Table of Contents
Abbreviationse5
Section 1: Introductione6
Section 2: Definitions, Mechanisms, and Rationale for AF Ablatione7
Definitione7
Demographic Profile of Patients with AF and Risk Factors for Development of AFe8
Natural History of AFe9
Genetic Contribution to AFe9
Genetic Determinants of Ablation Outcomee9
Significance of AFe10
Relationship Between Presence and Type of AF and Symptomse10
Anatomic and Electrophysiological Features of the Atria, Coronary Sinus, and Pulmonary Veinse10
Autonomic Nervous System and How It Relates to AF and AF Ablatione13
Cardiac Fibrosis: Etiology and How It Relates to AFe13
Atrial Electrical and Structural Remodelinge14
AF-Related Extracellular Matrix Remodelinge14
Atrial Amyloidosise14
Role of Intracellular Ca2+ Dysregulatione14
Ion Channels and Electrical Remodelinge15
Mechanisms of AF: Multiple Wavelet Hypothesis, Reentry, Spiral Waves, Rotational Activity, and Focal Triggers from the Pulmonary Veins and Other Sitese15
Mechanisms of Atrial Tachycardia and Atrial Fluttere18
Potential Benefits and Rationale for Eliminating AF with Ablatione19
Electrophysiological Basis of AF Ablatione20
The Mechanisms of AF Recurrence After Catheter Ablation or Surgical AF Ablatione20
Section 3: Modifiable Risk Factors for AF and Impact on Ablatione21
AF Risk Factors and Their Interaction with AF Management and Ablatione21
Obesitye21
Sleep Apneae22
Types, Assessment, and Treatment of Apneae22
AF Mechanisms in Sleep Apneae22
Sleep Apnea Treatment and AF Ablation Outcomese23
Hypertensione23
Diabetese23
Alcohole23
Exercisee24
Section 4: Indicationse24
Recommendations and General Considerationse24
Catheter Ablation of AF as First-Line Therapye30
Catheter Ablation of AF in Patients with Heart Failure and Reduced Cardiac Functione30
Catheter Ablation in Older Peoplee30
Catheter Ablation in Other Populations of Patients Not Well Represented in Clinical Trialse31
Catheter Ablation to Reduce Stroke Riske31
Catheter Ablation in Patients with Asymptomatic AFe31
Indications for Surgical Ablation of AFe33
Section 5: Strategies, Techniques, and Endpointse33
Historical Considerationse33
Ablation Approaches Targeting the PVs and Techniques to Obtain Permanent PVI Using RF Energye34
Optimal Initial Lesion Creation and Waiting Phasee34
Adenosine Testinge35
Isoproterenol Infusione35
Loss of Pace Capture on the Ablation Linee35
Exit Blocke35
Techniques for Obtaining Permanent PVI with Balloon Technologiese36
Obtaining Permanent PVI with the Cryoballoone36
Endoscopic Laser Balloon PVIe36
Adjunctive Ablation Strategies to Be Performed in Addition to PVI During AF Ablatione37
Cavotricuspid Isthmus Ablatione37
Linear Lesions Not Involving the Cavotricuspid Isthmuse37
Posterior Wall Isolatione37
Nonpulmonary Vein Triggerse38
LAA Focal Ablation, Isolation, and Ligation or Resectione39
Complex Fractionated Atrial Electrogram Ablatione39
Ablation of Fibrosis Identified by Voltage Mapping and/or MRI Mappinge40
Mapping and Ablation of Rotational Activitye41
Localization and Ablation of Left Atrial Ganglionated Plexie42
Dominant Frequency Mappinge43
Renal Denervatione43
Epicardial Ablation of AFe43
Nonablative Strategies to Improve Outcomes of AF Ablatione44
AAD Therapye44
Risk Factor Modificatione44
Mechanisms of Nonisthmus-Dependent Atrial Flutter and Approaches to Mapping and Ablatione44
Anesthesia During AF Ablatione45
Recurrent AF with or without PV Reconnectione46
Endpoints for Ablation of Paroxysmal, Persistent, and Long-Standing Persistent AFe46
Section 6: Technology and Toolse46
Radiofrequency Energye47
Biophysics and Irrigatione47
Contact Force-Sensing Catheters and Systemse48
Contact Forcee48
Cryoablatione49
Laser and Ultrasound Ablation Systemse50
Other Balloon Technologiese50
Multielectrode Circumferential Ablation Catheterse50
Electroanatomical Mapping Systemse51
Robotic and Magnetic Catheter Navigatione52
Ultrasounde52
PV Venographye52
CT and/or MRI Scans and Rotational Angiography to Define the Anatomy of the Atrium, PVs, and Antrume52
MRI of Atrial Fibrosis and Ablation Lesions and MRI-Guided AF Ablatione53
Section 7: Technical Aspects of Ablation to Maximize Safety and Anticoagulatione53
Prevention of Thromboembolism During and Following AF Ablatione53
Screening for LAA Thrombi Prior to Ablatione53
Transesophageal Echocardiographye53
Computer Tomographic Angiographye54
Intracardiac Echocardiographye54
Anticoagulatione54
Systemic Anticoagulation Prior to AF Ablatione54
Intraprocedural Anticoagulatione55
Early Postprocedural Anticoagulatione56
Anticoagulation Considerations Two or More Months Postablatione56
Anesthesia or Sedation During Ablatione58
General Anesthesiae58
Conscious and Deep Sedatione59
Jet Ventilatione59
Summarye59
Approaches to Minimize Risk of an AEFe59
Reduced Power Delivery on the Posterior Walle59
Esophageal Temperature Monitoringe59
Pharmacological Prophylaxise60
Role and Indications for Endoscopic Screening for Ulceration Following AF Ablatione60
Role and Indications for CT Imaging for Diagnosis of Atrioesophageal Fistulae60
Management of Atrial Esophageal Fistulae60
Summarye61
Section 8: Follow-up Considerationse61
Monitoring for Complications in the First Months After AF Ablatione61
Signs and Symptoms of Complications Within 1 Month Postablatione61
Signs and Symptoms of Complications More Than a Month Postablatione63
ECG Monitoring Pre- and Postablatione63
Available Methods for Arrhythmia Monitoringe64
Follow-up and Monitoring Guidelines for Routine Clinical Caree65
Early Recurrence After Ablatione66
Definition and Incidencee66
Causes of Recurrencese66
Early Recurrence as a Predictor of Failuree66
Antiarrhythmic Drugse66
Corticosteroidse66
Colchicinee67
Cardioversione67
Early Reablatione68
Conclusionse68
Atrial Tachycardias After AF Ablatione68
Antiarrhythmic and Other Pharmacological Therapy Postablatione68
Later-Term Repeat Ablation Procedurese69
Autonomic Alterationse69
Very Late Recurrence (More Than 1 Year) After AF Ablatione69
Section 9: Outcomes and Efficacye70
Overviewe70
Published Literature Review: Clinical Trials Performed for FDA Approvale70
AF Ablation as Second-Line Rhythm Control Therapye74
Outcomes and Efficacy of Catheter Ablation of AF as First-Line Rhythm Control Therapye74
Published Literature Review: Survey Resultse75
Outcomes of AF Ablation in Populations Not Well Represented in Clinical Trialse75
Outcomes of Catheter Ablation of Persistent and Long-Standing Persistent AFe75
Outcomes of AF Ablation in Elderly Patientse76
Outcomes of AF Ablation in Patients with Congestive Heart Failure and the Impact of Ablation on Left Ventricular Functione76
Outcomes of AF Ablation in Patients with Hypertrophic Cardiomyopathye77
Outcomes of AF Ablation in Young Patientse77
Outcomes of AF Ablation in Womene78
Outcomes of Cryoballoon Ablatione78
Outcome of Rotational Activity Ablation for AFe79
Outcomes of Laser Balloon Ablatione79
Long-Term Ablation Efficacye79
Impact of Catheter Ablation of AF on QOLe79
Impact of Catheter Ablation of AF on LA Size and Functione80
Impact of Catheter Ablation on Stroke Riske80
Predictors of Success Following AF Ablatione81
Cost Effectiveness of AF Ablatione81
Section 10: Complicationse82
Overviewe82
Cardiac Tamponadee82
PV Stenosise87
Atrial Esophageal Fistula, Atrial Pericardial Fistula, and Esophageal Hematomae88
Esophageal Hematomae88
AEF and Atrial Pericardial Fistulae88
Gastric Hypomotility and Periesophageal Vagal Nerve Injurye89
Phrenic Nerve Palsye90
Stroke, TIA, and Silent Microembolie91
Stroke and TIAe91
Asymptomatic Cerebral Embolie91
Air Embolisme92
Vascular Complicationse92
Acute Coronary Artery Occlusion and Stenosise93
Radiation Exposure During Catheter Ablation of AFe94
Pericarditise94
Mitral Valve Trauma and Curvilinear Catheter Entrapmente94
Mortality Risk with AF Ablatione95
Stiff Left Atrial Syndromee95
Coughe96
Increase in Heart Rate and/or Sinus Tachycardiae96
Section 11: Training Requirementse96
Overviewe96
Appropriate Selection of Patientse96
Anatomy of the Atria and Adjacent Structurese97
Conceptual Knowledge of Strategies to Ablate AFe97
Technical Competencee97
Procedural Experiencee97
Recognition, Prevention, and Management of Complicationse98
Appropriate Follow-up and Long-Term Managemente98
Section 12: Surgical and Hybrid AF Ablatione98
Historical Considerations and Development of the Cox-Maze Proceduree98
Surgical Ablation Technologye99
Surgical Technology for Appendage Ligation or Removal and Outcomes of These Procedurese100
Concomitant Surgical Ablatione101
Historical Considerationse101
Concomitant Surgical Ablatione101
Surgical Ablation at the Time of Concomitant Open Atrial Operationse101
Surgical Ablation at the Time of Concomitant Closed Atrial Operatione102
Stand-Alone Surgical Ablation of AFe102
Stand-Alone Operations for AF and Their Outcomese102
Catheter Ablation After AF Surgerye104
Hybrid Epicardial and Endocardial AF Ablation Procedurese105
Backgrounde105
The Futuree106
Section 13: Clinical Trial Designe106
Overviewe106
Types of Clinical Trials, Strengths, and Weaknessese106
Mortality Trialse106
Stroke and Thromboembolism Trialse107
Multicenter Outcome Studiese107
Industry-Sponsored Device Approval Studiese108
Registry Studiese108
Clinical Endpoint Considerationse108
Blanking Periode108
AF Recurrence Endpointse115
AF Burden Endpointse115
Endpoint Differences for Paroxysmal vs Nonparoxysmal AF Ablation Studiese118
Symptomatic vs Asymptomatic Recurrencee118
AF Monitoring Postablatione118
QOL Measuremente119
Other Endpoint Reportinge119
Unanswered Questions in AF Ablatione119
Section 14: Conclusione120
Acknowledgmentse120
Appendix Ae121
Appendix Be133
Referencese135
Abbreviations
- 3D
three-dimensional
- AADs
antiarrhythmic drugs
- AATAC
Ablation vs Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD
- ACE
asymptomatic cerebral emboli
- ACT
activated clotting time
- ADVICE
Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination study
- AEF
atrial esophageal fistula
- AF
atrial fibrillation
- AFACART
Non-Invasive Mapping of Atrial Fibrillation study
- AFACT
Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery study
- AFCL
atrial fibrillation cycle length
- AFEQT
Atrial Fibrillation Effect on QualiTy-of-Life questionnaire
- AFL
atrial flutter
- AH
arterial hypertension
- ANS
autonomic nervous system
- APD
action potential duration
- ARREST-AF
Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation study
- ASD
atrial septal defect
- ASTA
Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia
- AT
atrial tachycardia
- ATA
atrial tachyarrhythmia
- ATP
adenosine triphosphate
- AV
atrioventricular
- AVR
aortic valve replacement
- BIFA
box isolation of fibrotic areas
- BMI
body mass index
- BP
blood pressure
- bpm
beats per minute
- BSM
body surface mapping
- CABANA
Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial
- CABG
coronary artery bypass grafting
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CB
cryoballoon
- CBA
cryoballoon ablation
- CF
contact force
- CFAE
complex fractionated atrial electrogram
- CFS
contact force sensing
- CGCI
Catheter Guidance = Control = and Imaging
- CHASE-AF
Catheter Ablation of Persistent Atrial Fibrillation study
- CI
confidence interval
- CMAP
compound motor action potentials
- CPAP
continuous positive airway pressure
- CPVA
circumferential PV ablation
- Cryo-FIRST
Catheter Cryoablation vs Antiarrhythmic Drug as First-Line Therapy of Paroxysmal AF trial
- CS
coronary sinus
- CSA
central sleep apnea
- CT
computed tomography
- CV
conduction velocity
- DAD
delayed afterdepolarization
- DE
delayed enhancement
- DECAAF
Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation study
- DF
dominant excitation frequency
- DM
diabetes mellitus
- DW-MRI
diffusion-weighted magnetic resonance imaging
- EAM
electroanatomical mapping
- EAST
Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
- EAVM
electroanatomical voltage mapping
- ECG
electrocardiogram
- ECGI
noninvasive electrocardiographic imaging
- EF
ejection fraction
- ERAF
early recurrence of AF
- ERP
effective refractory period
- FACM
fibrotic atrial cardiomyopathy
- FAP
fractionated atrial potential
- FAST
AF Catheter Ablation Versus Surgical Ablation Treatment trial
- FDA
U.S. Food and Drug Administration
- FIRM
focal impulse and rotor modulation
- FLAIR
fluid-attenuated inversion recovery
- FTI
force-time integral
- GP
ganglionated plexi
- HCM
hypertrophic cardiomyopathy
- HDF
highest dominant excitation frequency
- HF
heart failure
- HFS
high-frequency stimulation
- HR
hazard ratio
- ICE
intracardiac echocardiography
- IRGP
inferior right ganglionated plexi
- ILR
implantable loop recorder
- INR
international normalized ratio
- LA
left atrial
- LAA
left atrial appendage
- LAD
left atrial dimension
- LALA
left atrial linear ablation
- LEGACY
Long-Term Effect of Goal Directed Weight Management on an Atrial Fibrillation Cohort study
- LGE
late gadolinium-enhanced
- LI
left inferior
- LICU
low-intensity collimated ultrasound
- LIPV
left inferior pulmonary vein
- LOE
Level of Evidence
- Look AHEAD
Action for Health in Diabetes
- LS
left superior
- LSPV
left superior pulmonary vein
- LVEF
left ventricular ejection fraction
- MANTRA-PAF
Medical ANtiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation
- MAP
mean arterial pressure
- MLWHF
Minnesota Living with Heart Failure questionnaire
- MRI
magnetic resonance imaging
- MVRR
mitral valve repair or replacement
- NCDR
National Cardiovascular Data Registry
- NCX
Na+-Ca2+ exchanger
- NOAC
novel oral anticoagulant
- NSAID
nonsteroidal anti-inflammatory drug
- OAC
oral anticoagulation
- OCEAN
Optimal Anticoagulation for Higher Risk Patients Post-Catheter Ablation for Atrial Fibrillation
- ODIn-AF
Prevention of Silent Cerebral Thromboembolism by Oral Anticoagulation With Dabigatran After PVI for Atrial Fibrillation trial
- OPC
objective performance criteria
- OR
odds ratio
- OSA
obstructive sleep apnea
- PA
peripheral artery
- PABA-CHF
Pulmonary Vein Antrum Isolation versus AV Node Ablation with Bi-Ventricular Pacing for Treatment of AF in Patients with Congestive Heart Failure
- PAF
paroxysmal AF
- PCC
prothrombin complex concentrates
- PCWP
pulmonary capillary wedge pressure
- PKA
protein kinase A
- PN
phrenic nerve
- PPIs
proton pump inhibitors
- PROTECT AF
WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation
- PS
phase singularity
- PSD
peak skin dose
- PV
pulmonary vein
- PVAC
pulmonary vein ablation catheter
- PVI
pulmonary vein isolation
- QALY
quality-adjusted life year
- QOL
quality of life
- RA
right atrium
- RAAFT
First Line Radiofrequency Ablation Versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment
- RAAFT-2
Radiofrequency Ablation versus Antiarrhythmic drugs as First-line Treatment of Paroxysmal AF
- RCA
right coronary artery
- RCT
randomized controlled trial
- RD
risk difference
- RE-CIRCUIT
Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy
- RF
radiofrequency
- RFA
radiofrequency energy ablation
- RFC
radiofrequency catheter
- RFCA
radiofrequency catheter ablation
- RI
right inferior
- RIPV
right inferior pulmonary vein
- RP
refractory period
- RR
relative risk
- RS
right superior
- RSPV
right superior pulmonary vein
- RVSP
right ventricular systolic pressure
- SARA
Study of Ablation Versus antiaRrhythmic Drugs in Persistent Atrial Fibrillation
- SMART-AF
ThermoCool SmartTouch Catheter for the Treatment of Symptomatic Paroxysmal Atrial Fibrillation
- SNP
single nucleotide polymorphism
- SPECULATE
Effect of Amiodarone on the Procedure Outcome in Long-standing persistent AF Undergoing PV Antral Isolation
- SR
sarcoplasmic reticulum
- STAR AF II
Substrate and Trigger Ablation for Reduction of AF Trial Part II
- STOP-AF
Sustained Treatment of Paroxysmal Atrial Fibrillation
- SVC
superior vena cava
- TEE
transesophageal echocardiogram
- TIA
transient ischemic attack
- VATS
video-assisted thoracoscopic surgery
- VKA
vitamin K antagonist
- WACA
wide-area circumferential ablation
- WL
wavelength
Section 1: Introduction
During the past three decades, catheter and surgical ablation of atrial fibrillation (AF) have evolved from investigational procedures to their current role as effective treatment options for patients with AF. Surgical ablation of AF, using either standard, minimally invasive, or hybrid techniques, is available in most major hospitals throughout the world. Catheter ablation of AF is even more widely available, and is now the most commonly performed catheter ablation procedure.
In 2007, an initial Consensus Statement on Catheter and Surgical AF Ablation was developed as a joint effort of the Heart Rhythm Society (HRS), the European Heart Rhythm Association (EHRA), and the European Cardiac Arrhythmia Society (ECAS).1 The 2007 document was also developed in collaboration with the Society of Thoracic Surgeons (STS) and the American College of Cardiology (ACC). This Consensus Statement on Catheter and Surgical AF Ablation was rewritten in 2012 to reflect the many advances in AF ablation that had occurred in the interim.2 The rate of advancement in the tools, techniques, and outcomes of AF ablation continue to increase as enormous research efforts are focused on the mechanisms, outcomes, and treatment of AF. For this reason, the HRS initiated an effort to rewrite and update this Consensus Statement. Reflecting both the worldwide importance of AF, as well as the worldwide performance of AF ablation, this document is the result of a joint partnership between the HRS, EHRA, ECAS, the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Society of Cardiac Stimulation and Electrophysiology (Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología [SOLAECE]). The purpose of this 2017 Consensus Statement is to provide a state-of-the-art review of the field of catheter and surgical ablation of AF and to report the findings of a writing group, convened by these five international societies. The writing group is charged with defining the indications, techniques, and outcomes of AF ablation procedures. Included within this document are recommendations pertinent to the design of clinical trials in the field of AF ablation and the reporting of outcomes, including definitions relevant to this topic.
The writing group is composed of 60 experts representing 11 organizations: HRS, EHRA, ECAS, APHRS, SOLAECE, STS, ACC, American Heart Association (AHA), Canadian Heart Rhythm Society (CHRS), Japanese Heart Rhythm Society (JHRS), and Brazilian Society of Cardiac Arrhythmias (Sociedade Brasileira de Arritmias Cardíacas [SOBRAC]). All the members of the writing group, as well as peer reviewers of the document, have provided disclosure statements for all relationships that might be perceived as real or potential conflicts of interest. All author and peer reviewer disclosure information is provided in Appendix A and Appendix B.
In writing a consensus document, it is recognized that consensus does not mean that there was complete agreement among all the writing group members. Surveys of the entire writing group were used to identify areas of consensus concerning performance of AF ablation procedures and to develop recommendations concerning the indications for catheter and surgical AF ablation. These recommendations were systematically balloted by the 60 writing group members and were approved by a minimum of 80% of these members. The recommendations were also subject to a 1-month public comment period. Each partnering and collaborating organization then officially reviewed, commented on, edited, and endorsed the final document and recommendations.
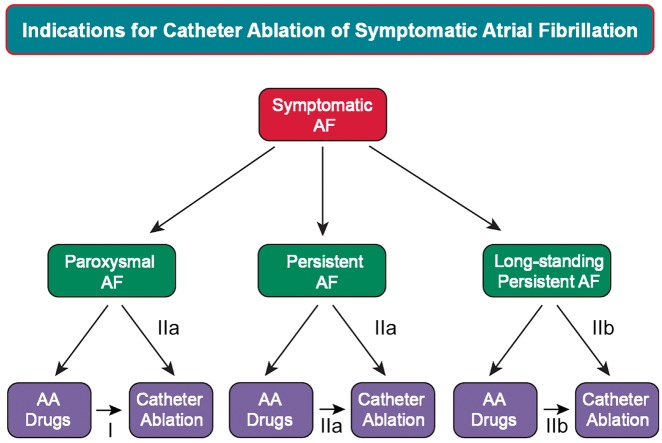
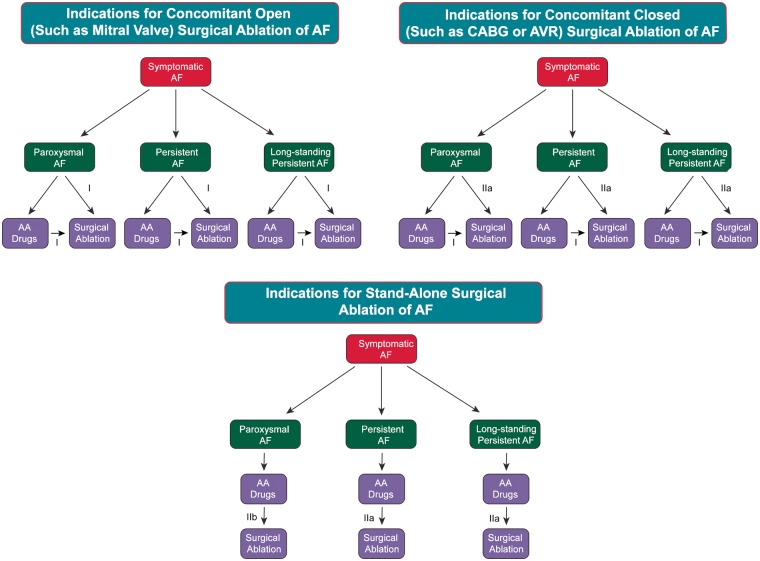
The grading system for indication of class of evidence level was adapted based on that used by the ACC and the AHA.3,4 It is important to state, however, that this document is not a guideline. The indications for catheter and surgical ablation of AF, as well as recommendations for procedure performance, are presented with a Class and Level of Evidence (LOE) to be consistent with what the reader is familiar with seeing in guideline statements. A Class I recommendation means that the benefits of the AF ablation procedure markedly exceed the risks, and that AF ablation should be performed; a Class IIa recommendation means that the benefits of an AF ablation procedure exceed the risks, and that it is reasonable to perform AF ablation; a Class IIb recommendation means that the benefit of AF ablation is greater or equal to the risks, and that AF ablation may be considered; and a Class III recommendation means that AF ablation is of no proven benefit and is not recommended.
The writing group reviewed and ranked evidence supporting current recommendations with the weight of evidence ranked as Level A if the data were derived from high-quality evidence from more than one randomized clinical trial, meta-analyses of high-quality randomized clinical trials, or one or more randomized clinical trials corroborated by high-quality registry studies. The writing group ranked available evidence as Level B-R when there was moderate-quality evidence from one or more randomized clinical trials, or meta-analyses of moderate-quality randomized clinical trials. Level B-NR was used to denote moderate-quality evidence from one or more well-designed, well-executed nonrandomized studies, observational studies, or registry studies. This designation was also used to denote moderate-quality evidence from meta-analyses of such studies. Evidence was ranked as Level C-LD when the primary source of the recommendation was randomized or nonrandomized observational or registry studies with limitations of design or execution, meta-analyses of such studies, or physiological or mechanistic studies of human subjects. Level C-EO was defined as expert opinion based on the clinical experience of the writing group.
Despite a large number of authors, the participation of several societies and professional organizations, and the attempts of the group to reflect the current knowledge in the field adequately, this document is not intended as a guideline. Rather, the group would like to refer to the current guidelines on AF management for the purpose of guiding overall AF management strategies.5,6 This consensus document is specifically focused on catheter and surgical ablation of AF, and summarizes the opinion of the writing group members based on an extensive literature review as well as their own experience. It is directed to all health care professionals who are involved in the care of patients with AF, particularly those who are caring for patients who are undergoing, or are being considered for, catheter or surgical ablation procedures for AF, and those involved in research in the field of AF ablation. This statement is not intended to recommend or promote catheter or surgical ablation of AF. Rather, the ultimate judgment regarding care of a particular patient must be made by the health care provider and the patient in light of all the circumstances presented by that patient.
The main objective of this document is to improve patient care by providing a foundation of knowledge for those involved with catheter ablation of AF. A second major objective is to provide recommendations for designing clinical trials and reporting outcomes of clinical trials of AF ablation. It is recognized that this field continues to evolve rapidly. As this document was being prepared, further clinical trials of catheter and surgical ablation of AF were under way.
Section 2: Definitions, Mechanisms, and Rationale for AF Ablation
Definition
AF is a common supraventricular arrhythmia that is characterized by rapid and irregular activation in the atria without discrete P waves on the surface electrocardiogram (ECG). AF can be diagnosed with a surface ECG, an intracardiac atrial electrogram, or both. An arrhythmia that has the ECG characteristics of AF and lasts sufficiently long for a 12-lead ECG to be recorded, or is otherwise documented to last for at least 30 seconds, should be considered to be an AF episode. The 30-second duration was selected based on previous published consensus statements and is used as the minimal duration to define recurrence of AF after catheter ablation.1,7 This duration of AF has not been linked to a specific outcome of AF. In addition to the duration requirements listed above, the diagnosis of AF requires an ECG or rhythm strip demonstrating: (1) “absolutely” irregular R-R intervals (in the absence of complete atrioventricular [AV] block); (2) no distinct P waves on the surface ECG; and (3) an atrial cycle length (when visible) that is usually less than 200 ms.2,7
Although there are several classification systems for AF, for this consensus document, we have adopted in large part the classification system that was presented in the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation.5 We recommend that this classification system be used for future studies of catheter and surgical ablation of AF. Paroxysmal AF (PAF) is defined as AF that terminates spontaneously or with intervention within 7 days of onset (Table 1); persistent AF is defined as continuous AF that is sustained beyond 7 days; and long-standing persistent AF is defined as continuous AF of greater than 12 months' duration. Early persistent AF is a new term we have defined as continuous AF of more than 7 days' duration but less than 3 months' duration. Within the context of AF ablation and clinical trials of AF ablation, early persistent AF defines a population of patients in whom better outcomes of AF ablation are anticipated as compared with persistent AF of more than 3 months' duration. The term permanent AF is defined as AF in which the presence of the AF is accepted by the patient and physician, and no further attempts will be made to either restore or maintain sinus rhythm. It is important, therefore, to recognize that the term permanent AF represents a therapeutic attitude on the part of a patient and their physician rather than on any inherent pathophysiological attribute of the AF. Such decisions can change as symptoms, the efficacy of therapeutic interventions, and patient and physician preferences evolve. If a rhythm control strategy is recommended after reevaluation, the AF should be redesignated as paroxysmal, persistent, or long-standing persistent AF. Within the context of any rhythm control strategy, including catheter and surgical AF ablation, the term permanent AF is not meaningful and should not be used. Silent AF is defined as asymptomatic AF diagnosed by an opportune ECG or rhythm strip. Paroxysmal, persistent, and long-standing persistent AF can be silent. We recognize that a particular patient might have AF episodes that fall into one or more of these categories; therefore, we recommended that patients be categorized by their most frequent pattern of AF during the 6 months prior to performance of an ablation procedure. Lone AF is a descriptor that has been applied to younger patients without clinical or echocardiographic evidence of cardiac disease. Because the definitions are variable, the term lone AF is potentially confusing, and should not be used to describe populations of patients with AF nor to guide therapeutic decisions.5 The term chronic AF also has variable definitions and should not be used to describe populations of patients with AF.
Table 1.
Atrial fibrillation definitions
| AF episode | An AF episode is defined as AF that is documented by ECG monitoring or intracardiac electrogram monitoring and has a duration of at least 30 seconds, or if less than 30 seconds, is present throughout the ECG monitoring tracing. The presence of subsequent episodes of AF requires that sinus rhythm be documented by ECG monitoring between AF episodes. |
| Chronic AF | Chronic AF has variable definitions and should not be used to describe populations of AF patients undergoing AF ablation. |
| Early persistent AF | Early persistent AF is defined as AF that is sustained beyond 7 days but is less than 3 months in duration. |
| Lone AF | Lone AF is a historical descriptor that is potentially confusing and should not be used to describe populations of patients with AF undergoing AF ablation. |
| Long-standing persistent AF | Long-standing persistent AF is defined as continuous AF of greater than 12 months’ duration. |
| Paroxysmal AF | Paroxysmal AF is defined as AF that terminates spontaneously or with intervention within 7 days of onset. |
| Permanent AF | Permanent AF is defined as the presence of AF that is accepted by the patient and physician, and for which no further attempts to restore or maintain sinus rhythm will be undertaken. The term permanent AF represents a therapeutic attitude on the part of the patient and physician rather than an inherent pathophysiological attribute of AF. The term permanent AF should not be used within the context of a rhythm control strategy with antiarrhythmic drug therapy or AF ablation. |
| Persistent AF | Persistent AF is defined as continuous AF that is sustained beyond 7 days. |
| Silent AF | Silent AF is defined as asymptomatic AF diagnosed with an opportune ECG or rhythm strip. |
AF, atrial fibrillation; ECG, electrocardiogram.
The writing group recognizes that these definitions of AF are very broad, and that additional details should be provided when describing a population of patients undergoing AF ablation. With the increased use of implantable loop recorders (ILRs), pacemakers, and implantable cardioverter-defibrillators for rhythm diagnosis, we urge the investigators to specify the duration of time patients have spent in continuous AF prior to an ablation procedure, including the 24-hour AF burden, when data are available. The investigators should also specify whether patients undergoing AF ablation have previously failed pharmacological therapy, electrical cardioversion, catheter and/or surgical ablation. Shown in Table 1 are a series of definitions of AF types that can be used for future trials of AF ablation and in the literature to help standardize reporting of patient populations and outcomes.
Demographic Profile of Patients with AF and Risk Factors for Development of AF
AF is an exceedingly common age-related arrhythmia. Among people of European descent, the lifetime risk of developing AF after age 40 is 26% for men and 23% for women.8 There are multiple risk factors for development of AF.5,7 Some of these risk factors are modifiable, including hypertension, obesity, endurance exercise, obstructive sleep apnea (OSA), thyroid disease, and alcohol consumption, whereas many others are not.5,7,9,10,11 Nonmodifiable risk factors include age, sex, family history, race, tall stature, and other types of heart and valvular disease.5,7 Among the many risk factors for development of AF, age is perhaps the most powerful.8,9 The relative risks (RRs) of AF development associated with a number of risk factors are provided in a recent systematic review.12 It is rare to develop AF prior to age 50; and by age 80, approximately 10% of individuals are diagnosed with AF. The precise pathophysiological basis of this link between AF and age is not completely understood; however, age-related fibrosis likely plays a key role.9 AF risk factors have also been shown to be of value in predicting progression of paroxysmal to persistent AF.13 It is notable that many of the risk factors that have been associated with development of AF also contribute to AF progression, recurrences of AF following ablation, and complications associated with AF (e.g., stroke).
Natural History of AF
The concept of “AF begets AF” remains a cornerstone in the understanding of the natural history of AF progression.14 Increasing AF burden is associated with progressive atrial remodeling and the development of atrial fibrosis, which can contribute to the long-term persistence of AF.15 A wealth of experimental data exist regarding structural and functional atrial changes that contribute to the development, maintenance, and progression of AF. In contrast, considerably less data exist regarding the natural history of AF.16,17 This is in large part related to the difficulty in accurately assessing the underlying burden of AF in individuals and large populations. Thus, estimates of the prevalence of clinical AF subtypes and their progression have evolved with the changes in population characteristics, associated comorbidities, and development of modern arrhythmia monitoring technology. For example, the rate of progression appears to be very low in individuals with an initial diagnosis of AF who are younger than 60 years of age and who have no concomitant heart disease. Among 97 individuals followed over three decades, 21% had an isolated AF event without further recurrence, 58% had recurrent AF, and 22% developed persistent AF.18 Other longitudinal studies have demonstrated a much higher rate of AF progression. One recent study examined the rate of progression to persistent AF among 1219 paroxysmal patients with AF.13 Progression to persistent AF was observed in 15% of the patients over 12 months of follow-up. Predictors of progression included age, hypertension, prior transient ischemic attack (TIA) or stroke, and chronic obstructive pulmonary disease. Similar results were reported in another recent study that examined AF progression while waiting for an AF ablation procedure.19 Among 564 patients with PAF, 11% progressed to persistent AF during a 10-month follow-up period. In this study, heart failure (HF) and a left atrial (LA) diameter >45 mm were predictive of progression. These findings raise the possibility that the clinical progression of AF could be driven by the development of associated comorbidities as opposed to the arrhythmia itself. Moreover, recent studies using pacemaker-documented AF burden have demonstrated a more complex natural history of the arrhythmia, with persistent AF reverting to paroxysmal forms, without intervention.20 This highlights our incomplete understanding of the natural history of clinical AF and the need for larger studies focusing on the accurate assessment of AF progression and regression.
Genetic Contribution to AF
It is now well recognized that AF is heritable.21,22,23 Individuals having a first-degree relative with AF have approximately a 40% increased risk for development of AF after accounting for established clinical AF risk factors.23 In the last decade, great progress has been made in identifying the genetic determinants of AF. Although studies of families with AF have led to the identification of mutations in a series of ion channels and molecules, these mutations are typically family-specific, rare, and do not explain a significant portion of the heritability of AF.24 Therefore, population-based or genome-wide studies have been used to identify many AF risk loci.25,26,27,28,29,30 The genes at these loci encode transcription factors and ion channels, and many are without a clear relation to AF at the present time.
There is interest in trying to use genetics to predict the onset of AF, to stratify the risk of AF outcomes such as stroke and HF, and to identify the response to treatments including antiarrhythmic medications or catheter ablation procedures. Interestingly, a genetic risk score consisting of the top 12 loci for AF can be used to identify as much as a 5-fold gradient in the risk of AF or those at greatest risk for a stroke.31,32 However, similar to other common diseases, the genetic risk for AF provides minimal additional predictive value after considering basic clinical risk factors such as age and sex.33,34 Future studies will be directed at using a comprehensive panel of genetic variants to identify those at greatest risk for AF, and also to predict stroke risk and outcomes to AF therapy, including AF ablation.35 Whether genetic testing will ultimately prove to be an important clinical marker of AF risk will become clear over time. An alternative and/or complementary strategy, which might be easier for clinicians to employ, will be the use of a clinical risk score.
Genetic Determinants of Ablation Outcome
Because many genetic determinants of AF have been identified, a logical question would be to ask whether genetics can help predict the outcome of an ablation procedure.35 At the present time, however, whether genetics will help predict outcomes remains an unanswered question. Although there have been a number of studies exploring the relation between a genetic variant or single nucleotide polymorphism (SNP) and AF ablation outcome, these studies have been challenged by small sample sizes, testing of a limited number of SNPs, and variable endpoints.
One recent study pooled ablation data from three different sites consisting of 991 individuals of European ancestry.36 They tested representative SNPs at the top three loci (PITX2, ZFHX3, and KCNN3) identified for AF in genome-wide association studies and related these SNPs to ablation outcome. The primary finding was that an SNP, rs2200733, at the chromosome 4q25 or the PITX2 locus for AF was associated with a 1.4-fold increased risk of late AF recurrence. In contrast, another recent study found differing results in a large Korean population of 1068 individuals undergoing catheter ablation for AF.37 This second study tested a similar set of SNPs, representing the PITX2, ZFHX3, and KCNN3 loci, yet they did not observe any long-term difference in AF recurrence after an ablation.
It is possible that the different outcomes noted in these two studies are due to a racial difference in the genetic influence on ablation outcome, although future studies will be necessary to resolve this issue. Larger, prospective, multiethnic studies that test a comprehensive number of SNPs will be necessary before genetic data can be considered clinically useful when considering AF ablation procedures.
Significance of AF
AF is an important arrhythmia for many reasons. First, it is common: current estimates reveal that more than 33 million individuals worldwide have AF.38 In the United States alone, it is estimated that between 3 and 5 million people have AF, and that by 2050 this number will exceed 8 million.39 Second, AF increases risk of stroke by an average of 5-fold.40 AF-related strokes are more severe than those not related to AF.41 Third, AF increases mortality, and has been linked to an increased risk of sudden death.42,43 Consistent with these prior studies, a recent Framingham study reported that those with recurrent or sustained AF had a higher multivariable-adjusted mortality compared with those with an isolated AF episode.44 Fourth, AF increases the risk of HF.45 Fifth, recent studies have linked AF with the development of dementia.46 Finally, AF causes a wide variety of symptoms, including fatigue and reduced exercise tolerance, and significantly impairs quality of life (QOL).47 It is notable that asymptomatic status is associated with similar (or worse) prognosis compared with symptomatic status.48 AF is also important when considered in terms of use of health care resources and cost. In the United States, AF accounts for more than 450,000 hospitalizations yearly and has contributed to more than 99,000 deaths.49,50 AF has been reported to increase annual health care costs by $8700 per patient, resulting in a $26 billion annual increase in U.S. health care costs. Although studies have not been performed to address the question of whether AF control with catheter ablation impacts the morbidity and mortality associated with AF, it is notable that emerging data have revealed that persistent forms of AF are associated with a significant increase in thromboembolism and death compared with PAF.51
The morbidity and mortality associated with AF provide a rationale to maintain sinus rhythm. Given the anticipated enormous public health impact of AF, proven interventions to reduce the risk of stroke, HF, cognitive impairment, and mortality are direly needed. Large, prospective, multicenter, randomized clinical trials will help address whether sinus rhythm achieved with ablation techniques lowers morbidity and mortality compared with rate control alone or treatment with antiarrhythmic therapy. These studies will also best define the patient population that will derive the most benefit. Until the results of these types of clinical trials are available, it must be recognized that the only proven benefit of AF ablation remains the reduction of symptoms and an improvement in QOL.
Relationship Between Presence and Type of AF and Symptoms
During the past 15 years, multiple studies have investigated the impact of rate vs rhythm control on stroke risk and mortality.52,53,54,55 These studies have demonstrated no difference in these endpoints. When interpreting the results of these studies, it is important to keep in mind the population of patients who were enrolled, the approach used for rhythm control, and the duration of follow-up. These studies enrolled predominantly elderly, minimally symptomatic patients with AF in whom either a rate or rhythm control strategy would be acceptable; the mean duration of follow-up was less than 4 years. The primary indication for catheter ablation is to reduce patient symptoms and improve QOL. Therefore, prior to undergoing catheter ablation, it is important to confirm that the patient's symptoms (palpitations, fatigue, or effort intolerance) result from AF and to assess their severity. In some patients with PAF, arrhythmia-monitoring tools (e.g., transtelephonic monitoring, Holter) are useful to establish the correlation between symptoms and rhythm. In patients with persistent AF who initially appear to be asymptomatic, a reassessment of symptoms after restoration of sinus rhythm with cardioversion often reveals that the patient does in fact feel better when in sinus rhythm. Because of this observation, many experienced clinicians routinely recommend cardioversion with a reassessment of symptoms in apparently asymptomatic patients with persistent AF. If the patient is ultimately demonstrated to be symptomatic, a rhythm control strategy becomes an attractive therapeutic approach. Conversely, if there is no change in symptoms postrestoration of sinus rhythm, a rate control strategy could be preferable.
Several AF ablation studies evaluated the relationship between patient characteristics and the presence of AF symptoms.56,57,58 It is well recognized that patients' perception of AF varies widely. One of the first studies to examine AF symptoms prior to and following ablation found that among 114 patients who underwent 7-day Holters prior to and following ablation, 38% of the patients had only symptomatic AF episodes, 57% had both symptomatic and asymptomatic episodes, and 5% of the patients had only asymptomatic episodes. Following the ablation, the percentage of patients with only asymptomatic episodes of AF increased to 37%.56 Asymptomatic AF is more frequent in men than in women.48,59,60 In two prospective registries and in one recent retrospective study, older age was associated with asymptomatic AF.48,60,61 Inconsistent results have been reported for the association between asymptomatic AF and cardiac and noncardiac comorbidities.48,59,60 Although any type of AF can be asymptomatic, asymptomatic AF is more common in patients with continuous persistent AF.48 In approximately half of the patients with highly symptomatic AF referred for catheter ablation, asymptomatic episodes are also present.45,50,57,62 Arrhythmia episodes are more likely to be asymptomatic following, as compared with prior to, AF ablation. Therefore, assessment of freedom from AF postablation cannot be based on freedom from symptoms alone.63
Anatomic and Electrophysiological Features of the Atria, Coronary Sinus, and Pulmonary Veins
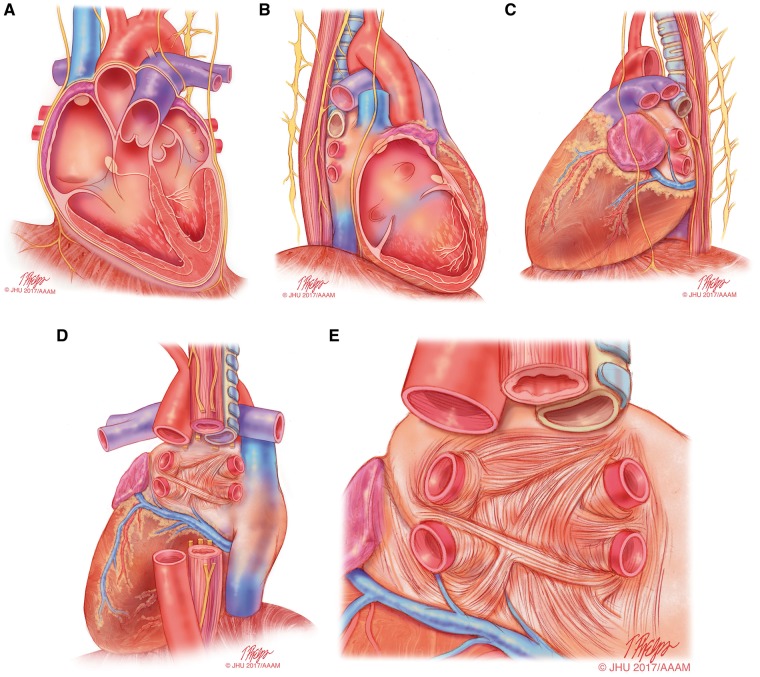
In recent decades, the development of catheter ablation of AF and other atrial arrhythmias has made it necessary to have a sound understanding of cardiac anatomy (Figure 1). Figure 1 shows the cardiac anatomy relevant for AF ablation when viewed from the anterior (Figure 1A), right lateral (Figure 1B), left lateral (Figure 1C), and posterior projections (Figure 1D, 1E).64 Viewed from the front, the right atrium (RA) is right and anterior, while the LA is situated to the left and mainly posteriorly, with the right pulmonary veins (PVs) adjacent to the intercaval area of the RA.65,66 Consequently, the plane of the atrial septum lies at an angle to the sagittal plane of the body. The front of the LA and the medial wall of the RA lie just behind the aortic root, separated only by the transverse pericardial sinus. The posterior wall of the LA is just in front of the tracheal bifurcation and the esophagus, with the fibrous pericardium separating the heart from these structures.
Figure 1.
Anatomical drawings of the heart relevant to AF ablation. This series of drawings shows the heart and associated relevant structures from four different perspectives relevant to AF ablation. This drawing includes the phrenic nerves and the esophagus. (A) The heart viewed from the anterior perspective. (B) The heart viewed from the right lateral perspective. (C) The heart viewed from the left lateral perspective. (D) The heart viewed from the posterior perspective. (E) The left atrium viewed from the posterior perspective. Illustration: Tim Phelps © 2017 Johns Hopkins University, AAM.
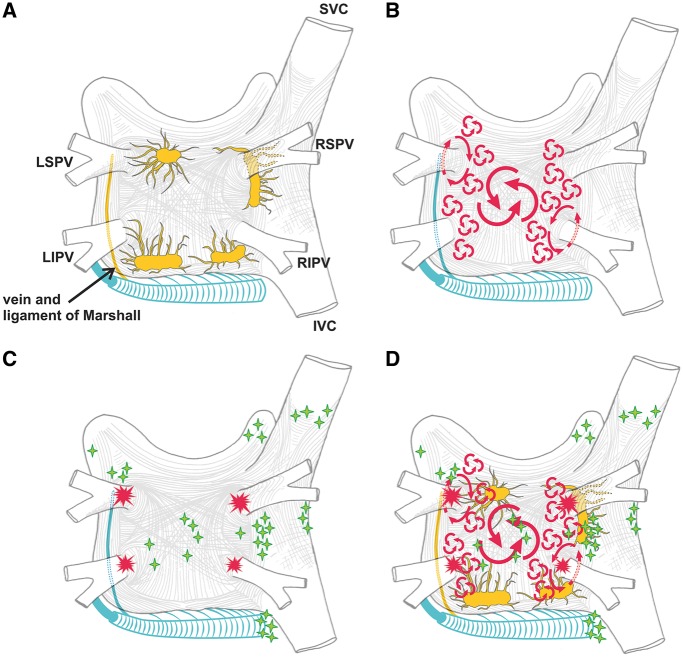
PV anatomy is highly variable between patients (Figure 2). Four distinct PV ostia are present in approximately 60% of patients, whereas variant anatomy is observed in 40% of patients undergoing ablation.67 In approximately 80% of cases, the anterior part of the ostium of the left PVs is common, separated from the appendage by a ridge.68,69 The most frequent type of variant anatomy is a left common PV, and the second most frequent variant anatomy is a right middle PV. Anomalous PVs can also be observed arising from the roof of the atrium. The orifices of the left PVs are located more superior than those of the right PVs. The right superior (RS) PV and the left superior (LS) PV project forward and upward, whereas the right inferior (RI) PV and the left inferior (LI) PV project backward and downward. The RSPV lies just behind the superior vena cava (SVC) or RA, and the left PVs are positioned between the left atrial appendage (LAA) and the descending aorta.
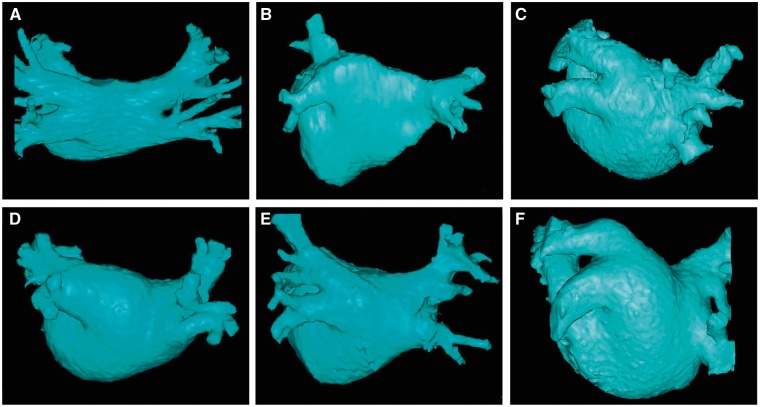
Figure 2.
This figure includes six CT or MR images of the left atrium and pulmonary veins viewed from the posterior perspective. Common and uncommon variations in PV anatomy are shown. (A) Standard PV anatomy with 4 distinct PV ostia. (B) Variant PV anatomy with a right common and a left common PV. (C) Variant PV anatomy with a left common PV with a short trunk and an anomolous PV arising from the right posterior left atrial wall. (D) and (E) Variant PV anatomy with a common left PV with a long trunk. (F) Variant PV anatomy with a massive left common PV.
Nathan and Eliakim first drew attention to the presence of sleeves of cardiac tissue that extend onto the PVs (Figure 1E).70 Myocardial muscle fibers extend from the LA into all the PVs for 1–3 cm; the thickness of the muscular sleeve is highest at the proximal ends (1–1.5 mm), and then gradually decreases distally.16,64,71 The orientation of the major atrial muscular bundles (e.g., Bachmann's bundle or Crista terminalis) has been recognized from anatomical dissections, with mostly circular bundles around the ostia of the PVs, AV valves, and LAA.72 Studies have described how premature firing from the PVs can initiate AF by interacting with tissue mechanisms, using diffusion tensor imaging (at present, in vitro).73,74 These findings have been reproduced by cardiac magnetic resonance imaging (MRI), highlighting the very variable individual pattern of fiber orientation.75 Future in vivo implementation (in addition to identification of fibrosis), combined with simultaneous mapping techniques, could allow individual tailoring of interruption of potential reentrant “pathways.”76,77
The greater coronary venous system drains approximately 85% of the venous flow into the RA, with the most proximal part being called the coronary sinus (CS). The great cardiac vein ascends into the left AV groove, where it passes close to the circumflex artery and under the cover of the LAA. The juncture between the great cardiac vein and the CS is marked by the entrance of the vein of Marshall (which is typically obliterated in adults and is referred to as the ligament of Marshall), which descends along the epicardium between the LAA and the LSPV and can contain sympathetic nerves and ganglia.78 Especially around the CS itself, muscular bundles are present that interconnect to the LA, thereby serving as additional interatrial electrical “conductors.”79,80
PV focal firing can trigger AF or act as a rapid driver to maintain the arrhythmia. During embryological development of the heart, the location of the precursors of the conduction system is defined by the looping process of the heart tube.81,82 Cell markers common to precursors of specialized conduction tissue derived from the heart tube have been found within myocardial sleeves.83 The presence of P cells, transitional cells, and Purkinje cells has been demonstrated in human PVs.84,85 PV-sleeve cardiomyocytes have discrete ion channel and action potential properties that predispose them to arrhythmogenesis.84,85 They have small background IK1, which could favor spontaneous automaticity,84 as could their reduced coupling to atrial tissue, a property common to pacemaking structures.86 Other studies show susceptibility to Ca2+-dependent arrhythmia mechanisms,87 possibly due to cells of melanocyte origin.88 Some, but not all, studies have reported that isolated cardiomyocytes from rabbit and canine PVs show abnormal automaticity and triggered activity during manipulations that enhance Ca2+ loading.87,88,89 These properties might explain the electrical activity within the PVs that is commonly observed after electrical disconnection of the PVs from the atrium.90
Other studies have provided evidence to suggest that the PVs and the posterior LA are also preferred sites for reentrant arrhythmias.90,91 One important factor could be the shorter action potential duration (APD) of the PVs vs the atrium84 due to larger delayed-rectifier K+ currents and smaller inward Ca2+ currents in the PV.89,92,93 In addition, PVs demonstrate conduction abnormalities that promote reentry due to abrupt changes in fiber orientation as well as Na+ channel inactivation by reduced resting potentials due to small IK1.84 Yet another study examined the impact of increasing atrial pressure on PV activation, finding that as LA pressure was increased above 10 cm H2O, the PV–LA junction became the source of dominant rotors.94 These observations help explain the clinical link between AF and increased atrial pressure. Several clinical studies have reported shorter refractory periods (RPs) inside PVs compared to the LA, decremental conduction inside PVs, and easy induction of PV reentry with premature stimulation from the PVs. Accordingly, rapid reentrant activity with entrainment phenomena have been described inside PVs after successful PV isolation (PVI).95,96 Electrophysiological evaluation of the PVs using multielectrode basket catheters has revealed effective refractory period (ERP) heterogeneity and anisotropic conduction properties within the PV and at the PV–LA junction, which can provide a substrate for reentry.97 The response of PV activity to adenosine administration in patients with PAF is more consistent with a reentrant than a focal-ectopic type of mechanism.98,99 In addition, dominant frequency analysis points to an evolution of mechanisms in patients with AF, with PV sources becoming less predominant as AF becomes more persistent and atrial remodeling progresses.95
Autonomic Nervous System and How It Relates to AF and AF Ablation
The cardiac autonomic nervous system (ANS) can be divided into the extrinsic and intrinsic ANS.100 The extrinsic cardiac ANS consists of sympathetic and parasympathetic components,101,102 and includes neurons in the brain and spinal cord and nerves directed to the heart. The intrinsic ANS primarily includes thousands of autonomic neurons and nerves located in ganglionated plexi (GP), which are transitioned to the epicardial fat pads outside the heart and along the great vessels in the thorax.100,103,104 There are 7 major GP, including 4 located in the LA around the PVs.103,105 The ligament of Marshall, which also contains GP, plays a coordination role between the extrinsic and intrinsic ANS.106 The GP predominantly contain parasympathetic neurons, but also sympathetic neurons. In humans, numerous autonomic nerves are located at the PV–LA junction. The nerve densities are much more pronounced within 5 mm of the PV–LA junction and are higher in the epicardial surface than in the endocardium.107,108 These data reveal that the areas of LA endocardial surface most suitable for ANS modification are located in the immediate vicinity of the PV–LA junction. Due to close relationship of the sympathetic and parasympathetic ANS components, it is difficult to perform selective radiofrequency (RF) ablation of a particular part of the ANS,109 and ablation of these sites can destroy both adrenergic and cholinergic nerves.
In an animal model of PAF, injection of parasympathomimetics into the fat pad adjacent to the PV-atrial junctions resulted in spontaneous or easily induced sustained AF, suggesting that a hyperactive ANS can play an important role in patients with focal AF arising from the PV.110,111 Stimulation of GP by pacing at the base of the PV can also provide a substrate of AF initiation from PV firing.112,113 Studies have shown that the intrinsic ANS has a potential impact on acute atrial electrical remodeling induced by rapid atrial pacing.113 Other studies have shown that synergic actions of both the sympathetic and parasympathetic neurotransmitters promote rapid PV firing in an experimental system.114 Another study demonstrated that stimulation of the right anterior GP converts isolated premature depolarization from the RSPV into AF-inducing premature depolarizations,115 indicating a link between GP activity and AF inducibility. The authors proposed a model of a highly integrated atrial neural network in which a GP hyperactive state could release a gradient of locally excessive concentrations of neurotransmitters that initiate AF, whereas activation of the axons can “retrogradely” excite the GP at a distance to cause the release of neurotransmitters to induce AF. Several studies have identified a link between the intrinsic cardiac nervous system and complex fractionated atrial electrograms (CFAEs) and AF triggers.113,116
The effectiveness of catheter ablation of GP in patients with AF remains controversial. One of the major challenges has been the lack of a sensitive and specific means to localize the GP in patients.117,118,119,120 Whereas several small studies have reported improved outcomes using an anatomically based approach to localize autonomic ganglia, these findings have not been replicated by other investigators.121,122 A recent prospective randomized surgical AF ablation study reported no improvement of outcomes by ablation of autonomic ganglia.123
The most commonly used approach to localize the major atrial GP is to apply high-frequency stimulation (HFS) to the presumed GP areas to elicit AV block. This method has low specificity and sensitivity because endocardial GP can be embedded in epicardial fat pads.106,124 Some investigators have suggested that HFS mainly reveals the afferent link of the ANS, suggesting that sites eliciting vagal responses do not coincide with sites where GP clusters and efferent autonomic nerves are located.125 Another issue is reinnervation of the ANS during follow-up.108,114 Whether reinnervation causes recurrent AF postablation remains uncertain. One study has reported that reinnervation of the ANS in patients after RF ablation is not directly related to AF recurrence.126
In summary, there is considerable evidence that the ANS contributes to the initiation and maintenance of AF. Whether ablation of the ANS impacts the outcomes of AF ablation remains uncertain. In the future, novel approaches for ANS modulation could increase the efficacy of AF ablation treatment.127,128,129
Cardiac Fibrosis: Etiology and How It Relates to AF
Atrial fibrosis is a common finding in patients with AF. The question of whether atrial fibrosis stems from AF itself, from AF-related risk factors, or from a specific fibrotic atrial cardiomyopathy (FACM) is under debate.130,131,132,133,134 Recently, a subgroup of patients with recent onset persistent AF have been described with a diffuse abnormal substrate and with poor outcome after ablation.135 There is great variability in the amount of fibrosis in patients with AF, in which some patients with PAF have massive fibrosis and some patients with persistent AF show mild fibrosis.134,136 Some morphological studies have shown that fibrosis in humans is related to the underlying disease rather than being caused by AF.73,137,138 The specific role of age and AF risk factors in atrial fibrosis was questioned by an autopsy study, in which only small amounts of fibrofatty tissue were described in atrial specimens from patients with a high mean CHA2DS2-VASc score of 4.3 but no AF.139 In addition, a low correlation between risk factors and the fibrotic substrate as estimated from electroanatomic voltage mapping in patients with non-PAF has been described.140 Similarly, cardiovascular risk factors were found to be equally distributed in various classes of LA fibrosis as described by MRI studies, and structural atrial remodeling was the same in patients with and without cardiovascular risk factors.130 On the other hand, there is extensive evidence that many AF risk factors do substantially increase atrial fibrosis content, and that AF itself might have a profibrotic effect.141,142,143 One study reported that elevated serum markers of collagen synthesis were associated with postsurgical AF, compared with those who stayed in sinus rhythm.144
It is possible that the fibrotic atrial substrate could be a result of a specific FACM.131,133,140 FACM has been described as a specific disease with various expressions, from mild, to moderate, to severe atrial fibrosis, and with a potentially progressive disease process. Consequently, AF—and other arrhythmias such as reentrant atrial tachycardia (AT) and sinus node disease—can be understood as a manifestation of the preexisting FACM.131,133,145,146
Atrial Electrical and Structural Remodeling
The pathophysiology of AF is complex, involving interaction among multiple factors, including triggers, which are responsible for AF initiation; substrate, which is necessary for AF maintenance; and perpetuators, which underlie the progression of the arrhythmia from paroxysmal to the persistent forms.146,147 The recently published EHRA/HRS/APHRS/SOLAECE expert consensus document on atrial cardiomyopathies provides a detailed review of the important topic of atrial cardiomyopathies and their interrelationship with AF.148 It is generally believed that some degree of structural remodeling must predate electrical remodeling. The trigger mechanisms can include focal enhanced automaticity or triggered activity. Initiation of AF can be favored by both parasympathetic and sympathetic activation, which also appear to play a role in maintaining AF.149 However, the central mechanisms governing AF initiation and perpetuation are poorly understood, which explains in part why treatment of patients with all forms of AF, and particularly long-standing persistent AF, remains suboptimal. Although AF usually starts with paroxysmal episodes, it can evolve to a persistent form in a significant number of patients.150 A few clinical factors have been associated with transition from paroxysmal to persistent AF.20,151,152 The transition likely reflects progressive structural and electrophysiological remodeling in both atria, making the sources of the arrhythmia more stable by fundamental mechanisms that have been incompletely explored.153,154,155,156
AF-Related Extracellular Matrix Remodeling
Persistent AF itself leads to electrical remodeling and fibrosis of the atria.157,158 Experimental and clinical data point to a complex pathophysiology involving diverse factors, including oxidative stress, calcium overload, atrial dilatation, microRNAs, inflammation, and myofibroblast activation.141,159,160,161,162 In a recent study of transcriptional changes associated with AF, susceptibility to the arrhythmia was associated with decreased expression of targets of several transcription factors related to inflammation, oxidation, and cellular stress responses.163 However, it is unknown to what extent and at which time points such alterations influence the remodeling process that perpetuates AF. Moreover, rapid atrial rates activate fibroblasts to increase collagen-gene activity, and AF in isolation might promote cardiac fibrosis.131,133,134
Cardiac fibrosis is part of the maladaptive cardiac remodeling in response to cardiac injury164,165 and has been implicated in initiation and maintenance of AF.166 The mechanisms that are responsible for fibrosis and its consequences comprise many phenomena occurring at various scales, including molecular, organelle, cellular, and tissue scales.167 At the molecular scale are dynamics changes in the genome, the transcriptome, and the signaling pathways underlying the generation of profibrotic molecules168; cellular changes involve interactions among the various cardiac cells, including myocytes, fibroblasts or myofibroblasts, and inflammatory cells such as macrophages and neutrophils169; and tissue changes relate to the dynamics of scar, angiogenesis, electrical conduction, and contractility.153 Fibrosis can certainly act as an electrically insulating obstacle. Profibrotic stimuli promote differentiation of fibroblasts into activated myofibroblasts, which electronically couple to myocytes in vitro20,150,151,152; whether this occurs to a significant extent in AF atria in vivo remains uncertain. Fibrosis affects electrical propagation through slow, discontinuous conduction with “zigzag” propagation,170,171 reduced regional coupling,172 abrupt changes in fibrotic bundle size,173 interruption of bundle continuity, and micro-anatomical reentry.174
Another potentially important factor in AF-related atrial remodeling is fatty infiltration, which is known to increase in a number of myocardial pathophysiological conditions and is regarded as arrhythmogenic.175,176,177 Obesity is a known AF risk factor, and the increasing incidence of AF could be related to increasing rates of obesity.177,178 Obesity frequently coexists with other AF risk factors that improve in response to weight loss, emphasizing the importance of weight loss in AF risk factor management.179 Epicardial fatty infiltration occurs with obesity180 and has been associated with AF.177 Biofactors released from fat might promote fibrosis and myocardial remodeling.
Atrial Amyloidosis
Over the past decade, a number of studies have called attention to a link between atrial amyloidosis and AF.74,181,182 Amyloidosis is characterized by the presence of extracellular proteinaceous deposits showing characteristic structural and tinctorial properties. The various types of amyloidosis are distinguished based on the fibril protein deposited and the clinical presentation. Amyloidosis can affect the heart as part of a systemic process, as in immunoglobulin-derived light-chain amyloidosis. Amyloid can also be deposited in the heart as a manifestation of aging (senile amyloidosis), with amyloid observed in cardiac vessels, in the ventricular interstitium, and in the atria. The heart can also be affected by an organ-limited variant called isolated atrial amyloidosis. The incidence of isolated atrial amyloidosis exceeds 90% in the ninth decade. Studies have shown that isolated atrial amyloidosis affects atrial conduction and increases the risk of AF. Notably, there is an inverse correlation between isolated atrial amyloidosis and atrial fibrosis.
Role of Intracellular Ca2+ Dysregulation
Spontaneous Ca2+ release promoting triggered activity is likely to be an important mechanism of AF initiation.183 During AF, the exceedingly high frequency of atrial excitation is expected to lead to RyR2 refractoriness184 and downregulation of Ca2+ handling proteins,158 acting to prevent triggered activity in the presence of persistent AF. RyR2 leakiness is therefore unlikely to contribute to persistent AF.185 However, such considerations do not apply in PAF, in which ectopic activity likely related to Ca2+-dependent ectopy could play an important role. There is evidence that Ca2+ released from the leaky RyR2 receptors in the sarcoplasmic reticulum (SR) is exchanged by the Na+-Ca2+ exchanger (NCX), which produces an arrhythmogenic depolarizing current that induces atrial ectopic activity.186,187 In a mouse model characterized by progressive AF, SR Ca2+ leak is enhanced in association with Ca2+/calmodulin-dependent protein kinase II (CaMKII)-dependent hyperphosphorylation of the ryanodine receptor.188 Genetic inhibition of the Ca2+ leak reduced structural remodeling and prevented the development of persistent AF.188 However, in isolated remodeled rabbit and human atrial myocytes, Ca2+ signaling was silenced through a variety of mechanisms.185 The authors suggested that Ca2+ silencing might be a protective mechanism against the Ca2+ overload that occurs during chronic AF, and challenged the notion that aberrant Ca2+ release contributes to the pathophysiology of persistent AF. However, during AF, the exceedingly high frequency of atrial excitation is expected to lead to RyR2 refractoriness and downregulation of Ca2+-handling proteins, acting to prevent triggered activity. Therefore, whether RyR2 leakiness contributes to persistent AF is now being disputed.158,184,185 A popular concept that had been promoted by some investigators over the last several years was that both initiation and maintenance of AF could be related to increased activity of protein kinase A (PKA) and/or Ca2+/CaMKII, with subsequent uncontrolled diastolic Ca2+ release from the SR.186 The idea is that Ca2+ released from the “leaky” RyR2 receptors in the SR would overactivate the NCX to extrude Ca2+ and produce an arrhythmogenic depolarizing current, thereby explaining both the contractile dysfunction and the high recurrence rate of the arrhythmia.186,187 In a recent study in mice with a mutation causing progressive AF, SR Ca2+ leak was reported to be enhanced in association with Ca2+/CaMKII–dependent hyperphosphorylation of the ryanodine receptor.188 Genetic inhibition of Ca2+/CaMKII-mediated RyR2-S2814 suppressed the Ca2+ leak, reduced structural remodeling, and prevented the development of persistent AF.188 However, recent studies in large animals and in humans have challenged the idea that Ca2+ dysfunction underlies AF maintenance and perpetuation. In isolated rabbit atrial myocytes, remodeling in response to sustained tachycardia for up to 5 days was shown to silence Ca2+ signaling through a failure of subcellular propagated Ca2+ release.185 The authors suggested that Ca2+ silencing might be a protective mechanism against the massive Ca2+ overload that occurs during chronic AF. In another study in human atrial myocytes, although CaMKII appeared to facilitate catecholamine-evoked arrhythmias in the atrial myocardium of patients with sinus rhythm, the same agonists failed to elicit arrhythmias in the atrial myocardium of patients with chronic AF, likely related to atrial remodeling, which included decreases in CaMKII-mediated processes.189
The above results in patients are consistent with data derived from western blot analyses in sheep, designed to test whether remodeling was related to altered intracellular calcium dysfunction.158 Although the Na+-Ca2+ exchange was increased in the LAA of animals with persistent AF, both total RyR2 and phosphorylated RyR2 proteins were decreased, and the ratio of phosphorylated RyR2 to total RyR2 phosphorylation was unaffected. Thus, the transition from paroxysmal to persistent AF in the sheep model of atrial tachypacing did not appear to depend on Ca2+ leak or delayed afterdepolarizations (DADs).
Ion Channels and Electrical Remodeling
Electrical remodeling, manifested as shortening of atrial refractoriness, develops within the first few days of AF.13,153,154,190 A number of ion channel modifications underlying such electrical changes have been described in animal models and humans.17,190,191,192 A recent study158 used a clinically relevant ovine model of intermittent RA tachypacing and demonstrated that, after the first AF episode, the dominant excitation frequency (DF) increased gradually during a 2-week period in both LA and RA until it stabilized at a time that coincided with the onset of persistent AF. The DF changes were associated with downregulation of ICaL and INa and upregulation of IK1, along with corresponding mRNA or protein changes, as described in extensive previous studies of atrial remodeling.17 Interstitial fibrosis developed at 6–12 months and coincided with persistent AF. This study highlighted progressive forms of atrial remodeling in the increasing tendency of AF to persist over time. Consistent with these findings, another study recently demonstrated that AF persistence was associated with numerous transcriptional changes in ion channel expression.163 Such changes included upregulation of KCNJ2 and KCNJ4 (encoding Kir2.1 and Kir2.3 subunits, respectively, which contribute to IK1) and downregulation of CACNA1C (encoding the ICaL α-subunit) and CACNAB2 (an ICaL β-subunit).163,193 Therefore, the progressive DF increase during PAF is also consistent with the fact that AF frequency is usually higher in patients with persistent than with PAF,98 a difference that is now clearly due to sustained AF-related electrical remodeling. Sustained AF shortens APD and the ERP, decreasing the wavelength and facilitating the acceleration and stabilization of sustained reentry. The primary determinants of APD shortening are the decrease in ICaL and increase in IK1.158
Mechanisms of AF: Multiple Wavelet Hypothesis, Reentry, Spiral Waves, Rotational Activity, and Focal Triggers from the Pulmonary Veins and Other Sites
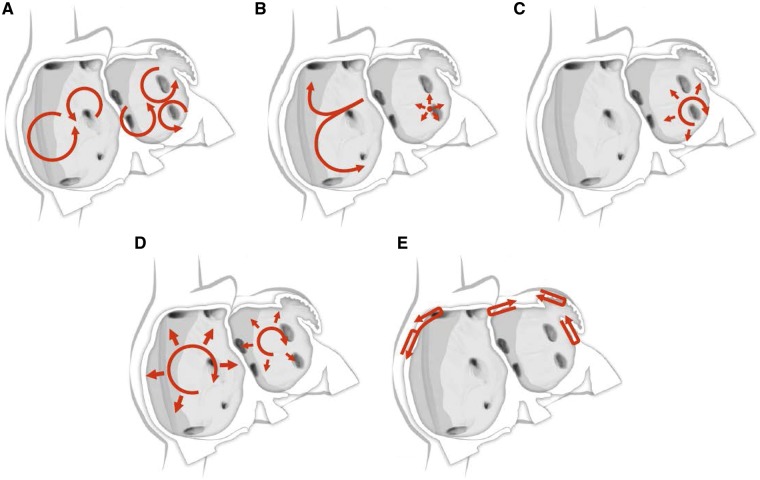
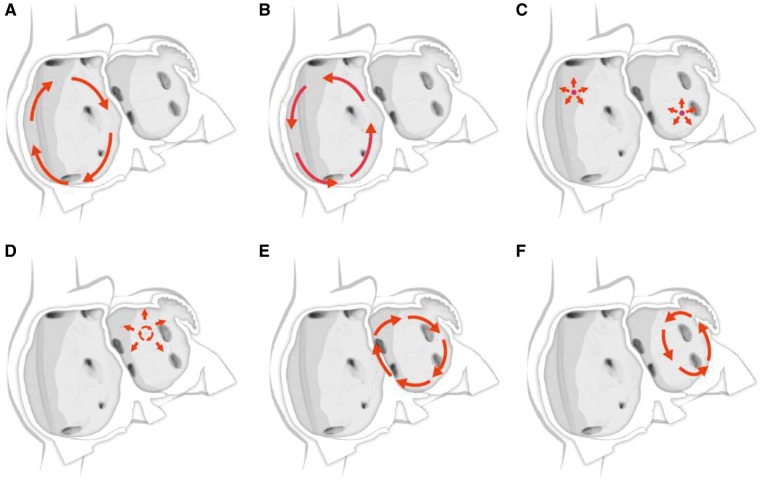
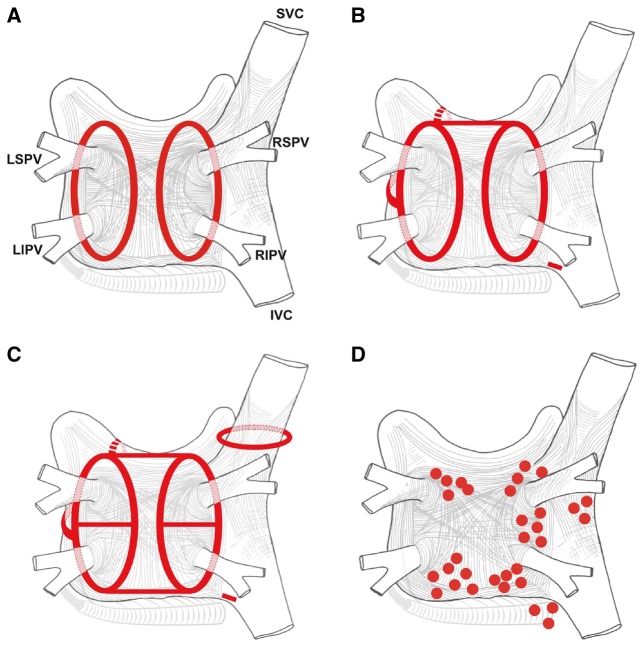
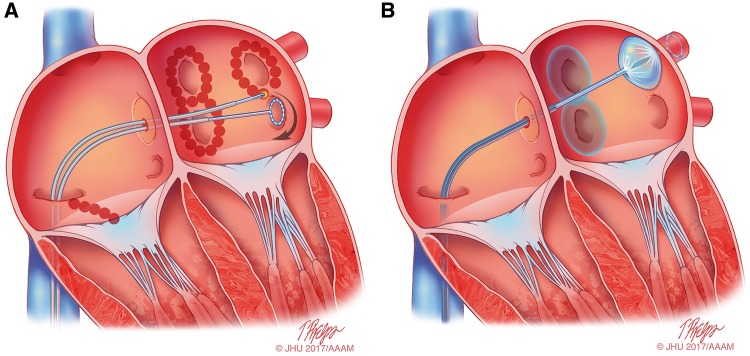
For many years, three concepts competed to explain the mechanism of AF: multiple reentrant wavelets (Figure 3A), rapidly discharging automatic foci (Figure 3B), and a single reentrant circuit with fibrillatory conduction (Figure 3C).194,195,196 Considerable progress has been made in defining the mechanisms underlying initiation, perpetuation, and progression of AF (Figures 3, 4).16,17 A key breakthrough was the recognition that in some patients, AF is triggered and/or maintained by rapidly firing foci and can be “cured” by local catheter ablation.197 This crucial observation focused attention on the PV cardiomyocyte sleeves. Subsequent work confirmed the key role of the PVs in AF, particularly paroxysmal forms, and showed that the PVs have features that make them favored zones to harbor both focal automatic and microreentrant activity.157
Figure 3.
