Abstract
Background
The expression of programmed death (PD) ligand 1 (PD-L1) protein expression assessed by immunohistochemistry (IHC) has been correlated with response and survival benefit from anti-PD-1/PD-L1 immune checkpoint inhibitor therapies in advanced non-small cell lung carcinoma (NSCLC). The efficacy of several agents appears correlated with PD-L1 expression. It remains controversial whether PD-L1 is prognostic in NSCLC. We assessed the prognostic value of PD-L1 IHC and its predictive role for adjuvant chemotherapy in early stage NSCLC.
Patients and methods
Tumor sections from three pivotal adjuvant chemotherapy trials (IALT, JBR.10, CALGB 9633) using the E1L3N antibody were studied in this pooled analysis. PD-L1 staining intensity and percentage in both tumor cells (TCs) and immune cells (ICs) were scored by two pathologists. The average or consensus PD-L1 expression levels across intensities and/or percent cells stained were correlated with clinicopathological and molecular features, patient survivals and potential benefit of adjuvant chemotherapy.
Results
Results from 982 patients were available for analysis. Considering staining at any intensities for overall PD-L1 expression, 314 (32.0%), 204 (20.8%) and 141 (14.3%) tumor samples were positive for PD-L1 staining on TCs using cut-offs at ≥1%, ≥10% and ≥25%, respectively. For PD-L1 expressing ICs, 380 (38.7%), 308 (31.4%) and 148 (15.1%) were positive at ≥ 1%, ≥10% and 25% cut-offs, respectively. Positive PD-L1 was correlated with squamous histology, intense lymphocytic infiltrate, and KRAS but not with TP53 mutation. EGFR mutated tumors showed statistically non-significant lower PD-L1 expression. PD-L1 expression was neither prognostic with these cut-offs nor other exploratory cut-offs, nor were predictive for survival benefit from adjuvant chemotherapy.
Conclusions
PD-L1 IHC is not a prognostic factor in early stage NSCLC patients. It is also not predictive for adjuvant chemotherapy benefit in these patients.
Keywords: immunotherapy, PD-L1, immune cells, checkpoint inhibitors, E1L3N antibody, LACE-Bio
Introduction
Anticancer immunotherapy targeting immune checkpoints with antibodies to programmed death-1 (PD-1) and its ligand PD-L1 is an established treatment modality for non-small cell lung cancer (NSCLC) [1–6]. PD-L1 protein expression assessed by immunochemistry (IHC) has emerged as a biomarker to select NSCLC patients for pembrolizumab therapy [2, 4, 5]. Data from the second-line phase 3 trials of nivolumab and atezolizumab in NSCLC showed increasingly greater efficacy compared with chemotherapy in patients whose tumors expressed PD-L1 on tumor cells (TCs) and/or immune cells (ICs) [3, 6]. Recently, first-line pembrolizumab has demonstrated significantly longer survival compared with chemotherapy for PD-L1 IHC positive NSCLC [5]. Trials of some of these agents have moved into the adjuvant setting for early stage resected NSCLC patients. In this setting, it will be important to know whether PD-L1 expression is a prognostic marker for these patients. To date, several institutional series assessing the latter have been reported, but the results have been inconsistent (Table 1). Furthermore, as adjuvant chemotherapy has become standard of care in stage II–IIIA patients, the potential impact of PD-L1 expression on adjuvant chemotherapy in operable lung cancer patients is also important to determine. There are different ways by which platinum may induce immunogenic cell death providing rational for immune checkpoint combinations with cisplatin therapy [7–9]. Recently, the phase 2 KEYNOTE 021 trial reported a potential enhancing effect of pembrolizumab with chemotherapy in first-line advanced NSCLC [10].
Table 1.
