Abstract
Background
Patients with EGFR-mutant lung cancers treated with EGFR tyrosine kinase inhibitors (TKIs) develop clinical resistance, most commonly with acquisition of EGFR T790M. Evolutionary modeling suggests that a schedule of twice weekly pulse and daily low-dose erlotinib may delay emergence of EGFR T790M. Pulse dose erlotinib has superior central nervous system (CNS) penetration and may result in superior CNS disease control.
Methods
We evaluated toxicity, pharmacokinetics, and efficacy of twice weekly pulse and daily low-dose erlotinib. We assessed six escalating pulse doses of erlotinib.
Results
We enrolled 34 patients; 11 patients (32%) had brain metastases at study entry. We observed 3 dose-limiting toxicities in dose escalation: transaminitis, mucositis, and rash. The MTD was erlotinib 1200 mg days 1–2 and 50 mg days 3–7 weekly. The most frequent toxicities (any grade) were rash, diarrhea, nausea, fatigue, and mucositis. 1 complete and 24 partial responses were observed (74%, 95% CI 60–84%). Median progression-free survival was 9.9 months (95% CI 5.8–15.4 months). No patient had progression of an untreated CNS metastasis or developed a new CNS lesion while on study (0%, 95% CI 0–13%). Of the 18 patients with biopsies at progression, EGFR T790M was identified in 78% (95% CI 54–91%).
Conclusion
This is the first clinical implementation of an anti-cancer TKI regimen combining pulse and daily low-dose administration. This evolutionary modeling-based dosing schedule was well-tolerated but did not improve progression-free survival or prevent emergence of EGFR T790M, likely due to insufficient peak serum concentrations of erlotinib. This dosing schedule prevented progression of untreated or any new central nervous system metastases in all patients.
Keywords: EGFR-mutant lung cancer, acquired resistance, evolutionary modeling, EGFR T790M, pulse dose erlotinib
Key Messages
Evolutionary modeling and pre-clinical studies suggested that twice weekly pulse and daily low-dose erlotinib would be superior at delaying acquired resistance to erlotinib. A phase 1 study of this dosing schedule did not delay acquired resistance or prevent emergence of EGFR T790M but did prevent progression of CNS metastases in all patients treated.
Introduction
The majority of patients with EGFR-mutant lung cancers will respond to erlotinib, gefitinib or afatinib, but in less than a year develop resistance to further therapy with these agents [1, 2]. The most common mechanism of resistance is acquisition of an EGFR T790M mutation, identified in 60% of patients with acquired resistance to EGFR TKIs [3, 4]. Third generation EGFR TKIs, such as osimertinib, inhibit EGFR T790M and are effective at the time of progression on erlotinib, afatinib or gefitinib [5, 6]. However, acquired resistance occurs with these agents as well [7–9], with the central nervous system being a frequent site of disease progression. A parallel strategy to improve outcomes in patients with EGFR-mutant lung cancers is to adjust initial treatment to delay or prevent acquired resistance. While some have investigated EGFR TKIs in combination with other agents [10–12], modulating EGFR TKI dosing to prevent resistance has not been assessed.
Erlotinib was initially developed to inhibit wild-type EGFR [13], and hence the 150-mg daily dose was not optimized to prevent the development of drug resistance in patients whose tumors harbor EGFR mutations. Mathematical modeling can predict the evolutionary dynamics that result in proliferation of resistant clones, and suggest potential alternative dosing schedules to delay resistance [14, 15]. We used these methods to evaluate different dosing schedules of erlotinib and identified twice weekly high dose plus daily low-dose erlotinib as better able to delay progression in the setting of pre-existing resistant, EGFR T790M positive cells. The modeling dosing was confirmed in pre-clinical studies using 20 µM erlotinib/1 µM erlotinib [14].
We previously conducted a study of weekly high-dose erlotinib in unselected patients with advanced lung cancers [16]. The MTD was not reached at erlotinib 2000 mg once weekly. A separate phase 1 study of twice weekly pulse dose erlotinib identified the MTD as erlotinib 1000 mg twice weekly with a DLT of rash seen at higher dose levels [17]. Lower daily doses of EGFR TKIs may be as effective as higher doses with less toxicity in patients with EGFR-mutant lung cancers. Series of patients who received erlotinib 25 mg daily and gefitinib 250 mg every other day demonstrated similar progression-free survival compared to standard doses of erlotinib and gefitinib [18, 19]. The available clinical data suggest that EGFR TKI high pulse dosing is tolerable and low daily dosing is effective, but these have not previously been administered together in patients.
