Abstract
Background
We adopted ABVD chemotherapy with risk-adapted radiation therapy (RT) as first-line therapy for children, adolescents and young adults with Hodgkin lymphoma (HL) in British Columbia in 2004.
Patients and methods
Patients ≤ 25 years diagnosed from 2004 to 2013 with all stages of HL who received ABVD as initial therapy were included.
Results
Among 55 children (age < 18 year) and 154 young adults (18–25 year), there were no significant differences among age groups for sex, histologic subtype, tumour bulk, B symptoms, prognostic risk groups or treatment received. The rates of complete response, partial response and progressive disease were 84%, 7% and 10% for children and 95%, 4% and 1% for young adults (P=0.01), respectively. Treatment failures in children all occurred within one year of completion, while 8/21 (38%) relapses in young adults occurred later (P=0.04). With a median follow-up of 66 months the 5-year progression-free (PFS) and overall survival (OS) were 85 ± 3% and 97 ± 1%, respectively. For limited stage disease, PFS was 90 ± 7% for children and 93 ± 3% for young adults (P=0.65); OS was 100% for both. For advanced stage patients, PFS and OS were also similar for the children and young adults (77 ± 7% versus 81 ± 4%; P=0.38 and OS 90 ± 6% versus 97 ± 2%; P=0.17). The rate of consolidative RT was low (21%) and did not differ between age groups.
Conclusion
ABVD is an effective treatment in children, adolescents and young adults with HL. Children were less likely to achieve complete response and demonstrated earlier relapses compared to young adults. RT may be omitted for the majority of patients while maintaining excellent 5-year OS.
Keywords: Hodgkin lymphoma, ABVD, paediatric, adolescent, young adult
Introduction
Hodgkin lymphoma (HL) is one of the most commonly diagnosed malignancies in adolescents and young adults [1, 2] with a peak incidence between ages 15 and 34 years. With current therapies, 5-year overall survival (OS) is greater than 90% and there is an increasing emphasis on minimizing late treatment-related morbidity. Treatment protocols differ between paediatric and adult centres and clinical trials typically do not target both populations. Without strong clinical evidence of biological differences in the underlying disease, therapy received by adolescents and young adults with HL is often dictated predominantly by referral preference and location of care.
The current standard of care in North America for treatment of HL in adulthood is ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) with varying use of consolidative radiotherapy (RT) at doses of 30–35 Gy. Published long-term outcomes document excellent survival outcomes in both limited and advanced stage disease with low rates of long-term toxicity [3, 4]. In paediatrics, HL therapy has evolved away from ABVD and outcomes in children have recently been reported to be similar to adults with 5-year OS of 96% and PFS of 79% [5].
In British Columbia (BC), Canada, ABVD with consolidative involved-field RT (IFRT) only for incomplete chemotherapy responders became standard therapy for young adults with HL in 2004, and was adopted for paediatric HL in 2006 [6]. We report the outcomes of ABVD with risk-adapted RT in children, adolescents and young adults with HL.
Methods
This is a retrospective population-based registry study of 209 patients with histologically confirmed classical HL of all stages, diagnosed in BC, Canada at age ≤ 25 years, who were treated with ABVD between 1 January 2004 and 1 July 2013 at an adult oncology centre, and 1 January 2006 and 1 July 2013 at a paediatric centre based on dates of adoption of ABVD as standard therapy. Nodular lymphocyte predominant was not included. From an initial 236 patients with classical HL, 27 were excluded (supplementary Figure S1, available at Annals of Oncology online). Patient characteristics in the excluded group did not differ from the study cohort. Patients were identified from clinical databases that are verified against the BC Provincial Cancer Registry to ensure inclusion. This approach captures ≥ 95% of all patients with HL in the province. Data collection was by chart review for patients treated at paediatric centres, and by use of the BC Cancer Agency Lymphoid Cancer Database for patients treated at adult centres. The study was approved by the Research Ethics Boards at the University of British Columbia.
