Abstract
Aims
This study aims to examine the association of clinical co-morbidities with the presence of left atrial (LA) late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR). Previous studies have established the severity of LA LGE to be associated with atrial fibrillation (AF) recurrence following AF ablation. We sought to determine whether baseline clinical characteristics were associated with LGE extent among patients presenting for an initial AF ablation.
Methods and results
The cohort consisted of 179 consecutive patients with no prior cardiac ablation procedures who underwent pre-procedure LGE-CMR. The extent of LA LGE for each patient was calculated using the image intensity ratio, normalized to the mean blood pool intensity, corresponding to a bipolar voltage ≤0.3 mV. The association of LGE extent with baseline clinical characteristics was examined using non-parametric and multivariable models. The mean age of the cohort was 60.9 ± 9.6 years and 128 (72%) were male. In total, 56 (31%) patients had persistent AF. The mean LA volume was 118.4 ± 41.6 mL, and the mean LA LGE extent was 14.1 ± 10.4%. There was no association with any clinical variables with LGE extent by quartiles in the multivariable model. Extent of LGE as a continuous variable was positively, but weakly associated with LA volume in a multivariable model adjusting for age, body mass index, AF persistence, and left ventricular ejection fraction (1.5% scar/mL, P = 0.038).
Conclusion
In a cohort of patients presenting for initial AF ablation, the presence of pre-ablation LA LGE extent was weakly, but positively associated with increasing LA volume.
Keywords: Atrial fibrillation, Cardiac magnetic resonance imaging, Delayed enhancement magnetic resonance, Ablation, Fibrosis
What's new?
• Atrial fibrosis provides the structural substrate for atrial fibrillation (AF) maintenance. Left atrial (LA) late gadolinium enhancement (LGE) on MRI, as a surrogate for atrial fibrosis, has been shown to associate with worse outcomes following AF catheter ablation. We found no patient level factor to be associated with LA LGE, except for a weak association between LA volume and LA LGE. Additional research is warranted to identify the mechanism and predictors of LA LGE in AF patients in order to properly identify the mechanistic role of fibrosis in the AF pathway and strategies for its prevention.
• Left atrial volume is not an adequate surrogate for LA LGE. Determining LA LGE is clinically important in order to properly communicate with patients their chances of success following catheter ablation of AF.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the world and is associated with significant morbidity as well as rising attributable mortality.1 At a structural level, AF is associated with electrical and mechanical atrial remodelling, which provide the substrate for AF initiation and maintenance.2,3 At a clinical level, increasing age, hypertension, obesity, metabolic syndrome, and obstructive sleep apnoea (OSA) are associated with incident AF.4,5 A recent study showed that obese sheep had more interstitial atrial fibrosis, increased fractionated left atrial signals, increased LA voltage heterogeneity, and more easily sustained AF compared with lean sheep.6
This new finding supports the well-established concept that ‘atrial fibrillation begets atrial fibrillation’. The scientific basis of this relationship was first reported by Wijffels et al.3 Using a goat model these authors demonstrated that when AF is artificially maintained there is a decrease in the AF cycle length, and increase in stability of AF and upon termination, and AF is more easily induced with a premature stimulus. However, more recently Kottkamp has come up with a new concept of ‘fibrotic atrial cardiomyopathy’ (FACM), which proposes that there is little relationship between the duration of AF and the extent of myocardial scar.7
There are two main objectives of our study. First, we sought to gain further insight into the competing theories of ‘atrial fibrillation begets atrial fibrillation’ and ‘FACM’ by examining the relationship between AF duration and atrial late gadolinium enhancement (LGE) as a surrogate of fibrosis. We also examined the association of clinical co-morbidities with atrial LGE extent. The second objective of this study was to determine the relationship between atrial scar and atrial size. If a close association between atrial LGE and atrial volume is present, the incremental value of determining LGE extent using cardiac magnetic resonance (CMR) would be limited.