Schematic drawing showing various hypotheses and proposals concerning the mechanisms of atrial fibrillation. (A) Multiple wavelets hypothesis. (B) Rapidly discharging automatic foci. (C) Single reentrant circuit with fibrillatory conduction. (D) Functional reentry resulting from rotors or spiral waves. (E) AF maintenance resulting from dissociation between epicardial and endocardial layers, with mutual interaction producing multiplying activity that maintains the arrhythmia.
The multiple wavelet concept was initially proposed by Garrey (Figure 3A), was later refined by Moe, and for at least 50 years became the dominant mechanistic framework for AF. Engelmann had earlier suggested that AF was maintained by rapidly discharging atrial ectopic foci,198,199,200 a notion that was subsequently rejected only to periodically resurface.201 Finally, Thomas Lewis suggested that a single rapidly rotating primary reentrant circuit (a “mother wave”) was the most likely mechanism underlying AF.202 For AF due to a single ectopic focus or a rapidly rotating single circuit, fibrillatory conduction is required to account for the irregular activation typical of AF.203 All three of these classical mechanisms were proposed in the early 20th century and continue to underlie much of the contemporary thinking about AF mechanisms.195
As mentioned above, the observations of early investigators who recognized the importance of the PVs in AF were critical. Their initial observations pointed to a critical role for very rapidly discharging PV foci in maintaining AF. Subsequent experimental studies indicated that the PVs could indeed represent sites of very rapid automatic activity, which is enhanced by the rapid activation caused by AF.204 Subsequent detailed studies of PV cardiomyocyte ion-current function84 and structure91 indicated that the PVs also have properties favoring local microreentry, which likely contribute to their participation in AF. Recent studies have implicated abnormal Ca2+ handling and DAD related to spontaneous ectopic activity of patients with paroxysmal or long-standing persistent AF.186,205 However, more recent studies strongly suggest that during long-term sustained AF, one should not expect an increase in the spontaneous release of Ca2+ from the SR, nor that DADs or triggered activity is involved in AF maintenance or in the progression to stable forms of the arrhythmia.158,206,207 Subsequent to recognition of the importance of the PVs, a variety of sites other than the PVs have been shown to potentially harbor AF-maintaining sources,208 but the critical importance of the PVs has withstood the test of time.
Allessie et al induced and mapped electrically induced tachycardia in isolated rabbit atria and documented the circular movement reentry in that model.209 Using a limited number of electrodes, the authors detected an activation sequence that suggested centripetal direction of wavelet propagation. The authors proposed that these centripetal wavelets activated tissue at the center of the circuit, resulting in double responses (double potentials) of subnormal amplitude. Because the centripetal wavelets were unable to propagate beyond the center, they prevented the impulse from shortcutting the circuit, resulting in the maintenance of reentry. This mechanism of reentry was named leading circle reentry by Allessie et al.209 Building on ideas put forward initially by Mines and later quantified by Wiener and Rosenblueth, Allessie et al suggested that functional reentry naturally establishes itself in the shortest circuit that can maintain reentry, defined by the distance a cardiac impulse travels during the RP.210,211,212 This distance determines the length of the shortest reentrant cardiac excitation wave (wavelength, WL) and is equal to the product of conduction velocity (CV) and RP (e.g., WL = CV × RP). If AF is maintained by multiple simultaneous reentrant waves, the likelihood of spontaneous termination is greatest when the atria are only large enough to maintain one reentrant wave; if the wavelength is shortened so that multiple waves can be maintained simultaneously, the chances of spontaneous termination will be greatly reduced and AF is likely to be sustained. Evidence to support this notion was obtained in a dog model by varying autonomic tone and administering antiarrhythmic drugs (AADs).211 However, some clinical observations were incompatible with the leading circle mechanism, notably the effectiveness of Na+ channel blockers in AF. According to the leading circle notion, Na+ channel blockers should decrease the wavelength by reducing CV and thereby promote, rather than terminate, AF. Furthermore, for many years, multiple numerical studies and high-density mapping studies in cardiac tissues have failed to confirm the idea of the leading circle or the presence of centripetal wavelets in the maintenance of reentrant excitation.
Computer simulations and experiments in multiple mammalian species suggest that functional reentry is better explained by rotors or spiral waves (Figure 3D). This idea was first conceptualized by Russian scientists in the 1960s, and later popularized by Arthur Winfree to explain the reentry in all excitable media.213,214,215 The rotor is the organizing center of the reentrant excitation215; it spins at exceedingly high frequencies, radiating spiral wavefronts with outwardly decreasing curvature, forming an Archimedean spiral, and resulting in wave fragmentation in its periphery.216,217 Because CV decreases as the wavefront curvature becomes steeper toward the center tip, it follows that at that site (sometimes called the phase singularity [PS]) the curvature reaches a critical value, the velocity becomes zero, and the PS follows a circular trajectory.215,218 At each point the direction of propagation is perpendicular to the wavefront and the velocity increases toward the periphery. The PS is a unique point where the wavefront and the wavetail converge and velocity is zero, preventing the impulse from extending toward the center of the rotation. Instead, the PS becomes the rotor, circling around a small center of unexcited but excitable tissue.218 The concept of rotor can also be applicable to anatomical reentry in the atria; a pectinate muscle or the orifice of a PV can stabilize a reentrant rotor.156,219 Unlike leading-circle reentry, spiral-wave reentry is not determined by the wavelength, but rather by the source-sink relationship between the activation wavefront and the tissue that must be excited in front of it to maintain activity. The rotor concept has been applied to AF, and subsequent studies have confirmed its ability to account for the AF-suppressing actions of Na+ channel blockers.119
Recent advances in electrophysiological recording and analysis have led to important advances in appreciating AF-maintaining mechanisms in patients. Interestingly, they have also led to new controversies. The application of advanced computing technology to the definition of detailed intracardiac electrical activity based on highly sophisticated body surface mapping (BSM), a technique called electrocardiographic imaging (ECGI), has led to the noninvasive analysis of underlying mechanisms in patients with AF.220,221 Both focal and reentrant rotor sources were visualized and tended to become more numerous as AF was maintained for longer periods.221 Detailed analysis of patients undergoing AF ablation indicates that rotors are localized to specific atrial regions and tend to be short-lasting, with rotor cores tending to occur at the interface between fibrotic tissue and more normal atria.222 Investigators have also applied intra-atrial basket catheters and complex mathematical analysis to define AF mechanisms and target them in the electrophysiology laboratory with a technique called focal impulse and rotor modulation (FIRM).77 FIRM procedures have identified rotational activity in patients with AF. A number of studies have shown the superiority of FIRM-based ablation over conventional ablation strategies.223 However, the success of targeted rotational activity ablation, as well as the meaning of rotors detected by FIRM technology, have been disputed in recent clinical studies. A prospective randomized clinical trial is now underway. It is notable that conventional mapping techniques using isochronal mapping have not been able to identify continuous rotational activation.224,225 It is also notable that detailed human atrial mapping studies have not observed discrete rotors, but rather suggest that AF is maintained by dissociation between epicardial and endocardial layers, with mutual interaction producing multiplying activity that maintains the arrhythmia (Figure 3E).226,227,228,229 Investigators have recorded more than 500 epicardial electrograms from both atria during cardiac surgery in patients with persistent AF and were unable to identify reentrant activity.229 They interpreted their results as suggesting predominance of focal activity and breakthroughs. Potential unifying findings were recently presented by investigators, who performed high-resolution endocardial and epicardial optical mapping in explanted diseased human hearts.227 They noted that AF was driven by stable transmural reentrant sources anchored to anatomical complexities and fibrotic regions. One limitation of their studies was a need for an action potential abbreviating drug (pinacidil) to observe AF, limiting the applicability of their findings to spontaneous AF.
In summary, although the presently available data leave a number of questions open, they do indicate that both ectopic activity and reentry play important roles in AF. The specific mechanisms and determinants remain to be elucidated, along with their implications for therapy.
Mechanisms of Atrial Tachycardia and Atrial Flutter
Atrial arrhythmias can be broadly classified as focal, small circuit, or macroreentry (Figure 5A–F). Focal ATs can originate from anywhere within the atria or venous structures but do have a classical anatomic distribution (Figures 4, 5C). In the absence of a prior LA catheter or surgical ablation procedure, approximately two-thirds of focal tachycardias have an RA origin and one-third occur from the LA. In the RA, the most common anatomic locations are the crista terminalis, tricuspid annulus, CS ostium, and perinodal regions. In the LA, the pulmonary venous ostia and mitral annulus are most common. Focal tachycardias also can arise from the LA and RA appendages, but these sites of origin are rare.
Figure 5.
Schematic drawing showing mechanisms of atrial flutter and atrial tachycardia. (A) Isthmus-dependent reverse common (clockwise) atrial flutter. (B) Isthmus-dependent common (counter clockwise) atrial flutter. (C) Focal atrial tachycardia with circumferential spread of activation of the atria (can arise from multiple sites within the left and right atrium). (D) Microreentrant atrial tachycardia with circumferential spread of activation of the atria. (E) Perimitral atrial flutter. (F) Roof-dependent atrial flutter.
Figure 4.
Structure and mechanisms of atrial fibrillation. (A) Schematic drawing of the left and right atria as viewed from the posterior perspective. The extension of muscular fibers onto the PVs can be appreciated. Shown in yellow are the five major left atrial autonomic ganglionic plexi (GP) and axons (superior left GP, inferior left GP, anterior right GP, inferior right GP, and ligament of Marshall). Shown in blue is the coronary sinus, which is enveloped by muscular fibers that have connections to the atria. Also shown in blue is the vein and ligament of Marshall, which travels from the coronary sinus to the region between the left superior PV and the left atrial appendage. (B) The large and small reentrant wavelets that play a role in initiating and sustaining AF. (C) The common locations of PV (red) and also the common sites of origin of non-PV triggers (shown in green). (D) Composite of the anatomic and arrhythmic mechanisms of AF. Adapted with permission from Calkins et al. Heart Rhythm 2012; 9:632–696.e21.2
Macroreentry is a broad term that encompasses what have been considered to be typical and atypical atrial flutters (AFLs). The hallmark of macroreentry is that two sites ≥2 cm apart demonstrate entrainment with a postpacing interval–tachycardia cycle length of ≤ 20 ms (i.e., within the circuit). The most common forms of atrial macroreentry are variants of classical common and reverse common cavotricuspid isthmus-dependent flutter (Figure 5A,B). These include both counterclockwise (common) and clockwise (reverse common) variants, with the circuit originally described as a broad active wavefront rotating around the tricuspid annulus. However, it is now recognized that many variants exist, such as lower loop reentry and forms in which the active wavefront crosses immediately anterior or posterior to the inferior vena cava. Rarely, intraisthmus reentry can occur. Classical AFL almost invariably coexists with AF. Studies of AFL onset and termination have demonstrated that both invariably require transitional AF, indicating that flutter is largely a downstream arrhythmia. Attempts to modify the natural history of AF by ablation of AFL have thus far largely been unsuccessful. Nevertheless, cavotricuspid isthmus ablation is a simple procedure with high efficacy and low risk that can provide good arrhythmia palliation in the appropriately selected patient. However, long-term follow-up studies following flutter ablation have demonstrated increasing prevalence of AF during long-term follow-up.230
Atypical forms of macroreentry can occur in both the LA and RA and are most common in the setting of prior atrial surgery or prior ablation for AF. They can also occur spontaneously. In the RA, these can occur in the free wall, where a surgical or spontaneous scar creates the central obstacle; or in the form of upper loop reentry in which the SVC is the central obstacle, often with some anchoring scar. Circuits have also been described around segments of the crista terminalis, which acts as a central barrier and creates regions of slow conduction. Reentrant circuits on the right septum, even in the context of surgical scars or prosthetic material, are uncommon. In the LA, macroreentry is most common in the context of prior ablation. The type of circuit varies according to the nature of prior ablation and to the underlying structural heart disease. Patients with more advanced atrial remodeling, such as those with persistent AF, will be more likely to have regions of slow conduction. Linear ablation particularly induces macroreentry due to the propensity for gaps in lines to develop. At the gap site, conduction can also be slowed due to the presence of damaged tissue. Common reentrant circuits are perimitral- or mitral isthmus-dependent or, alternately, roof-dependent circuits (Figure 5E,F), which occur around either the left- or right-sided PVs. Ablation of these circuits can be accomplished by creation of a linear ablation lesion in the form of a mitral or an anterior line for perimitral flutter or a roof line for roof-dependent flutters. Microreentrant AFL can be ablated with a focal lesion (Figure 5D). When flutter occurs through a gap in a preexisting line, focal ablation at that gap can often be sufficient to create complete conduction block. With the diminished use of linear ablation for persistent AF treatment, the prevalence of these circuits is expected to diminish. Whenever linear ablation is required for ablation of a macroreentrant circuit it is important to check for bidirectional conduction block. Macroreentrant circuits can also occur in the LA around large regions of scar. These can either occur spontaneously, particularly in the setting of structural heart disease and atrial enlargement, or be due to prior ablation. Simultaneous dual-loop reentry can also be observed in this situation. Left septal flutter has been described, but is an uncommon arrhythmia. When patients present with macroreentrant arrhythmias following AF ablation, it is important to also identify and ablate the trigger causing onset. Common sources of triggers include the PVs, reflecting PV reconnection, or non-PV triggers.
Small circuit reentry has been described more recently, and most classically occurs in the context of a prior catheter or surgical ablation procedure due to islands of scar that form a central obstacle and regions of slow conduction (Figure 5D). The definition of a small circuit as being less than 2 cm in diameter creates a rather arbitrary distinction from macroreentry, but it does have clinical relevance. In the majority of small circuits, a single focal isthmus of slow conduction can be found in which focal ablation eliminates the circuit.
Potential Benefits and Rationale for Eliminating AF with Ablation
As described earlier in this document, AF is associated with many adverse outcomes, including stroke, dementia, HF, impaired QOL, increased medical costs, and mortality. Understanding the effect of catheter ablation of AF on these outcomes is important in the overall assessment of the role of ablation in the long-term management of patients with AF. There have been a number of studies that have examined long-term outcomes with AF ablation. To date, however, none have prospectively randomized patients to ablation vs medical management and followed them longitudinally for more than 1 to 2 years. There are some long-term prospective registries of patients who have undergone AF ablation, with patients matched to those treated medically. Despite rigorous propensity matching, there could still be unrecognized differences in the populations treated with ablation compared with those receiving medical management. Thus, most of the evidence we have, while suggestive of benefit from ablation, cannot be taken as definitive with respect to major health outcomes. This lack is the rationale behind the Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) (ClinicalTrials.gov identifier NCT00911508), which is a prospective, randomized trial of ablation vs medical management of AF. The trial has completed enrollment, but it will be some time before the results are known.
It is widely recognized that AF ablation is effective in controlling AF and its associated symptoms. Multiple studies have demonstrated that AF ablation improves QOL in a patient with symptomatic AF, including those with HF.231,232 Many patients with AF have HF with reduced ejection fraction (EF). Multiple studies have examined the effect of ablation on EF.63,76,233,234,235,236 In a meta-analysis of nine studies of AF ablation in patients with HF, mean EF improved 11% (95% confidence interval [CI] 6.9–15.3, P <.001).237 The effect of ablation on the future risk of stroke is of great interest, partly because of the morbidity and mortality associated with stroke, but also because of the need to inform decisions regarding continuing anticoagulation in patients with apparently successful ablations. A number of studies, but not all, have reported a low stroke rate in patients who have undergone AF ablation when followed long term.238,239,240,241,242 Although the results of these studies taken as a whole report a lower than expected stroke rate, these results can be considered preliminary data because many of these trials enrolled patients with a CHA2DS2-VASc score of < 2, in whom stroke rates will be anticipated to be low. This reflects the fact that very few patients with a high stroke risk profile were followed long term after suspension of anticoagulation. Notably, it has recently been shown that patients with PAF have a lower stroke rate than those with persistent AF.51 These observations, while preliminary, are supportive of the emerging belief that AF ablation could in fact reduce stroke risk. The ultimate proof that elimination of AF by ablation lowers stroke risk will require a large, prospective, randomized clinical trial such as CABANA. Limited studies have evaluated the effect of ablation on the risk of dementia. Prior studies have reported that Alzheimer's dementia occurred in 0.2% of AF ablation patients compared with 0.9% of patients with AF who did not have ablation and 0.5% of patients without AF (P <.0001). Other types of dementia were also reduced significantly in patients who had undergone ablation.239 Although these findings are of interest, they must be considered preliminary because this was not a prospective randomized trial. The impact of AF ablation on mortality is also uncertain. Although a number of preliminary studies have reported encouraging results, not all studies have reported a reduction in mortality. These results must also be considered preliminary due to their study design.232,239,241
In summary, there is strong evidence that AF ablation improves QOL, and reasonable evidence that AF ablation improves ventricular function in those patients with AF who have HF. The impact of AF control with ablation on other endpoints, including stroke risk, dementia, and mortality, will require further study.
Electrophysiological Basis of AF Ablation
It is generally accepted that development of AF requires both a trigger and a susceptible substrate. Figures 3 and 4 summarize the many mechanisms of AF. Over time, AF progresses from a trigger-driven to a more substrate-mediated arrhythmia due to structural remodeling of the atria.153,243 Ablative therapy is therefore aimed at either eliminating the trigger initiating AF or modifying the arrhythmogenic substrate. The most commonly employed ablation strategy consists of electrical isolation of the PVs by creation of circumferential lesions around the right and the left PV.197,244,245 A schematic overview of common lesion sets created during an AF ablation procedure is shown in Figure 6. The effects of these lesions have been attributed to isolation of AF triggering PV foci, elimination of non-PV triggering foci, and/or as the result of modification of the arrhythmogenic substrate.246 The latter might include interruption of crucial pathways of conduction between pulmonary-atrial junctions, which play a role in sustenance of AF, reduction of the amount of mass available for the number of simultaneously circulating wavelets (atrial debulking), or partial vagal denervation by interruption of vagal stimulation from the autonomic ganglia.109,247,248,249 Adjuvant substrate modification is targeted at patient-specific AF sources in the RA and LA.250 AF recurrences after an initially successful AF ablation procedure are typically associated with PVI reconnection. A more recent strategy for AF ablation involves mapping and ablation of rotational activity.222,223
Figure 6.
Schematic of common lesion sets employed in AF ablation. (A) The circumferential ablation lesions that are created in a circumferential fashion around the right and the left PVs. The primary endpoint of this ablation strategy is the electrical isolation of the PV musculature. (B) Some of the most common sites of linear ablation lesions. These include a “roof line” connecting the lesions encircling the left and/or right PVs, a “mitral isthmus” line connecting the mitral valve and the lesion encircling the left PVs at the end of the left inferior PV, and an anterior linear lesion connecting either the “roof line” or the left or right circumferential lesion to the mitral annulus anteriorly. A linear lesion created at the cavotricuspid isthmus is also shown. This lesion is generally placed in patients who have experienced cavotricuspid isthmus-dependent atrial flutter clinically or have it induced during EP testing. (C) Similar to 6B, but also shows additional linear ablation lesions between the superior and inferior PVs resulting in a figure of eight lesion sets as well as a posterior inferior line allowing for electrical isolation of the posterior left atrial wall. An encircling lesion of the superior vena cava (SVC) directed at electrical isolation of the SVC is also shown. SVC isolation is performed if focal firing from the SVC can be demonstrated. A subset of operators empirically isolates the SVC. (D) Representative sites for ablation when targeting rotational activity or CFAEs are targeted. Modified with permission from Calkins et al. Heart Rhythm 2012; 9:632–696.e21.2
The Mechanisms of AF Recurrence After Catheter Ablation or Surgical AF Ablation
Although its efficacy has been established, both catheter and surgical ablation of AF are associated with a substantial risk of AF recurrence.251,252 It is important to recognize that late recurrences are often asymptomatic.56 Recurrences of AF after ablation are generally classified into three types according to the phase after ablation in which they appear: (1) early recurrence (within 3 months); (2) late recurrence (from 3 months to 1 year); and (3) very late recurrence (more than 1 year). The characteristics and optimal managements differ according to the type of recurrence.
Early recurrence, which is defined as recurrence within the first 3 months after the procedure, is observed in 50% or more of patients.253,254,255 Although its precise mechanisms have not been fully elucidated, the possible causes include (1) a transient stimulatory effect of the tissue inflammatory response to the application of RF256; (2) a transient imbalance of the ANS257; and (3) a delayed effect of the application of RF, likely due to the maturation of the ablation lesion soon after the procedure.258 A “blanking period” of 3 months after the procedure, during which reintervention should be avoided, is recommended because up to half of the patients with early recurrence remain AF-free during long-term follow-up.254,255,259 It has recently been shown that patients who experience multiple early recurrences, especially more than a month postablation, are more likely to have an unsuccessful response to AF ablation at 1-year follow-up. It is for this reason that some electrophysiologists recommend early reablation in this subset of patients.260
Late recurrence, during the first 9 months after the blanking period, occurs in 25%–40% of cases261,262; however, the incidence differs depending on the patient population (ratio of paroxysmal to persistent form) and the manner in which recurrence is screened for and detected. Studies have shown that the mechanism for late-term recurrence is predominantly linked to the recovery of electrical conduction between the PVs and the LA, irrespective of the type of AF.261,263 Accordingly, ongoing efforts are focused on identifying techniques to achieve permanent PVI during an initial AF ablation procedure.264,265
The incidence of very late recurrence (after more than 12 months postablation) has been shown to be higher than previously expected. Multiple studies of long-term follow-up data (more than 5 years) have demonstrated that the longer the follow-up postablation, the higher the recurrence rate.266,267,268 The predominant mechanism of very late recurrence includes PV reconnection, the development of non-PV triggers, and development and maturation of substrate.267,269,270 The predictors of the very late recurrence of AF appear to be the nonparoxysmal form of AF at baseline, organic heart disease (valvular heart disease and cardiomyopathy), advanced age, and obesity.268,271
One study investigated the relationship between time to recurrence of AF following AF ablation, response to therapy, and outcome.272 This study found that time to recurrence is a major determinant of outcome. Patients with later recurrences were more likely to have sporadic episodes and respond better to AADs and repeat ablation. This observation suggests pathophysiological differences based on time to recurrence, and have implications for clinical management.
Section 3: Modifiable Risk Factors for AF and Impact on Ablation
AF Risk Factors and Their Interaction with AF Management and Ablation
Management of patients with AF has traditionally consisted of three main components: (1) anticoagulation for stroke prevention; (2) rate control; and (3) rhythm control. With the emergence of large amounts of data, which have both defined and called attention to the interaction between modifiable risk factors and the development of AF and outcomes of AF management, we believe it is time to include risk factor modification as the fourth pillar of AF management.7,10,273,274,275 In this section of the document, we will review the link between modifiable risk factors and both the development of AF and their impacts on the outcomes of AF ablation.
Obesity
Data from population studies have demonstrated a significant dose relationship between increasing risk of developing AF and increasing severity of obesity.8 This relationship holds true even after multivariate adjustment for other known risk factors, with 3%–7% increased AF risk per unit increment of body mass index (BMI).8,276,277,278 Obesity is an important contributor to the burden of AF, explaining one-fifth of all AF cases.279
Obesity results in significant atrial remodeling, which predisposes an individual to the development of AF. Progressive weight gain has been associated with increasing atrial size, interstitial fibrosis, pericardial fat, heterogeneous and slowed conduction, and infiltration of the atrial myocardium by the adjacent pericardial fat.180,280,281 As a consequence of these changes, AF is more frequently induced and sustained.
There is increasing recognition that obesity can influence the risk of AF recurrence after catheter ablation procedures.268,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298 A recent meta-analysis identified 16 studies involving 5864 individuals reporting on the link between obesity and recurrence of AF after catheter ablation, identifying that there was a 3.1% greater risk of recurrent AF postablation for every one unit increase in BMI (RR 1.03; 95% CI 1.00–1.07).299
Much less information is available on the effect of weight management on reducing the risk of developing AF and on the impact of weight management on AF burden and the outcomes of ablation in those with AF. In light of the above discussion, it was somewhat surprising that the 5067-patient Action for Health in Diabetes (Look AHEAD) randomized trial of an intensive lifestyle intervention failed to reduce the risk of developing AF in individuals with type 2 diabetes.300 In a recent, randomized, controlled clinical study, patients with highly symptomatic AF were randomized to either physician-directed weight and cardiometabolic risk factor management or standard of care. Weight and risk factor management were associated with a reduction in AF symptom burden and reduced number and duration of AFs on ambulatory monitoring. Indeed, a dose-dependent improvement in arrhythmia-free survival has been observed in obese individuals with AF who underwent weight loss in the Long-Term Effect of Goal Directed Weight Management on an Atrial Fibrillation Cohort (LEGACY) study, with the best outcomes being observed in those having a linear loss of weight ≥10% and without weight fluctuation. The Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation (ARREST-AF) cohort study evaluated the impact of weight and risk factor management in the context of patients undergoing AF ablation. In this observational study, adjunctive weight and risk factor management resulted in improvement in AF symptoms and a 5-fold greater likelihood of maintaining sinus rhythm after ablation; at a 42-month follow-up, 87% in the intervention group, compared with 18% in the control group, were in sinus rhythm (P <.001).301
Although there are randomized data demonstrating the impact of weight and cardiometabolic risk factor management, data after catheter ablation remain observational and require confirmation. A survey of the writing group shows that 96% recommend weight loss as part of a comprehensive risk factor management strategy for patients with AF, including those who are being evaluated for an AF ablation procedure. Eighty-eight percent of the writing group consider a patient's BMI when discussing the risks, benefits, and outcomes of AF ablation with a patient being evaluated for an AF ablation procedure. One limitation to enacting a weight loss program is that only 34% of the writing group members currently have ready access to a weight loss clinic at their center.
Sleep Apnea
Types, Assessment, and Treatment of Apnea
Sleep-disordered breathing includes OSA, central sleep apnea (CSA), periodic breathing (including Cheyne-Stokes breathing), and sleep-related hypoventilation. OSA affects approximately 24% of men and 9% of women between 30 and 60 years of age. Several studies revealed that the prevalence of OSA is substantially higher among patients with AF (ranging from 32% to 39%), indicating that OSA could be contributing to the initiation and progression of AF.
OSA is caused by repeated upper airway collapse leading to oxygen desaturation and disrupted sleep. Pathogenesis varies; predisposing factors include small upper airway lumen, unstable respiratory control, low arousal threshold, and dysfunctional upper airway dilator muscles. Risk factors include obesity, male sex, age, menopause, fluid retention, adenotonsillar hypertrophy, and smoking. Continuous positive airway pressure (CPAP) is the treatment of choice for OSA, with adherence of 60%–70%. The positive pressure keeps the pharyngeal area from collapsing, and thus helps alleviate the airway obstruction.
Bi-level positive airway pressure or adaptive servoventilation can be used for patients who are intolerant of CPAP. Other treatments include mandibular advancement devices, upper airway surgery, and lifestyle modification (weight loss, avoidance of alcohol and sedatives).
AF Mechanisms in Sleep Apnea
A variety of mechanisms have been implicated in the pathogenesis of OSA-associated AF.302 Exposure of rats over 35 days to episodic hypoxia of the type caused by OSA causes a sustained increase in blood pressure (BP) due to activation of sympathetic nerves and the renin-angiotensin-aldosterone system.303 Although intrinsic endothelial sensitivity appears unaltered in OSA, the vasoconstrictor response to angiotensin is enhanced.304
A variety of lines of evidence point to an important role of the ANS. Prolonged apneic episodes in dogs (2 min) enhance ganglionated plexus neural activity and increase AF inducibility.305 Strong negative intrathoracic pressure applied to pigs during 2-minute apneic episodes reduces the atrial ERP and enhances AF inducibility, effects reversed by atropine or vagotomy.306 Renal denervation suppresses postapneic BP increases, AF inducibility and neurohumoral activation in pigs.307,308
That altered autonomic nerve activity is not the entire explanation for the influence of sleep apnea in AF occurrence was shown in a study of sleep apnea in a rat model of obesity.309 Obstructive apnea promoted AF induction in obese rats much more than in lean rats, with only partial protection (<50%) by autonomic blockade. On the other hand, obstructive episodes caused LA dilation that was enhanced in obese rats by obesity-associated left-ventricular diastolic dysfunction. Prevention of LA dilation fully prevented apnea-associated AF. In this model, neither obstructive apnea nor obesity was enough to cause significant AF vulnerability; the interaction appeared necessary to cause the degree of LA dilation needed to allow for AF induction.
Subsequent studies evaluated the effects of repeated obstructive apnea, as occurs in patients with OSA, on cardiac structure, function, and electrophysiology.310 In a rat model of repeated OSA for 4 weeks, atrial conduction slowed considerably in association with connexin downregulation and atrial fibrosis. Left ventricular dilation, hypertrophy, and diastolic dysfunction also occurred. In addition to the remodeling-induced AF substrate caused by repeated apneic episodes, AF inducibility was further enhanced by superimposed episodes of acute obstructive apnea in over 80% of the rats.310 The role of atrial remodeling by repeated OSA is supported by electroanatomical studies in the clinical electrophysiology lab, with OSA associated with prolonged atrial conduction times, slower conduction, reduced electrogram amplitudes, and widespread complex atrial electrograms.311
Finally, experiments in sheep demonstrated that hypercapnia per se causes profound atrial conduction slowing and increased AF inducibility, which persists following the return of CO2 levels to normal.312 This effect was independent of oxygen levels. Taken together, the results suggest that acute OSA episodes enhance AF vulnerability via a combination of LA dilation and autonomic and electrophysiological changes; however, these abnormalities alone are not enough to significantly enhance AF risk in normal hearts. Repeated nocturnal OSA activates neurohormonal systems that over time produce sustained hypertension and cardiac structural and electrophysiological remodeling. These render the atria susceptible to AF, particularly during an acute OSA episode.
Sleep Apnea Treatment and AF Ablation Outcomes
Several studies have observed associations between AF and OSA. Epidemiological data suggest AF prevalence and progression are linked with OSA severity,313,314 whereas observational data have linked OSA with a more severely remodeled atrial substrate.311 Treatment of OSA with CPAP, however, appears to favorably impact AF management, regardless of the rhythm control strategy adopted.315,316
Several studies have examined the impact of CPAP intervention on arrhythmia-free survival following catheter ablation of AF.283,289,290,291,316,317,318,319 Although OSA has been associated with poorer outcomes, CPAP appears to attenuate the deleterious impact of OSA. Pooled analysis suggests that although OSA increases the risk of AF recurrence following ablation (RR 1.31; 95% CI 1.16–1.48; P <.001), CPAP therapy improves ablation success to rates comparable with non-OSA populations (RR 1.25; 95% CI 0.77–2.03; P = .37).320 Nonuse of CPAP increases the risk of recurrent AF after ablation by 57% (RR 1.57; 95% CI 1.36–1.81; P <.001).320 One study demonstrated an increased prevalence of non-PV triggers in patients with OSA.290
The observational nature of the literature that evaluates CPAP use in OSA, however, limits its generalizability and precision. In most studies, formal sleep studies were not used to systematically screen all patients for sleep apnea. Clinical history or diagnostic questionnaires (e.g., Berlin questionnaire) formed the basis of OSA diagnosis in some, whereas the diagnosis was rarely excluded by sleep study in non-OSA groups.283,289,290,291,316 Similarly, treatment efficacy was assessed by self-reported CPAP use.289,290,291,316 These study deficiencies lead to a poorly defined treatment effect of CPAP on AF recurrence after ablation.
A survey of the writing group shows that 80% of the writing group members screen for signs and symptoms of sleep apnea when evaluating patients for an AF ablation procedure. This survey also revealed that 94% refer patients being evaluated for an AF ablation procedure, in whom signs and symptoms of sleep apnea are detected, to a sleep center for evaluation and management of sleep apnea. Eighty-six percent of the writing group members currently have ready access to a sleep program at their center.
Hypertension
Hypertension is a well-established, independent risk factor for AF,9,279,321,322 and this risk increases in patients with uncontrolled systolic BP,323 particularly in those with EF less than 40%.324 Even BPs that are near the upper limit of normal (systolic 130–139 mm Hg, diastolic 85–89 mm Hg) predict risk for AF in healthy middle-aged men325 and women.326 Hypertensive animal models have shown that elevated systolic and diastolic BP promote the development of the atrial substrate for AF by increasing LA pressure, promoting interstitial fibrosis and inflammatory infiltrates.327
Following AF ablation, hypertension has been shown to be an independent predictor of recurrence.268,328,329,330 Conversely, patients with pharmacologically controlled hypertension could have similar risk of AF recurrence postablation as those without hypertension.279 Pooled analysis of two small studies demonstrated that renal artery denervation resulted in both sustained lowering of BP and reduction in AF recurrence postablation.127,331 However, the antiarrhythmic effect of renal denervation is not well established, and whether it is mediated by remodeling of the hypertension-associated atrial substrate or decrease in neurohormonal activation remains uncertain.129 Whereas upstream therapy with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers might be effective for primary prevention of AF in patients with systolic LV dysfunction or left ventricular hypertrophy,332 their effect on secondary prevention postablation of AF is uncertain. Numerous, mostly retrospective, studies have shown that modulation of the renin-angiotensin-aldosterone system does not improve ablation outcome.289,290,291,333,334
In summary, studies have shown that hypertension predicts AF recurrence after AF ablation; however, it is not well established whether aggressive BP reduction with antihypertensive therapy, modulation of the autonomic system, or inhibition of the renin-angiotensin-aldosterone system is required for reducing AF reoccurrence in patients with hypertension undergoing AF ablation. Despite this, aggressive treatment of hypertension is warranted in all patients with AF due to the well-established link between hypertension and stroke risk.
Diabetes
Diabetes promotes atrial remodeling characterized by diffuse interstitial fibrosis and conduction slowing,335 and has been shown to be an independent risk factor for development of AF.9,336,337 At least 10 studies have evaluated whether diabetes or impaired glucose metabolism predicts AF recurrence following ablation, with varying results. A systematic review and meta-analysis reported that the risk of AF postablation was not elevated in patients with diabetes.338
Overall, studies have not consistently demonstrated differences in AF ablation outcomes in patients with diabetes, and whether aggressive glucose control is effective for secondary AF prevention following ablation is uncertain.
Alcohol
Alcohol consumption at varying degrees could increase the likelihood of incident AF and might also elevate the risk of thromboembolic events and postablation recurrence in patients with AF.339,340,341,342 Fibrotic changes in the myocardium caused by alcohol toxicity potentially facilitate development of LA scar and origin of non-PV triggers.301,343 A recent observational study demonstrated a lower AF ablation success rate in moderate and heavy drinkers.342 Furthermore, binge drinking has been reported to be associated with increase in the risk of postablation AF recurrence. Finally, the ARREST-AF study demonstrated significant reduction in symptom severity, burden, and recurrence rate in patients with risk factor management that included lowering alcohol intake to ≤ 30 g per week.344 Thus, limiting alcohol intake is a potential target to increase the success rate in AF ablation, but definitive evidence is required before stronger conclusions can be made.
Exercise
Recent studies have observed a U-shaped risk relationship of physical activity to AF. At one end of the spectrum, a large observational study of 64,561 people showed that those at the lowest levels of physical fitness had a 5-fold increased risk of AF.345 Increasing the physical activity of sedentary patients could help reduce the risk or burden of AF. For example, one randomized study demonstrated that just 12 weeks of moderate-intensity physical activity decreased the AF burden by 41%.346 Of the physically inactive with AF, the obese might benefit the most from moderate levels of physical activity.345 In contrast, a meta-analysis of 655 endurance athletes also demonstrated a 5-fold increased risk of AF.347 Of these studies, increased AF risk was generally only observed with the highest levels of physical activity over a prolonged period of time.348,349 One explanation for the exercise paradox is that both long-term endurance training and a sedentary lifestyle increase chronic systemic inflammation.
The risk of AF from sustained high levels of physical activity is likely modulated by age, given studies of young athletes have failed to show an increased risk.350,351 Indeed, AF is the primary arrhythmia observed in middle-aged athletes.352 AF in athletes tends to be paroxysmal, vagally mediated, and highly symptomatic.353 Risk is augmented in athletes who are better conditioned, participate more often, and have faster performance times.354
The mechanism of increased AF risk at either end of the physical activity spectrum likely includes autonomic, structural, inflammatory, and fibrotic changes to the heart. For example, increased vagal tone, which is often observed in the endurance athlete, has been shown to result in a short atrial RP, and thus initiates AF.355 Most studies have shown structural changes in endurance athletes, which have resulted in the term athlete's heart. These changes include dilatation of all four heart chambers, increase in left ventricular mass, and mild right ventricular hypertrophy.356 Initially, these adaptive changes were felt to be benign; however, emerging evidence suggests otherwise, with endurance athletes experiencing higher-than-expected rates of coronary artery calcium scores, myocardial fibrosis, AF, and sinus node dysfunction.357
Long-term endurance training, as well as a sedentary lifestyle,358 increase chronic systemic inflammation,359 which in turn could also facilitate AF.360 Studies show that moderate physical activity might reduce inflammatory markers.361,362,363
Extreme levels of exercise are a known cause of cardiac fibrosis, particularly in hinge point locations of the heart, such as the right ventricle; however, the significance of MRI-detected fibrosis remains controversial.349 Athletes who experience higher levels of fibrosis also have higher levels of coronary calcium.364 In turn, fibrosis is a well-established risk factor of AF.365 In one study, the fibrotic changes caused by vigorous exercise were reversed after an 8-week period of physical activity cessation.366 One study showed an increase in collagen and other fibrosis biomarkers in athletes.367 Murine models have found that losartan reduces all fibrosis biomarkers and the histologic findings of fibrosis induced by long-term intensive exercise.368
Although increasing physical activity might reduce AF in sedentary patients, decreasing physical activity levels in elite endurance athletes could also reduce AF.350 Professional athletes represent a unique treatment dilemma because medical therapy for AF might not only reduce athletic performance but it could also be prohibited in some sports.369
Furthermore, many athletes have marked resting bradycardia, limiting use of AAD therapy. Because most high-level endurance athletes are unwilling to give up or reduce their level of their sports participation, AF ablation might be the only viable treatment option for these patients. Although there are limited data on the efficacy of AF ablation in athletes, two small studies involving a total of 276 patients suggested equal benefit of ablation compared with AAD therapy for the endurance athlete.370,371 However, caution should be exercised when interpreting these data because a third small study reported that, although ablation can result in a durable arrhythmia-free benefit, athletes typically require multiple procedures—an average of 2.3—to achieve a long-term benefit.372
Although moderate levels of physical activity have not been independently shown to improve AF ablation outcomes in the sedentary patient, one observational study of 308 patients showed that increased physical fitness was associated with a dose-dependent reduction in AF burden. Moreover, the AF benefit of physical fitness provided a 12% incremental gain over weight loss, resulting in an improved AF risk factor profile, inflammatory state, and cardiac remodeling.373 These observed beneficial changes of moderate physical activity would predict a better AF ablation outcome in the sedentary patient. Given that interventions aimed at increasing physical activity could be more successful than those targeting weight loss,374 increasing physical activity could be an attractive option to prevent or treat AF. To date, however, definitive evidence of the impact of physical activity on ablation outcomes is lacking.
Section 4: Indications
Recommendations and General Considerations
Shown in Table 2, and summarized in Figures 7 and 8 of this document, are the Consensus Indications for Catheter and Surgical Ablation of AF. As outlined in the introduction section of this document, these indications are stratified as Class I, Class IIa, Class IIb, and Class III indications. The evidence supporting these indications is provided, as well as a selection of the key references supporting these levels of evidence. In making these recommendations, the writing group considered the body of published literature that has defined the safety and efficacy of catheter and surgical ablation of AF. Also considered in these recommendations is the personal lifetime experience in the field of each of the writing group members. Both the number of clinical trials and the quality of these trials were considered. In considering the class of indications recommended by this writing group, it is important to keep several points in mind. First, these classes of indications only define the indications for catheter and surgical ablation of AF when performed by an electrophysiologist or a surgeon who has received appropriate training and/or who has a certain level of experience and is performing the procedure in an experienced center (Section 11). Catheter and surgical ablation of AF are highly complex procedures, and a careful assessment of the benefit and risk must be considered for each patient. Second, these indications stratify patients based only on the type of AF and whether the procedure is being performed prior to or following a trial of one or more Class I or III antiarrhythmic medications. This document for the first time includes indications for catheter ablation of select asymptomatic patients. As detailed in Section 9, there are many other additional clinical and imaging-based variables that can be used to further define the efficacy and risk of ablation in a given patient. Some of the variables that can be used to define patients in whom a lower success rate or a higher complication rate can be expected include the presence of concomitant heart disease, obesity, sleep apnea, LA size, patient age and frailty, as well as the duration of time the patient has been in continuous AF. Each of these variables needs to be considered when discussing the risks and benefits of AF ablation with a particular patient. In the presence of substantial risk or anticipated difficulty of ablation, it could be more appropriate to use additional AAD options, even if the patient on face value might present with a Class I or IIa indication for ablation. Third, it is important to consider patient preference and values. Some patients are reluctant to consider a major procedure or surgery and have a strong preference for a pharmacological approach. In these patients, trials of antiarrhythmic agents including amiodarone might be preferred to catheter ablation. On the other hand, some patients prefer a nonpharmacological approach. Fourth, it is important to recognize that some patients early in the course of their AF journey might have only infrequent episodes for many years and/or could have AF that is responsive to well-tolerated AAD therapy. And finally, it is important to bear in mind that a decision to perform catheter or surgical AF ablation should only be made after a patient carefully considers the risks, benefits, and alternatives to the procedure.
Table 2.
Indications for catheter (A and B) and surgical (C, D, and E) ablation of atrial fibrillation
| Recommendation | Class | LOE | References | |
|---|---|---|---|---|
| Indications for catheter ablation of atrial fibrillation | ||||
| A. Indications for catheter ablation of atrial fibrillation | ||||
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Catheter ablation is recommended. | I | A | 261,262,462,489,503, 655,673,684,709, 1027–1029 |
| Persistent: Catheter ablation is reasonable. | IIa | B-NR | 245,262,515,527,733, 1015,1025–1030 | |
| Long-standing persistent: Catheter ablation may be considered. | IIb | C-LD | 245,262,515,527,733,1015,1025–1030 | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Catheter ablation is reasonable. | IIa | B-R | 370,372,377–383 |
| Persistent: Catheter ablation is reasonable. | IIa | C-EO | ||
| Long-standing persistent: Catheter ablation may be considered. | IIb | C-EO | ||
| B. Indications for catheter atrial fibrillation ablation in populations of patients not well represented in clinical trials | ||||
| Congestive heart failure | It is reasonable to use similar indications for AF ablation in selected patients with heart failure as in patients without heart failure. | IIa | B-R | 233–237,384,386–395,1042 |
| Older patients (>75 years of age) | It is reasonable to use similar indications for AF ablation in selected older patients with AF as in younger patients. | IIa | B-NR | 396–398,401–404 |
| Hypertrophic cardiomyopathy | It is reasonable to use similar indications for AF ablation in selected patients with HCM as in patients without HCM. | IIa | B-NR | 385,1043,1044 |
| Young patients (<45 years of age) | It is reasonable to use similar indications for AF ablation in young patients with AF (<45 years of age) as in older patients. | IIa | B-NR | 405,1045 |
| Tachy-brady syndrome | It is reasonable to offer AF ablation as an alternative to pacemaker implantation in patients with tachy-brady syndrome. | IIa | B-NR | 381–383 |
| Athletes with AF | It is reasonable to offer high-level athletes AF as first-line therapy due to the negative effects of medications on athletic performance. | IIa | C-LD | 370–372 |
| Asymptomatic AF∗∗ | Paroxysmal: Catheter ablation may be considered in select patients.∗∗ | IIb | C-EO | 416,418 |
| Persistent: Catheter ablation may be considered in select patients. | IIb | C-EO | 417 | |
| Indications for surgical ablation of atrial fibrillation | ||||
| C. Indications for concomitant open (such as mitral valve) surgical ablation of atrial fibrillation | ||||
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 |
| Persistent: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 | |
| Long-standing persistent: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 |
| Persistent: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 | |
| Long-standing persistent: Surgical ablation is recommended. | I | B-NR | 1290,1326–1338 | |
| D. Indications for concomitant closed (such as CABG and AVR) surgical ablation of atrial fibrillation | ||||
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is recommended. | I | B-NR | 1339–1344 |
| Persistent: Surgical ablation is recommended. | I | B-NR | 1339–1344 | |
| Long-standing persistent: Surgical ablation is recommended. | I | B-NR | 1339–1344 | |
| Symptomatic AF prior to initiation of antiarrhythmic therapy with a Class I or III antiarrhythmic medication | Paroxysmal: Surgical ablation is reasonable. | IIa | B-NR | 1339–1344 |
| Persistent: Surgical ablation is reasonable. | IIa | B-NR | 1339–1344 | |
| Long-standing persistent: Surgical ablation is reasonable. | IIa | B-NR | 1339–1344 | |
| E. Indications for stand-alone and hybrid surgical ablation of atrial fibrillation | ||||
| Symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication | Paroxysmal: Stand-alone surgical ablation can be considered for patients who have failed one or more attempts at catheter ablation and also for those who are intolerant or refractory to antiarrhythmic drug therapy and prefer a surgical approach, after review of the relative safety and efficacy of catheter ablation versus a stand-alone surgical approach. | IIb | B-NR | 123,601,1306,1339–1341,1349,1351–1361 |
| Persistent: Stand-alone surgical ablation is reasonable for patients who have failed one or more attempts at catheter ablation and also for those patients who prefer a surgical approach after review of the relative safety and efficacy of catheter ablation versus a stand-alone surgical approach. | IIa | B-NR | 123,601,1306,1339–1341,1349,1351–1361 | |
| Long-standing persistent: Stand-alone surgical ablation is reasonable for patients who have failed one or more attempts at catheter ablation and also for those patients who prefer a surgical approach after review of the relative safety and efficacy of catheter ablation versus a stand-alone surgical approach. | IIa | B-NR | 123,601,1306,1339–1341,1349,1351–1361 | |
| It might be reasonable to apply the indications for stand-alone surgical ablation described above to patients being considered for hybrid surgical AF ablation. | IIb | C-EO | 1361–1366 | |
AF, atrial fibrillation; LOE, Level of Evidence; HCM, hypertrophic cardiomyopathy.
A decision to perform AF ablation in an asymptomatic patient requires additional discussion with the patient because the potential benefits of the procedure for the patient without symptoms are uncertain.
Figure 7.
Indications for catheter ablation of symptomatic atrial fibrillation. Shown in this figure are the indications for catheter ablation of symptomatic paroxysmal, persistent, and long-standing persistent AF. The Class for each indication based on whether ablation is performed after failure of antiarrhythmic drug therapy or as first-line therapy is shown. Please refer to Table 2B and the text for the indications for catheter ablation of asymptomatic AF.
Figure 8.
Indications for surgical ablation of atrial fibrillation. Shown in this figure are the indications for surgical ablation of paroxysmal, persistent, and long-standing persistent AF. The Class for each indication based on whether ablation is performed after failure of antiarrhythmic drug therapy or as first-line therapy is shown. The indications for surgical AF ablation are divided into whether the AF ablation procedure is performed concomitantly with an open surgical procedure (such as mitral valve replacement), a closed surgical procedure (such as coronary artery bypass graft surgery), or as a stand-alone surgical AF ablation procedure performed solely for treatment of atrial fibrillation.
As demonstrated in a large number of published studies, the primary clinical benefit from catheter ablation of AF is an improvement in QOL resulting from elimination of arrhythmia-related symptoms, such as palpitations, fatigue, or effort intolerance (see Section 9). Thus, the primary selection criterion for catheter ablation should be the presence of symptomatic AF. The indications for catheter and surgical ablation of symptomatic AF shown in Table 2, and summarized in Figures 7 and 8, are for the most part consistent with the indications for AF ablation recommended in the recently published 2016 ESC Guidelines for the Management of AF, as well as in the 2014 ACC/AHA/HRS Guidelines for AF management.5,6 These recommendations for AF ablation present the indications for AF ablation as second-line therapy, after failure of a Class I or III antiarrhythmic agent and also the indications for AF ablation as first-line therapy. As shown in Table 2, catheter ablation is recommended for patients with symptomatic PAF who have failed AAD therapy (Class I, LOE A). For patients with symptomatic persistent AF who have failed AAD therapy, catheter ablation has a Class IIa, LOE B-NR indication, and for patients with symptomatic long-standing persistent AF who have failed drug therapy, catheter ablation has a Class IIb, LOE C-LD indication.
In preparing this document, the writing group has chosen to address for the first time the important issue of catheter ablation of AF in select asymptomatic patients. We have also addressed the important issues of AF ablation as first-line therapy, the role of AF ablation in patients with HF, and the role of AF ablation in subgroups of patients not well represented in clinical trials. Each of these important considerations is addressed in detail below.
Catheter Ablation of AF as First-Line Therapy
The role of catheter ablation as first-line therapy, prior to a trial of a Class I or III antiarrhythmic agent, is an appropriate indication for catheter ablation of AF in patients with symptomatic paroxysmal or persistent AF. These recommendations are consistent with the indications for AF ablation recommended in the recently published 2016 ESC guidelines for the management of AF as well as in the 2014 ACC/AHA/HRS Guidelines for AF management.5,6,375,376 For patients with paroxysmal symptomatic AF who have not failed drug therapy, catheter ablation has a Class IIa, LOE B-R indication; for patients with persistent symptomatic AF who have not failed drug therapy, catheter ablation has a Class IIa, LOE C-EO indication; and for patients with long-standing persistent AF who have not failed drug therapy, catheter ablation has a Class IIb, LOE C-EO indication.
There have been three prospective randomized clinical trials that have examined the relative efficacy and safety of first-line AF ablation vs pharmacological therapy.377,378,379 The outcomes of these three trials have recently been summarized in a meta-analysis.380 A total of 491 young, generally healthy patients with predominantly PAF were randomized to AF ablation vs pharmacological therapy. Catheter ablation of AF was associated with a significantly higher freedom from AF recurrence, as compared with drug therapy. The difference in the rate of symptomatic AF recurrences was not significant. There was one procedure-related death due to a stroke in the ablation arm. The main major complication was cardiac tamponade which occurred in 1.7% of patients in the ablation arm. Taken as a whole, these findings provide evidence to support the role of AF ablation as first-line therapy.
It is important to recognize that there are certain situations in which first-line AF ablation is a preferred treatment option. For example, first-line AF ablation is often recommended in patients with PAF who have symptomatic pauses (tachy-brady syndrome). For these patients, initiation of pharmacological therapy would be inappropriate in the absence of a permanent pacemaker. Over the past 15 years, a body of literature has been published demonstrating that catheter ablation is effective, without concomitant need of a permanent pacemaker in the great majority of patients with tachy-brady syndrome.381,382,383 Based on this body of literature and the cumulative experience of the writing group, we believe that it is appropriate to consider AF ablation as first-line therapy in this clinical situation. Another specific population of patients in whom first-line AF ablation is often recommended as an initial approach are high-level competitive athletes with paroxysmal or persistent AF. These individuals often are strongly against taking a medication, which could potentially reduce their peak heart rate and/or impair cardiac function; they often have a marked resting bradycardia. Several studies have reported favorable outcomes of AF ablation in this subgroup of patients.371,372,373
Catheter Ablation of AF in Patients with Heart Failure and Reduced Cardiac Function
AF and HF are closely related conditions. HF can predispose an individual to the occurrence of AF through various mechanisms, such as the increase of the left ventricular filling pressure or LA dilatation and fibrosis, each of which can lead to atrial structural and electrical remodeling. Conversely, AF with the loss of atrial contraction and potential uncontrolled heart rate secondary to arrhythmia can predispose an individual to the development of HF, thus leading to impaired contractility and reduced cardiac output. AF can increase mortality in patients with left ventricular dysfunction; therefore, the treatment of AF in this subset of patients is of pivotal importance.
Much controversy still exists regarding how to maintain sinus rhythm in patients with HF; undoubtedly, however, maintenance of sinus rhythm is beneficial in restoring atrial-ventricular coordination, thus favoring an improved left ventricular performance and a better QOL.
The published literature describing the safety and efficacy of AF ablation in patients with HF and/or a reduced EF is considerable. When viewed cumulatively, these published results describe the outcomes of AF ablation in more than 1000 patients with HF.233,234,235,236,237,384,385,386,387,388,389,390,391,392,393,394 Included within this large body of literature are four prospective, randomized clinical trials, the results of which were recently summarized in a meta-analysis.235,236,237,390,392 A total of 224 patients were randomized, among whom 83% had persistent AF. AF ablation resulted in an increase in left ventricular ejection fraction (LVEF) by a mean of 8.5% compared with rate control alone. AF ablation also was superior to rate control in improving QOL. Peak oxygen consumption and 6-minute walk test distance increased in ablation compared with rate control patients. Major adverse events were not different in the two treatment arms. The meta-analysis of these four studies concluded that catheter ablation is superior to rate control in improving LVEF, QOL, and functional capacity. This conclusion is consistent with other data that reveal that adequate rate control alone is insufficient to prevent AF-mediated cardiomyopathy in a subset of AF patients.395 Prior to accepting a rate control strategy, patients with HF and persistent AF or drug-refractory AF should be advised to consider AF ablation.
Based on this growing body of data, the writing group believes that catheter ablation of AF is a safe, effective, and clinically acceptable therapeutic option in patients with AF and HF. This applies both to patients suspected of having a cardiomyopathy that is strongly suspected to be due to AF, with a rapid ventricular response, as well as to other populations of patients with AF. The writing group recommends that it is reasonable to use similar indications for AF ablation in selected patients with HF as for patients without HF (Class IIa, LOE B-R).
Catheter Ablation in Older People
AF is in large part a disease of the older person. During the past decade, there have been a number of studies that have specifically focused on reporting the outcomes of AF ablation in older individuals.396,397,398,399,400,401,402,403,404 The age cutoff for defining elderly varied between age ≥70 years, age ≥75 years,397,402,404 and age ≥80 years.398,399,400,401 The number of older people in these studies was small, with five of the seven studies enrolling fewer than 100 patients and the largest reporting outcomes on 261 older people. Taken as a whole, the results of these studies provide evidence that catheter ablation of AF has an acceptable safety and efficacy profile in selected older individual over the age of 75 or 80 years. However, as shown in analysis of the relationship between age and 5-year outcomes of AF ablation,403 age significantly impacts long-term outcomes of AF ablation. This study reported that for every 10-year increase in age there was a higher multivariate-adjusted risk of AF recurrence, death, and major cardiac events.
The writing group recommends that it is reasonable to use similar indications for AF ablation in selected older people with AF as in younger patients (Class IIa, LOE B-NR). It is important to note that the complications of the procedure are somewhat increased in older individuals, the need for concomitant antiarrhythmic therapy postablation is greater, and the efficacy is somewhat reduced.396,405 It is also important to recognize that amiodarone, although not a good long-term pharmacological option in younger individuals, is an appropriate treatment strategy in older people.
Catheter Ablation in Other Populations of Patients Not Well Represented in Clinical Trials
Shown in Table 2 are indications for AF ablation in several additional subgroups of patients not well represented in clinical trials. These subgroups include patients with hypertrophic dilated cardiomyopathy, young patients (<45 years of age), high-level athletes, and patients with tachy-brady syndrome. The references supporting the recommendations of the writing group are shown.
Catheter Ablation to Reduce Stroke Risk
Patients with AF might seek catheter ablation to avoid long-term oral anticoagulation (OAC) therapy. Multiple studies reported a low thromboembolic risk in patients who discontinued OAC after successful AF ablation.238,406,407,408,409,410,411 However, an important limitation of these studies is the fact that only a small subset of patients had a CHA2DS2-VASc score ≥2, and almost no patients were at extreme increased stroke risk due to a prior stroke or TIA and/or age ≥75 years. Recent data from the German Ablation Registry412 showed a high thromboembolic risk after ablation in high-stroke-risk patients. Furthermore, it is well recognized that both symptomatic and asymptomatic AF can recur after AF ablation procedures,56,58,413 and late recurrence of AF is observed in 50% or more patients by 5 years. Absence of symptomatic AF after ablation does not necessarily indicate an absence of asymptomatic AF or a low risk of stroke.414 The writing group also recommends that systemic anticoagulation with warfarin or a novel oral anticoagulant (NOAC) is recommended for at least 2 months post-catheter ablation of AF (Class I, LOE C-EO). The writing group recommends that decisions regarding continuation of systemic anticoagulation more than 2 months postablation should be based on the patient's stroke risk profile and not on the perceived success or failure of the ablation procedure (Class I, LOE C-EO, Table 4). Anticoagulation is recommended for patients with a CHA2DS2-VASc score of 2 in men or 3 in women. For patients with one stroke risk factor (e.g., CHA2DS2-VASc 1 in men or 2 in women), the role of OAC is borderline, and can “be considered.” Anticoagulation is generally not recommended 2 or more months post-AF ablation in patients with a low stroke risk profile (e.g., CHA2DS2-VASc 0 in men or 1 in women) unless cardioversion is anticipated or has recently been performed.415
Table 4.
Anticoagulation strategies: pre-, during, and postcatheter ablation of AF
| Recommendation | Class | LOE | References | |
|---|---|---|---|---|
| Preablation | For patients undergoing AF catheter ablation who have been therapeutically anticoagulated with warfarin or dabigatran, performance of the ablation procedure without interruption of warfarin or dabigatran is recommended. | I | A | 400,532,829,830,833,834,837,841 |
| For patients undergoing AF catheter ablation who have been therapeutically anticoagulated with rivaroxaban, performance of the ablation procedure without interruption of rivaroxaban is recommended. | I | B-R | 842 | |
| For patients undergoing AF catheter ablation who have been therapeutically anticoagulated with a NOAC other than dabigatran or rivaroxaban, performance of the ablation procedure without withholding a NOAC dose is reasonable. | IIa | B-NR | 1395 | |
| Anticoagulation guidelines that pertain to cardioversion of AF should be adhered to in patients who present for an AF catheter ablation procedure. | I | B-NR | 5,6 | |
| For patients anticoagulated with a NOAC prior to AF catheter ablation, it is reasonable to hold one to two doses of the NOAC prior to AF ablation with reinitiation postablation. | IIa | B-NR | 835–840 | |
| Performance of a TEE in patients who are in AF on presentation for AF catheter ablation and who have been receiving anticoagulation therapeutically for 3 weeks or longer is reasonable. | IIa | C-EO | 5,6 | |
| Performance of a TEE in patients who present for ablation in sinus rhythm and who have not been anticoagulated prior to catheter ablation is reasonable. | IIa | C-EO | 5,6 | |
| Use of intracardiac echocardiography to screen for atrial thrombi in patients who cannot undergo TEE may be considered. | IIb | C-EO | 768,820–824 | |
| During ablation | Heparin should be administered prior to or immediately following transseptal puncture during AF catheter ablation procedures and adjusted to achieve and maintain an ACT of at least 300 seconds. | I | B-NR | 768,802–804,820,830,840,846–849 |
| Administration of protamine following AF catheter ablation to reverse heparin is reasonable. | IIa | B-NR | 851 | |
| Postablation | In patients who are not therapeutically anticoagulated prior to catheter ablation of AF and in whom warfarin will be used for anticoagulation postablation, low molecular weight heparin or intravenous heparin should be used as a bridge for initiation of systemic anticoagulation with warfarin following AF ablation.∗ | I | C-EO | |
| Systemic anticoagulation with warfarin∗ or a NOAC is recommended for at least 2 months postcatheter ablation of AF. | I | C-EO | 1,2 | |
| Adherence to AF anticoagulation guidelines is recommended for patients who have undergone an AF ablation procedure, regardless of the apparent success or failure of the procedure. | I | C-EO | 5,6 | |
| Decisions regarding continuation of systemic anticoagulation more than 2 months post ablation should be based on the patient's stroke risk profile and not on the perceived success or failure of the ablation procedure. | I | C-EO | 5,6 | |
| In patients who have not been anticoagulated prior to catheter ablation of AF or in whom anticoagulation with a NOAC or warfarin has been interrupted prior to ablation, administration of a NOAC 3 to 5 hours after achievement of hemostasis is reasonable postablation. | IIa | C-EO | 835–840 | |
| Patients in whom discontinuation of anticoagulation is being considered based on patient values and preferences should consider undergoing continuous or frequent ECG monitoring to screen for AF recurrence. | IIb | C-EO |
AF, atrial fibrillation; LOE, Level of Evidence; NOAC, novel oral anticoagulant; TEE, transesophageal electrocardiogram; ACT, activated clotting time.
∗Time in therapeutic range (TTR) should be > 65% – 70% on warfarin.
The writing group also recommends that adherence to AF anticoagulation guidelines is recommended for patients who have undergone an AF ablation procedure, regardless of the apparent success or failure of the procedure (Class I, LOE C-EO, Table 4). Patients in whom discontinuation of anticoagulation is being considered based on patient values and preferences should consider undergoing continuous or frequent ECG monitoring to screen for AF recurrence (Class IIb, LOE C-EO, Table 4).414 A patient's desire to eliminate the need for long-term anticoagulation by itself should not be considered an appropriate selection criterion for AF ablation. In arriving at this recommendation, the writing group recognizes that patients who have undergone catheter ablation of AF represent a new and previously unstudied population in trials of OAC therapy. Clinical trials are therefore needed to define the stroke risk of this patient population and to determine whether the risk factors identified in the CHA2DS2-VASc or other scoring systems apply to these patients. Please refer to Section 7 for a more detailed discussion of this topic and the writing group recommendations for long-term anticoagulation.
Catheter Ablation in Patients with Asymptomatic AF
The writing group believes that AF ablation may be considered in select asymptomatic patients with paroxysmal or persistent AF when performed by an experienced operator and following a detailed discussion of the risks and benefits of undergoing the procedure (Class IIb, LOE C-EO). It is recognized that a decision to perform AF ablation in an asymptomatic patient requires additional discussion with the patient, given the potential benefits of the procedure for the patient without symptoms are uncertain. AF ablation is not recommended for patients with asymptomatic long-standing persistent AF. The justification for making this recommendation is as follows. First, it is well established that the duration of time a patient is in continuous AF directly impacts the outcomes of AF ablation or other rhythm control strategies. Whereas AF ablation in patients with symptomatic PAF is associated with high efficacy, the efficacy of AF ablation in patients who have been in continuous AF for 2 or more years is dramatically reduced. Second, it is well established that AF is associated with an increased risk of stroke, HF, dementia, and mortality. It is also notable that asymptomatic status is associated with similar (or worse) prognosis compared with symptomatic status.48 Furthermore, the risk of stroke has been shown to be lower in patients with paroxysmal rather than continuous AF. Although large-scale, prospective, randomized clinical trials have not been performed to evaluate the impact of AF ablation on stroke risk, dementia, HF, and mortality, it is plausible that maintenance of sinus rhythm will ultimately be shown to reduce these risks. While awaiting the results of these trials, it is important to recognize that if a physician makes a decision to leave a patient with apparent asymptomatic continuous AF in AF rather than to pursue restoration of sinus rhythm, it will be extremely difficult or impossible to restore and maintain sinus rhythm later in this patient's life.
There have been three small studies that have described the safety and efficacy of AF ablation in patients with asymptomatic AF.416,417,418 The first compared the outcomes of 54 patients with asymptomatic subclinical AF with 486 patients with drug-refractory symptomatic AF.416 No difference in safety or efficacy of the procedure was observed at the 24-month follow-up. The second study reported the outcomes of 61 patients with asymptomatic long-standing persistent AF.417 At 50 ± 5 months' follow-up, 57% remained AF recurrence-free after removal from drugs. QOL improved, with the physical component summary and the mental component summary demonstrating substantial improvement. Improvement was also noted on metabolic stress testing. A third study compared the outcomes of 61 patients with asymptomatic persistent AF with 132 otherwise matched symptomatic patients with AF.418 In this study, the outcomes of ablation were superior in the symptomatic patients compared with the asymptomatic patients (71% vs 27% freedom from AF). Also of note was the fact that 16 patients (37%) in the asymptomatic group developed symptomatic AT. Perhaps not surprisingly, the asymptomatic patients showed less improvement in QOL than the symptomatic patients. The study concludes that the outcomes of AF ablation are worse in asymptomatic patients, predominantly due to the risk of developing a symptomatic AT postablation of asymptomatic AF. There are no prospective randomized clinical trials that determine the benefit and risk ratio of ablation in patients with asymptomatic AF.
While considering the issues of rhythm control and AF ablation in apparently asymptomatic AF patients, the writing group of this document feels it is important to note that many patients with apparently asymptomatic AF are in fact symptomatic once an assessment of how the patient feels in sinus rhythm has been carried out. It has become common practice, when faced with a relatively young person with apparently asymptomatic persistent AF, to cardiovert the patient, with or without concomitant use of antiarrhythmic medications, and then reassess the patients' symptom status while in sinus rhythm. In our experience, many patients subjected to this “trial of sinus rhythm” recognize that they feel better in sinus rhythm. This finding is important because a rhythm control strategy and/or catheter ablation then becomes a more acceptable therapeutic strategy.
At the end of the day, the writing group believes that in selected patients, after a careful discussion of the risks, benefits, and alternatives, that AF ablation may be considered in patients with asymptomatic paroxysmal or persistent AF (Class IIb, LOE C-EO) (Table 2). Patients in whom this management strategy should be entertained include patients whose clinical profile would be associated with a high efficacy and safety of AF ablation.
Indications for Surgical Ablation of AF
Indications for surgical ablation of AF are also shown in Table 2 and are summarized in Figure 8. Safety and efficacy of AF ablation have been well supported for a surgical approach, either performed in conjunction with another cardiac surgical procedure or when carried out as a stand-alone procedure. The rationale and detailed indications for surgical ablation of AF are discussed in more detail in Section 12.
Section 5: Strategies, Techniques, and Endpoints
Historical Considerations
Cox and colleagues are credited with developing and demonstrating the efficacy of surgical ablation of AF.419,420 Subsequent surgeons evaluated the efficacy of surgical approaches that limit the lesion set to PVI.421,422 The final iteration of the procedure developed by Cox, which is referred to as the MAZE-3 procedure, was based on a model of AF in which maintenance of the arrhythmia was shown to require a critical number of circulating wavelets for reentry. The success of the Maze-3 procedure in the early 1990s led some cardiac electrophysiologists to attempt to reproduce the procedure with RF catheter ablation. Swartz and colleagues reported replication of the MAZE-1 lesion set in a small series of patients using specially designed sheaths and standard RF ablation catheters.423 The efficacy was modest, the complication rate was high, and the procedure and fluoroscopy times were long. As a result, this approach was quickly abandoned. This report demonstrated a proof of concept that motivated others to try to improve catheter-based ablative treatment of AF. Subsequently, a large number of investigators attempted to replicate the surgical MAZE procedure through the use of either three-dimensional (3D) mapping systems or the use of multipolar ablation catheters. These clinical trials had limited success.424,425,426,427,428,429 Based on these observations and the rapid advances in ablation of AF targeting focal triggers that initiate AF in the PVs, electrophysiologists lost interest in linear non-PV-based approaches to catheter ablation of AF that attempted to replicate various aspects of the surgical MAZE procedure.
The identification by Haissaguerre and colleagues of triggers that initiate AF within the PVs led to prevention of AF recurrence by catheter ablation at the site of the origin of the trigger.197,430,431,432,433 Direct catheter ablation of the triggers was limited by the infrequency with which AF initiation could be reproducibly triggered. To overcome these limitations, an ablation approach was introduced433 that was designed to electrically isolate the PVs. This segmental PVI technique involved the sequential identification and ablation of the PV ostium close to the earliest sites of activation of the PV musculature. An ablation strategy of encircling the PVs guided by 3D electroanatomical mapping (EAM) was subsequently developed by Pappone et al.244,432
The recognition of PV stenosis as a complication of RF delivery within a PV, as well as the recognition that sites of AF initiation and/or maintenance were frequently located within the PV antrum, resulted in a shift in ablation strategies to target the atrial tissue located in the antrum rather than in the PV itself.434,435 Ablation at these sites was either performed segmentally, guided by a circular mapping catheter433,436 positioned close to the PV ostium, the so-called segmental PV ablation, or by wider continuous circumferential ablation lesions created to surround the right or left PVs,244,432 the so-called wide-area circumferential ablation (WACA). The circumferential ablation or isolation line was either guided by 3D EAM,244,437,438 by fluoroscopy,439 or by intracardiac echocardiography (ICE).435,440 Studies comparing these two procedures reported contradictory data.441,442 A subsequent randomized study compared isolation of each individual PV vs isolation of large areas around both ipsilateral PVs. This study reported that isolation of a large circumferential area around both ipsilateral PVs with verification of conduction block is a more effective treatment of AF than segmental isolation of each PV.443 The endpoint for this procedure was either amplitude reduction within the ablated area,244,437 elimination (or dissociation) of the PV potentials recorded from either one or two circular mapping catheters, or a basket catheter within the ipsilateral PVs435,438,439,441,442,444 and/or exit block from the PV.445
PVI is now widely accepted as the cornerstone of AF ablation procedures (Table 3).2 Electrical isolation of the PVs is recommended during all AF ablation procedures (Class I, LOE A). Elimination (or dissociation) of the PV potentials recorded from a multipolar electrode catheter is the primary endpoint for AF ablation procedures for 95% of the writing group members. A single-catheter approach to AF ablation, without employing a circular multipolar electrode catheter as an ablation endpoint, is used by 5% of the writing group members, whereas 95% employ both an ablation catheter and a mapping catheter in the LA when performing AF ablation using either RF energy or cryoballoon (CB) ablation (CBA). Due to the high recurrence rate observed in patients with persistent and long-standing persistent AF with PVI alone, efforts continued to identify additive strategies to improve the outcomes of AF ablation. These strategies have included linear RF lesions in the LA and RA, ablation of autonomic ganglia, ablation by CFAE, ablation of non-PV foci, isolation of the LAA, ablation of scar identified by voltage mapping or MRI, and most recently, ablation of rotational activity. Whether any or all of these strategies will emerge as standard proven components to AF ablation procedures will be determined over time. The results of the recent randomized Substrate and Trigger Ablation for Reduction of AF Trial Part II (STAR AF II) trial failed to demonstrate any reduction in AF recurrence by adding either linear or CFAE ablation to PVI in patients with persistent AF.245 This study represented a sobering landmark in the field of AF ablation, which has served to remind those in the field that rigorous clinical trials are needed to define the safety and efficacy of a particular ablation strategy before it is adopted widely as part of routine clinical care. Other advancements in the field include the introduction of the CBA system, as well as the introduction of force-sensing ablation catheters. In the following sections of this document we will go through the data supporting each of these approaches and technologies in detail. We will also provide input as to the importance of these approaches as assessed by this large international writing group.
Table 3.
Atrial fibrillation ablation: strategies, techniques, and endpoints
| Recommendation | Class | LOE | References | |
|---|---|---|---|---|
| PV isolation by catheter ablation | Electrical isolation of the PVs is recommended during all AF ablation procedures. | I | A | 245,261,262,456,462,489,503,515,527, 655,673,684,709,733,1015,1025,1026,1027,1030 |
| Achievement of electrical isolation requires, at a minimum, assessment and demonstration of entrance block into the PV. | I | B-R | 245,261,262,456,462,489,503,515,527,655,673, 684,709,733,1015,1025,1026,1027,1030 | |
| Monitoring for PV reconnection for 20 minutes following initial PV isolation is reasonable. | IIa | B-R | 263,265,448,450,451,452,457–461,462 | |
| Administration of adenosine 20 minutes following initial PV isolation using RF energy with reablation if PV reconnection might be considered. | IIb | B-R | 265,448,449–451,454,456,461,463–468 | |
| Use of a pace-capture (pacing along the ablation line) ablation strategy may be considered. | IIb | B-R | 264,472–475 | |
| Demonstration of exit block may be considered. | IIb | B-NR | 445,477–481 | |
| Ablation strategies to be considered for use in conjunction with PV isolation | If a patient has a history of typical atrial flutter or typical atrial flutter is induced at the time of AF ablation, delivery of a cavotricuspid isthmus linear lesion is recommended. | I | B-R | 230,504,511,1397 |
| If linear ablation lesions are applied, operators should use mapping and pacing maneuvers to assess for line completeness. | I | C-LD | 245,504,506–508,510–513,1397 | |
| If a reproducible focal trigger that initiates AF is identified outside the PV ostia at the time of an AF ablation procedure, ablation of the focal trigger should be considered. | IIa | C-LD | 96,197,208,257,530,531,533–535,537–539 | |
| When performing AF ablation with a force-sensing RF ablation catheter, a minimal targeted contact force of 5 to 10 grams is reasonable. | IIa | C-LD | 453,468,668,670–686 | |
| Posterior wall isolation might be considered for initial or repeat ablation of persistent or long-standing persistent AF. | IIb | C-LD | 522–529 | |
| Administration of high-dose isoproterenol to screen for and then ablate non-PV triggers may be considered during initial or repeat AF ablation procedures in patients with paroxysmal, persistent, or long-standing persistent AF. | IIb | C-LD | 96,197,208,257,530,531,533–535,537–539 | |
| DF-based ablation strategy is of unknown usefulness for AF ablation. | IIb | C-LD | 587–594 | |
| The usefulness of creating linear ablation lesions in the right or left atrium as an initial or repeat ablation strategy for persistent or long-standing persistent AF is not well established. | IIb | B-NR | 245,507–521 | |
| The usefulness of linear ablation lesions in the absence of macroreentrant atrial flutter is not well established. | IIb | C-LD | 245,507–521 | |
| The usefulness of mapping and ablation of areas of abnormal myocardial tissue identified with voltage mapping or MRI as an initial or repeat ablation strategy for persistent or long-standing persistent AF is not well established. | IIb | B-R | 140,522,553–561 | |
| The usefulness of ablation of complex fractionated atrial electrograms as an initial or repeat ablation strategy for persistent and long-standing persistent AF is not well established. | IIb | B-R | 245,514–517,540,545–552 | |
| The usefulness of ablation of rotational activity as an initial or repeat ablation strategy for persistent and long-standing persistent AF is not well established. | IIb | B-NR | 76,221–226,562–575 | |
| The usefulness of ablation of autonomic ganglia as an initial or repeat ablation strategy for paroxysmal, persistent, and long-standing persistent AF is not well established. | IIb | B-NR | 103,105,114,116,122–124,245,355,576–586 | |
| Nonablation strategies to improve outcomes | Weight loss can be useful for patients with AF, including those who are being evaluated to undergo an AF ablation procedure, as part of a comprehensive risk factor management strategy. | IIa | B-R | 8,180,268,276–301 |
| It is reasonable to consider a patient's BMI when discussing the risks, benefits, and outcomes of AF ablation with a patient being evaluated for an AF ablation procedure. | IIa | B-R | 8,180,268,276–301 | |
| It is reasonable to screen for signs and symptoms of sleep apnea when evaluating a patient for an AF ablation procedure and to recommend a sleep evaluation if sleep apnea is suspected. | IIa | B-R | 283,289–291,302–320 | |
| Treatment of sleep apnea can be useful for patients with AF, including those who are being evaluated to undergo an AF ablation procedure. | IIa | B-R | 283,289–291,302–320 | |
| The usefulness of discontinuation of antiarrhythmic drug therapy prior to AF ablation in an effort to improve long-term outcomes is unclear. | IIb | C-LD | 617–621 | |
| The usefulness of initiation or continuation of antiarrhythmic drug therapy during the postablation healing phase in an effort to improve long-term outcomes is unclear. | IIb | C-LD | 617–621 | |
| Strategies to reduce the risks of AF ablation | Careful identification of the PV ostia is mandatory to avoid ablation within the PVs. | I | B-NR | 434,505,778,927,928,1143–1160 |
| It is recommended that RF power be reduced when creating lesions along the posterior wall near the esophagus. | I | C-LD | 341,417,637,806,866,900,906–911,920,1162–1178,1398 | |
| It is reasonable to use an esophageal temperature probe during AF ablation procedures to monitor esophageal temperature and help guide energy delivery. | IIa | C-EO | 341,417,910,1398 |
AF, atrial fibrillation; LOE, Level of Evidence; PV, pulmonary vein; RF, radiofrequency; MRI, magnetic resonance imaging; BMI, body mass index.