Staging and treatment
Patients were staged using the Ann Arbor staging system [7]. Limited access to centralized PET/CT imaging has been available since July 2005 in BC. Bulky disease was defined as a mediastinal mass ≥ 1/3 of the maximum chest wall diameter or any mass with diameter ≥ 10 cm. Risk stratification based on adult criteria [8] was adopted for all ages: limited stage disease was stage IA, IB or IIA and the absence of bulky disease. All others had advanced stage disease.
Children under age 16 years had therapy coordinated by a single tertiary paediatric centre, adolescents aged 16–18 years were treated at either paediatric or adult centres and young adults over 18 years received care at one of the six cancer centres or regional clinics. All patients followed a common ABVD treatment protocol. Limited stage patients received 2 cycles of ABVD followed by PET or CT assessment. Consolidation for limited stage rapid early responders (RER) (negative after 2 cycles) consisted of 2 further cycles of ABVD across all age groups. Paediatric patients with slow early response (SER) (positive after 2 cycles) received 4 further cycles of ABVD, followed by IFRT if end of treatment imaging remained positive; adolescent and young adult patients with SER received IFRT to all sites of original disease. Advanced stage patients received 6 cycles of ABVD followed by repeat imaging. IFRT to areas of residual CT or PET positive disease was recommended for those patients with incomplete response if the area was radio-encompassable. The management of cases with inconclusive response on PET at any time-point was determined by the individual treating physician. The IFRT dose for all stages was 35 Gy for patients treated at an adult centre and 21 Gy for patients treated at the paediatric centre. Second-line therapy for patients with primary progressive disease or first relapse was a salvage chemotherapy regimen, followed by high-dose BEAM (carmustine, etoposide, cytarabine and melphalan) conditioning and autologous stem cell transplantation.
Patients treated at paediatric centres were followed during active care with uniform screening for acute toxicities, including echocardiogram and pulmonary function testing (PFT) every 2nd cycle. Any of this subset with follow-up greater than 2 years from diagnosis were further screened for late effects in specialized survivor clinics that utilize the Children’s Oncology Group Survivorship Guidelines [9]. Acute and late toxicity events were evaluated retrospectively using Common Terminology Criteria for Adverse Events v3.0 [10]. Toxicity data was not available for those treated at adult centres as it is not tracked in the BCCA Lymphoma Database.
Statistical methods
The response assessments were based on CT or by visual assessment of PET status as positive, inconclusive or negative by clinical radiologist. The clinical criteria used for evaluation of PET varied over time based on emerging evidence and the definitions of response to treatment were thus broadly guided by International Harmonization Project response criteria [11]: Complete response (CR) was defined as resolution of all measurable disease or negative PET; partial response (PR) as greater than 50% reduction of up to 6 largest masses and no increase in size of other nodes, with one or more PET positive lesions at previously involved sites; progressive disease (PD) as the appearance of new lesions > 1.5 cm or >50% increase in previously involved sites and PET positive; and stable disease (SD) as failure to achieve PR/CR and no PD. Primary progressive disease was defined as lack of response (SD) or PD while on treatment or within 3 months after completion of treatment; early relapse as progression detected 3 to <12 months after completion and late relapse was after 12 months.
Patient characteristics in childhood (<18 year) and young adult (18 to ≤ 25 year) age cohorts were compared using Pearson Chi-square tests. All comparisons were two-sided and significance level was P = 0.05. Any SD, PD, relapse or death due to any cause was considered an event. Progression-free survival (PFS) and overall survival (OS) were calculated from date of diagnosis to date of event or last follow-up. PFS and OS were estimated using the Kaplan–Meier method and compared by the log-rank tests at P = 0.05 significance level. Statistical analysis was performed was analyzed using SAS Statistical Software, version 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
The study cohort included 209 children, adolescents and young adults (age ≤25 year) diagnosed with HL between 2004 and 2013 who received ABVD as initial therapy. Children (<18 year) and young adults (18–25 year) had a similar distribution of sex, histological subtype, presence of systemic symptoms and tumour bulk (Table 1). Ann Arbor stage differed between age groups, with a higher proportion of stage III disease in the younger cohort likely due to more frequent baseline PET/CT imaging in the paediatric group (Table 2). However, age groups were similar in proportion of patients with limited and advanced disease.
