Abstract
Background
Recent breast cancer treatment guidelines recommend that higher-risk premenopausal patients should receive ovarian function suppression (OFS) as part of adjuvant endocrine therapy. If chemotherapy is also given, it is uncertain whether to select concurrent or sequential OFS initiation.
Design and methods
We analyzed 1872 patients enrolled in the randomized phase III TEXT and SOFT trials who received adjuvant chemotherapy for hormone receptor-positive, HER2-negative breast cancer and upon randomization to an OFS-containing adjuvant endocrine therapy, initiated gonadotropin-releasing-hormone-agonist triptorelin. Breast cancer-free interval (BCFI) was compared between patients who received OFS concurrently with chemotherapy in TEXT (n = 1242) versus sequentially post-chemotherapy in SOFT (n = 630). Because timing of trial enrollment relative to adjuvant chemotherapy differed, we implemented landmark analysis re-defining BCFI beginning 1 year after final dose of chemotherapy (median, 15.5 and 8.1 months from enrollment to landmark in TEXT and SOFT, respectively). As a non-randomized treatment comparison, we implemented comparative-effectiveness propensity score methodology with weighted Cox modeling.
Results
Distributions of several clinico-pathologic characteristics differed between groups. Patients who were premenopausal post-chemotherapy in SOFT were younger on average. The median duration of adjuvant chemotherapy was 18 weeks in both groups. There were 231 (12%) BC events after post-landmark median follow-up of about 5 years. Concurrent use of triptorelin with chemotherapy was not associated with a significant difference in post-landmark BCFI compared with sequential triptorelin post-chemotherapy, either in the overall population (HR = 1.11, 95% CI 0.72–1.72; P = 0.72; 4-year BCFI 89% in both groups), or in the subgroup of 692 women <40 years at diagnosis (HR = 1.13, 95% CI 0.69–1.84) who are less likely to develop chemotherapy-induced amenorrhea.
Conclusion
Based on comparative-effectiveness modeling of TEXT and SOFT after about 5 years median follow-up, with limited statistical power especially for the subgroup <40 years, neither detrimental nor beneficial effect of concurrent administration of OFS with chemotherapy on the efficacy of adjuvant therapy that includes chemotherapy was detected.
Clinicaltrials.gov
NCT00066690 and NCT00066703.
Keywords: adjuvant therapy, GnRH-agonist, hormone receptor-positive, ovarian function suppression, premenopausal, triptorelin
Introduction
Surgical ovarian ablation was the first systemic adjuvant treatment of breast cancer [1]. More recently, gonadotropin-releasing-hormone (GnRH) agonists provide a reversible method to achieve ovarian function suppression (OFS). Several trials have since compared ovarian ablation or GnRH-agonist-induced OFS to chemotherapy alone and/or with chemotherapy followed by OFS in premenopausal women with hormone receptor-positive (HR+) early breast cancer (with or without oral endocrine therapy) [2]. However, addition of OFS to endocrine adjuvant treatment of HR+ breast cancer has remained controversial for a number of reasons, including the confounding effect of chemotherapy-induced amenorrhea (CIA) and study designs including non-HR+ patients and omitting a tamoxifen-alone comparator. Current anthracycline/taxane-based regimens are associated with a lower incidence of CIA than older alkylating-based regimens such as cyclophosphamide/methotrexate/5-fluorouracil, especially in younger women [3]. Data from 9864 premenopausal patients treated with chemotherapy alone in cooperative group trials showed a significantly worse outcome for very young patients (<35 years) with HR+ tumors compared with older HR+ patients [4]. The age-related difference in outcome was much smaller for HR-negative patients. The lower incidence of CIA in very young patients translates into an attenuated endocrine effect of chemotherapy and suggests a potential impact of early initiation of adjuvant endocrine therapy [4, 5].
There are theoretical concerns about the concurrent use of endocrine therapy with chemotherapy based on laboratory and animal studies [6–8]. The only randomized evidence in the adjuvant setting derives from studies in postmenopausal women treated with tamoxifen [9–11]. The data for concurrent use of endocrine therapy and chemotherapy in premenopausal women are scanty: among 1096 patients (39% premenopausal) enrolled in two trials of adjuvant chemotherapy, in which tamoxifen was given concomitantly or sequentially by physician discretion, no difference in DFS was reported [12]. Subgroup analyses suggested greater benefit of concomitant versus sequential therapy among 427 premenopausal women, particularly those aged ≤40 years [12]. However, concomitant tamoxifen does increase the risk of thrombotic events during chemotherapy and is not recommended [11].
