: Amgen, Tragara and X4. DFCI and KLL have patents related to molecular diagnostics of cancer.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Liao P. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2014; 16(Suppl 4): iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 3. Rønning PA, Helseth E, Meling TR, Johannesen TB.. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol 2012; 14: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner AA. et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 2015; 314: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 5. Weller M, van den Bent M, Hopkins K. et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014; 15: e395–e403. [DOI] [PubMed] [Google Scholar]
- 6. Phillips HS, Kharbanda S, Chen R. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006; 9: 157–173. [DOI] [PubMed] [Google Scholar]
- 7. Parsons DW, Jones S, Zhang X. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Network CGAR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noushmehr H, Weisenberger DJ, Diefes K. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010; 17: 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brennan CW, Verhaak RG, McKenna A. et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155: 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frattini V, Trifonov V, Chan JM. et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet 2013; 45: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louis DN, Perry A, Reifenberger G. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 13. Verhaak RG, Hoadley KA, Purdom E. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshihara K, Wang Q, Torres-Garcia W. et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 2015; 34: 4845–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ceccarelli M, Barthel FP, Malta TM. et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 2016; 164: 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babu R, Adamson DC.. Rindopepimut: an evidence-based review of its therapeutic potential in the treatment of EGFRvIII-positive glioblastoma. Core Evid 2012; 7: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohle D, Popovici-Muller J, Palaskas N. et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013; 340: 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reardon DA, Wen PY, Mellinghoff IK.. Targeted molecular therapies against epidermal growth factor receptor: past experiences and challenges. Neuro Oncol 2014; 16(Suppl 8): viii7–vii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas AA, Brennan CW, DeAngelis LM, Omuro AM.. Emerging therapies for glioblastoma. JAMA Neurol 2014; 71: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 20. Gallego O, Cuatrecasas M, Benavides M. et al. Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry. J Neurooncol 2014; 116: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Stefano AL, Fucci A, Frattini V. et al. Detection, characterization and inhibition of FGFR-TACC fusions in IDH wild type glioma. Clin Cancer Res 2015; 21: 3307–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer M, Reimand J, Lan X. et al. Single cell-derived clonal analysis of human glioblastoma links functional and genomic heterogeneity. Proc Natl Acad Sci USA 2015; 112: 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prados MD, Byron SA, Tran NL. et al. Toward precision medicine in glioblastoma: the promise and the challenges. Neuro Oncol 2015; 17: 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snuderl M, Fazlollahi L, Le LP. et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 2011; 20: 810–817. [DOI] [PubMed] [Google Scholar]
- 25. Lass U, Nümann A, von Eckardstein K. et al. Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1- mutation as common tumor initiating event. PLoS One 2012; 7: e41298.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Little SE, Popov S, Jury A. et al. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer Res 2012; 72: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 27. Sottoriva A, Spiteri I, Piccirillo SG. et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 2013; 110: 4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson BE, Mazor T, Hong C. et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014; 343: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel AP, Tirosh I, Trombetta JJ. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014; 344: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francis JM, Zhang CZ, Maire CL. et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov 2014; 4: 956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nathanson DA, Gini B, Mottahedeh J. et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 2014; 343: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim H, Zheng S, Amini SS. et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res 2015; 25: 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim J, Lee IH, Cho HJ. et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell 2015; 28: 318–328. [DOI] [PubMed] [Google Scholar]
- 34. Yap TA, Gerlinger M, Futreal PA. et al. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med 2012; 4: 127ps110.. [DOI] [PubMed] [Google Scholar]
