Abstract
Background
Surrogate biomarkers of efficacy are needed for anti-PD1/PD-L1 therapy, given the existence of delayed responses and pseudo-progressions. We evaluated changes in serum IL-8 levels as a biomarker of response to anti-PD-1 blockade in melanoma and non-small-cell lung cancer (NSCLC) patients.
Patients and methods
Metastatic melanoma and NSCLC patients treated with nivolumab or pembrolizumab alone or nivolumab plus ipilimumab were studied. Serum was collected at baseline; at 2–4 weeks after the first dose; and at the time-points of response evaluation. Serum IL-8 levels were determined by sandwich ELISA. Changes in serum IL-8 levels were compared with the Wilcoxon test and their strength of association with response was assessed with the Mann–Whitney test. Accuracy of changes in IL-8 levels to predict response was estimated using receiver operation characteristics curves.
Results
Twenty-nine melanoma patients treated with nivolumab or pembrolizumab were studied. In responding patients, serum IL-8 levels significantly decreased between baseline and best response (P <0.001), and significantly increased upon progression (P = 0.004). In non-responders, IL-8 levels significantly increased between baseline and progression (P = 0.013). Early changes in serum IL-8 levels (2–4 weeks after treatment initiation) were strongly associated with response (P <0.001). These observations were validated in 19 NSCLC patients treated with nivolumab or pembrolizumab (P = 0.001), and in 15 melanoma patients treated with nivolumab plus ipilimumab (P <0.001). Early decreases in serum IL-8 levels were associated with longer overall survival in melanoma (P = 0.001) and NSCLC (P = 0.015) patients. Serum IL-8 levels also correctly reflected true response in three cancer patients presenting pseudoprogression.
Conclusions
Changes in serum IL-8 levels could be used to monitor and predict clinical benefit from immune checkpoint blockade in melanoma and NSCLC patients.
Keywords: IL-8, serum biomarkers, anti-PD-1 mAbs, anti-CTLA-4 mAbs, melanoma, NSCLC
Introduction
Targeting the Program Death 1 (PD-1)/Program Death Ligand 1 (PD-L1) pathway has demonstrated unprecedented success against diverse cancers [1]. Three drugs targeting this co-inhibitory pathway, nivolumab (anti-PD-1), pembrolizumab (anti-PD-1) and atezolizumab (anti-PD-L1) have been approved for the treatment of different tumor types [2–10]. This new treatment strategy stimulates the immune system to attack tumors instead of directly targeting tumor cells, a mechanism of action that implies different patterns of response to therapy [11]. Response to anti-PD-1/PD-L1 mAbs is usually seen by week 12, albeit some responses may also occur later in the course of treatment. In addition, up to 15% of patients may experience pseudoprogression, consisting of increases of ≥25% in the size of target tumor lesions or even the appearance of new lesions, that do not represent true tumor progression and are not confirmed as progressive disease (PD) on subsequent imaging assessments [12].
Such unconventional response patterns make it difficult to differentiate responders from non-responders early on in treatment; and there are no methods, at this early stage, with which to identify patients who may ultimately benefit from these immunotherapies. We have previously demonstrated that serum interleukin-8 (IL-8) levels reflect tumor burden and allow to monitor response to BRAF inhibitors (vemurafenib), and an anti-CTLA-4 mAbs (ipilimumab) in metastatic melanoma patients [13].
IL-8 is a member of the CXC chemokine family originally identified as a chemotactic factor for neutrophils [14]. IL-8 is secreted by malignant cells and tumor stroma cells across many different tumor types [15, 16]. Multiple mechanisms may be involved in the protumoral actions of IL-8, including direct effects on endothelial cells, malignant cells, cancer stem cells, as well as indirect effects attracting and/or modulating tumor-associated myeloid cells [17–21].
Here, we studied the association between serum IL-8 levels and clinical performance of patients with advanced melanoma and NSCLC patients during treatment with anti-PD-1 mAbs, used either as single-agents or in combination with anti-CTLA-4 mAbs.