CNS involvement is a major issue for patients with EGFR-mutant lung cancers, with up to 60% of these patients developing brain or leptomeningeal metastases during their disease course [20, 21]. Although a benefit is commonly seen with EGFR TKIs when CNS disease is already present, duration of CNS control is less well-described. Up to 33% of patients with EGFR-mutant lung cancers have CNS progression during initial EGFR TKI therapy and in many patients, the CNS progression occurs in the setting of continued systemic control [20–22]. CNS-only progression may be a result of low drug concentrations, with CSF concentrations of erlotinib 3–5% of that in plasma [23]. Consequently, the CNS becomes a common site of disease progression due to inadequate drug delivery, not acquired drug resistance. Pulse erlotinib produces higher CSF concentrations and may be more effective in the treatment of CNS metastases [24]. With erlotinib 1500 mg once weekly, a peak plasma concentration of 11 300 nM was reached with a concurrent CSF concentration of 120 nM which is above the IC50 of erlotinib [25]. In one series, patients with EGFR-mutant lung cancers with CNS involvement were treated with a median dose of erlotinib 1500 mg once weekly. Six of nine had a partial response in the CNS [24].
Based on our evolutionary mathematical modeling and preclinical data, we tested the schedule of twice weekly pulse (high dose) erlotinib and daily erlotinib as initial treatment in patients with EGFR-mutant lung cancers. With this unique dosing schedule, we aimed to delay the development of acquired resistance.
Patients and methods
This trial was a prospective, open-label, single-center phase 1 dose-escalation study in patients with EGFR-mutant lung cancers. The primary endpoint of the study was the identification of the maximum tolerated dose of the combination of twice weekly high-dose and daily low-dose erlotinib. Secondary endpoints included the measurement of progression-free survival, overall survival, overall response rate, determination of CNS progression and pharmacokinetic analyses.
Patients had stage IV or recurrent EGFR-mutant lung adenocarcinomas and no prior treatment with an EGFR TKI. Prior cytotoxic chemotherapy was allowed. Patients were required to have measurable disease per RECIST (version 1.1). Patients must have had adequate organ function and a Karnofsky Performance Status ≥ 70%. Patients with clinically stable brain metastases, either treated or untreated, were eligible.
Study design
The study used a standard 3 + 3 dose escalation design. Three patients were enrolled at each dose level and assessed for one full cycle before dose escalation. No intra-patient dose escalation was allowed. Patients received pulse dose erlotinib on days 1 and 2, and erlotinib 50 mg oral daily for 5 days on days 3–7 which was repeated weekly to complete 21 day cycles. Cycle 1 was 4 weeks in duration with 1 week of lead-in pulse dose erlotinib only without daily low dose. For each dose level, the dose of pulse erlotinib on days 1–2 was escalated (supplementary Table S1, available at Annals of Oncology online). Patients who did not experience a dose-limiting toxicity (DLT) continued treatment at the assigned dose until progression of disease, unacceptable toxicity, or withdrawal of informed consent. Dose reductions of the pulse dose were allowed for toxicity, in 150 mg increments. Once the MTD dosing schedule was determined, the study included a pre-planned dose expansion cohort of 10 patients who were treated at the MTD to further assess treatment tolerability and to obtain preliminary data regarding efficacy.
Study assessments
Patients were assessed 5 times during cycle 1 (28 days) and then every 21 days. Patient history, physical examination, complete blood count and serum chemistries were performed at each visit. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4. Dose-limiting toxicity was defined as any grade 4 hematologic toxicity lasting >5 days. All grade 3 or greater nonhematologic toxicities were considered DLTs with diarrhea, nausea and vomiting needing to be grade 3 for 72 h despite maximal supportive care to qualify as a DLT. Once the maximum tolerated dose (MTD) was determined, an additional 10 patients were enrolled at the MTD.
Response to therapy was assessed by interval imaging every 6 weeks with a CT scan with response evaluated per RECIST 1.1. After six cycles on treatment, patients could reduce the radiographic assessments to every 4 cycles (12 weeks).
