Abstract
Aims
Focal Impulse and Rotor Modulation (FIRM) uses 64-electrode basket catheters to identify atrial fibrillation (AF)-sustaining sites for ablation, with promising results in many studies. Accordingly, new basket designs are being tested by several groups. We set out to determine the procedural safety of adding basket mapping and map-guided ablation to conventional pulmonary vein isolation (PVI).
Methods and results
We collected 30 day procedural safety data in five US centres for consecutive patients undergoing FIRM plus PVI (FIRM-PVI) compared with contemporaneous controls undergoing PVI without FIRM. A total of 625 cases were included in this analysis: 325 FIRM-PVI and 300 PVI-controls. FIRM-PVI patients were more likely than PVI-controls to be male (83% vs. 66%, P < 0.001) and have long-standing persistent AF (26% vs. 13%, P < 0.001) reflecting patients referred for FIRM. Total ablation time was greater for FIRM-PVI (62 ± 22 min) vs. PVI-controls (52 ± 18 min, P = 0.03). The complication rate for FIRM-PVI procedures (4.3%) was similar to controls (4.0%, P = 1) for both major and minor complications; no deaths were reported. The rate of complications potentially attributable to the basket catheter was small and did not differ between basket types (Constellation 2.8% vs. FIRMap 1.8%, P = 0.7) or between cases in which basket catheters were and were not used (P = 0.5). Complication rates did not differ between centres (P = 0.6).
Conclusions
Procedural complications from the use of the basket catheters for AF mapping are low, and thus procedural safety appears similar between FIRM-PVI and PVI-controls in a large multicentre cohort. Future studies are required to determine the optimal approach to maximize the efficacy of FIRM-guided ablation.
Keywords: Atrial fibrillation, Focal impulse, Electrical rotor, Ablation, Procedural safety
What’s new?
Patients who received Focal Impulse and Rotor Modulation (FIRM) ablation in addition to pulmonary vein isolation had a similar complication rate compared with patients who had pulmonary vein isolation alone in a large series of 625 patients between five centres.
Complications rates were similar between enrolling centres and between basket catheter types.
Introduction
Current guidelines for ablation emphasize the importance of pulmonary vein isolation (PVI) to reduce the impact of atrial fibrillation (AF) triggers,1 but the overall success of this approach is suboptimal in many patients and is not improved by adding empirical lines or targeting complex fractionated atrial electrograms (CFAE)2,3 even in experienced centres. Recent single-4,5 and multicentre6 studies have shown improved AF ablation outcome sites using Focal Impulse and Rotor Modulation (FIRM) in addition to PVI mapping and ablation, which persist to 3 years.7 However, the safety of this approach compared with PVI-controls has not been systematically analysed.
The overall complication rate for AF ablation has been reported as 6.29%8 and 9.1%9 using different databases. These rates reflect the aggregate of bleeding, cardiac perforation and tamponade, atrio-oesophageal fistula, diaphragmatic paralysis, heart block, repeat hospitalization, severe bleeding, myocardial infarction, stroke, and death. In patients undergoing extensive atrial ablation, stiff left atrial syndrome10 and increased risk of stroke11 have recently been emphasized.
FIRM ablation was developed to directly target patient-specific AF sustaining mechanisms,12 whose endocardial stability is similar to AF-sustaining sources identified by optical mapping studies of human AF.13 To accomplish this, FIRM ablation incorporates the use of a multi-electrode, multi-spline endocardial catheter for mapping. Although initial trials showed promising efficacy5,6 with no safety concerns, the use of basket catheters theoretically may increase the risk of cardiac perforation, tamponade, or thrombo-embolism.
We therefore set out to determine the procedural complication rate for FIRM-PVI compared with PVI-controls. We also evaluated for risks potentially attributable to the basket catheter itself to test for potential differences between catheter types. We tested this hypothesis by collecting FIRM-PVI and PVI-control 30 day safety rates from five centres each performing > 30 FIRM + PVI ablation cases.