Materials and methods
Patients and study design
The study was conducted in two parts. In the first part, we aimed to define the cut-off point that better evaluated serum IL-8 levels to predict clinical response in metastatic melanoma patients treated with anti-PD-1 mAbs (identification melanoma cohort). In the second part, we aimed to validate the previous cut-off point in two independent cohorts: metastatic NSCLC patients treated with anti-PD-1 mAbs (independent NSCLC cohort) and metastatic melanoma patients treated with anti-PD-1 mAbs plus anti-CTLA-4 mAbs (independent melanoma cohort). Treatment and sample collection details are provided in the supplementary Appendix, available at Annals of Oncology online. The study protocol was approved by the Institutional Review Boards from both participating institutions. All patients signed written informed consents.
Sample collection and biochemical assays
Peripheral blood samples were sequentially obtained by venipuncture at baseline; at the time of first visit to the clinic (2–3 weeks) after starting treatment; and at each subsequent radiographic tumor evaluation [22]. Serum levels of IL-8 were measured by a commercial enzyme-linked immunosorbent assay (ELISA) (Human IL-8 ELISA set; BD Bioescience Pharmingen). Details are provided in the supplementary Appendix, available at Annals of Oncology online.
Quantitative in situ IL-8 mRNA measurement
In situ detection of IL-8 mRNA transcripts in FFPE tumor sections was carried out using the multiplex RNAscope paired-probe assay (Advanced Cell Diagnostics) coupled to quantitative immunofluorescence, as previously described [23].
Statistical analyses
Details are provided in the supplementary Appendix, available at Annals of Oncology online.
Results
Patient population
From November 2008 to May 2016, 63 patients were included in this study at Yale University and University Clinic of Navarra. The identification cohort included 29 metastatic melanoma patients treated with nivolumab or pembrolizumab (identification melanoma cohort). The validation cohorts included 19 metastatic NSCLC patients treated with nivolumab or pembrolizumab (independent NSCLC cohort) and 15 metastatic melanoma patients treated with ipilimumab plus nivolumab (independent melanoma cohort). Patients’ baseline and treatment characteristics are shown in supplementary Table S1, available at Annals of Oncology online. At a median-follow up of 15 months across the three cohorts, 13 of 29 patients (44.8%) in the identification melanoma cohort, 11 of 19 patients (57.9%) in the independent NSCLC cohort and 10 of 15 patients (66.7%) in the independent melanoma cohort had a confirmed objective response by RECISTv1.1, with disease control rates of 48.2%, 63.1% and 80%, respectively. Two of nine NSCLC responders presented pseudoprogression (i.e. >25% initial increase in tumor burden and subsequent imaging evaluations that fulfilled criteria of partial response). Additionally, a 58-year-old female patient with metastatic bladder cancer treated with atezolizumab (anti-PD-L1 mAb) who presented with pseudoprogression and subsequently fulfilled criteria of partial response, was studied. The response characteristics of the different patient cohorts are shown in supplementary Table S2, available at Annals of Oncology online.
