Abstract
Aims
Postural tachycardia syndrome (POTS), a common and debilitating cardiovascular disorder, is characterized by an exaggerated heart rate increase during orthostasis and a wide spectrum of adrenergic-related symptoms. To determine the aetiology of POTS, we examined a possible pathophysiological role for autoantibodies against α1-adrenergic (α1AR) and β1/2-adrenergic receptors (β1/2AR).
Methods and results
Immunoglobulin G (IgG) derived from 17 POTS patients, 7 with recurrent vasovagal syncope (VVS), and 11 normal controls was analysed for its ability to modulate activity and ligand responsiveness of α1AR and β1/2AR in transfected cells and to alter contractility of isolated rat cremaster arterioles in vitro. Immunoglobulin G activation of α1AR and β1/2AR was significantly higher in POTS compared with VVS and controls in cell-based assays. Eight, 11, and 12 of the 17 POTS patients possessed autoantibodies that activated α1AR, β1AR and β2AR, respectively. Pharmacological blockade suppressed IgG-induced activation of α1AR and β1/2AR. Eight of 17 POTS IgG decreased the α1AR responsiveness to phenylephrine and 13 of 17 POTS IgG increased the β1AR responsiveness to isoproterenol irrespective of their ability to directly activate their receptors. Postural tachycardia syndrome IgG contracted rat cremaster arterioles, which was reversed by α1AR blockade. The upright heart rate correlated with IgG-mediated β1AR and α1AR activity but not with β2AR activity.
Conclusion
These data confirm a strong relationship between adrenergic autoantibodies and POTS. They support the concept that allosteric-mediated shifts in the α1AR and β1AR responsiveness are important in the pathophysiology of postural tachycardia.
Keywords: Autoimmunity, Postural tachycardia syndrome, Vasovagal syncope, Adrenergic receptors, Allosteric activation
What's new?
Serum from patients diagnosed with postural tachycardia syndrome contains circulating autoantibodies with a direct stimulatory effect on adrenergic receptors.
The autoantibodies may also exert an allosterically mediated positive modulatory effect upon β1AR and a negative modulatory effect on α1AR activity.
These and possibly other yet-to-be identified immunoglobulins with different target epitopes might explain different constellations of symptoms in POTS.
This concept, if validated, would provide concrete support for novel therapeutic approaches against POTS based on immunotherapy.
Introduction
Postural tachycardia syndrome (POTS) is a functional cardiovascular disorder characterized by excessive heart rate increase and discomfort during orthostasis, usually accompanied by a spectrum of non-specific symptoms such as sporadic syncope, orthostatic intolerance, deconditioning, headache, cognitive impairment, and gastrointestinal dysfunction.1,2 The syndrome affects predominantly young women (70–80%) within a range of 15–40 years, first being defined in 1990s as a variant of orthostatic intolerance and dysautonomic syncope, but its aetiology remains unknown.3–5 Postural tachycardia syndrome is considered to be one of the most common autonomic disorders, with an estimated prevalence of 0.5 million in United States of America alone. Its onset may follow an acute infection or vaccination but more commonly appears without antecedent causative events.2,6 It often leads to debilitating symptoms and uncertain long-term prognosis.1 A number of treatment options have been proposed in POTS but their efficacy in the more severe forms is limited and often questioned.2,7 Since the pathophysiological basis for POTS is unclear, therapeutic interventions are empirical.
POTS-related syncope differs from classic vasovagal syncope (VVS), as the underlying symptoms are present constantly with occasional syncope associated with a preceding tachycardia and associated adrenergic symptoms. In contrast, VVS typically features episodic syncope and asymptomatic periods between attacks.1,4,7
Li et al. recently reported that activating autoantibodies (AAb) to the α1-adrenergic (α1AR) and β1/2-adrenergic receptors (β1/2AR) might play a pathophysiological role in POTS.8 In that pilot study, the investigators used a limited number of POTS subjects (n = 7) and controls from their institution; and added 7 additional POTS subjects and 4 controls submitted in a blinded fashion from an independent institution for a more rigorous confirmation of the discovery. This first study used a functional assay based on immunoglobulin G (IgG)-induced changes in α1AR and β2AR-mediated contractility of small arterioles in vitro. While effective, this technique is tedious and does not lend itself to screening larger number of patients. In the present study, we planned to examine the AAb profile of a larger, well-characterized, and representative cohort of patients with POTS from an independent European institution whose selection was completely separate from the originating laboratory. We chose to include two control groups, one being an autonomic control with patients presenting with classic recurrent VVS and the other as normal healthy volunteers without a history of syncope. The study was designed to examine the effect of patient-derived IgG on the activation of adrenergic receptors in transfected cells as well as IgG impact on the adrenergic receptor responsiveness to the well-characterized orthosteric ligands such as phenylephrine and isoproterenol.