Published reports that have studied the prognostic value of PD-L1 expression in non-small cell lung cancer or its subtypes
| Author (year) | Ethnicity (country) | PD-L1 Ab | Ab nature (epitope) | Patient number | Sample type | Tumor histology | Tumor stage | Method | Staining feature | Cut-off | PD-L1 +/high patient (%) | Prognostic (OS) for +/High PD-L1 patients | Correlated with PD-L1 expression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Velcheti (2014)c | Caucasian (USA) | 5H1 (JHU) | Mouse MC (extra-cellular) | 155 | TMA | NSCLC | I-IV | QIF/AQUA | Tumor cell membrane | >normal lung | 36.1 | Good | Tumor infiltrating lymphocytes (TIL) |
| Caucasian (Greece) | 303 | I-IV | 24.8 | Good | |||||||||
| Schalper (2015) | Caucasian (USA) | E1L3N (CST) | Rabbit PC (intra-cellular) | 202 | TMA | NSCLC | I-IV | QIF/AQUA | Tumor cell membrane | Not specified | 16.9 | No | CD8+ TIL |
| Caucasian (Greece) | 350 | I-IV | 20.0 | No | CD8+ TIL | ||||||||
| Tang (2015) | East Asian (China) | E1L3N (CST) | 170 | Full section | NSCLC | IIB-IV | IHC | Membrane | H-score ≥5% | 65.9 | No | None with EGFR | |
| Kim (2015) | East Asian (Korean) | E1L3N (CST) | 331 | TMA | SqCC | I-III | IHC | Membrane | ≥10% (any intensity) | 26.9 | No | CD8+ TIL | |
| Schmidt (2015) | Caucasian (German) | E1L3N (CST) | 321 | TMA | NSCLC | I-III | IHC | Membrane | ≥5% 2 + (moderate) | 24 | No | None | |
| Amera-tunga (2016) | Caucasian (Australia) | E1L3N (CST) | 420 | TMA | NSCLC | I-III | IHC | Membrane | ≥5% 2 + (moderate) | 43.6 | No (AdC and SqCC) Poor in N1 Good in N2 | Strong PD-L1 (≥50%): None with KRAS, EGFR Yes with FOXP3+ | |
| ≥50% 2 + (moderate) | 23.8 | ||||||||||||
| Cooper (2015) | Caucasian (Australian) | 22C3 (Merck) | Mouse MC (extra-cellular) | 678 | TMA | NSCLC | I-III | IHC | Membrane | ≥50% (any intensity) | 7.7 | Good | Not with ALK, EGFR and KRAS |
| Sun (2016) | East Asian (Korea) | 22C3 (Merck) | 1070 | Full section | NSCLC | I-IV | IHC | Membrane | ≥1% (any intensity) | 45 | No | Not analyzed | |
| ≥50% (any intensity) | 6 | Borderline worse (P = 0.05) | |||||||||||
| Chen (2012) | East Asian (China) | 236A/E7 (abcam) | FOXP3 Ab (unknown) | 120 | Full section | NSCLC | I-III | IHC | Membrane and/or cytoplasm | IRSa ≥3 | 57.5 | Poor | None reported |
| Zhang (2014) | East Asian (China) | SAB 2900365 (Sigma- Aldrich) | Rabbit PC (unknown) | 143 | Full section | AdC | I-III | IHC | Membrane and cytoplasm | ‘Quickscore’ ≥8 (range 0–16) | 49 | Poor | Not with EGFR,KRAS, ALK |
| Yang (2014) | East Asian (Taiwan) | Proteintech | Rabbit PC (unknown) | 163 | Full section | AdC | I | IHC | Membrane | ≥5% (any intensity) | 39.9 | None | None with EGFR,KRAS, ALK, BRAF |
| Azuma (2014) | East Asian (Japan) | Lifespan Bioscience | Rabbit PC (unknown) | 164 | Full section | NSCLC | I-III | IHC | Membrane and cytoplasm | H-score >30% (median) | 50 | Poor | Yes with never smokers, AdC and EGFR mutant |
| Lin (2015) | East Asian (China) | ab58810 (Abcam) | Mouse MC (Center of CD274) | 63 | Biopsy | EGFR mutant AdCb | Advanced | IHC | Membrane and cytoplasm | Mean IRS scorea | 53.6 | Good | Not with CD4/CD8 TIL |
AdC, adenocarcinoma; JHU, Johns Hopkin University; CST, cell signaling technology; TMA, tissue microarray; NSCLC, non-small cell lung carcinoma; MC, monoclonal; PC, polyclonal; SqCC, squamous cell carcinoma.
IRS, intensity (0–3) × percentage stained cells: 0, negative; 1: 1%–10%, 2: 11%–50%, 3: 51%–80%, 4: ≥80%. 3: +1 in >50% cells, or 2+ in >10% cells, or 3+ in ≥1% of cells.
EGFR TKI treated.
Full references are provided in Supplementary (S) Materials.
The Lung Adjuvant Cisplatin Evaluation Biomarker (LACE-Bio) collaborative group has conducted several pooled analyses or validation studies on promising biomarkers in a large cohort of patients who participated in four pivotal adjuvant chemotherapy trials: IALT [11], ANITA, JBR.10, and CALGB 9633. These prospectively randomized controlled phase 3 trials provided a unique opportunity to study both the prognostic and predictive value of PD-L1 in early stage NSCLC, in light of many biological relations of immune checkpoint PD-L1/PD1 and chemotherapy. This study also provided the opportunity to gain insight into potential relationships of PD-L1 expression with several immune-related factors that previously have been investigated in the LACE-Bio patients, including tumor histology [11], lymphocytic infiltration [12], and mutation status of EGFR [13], KRAS [14], and TP53 [15].