The TEXT and SOFT randomized phase III trials studied adjuvant endocrine therapy for premenopausal women with HR+ early breast cancer [13–15]. TEXT enrolled patients before starting any adjuvant therapy, including chemotherapy, whereas SOFT enrolled patients who were premenopausal after finishing adjuvant chemotherapy. Thus the timing of OFS and chemotherapy, if chemotherapy was given, differed in the two trials, being concurrent in TEXT and sequential in SOFT. Based largely on the results of SOFT and TEXT, guidelines recommend higher-risk patients should receive OFS as part of adjuvant endocrine therapy [16–19].
Several questions remain. Is there a best timing for initiating OFS when chemotherapy is also given? Is there a benefit to undergoing OFS as quickly as possible, concurrently with chemotherapy, or might concurrent administration be detrimental? Is it equivalent to wait and see if chemotherapy induces menopause thereby avoiding GnRH-agonist injections? Should age be taken into consideration when deciding when to start OFS? In the absence of a randomized clinical trial to address these questions, we used comparative-effectiveness methods for non-randomized treatment comparisons to conduct an exploratory, observational study of SOFT/TEXT patients treated with adjuvant chemotherapy and GnRH-agonist. We investigated whether there was evidence of differential efficacy between initiating adjuvant GnRH-agonist concurrently with chemotherapy versus sequentially after chemotherapy, once premenopausal status was reestablished.
Patients and methods
The designs and conduct of the TEXT/SOFT trials have been described previously [13–15]. The ethics committee at each participating center approved the study protocols; all patients provided written informed consent. In both trials, eligible premenopausal women had surgically resected, invasive early HR+ breast cancer (≥10% ER and/or PgR-expressing cells). Endocrine therapy, including OFS if it was assigned, was given for 5 years; oral endocrine therapy commenced after chemotherapy in both trials.
In TEXT, OFS was given from the start of adjuvant therapy. Between November 2003 and March 2011, 2660 women were randomized within 12 weeks after definitive surgery to exemestane + OFS or tamoxifen + OFS. OFS was by GnRH-agonist triptorelin; after at least 6 months of triptorelin, patients could opt for ovarian ablation. Chemotherapy was optional and planned for 1607 (60%) patients, started concurrently with triptorelin.
In SOFT, OFS was given sequentially after chemotherapy. Between December 2003 and January 2011, 3047 women were randomized to tamoxifen, tamoxifen + OFS or exemestane + OFS. A total of 1628 (53%) patients who received prior (neo)adjuvant chemotherapy were randomized within 8 months after the final dose of chemotherapy once a premenopausal level of estradiol was confirmed. Use of triptorelin or ovarian ablation, if OFS was assigned, was by patient preference.
Analysis population, endpoints and statistical considerations
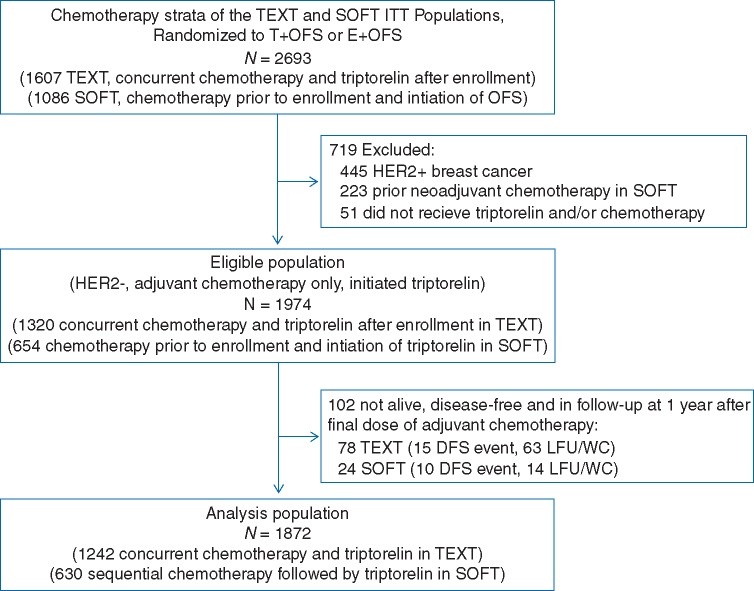
In the intention-to-treat populations, 2693 patients within the chemotherapy strata were randomized to an OFS-containing combination. After exclusions, 1320 patients from TEXT and 654 from SOFT with HER2-negative disease treated with adjuvant chemotherapy and triptorelin were analyzed (Figure 1). TEXT patients were enrolled a median of 1.8 months post-surgery. SOFT patients were enrolled after adjuvant chemotherapy, after demonstration of premenopausal estradiol level, at a median of 9.8 months post-surgery. To avoid guarantee-time bias, the observation periods for analysis were re-aligned forwards, by defining a landmark time point at 1 year after the final dose of chemotherapy, which ensured that all SOFT patients could have reached the first 3-month protocol visit (supplementary Figure S1, available at Annals of Oncology online). Among TEXT and SOFT patients, the landmark was a median of 15.5 months [interquartile range (IQR) 14.3–16.0 months] and 8.1 months (IQR 6.3–9.8 months) post-enrollment.