Table 1.
Baseline patient characteristics
| All patients | Age < 18year | Age 18 –25 year | P | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Total | 209 | 55 (26) | 154 (74) | – |
| Age (year) | ||||
| Range | 6 –25 | 6 –17 | 18 –25 | – |
| Median | 21 | 15 | 22 | |
| Follow up (m) | ||||
| Range | 16 –135 | 24 –110 | 16 –135 | – |
| Median | 66 | 61 | 67 | |
| Sex | ||||
| M | 112 (54) | 30 (55) | 82 (53) | 0.87 |
| F | 97 (46) | 25 (45) | 72 (47) | |
| Diagnosis | ||||
| NS | 177 (85) | 47 (85) | 130 (84) | 0.83 |
| MC | 16 (8) | 5 (9) | 11 (7) | |
| LR | 1 (0.5) | 0 | 1 (1) | |
| NOS | 15 (7) | 3 (6) | 12 (8) | |
| Stage | ||||
| I | 20 (10) | 2 (4) | 18 (12) | 0.006 |
| II | 117 (56) | 26 (47) | 91 (59) | |
| III | 38 (18) | 18 (33) | 20 (13) | |
| IV | 34 (16) | 9 (16) | 25 (16) | |
| B symptoms | ||||
| Yes | 83 (40) | 20 (36) | 63 (41) | 0.55 |
| No | 126 (60) | 35 (64) | 91 (59) | |
| Bulk | ||||
| Yes | 68 (33) | 19 (35) | 49 (32) | 0.71 |
| No | 141 (67) | 36 (65) | 105 (68) | |
| Risk Group | ||||
| Limited | 78 (37) | 20 (36) | 58 (38) | 0.86 |
| Advanced | 131 (63) | 35 (64) | 96 (62) |
NS, nodular sclerosing; MC, mixed cellularity; LR, lymphocyte rich; NOS, not otherwise specified.
Table 2.
Patient treatment and response by prognostic risk group within age subsets
| All patients | Limited |
Advanced |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) |
N (%) |
N (%) |
|||||||
| Total | Age | Age | P | Total | Age | Age | P | ||
| < 18 year | 18–25 year | < 18 year | 18–25 year | ||||||
| Total | 209 | 78 | 20 (26) | 58 (74) | 131 | 35 (27) | 96 (73) | ||
| Treatment site | |||||||||
| Paediatric centre | 35 (17) | ||||||||
| Adult centre | 174 (83) | ||||||||
| PET at diagnosis | |||||||||
| Yes | 51 (24) | 18 (23) | 13 (65) | 5 (9) | <0 .001 | 33 (25) | 23 (66) | 10 (10) | <0 .001 |
| No | 158 (76) | 60 (77) | (35) | 53 (91) | 98 (75) | 12 (34) | 86 (90) | ||
| RT in primary therapy | |||||||||
| Yes | 43 (21) | 19 (24) | 2 (10) | 17 (29) | 0 .08 | 24 (18) | 6 (17) | 18 (19) | 0 .83 |
| No | 166 (79) | 59 (76) | 18 (90) | 41 (71) | 107 (82) | 29 (83) | 78 (81) | ||
| Response | |||||||||
| CR | 192 (92) | 78 (100) | 20 (100) | 58 (100) | – | 114 (87) | 26 (74) | 88 (92) | 0 .01 |
| PR | 10 (5) | 0 | 0 | 0 | 10 (7) | 4 (11) | 6 (6) | ||
| SD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PD | 7 (4) | 0 | 0 | 0 | 7 (5) | 5 (14) | 2 (2) | ||