Methods
Data collection
Thirty day procedural complication data from FIRM-PVI cases and PVI-controls were collected from five US centres experienced in both approaches (Arizona Heart Rhythm Center, Baptist Health Lexington, KY; Indiana University, Indianapolis, IN; Stanford University, Stanford, CA; University of California San Diego/VA San Diego Healthcare System, CA). Data included patient demographics, AF type, procedural details, and specific complication. The collection dates were from May 2009 to May 2015. FIRM + PVI patients were consecutive patients receiving this procedure, and PVI-controls were cotemporaneous patients receiving PVI alone during the timeframe in which FIRM-guided cases were being performed at each centre.
Procedural details
Procedures were performed > 5 half-lives after discontinuing antiarrhythmic medications except amiodarone. Pre-procedural anticoagulation was managed in routine clinical fashion, but no patients were studied on uninterrupted non-vitamin K oral anticoagulant. Catheters were placed via femoral access in the coronary sinus, right, and left atrium per standard technique. Heparin was administered by infusion at each Centre to maintain activated clotting time (ACT) > 350 s. Periprocedural anticoagulation/bridging was determined according to each Institution’s protocol.
FIRM catheter placement
A 64-electrode basket catheter (Constellation, Boston Scientific Inc., Natick, MA, USA, 48 or 60 mm diameter or FIRMap, Topera, Inc., Palo Alto, CA, USA, 50, 60, or 70 mm diameter) was advanced to the right and left atria via 8.5 F sheaths. Basket size was matched to atrial dimensions from intracardiac echocardiography or computed tomography. Fluoroscopy, electrography, and intracardiac echocardiography were used to optimize contact. Spontaneous AF, or AF induced by rapid atrial pacing (cycle lengths: 500, 450, 400, 350, 300 ms, then in 10 ms steps to AF) with or without isoproterenol was recorded.5 Sustained AF was achieved in all mapped FIRM patients, and analysed after > 5 min for stabilization of AF. Unipolar electrograms were filtered at 0.05–500 Hz and exported digitally from electrophysiological recorders at each site for analysis on RhythmView (Abbott Laboratories, Abbott Park, IL, USA).
FIRM atrial fibrillation mapping details
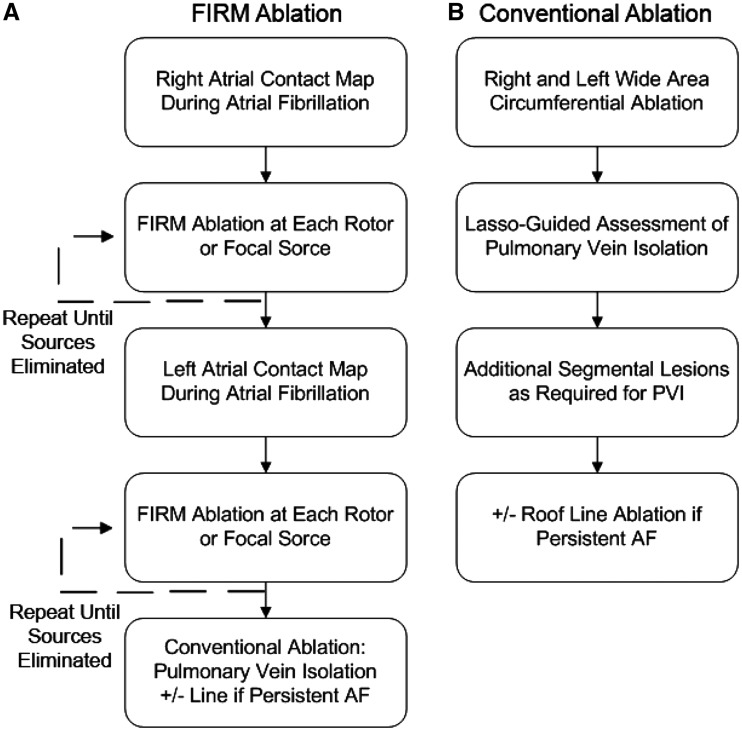
The FIRM-mapping and ablation workflow is shown in Figure 1A. First, 64-electrode basket catheters were placed in the right atrium and AF electrograms were exported for analysis. Next, exported electrograms were processed to create phase movies in order to identify AF rotors or focal sources in the right atrium (RA) in near-real-time. Ablation (see below) was applied until RA rotor sites were eliminated on remapping. Next, the basket catheter was positioned in the left atrium (LA), and this process was repeated until all LA sites were eliminated. If AF terminated during ablation, reinduction was attempted. In the first 11 cases at one centre (VA San Diego), ablation was applied to diagnosed AF sources without remapping.