Figure 1.
Flow diagram for defining the eligible population from TEXT and SOFT and the final analysis population.
The primary endpoint was breast cancer-free interval (BCFI), defined as the time interval beginning 1 year after final dose of adjuvant chemotherapy until the first appearance of invasive local-regional or distant recurrence or invasive contralateral breast cancer; in the absence of an event, BCFI was censored at date of last visit (or date of death without breast cancer event). The secondary endpoint was distant recurrence-free interval (DRFI), similarly defined beginning 1 year after final dose of adjuvant chemotherapy until the first appearance of invasive distant recurrence. BCFI was the chosen endpoint, rather than DFS, to disregard second (non-breast) invasive malignancies that occurred at similar frequencies across treatment groups.
Because timing of triptorelin initiation with chemotherapy was not randomized and there were differences in patient characteristics and prognostic features between TEXT and SOFT patients, the analysis used an inverse probability of ‘treatment’ weighting (IPTW) analysis using a propensity score [20]. The propensity score for the probability of concurrent versus sequential triptorelin (i.e. TEXT versus SOFT enrollment), conditional on measured patient, disease and treatment covariates, used logistic regression (supplementary Table S1, available at Annals of Oncology online). The covariates were pre-specified as those believed to be prognostic and potentially associated with enrollment into the trials, without use of variable-selection procedures [20], as presented in Table 1. The primary analysis used age at diagnosis; as sensitivity analysis, the analysis was repeated using age at enrollment (supplementary data, available at Annals of Oncology online). We calculated average treatment effect in the ‘treated’ (ATT) weights referenced to the TEXT population with concurrent triptorelin and used absolute standardized differences in characteristics between groups as diagnostic tool [21] (supplementary Table S2, available at Annals of Oncology online). An IPTW log-rank test and IPTW Kaplan–Meier estimates of time-to-event were estimated [22], as well as hazard ratios (HR) with 95% confidence intervals from an IPTW Cox model using robust standard errors.
Table 1.
Patient, disease and treatment characteristics of the analysis population, according to timing of triptorelin (GnRH-agonist) initiation with chemotherapy (trial)
| Timing of triptorelin initiation and chemotherapy (trial) |
Absolute | ||||||
|---|---|---|---|---|---|---|---|
| Concurrent (TEXT) |
Sequential (SOFT) |
All |
standardized difference | ||||
| N | % | N | % | N | % | ||
| N patients | 1242 | 100.0 | 630 | 100.0 | 1872 | 100.0 | |
| Race/ethnicity | |||||||
| Other/unknown | 54 | 4.3 | 47 | 7.5 | 101 | 5.4 | 0.132 |
| Black/African American | 30 | 2.4 | 25 | 4.0 | 55 | 2.9 | 0.088 |
| Hispanic/Latino/South American native | 119 | 9.6 | 41 | 6.5 | 160 | 8.5 | 0.113 |
| White/Caucasian | 1039 | 83.7 | 517 | 82.1 | 1556 | 83.1 | 0.042 |
| Age at diagnosis | |||||||
| <35 | 140 | 11.3 | 140 | 22.2 | 280 | 15.0 | 0.296 |