Methods
Study patients
SYSTEMA (Syncope Study of Unselected Population in Malmö) is an ongoing single-centre project aiming at studying syncope epidemiology, pathophysiology, and prognosis in the general population.9 Within the SYSTEMA cohort, we randomly selected 17 patients with a confirmed diagnosis, by clinical history and tilt testing, of POTS (16 females; age, 26 ± 9 years), and 7 with recurrent VVS (5 females; age: 31 ± 9 years). These patients were investigated at the Syncope Unit of Skåne University Hospital between February 2011 and March 2014. The POTS patients reported at least 3 months duration of characteristic symptoms such as orthostatic intolerance, tiredness, headache, and were unresponsive to conventional therapy with persistent symptoms of orthostatic intolerance during blood collection. In contrast, VVS patients were selected among those who reported recurrent syncope prior to diagnosis, and were free from symptoms between the episodes of syncope. Eleven normal controls (10 females; age, 31 ± 6 years) were recruited at the study site in Sweden among healthy individuals without any history of syncope, autoimmune disease, and with a normal postural haemodynamics during active standing (i.e. pulse increase <30 bpm and absence of orthostatic hypotension).10,11 All the patients and controls were recruited from the same community of Malmö or neighbouring municipalities in Skåne Region, Southern Sweden.
Examination protocol
The head-up tilt (HUT) test for SYSTEMA patients was performed according to the Italian protocol.12 The tilt table was raised at constant speed to 70° in ∼18 s, and had the same lowering time. The patients' haemodynamic parameters and electrocardiogram were monitored using a non-invasive beat-to-beat measurement device (Nexfin, BMEYE, Amsterdam, the Netherlands).13 Study participants were instructed to fast for 2 h before the test, and they were allowed to drink water ad libitum. Participants were also asked to complete a questionnaire, referring to their past medical history, duration, frequency and characteristics of syncope, smoking status, and current medication. The Regional Ethical Review Board in Lund, Sweden approved the study protocol (ref no 82/2008), and all study participants gave their written informed consent including serum analyses.
Diagnostic criteria of postural tachycardia syndrome and classic vasovagal syncope
The test was diagnostic when patients demonstrated a typical prodrome (such as palpitation, nausea, dizziness, lightheadedness, or intense perspiration) reproducing previous events experienced and/or precipitated syncope, and the haemodynamic parameters met the diagnostic criteria of POTS or VVS, respectively.4,11,14 Briefly, POTS was defined as characteristic symptoms of orthostatic intolerance (lightheadedness, dizziness, and/or discomfort) with a maximal and persistent heart rate increase of >30/min (or ≥40/min for those aged ≤19 years) or tachycardia of >120/min in standing position, and the absence of orthostatic hypotension, i.e. max systolic/diastolic BP drop was <20/10 mmHg on head-up tilt.2,11 Vasovagal syncope was defined as a reproduction of syncope during HUT associated with a characteristic pattern of pronounced hypotension, bradycardia, or asystole.4 In order to ascertain the accuracy of diagnoses, the digital test records were subsequently inspected off-line using Nexfin@PC (BMEYE, Amsterdam, the Netherlands), dedicated software provided by the manufacturer.
Sample preparation
Blood samples for the present study were obtained post-test (i.e. after the diagnostic HUT was performed) following overnight fasting. A trained nurse performed an antecubital venipuncture in a dedicated room after 10 min rest in supine position. Serum was separated by centrifugation and stored at −80°C prior to shipping of duplicate aliquots in dry ice to the laboratory in Oklahoma City. The frozen integrity of each aliquot was confirmed upon arrival and samples were immediately placed in a −80°C freezer prior to thawing of one aliquot for the first assay. Serum IgG was purified using the NAb Protein A/G Spin Kit (Pierce Biotechnology). Receipt and assay of these de-identified samples was approved by the University of Oklahoma Health Sciences Center Institutional Review Board.
Cell-based α1AR assay
Immunoglobulin G activation of α1AR in α1AR-NFAT-bla CHO-K1 cells was assessed using the GeneBLAzer FRET-based β-lactamase reporter assay (Invitrogen) according to manufacturer's instructions. Briefly, cells were plated in 96-well plates and incubated overnight. The individual IgG (0.1 mg/mL) samples and positive and negative controls were then added and incubated for 5 h. The α1AR blocker prazosin (10 µM) was used for specific blockade. The β-lactamase substrate CCF4-AM (LiveBLAzer-FRET B/G Loading Kit, Invitrogen) was then added and incubated for 2 h. The plates were read using a fluorescence microplate reader (BioTek Synergy 2 Multi-Detection Microplate Reader). All samples were assayed in triplicate. Negative (buffer) and positive (phenylephrine) controls were included in each assay. Data were expressed as the ratio of the emissions 460/530 nm (blue/green) after subtraction of the background values.
Full dose–response curves for phenylephrine (10−10–10−5 M) were generated in three known positive POTS subjects in the absence and presence of IgG (0.1 mg/mL) to examine the allosteric effect of IgG on the α1AR orthosteric ligand phenylephrine. We then used a simplified one dosage (10−6 M) phenylephrine response in the absence and presence of IgG from the POTS subjects and the normal controls to examine whether an apparent change in sensitivity existed irrespective of whether a direct activation of the receptor by the IgG was present.