Ablation Approaches Targeting the PVs and Techniques to Obtain Permanent PVI Using RF Energy
Permanent electrical isolation of the PVs is well established as the cornerstone of AF ablation. Despite the importance of this ablation endpoint, permanent electrical isolation of the PVs can rarely be achieved.263,446,447,448,449,450,451,452,453,454,455,456 Among patients returning to the electrophysiology laboratory for a repeat ablation procedure after failing an initial ablation procedure, most studies report that recurrence of PV conduction is observed in one or more PVs in more than 80% of patients.263,446,447,452,454
Studies have also been performed to define the rate of PV reconduction among patients who are AF-free after an initial PVI procedure. Although several of these series reported rates of PV reconduction less than 20%,263,447 most studies have reported far higher reconduction rates, varying from 62% to 90%.449,454,456 The time course of electrical reconduction appears to be rapid, with studies reporting acute reconduction rates of 33% at 30 minutes448,450,451 and 50% at 60 minutes.457 In this section of the document, we will review techniques that have been developed in attempt to improve the rate of permanent PVI. We will also provide input concerning which approaches are most widely used by the writing group members of this document.
Optimal Initial Lesion Creation and Waiting Phase
It is widely recognized that the likelihood of obtaining permanent PVI is related to the quality of ablation energy delivery and lesion formation. There are many factors that play a role in determination of lesion size. With RF energy it is well recognized that important variables that impact lesion size and transmurality include catheter stability, contact force (CF), power output, temperature, and duration of RF output. Please refer to Section 6 of this document for a more detailed discussion of these topics. Each of these variables is of importance. The writing group recommends that when performing AF ablation with a force-sensing RF catheter, that a minimum targeted CF of 5 to 10 grams is reasonable (Class IIa, LOE C-LD) (Table 3).
The writing group also recommends that it is reasonable to use an esophageal temperature probe during RF ablation procedures to monitor esophageal temperature and help guide energy delivery (Class IIa, LOE C-EO) (Table 3). In contrast to these two topics on which consensus was achieved, there is much less consensus regarding power output and the duration of energy delivery because a wide variety of approaches are used by members of the writing group.
One of the approaches that has been proposed as a technique to increase the rate of permanent PVI is to incorporate a 20- to 30-minute waiting period following initial isolation of each PV. The prevalence of time-induced PV reconnection is most frequent at 30 minutes, with a significant proportion of patients having further reconnection at 60 minutes and very few between 60 and 90 minutes.265,448,450,451,457,459,460,461 In a retrospective study including patients undergoing a repeat ablation procedure for recurrent AF, receiver operating characteristic analysis revealed a strong negative correlation between the observation time after complete PVI during the initial procedure and chronic PV reconnection.452 The optimal cutoff value was 35 minutes, although the diagnostic accuracy was not high (sensitivity 66.9%, specificity 60.6%). A small, prospective randomized trial comparing the outcomes of AF ablation in which no waiting period, a 30-minute, and a 60-minute waiting period was incorporated into the ablation procedure revealed a clear benefit of incorporating a 30-minute or longer waiting phase (60.7%, 84.3%, and 86.7%, respectively).457 It is notable that reevaluation of the need for a waiting phase has not been reassessed since the widespread availability of contact force-sensing (CFS) ablation catheters. The need for a waiting phase has been less completely assessed with CB AF ablation. The Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) Trial did require a 30-minute waiting period before termination of the observation period. It is unclear whether this contributed to the 69.9% success rate in elimination of AF, although those outcomes are similar to equivalent studies during that time frame.462
The above data would suggest that a 20- to 30-minute waiting phase is reasonable to incorporate into an AF ablation procedure. A limitation of a longer observation period is the impact on the total procedure duration and work flow in the electrophysiology laboratory. A survey of the writing group members shows that 80% incorporate at least a 20-minute waiting period following initial isolation of the PVs when performing AF ablation with RF energy. Based on this information, the published literature, and the experience of this writing group, the writing group recommends that monitoring for PV reconnection for 20 minutes following initial PVI “is reasonable” (Class IIa, LOE B-R) during AF ablation using RF energy (Table 3).
Adenosine Testing
Intravenous adenosine (or adenosine triphosphate [ATP]) can transiently restore cellular excitability and differentiate permanent conduction block from dormant conduction (e.g., viable but latently nonconducting tissue) across circumferential PV ablation (CPVA) lines.463,464,465,466,467 The ability of adenosine to unmask dormant PV reconnection is impacted by the adenosine dose as well as by the waiting time since initial documentation of PVI.466,467 The results of one recent study suggest that the demonstration of a physiological effect of adenosine (e.g., sinus tachycardia, hypotension) is inadequate, and that sufficient adenosine needs to be administered to demonstrate transient AV block.467 There have been more than a dozen studies that have investigated the role of adenosine as an adjunct to achieving permanent PVI and improving the outcomes of AF ablation; these are summarized in a recent review article.466 Among these studies are two large, prospective, randomized clinical trials.
The first study was a prospective, randomized clinical trial involving 534 patients with PAF. All the patients were administered adenosine 20 minutes following initial PVI. The initial dose was 12 mg, which was titrated until at least one blocked P wave or a 3-second pause was observed.265 The presence of dormant PV conduction was associated with an increased risk of arrhythmia recurrence. Patients with dormant PV conduction were randomly assigned to additional adenosine-guided or to no further ablation. Elimination of dormant PV conduction by additional targeted ablation significantly reduced recurrent atrial tachyarrhythmias (ATAs) by 56% during follow-up (P <.0001).265 The most recent study enrolled 2113 patients with paroxysmal, persistent, and long-standing persistent AF.461 In this study, 0.4 mg per kg of adenosine was administered after a variable waiting time (median time 43 minutes). Early reconduction after the waiting time alone was observed in 42% of the patients in both groups. Subsequent administration of adenosine demonstrated further reconduction in an additional 27% of the patients. Further ablation was then performed to eliminate dormant conduction. At the end of 1 year of follow-up, no difference in outcome was observed, with a success rate of approximately 65% in each group. Of note, given the lower observed 27.6% prevalence of dormant conduction in the latter trial, a risk reduction of 72.4% in those patients with dormant conduction would have been required to detect a significant overall difference.466 It is also notable that the use of force-sensing ablation catheters is associated with a significant reduction in the prevalence of dormant conduction, therefore limiting the impact of an adenosine-guided ablation strategy on clinical outcome.468
Although the above data would suggest that administration of adenosine 20 minutes after initial PVI at a dose titrated to achieve AV block or a 3-second pause could improve outcomes of AF ablation, the impact of such a strategy is limited. Furthermore, the addition of an adenosine testing strategy prolongs procedure time and increases costs. A survey of the writing group members shows that 61% routinely incorporate use of adenosine into their ablation procedure when using RF energy and 25% incorporate this approach into their ablation strategy when using CB. Based on this information, the published literature, and the experience of this writing group, the writing group recommends that administration of adenosine 20 minutes after initial PVI phase “may be considered” (Class IIb, LOE B-R) during AF ablation (Table 3).
Isoproterenol Infusion
Some studies reported the use of adenosine during isoproterenol infusion to unmask dormant PV conduction.460,469,470 A prospective clinical and experimental evaluation of various pharmacological strategies found that adenosine was superior to an isoproterenol infusion, with no significant additional value of combining the two.471 Therefore, although isoproterenol infusion can be used to identify non-PV triggers in paroxysmal and persistent AF, there is only a limited role of isoproterenol infusion alone to reveal dormant PV conduction after PVI. A survey of writing group members shows that less than one-third administer isoproterenol to search for PV reconnection.
Loss of Pace Capture on the Ablation Line
The use of a pace capture-guided ablation strategy as an additional endpoint in PVI procedures has been proposed as another method to improve the durability of PVI.264,472,473,474,475 Using this approach, after completion of PVI, high-output pacing (10 mA) from the ablation catheter's distal bipole is performed during sinus rhythm while slowly moving the catheter along the entire circumference of the ipsilateral PVI lines.264,472 Where local LA capture is identified, additional ablation is performed until loss of capture, with the goal of closure of the residual gaps. In a recent randomized study involving 102 patients at two centers, this technique significantly improved arrhythmia-free survival compared with conventional PVI.474 Another study compared an ablation strategy of loss of pace capture vs adenosine administration to identify dormant conduction. The outcomes were no different in the two groups.473 Another study revealed that a strategy of pace-capture-guided PVI was found to be associated with a significant reduction in dormant PV conduction revealed by adenosine.475 It is also notable that reevaluation of value of pace capture has not been reassessed since the widespread availability of CFS ablation catheters.
Although the above data would suggest that using pace capture might improve outcomes of AF ablation, the data are somewhat limited and have been published from a very small number of centers. Like other strategies to improve outcomes of AF ablation, this strategy also prolongs procedure time. A survey of the writing group members shows that 24% routinely incorporate the strategy of pace capture when using RF energy. This strategy is not applicable for the CB approach. Based on this information, the published literature, and the experience of this writing group, the writing group recommends that pace-capture-guided ablation strategy may be considered following PVI with RF energy (Class IIb, LOE B-R) (Table 3).
Exit Block
Although the demonstration of entrance conduction block is the standard endpoint of PVI procedures, permanent PV exit conduction block (or the “stable absence of PV-LA conduction”) is the ultimate goal for prevention of PV-induced AF. Exit block can be demonstrated either by spontaneous discharges recorded circumferentially around the PV or by continuing arrhythmia within the PV, which are dissociated from sinus rhythm or by pacing from within the PV.476 In the case of PV pacing, it is imperative to demonstrate local PV capture without conduction to the LA to prove exit block. It is also important to avoid inadvertent capture of adjacent far-field structures, which would result in misinterpretation of apparent exit conduction.477,478 The presence of entrance block appears to be effective in predicting bidirectional block across CPVA lines.141,142,143 Mechanistically, the most likely explanation is source–sink mismatch, which is defined as delay or block of conduction observed when the size of a given excited region supplying depolarizing current (the current source) is insufficient for the amount of depolarizing current necessary to excite the regions ahead (the current sink).479 Although an initial report has observed unidirectional entrance block in more than 40% of PVs,445 the incidence was much lower in more recent studies (1.5%–16%).477,479,480,481 Interestingly, in one of these studies, the presence of PV discharges conducted to the LA (exit conduction) was followed by recovery of entrance conduction during a 30-minute waiting period.480 None of the reported studies compared success rates of PVI using only verification of PV entrance block vs bidirectional PV conduction block.
The above data would suggest that demonstration of exit block is feasible, and is a reasonable endpoint for AF ablation when combined with the presence of exit block. A survey of the writing group members shows that 60% routinely pace in the PV and employ exit block as an endpoint during AF ablation using RF energy, and 46% routinely pace in the PV and employ exit block as an endpoint during CBA. Based on this information, the published literature, and group experience, the writing group recommends that demonstration of exit block may be considered following PVI with RF or CB energy (Class IIb, LOE B-NR) (Table 3).
Techniques for Obtaining Permanent PVI with Balloon Technologies
PVI is the cornerstone of all ablation strategies in AF. However, it is still challenging, and there exists a considerable learning curve to develop the skills needed to safely and effectively perform RF AF under 3D electroanatomical guidance. Therefore, novel catheter designs with alternative energy sources have been developed. These catheter technologies are balloon-based ablation systems using various energy modalities, such as cryoenergy (Arctic Front, Medtronic, Inc., Minneapolis, MN, USA), laser (Heartlight, CardioFocus, Marlborough, MA, USA), and radiofrequency catheter (RFC) (Hot Balloon Catheter, Hayama Arrhythmia Institute, Kanagawa, Japan). These technologies will be discussed in detail in the technologies section of this document. The present section will focus on some of the strategies that have been developed to facilitate the endpoint of achieving permanent PVI.
Obtaining Permanent PVI with the Cryoballoon
The first-generation CB was introduced approximately 10 years ago and consisted of a noncompliant balloon with two different diameters (23 mm and 28 mm), using N2O as the refrigerant. A stiff wire or a spiral mapping catheter (Achieve, Medtronic, Inc.) is inserted via a central lumen of the catheter shaft. Applying the first-generation CB and freeze cycle durations of 300 seconds, successful PVI was routinely followed by a bonus freeze cycle of the same duration. The 1-year success rate was approximately 70%–73%.462,482 More than 80% of the patients with AF recurrence after first-generation CB-based PVI demonstrated recurrence of conduction at the time of a repeat ablation procedure.483 The second-generation CB (Arctic Front Advance, Medtronic, Inc.) was introduced in 2012 and incorporates a modified refrigerant injection system characterized by 8 injection jets in a more distal balloon position. Thus, a more homogeneous cooling of the complete distal balloon hemisphere, including the distal tip, was achieved. Multiple studies have demonstrated clinical success rates of 65% or greater.484,485,486,487,488,489,490,491,492,493 The recently published FIRE AND ICE trial was the first to compare the acute and long-term efficacy as well as the safety profile of the second-generation CB with conventional RFC ablation in a prospective, randomized, multicenter fashion.489 The study demonstrated the noninferiority of CBA compared with RF-based ablation with respect to efficacy and safety of patients with drug-refractory PAF.494 However, secondary endpoints, such as the rate of rehospitalization and reablations, or the necessity of electrical cardioversion during follow-up were in favor of CB.490 Among patients who fail AF ablation with the CB2 system, recurrent PV conduction is observed in 27% to 65% of patients.487,491 The role of adenosine in revealing dormant conduction has also been evaluated. One study reported that administration of 12 mg or more of adenosine (titrated to AV block) 30 minutes postablation resulted in an 11% incidence of recurrent PV conduction.495 A second study reported similar findings, with a 12% incidence of recurrent conduction after a waiting phase plus adenosine administration. This small study of 90 patients reported a higher success rate (84% vs 79%) when a strategy of reablation based on adenosine-induced dormant conduction was targeted.495
A survey of the writing group shows that only 51% of the writing group members employ a 20-minute or longer waiting phase when using CBA. This survey also revealed that 46% of operators routinely pace in the PV and employ exit block as a secondary endpoint during AF ablation using CB energy.
Endoscopic Laser Balloon PVI
The endoscopic laser balloon is a recently introduced balloon-based ablation system incorporating a titratable laser source and a 2F endoscope. This ablation system received U.S. Food and Drug Administration (FDA) approval in the United States for ablation of PAF in 2016. It consists of a compliant balloon (9–35 mm) that can be adapted according to the individual PV diameter. The laser arc covers 30° of a complete circle and allows energy titration in five steps, from 5.5 W to a maximum of 12 W. The rate of acute PVI when applying this system is 98%–100%.496,497,498,499 A study in which 52 patients underwent chronic reassessment of PV conduction revealed that 14% of PVs demonstrated reconnection, translating to 38% of patients with reconduction of one or more veins.497 The application of higher energy levels (8.5 W or 10 W) was associated with a significant increase in the rate of acute PVI after a purely visually guided ablation circle.500,501,502 At the same time, the application of higher energy settings did not compromise the safety profile. One-year clinical follow-up data from two prospective, multicenter studies in patients with PAF demonstrated a single-procedure clinical success rate of 63% and 60% with no anti-arrhythmic medication.498,499 A prospective comparison with RF ablation revealed equivalent 1-year outcomes, with a success rate of 61%.503 There have been no studies that have examined how often vein reconnection is observed within the first 60 minutes post-PVI. There are also no data on the role of adenosine.
Adjunctive Ablation Strategies to Be Performed in Addition to PVI During AF Ablation
Cavotricuspid Isthmus Ablation
Catheter ablation of typical AFL involving the cavotricuspid isthmus is a safe, effective, and well-established ablation procedure. For patients undergoing AF ablation, creation of a cavotricuspid isthmus line can be performed safely, easily, and with only a slight prolongation in procedure time. A survey of the writing group members shows that ablation of the cavotricuspid isthmus is performed by 94% of the writing group members in patients undergoing AF ablation who have previously been documented by ECG to have experienced isthmus-dependent AFL, as well as those with inducible cavotricuspid isthmus-dependent AFL at the time of the ablation procedure. This is based on decades of experience demonstrating the safety and efficacy of catheter ablation of AFL as well as data from clinical trials comparing ablation with AAD therapy.230,504,511,1397 The writing group recommends that if a patient has a history of typical AFL or typical AFL is induced at the time of AF ablation, delivery of a cavotricuspid isthmus linear lesion is recommended (Class I, LOE B-R, Table 3).
Linear Lesions Not Involving the Cavotricuspid Isthmus
Circumferential isolation of PVs has become the standard therapy for PAF. However, due to the high recurrence rate observed in patients with persistent and long-standing persistent AF with PVI alone, continued efforts are underway to identify additive strategies to improve outcome. One of these strategies is to create additional linear lesions in the LA similar to those that are a part of that advocated with the Cox-Maze III lesion set (Figure 6).505,506 The most common sites for linear ablation are the LA “roof” connecting the superior aspects of the left and right upper PVI lesions, the region of tissue between the mitral valve and the LIPV (the mitral isthmus), and anteriorly between the roof line near the left or right circumferential lesion and the mitral annulus. A prior randomized, prospective trial of catheter ablation of PAF comparing segmental PVI vs CPVA plus LA linear ablation (CPVA-LALA) at the LA roof and myocardial infarction showed that significantly more patients had LA flutter in the CPVA-LALA group.507 A survey of the writing group shows that only 2% perform linear ablation during an initial AF ablation in patients with PAF. For redo ablation procedures in PAF, only 10% of the writing group members routinely employ linear ablation. Based on this information, the published literature, and the experience of this writing group, we recommend that creation of linear lesions not be performed during initial or redo AF ablation for PAF unless a macroreentrant AT is induced. The role of additional lines in cases of persistent AF remains controversial.508 The recently completed STAR-AF study of ablation strategies for persistent AF showed no improvement in ablation efficacy for linear lesions plus PVI vs PVI alone.245 The Catheter Ablation of Persistent Atrial Fibrillation (CHASE-AF) study also revealed that the addition of linear lesions and defragmentation of PVI did not improve outcomes for ablation of persistent AF compared with PVI alone.509 For patients with persistent or long-standing persistent AF, 25% of the writing group members perform linear ablation at the time of an initial ablation procedure, increasing to 45% when redo procedures are performed in patients with persistent and long-standing persistent AF. The writing group recognizes that the usefulness of linear ablation lesions in the absence of macroreentrant AFL is not well established (Class IIb, LOE C-LD). For patients with PAF, linear ablation should not be performed. The usefulness of creation of linear ablation lesions in the RA or LA as an initial or repeat ablation strategy for persistent or long-standing persistent AF is not well established (Class IIb, LOE B-NR) (Table 3). It has been widely demonstrated that incomplete block across the ablation lines can be responsible for AT recurrence.510,511,512,513 Therefore, if linear ablation lesions are applied, operators should use mapping and pacing maneuvers to assess for line completeness (Class I, LOE C-LD) (Table 3). Well-designed, prospective, randomized clinical trials must be the ultimate test of linear ablation techniques.
In patients with long-standing persistent AF, the stepwise approach has been proposed.514,515 The strategy starts by pulmonary isolation, followed by ablation of CFAE, looking for reversion to sinus rhythm or AT. If this endpoint is not achieved, additional linear lesions are deployed.514,516,517 Whether the endpoint of AF termination is associated with improved long-term results remains controversial.518 Despite encouraging acute outcomes, with termination of AF in 80% of patients, the follow-up data were less impressive with a 1-year single procedure efficacy of 35% and a 5-year efficacy of 17%. Arrhythmia-free survival rates after the last procedure (mean 2.1 ± 1.0 procedures) were 89.7% ± 2.5%, 79.8% ± 3.4%, and 62.9% ± 4.5%, at 1, 2, and 5 years, respectively.515 More recent data also suggest that linear ablation does not improve outcomes compared with PVI alone.245,515,519,520,521 The recently completed CHASE-AF study also reported no improvement in efficacy for ablation of persistent AF with PVI plus linear lesions and defragmentation compared with PVI alone.509 The clinical significance of these data, which appear to be contrary to our understanding of the mechanisms of AF, predicting that AF is less likely to be sustained in electrophysiologically smaller, segmented atria, is of clinical importance. Potential explanations for this discrepancy might be that the linear lesions are incomplete, are in the wrong place, or that our understanding of the AF mechanism is incorrect.
Posterior Wall Isolation
Some patients with PAF can be undertreated with PVI alone, and PVI might not be enough to control persistent and long-standing persistent AF. Further modification of the atrial substrate might be required. One of the strategies that has been proposed is electrical isolation of the posterior wall. This can be performed by creating a linear ablation of the LA roof joining the superior PVs and the LA floor joining the inferior PVs or by point-by-point ablation of the entire posterior wall (Figure 6). Entrance block of the box lesion is confirmed by lack of potentials in the box. Exit block of the box lesion is confirmed by pacing in the box and demonstrating exit block during sinus rhythm. Gaps along the ablation lines, if present, are mapped and ablated.522,523,524 With lack of LA capture outside the box, the lines are considered complete. The endpoint of box isolation is defined as bidirectional conduction block, i.e., both lack of potentials in the box and lack of LA capture.
Although some studies have been published that report improved outcomes,525,526 with posterior wall isolation, other clinical trials report no improvement on outcomes.527 The recently published BELIEF trial reported at 28% efficacy of an extensive ablation strategy, including ablation of the posterior wall, in patients with long-standing persistent AF.528,529 A survey of the writing group shows that 15% of the writing group members perform posterior wall isolation in patients with PAF during an initial AF ablation procedure, and 18% isolate the posterior wall during a repeat ablation procedure in a patient with PAF. The role of posterior wall isolation in cases of persistent AF also remains controversial.508 For patients with persistent or long-standing persistent AF, 22% of the writing group members perform posterior wall isolation at the time of an initial AF ablation procedure and 38% of the writing group members perform posterior wall isolation for repeat AF ablation procedures in patients with persistent and long-standing persistent AF. Based on this information and a review of the literature, the writing group recommends that posterior wall isolation might be considered during an initial or repeat AF ablation for paroxysmal, persistent, or long-standing persistent AF (Class IIb, LOE C-LD, Table 3).
Nonpulmonary Vein Triggers
Non-PV “triggers” can be identified in 10%–33% of unselected patients referred for catheter ablation of AF.96,197,208,257,530,531,532,533,534 The prevalence of non-PV triggers in different studies varies with the specific definition adopted, which ranges from repetitive atrial premature depolarizations without definitive AF initiation532,533 to reproducible sustained AF triggering.534 Supraventricular tachycardias, such as AV nodal reentry or accessory pathway-mediated AV reciprocating tachycardia, can also be identified in up to 4% of unselected patients referred for AF ablation and can serve as a triggering mechanism for AF.458 Non-PV triggers can be provoked in patients with both paroxysmal and more persistent forms of AF.531,534 In selected patients with reproducible non-PV triggers and without provocable PV AF triggers with high-dose isoproterenol, elimination of only the non-PV triggers has resulted in elimination of AF.96,458,535
The most common sites of origin for non-PV atrial triggers include the posterior wall of the LA, the SVC, the crista terminalis, the fossa ovalis, the CS, the eustachian ridge, the ligament of Marshall, and adjacent to the AV valve annuli (Figure 4).96,208,458,530,531,536 More recently, frequent and repetitive atrial premature depolarizations have been identified in the LAA in patients with more persistent AF, which have been targeted by LAA isolation techniques.532,533
Isoproterenol is the most commonly used agent to provoke non-PV triggers. Withholding of antiarrhythmic agents for five half-lives and withholding beta-blockers for at least 24 hours is important when a strategy of searching for non-PV triggers is employed. A typical protocol for initiating non-PV triggers includes:
Baseline infusion of a vasoconstrictor (e.g., phenylephrine) to maintain mean arterial pressure (MAP) >70 mm Hg and increase the bolus vasoconstrictor throughout infusion to maintain adequate perfusion. This is particularly important under general anesthesia. Careful titration of a vasoconstrictor allows for higher doses of isoproterenol infusion.
Graded infusion of isoproterenol using up to 20–30 μg per minute for at least 10 minutes is recommended. Most members of the non-PV trigger writing group felt that lower-dose isoproterenol infusion was frequently ineffective.
If no effect with isoproterenol infusion, burst pacing into AF and then cardioversion during low-dose isoproterenol infusion (2–6 μg per minute) may be considered.
Use of adenosine bolus or burst atrial pacing during lower-dose isoproterenol infusion to attempt to identify repetitive triggers after the drive train is an adjunctive technique employed by a subset of the non-PV trigger writing group. Localization of non-PV AF triggers can be challenging, particularly when only the first triggering beat is being targeted, and typically involves recognition of specific intra-atrial activation patterns on multipolar catheters placed in the RA and CS, together with information from the surface ECG to help regionalize an area of interest.458,534,535 Moving the circular mapping or ablation catheters around the LA and reinitiating AF can be useful to localize AF triggers, taking care to minimize ectopy with catheter manipulation. Placement of a multipolar catheter inside the SVC is important for identifying SVC triggers. The majority of the writing committee members perform SVC isolation if an SVC trigger is identified. To isolate the SVC, a circular mapping catheter is placed inside the SVC to identify SVC potentials. Ablation is performed proximally at the SVC/RA junction. While isolating the SVC, high-voltage pacing (at least 20 mA) is used before each RF application to check for phrenic nerve (PN) stimulation. Ablation is avoided in areas of PN capture, even if incomplete isolation is the result. SVC isolation should ideally be performed in sinus rhythm after isoproterenol infusion has worn off to avoid sinus node injury. RF application is ceased if sinus node acceleration or pauses are observed. The endpoint of SVC isolation is entry and exit block into the SVC, as is typically seen with PVI. Dissociated firing of the SVC can also be observed. In contrast to wide-area PVI and because of phrenic capture or risk of sinus node injury, a segmental approach targeting the earliest breakthrough on the circular mapping catheter is most commonly employed.
While ablating AF triggers on the LA posterior wall, the RF power is typically decreased to ≤ 30 W. An esophageal temperature probe is frequently used. The writing group also recommends that it is reasonable to use an esophageal temperature probe during RF ablation procedures to monitor esophageal temperature and to help guide energy delivery (Class IIa, LOE C-EO, Table 3). Some committee members isolate the entire posterior wall if numerous or multifocal posterior wall triggers are identified. This can be accomplished using a “box” lesion set, including a roof line (RSPV to LSPV) and floor line (RIPV to LIPV) after PVI. If triggers are observed to be originating from the CS or the LAA, some writing committee members perform isolation of these structures, while most prefer focal ablation. LAA triggers can be identified by observing far field LAA activity on a circular mapping catheter placed in the LSPV; placing the ablation catheter into the LAA should be avoided to minimize risk of perforation or catheter-induced ectopy. Isolation of the LAA should be performed only after prior discussion with the patient and consideration of the long-term need for thromboembolic prophylaxis, with consideration given to LAA closure by one of the available methods. For other non-PV triggers, such as AT, AV nodal reentry tachycardia or AV reentrant tachycardia, focal ablation is performed. Inability to provoke the trigger with repeat isoproterenol infusion is considered as the endpoint. Observational studies have shown improved arrhythmia-free survival when non-PV triggers are targeted for ablation and effectively eliminated at the time of PVI.537,538
In some redo ablation cases, if non-PV triggers cannot be provoked, empiric ablation of non-PV trigger sites may be attempted. The empiric targeting of frequently defined non-PV trigger sites can have more value in persistent forms of AF when triggers are not observed with provocative maneuvers.539 The most common empiric non-PV trigger ablation is SVC isolation. Other common sites for empiric non-PV trigger ablation include the mitral annulus, limbus of the crista terminalis, mid to inferior crista terminalis, and eustachian ridge.539 Some investigators also advocate empiric LAA and CS isolation.
Despite the suggested improved outcome with elimination of non-PV triggers, the minority of operators according to a recent European survey routinely perform non-PV trigger initiation and ablation.540 Ablation of non-PV triggers might be more important for patients with persistent forms of AF and for those patients who undergo repeat ablation procedures in whom all PVs are found to be isolated.
Additional investigation is needed on the optimum method for initiating and mapping infrequent non-PV triggers. Furthermore, the value of routine non-PV trigger identification and ablation with the initial ablation procedure and at the time of repeat procedure following recurrence warrants further study.
A survey of the writing group members shows that when ablating PAF with the CB system, 18% also search for non-PV triggers. Among those who use RF energy for AF ablation in patients with PAF, 41% routinely employ a strategy including administration of high-dose isoproterenol to screen for and then ablate non-PV triggers. When performing a repeat procedure in a patient with PAF, 57% of the writing group members search for non-PV triggers. When ablating persistent and long-standing persistent AF with RF energy, the percentage of the writing group members who use a non-PV trigger protocol are 35%, and 46% for first-time and redo AF ablation procedures, respectively. Based on this information and a review of the literature, the writing groups recommends that administration of high-dose isoproterenol to screen for and then ablate non-PV triggers may be considered during initial or repeat AF ablation procedures in patients with paroxysmal, persistent, or long-standing persistent AF (Class IIb, LOE C-LD).
LAA Focal Ablation, Isolation, and Ligation or Resection
A relatively new non-PV-based strategy for ablation of AF involves targeting non-PV triggers and reentrant tachycardias that arise from the LAA.541 Over the past 5 years, new information has been published showing promising outcomes using a variety of non-PV-based ablation strategies that target the LAA. These strategies include focal ablation of non-PV triggers arising in the appendage,541 electrical isolation of the LAA,541,542,543,544 and most recently, ligation of the LAA, although this approach is an off-label use of LA tissue ligation.532,533,535,536 LAA isolation has been described using a technique similar to that of PVI: with the circular mapping catheter positioned at the level of the LAA ostium, addressing the earliest LAA activation site (preferably during sinus rhythm). Care should be taken not to ablate inside the LAA (risk of perforation and PN injury). After LAA isolation, patients should be kept on long-term OAC or considered for LAA occlusion. This reflects the results of a recent study that has reported an increased stroke risk following LAA electrical isolation.544 The recently published BELIEF trial randomized 173 patients to start AF ablation or to start standard AF ablation with empirical electrical isolation of the LAA. After an average of 1.3 procedures, the cumulative success at 24 months' follow-up was 76% in the combined group vs 56% with standard AF ablation.528,529 One approach to address this potential issue is to combine LAA electrical isolation with placement of a Watchman Device.542,543 Recent animal and human studies have also reported the feasibility of this combined strategy.542,543 Currently, a prospective randomized clinical trial is being performed to determine if LAA ligation with the LARIAT device will improve the efficacy of PVI in patients with persistent AF. The outcome of this trial will be required to provide a clear indication for this approach. A survey of the writing group members shows LAA focal ablation, isolation, or ligation as an initial ablation strategy in patients with PAF is used by 2% of the writing group members, and 4% use the above for repeat AF ablation procedures in patients with PAF. For patients with persistent and long-standing persistent AF, LAA focal ablation, isolation, or ligation was used by 9% of the writing group members as an initial ablation strategy in patients with PAF, and was used by 11% of the writing group members for repeat AF ablation procedures in patients with persistent and long-standing persistent AF. There is need for additional well-performed, prospective, multicenter randomized trials in order to determine the safety and efficacy of this approach.
Complex Fractionated Atrial Electrogram Ablation
More than a decade ago, CFAEs were reported to potentially represent AF substrate sites and became target sites for AF.516,545 CFAEs are electrograms with highly fractionated potentials or with a very short cycle length (<120 ms). CFAEs are typically low-voltage multiple potential signals between 0.06 and 0.25 mV. The primary endpoints using this approach during RF catheter ablation strategies for AF are either complete elimination of the areas identified with CFAEs, conversion of AF to sinus rhythm (either directly or first to an AT), and/or noninducibility of AF. In patients with persistent AF, the endpoint of ablation with this approach is AF termination. Although use of the AF termination endpoint has been reported to be associated with improved short-term outcomes, the long-term results have been disappointing.245,515,517,546 When the areas identified with CFAEs are completely eliminated, but the arrhythmias continue as organized AFL or AT, the ATAs are mapped and ablated. In patients with long-standing persistent AF, a step-wise approach to ablation has been reported to successfully convert AF to either sinus rhythm or AT in greater than 80% of patients.514,547 Despite these encouraging acute outcomes, the follow-up data were disappointing, with a 1-year single procedure efficacy of 35% and a 5-year efficacy of 17%.515
One of the limitations of ablation targeting with CFAEs has been the extensive amount of ablation needed. As a result, some strategies for differentiating “active” from “passive” sites have been described. These include pharmacological interventions, the use of monophasic action potentials, limiting ablation to areas of continuous electrical activity, and activation mapping of AF.540,548,549,550,551 Unfortunately, improved outcomes with CFAE ablation in patients with persistent AF have not been uniformly reported, and the scientific basis for CFAE ablation is not universally accepted. Moreover, results from the STAR AF II trial have shown that the addition of further ablation (lines or CFAEs) to PVI increased ablation time but did not reduce the recurrence of AF in 589 patients with persistent AF.245 At 18 months, the percentage of patients who were free from AF recurrence after one procedure without antiarrhythmic medication did not significantly differ among groups. Similar findings were reported in the CHASE-AF trial, which reported that the addition of defragmentation and linear ablation to PVI did not improve ablation outcomes for persistent AF compared with PVI alone.509 This suggests that CFAE has clearly lost momentum, perhaps in favor of less extensive approaches for AF ablation.552
None of the writing group members routinely employ CFAE-based ablation as part of an initial ablation strategy in patients with PAF, and only 4% of the writing group members employ CFAE ablation during repeat procedures. Ten percent of the writing group members routinely employ CFAE-based ablation as part of an initial ablation strategy in patients with persistent and long-standing persistent AF, and 26% incorporate CFAE-based ablation for redo procedures in this subset of patients. Based on this information and a review of the literature, the writing group recognizes that the usefulness of CFAE-based ablation as an initial or repeat ablation strategy for persistent and long-standing persistent AF is not well established (Class IIb, LOE B-R). Focal ablation of sites targeted by CFAE without anchoring the lesion to a nonconducting neighboring area might simply lead to formation of a substrate prone to produce future AFLs. CFAE ablation is not recommended for ablation of PAF.
Ablation of Fibrosis Identified by Voltage Mapping and/or MRI Mapping
Another new ablation strategy that has been developed to improve the outcomes of ablation involves targeting detected areas of fibrosis, based either on voltage mapping or on MRI. The regional and individual extent of the fibrotic LA substrate in patients with AF can be visualized during ablation intervention in sinus rhythm by applying electroanatomical voltage mapping (EAVM). This technique led to the use of a patient-tailored ablation strategy called box isolation of fibrotic areas (BIFA), with circumferential isolation of the severely affected fibrotic areas (e.g., <0.5 mV), and with complete isolation confirmed by a circular mapping catheter.140,553 Early pilot data using this approach combined with PVI was encouraging.
This approach involves first isolating the PVs. A voltage map is then recorded during sinus rhythm, and the low voltage areas are identified and subsequently isolated according to the individual localization and extent. Efforts are made to connect these BIFA ablation lines to the initial PVI lines to prevent the production of small channels, such as is possible with CFAE or GP ablation. Using this approach, approximately one-third of the patients with persistent AF were identified as not having substantial LA fibrosis and, accordingly, only PVI was performed. This limited approach led to freedom from AF at 12 months with a single procedure in 69% of the patients and with only 1.2 procedures per patient in 85%.140 These results were comparable to patients with substantial fibrosis and subsequent BIFA ablation. In addition, in a small portion of patients with massive fibrosis (the strawberry), failure of the initial ablation was likely, and further ablation procedures were discouraged.140 Other investigators have also described a tailored substrate modification based on low-voltage areas.554 They used several strategies, including linear lines encircling of low-voltage areas, with demonstration of a significant reduction in local electrograms, defractionation, and/or loss of capture. Even earlier, isolation of the posterior wall at the level of the PVs without prior EAVM was introduced522; however, this strategy did not address the individual localization of the fibrotic disease at that time. Several other groups have recently reported on voltage-guided substrate modification in patients with persistent AF.555,556,557 The durability of RFC-induced isolating lesions is clearly still an issue. The same holds true for BIFA lines for substrate modification guided by EAVM. Recent technology improvements of point-by-point catheter approaches (e.g., CF measurement) could further pave the way for improving these approaches. However, the realization of the proposed strategies for BIFA substrate modification requires of the operator extensive manual skills and experience.
The methods to describe the fibrotic substrate with EAVM are also under intensive investigation, and several limitations yet remain. The voltage maps using point-by-point mapping not only take time, but the measured voltage also depends on the rhythm (sinus rhythm vs extrasystole or AF), the contact of the electrode to the tissue, the thickness of the atrial myocardium, the size of the mapping electrodes, interelectrode distance, and other variables. Investigators described preexistent LA scarring in patients undergoing PVI as an independent predictor of procedural failure.558 Low voltage for abnormal areas in their study was also defined as an amplitude ≤0.5 mV, and scar ≤0.05 mV, indistinguishable from noise. Other investigators recently described contact EAM-derived voltage criteria for characterizing LA scar in patients undergoing ablation for AF.559 In their study, a voltage range of 0.2 to 0.45 mV was found to delineate scar. Others used 0.2 to 0.5 mV as diseased and >0.5 mV as healthy.554 This differs from the experience of another investigator, in whose studies 0.5 to 1.5 mV presented an intermediate zone that did not denote substantial fibrosis but that also did not provide clear evidence for a normal atrial myocardium.140,553 The fragmented electrogram appearance of voltages in the range of 0.5 and 1.5 mV frequently argue in favor of mild fibrosis. Certainly, there is no “yes or no” with respect to atrial fibrosis, but various grades can be observed. In summary, atrial scar is proposed for sites with no discrete electrograms (apart from potential far-field electrograms) and no local capture during pacing, dense fibrosis for sites with voltages ≤0.5 mV, an intermediate zone of mild fibrosis for sites with voltages >0.5 to 1.5 mV, and normal for sites with voltages >1.5 mV. However, with some exceptions, mild fibrosis is even assumed at sites with voltages between 1.5 and 2.5 mV. Furthermore, criteria need to be developed when other diagnostic catheters are used for EAVM, e.g., the circular mapping catheter or the Pentaray catheter. Overall, these initial single-center observational studies on ablation or isolation of fibrotic areas need to be confirmed and extended in multicenter randomized studies.
Recently, the utility of delayed enhancement (DE) MRI has been introduced for detecting, quantifying, and localizing atrial fibrosis, including the definition of four categories of structural changes (Utah stages I–IV).130,560,561 The tissue characterization of the LA wall on DE MRI correlated with EAVM and with histology from surgical biopsy specimens.560,561 The association of atrial tissue fibrosis and AF catheter ablation outcomes, with more extensive fibrosis associated with lower efficacy, was confirmed in the multicenter Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation (DECAAF) study.365 On the other hand, these MRI findings at this point in time require extensive MRI experience, including specification of image contrast and continuity, required to set boundaries for the various degrees of fibrosis. The reproducibility of this approach is still under investigation. A major limitation of this approach is that the degree of scar identified depends strongly on the above thresholds used to define scar. At the present time, no uniform standard has been developed. This limitation hinders the reader-to-reader and day-to-day reproducibility of MRI-determined measurements of atrial scar. Once established, however, the DE MRI quantification and localization of atrial fibrosis might be used effectively to guide individually tailored substrate elimination comparable to EAVM-guided substrate modification. Finally, tissue visualization before and also during and directly after RF catheter ablation is the target for introducing real-time MRI into the clinical electrophysiological laboratory. Currently, the DECAAF-2 trial has been launched to test the hypothesis that ablation of scar detected on MRI improves ablation outcomes for persistent AF compared with PVI alone.
A survey of the writing group members shows that for patients undergoing an initial AF ablation for PAF, 7% of the writing group members employ an ablation strategy based in part on MRI or voltage mapping-detected scar, and 9% of the writing group members employ this strategy for repeat AF ablation procedures in patients with PAF. For an initial ablation procedure in patients with persistent and long-standing persistent AF, 15% of the writing group members employ an ablation strategy based in part on MRI or voltage mapping-detected scar. The proportion increases to 22% for repeat ablation in patients with persistent or long-standing persistent AF. Based on this information and a review of the literature, the writing group recognizes that the usefulness of mapping and ablation of areas of abnormal myocardial tissue identified with voltage mapping or MRI as an initial or repeat ablation strategy for persistent and long-standing persistent AF is not well established (Class IIb, LOE B-R, Table 3).
Mapping and Ablation of Rotational Activity
Several approaches have been developed to identify areas of rotational activity in the atria. The first identification and targeted ablation of rotational activity with fibrillatory activities was reported in 2005, using the noncontact mapping technique by Lin et al.562 The next system that was developed for clinical use employed two 64-pole basket catheters to obtain simultaneous unipolar endocardial electrograms from 128 locations in both atria of patients undergoing AF ablation.563 A computational mapping system (RhythmView, Topera, Inc.) was used to process the electrograms and generate activation movies of the atrial electrical activity. After considerable processing and interpolation, evidence was found for rotational activity in patients with paroxysmal, persistent, and long-standing persistent AF.564 MAPs established the minimum repolarization interval and provided physiologically feasible sequential activation paths. Movies of activation patterns and isochronal maps from individual cycles showed circulating activity around a center of rotation that was identified as rotational activity.564 Focal (centrifugal) activations were also identified. Both were considered drivers only if they sustained for ≥50 rotations or focal discharges.563 This approach has recently been validated by linking FIRM simultaneously with optically mapped rotational activity in the same human hearts.76 Although early reports documented findings showing that this approach to mapping and ablating rotational activity improved outcomes of AF ablation,223,226,563,565,566,567 more recent studies have failed to confirm these early findings.568 Issues associated with the FIRM-guided protocol that have contributed to uncertainty regarding its clinical value include difficulties with basket catheter placement and appropriate electrode contact, and the inability to identify atrial electrogram characteristics expected from rotational activity that differ quantitatively from surrounding tissue. At the time this document is being written, the approach of FIRM-guided ablation has not been universally adopted. Considerable debate continues concerning the efficacy of this ablation strategy. Further research is clearly needed.223,564,566,569 At the present time, several prospective, randomized clinical trials are underway to evaluate the long-term safety and efficacy of this approach.
Several other approaches have recently been developed to identify rotational activity as a potential target for ablation. One of these systems involves the use of high-density multipolar recordings with nonlinear analysis of the similarity index and phase mapping of rotational activity. This approach has resulted in improved ablation outcome for patients with persistent AF.224,225 Another system that has been developed to noninvasively map rotational activity is the ECGI mapping system.221,570,571,572 This system utilizes a multielectrode vest that records 224 body surface ECGs; electrical potentials, electrograms, and isochrones are then reconstructed on the heart's surface using geometrical information from computed tomography (CT). A mathematical algorithm combines the body surface potentials recorded by the electrodes and the geometric information provided by CT and solves the electrocardiographic inverse problem in order to noninvasively obtain estimated epicardial electrograms.221,570 An advantage of this approach is that it is noninvasive, and thus can be used to provide detailed follow-up information on AF recurrence. Disadvantages of the system are that it is limited to providing virtual electrograms of the atrial epicardium; activity on the interatrial septum, the PV-LAA ridge, etc., is not recorded. Another limitation has to do with workflow and the fact that CT imaging is required to obtain the torso geometry. An additional limitation of this system is that it requires the assumption that the torso has uniform electrical properties when, clearly, thoracic tissue conducts electricity nonuniformly. A clinical trial used ECGI combined with phase mapping to identify the drivers of persistent AF in 103 patients undergoing AF ablation. They observed continuously changing wavefronts and a wide variety of rotational activity behaviors.222 Reentrant drivers were unsustained and meandered substantially, but recurred repetitively within the same region. Computation of aggregated driver-density maps over a cumulative registering period allowed identification of a median of four driver domains per patient and helped to guide the ablation procedure. Of note, the longer the duration of sustained AF, the larger the number of driver regions. Ablation of driver domains alone terminated AF in 75% of patients with persistent AF and in 15% of patients with long-standing persistent AF. The onset or extinction of drivers during ablation was not assessed; thus, there is room for improving the ablation results if real-time data are used.222 At the 12-month follow-up, 83% of the patients with PAF and 75% of the patients with persistent or long-lasting AF were free from AF.222 At the present time, this system is not widely available, and few members of the writing group have clinical experience with this system.
Other investigators have also reported the ability of body surface potential mapping to detect rotational activity and stable propagation patterns during AF.573 Phase maps computed from the TQ intervals in 64 surface potentials showed complex patterns in which rotational activity could be identified, but they were unstable and lasted for a very short time. Noninvasive BSM methodology has recently started to gain momentum for the analysis of activation patterns during AF.574,575 These investigators used a custom-made 67-electrode vest that covered the whole torso of the patient; intracardiac signals at several locations were simultaneously recorded.574 They selected either segments without ventricular activity after adenosine infusion, or applied complex subtraction of QRST if such intervals were not found. After computing and performing comparisons between intracardiac and surface DF maps, the investigators demonstrated that high-frequency sources could be reflected on a small area of the body surface close to the atrium harboring the highest DF.574 More recently, investigators have used phase mapping to filter the unipolar signals with a narrow 2-Hz band-pass around the highest DF (HDF filtering) to significantly improve the detection of stable rotational activity.575 Prior to HDF band-pass filtering, phase maps displayed unstable reentries, likely as a result of superposition of the disorganized electrical activity coming from the rest of the atrial tissue. HDF filtering accentuated the organized activity of scroll waves, after which rotational activity was the main pattern of activation during AF (median of 2.8 rotations, present 73% of the time). Also, computer simulations showed that epicardial propagation is spatially smoothened when projected on the torso. For example, nearby epicardial rotational activity with opposing chirality might not be detected on the torso. This fact and the possibility of temporal intervals in which AF activity can be affected by ectopic foci, could explain the lack of detected rotational activity during the remaining 27% of the time.575
Improved understanding of underlying mechanisms improves therapy. High-frequency reentrant sources are an important mechanism of AF maintenance in humans, even if other mechanisms might also be involved in AF initiation and maintenance.17 Experimental data and ablation outcomes are making it increasingly clear that multielectrode approaches that provide simultaneous acquisition of tens or hundreds of recording sites from the fibrillating atria provide substantial improvement for the identification and eventual termination of AF sources. Although there is still substantial room for improvement, mapping technology is evolving at an accelerating pace, which gives hope that novel breakthroughs will enable panoramic assessment of the underlying mechanisms that underlie electrical turbulence in AF. Simultaneous high-resolution panoramic assessment of wave propagation from the body surface and the endocardium could help in tracking drifting or more stationary rotational activity trajectories over wide areas of the atria with better accuracy, and hopefully should advance ablation therapy. An important issue with these mapping forms is that they are critically dependent on electrogram acquisition, electrogram integration, and a variety of signal manipulations with mathematical techniques, including inverse solution, Hilbert, and phase transformations that produce an additional level of complexity in attempting to simplify mapping. Registration of the maps to anatomic structures, or CT or MRI and subsequent navigation, likewise add complexity to the process and underscore the need for additional translational and clinical studies to validate and clarify their utility.
A survey of the writing group shows that none of the members routinely employ ablation of rotational activity during initial or repeat ablation procedures in patients with PAF; 7% do so during initial ablation of persistent and long-standing persistent AF, and 9% do so during repeat ablation of persistent and long-standing persistent AF. Based on this information and a review of the literature, the writing group recognizes that the usefulness of ablation of rotational activity as an initial or repeat ablation strategy for persistent and long-standing persistent AF is not well established (Class IIb, LOE B-NR, Table 3).
Localization and Ablation of Left Atrial Ganglionated Plexi
Recent experimental and clinical studies have shown that the intrinsic cardiac ANS, which is formed by interconnected clusters of autonomic ganglia, known as GP, plays an important role in the initiation and maintenance of AF.103,105,114,116,124,355,576,577,578,579,580,581,582 Because the GP are consistently located within areas of highly fractionated atrial potentials (FAPs), also referred to as CFAEs,103,105,114,116,124,355,576,577,578,579,580,581,582,583,584 it is useful to begin with a fractionation map of the LA and PVs during AF. The LA FAPs are usually located in four areas: (1) LAA ridge FAP area (between LAA and left PVs); (2) superior left FAP area; (3) inferoposterior FAP area; and (4) anterior right FAP area (Figure 4). GP can be localized using HFS to identify sites exhibiting transient AV block during AF. In one approach,124,582 endocardial HFS (cycle length 50 ms, 12–15 V, 10 ms pulse width) is delivered through the distal pair of the electrodes on a mapping or ablation catheter to sites within FAPs in the LA. Sites exhibiting a positive HFS response (transient AV block, increase in mean R-R interval >50% during AF) identify the 5 major GP (Marshall tract GP, superior left GP, anterior right GP, inferior left GP, and inferior right GP) (Figure 4). HFS of a GP generally increases the degree of fractionation in the adjacent PV and frequently in distant PVs.
For endocardial catheter ablation of the GP, RF energy is applied to each site exhibiting a positive HFS response (usually 25–35 W for 30–60 seconds, but the RF power and/or time is reduced when close to the esophagus).105,124,582 HFS is repeated after each RF application. If the positive HFS response is still present, RF energy is reapplied until the response is eliminated (generally only one or two RF applications are required). Ablation of each of the five GP areas usually requires 2–12 (median 6) RF applications.124,582 A positive HFS response might not identify the entire GP area. HFS-induced transient AV block is driven by activation of the inferior right ganglionated plexi (IRGP). Therefore, activating the Marshall tract GP, superior left GP, inferior left GP, or anterior right GP by HFS is followed by activation of other GP, including the inferior right GP, which innervates the AV node. The positive response to HFS (transient AV block) might not occur, due to ablation of one of the intermediate GP along the line to the IRGP. To minimize the loss of a positive HFS response, ablation of the GP should be performed in the following order: Marshall Tract GP, superior left GP, anterior right GP, inferior left GP, and finally inferior right GP. Other signs of GP activation (such as the onset of PV firing other than the PV adjacent to the stimulated GP) are occasionally observed during HFS, which does not produce an AV block response, suggesting lower sensitivity of HFS in identifying GP. Some reports targeted GP without HFS, delivering RF applications to the presumed anatomical locations of the GP.245,576,583,584
The GP (identified by HFS) are consistently located within an area of FAPs, which is much larger than the GP area, suggesting that although GP ablation consistently produces CFAE (or FAP) ablation, CFAE ablation is not equivalent to GP ablation. In patients with either paroxysmal or persistent AF, GP ablation (before PVI) significantly reduced the inducibility of sustained AF. If AF remains inducible after GP ablation, GP ablation often eliminates the majority of CFAEs, despite ablating a much smaller area than the overall CFAE area.124,582
One clinical study randomized a total of 242 patients with PAF to conventional PVI, PVI plus GP ablation, and GP ablation alone.122 Freedom from ATAs (followed for at least 2 years) was achieved in a similar number of patients in the conventional PVI and GP ablation alone groups (56% and 48%, respectively), and in a significantly greater number of patients in the PVI plus GP ablation group (74%; P = .004). In another randomized study including 264 patients with persistent or long-standing persistent AF, GP ablation as an adjunct to PVI resulted in higher rates of sinus rhythm maintenance at 3 years (49%) compared with PVI plus LA linear lesions (34%).585,586 In addition, LA tachycardias were less common with PVI plus GP ablation than with PVI plus linear lesions. GP ablation alone was also tested in patients with drug-refractory long-standing persistent AF, resulting in a lower success rate (38% sinus rhythm maintenance at 2 years).583,584 A recent, prospective, randomized, surgical AF ablation study reported no improvement of outcomes by ablation of the autonomic ganglia.123 Again, anchoring the focal ablation of a GP to other nonconducting tissue produced by PVI or anatomic structures remains critical to prevent subsequent ATs.
A survey of the writing group showed that 7% of the writing group members routinely employ ablation of autonomic ganglia during initial or repeat ablation procedures in patients with PAF, and 7% do so during initial or repeat ablation of persistent and long-standing persistent AF. Based on this information and a review of the literature, the writing group recognizes that usefulness of ablation of autonomic ganglia as an initial or repeat ablation strategy for paroxysmal, persistent, and long-standing persistent AF is not well established (Class IIb, LOE B-NR, Table 3).
Dominant Frequency Mapping
An emergent property of the complex spatiotemporal dynamics is that during AF, the local cycle length (atrial fibrillation cycle length [AFCL]) varies depending on electrode location, with the shortest AFCLs usually localized in the LA.587,588 The combined use of phase mapping589 and DF mapping demonstrated that the highest DF corresponded with the location of rotational activity that was driving the arrhythmia.590,591 A subsequent study in patients with paroxysmal or persistent AF showed that ablation of PVs harboring high DF sites resulted in an increase in the AFCL (≥5 ms) within the CS in 89% of cases.98 Arrhythmia termination occurred during ablation in 15 of 17 patients (88%) with PAF, but in none with permanent AF. In 87% of patients with PAF, ablation at a high DF site terminated the arrhythmia. Subsequent studies supported the notion that the high DF (DFmax) sites play a role in the maintenance of AF in a significant number of patients.592,593
Based on these mechanistic studies, a small trial of 50 patients with paroxysmal and persistent AF was performed, combining PVI with ablation of DFmax sites. At a mean of 9.3 ± 5.4 months, freedom from AF after one or more ablation procedures was achieved in 88% and 56% of paroxysmal and persistent AF patients, respectively.593 A more recent prospective randomized clinical trial of 232 patients with paroxysmal and persistent AF reported no improvement in ablation outcomes with a DF-based approach compared with PVI alone.594 None of the writing group members incorporate DF mapping as a routine AF ablation strategy in initial or repeat ablation of PAF. One writing group member (2%) incorporates a DF-based approach during initial and repeat ablation of persistent and long-standing persistent AF. Based on this information and a review of the literature, the writing group recognizes that a DF-based ablation strategy is of unknown usefulness for AF ablation (Class IIb, LOE C-LD, Table 3).
Renal Denervation
Arterial hypertension (AH) is the most frequent comorbidity in patients with AF, and this condition is also an important risk factor for the triggering and maintenance of AF. The potential antiarrhythmic role of renal denervation was demonstrated in animal studies suggesting a beneficial effect on AF inducibility, maintenance, and progression.307,595,596,597 The positive impact of renal denervation on AF recurrence was demonstrated in a first-in human study, including 27 patients with paroxysmal or persistent AF and refractory hypertension. At 12-month follow up, the group of patients who underwent PVI plus renal denervation had a significantly higher success rate in terms of freedom from AF compared with PVI alone (69% vs 29%, respectively). Also, the reduction in BP was much more significant in the PVI-plus-renal-denervation group.331 Recently, data were reported from a combined analysis of two randomized studies with a large and diverse group of 80 patients with AF and hypertension. For the entire cohort, renal artery denervation significantly reduced the rate of AF recurrences; however, this result was more pronounced in patients with persistent AF and refractory hypertension.331 A case report using renal denervation instead of PVI in a patient with drug-refractory persistent AF was recently published, with no AF recurrence at 6-month follow-up. Moreover, the renal denervation resulted in a reduction of LA size.598 The mechanism by which renal denervation, when combined with PVI, can impact outcomes of AF ablation has not been well defined. One potential mechanism is through improved control of hypertension. An alternate mechanism is through a decrease of central sympathetic activity by renal denervation.599 The current body of evidence supporting a role of renal denervation in improving outcomes of AF ablation is extremely limited. This is an area in need of further investigation. At the present time, we do not advise renal denervation as a technique to improve outcomes of AF ablation outside of a clinical trial. This sentiment reflects not only the limited body of literature supporting this approach, but also the recent large prospective randomized SIMPLICITY HTN-3 trial that showed that renal artery denervation was safe, but was not effective in lowering hypertension.600
Epicardial Ablation of AF
More data concerning thoracoscopic epicardial ablation and combined epicardial-endocardial ablation procedures have been published since the last AF ablation consensus statement. In addition to a potentially more durable lesion set, other advantages of an epicardial approach include access to epicardial structures such as the ligament of Marshall and GP, management of the LAA, and avoidance of damaging collateral structures, such as the PN and esophagus.
To date, three randomized prospective trials have compared a video-assisted thoracoscopic surgical (VATS) approach to percutaneous endocardial catheter ablation for the treatment of patients with paroxysmal and non-PAF, most of whom had failed an initial catheter ablation.585,586,601,602 A meta-analysis of these and other observational studies demonstrated a significant improvement of arrhythmia-free survival for the VATS procedure (78.4 vs 53%; RR 1.54; 95% CI 1.50–2.14; I2 = 0%; P <.0001), with a clearer benefit for patients with persistent AF.603 Complications were three times more frequent in the VATS group, mostly due to pneumothorax and pleural effusion. VATS procedures can also provide a reasonable outcome for patients with large atria and long-standing persistent AF.604,605
Many observational studies have reported promising results for so-called hybrid ablation procedures, which combine elements of VATS and catheter ablation procedures; however, no randomized controlled trials (RCTs) have been performed to demonstrate effectiveness and safety relative to either procedure alone.606,607,608,609,610,611,612,613,614,615,616 Before these approaches are applied more widely, a streamlining of the workflow of the dual procedure approach will be required.
Nonablative Strategies to Improve Outcomes of AF Ablation
AAD Therapy
There is evidence that reverse remodeling occurs after cardioversion of AF. The evidence in support of this conclusion is based on changes in atrial APD, ERP, CV, and P wave duration when measured 1–4 weeks after achieving sinus rhythm.617,618,619,620 The changes in P wave duration occur more slowly and do not predict long-term maintenance of sinus rhythm.619 An important question is whether AAD therapy can be used to prevent or initiate reverse remodeling even prior to ablation, thereby improving the success rate for ablation procedures. AAD therapy would be beneficial if it prevented electrical and structural remodeling by reducing the burden of AF, but it might also affect the therapeutic endpoints of conduction into or out of the PVs and the automaticity of PV or non-PV triggers of AF. The effect of drugs on mapping putative rotational activity or repetitive wavelets in humans with AF is unknown.
Four trials have evaluated the effect of preprocedural amiodarone or dofetilide therapy on outcomes of ablation. One study reported that the recurrence rate of AF following ablation was lower when treatment with dofetilide prior to ablation reduced the burden of AF, and was associated with a decrease in P wave duration.617,618 Another single-center nonrandomized study found that amiodarone prolonged the cycle length of AF and reduced the time spent on CFAE ablation, but it did not have any effect on long-term outcomes.619 A second single-center study treated patients with Class I or III drugs for long-standing persistent AF and compared the outcomes of ablation in those who were successfully converted to sinus rhythm months prior to ablation as opposed to those who were not. They observed decreases in LA dimensions (LADs) in the group that achieved preprocedural sinus rhythm. Long-term freedom from recurrent atrial arrhythmias was higher in the group that achieved sinus rhythm prior to ablation.620 The multicenter randomized Effect of Amiodarone on the Procedure Outcome in Long-standing persistent AF Undergoing PV Antral Isolation (SPECULATE) trial studied the effect of preprocedural interventions in patients with long-standing persistent AF. Termination of AF was more common in the patients treated with amiodarone, and fewer non-PV triggers were identified. At 6 months, both groups had similar recurrence rates; however, at 12 months, arrhythmia-free survival was higher in the patients who were not treated with amiodarone. A significant limitation of the SPECULATE trial is that many patients who were assigned treatment with amiodarone remained in AF; thus, there was no opportunity for remodeling to occur.621
From a practical standpoint, patients often require AAD therapy prior to ablation to reduce the burden of symptomatic AF. Whether this delays the progression of remodeling or reverses it is not well established, although there is evidence from these studies that conversion to sinus rhythm affects the atrial electrophysiological properties. Studies that showed improved outcomes in patients who regained sinus rhythm might be interpreted to show that remodeling occurs and is beneficial, but the alternative interpretation could be that patients who regain sinus rhythm have less advanced disease and are better candidates for ablation. The data also suggest that amiodarone affects the cycle length of AF, has increased conversion rates to sinus during ablation, and might mask triggers of AF, which could adversely affect the success of the procedure.
Based on this information, the published literature, and the experience of this writing group, the writing group recognizes that the usefulness of discontinuation of AAD therapy prior to AF ablation in an effort to improve long-term outcomes is unclear (Class IIb, LOE C-LD, Table 3). The writing group also recognizes that the usefulness of initiation or discontinuation of AAD therapy during the postablation healing phase in an effort to improve long-term outcomes is unclear (Class IIb, LOE C-LD, Table 3).
Risk Factor Modification
In Section 3 of this document, we have summarized the emerging data supporting risk factor modification as an approach to improve outcomes of AF ablation. Based on this information, the published literature, and the experience of this writing group, for patients with AF, including those who are being evaluated to undergo an AF ablation procedure, it is felt that that weight loss can be useful as part of a comprehensive risk factor management strategy (Class IIa, LOE B-R, Table 3). The writing group also recommends that it is reasonable to screen for signs and symptoms of sleep apnea when evaluating a patient for an AF ablation procedure, and recommends a sleep evaluation if sleep apnea is suspected (Class IIa, LOE B-R). And finally, the writing group recommends that the treatment of sleep apnea can be useful for patients with AF, including those who are being evaluated to undergo an AF ablation procedure (Class IIa, LOE B-R, Table 3).
Mechanisms of Nonisthmus-Dependent Atrial Flutter and Approaches to Mapping and Ablation
The occurrence of AFL after AF ablation is common enough that all operators performing AF ablation should be skilled in mapping and ablating both typical and atypical AFL. The incidence of AFL after AF ablation depends on the type of ablation performed during the initial procedure, varying from 2.6% in centers performing PVI alone to 31% in centers performing linear ablation.622,623,624,625 AFL is less common after PVI with CB compared with RF-based PVI.489 The mechanism of AFL is reentry. Reentrant arrhythmias include focal reentry occurring through gaps in the prior PVI line (PV tachycardia) or macroreentry around anatomic obstacles created during ablation (mitral annular flutter, LA roof-dependent flutter, or septal flutters around areas of scar).447,506,626,627 Typical cavotricuspid isthmus-dependent flutter can also occur (Figure 5).
Determining the location of the reentry circuit starts with examination of the 12-lead ECG. A clear isoelectric line in all 12 leads suggests a focal AT involving a small reentrant circuit (e.g., microreentry), whereas continuous activation suggests a macroreentrant flutter involving a larger circuit.628 Typical cavotricuspid isthmus-dependent flutter frequently has an atypical appearance on the 12-lead ECG after prior LA ablation, and this should not be excluded based on an atypical ECG appearance alone.629 Although extensive LA ablation can limit interpretation of the 12-lead ECG, certain rules apply. Tachycardias arising near the PV ostia will typically have an inferior axis and positive F waves across the precordial leads. An “m”-shaped F-wave in lead V1 suggests a left PV exit. Right PV tachycardias are characterized by the ECG amplitude in lead II greater than that in lead III and a positive F wave in lead I. Mitral annular flutter has a similar appearance to left PV tachycardias, although an initial negative component in the precordial leads or amplitude in lead V2 less than that in V1 and V3 might be suggestive.630 These rules, however, could be less applicable in patients with extensive scarring, including that from prior ablations.
Initial localization of an atypical flutter can frequently be facilitated by simply observing the activation sequence on a CS catheter. Proximal to distal activation suggests cavotricuspid isthmus-dependent flutter, right PV tachycardia, or counterclockwise mitral annular flutter, whereas distal to proximal activation suggests a left PV tachycardia or clockwise mitral flutter. An “on time” or fused CS pattern could be suggestive of a roof-dependent flutter.631 When acquiring an electroanatomical activation map, each acquired point should be carefully annotated by the operator, taking care to tag and not include widely split potentials that might indicate a line of block. Multipoint mapping can decrease the time needed for acquiring a map, but automated electrogram annotation might lead to errors and confusing maps. A novel very automated high-density automated mapping system has recently become available.627,632 Early results using this system to determine activation sequences and perform ablation based solely on activation rather than entrainment mapping have been encouraging.627 However, the true clinical value of this type of system is unknown and will require a prospective randomized comparison with conventional electroanatomical and entrainment mapping. When performing ablation of AFL with a conventional 3D mapping system, scar should be labeled as such to identify anatomic obstacles. Focal microreentrant tachycardias can also occur and are typically indicated by centrifugal conduction away from a focal area of onset and by long fractionated potentials with duration >50% of the tachycardia cycle-length.628 The main strength of activation mapping is that it is unlikely that the tachycardia will terminate. The disadvantage is that these activation maps can be extremely difficult to interpret and might not translate to identifying successful ablation sites. When performing ablation of atypical AFL with a conventional 3D mapping system, especially when a stable reentrant circuit is present that allows entrainment, most operators find that entrainment mapping from multiple sites is a better and more accurate approach to localize the reentrant circuit and target ablation lesions. It is for this reason that entrainment mapping is the gold standard for mapping reentrant tachycardias and is the preferred mapping strategy employed by most writing group members at the present time. For atypical flutter, because fusion of the F wave can be difficult to interpret, the primary goal is to identify regions with a postpacing interval within 20 ms of the tachycardia cycle length. Care should be taken to pace at or near threshold, given high-output pacing can capture adjacent tissue that leads to an erroneous postpacing interval. High-output pacing can also lead to electrode polarization that obscures the return electrogram. Once the reentry circuit is delineated, an ablation strategy can be designed to connect anatomic obstacles and interrupt the tachycardia. Despite this current preference, new high-density automated mapping systems have been developed, as noted above, which allow development of successful ablation strategies based on high-density activation mapping alone, without the risk of entrainment pacing resulting in termination of the flutter under study or its degeneration into a different flutter or fibrillation. Prospective randomized clinical trials will need to be performed to determine the true clinical value of these new automated high-density mapping systems.627,632
For PV tachycardias, 2 gaps in the PVI line are typically present and reisolation of the PVs suffices to eliminate the tachycardia.447 For macroreentrant tachycardias, ablation that connects anatomic obstacles is required. The classic post-PVI macroreentrant tachycardia is mitral annular flutter; ablation between the mitral annulus and left lower PV (mitral isthmus) is typically performed (Figure 5), although an anterior line between the mitral annulus and the LSPV, the RSPV, or the roof line can also be performed. For mitral isthmus ablation, epicardial ablation within the CS is required approximately 80% of the time. The endpoint of linear ablation should be proof of bidirectional block using pacing maneuvers rather than simply tachycardia termination. Interruption of the clinical tachycardia should be performed first, because burst pacing might induce multiple tachycardias of unclear significance. After termination of the clinical tachycardia, reisolation of any reconnected PVs should always be performed.
Anesthesia During AF Ablation
The type of anesthesia used for AF ablation depends in part on the availability of anesthesia support for ablation procedures. Given the need to minimize patient movement to improve catheter and mapping system stability, deep sedation or general anesthesia is generally preferred. One prospective randomized clinical trial randomized patients with general anesthesia or conscious sedation. This study reported that use of general anesthesia increased the single procedure success rate, lowered the prevalence of PV reconnection among those who needed a redo procedure, and shortened fluoroscopy time and procedure time.633 Another nonrandomized clinical trial reported improved efficacy of AF ablation with use of jet ventilation.634 A survey of the writing group members performing AF ablations found that 73% use general anesthesia, 13% use deep sedation with an anesthesiologist, and 14% use moderate conscious sedation with an electrophysiology nurse. Jet ventilation was used by only 8%. The major reason cited for not using general anesthesia was lack of anesthesiologist availability. Some proponents of not employing general anesthesia believe that the risk of an atrial esophageal fistula (AEF) could be higher in patients in whom general anesthesia is employed.635,636,637,638,639
Recurrent AF with or without PV Reconnection
Some degree of PV reconnection is observed in more than 80% of patients who are returned to the electrophysiology laboratory for a clinically indicated electrophysiology procedure. PV reconnection is also observed in patients doing well post-PVI. The Gap-AF trial reported PV reconnection at 3 months in 70% of patients randomized to complete PVI and in 89% of patients in whom a PV “gap” was left intentionally. AF recurred during the first 3 months postablation in 62% of the patients with complete PVI vs 79% of the patients in whom a gap was left intentionally.456 When reconnection of the PVs is observed, it is recommended that the PVs be reisolated. This can be accomplished by a limited approach, which involves only targeting those PVs that demonstrate reconnection, and only targeting the segment of the PV circumference in which the PV reconnection is detected. Among the writing group members, 73% employ this strategy. An alternate approach is to be more liberal with ablation, with creation of a new circumferential lesion set around each of the PVs, which demonstrates reconnection. This approach is employed by 20% of the writing group members. An even more liberal approach is to repeat the entire WACA lesion set that was delivered the first time; this approach is employed by the remaining 7% of the writing group members. In the small proportion of patients in whom no PV reconnection is observed, there is agreement that a number of non-PV-based strategies should be considered, including searching for non-PV triggers, delivery of one or more linear lesions, isolation of the CS, isolation of the LAA, ablation of autonomic ganglia, CFAE ablation, and rotational activity ablation. A recent report suggested that the best outcomes following ablation of non-PV triggers are achieved in patients with a well-defined provocable target.640 Each of these strategies has been described in detail in the rest of this document.
Endpoints for Ablation of Paroxysmal, Persistent, and Long-Standing Persistent AF
PVI is the cornerstone of AF ablation. Among the writing group members, 95% employ this endpoint during all AF ablation procedures. PVI is demonstrated by entrance block alone by 35%, and both entrance and exit block by 65%.
Beyond PVI, other endpoints, particularly during ablation for persistent AF, are unclear. It has been suggested that regardless of other non-PV targets ablated, the endpoint for ablation of persistent AF should be the termination of AF either to a regular ATA, or to a sinus rhythm. Although termination of AF has been shown by some to be predictive of longer-term outcome, other studies have not confirmed this finding.399,400,401,515,621,641,642 It is unclear whether acute, intraprocedural termination is a true indication of procedural success, or simply might indicate patients with less persistent AF who are destined to do better regardless of the approach used. A substudy of the STAR AF II trial has suggested this latter point.643 Slowing of AF cycle length as measured from the CS or the LA or RA appendage has also been used as a surrogate for acute procedural success. However, AF cycle length prolongation can be difficult to measure reliably in AF, and prolongation is often used as a harbinger of acute termination. Again, longer baseline AF cycle length can be an indication of AF that is more likely to terminate or respond to ablation rather than indicating a procedural endpoint in and of itself.643 Thus, AF termination of cycle length prolongation might not be useful as a sole procedural endpoint.
Other non-PV targets have been suggested for ablation, particularly for persistent AF. CFAEs have been put forward as an important target, although many recent randomized studies and meta-analyses have not concluded that there is any benefit.644 Ablation of non-PV focal triggers identified via isoproterenol challenge, ablation of atrial scarred regions, or ablation of localized rotational activations (so-called rotational activity) have also been reported to have benefit over PVI alone.140,534,538,645 It appears that regardless of which target is chosen, complete local elimination of the target should be the goal, so as not to leave behind partially ablated tissue that could serve as a site for future AT recurrence. The best method of ablating a localized rotational activation is as yet unclear. Early descriptions suggested ablating the center of activation with several lesions and then remapping to confirm that the rotation is terminated.563 Others have suggested that central ablation should be combined with creation of a short line to an anatomical or ablated boundary that crosses and interrupts the rotational pathway. The choice at this point is unclear. Similarly, for scar-based ablation, the best methods of defining scar are not yet confirmed (late gadolinium enhancement vs voltage mapping), and even for voltage mapping, the appropriate voltage cutoffs have not yet been validated. Furthermore, it is unclear whether such scar regions should be surrounded by lesions to isolate them from the rest of the atrium; whether ablation within the scar to eliminate all residual electrograms (so-called homogenization) should be employed; or whether these regions should also be tied to anatomical boundaries by short linear ablations. It follows from earlier comments that these scar ablations should be anchored to other nonconducting anatomical structures. There will need to be much further research into the best ablative endpoint for these ancillary targets. Empiric linear ablation likely does not add much to ablation of persistent AF.646,647 However, if linear ablation along the roof or mitral annulus is added to target roof or mitral-dependent flutters, then bidirectional block is a prerequisite endpoint. Block across a line must be assessed in sinus rhythm and with differential pacing maneuvers, and these are described in detail in the following section.