| 35–39 | 220 | 17.7 | 192 | 30.5 | 412 | 22.0 | 0.302 |
| 40–44 | 435 | 35.0 | 197 | 31.3 | 632 | 33.8 | 0.080 |
| 45–49 | 383 | 30.8 | 83 | 13.2 | 466 | 24.9 | 0.436 |
| 50+ | 64 | 5.2 | 18 | 2.9 | 82 | 4.4 | 0.117 |
| Year of diagnosis | |||||||
| 2003–2006 | 687 | 55.3 | 293 | 46.5 | 980 | 52.4 | – |
| 2007–2011 | 555 | 44.7 | 337 | 53.5 | 892 | 47.6 | – |
| Age at enrollment | |||||||
| <35 | 136 | 11.0 | 122 | 19.4 | 258 | 13.8 | 0.236 |
| 35–39 | 214 | 17.2 | 173 | 27.5 | 387 | 20.7 | 0.247 |
| 40–44 | 432 | 34.8 | 205 | 32.5 | 637 | 34.0 | 0.047 |
| 45–49 | 394 | 31.7 | 106 | 16.8 | 500 | 26.7 | 0.353 |
| 50+ | 66 | 5.3 | 24 | 3.8 | 90 | 4.8 | 0.072 |
| Year of enrollment | |||||||
| 2003–2006 | 636 | 51.2 | 190 | 30.2 | 826 | 44.1 | – |
| 2007–2011 | 606 | 48.8 | 440 | 69.8 | 1046 | 55.9 | – |
| BMI at enrollment | |||||||
| Normal (<25) | 671 | 54.0 | 275 | 43.7 | 946 | 50.5 | 0.209 |
| Overweight (25 to < 30) | 318 | 25.6 | 168 | 26.7 | 486 | 26.0 | 0.024 |
| Obese (≥30) | 235 | 18.9 | 175 | 27.8 | 410 | 21.9 | 0.211 |
| Unknown | 18 | 1.4 | 12 | 1.9 | 30 | 1.6 | 0.035 |
| Menstruation status at enrollment | |||||||
| Normal | 1080 | 87.0 | 233 | 37.0 | 1313 | 70.1 | 1.201 |
| Irregular | 86 | 6.9 | 184 | 29.2 | 270 | 14.4 | 0.605 |
| Persistent amenorrhea | 56 | 4.5 | 201 | 31.9 | 257 | 13.7 | 0.759 |
| Unknown | 20 | 1.6 | 12 | 1.9 | 32 | 1.7 | 0.022 |
| Performance status at enrollment | |||||||
| Fully active (90–100) | 1199 | 96.5 | 579 | 91.9 | 1778 | 95.0 | 0.200 |
| Restricted/ambulatory(50–80)/unknown | 43 | 3.5 | 51 | 8.1 | 94 | 5.0 | 0.200 |
| Hormone receptor status | |||||||
| ER+/PR+ | 1081 | 87.0 | 526 | 83.5 | 1607 | 85.8 | 0.100 |
| Other | 161 | 13.0 | 104 | 16.5 | 265 | 14.2 | 0.100 |
| Tumor size (path) | |||||||
| ≤2 cm | 566 | 45.6 | 332 | 52.7 | 898 | 48.0 | 0.143 |
| >2 cm | 657 | 52.9 | 287 | 45.6 | 944 | 50.4 | 0.147 |
| Unknown | 19 | 1.5 | 11 | 1.7 | 30 | 1.6 | 0.017 |
| No. nodes positive | |||||||
| pN0 | 385 | 31.0 | 280 | 44.4 | 665 | 35.5 | 0.280 |
| pN + 1–3 | 548 | 44.1 | 241 | 38.3 | 789 | 42.1 | 0.119 |
| pN + 4+ | 309 | 24.9 | 109 | 17.3 | 418 | 22.3 | 0.187 |
| Tumor grade | |||||||
| 1 | 162 | 13.0 | 118 | 18.7 | 280 | 15.0 | 0.156 |
| 2 | 679 | 54.7 | 329 | 52.2 | 1008 | 53.8 | 0.049 |
| 3 | 401 | 32.3 | 183 | 29.0 | 584 | 31.2 | 0.070 |
| Lymphovascular invasion | |||||||
| No/unknown | 648 | 52.2 | 396 | 62.9 | 1044 | 55.8 | 0.217 |
| Yes | 594 | 47.8 | 234 | 37.1 | 828 | 44.2 | 0.217 |
| Local therapy | |||||||
| Mastectomy, no RT | 309 | 24.9 | 139 | 22.1 | 448 | 23.9 | 0.066 |
| Mastectomy with RT | 304 | 24.5 | 186 | 29.5 | 490 | 26.2 | 0.114 |
| BCS with RT | 623 | 50.2 | 303 | 48.1 | 926 | 49.5 | 0.041 |
| Other | 6 | 0.5 | 2 | 0.3 | 8 | 0.4 | 0.026 |
| Chemotherapy regimen | |||||||
| Anthracycline-based | 702 | 56.5 | 306 | 48.6 | 1008 | 53.8 | 0.160 |
| Taxane-based | 63 | 5.1 | 46 | 7.3 | 109 | 5.8 | 0.093 |
| Anthracycline+taxane-based | 448 | 36.1 | 273 | 43.3 | 721 | 38.5 | 0.149 |
| Other/unknown | 29 | 2.3 | 5 | 0.8 | 34 | 1.8 | 0.124 |
| Chemotherapy duration | |||||||
| ≤12 weeks | 497 | 40.0 | 190 | 30.2 | 687 | 36.7 | 0.208 |