Cell-based β1/2AR assay
Immunoglobulin G-mediated β1AR and β2AR activation of cAMP production in transfected CHO cells (with either β1AR or β2AR stimulation) was measured using the cAMP Hunter eXpress GPCR Assay kit (DiscoveRx) as described.8,15 Briefly, β1AR- or β2AR-CHO cells were dispensed into 96-well plates and incubated overnight. The medium was then removed and assay buffer containing the cAMP antibody and serum-derived IgG (0.1 mg/mL) was added in the presence and absence of the non-selective βAR blocker propranolol (1 µM) and incubated for 30 min. The cAMP standard, negative (buffer), and positive (isoproterenol) controls were included in each assay. All samples were tested in triplicate. Following sample treatment, cAMP detection reagent and solution were added, and chemiluminescent signal was read on a TD-20/20 Luminometer (Turner BioSystems). The cAMP values generated for each assay were expressed as relative luminescence unit (RLU).
Dosage response curves for isoproterenol (10−10–10−6M) in β1AR-CHO cells in the absence and presence of IgG (0.1 mg/mL) from three positive POTS subjects were constructed to examine the allosteric effect of IgG on the β1AR orthosteric ligand isoproterenol. Likewise, a simplified single dosage isoproterenol (10−6 M) response was examined using IgG from the POTS subjects and the normal controls to determine if an allosteric-mediated effect on the β1AR-CHO cells was present independent of any direct activation from the IgG.
Isolated cremaster arteriole assay
The vasoconstrictor effect of IgG activation of α1AR on resistance vessels was examined using an isolated rat cremaster arteriole assay as previously described.8 After equilibration in the presence of propranolol (1 µM) and the nitric oxide synthase inhibitor L-NAME (1 µM) to eliminate any β2AR- and M3 muscarinic receptor-mediated vasodilation and to achieve steady-state myogenic tone, the arterioles were perfused with IgG (0.05 mg/mL) and their effects on vessel diameter were recorded. Phentolamine (10 µM) was used to specifically block α1AR activity. The buffer baseline diameters were normalized to 100% and subsequent IgG-induced contractility was reported as percentage of baseline. This procedure was approved by the Oklahoma University Health Sciences Center Institutional Animal Care and Use Committee.
Statistical analyses
Data are expressed as mean ± SD. Group comparisons were performed using Student's t-test for comparison of two groups, or one-way ANOVA followed by post hoc Tukey's test for multiple group comparison. Pearson's χ2-analysis was used to compare categorical variables. The positivity of bioactive autoantibodies was defined as values above the mean + 2SD or below the mean − 2SD from the control group. A linear regression analysis was performed to examine the relationship between the haemodynamic parameters resting supine plus during head-up tilt and direct IgG-mediated receptor bioactivity. Analyses were performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P< 0.05.
Results
Clinical characteristics
The demographic and clinical characteristics for each group are shown in Table 1. The mean age for the POTS patients was not significantly different from those with VVS and control subjects. All patients in the POTS group had documented persistent increase in heart rate >30 bpm vs. supine during head-up-tilt with reproduction of typical orthostatic intolerance symptoms. There was no significant difference in BP response during the first 3 min of head-up tilt in the POTS compared with VVS and controls and there was no significant difference in catecholamine profile supine and 3-min HUT between POTS and VVS (see Supplementary material online, Table S1). The VVS group reported a longer history of syncopal symptoms (P= 0.017), but the number of syncope episodes, proportions of characteristic precipitating symptoms, and self-reported dizziness on standing did not differ between POTS and VVS patients.
Table 1.
Characteristics of patients with POTS, VVS and controls
| POTS (n = 17) | VVS (n = 7) | Controls (n = 11) | P-valuea VVS/Controls vs. POTS | |
|---|---|---|---|---|
| Age | 25 ± 7 | 31 ± 9 | 31 ± 6 | 0.17/0.063 |
| Sex M/F | 2/15 | 2/5 | 1/10 | 0.55/0.98 |
| Systolic BP supine | 121 ± 14 | 123 ± 6 | 112 ± 8 | 0.95/0.92 |
| Diastolic BP supine | 71 ± 8 | 71 ± 3 | 70 ± 9 | 1.0/0.97 |
| Heart rate supine | 73 ± 10 | 73 ± 8 | 70 ± 9 | 1.0/0.63 |
| Systolic BP upright 3 min | 122 ± 14 | 125 ± 6 | 112 ± 8 | 0.78/0.069 |
| Diastolic BP upright 3 min | 80 ± 8 | 78 ± 5 | 77 ± 10 | 0.84/0.63 |
| Heart rate upright 3 min | 100 ± 14 | 82 ± 10 | 78 ± 9 | 0.004/0.0002 |
| Heart rate max upright | 111 ± 13 | 90 ± 10 | – | 0.001 |
| Duration of symptoms (years, median, interquartile range) | 3 (1–6) | 10 (2–24) | – | 0.017 |
| No. of syncope (median, interquartile range) | 4 (1–30) | 10 (3–10) | – | 0.67 |
| Autonomic symptoms (nausea, perspiration) preceding syncope (n, %) | 8 (47) | 5 (71) | – | 0.34 |
| Palpitations preceding syncope (n, %) | 4 (24) | 3 (43) | – | 0.39 |
| Orthostatic dizziness (n, %) | 13 (77) | 4 (57) | – | 0.23 |
BP, blood pressure.
aOne-way ANOVA plus Tukey post hoc adjustment (for continuous variables) and Pearson's χ2 test (for categorical variables) were applied.