Changes in serum IL-8 levels reflect tumor response to anti-PD-1 mAbs in metastatic melanoma patients
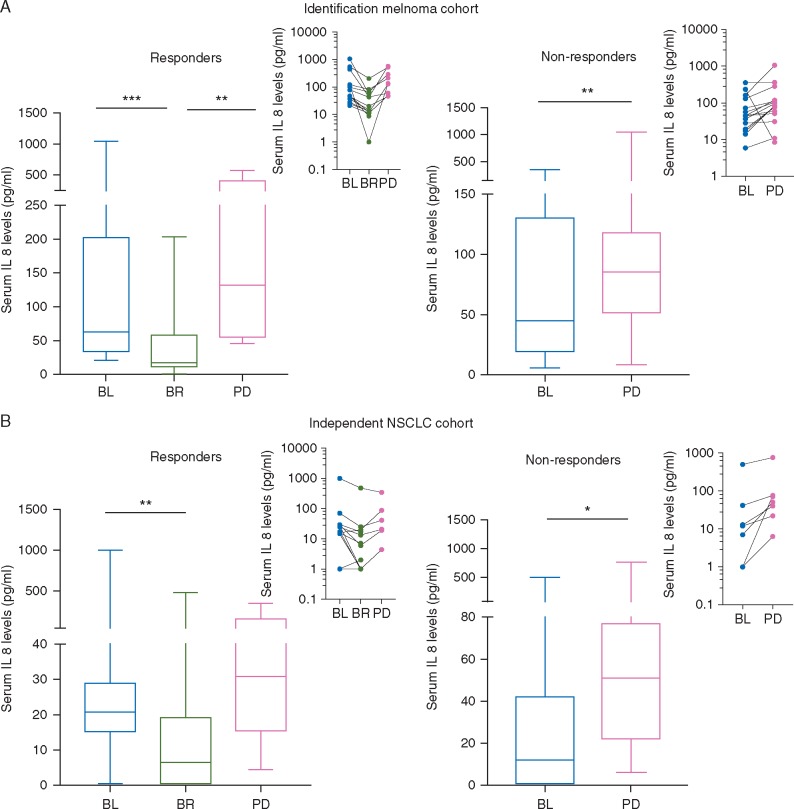
We assessed serum IL-8 levels during single-agent anti-PD-1 mAbs treatment of 29 metastatic melanoma patients. In responding patients (n = 14), median serum IL-8 levels decreased significantly at the moment of best response (BR), when compared with baseline levels [baseline: 63 pg/ml (Q1–Q3: 34.4–202) versus BR: 17.4 pg/ml (Q1–Q3: 11.7–57.7); P <0.001], and significantly increased upon progression in those cases in which it occurred [BR: 43 pg/ml (Q1–Q3: 11.5–73.8) versus PD 132 pg/ml (Q1–Q3: 55–405); P = 0.004] (Figure 1A, left). In non-responders (n = 15), the median serum IL-8 levels significantly increased upon progression [baseline 45 pg/ml (Q1–Q3: 19–130) versus PD 85 (Q1–Q3: 51.4–118); P= 0.013] (Figure 1A, right). Overall, 13 of 14 responders (92.8%) showed decreased plasma IL-8 at BR and 13 of 15 non-responders (86.6%) displayed elevation in IL-8 levels.
Figure 1.
Changes in serum IL-8 levels reflect tumor response in metastatic melanoma and NSCLC patients during treatment with anti-PD-1 mAbs. Metastatic melanoma (n = 29) and NSCLC (n = 19) patients were treated with anti-PD-1 mAbs and serum IL-8 levels were assessed at baseline (BL), at the moment of best response (BR), and at the moment of progressive disease (PD). (A) Results of IL-8 levels are plotted in the different time-points for metastatic melanoma responders (n = 14) (left) and non-responders (n = 15) (right) according to RECIST 1.1. (B) Results of IL-8 levels are plotted in the different time-points for metastatic NSCLC (n = 12) (left) and non-responders (n = 7) (right) according to RECIST 1.1. Insets show the progression of IL-8 values in each individual patient at the different time-points. Statistical comparisons across median IL-8 levels in different time-points were made by nonparametric Mann–Whitney U tests. *P <0.05, **P <0.01, ***P <0.001.
Changes in serum IL-8 levels reflect tumor response to anti-PD-1 mAbs in metastatic NSCLC patients
We validated our results in 19 NSCLC patients treated with single-agent anti-PD-1 mAbs (independent NSCLC cohort). In responding patients (n = 12) median serum IL-8 levels decreased significantly at the moment of BR [baseline 20.8 pg/ml (Q1–Q3: 15.1–29) versus BR 6.5 pg/ml (Q1–Q3: 0–19); P= 0.005] (Figure 1B, left). In non-responders (n = 7), median IL-8 serum levels significantly increased at the moment of progression, when compared with baseline levels [baseline 12 pg/ml (Q1–Q3: 0–42) versus PD 51 pg/ml (Q1–Q3: 22–77); P= 0.016] (Figure 1B, right).
Early changes in serum IL-8 levels predict response to anti-PD-1 mAbs in melanoma and NSCLC patients
Due to the observed association between serum IL-8 levels and tumor response, we hypothesized that early changes in serum IL-8 concentrations might serve as an early predictor of clinical benefit to anti-PD-1 treatment. Therefore, we measured serum IL-8 levels at baseline and 2–3 weeks after starting treatment in patients from the identification melanoma cohort.