| >12 to < 24 weeks | 588 | 47.3 | 323 | 51.3 | 911 | 48.7 | 0.079 |
| ≥24 weeks | 157 | 12.6 | 117 | 18.6 | 274 | 14.6 | 0.164 |
| Chemotherapy included cyclophosphamide | |||||||
| Tamoxifen before enrollment | 1229 | 99.0 | 618 | 98.1 | 1847 | 98.7 | – |
| No | 1242 | 100 | 346 | 54.9 | – | – | – |
| Yesa | 0 | 0 | 284 | 45.1 | – | – | |
| Endocrine therapy assignment | |||||||
| Tamoxifen+OFS | 630 | 50.7 | 319 | 50.6 | 949 | 50.7 | – |
| Exemestane+OFS | 612 | 49.3 | 311 | 49.4 | 923 | 49.3 | – |
Absolute standardized difference is the absolute value of the difference in sample proportions of the two groups divided by the pooled standard deviation.
Prior endocrine therapy was allowed in SOFT but not TEXT. Among the 284 patients, the median duration of prior tamoxifen was 17 weeks (interquartile range, 10–23 weeks).
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; BCS, breast-conserving surgery; RT, radiotherapy; OFS, ovarian function suppression.
Results
The eligible population included 1320 HR+/HER2-negative premenopausal patients who initiated triptorelin concurrently with chemotherapy in TEXT (concurrent), and 654 premenopausal patients who initiated triptorelin after adjuvant chemotherapy in SOFT (sequential; Figure 1). The estimated 5-year BCFI was 88.6% (±1.0%) and 86.4% (±1.4%) among TEXT and SOFT patients, respectively, based on data from time of trial enrollment (i.e. before introducing landmark and IPTW analyses). After introducing the landmark analysis, the final analysis population included 1872 patients who remained alive, disease-free and in follow-up at the landmark of 1 year since the final dose of adjuvant chemotherapy, 1242 and 630 who received concurrent and sequential triptorelin, respectively.
Differences between the two groups were evident in most characteristics. In particular, SOFT patients receiving sequential triptorelin were younger than TEXT patients receiving concurrent triptorelin (median age at diagnosis 39 and 43 years, respectively). The median duration of adjuvant chemotherapy was 18 weeks (IQR 12–18 weeks) in both groups. At the landmark time point, 85% of concurrent and 87% of sequential patients continued triptorelin, 11% and 8% had undergone ovarian ablation, respectively, and 5% had ceased OFS early. In total 93% of patients continued the assigned oral endocrine therapy (91% and 95%, respectively).
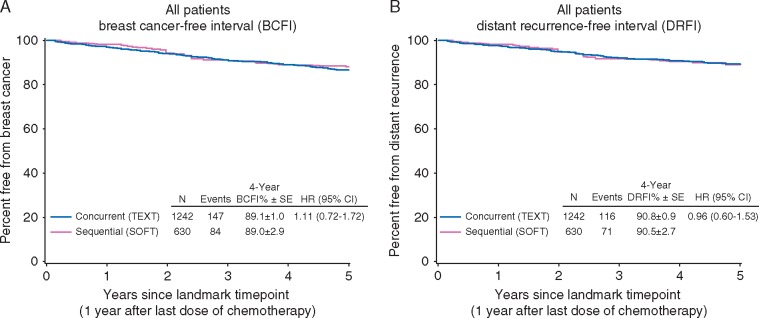
There were 231 (12%) breast cancer events in the 2 groups at post-landmark median follow-up of 4.7 and 4.9 years. The concurrent use of triptorelin with chemotherapy was not associated with a significant difference in BCFI (HR = 1.11, 95% CI 0.72–1.72; p = 0.72), with an estimated 89.1% (±1.0%) and 89.0% (±2.9%) patients breast cancer-free at 4 years post-landmark, with concurrent and sequential triptorelin initiation, respectively (Figure 2). The results were similar for DRFI; 187 distant recurrences were reported, with estimated 90.8% (±0.9%) and 90.5% (±2.7%) patients distant recurrence-free in the concurrent and sequential groups, respectively, at 4 years post-landmark (HR = 0.96, 95% CI 0.60–1.53; p = 0.86).