Methods
Patient population
The patient population comprised 179 patients referred for an initial AF ablation at the Johns Hopkins Hospital, who underwent a pre-procedure MRI scan, and consented to participate in a prospective AF ablation registry approved by the Johns Hopkins Institutional Review Board. Patients were categorized based on the AF type according to the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guideline definitions as well as the duration of time since first diagnosis of AF and based on the longest time in continuous AF prior to undergoing AF ablation.
Cardiac magnetic resonance
All patients underwent pre-procedural CMR using a 1.5 Tesla scanner (Avanto, Siemens, Erlangen, Germany) with a six-channel phased array torso coil as previously described.8 Contrast-enhanced three-dimensional (3D) magnetic resonance angiography images were used to define LA and pulmonary vein anatomy. Late gadolinium-enhanced CMR scans were acquired ∼18 min following 0.2 mmol/kg gadolinium injection (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ, USA). We utilized a 3D inversion recovery prepared fast spoiled gradient recalled sequence that is respiratory triggered and navigated, ECG gated, and fat suppressed (repetition time of 2.5–5.5 ms, echo time of 1.52 ms, flip angle at 10 degrees, in-plane resolution of 1.3 × 1.3, slice thickness of 2.0 mm, and inversion time of 240–290 ms).
Left atrial late gadolinium enhancement quantification
The LA myocardium was defined by placing epi- and endocardial contours using QMass MR Software (Version 7.2, Leiden University Medical Center, Leiden, The Netherlands). The image intensity ratio (IIR), a previously described LGE-CMR technique that normalizes myocardial image intensities by the mean blood pool intensity, corresponding to a bipolar voltage ≤0.3 mV was used to identify fibrosis. For this study, we chose a threshold of 0.3 mV to be consistent with prior studies of the extent of LA LGE.9 The methodology for calculation of the IIR and its validation against regional bipolar voltage has been previously described.8 The mean IIR of the entire LA and extent of fibrosis (as a percentage of total LA myocardium) were measured for all patients. Left atrial volume was calculated based upon the 3D reconstruction of the LA contours.
Statistical analysis
Categorical variables were summarized by number and percentage. Continuous variables were summarized as a mean and standard deviation. To examine non-linear associations, LGE extent was analysed as a categorical variable by quartiles: Group 1, ≤6%; Group 2, 7–12%; Group 3, 13–20%; and Group 4, >20%. Additionally, analyses were performed on LGE extent as a continuous variable. However, given the skewed nature of the LGE extent distribution, these analyses were performed after log transformation. The univariable association of LGE extent with clinical variables was assessed using non-parametric tests as appropriate. The multivariable association of clinical variables with LGE extent was examined using multivariable ordinal logistic regression and linear regression as appropriate. Finally, we examined the presence of interaction between LA volume and AF persistence using a multiplicative interaction term. Statistical analyses were performed using STATA Statistical Software (Version 12, StataCorp, LP, College Station, TX, USA). A two-sided P-value of <0.05 was considered statistically significant.
Results
Clinical characteristics
The clinical characteristics of the 179 patients in the cohort are summarized in Table 1. The mean age was 60.9 ± 9.6 years. One hundred and twenty-eight were men (72%) and 56 (31%) had persistent AF. The mean left ventricular ejection fraction (LVEF) was 56.6 ± 8.3. The median CHA2DS2-VASc score was 1 [interquartile range (IQR): 1–3]. Table 1 also shows the LA volume and the extent of LGE. The mean LA volume was 118.4 ± 41.6 mL, and the mean LA LGE extent was 14.1 ± 10.4%.
Table 1.