Figure 1.
Study flow for FIRM-PVI (A) and PVI-controls (B).
Atrial fibrillation maps were used to diagnose sustained rotors or focal sources if observed on multiple epochs (1 min recording segments). The core regions of AF sources are spatially reproducible within precession areas of ∼2 cm2 for tens of seconds14 (i.e. bounded by two electrodes in each axis) with spiral arms that disorganize via fibrillatory conduction. AF focal sources were diagnosed if they showed centrifugal activation from an origin to surrounding atrium with peripheral disorganization.5 Basket repositioning was used by these experienced centres to optimize basket contact in cases with very large atria or if regions of interest had no signals. AF propagation maps from 3D contact baskets were projected onto grids aligned to atrial anatomy. Map interpretation and ablation were performed by the operator at each site.
FIRM ablation
Ablation commenced at sources identified using FIRM mapping. Ablation was performed using usual safety precautions observed during AF ablation to minimize the risk of injury to the phrenic nerve via pacing, the use of temperature monitoring to prevent oesophageal injury, and other precautions. Specific ablation technology was chosen according to investigator preference: 3.5 mm tip irrigated radiofrequency catheter (ThermoCool, Biosense-Webster, Diamond Bar, CA, USA; Safire Blu, St. Jude Medical, Minnetonka, MN, USA) at 25–40 W, a cryoballoon at sources near the PV antra (Arctic Front, Medtronic, MN, USA) or, in early patients with heart failure, by an 8 mm non-irrigated radiofrequency catheter (Blazer, Boston Scientific, Sunnyvale, CA, USA) at 40–50 W, 52 °C target temperature. Most patients in this series were studied before the widespread use of contact force catheter technology. Ablation was applied to the basket grid coordinates of the centre of rotation or focal impulse origin (2 cm2 areas), referenced in all cases to electrode positions on electroanatomic shells (NavX, St Jude Medical, Sylmar, CA, USA; Carto, Biosense-Webster) with the goal of eliminating rotors on repeat mapping. In addition to FIRM-guided ablation, conventional PVI ablation was performed as described below.
Conventional pulmonary vein isolation ablation
Conventional PVI ablation for all patients was performed in combination with FIRM ablation for all patients in the FIRM-PVI cohort. PVI comprised wide-area antral PVI verified using a circular catheter (Lasso; Biosense-Webster or Optima, St. Jude Medical). Additional ablation included an optional left atrial roof line in persistent AF patients. Observed atrial tachycardias or flutters were also ablated, particularly those arising from termination of AF to atrial tachycardia (AT) by FIRM-guided termination as reported.4,6 Ablation power, temperatures, and duration were as noted above. If AF persisted after completion of the ablation protocol, cardioversion was used to restore sinus rhythm.
Post-procedure clinical management
Follow-up for arrhythmia recurrence followed current guidelines with in-clinic evaluation at 3 months, and 6 months at all centres. Complications were recorded as part of detailed safety monitoring protocols for each facility including all hospitalizations, patient calls, and clinic visits up to 30 days.