Autoantibody activity
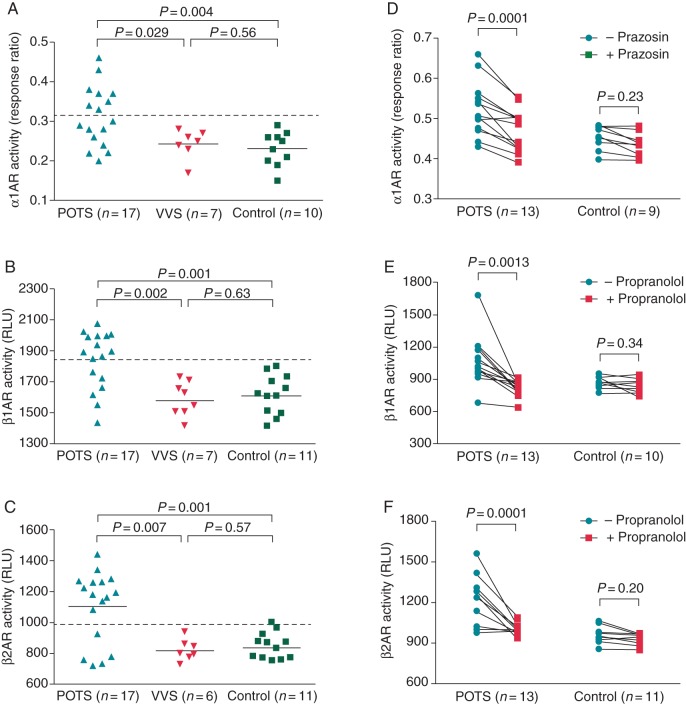
The activities of autoantibodies against the α1AR and β1/2AR were examined using IgG (0.1 mg/mL) in cell-based bioassays. These data are shown in Figure 1. Among the 17 patients with POTS, 8, 11, and 12 showed direct activation of α1AR (47%), β1AR (65%), and β2AR (71%), respectively (Figure 1A–C). Based on these direct assays, 6 of these 17 POTS patients (35%) harboured both α1AR- and β1AR-AAb (Table 2). None of these autoantibodies were found in the patients with VVS or healthy controls. The mean activity values of α1AR, β1AR and β2AR autoantibodies were all significantly higher in the POTS group than for both VVS and normal controls (α1AR: POTS 0.31 ± 0.07(SD) vs. VVS 0.24 ± 0.04 and control 0.23 ± 0.04, P= 0.029 and 0.004, respectively; β1AR: POTS 1840 ± 186 vs. VVS 1578 ± 117 and control 1608 ± 127, P= 0.002 and 0.001, respectively; β2AR: POTS 1104 ± 230 vs. VVS 816 ± 73 and control 837 ± 72, P= 0.007 and 0.001, respectively) (Figure 1A–C). There was no significant difference between the latter two groups. The mean activities of α1AR, β1AR, and β2AR autoantibodies in 13 POTS patients were markedly suppressed by the α1AR blocker prazosin (10 µM, from 0.52 ± 0.06 to 0.46 ± 0.05, P= 0.0001) and β-blocker propranolol (1 µM, β1AR: from 1068 ± 233 to 821 ± 71, P= 0.0013; β2AR: from 1221 ± 164 to 988 ± 37, P= 0.0001) (Figure 1D–F). No significant effect of these blockers was observed in the control group.
Figure 1.
Effects of serum IgG (0.1 mg/mL) from POTS and VVS patients and healthy control subjects on direct activation of α1AR, β1AR, and β2AR in cell-based bioassays. The α1AR activity is expressed as the FRET blue/green response ratio, and the β1/2AR activity is expressed as RLU. There was a significant increase in α1AR (A), β1AR (B), and β2AR (C) activity in the POTS group compared with the VVS and control groups. The addition of the α1 blocker prazosin (10 µM) or non-selective β-blocker propranolol (1 µM), respectively, suppressed the α1AR (D), β1AR (E), and β2AR (F) activity values to control levels. The dashed line is the threshold derived from the mean activity values + 2SD of the healthy controls.
Table 2.
