Abstract
Background
Radiotherapy is an effective treatment of intermediate/high-risk locally advanced prostate cancer, however, >30% of patients relapse within 5 years. Clinicopathological parameters currently fail to identify patients prone to systemic relapse and those whom treatment intensification may be beneficial. The purpose of this study was to independently validate the performance of a 70-gene Metastatic Assay in a cohort of diagnostic biopsies from patients treated with radical radiotherapy and androgen deprivation therapy.
Patients and methods
A bridging cohort of prostate cancer diagnostic biopsy specimens was profiled to enable optimization of the Metastatic Assay threshold before further independent clinical validation in a cohort of diagnostic biopsies from patients treated with radical radiotherapy and androgen deprivation therapy. Multivariable Cox proportional hazard regression analysis was used to assess assay performance in predicting biochemical failure-free survival (BFFS) and metastasis-free survival (MFS).
Results
Gene expression analysis was carried out in 248 patients from the independent validation cohort and the Metastatic Assay applied. Ten-year MFS was 72% for Metastatic Assay positive patients and 94% for Metastatic Assay negative patients [HR = 3.21 (1.35–7.67); P = 0.003]. On multivariable analysis the Metastatic Assay remained predictive for development of distant metastases [HR = 2.71 (1.11–6.63); P = 0.030]. The assay retained independent prognostic performance for MFS when assessed with the Cancer of the Prostate Assessment Score (CAPRA) [HR = 3.23 (1.22–8.59); P = 0.019] whilst CAPRA itself was not significant [HR = 1.88, (0.52–6.77); P = 0.332]. A high concordance [100% (61.5–100)] for the assay result was noted between two separate foci taken from 11 tumours, whilst Gleason score had low concordance.
Conclusions
The Metastatic Assay demonstrated significant prognostic performance in patients treated with radical radiotherapy both alone and independent of standard clinical and pathological variables. The Metastatic Assay could have clinical utility when deciding upon treatment intensification in high-risk patients. Genomic and clinical data are available as a public resource.
Keywords: prostate cancer, risk stratification, radiation therapy, prognostic, metastatic, biomarker
Introduction
In developed countries, prostate cancer is the most commonly diagnosed male cancer [1]. Clinicopathological parameters are used for risk stratification before therapy decisions. Recently, treatment options for localized prostate cancer have increased. Patients may be managed with active surveillance, while locally advanced and intermediate/high-risk patients are offered radical surgery or radiotherapy. Upfront docetaxel chemotherapy and abiraterone-acetate have recently been shown to improve failure-free survival in hormone-sensitive locally advanced prostate cancer [2, 3].
There is robust evidence supporting conventional, moderately hypofractionated or stereotactic radiotherapy, recognized in national treatment guidelines [4–7]. Furthermore, when combined with short (≤6 months) or long (>6–36 months) course androgen deprivation therapy (ADT), overall survival is prolonged [8–10]. However, ∼30% of patients will relapse within 5 years potentially due to undetectable metastatic disease, radio-resistant clones or insufficient treatment. Identifying patients at high-risk of relapse post-radiotherapy may inform treatment intensification used to reduce life-threatening disease. Routinely, there are no diagnostic tests used to identify these patients.
Gene expression (GE) biomarkers have shown promise for prostate cancer risk stratification, particularly in men undergoing radical prostatectomy [11, 12]. Few studies have assessed GE biomarkers in formalin-fixed paraffin-embedded (FFPE) diagnostic biopsies in men treated with primary radiotherapy, likely due to small tissue samples that may have degraded [13, 14].
A novel 70-gene Metastatic Assay was recently reported which identifies localized prostate cancer harbouring tumour gene expression patterns similar to metastatic disease. The Metastatic Assay was validated in a cohort of radical prostatectomy samples for biochemical and metastatic recurrence [15]. The objective of this study was to further independently validate the prognostic utility of the Metastatic Assay in FFPE diagnostic biopsies from patients receiving radical radiotherapy for localized or locally advanced prostate cancer.