| Timing of relapse | |||||||||
| Primary progressive | 11 (35) | 1 (17) | 0 | 1 (25) | 0 .22 | 10 (40) | 6 (75) | 4 (24) | 0 .03 |
| Early relapse | 12 (39) | 3 (50) | 2 (100) | 1 (25) | 9 (36) | 2 (25) | 7 (41) | ||
| Late relapse | 8 (26) | 2 (33) | 0 | 2 (50) | 6 (24) | 0 | 6 (35) | ||
| 5-year PFS ± SE (%) | 85 ± 3 | 92 ± 3 | 90 ± 7 | 93 ± 3 | 0 .65 | 80 ± 4 | 77 ± 7 | 81 ± 4 | 0 .38 |
| 5-year OS ± SE (%) | 97 ± 1 | 100 | 100 | 100 | – | 95 ± 2 | 90 ± 6 | 97 ± 2 | 0 .17 |
Analysis of the difference in treatment and response based on age for limited and advanced stage prognostic groups. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Treatment, response and outcomes
The median follow-up for living patients was 66 months (range 16–135 months). There was no difference between the age groups in the number of chemotherapy cycles (P=0.28) or proportion receiving RT (P=0.20). Radiotherapy was administered to 21% overall (24% limited versus 18% advanced stage). Overall, 192 of 209 patients achieved CR following initial therapy (92%), while 10 patients had PR, none had SD and seven patients developed PD. A greater proportion of young adults achieved a CR compared to children (95% and 84%; P = 0.01). Among children with advanced stage disease, only 74% achieved CR compared to 92% of the young adults (Table 2). Relapsed or refractory lymphoma developed in 31/209 patients (15%). Time to treatment failure differed between children and young adults: under age 18, 100% of treatment failures occurring during treatment or within 12 months of completion whereas in young adults, 8/21 (38%) of relapses occurred beyond a year off therapy (P=0.04).
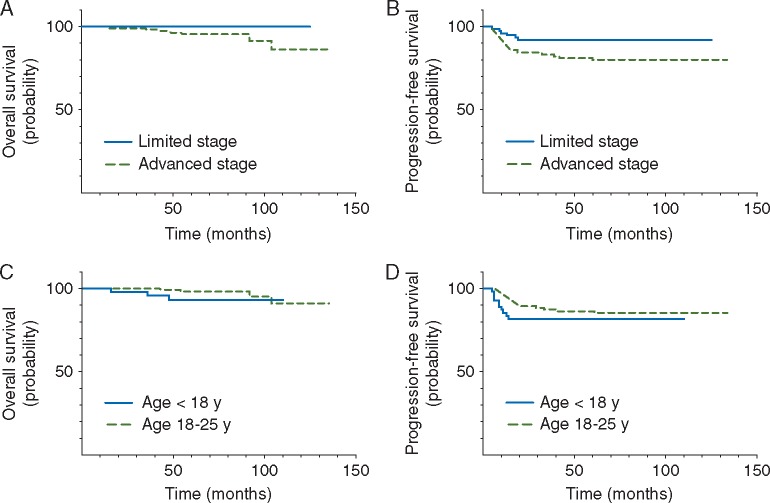
For the entire cohort, 5-year PFS was 84 ± 3% and OS was 97 ± 1%. Outcomes by the risk group and by the age are presented in Figure 1. Among patients with limited stage disease, the 5-year PFS was 90 ± 7% for the paediatric group and 93 ± 3% for young adults (P=0.65), with an OS of 100% for both groups (Table 2). For advanced stage patients, PFS and OS were also similar for the paediatric and young adult cohorts (77 ± 7% versus 81 ± 4%;P=0.38 and OS 90 ± 6% versus 97 ± 2%; P=0.17). On univariate analysis, Ann Arbor stage IV disease and bulk were significantly prognostic of PFS and OS, while the presence of B symptoms was prognostic for PFS alone (supplementary Table S1, available at Annals of Oncology online).