Figure 2.
Breast cancer-free interval (BCFI; A) and distant recurrence-free interval (DRFI; B) according to timing of triptorelin initiation with chemotherapy, from the landmark time point beginning 1 year after the final dose of adjuvant chemotherapy.
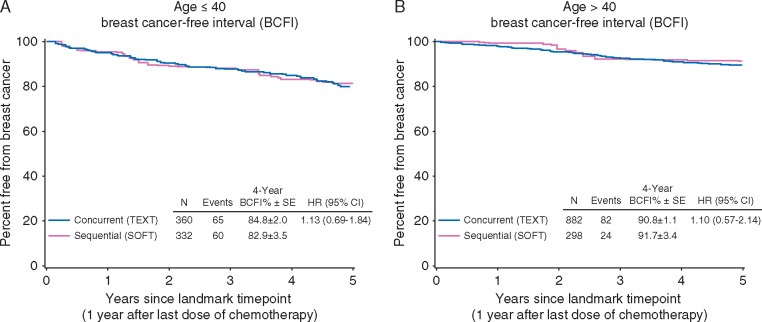
There was no evidence of differential effectiveness of concurrent triptorelin among younger women (<40 years at diagnosis; n = 692) than older premenopausal women at this point in follow-up (Figure 3). The estimated BCFI at 4 years post-landmark with concurrent and sequential triptorelin, respectively, was 84.8% (±2.0%) and 82.9% (±3.5%) for women aged <40 years at diagnosis (HR = 1.13, 95% CI 0.69–1.84), and 90.8% (±1.1%) and 91.7% (±3.4%) for those ≥40 years at diagnosis (HR = 1.10, 95% CI 0.57–2.14).
Figure 3.
Breast cancer-free interval (BCFI) for women aged <40 years (A) and ≥40 years (B) at diagnosis, according to timing of triptorelin initiation with chemotherapy, from the landmark time point beginning 1 year after the final dose of adjuvant chemotherapy.
Discussion
TEXT and SOFT demonstrated the benefit of adding OFS to tamoxifen alone in high-risk patients remaining premenopausal after adjuvant chemotherapy and of exemestane over tamoxifen when combined with OFS. When chemotherapy was also given, OFS initiation differed between TEXT and SOFT (concurrently or sequentially with chemotherapy, respectively) to accommodate different attitudes worldwide. The trials were not designed to elucidate an optimal strategy. The sequential administration, by postponing an effective targeted therapy in premenopausal patients at higher-risk of relapse, might reduce treatment efficacy. On the other hand, the concurrent strategy might interfere with the cytotoxic activity of chemotherapy and will mask and abrogate the therapeutic role of CIA, especially in older premenopausal women, resulting in 5 years of potentially unnecessary and costly GnRH-agonist therapy. To investigate this relevant clinical issue, we analyzed 1872 patients with HER2-negative breast cancer who received adjuvant triptorelin in SOFT/TEXT. Our analysis showed no difference in the BCFI between the concurrent and sequential triptorelin treatment groups, neither overall nor in the subgroup of women <40 years at diagnosis who are less likely to develop CIA, after about 5-years median follow-up.
Timing and sequencing of endocrine therapy and chemotherapy has not been adequately studied in early breast cancer [23]. The biologic evidence relates to tamoxifen, which works by a different endocrine mechanism than OFS [7, 24]. The tamoxifen-induced blockade in the G1-S phase of the cell cycle has been hypothesized to antagonize the antitumor effect of chemotherapy. The 2011 Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview of adjuvant tamoxifen showed significant recurrence risk (RR) reduction regardless of its concurrent (RR = 0.62) or sequential (RR = 0.71) administration with chemotherapy [25]. A small number of neoadjuvant studies compared sequential chemotherapy followed by OFS to concurrent therapy. The NSABP B-52 trial, in which 46% of patients were premenopausal, recently showed that adding endocrine therapy (GnRH-agonist + AI) to neoadjuvant immunochemotherapy in HR+/HER2-positive breast cancer was not antagonistic and did not increase toxicity [26]. The only large trials studying OFS concurrently with chemotherapy assessed ovarian/fertility preservation, but the majority were restricted to HR-negative breast cancer [27]. Reassuringly, the breast cancer outcomes have not been compromised by the addition of concomitant GnRH-agonist [27].