Clinical characteristics
| Total cohort (n = 179) | Group 1 (n = 49) | Group 2 (n = 45) | Group 3 (n = 44) | Group 4 (n = 41) | P-value (among groups) | |
|---|---|---|---|---|---|---|
| Extent of scar (%) | 14.1 ± 10.4 | 3.6 ± 1.7 | 9.5 ± 1.8 | 16.5 ± 2.4 | 29.2 ± 1.6 | |
| Age (years) | 60.9 ± 9.6 | 61.5 ± 9.0 | 60.7 ± 10.6 | 59.1 ± 9.3 | 62.3 ± 9.4 | 0.436 |
| Caucasian | 169 (94.4%) | 46 (93.9%) | 42 (93.3%) | 41 (93.2%) | 40 (97.6%) | 0.796 |
| Male gender | 128 (71.5%) | 33 (67.4%) | 28 (62.2%) | 34 (77.3%) | 33 (80.5%) | 0.198 |
| Body mass index (kg/m2) | 28.7 ± 5.5 | 29.1 ± 5.9 | 28.4 ± 5.4 | 29.6 ± 5.3 | 27.7 ± 5.4 | 0.415 |
| Coronary artery disease | 19 (10.6%) | 5 (10.2%) | 3 (6.7%) | 7 (15.9%) | 4 (9.8%) | 0.556 |
| Congestive heart failure | 22 (12.3%) | 6 (12.2%) | 2 (4.4%) | 10 (22.7%) | 4 (9.8%) | 0.064 |
| Peripheral vascular disease | 3 (1.7%) | 1 (2.0%) | 0 (0%) | 1 (2.3%) | 1 (2.4%) | 0.790 |
| Hypertension | 95 (53.1) | 26 (53.1%) | 16 (35.6%) | 26 (59.1%) | 27 (65.9%) | 0.031 |
| Obstructive sleep apnoea | 42 (23.5) | 12 (24.5%) | 9 (20%) | 13 (29.6%) | 8 (19.5%) | 0.661 |
| Dyslipidaemia | 76 (42.5%) | 20 (40.8%) | 15 (33.3%) | 18 (40.9%) | 23 (56.1%) | 0.191 |
| Insulin dependent diabetes | 4 (2.2%) | 1 (2.0%) | 0 (0%) | 1 (2.3%) | 2 (4.9%) | 0.461 |
| TIA/stroke | 14 (7.8%) | 7 (14.3%) | 1 (2.2%) | 2 (4.6%) | 4 (9.8%) | 0.129 |
| History of tobacco use | 48 (26.8%) | 17 (34.7%) | 10 (22.2%) | 11 (25%) | 10 (24.4%) | 0.526 |
| Persistent AF | 56 (31.3%) | 10 (24.5%) | 13 (28.9%) | 14 (31.8%) | 19 (46.3%) | 0.067 |
| Time in continuous AF (months) | 4.8 ± 7.1 | 4.1 ± 7.2 | 7.1 ± 11.1 | 3.6 ± 3.1 | 4.4 ± 5.9 | 0.380 |
| LA volume (mL) | 118.4 ± 41.6 | 113.1 ± 46.5 | 110.4 ± 32.0 | 121.8 ± 43.0 | 129.8 ± 41.6 | 0.087 |
| LVEF | 56.6 ± 8.3 | 57.4 ± 7.0 | 58.4 ± 6.8 | 55.2 ± 9.7 | 54.9 ± 9.3 | 0.153 |
| CHA2DS2-VASc | 0.890 | |||||
| 0 | 38 (21.2%) | 7 (14.3%) | 11 (24.4%) | 11 (25%) | 9 (22.0%) | |
| 1 | 60 (33.5%) | 17 (34.7%) | 16 (35.6%) | 12 (27.3%) | 15 (36.6%) | |
| 2 | 31 (17.3%) | 9 (18.4%) | 8 (17.8%) | 10 (22.7%) | 4 (9.8%) | |
| 3 | 25 (14.0%) | 7 (14.3%) | 5 (11.1%) | 7 (15.9%) | 6 (14.6%) | |
| 4 | 15 (8.4%) | 4 (8.2%) | 4 (8.9%) | 2 (4.6%) | 5 (12.2%) | |
| 5 | 4 (2.2%) | 2 (4.1%) | 1 (2.2%) | 1 (2.3%) | 0 (0%) | |
| 6 | 6 (3.4%) | 3 (6.1%) | 0 (0%) | 1 (2.3%) | 2 (4.9%) |
Values in bold are statistically significant (P ≤ 0.05).