Figure 1.
Kaplan–Meier analysis of 5-year OS and PFS by the prognostic risk group and by the age. Patients with limited and advanced stage disease had 5-year OS 100% and 95% ± 2%, P=0.045, and PFS 92% ± 3% and 80% ± 4%, P = 0.03, respectively (A and B). OS and PFS for the paediatric (<18 year) and young adult (18–25 year) groups were 93% ± 4% versus 98% ± 1%, P=0.14, and 82% ± 5% and 86% ± 3%, P=0.33, respectively (C and D).
Thirty-five paediatric patients were reviewed for acute toxicities and of those, 24 had follow-up beyond 2 years from diagnosis (range 27–93 months) to evaluate for late effects (supplementary Table S2, available at Annals of Oncology online). The rate of grade ≥2 acute pulmonary toxicity on PFT was 60%; the most common finding was isolated reduction in diffusion capacity. Only two patients had symptomatic lung disease: one had dyspnoea on exertion and the second developed pneumonitis syndrome that required ventilator support after undergoing autologous stem cell transplant for progressive disease. There were no cases of late onset pulmonary toxicity although 3 patients had persistent mild or moderate restrictive lung disease beyond 2 years. No patient met criteria for acute cardiac toxicity; however, one patient had left ventricular dilatation initially noted during therapy and progressed to grade 2 cardiomyopathy 5 years later.
Discussion
Adult studies have shown that ABVD is effective for limited (5-year PFS 86%–94%; OS 96%–98%) [12–14] and advanced stage (5-year PFS 71%–85%; OS 84%–90%) disease [15–17]. In our cohort, young adults aged 18–25 years achieved high rates of CR to upfront therapy and 5-year PFS and OS of 93% and 100%, respectively, in limited disease and 81% and 97% in advanced disease. In addition, with a risk-adapted approach, only 23% of young adults required consolidative RT. Our data suggest that for the young adults treated with ABVD, the majority of patients have ABVD-sensitive disease, with correspondingly excellent response rates, low utilization of RT and high rates of durable remission.
In children and adolescents with limited stage disease, we similarly report an excellent 5-year PFS and OS and a 10% rate of RT usage. However, the subset of children with advanced disease stood out as having poorer outcomes including significantly lower rates of CR and earlier treatment failures. This difference is unlikely to be a reflection of treatment at a paediatric versus adult centre, as we found no difference in treatment response or survival based on locus of care (data not shown). Contemporary paediatric approaches more commonly employ 3-level risk stratification models and use of broader multi-agent therapy to minimize cumulative doses. Outcomes of this approach by the European GPOH-HD 2002 [18] were 5-year PFS 93% in both low-risk (TG-1) and intermediate-risk (TG-2), and 87% in high-risk groups (TG-3). The Children’s Oncology group trials AHOD 0431 [19], 0031 [20] and 0831 [21] report 5-year PFS 84% in low-risk, 85% in intermediate-risk and 80% in high-risk groups. Reported 5-year OS were 95%–100% in all of these trials and rates of RT were between 65% and 100%. To facilitate comparison, we performed secondary analysis of our data as per inclusion criteria of COG trials into low risk (IA and IIA without bulk, N = 43), high risk (IIIB and IVB, N = 26) and intermediate risk (all others, N = 51). Five-year PFS for low and intermediate-risk groups were 93% ± 4% and 85% ± 5%, respectively, while 5-year OS was 100% for both groups. This suggests comparable outcomes for paediatric patients with low and intermediate risk HL following ABVD chemotherapy, with considerably less RT. However, for the small group with high-risk disease, our 5-year EFS and OS were only 69% ± 9% and 87% ± 7%, respectively. As such, we cannot support utilization of this approach in this high-risk subset who likely require intensive therapy, greater use of consolidative RT and may benefit most from the inclusion of novel targeted therapies.