Test positivity (direct-activating and/or ligand-modulating activity) among patients diagnosed with POTS
| Patient no. | α1AR Ab |
β1AR Ab |
β2AR Ab | ||
|---|---|---|---|---|---|
| Activating | Modulating | Activating | Modulating | Activating | |
| 1 | x | x | |||
| 2 | x | x | |||
| 3 | x | x | x | x | |
| 4 | x | x | x | x | |
| 5 | x | x | x | ||
| 6 | x | x | x | ||
| 7 | x | x | x | ||
| 8 | x | x | x | ||
| 9 | x | x | |||
| 10 | x | x | x | ||
| 11 | x | x | x | ||
| 12 | x | x | x | x | x |
| 13 | x | x | |||
| 14 | x | x | |||
| 15 | x | x | |||
| 16 | x | x | x | x | |
| 17 | x | x | x | x | x |
| Total | 8/17 | 8/17 | 11/17 | 13/17 | 12/17 |
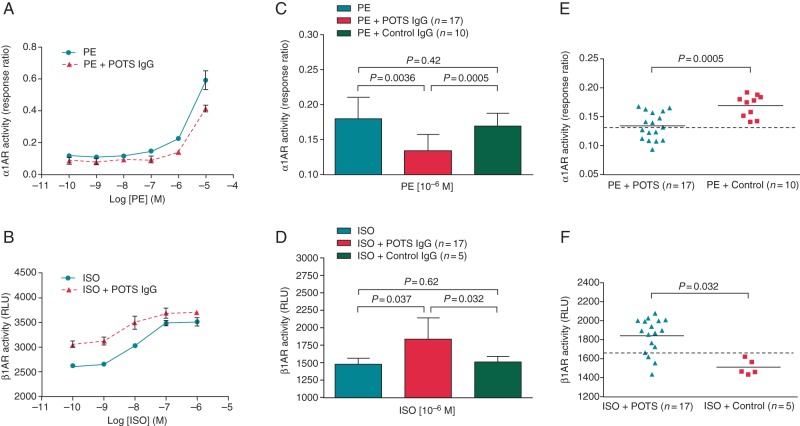
To examine the effect of autoantibodies on orthosteric ligand activation of α1AR and β1AR, dosage response curves for phenylephrine (10−10-10−5M) and isoproterenol (10−10–10−6M) with and without POTS IgG (0.1 mg/mL) were constructed and compared. Immunoglobulin G from three POTS patients who were positive for α1AR and β1AR autoantibodies were used for curve comparisons. In the presence of POTS IgG, the phenylephrine response curve was shifted to the right, demonstrating a decreased α1AR response (Figure 2A). In contrast, the isoproterenol response curve was significantly shifted to the left, demonstrating an enhanced β1AR response with β1AR autoantibodies (Figure 2B). Neither shift was observed using control IgG. We then tested the effect of IgG (0.1 mg/mL) from all 17 POTS subjects on the responses to phenylephrine (1 µM) and isoproterenol (1 µM). There was a significant decrease in phenylephrine-induced α1AR activation with POTS IgG (0.13 ± 0.02) compared with phenylephrine alone (0.18 ± 0.03, P< 0.0036) or IgG from normal controls (0.17 ± 0.02, P< 0.0005) (Figure 2C). The isoproterenol-stimulated β1AR activation, on the other hand, was significantly increased with POTS IgG (1835 ± 309) compared with isoproterenol alone (1479 ± 86, P< 0.037) or IgG from normal controls (1508 ± 80, P< 0.032) (Figure 2D). Control IgG showed minimal modulating effects.
Figure 2.
Effects of IgG (0.1 mg/mL) from POTS patients on PE and ISO responses in cell-based assays. IgG from 3 POTS patients with α1AR-activating activity shifted the PE dosage response curve to the right indicating an inhibitory allosteric effect (A). In contrast, IgG from 3 β1AR antibody-positive POTS patients shifted the ISO dosage response curve to the left compatible with a positive allosteric effect. Control IgG failed to alter these curves. When IgG from all 17 POTS patients was tested in the presence of PE (1 µM) or isoproterenol (1 µM), there was a significant decrease in the PE response compared with PE alone and control IgG (C) and a significant increase in the ISO response compared with ISO alone and control IgG (D). Control IgG showed minimal modulating effects. Individual data from (C) and (D) for the POTS and control subjects are shown in (E) and (F), respectively. The dashed line is the threshold derived from the control mean values—2SD for the α1AR-modulating effect or control mean values + 2SD for the β1AR-modulating effect.
When each individual data was plotted, 8 of the 17 POTS patients demonstrated an inhibitory effect on phenylephrine-induced α1AR activation using a cut-off value of the mean—2SD from the control group (Figure 2E). Thirteen of the 17 POTS patients were positive for an enhanced effect on isoproterenol-induced β1AR activation using a cut-off value of the mean + 2D from the control group (Figure 2F). There was some overlap for the α1AR and considerable overlap for the β1AR with the other tests (Table 2). Overall, all POTS subjects had at least one positive test.