Section 6: Technology and Tools
In this section, we provide an update on many of the technologies and tools that are employed for AF ablation procedures. It is important to recognize that this is not a comprehensive listing and that new technologies, tools, and approaches are being developed. It is also important to recognize that RF energy is the dominant energy source available for ablation of typical and atypical AFL. Although cryoablation is a commonly employed tool for AF ablation, it is not well suited for ablation of typical or atypical AFL. Other energy sources and tools are available in some parts of the world and/or are in various stages of development and/or clinical investigation. Shown in Figure 9 are schematic drawings of AF ablation using point-by-point RF energy (Figure 9A) and AF ablation using the CB system (Figure 9B).
Figure 9.
Schematic drawing showing catheter ablation of atrial fibrillation using either RF energy or cryoballoon AF ablation. (A) Shows a typical wide area lesion set created using RF energy. Ablation lesions are delivered in a figure of eight pattern around the left and right PV veins. Also shown is a linear cavotricuspid isthmus lesion created for ablation of typical atrial flutter in a patient with a prior history of typical atrial flutter or inducible isthmus-dependent typical atrial flutter at the time of ablation. A multielectrode circular mapping catheter is positioned in the left inferior PV. (B) Shows an ablation procedure using the cryoballoon system. Ablation lesions have been created surrounding the right PVs, and the cryoballoon ablation catheter is positioned in the left superior PV. A through the lumen multielectrode circular mapping catheter is positioned in the left superior PV. Illustration: Tim Phelps © 2017 Johns Hopkins University, AAM.
Radiofrequency Energy
Biophysics and Irrigation
The presumed basis of successful AF ablation is production of myocardial lesions that block the propagation of rapidly firing PV triggers or modification of the arrhythmogenic substrate responsible for reentry. Successful ablation depends on achieving lesions that are reliably transmural.648,649 The conventional approach employed by cardiac electrophysiologists to reach the goal of AF ablation is RF energy delivery by way of a transvenous electrode catheter. RF energy achieves myocardial ablation by causing resistive heating of the tissue with subsequent heat conduction to deeper tissue layers. Most RF energy is delivered in a unipolar fashion between the tip of the ablation catheter and a large surface-area dispersive electrode applied to the patient's thorax or thigh. The position of the dispersive electrode does not greatly affect lesion size or geometry. If a high-power system is used, two dispersive electrodes should be employed to avoid skin burns. With bipolar RF delivery, there is no dispersive electrode, and both electrodes are active. One commercial system delivers RF energy simultaneously through multiple electrodes in a unipolar, blended, or bipolar fashion, using either continuous unipolar delivery with an offset of the phase of the RF wave between electrodes (phased RF delivery), or field sequential unipolar and bipolar delivery between contiguous electrodes in a prespecified ratio.650 Although bipolar ablation can be effective in heating tissue between contiguous electrodes, the lesions are not as deep as those using unipolar ablation.
Factors that will determine the size and depth of RF energy ablative lesions are power, impedance, temperature, duration, and CF.651,652,653 High-power delivery and good electrode–tissue contact promote the formation of larger lesions and improve procedure efficacy. However, if the temperature of the electrode–tissue interface exceeds 100 °C, then blood will boil and the blood proteins will form char and coagulum. As coagulum adheres to the electrode, less surface area is available for electrical conduction and the current density rises, resulting in more tissue and blood heating in a positive feedback spiral leading to a rapid rise in electrical impedance. Higher power delivery can be achieved with saline-irrigated tip catheters that cool the endocardial surface and prevent char and impedance rise. Increased convective cooling can also be achieved passively by using electrode material with high thermal conductivity, such as gold.653 The higher power delivery achieved with tip irrigation results in greater depth of resistive heating, with significant increase in lesion size. If intramural temperatures exceed 100 °C, steam expansion can suddenly vent through the endocardium or epicardium (pop lesion) and potentially cause a perforation.654 Because of more reliable creation of transmural lesions, and reduced risk of formation of endocardial thrombus and char, AF ablation with RF catheters is most commonly performed with tip irrigation. Optimal catheter–tissue contact is achieved by a combination of steerable catheter selection, guide sheath manipulation, operator skill, and monitoring catheter–tissue CF.655 Significant complications can occur during AF ablation if high RF power is administered in an uncontrolled fashion. The increased risk of AF ablation compared with ablation of other arrhythmias can be attributable to the great surface area of tissue ablated, the large cumulative energy delivery, the risk of systemic thromboembolism, and the close location of structures susceptible to collateral injury, such as the PN, PVs, and esophagus. Thrombus and char can be minimized by limiting power and/or target temperature by monitoring the production of steam microbubbles at the catheter tip with ICE, and by cooling the electrode–tissue interface with saline-irrigated tips.656,657,658,659 Intramural steam pops can be reduced by limiting both power and the electrode–tissue contact pressure. Duration of energy delivery affects the tissue temperature profile. The half-time of lesion growth is approximately 5–15 seconds, depending on the power used; thus, maximum lesion size is usually achieved within 1 minute. A long ablation duration will allow the heat generated in the region of resistive heating to conduct to deeper tissue layers, with maximum lesion size being achieved when the system has reached steady state. A short duration will yield maximal heating close to the source, with a steep drop in temperature in deeper layers, and might be preferred when ablating thinner regions such as the posterior LA when heating of contiguous structures (esophagus) needs to be avoided.
Immediately postablation, lesions show typical coagulation necrosis, hemorrhage, and edema. Subacute lesions examined 2–7 days later show infiltration of inflammatory cells, and early chronic lesions show replacement of myocardium with granulation tissue at 4 weeks.660 Myocardium exposed to temperatures of 50 °C or higher for more than several seconds will show irreversible coagulation necrosis and evolve into nonconducting myocardial scar.652 The mechanism of acute injury to myocardium is attributed to thermal injury to the sarcolemmal membrane with resultant depolarization and intracellular calcium overload.661,662 In the border zone region of lesion formation, myocytes can become inactive or dormant, but then subsequently reestablish a normal resting membrane potential and normal electrical conduction. These dormant zones can be reactivated by the hyperpolarizing effects of adenosine.465 Conversely, the inflammatory response to the acute injury and damage to the microvasculature can lead to lesion progression.
Various techniques have been proposed to minimize collateral injury. Temperature sensors at the electrode catheter tip can provide gross feedback of surface temperature, but because of passive convective cooling from circulating blood flow or active cooling in a cooled tip catheter, temperatures measured at the catheter tip significantly underestimate peak tissue temperatures. Limiting power and shortening duration of energy delivery will limit collateral injury, but at the expense of reliably creating transmural lesions. ICE has been used to monitor lesion formation. If the tissue shows evidence of increased echogenicity, or if small gas bubbles are observed, then power should be reduced or terminated.663,664
Contact Force-Sensing Catheters and Systems
Contact Force
During RF catheter ablation, electrode–tissue CF is one of the primary determinants of lesion size.636,665,666,667 No effective lesion is formed without adequate CF, and excessive CF is associated with excessive deep tissue heating and an increased risk of deep steam pop (and perforation) and injury outside the heart, such as esophageal, pulmonary, and PN injury.
Ablation catheters using two different technologies have been developed recently to measure real-time catheter–tissue CF during mapping and RF ablation. One catheter uses three optical fibers to measure the microdeformation of a deformable body in the catheter tip (TactiCath, St. Jude Medical, Inc.), which correlates with tip force.668,669,670 The second catheter uses a small spring between the ablation tip electrode and the catheter shaft, with a tiny magnetic transmitter in the tip and magnetic sensors proximal to the tip to measure microdeflection of the spring (ThermoCool SmartTouch, Biosense Webster, Inc.), corresponding to tip force.671,672,673 Both systems have high resolution (<1 gram) in bench testing and accurately display the direction of force. These two catheters, equipped with saline-irrigated tip electrodes, underwent extensive preclinical studies and were introduced for clinical use, beginning in 2010. The surrogate measures of contact used previously, including the fluoroscopic appearance of catheter motion, intracardiac electrogram amplitude, and impedance, have been found to be very poor predictors of CF.668,670,671,672,674
Preclinical experimental studies have shown that (1) at constant RF power and application time, RF lesion size significantly increases with increasing CF; (2) the incidence of steam pop and thrombus also increase with increasing CF; and (3) modulating RF power based on CF (e.g., high RF power at low CF and lower RF power at high CF) results in a similar and predictable RF lesion size.668,670,672
AF ablation studies using CFS catheters have provided important insight into the spatial distribution of CF during PVI. When the operator was blinded to CF measurements during catheter manipulation and ablation, sites of high CF were identified to be the RS aspect of the anterior LA wall, the posterior antrum of the RSPV, the inferior posterior LA wall, the posterior antrum of the LSPV, and the LA roof.671 Sites of low CF have been identified to be anterior to the left PVs and right carina.675,676,677 These low CF sites have correlated with sites of PV reconnection.677 CFS catheters provide operator feedback to allow for more homogeneous force delivery and reduce impedance rise, cardiac perforation, steam pops, and thrombus formation while at the same time improving effective lesion formation.453,668,671,676,678,679,680
Several clinical studies have compared circumferential antral PVI using the ThermoCool SmartTouch CFS catheter with ablation using a non-CFS catheter. The SmartTouch catheter has been shown to reduce gaps, prevalence of adenosine-induced dormant conduction, fluoroscopy time, and AF recurrence.468,673,679,681,682,683 One of the largest studies was a retrospective, case-control study of 600 patients followed for mean duration of 11.4 ± 4.7 months. The use of the SmartTouch CFS catheter predicted freedom from ATA in patients with PAF (hazard ratio [HR] 2.24; 95% CI 1.29–3.90; P = .004), but not in those with non-PAF (HR 0.73; 95% CI 0.41–1.30; P = .289). These findings could be due to differences in the AF substrate in which recurrence in patients with PAF is attributed to gaps in lesion sets rather than advanced AF substrate, and CFS improves lesion formation. There was no difference in complication rate between the CFS and non-CFS catheters. Another evaluation of the SmartTouch catheter was the ThermoCool SmartTouch Catheter for the Treatment of Symptomatic Paroxysmal Atrial Fibrillation (SMART-AF) trial, which was a multicenter, prospective, randomized clinical trial performed for FDA approval of this catheter.673 The outcomes were compared with the earlier clinical trial for ThermoCool approval,684 in which 170 patients were enrolled. The 12-month freedom from AF/AT/AFL was 72.5% at 1-year follow-up, compared with 66% efficacy for the ThermoCool noncontact-force catheter.684 The average CF per procedure was 18 grams. Four patients experienced cardiac tamponade. A post hoc analysis revealed that when the CF employed was between the investigator-selected working ranges >80% of the time, outcomes were 4.25 times more likely to be successful.673 The most recent study to evaluate the efficacy of CF catheters randomized 117 patients with PAF to AF ablation with the Smart-Touch Catheter. Patients were randomized to having the CF information available or not available to the operator. The availability of CF information resulted in a lower incidence of acute reconnection (22% vs 32%); however, there was no difference in long-term efficacy, fluoroscopy times, or complications.685
The efficacy of the TactiCath CFS catheter for AF ablation has been evaluated in a number of clinical trials, one of which resulted in FDA approval of this device. The TOCCATA study enrolled 35 patients with PAF and demonstrated that CF predicted freedom from AF postablation.676 All the patients in whom the average ablation CF was less than 10 grams (n = 5) had recurrent AF by 1-year follow-up; whereas, 80% of the patients (n = 8 of 10) were free from AF at 1 year when the average CF was greater than 20 grams. The EFFICAS I study enrolled 46 patients with PAF and correlated CF with incidence of gaps in PVI lines 3 months after the initial PVI procedure.453 The number of ablation lesions, minimum CF, and minimum force time integral <400 grams were highly predictive of gap presence and PV reconnection 3 months postablation.
A small study enrolling 6 patients used late gadolinium-enhanced (LGE) cardiac MRI to assess scar formation 3 months postablation following PVI. Increasing force-time integral (FTI) correlated with increased LGE signal intensity.680 This was particularly so when the FTI was >1200 grams. Segments with FTI <1200 grams showed less scar formation 3 months postablation. In addition to being able to provide feedback to improve durability of AF ablation lesions sets, the TactiCath catheter has been shown to decrease fluoroscopy time and reduce the number of RF applications for PVI compared with a non-CFS catheter.686 The most recent and most important clinical trial of the TactiCath catheter was the TOCCASTAR clinical trial performed for FDA approval of this device.655 In this prospective, randomized clinical trial, 300 patients with PAF were randomized to ablation with the TactiCath catheter or to the non-CFS ThermoCool ablation catheter. No difference in efficacy was observed, with success rates of 67.8% and 69.4% in the CF and control arms, respectively. When the CF arm was stratified into optimal CF and nonoptimal CF groups, effectiveness was achieved in 75.9% vs 58.1%, respectively. There was no difference in the rate of complications in the two groups. Cardiac tamponade occurred in one patient in each group.
Despite the absence of prospective clinical trials that have proven that CF monitoring improves not only the efficacy but the safety of AF ablation, operators worldwide have quickly adopted these new advanced ablation tools. Many of these operators who employ CFS during AF ablation believe that CF monitoring provides important biomechanical feedback to improve effective lesion formation, durability of PVI, and clinical outcomes. Future systems combining CF, RF power, and application time (such as the Force-Power-Time Index) could provide real-time assessment of lesion formation to increase the efficacy and safety of RF ablation. It should be remembered that CF is only one of the surrogate markers of ablative energy delivery. Power, impedance, temperature, and other factors remain in place and interact with the CF measurements. Several systems now employ a monitoring system that uses an integral of two or more of these factors. It is also possible that when higher CFs are realized, the power of RF delivery might need to be reduced. Achieving an adequate CF does not eliminate the user's responsibility to maintain awareness of other factors, such as power or other matrix components of ablation.
A survey of the writing group shows that among those who perform AF ablation with RF energy, 70% routinely use CFS. Two-thirds of the writing group members allow at least 20 seconds at a given ablation site to elapse before moving the ablation catheter to a new site. The target minimal CF used by the writing group members is > 5 grams by 28%, >10 grams by 62%, >15 grams by 8%, and >20 grams by 3%. A CF upper limit of ≤ 30 grams is employed by 48% of the writing group members, less than 40 grams by 36%, and <50 grams by 15%. As noted in Section 5 and Table 3, the writing group recommends that when performing AF ablation with a force-sensing RF catheter, that a starting point minimum targeted CF of 5 to 10 grams is reasonable (Class IIa, LOE C-LD, Table 3).
Cryoablation
In recent years, CBA has become the most efficient alternative to RF catheter ablation (RFCA) for the treatment of AF (Figure 9B).
The CB single shot ablation approach to AF has been designed to shorten and simplify the ablation procedure for achieving an effective PVI. Preclinical and clinical studies have shown that CB is effective in achieving PVI, offering a valid alternative to RF's point-by-point approach to PAF treatment.494,687,688 The multicenter, prospective randomized controlled Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) trial has reported that PVI with the first-generation CB achieved 69.9% freedom from AF at 12 months compared with 7.3% with AADs.462 More recently, the second-generation CB has become available to overcome some of the limitations of the first CB generation.689 These improvements include expansion of the cooling zone from the equatorial surface of the balloon to the entire distal half, leading to a more uniform circumferential ablation.473,484,690,691,692 Composite circumferential lesion size could be a second factor in this process.
In a prospective, multicenter registry, there was no difference between conventional RFCA and CB in terms of acute success rate and overall complications; however, fluoroscopy times were longer in CBA procedures.693 Many studies (mostly nonrandomized) showed that CBA yields similar or higher success rates in comparison to RF in patients presenting with drug-resistant PAF, and that the procedure is somewhat less time-consuming and might be associated with a safer profile.482,694,695,696 More recently, the multicenter randomized clinical trial FIRE AND ICE, comparing conventional PVI with RFCA (force-sensing catheters were used in approximately 25% of the procedures) to CBA (CB-2 in approximately 75% of the procedures) in drug-refractory PAF has shown that CBA is noninferior to RFCA with respect to efficacy and overall safety.489,697 More specifically, the primary efficacy endpoint (any atrial arrhythmia recurrence, use of AADs, or repeat ablation at 1 year) was not different between the CBA and RFCA groups; neither was the primary safety endpoint. PN injury at the time of discharge was the most frequent adverse event reported in the CBA group (2.7%), but lower than what was observed in the STOP-AF trial (13.5%). Permanent PN injury occurred in 0.3% of patients. A recent analysis of the secondary endpoints of FIRE AND ICE has shown that CBA had significantly fewer repeat ablations, direct current cardioversions, all-cause rehospitalizations, and cardiovascular rehospitalization during the follow-up compared with RFCA, with a similar improvement in QOL.490
A recent trial reported their pivotal findings on CBA as first-line therapy in a selected population suffering from PAF; the authors suggest that it might be appropriate to consider CBA therapy for PVI as first-line therapy in patients with PAF in the absence of significant structural heart disease.698 Yet, the decision whether to perform a catheter-based intervention in a symptomatic patient still takes into account the stage of atrial disease, the presence and severity of any underlying cardiovascular disease, potential treatment alternatives, and—in particular—patient preference and operator experience.7 Cryoballoon AF ablation requires a shorter learning curve than point-by-point RFCA. The results of the currently ongoing Catheter Cryoablation vs Antiarrhythmic Drug as First-Line Therapy of Paroxysmal AF (Cryo-FIRST) trial (NCT01803438), expected in 2017, will help answer the question of whether to propose CBA as a first-line therapy in highly symptomatic patients with PAF.
Laser and Ultrasound Ablation Systems
A laser balloon ablation system transmits light energy through a balloon filled with deuterium oxide (D2O, or “heavy water”) to perform PVI.498,508,699,700,701 The unique part of this system is that the lumen of the catheter contains a fiber optic endoscope that allows PVI under direct visualization. The balloon is compliant, allowing a variable inflation diameter from 25–32 mm, depending on PV size, and is delivered via a 16 Fr outer-diameter steerable sheath. Once the balloon is inflated, the diode laser emits energy in a 30-degree arc of overlapping lesions that can be rotated around the circumference of the PV and tracked visually using special software. The power of the laser ablation energy can be titrated from 5.5 to 12 W, lasting for 20–30 seconds for each ablation lesion. Lower power is used when blood is present in the field of view or when the laser is overlying the posterior LA wall, and higher power is favored over remaining PV segments in order to achieve persistent PVI.502 The endoscope has a 115-degree field of view (partially blocked by the lesion generator), and the balloon catheter is then rotated to complete the isolation. Each PV is typically individually isolated, as opposed to the individual or pairwise isolation used during point-by-point RF ablation. A multicenter, prospective pivotal trial of the laser balloon for treating PAF found that freedom from AF after a single laser balloon ablation was noninferior, and nearly identical, to the success rate using irrigated RF ablation (61.1% laser vs 61.7% RF; P = .003 for noninferiority), with a similar safety profile.503 PN injury was more common using the laser balloon (3.5% vs 0.6%; P =.05), but PV stenosis was less common (0.0% vs 2.9%; P =.03). In a single-center randomized study, AF recurrence after ablation was similar using the laser balloon compared with the first-generation CB (27% vs 37%; P = .18).501,502 The laser balloon has been used commercially in Europe and has received FDA approval for use in the United States to treat patients with drug refractory recurrent symptomatic PAF.
A novel automated low-intensity collimated ultrasound ablation system is in development.702 This system uses low-intensity collimated ultrasound (LICU) to automatically create a 3D anatomical map of the LA. A graphical interface allows the operator to define a desired lesion set on the 3D map, then delivers computer-controlled LICU along the desired ablation path to create a contiguous lesion. The lesions are created without contacting the atrial wall and are calibrated with respect to detected tissue thickness. Ultrasonic power is varied along the ablation path to achieve transmurality while reducing the risk of damage to extracardiac structures. Animal studies show that PVs are electrically isolated with a single planned set of lesions. Initial phase clinical trials are being performed at this time, and the results are pending.
Other Balloon Technologies
Balloon-based ultrasound and RF ablation systems have also been developed for AF ablation.703,704,705 The first of these balloon ablation systems to be approved for clinical use in Europe was the focused ultrasound ablation system.703,704,705 Although this balloon-based ablation system was demonstrated to be effective, it was removed from the market because of a high incidence of AEFs, some of which resulted in patient death.
The hot balloon ablation catheter employs a compliant balloon filled with saline that is inflated to occlude the PV.706 A central electrode delivers RF energy to the saline in the balloon, and a unique mixing system creates turbulent flow, promoting uniform distribution of the heated saline throughout the balloon. The balloon surface directly heats the PV wall circumferentially. Tissue heating occurs through direct conductive heating.707 This technology has been used to successfully treat patients with PAF, with a reported 65% long-term single-procedure success rate without AADs. The main reported complication with this technology was PN palsy (3.4%) and PV stenosis (1.7%).708 A recent prospective multicenter clinical trial compared the outcomes of hot balloon ablation vs AAD therapy for PAF. Freedom from atrial arrhythmias was achieved at 12-month follow-up in 59% of patients undergoing ablation vs 4.7% with AAD therapy. Serious adverse events occurred in 10% of hot balloon patients. The incidence of PV stenosis was 5.2%, and the incidence of PN injury was 3.7%. The clinical availability of this ablation technology is limited at the present time.709
Multielectrode Circumferential Ablation Catheters
Currently, two multielectrode circumferential catheter systems are in clinical use: The PV ablation catheter (PVAC) and the nMarq system. From a historical standpoint, the first catheter using a circumferential multielectrode approach for sensing and simultaneous ablation with the same electrodes was the Mesh ablator.710 However, due to technical limitations of the system and poor clinical outcome, this system is no longer available. The nMARQ catheter is a combined decapolar irrigated ablation and mapping RF system.711,712,713,714 The catheter has a circular design and consists of 10 platinum-coated 3 mm electrodes with a 4 mm interelectrode spacing. Irrigation is performed via 10 holes in each electrode. RF ablation can be performed via all 10 electrodes simultaneously, up to a maximum of 25 W in unipolar and 15 W in bipolar mode. In an initial evaluation phase in smaller patient cohorts, a high acute success rate of PV disconnection was reported.715,716,717 A multicenter prospective registry reported data on approximately 180 consecutive patients with paroxysmal (140 patients) and persistent (40 patients) AF who underwent nMARQ AF ablation. Aside from a high acute success rate, acute complications and rate of AF relapse (e.g., 27% in the PAF group) were comparable to other ablation techniques after a mean follow-up of 13.9 months.718 Esophageal injury has been reported in several studies using the nMARQ AF ablation catheter.719,720 Comparing the intracardiac signals from the nMARQ to a standard “lasso”-like mapping resulted in both underestimation of PV potentials with the nMARQ postablation (which were still detectable in the lasso) and overestimation by sensing fragmented electrograms that could not be verified with a lasso mapping.721 This appears to be an area with a need for further investigation, because underestimation of remaining PV potentials can lead to a pseudo-high acute success rate but higher AF relapses during long-term follow-up. The nMARQ is not currently available for clinical use in the United States. A prospective clinical trial is underway to demonstrate the safety and efficacy of this ablation system, needed for FDA approval.722
The PVAC in its first version used 10 platinum-iridium electrodes in a circular fashion to deliver duty-cycled bipolar or unipolar phased RF energy via selected or even all electrodes (temperature-controlled and power-limited: 60 °C/10 W/60 second delivery time). Following initial, mostly single-center, experiences that demonstrated excellent clinical efficacy, several studies reported a high incidence of asymptomatic cerebral emboli (ACE) lesions after PVI with the PVAC compared with irrigated focal RF and CBA.650,723,724,725,726,727 These reports triggered an evaluation of the underlying mechanisms; the Endovascular Revascularization and Supervised Exercise Therapy in Patients with Peripheral Artery Disease and Intermittent Claudication trial subsequently demonstrated that through modifications in the catheter design, including the elimination of overlying pole 1 and 10 ablation, and protocol for use, it was possible to reduce the incidence of ACE lesions to 1.7%.722,727,728,729,730 These findings were confirmed in the PRECISION GOLD trial.731,732 This ablation system was not approved for clinical use by the FDA due in part to this initial safety signal and the occurrence of 4 strokes (2.9% of patients randomized to ablation) following ablation in the pivotal Tailored Treatment of Permanent Atrial Fibrillation study.733,734 An additional single-arm study to again elucidate the safety of PVAC technology, the Evaluation of Multielectrode Phased RF Technology in Persistent AF (NCT01693120), was subsequently launched but has recently been closed due to lack of enrollment. In summary, there is considerable reason and data to believe that PVAC technology in its redesigned format has achieved at least a similar safety profile as CB or irrigated tip catheter ablation. The relative efficacy of this ablation system compared with the CB system or point-by-point RF ablation will require an adequately powered prospective, randomized clinical trial.734 Several prospective and randomized data collections are starting, but results will not soon be available, including the Efficiency Study Evaluating the Use of the PVAC Catheter Technology for Performing Ablation in Patients with AF (CAPCOST) (NCT01562912) and PVAC GOLD Versus Irrigated RF Single Tip Catheter with Contact FORCE Ablation of the PVs for Treatment of Drug Refractory Symptomatic Paroxysmal and Persistent AF (GOLD FORCE) (NCT02463851).
Electroanatomical Mapping Systems
AF is a disease frequently progressing from paroxysmal to persistent AF. The mechanisms underlying the process of arrhythmia perpetuation are complex. Major contributions to the understanding of the initiating and perpetuating factors derive from mapping studies in both patients and in animal models of AF. Mapping and ablation of AF require accurate navigation in the LA within the context of the underlying microstructures and physiology of the electrogram formation. This can be obtained using standard fluoroscopy or, more commonly, with EAM systems that combine anatomical and electrical information by catheter sequential point-by-point or simultaneous multielectrode mapping, allowing an accurate anatomical reconstruction of the 3D shell of the targeted cardiac chamber. There are several different EAM systems that are widely used in clinical practice. The current generation of the CARTO mapping system (CARTO 3; Biosense Webster, Diamond Bar, CA, USA) relies both on a magnet-based system for accurate localization of dedicated mapping or ablation catheters and an electrical impedance-based system that allows for visualization of electrodes and shaft of various types of electrophysiological catheters. The second EAM system is the electrical mapping system EnSite NavX (current version, Velocity; St. Jude Medical, Minneapolis, MN, USA), which uses both voltage and impedance for localization of proprietary and nonproprietary diagnostic and ablation catheters. This system has now been modified to also provide magnetic-based navigation, which is anticipated to increase the precision of this system. Another 3D mapping system that has been developed is the 3D MediGuide system.735 This sensor-based electromagnetic navigation system allows real-time catheter tracking in the environment of prerecorded X-ray loops. This system has been shown to easily integrate into the workflow of a standard AF ablation and allows for high-quality catheter tracking. Studies have shown that this system is useful in reducing fluoroscopy exposure for patients and staff. A third EAM system that is available for clinical use is the magnetic electrical Rhythmia mapping system (Boston Scientific, Marlborough, MA, USA), which is an EAM system that allows for automated high-density mapping using a dedicated steerable 64-electrode mini basket catheter.627,632,736 This system has only become available in the past several years, and as a result, the experience with this system is limited. A recent report describes the value of this system for activation mapping and ablation of complex left AFLs.627
To further improve the anatomical accuracy of the maps, integration of 3D images obtained by CT or MRI and of images acquired with intracardiac ultrasound during the procedure has become available.737,738 Another approach involves use of 3D rotational angiography images that can be merged with live two-dimensional fluoroscopy.739 This approach is rarely used at the present time. It is important to recognize that CT and MRI are not real-time images, and that the accuracy of the use of multiple imaging modalities is dependent on the accuracy of the image fusion. This requires the matching of fiducial points from both image sets, and not simply their display in the same user interface space. In addition, the utility of multimodal approaches to ablation depends on the quality of electrogram acquisition and the registration of that physiology onto the 3D images. Again, as more electrodes are simultaneously used in mapping, synthesizing information from that number of electrograms might require the use of electrogram single processes made simpler by mathematical manipulations, such as fast Fourier transform, inverse solution, Hilbert, and phase transformations. The positive and negative benefits of these manipulations require increased understanding on the part of the user and do not excuse a clear understanding of the first principles of cardiac electrophysiology.
The use of these 3D mapping systems has been demonstrated to reduce fluoroscopy duration.740,741,742 However, several studies performed to define the clinical benefit of EAM systems have generated mixed results: whereas some studies have reported that use of these mapping systems with or without image integration improves the safety and efficacy of AF ablation, other studies have reported contradictory findings.743,744,745,746,747 Obviously, the use of 3D mapping systems will increase the cost of the procedure.
A survey of the writing group members showed that 93% routinely employ 3D EAM when performing AF ablation with RF energy.
Robotic and Magnetic Catheter Navigation
The concept of remote catheter navigation is appealing for the operator because these systems can reduce radiation exposure and the risk to the physician of developing orthopedic problems related to prolonged use of protective lead aprons during protracted cases. They also can facilitate analysis of intracardiac electrograms and 3D images because the catheter navigation and analysis can be performed from the workstation where the operator is seated. The four technologies developed to meet these objectives include the magnetic navigation system designed by Stereotaxis Inc; a second magnetic system referred to as the Catheter Guidance, Control, and Imaging (CGCI) system, for which there is limited experience; a third robotic-controlled catheter system manufactured by Hansen Medical; and the remote catheter system developed by Catheter Robotics.748,749,750,751 These technologies have been used to ablate AF, and there is evidence that they are safe, effective, and result in a significant reduction in fluoroscopy time and radiation exposure; however, the studies are relatively small, not randomized, and the populations of patients are not uniform.646,647,748,749,750,751,752,753,754,755,756,757,758,759 Use of these technologies is a matter of operator preference. The potential advantages of the systems are offset by additional costs for the navigation systems, disposables, and maintenance contracts. A survey of the writing group members shows that 10% routinely employ a robotic or magnetic system when performing AF ablation procedures.
Ultrasound
In the electrophysiology lab, ultrasound is a valuable tool, used both for guiding vascular access and during the procedure. With a linear probe, central venous access to the femoral, internal jugular, and subclavian veins can be obtained safely, reducing complications, the number of attempts, and the time required to gain access.760,761 The impact of real-time ultrasound guidance is even greater in obese patients, in procedures with less experienced operators, and in patients undergoing anticoagulation therapy.
ICE, which allows for real-time imaging of cardiac anatomy, is used in many electrophysiology laboratories throughout the world to facilitate AF ablation procedures.762,763,764,765,766,767 Advocates of the use of ICE find it to be of value because it can (1) help identify anatomical structures relevant to ablation, including the PVs and esophagus; (2) facilitate transseptal access and allow selective puncture in various regions of the fossa; (3) guide accurate placement of the multielectrode circular ablation catheter and/or balloon-based ablation system; (4) allow titration of the delivered energy; (5) provide feedback about catheter contact; (6) allow for recognition of thrombus formation on sheaths and catheters; and (7) allow early recognition of cardiac perforation and/or development of a pericardial effusion.767,768 Some centers also use ICE to screen for the presence of LAA thrombus, because it has been shown to be comparable to transesophageal echocardiogram (TEE) when performed by experienced operators. A survey of the writing group members shows that 53% of the members routinely employ ICE imaging during AF ablation. Our survey revealed that ICE was being used routinely by 87% of the writing group members in the United States and Canada compared with 13% of the writing group members from other countries. Among those who employ ICE imaging, 37% use ICE to screen for LA thrombi prior to performing the transseptal stick.
PV Venography
PV venography is commonly performed at the time of AF ablation procedures.769,770 The purpose of PV venography is to help guide catheter manipulation, determine the size and location of the PV ostia, and assess PV stenosis, particularly among patients undergoing repeat ablation procedures. Among the writing group members, 25% routinely use PV venography during their AF ablation procedures. There are three techniques that have been described for PV venography. The first technique involves selective delivery of contrast media into each of the PV ostia. This can be accomplished by positioning the transseptal sheath in the region of the right and left PV trunks and injecting contrast, or by selectively engaging each of the four PV ostia using a deflectable catheter or a multipurpose angiography catheter.769 A limitation of the selective PV venography approach is that noncatheterized PVs can be missed if a preacquired CT or MRI scan is not available to ensure that all the PVs are identified. The second technique is performed by injection of contrast medium into the left and right pulmonary arteries or the pulmonary trunk. The location of the PVs can then be assessed during the venous phase of pulmonary arteriography. The third technique involves the injection of contrast media in the body of the LA or at the roof of the right or left superior PV ostium immediately after delivery of a bolus of adenosine to induce AV block. The contrast media will fill the LA body, the PV antrum, and the proximal part of the PV during the phase of ventricular asystole.
CT and/or MRI Scans and Rotational Angiography to Define the Anatomy of the Atrium, PVs, and Antrum
The complex anatomy of the LA plays a major role in the pathophysiology of AF.771 A detailed understanding of this anatomy is essential for a safe and effective AF ablation procedure.772 There is a significant inter- and intrapatient variability in the number, size, and bifurcation of the PVs67,773,774,775,776,777,778 (Figure 2). Common variations include supernumerary right PVs (18%–29%) and common trunk (>30%), mainly located on the left side and right middle or right top PV.69,776 Knowledge of the presence of additional veins prevents placing ablation lesions over their ostia, which could result in PV occlusion, whereas knowledge of the bifurcation pattern is essential during CB PVI, in which wiring of various branches might be needed to ensure optimal occlusion.779 LA imaging facilitates ablation by providing detailed anatomical description of the PVs, antrum, and the remainder of the LA, enabling selection of the most suitable ablation technique prior to the procedure.772,780 During the procedure, integration of LA images obtained by CT or MRI reduces procedural time because it enables a more accurate reconstruction of the anatomy.41 However, this requires accurate registration. Prior to RF ablation, imaging of LA anatomy with either MRI or CT imaging is performed routinely by 59% of the writing group members. Prior to CB, AF ablation imaging of the LA anatomy with either MRI or CT imaging is performed routinely by 56% of the writing group members.
Another method of intraprocedural 3D imaging of the LA is rotational angiography. After contrast medium injection in the right heart chambers, the fluoroscopy c-arm is rapidly rotated around the patient, and images are acquired throughout the rotation to generate 3D volumetric anatomical rendering of the LA. These images can then be integrated into an EAM system or superimposed on the fluoroscopic projections of the heart.781,782 A survey of the writing group members shows that rotational angiography is routinely performed prior to AF ablation by 0% of the writing group members.
After the procedure, LA imaging is valuable in detection of postprocedural complications such as PV stenosis or AEF.783
MRI of Atrial Fibrosis and Ablation Lesions and MRI-Guided AF Ablation
AF is associated with various degrees of structural remodeling of the atrial myocardium.134,136,139,161,784 In the ventricular myocardium, MRI is an established modality to visualize myocardial inflammation and fibrous tissue by using LGE.134,560,561,785,786 However, high-resolution imaging of atrial fibrosis remains technically challenging, with limited reproducibility of accuracy of MRI measures of fibrosis by different centers.787 In a recent study, MRI data of 17% of patients were excluded due to poor quality.365 MRI may be performed before catheter ablation of AF to identify atrial fibrosis, or after ablation to visualize RF lesions.134,784,788,789 Several studies have demonstrated that the extent of atrial fibrosis evaluated by LGE prior to ablation can predict the outcomes of catheter ablation of AF.789 Other studies have reported contradictory results.790 In the multicenter prospective DECAAF trial, the extent of atrial fibrosis found on preablation MRI was categorized as stage 1 (<10%), stage 2 (10%–20%), stage 3 (20%–30%), and stage 4 (>30%). AF recurrence 325 days after ablation was independently associated with the extent of atrial fibrosis (15% for stage 1, 33% for stage 2, 46% for stage 3, and 51% for stage 4).365 These preliminary results suggest that the extent of fibrosis can be useful to predict arrhythmia recurrences and to guide the decision to perform catheter ablation in selected patients with AF. Studies evaluating whether LGE can visualize scar lesions induced by catheter ablation with RF cryoablation or laser ablation in atrial tissue, or identify PV reconnection sites have reported conflicting results.784,791 Overall, despite the promise of MRI techniques to improve the outcomes of AF ablation, further investigation is needed before advocating the systematic use to assist catheter ablation of AF. The DECAAF-2 study has just been launched for this purpose. This randomized, prospective, multicenter clinical trial is designed to test the hypothesis that PVI plus consolidation of fibrotic areas with RF ablation is superior to PVI alone.791 A survey of the writing group members shows that MRI for detection of scar is routinely performed prior to AF ablation by 8% of the writing group members.
During the past decade, a number of centers have developed the technology to allow real-time MR-guided electrophysiology intervention. Advantages of this approach include the absence of ionizing radiation and the ability to monitor lesion development in real time. Although these systems are still under development and are not available with routine clinical use at this time, this is an area of considerable interest that could emerge as an important ablation monitoring and guidance strategy in the future.792,793,794,795
Section 7: Technical Aspects of Ablation to Maximize Safety and Anticoagulation
Prevention of Thromboembolism During and Following AF Ablation
Patients with AF are at increased risk of thromboembolism during, immediately following, and for several days to months after their ablation.796,797,798,799 ACE lesions have also been observed after AF ablation.800 The prothrombotic state associated with AF ablation results in a higher, but transient, thromboembolism risk in patients with AF who were identified as low-risk before ablation. Careful attention to anticoagulation of patients before, during, and after ablation for AF is critical to avoid the occurrence of a thromboembolism event. Consensus recommendations for anticoagulation prior to, during, and following ablation are summarized in Table 4. The ablation procedure leaves patients with substantial areas of damaged LA endothelium that can become a nidus for thrombus formation. Transseptal sheath placement and insertion of electrode catheters can precipitate thrombus formation on the catheter or on or within the sheath during the procedure.768,801,802,803,804 The atrial tissue can be stunned for weeks or months postprocedure, leading to impairment of normal contraction.805 Anticoagulation, in turn, contributes to some of the most common complications of the procedure, including hemopericardium, pericardial tamponade, and vascular complications.806,807,808 Therefore, attention must be paid to achieving the optimal safe level of anticoagulation throughout the process.
Screening for LAA Thrombi Prior to Ablation
Transesophageal Echocardiography
Thromboembolic stroke after AF ablation is a devastating consequence of an invasive procedure. One of the mechanisms could be dislodgement of a pre-existing clot that could be identified by a screening TEE. The risk of a thromboembolic event at the time of an AF ablation procedure varies, depending on a number of factors, including (1) the type of AF; (2) the presence, absence, and duration of AF as the presenting rhythm on the day of ablation; and (3) the patient's stroke risk profile, including LA size and CHA2DS2-VASc score. With careful, multiplanar inspection of the LAA and the number of LAA lobes, the TEE can also provide additional information to help guide the procedure, such as identification of a pre-existing pericardial effusion, globally impaired cardiac function, presence of an atrial septal defect (ASD) or persistent foramen ovale, or fibrosis of the interatrial septum after previous ablation.809,810,811 In addition, LA anatomical features, such as a thickened ridge toward the left PVs, PV stenosis or occlusion, or cor triatriatum, can be identified.
Because many centers perform their procedures on uninterrupted OAC, one could argue that TEE is unnecessary; however, studies evaluating the incidence of LA thrombus on TEE among patients undergoing AF ablation who have been therapeutically anticoagulated have consistently demonstrated that 1.6% to 2.1% of patients will have a thrombus or “sludge” in the LAA.796,812,813 The probability of identifying a thrombus was related to the CHA2DS2-VASc score in some but not in every case. Other risk factors for thrombus were LA size and a history of persistent AF. Among patients with a CHA2DS2-VASc score of zero, a thrombus was identified in < 0.3% of patients compared with >5% of patients with a CHA2DS2-VASc score of ≥ 2. The practice of routine vs selective TEE surveillance for LAA or intracavitary thrombus prior to PVI varies widely, given evidence to guide this decision is limited in terms of important clinical outcomes.796,809,810,811,812,813,814
A survey of the writing group members shows that 51% perform a TEE in all patients presenting for AF ablation regardless of presenting rhythm and anticoagulation status. This survey also revealed that 71% of the writing group members perform a TEE in patients presenting AF who have been therapeutically anticoagulated for 3 or more weeks prior to ablation. Among patients who present for AF ablation in sinus rhythm who have not been previously anticoagulated, 78% of the writing group members routinely perform a TEE. Among patients presenting for AF ablation who are chronically anticoagulated with warfarin, 87% of the writing group members perform AF ablation on uninterrupted warfarin. Among patients undergoing AF ablation who are chronically maintained on a NOAC, 38% of the writing group members perform AF ablation on a patient receiving uninterrupted NOAC without withholding a dose. For patients not anticoagulated prior to ablation or in whom NOAC therapy is interrupted prior to ablation, 16% of the writing group members reinitiate the NOAC at 2 hours, 12% at 3 hours, 37% at 4 hours, and 35% at 4 or more hours after initially achieving hemostasis. It is important to recognize that this is a rapidly evolving area in AF ablation. The results of the above survey were obtained prior to publication of the results of the Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy (RE-CIRCUIT) trial, which demonstrated that performance of AF ablation on patients receiving uninterrupted dabigatran results in a lower rate of major bleeds compared with the uninterrupted warfarin strategy.815,841 Shown in Table 4 are the writing group recommendations concerning anticoagulation strategies prior to ablation. As with the anticoagulation guidelines for cardioversion of AF, if a thrombus is identified in the LAA prior to catheter ablation of AF, the AF ablation procedure should not be performed.
Computer Tomographic Angiography
Data are emerging to suggest that CT imaging can be valuable in detecting thrombi prior to an AF ablation procedure. Several studies have investigated whether CT imaging can be used to screen for LA thrombi, with the hope of obviating the need for a screening TEE in at-risk patients. Compared with TEE as a gold standard, several studies and one meta-analysis have reported a high diagnostic accuracy of CT to detect LAA thrombi.780,816,817 Other studies have reported lower diagnostic accuracy and high inter-reader variability in detecting LA thrombi with CT imaging.818,819 In a meta-analysis of studies using delayed imaging protocols, the diagnostic accuracy for detection of LAA thrombi was reported to be 99%.817 These findings suggest that cardiac CT (with an acceptable radiation dose) could be of value in detecting LA thrombi. It is important to note that in many centers the CT is obtained days to weeks prior to ablation, rendering this imaging modality of no value because of this time delay.
The writing group members believe that the data are currently insufficient to recommend widespread use of CT imaging as an alternative to TEE for preablation screening for LA thrombi. This sentiment reflects in large part a great variability in CT detector imaging equipment and protocols. Further large-scale studies will be required before CT imaging can be considered an alternative for TEE screening prior to AF ablation. A survey of the writing group members shows 49% of the members employ CT imaging on a routine basis prior to AF ablation. Among those who obtain CT imaging, 32% use the CT image to identify LAA thrombi.
Intracardiac Echocardiography
Data are also emerging to suggest that ICE can be valuable in detecting LAA thrombi prior to an AF ablation procedure. Imaging from the pulmonary artery is preferred. Whereas the ICE-CHIP study demonstrated that ICE imaging from the RA had reduced sensitivity in the detection of LA thrombi compared with standard TEE, other studies showed that ICE imaging from the pulmonary artery can be used safely and effectively (compared with TEE) for the evaluation of the LAA in patients undergoing ablation.768,820,821,822,823 Of interest, ICE has been shown to have complementary value in rescreening the LA and the LAA for thrombus after a recent negative or equivocal TEE.824 These findings suggest that ICE could be of value in detecting LA thrombi. However, the writing group members believe that the data are currently insufficient to recommend widespread use of ICE imaging as an alternative to TEE for preablation screening for LA thrombi. This sentiment reflects in large part a great variability in the skills needed to both perform and interpret the results of ICE imaging for thrombi detection. Further large-scale studies will be required before ICE imaging can be considered to be a standard and proven alternative for TEE screening prior to AF ablation. A survey of the writing group members shows that 53% of the members routinely employ ICE imaging during AF ablation. Our survey revealed that ICE was being used routinely by 87% of the writing group members in the United States and Canada compared with 13% of the writing group members from other countries. Among those who employ ICE imaging, 37% use ICE to screen for LA thrombi prior to performing the transseptal stick. Based on this information and a review of the literature, the writing group recommends that use of ICE to screen for atrial thrombi in patients who cannot undergo TEE imaging may be considered (Class IIb, LOE C-EO, Table 4).
Anticoagulation
Systemic Anticoagulation Prior to AF Ablation
Many patients who are undergoing AF ablation have an elevated risk of stroke as assessed using the CHA2DS2-VASc score and are therefore systemically anticoagulated with warfarin or with a direct thrombin or factor Xa inhibitor.825,826,827,828 Most operators initiate therapeutic anticoagulation for at least 3 weeks prior to ablation in patients with a CHA2DS2-VASc risk score of 2 or greater, especially if they are likely to present for the procedure in AF. Because of the slow offset and onset of warfarin, these patients were historically transitioned or “bridged” with heparin or low molecular weight heparin before and after the ablation procedure. An increased recognition of bleeding complications associated with this practice, especially at the site of vascular access, has led to the use of uninterrupted warfarin, which has been shown to have a better safety profile, provided the international normalized ratio (INR) is within the target range.399,400,401,532,533,829,830,831,832,833,834
Dabigatran and the factor Xa inhibitors (apixaban, rivaroxaban, edoxaban) have a more rapid onset of action, a shorter half-life, and a more predictable dose response compared with warfarin. Accumulating evidence and several meta-analyses have demonstrated similar efficacy and safety of dabigatran and the factor Xa inhibitors compared with warfarin in the setting of catheter ablation.835,836,837,838,839,840,841,842,843 These data provide reassurance; however, several methodological considerations warrant mention. In most of these studies, one or two doses of the NOACs were held prior to AF ablation. Nearly all of the included studies were observational in design, and are therefore subject to confounding and selection bias. The sample sizes of the individual treatment arms were small, and study heterogeneity precludes statistically robust comparisons. In addition, the study populations were predominantly male, largely characterized by normal renal function, and the mean patient age was 61 years, a decade younger than the stroke prevention trial populations.
The results of the RE-CIRCUIT study were recently published, which was a head-to-head comparison of performing AF ablation on patients receiving uninterrupted dabigatran vs uninterrupted warfarin.841 This study randomized 704 patients across 104 sites to these two anticoagulation strategies. The incidence of major bleeding events during and up to 8 weeks postablation among the 635 patients who underwent AF ablation was significantly lower with dabigatran than with warfarin (5 patients [1.6%] vs 22 patients [6.9%]); absolute risk difference [RD] −5.3%, RR reduction 77%. There were six patients with cardiac tamponade in the warfarin arm vs one in the dabigatran arm. No strokes or other thromboembolic events occurred in the dabigatran arm compared with one TIA in the warfarin arm. No patients in the dabigatran arm required the specific reversal agent idarucizumab. There has been one other smaller head-to-head comparison published of uninterrupted rivaroxaban vs uninterrupted warfarin (Venture-AF, N = 248).842 This study reported one major bleeding event, one ischemic stroke, and one vascular death, each of which occurred in the warfarin arm of the study. A third trial of apixaban vs coumadin is also underway (NCT02227550).
Based on these clinical trials, it is now apparent that a strategy of performing AF ablation on patients receiving uninterrupted anticoagulation can be performed safely and minimizes the risk of thromboembolic events. Specific recommendations for pre- and intraprocedure anticoagulation are shown in Table 4. Although further studies are needed to further define the efficacy and safety of performing AF ablation on uninterrupted Factor XA inhibitors or direct thrombin inhibitors, the writing committee believes that the data and worldwide experience are now sufficient to provide a Class I recommendation for performing AF ablation with uninterrupted dabigatran (Class I, LOE A) or rivaroxaban (Class I, LOE B-R), and a 2A recommendation for the other XA inhibitors for which specific clinical studies have not been performed at this time. Further studies are needed to determine if a TEE can be omitted in patients with a high stroke risk profile who present for ablation in AF and are undergoing ablation on uninterrupted anticoagulation. Data will also be needed on outcomes and use of specific reversal agents in this setting, particularly for management of serious procedural bleeding complications.844,845
Table 4 summarizes the recommendations for anticoagulation pre-, during, and post-AF ablation, both for warfarin and for the NOACs.
Intraprocedural Anticoagulation
Optimal anticoagulation using heparin with close attention to maintaining therapeutic dosing during the procedure is important. It is recommended that heparin be administered prior to or immediately following transseptal puncture during AF ablation procedures and adjusted to achieve and maintain a target activated clotting time (ACT) of 300 seconds or greater (Class I, LOE B-NR, Table 4). It has been observed that thrombi can form on the transseptal sheath and/or the electrode catheter almost immediately after crossing the septum and that early heparinization substantially decreases this risk.768,802,803,804,846,847,848 A recent meta-analysis of more than 7000 patients supports this recommendation, showing that performing ablation of AF with a target ACT >300 seconds decreases the risk of thromboembolic complications without increasing the risk of bleeding.849 Seventy-seven percent of the writing group members administer heparin prior to the transseptal puncture. A heparin loading dose should be administered initially, followed by a standard heparin infusion. The ACT level should be checked at 10–15 minute intervals until therapeutic anticoagulation is achieved, and then at 15–30 minute intervals for the duration of the procedure. Patients receiving a vitamin K antagonist (VKA) require less heparin and reach the target ACT faster compared with NOACs; thus, when using anticoagulation strategies with the latter, more frequent ACT monitoring and higher heparin doses should be used.840,849 This recent report from a large-volume medication center employs an initial heparin bolus of 50 units per kg in patients who are therapeutically anticoagulated with warfarin, 75 units per kg in patients who are not anticoagulated prior to ablation, and 120 units per kg for patients who are anticoagulated on a NOAC and have held one to two doses. A survey of the writing group showed great variability in loading protocols for heparin prior to an ablation procedure. The heparin dose should be adjusted to maintain an ACT of at least 300–350 seconds throughout the procedure. One-third of the writing group members routinely employ a target ACT of > 350 seconds.820,830,846 Heparinized saline should be infused continuously through each transseptal sheath to further reduce the risk of thrombi.802 The risk of systemic embolization of thrombus formed on a sheath can be reduced by withdrawing the sheath to the RA once a catheter is positioned in the LA. Heparin infusion can be discontinued once all catheters are removed from the LA, and the sheaths removed from the groin when the ACT is less than 200–250 seconds. Sheaths can be removed during full anticoagulation by employing a figure-of-eight suture.850 Alternatively, the heparin effect can be reversed with protamine (Class IIa, LOE B-NR, Table 4).851 This approach is used by 70% of the writing group members.
In the event of persistent bleeding or cardiac tamponade, protamine should be administered to reverse heparin. If bleeding resolves, then reversal of the oral anticoagulant is not recommended, because this continues to offer protection from thromboembolic complications postprocedure. However, if pericardial or other bleeding persists with the above measures, fresh frozen plasma can be administered for reversal of warfarin. Dabigatran can be reversed with idarucizumab.844 Development of a reversal agent for Factor Xa inhibitors is underway but is not yet available on a clinical basis.845 Until these agents are available, it is recommended that prothrombin complex concentrates (PCC: Factors II, VII, IX, and X) or recombinant activated factor VII (rFVIIa) be administered.852 The increasing availability of specific reversal agents for factor IIa and Xa inhibitors will certainly encourage the adoption of continuous anticoagulation with the newer oral anticoagulants during AF ablation.
Early Postprocedural Anticoagulation
There is a prothrombotic milieu following RF ablation for AF due to reduced contraction of the atria, endothelial damage from ablation lesions, and a thrombogenic state. Therefore, it is the consensus recommendation of the writing group members that patients should be anticoagulated for at least 2 months postablation, regardless of their CHA2DS2-VASc score or rhythm status (Class I, LOE C-EO, Table 4). In patients treated with warfarin who have a subtherapeutic INR the day of the procedure, there are two options. First, a direct thrombin or Factor Xa inhibitor can be administered several hours following ablation.826,827,853,854 Second, low molecular weight heparin (enoxaparin 0.5–1.0 mg per kg twice daily) or intravenous heparin can be used as a bridge to resumption of INR 2.0–3.0. For most patients, other than those with prosthetic valves who will need to remain indefinitely on warfarin, initiation of a NOAC postablation is a preferred strategy to use instead of heparin or low molecular weight heparin due to the increased bleeding risk with these agents.
It is expected that patients will have their sheaths removed immediately after ablation, either with or without the use of protamine to reverse the intravenous heparin used during the procedure. Hemostasis can be achieved by either direct pressure or the use of a figure-of-8 suture. Evidence for the safety of uninterrupted NOAC therapy has increased with the recent publication of Venture AF and RE-CIRCUIT.841,842 Despite these new data, some centers have the patient withhold one to two doses of NOACs in the days prior to their ablation procedure. For these patients, reinitiation of the NOAC should take place as soon as the clinician is satisfied that there is no significant pericardial effusion or vascular bleeding following the ablation. Similarly, for the small subset of low-risk patients who were not being treated with anticoagulation before the procedure, a NOAC can be administered immediately following ablation. The writing group members advise that readministration of a NOAC be given 3 to 5 hours after completion of the procedure and removal of the vascular sheaths, provided there is no evidence of ongoing bleeding, or a significant pericardial effusion or cardiac tamponade is reasonable (Class IIa, LOE C-EO, Table 4).
Anticoagulation Considerations Two or More Months Postablation
Whether elimination of AF or reduction of AF burden by catheter ablation results in a significant reduction in stroke risk is an important, and as yet unanswered, question. Until this important question is addressed by an adequately designed clinical trial, adherence to the AF anticoagulation guidelines is recommended for patients who have undergone AF ablation procedures, regardless of the apparent success or failure of the procedure (Class I, LOE C-EO, Table 4). The writing group advises that decisions regarding continuation of systemic anticoagulation more than 2 months postablation should be based on a patient's stroke risk profile and not on the apparent success or failure of the ablation procedure (Class I, LOE C-EO, Table 4). And finally, the writing group recommends that for patients in whom discontinuation of anticoagulation is being considered based on the patient's values and preferences, they should consider undergoing continuous or frequent ECG monitoring to screen for AF recurrence (Class IIb, LOE C-EO, Table 4). This recommendation is based on the following: (1) recurrences of AF are common both early and late following AF ablation; (2) asymptomatic AF is common, and is more common following AF ablation than prior to AF ablation; (3) AF ablation destroys a portion of the atria and the impact of this on stroke risk is uncertain; (4) there have been no large, randomized prospective trials that have assessed the safety of discontinuing anticoagulation in this patient population; (5) studies have shown that strokes in patients with AF might not be temporaneously related to an AF event855; and (6) the use of direct thrombin inhibitors or Factor Xa inhibitors, such as dabigatran, rivaroxaban, edoxaban and apixaban, is more convenient than warfarin.825,826,827,828
The small subset of writing group members who support the discontinuation of systemic anticoagulation in patients with an increased stroke risk profile make the argument that (1) continuing anticoagulation exposes patients to the risks for hemorrhage and the unfavorable effects of anticoagulation on long-term QOL; (2) several large outcome studies have reported a lower-than-expected stroke risk in patients who undergo AF ablation compared with control populations239; and (3) one center has reported a low stroke risk in patients postablation who screen for recurrence by pulse assessment or ECG monitoring.238,407,409,545,856,857,858,859,860
In considering these consensus recommendations, it is worth commenting that some patients who have multiple stroke risk factors are highly motivated to discontinue systemic anticoagulation and are willing to accept a possible increased risk of stroke. It is for these patients that we recommend that some type of continuous monitoring be performed to screen for silent AF at regular intervals as long as they remain untreated with systemic anticoagulation. A survey of the writing group members shows that 77% continue anticoagulation indefinitely in patients who have undergone AF ablation and who have a CHA2DS2-VASc score of 2 or greater. It is possible that the outcomes of the CABANA and Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST) (NCT01288352) will help clarify this issue. In selected patients with ECG, evidence of AF control, and diligent follow-up for AF recurrences, 23% of the writing group members indicated that they would consider discontinuing anticoagulation after a conversation with the patients in which risks and benefits were discussed. This survey also shows that only 1 writing committee member (2%) routinely discontinues anticoagulation in all patients following AF ablation who are AF-free.
It is important to recognize that the above discussion has focused on patients at high risk of stroke (i.e., CHA2DS2-VASc score ≥2). There is far greater flexibility as to how anticoagulation is managed in patients at a low or moderate risk of stroke because current guidelines do not mandate systemic anticoagulation. Another important consideration is that patient preference plays a large role in this decision. It is our belief that patients should be made aware of the available data and consensus recommendations, and then should be encouraged to consider the risks and benefits of continuing vs discontinuing systemic anticoagulation. Some patients who are at increased risk of stroke are highly motivated to discontinue systemic anticoagulation and are willing to accept an increased risk of stroke. For these patients, we recommend diligent pulse assessment at least twice daily and strong considerations that some type of continuous monitoring be performed to screen for silent AF at regular intervals as long as they remain free from systemic anticoagulation. A final comment worth mentioning is that the mechanisms of stroke are not limited to cardioembolism due to AF; thus, other sources of emboli should also be considered, such as paradoxical embolism and atheromas from the aortic arch. In the remainder of this section, we will briefly review some of the available data.
In multiple randomized trials, AF ablation was superior to AADs in reducing AF recurrence in drug-refractory patients.861 However, stroke prevention among these strategies has been largely similar. One investigator recently undertook a meta-analysis to evaluate whether AF ablation reduces the long-term risk of stroke compared with AAD therapy.857 Thirteen RCTs were analyzed, with 1097 patients treated by catheter ablation and 855 patients receiving AAD therapy. Overall, seven patients (0.64%) in the catheter ablation group had ischemic stroke or TIAs vs two patients (0.23%) in the drug therapy group. No difference was shown in the rate of stroke or TIA between ablation and drug therapy. To date, however, no AF ablation trial has evaluated whether successful ablation obviates the need for long-term OAC, but there are reports from large administrative registries and observational studies addressing this issue.
One study evaluated the long-term results of OAC cessation after successful catheter ablation of AF.857 OAC and AADs were discontinued irrespective of AF type or baseline CHA2DS2-VASc risk score in 327 patients with drug-refractory AF after catheter ablation. Patients with a CHA2DS2-VASc score of 2 (45.4%) and 3 (23.2%) accounted for 68.8% of this cohort. In the patients with a high risk of recurrence or prior thromboembolic complications, OAC was continued for up to 6 to 12 months postablation, and antiplatelet therapy was administered to all patients who maintained sinus rhythm upon OAC interruption. After a follow-up of 46 months, 82% remained AF-free (free from AADs). No symptomatic ischemic cerebrovascular events were detected during follow-up, despite interruption of OAC in 298 (91%) patients and AADs in 293 (89%) patients.
Another study reported the patterns of anticoagulation use and cardioembolic risk after catheter ablation for AF.862 They found an increased use of NOACs after ablation from 0% in 2005 to 69.8% in 2014. OAC discontinuation was high, with only 60.5% and 31.3% of patients remaining on OAC at 3 and 12 months, respectively. The rate of discontinuation was higher in low-risk patients (82% vs 62.5% at 12 months for CHA2DS2-VASc 0–1 vs ≥ 2, respectively; P <.001). Stroke occurred in 1.4% of the patients with CHA2DS2-VASc ≥2 and in 0.3% of those with a CHA2DS2-VASc of 0 or 1 over the study follow-up. The risk of cardioembolism in the first 3 months after ablation was increased among those with any time free from OAC (HR 8.06; 95% CI 1.53–42.3; P <.05). The risk of cardioembolism beyond 3 months was increased with OAC discontinuation among high-risk patients (HR 2.48; 95% CI 1.11–5.52; P <.05) but not low-risk patients, suggesting that continuing OAC for at least 3 months in all patients and indefinitely in high-risk patients appears to be the safest strategy in the absence of effective monitoring and AF detection.
An AF ablation registry reported data of patients followed up after ablation of PAF in a high-risk group (previous stroke; group 1) and a low-risk group (no previous stroke; group 2) based on data from the German Ablation Registry, to reveal real-life prescription behavior.412 Between April 2008 and April 2011, 83 patients in group 1 and 377 patients in group 2 with a first ablation of PAF were included in the registry. The results showed a mean CHA2DS2-VASc score of 4.2 ± 1.4 (group 1) vs 1.6 ± 1.2 (group 2) (P <.0001). OAC was discontinued in 38.6% of the patients in group 1 vs 66.3% of those in group 2 (P <.0001) during follow-up. Thromboembolism occurred more often in group 1 than in group 2 (4.3% vs 0.3%, P <.05), arguing against OAC discontinuation in a high-risk population without ongoing pulse assessment and ECG monitoring.
Another study in a large Danish cohort410 evaluated the long-term risk of thromboembolism and serious bleeding associated with OAC therapy beyond 3 months after RF ablation of AF. During a median follow-up of 3.4 years, 71 (1.8%) thromboembolism cases were identified, in which incidence rates with and without OAC were similar at 0.56 (95% CI 0.40–0.78) and 0.64 (95% CI 0.46–0.89), respectively. OAC therapy was significantly associated with serious bleeding risk (HR 2.05; 95% CI 1.25–3.35). Of note, half the patients received OAC for at least 1 year after catheter ablation, including 56% of the CHA2DS2-VASc = 0 patients and 67% of the CHA2DS2-VASc = 1 patients. As expected, bleeding events were higher in the patients who remained on anticoagulation after AF ablation (HR 2.05).
Another study assessed the feasibility for discontinuation of OAC after ablation based on the AF burden documented by implantable cardiac monitors.859 During a follow-up time of 32 ± 12 months (126 patient-years), 41 of the 65 patients (63%) had an AF burden <1 hour per day and were able to stay off OAC. Twenty-one patients (32%) had to reinitiate OAC due to an AF burden >1 hour, and three patients reinitiated OAC due to other reasons. No stroke, TIA, or other thromboembolic event was observed during follow-up. These are important data for those patients who decide not to receive chronic OAC, and we suggest consideration of an anticoagulation strategy based on AF burden measured by monitoring.
Another single-center report described outcomes in 635 patients with one or more risk factors for stroke during a mean follow-up of 836 ± 605 days after an AF procedure.545 Anticoagulation was discontinued in 434 of 517 patients who remained in sinus rhythm, and aspirin and/or clopidogrel was prescribed. There were three ischemic strokes and two TIAs in the anticoagulation discontinuation group. The estimated 5-year stroke rate in this group was 3%.
An observational study from five large AF ablation centers included data from 3344 patients who underwent AF ablation.238 Oral anticoagulant therapy was typically discontinued regardless of the CHA2DS2-VASc score if patients did not manifest one of the following: (1) any recurrence of ATAs; (2) severe PV stenosis; or (3) severe LA mechanical dysfunction. After discontinuation of anticoagulation, the patients were treated with aspirin. If AF recurred, anticoagulation was restarted in those with a CHA2DS2-VASc score of one or more. There were 347 patients who had a CHA2DS2-VASc score of >2. Among these 347 patients, no thromboembolic events occurred.
One of the most recent studies to be published reports data from the Swedish national health registry.863 Among 1175 individuals followed for more than a year post-AF ablation, 30% discontinued warfarin treatment during the first year. In patients with a CHA2DS2-VASc score >2, the patients discontinuing warfarin had a higher rate of ischemic stroke (1.6% per year vs 0.3% per year for those who continued warfarin). Patients with a CHA2DS2-VASc score >2 and who had had a prior ischemic stroke displayed an especially high risk of stroke if warfarin was discontinued (HR 4.6). It is important to note that in this registry, recurrence rates of AF after ablation were quite high. Sixty percent of the entire cohort and 8 of the 11 patients with stroke (72.7%) underwent cardioversion of AF or a second PVI. The study convincingly demonstrated that in patients with recurrent AF after catheter ablation, a high CHA2DS2-VASc score, and/or a history of stroke, OAC therapy should not be discontinued. Because there were so few patients without AF recurrence, the question of whether “successful” AF ablation might convey a lower risk was not adequately addressed.
As stated above, there is a lack of randomized trials evaluating this important clinical challenge; however, there are some ongoing trials, such as EAST and CABANA, which will address the prognostic impact of rhythm control therapy, including AF ablation and the effect of rhythm control therapy on stroke. Other trials are needed to define the optimal anticoagulation during AF ablation procedures in an era in which novel anticoagulants are increasingly used. We are optimistic about the ongoing Optimal Anticoagulation for Higher Risk Patients Post-Catheter Ablation for Atrial Fibrillation (OCEAN) trial (NCT02168829), which will effectively determine whether successful long-term reduction or elimination of AF with catheter ablation will reduce stroke risk sufficiently to obviate the need for long-term OAC. Additionally, the ongoing Prevention of Silent Cerebral Thromboembolism by Oral Anticoagulation With Dabigatran After PVI for Atrial Fibrillation (ODIn-AF) trial (NCT02067182) will address the effect of dabigatran compared with no OAC on the incidence of silent cerebral embolic events in patients with a high risk for embolic events, but who are free from symptomatic AF after successful PV ablation.
Until the outcomes of such trials are available, our current treatment recommendations to continue OAC after catheter ablation of AF in patients at high risk for stroke should continue. In patients who desire to discontinue anticoagulants because of ECG-documented AF elimination who remain at risk because of high CHA2DS2-VASc score, an individualized approach after full disclosure is warranted. It is important that patients who are considering discontinuation of anticoagulation in the setting of a stroke risk profile have a complete discussion of the potential risks of this strategy. As noted above, the writing group recommends that, for patients in whom discontinuation of anticoagulation is being considered based on the patient's values and preferences, they should consider undergoing continuous or frequent ECG monitoring to screen for AF recurrence, although recurrence of AF is only one of many reasons for stroke events after discontinuation of anticoagulation (Class IIb, LOE C-EO, Table 4). Whether this strategy results in a significant reduction of stroke risk remains uncertain at this time.
Less information is available concerning the optimal approaches to anticoagulation following surgical ablation of AF. Many variables need to be considered, including whether the patient underwent ligation of their LAA and the patient's stroke risk profile. At the present time, there is little to no evaluable evidence for or against the merits of anticoagulation following surgical ablation when the LAA has been surgically obliterated. In the absence of current evidence, the decision to anticoagulate and the duration of treatment should be made on an individual basis weighing the risks and benefits of anticoagulation in the postsurgical patient. It is, however, not unreasonable to anticoagulate for several months following surgical ablation, provided there are no other bleeding risks. For patients in whom appendage closure or ligation was performed at the time of surgical ablation, and in whom discontinuation of anticoagulation is being considered, TEE-based assessment of whether complete appendage closure has been accomplished is recommended because incomplete closure of the LAA is not uncommon.
Anesthesia or Sedation During Ablation
Patients undergoing catheter ablation of AF are required to lie motionless on the procedure table for several hours, and repeated stimuli from ablation are sometimes painful. For these reasons, most patients are treated with conscious sedation or general anesthesia. The choice of approach is determined by the institutional preference and by assessment of the patient's suitability for conscious sedation.
General Anesthesia
AF ablation procedures are commonly performed under general anesthesia. Not only does use of general anesthesia improve the safety of the procedure for patients at risk of airway obstruction, but it also improves patient comfort and might improve efficacy by preventing patient movement during the procedure. Given the need to minimize patient movement to improve catheter and mapping system stability, general anesthesia or deep sedation are generally preferred. One prospective randomized clinical trial randomized patients with general anesthesia or conscious sedation. This study reported that use of general anesthesia increased the single procedure success rate, lowered the prevalence of PV reconnection among those who needed a redo procedure, and shortened fluoroscopy time and procedure time.633 General anesthesia is of particular importance for patients at risk of airway obstruction, those with a history of sleep apnea, and those at increased risk of pulmonary edema. General anesthesia may also be employed electively in healthy patients in order to improve patient tolerance of the procedure. Anesthesia or analgesia needs to be administered by well-trained and experienced individuals with monitoring of heart rate, noninvasive or arterial line BP, and oxygen saturation. Guidelines for assessing levels of anesthesia and training requirements for administration of intravenous sedation during procedures have been developed by the American Society of Anesthesiologists, which can be found on their website. A survey of the writing group members shows that in the United States and Canada, 85% routinely employ general anesthesia. Outside the United States and Canada, 45% routinely employ general anesthesia. (Please also see discussion of anesthesia on page e51).
Conscious and Deep Sedation
Deep sedation is a step beyond conscious sedation and just before general anesthesia. Generally, only anesthesia providers or specially trained physicians can provide deep sedation because airway and hemodynamic management might be required. The major limitation to deep sedation is the need for the patient to lie on the procedure table with minimal movement during the entire procedure. RF lesions can be associated with intense pain, resulting in patient movement. The location of sites eliciting pain with RF lesions are not predictable, although are most often located on the posterior wall. Monitoring esophageal temperature during deep sedation is possible, but more cumbersome, due to intact airway reflexes that are abolished during general anesthesia. Patient movement with right phrenic stimulation during CB procedures is also a common occurrence with deep sedation, and is largely absent with the use of general anesthesia.
Jet Ventilation
Catheter stability and catheter contact during LA ablation are crucial for effective lesion creation. Both catheter stability and catheter–tissue CF can be further increased by reduced respiratory thoracic excursions. Data from one institution suggest improved clinical outcome as a result of enhanced lesion quality and reduction of PV reconnection when applying high-frequency jet ventilation in general anesthesia during PVI.634,864,865 Further data from other centers are needed, however, before final conclusions can be drawn. A survey of the writing group members reveals that in the United States and Canada, 14% routinely employ high-frequency jet ventilation. Outside the United States and Canada, 4% routinely employ high-frequency jet ventilation during AF ablation procedures.
Summary
The type of anesthesia used for AF ablation depends in part on the availability of anesthesia support for ablation procedures. Given the need to minimize patient movement to improve catheter and mapping system stability, deep sedation or general anesthesia is generally preferred.
Approaches to Minimize Risk of an AEF
A rare but potentially devastating complication of AF ablation is injury to the esophagus, with the possible outcome of AEF or esophageal perforation leading to mediastinal infection, stroke, and/or death.866,867,1398 Another complication that is thought to be related to thermal injury to the periesophageal vagal plexus is gastroparesis.868 More information concerning the incidence, presentation, and management of these complications is presented under Section 10. Because of the serious consequences of an AEF, it is important to attempt to prevent severe esophageal and periesophageal injury. Some operators design the ablation lesions to avoid the esophagus. The location of the esophagus can be visualized using a variety of approaches, including multidetector CT, topographic tagging of the esophageal position with an EAM system, barium paste, and ICE.869,870,871,872,873,874,875,876 It is important to know that esophagus location can change during the procedure, and repeated imaging or visualization is needed to account for the motion of the esophagus. However, it is difficult to accomplish complete PV ablation without some ablation in close proximity to the esophagus. Strategies to prevent and treat esophageal injury follow.
Reduced Power Delivery on the Posterior Wall
Higher power and greater depth of tissue heating or cooling are associated with increased risk of esophageal injury. In order to minimize injury to the esophagus during RF applications on the posterior wall close to the esophagus, several approaches can be employed, including (1) reduction of RF power (e.g., ≤25 W); (2) shortening RF application time (e.g., ≤20 seconds); and/or (3) decreasing CF (e.g., ≤10 grams). The writing group recommends that RF power be reduced when creating lesions along the posterior wall near the esophagus (Class I, LOE C-LD, Table 3). Some reports employed the use of light conscious sedation to use pain to identify potential esophageal injury. However, there are conflicting data on the specificity of the pain response. It has been proposed that an alternative energy source, such as the CB for PVI, could minimize esophageal injury877,878; however, AEF or periesophageal vagal plexus injury after CBA has been reported.879,880 There are also data that other heat-based energy sources, such as high-intensity focused ultrasound or laser energy, can damage the esophagus.501,502,700,701,705,881 Although each of these approaches is variously adopted by different ablation centers, each remains largely unproven due to the rarity of an AEF as a complication.
Esophageal Temperature Monitoring
A strategy to avoid esophageal injury employed by 65% of the writing group members is luminal esophageal temperature monitoring, used to identify potentially dangerous heating of the esophagus.882,883,884,885 Unfortunately, because the esophagus is broad, the lateral position of the temperature probe or mapping electrode might not align with the ablation electrode, and the operator could receive a false impression of safety.1398 There is general agreement among those operators who employ temperature probes that an increase in esophageal temperature should trigger interruption of RF energy delivery. Three-quarters of the writing group members terminate ablation if they observe a 1 °C or 2 °C rise in temperature from baseline, or a recorded temperature of 39 °C–40 °C. During CBA, two-thirds of the writing group members monitor esophageal temperature, and terminate cooling if the esophageal temperature reaches 20 °C–25 °C. A variety of esophageal temperature probes are available for clinical use.886 A recent study has shown the superior thermodynamic profile of multisensor esophageal recording systems; however, no clinical trial has demonstrated superiority in terms of reducing AEFs.646,647 This type of study would be impossible to perform due to the very low event rate of this complication. Among the writing group members who employ esophageal temperature monitoring, single thermocouple probes are used by two-thirds and multithermocouple probes are employed by one-third. The potential benefit of multithermocouple probes must be weighed against their increased complexity and cost.886,887,888,889 The writing group recommends that it is reasonable to use an esophageal temperature probe during RF ablation procedures to monitor esophageal temperature to help guide energy delivery (Class IIa, LOE C-EO, Table 3).