Patients and methods
Patients and pathology materials
This study included only LACE-Bio patients from the IALT, JBR.10, and CALGB 9633 trials. Among 1608 patients in this cohort, 1008 patients had one representative formalin-fixed paraffin embedded tumor block available for assessment. Attrition due to lack of adequate tumor tissue in the block or technical failure, resulted in 982 patients with evaluable PD-L1 stained sections (supplementary Figure S1, available at Annals of Oncology online).
PD-L1 immunohistochemistry and scoring
PD-L1 immunohistochemistry (IHC) was carried out on 4 μm sections, using the E1L3N rabbit monoclonal antibody (Cell Signaling, Danvers, MA) on BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ). The details of staining protocols are described in Supplementary (S) Materials. Slides were assessed independently by the two study pathologists (EB, MST) for percent TCs showing membranous PD-L1 staining (TC) and for percent area of tumor infiltrating ICs showing PD-L1 staining (IC), at any intensity. IALT cases were screened by EB, and JBR.10/CALGB cases were screened by MST. Cases that showed TC or IC PD-L1 stained cells were identified for cross evaluation by the second pathologist (EB: JBR.10/CALGB; MT: IALT). When the scores of the two readers showed >20% difference, the slides were re-assessed independently by both pathologists. The final scores were the average of two closest scores by the two pathologists. The scoring was carried out blinded to clinical data/endpoints.
Statistical analysis
Overall survival (OS), the main endpoint, was defined as the time from randomization to death from any cause and disease-free survival (DFS) as the time from randomization to disease recurrence or death from any cause, whichever came first. The association of clinico-pathological variables and tumor and IC PD-L1 staining was studied using logistic regression stratified by trial. The prognostic value was estimated in the observation arm and its heterogeneity across histology and trial investigated by interaction terms. The predictive value was also estimated by adding an interaction term between treatment and PD-L1 and its heterogeneity across trials investigated. To be consistent with the PD-L1 cut-offs evaluated/adopted in various anti-PD1/PD-L1 therapeutic trials [2–6, 20] TC (1%, 25%, and 50%) and IC (1%, 10%, and 25%) were evaluated. P-values were two-sided and alpha level set to 5% for correlation analyses and 1% for prognostic and predictive analyses (pooled analysis). Additional details are provided in Supplementary Materials.
Results
Altogether 982 NSCLC patients had PD-L1 staining results available for the current analyses (supplementary Table S1, available at Annals of Oncology online). For these patients, the median follow-up was 5.3 years [range: 0.25–11.28] with 457 (46.5%) deaths and 520 (53.0%) events. The cohort had more males (72.6%) and T2 (76.1%), N0 (51.0%) patients, but had comparable proportions of squamous carcinoma and adenocarcinoma. However, 14% of patients were classified as other (not squamous or adenocarcinoma) NSCLC. EGFR mutation was found in 35 of the 300 (11.7%) adenocarcinoma patients successfully assayed, while KRAS mutation prevalence was at 33.0% (127/385) in adenocarcinoma. The characteristics of these 982 patients did not differ from 626 patients excluded because of lack of tissue or unsuccessful PD-L1 assessment, except for WHO PS (P ≤ 0.001), T-stage (P ≤ 0.001), and N-stage (P = 0.01) (supplementary Table S2, available at Annals of Oncology online). Excluded patients had more N0 tumors, WHO PS = 0.
PD-L1 IHC
Among the 982 patients, 314 (32.0%), 204 (20.8%), and 141 (14.3%) had TC of ≥1%, ≥25%, and ≥50%, respectively. For IC, 380 (38.7%), 308 (31.4%), and 148 (15.1%) had ≥1%, ≥10%, and ≥25%, respectively (supplementary Table S3, available at Annals of Oncology online).
Association of PD-L1 expression and clinical pathological factors
PD-L1 expression for TC and IC were not significantly associated in both univariate and multivariable analyses with sex, age, WHO PS, type of surgery, T and N of TNM, and stage, except for univariate N stage for IC 1% (supplementary Table S4, available at Annals of Oncology online). PD-L1 TC expression at all three cut-offs was significantly more prevalent in squamous than adenocarcinoma (univariate analysis). Similarly, significantly higher expression in squamous carcinoma also was observed for IC infiltrate at 1% and 10% cut-offs. Higher TC expression was noted in poorly differentiated NSCLC including large cell/adenosquamous/pleomorphic/sarcomatoid carcinomas (supplementary Table S4, available at Annals of Oncology online), but lower expression in large cell neuroendocrine carcinoma. Among adenocarcinoma subtypes, solid predominant tumors demonstrated significantly higher PD-L1 TC staining than other adenocarcinoma subtypes, for TC cut-offs 1%, 25%, and 50%, and IC at 1% and 10% (supplementary Table S5, available at Annals of Oncology online). Marked or intense tumor lymphocytic infiltration correlated marginally with greater frequency of PD-L1 expression on TC only in univariate analysis and significantly on IC in a multivariable analyses.