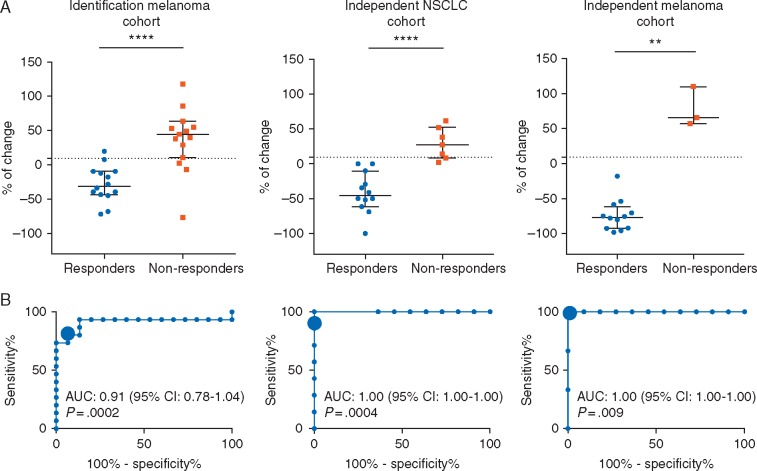
Early changes in serum IL-8 levels were significantly associated with response to anti-PD-1 mAbs [median change (%): responders −31.3% [Q1–Q3: −43.8 to (−31.3)]; non-responders 44.4% (Q1–Q3: 10.7–63.2); P <0.0001], with an area under curve (AUC) in the receiver operation characteristics (ROC) function of 0.91 (95% CI 0.78–1.04, P= 0.0002) (Figure 2A and B, left). Using the ROC curve to determine the value of serum IL-8 levels change to predict response, we chose >9.2% change over baseline as the cut-off point that combined maximal sensitivity (80%, 95% CI 51.9%–95.67%) with best specificity (92.9%, 95% CI 66.1%–99.8%).
Figure 2.
Early changes in serum IL-8 levels are associated with response in melanoma and NSCLC patients treated with single-agent anti-PD-1 mAbs or in melanoma patients treated with the combination of anti-PD-1 plus anti-CTLA-4 mAbs. (A) Percentages of change in serum IL-8 levels in melanoma patients treated with single agent anti-PD-1 mAbs (left panel); NSCLC patients treated with single agent anti-PD-1 mAbs (middle panel); and melanoma patients treated with anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) mAbs (right panel), from baseline levels to 2–3 weeks after start of treatment. Statistical comparisons across median IL-8 levels in responders versus non-responders were made by nonparametric Wilcoxon test. (B) ROC curves for the correlation of early changes in serum IL-8 levels with response to treatment in the identification melanoma cohort (left panel); the independent NSCLC cohort (central panel); and the independent melanoma cohort (right panel). The cut-off point of ≥9.2% increase in serum IL-8 levels is designated by a larger circle. AUC and null hypothesis P-value are indicated inside the graph in each case. **P <0.01; ****P <0.0001.
We validated this cut-off point in the independent NSCLC cohort, confirming association with response [median change (%): responders: −45.6% (Q1/Q3: −59.1/−15.1)], non-responders 27.0% (Q1–Q3: 8.3–52.1); P <0.0001] with an AUC of 1.00 (95% CI 1.00–1.00, P= 0.0004) (Figure 2A and B, middle). In NSCLC patients, the cut-off point of a >9.2% change showed a sensitivity and specificity to predict response of 85.7% (95% CI 42.1–99.6) and 100% (95% CI 73.5–100), respectively.
Changes in serum IL-8 levels reflect and predict response in metastatic melanoma patients treated with the combination of anti-PD-1 plus anti-CTLA-4 mAbs
We studied serum IL-8 levels in 15 metastatic melanoma patients treated with anti-PD-1 plus anti-CTLA-4 mAbs (independent melanoma cohort). We found that in responsive patients (n = 12) median serum IL-8 levels decreased at the moment of BR [baseline 42.5 pg/ml (Q1–Q3: 20.7–131.5) versus BR 5.5 pg/ml (Q1–Q3: 1.7–8.7), P <0.001] (supplementary Figure S1, available at Annals of Oncology online). Since most patients remained in response at the time of data analysis and only 3 of the 15 patients showed no response [median serum IL-8 levels: baseline 32 pg/ml versus PD 81 pg/ml], we were unable to study the significance of serum IL-8 changes at progression. Early changes in serum IL-8 levels in this group were also significantly associated with the eventual onset of response [median change (%): responders −76.7% [Q1–Q3: −92.5 to (−61.8)], non-responders 65.6% (Q1–Q3: 57.1–110); P= 0.004] with an AUC of 1.00 (95% CI 1.00–1.00, P= 0.009). Using the 9.2% change as stratification threshold, the sensitivity was 100% (95% CI 29.2–100) and specificity 100% (95% CI 73.5–100) (Figure 2A and B, right).