Rebiopsy samples at acquired resistance were assessed for EGFR T790M using a locked nucleic acid based PCR sequencing, and also by our institutional next-generation sequencing platform; the details of both have been previously discussed [4, 26].
Statistical analysis
Progression-free survival was estimated using the Kaplan–Meier method, and defined as the time from start of study therapy until progression or death. Patients who did not experience the event of interest were censored at the date they came off study or date of last assessment if still receiving study therapy. Response rates were calculated using binomial proportions and exact 95% CIs. All statistical analyses were performed using R 3.2.2 (R Development Core Team) including the ‘survival’ and ‘Hmisc’ packages.
Pharmacokinetic analysis
Please see the Supplementary Material.
Modeling tumor evolutionary dynamics
Please see the Supplementary Material.
Res ults
Pa tients
From November 2013 to January 2015, 34 patients were enrolled onto this study, including 24 patients in the dose escalation portion and 10 patients in an expansion cohort at the MTD. In total, 16 patients were treated at the MTD. The clinical characteristics of all patients are listed in Table 1.
Table 1.
Baseline patient and disease characteristics
| Characteristic | n=34 |
|---|---|
| Age, Median (range), years | 61 (33–77) |
| Sex | |
| Female | 18 |
| Male | 16 |
| KPS (%) | |
| ≥90 | 16 |
| 80 | 15 |
| 70 | 3 |
| Smoking status | |
| Former (pack-year range) | 12 (<1–35) |
| Never | 22 |
| EGFR sensitizing mutation | |
| L858R | 12 |
| Exon 19 deletion | 21 |
| G719A | 1 |
| Prior chemotherapy | |
| Yes | 5 |
| No | 29 |
| CNS involvement at diagnosis | |
| No | 23 |
| Yes (untreated/treated) | 11 (6/5) |
Determination of the maximum tolerated dose
There were no DLTs seen at the 600, 750 and 900 mg pulse dose levels. At the erlotinib 1050 mg pulse dose, there was one DLT of grade 3 transaminitis (supplementary Table S1, available at Annals of Oncology online). At 1350 mg pulse dose level, there were two DLTs: grade 3 rash and grade 3 mucositis. The 1200 mg pulse on days 1 and 2 and 50 mg erlotinib on days 3–7 was determined to be the MTD.
Toxicity
All 34 patients were evaluable for toxicity (Table 2). No grade 4 or greater toxicities were reported, with no deaths on study. Most drug-related toxicities were grade 1 and 2. The most common (≥25%) drug-related adverse events were rash, diarrhea, nausea, fatigue, mucositis, pruritus, vomiting, increased bilirubin and dry skin (Table 2). Six patients were removed from the study for toxicity: hyperbilirubinemia (1), possible pneumonitis (1), diarrhea (2), transaminitis (1) and mucositis (1).
Table 2.
Study drug-related adverse events seen in ≥10% of patients