Patients and methods
Study design
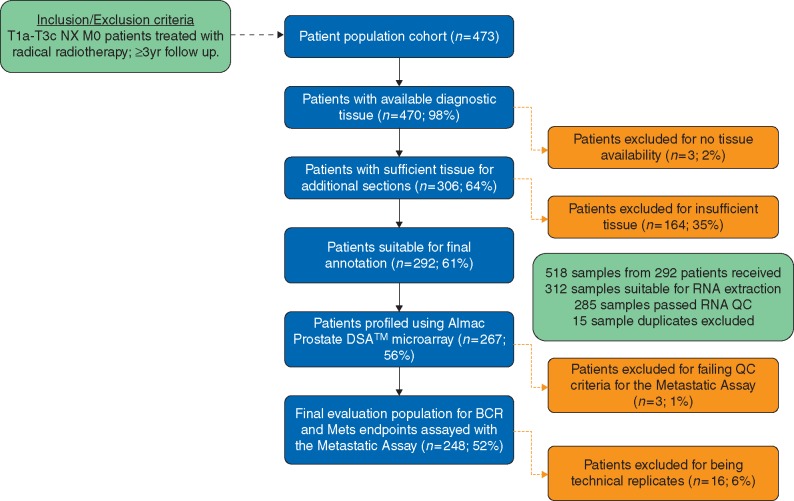
This study was designed and conducted in accordance with the Reporting Recommendations for tumour marker prognostic studies (REMARK) guidelines (Figure 1 and supplementary Appendix S1, available at Annals of Oncology online) [16]. Ethical approval for this study was obtained from the Northern Ireland Biobank (NIB Reference 15-0169) under the remit of the NIB's ethical approval from the Office of Research Ethics Committees Northern Ireland for the collection, storage and release of tissue (ORECNI Reference 16/NI/0030).
Figure 1.
REMARK study design flow diagram and resultant cohort for validation of the Metastatic Assay. Inclusion/exclusion criteria for the independent validation cohort is outlined. Critical steps within the design are highlighted in blue and sample failures at each step are highlighted in orange.
Patients
A bridging cohort of 75 FFPE diagnostic biopsies from patients with localized prostate cancer was collected to optimize the Metastatic Assay threshold in biopsy material (supplementary Table S1, available at Annals of Oncology online).
For the independent validation cohort, 473 localized/locally advanced prostate cancer patients commencing radical radiotherapy (with/without ADT) at the NI Cancer Centre, Belfast Health and Social Care Trust (BHSCT), between 1 January 2005 and 31 December 2009 were considered for inclusion (Figure 1; supplementary Table S2, available at Annals of Oncology online). Patients were treated with 70–74 Gy external beam radiation therapy (EBRT) in 2 Gy fractions with 3D conformal or intensity modulated techniques over 7–7.5 weeks. Node-negative patients received elective pelvic nodal irradiation at the physician’s discretion; node-positive patients had radiotherapy to pelvic nodal regions. Short (≤6 months) or long (>6–36 months) course ADT commenced at least 3 months before radiation with LHRH agonists or antiandrogens.
A cohort of 22 FFPE diagnostic biopsies representing 11 localized prostate cancer patients were identified from The Prostate Biobank, Oslo University Hospital and used to assess intra-tumour heterogeneity.
Gene expression profiling
As previously described [15] microarray profiling was carried out in a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory (Almac Diagnostics, UK). Stratagene UHR samples and ES-2 cell lines were used as process control measures, monitored using statistical process control charts. Quality control analysis was carried out in each cohort (supplementary Methods, available at Annals of Oncology online).
Generation of Metastatic Assay scores
Samples were pre-processed independently including RMA background correction; median summarization of probes to probesets; median summarization of probesets to Entrez Gene ID and quantile normalization using a pre-defined model. Scores were calculated on a per sample basis using the Metastatic Assay parameters and algorithm [15]. Metastatic Assay calls were derived by applying the optimized biopsy threshold (0.5505), where scores >0.5505 were classified as ‘Assay Positive’, otherwise ‘Assay Negative’.
Outcomes
Primary outcome measures were time to biochemical failure-free survival (BFFS) and time to metastatic recurrence (MFS). BFFS was defined as a PSA rise >2.0 ng/ml above nadir PSA followed by a subsequent rise [17]. MFS was defined as radiological evidence of metastatic disease, including non-pelvic lymph nodes, bone and visceral metastases using radiological imaging. Follow-up times started on the date of commencement of EBRT and were censored on the date of last follow-up or recurrence.