- 35. Burrell RA, McGranahan N, Bartek J, Swanton C.. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013; 501: 338–345. [DOI] [PubMed] [Google Scholar]
- 36. McGranahan N, Favero F, de Bruin EC. et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med 2015; 7: 283ra254.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Touat M, Dhermain F, André F, Sanson M.. Adapting the drivers to the road: a new strategy for cancer evolution? Ann Oncol 2015; 26: 827–829. [DOI] [PubMed] [Google Scholar]
- 38. Wang J, Cazzato E, Ladewig E. et al. Clonal evolution of glioblastoma under therapy. Nat Genet 2016; 48: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stommel JM, Kimmelman AC, Ying H. et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007; 318: 287–290. [DOI] [PubMed] [Google Scholar]
- 40. Szerlip NJ, Pedraza A, Chakravarty D. et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci USA 2012; 109: 3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stevens MM, Maire CL, Chou N. et al. Drug sensitivity of single cancer cells is predicted by changes in mass accumulation rate. Nat Biotechnol 2016; 34: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishikawa R, Ji XD, Harmon RC. et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 1994; 91: 7727–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frederick L, Wang XY, Eley G, James CD.. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000; 60: 1383–1387. [PubMed] [Google Scholar]
- 44. Maire CL, Ligon KL.. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro Oncol 2014; 16(Suppl 8): viii1–viii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vivanco I, Robins HI, Rohle D. et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2012; 2: 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schulte A, Liffers K, Kathagen A. et al. Erlotinib resistance in EGFR-amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110δ. Neuro Oncol 2013; 15: 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sampson JH, Heimberger AB, Archer GE. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010; 28: 4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schuster J, Lai RK, Recht LD. et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol 2015; 17: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reardon DA, Desjardins A, Schuster J. et al. IMCT-08ReACT: long-term survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. Neuro Oncol 2015; 17(Suppl 5): v109. [Google Scholar]
- 50. Weller M, Butowski N, Tran D. et al. ATIM-03. ACT IV: an international, double-blind, phase 3 trial of rindopepimut in newly diagnosed, EGFRvIII-expressing glioblastoma. Neuro-Oncology 2016; 18(Suppl 6): vi17–vi18. [DOI] [PubMed] [Google Scholar]
- 51. Phillips AC, Boghaert ER, Vaidya KS. et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther 2016; 15: 661–669. [DOI] [PubMed] [Google Scholar]
- 52. van den Bent M, Gan H, Lassman A. et al. ACTR-07. Efficacy of a novel antibody-drug conjugate (ADC), ABT-414, as monotherapy in epidermal growth factor receptor (EGFR) amplified (EGFRamp), recurrent glioblastoma (rGBM). Neuro-Oncology 2016; 18(Suppl 6): vi2. [Google Scholar]
- 53. Rich JN, Reardon DA, Peery T. et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 2004; 22(1): 133–142. [DOI] [PubMed] [Google Scholar]
- 54. Franceschi E, Cavallo G, Lonardi S. et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer 2007; 96(7): 1047–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van den Bent MJ, Brandes AA, Rampling R. et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol 2009; 27: 1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neyns B, Sadones J, Joosens E. et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 2009; 20: 1596–1603. [DOI] [PubMed] [Google Scholar]
- 57. Thiessen B, Stewart C, Tsao M. et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol 2010; 65: 353–361. [DOI] [PubMed] [Google Scholar]
- 58. Raizer JJ, Abrey LE, Lassman AB. et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol 2010; 12: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yung WK, Vredenburgh JJ, Cloughesy TF. et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol 2010; 12: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uhm JH, Ballman KV, Wu W. et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int J Radiat Oncol Biol Phys 2011; 80: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lv S, Teugels E, Sadones J. et al. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int J Oncol 2012; 41: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 62. Reardon DA, Nabors LB, Mason WP. et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro Oncol 2015; 17: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sathornsumetee S, Desjardins A, Vredenburgh JJ. et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol 2010; 12: 1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hasselbalch B, Lassen U, Hansen S. et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol 2010; 12: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reardon DA, Groves MD, Wen PY. et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res 2013; 19: 900–908. [DOI] [PubMed] [Google Scholar]
- 66. Reardon DA, Quinn JA, Vredenburgh JJ. et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res 2006; 12: 860–868. [DOI] [PubMed] [Google Scholar]