| CTCAE term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total (any grade) |
|---|---|---|---|---|---|
| Rash | 21 (62%) | 8 (24%) | 1 (3%) | 0 | 30 (88%) |
| Diarrhea | 24 (71%) | 1 (3%) | 4 (12%) | 0 | 29 (85%) |
| Nausea | 10 (29%) | 3 (9%) | 2 (6%) | 0 | 15 (44%) |
| Fatigue | 8 (24%) | 4 (12%) | 0 | 0 | 12 (35%) |
| Mucositis | 8 (24%) | 3 (9%) | 1 (3%) | 0 | 12 (35%) |
| Pruritis | 11 (32%) | 0 | 0 | 0 | 11 (32%) |
| Vomiting | 9 (26%) | 1 (3%) | 1 (3%) | 0 | 11 (32%) |
| Bilirubin increased | 4 (12%) | 5 (15%) | 2 (6%) | 0 | 11 (32%) |
| Dry skin | 10 (29%) | 0 | 0 | 0 | 10 (29%) |
| ALT elevated | 6 (18%) | 1 (3%) | 1 (3%) | 0 | 8 (24%) |
| Alopecia | 7 (21%) | 0 | 0 | 0 | 7 (21%) |
| AST increased | 5 (15%) | 1 (3%) | 0 | 0 | 6 (18%) |
| Paronychia | 4 (12%) | 2 (6%) | 0 | 0 | 6 (18%) |
| Anorexia | 3 (9%) | 2 (6%) | 0 | 0 | 5 (15%) |
| Anemia | 2 (6%) | 1 (3%) | 1 (3%) | 0 | 4 (12%) |
Efficacy
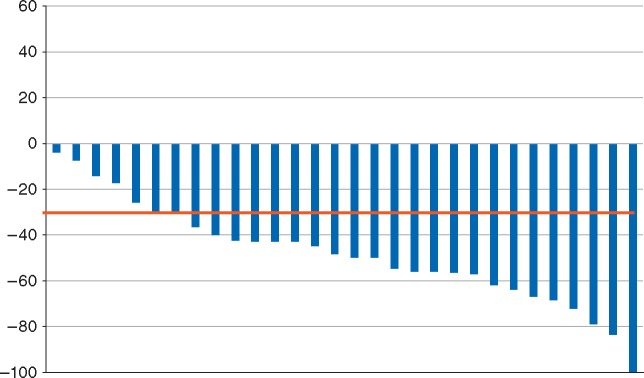
All 34 patients had baseline radiographic assessments. Four patients came off study prior to the first follow-up radiographic assessment (three due to toxicity, one due to noncompliance) and were counted as nonresponders in our intent to treat analysis. Twenty-four patients had confirmed partial responses and one patient had a confirmed complete response. The overall response rate was 74% (95% CI: 60–84%). All patients had a decrease in the sum of their target lesions (Figure 1). The median progression-free survival was 9.9 months (95% CI 5.8–15.4 months) (supplementary Figure S3, available at Annals of Oncology online). The overall survival estimate is not yet mature (with a median follow-up of 18 months). Six subjects have died: four due to lung cancer progression, one due to Parkinson’s disease, and one due to a secondary primary gastric cancer. None of the deaths were study-related.
Figure 1.
Best response of target lesions (RECIST 1.1) in all patients with a radiographic assessment of response. Four patients without follow-up imaging were excluded.
Central nervous system activity
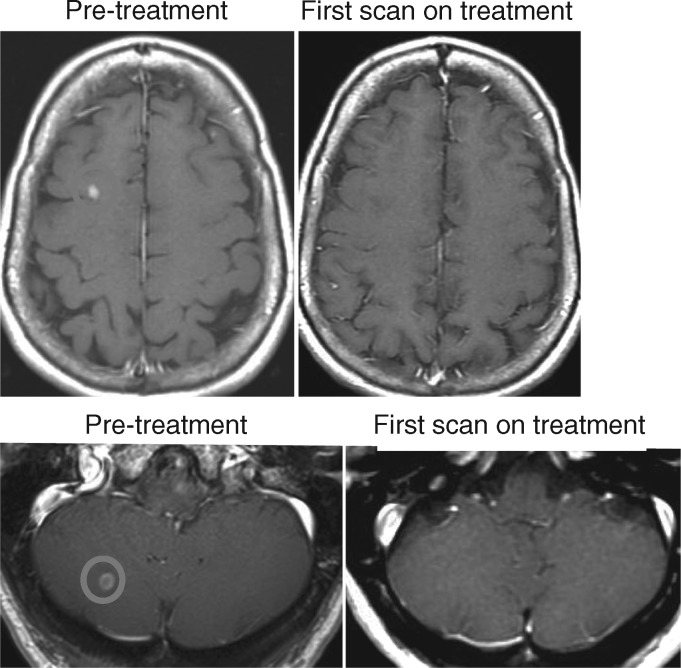
Eleven patients (32%) had brain metastases at study entry (baseline brain MRI was required). No patient had leptomeningeal disease. Prior to the trial, three patients had whole brain radiation, one had stereotactic radiosurgery and one had a surgical resection. All six patients with untreated brain metastases had tumor regression in the CNS (Figure 2). No patients had clinical CNS progression on study treatment. Of the 19 patients who came off study for progression of disease, 16 had CNS imaging when coming off study and none had radiographic evidence of new or progressing brain metastases.
Figure 2.
CNS responses in two patients with untreated brain metastases on pulse-continuous erlotinib.