Immunoglobulin G (0.05 mg/mL) from each subject with POTS and 8 controls were tested for α1AR autoantibody-mediated contractile activity using an isolated rat cremaster arteriole assay. A total of 13 among the 17 POTS patients demonstrated significantly increased contractility (Figure 3A). There was a significant difference in the mean contractile activity between the POTS group and control group (91.0 ± 3.1% vs. 96.5 ± 1.3%, P< 0.0001). The control subjects failed to produce any significant vasoactivity. Six POTS subjects with positive α1AR autoantibodies and 6 controls were then selected to test the effect of specific α1AR blockade. As shown in Figure 3B, POTS IgG-induced contractility was largely returned to baseline level by addition of the α1AR blocker phentolamine (10 µM, from 88.9 ± 3.0% to 96.5 ± 2.1%, P= 0.0006). Phentolamine had no significant impact on control IgG activity.
Figure 3.
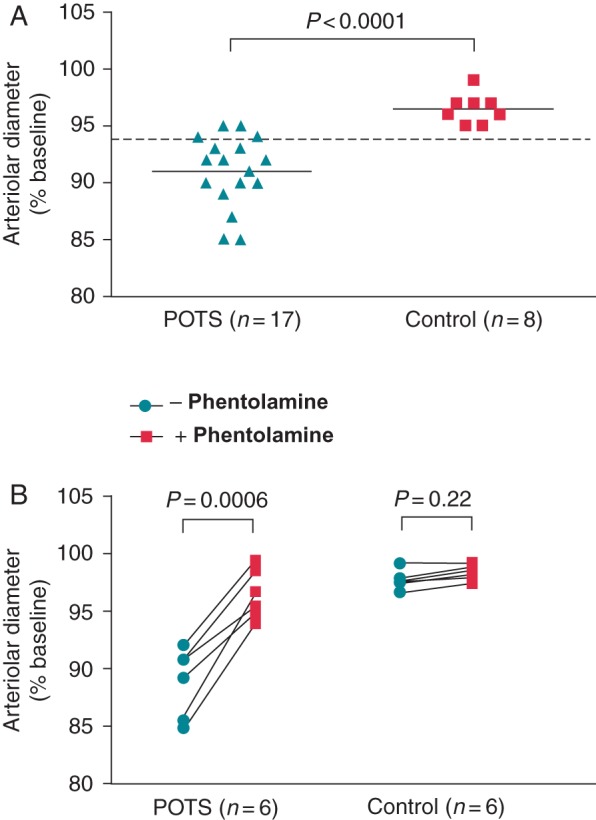
Effects of IgG (0.05 mg/mL) from POTS patients and healthy control subjects on arteriolar contractility in isolated rat cremaster arteriole assay. Values are expressed as % of buffer baseline. IgG was tested in the presence of propranolol (1 µM) and the nitric oxide synthase inhibitor L-NAME (1 µM) to eliminate any β2AR- and M3 muscarinic receptor-mediated vasodilation. Thirteen of the 17 POTS patients demonstrated significant vasoconstrictive activity compared with eight control subjects (A). No significant vasoconstriction was observed for the control subjects. The dashed line is the threshold derived from the mean contractility values—2SD of the healthy controls. The addition of the α1AR blocker phentolamine (10 µM) markedly suppressed and reversed POTS IgG-mediated contractility to control levels (B). There was no significant change in contractile activity with phentolamine for the control IgG.
In the linear regression analysis, IgG-mediated β1AR activity was strongly correlated with both 3 min (P= 0.0009) and maximal upright heart rate (P= 0.0006), whereas the latter was also correlated with α1AR activity (P= 0.039) but not with β2AR activity (P= 0.11). Neither SBP nor DBP were correlated with adrenergic receptor stimulation.
Discussion
We have demonstrated that serum from subjects with POTS contains circulating autoantibodies, some with a direct stimulatory effect on adrenergic receptors. A majority also exerted an allosterically mediated positive modulatory effect upon β1AR and a negative modulatory effect on α1AR activity. These and possibly other yet-to-be identified immunoglobulins with different target epitopes may exert varying biological effects in particular patients, which may explain different constellations of symptoms in POTS. It is of interest that IgG-mediated β1AR and α1AR activation were correlated with the heart rate increase on standing. The relatively high correlation with the β1AR autoantibody activity would appear to be clinically relevant. The lower but still significant relationship of the allosterically mediated inhibitory action of the α1AR response would secondarily lead to the increase in heart rate by reflexly increasing circulating catecholamines.
The primary objective of this study was to determine if autoantibodies with the capacity to modify the activity of autonomic adrenergic receptors, previously reported in POTS patients in the USA,8 were likewise present in a cohort of POTS subjects from a European location. A secondary goal included usage of an ‘autonomic’ control group characterized by subjects with recurrent VVS. This permitted us to determine if such autoantibodies were different between normal subjects and POTS; and as well between POTS and a group with recurrent VVS. These latter two groups shared somewhat broadly based autonomic disorders but clearly were different from each other and from normal controls. A third goal was to develop bioactivity assays that might permit larger scale testing for pathophysiological and therapeutic study.