- 67. Doherty L, Gigas DC, Kesari S. et al. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology 2006; 67: 156–158. [DOI] [PubMed] [Google Scholar]
- 68. Reardon DA, Desjardins A, Vredenburgh JJ. et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol 2010; 96: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wen PY, Chang SM, Lamborn KR. et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol 2014; 16: 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kreisl TN, Lassman AB, Mischel PS. et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol 2009; 92: 99–105. [DOI] [PubMed] [Google Scholar]
- 71. Peereboom DM, Ahluwalia MS, Ye X. et al. NABTT 0502: a phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol 2013; 15: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Prados MD, Lamborn KR, Chang S. et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol 2006; 8: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Prados MD, Yung WK, Wen PY. et al. Phase-1 trial of gefitinib and temozolomide in patients with malignant glioma: a North American brain tumor consortium study. Cancer Chemother Pharmacol 2008; 61: 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brown PD, Krishnan S, Sarkaria JN. et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol 2008; 26: 5603–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krishnan S, Brown PD, Ballman KV. et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int J Radiat Oncol Biol Phys 2006; 65: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 76. Prados MD, Chang SM, Butowski N. et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 2009; 27: 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peereboom DM, Shepard DR, Ahluwalia MS. et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol 2010; 98: 93–99. [DOI] [PubMed] [Google Scholar]
- 78. Chakravarti A, Wang M, Robins HI. et al. RTOG 0211: a phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys 2013; 85: 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Clarke JL, Molinaro AM, Phillips JJ. et al. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol 2014; 16: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Seystahl K, Wick W, Weller M.. Therapeutic options in recurrent glioblastoma—an update. Crit Rev Oncol Hematol 2016; 99: 389–408. [DOI] [PubMed] [Google Scholar]
- 81. Singh D, Chan JM, Zoppoli P. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012; 337: 1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tabernero J, Bahleda R, Dienstmann R. et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 2015; 33: 3401–3408. [DOI] [PubMed] [Google Scholar]
- 83. Touat M, Ileana E, Postel-Vinay S. et al. Targeting FGFR Signaling in Cancer. Clin Cancer Res 2015; 21: 2684–2694. [DOI] [PubMed] [Google Scholar]
- 84. Lassman AB, Pugh SL, Gilbert MR. et al. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol 2015; 17: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wen PY, Yung WK, Lamborn KR. et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res 2006; 12: 4899–4907. [DOI] [PubMed] [Google Scholar]
- 86. Reardon DA, Dresemann G, Taillibert S. et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer 2009; 101: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Razis E, Selviaridis P, Labropoulos S. et al. Phase II study of neoadjuvant imatinib in glioblastoma: evaluation of clinical and molecular effects of the treatment. Clin Cancer Res 2009; 15: 6258–6266. [DOI] [PubMed] [Google Scholar]
- 88. Dresemann G, Weller M, Rosenthal MA. et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol 2010; 96: 393–402. [DOI] [PubMed] [Google Scholar]
- 89. Holdhoff M, Supko JG, Gallia GL. et al. Intratumoral concentrations of imatinib after oral administration in patients with glioblastoma multiforme. J Neurooncol 2010; 97: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li Y, Li A, Glas M. et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci USA 2011; 108: 9951–9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jahangiri A, De Lay M, Miller LM. et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 2013; 19: 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Petterson SA, Dahlrot RH, Hermansen SK. et al. High levels of c-Met is associated with poor prognosis in glioblastoma. J Neurooncol 2015; 122: 517–527. [DOI] [PubMed] [Google Scholar]
- 93. Johnson J, Ascierto ML, Mittal S. et al. Genomic profiling of a Hepatocyte growth factor-dependent signature for MET-targeted therapy in glioblastoma. J Transl Med 2015; 13: 306.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wen PY, Schiff D, Cloughesy TF. et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 2011; 13: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chi AS, Batchelor TT, Kwak EL. et al. Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal-epithelial transition inhibitor. J Clin Oncol 2012; 30: e30–e33. [DOI] [PubMed] [Google Scholar]
- 96. Le Rhun E, Chamberlain MC, Zairi F. et al. Patterns of response to crizotinib in recurrent glioblastoma according to ALK and MET molecular profile in two patients. CNS Oncol 2015; 4: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cloughesy T, Finocchiaro G, Belda-Iniesta C. et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O(6)-methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol 2016; 35: 343–351. [DOI] [PubMed] [Google Scholar]