Given the high rates of survival in HL, the balance of risk for late effects must be factored into decision-analysis regarding optimal care. ABVD chemotherapy confers elevated risk of pulmonary toxicity from bleomycin [22] and of cardiomyopathy from anthracycline [23], but has potentially less risk of secondary malignancies and infertility based on absence of alkylators and etoposide. In our cohort, we identified a high rate of asymptomatic acute pulmonary toxicity but the majority (86%) had subsequent resolution by 2 years post-diagnosis, and there was only one case of asymptomatic cardiomyopathy among paediatric survivors with a median follow up of almost 5 years. The addition of RT is a further independent risk factor for late effects including increased risk of secondary malignancy [24], cardiac, pulmonary, thyroid and gonadal toxicity [22, 25–27]. ABVD without consolidative RT in young adults with favourable, limited stage HL has been reported to achieve 5-year PFS and OS of 92% and 100%, respectively [28]. The North American HD.6 trial [4, 12] compared ABVD alone versus ABVD plus extended field RT in adults with limited stage HL and showed that long-term OS was superior for those treated without RT, owing to fewer late effects leading to excess mortality. Our rates of consolidative RT are among the lowest for current risk-adapted protocols. In young patients with low- and intermediate-risk HL, a treatment strategy of ABVD with risk-adapted RT presents an appealing approach that allows for minimization of RT in a group with a high lifetime risk of treatment-related morbidity and mortality, without compromising survival outcomes.
As a population-based study that spans paediatric and adult sites, our study is able to report response and survival outcomes in children, adolescents and young adults treated with a common approach. Limitations include a retrospective design and restriction to registry data for adult centres, including the absence of toxicity data and incidence of late effects. Further limitations are the application of some accepted adult criteria to paediatric cohort, including the adult definition of bulk, which may underestimate its incidence and overestimate its prognostic importance. The paediatric group also had increased access to staging PET scans, which may lead to stage drift, although there was no difference in frequency of limited or advanced disease between age groups.
In conclusion, we report that ABVD with risk-adapted RT results in excellent survival outcomes in young adults with both limited and advanced stage disease, as well as children with limited stage diseases. These results were achieved with a markedly lower use of RT compared with standard protocols, thus limiting the potential risk of radiation related long-term toxicities. However, among children with high-risk, advanced stage disease, our results suggest that this approach may not be optimal.
Supplementary Material
Acknowledgements
We would like to recognize Dr Ruth Milner, Dr Boris Kuzeljevic and Dr Jim Potts for their contributions to the statistical analysis.
Funding
We gratefully acknowledge funding support from the Terry Fox Research Institute (JMC), Genome Canada (JMC), Genome British Columbia (JMC), the Canadian Institutes of Health Research (JMC), the British Columbia Cancer Foundation (JMC) and the Michael Cuccione Childhood Cancer Research Program (RJD). No grant numbers apply.
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Bazzeh F, Rihani R, Howard S, Sultan I.. Comparing adult and pediatric Hodgkin lymphoma in the Surveillance, Epidemiology and End Results Program, 1988–2005: an analysis of 21 734 cases. Leuk Lymphoma 2010; 51: 2198–2207. [DOI] [PubMed] [Google Scholar]
- 2. Gatta G, Zigon G, Capocaccia R. et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer 2009; 45: 992–1005. [DOI] [PubMed] [Google Scholar]
- 3. Canellos GP, Niedzwiecki D.. Long-term follow-up of Hodgkin's disease trial. N Engl J Med 2002; 346: 1417–1418. [DOI] [PubMed] [Google Scholar]
- 4. Meyer RM, Gospodarowicz MK, Connors JM. et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med 2012; 366: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Kapoor G, Bajpai R.. ABVD-based therapy for Hodgkin lymphoma in children and adolescents: lessons learnt in a tertiary care oncology center in a developing country. Pediatr Blood Cancer 2016; 63: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 6. Foltz LM, Song KW, Connors JM.. Hodgkin's lymphoma in adolescents. J Clin Oncol 2006; 24: 2520–2526. [DOI] [PubMed] [Google Scholar]
- 7. Carbone PP, Kaplan HS, Musshoff K. et al. Report of the committee on Hodgkin's disease staging classification. Cancer Res 1971; 31: 1860–1861. [PubMed] [Google Scholar]
- 8. Hapgood G, Zheng Y, Sehn LH. et al. Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol 2016; 34: 2493–2500. [DOI] [PubMed] [Google Scholar]
- 9. Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. Published October 2013. Internet. Monrovia, CA: Children's Oncology Group 2013.