Mechanisms of acquired resistance
Of the 19 patients that progressed on therapy, 6 continued daily erlotinib at standard doses for ≥ 2 months after discontinuation of study therapy. Nineteen patients had a tumor re-biopsy at the time of progression. One patient’s biopsy sample was inadequate for molecular analysis. 14 of 18 (78%, 95% CI 54–91%) patients had EGFR T790M identified in their rebiopsy specimen. Of the 18 patients, 11 had next generation sequencing on their re-biopsy sample, and four had a paired pre-treatment tumor sample as well (supplementary Table S2, available at Annals of Oncology online). Multiple concurrent mutations were found in both baseline and acquired resistance tumor samples in addition to the sensitizing EGFR mutation, which was identified in all samples. No patients had evidence of pre-treatment EGFR T790M. Acquired molecular alterations not present in baseline samples included previously reported HER2 and MET amplification [27, 28], as well as amplification in RAD21, SDHA, TERT, IL7R and RICTOR, and mutations in RET E164K, CENPA E50fs, and PLCG2 P999T.
Patient disposition
As of 1 December 2015, six patients remain on study. Nineteen patients discontinued study therapy because of progressive disease, and six others for adverse events. Two patients stopped study therapy due to noncompliance and one due to an unrelated gastric adenocarcinoma.
Pharmacokinetic analysis
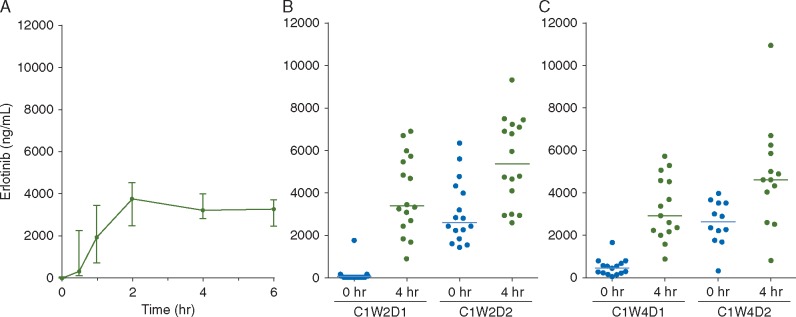
Median plasma concentration–time profiles are shown in Figure 3 with individual patients’ data in supplementary Figure S1, available at Annals of Oncology online. There was significant inter-patient variability in erlotinib plasma concentrations. No significant increases in peak plasma concentration was observed with increasing pulse doses of erlotinib from 600 to 1350 mg, at any time point examined during Cycle 1. This finding was also confirmed by analysis of the major erlotinib drug metabolites (OSI-420 and M11), which showed no dose-dependence with increasing pulse doses of erlotinib. The peak plasma concentration occurred after the second day of weekly pulse dose at Week 2, with median plasma concentration at 4-h post-administration reaching 5393 ng/ml (range 2600–9355 ng/ml), ∼13.7 μM, >5 times the peak plasma concentration seen with standard erlotinib 150 mg daily dosing [29]. The median plasma trough concentration before the pulse dosing at the start of week 4 was 435 ng/ml (range: 29–1655 ng/ml), a concentration of 1.1 µM, consistent with the 24-h trough plasma concentrations observed following standard erlotinib 150 mg daily dosing [29].
Figure 3.
Plasma concentration curves for erlotinib after multiple dosing. Blood samples were collected during (A) cycle 1 week 1 day 1 at 0.5, 1, 2, 4, and 6 h after the first dose; before and 4 h after erlotinib dosing on (B) cycle 1 week 2 day 1 and 2 and (C) cycle 1 week 4 day 1 and 2.