Statistical methods
Cox proportional hazards (PH) regression was used to investigate prognostic effects of the Metastatic Assay on time to BFFS/MFS. The estimated effect of the Metastatic Assay was adjusted for clinical covariates by fitting a multivariable analysis (MVA) model, including Gleason, age, PSA, T-stage and ADT; and secondly by evaluating adjustment for CAPRA. The Cox PH assumption was verified using statistical tests based on the Schoenfeld residuals [18].
Intra-tumour heterogeneity was assessed by calculating overall percentage agreement of the dichotomous assay call between different biopsies from the same patient.
Samples with missing clinical information were excluded. All statistical tests were two-sided at a 5% significance level.
Results
Threshold optimization and application of the Metastatic Assay in diagnostic biopsies
A bridging cohort of 75 diagnostic specimens from localized prostate cancer patients was used to optimize the Metastatic Assay biopsy threshold (supplementary Table S1, available at Annals of Oncology online). Semi-supervised hierarchical clustering identified four sample clusters. Metastatic Assay scores were highest within sample cluster S1, characterized predominantly by down-regulation of GE, indicative of our pre-defined Metastatic biology subgroup (supplementary Figure S1, available at Annals of Oncology online). A suitable biopsy threshold was derived where all performance metrics (sensitivity, specificity, NPV, PPV and accuracy) were deemed optimal (y = 0.93) (supplementary Figure S2, available at Annals of Oncology online). A threshold of 0.5505 was selected and tested initially within this bridging cohort and predicted BFFS [HR = 3.31 (0.93–11.82); P = 0.003] and MFS [HR = 8.05 (0.96–67.12); P = 0.001] (supplementary Figure S3, available at Annals of Oncology online).
Metastatic Assay performance in an independent primary prostate cancer radiation therapy cohort
We identified 473 patients for possible inclusion in the study. In 35%, residual tissue was absent or insufficient due to previous diagnostic procedures (Figure 1). In total, 248 patients (52% of original cohort, 93% of profiled cohort) had successful GE profiling and generation of a Metastatic Assay result (Table 1) (Original cohort, supplementary Table S2, available at Annals of Oncology online). Median follow-up of the analysable cohort was 99 months with one patient lost to follow-up. Median age at diagnosis was 68 years, 107 (43%) patients had Gleason 8–10 disease, 9 (4%) were node-positive and 78% had NCCN high-risk disease. Median radiation dose was 74 Gy with 27% of patients receiving pelvic nodal irradiation. A total of 141 patients (57%) received long-course ADT, 184 patients (74%) with LHRH agonists.
Table 1.
Summary of clinical characteristics for the independent radiation cohort
| Cases included in final analysis |
|||
|---|---|---|---|
| No. of patients (n) |
|||
| 248 |
|||
| n | % | ||