Serum IL-8 levels decrease during pseudoprogression
We tested serum IL-8 levels in three cancer patients (two NSCLC, one bladder urothelial carcinoma) that presented pseudoprogression according to investigator’s criteria. Serum IL-8 levels were determined at baseline, at the moment of pseudoprogression (first increase >25% in tumor burden) and in subsequent imaging evaluations that fulfilled criteria of partial response. Interestingly, despite imaging-assessed increases in the target lesion size during pseudoprogression, serum IL-8 levels decreased and remained lower than baseline at subsequent imaging evaluations. Moreover, serum IL-8 levels steadily increased when one of these three cancer patients eventually developed PD (supplementary Figure S2, available at Annals of Oncology online).
Serum IL-8 levels reflect tumor IL-8 production
Using multiplexed quantitative fluorescence and the RNAscope paired-probe assay, we validated an assay for in situ IL-8 mRNA hybridization using a human colon cancer (HT-29) cell line as a positive control and a human embryonic kidney 293 (HEK-293) cell line as a negative control (Figure 3A and B). Then, on NSCLC patient biopsies we multiplexed in situ IL-8 mRNA with positive/negative mRNA controls and a panCK protein staining to determine the level of IL-8 mRNA in the tumor (e.g. cytokeratin-positive) and stromal (cytokeratin-negative) tissue compartments. Interestingly, we found that IL-8 was expressed in the lung cancer tissues and was more abundant in the tumor compartment than in the stromal compartment (Figure 3C and D). Additionally, we observed that IL-8 expression in the tumor compartment was significantly higher in patients with markedly elevated serum IL-8 levels than in cases with low or undetectable serum IL-8 (Figure 3C).
Figure 3.
In situ IL-8 mRNA measurement using quantitative fluorescence. (A) IL-8 mRNA transcripts were detected using multiplexed in situ hybridization in FFPE preparations containing human colon cancer HT-29 cells as a positive control and HEK-293 cells as negative control. The signal in each preparation was measured using quantitative fluorescence. (B) Representative fluorescence captions showing the IL-8 mRNA signal (red channel) in HT-29 cells (upper panels) and HEK-293 cells (lower panels). Nuclei were highlighted with DAPI (blue channel). (C) Level of IL-8 mRNA in the tumor (cytokeratin-positive) and stromal (cytokeratin-negative) compartment of NSCLC samples with high and low levels of serum IL-8. (D) Representative fluorescence caption showing the localized IL-8 mRNA signal (red channel), cytokeratin positivity (green channel) and DAPI (blue channel) staining. The number of independent 20× fields of view analyzed is indicated within each bar and scores are expressed as arbitrary units (AU). Error bars represent SEM. *P <0.05, **P <0.01, ***P <0.001.
Changes in serum IL-8 levels correlate with overall survival in metastatic melanoma and NSCLC patients treated with anti-PD-1
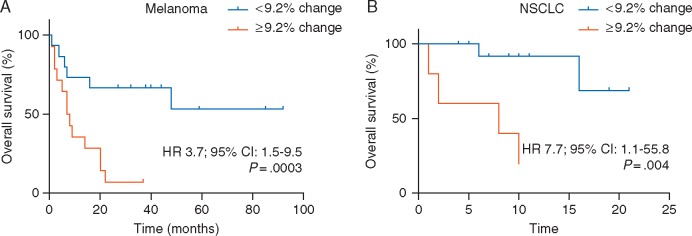
Using the same stratification cut-off point as in the assessment of response, overall survival was significantly longer in PD-1-treated melanoma patients presenting early decreases in serum IL-8 levels (median OS: not reached) than in patients showing early increases (median OS: 7 months, 95% CI: 3.3–10.7; log-rank P= 0.003) (Figure 4A). Similar results were observed in the independent NSCLC cohort [median OS (decrease): not reached versus median OS (increase): 8 months, 95% CI: 0–19.7; log-rank P= 0.004] (Figure 4B). Additionally, univariate analyses (Cox regression model) showed that early increases in serum IL-8 levels were associated with poorer OS in melanoma patients (HR 3.7; 95% CI: 1.5–9.5; P= 0.003) and NSCLC patients (HR 9.78; 95% CI: 1.0–90.1, P= 0.004) treated with anti-PD-1 mAbs.