Clinical predictors of fibrosis
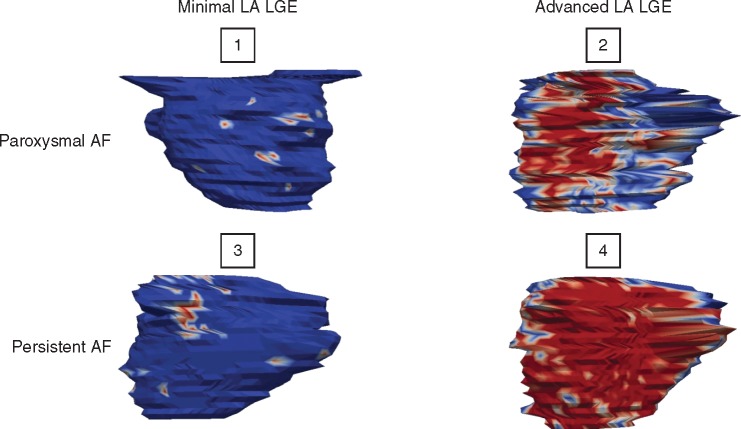
Shown in Figure 1 are four representative LA CMR images. It is notable that no apparent relationship exists between AF type and the degree of LA LGE. Patient 1 is a 67-year-old man with morbid obesity, hypertension, diabetes, dyslipidaemia, OSA, and paroxysmal AF, but has only 2.2% LA LGE. In contrast, Patient 2 is a 50-year-old man with obesity, hypertension, dyslipidaemia, diabetes, OSA, and paroxysmal AF, with 29.7% LA LGE. Patient 3 is a 67-year-old man with dyslipidaemia, OSA, and persistent AF, with only 5.4% LA LGE. Finally, Patient 4 is a 57-year-old man with persistent AF and only a history of hypertension, yet has 32.4% LA LGE.
Figure 1.
Posterior wall of the left atrium of four different patients showing varying degrees of LA LGE independent of AF persistence.
Table 1 summarizes the comparison of clinical characteristics among groups formed by LGE extent quartiles. The mean percentage LGE in the four quartiles was 3.6 ± 1.7%, 9.5 ± 1.8%, 16.5 ± 2.4%, and 29.2 ± 1.6%, respectively. Among the clinical variables, which were examined, the only difference was in the percentage of baseline hypertension among the groups (P = 0.031). In the univariable analysis, there was no other difference in clinical characteristics between the groups. After adjustment for age, body mass index (BMI), coronary artery disease, diabetes, OSA, LVEF, left atrial volume, and type of AF in a multivariable model, there was no longer a difference in the percentage of hypertension between the four groups (P = 0.155). Of particular note, no difference in percentage LGE was observed in patients with paroxysmal vs. persistent AF.
To further explore the relationship between AF duration and LGE, we plotted the association between the maximal AF duration and percentage LGE in Figure 2. No association was noted between time in continuous AF and LGE quartiles (one-way ANOVA P = 0.248). Further, there was no association between time in continuous AF and LGE extent as a continuous variable (Spearman's rho 0.042, P = 0.757). Many patients with persistent AF of only short duration had a marked degree of LGE, whereas other patients who had been in AF for many months had relatively little LGE.
Figure 2.
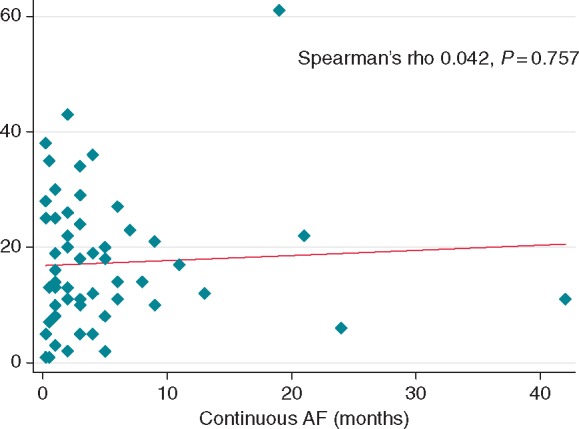
Relationship between LA LGE extent and time in continuous AF among patients with persistent AF.