| Age at diagnosis, median (IQR) | 68 (62–72) | ||
| ECOG performance status (%) | 0 | 212 | 86% |
| 1 | 35 | 14% | |
| ≥2 | 1 | <1% | |
| Age-adjusted Charlson comorbidity index, median (IQR) | 0–2 | 95 | 38% |
| 3–4 | 140 | 57% | |
| ≥5 | 13 | 5% | |
| Baseline PSA (ng/ml), median (IQR) | 18 (11.1–27.4) | ||
| Clinical T-stage | T1 | 51 | 20% |
| T2 | 76 | 31% | |
| T3 | 92 | 37% | |
| T4 | 4 | 2% | |
| Unknown | 25 | 10% | |
| N-stage | N0 | 239 | 96% |
| N1 | 9 | 4% | |
| Gleason score | < 7 | 41 | 17% |
| 7 | 100 | 40% | |
| 3 + 4 | 60 | 24% | |
| 4 + 3 | 40 | 16% | |
| >7 | 107 | 43% | |
| Percent positive cores | N (%) (total) | 203 | 82% |
| Median (IQR) | 56% | 38%–83% | |
| Modified D’Amico risk group (%) | Low | 4 | 2% |
| Intermediate | 47 | 19% | |
| High | 197 | 79% | |
| NCCN risk group (%) | Low | 4 | 2% |
| Intermediate | 51 | 20% | |
| High | 193 | 78% | |
| CAPRA score (%) | 0–2 | 3 | 1% |
| 3–5 | 57 | 23% | |
| 6–10 | 120 | 49% | |
| Unknown | 68 | 27% | |
| MB score (%) | Positive | 78 | 31% |
| Radiation dose (Gy2), median | 74 | ||
| Treatment site (%) | Prostate alone | 182 | 74% |
| Prostate and pelvic lymph nodes | 66 | 27% | |
| ADT duration (%) | None | 1 | <1% |
| Short-term | 106 | 43% | |
| Long-term | 141 | 57% | |
| ADT subtype (%) | Total patients | 247 | 99% |
| Antiandrogen | 63 | 26% | |
| LHRH agonist | 184 | 74% | |
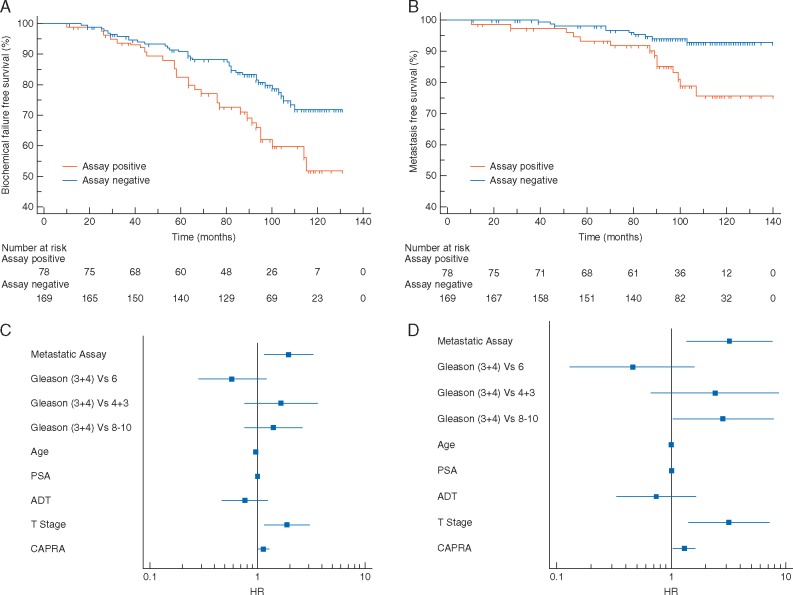
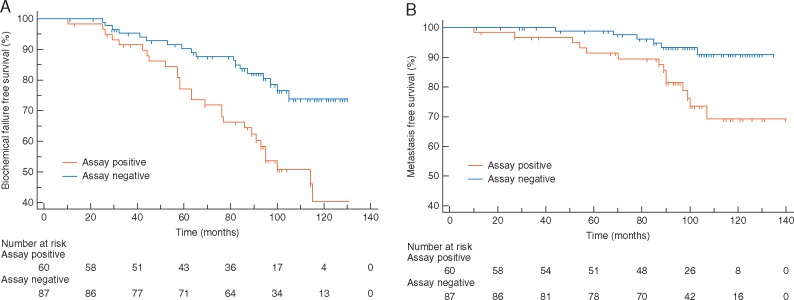
In total, 31.5% of patients were Assay Positive with confirmed clinical failure occurring in 65/248 (26.2%) patients. At 10 years, BFFS was 50% for Assay Positive patients compared with 71% for Assay Negative [HR = 1.96 (1.15–3.34); P = 0.006] (Figure 2A). In total, 24 patients (9.7%) developed distant metastases. Assay Positive patients were more likely to develop distant metastases than Assay Negative patients, with 10-year MFS of 72% compared with 94% [HR = 3.21 (1.35–7.67); P = 0.003] (Figure 2B). Similar results were observed in patients with stratification for Gleason ≥ 4 + 3 [BFFS: HR = 2.59 (1.43–4.71); P = 0.001 and MFS: HR = 3.59 (1.46–8.80); P = 0.005] (Figure 3). Low numbers of events precluded subset analysis within Gleason ≤ 4 + 3 patients.
Figure 2.
Metastatic Assay validation in the radiation biopsy cohort. Kaplan–Meier survival analysis for the Metastatic Assay predicting biochemical failure-free survival (A) in Metastatic Assay positive patients (orange) compared with Metastatic Assay negative patients (blue) [HR 1.96 (1.15–3.34); P = 0.006] and metastasis-free survival (B) in Metastatic Assay positive patients (orange) compared with Metastatic Assay negative patients (blue) [HR 3.21 (1.35–7.67); P = 0.003]. Forest plot representing the UVA performance of standard clinical factors for biochemical failure-free survival (C) and metastasis-free survival (D). Factors included are Gleason grade, age at diagnosis, PSA at diagnosis, T-stage, ADT treatment group and CAPRA.