- 98. Galanis E, Buckner JC, Maurer MJ. et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 2005; 23: 5294–5304. [DOI] [PubMed] [Google Scholar]
- 99. Lassen U, Sorensen M, Gaziel TB. et al. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res 2013; 33: 1657–1660. [PubMed] [Google Scholar]
- 100. Ma DJ, Galanis E, Anderson SK. et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol 2015; 17: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pitz MW, Eisenhauer EA, MacNeil MV. et al. Phase II study of PX-866 in recurrent glioblastoma. Neuro Oncol 2015; 17: 1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Massacesi C, Di Tomaso E, Urban P. et al. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther 2016; 9: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dias-Santagata D, Lam Q, Vernovsky K. et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One 2011; 6: e17948.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol 2013; 114: 237–240. [DOI] [PubMed] [Google Scholar]
- 105. Hyman DM, Puzanov I, Subbiah V. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015; 373: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hainsworth JD, Ervin T, Friedman E. et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer 2010; 116: 3663–3669. [DOI] [PubMed] [Google Scholar]
- 107. Galanis E, Anderson SK, Lafky JM. et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res 2013; 19: 4816–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Reardon DA, Vredenburgh JJ, Desjardins A. et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol 2011; 101: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zustovich F, Landi L, Lombardi G. et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: a phase II study. Anticancer Res 2013; 33: 3487–3494. [PubMed] [Google Scholar]
- 110. Lee EQ, Kuhn J, Lamborn KR. et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol 2012; 14: 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hottinger AF, Aissa AB, Espeli V. et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br J Cancer 2014; 110: 2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vousden KH, Lane DP.. p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283. [DOI] [PubMed] [Google Scholar]
- 113. Sidransky D, Mikkelsen T, Schwechheimer K. et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature 1992; 355: 846–847. [DOI] [PubMed] [Google Scholar]
- 114. Bögler O, Huang HJ, Cavenee WK.. Loss of wild-type p53 bestows a growth advantage on primary cortical astrocytes and facilitates their in vitro transformation. Cancer Res 1995; 55: 2746–2751. [PubMed] [Google Scholar]
- 115. Reifenberger G, Liu L, Ichimura K. et al. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 1993; 53: 2736–2739. [PubMed] [Google Scholar]
- 116. Duffy MJ, Synnott NC, McGowan PM. et al. p53 as a target for the treatment of cancer. Cancer Treat Rev 2014; 40: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 117. Costa B, Bendinelli S, Gabelloni P. et al. Human glioblastoma multiforme: p53 reactivation by a novel MDM2 inhibitor. PLoS One 2013; 8: e72281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Verreault M, Schmitt C, Goldwirt L. et al. Preclinical efficacy of the MDM2 inhibitor RG7112 in MDM2-amplified and TP53 wild-type glioblastomas. Clin Cancer Res 2016; 22: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ohgaki H, Kleihues P.. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci 2009; 100: 2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Michaud K, Solomon DA, Oermann E. et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 2010; 70: 3228–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wiedemeyer WR, Dunn IF, Quayle SN. et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci USA 2010; 107: 11501–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cen L, Carlson BL, Schroeder MA. et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol 2012; 14: 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Barton KL, Misuraca K, Cordero F. et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One 2013; 8: e77639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mir SE, De Witt Hamer PC, Krawczyk PM. et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 2010; 18: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Witt Hamer PC, Mir SE, Noske D. et al. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res 2011; 17: 4200–4207. [DOI] [PubMed] [Google Scholar]
- 126. Toledo CM, Ding Y, Hoellerbauer P. et al. Genome-wide CRISPR-Cas9 Screens Reveal Loss of Redundancy between PKMYT1 and WEE1 in Glioblastoma Stem-like Cells. Cell Rep 2015; 13: 2425–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gupta SK, Mladek AC, Carlson BL. et al. Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomide-sensitive versus -resistant glioblastoma multiforme xenografts. Clin Cancer Res 2014; 20: 3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Venere M, Hamerlik P, Wu Q. et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ 2014; 21: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Robins HI, Zhang P, Gilbert M. et al. ATCT-27NRG Oncology/RTOG 0929: a randomized phase I/II study of ABT-888IN combination with temozolomide in recurrent temozolomide resistant glioblastoma. Neuro Oncol 2015; 17(Suppl 5): v7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yan H, Parsons DW, Jin G. et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hartmann C, Meyer J, Balss J. et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009; 118: 469–474. [DOI] [PubMed] [Google Scholar]