Association of PD-L1 expression and EGFR, KRAS and TP53 mutation
TC and IC PD-L1 staining was lower in EGFR mutant compared with wild-type (WT) adenocarcinoma, although the differences were not significant (supplementary Table S4, available at Annals of Oncology online). Conversely, KRAS mutant tumors showed significantly higher frequency of tumor PD-L1 expression (1% and 25%) compared with WT KRAS in multivariable analyses (see footnote of supplementary Table S4, available at Annals of Oncology online). PD-L1 staining was not significantly different between TP53 mutant and WT NSCLC.
Prognostic analyses of PD-L1 IHC
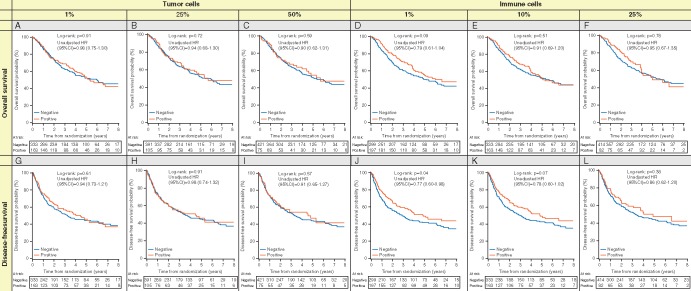
At 1% cut-off, the unadjusted OS curves in the observation arm (Figure 1A) showed no significant difference (TC PD-L1 negative vs positive log rank P = 0.91). Similar results were observed for DFS (Figure 1G; log rank P = 0.61). A marginal effect was found with IC for both OS (HR = 0.79, 95% CI 0.69–1.04, P = 0.09) and DFS (HR = 0.77, 95% CI 0.60–0.98, P = 0.04) (Figure 1D and J). Multivariable analyses confirmed the lack of significant prognostic value (Table 2). The proportional hazards assumption was not violated (data not shown). These results were homogeneous across histologies (supplementary Table S6, available at Annals of Oncology online) and trials (supplementary Table S7, available at Annals of Oncology online). With 10%, 25%, or 50% cut-off, no prognostic effect was observed either for TC (Figure 1B, C, H, and I) or IC (Figure 1E, F, K, and L), for OS (Figure 1B, C, E, and F) and DFS (Figure 1H, I, K, and L), respectively. Multivariable analyses confirmed the lack of prognostic value (Table 2) with a marginal heterogeneity across trials for OS and IC (1%) (P = 0.04) (supplementary Table S7, available at Annals of Oncology online).
Figure 1.
Kaplan–Meier plots showing lack of prognostic value of PD-L1 staining on tumor cell (TC) or immune cell (IC), using different pre-specified cut-offs (25% and 1%), in the observation arm patients (n = 496) with positive vs. negative PD-L1 staining. (A–D) overall survival; (E–H) disease-free survival.
Table 2.
Prognostic values of PD-L1-based markers (tumor and immune cells) estimated from a multivariable Cox regression model stratified by trial in the observation arm (n = 478)a
| Overall survival |
Disease-free survival |
|||||
|---|---|---|---|---|---|---|
| Cut-offs | No. deaths/ no. patients | HR for death [95% CI] | P-value | No. events/ no. patients | HR for event [95% CI] | P-value |
| Tumor cells (N = 478) | ||||||
| 1% | ||||||
| Negative | 151/319 | 1.00 | 0.93 | 179/319 | 1.00 | 0.82 |
| Positive | 73/159 | 1.01 [0.76–1.35] | 85/159 | 0.97 [0.74–1.27] | ||
| 25% | ||||||
| Negative | 178/375 | 1.00 | 0.96 | 208/375 | 1.00 | 0.62 |
| Positive | 46/103 | 0.99 [0.71–1.39] | 56/103 | 1.08 [0.80–1.47] | ||
| 50% | ||||||
| Negative | 193/405 | 1.00 | 0.86 | 226/405 | 1.00 | 0.98 |
| Positive | 31/73 | 0.96 [0.65–1.43] | 38/73 | 1.00 [0.70–1.44] | ||
| Immune cells (N = 478) | ||||||
| 1% | ||||||
| Negative | 144/286 | 1.00 | 0.10 | 169/286 | 1.00 | 0.10 |
| Positive | 80/192 | 0.79 [0.59–1.05] | 95/192 | 0.80 [0.62–1.05] | ||
| 10% | ||||||
| Negative | 155/320 | 1.00 | 0.50 | 187/320 | 1.00 | 0.16 |
| Positive | 69/158 | 0.90 [0.67–1.22] | 77/158 | 0.82 [0.62–1.08] | ||
| 25% | ||||||
| Negative | 187/400 | 1.00 | 0.97 | 224/400 | 1.00 | 0.54 |
| Positive | 37/78 | 0.99 [0.69–1.44] | 40/78 | 0.90 [0.63–1.27] | ||
HR, hazard ratio; CI, confidence interval.