Figure 3.
Metastatic Assay validation in the radiation biopsy cohort in high-risk patients with Gleason ≥4 + 3. Kaplan–Meier survival analysis for the Metastatic Assay predicting biochemical failure-free survival (A) in Metastatic Assay positive patients (orange) compared with Metastatic Assay negative patients (blue) [HR 2.59 (1.43–4.71); P = 0.001] and metastasis-free survival (B) in Metastatic Assay positive patients (orange) compared with Metastatic Assay negative patients (blue) [HR 3.59 (1.46–8.80); P = 0.005].
Correlation of standard clinical variables with the Metastatic Assay
In univariate analysis, aside from the Metastatic Assay, PSA and T-stage were significantly associated with BFFS [HR = 1.01 (1.00–1.02); P = 0.029 and HR = 1.00 (1.14–3.09); P = 0.023, respectively) (Figure 2C). However, PSA [HR = 1.01 (1.00–1.02); P < 0.007], T-stage [HR = 3.20 (1.41–7.26); P = 0.025] and CAPRA [HR = 1.30 (1.03–1.63); P = 0.027] were also significantly associated with MFS (Figure 2D). Importantly, the Metastatic Assay performance was better than clinical variables at predicting BFFS [HR = 1.96 (1.15–3.34); P = 0.006] and MFS [HR = 3.21 (1.35–7.67); P = 0.003]. Proportions of clinical factors within Metastatic Assay groupings indicated that Assay Positive patients had higher Gleason score (67% Gleason 8–10), PSA levels (median 21.4 ng/ml) and CAPRA scores (86% high risk) than Assay Negative patients. All clinical factors with the exception of age were significantly correlated with the Metastatic Assay result (Gleason P < 0.0001, CAPRA P = 0.0007, ADT P < 0.0001, PSA P = 0.0047 and T-stage P = 0.0001) (supplementary Table S3, available at Annals of Oncology online).
Comparison of the Metastatic Assay with clinical risk stratification
On MVA the Metastatic Assay significantly predicted for BFFS and MFS [HR = 1.94, (1.13–3.31); P = 0.016, HR = 2.71 (1.11–6.63); P = 0.030, respectively]. For BFFS, age was significant in MVA whilst for MFS, ADT was significant. All other variables were not significant following adjustment for other prognostic factors and the Metastatic Assay.
When standard clinicopathological variables were combined using the CAPRA tool, the CAPRA score did not significantly predict for BFFS [HR = 1.24 (0.61–2.55); P = 0.550] or MFS [HR = 1.88 (0.52–6.77); P = 0.332] (Table 2). The Metastatic Assay remained prognostic independent of CAPRA for BFFS and MFS [HR = 2.46 (1.31–4.62); P = 0.005 and HR = 3.23 (1.22–8.59); P = 0.019, respectively].
Table 2.
Multivariable analysis for the Metastatic Assay validation in the radiation biopsy cohort for biochemical failure-free survival (left) and metastasis-free survival (right)
| Biochemical failure-free survival (BFFS) |
Metastatic-free survival (MFS) |
||||||
|---|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | P | Covariate | HR | 95% CI | P |
| Multivariate model 1 | Multivariate model 1 | ||||||
| Metastatic Assay (negativea versus positive) | 1.94 | 1.13 to 3.31 | 0.0163 | Metastatic Assay (negativea versus positive) | 2.71 | 1.11–6.63 | 0.0300 |
| Gleason: (a3+4) | Gleason: (a3+4) | ||||||
| 6 | 0.66 | 0.25–1.74 | 0.3506 | 6 | 0.57 | 0.06–5.46 | 0.6240 |
| 4+3 | 1.71 | 0.79–3.71 | 0.1290 | 4+3 | 2.34 | 0.55–10.03 | 0.2545 |
| 8–10 | 1.33 | 0.60–2.95 | 0.4290 | 8–10 | 3.13 | 0.71–13.88 | 0.1349 |
| Age | 0.96 | 0.93–1.00 | 0.0505 | Age | 0.99 | 0.93–1.05 | 0.7259 |
| PSA | 1.00 | 1.00–1.01 | 0.2430 | PSA | 1.01 | 1.00–1.02 | 0.0914 |
| ADT (long coursea versus short course) | 1.53 | 0.77–3.04 | 0.2304 | ADT (long coursea versus short course) | 3.01 | 0.99–9.15 | 0.0538 |
| T-stage (1 and 2a versus 3 and 4) | 1.62 | 0.86–3.03 | 0.1345 | T-stage (1 and 2a versus 3 and 4) | 1.91 | 0.58–6.30 | 0.2912 |
| Multivariate model 2 | Multivariate model 2 | ||||||
| Metastatic Assay (negativea versus positive) | 2.46 | 1.31–4.62 | 0.0051 | Metastatic Assay (negativea versus positive) | 3.23 | 1.22–8.59 | 0.0185 |
| CAPRA (low riska versus high risk) | 1.24 | 0.61–2.55 | 0.5499 | CAPRA(low riska versus high risk) | 1.88 | 0.52–6.77 | 0.3320 |
MVA model 1 includes adjustment for the following clinical factors, age, PSA, Gleason, T-stage and ADT (dichotomized by treatment group). MVA model 2 includes adjustment for the CAPRA tool. In all models, P-values, hazard ratios and 95% confidence intervals are indicated.