Relationship between left atrial volume and fibrosis
Shown in Figure 3 is the relationship between LA volume and LGE. Patients with paroxysmal AF are identified by triangles, whereas patients with persistent AF are marked with open squares. It can be appreciated that the association between LA LGE and volume, while present, is weak. The LA volume overall was 118.4 ± 41.6 mL. The LA volume measures corresponding to quartiles of LGE were 113.1 ± 46.5 mL, 110.4 ± 32 mL, 121.8 ± 43.0 mL, and 129.8 ± 41.6 mL, respectively. Log LGE extent as a continuous variable was weakly associated with LA volume (Spearman's rho 0.208, P = 0.005) and LVEF (Spearman's rho −0.190, P = 0.012). Further, those with persistent AF (17.2 ± 11.8%) had a higher LGE extent compared with those with paroxysmal AF (12.7 ± 9.3%) (P = 0.012). After adjusting for age, BMI, AF persistence, and LVEF, LA volume was the only variable associated with LGE extent in a multivariable linear regression model (1.5% scar/mL, P = 0.046) (Table 2, Figure 3).
Figure 3.
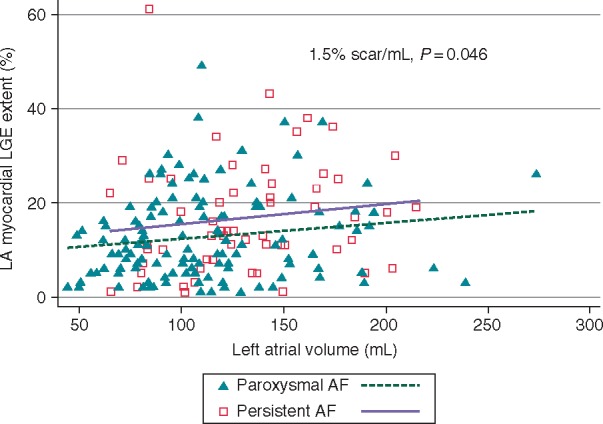
Relationship between LA LGE extent and LA volume.
Table 2.
Association of clinical characteristics with late gadolinium enhancement
| Univariable P-value | Multivariable analysis, OR (95% CI) | Multivariable P-value | |
|---|---|---|---|
| Age (years) | 0.725 | ||
| Caucasian | 0.320 | ||
| Male gender | 0.054 | ||
| Body mass index (kg/m2) | 0.853 | ||
| Coronary artery disease | 0.524 | ||
| Congestive heart failure | 0.337 | ||
| Peripheral vascular disease | 0.540 | ||
| Hypertension | 0.058 | ||
| Obstructive sleep apnoea | 0.827 | ||
| Dyslipidaemia | 0.127 | ||
| Insulin dependent diabetes | 0.319 | ||
| TIA/stroke | 0.709 | ||
| History of tobacco use | 0.527 | ||
| Persistent AF | 0.012 | 1.2 (0.87–1.59) | 0.293 |
| Time in continuous AF (months) | 0.758 | ||
| LA volume (mL) | 0.005 | 1.5 (1.01–2.26) | 0.046 |
| LVEF | 0.012 | 0.53 (0.24–1.60) | 0.111 |
| CHA2DS2-VASc | 0.834 |
Univariable model based on Spearman's rho, Mann–Whitney test, or Kruskal–Wallis test as appropriate.
Multivariable model adjusted for age, BMI, AF persistence, and LVEF.
In the multivariable model for the association of LGE extent with age, BMI, AF persistence, LVEF, and LA volume, there was no evidence for statistical interaction between AF persistence and LA volume (P = 0.506).
Discussion
Main findings
The purpose of this study was to explore the clinical predictors of LA LGE as a surrogate of fibrosis and also to examine the association between LA volume and LGE. Identification of the clinical factors that associate with atrial fibrosis is important not only for screening potential candidates for AF ablation, but also for understanding the pathophysiology of AF and its substrate.
There were two main findings of this study. First, the results of this study showed that no clinical variable predicts the degree of LA LGE on CMR. Second, the association between LA LGE and volume, while present, is weak.