Eighteen observations were excluded because of missing values for covariates.
Predictive analyses for adjuvant chemotherapy benefit
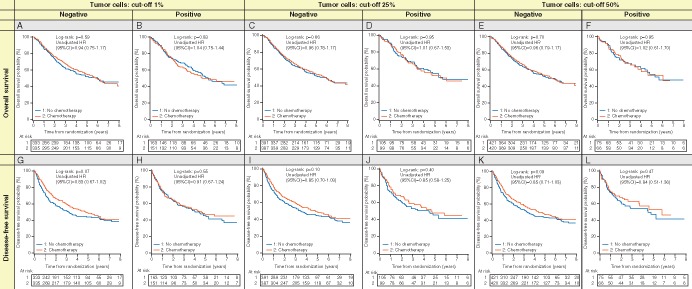
At 1% cut-off, interaction P-value TC was 0.78 and 0.83 for OS and DFS, respectively and for IC, was 0.13 and 0.12 for OS and DFS, respectively (Table 3; Figure 2A, B, G, and H). However, we observed marginal heterogeneity across trials for OS and IC (1%) (P = 0.06) (supplementary Table S8, available at Annals of Oncology online). For TC ≥25% and ≥50%, the OS multivariable hazard ratio (HR) for post-operative chemotherapy compared with observation for positive was 0.90 (95% CI 0.59–1.37) and 0.78 (95% CI 0.46–1.32), while the HR for negative was 1.00 (95% CI 0.81–1.23) and 1.01 (95%CI 0.83 = 1.24), respectively (Table 3; Figure 2C, D, E, and F). The interaction P-value was not significant (P = 0.67 and 0.37). Similar results were obtained for DFS (P-interaction 0.33 and 0.18, respectively) (Table 3; Figure 2I, J, K, and L). These results were homogeneous across trials (supplementary Table S8, available at Annals of Oncology online). No differences in HRs were observed for IC at 10% and 25% cut-offs, both for OS and DFS with interaction P-values ranging from 0.25 to 0.94 (Table 3; supplementary Figure S2C–F and I–L, available at Annals of Oncology online).
Table 3.
Predictive values of PD-L1-based markers (tumor and immune cells) estimated from a multivariable Cox regression model stratified by trial (n = 947)a
| Overall survival |
Disease-free survival |
|||||
|---|---|---|---|---|---|---|
| Chemotherapy |
Observation |
HR for death CT vs. no CT [95% CI] | Chemotherapy |
Observation |
HR for event CT vs. no CT [95% CI] | |
| No. deaths/No. patients | No. events/No. patients | |||||
| Tumor cells (N = 947) | ||||||
| 1% | ||||||
| Negative | 147/319 | 151/319 | 0.96 [0.76–1.21] | 163/319 | 179/319 | 0.86 [0.69–1.07] |
| Positive | 70/150 | 73/159 | 1.02 [0.73–1.41] | 74/150 | 85/159 | 0.90 [0.65–1.23] |
| HR for event Pos vs. neg [95% CI] | 1.08 [0.81–1.45] | 1.02 [0.77–1.36] | Interaction: P = 0.78 | 1.03 [0.78–1.35] | 0.99 [0.76–1.28] | Interaction: P = 0.83 |
| 25% | ||||||
| Negative | 173/371 | 178/375 | 1.00 [0.81–1.23] | 190/371 | 208/375 | 0.91 [0.75–1.11] |
| Positive | 44/98 | 46/103 | 0.90 [0.59–1.37] | 47/98 | 56/103 | 0.73 [0.49–1.08] |
| HR for event Pos vs. neg [95% CI] | 0.89 [0.64–1.25] | 0.99 [0.71–1.37] | Interaction: P = 0.67 | 0.88 [0.64–1.22] | 1.10 [0.81–1.48] | Interaction: P = 0.33 |
| 50% | ||||||
| Negative | 190/403 | 193/405 | 1.01 [0.83–1.24] | 209/403 | 226/405 | 0.92 [0.76–1.11] |
| Positive | 27/66 | 31/73 | 0.78 [0.46–1.32] | 28/66 | 38/73 | 0.64 [0.39–1.05] |
| HR for event Pos vs. neg [95% CI] | 0.76 [0.50–1.14] | 0.98 [0.66–1.45] | Interaction: P = 0.37 | 0.72 [0.48–1.07] | 1.04 [0.73–1.47] | Interaction: P = 0.18 |
| Immune cells (N = 947) | ||||||
| 1% | ||||||
| Negative | 132/288 | 144/286 | 0.87 [0.68–1.10] | 145/288 | 169/286 | 0.78 [0.62–0.97] |
| Positive | 85/181 | 80/192 | 1.17 [0.86–1.60] | 92/181 | 95/192 | 1.04 [0.78–1.39] |