Another strategy to protect the esophagus uses active cooling.890,891,892,893 This technique has not been tested on a large scale, and the data describing this technique are limited. Selected operators use mechanical displacement of the esophagus.894,895 This technique appears to be promising, but its use has been limited to a small number of patients and is therefore an unproven approach.
Pharmacological Prophylaxis
Esophageal ulcers are found in a 5%–40% of patients following AF ablation. It is hypothesized that AEF occurs because there is transmural necrosis of both the atrium and esophagus with subsequent ulcer erosion from gastroesophageal reflux.896,897 To prevent ulcer erosion, proton pump inhibitors (PPIs) have been employed, and are used by 65% of the writing group members after ablation. PPIs are highly effective in gastroesophageal reflux disease by reducing the acidity of the gastric juice and healing esophagitis.898,899,900 PPIs are effective in reducing the size of iatrogenic-induced ulcers, therefore could also be helpful for ablation-induced ulcers.901 Other mechanisms, such as traumatic injury of the esophageal wall, could also play a potential role in fistula formation, although there is no proof of this concept. Prophylactic short-term use of PPIs after AF ablation is assumed to be effective; however, further large randomized studies are required to determine whether PPIs reduce AEFs. Because of the low event rate of AEFs, such a study will not likely be performed. At the moment, PPI therapy is justified as a singular preventive treatment.
Role and Indications for Endoscopic Screening for Ulceration Following AF Ablation
Because AEF can cause septicemia and air embolism leading to death, early detection of esophageal tissue injuries is essential. Data evaluating the role of gastrointestinal endoscopy for detection of esophageal tissue lesions are limited. In 185 patients who underwent gastrointestinal endoscopy after LA RF ablative therapy, ulcer-like or hemorrhagic esophageal thermal lesions (diameter: 2–16 mm) were observed in 14.6% of the patients.902 These lesions only occurred when the intraluminal esophageal temperature had reached more than 41 °C. The odds of an esophageal lesion increased by a factor of 1.36 (95% CI 1.07–1.74; P = .012) for every 1 °C rise in temperature.
Gastrointestinal endoscopy in a cohort of 425 patients 1 to 3 days after AF catheter ablation, in whom intraluminal esophageal temperatures higher than 41 °C were recorded, revealed esophageal tissue lesions in 11.6% of asymptomatic patients.903 Hence, these observations suggest that asymptomatic patients could benefit from routine gastrointestinal endoscopy after RF catheter ablative therapy when the intraluminal esophageal temperature during the procedure has reached a certain target temperature, such as 41 °C. However, there are no reports on the value of this type of follow-up endoscopic examination after ablative therapy. Only one study did a follow-up endoscopy at least 7 days after the first examination in patients with an esophageal lesion diameter >5 mm and found regression of all 3168 lesions.903 A PPI was used in all the patients for 4 weeks after ablation.
Role and Indications for CT Imaging for Diagnosis of Atrioesophageal Fistula
After ablation, symptoms and findings suggesting the possibility of evolving AEF include chest pain, painful swallowing, fever, leukocytosis, TIA, and/or stroke typically occurring between 1 and 3 weeks postablation. If esophageal injury is suspected, CT imaging with intravenous and water-soluble oral contrast is recommended.904,905,906 Findings on CT imaging on an AEF include mediastinal or pericardial free air, evidence of free communication between the esophagus and pericardium or atrium, and inflammatory phlegmon between the esophagus and the heart. Unfortunately, these CT findings are usually observed late in the progression of AEF. The appearance of the CT scan early in the course of this complication can be entirely normal. If esophageal injury postablation is suspected, but if the CT scan is normal, the physician must continue to have a high index of suspicion and repeat imaging if symptoms or findings do not resolve. Esophageal ultrasound can also be useful in this setting to disclose muscle and external injury, beyond a simple ulcer. Although a barium swallow can detect a fistula, its sensitivity is low. If an AEF is suspected, endoscopy with air insufflation should be avoided, given that insufflation of the esophagus with air can result in a large air embolus, producing stroke or death. An alternative strategy, which some members of the writing group employ and which appears to have lower risk is to use CO2 instead of air for insufflation in this setting. If CO2 were introduced into the LA, there would be little adverse consequence. The early recognition of an AEF can be missed due to the low awareness of this rare complication. It is important for patients to be educated as to warning signs and to contact their AF ablation center should any suggestive symptoms develop.
Management of Atrial Esophageal Fistula
The management of AEF following catheter ablation for AF includes preventive measures and therapeutic options. If AEF is diagnosed, available therapeutic options are as follows:
surgical repair of the fistula via thoracotomy (combined LA and esophageal repair with an intercostal muscle flap inserted in between to prevent future recanalization of the fistula tract) via thoracotomy;
the less invasive esophageal stenting, followed by long-term antibiotic therapy; and
conservative management with aggressive chest tube drainage and treatment of sepsis.341,417,907,908,909,910,911
Of the above three, conservative treatment of AEF is associated with a high mortality rate. 907,1398 Similarly, with esophageal stenting, earlier studies have reported fatality in the majority and survival in very few only after undergoing emergency surgical repair.341,417,896,897,907,910,911,912,913,914,1398 Mixed results have also been shown for surgical repair of AEF complicating RFCA, some with positive outcome and others with fatal ending.341,417,910,911 However, the only reported survival in patients thus far underwent surgical fistula repair, and failure of surgery has been mostly attributed to delay in diagnosis and intervention.341,417,910,911 Thus, based on currently available clinical information, it is apparent that early surgical intervention is critical for survival in AEF manifesting as a complication of AF ablation. Of note, there are few reports on successful resolution of the fistula with stenting in patients with cardioesophageal (connecting to CS) and esophagopericardial fistula.905,915,916 In cases of perforation (not thermal injury) before the fistula has formed, closure with stent or endoscopic clip can be considered.917,918,919
Summary
Although all of the approaches described above for the prevention of AEF have been variously adopted by different ablation centers, each remains largely unproven due to the rarity of an AEF as a complication. Among the writing group members, 67% employ an esophageal temperature probe (single thermocouple for two-thirds, multiple thermocouple for one-third), 36% use 3D image integration and import the esophagus location into the electroanatomical map, 91% decrease RF power when ablating on the posterior wall of the atrium, 7% use barium paste, and none (0%) mechanically displace the esophagus. Among the writing group members, 30% limit power to ≤ 20 W on the posterior wall, 45% limit it to 25 W, 18% to 30 W, and 7% use powers of > 30 W. The writing group recommends that it is reasonable to use an esophageal temperature probe during RF ablation procedures to monitor esophageal temperature and to help guide energy delivery (Class IIa, LOE C-EO, Table 3). The writing group recommends that RF power be reduced when creating lesions along the posterior wall near the esophagus (Class I, LOE C-LD, Table 3).
Despite its rarity, the devastating consequences of AEF demand that the operator maintain a high index of suspicion for this diagnosis. Presenting symptoms, including fever, dysphagia, and neurological deficits, often occur in the several weeks after the procedure.918 Therefore, early signs of these symptoms should be reported by patients to their treating electrophysiologist to avoid the delayed diagnosis.908 If AEF is suspected, standard transesophageal endoscopy should be avoided, because esophageal perforation can be exacerbated and air embolism promoted by required air insufflation. An alternative strategy, which some members of the writing group employ and which appears to have lower risk is to use CO2 instead of air for insufflation in this setting. If CO2 were introduced into the LA, there would be little adverse consequence. In patients diagnosed with an AEF, surgical treatment is recommended.
Section 8: Follow-up Considerations
Monitoring for Complications in the First Months After AF Ablation
AF ablation is an invasive procedure that entails risks, most of which are present during the acute procedural period. However, complications can also occur in the weeks or months following ablation.920,921,922 Recognizing common symptoms after AF ablation and distinguishing those that require urgent evaluation and referral to an electrophysiologist is an important part of follow-up after AF ablation. Symptoms and complications can be divided into those that occur immediately after ablation (0–3 days), early (1–4 weeks), and those that can occur late (>4 weeks) after ablation.
Signs and Symptoms of Complications Within 1 Month Postablation
Shown in Table 5 is a list of signs and symptoms that can occur within the first several months following ablation. These signs and symptoms are divided into those that occur within 30 days of AF ablation and those that occur more than 30 days postablation. Some complications, such as a stroke or development of an AEF, might present within the first month or following the first postablation month and therefore are listed in both sections of this table. The differential diagnosis, which should be considered, as well as the recommended evaluation, are also shown. AF ablation is often performed under general anesthesia. Some patients might feel fatigued for several days after prolonged general anesthesia. Mechanical complications from endotracheal intubation and transesophageal echocardiography, such as hoarseness and difficulties swallowing, might also occur and typically resolve with time.
Table 5.
Signs and symptoms following AF ablation
| Differential | Suggested evaluation | |
|---|---|---|
| Signs and symptoms of complications within a month postablation | ||
| Back pain | Musculoskeletal, retroperitoneal hematoma | Physical exam, CT imaging |
| Chest pain | Pericarditis, pericardial effusion, coronary stenosis (ablation related), pulmonary vein stenosis, musculoskeletal (after cardioversion), worsening reflux | Physical exam, chest X-ray, ECG, echocardiogram, stress test, cardiac catheterization, chest CT |
| Cough | Infectious process, bronchial irritation (mechanical, cryoballoon), pulmonary vein stenosis | Physical exam, chest X-ray, chest CT |
| Dysphagia | Esophageal irritation (related to transesophageal echocardiography), atrioesophageal fistula | Physical exam, chest CT or MRI |
| Early satiety, nausea | Gastric denervation | Physical exam, gastric emptying study |
| Fever | Infectious process, pericarditis, atrioesophageal fistula | Physical exam, chest X-ray, chest CT, urinalysis, laboratory blood work |
| Fever, dysphagia, neurological symptoms | Atrial esophageal fistula | Physical exam, laboratory blood work, chest CT or MRI; avoid endoscopy with air insufflation |
| Groin pain at site of access | Pseudoaneurysm, AV fistula, hematoma | Ultrasound of the groin, laboratory blood work; consider CT scan if ultrasound negative |
| Headache | Migraine (related to anesthesia or transseptal access, hemorrhagic stroke), effect of general anesthetic | Physical exam, brain imaging (MRI) |
| Hypotension | Pericardial effusion/tamponade, bleeding, sepsis, persistent vagal reaction | Echocardiography, laboratory blood work |
| Hemoptysis | PV stenosis or occlusion, pneumonia | Chest X-ray, chest CT or MR scan, VQ scan |
| Neurological symptoms | Cerebral embolic event, atrial esophageal fistula | Physical exam, brain imaging, chest CT or MRI |
| Shortness of breath | Volume overload, pneumonia, pulmonary vein stenosis, phrenic nerve injury | Physical exam, chest X-ray, chest CT, laboratory blood work |
| Signs and symptoms of complications more than a month postablation | ||
| Fever, dysphagia, neurological symptoms | Atrial esophageal fistula | Physical exam, laboratory blood work, chest CT or MRI; avoid endoscopy with air insufflation |
| Persistent cough, atypical chest pain | Infectious process, pulmonary vein stenosis | Physical exam, laboratory blood work, chest X-ray, chest CT or MRI |
| Neurological symptoms | Cerebral embolic event, atrial esophageal fistula | Physical exam, brain imaging, chest CT or MRI |
| Hemoptysis | PV stenosis or occlusion, pneumonia | CT scan, VQ scan |
AF, atrial fibrillation; ECG, electrocardiogram; CT, computed tomography; MRI, magnetic resonance imaging; VQ, ventilation-perfusion.
Tenderness at the vascular access sites is common; hematomas present after sheath removal will typically extend inferiorly (due to gravity) and might result in extensive ecchymosis after ablation. Prompt ultrasound Doppler investigation should be performed if an AV fistula or pseudoaneurysm is suspected. Worsening of back or buttock pain is also common from prolonged supine positioning during the procedure. However, more severe back pain or flank ecchymosis should prompt an evaluation for retroperitoneal hematoma with CT imaging. Significant bleeding into the leg can also result in compartment syndrome.
Shortness of breath soon after ablation might have several causes. The patient should be examined after ablation for evidence of volume overload related to irrigated ablation and diuresed as necessary. Volume overload can be observed in patients with normal or reduced cardiac function, perhaps due to atrial stunning. If dyspnea persists or occurs in the absence of volume overload, a chest X-ray should be obtained to exclude an infectious process or elevation of the respective hemi-diaphragm. PN injury most commonly occurs after balloon-based ablation, but can also occur after RF ablation.503 Lack of diaphragmatic movement during inspiration under fluoroscopy (the sniff test) is diagnostic of PN injury. Right PN injury is much more common after AF ablation and is due to ablation near the RSPV or SVC (Figure 1). Left PN injury less commonly occurs when ablating near the LAA. Although most cases of phrenic injury recover with reinnervation over a 6–12 month period after ablation, permanent diaphragmatic paralysis has been reported.
Chest pain is common after ablation; the causes include pericarditis, coronary ischemia, and musculoskeletal pain. Symptoms of pericarditis (pleuritic chest pain) are the most common (>75% of patients) and typically persist for up to a week postablation. In the absence of evidence of hemodynamic compromise, an ECG is of little value. It is important to recognize that nearly all patients will demonstrate a small pericardial effusion following AF ablation as a result of edema. Nonsteroidal anti-inflammatory agents are recommended for symptom control. Colchicine can also be used to treat pericardial symptoms. Oral steroids should be avoided after catheter ablation unless pericardial symptoms persist or are recurrent. Chest pain that is associated with ECG changes or that occurs with exertion should prompt evaluation of coronary ischemia. In particular, if ablation has been performed inside the CS to target the epicardial portion of the mitral isthmus, or for isolation of a CS tachycardia, circumflex artery stenosis should be considered.923
Any unexplained hypotension during or following ablation should be evaluated promptly. Transthoracic echocardiography or ICE (if during ablation) should be performed urgently to exclude pericardial effusion or cardiac tamponade. A complete blood count should be performed to exclude bleeding or infection.
Fever might occur early after ablation. We should exclude infectious sources such as a urinary tract infection related to bladder instrumentation or pneumonia related to intubation. Low-grade fever might also be related to pericarditis. In addition, fever might be the first marker of an impending AEF formation. Chest imaging should be considered if fever persists, an AEF is suspected, and no other clear infectious source is identified.
Any neurological symptoms occurring shortly after ablation should be taken seriously, with brain imaging performed to exclude an embolic event. Migraine-like signs and symptoms have been reported and are most commonly benign and are attributed to the residual ASD following transseptal puncture. As noted above, an AEF might also present with neurological symptoms. It is also important to recognize that an AEF might present as a neurological event and therefore must be considered the differential diagnosis of neurological symptoms that develop post AF ablation.
Symptoms of pericarditis typically persist up to a week after ablation (Table 5). If symptoms persist for >1 week or are associated with lightheadedness or shortness of breath, further evaluation is warranted. Groin pain that persists past 7 days or is getting worse should prompt a physical exam and vascular ultrasound to exclude femoral access complications. A persistent nagging dry cough might also be observed for up to 6 weeks after ablation. This complication is more common with CB than with RF ablation and is likely related to direct bronchial or lung injury. This type of cough is generally treated with antitussives and will typically subside over 4–6 weeks.
Some patients, particularly those with a history of migraines, might experience migraine headaches in the first few weeks after ablation.924,925 These headaches might be related to the residual ASD present after transseptal puncture and will typically improve over several weeks. Hemoptysis is rare but might result from pneumonia or pulmonary infarction due to an occluded PV, typically occurring 3–6 months after ablation. Dysphagia in the first days after ablation is most likely related to irritation from transesophageal echocardiography or intubation. If dysphagia persists, then imaging (chest CT or MRI) should be performed to exclude an AEF (see late complications). The differential diagnosis of dyspnea occurring early after ablation should include volume overload, pneumonia, or PN injury as outlined above. A chest roentgenogram should be obtained. If symptoms persist with a normal chest roentgenogram, we should also consider PV stenosis (see late symptoms, below). Vagal denervation of the esophagus or stomach can occur after ablation due to ablation lesions placed in the vicinity of the esophagus, particularly if extensive ablation is performed along the LA posterior wall.536,926 Symptoms can include nausea and early satiety. Patients should be advised to eat small, frequent meals. Symptoms will typically improve over 4–6 weeks. If symptoms are profound or persist, a gastric emptying study can be diagnostic. Pain at the site of sheath insertion can result from an pseudoaneurysm, an AV fistula, or a hematoma. Evaluation usually starts with a vascular ultrasound. Bloodwork and a CT scan might be appropriate.
Signs and Symptoms of Complications More Than a Month Postablation
Late symptoms of dysphagia and/or fever, particularly in the presence gastrointestinal bleeding or any neurological symptoms, should prompt an urgent evaluation for an AEF, a rare but potentially lethal complication after AF ablation (see Section 10).341,417,866,910 If AEF is suspected, esophagogastroduodenoscopy should not be performed, because increased pressure in the esophagus can lead to the introduction of air into the LA and stroke. Imaging with CT or MR is preferred, with the presence of air in the mediastinum or LA considered diagnostic. Although barium should not be introduced into the esophagus, a small amount of water-soluble contrast can help identify the location of the fistula. The recommended treatment for AEF at any stage is surgical exploration and resection of the fistulae, typically requiring resection of the involved esophagus and repair of the posterior LA wall with a pericardial patch. There have been reports of treatment of early fistulae with covered esophageal stents; however, surgical treatment is generally preferred. A persistent cough >6 weeks after ablation, particularly if associated with atypical chest pain, recurrent pneumonia or hemoptysis, should prompt an evaluation for PV stenosis.927,928 A chest roentgenogram might also show evidence of atelectasis or infiltrate localized to one lobe of the lung, which is typically related to focal pulmonary edema. Many patients have received repeated courses of antibiotics for lung infection before the correct diagnosis is reached. If PV stenosis is suspected, a chest contrast CT angiogram or MR angiogram should be performed to examine PV anatomy and exclude PV stenosis or occlusion. If PV stenosis or occlusion is detected, a ventilation or perfusion scan is typically performed to quantify lung perfusion. Referral to a center with expertise in PV stenting should be recommended early in the course of PV stenosis, because dilatation is more difficult and has a higher incidence of pulmonary hypertension, lung infarct, and hemoptysis once high grade stenosis has occurred (see Section 10: Complications). Hemoptysis should trigger an evaluation for PV stenosis and usually indicates the presence of complete branch or PV occlusion. Other late complications include a stroke or embolic event related to recurrent AF or deep vein thrombosis or pulmonary embolus related to femoral vein instrumentation. These complications are uncommon because anticoagulation is typically reinstated after ablation.
ECG Monitoring Pre- and Postablation
Arrhythmia monitoring is an important component of the initial evaluation of patients who are to undergo catheter ablation procedures for AF. Prior to undergoing a catheter ablation procedure, it is important to confirm that a patient's symptoms result from AF and to determine whether a patient has paroxysmal or persistent AF. The choice of ablation technique, expectations with respect to the procedure's outcome, anticoagulation strategies employed, and the need for TEE prior to the procedure might be impacted by the accurate characterization of the AF type and burden. Preprocedure arrhythmia monitoring is also useful to determine whether a patient has evidence of regular supraventricular tachycardia that degenerates into AF as a triggering mechanism or has a pattern of repetitive “focal firing,” characterized by the presence of frequent atrial premature beats (>1000 per 24 hours) with frequent rapid salvos of nonsustained AT.458 Focal AF is characterized by localized triggers arising from the PVs.929 Either of these triggering patterns of AF initiation identifies a patient in whom a more limited ablation, targeted at only the triggering arrhythmia focus or PV(s) might be appropriate.406,458,930 An assessment of the adequacy of heart rate control is particularly important in patients with depressed left ventricular function who might show evidence of a reversible tachycardia-induced cardiomyopathy.234
ECG monitoring also plays an important role in the follow-up after an ablation procedure. Early recurrences of AF are common during the first 3 months following a catheter ablation procedure.931,932 For this reason, arrhythmia monitoring to assess the efficacy of catheter ablation is typically delayed for at least 3 months following catheter ablation unless required to evaluate arrhythmia symptoms during the early postablation period. However, recurrences particularly after the first month following an ablation procedure are predictive of later recurrence of AF, and therefore monitoring may be used to identify patients at higher risk of needing a second ablation procedure or ongoing AAD therapy.272,329,933,934,935
The two main reasons to perform arrhythmia monitoring following catheter ablation are clinical care and as part of a clinical research trial. From a purely clinical perspective, arrhythmia monitoring is useful to determine whether a patient's complaints of palpitations result from recurrent AF or other ATA. Complaints of palpitations often result from atrial or ventricular premature beats and are not an accurate predictor of recurrent AF.57,936 Arrhythmia monitoring can also be of value in asymptomatic patients and can influence decision making regarding anticoagulant therapy after ablation. Multiple studies have demonstrated that asymptomatic AF commonly occurs in patients following catheter ablation.56,57,63,413,442,936,937,938 Detection of these asymptomatic episodes of AF impact the characterization of the procedure as “successful.” Arrhythmia monitoring is an essential component of clinical trials aimed at assessing the outcomes of catheter ablation procedures and should be incorporated into all clinical trials designed to assess the efficacy of AF catheter ablation tools and techniques. The suggested monitoring strategies and minimum standards to be used as part of clinical trials are discussed in Section 13: Clinical Trial Design. These strategies and standards can be useful in tracking the outcome of clinical care when assessing an institution's performance standards related to success and complications of AF ablation procedures. However, it is recognized that clinical endpoints and clinical trial secondary endpoints for defining success can include the elimination of symptomatic AF and control of AF with previously ineffective AADs after the AF ablation procedure.
Available Methods for Arrhythmia Monitoring
Use of ECG monitoring tools is essential to assess AF ablation success, and the monitored results can have important implications in terms of clinical care and research outcomes. Arrhythmia monitoring can be performed with the use of noncontinuous or continuous ECG monitoring tools (Table 6). The choice of either method depends on individual needs and the consequences of arrhythmia detection. More intensive monitoring is associated with a greater likelihood of detecting both symptomatic and asymptomatic AF.57,414,937,938,939,940,941,942,943,944 The proportion of asymptomatic compared with symptomatic events might be higher after AF ablation; two studies reported that the proportion of AF events that were asymptomatic was 11%–35% prior to and 53%–65% after ablation.63,945,946 Another study reported that for patients in sinus rhythm, 53.8% of AF episodes were asymptomatic, with an increase in asymptomatic episodes changing from the acute to the chronic period after ablation, demonstrating that AF success cannot be based on the absence of symptoms alone.936
Table 6.
Types of ambulatory cardiac monitoring devices
| Type of recorder | Typical monitoring duration | Continuous recording | Event recording | Auto trigger | Unique features |
|---|---|---|---|---|---|
| Holter monitor | 24–48 hours, approximately 7–30 days | Yes | Yes | N/A | Short term, provides quantitative data on arrhythmia burden |
| Patch monitor | 1–3 weeks | Yes | Yes | N/A | Intermediate term, can provide continuous data for up to several weeks; improved patient compliance without lead wires |
| External loop recorder | 1 month | Yes | Yes | Variable | Good correlation between symptoms and even brief arrhythmias |
| External nonloop recorder | Months | No | Yes | No | May be used long term and intermittently; will not capture very brief episodes |
| Smartphone monitor | Indefinite | No | Yes | No | Provides inexpensive long-term intermittent monitoring; dependent on patient compliance; requires a smartphone |
| Mobile cardiac telemetry | 30 days | Yes | Yes | Yes | Real time central monitoring and alarms; relatively expensive |
| Implantable loop recorder | Up to 3 years | Yes | Yes | Yes | Improved patient compliance for long-term use; not able to detect 30-second episodes of AF due to detection algorithm; presence of AF needs to be confirmed by EGM review because specificity of detection algorithm is imperfect; expensive |
| Pacemakers or ICDs with atrial leads | Indefinite | Yes | Yes | Yes | Excellent AF documentation of burden and trends; presence of AF needs to be confirmed by electrogram tracing review because specificity of detection algorithms is imperfect; expensive |
| Wearable multisensor ECG monitors | Indefinite | Yes | Yes | Yes | ECG 3 leads, temp, heart rate, HRV, activity tracking, respiratory rate, galvanic skin response |
AF, atrial fibrillation; ICD, implantable cardioverter defibrillator; ECG, electrocardiogram; HRV, heart rate variability.
The identification of AF and the assessment of AF burden with intermittent monitoring have been shown to depend on a patient's actual AF burden and improve with an increasing frequency or duration of intermittent monitoring.943,947,948,949 Conversely, the more complex and longer the method of monitoring used, the lower the patient compliance.
Traditional AF detection tools for intermittent monitoring after AF ablation include scheduled or symptom-initiated standard ECGs, Holter monitors, patient-activated and automatically activated full disclosure external loop recorders, and transtelephonic recordings. More recently, implanted loop recorders and external recordings with wireless connection via smartphone applications have been used for longer-term monitoring to detect AF after ablation.
The intermittent, scheduled use of continuous short-term ECG monitors after AF ablation has utilized traditional Holter monitors and more recently patch ECG monitors. Holter monitors use single- or multi-lead external recorders connected via wires to small recording devices. Typical Holter monitors record 2 or 3 channels for 24–48 hours, but some can record continuous 12-lead ECGs or for periods of 7–30 days. Patients can record symptoms on a diary and/or by activating an event button. Because Holter monitors are analyzed by trained technicians and are read by experienced physicians, these approaches might represent the standard monitoring method against which other methods should be compared. Newer wearable patch ECG monitors record from closely spaced electrodes, removing the need for wires and typically generating up to 2 channel recordings. These are water resistant, wearable for up to 30 days, and have enjoyed superior patient acceptance over conventional wired monitor systems. Symptoms can be recorded by an event button. Future devices are being developed with multiple sensors that can record body temperature, activity, respiratory rate, and galvanic skin responses.
Patient- or event-activated external loop recorders can be used for longer or intermediate duration monitoring, typically over weeks to months.254 These memory loop recorders can be programmed to record ECGs for seconds to minutes before and after the detection of an arrhythmia or a patient-triggered event and thus can detect and correlate rhythms with even brief symptoms. External loop recorders should be worn continuously to capture such events and typically are connected via wires to skin electrodes.
Nonloop external event recorders can be used for intermittent transtelephonic recordings that can be initiated by patients with symptoms or on a schedule. These recorders are applied to the chest or held by hand. Older conventional transtelephonic monitors required the recording of rhythm strips while connected in real time over the phone, but more recent monitors allow the storage of rhythm strips with transmission at a later time. Event recording occurs after an event is detected by the patient; the diagnostic yield is dependent on the recognition of symptoms, the duration of symptomatic episodes, or on scheduled or more frequent use to detect asymptomatic arrhythmias.
More recently, smartphone-based ECG monitors have been developed that can be helpful for long-term intermittent surveillance.950,951 Recordings from electrodes embedded in a smartphone case or a card are connected via low-energy Bluetooth technology to smartphone applications. These monitors are nonlooping; patients can record during symptoms that persist long enough to activate the application. Recordings are stored and can be transmitted via wireless or cellular networks. In a study conducted after AF ablation, a smartphone-based single-lead system was compared to transtelephonic monitor ECGs with 100% sensitivity and 97% specificity in detecting AF or flutter.951 Multi-lead and reconstructed 12-lead recording devices are being developed, but have not been studied in the setting of AF ablation. Continuous ECG monitoring technology using such applications are also in development.
Mobile cardiac outpatient telemetry devices provide real-time monitoring and wireless transmission to trained personnel at a central monitoring center with activation of alarms to caregivers for specified significant arrhythmias. These monitors are typically worn continuously for a period of 2–4 weeks and can record 1–3 leads connected to a small device via conventional wires or embedded in a patch. The advantage of these systems is their ability to capture and identify potentially severe or significant arrhythmias in an immediate or timely fashion.
Continuous ECG monitoring for longer periods (1–3 years) can be facilitated with the use of implantable devices. Long-term subcutaneous implantable loop monitors can facilitate continuous AF monitoring based on R-R interval analysis over a time period of up to 3 years.952,953 These types of continuous ECG monitoring devices have been used in several studies to evaluate the results of surgical or catheter AF ablation.127,607,938,953,954,955,956,957,958,959,960,961 Although implantable subcutaneous monitors hold promise for the determination of AF burden in the long term, AF detection algorithms are primarily based on R-R interval regularity, and important limitations include reduced specificity due to undersensing of beats, oversensing of myopotentials, and irregular atrial and ventricular premature beats, as well as limited memory resulting in electrograms not being retrievable to verify the correct rhythm diagnosis.941,944,962 Nevertheless, implantable continuous monitors can ameliorate patient compliance issues and provide an assessment of long-term AF burden and late recurrences, including asymptomatic episodes that might have implications for continuation of anticoagulation. In one study after concomitant surgical ablation, ILRs compared with conventional Holter monitoring facilitated more follow-up antiarrhythmic management, including cardioversions and catheter ablation procedures, which were associated with a trend toward higher sinus rhythm rates at 1 year.942
Implantable pacemakers or defibrillators with atrial leads allow the burden of AF to be assessed by tracking the number and duration of mode-switch episodes.963,964 These devices can also assess long-term AF burden, burden trends, and late or asymptomatic recurrences.940,965,966 The ability to record intracardiac atrial electrograms provides excellent sensitivity and specificity for the diagnosis of atrial arrhythmias, especially with durations exceeding a few minutes.937,967,968
Follow-up and Monitoring Guidelines for Routine Clinical Care
There is a consensus among the writing group members that all patients who undergo catheter ablation of AF, regardless of whether they are enrolled in a clinical trial, should be seen in follow-up a minimum of 3 months following the ablation procedure. There is also consensus that all patients who undergo catheter ablation should be seen by some type of physician (family physician, internist, cardiologist, or electrophysiologist) on an annual basis thereafter. These ongoing interactions with the medical profession allow the patient's clinical status to be evaluated, including an assessment of the presence or absence of AF as well as their stroke risk profile and anticoagulation needs. These interactions also provide an opportunity to focus on the treatment of associated diseases and lifestyle modifications. These recommendations are slightly modified from the previous edition of this document, which advised that all patients who undergo catheter ablation of AF, regardless of whether they are enrolled in a clinical trial, should be seen in follow-up at a minimum of 3 months following the ablation procedure, and then every 6 months for at least 2 years. A 12-lead ECG was recommended at all follow-up visits and more intense monitoring driven mainly by the clinical impact of AF detection with strict monitoring necessary (suspected rate-related cardiomyopathy). This modification of our writing group recommendations reflects, in part, data from real life clinical practice.969 This European study revealed that one-third of the 12-month follow-up evaluations were performed by telephonic contact, only 87.2% of the patients had at least one ECG during the follow-up, and the patients with continuous monitoring of ≥ 24 hours (Holter- or implanted monitoring systems) represented only 57.4% of the population.
Explanations of this gap between prior expert consensus recommendations and routine clinical practice might reflect the current disconnect between indications for catheter ablation and clinical outcomes of the procedure. Another factor can be cost. On one hand, the main indication for catheter ablation is symptomatic AF and decisions regarding continuation of anticoagulation therapy should be based on the patient's risk factors for stroke and not on the presence of or type of AF. At the same time, transtelephonic or long-term monitoring is at times recommended after ablation to capture even asymptomatic episodes of AF to evaluate the need to continue anticoagulation. The majority of writing group members do not believe that data currently exist to support this common practice of making decisions regarding anticoagulation based on the presence or absence of AF (see Section 7).
A significant amount of information has accumulated showing that cardiac risk factors such as obesity, sleep apnea, and hypertension are associated with structural and electrical remodeling of the atria, which forms the substrate leading to AF development and progression (see Section 3). The recommended indefinite annual follow-up visits with a health care professional allow for the evaluation and treatment of associated diseases and lifestyle modification rather than monitoring of the rhythm itself.
Early Recurrence After Ablation
Definition and Incidence
Early recurrences of AF after AF ablation has been defined as any recurrence of AF > 30 seconds during the first 3 months of follow-up. Late recurrence has been defined as any recurrence of AF > 30 seconds between 3 and 12 months after AF.141,142,143 In using the term early recurrence of AF (ERAF) it is recognized that the early recurrence might be AFL or AT. Although we considered defining a new term, early recurrence of ATAs, post-AF ablation, for simplicity we have employed the term early recurrence of AF. Throughout the document and this section of the document, we note that recurrences can present in the form of AF, flutter, or tachycardia.
Early recurrences of AF after RF catheter ablation have been reported in up to 50% of patients within the first 3 months of AF ablation.253,329,436,684,932,935,970,971,972 Because these arrhythmias do not definitively indicate therapy failure over the long term (only half of these patients will manifest later recurrences), this period is also referred to as the blanking or therapy stabilization period.935,973 It is also important to recognize that the later AF recurrences are observed during the blanking phase, the lower the chance of long-term success.935
Causes of Recurrences
The pathophysiological mechanisms of these early recurrences are attributed to various mechanisms: primarily incomplete isolation of the PVs,973,974 acute inflammatory changes owing to energy delivery,755 recovery of conduction in a previously isolated PV,448,622,975 modification of the ANS, changes in the atrial substrate, and delayed effect of RF ablation due to lesion consolidation.257,258
Early Recurrence as a Predictor of Failure
The occurrence of atrial arrhythmias early after ablation does not necessarily indicate treatment failure later during follow-up.974 Nevertheless, early recurrences have been shown to predict arrhythmia recurrences late after catheter ablation of AF in some patients.260,329,935,976,977,978
Management of early recurrences is controversial and has been treated by AADs, corticosteroids, early cardioversion, or repeat catheter ablation.
Antiarrhythmic Drugs
Because early AF recurrence usually peaks within the first few weeks following PVI, the temporary routine administration of AADs in the immediate postablation period has been proposed as a potential preventive strategy.1,979 Although the true efficacy of this approach is unknown, studies have suggested that transient AAD use does not prevent late arrhythmia relapses.935,980 The 5A study randomized 110 consecutive patients with PAF undergoing ablation to empirical AAD therapy vs no AAD therapy for the first 6 weeks after RF catheter ablation.980 The authors noted a significantly lower incidence of clinically significant atrial arrhythmias (AF > 24 hours or associated with severe symptoms), cardioversions, and arrhythmia-related hospitalization during the 6-week treatment period (13% vs 28% in the AAD vs non-AAD group; P <.05); however, there was no difference in the 6-month freedom from recurrent AF (72% vs 68%; P = .84).980 As noted earlier in this document, the writing group also recognizes that the usefulness of initiation or discontinuation of AAD therapy during the postablation healing phase in an effort to improve long-term outcomes is unclear (Class IIb, LOE C-LD, Table 3).
Corticosteroids
Given the association between AF recurrence and RF-induced inflammation, it has been postulated that empiric pretreatment with high-dose corticosteroids could reduce the incidence of ERAF and long-term recurrence. One study examined this hypothesis in a population of 125 patients undergoing PV ablation for symptomatic PAF.981 Corticosteroid therapy resulted in a significant reduction in the early AF recurrence rate (27% vs 49% at 1 month with corticosteroids vs placebo, respectively), which was driven by a marked reduction in the immediate recurrence rate (7% vs 31% within 72 hours, respectively). Interestingly, despite the lack of difference in the rate of recurrence between 3 and 30 days (20% vs 18% in the corticosteroid and placebo groups, respectively), the long-term freedom from AF without any AAD was significantly higher in the corticosteroid group (85% vs 71% freedom from AF at 14 months, respectively).
Another study was published recently to evaluate the efficacy of corticosteroids to prevent early and late recurrence. The authors enrolled 138 patients who were randomly assigned to two groups (a steroid group and a control group). The primary endpoint was ERAF during the blanking period (3 months postablation). During the blanking period, 51 of the 138 (37.0%) patients experienced ERAF after AF ablation. The steroid group had a lower rate of ERAF than the control group (15 of 64 [23.4%] vs 36 of 74 [48.6%]; P =.003). There was no difference between the two groups in late recurrence during a 24-month follow-up (log-rank test, P = .918). In a multivariate analysis, short-term steroid therapy was independently associated with a lower rate of ERAF during the blanking period (adjusted odds ratio [OR] 0.45; 95% CI 0.25–0.83; P = .01). The authors concluded that periprocedural short-term moderate intensity steroid therapy reduces early recurrence of ATA (approximately 3 months) after catheter ablation of AF; however, it is not effective in preventing late (3–24 months) AF recurrence.982
Additional information regarding optimum dosing, and safety and tolerability of corticosteroid therapy post-AF ablation is needed before it can be recommended.
Colchicine
Colchicine, an anti-inflammatory agent, has been used post-AF ablation both to reduce pericarditis-related pain, but also to reduce AF. Colchicine has been shown to reduce postoperative AF following cardiac surgery,983,984 and has also been studied following AF ablation. The first major study was a prospective randomized trial in 161 patients undergoing ablation of PAF. Patients were randomized to receive colchicine 0.5 mg bid or placebo.985 At 3 months of follow-up, AF recurred in 34% of the placebo patients vs 16% of the patients treated with colchicine. Colchicine led to a reduction in C-reactive protein and IL-6. A subsequent randomized study of 233 patients with PAF demonstrated a long-term recurrence rate of 31% among the patients treated with colchicine vs 49% among the placebo patients.986 A survey of the writing group members shows that 6% of the members routinely administer colchicine for 1–3 months postablation. Ninety-four percent of the writing group members do not routinely administer colchicine.
Cardioversion
Three studies examined long-term outcomes of patients who required cardioversion for early recurrence of ATAs following RF catheter ablation. One study examined 55 patients who underwent cardioversion 2.7 ± 1.4 months after the index procedure.987 Sinus rhythm was restored in 39 of 45 patients with persistent AF (87%) and 9 of 10 patients (90%) with AFL (P = .77). After a mean follow-up of 15 ± 8 months postablation, only eight patients (15%) remained completely free of AF in the absence of AAD therapy. An additional 11 patients (20%) achieved partial success, as defined by a ≥ 90% reduction in arrhythmia burden, whereas the remaining 36 patients (65%) were considered to have failed ablation. Surprisingly, no differences were noted in acute efficacy or long-term outcomes based on timing of cardioversion (e.g., cardioversion performed during or following the 90-day blanking period).987 Another study reported on outcomes of 384 consecutive patients undergoing AF ablation, of whom 93 had cardioversion at a mean of 88 ± 72 days after ablation (74 for AF, 19 patients for AFL).988 A mean of 16 ± 10 months after the index ablation procedure and 15 ± 10 months after cardioversion, 25 of 93 patients (27%) remained free from recurrent atrial arrhythmias in the absence of AAD therapy. In contrast to the earlier study, the patients in the more recent study who underwent early cardioversion (within 30 days of arrhythmia recurrence) were more than 20 times more likely to remain in sinus rhythm than patients who were cardioverted after 30 days, regardless of the timing of recurrence or whether concomitant AAD therapy was used. In those with a delayed cardioversion, only 2 of 47 patients (4%) remained in sinus rhythm without AAD therapy. In the multivariate analysis, the time from atrial arrhythmia recurrence to cardioversion was the only independent predictor of maintenance of sinus rhythm after a single ablation procedure in the absence of an AAD (P <.001). Interestingly, these two studies reported similar outcomes for patients who underwent cardioversion after 30 days, suggesting that if a benefit is to be gained from early cardioversion, it must be performed within the first month after arrhythmia recurrence. A larger study included consecutive catheter ablations for AF.989 Prompt electrical cardioversion was performed if AF or AFL was confirmed and sustained, using a standard approach with the aim of performing cardioversion within 24 hours of arrhythmia onset. Of the ablations performed, a total of 515 (29%; age: 65.6 ± 11.2 years; male: 57.9%) developed AF or AFL that required cardioversion. The majority of these arrhythmias first occurred in the initial 90 days (63.7%) postablation. During this period, 62.8% were being treated with an AAD. Only 25.1% were using an AAD at 3 months. The majority of patients postablation (75.6%) who experienced AF or AFL within the first 90 days after ablation were in sinus rhythm, requiring no AAD at 1 year. Further, 48% of those patients with the first recurrence from 90 to 180 days were in sinus rhythm with no AAD at 1 year. Thus, it appears that patients undergoing their first cardioversion early after ablation (<3 months) were more likely to remain free from arrhythmia at 1 year (75%).989 An aggressive approach with early electrical cardioversion after LA catheter ablation appears important to maintain sinus rhythm in order to minimize late arrhythmia recurrences, reduce chronic AAD use, and prevent reablation procedures. When comparing an aggressive rhythm control strategy with amiodarone and repetitive use of cardioversion vs amiodarone and infrequent cardioversion in surgical RF ablated patients, systematic and repetitive use of cardioversion resulted in a significantly higher portion of patients in sinus rhythm during follow-up.990 Although the development of a persistent atrial arrhythmia post-AF ablation is a sign of poor prognosis, it is currently recommended to cardiovert those patients preferably within 30 days of arrhythmia onset. Pathophysiological findings supporting rapid functional and structural remodeling during AF encourage the clinician to cardiovert persistent arrhythmias early post-AF ablation. However, the clinical data available supporting this approach remain limited. The number of electrical cardioversions needed to treat repetitive persistent AF recurrences postablation of persistent AF was investigated.991,992 In this small trial of 40 patients, the number of electrical cardioversions ≥3 was the only independent predictor of an ablation failure. Therefore, currently, reablation should be considered in clinical practice after two cardioversions have been performed, because of the high likelihood of recurrent arrhythmias.
Early Reablation
Performance of early reablation reduces the incidence of further recurrences, but the overall number of procedures is higher in the medium-term follow-up. Two studies evaluated the use of early reablation on long-term freedom from AF in patients with ERAF.141,142,143,989,993 In 302 consecutive patients with RF ablation for medically refractive AF, 151 experienced an ERAF, 61 of whom underwent reablation within the first month (e.g., early reablation group). The remaining 90 patients had a repeat procedure at least 1 month after the index ablation. During a mean follow-up of 11 ± 11 months, patients with early reablation had a lower rate of recurrences (51% vs 91%, P <.0001), symptomatic improvement, and improved QOL. However, the total number of procedures required over the entire duration of follow-up was greater in the patients who underwent early reablation (2.5 ± 0.7 vs 2.2 ± 0.6, P = .02).993 The STOP-AF trial randomized 245 patients with PAF to medical therapy versus CB-based PV ablation. Patients were followed for 12 months. Of the 163 patients randomized to cryoablation, 84 patients experienced ERAF (51.5%). The only significant factor associated with ERAF was male sex (HR 2.18; 95% CI 1.03–4.61; P = .041). Late recurrence was observed in 41 patients (25.1%), and was significantly related to ERAF (55.6% late recurrence with ERAF vs 12.7% without ERAF; P <.001). Among the patients with ERAF, only current tobacco use (HR 3.84; 95% CI 1.82–8.11; P <.001) was associated with late recurrence. Conversely, early reablation was associated with greater freedom from late recurrence (3.3% late recurrence with early reablation vs 55.6% without; HR 0.04; 95% CI 0.01–0.32; P = .002).141,142,143 Although the clinical benefit of early reablation was demonstrated, the first month following the procedure might not be the optimal time for a repeat intervention. On the other hand, up to 60% of the patients experiencing this event within the first months postablation will not have any further arrhythmias during long-term follow-up.253,436,970,971,994 Therefore, reablation is not recommended in an ERAF that might be a transient phenomenon.2
Conclusions
Theoretically, aggressive treatment of early recurrences of AF might prevent electrical and structural remodeling and improve long-term outcome. Larger studies with more reliable follow-up methods are needed to clarify the relevance and optimal management of early recurrences.
Atrial Tachycardias After AF Ablation
ATs of new onset make up to 50% of all arrhythmias observed following catheter-based ablation of AF.253,436,507,508,622,623,624,625,630,870,871,995,996,997,998,999,1000,1001,1002 Most of these tachycardias originate in the LA, although RA cavotricuspid isthmus (CTI)-dependent flutters might also occur. Patients with a regular AT of new onset might complain of worsening symptoms due to a faster mean ventricular rate (frequently 2:1 ventricular response) than that during AF preablation. Rhythm control is often difficult with AADs.
The mechanisms underlying regular LA tachycardias following AF ablation include focal microreentrant tachycardias originating from reconnected PV ostia or macroreentrant tachycardias around anatomic obstacles or scar from intrinsic LA disease or prior ablation(s) (Figure 5).447,508,933,998 Occurrence of early AT within 3 months after ablation predicts occurrence of both late AT and AF.1003,1004,1005 However, because up to 49% of ATs resolve with time, ablation should not be undertaken for early AT occurrence unless symptoms cannot be controlled.1003 Initial treatment should include electrical cardioversion and AADs. Because Vaughan Williams Class Ic antiarrhythmic agents promote slow conduction that can facilitate macroreentrant tachycardias, Class III antiarrhythmic agents (dofetilide, sotalol, or amiodarone), together with negative dromotropic agents, are typically preferred. For those with intolerable symptoms or continued late AT recurrence, detailed activation and entrainment mapping of the tachycardia results in effective ablation in approximately 90% of patients.447,622,623,624,1006,1007,1008
Antiarrhythmic and Other Pharmacological Therapy Postablation
AF recurrences during the first 3 months after ablation are rather common. It is generally believed that the mechanisms of AF in this setting are different from that of the patient's clinical arrhythmia. Acute inflammatory changes owing to energy delivery1009; modification of the ANS with consecutive changes in the atrial substrate257; or delayed effect of radiofrequency ablation due to lesion consolidation have been considered.258 It is also suggested that AF might resolve completely upon resolution of the transient factors promoting early AF recurrences. Accordingly, suppressive antiarrhythmic agents are frequently prescribed for patients with AF recurrences during the first 1–3 months following ablation.253,436,988,1010,1011 Because ATs can also occur shortly after ablation, negative dromotropic agents (beta or calcium channel blockers) are commonly continued for at least the first month after ablation. The impact of empirical AAD therapy for 6 weeks after AF ablation on the occurrence of AF was investigated in several randomized studies.934,979,980 The drugs employed for this purpose vary, but most commonly are those that have been used unsuccessfully prior to ablation; they include flecainide, propafenone, sotalol, dofetilide, dronedarone, and amiodarone. The short-term use of AADs after AF ablation decreased early recurrences of atrial arrhythmias and need for hospitalization or cardioversion, but had no effect on the prediction or prevention of arrhythmia recurrence at 6 and 12 months.934,979,980 As noted earlier in this document, the writing group recognizes that the usefulness of initiation or discontinuation of AAD therapy during the postablation healing phase in an effort to improve long-term outcomes is unclear (Class IIb, LOE C-LD, Table 3).
Because an inflammatory process after AF ablation can be one specific cause leading to early recurrences, the efficacy of corticosteroids for preventing early postablation atrial arrhythmias was investigated in several studies.981,982 The prevalence of immediate AF recurrences (≤3 days after PVI) was significantly lower in the corticosteroid group compared with the placebo group (7% vs 31%). However, few investigators routinely administer steroids during or following AF ablation. The use of PPIs or H2 blockers for 1–4 weeks following ablation has been suggested to avoid esophageal ulcerations observed on endoscopy following AF ablation.896,897 However, there are no randomized data available to demonstrate that this approach reduces the incidence of esophageal symptoms or the development of an AEF. Early diagnosis of AEF, with early employment of operative intervention, is the best treatment option for AEF (please refer to Section 10 for more information). Attention to the control of hypertension and addressing other AF risk factors such as sleep apnea and obesity remain an integral part of AF management after the ablation procedure.929 The impact of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the long-term outcome of AF ablation was investigated in a prospective registry of consecutive patients undergoing catheter ablation of paroxysmal or persistent AF.334 In that study, however, modulation of the renin-angiotensin aldosterone system did not appear to affect maintenance of sinus rhythm after catheter ablation of AF. Thus, the hypothesis that so-called medical upstream therapy can positively influence the reverse atrial remodeling after catheter ablation of AF remains unproven.
Later-Term Repeat Ablation Procedures
Recurrences of AF (or AT) after index AF ablation procedures lead to repeat ablation in a considerable number of patients. Recent data show a repeat procedure rate of 15% and 50% depending on the duration of follow-up and patient characteristics.1012 Since early recurrences of AF and/or the development of AT are common during the first 2–3 months after AF ablation and might resolve spontaneously, repeat ablation procedures should be deferred for at least 3 months following the initial procedure if possible. Nevertheless, such early recurrences are associated with decreased long-term success of the procedure.260 It is also recognized that some patients will develop highly symptomatic early recurrence of atrial arrhythmias that cannot be controlled with antiarrhythmic therapy or slowed with rate controlling medications and are best managed with a reablation procedure within the first 3 months post-AF ablation. Most studies have reported that patients who fail an initial attempt at ablation and undergo a repeat ablation procedure demonstrate resumption of electrical conduction of the previously isolated PVs rather than new arrhythmogenic foci from nontargeted PVs or outside of the PVs.263,440,446,1013 This outcome appears to be overwhelmingly the case for the first reablation procedure, whereas in further redo procedures (e.g., second or third redo procedure) other mechanisms also appear to play a more important role.1014
Consequently, the first step when performing a second AF ablation procedure is to check each PV for reconduction of electrical activity. If reconduction is found, the primary goal should be reisolation of the PVs. If, however, there is no evidence of PV reconduction, the decision on the best ablation technique is more complex. Several targets have been proposed in this setting, such as LA substrate mapping and tailored ablation guided by electrogram voltage, ablation with complex fractionated electrograms, ablation of provoked non-PV triggers or sites commonly associated with non-PVs triggers such as the SVC, or targeting of focal impulse and rotational activity mapping. However, definitive evidence of the benefit or superiority of any of these techniques over the others is lacking.126,223,247,257,567,1015,1016 Data on current clinical practice confirm the prevailing uncertainty regarding the best reablation technique.540
High-dose isoproterenol infusions have been shown to be helpful in the provocation of PV and non-PV triggers.440,1013 A recent randomized trial showed that adenosine administration during the ablation procedure might unmask dormant PV conduction and reduce the recurrence rate of the procedure, but results were not confirmed in another trial evaluating ATP.265,1017 The value of adenosine administration at the time of the redo procedure to demonstrate latent PV conduction has not been demonstrated.461
Autonomic Alterations
Potential side effects of AF ablation include transient and permanent alterations in autonomic nerve activity. Transient (<6 months) elevation of heart rate, inappropriate sinus tachycardia and reduction of heart rate variability have been observed after PVI.223,257 Others reported an immediate decrease in autonomic function such as deceleration capacity and acceleration capacity after PVI. Some of these changes can last for over a year.126,1016 Although most autonomic alterations associated with PVI are transient and are not associated with significant symptoms, more severe autonomic alterations can occur in cases of periesophageal vagal nerve injury.265,461,868,1017,1018,1019,1020 A prospective observational study showed a high incidence (33%–48%) of transient (<6 months) new onset alterations in esophageal motility after AF ablation.536 Although most patients recover within several months, gastric hypomotility can persist for over 28 months after the procedure in rare cases.1020 A case of achalasia cardia has recently been reported to occur after PVI.252,1021 Another study showed a 7.9% prevalence of gastric hypomotility after high output (25–30 W) posterior LA ablation. Reduction of the output to 20–25 W at sites where the ablation line transversed the esophagus eliminated the postablation esophageal hypomotility.1020 In additional to the watts used during ablation, it is possible that both time and CF are factors that determine periesophageal vagal nerve injury. However, the CF was not evaluated in the latter study. In summary, most autonomic alterations associated with AF ablation were self-terminating and asymptomatic. However, severe symptomatic periesophageal vagal nerve injury can occur after LA posterior wall ablation.
Very Late Recurrence (More Than 1 Year) After AF Ablation
Many groups have reported the incidence of very late AF recurrences occurring up to 10 years postablation, even after an initially successful procedure at 1 year.63,270,536,1022,1023,1024 A recent meta-analysis analyzed 19 studies including 6167 patients, describing outcomes ≥3 years after AF ablation with a mean follow-up ≥24 months after the index procedure. Single procedure and multiple procedure freedom from atrial arrhythmias has been reported to be 53% and 80%, respectively, with substantial heterogeneity noted for single-procedure outcomes.
Very late recurrences have been noted after an initial freedom from AF at 1 year postablation, with an annual recurrence rate estimated at 7.6%, reaching attrition rates of 16%–46% and 30%–54% at 5 and 10 years, respectively. Interestingly, despite the recurrence rates, a low incidence of progression (0.3% per year) from paroxysmal to persistent AF as well as stroke rates <1% have been reported. Also noteworthy is the fact that time to recurrence might influence outcomes. Patients with very late recurrences are more likely to have sporadic episodes and a better response to AADs and repeat ablation procedures than those with earlier recurrences.
The most consistent predictor of late recurrence is persistent AF. Other predictors include hypertension, age, LA size, diabetes, valvular heart disease and left ventricular dysfunction, and higher thromboembolic risk scores.63 Recurrences in patients undergoing repeat ablation procedures have been noted to be due mostly to PV reconnection. However, recent evidence pointing to the importance of non-PV foci and gaps in prior ablation lines can also play a role. In particular, the LAA and LA posterior wall have been shown to contain significant triggers in patients with non-PAF and isolation strategies are suggested to be of benefit in improving long-term outcomes.
Section 9: Outcomes and Efficacy
Overview
AF ablation is a maturing field. Prior to the publication of the initial consensus report in 2007, the majority of the published literature on AF ablation consisted of uncontrolled single- and multicenter reports.1 Further, there had been no standardization in the design of clinical trials of AF ablation, and the 2007 and 2012 consensus documents were developed in part to generate standard terminology, definitions, and recommendations for end points, follow-up procedures, and outcome reporting in an effort to make studies more rigorous and consistent.1,2
Over the past 10–12 years, a large number of randomized trials have been completed addressing various aspects of AF ablation. Many have compared AF ablation with AAD therapy in both “first-line” (AAD-naïve patients) and “second-line” (following the failure of 1 or more drugs) settings.261,377,378,379,462,529,684,733,1025,1026,1027,1028,1029,1030 In some cases, these trials have supported regulatory approval of specific ablation technologies.462,503,655,673,684,733 Some trials have compared AF ablation with other standard pharmacological or nonpharmacological approaches to rate control.235,236,237,390 Many other trials have compared various ablation techniques or alternative ablation systems with each other. Table 7 provides a summary of the outcomes of a selected group of clinical trials of AF ablation. This table includes a summary of the clinical trials that have been performed for FDA approval, clinical trials of AF ablation as first-line therapy, as well as randomized clinical trials of AF ablation for PAF, persistent AF, mixed trials, and randomized trials of AF ablation in patients with HF.
Table 7.
Selected clinical trials of catheter ablation of atrial fibrillation and/or for FDA approval
| Trial | Year | Type | N | AF type | Ablation strategy | Initial time frame | Effectiveness endpoint | Ablation success | Drug/Control success | P value for success | Ablation complications | Drug/Control complications | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Trials Performed for FDA Approval | |||||||||||||
| JAMA 2010; 303: 333-340 (ThermoCool AF)684 | 2010 | Randomized to RF ablation or AAD, multicenter | 167 | Paroxysmal | PVI, optional CFAEs and lines | 12 months | Freedom from symptomatic paroxysmal atrial fibrillation, acute procedural failure, or changes in specified drug regimen | 66% | 16% | <0.001 | 4.9% | 8.8% | FDA approval received |
| JACC 2013; 61: 1713-1723 (STOP AF)462 | 2013 | Randomized to cryoballoon ablation or AAD, multicenter | 245 | Paroxysmal | PVI | 12 months | Freedom from any detectable AF, use of nonstudy AAD, or nonprotocol intervention for AF | 70% | 7% | <0.001 | 3.1% | NA | FDA approval received |
| Heart Rhythm 2014; 11: 202-209 (TTOP)733 | 2014 | Randomized to phased RF ablation or AAD/cardioversion, multicenter | 210 | Persistent | PVI + CFAEs | 6 months | Acute procedural success, ≥90% reduction in AF burden, off AAD | 56% | 26% | <0.001 | 12.3% | NA | Not FDA approved |
| JACC 2014; 64: 647-656 (SMART-AF)673 | 2014 | Nonrandomzied multicenter study of contact force-sensing RF catheter, comparing to performance goals | 172 | Paroxysmal | PVI, optional CFAEs and lines | 12 months | Freedom from symptomatic AF, flutter, tachycardia, acute procedural failure, or changes in AAD | 72.5% | N/A | <0.0001 | 7.5% | NA | FDA approval received |
| Circulation 2015; 132: 907-915 (TOCCASTAR)655 | 2015 | Randomized to contact force sensing RF catheter or approved RF catheter, multicenter | 300 | Paroxysaml | PVI, optional triggers, CAFEs and lines in both arms | 12 months | Acute procedural success + Freedom from Symptomatic AF/Flutter/Tachycardia off AAD | 67.8% | 69.4% | 0.0073 for noninferiority | 7.2% | 9.1% | FDA approval received |
| JACC 2015; 66: 1350-1360 (HeartLight)503 | 2015 | Randomized to laserballoon or approved RF catheter, multicenter | 353 | Paroxysmal | PVI ± CTI ablation vs PVI, optional CFAEs, and Lines | 12 months | Freedom from Symptomatic AF/Flutter/Tachycardia, acute procedural failure, AAD, or non-prototocol intervention | 61.1% | 61.7% | 0.003 for noninferiority | 5.3% | 6.4% | FDA approval received |
| First-Line Therapy Trials | |||||||||||||
| JAMA 2005; 293: 2634-2640 (RAAFT)377 | 2005 | Randomized to drug, multicenter | 70 | Paroxysmal (N=67), persistent (N= 3) | PVI | 12 months | Freedom from detectable AF | 84% | 37% | <0.01 | 9% | 11% | |
| NEJM 2012; 367:1587-1595 (MANTRA-PAF)378 | 2012 | Randomized to drug, multicenter | 294 | Paroxysmal AF | PVI, roof line, optional mitral and tricuspid line | 24 months | Cumulative AF burden | 13% AF burden | 19% AF burden | NS | 17% | 15% | |
| JAMA 2014; 311: 692-700 (RAAFT-2)379 | 2014 | Randomized to drug multicenter | 127 | Paroxysmal AF | PVI plus optional non-PVI targets | 24 months | Freedom from detectable AF, flutter, tachycardia | 45% | 28% | 0.02 | 9% | 4.9% | |
| Other Paroxysmal AF Ablation Trials | |||||||||||||
| JACC 2006; 48: 2340-2347 (APAF)1027 | 2006 | Randomized to drug single center | 198 | Paroxysmal AF | PVI, mitral line and tricuspid line | 12 months | Freedom from detectable AF, flutter, tachycardia | 86% | 22% | <0.001 | 1% | 23% | |
| Circulation 2008; 118: 2498-2505 (A4)261 | 2008 | Randomized to drug | 112 | Paroxysmal | PVI (optional LA lines, CTI, focal) | 12 months | Freedom from AF | 89% | 23% | <0.0001 | 5.7% | 1.7% | |
| NEJM 2016; 374: 2235-2245 (FIRE AND ICE)489 | 2016 | Randomized RF vs Cryo, multicenter | 762 | Paroxysmal AF | PVI | 12 months | Freedom from detectable AF, flutter, tachycardia | 64.1% (RF) | 65.4% (cryo) | NS | 12.8% | 10.2% | |
| JACC 2016; 68: 2747-2757709 | 2016 | Randomized to hot balloon or drug, multicenter | 100 | Paroxysmal AF | PVI | 12 months | Freedom from AF | 59% | 5% | <0.001 | 10.4% | 4.7% | |
| Other Persistent AF Ablation Trials | |||||||||||||
| NEJM 2006; 354: 934-9411026 | 2006 | Randomized to RF ablation or to CV and short term amio | 146 | Persistent | PVI, roof, mitral line | 12 months | No AF or flutter month 12 | 74% | 58% | 0.05 | 1.3% | 1.4% | |
| EHJ 2014; 35: 501-507 (SARA)1030 | 2014 | Randomized to drug (2:1 ablation to drug), multicenter | 146 | Persistent | PVI (optional LA lines, CFAEs) | 12 months | Freedom from AF/flutter lasting >24h | 70% | 44% | 0.002 | 6.1% | 4.20% | |
| NEJM 2015; 372: 1812-1822245 | 2015 | Randomized ablation strategies, multicenter | 589 | Persistent | PVI alone versus PVI & CFAEs or PVI & lines | 18 months | Freedom from afib with or without drugs | 59% (PVI alone) | 49% & 46% | NS | 6% | 4.3% & 7.6% | |
| Other Mixed Paroxysmal and Persistent AF Ablation Trials | |||||||||||||
| J Med Assoc Thai 2003; 86 (Suppl 1): S8-S161025 | 2003 | Randomized to RF ablation or amiodarone | 30 | Paroxysmal (70%), Persistent (30%) | PVI, mitral line, CTI, SVC to IVC | 12 months | Freedom from AF | 79% | 40% | 0.018 | 6.70% | 47% | |
| EHJ 2006; 27: 216-2211028 | 2006 | Randomized to RF ablation or drug, multicenter | 137 | Paroxysmal (67%), Persistent (33%) | PVI, mitral line, CTI | 12 months | Freedom from AF, flutter, tachycardia | 66% | 9% | <0.001 | 4.40% | 2.90% | |
| JCVEP 2009, 20: 22-281029 | 2009 | Randomized to RF ablation or drug, multicenter | 70 | Paroxysmal (41%), Persistent (59%) & type 2 DM | PVI, CTI, optional mitral line and roof line | 12 months | Freedom from AF and atypical atrial flutter | 80% | 43% | 0.001 | 2.90% | 17% | |
| Randomized Trials of AF Ablation in Patients with Heart Failure | |||||||||||||
| NEJM 2008; 359: 1778-1785 (PABA-HF)235 | 2008 | Randomized to RF ablation of AVJ abl and BiV pacing | 81 | Persistent (50%), Paroxysmal (50%), EF 27% abl, 29% AVJ | PVI, optional linear abl and CFAEs | 6 months | Composite EF, 6 min walk, MLWHF score; freedom from AF (secondary, mult proc, +/- AA drugs) | 88% AF free, EF 35% abl, 28% AVJ (P <.001), > QOL and 6 min walk increase with abl | <0.001 | 14.60% | 17.50% | ||
| Heart 2011; 97: 740-747236 | 2011 | Randomized to RF ablation or pharmacological rate control | 41 | Persistent, EF 20% abl, 16% rate control | PVI, roof line, CFAEs | 6 months | Change in LVEF, sinus rhythm at 6 months (secondary) | 50% in NSR, LVEF increase 4.5% | 0% in NSR, LVEF increase 2.8% | 0.6 (for EF increase) | 15% | Not reported | |
| JACC 2013; 61: 1894-1903390 | 2013 | Randomized to RF ablation or pharmacological rate control | 52 | Persistent AF (100%), EF 22% abl, 25% rate control | PVI, optional linear abl and CFAEs | 12 months | Change in peak O2 consumption (also reported single procedure off drug ablation success) | Peak O2 consumption increase greater with abl, 72% abl success | 0.018 | 15% | Not reported | ||
| Circ A and E 2014; 7: 31-38237 | 2014 | Randomized to RF ablation or pharmacological rate control | 50 | Persistent AF (100%), EF 32% abl, 34% rate control | PVI, optional linear abl and CFAEs | 6 months | Change in LVEF at 6 months, multiple procedure freedom from AF also reported | LVEF 40% with abl, 31% rate control, 81% AF free with abl | 0.015 | 7.70% |
AF, atrial fibrillation; RF, radiofrequency; AVJ, atrioventricular junction; abl, ablation; BiV, biventricular; EF, ejection fraction; PVI, pulmonary vein isolation; CFAEs, complex fractionated atrial electrograms; MLWHF, Minnesota Living with Heart Failure; LVEF, left ventricular ejection fraction; QOL, quality of life; NSR, normal sinus rhythm.
In this section, we will focus our review of this large and growing body of literature on trials comparing AF ablation with alternative treatment approaches—primarily AADs—for AF, to provide support for recommendations on the role of AF ablation in various patient groups. Outcomes for specific ablation systems (CBA, rotational activity ablation, and laser balloon ablation) will also be reviewed. Studies comparing ablation techniques or lesion sets with each other are primarily discussed in Section 5: Strategies, Techniques, and Endpoints.
Previous versions of the consensus report included a section on nonrandomized studies of AF ablation. Due to the very large number of studies reported, the lack of standardization among them, and their generally early time frame in the evolution of AF ablation, we will not review that literature in the current document, and instead we refer readers to the prior consensus documents and to a large meta-analysis on the topic.262 Useful insights into AF ablation outcomes outside the setting of RCTs have also been obtained from worldwide surveys on AF ablation, and these will also be reviewed.
In addition to a broad overview of AF ablation trials among commonly treated patient groups, this section will also review outcomes of AF ablation in populations not well represented in clinical trials and with specific ablation systems. Additionally, end points beyond maintenance of sinus rhythm of considerable interest to the field (e.g., QOL, stroke, cost-effectiveness) will be examined.
Published Literature Review: Clinical Trials Performed for FDA Approval
When AF ablation began, procedures were performed using standard 4 mm and later 8 mm tipped, nonirrigated RF catheters that had been developed and approved for the treatment of other arrhythmias. The first two classes of devices to seek and achieve FDA approval for ablation of AF were irrigated RF catheters and CBA catheters (Table 7). In consultation with the FDA, the manufacturers of these devices were required to conduct randomized trials comparing AF ablation to AADs and chose to evaluate patients with PAF who had previously failed treatment with one or more drugs.
Although the two initial device approval studies had narrow entry criteria and enrolled primarily young and healthy AF patients, they were conducted with great rigor and contributed substantially to the previous literature comparing ablation to AAD therapy.462,684 In both cases, protocol-defined success with ablation (66% and 70%, respectively) was much higher than with drugs (16% and 7%), and acceptable rates of serious adverse events occurred.
Given the overwhelmingly superior efficacy of AF ablation in this patient population, ablation has become the standard of care in many centers. Accordingly, subsequent FDA-regulated device approval studies of novel ablation technologies have not required randomization against AADs, because doing so would be not be feasible. Instead, several new technologies have been either compared with a previously approved device with the same indications for use in a randomized, controlled noninferiority study or, in the case of a second generation RF ablation catheter, compared with predefined performance goals in a single-arm study. Examples of this pathway include recent trials of force-sensing655,673 and the laser balloon ablation system.503,673 Each of these trials met its prespecified end points, generally confirming the safety and efficacy of AF ablation in patients with PAF, but none demonstrated the superiority of new technologies in the full study populations. Protocol-defined success rates at 12 months in these studies ranged from 61% to 72.5% (primary effectiveness definitions were not identical across studies). Because these studies were designed to demonstrate noninferiority to an approved device with the same indications for use, they have been subject to the same limitations in terms of patient population, follow-up duration, etc., as the earlier studies.
An additional study of a phased array multielectrode RF ablation system performed under regulatory supervision by the FDA in patients with persistent AF refractory to ≥ 1 AAD has also been completed.733 In this study, patients were randomized to ablation vs treatment with an alternative AAD or increased dose of a previously ineffective drug and DC cardioversion. As expected, protocol-defined success was higher following ablation at 6 months (56% vs 26%). However, the study did not meet its prespecified safety end point, partly due to the occurrence of 4 strokes (2.9% of the patients randomized to ablation) that mainly occurred during early experience with the ablation system. As a result, this system has not been approved in the United States.
For the purpose of regulatory approval, it is expected that future ablation technologies designed to treat PAF will continue to be compared with previously approved ablation systems in randomized studies. We believe that this is appropriate, although there should be careful consideration of the possibility of a downward “creep” in acceptable effectiveness (if each device is numerically inferior but statistically equivalent to the prior comparator device). In the future, we expect that devices designed to treat patients with symptomatic PAF might alternatively be evaluated in nonrandomized trials, comparing prespecified performance goals or objective performance criteria (OPC), if uniformly established and applied. However, given the rapid evolution of the field of AF management, it should be understood that such performance criteria are potentially subject to change over time. An OPC refers to a numerical target value derived from historical data from clinical studies and/or registries and can be used in a dichotomous (pass or fail) manner by the FDA for the review and comparison of safety or effectiveness endpoints. Currently, no such OPC has been validated with respect to catheter ablation of persistent AF or PAF. If an OPC is employed, it is important to clarify the patient population to which it applies. It is anticipated that the patient population should be similar to predicate patient populations. However, in the clinical trials section of this document we have provided what we believe are acceptable OPC for AF ablation clinical trials.
Studies seeking regulatory approval for the treatment of persistent and long-standing persistent AF can follow one of two potential approaches. As in the past, future studies might compare novel ablation systems against medical management because, at this point, no ablation system is expressly approved for persistent or long-standing persistent AF in the United States. Alternatively, a novel ablation system could be evaluated in single-arm trials with prespecified OPCs.
AF Ablation as Second-Line Rhythm Control Therapy
At the time of this writing, at least 16 randomized clinical trials have been completed comparing AF ablation with AADs in patients with AF refractory to one or more AADs. Each of these trials is summarized in Table 7. In addition to the trials for FDA approval listed in Table 7, four of these trials exclusively enrolled patients with paroxysmal (or “early persistent”) AF, three trials enrolled only patients with persistent AF, and three trials enrolled patients with either AF pattern.261,462,684,733,1025,1026,1027,1028,1029,1030 Additional randomized trials in patients with HF and persistent AF have been completed, comparing ablation with rate control, amiodarone, or AV junction ablation with biventricular pacing.235,236,237,390,529 The HF trials will be reviewed in a later section of this document.
In general, the trials involving PAF focused on PVI (although adjunctive ablation was allowed or encouraged to varying degrees), and reported success rates at 12 months ranged from 59%–89%. In all cases, freedom from arrhythmia at 12 months was significantly higher than with drug therapy, which had reported success rates of 5%–23%. Among trials that included patients with persistent AF or combined paroxysmal and persistent populations, the ablation techniques more frequently incorporated linear lesion sets or ablation with CFAEs. Reported success rates with ablation ranged from 59%–80% at 6 or 12 months, whereas success rates with drug therapy ranged from 9%–58%. In all cases, maintenance of sinus rhythm was significantly higher with ablation.
It is difficult to directly compare adverse events from AF ablation to those from AADs. In most of the above trials, low rates of serious procedural complications were reported. With the exception of the HF trials, most of the second-line rhythm control trials enrolled relatively healthy and young patients with AF. Mortality rates in these relatively small trials have therefore been very low, precluding any meaningful attempts to determine whether ablation has any impact on mortality.
The many trials comparing AF ablation with AADs for second-line rhythm control have been evaluated in a number of meta-analyses and technology assessments.262,1031,1032 Pooling of results across trials indicates that AF ablation is clearly superior to AAD therapy for the maintenance of sinus rhythm. The impact of AF ablation on other key outcomes, including HF, stroke, and QOL, will be reviewed in subsequent sections of this document.
Outcomes and Efficacy of Catheter Ablation of AF as First-Line Rhythm Control Therapy
There have been several studies performed to investigate the role of AF ablation as first-line therapy, prior to a trial of a membrane-active antiarrhythmic medication.377,378,379 The Medical ANtiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation (MANTRA-PAF)494 trial compared catheter ablation with AAD therapy for the first-line therapy of symptomatic PAF.378 The trial did not show a reduction in the cumulative AF burden over 2 years; however, catheter ablation was associated with a lower rate of AF recurrence (15% vs 29%, P =.004) and a similar rate of complications (17% vs 15%) compared with AAD. The unexpected result in the MANTRA-PAF might be explained by the ablation techniques with discretional circumferential ablation without confirmation of PVI with a circular mapping catheter as well as by the choice of reduction in AF burden on 7-day Holter as a primary endpoint. Reductions in AF burden on a short 7-day Holter can be difficult to demonstrate in a paroxysmal population. In the Radiofrequency Ablation versus Antiarrhythmic drugs as First-line Treatment of Paroxysmal AF (RAAFT-2) trial, PV antrum isolation was performed using irrigated-tip ablation catheters confirmed by recordings from a circular mapping catheter.379 The results of the RAAFT-2 demonstrated that AF or AT recurred in 55% of the catheter ablation group compared with 72% of the patients in the AAD group after a 2-year follow-up (P = .016).
Moreover, a meta-analysis showed that first-line therapy with catheter ablation was more effective than AAD for long-term maintenance of sinus rhythm and was associated with comparable rates of adverse events in relatively young patients with PAF and minimal structural heart disease.380 A preliminary analysis based on the results of the first RAAFT trial suggests that catheter ablation as a first-line therapy also has a better cost-effectiveness profile.1033 These results provide some support for the role of catheter ablation as first-line therapy for PAF. Whether such benefits extend to elderly patients with PAF, patients with associated structural heart disease, or non-PAF is still controversial.
One small, prospective, multicenter randomized study was performed to evaluate whether catheter ablation is superior to AAD in patients with HF and persistent AF.529 The main goal of the ablation procedure was PV antrum isolation and LA posterior wall isolation. The results showed that catheter ablation was superior to amiodarone in achieving freedom from AF at the long-term follow-up (70% vs 34%, P <.001). Moreover, ablation improved QOL and exercise capacity, and reduced hospitalization (31% vs 57%, P <.001) and mortality (8% vs 18%, P = .037). These data provide some support for the notion of AF ablation as first-line therapy; however, further studies are needed.