Study endpoints
The primary endpoint was the overall rate of 30 day complications for FIRM-PVI vs. PVI-controls, defined as any of the following: stroke/transient ischaemic attack (TIA), cardiac perforation/tamponade, rehospitalization, heart block, myocardial infarction, phrenic nerve injury, atrial oesophageal fistula, bleeding requiring transfusion, and death.8 Secondary endpoints were the individual rates for each complication type. Complications potentially attributable to the basket catheter were defined as stroke/TIA, cardiac perforation/tamponade, or peripheral thrombo-embolism, and were also reported.
Statistical analysis
Continuous data are represented as mean ± standard deviation. The Student’s t-test was used to compare continuous variables between two groups, such as age. The χ2 test was applied to contingency tables for categorical variables; Fisher’s exact test was used when expected values were ≤5. A P-value <0.05 was considered statistically significant.
Study power calculation
We based our power calculations for this study upon the work of Deshmukh et al.8 and Ellis et al.9 in which AF ablation complications rates were 6.29% and 9.1%, respectively. Because the electrophysiologists participating in this study were experienced AF ablation providers, we estimated a PVI-control complication rate of 6%. We powered the study to detect a complication rate greater than what we would consider the upper limit of normal, 9% (as reported by Ellis et al.). We calculated that 275 patients in each arm (total 550 patients) would be required to provide 80% power to detect this difference at an alpha of 0.05. Our study size (total 625 patients) exceeded this target by 14%, providing additional power to detect a difference in complication rate.
Results
Patient characteristics
FIRM-PVI cases from each institution are included up to the data collection cutpoint, including their initial procedures. A total of 625 patients are included in this analysis: 325 FIRM-PVI and 300 PVI-controls. Demographics of studied patients are shown in Table 1. FIRM-PVI patients were similar to PVI-controls in age, but more likely to be male and have long-standing persistent AF.
Table 1.
Patient demographics
| Demographics | FIRM-PVI | PVI-controls | P-value |
|---|---|---|---|
| Number of patients | 325 | 300 | — |
| Age (years) ± SD | 60 ± 11 | 59 ± 11 | 0 .12 |
| Female (%) | 55 (17) | 102 (34) | <0 .001 |
| Paroxysmal AF (%) | 130 (40) | 162 (54) | <0 .001 |
| Persistent AF (%) | 111 (34) | 99 (33) | 0 .8 |
| Long-standing Pers. AF (%) | 85 (26) | 39 (13) | <0 .001 |
FIRM, Focal Impulse and Rotor Modulation; PVI, pulmonary vein isolation; SD, standard deviation; AF, atrial fibrillation; Pers., persistent.
Procedural details
Procedural details are shown in Table 2. In summary, the addition of FIRM to PVI added 22 min to total procedure time, 10 min to radiofrequency ablation time, and 4 min to fluoroscopy time in this study (P < 0.05 for each). The Constellation catheter was used in 106 (32.6%) of the FIRM-PVI cases and the FIRMap catheter in the remainder (219, 67.4%).
Table 2.
Procedural details
| FIRM-PVI | PVI-controls | P-value | |
|---|---|---|---|
| Number of patients | 325 | 300 | — |
| Procedure time (min) ± SD | 345 ± 62 | 323 ± 81 | <0 .001 |
| Ablation time (min) ± SD | 62 ± 22 | 52 ± 18 | 0 .03 |
| Fluoroscopy time (min) ± SD | 47 ± 24 | 43 ± 21 | 0 .03 |
FIRM, Focal Impulse and Rotor Modulation; PVI, pulmonary vein isolation; SD, standard deviation.
Complications
Complication details are reported in Table 3. Overall, the complication rate for FIRM-PVI (4.3%) was similar to PVI-controls (4.0%, P = 1). The specific risks for stroke, cardiac perforation/tamponade, rehospitalization, heart block, myocardial infarction, phrenic nerve injury, atrial oesophageal fistula, bleeding requiring transfusion, and death were also similar between FIRM-PVI and PVI-controls. No instances of cardiac tamponade were attributed to the basket catheter by the attending physician for the case, but instead were attributed to transseptal cannulation or movement of the ablation catheter or sheath. Importantly, no atrio-oesophageal fistulae or deaths occurred in either arm of the study in follow-up to a minimum of 3 months.