- 10. National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology for Adverse Events version 3.0. Internet. Published August 2006.
- 11. Cheson BD, Pfistner B, Juweid ME. et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 12. Meyer RM, Gospodarowicz MK, Connors JM. et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin's lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 2005; 23: 4634–4642. [DOI] [PubMed] [Google Scholar]
- 13. von Tresckow B, Plutschow A, Fuchs M. et al. Dose-intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 2012; 30: 907–913. [DOI] [PubMed] [Google Scholar]
- 14. Radford J, Illidge T, Counsell N. et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med 2015; 372: 1598–1607. [DOI] [PubMed] [Google Scholar]
- 15. Gobbi PG, Levis A, Chisesi T. et al. ABVD versus modified Stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin's lymphoma: final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol 2005; 23: 9198–9207. [DOI] [PubMed] [Google Scholar]
- 16. Hoskin PJ, Lowry L, Horwich A. et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin's lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol 2009; 27: 5390–5396. [DOI] [PubMed] [Google Scholar]
- 17. Viviani S, Zinzani PL, Rambaldi A. et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med 2011; 365: 203–212. [DOI] [PubMed] [Google Scholar]
- 18. Mauz-Korholz C, Hasenclever D, Dorffel W. et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol 2010; 28: 3680–3686. [DOI] [PubMed] [Google Scholar]
- 19. Keller FG, Nachman J, Constine L. et al. A Phase III Study for the Treatment of Children and Adolescents with Newly Diagnosed Low Risk Hodgkin Lymphoma (HL). ASH Annual Meeting Abstracts 2010; 116: Abstract 767.
- 20. Friedman DL, Chen L, Wolden S. et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. J Clin Oncol 2014; 32: 3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly KM, Cole PD, Chen L. et al. Phase III Study of Response Adapted Therapy for the Treatment of Children with Newly Diagnosed Very High Risk Hodgkin Lymphoma (Stages IIIB/IVB) (AHOD0831): A Report from the Children's Oncology Group. In Annual Meeting of American Society of Hematology. Orlando, FL: Blood 2015.
- 22. Mertens AC, Yasui Y, Liu Y. et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95: 2431–2441. [DOI] [PubMed] [Google Scholar]
- 23. Bhakta N, Liu Q, Yeo F. et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016; 17: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castellino SM, Geiger AM, Mertens AC. et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2011; 117: 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schellong G, Riepenhausen M, Bruch C. et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer 2010; 55: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 26. Sklar C, Whitton J, Mertens A. et al. Abnormalities of the thyroid in survivors of Hodgkin's disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 2000; 85: 3227–3232. [DOI] [PubMed] [Google Scholar]
- 27. Sklar CA, Mertens AC, Mitby P. et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 2006; 98: 890–896. [DOI] [PubMed] [Google Scholar]
- 28. Canellos GP, Abramson JS, Fisher DC, LaCasce AS.. Treatment of favorable, limited-stage Hodgkin's lymphoma with chemotherapy without consolidation by radiation therapy. J Clin Oncol 2010; 28: 1611–1615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.