Published Literature Review: Survey Results
A worldwide survey on the methods, efficacy, and safety of catheter ablation of AF was first published in 2005.806 The outcomes of nearly 9000 AF ablation procedures were reported. More than one ablation procedure was performed in 27% of patients. The success rate, defined as freedom from symptomatic AF in the absence of antiarrhythmic therapy, was 52%. An additional 24% of patients were free of symptomatic AF in the presence of a previously ineffective AAD. The incidence of major complications was 6%.
In a subsequent survey from the same group, the clinical outcome and safety of AF ablation performed between the years 2003 to 2006 in 85 participating centers proved to be better than in the previous years.920 During a follow-up of 10 ± 8 months, 192 procedures per center were reported with a 70% efficacy rate free of AADs, and an additional 10% efficacy rate in the presence of previously ineffective AADs. Ablation of PAF was associated with a 35% and 66% larger probability of success compared with ablation of persistent and long-standing persistent AF, respectively. Despite a larger prevalence of centers reporting catheter ablation of persistent and long-standing persistent AF, the overall complication rate was 4.5%. There were 25 procedure-related deaths (0.15%), 37 strokes (0.23%), 115 TIAs (0.71%), and 213 episodes of tamponade (1.31%).
In a subsequent report analyzing the risk of periprocedural death by means of an aggregate calculation from the previous two surveys, 32 fatal events were observed (0.98 per 1000 patients) during 45,115 procedures in 32,569 patients.908 Cardiac tamponade was found to be the most frequent cause of death, with 8 patients (1 more than 30 days) suffering a fatal outcome as a consequence of this complication. Stroke was reported as the cause of death in 5 patients (2 more than 30 days), AEF in 5 patients, and massive pneumonia in 2 patients.
More recently, the same authors provided a systematic analysis of 45 delayed tamponade events (e.g., cardiac tamponade occurring at least 1 hour after procedure termination) in 21,478 patients undergoing 27,921 procedures (0.2%).1034 The median time to tamponade was 12 days (range: 0.2–45 days) after procedure termination, with only 4 patients experiencing this event prior to discharge. The mode of clinical presentation varied, with 39 patients exhibiting gradual progression to cardiac tamponade and 6 patients experiencing severe symptoms within minutes. Two patients died from this complication (risk of death 1 per 10,000 patients).
In January 2014, another survey reported on the first prospective series providing preprocedural, procedural, and 1-year follow up data on 72 centers enrolling about 1400 patients undergoing a median of 1.2 procedures.969 In their registry, the authors reported a 1-year success rate free from AAD therapy of only 40% (44% in PAF; 30% in persistent AF; 37% in long-term persistent AF). Adding AAD therapy increased the successful control rate to 72%. This result was achieved with 20% of the patients having undergone at least a second procedure. Using a multivariate analysis, the authors found that AF recurrence during the 3-month blanking period was the only predictor of failure at the 1-year follow-up.
In 2014, the Prospective European Survey on AF Ablation investigators published a prospective consecutive series of 946 consecutive patients enrolled in 35 centers.1035 AF patterns were paroxysmal, persistent, and long-standing persistent in 52%, 36%, and 12% of patients, respectively, with 12% of the centers offering AF ablation as first-line therapy. PVI was performed in all the centers, with empiric linear lesions and/or ablation of CFAEs being delivered as adjunctive approaches in patients with non-PAF. RF was the dominant energy form used (more than 95% of procedures), with cryoenergy and laser offered in 4% and less than 1% of procedures, respectively.
In a recent survey led by the EHRA, the strategy used by 30 European centers for treating persistent AF was reported.540 Almost half of the recruiting centers were performing more than 400 catheter ablations per year and more than 200 LA ablations. PVI was the main technique in patients undergoing first-time ablation for persistent, but not long-standing persistent, AF in the majority of the centers (67%), with ablation using fractionated electrograms, either as an addition to PVI or as a stand-alone procedure, in 13% and 3% of centers, respectively. A stepwise AF ablation technique was used in only 3% of centers. In patients with long-standing persistent AF, stand-alone PVI was adopted in only one-third of centers. In the remaining two-thirds, ablation with fractionated electrograms, stepwise ablation until AF was terminated, and PVI plus linear lesions at the LA roof or the mitral isthmus were the most frequently reported techniques. When PVI was the only technique used in patients with persistent or long-standing persistent AF, 20% and 10% of procedures were performed with CB, respectively. The 1-year success rate after a single procedure at 1 year was found to be 50%–60% in 40% of the centers, with three centers reporting a success rate of less than 40% and three centers reporting a success rate higher than 80%.
Outcomes of AF Ablation in Populations Not Well Represented in Clinical Trials
Outcomes of Catheter Ablation of Persistent and Long-Standing Persistent AF
Persistent AF is quite heterogeneous regarding the pathophysiological mechanisms responsible for electrical and structural remodeling of the atria. In addition, persistent AF itself is an independent predictor of recurrence, and catheter ablation has reduced success compared with PAF.268 Success rates vary according to the heterogeneity of the patient population and ablation strategies that are encompassed under the umbrella “non-PAF.” It is now increasingly well recognized that the duration of continuous AF is an important predictor of the efficacy of AF ablation. Patients with continuous AF of 12 months or less duration are very different from patients who have been in continuous AF for years.
The quality and quantity of data concerning the outcomes of AF ablation in patients with non-PAF, including both persistent and long-standing persistent AF, is limited.529,733,1015,1030Table 7 shows the outcomes of four trials of catheter ablation for persistent AF.245,733,1026,1030 Despite the widespread performance of AF ablation on patients with persistent AF, no ablation catheters have received FDA or CE Mark labeling specifically for the indication of ablation of persistent AF. One completed clinical trial was performed with a goal of obtaining FDA labeling for ablation of persistent AF using a novel, phased RF multielectrode ablation system.733 In this study, patients were randomized to ablation or to treatment with an alternative AAD or an increased dose of a previously ineffective drug, and DC cardioversion. As expected, protocol-defined success was higher following ablation at 6 months (56% vs 26%). However, the study did not meet its prespecified safety endpoint, partly due to the occurrence of 4 strokes (2.9% of the patients randomized to ablation) that mainly occurred during early experience with the ablation system. As a result, this system has not been approved in the United States. There have been a number of studies performed comparing ablation strategies in patients with persistent AF. The largest was the recently published STAR-AF trial.245 This well-performed and adequately powered study randomized patients with persistent AF to ablation with PVI alone, PVI alone plus linear ablation, or PVI plus ablation of CFAEs. No difference in efficacy was observed, and there was a trend toward superiority of PVI alone.
It is important to note, however, that several prospective clinical trials are planned or have begun in an effort to obtain FDA labeling for the use of point-by-point RF ablation and cryoablation. Both these trials have chosen to enroll patients with early persistent AF (and to exclude patients with long-standing persistent AF) and to employ OPC. In addition to these trials, there has been one small, prospective, randomized clinical trial that compared the outcomes of ablation with AAD therapy in 146 patients with persistent AF. The efficacy of AF ablation in this study was superior to AAD therapy.1030
The writing group recommendations for techniques to be used for ablation of persistent and long-standing persistent AF are shown in Table 3. PVI remains the cornerstone of all AF ablation procedures and is recommended. Several new ablation strategies are being explored for use in patients with persistent AF. These approaches include mapping and ablation of rotational activity, ablation of areas of low voltage, ablation of areas identified on MRI as showing fibrosis, ablation of non-PV triggers, as well as LAA focal ablation, isolation, and/or ligation. Each of these techniques is described elsewhere in this document.
In this regard, there is considerable debate as to which of these techniques, if any, should be employed during an initial or repeat AF ablation procedure in patients with long-standing persistent AF. An important study was a report of the 5-year outcomes of the “stepwise” approach to AF ablation515 The single-procedure efficacy of this approach at 1 year was 35%, falling to 17% at year 5. With repeated procedures, the arrhythmia-free survival after the last procedure at the 5-year follow-up was 63%. These and other trials are an important reminder that new ablation strategies should not be widely adopted into routine clinical practice until the safety, efficacy, and true clinical value of these new strategies have been demonstrated in well-designed and adequately powered prospective randomized clinical trials. As a result of these and other studies, use of the stepwise ablation strategy, empiric linear ablation, and ablation of CFAEs are much less commonly performed today than in the past, when this was a popular ablation strategy for patients with persistent AF.
Outcomes of AF Ablation in Elderly Patients
Several studies have been published describing the outcomes of AF ablation in elderly patients. One study compared the safety and efficacy of catheter ablation in three groups of patients: <65 years, 65–74 years, and ≥75 years. Although the total study population included 1165 patients, the group ≥75 years only included 32 patients. Over a mean follow-up of 27 months, AF control defined as no AF on or off AADs or “rare” AF was comparable in the older and younger patients. The older patients were less likely to undergo repeat ablation and were more likely to remain on AADs. Complication rates were similar.1036 In another study of 174 patients >75 years, 55% of whom had PAF, 127 (73%) maintained sinus rhythm after a single procedure, with an acute major complication rate of 1%.1037 Another study evaluated catheter ablation for AF in 103 octogenarians with paroxysmal, persistent, or long-standing persistent AF compared with patients <80 years.399,400,401 The proportion of patients with the different types of AF was similar in both groups. A higher rate of non-PV triggers (84% vs 69%, P = .001) was found in the octogenarians. After a mean follow-up of 18 ± 6 months, 71 (69%) of the octogenarians remained free from AF off AADs after a single procedure vs 71% in patients <80 years (P = NS). Complication rates did not differ between the two groups. Other studies of octogenarians have found similar results.398,1038
In a retrospective cohort study involving Medicare claims for 15,423 patients who underwent ablation procedures associated with a primary diagnosis of AF, it was found that advanced age was a major risk factor for all adverse outcomes. However, the overall rate of adverse outcomes was fairly small. Only 11% of patients underwent a second ablation procedure by 1 year after the index procedure, a low rate that has been observed in other studies.1039 In another analysis of a large commercial claims database, acute complications in patients over and under the age of 65 years were nearly identical.1040 Although comparable rates of periprocedural strokes have generally been found, late stroke might be more common in older patients.1041
In general, studies have shown similar success rates with catheter ablation for AF in older patients compared with younger patients, with comparable complication rates. However, the small number of elderly patients in most studies compared with the much greater prevalence of AF in the elderly indicates that ablation is being performed in a highly selected group of older patients. A consistent finding is that older patients are less likely to undergo a second procedure if the index procedure fails to eliminate the arrhythmia. The recommendations for AF ablation in elderly patients are shown in Table 2.
Outcomes of AF Ablation in Patients with Congestive Heart Failure and the Impact of Ablation on Left Ventricular Function
A number of clinical trials have examined the role of catheter ablation of AF in patients with HF. The initial study to address this important topic was published in 2004.232,1042 This study examined the role of catheter ablation in 58 patients with HF with an EF of less than 45% and 58 controls. During a mean follow-up of 12 ± 7 months, 78% of patients with HF and 84% of controls remained in sinus rhythm. Of particular note is that the EF improved by 21% ± 13%. Improvements also were seen in exercise capacity and in QOL. Another study is the Pulmonary Vein Antrum Isolation versus AV Node Ablation with Bi-Ventricular Pacing for Treatment of AF in Patients with Congestive Heart Failure (PABA-CHF) study that compared the efficacy of AF ablation with AV node ablation and pacemaker implantation.235 The primary endpoint of this prospective, multicenter clinical trial was a composite of EF, distance on a 6-minute walk, and Minnesota Living with Heart Failure (MLWHF) questionnaire score after a 6-month follow-up. This study demonstrated an overall superiority of PVI to AV node ablation and pacing given by a lower score on the MLWHF questionnaire (60 vs 82), longer walking distance (340 m vs 297 m), and higher EF (35% vs 28%). A third case-controlled series reported that the efficacy of AF ablation was similar in patients with and without LV systolic dysfunction and reported an improvement in EF at the 6-month follow-up.388 Since publication of the last consensus document, four additional prospective randomized clinical trials have been published focusing on the outcomes of AF ablation in patients with HF.236,237,390,529 The first three were included in a recent meta-analysis.392 The meta-analysis reported data from 4 randomized trials involving a total of 224 patients, 83% of whom had persistent AF. AF ablation was associated with an increase in LVEF of 8.5% compared with rate control. AF ablation was also superior in improving QOL as well as peak oxygen consumption and 6-minute walk distance. Major adverse events were not significantly different. The most recent trial randomized patients with persistent AF and HF (Ablation vs Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD [AATAC] trial) to AF ablation or treatment with amiodarone. Catheter ablation was more effective than amiodarone in preventing recurrent AF (70% after a mean of 1.4 procedures vs 34%) and was associated with a lower rate of unplanned hospitalization.529 Taken as a whole, the results of these studies suggest that catheter ablation of AF is safe and effective in selected patients with HF. As compared with rate control alone, catheter ablation results in a greater improvement in EF. The recommendations for AF ablation in patients with HF are shown in Table 2.
Outcomes of AF Ablation in Patients with Hypertrophic Cardiomyopathy
AF is a commonly reported complication of hypertrophic cardiomyopathy (HCM) with a prevalence and annual incidence of 22.5% and 3.1%, respectively.1043 The substrate for AF is complex and determined by atrial fibrosis, atrial dilatation, or intrinsic atrial myopathy. In patients with HCM, development of AF is associated with marked exacerbation of symptoms, increased risk of stroke, and excess HCM-related mortality.1043
Due to the association of AF with HCM-related morbidity and mortality, there is general agreement that vigorous maintenance of sinus rhythm should be attempted.273,274 Randomized data regarding the efficacy of AADs are not available for patients with HCM; in daily practice, however, drugs are frequently ineffective in eliminating AF recurrence. In addition, the efficacy and safety of catheter ablation in the setting of HCM is poorly characterized, with studies in small patient cohorts, observational in nature, and providing contradictory results. A recent systematic review and meta-analysis of these studies aimed to determine the efficacy and safety of catheter ablation of AF in patients with HCM.1044 Single-procedure success (freedom from AF or AT recurrence) was 38.7% in patients with HCM (vs 49.8% in controls; OR 2.25; 95% CI 1.09–4.64; P = .03). Outcome after ≥1 procedure amounted to 51.8% (vs 71.2% in controls; OR 2.62; 95% CI 1.52–4.51, P = .0006). Repeat procedures (mean difference = 0.16; 95% CI 0.0–0.32, P = .05) and AADs (OR 4.70; 95% CI 2.31–9.55, P <.0001) were more frequently needed in patients with HCM. Sensitivity analyses suggested that the outcome in patients with HCM with less dilated atria and PAF might be more comparable to the general population. Overall, the risk of procedure-related adverse events was low. In summary, even though the likelihood of recurrence is twofold higher, catheter ablation can be effective in patients with HCM and AF, particularly in patients with PAF and smaller atria. The recommendations for AF ablation in patients with HF are shown in Table 2.
Outcomes of AF Ablation in Young Patients
As an age-related condition, AF is uncommon in young adults. However, younger patients with AF are often highly symptomatic and might have a desire to avoid long-term medical therapy, making catheter ablation a potentially attractive treatment option.
Limited information is available regarding the outcomes of AF ablation in unusually young patients. At least two single-center studies and one multicenter study have reported on the outcomes of AF ablation in unusually young patients.405,1045 Two of these three studies defined young ablation patients as those under the age of 45 years; the other reported on outcomes of AF ablation for lone AF, defined as age <65 with no cardiac, pulmonary, or structural heart disease (mean age 45).
In a 2010 single-center study, 232 patients under age 45 were identified from an overall ablation cohort.405 The authors reported that younger patients had lower rates of major complications compared with more typically aged AF ablation patients. The rate of the author-defined primary outcome of AF control was similar across age groups, but a higher proportion of young patients (76%) were AF-free in the absence of AADs after 1 or more ablation procedures than older patients (from 53%–68%). A 2016 single-center study reported on ablation outcomes in 76 patients with lone AF (9% of their overall ablation population). Freedom from atrial arrhythmia after one procedure was 74%, whereas freedom from atrial arrhythmia after the last procedure without AADs was 96%. The largest study on AF ablation in younger patients was a multicenter German registry in which 593 patients aged ≤45 years were compared with 6650 patients aged >45 years. In this study, the younger patients had lower rates of complication, shorter hospital stays, and lower rates of AF recurrence and AAD than older patients. Together, these studies suggest that AF ablation might be both safer and more effective in younger patients compared with “average” or older AF patients, although this result could be due in part to a lower burden of cardiac and noncardiac comorbid diseases. It has been suggested that AF ablation might more readily be considered first-line rhythm control therapy in younger rather than older patients; however the evidence base for making such a recommendation is not strong. The recommendations for AF ablation in young patients are shown in Table 2.
Outcomes of AF Ablation in Women
Multiple studies have found that women are more symptomatic from AF, have a lower QOL, and are less tolerant of AADs than men.806,1046,1047,1048,1049 However, the rate of referral of women for catheter ablation of AF is significantly lower than men, and women are referred much later after failing more AADs.920 There has not been consistent evidence to support female sex as a predictor of recurrence after AF ablation, based on multiple univariate and multivariate analyses.252,1050,1051 A systematic review of predictors of AF recurrence after catheter ablation reported that none of the 23 studies found female sex to be a predictor of recurrence.1050
At least four major studies have specifically examined outcomes after ablation of AF in women. A large, retrospective multicenter study involved 3265 consecutive patients with drug-refractory AF who underwent PVI.289,290,291 Women constituted a much lower percentage of the patients referred for ablation, were referred later for ablation, had failed more AADs, more often had hypertension, and were older at the time of the procedure. After 24 ± 16 months of follow-up, the women had significantly lower success rates than the men, defined as single-procedure freedom from recurrent AF off AADs (68.5 vs 77.5%; P <.001). Another study also found a lower success rate in women after a single catheter ablation procedure (35.6% in women vs 57.1% in men; P = .003); however, once repeat procedures were taken into account, there was no significant difference in outcome.1052
Other studies have not shown a difference in outcomes between men and women.1053,1054 Most recently, a large-scale prospective analysis of sex-related differences in catheter ablation of AF that enrolled 1124 patients with PAF was reported from Japan.1054 After a mean follow-up of 31.7 ± 24.4 months following the index ablation, there was no significant difference in success rates or complication rates between women and men.
Sex-related recurrence rates have been reported as nonprimary end points in at least 17 other studies, most of which did not reveal significant sex-related differences.1055
Female sex has been reported as a predictor of complications after AF ablation, and higher complication rates from AF ablation in women have repeatedly been found.252,289,290,291,806,808,920,1050,1051,1052,1053,1054,1056,1057,1058,1059 A multicenter U.S. retrospective study reported total complications of 3265 (518 in women vs 2747 in men), with a 5% complication rate in women vs 2.4% in men (P <.001). This study found more hematomas and pseudoaneurysms in women.289,290,291 A large multicenter registry from Italy that enrolled 2323 patients also reported a significantly higher complication rate in women (7% vs 4.4%), and female sex was reported to be an independent predictor of a higher risk of complications by univariate analysis (OR 2.643; 95% CI 1.686–4.143; P <.0001).1059
Overall, studies have not shown a significant sex-related difference in outcomes with AF ablation in women compared with men, but complication rates are consistently higher in women.
Outcomes of Cryoballoon Ablation
Within the past 10 years, CB-based catheter ablation has emerged as an alternative technique to RF ablation for the treatment of patients with symptomatic AF, especially for those with PAF. This change is not only related to the simplified handling of the cryoablation catheter when compared with point-by-point RF ablation, but also to technological developments and the steadily increasing number of clinical trials consistently reporting an overall comparable efficacy to RF ablation. It is important to note that, as in the whole field of AF ablation, the reported efficacy outcomes must be interpreted with caution due to differences in the intensity of follow-up and endpoint definitions.
In 2012, the second-generation CB was introduced. A modified refrigerant injection system allows for a more uniform cooling across the distal balloon hemisphere.489,691,692,1060,1061,1062,1063,1064,1065
Since the 2012 update of this consensus paper, multiple randomized and nonrandomized studies including a large-scale registry have been published that compared CBA against point-by-point RF ablation with respect to rhythm outcome in patients with PAF.490,492,493,695,696,1066,1067,1068,1069,1070,1071,1072 The majority of these studies revealed that CBA was similarly effective in the prevention of arrhythmia recurrences, with arrhythmia-free survival ranging from 54% to 85% in patients undergoing cryoablation and from 55% to 88% in patients undergoing RF ablation after 1 to 2 years of follow-up. In particular, there was no difference in efficacy when the second-generation CB was compared with advanced-generation RF catheters featuring CF measurements for improved wall contact.1068,1070
In the FREEZE-AF study, 315 patients with PAF were randomized to open irrigated radiofrequency ablation or CBA for PVI.1069 Cryoablation was exclusively performed with the first-generation CB catheter. The primary endpoint was freedom from atrial arrhythmia recurrence with absence of persistent complications. At 12 months, the primary endpoint was met by 70.7% of the patients in the RF ablation group and by 73.6% of the patients in the cryoablation group after at least one ablation procedure with similar rates of redo procedures in both groups (19.5% vs 19.9%). Periprocedural complications occurred more frequently in the cryoablation group compared with the RF ablation group (12.2% vs 5.0%), which was largely driven by 9 transient PN injuries (5.8%) in the cryoablation arm.
The most robust data are provided by the FIRE AND ICE trial that, to date, is the largest randomized trial comparing both technologies in patients with symptomatic drug-refractory PAF.490 In this multicenter trial, 762 patients were randomly assigned to undergo PVI by open irrigated RF (approximately one-fourth with CF) or by CBA (approximately three-fourths with CB-2). The primary efficacy endpoint was defined as first documented clinical failure, a composite of recurrent AF, occurrence of AFL or AT, AAD use, or repeat ablation. After a mean follow-up of 1.5 years, CBA was noninferior to RF ablation with regard to efficacy, with the primary endpoint occurring in 34.6% and 35.9% of patients in the cryoablation group and in the RF ablation group, respectively. There was no statistically significant difference in the primary safety endpoint: a composite of death, cerebrovascular events, and serious treatment-related adverse events (10.2% vs 12.8% for cryoablation vs RF ablation, respectively). There were 10 PN injuries (2.7%) in the cryoablation group, with nine of these injuries resolving by 6 months after ablation. Thus, the incidence of permanent PN paralysis was 0.3%. A subsequent analysis reported that the patients who underwent CBA had fewer repeat AF ablation procedures, direct current cardioversions, all-cause hospitalizations, and cardiovascular hospitalizations during follow-up compared with the group randomized to RF ablation.489 Limitations of this subsequent analysis, which need to be considered when interpreting the results, included the inclusion of nonprespecified endpoints, as well the fact that this analysis was industry sponsored.
The role of cryoablation in patients with persistent AF is less well established. To date, data are predominantly derived from relatively small, noncontrolled trials and mostly reflect a single-center experience.486,1073,1074,1075,1076,1077 Overall, cryoablation appears to be associated with a favorable long-term outcome in patients with persistent AF, with arrhythmia-free survival ranging from 56% to 82%. In one non-randomized study, arrhythmia-free survival off AADs was similar when cryoablation was compared with RF ablation at 1-year follow-up after a single procedure (60% vs 50%).1078 Preliminary results from a single-center study demonstrated the feasibility of extra-PV CBA in patients with long-standing persistent AF.1077 Future prospective randomized trials are needed to more precisely define the role of CBA in patients with persistent and long-term persistent AF.
Outcome of Rotational Activity Ablation for AF
Several studies have used phase mapping techniques to identify the rotational activity in patients with AF. Furthermore, catheter ablation of AF guided by phase mapping targeting rotational activity has been found to be effective at eliminating AF in some reports. However, the prevalence of rotational activity and the outcome of rotational activity ablation have varied widely, and long-term outcomes of rotational activity ablation are still lacking. An early investigator used the 64-pole basket catheter to map rotational activity in patients with paroxysmal or persistent AF, and found a high prevalence of rotational activity; application of RF energy on the rotational activity eliminated AF in more than 90% of the patients.563 A more recent study reported favorable results when using an ablation strategy that combined PVI and ablation or rotational activity in patients with non-PAF. However, another investigator1079 used the same mapping tool and techniques for AF ablation and identified rotational activity in less than 20% of AF patients.569 Other investigators could not reproduce a high percentage of rotational activity in patients with AF and found disappointing results with rotational activity-based ablation.1080 Another investigator used the body-surface high-density mapping technique and identified reentrant drivers in 80.5% of paroxysmal and persistent patients with AF, and AF was eliminated in 75% of the AF with reentry drivers.222 In another study, high-density activation mapping identified rotational activity in 15% of the patients with persistent AF.224 Although the Non-Invasive Mapping of Atrial Fibrillation (AFACART) study also showed an 80% success rate in terminating AF, the long-term success rate for eliminating AF was significantly less. However, the duodecapolar mapping catheter with phase mapping identified rotational activity in 65% of persistent AF and long-term persistent AF; application of RF energy on these areas of rotational activity after PVI rendered 65% of the AF free (mean follow-up 18 months).497 Although the application of phase mapping facilitates identification of rotational activity, conventional activation mapping might not see rotational activity clearly. Due to the controversy around the various ablation techniques for persistent and long-term persistent AF, the prevalence and outcome of rotational activity ablations need further investigation.1081
Outcomes of Laser Balloon Ablation
Visually guided PVI using the laser balloon is a recently developed technology. It was first introduced in Europe and was approved in the United States in 2016. Since 2010, there have been a number of publications describing its use.497,498,499,501,502,503,1082,1083,1084,1085 The number of patients included in these studies has ranged from 50 to 200.498,1084 The patients in all these studies had PAF, except for one study that included patients with persistent AF.1085
The laser balloon is highly effective in achieving PVI. The rate of acute PVI ranged from 98% to 100%.498,501,502 Remapping studies also demonstrated a highly durable rate of isolation of the PVs using this technology.503 The freedom from AF at follow-up ranged from 60% to 88%, which is comparable to the outcome of PVI using RF energy in similar populations.503,1085 The patients in all the published studies were followed for at least 12 months.
The laser beam positioning during PVI is executed under direct visualization. However, manipulating the catheter in the LA is guided by X-ray imaging. The fluoroscopy time for laser balloon PVI has been reported to range between 13 and 36 minutes, with a total procedure time range of 2–4 hours.498,1082
The FDA-reviewed HeartLight study was a prospective, multicenter randomized trial comparing laser balloon PVI with conventional RF ablation.503,1082 The 1-year success rate of the laser balloon did not differ from ablation with the ThermoCool System (61.1% vs 61.7%, respectively). The rates of stroke (1.2%) and tamponade (1.2%) were similar to RF ablation. Diaphragmatic paralysis secondary to PN injury occurred in 3.6% of the patients with the laser balloon, which was more common than with RF ablation. Persistent PN paralysis at 1 year occurred in 1.8% of patients. PV stenosis was observed only in patients randomized to RF ablation (2.9%). There were no deaths or AEFs in the study. This ablation system is now approved for clinical use in Europe and the United States.
Long-Term Ablation Efficacy
During the past decade, a large number of studies have been published that have examined the important issue of the long-term efficacy of AF ablation. Prior to this time, most clinical studies presented data from short-term follow-ups, often less than 12 months in duration. The first of these studies was published 5 years ago and described the long-term outcomes of a series of 264 patients who were AF-free and off AAD therapy at the 12-month point following an initial ablation procedure.284 During a mean follow-up of 28 ± 12 months, AF recurred in 23 patients (8.7%). The actuarial recurrence rate of AF at 5 years was 25.5%. Similar findings have been reported in each of the subsequent trials.266,267,268,1022,1086,1087 The predictors of late recurrence most commonly identified include persistent AF as well as comorbid conditions. Despite the low single-procedure, long-term success rate reported in virtually all of these clinical trials, they also reveal that with the use of repeat AF ablation procedures and/or AAD therapy, much higher rates of freedom from recurrent AF as well as concomitant reductions in AF burden can be achieved.
Impact of Catheter Ablation of AF on QOL
Because symptomatic improvement is a primary objective in the treatment of patients with AF, formal assessments of QOL have played an increasingly important role in the evaluation of ablation outcomes.47,63,1088 These measures can provide a more global reflection of symptom change, symptomatic arrhythmia burden, and the difference between actual and desired health and function than more focused endpoints of rhythm status at specific points in time. Generic tools, such as the SF-36 health survey,1089 which is applicable to a broad range of disease states and health conditions, and disease-specific questionnaires1090,1091 developed to assess symptom burden in patients with arrhythmias, have been most widely employed.
Patients with AF, as reflected by standardized SF-36 scores, have substantially impaired QOL, below population norms and comparable to patients with coronary artery disease and congestive HF.47,1088,1092 A number of single-center, nonrandomized observational studies of AF ablation have demonstrated significant and sustained improvements in QOL scores following catheter ablation.63,1088 Taken alone, these findings need to be interpreted cautiously, because in the absence of a comparison group or treatment blinding, placebo effects cannot be excluded. Two studies demonstrated that over a 12-month period following treatment, changes in QOL scores were strongly related to the presence or absence of documented AF recurrence within the previous 30 days.1093,1094
More important are the results of randomized clinical trials that compared catheter ablation with AAD therapy in patients with PAF, and evaluated QOL as an outcome measure.261,377,378,379,684 Investigating catheter ablation as second-line therapy after failed AAD treatment, catheter ablation was associated with significant improvements in SF-36 scores relative to baseline, with restoration to levels at or above population norms.261,684 QOL scores were significantly higher for patients treated with catheter ablation than for patients treated with drug therapy, in whom there was little change from baseline scores.
In the three trials investigating catheter ablation as first-line therapy for AF, QOL improved with both AAD treatment and catheter ablation, and significantly more with catheter ablation, using the SF-36,377,378,1091 or 4312 EQ-5D379 instruments (EuroQOL five dimensions questionnaire).
A recent meta-analysis included data from 12 RCTs comparing catheter ablation (as first- or second-line therapy) and AAD treatment, and including a total of 1707 patients with symptomatic AF. In this analysis, catheter ablation led to greater improvements in several areas of the SF-36 questionnaire and in the symptom frequency score from baseline to 3 months follow-up. However, for all QOL metrics as well as for symptom frequency and severity scores, the differences between catheter ablation and AAD treatment diminished with increasing duration of follow-up, and no significant differences remained beyond 9 months of follow-up.1032 In the randomized trials, an impact of crossover from AAD treatment to catheter ablation cannot be excluded. However, in an on-treatment analysis of data from a randomized trial comparing catheter ablation and AAD treatment as first-line therapy, no differences in QOL were observed between patients treated with catheter ablation, patients treated with AADs, and patients treated with a combination of both.494
Concerns have been raised that generic QOL instruments such as SF-36 are not sufficiently sensitive or focused to detect changes in disease-specific symptoms such as those associated with AF.63,974 AF-specific QOL measures, including the AF Effect on QOL (AFEQT) questionnaire,1095 the Mayo AF Symptom Inventories,63 and the Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA),1096 have been developed and are in the process of validation. A recent study reported that disease-specific assessments of QOL are superior to generic questionnaires.1097 Preliminary findings indicate that these tools also demonstrate substantial improvements in QOL with ablation, and can more accurately reflect ablation efficacy. However, there is currently no general agreement that any of the AF-specific QOL instruments are superior to others or to the general QOL instruments. The use of QOL measures will be discussed further in the section.
Impact of Catheter Ablation of AF on LA Size and Function
Experimental and clinical research has demonstrated that in some settings AF results in, or is accompanied by, electrical, structural, and functional remodeling of the atrium.14,587,1098,1099 The results of a subset of these studies suggest that AF can be viewed, in some patients, as a rate-related atrial cardiomyopathy. As discussed elsewhere in the document, AF can also follow and be the consequence of prior atrial damage and fibrosis (atrial myopathy). To the extent that rate-related cardiomyopathies lead to reversible chamber dilatation and dysfunction, it was anticipated that reverse remodeling might also occur in a subset of patients who underwent AF catheter ablation.
Several studies have examined LA size before and after catheter ablation.437,1100,1101,1102 These studies have demonstrated a significant decrease in the size of the LA after PVI of PAF, regardless of whether echocardiography, MRI, or CT was used for LA imaging. The reverse remodeling of LA was more pronounced when sinus rhythm had been successfully restored.1103 Although the precise mechanism of this decrease in size is not clear, it appears consistent with reverse remodeling due to the decreased burden of AF and scar formation from the ablation procedure.
The impact of catheter ablation of AF on LA transport function was investigated in patients with paroxysmal and persistent AF, with conflicting results.1104,1105 A meta-analysis showed a significant decrease in LA volume but did not find significant changes in active LA function, including studies with persistent as well as PAF ablation procedures.1106 However, because AF eliminates essentially all contractility of the LA, there is general agreement that restoration of sinus rhythm in patients with persistent AF improves atrial function if sinus rhythm is maintained.391,1107,1108,1109
However, ablation-related scarring with the risk of causing persistent atrial dysfunction still remains a major concern after extensive ablation for persistent AF. The long-term outcome after stepwise approach for persistent AF demonstrated the impaired contractility and compliance of LA, which was related to scar burden.1110 Moreover, a recent study has reported a series of patients who developed LA diastolic dysfunction and pulmonary hypertension following AF ablation.1111 The precise cause of “stiff LA syndrome” or a “noncompliant LA” and methods to prevent it will clearly be an area for further study going forward.
Impact of Catheter Ablation on Stroke Risk
Conceptually, it appears logical that catheter ablation—by elimination of or reductions in AF burden—lowers the risk of stroke or TIA; initial reports from single-center, nonrandomized studies did demonstrate a relatively low rate of stroke or TIA after catheter ablation.231,545 To date however, there are no RCTs verifying the hypothesis that ablation lowers the long-term incidence of stroke or TIA. Please refer to Section 7 for a more detailed discussion of the recommendations made by the writing group for long-term anticoagulation post-AF ablation.
Indirect evidence stems from four large, health administrative databases using propensity-score matching to create a “control” population and to even out differences between patient groups.239,1112,1113,1114 In a very large, prospectively collected registry (the Intermountain Healthcare Database in Utah), investigators reported a significantly lower rate of stroke in 4212 ablated patients (follow-up 3.1 ± 2.4 years), compared with those who did not undergo ablation.239 Moreover, ablated patients had comparable stroke rates when compared with age- and sex-matched patients who did not have a history of AF. Both observations were independent of baseline stroke risk score. Another propensity score-matched analysis of medically treated and ablated patients within the U.S. MarketScan Research Database (n = 805 in each group with follow-up of up to 3 years), showed that AF ablation was associated with a reduced risk of stroke or TIA compared with AAD therapy (HR 0.62; 95% CI 0.44–0.86, P = .005).1112 Similarly, data from the Taiwanese national health insurance claims database reported lower risk of stroke in 846 ablated patients compared with the control group (HR 0.57; 95% CI 0.35–0.94; P = .026).1113 Recently, a retrospective, propensity score-matched analysis (using as many as 51 parameters) of medically treated and ablated patients from Swedish health registries (n = 2836 in each group during a follow-up period of 4.4 years) confirmed that ablation was associated with a lower incidence of ischemic stroke than nonablated patients (HR 0.69; 95% CI 0.51–0.93; P = .016).1114 This association between ablation and stroke was pronounced in patients with a CHA2DS2-VASc score ≥2 points, but was not discernible among patients with low stroke risk.
Three other observational studies determined possible predictors for stroke-free survival within the ablated patient group.241,242,408 Multivariate analysis in 174 ablated Taiwanese patients showed that ablation outcome was the strongest independent predictor for survival free of major adverse cardiovascular events, including stroke (HR 0.225; 95% CI 0.076–0.671; P = .007).241 A Kaplan-Meier survival analysis demonstrated that ablation-treated patients without AF recurrence had a lower incidence of ischemic strokes and TIAs (P = .015) compared with patients with AF recurrence or medically treated patients. Of interest, a retrospective analysis of 3058 ablated low-risk patients from a single-center registry showed only a modest, non-significant reduction in the risk of cerebrovascular events in patients who maintained sinus rhythm when compared with those who had AF recurrence.242
The above studies suggest that catheter ablation can lower the risk of stroke via maintenance of sinus rhythm. However it is recognized that the retrospective nature of these studies makes them prone to bias. The above studies are limited by a lack of detailed data on rhythm and/or anticoagulation status, selection bias (low stroke risk at baseline), relatively short follow-up, and the extent to which patient groups can be matched. Despite the fact that propensity score matching was successful in creating a control population that was similar to the ablated group, adjustment is only possible for observable factors. Unknown confounding factors could account for why patients treated with ablation had lower rates of stroke or TIA (post hoc ergo propter hoc). Bias of unmeasured variables can only be fully neutralized in RCTs. Finally, it should be noted that some of the above studies made comparisons to historical cohorts whose risk of stroke appears to be much higher than the risk reported in recent studies.
Therefore, the above findings cannot be viewed as definitive and do not provide sufficient evidence that ablation reduces stroke risk. Instead, they reinforce the hypothesis behind studies like the CABANA trial or the EAST trial, which will provide more definitive evidence. However, the results will not be available until 2018, and, as with most long-term studies in ablation, their relevance could be challenged by the rapidly evolving nature of the ablation field.
Predictors of Success Following AF Ablation
A large number of studies have been performed to examine clinical predictors of the efficacy of AF ablation.328,329,365,1050,1115,1116,1117 Factors that have been identified as predictors of a poorer outcome, at least in some studies, include (1) non-PAF and particularly long-term persistent AF; (2) sleep apnea and obesity; (3) increased LA size; (4) increased age; (5) hypertension; and (6) LA fibrosis as detected by cardiac MRI.365
A systematic review of predictors of AF recurrence after AF ablation analyzed data from 45 studies, 25 of which included a multivariable analysis of predictors of recurrence.1050 Among the 17 studies that examined AF type as a predictor of recurrence, 11 studies reported no impact of AF type on recurrence, whereas six studies reported that the presence of non-PAF was an independent predictor of a higher rate of recurrence (HR ranging from 1.8 to 22). Seventeen studies evaluated EF as a predictor of recurrence. Very few patients in any of these studies had an EF less than 40%. Among these 17 studies, only five reported a significant association between lower EF and a higher rate of AF recurrence. Twenty studies examined LA diameter as a predictor of AF recurrence. Very few patients in any study had LAD >60 mm. Among these 20 studies, four reported a significant association between larger LAD and a higher rate of recurrence of AF. Among 21 studies that examined the presence of structural heart disease as a predictor, only one reported a significant association at 12 months of follow-up. Most studies examined sex, and no association between recurrence and sex was found. Only one of 22 studies reported an independent association between age and recurrence.
Cost-Effectiveness of AF Ablation
The cost-effectiveness of AF ablation has been evaluated in a number of individual studies and several systematic reviews.1118,1119,1120,1121,1122,1123,1124,1125 The costs of AF ablation procedures can vary widely, depending on the treatment setting and the actual equipment used.409,1126 Estimates of the cost-effectiveness of AF ablation can vary further based on a number of additional factors, including the patient population, the severity of symptoms, the analytic time horizon, and assumptions about the impact of AF ablation on QOL, stroke, and other clinical outcomes. One issue supporting the potential cost-effectiveness of AF ablation is that the costs of ablation are at least partly offset over time by reducing long-term, arrhythmia-related health care resource utilization for patients not treated with ablation, as supported by some empirical evidence.90,476,1033,1127 However, most formal cost-effectiveness studies have not found AF ablation to be cost neutral or cost saving in the short to intermediate term.
The majority of published cost-effectiveness studies have compared AF ablation to AADs as second-line therapy in patients with PAF.1119,1120,1122,1124,1128,1129 In general, these studies have reported acceptable cost-effectiveness ratios—in the range of $27,000 to $59,000 (Canadian) per quality-adjusted life year (QALY) gained over 5-year time horizons.1122,1128 Results would be more favorable if ablation were found to significantly reduce the risk of stroke.1118 U.S. experts have recently indicated that cost-effectiveness ratios below $50,000 per QALY indicate high value, and between $50 and $150,000 indicate intermediate value.1130
Less is known about the cost-effectiveness of ablation in the first-line setting or in patients with persistent or long-term persistent AF. One report based on the First Line Radiofrequency Ablation Versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment (RAAFT) pilot study suggested that costs for patients initially treated with drugs would catch up to those for patients treated with ablation within 2 years due to a very high rate of crossover. However, another more detailed cost-effectiveness study modeled after the MANTRA-PAF trial population indicated that AF ablation might only be cost-effective as first-line therapy in younger patients.1125
Assessments of cost-effectiveness at present rely greatly on extrapolations from clinical trials with limited follow-up duration and sample sizes, necessitating assumptions about key clinical benefits. Robust data from larger, longer studies will be needed to refine cost-effectiveness estimates.
Section 10: Complications
Overview
Catheter ablation of AF is one of the most complex interventional electrophysiological procedures. AF ablation by its nature involves catheter manipulation and ablation in the delicate thin-walled atria, which are in close proximity to other important organs and structures that can be impacted through collateral damage. It is therefore not surprising that AF ablation is associated with a significant risk of complications, some of which might result in life-long disability and/or death. In this section of the document we will review the complications associated with catheter ablation procedures performed to treat AF. The complications are defined and their mechanisms explored. Emphasis is placed on both those complications that occur most frequently as well as those very infrequent complications that have the potential to result in the greatest disability and/or death. Means of avoiding complications are described and recommendations are made regarding management should the complications occur.
It is noteworthy that the publications from which these data are derived come from high-volume centers where one would expect the incidence of complications to be lower than in lower-volume centers. As the practice of AF ablation grows with an increasing number of low-volume centers performing these procedures, it is likely that the true complication rate of AF ablation will be higher than described here. Furthermore, other data such as those derived from the two worldwide surveys of catheter ablation of AF were provided voluntarily and, again, are therefore likely to underestimate the true complication rate.806,920 It is notable that a recent paper reported on the trends in hospital complication rates associated with AF ablation between 2000 and 2010 based on the Nationwide Inpatient Sample involving 93,801 procedures.921 The overall incidence of complications was 6.29%—increasing from 5.3% in 2000 to 7.5% in 2010. The in-hospital mortality was 0.46%. Not surprisingly, lower operator and hospital procedure volume was an important predictor of complications. These data are a stark reminder that our efforts to eliminate complications associated with AF ablation are incomplete and there is more work to do.
As our experience with AF ablation continues to grow, new complications are recognized and are reviewed here. These include stiff LA syndrome, cough, pulmonary injury, gastric hypomotility, and sinus tachycardia. Once again, the writing group strongly recommends that standardized reporting of complications be part of all published reports on AF ablation. In this document, we have provided definitions of the most important complications associated with AF ablation (Table 8). We hope these definitions and reporting standards can be incorporated in the design of future clinical trials of AF ablation. Shown in Table 9 is an overview of the incidence, prevention, diagnosis, and management of selected complications, and Table 5 presents signs and symptoms associated with various complications early and late postablation.
Table 8.
Definitions of complications associated with AF ablation
| Asymptomatic cerebral embolism | Asymptomatic cerebral embolism is defined as an occlusion of a blood vessel in the brain due to an embolus that does not result in any acute clinical symptoms. Silent cerebral embolism is generally detected using a diffusion weighted MRI. |
| Atrioesophageal fistula | An atrioesophageal fistula is defined as a connection between the atrium and the lumen of the esophagus. Evidence supporting this diagnosis includes documentation of esophageal erosion combined with evidence of a fistulous connection to the atrium, such as air emboli, an embolic event, or direct observation at the time of surgical repair. A CT scan or MRI scan is the most common method of documentation of an atrioesophageal fistula. |
| Bleeding | Bleeding is defined as a major complication of AF ablation if it requires and/or is treated with transfusion or results in a 20% or greater fall in hematocrit. |
| Bleeding following cardiac surgery | Excessive bleeding following a surgical AF ablation procedure is defined as bleeding requiring reoperation or ≥ 2 units of PRBC transfusion within any 24 hours of the first 7 days following the index procedure. |
| Cardiac perforation | We recommend that cardiac perforation be defined together with cardiac tamponade. See “Cardiac tamponade/perforation.” |
| Cardiac tamponade | We recommend that cardiac tamponade be defined together with cardiac perforation. See “Cardiac tamponade/perforation.” |
| Cardiac tamponade/perforation | Cardiac tamponade/perforation is defined as the development of a significant pericardial effusion during or within 30 days of undergoing an AF ablation procedure. A significant pericardial effusion is one that results in hemodynamic compromise, requires elective or urgent pericardiocentesis, or results in a 1-cm or more pericardial effusion as documented by echocardiography. Cardiac tamponade/perforation should also be classified as “early” or “late” depending on whether it is diagnosed during or following initial discharge from the hospital. |
| Deep sternal wound infection/mediastinitis following cardiac surgery | Deep sternal wound infection/mediastinitis following cardiac surgery requires one of the following: (1) an organism isolated from culture of mediastinal tissue or fluid; (2) evidence of mediastinitis observed during surgery; (3) one of the following conditions: chest pain, sternal instability, or fever (>38 °C), in combination with either purulent discharge from the mediastinum or an organism isolated from blood culture or culture of mediastinal drainage. |
| Esophageal injury | Esophageal injury is defined as an erosion, ulceration, or perforation of the esophagus. The method of screening for esophageal injury should be specified. Esophageal injury can be a mild complication (erosion or ulceration) or a major complication (perforation). |
| Gastric motility/pyloric spasm disorders | Gastric motility/pyloric spasm disorder should be considered a major complication of AF ablation when it prolongs or requires hospitalization, requires intervention, or results in late disability, such as weight loss, early satiety, diarrhea, or GI disturbance. |
| Major complication | A major complication is a complication that results in permanent injury or death, requires intervention for treatment, or prolongs or requires hospitalization for more than 48 hours. Because early recurrences of AF/AFL/AT are to be expected following AF ablation, recurrent AF/AFL/AT within 3 months that requires or prolongs a patient's hospitalization should not be considered to be a major complication of AF ablation. |
| Mediastinitis | Mediastinitis is defined as inflammation of the mediastinum. Diagnosis requires one of the following: (1) an organism isolated from culture of mediastinal tissue or fluid; (2) evidence of mediastinitis observed during surgery; (3) one of the following conditions: chest pain, sternal instability, or fever (>38 °C), in combination with either purulent discharge from the mediastinum or an organism isolated from blood culture or culture of mediastinal drainage. |
| Myocardial infarction in the context of AF ablation | The universal definition of myocardial infarction1399 cannot be applied in the context of catheter or surgical AF ablation procedures because it relies heavily on cardiac biomarkers (troponin and CPK), which are anticipated to increase in all patients who undergo AF ablation as a result of the ablation of myocardial tissue. Similarly, chest pain and other cardiac symptoms are difficult to interpret in the context of AF ablation both because of the required sedation and anesthesia and also because most patients experience chest pain following the procedure as a result of the associated pericarditis that occurs following catheter ablation. We therefore propose that a myocardial infarction, in the context of catheter or surgical ablation, be defined as the presence of any one of the following criteria: (1) detection of ECG changes indicative of new ischemia (new ST-T wave changes or new LBBB) that persist for more than 1 hour; (2) development of new pathological Q waves on an ECG; (3) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. |
| Pericarditis | Pericarditis should be considered a major complication following ablation if it results in an effusion that leads to hemodynamic compromise or requires pericardiocentesis, prolongs hospitalization by more than 48 hours, requires hospitalization, or persists for more than 30 days following the ablation procedure. |
| Phrenic nerve paralysis | Phrenic nerve paralysis is defined as absent phrenic nerve function as assessed by a sniff test. A phrenic nerve paralysis is considered to be permanent when it is documented to be present 12 months or longer following ablation. |
| Pulmonary vein stenosis | Pulmonary vein stenosis is defined as a reduction of the diameter of a PV or PV branch. PV stenosis can be categorized as mild <50%, moderate 50%–70%, and severe ≥70% reduction in the diameter of the PV or PV branch. A severe PV stenosis should be considered a major complication of AF ablation. |
| Serious adverse device effect | A serious adverse device effect is defined as a serious adverse event that is attributed to use of a particular device. |
| Stiff left atrial syndrome | Stiff left atrial syndrome is a clinical syndrome defined by the presence of signs of right heart failure in the presence of preserved LV function, pulmonary hypertension (mean PA pressure >25 mm Hg or during exercise >30 mm Hg), and large V waves ≥10 mm Hg or higher) on PCWP or left atrial pressure tracings in the absence of significant mitral valve disease or PV stenosis. |
| Stroke or TIA postablation | Stroke diagnostic criteria
|
| Stroke definitions | |
| |
| Minor—Modified Rankin score <2 at 30 and 90 days† | |
| Major—Modified Rankin score ≥2 at 30 and 90 days | |
| Unanticipated adverse device effect | Unanticipated adverse device effect is defined as complication of an ablation procedure that has not been previously known to be associated with catheter or surgical ablation procedures. |
| Vagal nerve injury | Vagal nerve injury is defined as injury to the vagal nerve that results in esophageal dysmotility or gastroparesis. Vagal nerve injury is considered to be a major complication if it prolongs hospitalization, requires hospitalization, or results in ongoing symptoms for more than 30 days following an ablation procedure. |
| Vascular access complication | Vascular access complications include development of a hematoma, an AV fistula, or a pseudoaneurysm. A major vascular complication is defined as one that requires intervention, such as surgical repair or transfusion, prolongs the hospital stay, or requires hospital admission. |
AF, atrial fibrillation; CT, computed tomography; MRI, magnetic resonance imaging; PRBC, packed red blood cell; AFL, atrial flutter; AT, atrial tachycardia; CPK, creatine phosphokinase; ECG, electrocardiogram; LBBB, left bundle branch block.
^Patients with nonfocal global encephalopathy will not be reported as a stroke without unequivocal evidence based on neuroimaging studies.
Modified Rankin score assessments should be made by qualified individuals according to a certification process. If there is discordance between the 30- and 90-day modified Rankin scores, a final determination of major versus minor stroke will be adjudicated by the neurology members of the clinical events committee.
Table 9.
Incidence, prevention, diagnosis, and treatment of selected complications of AF ablation
| Complication | Incidence | Selected prevention techniques | Diagnostic testing | Selected treatment options | References |
|---|---|---|---|---|---|
| Air embolism | <1% | Sheath management | Nothing or cardiac catheterization | Supportive care with fluid, oxygen, head down tilt, hyperbaric oxygen | 803,1218–1223 |
| Asymptomatic cerebral emboli (ACE) | 2% to 15% | Anticoagulation, catheter and sheath management, TEE | Brain MRI | None | 723,724,728,731,800,1205–1217 |
| Atrial esophageal fistula | 0.02% to 0.11% | Reduce power, force, and RF time on posterior wall, monitor esophageal temp, use proton pump inhibitors; avoid energy delivery over esophagus | CT scan of chest, MRI; avoid endoscopy with air insufflation | Surgical repair | 637,705,806,866,877–920,1162–1178,1398 |
| Cardiac tamponade | 0.2% to 5% | Cather manipulation, transseptal technique, reduce power, force, and RF time | Echocardiography | Pericardiocentesis or surgical drainage | 482,806,908,920,921,1034,1131–1135,1139–1141 |
| Coronary artery stenosis/occlusion | <0.1% | Avoid high-power energy delivery near coronary arteries | Cardiac catheterization | PTCA | 923,1233–1240 |
| Death | <0.1% to 0.4% | Meticulous performance of procedure, attentive postprocedure care | NA | NA | 921,806,908,920,1039 |
| Gastric hypomotility | 0% to 17% | Reduce power, force, and RF time on posterior wall | Endoscopy, barium swallow, gastric emptying study | Metoclopramide, possibly intravenous erythromycin | 536,1017–1021,1179–1185 |
| Mitral valve entrapment | <0.1% | Avoid circular catheter placement near or across mitral valve; clockwise torque on catheter | Echocardiography | Gentle catheter manipulation, surgical extraction | 1263–1269,1396 |
| Pericarditis | 0% to 50% | None proven | Clinical history, ECG, sedimentation rate, echocardiogram | NSAID, colchicine, steroids | 985,986,1257–1262 |
| Permanent phrenic nerve paralysis | 0% to 0.4% | Monitor diaphragm during phrenic pacing, CMAP monitoring, phrenic pacing to identify location and adjust lesion location | CXR, sniff test | Supportive care | 462,482,490,503,532,533,536,706,707,779,808,903,920,1017,1075,1182–1201 |
| Pulmonary vein stenosis | <1% | Avoid energy delivery within PV | CT or MRI, V/Q wave scan | Angioplasty, stent, surgery | 244,434,462,482,498,503,927,928,1142–1160 |
| Radiation injury | <0.1% | Minimize fluoroscopy exposure, especially in obese and repeat ablation patients, X-ray equipment | None | Supportive care, rarely skin graft | 747,749,763,1186,1241–1256 |
| Stiff left atrial syndrome | <1.5% | Limit extent of left atrial ablation | Echocardiography, cardiac catheterization | Diuretics | 1110,1111,1270–1275 |
| Stroke and TIA | 0% to 2% | Pre-, post-, and intraprocedure anticoagulation, catheter and sheath management, TEE | Head CT or MRI, cerebral angiography | Thrombolytic therapy, angioplasty | 242,489,503,532,655,673,796,798,799,806,920,921,1202–1204 |
| Vascular complications | 0.2% to 1.5% | Vascular access techniques, ultrasound-guided access, anticoagulation management | Vascular ultrasound, CT scan | Conservative treatment, surgical repair, transfusion | 806–808,834,840–842,920,921,1224–1232 |
AF, atrial fibrillation; CT, computed tomography; MRI, magnetic resonance imaging; TEE, transesophageal electrocardiogram; RF, radiofrequency; PTCA, percutaneous transluminal coronary angioplasty; NA, not applicable; ECG, electrocardiogram; NSAID, nonsteroidal anti-inflammatory drug; CMAP, compound motor action potentials; CXR, chest X-ray; TIA, transient ischemic attack.
Cardiac Tamponade
Cardiac tamponade remains the most common potentially life-threatening complication associated with AF ablation.921 A recent paper reported on the trends in in-hospital complication rates associated with AF ablation between 2000 and 2010 based on the Nationwide Inpatient Sample involving 93,801 procedures.921 In this analysis, the overall incidence of a “pericardial complication” was 1.5%. The incidence of pericardial complications increased from 0.74% in 2000 to 2.24% in 2010.921 The markedly higher incidence of cardiac tamponade during AF ablation compared with routine cardiac electrophysiology procedures can be attributed to a number of important procedural differences, including extensive intracardiac catheter manipulation and ablation, the common need for two or more transseptal punctures, and the need for systemic anticoagulation.806,920,921,1131,1132,1133,1134,1135 The most common causes of cardiac perforation leading to cardiac tamponade during AF ablation are (1) misdirected transseptal punctures either with punctures performed too posteriorly exiting the RA into the pericardium before entering the LA or punctures exiting the LA via the roof, LAA, or the lateral LA wall; (2) direct mechanical trauma, especially through the LAA; and (3) overheating during RF energy delivery, with or without the development of a steam pop. Excessive power, temperatures, and CF might also be contributory.
The need for periprocedural anticoagulation (with the use of interrupted or uninterrupted OAC strategies) and for intraprocedural anticoagulation (with the infusion of intravenous heparin to achieve a stable ACT above 300 seconds throughout the procedure duration) can exacerbate the bleeding risk and increase the volume of bleeding following the occurrence of one or more of the causes above. One initial large study reported that uninterrupted VKA anticoagulation therapy did not result in a higher incidence of tamponade compared with interrupted VKA anticoagulation therapy with bridging heparin.532,533 This observation was further corroborated by two meta-analyses.399,400,401,1136 Another study compared the outcomes of 23 patients who developed pericardial tamponade with an INR <2 to 17 patients on warfarin with an INR >2. No difference was observed in the initial pericardial drainage, or the duration of drainage; no patients required surgery.1137 A more recent shift in periprocedural anticoagulation strategies during AF ablation involves performing AF ablation on uninterrupted NOAC therapy. The results of the RE-CIRCUIT study, which was a head-to-head comparison of performing AF ablation on uninterrupted dabigatran vs uninterrupted warfarin, were recently published.841 This study randomized 704 patients across 104 sites to these two anticoagulation strategies. The incidence of major bleeding events during and up to 8 weeks postablation among the 635 patients who underwent AF ablation was significantly lower with dabigatran than with warfarin (5 patients [1.6%] vs 22 patients [6.9%]; absolute RD -5.3%; RR reduction 77%). It is notable that there were six patients with cardiac tamponade in the warfarin arm vs one in the dabigatran arm. All the patients with cardiac tamponade underwent successful pericardiocentesis with no need for surgical drainage. No strokes or other thromboembolic events occurred in the dabigatran arm compared with one TIA in the warfarin arm. No patients in the dabigatran arm required the specific reversal agent idarucizumab. Another smaller prospective trial of 250 patients that randomized patients to undergoing AF ablation on uninterrupted rivaroxaban versus uninterrupted warfarin has been published.842 The incidence of major bleeding was low (0.4%), and no patient developed pericardial tamponade. A recent meta-analysis reported that performance of AF ablation on NOACs was associated with a lower risk of minor bleeding and no major differences in the risk of stroke or TIA, cardiac tamponade, or groin hematomas.1138 Another recent study described the outcomes of 16 patients who developed a pericardial effusion while taking an uninterrupted Xa inhibitor. Eleven occurred in the periprocedural setting and 5 occurred between 1 and 28 days postprocedure. All the patients underwent pericardiocentesis. Protamine and 4-factor prothrombin complex concentrate were given to all periprocedure cases. Two patients required surgery. There were no deaths in this series.
The incidence of tamponade might be as high as 6%1135 and as low as 0%. The risk factors for tamponade identified in this study were linear ablation lesions and higher ablation power. A “pop” was heard during eight of these 10 cases of tamponade. Another large series reported cardiac tamponade during 15 of 632 ablation procedures (2.4%).1134 Two of these patients required surgical intervention. In contrast to the prior study, no “pop” was reported. The two worldwide surveys of AF ablation reported a 1.2% and a 1.3% incidence of cardiac tamponade, respectively.806,920 A recent meta-analysis of ablation procedures reported a 0.9% incidence of tamponade.1139 Women were 1.83-fold more likely to develop tamponade compared with men. A reciprocal relationship between center volume and the incidence of outcomes of cardiac tamponade was observed. Overall, 16% of tamponade cases required surgery, with lower rates of surgery in high-volume centers.1139 A meta-analysis of CBA with data on 1308 procedures reported an overall incidence of cardiac effusion or tamponade of 1.5%.482 A more recent prospective RCT of CBA vs RF ablation reported an incidence of tamponade of 1.3% in the RF arm and of 0.3% in the CB arm.489 Although it was hoped the recent introduction of force-sensing catheters would reduce the rate of tamponade, this has not been confirmed in clinical trials. The incidence of cardiac tamponade was 2.5% among 161 patients in the safety cohort of the recently published SMART-AF trial of the Smart Touch catheter.673 And in the TOCCASTAR trial, which randomized patients to ablation with a force-sensing catheter (Endosense) or a standard irrigated RF catheter, no difference in the incidence of cardiac tamponade was observed in the two arms (0.66% vs 0.7%, P = NS).655 It is important to recognize that the presentation of cardiac tamponade might be delayed and can occur any time from an hour after the procedure to weeks later.1034,1139 The incidence of delayed tamponade was 0.2% in the worldwide survey report.1034 Most, but not all, patients presented with warning symptoms and 13% of patients presented with hypotension and shock.
Cardiac tamponade presents either as an abrupt dramatic fall in BP, or more insidiously, as a gradual decrease in BP. In the latter case, administration of fluid might return the BP to normal before it subsequently declines. However, it is vital that operators and staff be vigilant to the development of cardiac tamponade, as a delay in diagnosis can be fatal. Sixty percent of the writing group members use an arterial line for BP monitoring during the AF ablation procedure. The development of hypotension in any patient should be assumed to indicate tamponade until proven otherwise by immediate ECG. An early sign of cardiac tamponade is a reduction in the excursion of the cardiac silhouette on fluoroscopy with a simultaneous fall in systemic BP. Ninety percent of the writing group members have an echo machine in their EP laboratory. Sixty percent of the writing group members routinely image the heart with an echocardiogram prior to the patient leaving the procedure room. Twenty percent of the writing group members routinely obtain an echocardiogram of the heart prior to discharge. ICE has been reported to allow earlier detection of pericardial effusion. It is important to recognize that small, asymptomatic pericardial effusions are commonly observed following AF ablation procedures. ICE imaging has the potential to detect pericardial effusion earlier. A survey of writing group members reveals that 53% of members routinely employ ICE imaging during AF ablation. Our survey revealed that ICE was being used routinely by 87% of the writing group members in the United States and Canada as compared with 13% of the writing group members from other countries. Monitoring filling pressures in the LA and RA can be helpful in order to evaluate progression of the effusion and/or effective drainage of the pericardial collection. Ninety-three percent of the writing group members hospitalize their AF ablation patients for at least one night following their procedure.
The majority of episodes of cardiac tamponade can be managed successfully by immediate percutaneous drainage and reversal of anticoagulation with protamine. In patients anticoagulated with warfarin, fresh frozen plasma is often administered. And in patients on an Xa inhibitor, 4-factor prothrombin complex concentrate is often appropriate. For patients on dabigatran, the reversal agent idarucizumab is now available worldwide and provides the opportunity to immediately reverse the anticoagulant effects of dabigatran.844 Factor Xa inhibitors can be reversed with andexanet alfa (currently not approved for clinical use).845 Percutaneous drainage is best achieved by subxiphoid Seldinger puncture of the pericardial sac and placement of an intrapericardial catheter. The pericardial tap can be performed either with fluoroscopic guidance based on anatomic landmarks or with echo guidance.1140 After initial aspiration, the BP promptly returns to normal. Once the pericardial space has been drained, the patient needs to be monitored for ongoing bleeding with the drainage catheter. The drainage catheter is typically left in place for at least 12 hours postablation. In rare cases, if there has been a tear, percutaneous drainage might be inadequate, and surgical drainage and repair could be necessary.1134 One recent meta-analysis reported that 16% of cases of cardiac tamponade required surgical intervention.1139 It is for this reason that AF ablation procedures should only be performed in hospitals equipped or prepared to manage these types of emergencies with access to emergency surgical support when required. Three cases have been reported of emergent drainage of a pericardial effusion through a sheath, either inadvertently or purposely placed into the pericardial space using an endocardial approach, although this would not be considered to be a standard approach.532,533,1132,1141 Early recognition and rapid appropriate treatment of cardiac tamponade is mandatory to prevent irreversible deterioration in perfusion of the brain and other important organs. In a dedicated worldwide survey, cardiac tamponade was reported to be the most frequent cause of periprocedural death, with 25% of all fatalities occurring in association with this complication.908
PV Stenosis
PV stenosis is a well-recognized complication of AF ablation that results from thermal injury to the PVs, including the media, intima, adventitia, and PV musculature. Since first reported in 1998, numerous studies have sought to determine the incidence, cause, diagnostic strategy and treatment approach for PV stenosis.434,927,1142,1143,1144,1145 Although the precise pathophysiological mechanisms are still uncertain, a progressive neointimal proliferation and myocardial fibrosis resulting in endovascular contraction has been reported after extensive radiofrequency energy ablation (RFA) to canine PVs.1146 PV stenosis has been described for both point-by-point RF ablation as well as CBA.244,462,482,928,1146,1147 To the best of our knowledge, significant PV stenosis has not been reported with the laser balloon system.498,503 There are controversial data regarding any impact that RF power output has on the rate of PV stenosis.244,1147 The incidence of PV stenosis might be somewhat lower with CB AF ablation than with RFA.1148,1149 In experienced hands, however, PV stenosis has become an increasingly uncommon complication with either ablation technology.489 The highest risk for PV stenosis is associated with RFA close to the PV orifices and/or within the PVs, with a 5.6-fold higher incidence in comparison with antral ablation.1147 Ablation within the PVs should be avoided, but can occur due to shifts in the 3D electroanatomic map, respiratory motion, poor catheter stability, and/or an inexperienced operator.
The published incidence of PV stenosis varies widely, from 0% to 40%.434,505,778,1142,1144,1150,1151 This variation results from differences in the ablation technique, definitions of PV stenosis, the intensity of screening for this complication, and the date the study was performed. When PV ablation for treating AF began in the late 1990s, investigators were unaware that PV stenosis was a potential complication. In contrast, operators today understand that PV stenosis can be prevented by avoiding RF energy delivery within a PV. This increased awareness and improvements in imaging modalities have enabled better identification of the true PV ostium and have resulted in a dramatic reduction in the incidence of PV stenosis.1141,1147 The incidence of symptomatic PV stenosis in experienced hands approaches zero, although the incidence of asymptomatic PV stenosis or PV narrowing might be higher.
Symptoms usually occur weeks to months after the ablation procedure.927,1152 Prominent symptoms are dyspnea, hemoptysis, cough, (recurrent) pulmonary infections or pneumonia, and chest pain.1142,1143,1152 These have often led to a misdiagnosis of pneumonia, pulmonary embolism, or even lung cancer; thus, patients should be told of the importance of returning to their ablation center if such signs or symptoms develop. There are data showing a progression of stenosis during 3 months after RFA despite a normal imaging examination at 1 month after the index procedure. Furthermore, severe stenosis can also remain asymptomatic.927 According to the percentage reduction of the luminal diameter, the severity of PV stenosis is generally defined as mild (<50%), moderate (50%–70%), or severe (>70%). In this consensus statement, we recommend that a significant PV stenosis be defined as a > 70% reduction in luminal diameter. PV stenosis can develop in any PV; and in some patients, multiple PV stenoses occur.927,928,1143,1152 It is unclear whether such patients are more prone to develop PV stenosis compared with others.
PV stenosis can be diagnosed by CT imaging, MRI, perfusion scans, TEE, or pulmonary venography. The preferred imaging modality is MRI or CT because location and severity of PV lesions can be precisely visualized. Advantages of MRI include the fact that pulmonary perfusion data can be obtained simultaneously and that the diagnostic procedure is free of radiation. Eleven percent of the writing group members routinely obtain a CT or MR scan several months postablation to screen for asymptomatic PV stenosis.
Although the incidence of PV stenosis has decreased over recent years, it remains a significant complication because it is difficult to treat and, rarely, it can lead to death. It is notable that 51% of the writing group members report having had a patient at their center develop PV stenosis requiring intervention. Most of these procedures were performed more than a decade ago. The indication for intervention is guided predominantly by the presence or absence of symptoms. Asymptomatic or mild symptomatic PV stenosis should be managed conservatively with watchful waiting, given symptomatic amelioration has been observed after PV stenosis or occlusion without treatment and indicates collateral formation or recruitment.1153 For symptomatic patients, PV angioplasty should be considered. In patients with more than one PV stenosis, perfusion imaging may be applied to identify the “culprit” lesion. The dilation procedure is often complex, especially if the target PV is completely occluded with failed visualization from direct angiography via the LA as well as antegradely via pulmonary artery angiography. Electroanatomical 3D mapping with registration of the anatomy of the LA and the PVs, as well as fusion with the reconstructed LA from the imaging scan before the index procedure, enables a precise localization of the occluded PV.1154 Baseline CT or MRI is more helpful in defining the PV anatomy.
Many PV stenoses are rigid and difficult to dilate. Even after acutely successful angioplasty, PV restenosis occurs in up to 50% of cases.927,1142,1143,1152,1155 Stent sizes of 9 mm or more, and especially drug-eluting stents, revealed significantly better results, although drug-eluting stents of this size are not available.1142,1143,1144,1156 Whether or not primary stenting of PV stenosis offers better results than angioplasty alone has now been systematically studied by several groups.1144,1155 The risk of restenosis is significantly less with PV stenting, providing a stent of 8–10 mm in diameter can be used. A further problem is the small sample size of the published case series. Complications of interventional treatment of PV stenosis include LA perforation with or without tamponade, but also PV dissection with massive bleeding, stent embolization, and stent thrombosis.1142,1143 There are limited data regarding the need for and intensity of anticoagulation and antiplatelet therapy. For cases in which anticoagulation is otherwise indicated for AF, a regimen including the addition of clopidogrel is most commonly used. Without the indication for anticoagulation, warfarin and clopidogrel should be combined. The duration of anticoagulation needed remains unclear. In the case of restenosing PVs, anticoagulation for life might be necessary. In the setting of stable PV stents over the course of 1–2 years, clopidogrel and, subsequently, warfarin can be discontinued. The role of NOACs in PV stenosis has not been readily studied. Surgical patch repair of primary PV stenosis in children reveals a 5-year success rate of 67%, with an in-hospital mortality of 10%.1157 Only one case of surgical treatment of severe PV stenosis with patch implantation after catheter ablation has been reported.1158 Thus, it remains unclear whether the results are better than with conventional interventional treatment. Connecting the patch to the proximal end of the stenosis is difficult, because this end is buried in the lung parenchyma. Given this challenge, and the excessive risk, there is no foundation for recommending its use in patients with recurrent PV stenosis after AF ablation, and decision making cannot be based on a single case report. Even for patients with recurrent severe and persistent problems due to restenosis despite interventional treatment, recurrent infection and hemoptysis are uncommon, readily manageable, and the need for lobectomy or pneumonectomy is very rare. In the largest series of PV stenosis to date, both patients who underwent subsequent pneumonectomy at outside institutions died during or after surgery.1155 Although lung transplantation can be considered in a case of congenital PV stenosis,1159 this has never been required in AF-ablation patients. Dealing with patients with fibrosing mediastinitis and PV or peripheral artery (PA) stenosis is, in contrast, exceptionally difficult.1160
Successful PV angioplasty or stenting usually results in a significant relief of symptoms.1142,1143,1144,1145,1155,1156 Thus, follow-up strategies and intensity should be based on symptoms. Patients with restenosis usually report an increase of complaints existing prior to the intervention. In such cases, MRI is recommended. There is an additional critical consideration in dealing with PV stenosis. With the decline in follow-up CT scans after AF ablation, the occurrence of serious stenosis, hemoptysis, permanent PV occlusion, scarring, lung infarction, and intraparenchymal hemorrhage has increased. Many such patients are being inappropriately evaluated for lung cancer because of the appearance of intraparenchymal hemorrhage. Candidate veins for intervention are also increasingly problematic. These are more difficult to open and have a higher restenosis rate, requiring repetitive reintervention. Because of this, it is recommended that if a patient does not undergo a routine follow-up 3-month CT or MR, at a minimum, those with recurrent pulmonary symptoms after AF ablation should be scanned to exclude PV stenosis. Patients should also be routinely screened for symptoms at the time of follow-up evaluations. The take-home message is to identify PV stenosis before it becomes a serious problem.
Atrial Esophageal Fistula, Atrial Pericardial Fistula, and Esophageal Hematoma
Esophageal injury is one of the most important complications associated with catheter and surgical ablation of AF. In this section of the document we will focus on three types of esophageal injury: (1) esophageal hematoma, (2) atrial pericardial fistula, and (3) AEF. We will consider esophagopericardial fistula and AEF as one topic, and will focus mainly on this serious and often lethal complication of AF ablation. However, to be complete, we will also comment on the recently described complication of an esophageal hematoma.
Esophageal Hematoma
The esophagus can be injured directly as a result of trauma from a transesophageal probe. Esophageal hematoma is a recognized complication after a transesophageal echo study, which can be performed in association with the ablation procedure.1161 A recent study reported that 0.27% of the patients who underwent an AF ablation with a preprocedure TEE experienced this complication. The predominant symptoms were pain on swallowing, regurgitation, and hoarseness, with an onset within 12 hours of the procedure. Fever and neurological symptoms were not present. The diagnosis was established by a CT scan, which ruled out an AEF and revealed a hematoma localized to either the upper esophagus or extending the length of the esophagus. Endoscopy can further confirm the diagnosis. Conservative management is advised. Long-term consequences of this complication include an esophageal stricture, esophageal dysmotility, and vocal cord paralysis.1161
AEF and Atrial Pericardial Fistula
Esophageal ulceration, perforation, or development of a left AEF or atrial pericardial fistula, have been reported after both catheter ablation of AF and surgical ablation of AF using unipolar RF current.806,866,920,1162,1163,1164,1165,1166,1167,1168,1169 It is a possible complication after catheter ablation using any energy modality that produces transmural atrial lesions. Although early reports showed AEF resulting from RF ablation, more recently, AEFs have also been reported after CBA.877,878,879,1170 An adequately powered study examining the relative frequency of this complication with the two primary ablation modalities has not yet been performed. AEFs have also been reported following ablation with a focal ultrasound balloon ablation system that is no longer clinically available.705,1167 Esophageal erosion has also been reported with a circular multielectrode irrigated RF ablation system.1171 It is notable that 51% of the writing group members report having had a patient at their center develop an AEF following AF ablation. It should be clear, however, that the occurrence of an esophageal ulcer is not the same as an AEF. Although AEFs can be accompanied by ulcers, the presence of an ulcer is not predictive of an AEF. Occurring in 10%–40% of patients undergoing an AF ablation, the prevalence of an AEF is closer to one in one thousand in those with an ulcer.
Although the precise mechanism of esophageal tissue injury is not understood, potential mechanisms include direct thermal injury, acid reflux, infection from the lumen, and ischemic injury through thermal occlusion of end arterioles. It has been hypothesized that vagal damage resulting from ablation on the posterior LA wall can cause gastroesophageal reflux by damaging the vagal nerves that run along the esophagus, altering the lower esophageal sphincter pressure. This hypothesis proposes that high esophageal acid production could contribute indirectly to the formation of AEFs.1172,1173 This hypothesis is attractive; however, one study that attempted to validate it by measuring esophageal acid levels post-AF ablation was negative.1173 The prevalence of esophageal reflux and an AEF are also very different.