Table 3.
Complication rates
| FIRM-PVI | PVI-controls | P-value | |
|---|---|---|---|
| Number of patients | 325 | 300 | |
| Overall 30 day complication (%) | 4.3% (14) | 4.0% (12) | 1 |
| Stroke/TIA (%) | 1 (0 .3) | 1 (0 .3) | 1 |
| Tamponade/Pericard. drain (%) | 6 (1.8) | 3 (1.0) | 0 .5 |
| Rehospitalization (%) | 4 (1.2) | 3 (1.0) | 1 |
| Heart block (%) | 1 (0 .3) | 1 (0 .3) | 1 |
| Myocardial infarction (%) | 0 | 1 (0 .3) | 0 .48 |
| Phrenic nerve injury (%) | 0 | 1 (0 .3) | 0 .48 |
| Atrio-oesophageal fistula | 0 | 0 | — |
| Bleeding Req. transfusion (%) | 2 (0 .6) | 2 (0 .7) | 1 |
| Death | 0 | 0 | — |
FIRM, Focal Impulse and Rotor Modulation; PVI, pulmonary vein isolation; TIA, transient ischaemic attack; Pericard., pericardial; Req., requiring.
Complications according to basket type and institution
The rate of complications which could potentially be attributable to the basket catheter (stroke/TIA, cardiac perforation/tamponade, or peripheral thrombo-embolism) was 2.2% (7/325 cases) in FIRM-PVI cases vs. PVI-control cases in which baskets were not used [1.3% (4/300 cases), P = 0.5]. Thus, the incidence of these complications did not appear to be increased by insertion of the basket.
The rate of these complications did not differ for cases performed using the Constellation (3/106 cases, 2.8%) or FIRMap (4/219 cases, 1.8%, P = 0.7) catheters.
When considered by case types, overall complication rates for FIRM-PVI cases were similar when dichotomized by the type of basket catheter used in the case; complications occurred in 5.7% of cases when the Constellation catheter was used for the FIRM portion and were slightly lower at 3.7% when the FIRMap catheter was used for the FIRM portion, although this did not reach statistical significance (P = 0.4). Complications were similar between institutions (range 1.4–5.9%, P = 0.55).
Discussion
The major finding from this study is that the addition of atrial basket mapping to PVI did not significantly increase overall or specific AF ablation procedural risks compared with PVI-controls in a large, multicentre cohort. A second finding is that there was no difference in complication rate between early cases and later cases using two types of basket catheter. Finally, the rate of complications was similar between the five centres, and consistent with prior data. These findings give reassurance to the increasing use of multi-electrode catheters for AF mapping and ablation the introduction of new basket designs.
Thrombo-embolic complications
In prior literature, diagnostic catheters have not been shown to carry an increased risk of thrombo-embolism. Nevertheless, theoretical risks and a small series15 are cause for potential concern. While studies performing ablation using non-irrigated catheters in the left atrium have reported an increased risk for cerebral thrombo-embolism,16 a variety of multipolar diagnostic catheters (Pentaray, Biosense-Webster, decapolar, basket,4 and non-contact balloon catheters) have been used in both atria with few reports of additional thrombo-embolic complications using standard-of-care anticoagulation regimens.
Importantly, there was no evidence of increased thrombo-embolic risk for patients receiving FIRM-PVI in this study. Specifically, one cerebrovascular accident/TIA was reported in each arm (0.3% risk for both, P = 1). This rate compares favourably with pooled national data;8 likely reflecting contemporary anticoagulation strategies such as uninterrupted warfarin in the periprocedural timeframe. Of note, no cases were performed with uninterrupted non-vitamin K oral anticoagulants. Currently, all AF ablation approaches (PVI, FIRM, other) combine several strategies to reduce the risk of thrombo-embolism including standard-of-care anticoagulation regimens, appropriate screening with transoesophageal echocardiography, careful intra-procedure anticoagulation using heparin (with target ACT > 350 s), and appropriate post-procedure care with either uninterrupted warfarin or heparin bridging when indicated.