Our analysis has limitations, mainly its non-randomized nature and the planned difference in enrollment timing in SOFT and TEXT, which led to inherent differences in the populations. Despite the use of IPTW and landmark analysis to balance the characteristics between concurrent and sequential OFS groups and align the periods of observation as in a randomized trial, the methodology may not have adequately overcome these issues. Moreover, median follow-up for SOFT/TEXT was <6 years, and differences in concurrent versus sequential OFS and chemotherapy could appear only later in follow-up. Statistical power was limited, especially for the subgroup <40 years, and real differences between the strategies may not have been detected.
From a clinical perspective, as no randomized trial will be conducted to properly answer this question, when chemotherapy will also be given, clinicians and patients need to select the concurrent or sequential strategy of OFS on an individual basis. Which considerations may help in guiding this decision? Concurrent administration does not increase chemotherapy-related adverse events [15] and the possibility to avoid permanent menopause and its consequences is attractive for younger premenopausal women. The rate of CIA is age- and regimen-dependent: most very young women (<35 years) resume menses after chemotherapy and could consider concurrent OFS thus receiving and completing a therapy that has proved to be particularly effective in this age group [14] 6 months earlier. In premenopausal women, the possibility to preserve fertility in addition to the adjuvant effect is especially attractive for those not having completed family planning. In contrast, in women already approaching menopause, delaying GnRH-agonist administration until resumption of menses after chemotherapy may avoid unnecessary and costly drug administration. For women on tamoxifen following chemotherapy, the evaluation of ovarian function can be challenging, especially in patients developing amenorrhea [28]. SOFT/TEXT data represent the only evidence available in premenopausal patients from a large sample within controlled clinical trials: they support clinicians and patients selecting the concurrent or sequential strategy of chemotherapy and OFS on an individual patient basis.
Supplementary Material
Acknowledgements
We thank the patients, physicians, nurses and trial coordinators who participated in the TEXT and SOFT clinical trials. The trials were coordinated by the International Breast Cancer Study Group (IBCSG), in collaboration with the Breast International Group (BIG), BIG cooperative groups, and US National Cancer Institute National Clinical Trials Network cooperative groups.
Funding
TEXT and SOFT received financial support for trial conduct from Pfizer, the IBCSG and the US National Cancer Institute at the National Institutes of Health (NIH). Pfizer and Ipsen provided drug supply. The pharmaceutical companies have no role in the reporting or interpretation of the trials, other than a minority representation on the Steering Committee. Support for the coordinating group, IBCSG: Frontier Science and Technology Research Foundation [no grant number], Swiss Group for Clinical Cancer Research [SAKK; no grant number], Cancer Research Switzerland/Oncosuisse [no grant number], the Foundation for Clinical Cancer Research of Eastern Switzerland [OSKK; no grant number], US National Institutes of Health [grant number CA075362], Breast Cancer Research Foundation [BCRF; grant number 16-185]. Grant support of cooperative groups: Australia and New Zealand Breast Cancer Trials Group [National Health and Medical Research Council grant numbers 351161, 510788 and 1105058]; SWOG [US National Institutes of Health grant number CA32102]; Alliance for Clinical Trials in Oncology [US National Institutes of Health grant number CA180821]; ECOG-ACRIN Cancer Research Group [US National Institutes of Health grant numbers CA21115, CA16116]; NSABP/NRG Oncology [US National Institutes of Health grant numbers U10-CA12027, U10-CA69651, U10-CA37377, U10-CA69974]; NCIC-CTG [US National Institutes of Health grant number CA077202; and Canadian Cancer Society Research Institute grant numbers 015469, 021039]; ICR-CTSU on behalf of the National Cancer Research Institute Breast Clinical Studies Group United Kingdom (NCRI-BCSG—ICR-CTSU Partnership) [Cancer Research UK grant numbers CRUKE/03/022, CRUKE/03/023, A15955; National Institute for Health Research/Institute of Cancer Research Biomedical Research Centre (no grant number); National Institute for Health/Cambridge Biomedical Research Centre (no grant number)].
Disclosure
IBCSG receives funding (and/or provision of drug supply for clinical trials) from Pfizer and Ipsen. MMR: research funding (institution) from Veridex, Merck (individual). PAF: Uncompensated presentation of SOFT/TEXT trial results for Pfizer at international meeting and advisory board; honorarium AstraZeneca; conference travel support from Roche and Amgen. All remaining authors have declared no conflicts of interest.