| HR for event Pos vs. neg [95% CI] | 1.06 [0.80–1.41] | 0.79 [0.59–1.04] | Interaction: P = 0.13 | 1.06 [0.81–1.39] | 0.80 [0.62–1.03] | Interaction: P = 0.12 |
| 10% | ||||||
| Negative | 152/325 | 155/320 | 0.94 [0.75–1.18] | 166/325 | 187/320 | 0.81 [0.66–1.00] |
| Positive | 65/144 | 69/158 | 1.06 [0.75–1.49] | 71/144 | 77/158 | 1.02 [0.74–1.41] |
| HR for event Pos vs. neg [95% CI] | 1.03 [0.77–1.39] | 0.92 [0.68–1.23] | Interaction: P = 0.57 | 1.01 [0.76–1.35] | 0.81 [0.61–1.06] | Interaction: P = 0.25 |
| 25% | ||||||
| Negative | 190/403 | 187/400 | 0.98 [0.80–1.20] | 208/403 | 224/400 | 0.86 [0.71–1.04] |
| Positive | 27/66 | 37/78 | 0.96 [0.58–1.59] | 29/66 | 40/78 | 0.90 [0.55–1.46] |
| HR for event Pos vs. neg [95% CI] | 0.96 [0.64–1.44] | 0.98 [0.68–1.40] | Interaction: P = 0.94 | 0.90 [0.60–1.33] | 0.86 [0.61–1.21] | Interaction: P = 0.87 |
HR, hazard ratio; CI, confidence interval; CT, chemotherapy; no CT, observation; neg, negative (defined as < cut-off); pos, positive (defined as ≥ cut-off).
Thirty-five observations were excluded because of missing values for covariates.
Figure 2.
Kaplan–Meier plots showing lack of predictive value of PD-L1 staining on tumor cell, between chemotherapy and observation arms by tumor cells PD-L1 staining status (negative, positive). (A–D) overall survival; (E–H) disease-free survival.
Discussion
In this large and unique cohort of PD-L1 assessment in early stage resected NSCLC, we have demonstrated that PD-L1 expression in tumor and ICs is neither prognostic nor predictive for survival benefit from adjuvant platinum-based chemotherapy, using 1%, 25%, and 50% cut-offs for TC, and 1%, 10%, and 25% for IC staining. We also found that in multivariable correlation analyses, more frequent TC PD-L1 staining (1% and 25% cut-offs) was significantly associated with non-adenocarcinoma NSCLC and KRAS mutation, but not with EGFR or TP53 mutation; the latter 2 markers might be affected by the high number of missing data. While EGFR mutated tumors appear to show lower PD-L1 expression, the difference did not reach statistical significance. Greater IC PD-L1 expression was significantly associated only with intense lymphocytic infiltrate.
Many PD-L1 antibodies are available to study PD-L1 expression in tumors, but not all antibodies have been well characterized for their specificity. McLaughlin et al. [16] reported that among 9 antibodies (5H1, E1L3N, E1J2J, Abcam 58810, 29E.2A3, MIH1, 27A2, GTX89590, 015) tested for staining specificities, only three (5HI, E1L3N and E1J2J) were specific for PD-L1. However, four other antibodies (22C3, 28-8, SP142, and SP263) have been developed independently by different pharmaceutical/diagnostic companies as PD-L1 biomarker for their respective anti-PD1/PD-L1 agents [17]. Until recently, these latter antibodies except SP142 (Ventana, Tucson, AZ) were not available commercially for academic studies and evaluation. However, recently, Rimm et al. [18] compared the staining performance of E1L3N with 28-8 and 22C3 and found them to be comparable. We used the E1L3N antibody in our study, as when our study was initiated, the 22C3, 28-8, and SP-263 clones were not commercially available.