Modeling tumor evolutionary dynamics
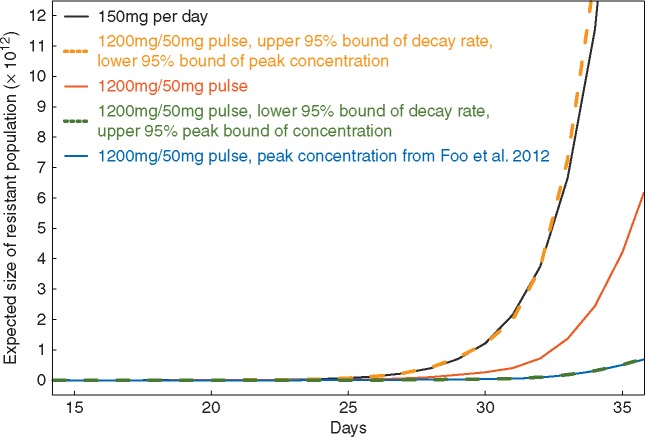
We utilized a multi-scale modeling platform consisting of a time-inhomogeneous stochastic birth–death process describing the behavior of sensitive and resistant tumor cells [30, 31] as well as a pharmacokinetic model [32] to monitor the expected number of resistant cells for two different treatment regimens: 150 mg daily versus 1200 mg twice weekly pulse and 50 mg daily dosing. The in vivo drug concentrations for 1200 mg pulse doses were calculated based on the erlotinib decay rate and peak concentration parameters estimated from our patient cohort (supplementary Figures S1 and S2, available at Annals of Oncology online). Since insufficient data was available for the 150 and 50 mg doses, we utilized PK parameters for those concentrations from previous work [32]. We then calculated the expected number of resistant cells over time for the pulse continuous dosing regimen for both the parameter point estimates from our current cohort and previous work [32] (Figure 4). Furthermore, we explored how the dynamics varied when using (i) the lower 95% bound of the decay rate and upper 95% bound of the peak concentration and (ii) the upper 95% bound of the decay rate and lower 95% bound of peak concentration (Figure 4). Interestingly, when investigating the upper 95% bound of the decay rate and lower 95% bound of peak concentration after a single dose of 1200 mg erlotinib, we found that the expected number of resistant cells exceeds the predicted number for the 150 mg daily dosing regimen. This effect arises since there is such a large extent of variability in pharmacokinetics across our patients. The varied dynamics due to the heterogeneous drug concentrations among patients suggests the necessity of identifying optimum treatment regimens on a patient-per-patient basis as well as evaluating larger patient cohorts for efficacy.
Figure 4.
Mathematical modeling prediction of the expected number of resistant tumor cells under different dosing regimens. Black: 150 mg/day; Blue: 1200 mg/50 mg pulse dose with peak concentration after single dose of 1200 mg inferred from Foo et al. [32]; Red: 1200 mg/50 mg pulse dose with decay rate and peak concentration after single dose of 1200 mg inferred from the current cohort; Orange: 1200 mg/50 mg pulse dose with upper 95% bound of decay rate and lower 95% bound of peak concentration inferred from the current cohort; Gold: 1200 mg/50 mg pulse dose with lower 95% bound of decay rate and upper 95% bound of peak concentration inferred from the current cohort.
Discussion
This work represents the first time evolutionary cancer modeling has been used to optimize dosing of a targeted therapy. This dosing schedule did not delay the development of resistance or prevent the emergence of EGFR T790M despite validation in vitro and in vivo pre-clinical models. A likely explanation is that we were unable to obtain high enough peak plasma concentrations in patients to correspond to the peak concentrations utilized in the pre-clinical models. The pulse and low-dose concentrations used in the pre-clinical models were 20 and 1 µM, while the median peak and trough concentration obtained at the MTD in our study was 13.7 and 1.1 µM, respectively. While toxicity prevented exploration of higher pulse dose levels, there was no significant increase in plasma peak concentration above the pulse dose of 600 mg, possibly due to limitations in drug absorption, clearance, or metabolism. Even if toxicity were not an issue at higher dose levels, it is uncertain whether shorter dosing intervals or higher doses would result in the peak plasma concentrations required to delay EGFR T790M. The peak concentrations of erlotinib required to delay EGFR T790M in the pre-clinical model do not appear achievable clinically. Furthermore, there was a significant inter-patient PK variability, resulting in difficulty identifying significant effects in a relatively small patient cohort. PK assessments were only completed during cycle 1 so inter-patient PK variability was not assessed and is a limitation of our analysis.
Pulse-continuous erlotinib had moderate toxicity when compared to studies of standard daily dose erlotinib [33, 34]. Toxicity was not markedly increased despite patients receiving 2.5 times the standard weekly dose and a 5-times increase in the median peak plasma concentration of erlotinib compared to standard dosing [29]. Pulse dosing may result in decreased toxicity compared to the same large amount of drug divided among equal daily doses, as side effects may be related to drug trough or steady state concentrations, rather than peak concentrations. This observation is corroborated by our previous study of once weekly pulse dose erlotinib at 2000 mg which also showed a similar toxicity profile to standard daily dose erlotinib . Our current study demonstrates that pulse-continuous erlotinib is a feasible dosing schedule that is tolerated by most people.