Figure 4.
Changes in serum IL-8 levels are associated with overall survival in melanoma and NSCLC patients treated with anti-PD-1 mAbs. Kaplan–Meier curves of overall survival (OS) stratified by early changes in serum IL-8 levels in (A) melanoma and (B) NSCLC patients treated with single-agent anti-PD1 mAbs. Data were analyzed using the log-rank (Mantel–Cox) test.
Discussion
Recent experience has demonstrated that traditional criteria for evaluation of response underestimate the effect of some new therapies such as checkpoint inhibitors, due to delayed kinetics and atypical patterns of response [24]. To address this vital problem, alternative criteria, termed the immune-related response criteria (irRC), have been implemented [12]. These new response criteria open the possibility of continuing treatment following the first progression until a new progression in a second assessment is observed. The rationale is to avoid premature treatment cessation in patients who will eventually experience clinical benefit. However, irRC also rely on imaging evaluation and suffer from the inherent limitations of these methods, especially for early evaluation of treatment results, when it is more difficult to define response or to declare progression. Early identification of responders and non-responders could help to avoid unnecessarily prolonged treatments, thus limiting the associated toxicities and costs.
In this study, we demonstrate that changes in serum IL-8 levels are associated with response to anti-PD-1 therapy in patients with metastatic melanoma and NSCLC. Moreover, we show that early changes in serum IL-8 levels, measured only 2–3 weeks after starting therapy (before radiologic evaluation) can predict response and overall survival. These results are in agreement with the fact that serum IL-8 concentrations actually reflect tumor burden [13]. Therefore, these early biochemical changes detected in peripheral blood could be indicative of changes in tumor burden in the initial weeks of treatment when imaging evaluation is not conclusive.
IL-8 levels may also be helpful to identify pseudoprogression. During pseudoprogression, increases in immune tumor infiltration impact on the overall tumor lesion volume. Current imaging techniques cannot differentiate the composition of cells inside the growing lesions. However, serum IL-8 levels might reflect more accurately changes in the tumor-cell compartment. In this regard, we present the results of three patients who developed true pseudoprogression, i.e. initial tumor growth followed by an objective partial response, during treatment. During the increase in tumor size as shown by imaging evaluation, serum IL-8 levels significantly decreased and remained lower than baseline when in subsequent imaging evaluations the overall tumor mass decreased. These results generate the hypothesis that changes in serum IL-8 levels might become a useful tool to diagnose and follow pseudoprogression. However, sequential samples of larger series of patients experiencing pseudoprogression need to be studied to confirm these findings.