As anti-PD1 clinical trials are adopting their cut-offs regardless of staining intensity [3, 4], we focused our analyses using this approach and selecting 1% (second line nivolumab, pembrolizumab), 25% (proposed for durvalumab), and 50% (first line pembrolizumab) as cut-offs. With these cut-offs, TC was 32%, 20.8%, and 14.4% PD-L1 positive, respectively. While these rates were lower than those reported in advanced NSCLC from phase 3 nivolumab (CheckMate 017 and 057) [1, 3] and pembrolizumab (KEYNOTE 10) trials [4], they were within range of positivity rates reported in other studies that evaluated the prognostic value of PD-L1 staining in early stage NSCLC (Table 1; supplementary Table S9, available at Annals of Oncology online). Nevertheless, proper comparison of PD-L1 expression in advanced vs. early stage NSCLC tumors is warranted.
Using the 1%, 25%, and 50% cut-offs for TC and 1%, 10%, and 25% for IC, PD-L1 expression was not prognostic in the LACE-Bio patients who received surgery alone. Exploratory analyses were carried out to study the prognostic value of PD-L1 using different approaches (design variables formed from the quartiles, martingale residuals and data-oriented approach). In fact, exploratory analyses with 10% increments, as well as design variables formed from the quartiles, martingale residuals and data-oriented approach for studying the form of continuous markers (non-linear association), did not identify any cut-off that made PDL1 a prognostic factor (supplementary Figure S3, available at Annals of Oncology online). As additional prognostic analyses, we considered the proposed adenocarcinoma grading in 3 groups (low, intermediate and high grade).[19] While no patient was classified into low grade, 174 (17.7%) and 215 (21.9%) patients were classified in intermediate and high grade, respectively. Consistent with our previous report [11], we observed a significant overall prognostic value of histology (squamous cell carcinoma, adenocarcinoma intermediate grade, adenocarcinoma high grade, and other NSCLC) for DFS but not for OS, with high grade adenocarcinoma patients having significantly poorer prognosis. However, the prognostic value of PD-L1-based markers remains not significant when considering histology with adenocarcinoma histological subtypes (data not shown).
This study presented a unique opportunity to evaluate the potential role of PD-L1 expression in the benefit of adjuvant chemotherapy, a standard of care for stage II-IIIA NSCLC. We have shown that PD-L1 expression has no differential effect on the survival benefit from adjuvant chemotherapy in early stage completely resected NSCLC patients (Table 3). This information may be useful in the future when interpreting the results of immune checkpoint therapies in the adjuvant setting in the BR.31 [NCT02273375], KEYNOTE-091/PEARLS [NCT02504372], and ANVIL [NCT02595944] trials.
Our data showed that PD-L1 positivity was lower in EGFR mutant than in WT adenocarcinoma, although the differences did not reach statistical significance. In contrast, PD-L1 positivity at 1% and 25% TC cut-off was more frequent in patients with KRAS mutant NSCLC after multivariable adjustment. In CheckMate 057 [3], among the non-squamous NSCLC patients, subgroup analyses showed that nivolumab did not demonstrate survival benefit in patients with EGFR-mutated tumors compared with docetaxel. In contrast, survival benefit appeared to be greater in patients with KRAS mutant than WT tumors. However, it was unclear whether these differences were related to PD-L1 expression, as relationships between PD-L1 expression and EGFR and KRAS mutation status were not reported. In KEYNOTE-001 [2], PD-L1 expression was not different in EGFR mutant or WT tumors, while it was higher in patients with KRAS mutated (44.2%) compared with WT (26.8%) tumors. Among studies that used a validated PD-L1 antibody (Table 1), Cooper et al. [20] also observed lower frequency of PD-L1 positivity in EGFR mutated and higher positivity in KRAS mutant NSCLC, although the differences were not statistically significant due to low sample size. Calles et al. [21] recently reported that in KRAS-mutant lung cancer, strong PD-L1 expression was positively correlated with smoking status including pack-years. Interestingly, we found that TP53 mutation, which is commonly regarded as a guardian of genomic stability and DNA repair, has no relationship with PD-L1 expression.
In summary, using the pooled cohort of four pivotal randomized adjuvant chemotherapy trials, we have demonstrated that PD-L1 expression is neither prognostic nor predictive of adjuvant chemotherapy benefit in early stage NSCLC patients, regardless of the cut-offs chosen.
Supplementary Material
Acknowledgements
The authors thank Nicolas Lemaitre, Celia Barrachina, Ni Liu, and Jing Xu for technical assistance in performing the PD-L1 immunohistochemical staining.