- 132. Mondesir J, Willekens C, Touat M. et al. IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. J Blood Med 2016; 7: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Turcan S, Rohle D, Goenka A. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012; 483: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mellinghoff IK, Touat M, Maher E. et al. ACTR-46. AG120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: results from the phase 1 glioma expansion cohorts. Neuro-Oncology 2016; 18(suppl 6): vi12. [Google Scholar]
- 135. Schumacher T, Bunse L, Pusch S. et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014; 512: 324–327. [DOI] [PubMed] [Google Scholar]
- 136. Pellegatta S, Valletta L, Corbetta C. et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun 2015; 3: 4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Xu J, Sampath D, Lang FF. et al. Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J Neurooncol 2011; 105: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cornago M, Garcia-Alberich C, Blasco-Angulo N. et al. Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis 2014; 5: e1435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grasso CS, Tang Y, Truffaux N. et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 2015; 21: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Galanis E, Jaeckle KA, Maurer MJ. et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 2009; 27: 2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee EQ, Puduvalli VK, Reid JM. et al. Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American Brain Tumor Consortium Study 04-03. Clin Cancer Res 2012; 18: 6032–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Puduvalli VK, Wu J, Yuan Y, Armstrong TS. et al. Brain Tumor Trials Collaborative Bayesian Adaptive Randomized Phase II trial of bevacizumab plus vorinostat versus bevacizumab alone in adults with recurrent glioblastoma (BTTC-1102). 2015 ASCO Annual Meeting. J Clin Oncol 2015; 33(suppl; abstr 2012). [Google Scholar]
- 143. Lee EQ, Reardon DA, Schiff D. et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol 2015; 17: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cheng Z, Gong Y, Ma Y. et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res 2013; 19: 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. de Vries NA, Hulsman D, Akhtar W. et al. Prolonged Ezh2 depletion in glioblastoma causes a robust switch in cell fate resulting in tumor progression. Cell Rep 2015; 14: S2211–S1247. [DOI] [PubMed] [Google Scholar]
- 146. Pastori C, Kapranov P, Penas C. et al. The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc Natl Acad Sci USA 2015; 112: 8326–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bai H, Harmancı AS, Erson-Omay EZ. et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet 2016; 48: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Friedman HS, Prados MD, Wen PY. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27: 4733–4740. [DOI] [PubMed] [Google Scholar]
- 149. Kreisl TN, Kim L, Moore K. et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009; 27: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. de Groot JF, Lamborn KR, Chang SM. et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol 2011; 29: 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Neyns B, Sadones J, Chaskis C. et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol 2011; 103: 491–501. [DOI] [PubMed] [Google Scholar]
- 152. Kreisl TN, Smith P, Sul J. et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol 2013; 111: 41–48. [DOI] [PubMed] [Google Scholar]
- 153. Hutterer M, Nowosielski M, Haybaeck J. et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neuro Oncol 2014; 16: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Batchelor TT, Duda DG, di Tomaso E. et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 2010; 28: 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Batchelor TT, Mulholland P, Neyns B. et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 2013; 31: 3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lee EQ, Kaley TJ, Duda DG. et al. A multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma patients. Clin Cancer Res 2015; 21: 3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Muhic A, Poulsen HS, Sorensen M. et al. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol 2013; 111: 205–212. [DOI] [PubMed] [Google Scholar]
- 158. Stupp R, Hegi ME, Gorlia T. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 159. Nabors LB, Fink KL, Mikkelsen T. et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol 2015; 17: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wick W, Puduvalli VK, Chamberlain MC. et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010; 28: 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Taal W, Oosterkamp HM, Walenkamp AM. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 2014; 15: 943–953. [DOI] [PubMed] [Google Scholar]