Recently, other biomarkers sequentially tested in blood have been shown to be potentially useful to monitor response in melanoma [25] and NSCLC [26] patients treated with anti-PD-1 therapies. These biomarkers, as occurs with serum IL-8 levels, have the advantage that they are noninvasive, and in contrast to biopsies, they can be repeated and sequentially studied, and thus reflect changes in cancer progression during treatment. This could be a major advantage in the case of immunotherapies, due to the dynamic nature of the antitumor immune response. Moreover, serum biomarkers reflect whole tumor burden and they may represent disease progression in an all-inclusive way, when compared with methods that only study a fraction of a single lesion. Serum IL-8 changes showed a very high specificity and sensitivity (mean AUC = 0.97 among the three different patient groups) to predict clinical outcome in melanoma and NSCLC patients treated with anti-PD-1 mAbs. Additionally, we have found that analyzing the three cohorts individually or mixing all patients together, the correlation between serum IL-8 levels and clinical response remains significant, supporting the strength of the biomarker (supplementary Figure S3, available at Annals of Oncology online). This could be explained because of a tight control of IL-8 expression in normal tissues given the potent pro-inflammatory effect of this cytokine and its easy access to the bloodstream given its relatively low molecular weight and solubility [27]. However, our results should be confirmed in larger, independent studies. Moreover, the possibility of artifacts due to concurrent inflammatory conditions [28] should be studied in detail. In this regard, we are considering combining IL-8 serum levels with inflammation-denoting analytes to help distinguish IL-8 increases related to cancer progression and those potentially caused by an inflammatory condition such as concurrent infections or autoimmune adverse events. However, we have found that in our NSCLC cohort only serum IL-8 levels change, but not other tested inflammation-associated analytes, are significantly associated with clinical response (supplementary Figure S4, available at Annals of Oncology online).
We did not observe any significant association between baseline serum IL-8 levels and response to anti-PD-1 mAbs (supplementary Figure S5, available at Annals of Oncology online), thus frustrating our expectation that this parameter could be a useful biomarker to select patients more likely to respond to anti-PD1 therapies. Nonetheless, the fact that response correlates with early changes in the serum concentration of IL-8 and not with baseline levels suggests that IL-8 could be more than a mere surrogate of tumor burden, since it may functionally reflect important changes in the composition of the tumor microenvironment (TME) after PD-1 or PD-L1 blockade. Moreover, we did not find any association between serum IL-8 levels (baseline or early change) and treatment-related toxicity (supplementary Figure S6, available at Annals of Oncology online), reinforcing the idea that serum IL-8 levels are specifically reflecting PD-pathway blockade effects at the TME.
In this study, we have demonstrated that IL-8 is produced preferentially by cytokeratin-positive tumor cells in biopsies from five NSCLC patients. Of note, relatively low/focal IL-8 signal was also observed in stromal cells. In this regard, tumor-associated myeloid cells are also a source of IL-8 and changes in serum IL-8 concentration might be reflecting changes in number and/or function in this leukocyte compartment. Interestingly IL-8 itself has been recently demonstrated to attract myeloid suppressor cells to tumors [21].
In conclusion, we have shown that changes in serum IL-8 levels accurately correlate with tumor burden changes in metastatic melanoma and NSCLC patients during treatment with anti-PD-1 mAbs and in metastatic melanoma patients during treatment with anti-PD-1 plus anti-CTLA-4 mAbs. Moreover, early changes in serum IL-8 levels, measured before responses were evaluated by radiologic imaging, also correlated with subsequent clinical responses. We also found that early changes in serum IL-8 concentrations during anti-PD-1 therapy correlate with overall survival in patients with metastatic melanoma and NSCLC. Finally, IL-8 serum levels were able to discern response in a small number of patients presenting pseudoprogression. All these data suggest that, following confirmation of our results in independent series, IL-8 serum levels may become a useful tool for the clinical management of metastatic melanoma and NSCLC patients treated with immune checkpoint blockade.
Supplementary Material
Acknowledgements
The authors particularly acknowledge the patients for their participation, the Biobank of the University of Navarra for its collaboration, the nurse Lourdes Soria from Oncology Department of Clínica Universidad de Navarra for their collaboration in the collection of the samples and Dr Paul Miller for editing of the article.
Funding
This work was supported by the Yale SPORE in Skin Cancer, funded by the National Cancer Institute, US National Institutes of Health, under award number 1 P50 CA121974 (RH) and by grants from MICINN (SAF2011-22831 and SAF2014-52361-R) (IM). MFS is a recipient from a Spanish Society of Clinical Oncology Fellowship. IM is funded by the Departamento de Salud del Gobierno de Navarra, Redes temáticas de investigación cooperativa RETICC, European Commission VII Framework and Horizon 2020 programs (AICR and PROCROP), SUDOE-IMMUNONET, Fundación de la Asociación Española Contra el Cáncer (AECC), Fundación BBVA and Fundación Caja Navarra. MER-R receives a Rio Hortega contract (ISCIII). CA receives a Sara Borrell contract from ISCIII. Department of Defense Lung Cancer Research Program Career Development Award #LC150383 (KAS) and Stand Up To Cancer - American Cancer Society Lung Cancer Dream Team Translational Research Grant SU2C-AACR-DT17-15 (KAS).