Reference category.
HR, hazard ratio; CI, confidence intervals; PSA, prostate specific antigen; CAPRA, Cancer of the Prostate Risk Assessment; ADT, androgen deprivation therapy.
Application of the Metastatic Assay as a continuous predictor of BFFS and MFS
When considered as a continuous variable the Metastatic Assay demonstrated improved AUCs, HRs and concordance-index (CI) performance than CAPRA alone for both BFFS [AUC = 0.62, HR = 1.25 (1.09–1.43); P = 0.001, CI = 0.62 and AUC = 0.58, HR = 1.13 (0.99–1.30); P = 0.080, CI = 0.52 for Metastatic Assay and CAPRA, respectively] and MFS [AUC = 0.69, HR = 1.44 (1.16–1.78); P = 0.001, CI = 0.65 and AUC = 0.66, HR = 1.30 (1.03–1.63); P = 0.028, CI = 0.57 for Metastatic Assay and CAPRA, respectively] (supplementary Table S4, available at Annals of Oncology online). Furthermore, the performance improved from [AUC = 0.66, HR = 1.30 (1.03–1.63); P = 0.028, CI = 0.57 using CAPRA alone to AUC = 0.72, HR = 2.20 (1.28–3.79); P = 0.005, CI = 0.70] when combining CAPRA and the Metastatic Assay in a continuous model to assess metastatic recurrence (supplementary Table S4, available at Annals of Oncology online).
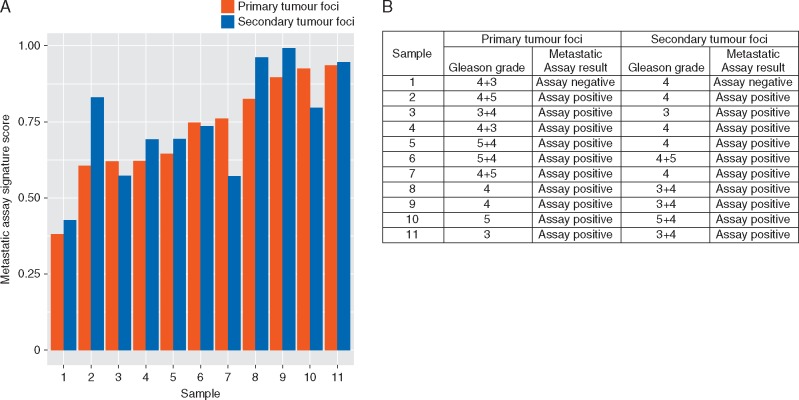
Assessment of intra-patient heterogeneity
The Metastatic Assay was applied to 22 diagnostic biopsy samples representing 2 tumour foci from 11 patients to assess intra-tumour heterogeneity both as a dichotomous (Figure 4B) and continuous result (Figure 4A). The overall agreement across tumour foci of the cohort was calculated as 100% (95% CI: 61.5% to 100%), indicating a low level of heterogeneity for the Metastatic Assay, albeit in a small cohort.
Figure 4.