References
- 1. Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet 1896; 2: 104–107. [PMC free article] [PubMed] [Google Scholar]
- 2. LHRH-Agonists in Early Breast Cancer Overview Group. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 2007; 369: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 3. Fornier MN, Modi S, Panageas KS. et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer 2005; 104: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 4. Goldhirsch A, Gelber RD, Yothers G. et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr 2001; 44–51. [DOI] [PubMed] [Google Scholar]
- 5. Aebi S, Gelber S, Castiglione-Gertsch M. et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000; 355: 1869–1874. [DOI] [PubMed] [Google Scholar]
- 6. Emons G, Grundker C, Gunthert AR. et al. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer 2003; 10: 291–299. [DOI] [PubMed] [Google Scholar]
- 7. Osborne CK, Kitten L, Arteaga CL.. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J Clin Oncol 1989; 7: 710–717. [DOI] [PubMed] [Google Scholar]
- 8. Sutherland RL, Green MD, Hall RE. et al. Tamoxifen induces accumulation of MCF 7 human mammary carcinoma cells in the G0/G1 phase of the cell cycle. Eur J Cancer Clin Oncol 1983; 19: 615–621. [DOI] [PubMed] [Google Scholar]
- 9. Pico C, Martin M, Jara C. et al. Epirubicin-cyclophosphamide adjuvant chemotherapy plus tamoxifen administered concurrently versus sequentially: randomized phase III trial in postmenopausal node-positive breast cancer patients. A GEICAM 9401 study. Ann Oncol 2004; 15: 79–87. [DOI] [PubMed] [Google Scholar]
- 10. Bedognetti D, Sertoli MR, Pronzato P. et al. Concurrent vs sequential adjuvant chemotherapy and hormone therapy in breast cancer: a multicenter randomized phase III trial. J Natl Cancer Inst 2011; 103: 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albain KS, Barlow WE, Ravdin PM. et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009; 374: 2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Mastro L, Dozin B, Aitini E. et al. Timing of adjuvant chemotherapy and tamoxifen in women with breast cancer: findings from two consecutive trials of Gruppo Oncologico Nord-Ovest-Mammella Intergruppo (GONO-MIG) Group. Ann Oncol 2008; 19: 299–307. [DOI] [PubMed] [Google Scholar]
- 13. Regan MM, Pagani O, Fleming GF. et al. Adjuvant treatment of premenopausal women with endocrine-responsive early breast cancer: design of the TEXT and SOFT trials. Breast 2013; 22: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francis PA, Regan MM, Fleming GF. et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015; 372: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pagani O, Regan MM, Walley BA. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burstein HJ, Lacchetti C, Anderson H. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016; 34: 1689–1701. [DOI] [PubMed] [Google Scholar]
- 17. Coates AS, Winer EP, Goldhirsch A. et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paluch-Shimon S, Pagani O, Partridge AH. et al. Second international consensus guidelines for breast cancer in young women (BCY2). Breast 2016; 26: 87–99. [DOI] [PubMed] [Google Scholar]
- 19. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Breast Cancer (Version 2.2016); https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (1 December 2016, date last accessed).
- 20. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist Med 2015; 34: 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie J, Liu C.. Adjusted Kaplan–Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005; 24: 3089–3110. [DOI] [PubMed] [Google Scholar]
- 23. Pritchard KI. Combining endocrine agents with chemotherapy: which patients and what sequence? Cancer 2008; 112: 718–722. [DOI] [PubMed] [Google Scholar]
- 24. Sertoli MR, Scarsi PG, Rosso R.. Rationale for combining chemotherapy and hormonal therapy in breast cancer. J Steroid Biochem 1985; 23: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 25. Chia SK, Wolff AC.. With maturity comes confidence: EBCTCG tamoxifen update. Lancet 2011; 378: 747–749. [DOI] [PubMed] [Google Scholar]
- 26. Rimawi MF, Cecchini RS, Rastogi P. et al. A phase III trial evaluating pCR in patients with HR+, HER2-positive breast cancer treated with neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) +/− estrogen deprivation: NRG Oncology/NSABP B-52 (S03-06). San Antonio, TX: SABCS, 2016.
- 27. Munhoz RR, Pereira AA, Sasse AD. et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol 2016; 2: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berliere M, Duhoux FP, Dalenc F. et al. Tamoxifen and ovarian function. PLoS ONE 2013; 8: e66616.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.