Cardiac perforation or tamponade
Cardiac tamponade is a potential complication of AF ablation, and may occur with greater frequency during the introduction of new mapping and ablation technologies. It may occur during transseptal catheterization, mapping, or ablation. Recent studies have provided reassurance that tamponade management is similarly effective whether anticoagulation is continued throughout the peri-ablation period.
In this analysis, the rate of tamponade rate in the FIRM-PVI group was similar to PVI-controls. No instance of tamponade in the FIRM-PVI group was directly attributed to the basket catheter by the attending physician for the case.
Overall risk
This large, multicentre study found that complications with FIRM-PVI were similar to prior data for experienced operators in high volume centres.8 A small single-centre study (n = 24 patients) showed a higher rate15 which may reflect early experience in the several operators in that study in early cases (2011–12) before current workflows were established. Other factors include periprocedural management, oesophageal temperature monitoring strategy, or patient selection. There was no signal for increased complications in other small studies of FIRM-guided ablation from other centres,17,18 or larger FIRM-guided studies independent of the initial centre.4,6,19 Notably, FIRM-guided ablation may offer an approach to reduce complications associated with extensive posterior wall ablation used in other approaches.10 In the work by Benharash et al.15 an atrio-oesophageal fistula was attributed to PVI lesions at the left inferior pulmonary vein, distant from FIRM lesions at the anterior left atrial roof. Whether the extent of posterior left atrial wall ablation can be reduced by eliminating rotor sites is a testable hypothesis. Further work is required to define the optimal use of FIRM ablation in complex cases to reduce complications and improve ablation success.
Impact of basket mapping on ablation procedures
Use of the basket catheter added approximately 22 min to the total procedure, 10 min to ablation time, and 4 min of fluoroscopy use. Although each increase is statistically significant, the increases are less than the standard deviation between cases in this study. Notably, this study incorporated the initial cases from each participating centre; ongoing improvements in workflow may further minimize the temporal impact of basket catheter mapping of AF.
Limitations
Limitations for this study include differences in clinical management between centres, reflecting differences in anticoagulation protocols, transseptal technique, PVI ablation strategy, and linear ablation approach between individual operators. However, this reflects real-world performance of any strategy, including FIRM-PVI, PVI-control, anti-arrhythmic or rate-control therapy, and other strategies. Debate continues on the benefit of basket mapping of human AF based on disappointing outcomes in small series from some centres.18,20 However, even these studies show termination of AF to AT by FIRM-guided ablation alone in 34% of cases,17 which strongly supports a mechanistic role of these sites. Moreover, FIRM-guided ablation has shown promising results in studies by Sommer et al.,4 Miller et al.,6 Tomassoni et al.,19 and Rashid et al.21 Additional data are required to determine the precise benefit of rotor and focal source mapping and ablation; randomized trials of FIRM + PVI vs. PVI are currently underway. Finally, in any multicentre study, complication rates are detected by varying protocols between Institutions; every effort was made to collect all relevant data, and the impact of such differences would be minimized by including PVI-controls from each centre for comparison.
Conclusions
A FIRM-PVI ablation strategy using basket mapping does not increase 30 day procedural adverse events compared with PVI-controls. Additionally, neither basket type nor individual institution demonstrated increased risk. Future studies are required to determine the optimal approach for periprocedural anticoagulation, mapping and ablation strategy, and post-procedural care to minimize complications, reduce procedural time, and maximize ablation effectiveness. Such studies are currently underway.