- 162. Chinot OL, Wick W, Mason W. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014; 370: 709–722. [DOI] [PubMed] [Google Scholar]
- 163. Gilbert MR, Dignam JJ, Armstrong TS. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Galanis E, Anderson SK, Anastasiadis P. et al. NCCTG N0872 (Alliance): a randomized placebo-controlled phase II trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma (GBM). 2015 ASCO Annual Meeting. J Clin Oncol 2015; 33(suppl; abstr 2004). [Google Scholar]
- 165. Duerinck J, Du Four S, Vandervorst F. et al. Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neurooncol 2016; 128: 147–155. [DOI] [PubMed] [Google Scholar]
- 166. Tanaka S, Louis DN, Curry WT. et al. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol 2013; 10: 14–26. [DOI] [PubMed] [Google Scholar]
- 167. Batchelor TT, Reardon DA, de Groot JF. et al. Antiangiogenic therapy for glioblastoma: current status and future prospects. Clin Cancer Res 2014; 20: 5612–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wick W, Platten M, Wick A. et al. Current status and future directions of anti-angiogenic therapy for gliomas. Neuro Oncol 2016; 18: 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wick W, Brandes A, Gorlia T. et al. LB-05phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol 2015; 17(Suppl 5): v1. [Google Scholar]
- 170. Sandmann T, Bourgon R, Garcia J. et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol 2015; 33: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hatterer E, Davoust N, Didier-Bazes M. et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood 2006; 107: 806–812. [DOI] [PubMed] [Google Scholar]
- 172. Iliff JJ, Wang M, Liao Y. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4: 147ra111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Louveau A, Smirnov I, Keyes TJ. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Aspelund A, Antila S, Proulx ST. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 176. Robert C, Schachter J, Long GV. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 177. Berghoff AS, Kiesel B, Widhalm G. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 2015; 17: 1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Nduom EK, Wei J, Yaghi NK. et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol 2016; 18: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fecci PE, Ochiai H, Mitchell DA. et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res 2007; 13: 2158–2167. [DOI] [PubMed] [Google Scholar]
- 180. Agarwalla P, Barnard Z, Fecci P. et al. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother 2012; 35: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Preusser M, Lim M, Hafler DA. et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 2015; 11: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Reardon DA, Gokhale PC, Klein SR. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res 2016; 4: 124–135. [DOI] [PubMed] [Google Scholar]
- 183. Reardon DA, Sampson JH, Sahebjam S. et al. Safety and activity of nivolumab (nivo) monotherapy and nivo in combination with ipilimumab (ipi) in recurrent glioblastoma (GBM): updated results from checkmate-143. 2016 ASCO Annual Meeting. J Clin Oncol 2016; 34(Suppl): abstr 2014. [Google Scholar]
- 184. Reardon DA, Kim T-M, Frenel J-S. et al. ATIM-35. Results of the phase IB KEYNOTE-028 multi-cohort trial of pembrolizumab monotherapy in patients with recurrent PD-L1-positive glioblastoma multiforme (GBM). Neuro-Oncology 2016; 18(Suppl 6): vi25–vi26. [Google Scholar]
- 185. Reardon D, Kaley T, Dietrich J. et al. ATIM-04. Phase 2 study to evaluate the clinical efficacy and safety of MEDI4736 (durvalumab [DUR]) in patients with glioblastoma (GBM): results for cohort B (DUR monotherapy), bevacizumab (BEV) naïve patients with recurrent GBM. Neuro-Oncology 2016; 18(Suppl 6): vi18. [Google Scholar]
- 186. Omuro A, Vlahovic G, Baehring J. et al. ATIM-16. Nivolumab combined with radiotherapy with or without temozolomide in patients with newly diagnosed glioblastoma: results from phase 1 safety cohorts in checkmate 143. Neuro-Oncology 2016; 18(suppl 6): vi21. [Google Scholar]
- 187. Snyder A, Makarov V, Merghoub T. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Van Allen EM, Miao D, Schilling B. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015; 350: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Rizvi NA, Hellmann MD, Snyder A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bouffet E, Larouche V, Campbell BB. et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 2016; 34: 2206–2211. [DOI] [PubMed] [Google Scholar]
- 192. Ardon H, Van Gool SW, Verschuere T. et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 2012; 61: 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Phuphanich S, Wheeler CJ, Rudnick JD. et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 2013; 62: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bloch O, Crane CA, Fuks Y. et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol 2014; 16: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hdeib A, Sloan AE.. Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax® platform in the treatment of glioblastoma multiforme. CNS Oncol 2015; 4: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Phuphanich S, Wheeler C, Rudnick J. et al. ATIM-25. Ten-year follow up with long term remission in patients with newly diagnosed glioblastoma (GBM) treated with ICT-107 vaccine (phase I). Neuro-Oncology 2016; 18(Suppl 6): vi23. [Google Scholar]