Funding
The work was supported by grants from AstraZeneca Inc. (Cambridge, UK), Ligue Nationale Contre le Cancer (France), le Programme National d’Excellence Specialisé cancer du poumon de l’Institut National du Cancer (INCa) (France), technical platform of Direction de la Rechersche Clinique (DCRI), CHUGA, Grenoble (France), National Cancer Institute (USA), Canadian Cancer Society Research Institute (Canada), the Gustave Roussy Foundation, the Princess Margaret Cancer Foundation and the European contract EU—FP7 Curelung. No grant numbers apply.
Disclosure
MST: Consultancy honoraria from Merck Canada, AstraZeneca, Hoffmann-LaRoche, Bristol-Meyers Squibb. EB, GLT, CL, PH, TLC, SG, RK, and JCS: none; MF: Consultancy honoraria from AstraZeneca, Merck Sharpe & Dome, Novartis Boehringer-Ingelheim; RP: Consultancy honoraria from Merck Sharpe & Dome, Syntha; JPP: Research funding from Sanofi Aventis; LS: Stock ownership of AstraZeneca; Consultancy honoraria from Innate Pharma and Boehringer-Ingelheim; Research grants from Pfizer, AstraZeneca, Novartis, Innate Pharma, Astex Pharmaceutical. FAS: Stock ownership of Lilly; consultancy honoraria from Lilly, AstraZeneca, Merck; Research grant from Boehringer-Ingelheim. All remaining authors have declared no conflicts of interest.
Key message
The prognostic impact of PD-L1 immunohistochemistry in NSCLC patients is controversial. We assessed PD-L1 in tumors of patients involved in three pivotal adjuvant chemotherapy trials (LACE-Bio). PD-L1 expression is correlated with squamous histology, intense lymphocytic infiltrate, KRAS mutation but not TP53 mutation. PD-L1 expression is not prognostic or predictive for benefit from adjuvant chemotherapy.
Footnotes
These authors contributed equally to this work.
References
- 1. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Rodríguez-Abreu D, Robinson AG. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 6. Fehrenbacher L, Spira A, Ballinger M. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 7. Champiat S, Ileana E, Giaccone G. et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014; 9: 144–153. [DOI] [PubMed] [Google Scholar]
- 8. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ.. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014; 20: 2831–2837. [DOI] [PubMed] [Google Scholar]
- 9. Kodumudi KN, Woan K, Gilvary DL. et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010; 16: 4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langer CJ, Gadgeel SM, Borghaei H. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsao MS, Marguet S, Le Teuff G. et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in completely resected patients. J Clin Oncol 2015; 33: 3439–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brambilla E, Le Teuff G, Marguet S. et al. Prognostic effect of tumor-lymphocytic infiltration in resectable non-small cell lung cancer. J Clin Oncol 2016; 34: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soria JC, Brambilla E, Le Teuff G. et al. A pooled analysis to the prognosis and predictive value of EGFR mutation on survival in patients with KRAS wild-type lung adenocarcinoma. J Thorac Oncol 2011; 6(Suppl 6): S617. [Google Scholar]
- 14. Shepherd FA, Domerg C, Hainaut P. et al. A pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early stage resected non-small cell lung cancer infour trials of adjuvant chemotherapy. J Clin Oncol 2013; 31: 2173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma X, Le Teuff G, Lacas B. et al. Prognostic and predictive effect of TP53 mutations in non-small cell lung cancer patients from adjuvant cisplatin-based therapy randomized trials: a LACE-bio pooled analysis. J Thorac Oncol 2016; 11: 850–861. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin JF, Schalper K, Carvajal-Hausdorf DE. et al. Domain-specific PD-L1 protein measurement in non-small cell lung cancer (NSCLC). J Clin Oncol 2014; 32(Suppl): 5s (abstr 8064). [Google Scholar]
- 17. Kerr KM, Tsao M-S, Nicholson AG. et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 2015; 10: 985–989. [DOI] [PubMed] [Google Scholar]
- 18. Rimm D, Han G, Taube JM. et al. A prospective, multi-institutional assessment of four assays for PD-L1 expression in NSCLC by immunohistochemistry. J Thorac Oncol 2016; 11: S249. [Google Scholar]
- 19. Yoshizawa A, Motoi N, Riely GJ. et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011; 24: 653–664. [DOI] [PubMed] [Google Scholar]
- 20. Cooper WA, Tran T, Vilain RE. et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015; 89: 181–188. [DOI] [PubMed] [Google Scholar]
- 21. Calles A, Liao X, Sholl LM. et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol 2015; 10: 1726–1735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.