Impact of intra-patient tumour heterogeneity on the Metastatic Assay. Bar chart depicting Metastatic Assay continuous signature scores for each of the 11 patients in both the primary and secondary tumour foci (A) and table outlining the Metastatic Assay dichotomous calls of 11 patients in both primary and secondary tumour foci representative of different Gleason grades (B).
Gene expression and pathway analysis of the metastatic subgroup
Differential gene analysis between Assay Positive and Assay Negative samples identified 1039 genes, encompassing 138 that were significantly overexpressed and 901 that were significantly underexpressed (supplementary Table S5a, available at Annals of Oncology online). Functional analysis using GO and DAVID identified seven key pathways defined by negatively regulated genes in the Metastatic Assay positive subgroup following Bonferroni multiple-test-correction (supplementary Table S5b, available at Annals of Oncology online). Of significant relevance were PI3K-AKT signalling (P = 0.04), protein digestion and absorption (P < 0.001) and focal adhesion (P < 0.001).
Discussion
Molecular diagnostic tests have potential to tailor therapeutic decision-making, including treatment intensification in high-risk patients. In this study, we have demonstrated that the Metastatic Assay is independently predictive of recurrence in men treated with EBRT whereby Assay Positive patients were more likely to develop BFFS and MFS in this dataset. Importantly, the Metastatic Assay was superior to conventional clinical parameters in predicting outcomes. Additionally, when combined with CAPRA, performance was superior to either alone when assessed as continuous variables. Key strengths of this study include successful application of the assay to diagnostic FFPE biopsies with low levels of heterogeneity, large cohort (n = 248), follow-up (median 99 months) and generation of high-quality GE data (93% pass-rate).
Currently, three tissue-based prognostic assays are commercially available: cell cycle progression (CCP) score (Prolaris® Myriad Genetics), Genomic Prostate Score (Oncotype Dx®, Genomic Health Inc.) and Genomic Classifier (GC) (Decipher™, Genome DX Biosciences). Most studies evaluating these panels have been in the context of radical prostatectomy. Two studies evaluated patients treated with primary radiotherapy.
In 141 men treated with radical radiotherapy, with a median follow-up of 4.8 years, the CCP assay predicted for BFFS (HR = 2.55 one-unit increase in CCP score; P = 0.03) [13]. In 100 intermediate/high-risk men treated with radiotherapy and ADT, with a median follow-up of 5.1 years, the Genomic Classifier predicted MFS (HR = 1.36 per 10% increase in score) [14]. The Metastatic Assay compares favourably to these assays and increases confidence in applying genomic biomarkers to prostate cancer clinical management.
Additional value of the Metastatic Assay is also supported by the observed failure of Gleason grade to demonstrate independent prognostic utility. Given the nature of its derivation, identification of a molecular subgroup of primary prostate cancer similar to metastatic disease [15], the Metastatic Assay has superior predictive value for MFS compared with BFFS. This may be explained by BFFS being a non-specific end point which cannot discriminate between local and distant failure. A proposed further utility of the Metastatic Assay may be identification of M0 patients who already have occult metastatic disease at presentation. The provision of localized therapy alone to Assay positive patients will likely be insufficient, therefore we propose treatment intensification using of systemic therapy, including brachytherapy [19], stereotactic radiotherapy [5] with ADT, adjuvant chemotherapy [5] or extended adjuvant ADT [9, 10]. Importantly, the Metastatic Assay also retained prognostic performance in high-risk prostate cancer (Gleason ≥ 4 + 3), consistent with its application to support treatment intensification.
A previously reported feature of Metastatic Assay positivity was significant loss of gene expression post-surgery, predominantly related to epigenetic silencing [15]. Supporting this findings, we detected a similar proportion of underexpressed (n = 901) to overexpressed genes (n = 138) in this study. Functional analysis identified PI3K-AKT and FAK signalling enrichment which provide a biological foundation to support the Metastatic Assay and the emergence of clinical relapse.
Limitations of this study include the retrospective validation within a single-centre cohort. Prostate cancer studies are restricted by timescales required to accurately quantify clinical end points. Another consideration is the pathology attrition-rate before GE profiling in archived biopsy samples [20, 21]. However, routinely clinical samples will be a few weeks old and better quality. In addition, the small sample size of the intra-tumour heterogeneity cohort and the differences of these samples compared with the validation cohort are considered to be limitations. Finally, there was an increased proportion of higher-risk disease in the final cohort when compared with the original. This was expected as there was a higher attrition rate of low-volume Gleason 6 disease.