For the primary objective, we found that antiadrenergic α1AR autoantibody activity was evident in POTS, while the VVS subjects were not different from normal subjects. In contrast to our initial publication,8 nearly half of the POTS subjects had direct activation of the α1AR autoantibody activity. The α1AR activity in our previous study was assayed using IgG in an arteriolar assay and not the present cell-based assay. For this reason, we re-assayed IgG from each POTS patient and a representative group of the controls in the rat cremaster arteriole assay. These data showed a heterogeneous response in which the majority with increased α1AR activity from the cell-based assay also demonstrated arteriolar contractile activity. Eight subjects with no α1AR direct activity from the cell-based assay also demonstrated positive contractile activity, while three subjects with positive α1AR activity from the cell-based assay demonstrated no arteriolar contractile activity.
We then examined the impact of representative IgG from POTS and normal controls on phenylephrine dosage response curves. As previously reported,8 these data confirmed the active α1AR autoantibodies inhibited the phenylephrine response and shifted the phenylephrine dosage response curve to the right. These autoantibodies appear to function as partial agonists and the absence of measurable direct activation of the transfected receptors in vitro in the absence of their normal ligand does not exclude their presence. It is possible this allosteric impact on the activity of the α1AR ligand norepinephrine represents the distinct pathophysiological feature characteristic of POTS. Consequently, identification of the antiadrenergic autoantibodies may additionally require testing for altered dosage response curves using established receptor orthosteric agonists.
β1/2AR autoantibody activity was increased in POTS but not in VVS. In the present study, approximately two-thirds activated β1/2AR in cell-based assays. Moreover, β1AR autoantibodies enhanced the isoproterenol response and most importantly shifted the isoproterenol dosage response curves to the left, confirming our previous observations.8
Postural tachycardia syndrome continues to be a vexing dysautonomia2 that has long challenged afflicted patients, their families, and caregivers. The variable presentation and seemingly associated autonomic disorders, and other non-specific symptoms including gastroparesis, migraine headaches, chest pain, chronic fatigue, and mental confusion (brain-fog) have perplexed medical personnel. Thus, it is important to define the aetiology and develop a rationale for specific interventions for controlling the underlying pathophysiology and thereby providing symptomatic relief. There has long been speculation that the predilection for POTS in younger females and occasional association with a viral/bacterial infection, vaccination or a stress event would support an autoimmune component in its aetiology.6,16,17
Recognition that circulating autoantibodies interact with G protein-coupled receptor targets and modify the autonomic system has led our group to identify AAb to the β2AR and M3 muscarinic acetylcholine receptor in a group of subjects with idiopathic orthostatic hypotension.15 The present study focused on the activity expected with these receptors; but identified a unique property of the studied IgG directed toward the α1AR which exerted allosteric effects characteristic of a partial agonist since its presence shifted the phenylephrine dosage response curve to the right. In contrast, the β1AR effect was just the opposite and shifted the β1AR agonist dosage response curve to the left. It is likely that under these conditions, the primary action of the autoantibodies is not an orthosteric direct activation or inhibition of the receptor but rather modulation of the activity and action of the ligand norepinephrine/epinephrine that normally is involved in the particular receptor transduction. These contrasting effects provide an appealing explanation for the cardiovascular effects associated with upright posture in those so afflicted.1,8
We believe that the present study helps affirm our concept that common cardiovascular dysautonomias may express a spectrum of autoantibodies, which contribute to a variety of clinical manifestations. This spectrum would certainly range from subclinical to overt expressions that may overlap with other entities such as inappropriate sinus tachycardia (IST), where circulating antibodies against cardiac β-receptors have been previously reported.18 Both POTS and IST share abnormal sinus tachycardia tendency, and differ in regard to resting heart rate control that is usually preserved in POTS but not in IST.2 Thus, some subjects who do not formally meet the diagnostic criteria of POTS may have partial presentation of such autoantibodies. From our studies, subjects with recurrent VVS and normal orthostatic tolerance would not be likely candidates for inclusion in this grouping. We have recently demonstrated an exaggerated catecholamine response in postural tachycardia among patients with syncope and dysautonomic cardiovascular response to orthostasis.19 The catecholamine surge may be seen as a compensatory mechanism to override the α1AR malfunction associated with the proposed autoimmune blockade as present in POTS patients but this explanation is unlikely in those with recurrent VVS.
Although it is likely that POTS has multiple causes, there has been a paucity of concrete evidence for other pathophysiological bases for an underlying pathophysiology to date for the vast majority of patients. It seems apparent that the high percentage of POTS subjects examined by our group present with an apparent autoimmune diathesis that supports the concept that these autoantibodies play important role in the pathophysiology of this entity.