The efficacy of pulse-continuous erlotinib was similar to standard dose erlotinib. The overall response rate was 74% (95% CI 60–84%) and the median progression free survival was 9.9 months (95% CI 5.8–15.4 months). The dose and schedule of erlotinib assessed in this clinical trial did not improve response rates, prolong progression-free survival or delay systemic resistance. The 78% (95% CI 54–91%) frequency of EGFR T790M at the time of acquired resistance was comparable to what is seen with standard dosing. A larger study sample would be required to assess whether the frequency of EGFR T790M was significantly different with pulse continuous dosing compared to standard dosing. The aim of this study was to delay EGFR T790M-mediated resistance, and a limitation of the study is that it did not attempt to address other mechanisms of resistance to EGFR inhibition.
This is the first trial that reports the pre-treatment incidence of CNS involvement in study subjects and also the rate of CNS progression on an EGFR TKI therapy. None of the reports of large prospective studies of EGFR tyrosine kinase inhibitors include this information [1, 2, 34, 35]. As site of failure has prognostic as well as treatment implications, we recommend that CNS failure should be explicitly reported in all future studies in lung cancers. For EGFR-mutant lung cancers, understanding the CNS efficacy of drugs is critically important and will help us chose among drugs that may have similar systemic efficacy.
Patients with EGFR-mutant cancers may be at greater risk for CNS involvement with the cumulative incidence of CNS metastases approaching 60% [20–22, 36–38]. New EGFR TKIs such as AZD3759 are being developed specifically for their superior CNS penetration and activity, but robust efficacy data is not yet available [39]. Our preliminary data suggests that the CNS efficacy of this dosing schedule may be superior to standard dosing; this finding needs to be tested in a larger cohort of patients. No patients had progression in pre-existing brain metastases or developed new brain metastases while on study. The pulse dosing allows for increased CNS penetration and continued daily dosing maintains systemic control of disease. Since our dosing schedule may delay or prevent CNS progression in patients with EGFR-mutant lung cancers, we are currently studying this regimen in a cohort of patients with EGFR-mutant lung cancers with untreated measurable systemic and CNS metastases.
Funding
This work was supported, in part, by Astellas Pharmaceutical Global Development, Inc, the National Institutes of Health [U54 CA193461 to F.M.,P01 CA129243 to M.K., P30 CA008748 to MSKCC], the Dallepezze Foundation and a Young Investigator grant from Free to Breathe.
Disclosure
HAY reports research funding given to her institution from Astellas, AstraZeneca, Clovis Oncology, Incyte and has done consulting for AstraZeneca and Boehringer Ingelheim.GJR reports research funding given to his institution from Novartis, Millenium, GSK, Pfizer, Infinity Pharmaceuticals and Ariad and has done consulting for Novartis and Roche. HAY and GJR are named on a patent pending for pulse and continuous erlotinib for CNS metastases pending to Astellas. MGK has done consulting for Ariad, AstraZeneca and Genentech/Roche. WP is currently employed by Roche and he also has rights to EGFR T790M testing that were licensed on his behalf and others by MSKCC to Molecular MD. CMR has done consulting for BMS, Celgene, Medivation, and Novartis. CS is employed by Genentech/Roche. All other authors have nothing to disclose.