In summary, the Metastatic Assay provides independent prognostic information for localized or locally advanced prostate cancer patients treated with radical radiotherapy and ADT who might benefit from treatment intensification. Future prospective studies could be designed to demonstrate potential benefit in patients including upstaging and intensification of treatment.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contribution of the Scientific Advisory Board Members, Prof. Kenneth J. Pienta (Johns Hopkins) and Prof. Robert G. Bristow (Princess Margaret Hospital) for their advice and discussion of this work.
Funding
Movember Prostate Cancer UK Centre of Excellence (CEO13_2-004); the Research and Development Division of the Public Health Agency of NI (COM/4965/14); the Simms Family Bequest (to CAL, JOS and SJ) and an investigator award from Friends of the Cancer Centre to SJ (NIC101345). The samples used in this research were received from the Northern Ireland Biobank which is funded by HSC Research and Development Division of the Public Health Agency in Northern Ireland and Cancer Research UK through the Belfast CR- UK Centre and the Northern Ireland Experimental Cancer Medicine Centre; additional support was received from the Friends of the Cancer Centre.
Disclosure
SJ and DW: consultancy for Almac Diagnostics. SW, AM, LK, PH and RK: employment at Almac Diagnostics, patent or IP ‘Molecular Test for Prostate Cancer’. CS, PM and GL: employment at Almac Diagnostics. All remaining authors have declared no conflicts of interest.
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. James ND, Sydes MR, Clarke NW. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387(10024): 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James ND, de Bono JS, Spears MR. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017; 377: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dearnaley D, Syndikus I, Sumo G. et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol 2012; 13(1): 43–54. [DOI] [PubMed] [Google Scholar]
- 5. King CR, Brooks JD, Gill H, Presti JC Jr. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82(2): 877–882. [DOI] [PubMed] [Google Scholar]
- 6. Kuban DA, Levy LB, Cheung MR. et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys 2011; 79(5): 1310–1317. [DOI] [PubMed] [Google Scholar]
- 7. NCC Network. Prostate Cancer, 2007; https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (June 2017, last accessed date)
- 8. Bolla M, Maingon P, Carrie C. et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC Trial 22991. J Clin Oncol 2016; 34: 1748–1756. [DOI] [PubMed] [Google Scholar]
- 9. Bolla M, Van Tienhoven G, Warde P. et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010; 11(11): 1066–1073. [DOI] [PubMed] [Google Scholar]
- 10. Horwitz EM, Bae K, Hanks GE. et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008; 26: 2497–2504. [DOI] [PubMed] [Google Scholar]
- 11. Cuzick J, Swanson GP, Fisher G. et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol 2011; 12(3): 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erho N, Crisan A, Vergara IA. et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 2013; 8(6): e66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedland SJ, Gerber L, Reid J. et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86(5): 848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen PL, Martin NE, Choeurng V. et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis 2017; 20(2): 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker SM, Knight LA, McCavigan AM. et al. Molecular subgroup of primary prostate cancer presenting with metastatic biology. Eur Urol 2017; 72(4): 509–518. [DOI] [PubMed] [Google Scholar]
- 16. McShane LM, Altman DG, Sauerbrei W. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97(16): 1180–1184. [DOI] [PubMed] [Google Scholar]
- 17. Roach M III, Hanks G, Thames H Jr. et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65(4): 965–974. [DOI] [PubMed] [Google Scholar]
- 18. Grambsch PM. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res 1995; 75: 95–112. [DOI] [PubMed] [Google Scholar]
- 19. Morton GC, Loblaw DA, Sankreacha R. et al. Single-fraction high-dose-rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate-risk prostate cancer: analysis of short- and medium-term toxicity and quality of life. Int J Radiat Oncol Biol Phys 2010; 77(3): 811–817. [DOI] [PubMed] [Google Scholar]
- 20. Gnanapragasam VJ. Unlocking the molecular archive: the emerging use of formalin-fixed paraffin-embedded tissue for biomarker research in urological cancer. BJU Int 2010; 105(2): 274–278. [DOI] [PubMed] [Google Scholar]
- 21. Xie R, Chung JY, Ylaya K. et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 2011; 59(4): 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.