Disclosure
HC received consulting fees from Regeneron, Prometheus and Alexion Pharmaceuticals and research funds from Merck. LC is a consultant/advisory board member/receives consulting fees from MedImmune, Pfizer, NextCure and GenomiCare; and currently has sponsored research grants from Boehringer Ingelheim, Pfizer and NextCure. MS has served as a consultant/advisory for Bristol-Myers Squib, Medimmune, Amgen, Merus, Astra-Zeneca, Roche-Genetech, Lilly, Merck, Nektar, Symphogen, Baxalta, Lycera, Novartis, Pfizer, Alexion, Vaccinex, Janssen/Johnson and Johnson; IM has served as a consultant for Bristol Myers Squibb, Astra-Zeneca, Roche-Genetech, Novartis, Lilly, Alligator, tusk therapeutics, Boehringer Ingelheim, Merck-Serono. All remaining authors have declared no conflicts of interest.
References
- 1. Chen L, Han X.. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125(9): 3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansell SM, Lesokhin AM, Borrello I. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372(4): 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powles T, Eder JP, Fine GD. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515(7528): 558–562. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brahmer JR, Tykodi SS, Chow LQM. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366(26): 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamid O, Robert C, Daud A. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369(2): 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Sznol M, McDermott DF. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32(10): 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nghiem PT, Bhatia S, Lipson EJ. et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016; 374(26): 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373(2): 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou W, Wolchok JD, Chen L.. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8(328): 328rv4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolchok JD, Hoos A, O’Day S. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res 2009; 15(23): 7412–7420. [DOI] [PubMed] [Google Scholar]
- 12. Hodi FS, Hwu W-J, Kefford R. et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34(13): 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanmamed MF, Carranza-Rua O, Alfaro C. et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res 2014; 20(22): 5697–5707. [DOI] [PubMed] [Google Scholar]
- 14. Walz A, Peveri P, Aschauer H, Baggiolini M.. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun 1987; 149(2): 755–761. [DOI] [PubMed] [Google Scholar]
- 15. Waugh DJJ, Wilson C.. The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14(21): 6735–6741. [DOI] [PubMed] [Google Scholar]
- 16. Zarogoulidis P, Katsikogianni F, Tsiouda T. et al. Interleukin-8 and interleukin-17 for cancer. Cancer Invest 2014; 32(5): 197–205. [DOI] [PubMed] [Google Scholar]
- 17. Mantovani A, Germano G, Marchesi F. et al. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41(9): 2522–2525. [DOI] [PubMed] [Google Scholar]
- 18. Yuan A, Chen JJW, Yao P-L, Yang P-C.. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 2005; 10: 853–865. [DOI] [PubMed] [Google Scholar]
- 19. Infanger DW, Cho Y, Lopez BS. et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res 2013; 73(23): 7079–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellmunt J, González-Larriba JL, Prior C. et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol 2011; 22(12): 2646–2653. [DOI] [PubMed] [Google Scholar]
- 21. Alfaro C, Teijeira A, Oñate C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res 2016; 22(15): 3924–3936. [DOI] [PubMed] [Google Scholar]
- 22. Perez-Gracia JL, Sanmamed MF, Bosch A. et al. Strategies to design clinical studies to identify predictive biomarkers in cancer research. Cancer Treat Rev 2017; 53: 79–97. [DOI] [PubMed] [Google Scholar]
- 23. Velcheti V, Schalper KA, Carvajal DE. et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014; 94(1): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoos A, Eggermont AMM, Janetzki S. et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010; 102(18): 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dronca RS, Liu X, Harrington SM. et al. T cell Bim levels reflect responses to anti-PD-1 cancer therapy. JCI Insight 2016; 1(6): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicolazzo C, Cristina R, Mancini M. et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep 2016; 6: 31726.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 1999; 19(5): 429–438. [DOI] [PubMed] [Google Scholar]
- 28. Harada A, Sekido N, Akahoshi T. et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994; 56(5): 559–564. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.