Supplementary Material
References
- 1. Sequist LV, Yang JC, Yamamoto N. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 2. Janne PA, Wang X, Socinski MA. et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 2012; 39: 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kobayashi S, Boggon TJ, Dayaram T. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005; 352(8): 786–792. [DOI] [PubMed] [Google Scholar]
- 4. Yu HA, Arcila ME, Rekhtman N. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sequist LV, Soria JC, Goldman JW. et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015; 372(18): 1700–1709. [DOI] [PubMed] [Google Scholar]
- 6. Janne PA, Yang JC, Kim DW. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372(18): 1689–1699. [DOI] [PubMed] [Google Scholar]
- 7. Piotrowska Z, Niederst MJ, Karlovich CA. et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov 2015; 5(7): 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thress KS, Paweletz CP, Felip E. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21(6): 560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu HA, Tian SK, Drilon AE. et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol 2015; 1(7): 982–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson ML, Yu HA, Hart EM. et al. Phase I/II study of HSP90 inhibitor AUY922 and erlotinib for EGFR-mutant lung cancer with acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol 2015; 33: 1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reguart N, Rosell R, Cardenal F. et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer 2014; 84(2): 161–167. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg SB, Supko JG, Neal JW. et al. A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J Thorac Oncol 2012; 7(10): 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hidalgo M, Siu LL, Nemunaitis J. et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001; 19(13): 3267–3279. [DOI] [PubMed] [Google Scholar]
- 14. Chmielecki J, Foo J, Oxnard GR. et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011; 3(90): 90ra59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foo J, Michor F.. Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput Biol 2009; 5(11): e1000557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milton DT, Miller VA, Azzoli CG. et al. Weekly high-dose erlotinib in patients with non-small cell lung cancer (NSCLC): a phase I/II study. Cancer 2006; 107: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 17. Chia S, Chi KN, Kollmannsberger C. et al. A Phase 1 dose escalation pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly and twice weekly erltoinib in advanced stage malignancies. J Clin Oncol 2007; 25(Suppl;abstract 3594). [Google Scholar]
- 18. Yeo WL, Riely GJ, Yeap BY. et al. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J Thorac Oncol 2010; 5(7): 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satoh H, Inoue A, Kobayashi K. et al. Low-dose gefitinib treatment for patients with advanced non-small cell lung cancer harboring sensitive epidermal growth factor receptor mutations. J Thorac Oncol 2011; 6(8): 1413–1417. [DOI] [PubMed] [Google Scholar]
- 20. Heon S, Yeap BY, Lindeman NI. et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012; 18(16): 4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omuro AM, Kris MG, Miller VA. et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005; 103(11): 2344–2348. [DOI] [PubMed] [Google Scholar]
- 22. Lee YJ, Choi HJ, Kim SK. et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 2010; 116(5): 1336–1343. [DOI] [PubMed] [Google Scholar]
- 23. Togashi Y, Masago K, Fukudo M. et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol 2010; 5(7): 950–955. [DOI] [PubMed] [Google Scholar]
- 24. Grommes C, Oxnard GR, Kris MG. et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro-Oncology 2011; 13(12): 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarke JL, Pao W, Wu N. et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010; 99(2): 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng DT, Mitchell TN, Zehir A. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17(3): 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engelman JA, Zejnullahu K, Mitsudomi T. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316(5827): 1039–1043. [DOI] [PubMed] [Google Scholar]
- 28. Takezawa K, Pirazzoli V, Arcila ME. et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR mutant lung cancers that lack the second-site EGFR T790M mutation. Cancer Discov 2012; 2: 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamilton M, Wolf JL, Rusk J. et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 2006; 12(7 Pt 1): 2166–2171. [DOI] [PubMed] [Google Scholar]
- 30. Foo J, Michor F.. Evolution of resistance to anti-cancer therapy during general dosing schedules. J Theor Biol 2010; 263(2): 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu LL, Li F, Pao W, Michor F.. Dose-dependent mutation rates determine optimum erlotinib dosing strategies for EGFR mutant non-small cell lung cancer patients. PLoS One 2015; 10(11): e0141665.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foo J, Chmielecki J, Pao W, Michor F.. Effects of pharmacokinetic processes and varied dosing schedules on the dynamics of acquired resistance to erlotinib in EGFR-mutant lung cancer. J Thorac Oncol 2012; 7(10): 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shepherd FA, Rodrigues Pereira J, Ciuleanu T. et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353(2): 123–132. [DOI] [PubMed] [Google Scholar]
- 34. Rosell R, Carcereny E, Gervais R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13(3): 239–246. [DOI] [PubMed] [Google Scholar]
- 35. Zhou C, Wu YL, Chen G. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12(8): 735–742. [DOI] [PubMed] [Google Scholar]
- 36. Heon S, Yeap BY, Britt GJ. et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010; 16(23): 5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rangachari D, Yamaguchi N, VanderLaan PA. et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015; 88(1): 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kris MG, Johnson BE, Berry LD. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. J Am Med Assoc 2014; 311(19): 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahn MJKD, Kim TM, Lin CC, Rathayake J. et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol 2016; 34(Suppl):abstract 9003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.