Although the development of an AEF following AF ablation is a very uncommon complication, its importance rests in the lethality of this complication. The Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation reported an AEF in six patients (0.04%).920 This incidence was similar to a separate survey of members of the Heart Rhythm Society. In this survey, an AEF was reported in 6 of 20,425 patients (0.03%).1168 All six of these patients experienced major cerebrovascular events, and five (83%) died. In contrast to an AEF, which is very rare, subclinical injury to the esophagus is extremely common following AF ablation. A more recent study reported an AEF incidence of 0.11%. In a number of studies, an endoscopy has been performed to screen for esophageal injury 1–3 days following AF ablation.1174 Esophageal tissue injury has been reported in up to 50% of patients.637,882,1175 Observed asymptomatic esophageal ulcers were usually healed on repeat endoscopy at 2–3 weeks.1176 One study reported endoscopy performed on 267 patients who underwent RF ablation. The power on the posterior wall was limited to 25 W. Among these patients, 6 (2.2%) had either erythema (n = 2) or a necrotic ulcer (n = 4) on endoscopy. Multivariate analysis revealed that the distance between the LA and the esophagus was the only independent predictor, although an LA isthmus line and CS ablation showed a trend.900 After treatment with a PPI (pantoprazole or esomeprazole) and sucralfate, all recovered without development of an AEF. One study reported a higher incidence of esophageal injury among patients undergoing AF ablation with general anesthesia compared with conscious sedation.637 It has been proposed that this relationship reflects the absence of pain feedback and reduced esophageal motility resulting from general anesthesia.
The clinical manifestations of an AEF usually present 2–4 weeks after the ablation procedure. The most common symptoms are fever and recurrent neurological events (septic emboli), but patients can present with septic shock, esophageal bleeding, or death. A recent case series of 53 patients who developed an AEF following AF ablation reported a mean interval between the procedure and presentation of 20 ± 12 days, ranging from 2 to 60 days. In this series, fever was the most common presenting symptom, followed by neurological deficits and hematemesis.1176 The preferred diagnostic modality is a chest CT scan.1169,1176 It is important to recognize that a normal chest CT scan does not rule out the presence of an AEF with 100% sensitivity. Ongoing vigilance and evaluation are important if the clinical suspicion is high. Although a barium swallow can detect a fistula, its sensitivity is low. IV contrast is much more likely to demonstrate a lesion passing from the esophagus to the mediastinum, the pericardium, or the LA. If an AEF is suspected, endoscopy with air insufflation should be avoided, given that insufflation of the esophagus with air can result in a large air embolus, producing stroke or death. An alternative strategy, which some members of the writing group employ and which appears to have lower risk, is to use CO2 instead of air for insufflation in this setting. If CO2 were introduced into the LA, there would be little adverse consequence. The early recognition of an AEF can be missed due to the low awareness of this rare complication. It is important for patients to be educated as to warning signs and to contact their AF ablation center should any suggestive symptoms develop.
Considerable efforts have been made to reduce the frequency of this complication. Approaches that have been proposed include avoiding ablation on the posterior wall of the atrium (or at least over the trajectory of the esophagus), reducing RF power on the posterior wall (to 25 W or less), using ICE to image the esophagus, and using an esophageal temperature sensor.637,900 Many institutions use an esophageal temperature probe to prevent thermal injury; however, it is widely acknowledged that use of an esophageal temperature probe does not eliminate the risk of esophageal injury.341,417,910 A survey of the writing group members shows that 87% use lower RF power on the posterior wall. This survey also reveals that two-thirds routinely use an esophageal temperature probe. Among those who use a temperature probe, one-third report using a temperature probe with multiple temperature sensors, whereas two-thirds use a probe with only one temperature sensor. It is important to recognize that the temperature probe should be as close as possible to the ablation catheter at all times during the procedure. Another variable concerns when to stop power delivery. Whereas some operators ablate until a predefined temperature has been met (e.g., 39 °C or 40 °C), other operators use a more conservative approach and terminate power when the esophageal temperature increases by as little as 0.2°. Esophageal temperature monitoring is also commonly used during CB AF ablation. Energy delivery is generally stopped when the esophageal temperature is lower than -20 °C. An alternative approach to the prevention of this complication is to move the esophagus away from the site of ablation using an endoscope or stylet positioned through a chest tube.894,895,1177
None of the writing group members employ this strategy. Other widely used strategies include the use of PPIs; although this approach is unproven, it has become a common approach. Seventy-two percent of the writing group members employ a PPI for 1–4 weeks following AF ablation. Use of PPIs is more common following RF ablation (95%) compared with CBA (54%). It is important to note, however, that this practice is based on the observation that esophageal ulcerations can be observed on endoscopy following ablation. There is no proof that this approach reduces the development of an AEF.
Treatment of an AEF is a medical emergency that requires urgent surgical repair.341,417,906,910,911,1169,1176,1178 Recent case series have reported an 83% to 100% mortality without surgical repair compared with a 34% mortality with surgical repair.341,417,906,910,1176 Although several case reports have been published describing favorable outcomes with esophageal stent placement for treatment of an esophageal perforation or an esophageal pericardial fistula, the mortality rate for stent placement in a patient with a true AEF approaches 100%.341,417,905,907,910,911
In summary, AEF is a rare but unpredictable complication with severe consequences that might only be mitigated by cautious use of energy on the posterior wall of the LA, early detection, and intervention. Prompt diagnosis and surgical treatment are typically required. Support for the use of esophageal stenting is limited, and progression of the AEF process can still occur despite this stenting procedure.
Gastric Hypomotility and Periesophageal Vagal Nerve Injury
Injury to the vagal anterior esophageal plexus can occur when RF energy is applied to the posterior wall of the LA, which can cause acute pyloric spasm and gastric hypomotility. Common symptoms include nausea, vomiting, bloating, and abdominal pain developing within a few hours to a few weeks after the ablation procedure.1018,1020,1179,1180,1181 Some patients also experience sinus tachycardia.1180 The incidence of symptomatic gastric problems can be as high as 17%.1020,1181 One recent study reported that asymptomatic functional impairment of the upper GI tract occurred in 74% of patients. After AF ablation, although the abnormality is often asymptomatic, the time to recovery is variable, with some patients recovering within 2 weeks, but others requiring a much more protracted time to recovery.536
The initial evaluation can include endoscopy or a barium swallow study to look for residual food after an overnight fast. CT shows marked gastric dilation. Solid food labeled with technetium-99 can demonstrate delayed gastric emptying. The 13C-acetate breath test has been reported to be a noninvasive alternative to scintigraphy.1182 Real-time MRI has been used to assess gastric motility and pyloric spasm.1179 In addition, electrogastrography can reveal gastric dysrhythmia with bradygastria in patients after ablation.1183 The integrity of the vagal innervation to the gastrointestinal system can be assessed by the pancreatic polypeptide response to sham feeding. Patients with this complication exhibit an abnormal kinetic and peak response. The normal response is a biphasic increase in pancreatic polypeptide. Injury to the vagus nerve impairs the first phase of the response.1017 After sham feeding, pancreatic polypeptide level elevation by less than 50% from baseline was considered as abnormal.
Management of this complication depends on the severity of symptoms and whether gastric immotility or pylorospasm predominates. Small, low-fat, and low-fiber meals can alleviate symptoms. Intravenous erythromycin can be effective in the acute stage to improve diabetic gastroparesis but has not been evaluated post-AF ablation.1184 Metoclopramide can be used to promote gastric motility for 1–3 months, but long-term treatment is associated with a risk of movement disorders. Botulinum injections or surgery might be required to alleviate pyloric spasm.1185 In severe cases, surgery or gastric pacing might be required.1185
Although there is no established method to prevent injury to the vagal nerves, the risk can be reduced by using the same techniques used to avoid an AEF, described earlier in this document. A recent report identifies higher BMI and limiting the power to 20–25 W on the posterior LA wall as protective against periesophageal nerve injury during AF ablation.1020
Phrenic Nerve Palsy
PN palsy is an important complication of AF ablation and results from direct thermal injury.536,903,1017,1182,1183,1184,1185 The right PN is most commonly affected, given it descends in close proximity to sites of ablation in the SVC and both right-sided PVs (Figure 1).536,903,1184,1185,1187 It courses slightly further from the RIPV so that injury during treatment of this vein is less common than that occurring with RSPV ablation. PN palsy is observed with all technologies for AF ablation, including RF, cryoablation, ultrasound, and laser ablation.490,536,903,1017,1182,1183,1184,1185,1187 PN palsy can be asymptomatic or can cause dyspnea, tachypnea, cough, hiccups, and thoracic pain. The diagnosis is suggested when newly elevated hemidiaphragm with atelectasis of the ipsilateral lung base is observed on postprocedure chest radiograph. When suspected, diaphragm excursion should be evaluated using fluoroscopy (sniff test) or ultrasound to confirm the diagnosis. Of the writing group members, 64% report having had a patient at their center develop permanent PN palsy, and 36% of the writing group members report having had a patient at their center develop permanent PN palsy following AF ablation with RF energy.
The most common scenario in which PN injury occurs is with CBA, with an incidence of transient PN palsy of 3.5%–11.2%.462,1075,1188,1189 Permanent PN palsy resulting from CBA is far less common, with an incidence of 0.3% in the recently completed FIRE AND ICE trial.490 PN palsy has also been reported with the laser-balloon ablation system. In the HeartLight study of the laser balloon, diaphragmatic paralysis secondary to PN injury occurred in 3.6% of the patients with the laser balloon and was more common than with RF ablation. Persistent PN paralysis at 1 year occurred in 1.8% of the patients.503 The hot balloon ablation catheter employs a compliant balloon filled with saline that is inflated to occlude the PV.706 Because of the mechanism of balloon heating, the possibility of hot spots forming in deeper tissue planes or in collateral structures such as the esophagus is unlikely.707 The main reported complications with this technology were PN palsy (3.4%) and PV stenosis (1.7%).708 A recent, prospective, multicenter clinical trial compared the outcomes of hot balloon ablation vs AAD therapy for PAF.706 The incidence of PN injury was 3.7%.
Several mechanisms have been proposed to explain the increased incidence of balloon-based (CB, laser balloon, hot balloon) AF ablation and PN injury. First, wedging or exerting force to direct the balloon into the RSPV for complete PV occlusion can distort the anatomy and decrease the distance between the RSPV endocardium and the right PN.1190 Second, a small balloon size relative to PV diameter can increase the likelihood of distal ablation in the vein.779 Third, the broader, circumferential thermal gradient and use of additional freeze cycles can increase risk of dose-dependent nerve palsy.1075 Studies have shown a higher risk of PN injury associated with the smaller 23-mm balloon compared with the larger 28-mm balloon with more proximal energy application.462,482 The smaller balloon is potentially advanced further within the PV, causing distortion of the anatomy, creating a higher susceptibility to PN thermal injury. PN palsy can also occur during WACA using RF energy. This likely results from thermal injury to the PN as it courses anterior to the right PVs.
The second most common scenario of PN palsy is during electrical isolation of the SVC using point-by-point RF ablation; the reported incidence is 2.1%–10%.1191,1192 Ablation within a persistent left SVC can result in left PN paralysis, but appears rarely, and has been associated with the use of CB.1193 Injury to the left PN during isolation of a persistent left SVC was not observed in several case series using RF energy.232,1042,1194,1195 Very rarely, ablation at the roof of the LAA can result in left PN damage1184; however, it was not observed in a large study in which LAA isolation was performed using RF ablation.532,533 The incidence of PN palsy is 0.17%–0.48% with PV antrum isolation using RF ablation, even though the PN is found within the typical WACA and carina lines of the right-sided PVs in 30% of patients.808,920,1184,1196 This highlights the importance of factors other than anatomic proximity alone contributing to the higher incidence of injury with CB.
A number of strategies have been employed to prevent PN palsy. These include limiting ablation to antral regions with various balloon maneuvers; preablation high-output pacing to establish whether the PN can be captured from the proposed ablation site before ablation; PN mapping with anatomic tagging of its course using an EAM system to guide the modification of the ablation lesion set; and monitoring of diaphragmatic excursion with abdominal palpation, fluoroscopy, or intracardiac ultrasound while pacing the PN from the SVC or subclavian vein during ablation.1196 Monitoring the effects of pacing the right PN is now considered a standard part of CBA and should be considered during SVC isolation using RF energy. Of the writing group members, 96% report employing this technique when performing CB AF ablation. Finally, diaphragmatic electromyography for direct monitoring of diaphragmatic compound motor action potentials (CMAP) during ablation is a technique for early detection of PN palsy that has been reported to reduce incidence of palsy.1197,1198 CMAPs are recorded using body surface electrodes, esophageal electrodes, or a diagnostic catheter positioned in the hepatic vein. A decrease in the amplitude of the myopotential by 30% is more sensitive than abdominal palpation for predicting the subsequent reduction in diaphragmatic excursion and nerve palsy.1199 Energy delivery should be interrupted immediately at the first sign of PN injury. One-third of the writing group members report employing this technique when performing CB AF ablation. One-third of the writing group members also report pacing anterior to the right PVs to tag the PN when performing AF ablation using RF energy.
PN palsy can be asymptomatic or can cause dyspnea, tachypnea, cough, hiccups, and thoracic pain.903,1017,1184,1187 The diagnosis is suggested when newly elevated hemidiaphragm with atelectasis of the ipsilateral lung base is observed on postprocedure chest radiograph. When suspected, diaphragm excursion should be evaluated using fluoroscopy (sniff test) or ultrasound to confirm the diagnosis.
There are various stages of PN palsy, ranging from detectable decrease in CMAP before a reduction in diaphragmatic excursion is perceived to persistent paralysis. With CBA, most PN injuries are transient and resolve within minutes.903,1184 In patients with persistent nerve palsy, most recover nerve function within weeks and almost all by 12 months, although 18–24 months might be required in some patients.1200 In a large meta-analysis of 22 studies enrolling 1308 patients undergoing CBA, 4.7% had persistent PN paralysis after the ablation procedure, but only 0.37% had paralysis lasting longer than 1 year.482 The pathophysiology of the palsy differs by type of ablation energy. With RF ablation, there is a dose-dependent response, and permanent palsy is characterized acutely by edema, coagulation, and homogenization of cytoplasmic contents and smearing of nuclear chromatin.536 With CB, the palsy is also dose-dependent; however, histopathology studies have shown Wallerian degeneration of large myelinated axons, and that axonal regeneration accounts for late recovery of nerve function.1201 There is no active treatment known to facilitate PN healing; however, in symptomatic patients with permanent nerve palsy, diaphragmatic plication can improve dyspnea and functional status.
Stroke, TIA, and Silent Microemboli
Stroke and TIA
Embolism of air or thrombus is one of the most significant complications of AF ablation, and both are potential causes of cerebral, coronary, and peripheral vascular compromise.
The incidence of thromboembolism associated with AF ablation is reported to be between 0% and 7%.242,489,503,532,533,655,673,796,798,799,806,920,921,1202,1203,1204 More than two-thirds of the clinical trials reviewed for preparation of this document reported one or more cerebrovascular events. Thromboembolic events typically occur within 24 hours of the ablation procedure, with the high-risk period extending for the first 2 weeks following ablation.798,1204 In one series that surveyed 26 embolic stroke events that occurred in a series of 3060 patients, long-term neurological outcomes were as follows: severe impairment (3 patients, with 2 possibly related deaths); moderate impairment (10 patients); mild impairment (9 patients); and unknown (4 patients).1202
A number of potential explanations for the development of thromboembolic complications have been proposed. These include the development of thrombi on or within stationary sheaths or ablation catheters positioned within the LA, char formation at the tip of the ablation catheter and at the site of ablation, disruption of a thrombus located in the atrium prior to the ablation procedure, and electrical cardioversion during procedures.875 Incidence of these events can be reduced by a combination of detailed preprocedural imaging, a strict anticoagulation protocol, meticulous attention to sheath management, and careful control of RF energy to minimize the risk of char formation. Of the writing group members, 68% report maintaining a constant heparinized flush through all long sheaths with access to the LA, and most heparinize to an ACT >300 seconds before transseptal catheterization.
Diagnosis of a symptomatic thromboembolic event is usually straightforward when ischemia or infarction results from arterial occlusion interrupting perfusion of dependent tissue. The potential manifestations depend on where the occlusion occurs, whether it be intracranial, coronary arterial, abdominal, or in other peripheral arterial beds. We have previously discussed the prevention of thromboembolism by intraprocedural and postprocedural anticoagulation in Section 7: Technical Aspects of Ablation to Maximize Safety and Anticoagulation. Treatment of a thromboembolic event will vary according to the location of the embolus. Peripheral arterial embolization might be amenable to surgical thrombectomy, whereas cerebral embolization has traditionally been managed conservatively and the consequences accepted. There is growing interest, however, in aggressive early management of such events, using either thrombolytic drugs or percutaneous interventional techniques. Some delay in diagnosis of a thromboembolic event that occurs during an ablation procedure while a patient is under general anesthesia cannot be avoided.
Asymptomatic Cerebral Emboli
ACE is defined as an occlusion of a blood vessel in the brain due to an embolus that does not result in any acute clinical symptoms and is therefore “silent.”800,1205 Emboli can result from a thrombus, air, gas, tissue, or fat. During an AF ablation procedure, potential sources of these microemboli include thrombi, which can develop on intracardiac catheters; sheath materials; air introduction through a sheath during catheter insertion or exchanges; dislodgement of thrombi in the heart; or as a result of thrombi or gas that forms during the ablation process. Diffusion-weighted MRI (DW-MRI), with or without fluid-attenuated inversion recovery (FLAIR) imaging, is very sensitive for identifying acute ischemic injury and can detect a cerebral lesion created by an embolus as early as 30 minutes postablation.
The first report of ACE lesions following AF ablation was published in 2006.1206 In this report, 2 of 20 patients developed new asymptomatic cerebral lesions on MRI, following AF ablation. Subsequent to this report, multiple studies have reported that DW-MRI can detect new acute lesions created by emboli, following up to 50% of AF ablation procedures.723,724,800,1205,1207,1208,1209
The incidence of this complication initially appeared to vary according to the system used for ablation, and was reported to be highest with the use of nonirrigated circumferential multielectrode ablation catheters with duty cycled phased RF energy.1209,1210 Based on these findings, modifications were made in anticoagulation, sheath management, and energy delivery protocols. Following introduction of these modifications, two subsequent studies reported a 2% or lower incidence of ACE lesions with use of this same circular phased RF ablation catheter.728,731,1211 One study examined the important question concerning whether these lesions persist on repeat DW-MRI and T2 FLAIR scanning. In this study, 14 patients who had 50 new silent cerebral emboli detected post-AF ablation had a repeat MRI a median of 3 months later. It was notable that 47 of the 50 lesions (94%) resolved in the interim. The three lesions in three patients that produced a residual defect at repeat scanning were initially >10 mm in size, and one of these patients had neurological symptoms. When considering the significance of the ACE lesions that have been observed following AF ablation, it is important to note that cerebral embolism has also been observed after most types of cardiac invasive procedures, including coronary angiography, carotid artery stenting, and cardiac valve replacement.1212,1213 Importantly, as of now, a direct link between silent cerebral embolism and a decline in neurocognitive function has not been proven.800,1205,1211,1212 However, one study has reported mild postoperative neurocognitive dysfunction in 13% of patients undergoing ablation for PAF and in 20% undergoing ablation for persistent AF. The precise mechanism of this neurocognitive dysfunction and its possible link to ACE lesions needs to be explored further.1214
A decade after the first description of ACE lesions following AF ablation, a tremendous amount of new knowledge has been generated concerning this important complication of AF ablation.800,1205,1211,1215,1216 These efforts have resulted in a striking decrease in the incidence of this complication. During this period of time, studies have identified a number of techniques to lower the risk of ACE lesions, including (1) aggressive anticoagulation prior to, during, and following ablation; (2) careful sheath management; (3) modifications in the delivery of phased RF energy; and (4) choice of ablation energy source and lesion sets. The long-term prognostic implications of ACE following AF ablation remain unclear. Because multiple studies have reported that the majority of acute lesions regress without evidence of chronic glial scar when reassessed several weeks to months later, the occurrence of long-term sequelae appears unlikely.1205 Nevertheless, there is a possibility of long-term sequelae, given the association between silent cerebral infarcts and an increased long-term risk of dementia.1217 While further work remains, the amount of progress is striking and will benefit our patients in the long term.
Air Embolism
The most common cause of air embolism is introduction of air via the transseptal sheath. Although this can be introduced through the infusion line, it can also occur with suction when catheters are removed. Air embolism has been reported with coronary angiography, percutaneous interventions requiring access to the LA, and during ablation procedures.803,1218,1219,1220,1221 Air embolism to the cerebral vasculature can be associated with altered mental status, seizure, and focal neurological signs. Central nervous system dysfunction is attributable to both mechanical obstruction of the arterioles and thrombotic-inflammatory responses of air-injured epithelium.1219,1220 Although immediate diagnosis and treatment is based on clinical suspicion, prompt MRI or CT scans obtained before the intravascular air is absorbed might show multiple serpiginous hypodensities representing air in the cerebral vasculature, with or without acute infarction.803,1221 Most importantly, AEF should be ruled out if air embolism is documented after the ablation. A common presentation of air embolism during AF ablation is acute inferior ischemia and/or heart block. This reflects the preferential downstream migration of air emboli into the right coronary artery (RCA). The preferential manifestation of air emboli into the RCA territory might reflect the superior position of the RCA ostium in the supine patient. Supportive care usually results in complete resolution of symptoms and signs within minutes. However, pacing and cardiopulmonary resuscitation might be needed if the hypotension and AV block persist. A recent study reported the clinical characteristics and outcomes of 5 out of a series of 2976 patients who underwent AF ablation who experienced a massive air embolism during the procedure. Hemodynamic collapse and hypoxemia occurred in all the patients and persisted for 10–35 minutes. Despite this, all the patients had complete recovery.1221 it is imperative, however, that all infusion lines be monitored closely for bubbles. Whenever catheters are removed, they should be withdrawn slowly to minimize suction effects, and the fluid column within the sheath should be aspirated simultaneously. Particular care is advised when inserting and removing balloon catheters through large sheaths.1222 Treatment should be initiated immediately in the laboratory if cerebral air embolism is suspected. The most important initial step is to maximize cerebral perfusion by the administration of fluids and supplemental oxygen, which increases the rate of nitrogen absorption from air bubbles. For large air emboli, it might be beneficial to briefly suspend the patient in a head-down position.1218,1219 Treatment with hyperbaric oxygen can reverse the condition and minimize endothelial thromboinflammatory injury if it is started within a few hours.1220 Heparin appears to limit injury in animal models of cerebral arterial air embolism.1223
Vascular Complications
Vascular complications, including groin hematoma, retroperitoneal bleed, femoral artery pseudoaneurysm, or arteriovenous fistula, are the most common complications of AF ablation. The incidence of the more significant of these complications (femoral pseudoaneurysm, arteriovenous fistula, and retroperitoneal bleeding) varies from 0.2% to 1.5%.806,808,920,921,1224,1225,1226 The first and updated worldwide surveys of AF ablation in 2005 and 2010, respectively, reported that the incidence of vascular complications was 0.95% (84 of 8745 patients) and 1.5% (240 of 16309 patients), respectively.806,920 A report from the United States analyzing an estimated 93,801 AF ablations between 2000 and 2010 showed the overall incidence of vascular complications requiring blood transfusion or surgical repair was 1.53%, which remained statistically unchanged from year 2000 to 2010.921 More recent reports from Czechia, Belgium, Japan, and the United States reported the incidence of these complications as 1.1% (13 of 1192 procedures in 959 patients), 1.2% (15 of 1233 procedures in 947 patients), 0.2% (7 of 3373 patients), and 1.5% (18 of 1190 patients), respectively.808,1224,1225,1226 The incidence of vascular complications that result from AF ablation is lower than those reported for ventricular tachycardia ablation (range, 3.6%–6.9%), in which femoral arterial access is used in many cases.1227,1228 Most groin hematomas can be managed conservatively or with ultrasound-guided compression. However, complications such as femoral pseudoaneurysm, arteriovenous fistula, and retroperitoneal bleeding might require blood transfusion and/or surgical or percutaneous repair, which leads to increased morbidity and prolonged hospital stay.1229 Rarely, a large dense hematoma can lead to femoral neurological sequelae.
The incidence of these complications can be related to the number and size of the venous sheaths used, insertion of an arterial pressure line, and perhaps to the intense anticoagulation management before, during, and after the procedure. Recent studies have suggested uninterrupted warfarin as an optimal anticoagulation regimen because it reduces stroke and nonmajor bleeding complications compared with interrupted warfarin with heparin bridging.834 Further, uninterrupted or briefly interrupted use of a direct oral anticoagulant was shown to be as safe and effective as uninterrupted warfarin.840,842,1230 The results of the RE-CIRCUIT study were recently published, which was a head-to-head comparison of performing AF ablation on patients receiving uninterrupted dabigatran vs uninterrupted warfarin.841 This study randomized 704 patients across 104 sites to these two anticoagulation strategies. The incidence of major bleeding events during and up to 8 weeks postablation among the 635 patients who underwent AF ablation was significantly lower with dabigatran than with warfarin (5 patients [1.6%] vs 22 patients [6.9%]); absolute RD -5.3%, RR reduction 77%). There has been one other smaller head-to-head comparison published of uninterrupted rivaroxaban vs uninterrupted warfarin (Venture-AF, N = 248).842 This study reported one major bleeding event, one ischemic stroke, and one vascular death, each of which occurred in the warfarin arm of the study.
The approach used for femoral venous access can impact on the risk of vascular complications. When an inferior approach to femoral vein access is used, small medial branches of the femoral artery, which can run across and superficial to the femoral vein, might be penetrated before entry to the femoral vein, possibly leading to a femoral pseudoaneurysm and arteriovenous fistula. When a superior approach is used, there is an increased risk of retroperitoneal bleeding. To prevent these vascular complications, real-time ultrasound-guided venipuncture is useful and can be recommended because it reduces both major and minor vascular complications in patients undergoing AF ablation and/or electrophysiological procedures.1231,1232 Among the writing group members, two-thirds routinely use ultrasound imaging to guide vascular access.
Acute Coronary Artery Occlusion and Stenosis
Injury to the coronary arteries during AF ablation is rare. In a consecutive series of 5709 patients undergoing ablation of AF, coronary arterial injury was observed to occur in eight patients (0.14%).1233
The circumflex artery is in close proximity to the lateral LA and can potentially be injured during ablation at sites adjacent to its course within the CS, the lateral mitral isthmus, or the base of the LAA. Occlusion of the circumflex accounted for three of the eight cases in the above series, all presenting with ventricular fibrillation 20 and 60 minutes after mitral isthmus ablation and 6 hours after ablation at the LAA base, respectively.1233 Others have also described features of acute myocardial infarction with ST segment changes occurring during ablation at the mitral isthmus.923 These patients have variably undergone unsuccessful intracoronary vasodilators or thrombectomy and have had to progress to coronary stenting. A single case presenting 48 hours after mitral isthmus ablation with total circumflex occlusion and ventricular arrhythmia storm is described as having ongoing ventricular arrhythmia requiring ablation and defibrillator implantation, highlighting the potential for ongoing consequences as a result of coronary artery injury.1234
The sinus node artery originates from the proximal circumflex artery in one-third of cases and then courses along the anterior LA and then the septal SVC, and could therefore be susceptible to injury during ablation. In the above series, five of the eight patients presented with acute sinus node dysfunction.1233 In most of these cases, the culprit site was adjacent to the sinus node artery (per CT) at the anterior LA or septal RA. All these cases presented with sinus arrest during or within 1 hour of ablation and with no evidence of any other electrocardiographic changes associated with coronary occlusion. Two of these patients eventually required permanent pacemaker insertion with significant atrial pacing during follow-up. Others have described a more transient sinus node dysfunction due to occlusion of the sinus node artery.1235
The cavotricuspid isthmus can be ablated in conjunction with AF ablation. This region is in close proximity to the RCA, and injury to this vessel has been described.1236,1237 These have occurred both acutely and later during the case, and with both septal and lateral approaches to the ablation.
In addition to the direct injury and occlusion at the sites of ablation, a single case of thromboembolic occlusion of the left anterior descending artery the day after ablation has been described.1238 This case was known to have factor V Leiden mutation, was therapeutically anticoagulated with an INR of 2, and the activated clotting time had been maintained between 280 and 390 seconds. Angiography demonstrated thrombus in the left anterior descending artery, requiring intervention. This case highlights the need for meticulous anticoagulation, sheath management, and physician awareness of the potential for thromboembolism to present as coronary occlusion. Although presentation with acute coronary artery occlusion is low, the possibility of thermal injury without occlusion and the possibility of subsequent remodeling leading to stenosis of a coronary artery should be considered. The most vulnerable location for this would appear to be the circumflex vessel during ablation of the mitral isthmus. In a series of 54 patients who had undergone mitral isthmus ablation, coronary angiography was performed before and after ablation.1239 Fifteen patients (28%) had angiographic changes following ablation, eight with midcircumflex narrowing, one with circumflex and obtuse marginal narrowing, one with obtuse marginal narrowing only, and five with distal circumflex narrowing or occlusion. A further five had significant narrowing that resolved with intracoronary vasodilators. Patients with such coronary arterial changes had a significantly longer ablation time within the CS. Therefore, limiting excessive ablation, particularly in areas adjacent to the coronary vasculature, should be a consideration in planning the ablation strategy. In the intraoperative setting, late coronary stenosis has been described at sites of previous ablation.1240
There can be several determining factors in the development of coronary artery injury during AF ablation. These include the degree of protective epicardial adiposity, coronary blood flow, and the intensity and duration of ablation; however, the most predictable is that of the location of ablation adjacent to the course of the coronary artery. Careful monitoring and avoiding high-power energy delivery in the vicinity of these vessels are potentially important in minimizing the risk of arterial injury.
Radiation Exposure During Catheter Ablation of AF
An important, less easily recognized, and rarely considered potential complication of AF ablation is the delayed effect of the radiation received by the patients, including acute and subacute skin injury, malignancy, and genetic abnormalities.1,1241,1242,1243,1244,1245,1246,1247 Fluoroscopy is required for most components of the procedure, including catheter placement, positioning a multielectrode catheter into the CS, double transseptal catheterization, PV angiography, and LA ablation. A survey of the writing group members reveals that two-thirds use single-plane fluoroscopy, whereas one-third employ biplane. One study reported a mean fluoroscopy time >60 minutes, with corresponding higher effective radiation doses in obese patients.1244,1246 By using a vest containing 50–60 dosimeters to measure peak skin doses (PSDs), another study reported a mean PSD of 1.0 ± 0.5 Gy in the right anterior oblique and 1.5 ± 0.4 Gy in the left anterior oblique projection, during a mean fluoroscopy time of 67.8 ± 21 minutes.1245 They estimated an overall lifetime risk of excess fatal malignancies normalized to 60 minutes of fluoroscopy of 0.07% for women and of 0.1% for men.1245 The relatively low radiation exposure to the patients in this study despite the prolonged fluoroscopy durations was attributable to the state-of-the-art very low pulsed fluoroscopy frame rate, the avoidance of magnification, and the optimal adjustments of fluoroscopy exposure rates. The resulting lifetime risk of malignancy was thus within the range previously reported for ablation of supraventricular tachycardias. However, this study demonstrated that catheter ablation of AF required significantly greater fluoroscopy duration and radiation exposure than simpler catheter ablation procedures. Thus, and especially because AF ablation procedures often need to be repeated, electrophysiologists should make every attempt to minimize radiation exposure.
Increasing availability and familiarity of electrophysiologists with 3D mapping systems, as well as the availability of CF monitoring, have significantly reduced fluoroscopy time and the need for fluoroscopy in recent years.747,1186,1248,1249 This can only be achieved, however, by an awareness of the importance of reducing fluoroscopy time, and therefore radiation exposure, by the operator.1250 It has been shown that use of optimized conventional fluoroscopy and optimized use of 3D mapping can result in a marked reduction in radiation exposure.1251 It is also important to recognize that fluoroscopy time is only weakly linked to true radiation exposure, because it does not reflect the fluoroscopy equipment being used, nor patient-specific factors such as obesity. The use of remote navigation for PVI appears to be effective, with fewer periprocedural complications and significant reductions in fluoroscopy exposure for both patient and operator.749,1252,1253 Another interesting option to minimize radiation exposure to the operator and to alleviate the orthopedic implications of conventional lead aprons is the use of a radioprotection cabin or a suspended lead apron.1254
More recently, it has been shown that PVI is feasible without using fluoroscopy or with extremely limited fluoroscopy. To safely navigate catheters in the heart with no fluoroscopy, intracardiac ultrasound is mandatory, as well as imaging integration with preacquired CT or MRI.763,1255,1256
Pericarditis
More than 50% of the patients who undergo catheter ablation of AF note some pleuritic chest pain in the first several days following their procedure. It is also common to observe a “trace” pericardial effusion following AF ablation. These largely self-limited manifestations of AF ablation-induced pericarditis are so common and of so little consequence that they are considered as part of the standard clinical course for patients who undergo AF ablation rather than as a complication of the procedure. A small subset of these patients will go on to develop more severe and clinically significant manifestations of pericarditis. In two recent multicenter registries, pericarditis has been reported to occur in 0.1% and 0.6% of patients, respectively.1059,1257 When transmural lesions are generated during catheter ablation of AF, some epicardial inflammation, and therefore some pericarditis, is inevitable. However, more extensive pericarditis can complicate AF ablation procedures both acutely and at some delay. These presentations include Dressler syndrome, pericarditis leading to delayed cardiac tamponade, and constrictive pericarditis.1258,1259,1260 These severe manifestations and consequences of pericarditis presented between 18 days and 3 months after their ablation procedures. The standard international practice for a short hospital stay after AF ablation procedures can contribute to an underappreciation of early postablation pericarditis.
There is currently no evidence to support the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or steroids to prevent AF recurrences. A single bolus injection of low-dose hydrocortisone (100 mg) reduced the incidence of pericarditis from 2.5% to 1.1% in one recent series from Japan, but no difference in early or late recurrences was found after AF ablation.1261 Another Japanese study also failed to demonstrate a reduction in immediate, early, or midterm AF recurrence with either a low-dose (hydrocortisone 100 mg) or moderate-dose (methylprednisolone 125 mg) single steroid bolus.
Colchicine is currently the cornerstone of pericarditis treatment that occurs outside of the AF ablation setting, although specific data after AF ablation are lacking. In one trial, however, in which patients were randomized to a 3-month course of colchicine (0.5 mg twice daily) or placebo, early recurrence was significantly reduced (33.5% of placebo patients vs 16% for colchicine), and this was strongly associated with a reduction in inflammatory mediators such as IL-6 and C-reactive protein. After a 15-month follow-up, a 37% reduction in the RR of AF recurrence was observed (number needed to treat = 6).985 In a subsequent randomized study of 233 patients with PAF, these investigators reported that the long-term recurrence rate was 31% among the patients treated with colchicine vs 49% among the placebo patients. Colchicine also resulted in an improvement in QOL.986
Mitral Valve Trauma and Curvilinear Catheter Entrapment
Entrapment of a circular multielectrode mapping catheter by the mitral valve apparatus is an uncommon but established complication of AF ablation.1262,1263,1264,1265,1266,1267,1268,1396 It results from inadvertent positioning of a circular electrode catheter close to the mitral valve or into the left ventricle, often during attempts to position the catheter into the LIPV or when using such catheters to create electroanatomical maps of the LA. This complication should be suspected when attempts to reposition the catheter into another PV are met with resistance. When suspected, it is important to confirm the diagnosis with echocardiography. Although successful freeing of the catheter has been reported with gentle clockwise catheter manipulation and advancing the sheath into the ventricle in two patients, there have also been a number of cases reported in which the mitral valve apparatus and/or papillary muscles are torn during attempts to free the catheter.1263,1264,1268,1269,1396 There have also been several cases reported in which the distal tip of the circular catheter broke off during attempts at catheter removal and had to be subsequently removed either with a snare or with an open surgical procedure.1263,1265,1266 We recommend that if gentle attempts to free the catheter fail, elective surgical removal of the catheter should be performed.
It is important for all electrophysiologists who perform AF ablation to be aware of this potentially serious complication. Every effort should be made to be certain that the circular catheter is kept safely away from the mitral valve and that only clockwise torque be applied to the catheter, with particular care taken when approaching the LIPV. The incidence of this rare complication is unknown, but might have decreased in recent years due to improved awareness.
Limited data are available regarding outcomes of AF ablation in patients with prosthetic valves. One small, matched cohort study suggested that long-term outcomes might be similar, but ablation procedures were longer and were associated with a numerically higher rate of complications in patients with prior mitral or aortic valve replacement (AVR).908 The development of new perivalvular leak following AF ablation in a patient with a mitral prosthesis has been reported, suggesting that care should be taken when ablating near the annulus in such patients.921
Mortality Risk with AF Ablation
Although AF ablation is generally considered to be safe, devastating complications can occur rarely, some being fatal. In a recent survey, death was reported in 32 of 32,569 (or 1 in 1017) patients undergoing AF ablation procedures worldwide.1039 The most frequent cause of death was cardiac tamponade, accounting for 25% of the deaths, of which 3% occurred later than 30 days after the procedure. Stroke was responsible in 16% of the cases, of which 6% occurred later than 30 days. AEF also accounted for 16% of the deaths, with extensive pneumonia responsible for 6%. Less common causes of death observed in the periprocedural phase included myocardial infarction, irreversible torsades de pointes, septicemia, sudden respiratory arrest, extrapericardial PV perforation, occlusion of both lateral PVs, hemothorax, and anaphylaxis, which were each responsible for 3% of early deaths.
Twenty-two percent of all deaths occurred more than 30 days after the procedure. Among the identified causes of late death were asphyxia from tracheal compression secondary to subclavian hematoma, intracranial bleeding, acute respiratory distress syndrome, and esophageal perforation by the intraoperative TEE probe, with each cause contributing to 3% of all late deaths.
It should be noted that these reported mortality risks of AF ablation came mostly from experienced operators and centers. In the community setting, the mortality risk of AF ablation can be much higher. Indeed, one study of 93,801 patients undergoing AF ablation in the United States between 2000 and 2010 showed that one in 238 AF ablation patients were never discharged alive following their procedure. These mortality risks were due primarily to inexperienced operators who performed fewer than 25 procedures annually, and to low-volume hospitals that performed fewer than 50 procedures annually.921
When a 30-day all-cause mortality definition is used for AF ablation, AF ablation mortality rises to 1 in 125 patients within the Medicare population (mean age of 72).921 Awareness about the risk of death and the possible causes might help physicians set more appropriate and efficient standards for procedural safety, and need to be considered in the patient's decision-making process.
Stiff Left Atrial Syndrome
First described after mitral valve surgery in 1988, stiff LA syndrome was recognized as a rare complication of LA catheter ablation in 2011.1110,1111,1270,1271,1272,1273 One early report described a series of three patients with unexplained exertional dyspnea, LA hypertension, and large V waves on LA pressure or pulmonary capillary wedge pressure (PCWP) tracings after multiple surgical LA ablation procedures.1271 A subsequent study prospectively collected 1380 consecutive patients undergoing ablation, obtaining echocardiograms before and after ablation to assess for pulmonary hypertension.1272 Excluding patients with PV stenosis or significant mitral valve disease, there were 19 patients (1.4%) with new or worsening pulmonary hypertension, LA diastolic abnormalities, and clinical findings of dyspnea, HF, pulmonary hypertension (mean PA pressure ≥25 mm Hg or during exercise ≥30 mm Hg), and large V waves (≥10 mm Hg and higher than mean LA pressure tracings) on PCWP or LA pressure tracings. Other authors reported worsened pulmonary hypertension (echocardiographic right ventricular systolic pressure (RVSP) >35 mm Hg with increases of > 10 mm Hg) in 41 of 499 patients (8.2%) by 3 months after ablation.1110 These studies were flawed, however, by the low cutoff for diagnosing PA hypertension, particularly after an ablation with excess volume delivery. Stiff LA syndrome was also reported in 9 patients after surgical maze procedures, presenting with unexplained dyspnea, severe pulmonary and LA hypertension, giant LA V waves, absent LA or LV A waves, blunted X descents, and elevated left ventricular end-diastolic pressure attributed to abnormal LA compliance and contractility.1274
Studies have identified small LA size (≤45 mm), high mean LA pressure, severe LA scarring (>60%), diabetes mellitus (DM), and OSA as independent predictors of pulmonary hypertension or stiff LA syndrome postablation.1272 The potential importance of scar burden and the extent of RF ablation to LA stiffness or function has also been noted by other investigators. In another study of 26 patients with mean follow-up of 80 months, LA scar by MRI was related to the number of procedures, total RF duration, LAA EF, and expansion index.1275 LAA EF correlated with exercise capacity at follow-up, and LA scar extent had a negative correlation with exercise capacity. Another study reported that LA stiffness index, derived from invasive pressure measurements and cardiac MRI volumes during sinus rhythm (ΔP/ΔV) was higher in patients with persistent rather than PAF, older age, and prior LA ablation.1276 A subsequent study reported that in 70 patients with 12-month follow-up, LV diastolic dysfunction worsened in 27% and correlated with total ablation time, concluding that more aggressive ablation might aggravate diastolic dysfunction.1145
The stiff LA syndrome fortunately appears to be largely responsive to diuretic therapy. One study reported that all 19 of their patients had symptomatic improvement after diuretic therapy, noting that diuretics appeared more effective for this syndrome than for other forms of pulmonary hypertension.1272 In contrast, another study reported a case of stiff LA syndrome after two AF catheter ablation procedures that failed with furosemide and spironolactone, but which responded to sildenafil.927
In summary, stiff LA syndrome or worsened pulmonary hypertension appears to occur in 1.4%–8% of patients after AF RF catheter ablation. The diagnosis of stiff LA syndrome after AF or LA ablation should be sought for patients who present with unexplained dyspnea with signs of right HF. Diagnosis can be made by signs of right HF in the presence of preserved left ventricular function, pulmonary hypertension (mean PA pressure ≥25 mm Hg or during exercise ≥30 mm Hg), and large V waves (≥20 mm Hg and higher than mean LA pressure tracings) on PCWP or LA pressure tracings in the absence of significant mitral valve disease or PV stenosis. We also recommend that to reduce the risk of stiff LA syndrome, judicious use of extensive LA ablation be considered in patients with small LA size, high LA pressures, preexisting severe LA scarring, DM, or OSA. Patients with stiff LA syndrome usually respond well to diuretics.
Cough
Cough is a specific respiratory symptom that can occur after catheter ablation of AF. It might be a sign of underlying PV stenosis, PN injury, direct bronchial injury, stiff LA syndrome, gastroesophageal reflux, pulmonary embolism, pericarditis, or other iatrogenic respiratory complications such as ventilator-associated pneumonia or postprocedure aspiration pneumonia. Although there is a paucity of data on the incidence and mechanisms of postprocedure cough, the underlying mechanisms can vary according to the ablation technology.
After RF ablation, cough might point to the presence of RF-induced PV stenosis. Whereas mild PV stenosis is frequently asymptomatic, patients with more extensive and severe PV narrowing can present with cough, dyspnea, chest pain, or hemoptysis.1152,1200 Similarly, another study reported that in 18 patients with severe PV stenosis, 7 (39%) reported cough.462 Cough might also be a sign of RF-induced PN injury. Although a rare complication (0.48%), RF-induced PN injury is frequently (9 of 22 patients) associated with immediate features of dyspnea, cough, hiccup, and/or sudden diaphragmatic elevation.1277
Cough following CBA is more frequent. In fact, as many as 1 in 6 patients can develop a dry cough following CBA, which is usually self-limiting in 91%.1278 Whereas the most evident mechanism for postprocedure cough is that of CBA-induced PN injury (up to 11%), some reports suggest that the cough is caused by direct upper airway irritation during CBA (bronchial or pulmonary injury).1278 In an experimental model, Aryana et al showed that CBA can elicit direct and acute bronchial inflammation, bleeding, and mucosal injury.1277 A recent study reported ice formation within the left main-stem bronchus using real-time bronchoscopy during CBA.583,584 Given the increasing number of case reports detailing respiratory complaints after CBA, a systematic examination of the short- and long-term consequences of CBA on normal bronchial tissue during PVI is warranted.
Increase in Heart Rate and/or Sinus Tachycardia
A subset of patients will experience a significant increase in their resting sinus heart rate following AF ablation.110 Although this typically results in a 10–20 beats per minute (bpm) increase in heart rate (well below the 100 bpm threshold to classify the increase as sinus tachycardia), the resulting increase in heart rate can exceed 100 bpm in a very small subset of patients. This phenomenon is related to shifts in autonomic tone following ablation and is predictive of ablation success. This shift in autonomic tone results from ablation of GP that are commonly located near the PV antra, as previously discussed.110,121 Stimulation of GP has been shown to elicit AF by producing repetitive bursts of rapid focal PV firing, and ablation of GP can play a role in AF treatment.257,577,1279 Following ablation of GPs, signs of parasympathetic withdrawal such as increased heart rate and attenuated heart rate variability can be observed, and these signs have been associated with improved procedural outcomes.118,126,577,1280,1281 Although the increase in heart rate and reduction in heart rate variability after ablation typically follow a transient time course, with resolution within 3 months, some studies have shown that the long-term persistence of these autonomic changes is associated with improved clinical outcomes.126 These clinical data are consistent with experimental findings demonstrating a reduction in stellate ganglion nerve activity and subsequent AF with continuous low-level vagal nerve stimulation.1200 Thus, the observation of increased heart rate following ablation can be a normal finding with potential positive prognostic implications regarding outcomes and is not necessarily a procedural complication per se.
Section 11: Training Requirements
Overview
The strategies, specific methods, and technology pertaining to AF ablation are evolving. Accordingly, the guidelines for training to perform this procedure must be flexible in recognition of various approaches and technologies that will change with advances in the field. Training for AF ablation should encompass six fundamental principles: (1) appropriate selection of patients; (2) knowledge of the anatomy of the atria and adjacent structures; (3) conceptual knowledge of strategies to ablate AF; (4) technical competence; (5) recognition, prevention, and management of complications; and (6) appropriate follow-up and long-term management.
The training required in each of these areas differs from other ablation procedures because, in comparison, ablation of AF is technically more difficult, is associated with greater risks, and requires more careful follow-up.
Appropriate Selection of Patients
Trainees should recognize clinical attributes that can increase the difficulty of a transseptal puncture, increase the risk of the procedure, and affect short- and long-term outcomes. These factors are discussed in Sections 8 and 9 of this document. The trainee should also develop the judgment to decide whether conscious sedation or general anesthesia would be most appropriate for the case under consideration. It is also important to assess the severity of symptoms related to AF and the potential benefit of an ablation procedure. Trainees should be experienced in counseling patients about the potential risks and benefits of, as well as the alternatives to, an ablation procedure and should be able to apply this knowledge for recommendations specific to the needs of individual patients. They should also take into consideration the prior use of AADs and pharmacological alternatives to AF ablation.
It is also important for electrophysiologists involved with catheter ablation to be knowledgeable about surgical ablation techniques for AF. In particular, electrophysiologists who perform AF ablation procedures must be aware of the indications, techniques, and outcomes of surgical approaches for AF ablation. This applies both to the new minimally invasive surgical approaches, AF surgery combined with other cardiac surgical procedures, and the Cox-Maze III procedure (see Section 12).
Anatomy of the Atria and Adjacent Structures
Detailed knowledge about the anatomy of the LA and its adjacent structures is crucial for performing the technical aspects of transseptal puncture and cannulation, LA mapping, and isolation of the PVs or modification of the substrate that sustains AF. The trainee must recognize the anatomic relationship of the atria, SVC, and PVs to the pulmonary arteries, aorta, mitral annulus, PNs, sympathetic and parasympathetic innervation, esophagus, and other mediastinal structures (Figure 1). These anatomic relationships affect the ability to perform the procedure successfully and to avoid complications.
Conceptual Knowledge of Strategies to Ablate AF
Trainees should understand the pathophysiology of AF and its implications for strategies to ablate AF. This includes the role of the PVs, the SVC, the musculature of the LA, and the potential impact of autonomic stimulation. They should understand the rationale for isolation of the PVs and elimination of the foci that trigger AF, as well as the basis for broad circumferential ablation of tissue or elimination of fractionated potentials or other technologies that appear to alter the substrate that sustains AF.
Technical Competence
The technical skills needed for ablation of AF are substantial. These include anticoagulation management, transseptal needle puncture and cannulation of the LA, precise manipulation of the catheter for mapping and ablation, identification of the pulmonary ostia, adjustment of the energy used for ablation, and the appropriate use of fluoroscopy, radiographic contrast for imaging, 3D mapping systems and/or ICE. Simulation technologies are evolving that could help trainees gain experience with fundamental techniques in the early phase of learning procedural skills or the recognition and management of acute complications such as cardiac tamponade.1250,1282,1283 There are substantial differences among laboratories in the use of radiographic contrast imaging, EAM or echocardiography, and the number and types of catheters used to identify electrical endpoints and to perform ablation. The degree of expertise gained in the use of a specific technology will depend on where training is completed, as well as the duration of training. Nonetheless, trainees should be expected to understand the potential advantages and limitations of these systems and should have the ability to interpret basic images and electrical recordings obtained from these various methodologies. They should be well versed in the principles of radiation safety for patients and the medical personnel who perform ablation procedures.
Training programs should emphasize the interpretation of intracardiac electrograms for recognition of PV potentials and determination of when electrical isolation of a PV has been achieved, the role of CS and LAA pacing in the differentiation of far field electrograms from PV potentials, identification of fractionated low-amplitude LA potentials, and techniques required to map and ablate right and/or LA tachycardias or AFL. Concepts related to entrainment are especially important. Trainees need to be skilled in identifying the presence, mechanism, origin, and ablation of other supraventricular tachycardias that could act as triggering mechanisms for AF, such as AV nodal reentrant tachycardia and AV reentrant tachycardia. Training and competence in RF catheter ablation are essential because this ablation technology is needed for ablation of typical and atypical AFL. Many electrophysiology laboratories also use RF energy as the preferred energy source for ablation of AF. Many other electrophysiologists prefer CBA for their AF ablation procedures. Other ablation technologies that are currently available in some parts of the world include laser balloon ablation and ablation using circular multielectrode RF ablation catheters. Trainees should be familiar with the advantages and limitations of each energy source and associated delivery system.
Procedural Experience
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society Advanced Training Statement on Clinical Cardiac Electrophysiology proposed a minimum of 5 five focal ATs, 30 macroreentrant ATs (including 20 isthmus- and 10 nonisthmus-dependent/complex macroreentry) and 50 AF ablation procedures for those who undergo fellowships in clinical cardiac electrophysiology.1284 The writing group members are supportive of the requirement that trainees perform at least 50 AF ablation procedures and at least 30 macroreentrant ATs (including 20 isthmus- and 10 nonisthmus-dependent/complex macroreentry) during fellowship training. Furthermore, the writing group recommends that those performing the procedure perform at least several AF ablation procedures per month to maintain competence.
These numbers underestimate the experience required for a high degree of proficiency.991,992,1082,1285,1286 Exact numerical values are difficult to specify because technical skills develop at different rates. Nonetheless, comparisons of high- and low-volume centers suggest that outcomes are better at centers that have performed more than 100 procedures.806 Other data report improved outcomes for operators with an annual procedure volume of at least 25 cases and for centers with an annual procedure volume of at least 50 cases.921 Moreover, the selection of patients and interpretation of AFL and other ATs that are often observed in patients with AF require training that is unique to electrophysiology fellowships. Trainees who intend to perform AF ablation independently should receive additional training after the standard fellowship is completed if they performed fewer than 50 AF ablation procedures during training.
Recognition, Prevention, and Management of Complications
As previously discussed, ablation of AF is associated with substantial risks that must be recognized. Training programs must emphasize techniques that reduce these risks. This includes careful manipulation of catheters, appropriate use of anticoagulation, modification of energy delivered on the posterior wall of the LA, and the risk of applying energy within the PVs or LAA. Fellows should be trained to suspect cardiac tamponade or internal bleeding as a common cause of hypotension. Training should also include management of these complications. The skills to perform an emergent echocardiogram when cardiac tamponade is suspected are important. It is preferable for fellows to undergo training in pericardiocentesis. If trainees do not gain proficiency in pericardiocentesis, they must be able to recognize and diagnose cardiac tamponade and have immediate access to a physician who can perform an emergency pericardiocentesis. They should understand the risks of conscious sedation, which include hypoventilation, aspiration, and respiratory arrest. They should also recognize the delayed time course associated with the development of AEFs or PV stenosis, as well as the appropriate steps needed to diagnose and manage these complications.
Appropriate Follow-up and Long-Term Management
Management of patients after hospital discharge can be complex and requires commitment from the physician (cardiologist or internist) who will be following the patient on an ongoing basis. Individuals undergoing training in AF ablation should participate in a longitudinal clinic in which these patients are followed. Experience must be gained in diagnosis and management of postprocedure complications, including esophageal injury, PV stenosis, and late tamponade, pseudoaneurysm, or arteriovenous fistula. Because the prevalence of some of these complications is very low, it is possible that the trainee will not have first-hand experience with patients. Therefore, supplementation of clinical experience with didactic presentations on diagnosis and management of postablation complications is required. Prophylaxis against and management of postprocedure atrial arrhythmias, including timing of repeat ablation and use of concomitant AADs, must be taught to trainees. Finally, the training experience must address the risk-benefit decision-making regarding the use of intermediate and long-term anticoagulation therapy. Given the complexity of these issues, it would be ill-advised for cardiologists who are not trained in electrophysiology to consider performing ablation procedures for AF. Due to these issues and prerequisites for obtaining and maintaining competency, this statement should also extend to the performance of cryoablation or other balloon ablation.
Section 12: Surgical and Hybrid AF Ablation
Historical Considerations and Development of the Cox-Maze Procedure
There is a rich history of surgery for AF. Initial procedures were aimed at controlling the ventricular response rate. Later procedures were directed at converting AF to a normal sinus rhythm. Following experimental investigation, the Maze procedure was introduced for the surgical treatment of AF in 1987 by James Cox. This procedure was designed to interrupt macroreentrant circuits, thereby reducing the ability of the atrium to fibrillate. Fortuitously, the surgery also isolated all of the PVs and the posterior LA. In contrast to previous procedures, such as the corridor procedure and LA transection procedures, the Cox-Maze procedure successfully restored both AV synchrony and sinus rhythm and decreased the incidence of late stroke.1287 This effect was attributed to both AF control and amputation of the LAA. The surgery involved creating multiple strategically placed incisions across both the RA and LA. The surgical incisions were placed so that the sinus node could “direct” the propagation of the sinus impulse throughout both atria. It also allowed most of the atrial myocardium to be activated, resulting in preservation of atrial transport function in most patients.1288 The final iteration of this procedure, the Cox-Maze III, became the standard for the surgical treatment of AF.
Long-term outcomes of 198 patients who underwent the Cox-Maze III procedure for treatment of paroxysmal (n = 113) or persistent or long-standing persistent AF (n = 85) have been reported.1289 The mean follow-up was 5.4 ± 2.9 years. Among the 112 patients who underwent surgery only for AF treatment, 96% were in sinus rhythm with or without AAD therapy and 80% were in sinus rhythm and free of AAD therapy at the last follow-up. Among the 86 patients who underwent AF surgery in conjunction with other cardiac surgery, 97% were in sinus rhythm with or without AAD therapy and 73% were in sinus rhythm free of AAD therapy. The incidence of major complications among the 112 patients who only underwent AF surgery was 11%. Among these were two perioperative deaths and two perioperative strokes or TIAs. Nine patients (8%) required pacemaker placement. The incidence of major complications among the 86 patients who underwent AF surgery at the time of other cardiac surgical procedures was 14%. Among these were one perioperative death and one perioperative stroke. Twenty patients (23%) required pacemaker placement.
In considering the results of these early reports of cardiac surgery for treatment of AF, it is now recognized that these patients did not undergo rigorous follow-up by present standards. Rhythms were documented by means of a mailed questionnaire, telephone interview, and/or an ECG for documentation. It is clear that the pioneering work of Cox and his team paved the way for the current, less invasive Cox-Maze IV surgery and other surgical approaches for AF ablation, as well as the field of endocardial catheter ablation of AF.
The term lone AF holds different meanings in EP jargon compared with surgical jargon. Electrophysiologists refer to lone AF when there is no other structural heart disease present. Surgeons often refer to a lone AF procedure as one in which the only surgical procedure performed is the ablation as opposed to a concomitant procedure. To eliminate confusion, we recommend that surgeons avoid using lone AF to describe populations of AF patients, and furthermore, we recommend the term stand-alone ablation when no other concomitant procedure is performed at the same operative encounter. As noted earlier in the document, the writing group recommends that the term lone AF not be used in any context related to AF or AF ablation.
Surgical Ablation Technology
Despite its efficacy, the Cox-Maze procedure did not gain widespread application due to its complexity, technical difficulty, and morbidity. The development and subsequent availability of technology to perform atrial ablation allowed surgeons to replace some of the traditional cut-and-sew lesions with ablation lines using this technology. The simplified Cox-Maze procedure lessened procedural morbidity, thus leading to wider adoption and extending its benefits to more patients. Although a variety of energy sources for ablation were initially developed, only cryothermy and RF energy delivery have emerged as practical and efficacious. The only surgical ablation system approved and specifically labeled for surgical AF ablation is the Atricure Ablation System, which includes a number of ablation tools, including a bipolar RF clamp.1290
Cryothermy can be thought of as nondirectional (although shielding mechanisms can be employed), whereas RF is a directional source. The RF technologies can be organized into two major groups: unipolar and bipolar. Bipolar RF can be directional bipolar or constrained bipolar. The directional bipolar devices have two side-by-side poles that are applied to the tissue surface, with the energy passing through the tissue between them. As the tissue between the poles desiccates and the impedance rises, the energy passes deeper into healthy tissue, with the goal of tissue transmurality.
The constrained bipolar devices consist of a clamp with two jaws, which are applied on opposite sides of the atrial tissue. The energy passes through the tissue between the two jaws. When conductance falls, transmurality is inferred. The unipolar devices do not provide the surgeon with a transmurality indicator. Since most of these ablation systems were released clinically without dose-response studies, their use has led to occasional collateral cardiac and extracardiac damage.1162,1291,1292 Moreover, both unipolar and directional bipolar energy sources have had difficulty creating transmural lesions when used from the epicardial surface on the beating heart.1293,1294,1295,1296,1297,1298 This difficulty occurs because the circulating intracavitary blood pool produces convective cooling, which makes transmural lesions difficult to achieve.1299 In an attempt to obviate this problem, one device provides suction to pull two walls of atrial tissue into apposition in a shallow trough, thus excluding the circulating heat sink of intracavitary blood while the energy is applied. All of these energy sources have a fixed depth of penetration, which makes their use in pathologically thickened atria problematic.
Bipolar RF ablation has overcome some of these shortcomings. Because energy is delivered between two closely approximated electrodes embedded in the jaw of a clamp device, the energy is focused and results in discrete lesions. The energy is confined to between the jaws of the clamp, reducing the possibility of collateral cardiac or extracardiac damage. By measuring the tissue conductance between the two electrodes, algorithms have been developed that help predict lesion transmurality in the experimental laboratory. The weakness of these devices is that they can only ablate tissue that can be clamped within the jaws of the device. This problem has limited the potential lesion sets, particularly in the beating heart. Moreover, in the clinical situation, multiple ablations have often been required to achieve entrance and exit block. These devices have been incapable of fully ablating the RA and LA isthmus and have required adjunctive cryothermy, or unipolar or directional bipolar RF ablation to perform a complete Cox-Maze III lesion set.
Nevertheless, the development of these new ablation technologies has benefited the surgical treatment of AF by making a technically difficult and time-consuming surgery easier for all cardiac surgeons to perform. At present, more than 50% of the patients undergoing open-heart surgery who have AF are offered concomitant AF surgery.1300 Replicating the full Cox-Maze lesion set with linear lines of ablation has been shown to be both feasible and clinically effective. A number of groups have reported excellent results with ablation-assisted Cox-Maze procedures.1301,1302,1303,1304,1305,1306
The largest of these experiences included 282 patients who underwent the Cox-Maze IV procedure over a 7-year period with either paroxysmal (n = 118), persistent (n = 28), or long-standing persistent AF (n = 135).1301 A total of 124 patients (44%) underwent surgery only for AF treatment, and 158 patients (56%) had other cardiac surgery performed, which included mitral valve surgery in approximately 50% of patients. Among the entire patient cohort, 89% of the patients were in sinus rhythm with or without AAD therapy, and 78% were in sinus rhythm and free of AAD therapy at 12 months of follow-up. In contrast to early studies on surgical AF ablation, more intensive monitoring was performed with Holter monitors every 3 months in 70% of the patients. The incidence of major complications was 11%, including an operative mortality of 2% and a 1.7% incidence of stroke. Pacemakers were implanted in 9% postoperatively. A propensity analysis, matching patients who underwent an ablation-assisted Cox-Maze with those having had a traditional cut-and-sew Cox-Maze III, showed no differences in freedom from AF at 3, 6, and 12 months of follow-up.1307 Further recent work has shown significantly improved results when the entire posterior LA is excluded by the so-called box lesion.1306,1308
A long-term study followed 576 patients from 2002 to 2014 with long-term monitoring.1306 At 5 years, freedom from ATAs was 73% (102 of 139) and freedom from ATAs off AADs was 61% (80 of 135). There was no difference in outcomes between patients with PAF or the more persistent forms. There was also no difference between outcomes for those patients who had stand-alone procedures and those who had concomitant procedures. Because outcomes were significantly better at 12 months of follow-up (92% freedom from ATAs overall and 88% freedom from ATAs off AADs), this paper highlights the importance of long-term follow-up. Currently, the limitations of the energy delivery devices and the attempt to deploy them through minimal access incisions or ports place constraints on the location and number of ablation lesions that can be performed. The impact on results of these alternative lesion patterns and the less invasive surgical approaches requires further observational prospective analysis and randomized trials.
There has only been one completed trial of concomitant surgical AF ablation that has resulted in specific FDA labeling for clinical treatment of AF.1290 This was the Atricure Synergy Ablation System trial intended for the ablation of persistent and long-standing persistent AF in patients who are undergoing open concomitant coronary artery bypass grafting (CABG) and/or valve replacement or repair. The principal device used in this trial was an Atricure Synergy Ablation clamp. This system had originally been approved by the FDA for soft tissue ablation without specifically labeling for AF ablation. This prospective nonrandomized clinical trial, using a Bayesian adaptive design with prespecified early stopping rules, enrolled 55 patients between February 2008 and June 2009. Along with concomitant cardiac surgery, investigators performed the Cox-Maze IV lesion set. The median patient age was 72 years, the median EF was 50%, and the median LA size was 6 cm. 56% of patients underwent valve surgery alone or in conjunction with CABG. The incidence of major adverse events was 9%, including death in 2 patients (3.6%), major bleeding in 2 patients (3.6%), and stroke in one patient (1.8%). In addition to these major complications, 25% of the patients required implantation of a permanent pacemaker for AV node dysfunction (8.3%) or sinus node dysfunction (17%). The effectiveness of the procedure was assessed in 50 evaluable patients, excluding four patients who died and one withdrawal. At 6 months of follow-up, 74% of the patients were AF-free and off AAD therapy, and 84% of the patients were free of AF on or off AAD therapy. The freedom from AF at 12 months of follow-up was also 75%. The results of this study were reviewed at an FDA panel meeting, leading to approval for clinical use in 2011. This surgical ablation system is currently the only system specifically labeled for treatment of AF.
We recommend that the term Maze procedure is appropriately used only to refer to the biatrial lesion set of the Cox-Maze surgery. It requires ablation of the RA isthmus and the LA isthmus. Less extensive lesion sets should not be referred to as a Maze procedure, but rather as a surgical AF ablation procedure. In general, surgical ablation procedures for AF can be grouped into three different groups: (1) a full biatrial Cox-Maze procedure, (2) PVI alone, and (3) PVI combined with LA lesion sets.
Surgical Technology for Appendage Ligation or Removal and Outcomes of These Procedures
The LAA is a site of thrombus formation in patients with AF. Retrospective evaluation has suggested that the LAA is responsible for up to 90% of the strokes in patients with AF and nonrheumatic heart disease.1309 Accordingly, it has been the target of elimination in the original Cox-Maze, as well as in the majority of its modifications. Early evaluation of the cut-and-sew Cox-Maze suggested a reduction of stroke late after surgery.1287 Other small, retrospective series subsequently suggested a lower-than-expected incidence of late neurological event (stroke or TIA) after a Maze, possibly independent of CHA2DS2-VASc score.1287,1310,1311 The reduction in stroke has been attributed to a combination of sinus restoration and LAA elimination. The role of the LAA has been clouded by small numbers of patients and the continuation of anticoagulation in a minority of postoperative patients, as well as a retrospective series suggesting a persistent stroke risk in postoperative patients who are in sinus rhythm, with large atria, and poor atrial contraction leading to effective LA asystole.1312
The strongest evidence that LAA elimination decreases stroke comes from the WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation (PROTECT AF) and WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trials that randomized patients to either anticoagulation or implantation of a WATCHMAN device in the LAA. The 4-year results of the PROTECT AF trial suggested that elimination of the LAA was superior to anticoagulation for the composite endpoint of cardiovascular death, all stroke, and systemic embolization.1313,1314
An important concern for surgical excision has been the complication of bleeding. This complication is especially important in older patients and those with enlarged atria in whom the tissue may be more friable. This has led to several different techniques for LAA elimination at the time of surgery. The most common have been internal ligation (e.g., sewing the LAA orifice closed from the inside) and stapled excision. There is a paucity of data that examines effectiveness of any surgical technique. However, with stapled excision, reported rates of tears requiring repair have been approximately 10%.1315,1316
Another issue is the potential for arrhythmia generation from the LAA. One study demonstrated LAA firing in 29% of patients and the only site of recurrence in 8.7% of patients who had undergone catheter ablation of paroxysmal or persistent AF; additional LAA isolation could increase the freedom from AF.532,533 Thus, the isolation or surgical excision of the LAA could influence procedure efficacy and reduce the risk of thromboembolic events.
However, a randomized study including 176 patients with persistent AF who were undergoing surgical ablation via thoracoscopic approach reported that additional LAA amputation did not reduce the rate of any atrial arrhythmias compared with the standard surgical ablation set. The follow-up period of this study was 18 months and the results cannot be extrapolated to the long-term maintenance of sinus rhythm or thromboembolic events prevention.1317
An emerging concern is the effectiveness of these alternative techniques. Most evidence is anecdotal and revolves around case reports because the LAA is not routinely evaluated late after surgery unless there is a clinical indication. In a series of 137 such patients, the LAA was incompletely ligated (either leaving a stump greater than 1 cm or a gap with flow) in 27% of patients after surgical excision. In internally ligated patients, the failure rate was 77%. There were no successes when the LAA was stapled without amputation of the distal remnant.1318 One limitation of this small series is that it only looked at the 5% of patients who received intervention who had an indication for late TEE, which included only 12 in the stapled group. A more recent small, randomized trial of internal ligation, surgical excision, and stapled excision reported that, at TEE evaluation in follow-up of 5 months, all three of these techniques left either a stump or a gap at least 50 percent of the time.1319
Epicardial LAA ligation with a LARIAT device has been developed through the combined transseptal and subxiphoid approach.1320 The results from the multicenter registry demonstrated a high acute closure rate, but procedural success was limited by bleeding.1321 More recent results showed that LARIAT device implantation was associated with a lower rate of leaks at 1 year of follow-up and a 1.1% rate of TIA or stroke.1322
Newer techniques include an external clip (Atriclip) that was approved by the FDA in 2011 for the occlusion of the LAA under direct visualization in patients undergoing other open cardiac surgical procedures, as indicated in the approved Indication statement of the AtriClip device. This study reported 98% success in 60 of 71 patients available for follow-up.1323 A longer-term study followed 36 patients with annual CT scans.1324 At 3.5 years of follow-up, all the clips were stable with no thrombi, no LAA perfused, no neck >1 cm, and no neurological events. The use of an endoloop has been described, as well as a silicone fastener, which is not currently available (Tiger paw). The true efficacy of any single technique is unknown and will require more investigation before any recommendations can be made.
There are data that suggest that despite the limitations of all these techniques, a reduction in strokes might occur. One series of 773 patients undergoing surgery for AF compared surgical excision with alternative techniques. The annual rates of late neurological events was approximately 1% using alternative techniques, and only one event was fatal.1325 This suggested at least a reduction of clot burden even in incompletely successful techniques. Our understanding of surgical elimination continues to rapidly evolve, and current studies are inadequate to make a distinction between LAA excision or exclusion techniques. It is reasonable and probably helpful to eliminate the LAA with any technique at the time of AF surgery, but late evaluation should be performed prior to cessation of anticoagulation. We have elected not to make recommendations regarding appendage occlusion, resection, or ligation in this document, because this is beyond the scope of this document and available data.
Concomitant Surgical Ablation
Historical Considerations
Surgical ablation is most commonly applied as a concomitant procedure during valve or CABG surgeries. Prior consensus recommendations referred to cardiac surgery as a whole, grouping data from multiple studies to derive IIa LOE C recommendations.2 However, that document went on to say, “It is advisable that all patients with documented AF referred for other cardiac surgeries undergo a left or biatrial procedure for AF at an experienced center, unless it… will add significant risk….”2
More recent AHA/ACC/HRS Guidelines continued this procedural grouping but included more recent randomized comparisons to determine that surgical ablation at the time of another surgery is a IIa LOE B recommendation. The frequencies of surgical ablation performance and durable rhythm success have steadily increased. Furthermore, as noted above, the FDA has now approved an ablation system for treatment of persistent AF in patients undergoing concomitant cardiac surgical procedures.1290 Recently, more information has become available on AF mechanisms and the potential influence of specific structural heart abnormalities on outcome. Therefore, this surgical section provides updated recommendations for three operation categories for which more data are now available: primary open atrial operations, primary closed atrial operations, and stand-alone operations for AF.
Concomitant Surgical Ablation
Open concomitant cardiac surgical operations, in which a left atriotomy is being performed for the primary procedure, commonly include patients receiving mitral valve repair or replacement (MVRR), with or without concomitant tricuspid valve repair or replacement, or closure of an ASD. Closed concomitant surgical ablation operations, in which a left atriotomy is not otherwise performed, commonly include patients undergoing prosthetic AVR, CABG, or AVR+CABG.
The prevalence of preoperative AF and frequency of concomitant cardiac surgical operations varies between these procedure classes. AF is found in one-third of patients presenting for mitral valve surgery, but in only 6% of patients undergoing isolated CABG, and in 14% of patients at the time of AVR. Mitral valve repair for primary regurgitation has largely supplanted mitral valve replacement and does not require lifelong anticoagulation. Thus, successful surgical ablation concomitant to mitral repair can mitigate the need for long-term anticoagulation or medicinal therapy for AF. The performance rate of concomitant cardiac surgery in patients with AF at the time of mitral operations has risen from 52% to 62%. In an analysis of operations performed in the early 2000s, the likelihood of surgical ablation performed for AF at the time of AVR was 31%, and only 26% at the time of CABG. Although differential application of surgical AF ablation exists among operative procedures, more recent information suggests an acceleration of surgical AF ablation, especially in the mitral subgroup.
Surgical Ablation at the Time of Concomitant Open Atrial Operations
At the time of a primary atriotomy, AF surgery can be performed during concomitant MVRR with or without tricuspid surgery, with or without closure of ASD, and with or without other concomitant procedures such as CABG.1326 The results of the only prospective study performed to achieve FDA labeling for AF ablation reported a 9% major complication rate, a 25% rate of pacemaker implantation, and a 75% freedom from AF at 12 months of follow-up among 54 consecutive patients with persistent AF undergoing other types of cardiac surgery who were enrolled in this clinical trial.1290 Several other RCTs and meta-analyses are available to evaluate AF surgery at the time of concomitant mitral procedures.1327,1328,1329,1330,1331,1332,1333,1334 Large LA, AF duration, advanced age, and failure to isolate the entire posterior LA are common predictors of reduced long-term efficacy. High baseline comorbid risk is a common reason cited for not performing surgical ablation, though many institutional studies note that this is not a contraindication to surgical ablation. The safety of concomitant surgical ablation has been established in the literature and in updated valve risk models from the STS database.
A multivariable regression and propensity matched cohort, composed of 52% mitral procedures from the STS database, demonstrated no impact on 30-day mortality with surgical AF ablation.1300 However, patients undergoing surgical AF ablation had a 26% higher chance of requiring a permanent pacemaker (OR 1.26). In a recent randomized trial of mitral valve operations, there was no increase in major complications associated with the addition of surgical ablation other than a doubling of pacemaker risk.1331,1333 Conversely, recent large meta-analyses confirmed the safety of concomitant surgical ablation, but did not find a significant increase in pacemaker use. The incidence and outcome relevance of pacemaker implantation remains a point of controversy. In analyses of more recent STS data, risk-adjusted mortality was either not impacted or actually decreased with surgical AF ablation in the mitral and multiple valve populations.1335 A longitudinal study (up to 120 months) demonstrated that restoration of sinus rhythm by a Cox-Maze procedure combined with heart surgery markedly increased long-term survival.1336
Despite previously published variability of efficacy of surgical ablation in heterogeneous populations, the longitudinal benefits of concomitant surgical AF ablation at the time of MVRR are now becoming clearer. Several recent RCTs and meta-analyses indicate that concomitant surgical ablation at the time of MVRR reduces the longitudinal incidence of postoperative AF greater than 50% for at least 1 year, with results ranging from 60%–90%.1327,1328,1329,1330,1331,1333,1334,1337,1338 In addition to LA size and preoperative AF duration, there is a procedural learning curve that can impact efficacy, and thus surgeons should seek appropriate training prior to performing surgical ablation.