Acknowledgements
We would like to thank Kathleen Mills, BA for coordinating this study. We also wish to thank Donna Cooper, RN, Elizabeth Greer, RN, Stephanie Yoakum, RNP, Ken Hopper, CVT, Tony Moyeda, CVT, Judy Hildreth, RN, and Cherie Jaynes, RN for their assistance with the clinical portion of this study.
Conflict of interest: D.E.K. has served as a consultant to Topera Inc./Abbott Laboratories, and receives fellowship programme support from Medtronic, Boston Scientific, St. Jude, Biotronik, and Biosense-Webster. A.A.S. receives fellowship programme support from Medtronic, Boston Scientific, St. Jude, Biotronik, and Biosense-Webster. V.S. has received consulting fees/honoraria from Biosense Webster and research grants from Biosense Webster, Medtronic, Boston Scientific, St. Jude Medical, and Biotronik. J.M.M. reports consulting fees/honoraria from Topera, Stereotaxis, Biosense Webster, Biotronik, and Medtronic, and fellowship support from Medtronic, Boston Scientific, Biotronik, and Biosense-Webster. G.F.T. reports consulting fees/honoraria from Topera, Stereotaxis, Biosense Webster, St. Jude Medical, Boston Scientific, Pfizer, and Atricure. He also serves as CMO of Stereotaxis. S.M.N. is co-author of intellectual property owned by the University of California Regents and licensed to Abbott Laboratories. He also received honoraria from Medtronic and St. Jude Medical. T.B., C.A.B.K., S.P., M.N.V., and P.J.W. have no disclosures.
Funding
D.E.K. has received grant support from the American Heart Association (10 BGIA 3500045). S.M.N. has received grant support from the National Institutes of Health (HL83359, HL103800).
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA. et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 2. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 3. Scott PA, Silberbauer J, Murgatroyd FD. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace 2016;18:359–67. [DOI] [PubMed] [Google Scholar]
- 4. Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B. et al. Successful repeat catheter ablation of recurrent longstanding persistent atrial fibrillation with rotor elimination as the procedural endpoint: a case series. J Cardiovasc Electrophysiol 2016;27:274–80. [DOI] [PubMed] [Google Scholar]
- 5. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM.. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD. et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25: 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE. et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 2014;63:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K. et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104–12. [DOI] [PubMed] [Google Scholar]
- 9. Ellis ER, Culler SD, Simon AW, Reynolds MR.. Trends in utilization and complications of catheter ablation for atrial fibrillation in medicare beneficiaries. Heart Rhythm 2009;6:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J. et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364–71. [DOI] [PubMed] [Google Scholar]
- 11. Rillig A, Tilz RR, Lin T, Fink T, Heeger CH, Arya A. et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol 2016;9:e003461. [DOI] [PubMed] [Google Scholar]
- 12. Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel WJ.. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol 2013;6:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA. et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swarup V, Baykaner T, Rostamian A, Daubert JP, Hummel J, Krummen DE. et al. Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol 2014;25:1284–92. [DOI] [PubMed] [Google Scholar]
- 15. Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K. et al. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera Siklody C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S. et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58:681–8. [DOI] [PubMed] [Google Scholar]
- 17. Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gokoglan Y. et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016;13:830–5. [DOI] [PubMed] [Google Scholar]
- 18. Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J. et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm 2013;13:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomassoni G, Duggal S, Muir M, Hutchins L, Turner K, McLoney AM. et al. Long-term follow-up of FIRM-guided ablation of atrial fibrillation: a single-center experience. J Innov Heart Rhythm Manage 2015;6:2145–51. [Google Scholar]
- 20. Berntsen RF, Haland TF, Skardal R, Holm T.. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: a within-patient controlled study with implanted cardiac monitoring. Heart Rhythm 2016;13:1768–74. [DOI] [PubMed] [Google Scholar]
- 21. Rashid H, Sweeney A.. Approaches for focal impulse and rotor mapping in complex patients: a US private practice perspective. J Innov Heart Rhythm Manage 2015;6:2193–8. [Google Scholar]