Clinical predictors of scar
There have been several prior studies that have examined predictors of LA LGE. Among these, the largest was of 333 patients undergoing first time AF ablation in which there was no significant difference in the degree of LA LGE by Utah Group staging (Utah 1: <5%, Utah 2: 5–20%, Utah 3: 20–35%, and Utah 4: >35%) among patients with lone AF (defined as<60 years of age, absence of structural heart disease, coronary artery disease, diabetes mellitus, or hypertension) and non-lone AF.10 In another study of 144 patients undergoing pulmonary vein isolation, there was no difference between the Utah Stage groups in regards to age, hypertension, diabetes, coronary artery disease, congestive heart failure, LVEF, or type of AF.11
The results of our study confirm prior reports that no patient clinical co-morbidity is associated with LA LGE. Further, our study, with its sizable population of AF patients, adds to the current literature because the approach used to define delayed enhancement is based on a novel method based on the IIR, which decreases both inter-patient and inter-scan variability.8
The pathophysiology of AF is complex and is an end product of a number of changes including a decrease in the effective refractory period, increasing conduction velocity, and haemodynamic alterations leading to LV dysfunction, increased LA pressure, and impaired atrial contractility. Finally, structural remodelling promotes fibrosis and tissue heterogeneity, which provides a substrate to maintain AF.2 It is thus interesting that while clinical markers such as advanced age, left ventricular dysfunction, and hypertension are associated with the development of AF, they are not associated with LGE extent. The results of this study provide more support to the FACM hypothesis in which a chronic bi-atrial fibrotic substrate exists even among those with ‘lone’ AF. Further, FACM may be a precursor to the development of AF as a recent study of 190 patients referred for cardiac MR showed there is considerable LA LGE among a general cardiology population without AF.12 Further, the presence of AF was independently associated with increasing LA LGE. Whether the association of certain genotypes linked with AF, such as single nucleotide polymorphisms on chromosome 4q25,13 is mediated through LA fibrosis is currently unknown. Recent work by Wang et al.14 in patients with paroxysmal AF has revealed increased expression of micro RNA 146b-5p, which targets tissue inhibitor of metalloproteinase 4 resulting in an increase in collagen content. Further investigation is warranted in the area of genetic modifiers for atrial fibrosis.
Time in continuous AF, which is a marker of AF persistence, has been shown to be associated with worse clinical outcomes, including a higher risk of stroke.15 Prior work has shown that those with persistent AF have lower mean LA bipolar voltage, slowed LA conduction time, and higher complex signals compared with patients without AF.16 In this study, however, we found no association between AF persistence and LA LGE. In agreement with the results of the DECAAF trial,17 these results suggest that the association between LA LGE, AF persistence, and AF recurrence post-ablation may be mediated by different factors. Left atrial LGE is a surrogate for structural remodelling that provides the substrate to maintain AF and can lead to LA dysfunction.18 However, persistent AF may lead to more electrical remodelling (changes in the atrial ERP, slowing of conduction velocity) allowing for AF recurrence. Further, among patients with persistent AF, there was no significant relationship between time in continuous AF and LA LGE extent. The results of this study, which show the duration of AF has little impact on scar, further supports the concept put forth by Kottkamp.
Relationship between left atrial volume and extent of scar
Prior studies have demonstrated that LGE predicts outcomes of AF ablation.11,17,19 In the DECAAF study, a multicentre, prospective, observational cohort study of 272 patients undergoing initial catheter ablation for AF, the extent LA LGE was an independent predictor of recurrent atrial arrhythmia. Compared with those in the Utah Stage 1 fibrosis group, those in Utah Stage 4 were more than four times as likely to develop recurrent atrial arrhythmias after 475 days of follow-up.
Studies have also reported that LA volume predicts outcomes. Among 146 patients with symptomatic AF undergoing catheter ablation, LA volume was found to be an independent predictor of long-term freedom from AF.20 For every 10 mL increase in LA volume, there was 14% increased odds of AF recurrence. Interestingly, in a study of 50 paroxysmal AF patients undergoing pre-procedural cardiac MR prior to pulmonary vein isolation, there was a significant association of LA LGE with AF recurrence at 12 months; however, increasing LA size (which was associated with increasing LA LGE) was not associated with AF recurrence.21 One of the goals of our study was to examine the relationship between LA LGE and LA volume. If a close correlation exists, one could argue that there is little value in determining LA LGE and LA volume may serve as a surrogate. The results of this study suggest a weak association between LA volume and LGE extent. This observation suggests that MRI imaging for detection of LGE provides unique information over and beyond the prognostic value of LA volume. Further trials are needed to more precisely define the relative importance of LA volume and fibrosis in determining outcomes of AF ablation.