- 197. Reardon D, Peereboom D, Nabors B. et al. ATIM-11. Phase 2 trial of SL-701, a novel immunotherapy comprised of synthetic short peptides against GBM targets IL-13Rα2, EphA2, and survivin, in adults with second-line recurrent GBM: interim results. Neuro-Oncology 2016; 18(Suppl 6): vi20. [Google Scholar]
- 198. Johnson LA, Scholler J, Ohkuri T. et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015; 7: 275ra222.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. O'Rourke D, Desai A, Morrissette J. et al. IMCT-15PILOT study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. Neuro-Oncology 2015; 17(Suppl 5): v110–v111. [Google Scholar]
- 200. Rosenberg S, Verreault M, Schmitt C. et al. Multi-omics analysis of primary glioblastoma cell lines shows recapitulation of pivotal molecular features of parental tumors. Neuro Oncol 2016. [epub ahead of print]; now160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mellinghoff IK, Wang MY, Vivanco I. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 2005; 353: 2012–2024. [DOI] [PubMed] [Google Scholar]
- 202. Haas-Kogan DA, Prados MD, Tihan T. et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst 2005; 97: 880–887. [DOI] [PubMed] [Google Scholar]
- 203. Touat M, Duran-Peña A, Alentorn A. et al. Emerging circulating biomarkers in glioblastoma: promises and challenges. Expert Rev Mol Diagn 2015; 15: 1311–1323. [DOI] [PubMed] [Google Scholar]
- 204. Pentsova EI, Shah RH, Tang J. et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol 2016; 34: 2404–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Klingler S, Guo B, Yao J. et al. Development of resistance to EGFR-targeted therapy in malignant glioma can occur through EGFR-dependent and -independent mechanisms. Cancer Res 2015; 75: 2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hegi ME, Diserens AC, Bady P. et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib–a phase II trial. Mol Cancer Ther 2011; 10: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 207. Yu C, Mannan AM, Yvone GM. et al. High-throughput identification of genotype-specific cancer vulnerabilities in mixtures of barcoded tumor cell lines. Nat Biotechnol 2016; 34: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Xiu J, Piccioni D, Juarez T. et al. Multi-platform molecular profiling of a large cohort of glioblastomas reveals potential therapeutic strategies. Oncotarget 2016; 7: 21556–21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Oberoi RK, Parrish KE, Sio TT. et al. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro Oncol 2016; 18: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lassman AB, Rossi MR, Raizer JJ. et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res 2005; 11: 7841–7850. [DOI] [PubMed] [Google Scholar]
- 211. de Vries NA, Buckle T, Zhao J. et al. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs 2012; 30: 443–449. [DOI] [PubMed] [Google Scholar]
- 212. Dréan A, Goldwirt L, Verreault M. et al. Blood–brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev Neurother 2016; 16: 1285–1300. [DOI] [PubMed] [Google Scholar]
- 213. Carpentier A, Canney M, Vignot A. et al. Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci Transl Med 2016; 8: 343re342.. [DOI] [PubMed] [Google Scholar]
- 214. Andre F, Mardis E, Salm M. et al. Prioritizing targets for precision cancer medicine. Ann Oncol 2014; 25: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 215. Aldape KD, Ballman K, Furth A. et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol 2004; 63: 700–707. [DOI] [PubMed] [Google Scholar]
- 216. Capper D, Zentgraf H, Balss J. et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol 2009; 118: 599–601. [DOI] [PubMed] [Google Scholar]
- 217. Capper D, Weissert S, Balss J. et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 2010; 20: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Capper D, Preusser M, Habel A. et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 2011; 122: 11–19. [DOI] [PubMed] [Google Scholar]
- 219. Fischer I, de la Cruz C, Rivera AL, Aldape K.. Utility of chromogenic in situ hybridization (CISH) for detection of EGFR amplification in glioblastoma: comparison with fluorescence in situ hybridization (FISH). Diagn Mol Pathol 2008; 17: 227–230. [DOI] [PubMed] [Google Scholar]
- 220. Nikiforova MN, Wald AI, Melan MA. et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol 2016; 18: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Herbst RS, Gandara DR, Hirsch FR. et al. Lung master protocol (Lung-MAP)-a biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res 2015; 21: 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ramkissoon SH, Bi WL, Schumacher SE. et al. Clinical implementation of integrated whole-genome copy number and mutation profiling for glioblastoma. Neuro Oncol 2015; 17: 1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.