Strengths and limitations
This report more than doubles the number of our previous observations, and focuses on a tightly characterized group of patients and controls. We have introduced a new direct assay to measure α1AR activity, which although similar in principle to the assay for β1/2AR in transfected cells was only positive in half of the POTS patients compared with the more demanding previous bioassay that measured contractility in the cremaster arterioles in vitro. This cremaster arteriolar bioassay is not suitable for use in large numbers of samples and the specific cell-based assays are an improvement with regard to numbers. Diagnostic criteria based on measuring the autoantibodies' impact on an allosteric-mediated receptor response to the natural ligand may provide a more favourable estimate of autoantibody involvement in the complex pathophysiology of POTS. This study has an important limitation as the parasympathetic tonus was not assessed during HUT, and possible autoantibodies against muscarinic receptors and/or acetylcholine system were not investigated. The vagal drive may have an important implication in related POTS symptomatology.20 Finally, the study groups were not strictly age matched, although the age difference was not significant.
Conclusion
The marked presence of either or both α1AR and β1/2AR autoantibodies with their potent allosteric impact on the relevant orthosteric ligands provides an appealing framework to support an autoimmune pathophysiology of POTS. This concept, if validated, would provide concrete support for novel therapeutic approaches against POTS based on immunotherapy. These could currently include suppression of autoantibody production using immunoglobulin infusions and in the foreseeable future by development of decoy peptides that are specific for autoantibodies that target the identified receptor epitopes.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.
Funding
This work was supported in part by the European Research Council (StG 282225), Swedish Medical Research Council, Swedish Heart and Lung Foundation, Medical Faculty of Lund University, Ernhold Lundströms Research Foundation, Hulda and Conrad Mossfelt Foundation, Wallenberg Foundation, Anna Lisa and Sven-Erik Lundgrens Foundation (Sweden), and by funding from the National Institute of Health (R56HL128393), a Veterans Affairs Merit Review Award, anonymous individual grants directed through Dysautonomia International, the American Heart Association, and an individual grant from Will and Helen Webster. This work was presented in part at the Annual Meeting of the European Society of Cardiology in London, UK, August 29–September 2, 2015 and the International Society for Autonomic Neuroscience (ISAN) 2015 Meeting in Stresa, Italy, September 26–29, 2015.
Supplementary Material
References
- 1. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 2012;87:1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M et al. 2015 Heart Rhythm Society Expert Consensus Statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R et al. Postural tachycardia syndrome (POTS). Neurology 1995;45:S19–25. [PubMed] [Google Scholar]
- 4. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000;2:66–76. [DOI] [PubMed] [Google Scholar]
- 6. Brinth LS, Pors K, Theibel AC, Mehlsen J. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine 2015;33:2602–5. [DOI] [PubMed] [Google Scholar]
- 7. Fedorowski A, Melander O. Syndromes of orthostatic intolerance: a hidden danger. J Intern Med 2013;273:322–35. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014;3:e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fedorowski A, Burri P, Juul-Moller S, Melander O. A dedicated investigation unit improves management of syncopal attacks (Syncope Study of Unselected Population in Malmo – SYSTEMA I). Europace 2010;12:1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol 1994;34:375–86. [DOI] [PubMed] [Google Scholar]
- 11. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 2011;161:46–8. [DOI] [PubMed] [Google Scholar]
- 12. Bartoletti A, Alboni P, Ammirati F, Brignole M, Del Rosso A, Foglia Manzillo G et al. ‘The Italian Protocol’: a simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace 2000;2:339–42. [DOI] [PubMed] [Google Scholar]
- 13. Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH, Wesseling KR, Blanc S, Wieling W et al. Nexfin noninvasive continuous blood pressure validated against Riva-Rocci/Korotkoff. Am J Hypertens 2009;22:378–83. [DOI] [PubMed] [Google Scholar]
- 14. Parry SW, Reeve P, Lawson J, Shaw FE, Davison J, Norton M et al. The Newcastle protocols 2008: an update on head-up tilt table testing and the management of vasovagal syncope and related disorders. Heart 2009;95:416–20. [DOI] [PubMed] [Google Scholar]
- 15. Li H, Kem DC, Reim S, Khan M, Vanderlinde-Wood M, Zillner C et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension 2012;59:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimpinski K, Figueroa JJ, Singer W, Sletten DM, Iodice V, Sandroni P et al. A prospective, 1-year follow-up study of postural tachycardia syndrome. Mayo Clin Proc 2012;87:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R. Postural tachycardia syndrome – current experience and concepts. Nat Rev Neurol 2012;8:22–34. [DOI] [PubMed] [Google Scholar]
- 18. Chiale PA, Garro HA, Schmidberg J, Sanchez RA, Acunzo RS, Lago M et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm 2006;3:1182–6. [DOI] [PubMed] [Google Scholar]
- 19. Nilsson D, Sutton R, Tas W, Burri P, Melander O, Fedorowski A. Orthostatic changes in hemodynamics and cardiovascular biomarkers in dysautonomic patients. PLoS ONE 2015;10:e0128962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshida S, Tanaka H, Nakao R, Okamoto N, Kajiura M, Kanbara Y et al. Variant cardiovascular regulation in children with postural tachycardia syndrome. Pediatr Int 2014;56:328–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.