Limitations
The cohort under study was formed exclusively of patients referred for catheter ablation of AF and thus reflects a selected population with limited generalizability to the overall source population of those with AF. Second, LA wall thickness is near the limit of LGE image resolution and thus it is possible that parts of the blood pool or pericardium were encompassed in the LA wall LGE analysis in some cases. Finally, the classification of paroxysmal vs. persistent AF may not always be clear in certain patients, as it is possible for a patient with documented persistent AF to also have periods of paroxysmal AF.
Conclusions
Left atrial LGE as a surrogate of left atrial remodelling and fibrosis is only one contributor to the pathophysiology of AF. This is evident by the fact that control of other haemodynamic attributing risk factors such as obesity, hypertension, and diabetes decreases AF recurrence after AF ablation.22 Based on the results of this study showing a lack of association between LA LGE and traditional co-morbidities associated with AF, one must begin to think of LA LGE as a phenomenon independent of clinical risk factors,7 which may be modifiable in order to reduce AF burden supporting the concept of Kottkamp's FACM. The results of this study also reveal that only a weak association exists between LA volume and LA LGE, thus making LA volume an un-reliable surrogate for determining LA LGE extent. Further research is warranted to determine whether these findings can be extrapolated to the general AF population, whether other clinical characteristics, genetic variants, or biomarkers are associated with the development of LA fibrosis, and whether baseline LA LGE in healthy individuals predicts future development of AF.
Funding
The study was funded by NIH grants 5T32HL007227-38 to J.C.; NIH grants K23HL089333 and R01HL116280 as well as a Biosense-Webster grant to S.N.; and The Roz and Marvin H Weiner and Family Foundation, The Dr. Francis P. Chiaramonte Foundation, The Marilyn and Christian Poindexter Arrhythmia Research Fund, and The Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
Conflict of interest: S.N. is a scientific advisor to Medtronic, CardioSolv, and Biosense Webster, Inc., and principal investigator for research funding to Johns Hopkins University from Biosense Webster, Inc.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al. . Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 3. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 4. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T et al. . Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–71. [DOI] [PubMed] [Google Scholar]
- 5. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ et al. . Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP et al. . Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J 2013;34:2731–8. [DOI] [PubMed] [Google Scholar]
- 8. Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T et al. . Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm 2014;11:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison JL, Jensen HK, Peel SA, Chiribiri A, Grondal AK, Bloch LO et al. . Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J 2014;35:1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CT et al. . Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm 2010;7:1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S et al. . Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011;22:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A et al. . Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26:484–92. [DOI] [PubMed] [Google Scholar]
- 13. Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J et al. . Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2015;8:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Wang Y, Han J, Li Y, Xie C, Xie L et al. . Integrated analysis of microRNA and mRNA expression profiles in the left atrium of patients with nonvalvular paroxysmal atrial fibrillation: role of miR-146b-5p in atrial fibrosis. Heart Rhythm 2015;12:1018–26. [DOI] [PubMed] [Google Scholar]
- 15. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C et al. . The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
- 16. Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ et al. . Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol 2012;23:232–8. [DOI] [PubMed] [Google Scholar]
- 17. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F et al. . Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 18. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K et al. . Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging 2015;8:e002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akkaya M, Higuchi K, Koopmann M, Burgon N, Erdogan E, Damal K et al. . Relationship between left atrial tissue structural remodelling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace 2013;15:1725–32. [DOI] [PubMed] [Google Scholar]
- 20. Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S et al. . Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol 2009;20:1005–10. [DOI] [PubMed] [Google Scholar]
- 21. Malcolme-Lawes LC, Juli C, Karim R, Bai W, Quest R, Lim PB et al. . Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2-center study. Heart Rhythm 2013;10:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D et al. . Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]