Therefore, based on the literature and the experience of the writing group members, surgical ablation for AF is recommended at the time of concomitant open atrial procedures, such as mitral valve surgery in patients with symptomatic AF (Class I, LOE B-NR) (Table 2, Figure 8).
Surgical Ablation at the Time of Concomitant Closed Atrial Operation
Concomitant surgical ablation of AF at the time of primary nonatriotomy operations includes patients undergoing isolated AVR, isolated CABG, or AVR+CABG. The presence of AF at the time of these operations, especially if left untreated, is associated with increased risk of early and late mortality and morbidity. When no intracardiac pathology exists in the setting of AF, further surgical decision-making is required. Although full open Cox-Maze IV has been shown to be safe and effective in these cases, surgeons are reluctant to add a left atriotomy to address AF. If less aggressive approaches, such as epicardial PVI or the Dallas lesion set are to be applied, care should be taken to note the mechanism and type of AF being treated.1339,1340,1341 Recent randomized and matched cohort studies of surgical ablation and concomitant AVR, AVR+CABG, and isolated CABG all consistently show no differences in 30-day or in-hospital morbidity or mortality.1342,1343,1344
We have known that at the time of isolated CABG operations, the open atrial Cox-Maze procedure is effective upwards of 90% at 5 years of follow-up.1345 The application of bipolar RF clamps to perform PVI has shown variable 50%-89% 1-year success superior to AAD alone in patients with paroxysmal and persistent AF.1346,1347,1348,1349,1350 A recent meta-analysis of 16 RCTs of surgical ablation and concomitant operations evaluated predominantly mitral operations, but included both AVR and CABG operations.1333 There were no significant differences in mortality, stroke, or pacemaker requirement between surgical ablation compared with no ablation; however, the surgical ablation groups demonstrated superior 1-year freedom from AF in AVR and AVR+CABG.
Therefore, based on the literature and the experience of the writing group members, surgical ablation for AF is recommended at the time of concomitant closed atrial procedures such as isolated AVR, isolated CABG, and AVR+CABG in patients with symptomatic AF refractory or intolerant to at least one Class I or III antiarrhythmic medication (Class I, LOE B-NR) (Table 2, Figure 8). For symptomatic patients with AF who have not previously been treated with antiarrhythmic therapy, concomitant closed AF surgery is recommended with a Class IIa indication, LOE B-NR (Table 2, Figure 8).
At the time of a planned cardiac operation for symptomatic structural pathology, it should be noted that interpreting symptoms of concomitant AF as distinct might or might not be feasible because these could be masked by symptoms prompting the primary cardiac operation (i.e., valvular or coronary disease). Therefore, in the setting of existing symptomatic surgical pathology, the presence or absence of AF symptoms should not be the only factor involved in surgical decision making on the concomitant performance of surgical ablation. It should be noted that the surgeon members of the writing committee, as well as other surgeon reviewers, felt that the evidence might warrant a Class I indication for this patient subgroup; however, among the larger group, consensus was attained for a level IIA recommendation.
Stand-Alone Surgical Ablation of AF
Stand-Alone Operations for AF and Their Outcomes
The primary indication for stand-alone surgery that was described in the 2012 Consensus Document was the presence of symptomatic AF, refractory or intolerant to at least one Class I or Class III AAD.2 In current practice, most patients also have experienced at least one unsuccessful catheter ablation before referral, unless the patient has a strong preference for a cure with a single procedure.
There has been over two decades of experience with operations performed solely for treatment of AF (stand-alone operations). The wide use of these procedures has been limited by a reluctance to refer patients to surgery for AF, procedural complexity, and limited data regarding outcomes. Moreover, the types of procedures and the technologies used to perform them have multiplied and are variable between operators. This has led to relatively modest-sized single-site case series, or at best multicenter series without comparison groups. In addition, the rigor and methodology of follow-up have changed dramatically over time and have further limited comparisons of outcomes. Lastly, the development of hybrid procedures, especially when staged, make comparisons even more difficult. This section will focus only on single-stage surgeries as sole AF therapies. A discussion of hybrid procedures will follow. Perhaps the best way to distinguish the types of surgeries is by those that require cardiopulmonary bypass and cardiac arrest and those that do not. In order to effectively create a lesion down to the mitral annulus, an open heart is required.
The earliest—and one of the largest—reported study of stand-alone operations for AF has been the 112 patients who underwent the cut-and-sew Cox-Maze III procedure by James Cox.1351 This procedure is performed through a sternotomy on cardiopulmonary bypass on an arrested heart and physically cuts and re-sews the atria to create a collection of lines of block. Cryothermia is used to destroy the tissue down to the mitral annulus. Among the 112 patients, 96% were in sinus rhythm with or without AAD therapy, and 80% were in sinus rhythm and free of AAD therapy at last follow-up. There was one late stroke in this group, and 88% of the patients were off chronic anticoagulation at last follow-up. The only risk factor for late recurrence was the preoperative duration of AF.1351 There have been several other published series with similar results that combine both stand-alone and concomitant patients with smaller numbers of patients. This procedure requires a sophisticated level of training and skill. As such, it is performed rarely, and only by experienced surgeons. Ideal patients for stand-alone AF ablation have failed other therapies, want definitive cures, or have clots in the LAA, making other approaches not using cardiopulmonary bypass risk prohibitive.
With the introduction of new ablation technology, including bipolar RF energy and new cryoablation systems, there has been renewed interest in less invasive procedures for stand-alone AF ablation. These new tools can be used in the open chest or through small incisions between the ribs. When used in the open chest with a full biatrial Cox-Maze lesion set performed, the procedure has been termed the Cox-Maze IV procedure. Techniques for a Cox-Maze IV procedure through a small, right inframammary incision have also been perfected. As noted in the earlier section on new surgical ablation technology, the outcomes achieved with the Cox-Maze IV procedure are similar to those achieved with the earlier Cox-Maze procedure. Importantly, the cross-clamp times are shorter with the Cox-Maze IV procedure.1301 The advantage of these approaches includes the ability to reliably create the endocardial lesions of the Maze, down to the mitral annulus. Late evaluation of this procedure in 146 stand-alone patients has shown a 72% freedom from AF at 5 years of follow-up and a 59% freedom from AF off antiarrhythmic medications.1306 Cryothermia alone has been used and described in a series of 77 patients, with a 6-month result of 88% freedom from AF, antiarrhythmic medications, and anticoagulants, but late follow-up is lacking.1306,1352
Other approaches have limited the lesions to only those that can be created from the epicardium without the need to open the heart, and use both cardiopulmonary bypass and cardiac arrest. This approach has limited the extent of lesions from the Maze that can be created, because the mitral line is buried by an epicardial fat pad that makes destruction of tissue in this area unreliable. The minimally invasive surgical approach using video-assisted PV ablation and exclusion of the LAA was first described in 2005.1353 A bipolar RF clamp was used for PVI on the beating heart in 27 patients, among whom 18 had PAF. Among the 23 patients followed for more than 3 months, 21 (91%) were free of AF and 65% were off all AADs. There were four major complications, but no deaths, and no pacemakers were implanted. An additional ablation strategy that has been reported is minimally invasive PVI and partial autonomic denervation.1354 In a study of 74 patients undergoing this approach, 84% of the patients with PAF were free of AF and 57% of patients with persistent or long-standing persistent AF were free of AF at 6 months. There was one death, one hemothorax, one case of transient renal insufficiency, and one patient with a transient brachial plexopathy. A second, larger report from this group in 114 patients reported that 72%, 46.9%, and 32% of patients with paroxysmal, persistent, and long-standing persistent AF, respectively, were free of AF and off antiarrhythmic medications at 195 days of follow-up.1355 Another multi-center series of 100 patients with a similar approach and mean follow-up of 13.6 months reported a sinus restoration rate of 87%, with 64% of patients free from AADs.1356 The results of these and other trials cited earlier in this section have made it clear that a more extensive lesion set than PVI alone is required for successful surgical treatment of persistent and long-standing persistent AF. Most surgeons who still perform this type of procedure have moved toward a hybrid approach in either a single or staged operation. However, a PVI alone remains a reasonable approach for patients with PAF.
The Dallas Lesion Set was developed to create a complete approach, which can be performed on a beating heart without cardiopulmonary bypass.1339,1340,1341 The set replicates the LA lesions of the Cox-Maze III, but changes the connection of the PVI to the aortic annulus in continuity with the mitral. Early results have been published on 30 patients.1339,1340 The group included 10 patients with persistent AF and 20 patients with long-standing persistent AF. Electrocardiographic long-term monitoring and the use of AAD data were collected 6 months postprocedure, and follow-up was 100%. Procedure-related complications did not occur during follow-up, nor were there any deaths. At 6 months of follow-up, 90% of the patients with persistent AF and 75% of the patients with long-standing persistent AF were in sinus rhythm. AAD therapy was continued in 22% of the patients with persistent AF and 53% of the patients with long-standing persistent AF. In a series of 100 paroxysmal patients randomized to include the Dallas Lesion Set or not, the additional lesion, as expected in a paroxysmal population, did not impact success at 16 months of follow-up.520 Much like the results of catheter ablation, this suggests that the type of AF will influence the success of the procedure. Persistent AF is likely to require a more extensive lesion set. An important area of interest is the decision to offer a patient surgery or catheter ablation.
The AF Catheter Ablation Versus Surgical Ablation Treatment (FAST) trial sought to compare catheter ablation with minimally invasive surgery.601 A total of 124 patients who had drug-refractory AF with dilated atria or failed catheter ablation were randomized to either catheter ablation or minimally invasive surgery using bipolar clamps, with or without additional connecting lesions. At 1 year of follow-up, freedom from AF was 37% in the catheter ablation group and 66% in the surgical group. Although this was somewhat offset by the increased adverse events in the surgical group (34% vs 16%), the only death was in the catheter ablation group.601 A different analysis of 7 studies, including two RCTs, suggested superior freedom from AF in the surgical group, with similar complication rates, except for an increase in pacemaker implantation in surgical patients.1357 However, the technologies and groups were fairly heterogeneous.
Other approaches, such as epicardial box lesions with suction-assisted unidirectional uni- and bipolar RF and a complete box lesion with bipolar clamps, have been described in numbers insufficient to draw any conclusion. As the new techniques have been introduced, there has been appropriate concern regarding the safety of minimally invasive stand-alone surgery. Although safety is dependent on procedure and site, it has been examined in a systematic review that compiled results from 23 observational studies with 752 patients who underwent minimally invasive stand-alone procedures.1349 Operative mortality was 0.4%. Complication rates attributed to surgery were only 3.2%. Reports from the STS National Database showed an operative mortality rate of 0.74%. The complication rate was considerably higher at 16.43%, although major morbidities such as stroke (0.72%), renal failure (2.45%), and bleeding (0.99%) were low. Pacemakers were implanted in 1.03% of patients.
The outcomes of stand-alone AF ablation from the STS database were recently reported.1358 Between 2005 and 2010, a total of 91,801 surgical AF ablations were performed, of which 4893 (5.3%) were stand-alone. During this period of time, the number of stand-alone AF surgeries increased from 552 cases in 2005 to 1041 cases in 2010. The mean age of the stand-alone group was 60 years, and 71% were men. Some 80% of the stand-alone procedures were off pump. The overall operative mortality was 0.74% (1.7% on pump vs 0.5% off pump), the rate of pacemaker implantation was 1%, and the overall complication rate was 16% (28% on pump vs 13% off pump).1358
The Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery (AFACT) study compared the outcomes of thoracoscopic surgical AF ablation in 240 patients with advanced AF at a single European center.123 A total of 59% of the patients had persistent AF and 68% had an enlarged LA. One-fourth of these patients had previously failed catheter AF ablation. Patients were randomized to undergo surgical AF ablation alone or combined with epicardial ablation of the four major GP. At 12 months follow-up, no recurrences of AF were observed in 71% and 68% in the GP and control groups, respectively; the incidence of major complications was greater in the group that underwent GP ablation (19% vs 8%, respectively). Major bleeding occurred in nine patients in the GP group, one of whom required sternotomy. Sinus node dysfunction occurred in 12 patients in the GP group and in 4 controls. The authors concluded that GP ablation during thoracoscopic surgery for advanced AF is associated with higher risk and no appreciable improvement in AF control. This center also recently examined the 5-year outcomes of thoracoscopic surgery for AF in 66 patients. A total of 50% of patients experienced no AF recurrences and discontinued AAD therapy at the 5-year follow-up, and 88% of the patients were in sinus rhythm. In this cohort, persistent AF and previous failure of catheter ablation were independently associated with AF recurrence.1359
Superior efficacy of a single approach has also been difficult to establish. A systematic review of 48 studies including 3832 patients suggested that the efficacy of bipolar RF was equivalent to the cut-and-sew Maze III technique for stand-alone surgical ablation, as long as both were applied meticulously.1360 Another meta-analysis of 16 published randomized trials indicated that the cut-and-sew Maze III produced slightly better recovery of SR and stroke prevention, but with increased perioperative risk.1361 Definitive recommendations for a surgical approach with or without cardiopulmonary bypass await more data.
Using a surgical approach with or without cardiopulmonary bypass that creates all, some, or a modification of the maze is reasonable, especially in patients in whom catheter ablation has failed or who are at high risk for an unsuccessful catheter-based result. However, a stand-alone operation should have the ability to create the complete full Maze lesion set, whether it is in a single operation or staged. This approach is especially important for those patients who have persistent AF.
Therefore, based on the literature and the experience of the writing group members, stand-alone surgical ablation of paroxysmal AAD-refractory AF can be considered for patients who have failed one or more attempts at catheter ablation, and after review of the relative safely and efficacy of catheter ablation vs a stand-alone surgical approach for those who are intolerant or refractory to AAD therapy and prefer a surgical approach (Class IIb, LOE B-NR). For patients with persistent and long-standing persistent AF, stand-alone surgical ablation is reasonable for patients who have failed one or more attempts at catheter ablation, and after review of the relative safely and efficacy of catheter ablation vs a stand-alone surgical approach for those who are intolerant or refractory to AAD therapy and prefer a surgical approach (Class IIa, LOE B-NR, Figure 8). Stand-alone surgical AF ablation is not recommended for patients who have not failed a trial of at least one antiarrhythmic medication.
Catheter Ablation After AF Surgery
The idea of a “touch-up” ablation for AF recurrence is not new to catheter ablation. It is, however, relatively new to the treatment algorithms of failure after cardiac surgery for AF, which has historically been considered the end of the road for sinus restoration. Now, catheter ablation can be a critical adjuvant for patients who undergo surgical AF ablation yet still suffer from residual AF. The potpourri of surgical AF treatment—ranging from PVI through complete LA to complete biatrial lesions and combinations in between—makes standardized conclusions difficult in this area. This endpoint is further obscured by the myriad of technologies used to create the lines of block, as well as the underlying type of fibrillation treated and the limited number of patients in published series. However, there are several publications that offer some guidance, which will be reviewed below. What has become clear over time is that, as with redo-catheter AF ablation procedures, finding a reconnection of the PVs is also to be expected in a patient undergoing a catheter-based AF ablation procedure following a surgical AF ablation procedure.
Because the cut-and-sew Maze was the earliest described procedure, initial reports focused on patients who underwent that specific procedure. One of the earliest studies reported on 23 patients who presented a mean 14 months after a cut-and-sew Maze. In this report, 8 patients had only undergone a Maze, and 15 had undergone a concomitant procedure.1361 The most common site of failure was around the PVs, which occurred in eight (35%) patients. Five patients had focal tachycardia (3 in the CS and 1 each in the posterior lateral RA and LA septum). Four patients had RA incisional flutter and six had left AFL, which mapped around the mitral valve annulus in four patients and around the PVs in two. One year after ablation, 19 of the 23 patients were both arrhythmia-free and off AADs.
The vulnerability of PVI was supported by another study that followed 20 patients with arrhythmias after surgical ablation.1362 This group, however, was much more heterogeneous: alternative energy sources were used to create the initial lines of block, including microwave, RF, cryothermy, and laser; most patients had only LA lesion sets at the time of initial surgery, and nearly half the patients had more than one mechanism of tachycardia. This report also highlighted the involvement of the mitral isthmus, including the CS and the LAA. The vulnerability of the mitral isthmus, especially at the CS, was also highlighted in a series of 22 patients failing after the Cox-Maze III.1363 Of note, this outcome represented a 15% failure rate among a total of 143 patients in whom lesions were created using a combination of cut-and-sew and cryothermy. Frequently, out of concern for injury to the circumflex, cryothermy is used at the mitral annulus and is often used to connect the PVs in a small area so that reapproximation of the tissue is easier.
In a series of patients with five different surgical types, various rates of failure were identified.1364 High-intensity focused ultrasound was associated with a 37.5% need for touch-up catheter ablation, which was much more frequent than the other groups. This group had failures primarily around the PVs, suggesting an incomplete lesion creation at initial operation. The other groups—consisting of cut-and-sew Maze, biatrial Maze using primarily RF, LA maze alone, and PVI alone—had no significant difference in success, ranging from 90% for the cut-and-sew to 69% for PVI alone. When the RA was not addressed at the time of initial operation, it was the site of failure in 75% of those who had recurrent AF. In the other groups, the mitral isthmus was again identified as an area for failure. Successful ablation was achieved in approximately 70% of patients.
These findings have relevance as new paradigms for treatment evolve. Using hybrid strategies with technology that replaces cut-and-sew and new lesion sets might require a more individualized approach to each patient. New technology can introduce an area of vulnerability around the PVs. In one series of 154 patients undergoing minimally invasive PVI, eight failures were studied. Half had gaps in the lesions created with new enabling technology.1365 The remainder had flutters around the mitral isthmus. In a series that compared a cut-and-sew Maze to a hybrid approach, only 8% of the patients needed ablation after a cut-and-sew Maze.1366 However, after PVI using bipolar RF, 7 of 25 (29%) patients needed a second-stage catheter ablation. All seven had at least one failure around the PVs, for a total of 15 veins. Reconnection was most common in the RI region. Interestingly, there were no RA failures in this group.
These reports suggest that there are many factors for surgical failures after AF treatment. It is likely that catheter ablation can help selected patients restore sinus rhythm after failures. These treatments should be performed at experienced centers by experienced individuals who will tailor the procedure to the individual patient based on initial lesion set, the ablation technology and strategy used during initial AF surgery, and the results of extensive mapping and provocative testing at the time of the redo ablation procedure. As the experience with new hybrid approaches evolve, more definitive conclusions should arise.
Hybrid Epicardial and Endocardial AF Ablation Procedures
Background
Forward-thinking practitioners view catheter AF ablation and minimal access surgical ablation as complementary rather than competitive techniques, having found that patients who fail a surgical ablation usually fail as paroxysmal with a relatively low burden of AF. Whereas they might not have been candidates for catheter AF ablation preoperatively, they are now ideal candidates for a “touch-up” catheter AF ablation. The electrophysiologist will frequently find a single small break in a line, which is easily completed with a catheter; thus, the procedure is converted to a success. This realization of the complementary nature of these disciplines has led some to believe that perhaps combining these approaches could lead to better outcomes than either approach alone.
There are other reasons why surgical (epicardial) and catheter (endocardial) ablation can be viewed as complementary. Surgical devices can fail to penetrate the endocardium; catheter devices can fail to penetrate to the epicardium. Surgeons are skilled at making lines; the tools are designed for it, the smooth epicardial surface is ideal for it, and visual imaging can reveal breaks in a line. Electrophysiologists excel at “spot welding.” The catheter tip is punctuated by design, so it can slip off of endocardial ridges or trabeculations, resulting in breaks, and nonvisual imaging does not show continuity of burns. Surgeons might have difficulty mapping for completeness; they are constrained by pericardial reflections, they might lack formal training, and their tools are first- or second-generation. Electrophysiologists excel at mapping for success; they have full access to the entire endocardial surface, they are formally trained in the techniques, and they have mature enabling technology. In addition, each specialty has its own unique contributions. Surgeons can fully divide the ligament of Marshall, eliminate the atrial appendage, perform targeted ablation of GP, and isolate the SVC with little risk of injury to the PN. Electrophysiologists can easily make a cavotricuspid isthmus line, map for flutters, ablate within the CS, and map and ablate focal triggers.1367,1368 Recognition of the complementary nature of these techniques has led some centers to explore “hybrid” procedures (combined surgical and catheter ablation), with early promising results.606
The advent of minimal access surgical ablation laid the groundwork for hybrid ablation. Seeking to advance the success of the Cox-Maze III yet lessen the morbidity, surgeons began exploring minimal-access approaches. Three things led to the expansion of minimal-access techniques: First was the focus on the PVs as the seminal goal of ablation; second, advances in enabling technology allowed lesion creation using RF energy and cryothermy; and third was the published data revealing modest success for catheter ablation of the persistent forms of AF.2,931
Thus, with the focus on the PV triggers, surgeons began performing an increasing number of minimal-access PVI procedures.197,1369,1370,1371 However, investigators showed that this treatment was inadequate for patients with persistent and long-standing persistent AF.1355 This led to the belief that the persistent forms of AF needed both substrate modification and trigger isolation, and this provided the impetus to develop the Dallas lesion set, which replicated all the LA lesions of the Cox-Maze III, yet allowed them to be placed on the surface of the full-beating LA. Although it was a major step forward, with a success rate of 79%, this approach failed to reach the success rates of the Cox-Maze III.1339,1340 To enhance the robustness of lesion formation, the complementary processes of performing a catheter-based endocardial ablation in combination with surgical epicardial ablation were contemplated, and this led to hybrid approaches.1372,1373,1374
Though these hybrid techniques are under active investigation, the published literature is limited to a few early feasibility studies. Early investigators used a unilateral right thoracoscopic approach to isolate the PVs with a single encircling box lesion. The energy source for the surgical ablation was monopolar RF (Cobra Adhere, Estech, San Ramon, CA). Nineteen consecutive patients underwent a right unilateral minimally invasive hybrid procedure. Ten patients (52.6%) had long-standing persistent AF, whereas four (21.1%) had persistent and five (26.3%) PAF.1375 In 17 patients, one or more PVs (mostly the LSPV) were not isolated, and an endocardial touch-up was needed. It was possible to complete all the procedures as planned, without any conversion to cardiopulmonary bypass. No patient died during the follow-up. At 1 year, 7 of 19 (36.8%) patients were in sinus rhythm with no episode of AF and off AADs. Among the patients with long-standing persistent AF, 20% (2 of 10) were in sinus rhythm and off AAD, 50% (2 of 4) in persistent and 60% (3 of 5) in PAF. Disappointing 1-year results were attributed to an inadequate energy source. Thus, the surgical portion of the procedure was converted to use a bipolar RF clamp (AtriCure Inc., West Chester, OH), which had been shown to be more effective.1376 This approach provided improved results, and in most cases, gaps in surgical lesions could be completed by endocardial catheter ablation during the same procedure.608 A sequential hybrid approach was subsequently developed.606 There are advantages and disadvantages to simultaneous and staged hybrid procedures.
An important concern of single-stage hybrid is that edema and stunning induced by surgical ablation might produce block on testing, but these areas might recover later, when edema has subsided. This presence of an incomplete block at delayed catheter mapping was reported by an investigator who performed bilateral PVI box lesion and an additional roofline and LAA exclusion with clips in 30 patients with persistent AF. At staged catheter hybrid 3 months later, they found gaps in 77%–87% of the PVI lesions, nearly 70% of the rooflines, and 40% of the floor lines, requiring endocardial touch-up ablation. Nevertheless, they were able to obtain a 1-year freedom from AF and AAD by 7-day Holter of 90% (27 of 30). Other surgeons compared 25 staged hybrid procedures using bipolar RF with 38 classic cut-and-sew Maze III procedures.1366 At 1 year of follow-up, freedom from AF and antiarrhythmic medication was 52% for the staged hybrid and 87.5% for the Maze III (P = .004). Other approaches included a unilateral thoracoscopic approach using the monopolar RF suction Estech Cobra Adhere XL device (AtriCure Inc., West Chester, OH) without atrial appendage occlusion, applied to 19 patients.1375 At immediate hybrid catheter ablation, every lesion required touch-up and 1-year freedom from AF and AAD was 36%.
An innovative approach has been the passage of a scope from the subxiphoid, transperitoneal and transdiaphragmatic region to approach the posterior LA (the convergent procedure). The surgeon uses the nContact monopolar RF coagulation system to produce a comprehensive biatrial lesion pattern on the outside of a beating heart while eliminating chest incisions. Then, the electrophysiologist uses an ablation catheter endocardially to finish the lesion pattern and ensure that all reentrant circuits are interrupted.
Reported success rates have varied, from 16.7% to 100%; however, there has been an elevated adverse event rate in most published series, with an associated mortality of up to 12.5%, mostly related to AEF and sudden death.607,609,613,1368,1377,1378,1379,1380,1381,1382,1383 This procedure has been largely redesigned to prevent these adverse results, and two papers have reported no mortality and no AEF.613,1381
A recent meta-analysis compared the Cox-Maze to hybrid procedures. The overall freedom from AF and freedom from AF off AAD at 1-year of follow-up was 87% vs 71%, respectively, but the complication rates were higher with hybrid procedures.1384 Based on current literature, the hybrid approach with the most effective outcomes and safety profile appears to be the bilateral PVI procedures with LAA management. Available published data on the monopolar convergent procedure do not indicate an adequate safety and efficacy profile.
Currently, there is investigation into both simultaneous and staged hybrid procedures, with no clinical trials showing one strategy superior to the other. The Dual Epicardial Endocardial Persistent Atrial Fibrillation trial is a prospective randomized staged hybrid study using bipolar RF. The CONVERGE trial is a set of prospective randomized simultaneous hybrid trials using monopolar RF. These trials also use different operative approaches. There are a number of other ongoing multicenter trials that are likely to define the roles and lesion sets for treatment of patients with persistent AF using these strategies.
The hybrid approach could hold significant promise for those patients with persistent or long-standing persistent, drug-resistant AF to offer improved results over minimal access surgical ablation or catheter ablation alone. Based on the literature and the experience of the writing group members, we believe that it might be reasonable to apply the indications for stand-alone surgical ablation described above to patients being considered for hybrid surgical ablation (Class IIb, LOE C-EO, Table 2).
The Future
The most successful programs in the future might be those that employ an interdisciplinary, collaborative team approach to the treatment of AF, resulting in higher success rates for patients. Many of these patients are well read and mobile and will seek out such centers, thus increasing both catheter and surgical volumes. Practitioners in the future will likely find value to working as part of a multidisciplinary team. The precedent is set for this type of collaboration. The STS, the ACC, the FDA, and the Centers for Medicare and Medicaid Services have joined together to collaboratively introduce transcatheter AVR as a mandatory multidisciplinary team approach with mandatory long-term follow-up. More work is needed in the area of collaborative ablation of AF.
Section 13: Clinical Trial Design
Overview
Although there have been many advances made in the field of catheter and surgical ablation of AF, there is still much to be learned about the mechanisms of initiation and maintenance of AF and how to apply this knowledge to the still-evolving techniques of AF ablation. Although single-center, observational reports have dominated the early days of this field, we are quickly moving into an era in which hypotheses are put through the rigor of testing in well-designed, randomized, multicenter clinical trials. It is as a result of these trials that conventional thinking about the best techniques, success rates, complication rates, and long-term outcomes beyond AF recurrence—such as thromboembolism and mortality—is being put to the test. The ablation literature has also seen a proliferation of meta-analyses and other aggregate analyses, which reinforce the need for consistency in the approach to reporting the results of clinical trials. This section will review the minimum requirements for reporting on AF ablation trials. It will also acknowledge the potential limitations of using specific primary outcomes and emphasize the need for broad and consistent reporting of secondary outcomes to assist the end-user in determining not only the scientific, but also the clinical relevance of the results.
Types of Clinical Trials, Strengths, and Weaknesses
Mortality Trials
Large, randomized, controlled multicenter trials are considered the “gold standard” for many therapies in cardiovascular medicine. They are most likely to provide an unbiased understanding of the outcomes of specific aspects of ablative intervention. Although AF is associated with increased mortality and morbidity from stroke, HF, and recurrent hospitalization, most of the AF ablation literature is focused on AF recurrence and symptomatic improvement. It remains unclear whether ablation can affect AF burden sufficiently to have a positive outcome with respect to mortality and stroke endpoints. Trials powered to demonstrate a benefit for ablation with regard to these “hard” endpoints require large numbers of patients with extensive follow-up and its accompanying expense; however, the need for such trials cannot be understated. The CABANA trial was powered to examine stroke and mortality outcomes of AF ablation compared with pharmacological rate and rhythm control strategies. CABANA, which recently completed enrollment, requires a minimum of 5 years of follow-up; thus, results will not be available until 2018. In the meantime, EAST is a study that is currently enrolling and is designed to compare standard care vs a strategy of early rhythm control with ablation and/or AADs with endpoints including a composite outcome of cardiovascular death, stroke, and hospitalization due to worsening of HF or acute coronary syndrome. Although it is unclear whether these trials will demonstrate a mortality benefit of AF ablation, both are designed to examine a host of prespecified secondary endpoints. Secondary endpoints such as HF hospitalizations are especially important for patients with uncontrolled rates and HF with preserved EF or tachycardia-induced cardiomyopathy. Finally, because both trials will include larger numbers of patient ablations with more novel technologies such as cryoablation and CFS than have been available in any other study to date, significant advances in the understanding of ablation procedure with these systems should be possible. Nevertheless, it remains imperative to continue with designs of large mortality trials that reflect shifting global ablation techniques, technologies, and patient selection. There are currently 45 trials that meet the search criteria of “ablation mortality AF” on ClinicalTrials.gov; however, fewer than 10 have mortality as part of the primary endpoint.
Stroke and Thromboembolism Trials
Reductions in stroke and thromboembolism remain the most important goals of AF treatment. It is unclear, however, if elimination of or reductions in AF will necessarily reduce the associated risk of stroke, and whether such outcomes exceed those possible with NOAC agents. Although an increased risk of stroke appears to be associated with brief episodes of AF detected by implanted cardiac devices, multiple large randomized trials have demonstrated that there might be no temporal relationship between AF episodes and AF thromboembolic events. This possibility has cast significant doubt regarding the direct causal role that AF plays in stroke. On the other hand, some cohort studies of AF ablation have reported a lower risk of stroke postablation compared with matched, nonablated AF populations. The impact of AF ablation on stroke and thromboembolism is an important topic of future study and will likely require a combination of very large studies with long durations of follow-up akin to CABANA and EAST. The OCEAN study is currently getting started, and will examine the optimal strategy for ongoing antithrombotic therapy 1 year after successful ablation in a moderate-risk profile population with a primary endpoint of overt and covert stroke. It is important to stress that until the results of these trials are known, the current recommendations are to continue anticoagulation indefinitely in patients with CHA2DS2-VASc ≥2, regardless of the success of the ablation procedure.
Periprocedural stroke reduction is an important topic that is actively being studied, with various strategies of anticoagulation, particularly continuous administration of VKA and non-VKA oral anticoagulants through the ablation procedure.834,841,842 In addition, concomitant LAA occlusion is being tested. In percutaneous procedures, there are few if any studies powered for stroke alone; most primarily evaluate AF recurrence.
Finally, multiple studies have demonstrated small ACE on MR brain imaging after ablation.724,728,1207 The clinical significance of such ACE lesions is not known, and many will resolve to the point of being undetectable after weeks or months. The impact on cognitive function, if any, is not clear. At this point, there are no mandates for performing periprocedural brain imaging for novel technologies to evaluate the incidence of silent cerebral embolism, in large part because of its unknown clinical significance and the cost and burden of MRI on patients. However, further evaluation of the significance of such findings remains an important area of study.
Screening substudies could be reasonable for high-risk devices and should be combined with clinical neurological and cognitive assessments. These silent cerebral emboli are to be distinguished from covert embolic strokes secondary to long-term AF, which have been linked with long-term cognitive decline, and are much larger than the silent emboli seen periprocedurally.1385,1386,1387,1388
Multicenter Outcome Studies
There has been a proliferation of multicenter, randomized studies primarily geared toward the outcome of AF recurrence in the last several years. Many of these studies have had the appropriate size and power to make some important statements on the appropriate techniques for AF ablation. Because of the endpoint of AF recurrence, these studies can be performed with smaller sample sizes and shorter follow-up periods compared with mortality- or stroke-driven trials. A number of randomized trials have demonstrated the superiority of AF ablation over AADs in drug-refractory patients. First-line catheter ablation has shown mixed results over first-line drug therapy in the MANTRA-PAF and RAAFT-2 studies.378,379 STAR AF 2, Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination (ADVICE), FIRE AND ICE, and TOCCASTAR are just a few examples of multicenter randomized studies that have included hundreds of patients per study and added important contributions to the daily practice of AF ablation.245,265,378,379,489,655 STAR AF 2, for example, challenged the long-held belief that additional ablation beyond PVI is important for ablation of persistent AF and has launched a new search for alternative targets to CFAE and empiric lines. It remains possible that incomplete ablation in these arenas is more problematic than the ablation format itself. As reported recently, the FIRE AND ICE trial has shown an equivalence of evolving cryoablation technologies to traditional RF. The ADVICE trial showed that systematic use of adenosine to search for dormant conduction can improve durability of PVI and associated 1-year outcome, although studies reported earlier in this document raise questions about the overall utility of adenosine or isoproterenol. There are many more studies planned to examine various aspects of AF ablation, primarily around the comparison of techniques in certain patient populations to improve ablation outcomes. As expected, a criticism of all such trials is that the technology and techniques are outdated prior to trial completion. STAR AF 2 did not use CFS and FIRE AND ICE used a mixture of first- and second-generation CB technologies. Therefore, ongoing trials comparing the most up-to-date technologies will always be required. Larger-scale surgical ablation trials are lacking, and the consensus group believes that the development of well designed, highly agile, large-scale multicenter surgical trials with similar monitoring regimens need to be encouraged and performed. As with catheter-based studies and registries, the use of patient-reported outcome measures as part of the study endpoints is highly recommended.
Industry-Sponsored Device Approval Studies
There have been a number of prospective randomized studies performed to evaluate the safety and efficacy of investigational devices used for AF ablation. These studies, such as THERMOCOOL IDE and STOP AF, have all provided important, high-quality data demonstrating the superiority of catheter ablation over drug therapy in drug refractory patients.462,684 Now that the utility of ablation over drug therapy in such patients has been accepted, many of the current studies are focused on comparing new technologies against approved devices in a noninferiority design. Although these studies are important from a safety and efficacy perspective and are often mandated by health approval bodies such as the FDA, the incremental yield in knowledge could be limited. Prespecified subgroup analyses, or the use of novel endpoints. could therefore be important to determine whether incremental value is added by the newer technology. TOCCASTAR, for example, demonstrated statistical noninferiority of CF-driven RF ablation to traditional RF. However, only in a post-hoc analysis did the trial show that optimal CF was associated with better outcomes, findings which should be viewed with caution due to the limitations of post hoc analyses. Testing of the durability of lesion sets such as PVI either after delayed waiting, drug (adenosine) challenge, or repeat electrophysiology study after 3 months might also help assess comparative efficacy more accurately. Industry must also look to see whether safety and efficacy parameters demonstrated in PAF also apply to nonparoxysmal populations. Several industry-sponsored studies are either being planned or are in progress to assess outcomes in this challenging population.
Registry Studies
AF ablation registries offer a unique opportunity to collect data from large numbers of patients to examine outcomes. In particular, registries might help assess how ablation is being performed in the “real world” compared with controlled clinical trials that are often performed on a highly selected patient population in very experienced centers. The definition of real world remains problematic, however, because recent studies have shown reasonable congruence between the outcomes of RCTs and registries. Registries are well suited to determining early complication rates of ablation, particularly for less common ones such as PV stenosis, esophageal injury, or mortality. Appropriateness of patient selection and outcomes in patient subgroups that are underrepresented in studies, such as women or patients with underlying structural heart disease, can also be assessed in sizable registries. The collection of this kind of information, by itself, makes registries worthwhile if they can be performed with sufficient representation of a majority of centers. Still, well-controlled efforts such as the STS database have shown an even-handed approach to collecting this kind of material. Worldwide surveys of AF ablation have been published, and ongoing efforts are being made to harmonize various centers or national databases to pool ablation information. Many countries are now setting up provincial or national registries to examine the use and outcomes of AF ablation. In the United States, for example, the older Safety of Atrial Fibrillation Ablation Registry Initiative registry project was discontinued, but another started by the National Cardiovascular Data Registry (NCDR) has been launched nationally, with voluntary participation. The HRS is also collaborating with the AHA to develop an additional AF ablation registry. Surgical data are currently being collected in the STS database; however, although data on safety and outcome are available, lesion-specific information for surgical ablation remains preliminary. Collection of longitudinal data, particularly longer-term outcomes, can be limited by a lack of patient follow-up at the same center and a lack of consistent monitoring protocols. The need for informed consent to collect follow-up data also remains an obstacle to obtaining outcome data. The burden of data entry can also lead to inadequate reporting, and the cost of auditing data can be very expensive and tedious. The purpose of establishing a registry and the realistic goals of data collection must be stated outright upon establishment, because the opportunity and financial costs could be alternatively spent on well-designed clinical trials. Comparison of performance among sites, for example, must be based on the stated purposes and strengths of the registry. If the main purpose is to report acute complications, then long-term outcomes cannot be compared. Comparisons must also be corrected for patient characteristics, referral patterns to the institution, and community-based versus advanced academic practices. Finally, once the stated goals of the registry are accomplished, there should be specific timeframes for termination of the registry to avoid indefinite data collection with no specific stated purpose.
Clinical Endpoint Considerations
Early data in the field of AF ablation were limited by the multitude of different endpoints used in the trials, including multiple definitions of success, complications, and minimum monitoring postablation. Prior consensus statements sought to create consistency in the reporting of clinical trials by adopting standardized definitions for AF type, blanking periods, definitions of success, recommendations for minimal monitoring postablation, major complications, and device-related complications.1,2 Again, this document outlines the definitions of various types of AF (Table 1), definitions of efficacy (Table 10), QOL measures (Table 11), non-AF recurrence endpoints (Table 12), and definitions of complications (Table 8).
Table 10.
Definitions for use when reporting outcomes of AF ablation and in designing clinical trials of catheter or surgical ablation of AF
| Acute procedural success(pulmonary vein isolation) | Acute procedural success is defined as electrical isolation of all pulmonary veins. A minimal assessment of electrical isolation of the PVs should consist of an assessment of entrance block. If other methods are used to assess PVI, including exit block and/or the use of provocative agents such as adenosine or isoproterenol, they should be prespecified. Furthermore, it is recommended that the wait time used to screen for early recurrence of PV conduction once initial electrical isolation is documented be specified in all prospective clinical trials. |
| Acute procedural success (not related by pulmonary vein isolation) | Typically, this would apply to substrate ablation performed in addition to PVI for persistent AF. Although some have proposed AF termination as a surrogate for acute procedural success, its relationship to long-term success is controversial. Complete elimination of the additional substrate (localized rotational activation, scar region, non-PV trigger, or other target) and/or demonstration of bidirectional conduction block across a linear ablation lesion would typically be considered the appropriate endpoint. |
| One-year success∗ | One-year success is defined as freedom from AF/AFL/AT after removal from antiarrhythmic drug therapy as assessed from the end of the 3month blanking period to 12 months following the ablation procedure. Because cavotricuspid isthmus-dependent atrial flutter is easily treated with cavotricuspid isthmus ablation and is not an iatrogenic arrhythmia following a left atrial ablation procedure for AF, it is reasonable for clinical trials to choose to prespecify that occurrence of isthmus-dependent atrial flutter, if confirmed by entrainment maneuvers during electrophysiology testing, should not be considered an ablation failure or primary effectiveness endpoint. |
| Alternative one-year success | Although the one-year success definition provided above remains the recommended end point that should be reported in all AF ablation trials, and the endpoint for which the objective performance criteria listed below were developed, the Task Force recognizes that alternative definitions for success can be used if the main goal of therapy in the study is to relieve AF-related symptoms and to improve patient QOL. In particular, it is appropriate for clinical trials to define success as freedom from only symptomatic AF/AFL/AT after removal from antiarrhythmic drug therapy as assessed from the end of the 3-month blanking period to 12 months following the ablation procedure if the main goal of therapy in the study is to relieve AF-related symptoms and to improve patient QOL. However, because symptoms of AF can resolve over time, and because studies have shown that asymptomatic AF represents a greater proportion of all AF postablation than prior to ablation, clinical trials need to continue to report freedom from both symptomatic and asymptomatic AF even if this alternative one year success definition is used as the primary trial endpoint. |
| Clinical/partial success∗ | It is reasonable for clinical trials to define and incorporate one or more secondary definitions of success that can be referred to as “clinical success” or “partial success.” If these alternative definitions of success are included, they should be defined prospectively. In prior Consensus Documents the Task Force has proposed that clinical/partial success be defined as a “75% or greater reduction in the number of AF episodes, the duration of AF episodes, or the % time a patient is in AF as assessed with a device capable of measuring AF burden in the presence or absence of previously ineffective antiarrhythmic drug therapy.” Because there is no firm scientific basis for selecting the cutoff of 75% rather than a different cutoff, this prior recommendation is provided only as an example of what future clinical trials may choose to use as a definition of clinical/partial success. |
| Long-term success∗ | Long-term success is defined as freedom from AF/AFL/AT recurrences following the 3-month blanking period through a minimum of 36-month follow-up from the date of the ablation procedure in the absence of Class I and III antiarrhythmic drug therapy. |
| Recurrent AF/AFL/AT | Recurrent AF/AFL/AT is defined as AF/AFL/AT of at least 30 seconds' duration that is documented by an ECG or device recording system and occurs following catheter ablation. Recurrent AF/AFL/AT may occur within or following the post ablation blanking period. Recurrent AF/AFL/AT that occurs within the postablation blanking period is not considered a failure of AF ablation. |
| Early recurrence of AF/AFL/AT | Early recurrence of AF/AFL/AT is defined as a recurrence of atrial fibrillation within three months of ablation. Episodes of atrial tachycardia or atrial flutter should also be classified as a “recurrence.” These are not counted toward the success rate if a blanking period is specified. |
| Recurrence of AF/AFL/AT | Recurrence of AF/AFL/AT postablation is defined as a recurrence of atrial fibrillation more than 3 months following AF ablation. Episodes of atrial tachycardia or atrial flutter should also be classified as a “recurrence.” |
| Late recurrence of AF/AFL/AT | Late recurrence of AF/AFL/AT is defined as a recurrence of atrial fibrillation 12 months or more after AF ablation. Episodes of atrial tachycardia or atrial flutter should also be classified as a “recurrence.” |
| Blanking period | A blanking period of three months should be employed after ablation when reporting efficacy outcomes. Thus, early recurrences of AF/AFL/AT within the first 3 months should not be classified as treatment failure. If a blanking period of less than 3 months is chosen, it should be prespecified and included in the Methods section. |
| Stroke screening | A risk-based approach to determine the level of postablation stroke screening in clinical trials is recommended by the Task Force. For ablation devices with a lower risk of stroke and for which a stroke signal has not been reported, a minimum standardized neurological assessment of stroke should be conducted by a physician at baseline and at hospital discharge or 24 hours after the procedure, whichever is later. If this neurological assessment demonstrates new abnormal findings, the patient should have a formal neurological consult and examination with appropriate imaging (i.e., DW-MRI), used to confirm any suspected diagnosis of stroke. For devices in which a higher risk of stroke is suspected or revealed in prior trials, a formal neurological examination by a neurologist at discharge or 24 hours after the procedure, whichever is later, is recommended. Appropriate imaging should be obtained if this evaluation reveals a new neurological finding. In some studies in which delayed stroke is a concern, repeat neurological screening at 30 days postablation might be appropriate. |
| Detectable AF/AFL/AT | Detectable AF is defined as AF/AFL/AT of at least 30 seconds' duration when assessed with ECG monitoring. If other monitoring systems are used, including implantable pacemakers, implantable defibrillators, and subcutaneous ECG monitoring devices, the definition of detectable AF needs to be prespecified in the clinical trial based on the sensitivity and specificity of AF detection with the particular device. We recommend that episodes of atrial flutter and atrial tachycardia be included within the broader definition of a detectable AF/AFL/AT episode. |
| AF/AFL/AT burden | It is reasonable for clinical trials to incorporate AF/AFL/AT burden as a secondary endpoint in a clinical trial of AF ablation. In stating this it is recognized that there are no conclusive data that have validated a rate of AF burden reduction as a predictor of patient benefit (i.e. reduction in mortality and major morbidities such as stroke, CHF, QOL, or hospitalization). If AF burden is included, it is important to predefine and standardize the monitoring technique that will be used to measure AF burden. Available monitoring techniques have been discussed in this document. Should AF burden be selected as an endpoint in a clinical trial, the chosen monitoring technique should be employed at least a month prior to ablation to establish a baseline burden of AF. |
| Entrance block | Entrance block is defined as the absence, or if present, the dissociation, of electrical activity within the PV antrum. Entrance block is most commonly evaluated using a circular multielectrode mapping catheter positioned at the PV antrum. Entrance block can also be assessed using detailed point-by-point mapping of the PV antrum guided by an electroanatomical mapping system. The particular method used to assess entrance block should be specified in all clinical trials. Entrance block of the left PVs should be assessed during distal coronary sinus or left atrial appendage pacing in order to distinguish far-field atrial potentials from PV potentials. It is recommended that reassessment of entrance block be performed a minimum of 20 minutes after initial establishment of PV isolation. |
| Procedural endpoints for AF ablation strategies not targeting the PVs | Procedural endpoints for AF ablation strategies not targeting the PVs: The acute procedural endpoints for ablation strategies not targeting the PVs vary depending on the specific ablation strategy and tool. It is important that they be prespecified in all clinical trials. For example, if a linear ablation strategy is used, documentation of bidirectional block across the ablation line must be shown. For ablation of CFAEs, rotational activity, or non-PV triggers, the acute endpoint should at a minimum be elimination of CFAEs, rotational activity, or non-PV triggers. Demonstration of AF slowing or termination is an appropriate procedural endpoint, but it is not required as a procedural endpoint for AF ablation strategies not targeting the PVs. |
| Esophageal temperature monitoring | Esophageal temperature monitoring should be performed in all clinical trials of AF ablation. At a minimum, a single thermocouple should be used. The location of the probe should be adjusted during the procedure to reflect the location of energy delivery. Although this document does not provide formal recommendations regarding the specific temperature or temperature change at which energy delivery should be terminated, the Task Force does recommend that all trials prespecify temperature guidelines for termination of energy delivery. |
| Enrolled subject | An enrolled subject is defined as a subject who has signed written informed consent to participate in the trial in question. |
| Exit block | Exit block is defined as the inability to capture the atrium during pacing at multiple sites within the PV antrum. Local capture of musculature within the pulmonary veins and/or antrum must be documented to be present to make this assessment. Exit block is demonstrated by a dissociated spontaneous pulmonary vein rhythm. |
| Nonablative strategies | The optimal nonablative therapy for patients with persistent and long-standing persistent AF who are randomized to the control arm of an AF ablation trial is a trial of a new Class I or III antiarrhythmic agent or a higher dose of a previously failed antiarrhythmic agent. For patients with persistent or long-standing persistent AF, performance of a direct-current cardioversion while taking the new or dose adjusted antiarrhythmic agent should be performed, if restoration of sinus rhythm is not achieved following initiation and/or dose adjustment of antiarrhythmic drug therapy. Failure of pharmacological cardioversion alone is not adequate to declare this pharmacological strategy unsuccessful. |
| Noninducibility of atrial fibrillation | Noninducibility of atrial fibrillation is defined as the inability to induce atrial fibrillation with a standardized prespecified pharmacological or electrical stimulation protocol. The stimulation protocol should be prespecified in the specific clinical trial. Common stimulation approaches include a high-dose isoproterenol infusion protocol or repeated atrial burst pacing at progressively more rapid rates. |
| Patient populations for inclusion in clinical trials | It is considered optimal for clinical trials to enroll patients with only one type of AF: paroxysmal, persistent, or long-standing persistent. If more than one type of AF patient is enrolled, the results of the trial should also be reported separately for each of the AF types. It is recognized that “early persistent” AF responds to AF ablation to a similar degree as patients with paroxysmal AF and that the response of patients with “late persistent AF” is more similar to that in those with long-standing persistent AF. |
| Therapy consolidation period | Following a 3-month blanking period, it is reasonable for clinical trials to incorporate an additional 1- to 3-month therapy consolidation period. During this time, adjustment of antiarrhythmic medications and/or cardioversion can be performed. Should a consolidation period be incorporated into a clinical trial design, the minimum follow-up duration should be 9 months following the therapy consolidation period. Performance of a repeat ablation procedure during the blanking or therapy consolidation period would “reset” the endpoint of the study and trigger a new 3-month blanking period. Incorporation of a therapy consolidation period can be especially appropriate for clinical trials evaluating the efficacy of AF ablation for persistent or long-standing persistent AF. The challenge of this approach is that it prolongs the overall study duration. Because of this concern regarding overall study duration, we suggest that the therapy consolidation period be no more than 3 months in duration following the 3-month blanking period. |
| Recommendations regarding repeat ablation procedures | It is recommended that all clinical trials report the single procedure efficacy of catheter ablation. Success is defined as freedom from symptomatic or asymptomatic AF/AFL/AT of 30 seconds or longer at 12 months postablation. Recurrences of AF/AFL/AT during the first 3-month blanking period post-AF ablation are not considered a failure. Performance of a repeat ablation procedure at any point after the initial ablation procedure should be considered a failure of a single procedure strategy. It is acceptable for a clinical trial to choose to prespecify and use a multiprocedure success rate as the primary endpoint of a clinical trial. When a multiprocedure success is selected as the primary endpoint, efficacy should be defined as freedom from AF/flutter or tachycardia at 12 months after the final ablation procedure. In the case of multiple procedures, repeat ablation procedures can be performed at any time following the initial ablation procedure. All ablation procedures are subject to a 3-month post blanking window, and all ablation trials should report efficacy at 12 months after the final ablation procedure. |
| Cardioversion definitions | |
| Failed electrical cardioversion | Failed electrical cardioversion is defined as the inability to restore sinus rhythm for 30 seconds or longer following electrical cardioversion. |
| Successful electrical cardioversion | Successful electrical cardioversion is defined as the ability to restore sinus rhythm for at least 30 seconds following cardioversion. |
| Immediate AF recurrence postcardioversion | Immediate AF recurrence postcardioversion is defined as a recurrence of AF within 24 hours following cardioversion. The most common time for an immediate recurrence is within 30–60 minutes postcardioversion. |
| Early AF recurrence postcardioversion | Early AF recurrence postcardioversion is defined as a recurrence of AF within 30 days of a successful cardioversion. |
| Late AF recurrence postcardioversion | Late AF recurrence postcardioversion is defined as recurrence of AF more than 30 days following a successful cardioversion. |
| Surgical ablation definitions | |
| Hybrid AF surgical ablation procedure | Hybrid AF surgical ablation procedure is defined as a joint AF ablation procedure performed by electrophysiologists and cardiac surgeons either as part of a single “joint” procedure or performed as two preplanned separate ablation procedures separated by no more than 6 months. |
| Surgical Maze ablation procedure | Surgical Maze ablation procedure is defined as a surgical ablation procedure for AF that includes, at a minimum, the following components: (1) line from SVC to IVC; (2) line from IVC to the tricuspid valve; (3) isolation of the PVs; (4) isolation of the posterior left atrium; (5) line from MV to the PVs; (6) management of the LA appendage. |
| Stand-alone surgical AF ablation | A surgical AF ablation procedure during which other cardiac surgical procedures are not performed such as CABG, valve replacement, or valve repair. |
| Nomenclature for types of surgical AF ablation procedures | We recommend that the term “Maze” procedure is appropriately used only to refer to the biatrial lesion set of the Cox-Maze operation. It requires ablation of the RA and LA isthmuses. Less extensive lesion sets should not be referred to as a “Maze” procedure, but rather as a surgical AF ablation procedure. In general, surgical ablation procedures for AF can be grouped into three different groups: (1) a full biatrial Cox-Maze procedure; (2) PVI alone; and (3) PVI combined with left atrial lesion sets. |
| Hybrid epicardial and endocardial AF ablation | This term refers to a combined AF ablation procedure involving an off-pump minimally invasive surgical AF ablation as well as a catheter-based AF ablation procedure designed to complement the surgical lesion set. Hybrid ablation procedures may be performed in a single-procedure setting in a hybrid operating room or a cardiac catheterization laboratory environment, or it can be staged. When staged, it is most typical to have the patient undergo the minimally invasive surgical ablation procedure first following by a catheter ablation procedure 1 to 3 months later. This latter approach is referred to as a “staged Hybrid AF ablation procedure.” |
| Minimum AF documentation, endpoints, TEE performance, and success rates in clinical trials | |
| Minimum documentation for paroxysmal AF | The minimum AF documentation requirement for paroxysmal AF is (1) physician's note indicating recurrent self-terminating AF and (2) one electrocardiographically documented AF episode within 6 months prior to the ablation procedure. |
| Minimum documentation for persistent AF | The minimum AF documentation requirement for persistent AF is (1) physician's note indicating continuous AF > 7 days but no more than 1 year and (2) a 24-hour Holter within 90 days of the ablation procedure showing continuous AF. |
| Minimum documentation for early persistent AF | The minimum AF documentation requirement for persistent AF is (1) physician's note indicating continuous AF > 7 days but no more than 3 months and (2) a 24-hour Holter showing continuous AF within 90 days of the ablation procedure. |
| Minimum documentation for long-standing persistent AF | The minimum AF documentation requirement for long-standing persistent AF is as follows: physician's note indicating at least 1 year of continuous AF plus a 24-hour Holter within 90 days of the ablation procedure showing continuous AF. The performance of a successful cardioversion (sinus rhythm >30 seconds) within 12 months of an ablation procedure with documented early recurrence of AF within 30 days should not alter the classification of AF as long-standing persistent. |
| Symptomatic AF/AFL/AT | AF/AFL/AT that results in symptoms that are experienced by the patient. These symptoms can include but are not limited to palpitations, presyncope, syncope, fatigue, and shortness of breath. For patients in continuous AF, reassessment of symptoms after restoration of sinus rhythm is recommended to establish the relationship between symptoms and AF. |
| Documentation of AF-related symptoms | Documentation by a physician evaluating the patient that the patient experiences symptoms that could be attributable to AF. This does not require a time-stamped ECG, Holter, or event monitor at the precise time of symptoms. For patients with persistent AF who initially report no symptoms, it is reasonable to reassess symptom status after restoration of sinus rhythm with cardioversion. |
| Minimum effectiveness endpoint for patients with symptomatic and asymptomatic AF | The minimum effectiveness endpoint is freedom from symptomatic and asymptomatic episodes of AF/AFL/AT recurrences at 12 months following ablation, free from antiarrhythmic drug therapy, and including a prespecified blanking period. |
| Minimum chronic acceptable success rate: paroxysmal AF at 12-month follow-up | If a minimum chronic success rate is selected as an objective effectiveness endpoint for a clinical trial, we recommend that the minimum chronic acceptable success rate for paroxysmal AF at 12-month follow-up is 50%. |
| Minimum chronic acceptable success rate: persistent AF at 12-month follow-up | If a minimum chronic success rate is selected as an objective effectiveness endpoint for a clinical trial, we recommend that the minimum chronic acceptable success rate for persistent AF at 12-month follow-up is 40%. |
| Minimum chronic acceptable success rate: long-standing persistent AF at 12-month follow-up | If a minimum chronic success rate is selected as an objective effectiveness endpoint for a clinical trial, we recommend that the minimum chronic acceptable success rate for long-standing persistent AF at 12-month follow-up is 30%. |
| Minimum follow-up screening for paroxysmal AF recurrence | For paroxysmal AF, the minimum follow-up screening should include (1) 12-lead ECG at each follow-up visit; (2) 24-hour Holter at the end of the follow-up period (e.g., 12 months); and (3) event recording with an event monitor regularly and when symptoms occur from the end of the 3-month blanking period to the end of follow-up (e.g., 12 months). |
| Minimum follow-up screening for persistent or long-standing AF recurrence | For persistent and long-standing persistent AF, the minimum follow-up screening should include (1) 12-lead ECG at each follow-up visit; (2) 24-hour Holter every 6 months; and (3) symptom-driven event monitoring. |
| Requirements for transesophageal echocardiogram | It is recommended that the minimum requirement for performance of a TEE in a clinical trial should be those requirements set forth in ACC/AHA/HRS 2014 Guidelines for AF Management pertaining to anticoagulation at the time of cardioversion. Prior to undergoing an AF ablation procedure a TEE should be performed in all patients with AF of > 48 hours' duration or of unknown duration if adequate systemic anticoagulation has not been maintained for at least 3 weeks prior to AF ablation. If a TEE is performed for this indication, it should be performed within 24 hours of the ablation procedure. |
AF, atrial fibrillation; DW-MRI, diffusion-weighted magnetic resonance imaging; CHF, congestive heart failure; QOL, quality of life; ECG, electrocardiogram; CABG, coronary artery bypass grafting; PV, pulmonary vein; SVC, superior vena cava; IVC, inferior vena cava; CFAE, complex fractionated atrial electrogram; PVI, pulmonary vein isolation; AFL, atrial flutter; AT, atrial tachycardia; ACC, American College of Cardiology; AHA, American Heart Association; HRS, Heart Rhythm Society.
When reporting outcomes of AF ablation, the development of atrial tachycardia or atrial flutter should be included in the broad definition of recurrence following AF ablation. All studies should report freedom from AF, atrial tachycardia, and atrial flutter. These endpoints can also be reported separately. All studies should also clearly specify the type and frequency of ECG monitoring as well as the degree of compliance with the prespecified monitoring protocol.
Table 11.
Quality-of-life scales, definitions, and strengths
| Scale | Definition/Details | Strengths/Weaknesses |
|---|---|---|
| Short Form (36) Health Survey (SF36)38 | Consists of 8 equally weighted, scaled scores in the following sections: vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, mental health. Each section receives a scale score from 0 to 100. | Advantages: extensively validated in a number of disease and health states. Might have more resolution than EQ-50 for AF QOL. |
| (General) | ||
| Physical component summary (PCS) and mental component summary (MCS) is an average of all the physically and mentally relevant questions, respectively. | Disadvantages: not specific for AF, so might not have resolution to detect AF-specific changes in QOL. | |
| The Short Form (12) Health Survey (SF12) is a shorter version of the SF-36, which uses just 12 questions and still provides scores that can be compared with SF-36 norms, especially for summary physical and mental functioning. | ||
| Gives more precision in measuring QOL than EQ-5D but can be harder to transform into cost utility analysis. | ||
| EuroQol Five Dimensions Questionnaire (EQ-5D)39 (General) | Two components: Health state description is measured in five dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression. Answers may be provided on a three-level (3L) or five-level (5L) scale. In the Evaluation section, respondents evaluate their overall health status using a visual analogue scale (EQ-VAS). Results can easily be converted to quality-adjusted life years for cost utility analysis. | Advantages: extensively validated in a number of disease and health states. Can easily be converted into quality-adjusted life years for cost-effectiveness analysis. |
| Disadvantages: might not be specific enough to detect AF-specific changes in QOL. Might be less specific than SF-36. | ||
| AF effect on Quality of Life Survey (AFEQT)40 (AF specific) | 20 questions: 4 targeting AF-related symptoms, 8 evaluating daily function, and 6 assessing AF treatment concerns. Each item scored on a 7-point Likert scale. | Advantages: brief, simple, very responsive to AF interventions. Good internal validity and well validated against a number of other global and AF-specific QOL scales. Used in CABANA. |
| Disadvantages: validation in only two published studies (approximately 219 patients). | ||
| Quality of Life Questionnaire for Patients with AF | 18-item self-administered questionnaire with three domains: psychological, physical, and sexual activity. Each item scores on a 5-point Likert scale. | Advantages: brief, simple, responsive to AF interventions; good internal validity; used in SARA trial. |
| (AF-QoL)41 | Disadvantages: external validity compared only to SF-36; formal validation in 1 study (approximately 400 patients). | |
| (AF specific) | ||
| Arrhythmia-Related Symptom Checklist (SCL)42 (AF specific) | 16 items covering AF symptom frequency and symptom severity. | Advantages: most extensively validated in a number of arrhythmia cohorts and clinical trials. |
| Disadvantages: time-consuming and uncertain generalizability. | ||
| Mayo AF Specific Symptom Inventory (MAFSI)43 (AF specific) | 10 items covering AF symptom frequency and severity. Combination of 5- point and 3-point Likert scale responses. | Advantages: validated in an AF ablation population and responsive to ablation outcome; used in CABANA trial. |
| Used in CABANA trial. | Disadvantages: external validity compared only to SF-36; 1 validation study (approximately 300 patients). | |
| University of Toronto Atrial Fibrillation Severity Scale (AFSS) (AF specific)44 | 10 items covering frequency, duration, and severity. 7-point Likert scale responses. | Advantages: validated and reproducible; used in CTAF trial. |
| Disadvantages: time-consuming and uncertain generalizability. | ||
| Arrhythmia Specific Questionnaire in Tachycardia and Arrhythmia (ASTA)45 (AF specific) | Records number of AF episodes and average episode duration during last 3 months. 8 symptoms and 2 disabling symptoms are recorded with scores from 1–4 for each. | Advantages: validated in various arrhythmia groups; external validity compared with SCL, EQ5D, and SF-36; used in MANTRA-PAF; brief; simple. |
| Disadvantages: one validation study (approximately 300 patients). | ||
| European Heart Rhythm Association (EHRA)46 (AF specific) | Like NYHA scale. I, no symptoms, II, mild symptoms not affecting daily activity, III, severe symptoms affecting daily activity, and IV, disabling symptoms terminating daily activities. | Advantage: very simple, like NYHA.Disadvantages: not used in studies and not well validated; not very specific; unknown generalizability. |
| Canadian Cardiovascular Society Severity of Atrial Fibrillation Scale (CCS-SAF)47 (AF specific) | Like NYHA scale. O, asymptomatic, I, AF symptoms have minimal effect on patient's QOL, II, AF symptoms have minor effect on patient QOL, III, symptoms have moderate effect on patient QOL, IV= AF symptoms have severe effect on patient QOL. | Advantages: very simple, like NYHA; validated against SF-36 and University of Toronto AFSS. |
| Disadvantages: poor correlation with subjective | ||
| AF burden; not very specific. |
AF, atrial fibrillation; QOL, quality of life; CABANA, Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation; SARA, Study of Ablation Versus antiaRrhythmic Drugs in Persistent Atrial Fibrillation; CTAF, Canadian Trial of Atrial Fibrillation; MANTRA-PAF, Medical ANtiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation; NYHA, New York Heart Association; AFSS, atrial fibrillation severity scale.
Table 12.
Non-AF recurrence–related endpoints for reporting in AF ablation trials
| Stroke and bleeding endpoints | Definitions/Details |
|---|---|
| Stroke (2014 ACC/AHA Key Data Elements) | An acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of hemorrhage or infarction. Symptoms or signs must persist ≥24 hours, or if documented by CT, MRI or autopsy, the duration of symptoms/signs may be less than 24 hours. Stroke may be classified as ischemic (including hemorrhagic transformation of ischemic stroke), hemorrhagic, or undetermined. Stroke disability measurement is typically performed using the modified Rankin Scale (mRS). |
| Transient ischemic attack (2014 ACC/AHA Key Data Elements) | Transient episode of focal neurological dysfunction caused by brain, spinal cord, or retinal ischemia without acute infarction and with signs and symptoms lasting less than 24 hours. |
| Major bleeding (ISTH definition) | Fatal bleeding AND/OR symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome AND/OR bleeding causing a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of two or more units of blood. |
| Clinically relevant nonmajor bleed (ISTH definition) | An acute or subacute clinically overt bleed that does not meet the criteria for a major bleed but prompts a clinical response such that it leads to one of the following: hospital admission for bleeding; physician-guided medical or surgical treatment for bleeding; change in antithrombotic therapy (including interruption or discontinuation). |
| Minor bleeding (ISTH definition) | All nonmajor bleeds. Minor bleeds are further divided into clinically relevant and not. |
| Incidence and discontinuation of oral anticoagulation | The number of patients receiving oral anticoagulation and the type of oral anticoagulation should be documented at the end of follow-up. If patients have their oral anticoagulation discontinued, the number of patients discontinuing, the timing of discontinuation, and the reasons for discontinuation of oral anticoagulation, as well as the clinical characteristics and stroke risk profile of the patients should be reported. |
AF, atrial fibrillation; CT, computed tomography; MRI, magnetic resonance imaging.
Clinical endpoints for AF ablation trials may either consist of clinical events like mortality, stroke, re-initiation of AAD treatment, need for cardioversion, reablation and rehospitalization, or of patient-reported outcomes such as symptom severity or QOL. AF recurrence or change in AF behavior is a very important endpoint to report in trials targeting AF elimination. The following section will focus on recommendations and definitions for AF-related measurements used in clinical ablation trials.
Blanking Period
It has long been recognized that in the weeks immediately following AF ablation, early recurrences of atrial arrhythmia can occur that subsequently subside over time.253,254,255,436 Whether this is due to an early “inflammatory” response in the atrium or pericardium remains hypothetical. Based on these observations, prior consensus statements, and the present consensus document, the writing group recommends the use of a 3-month blanking period immediately postablation, during which arrhythmia recurrences are not counted toward the primary recurrence endpoint (Table 10). The use of a blanking period is not without limitations. Although half of all early recurrences might subside, early recurrence remains a very significant predictor of late recurrence of AF.141,142,143,255 Furthermore, some studies have shown that recurrences occurring early in the blanking period (within 1–2 months) are less predictive of late recurrence, whereas those occurring in the third month have a very high predictive value for later recurrence.933,977,1389 Blanking periods can also be applied inconsistently, typically after the initial ablation, but not typically after repeat procedures, particularly when there is only a limited duration of follow-up. Despite these limitations, the writing group consensus continues to recommend the use of a 3-month blanking period for atrial arrhythmia recurrences post-initial ablation for AF. If alternate durations of blanking are employed, they should be prespecified in the trial methodology. Clinical trials should also consider routine discontinuation of AADs after the blanking period to determine off-drug success rates of ablation. Large clinical trials such as CABANA have also employed extensive ongoing monitoring, which could shed light on more robust blanking period definitions. The currently recommended definitions of the blanking periods, monitoring standards, complications, and other AF ablation clinical trial definitions are provided in Tables 8 and 10.
AF Recurrence Endpoints
The selection of a primary endpoint depends on the objectives of the trial. As mentioned earlier in this section, trials with mortality, stroke, or hospitalization outcomes are of particular interest in advancing the field of AF ablation. However, now and in the foreseeable future, recurrence of AF will remain of primary interest for most clinical trials. A summary of AF-related endpoints is listed in Table 13, along with the advantages and disadvantages of each endpoint.
Table 13.
Advantages and disadvantages of AF-related endpoints in AF ablation trials
| Endpoint | Advantages | Disadvantages | Relevance and Comments |
|---|---|---|---|
| Freedom from AF/AFL/AT recurrence “gold standard” is 30 seconds |
|
|
|
| Freedom from stroke-relevant AF/AFL/AT-duration cutoff of 1 hour |
|
|
|
| Freedom from AF/AFL/AT requiring intervention (emergency visits, cardioversion, urgent care visit, reablation, etc.) |
|
|
|
| Freedom from persistent AF/AFL/AT-duration cutoff of 7 days |
|
|
|
| Freedom from AF/AFL/AT on previously ineffective antiarrhythmic therapy |
|
|
|
| Significant reduction in AF burden: >75% reduction from pre- to postablation and/or total postablation burden <12% |
|
|
|
| Prevention in AF progression: time to first episode of persistent AF (>7 days) |
|
|
|
| Regression of AF: reduction in burden to a given threshold or conversion of persistent to paroxysmal AF |
|
|
|
| Acute AF termination during ablation procedure |
|
|
|
AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia.
The consensus statement reaffirms the use of freedom from any atrial arrhythmia (e.g., AF, AT, or AFL) greater than 30 seconds off antiarrhythmic therapy as the gold standard for reporting the efficacy of AF ablation (Table 10). The writing group also believes that all trials should report single-procedure, off AAD therapy efficacy for ablation with a minimum of 12 months follow-up. Slight variations in this endpoint have been used in several clinical trials, but ideally, all categories of recurrence should be reported transparently, such as freedom from AF separately from other atrial arrhythmia, one- and multiple-procedure success rates, and success on and off antiarrhythmic therapy. By reporting all of these variations, the reader can determine the most relevant outcome for themselves and can also easily compare results between clinical trials. A recent study that reported outcomes using a wide variety of endpoints can serve as an excellent example of this approach to reporting outcomes.245 The inclusion of all atrial arrhythmias compared with AF in isolation recognizes the fact that ablation can result in iatrogenic macro- and microreentrant tachycardias caused by incomplete scar formation from the procedure itself. Furthermore, patients might present with mixed pictures of both AFL and fibrillation, and elimination of one but not the other will not improve patient outcomes.
The consensus statement recognizes that the 30-second cutoff for arrhythmia recurrence is stringent and might not accurately reflect more clinically relevant endpoints, such as reduction in total AF burden, symptom abatement, and improvement in QOL. A strict cutoff might also underestimate the true benefit of ablation, especially when presented in the format of a Kaplan-Meier analysis. Isolated, brief recurrences can result in a patient being considered a “procedural failure,” although the overall reduction in AF burden has been substantial. Patients with preablation high-burden PAF might continue to experience AF episodes, but with a reduced frequency and duration and a significant improvement in QOL. More liberal cutoff points have been suggested based on implantable monitoring technology detection limits (>2 minutes) or based on hypothesized thresholds for stroke risk (>6 minutes or > 5–6 hours). However, selection of any other cutoff would be as arbitrary as the initial selection of 30 seconds, which has now been in place since 2007. Keeping the same endpoint threshold will therefore allow for comparison of future studies against those performed in the past. It also remains unclear whether the selection of a somewhat more generous threshold would actually significantly alter reported success rates in a time to event analysis.
Arrhythmia recurrence is often reported as time to first AF episode of a particular type, such as any episode of an ATA lasting more than 30 seconds, verified by surface ECG (loop recorder) or an intracardiac electrogram. This parameter might best reflect differences in lesion quality around PVs for electrical isolation. Ineffective ablation and early gap formation could result in an earlier time to first recurrent AF. Even the time to the second or third AF recurrence might further allow insights into such ablation effects and could therefore be used as a secondary outcome measurement.
Cutoffs of more than 30 seconds can be reported in addition to the 30-second primary endpoint to show how procedural success might change. In fact, the consensus group encourages such reporting routinely in all clinical trials to better assess the most clinically relevant outcomes for future clinical trials. In particular, higher cutoffs can be used for patients with persistent or long-standing persistent AF because of the very high burden of preablation AF and the lower likelihood that ablation will result in a full “cure” of the arrhythmia. It is strongly suggested that other cutoffs be prespecified and reported in secondary outcomes of trials so the true effects of catheter ablation on various types of AF can be put into proper context outside of the 30-second cutoff.
A cutoff that can be used in addition to 30 seconds would be the time to first clinical or stroke-relevant AF duration (e.g., more than 1 hour or 5.5 hours). As already described, the SOS trial revealed AF activity of more than 1 hour per day as a cutoff for an increased risk of stroke, whereas other investigation revealed various AF burden levels, such as a marker of an increased risk for thromboembolism. This parameter might be used preferentially in studies in which the potential of ablation to reduce outcomes such as thromboembolism might be the primary interest. “Time to first persistent AF” could be considered for trials of persistent AF ablation in which time to the first episode of more than 7 days might be a relevant parameter while investigating substrate modifying ablation therapies such as atrial lines or localized rotational activity elimination.
AF Burden Endpoints
Rather than report time to an AF recurrence of a specific duration, many feel that AF burden is a more optimal endpoint for assessing ablation efficacy. AF burden can be estimated based on serial long-term monitoring results and patient symptom reporting, but only continuous monitoring through a cardiac implantable electronic device (loop, pacemaker, ICD) can truly define the burden. Furthermore, placement of such an implantable recording device should ideally be performed preablation so that pre- and postablation outcomes can be compared. Use of such devices, however, can be quite costly and impose undue difficulty in performing clinical trials. AF burden can be used in various ways in AF ablation trials. Freedom from relevant AF—classically defined as an absence of any ATA of more than 30 seconds—might be defined, for example, as a low daily AF burden less than 1%–2%. This approach would recognize the fact that occasional and short-lasting atrial arrhythmias over a few minutes might be an acceptable outcome. It should be noted, however, that there is a substantial difference between long-term, daily monitoring of AF burden versus a detection period of 3 months, as in the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial, in which short-lasting AF was likely a marker for future long-lasting AF outside the monitoring period.1390Reduction in AF burden more than 75% could be considered as clinical success just as much as reduction in both the number and duration of AF episodes. However, the number and duration of episodes are significantly more sensitive to under- or oversensing with subcutaneous devices but also implanted pacemakers or defibrillators for various technical reasons. In contrast, the number of episodes necessitating urgent or emergency care visits might not only be clinically relevant, but might also help demonstrate the cost-effectiveness of the procedure. Furthermore, because there is no firm scientific basis for selecting the cutoff of 75%, this prior recommendation is provided only as an example of what future clinical trials might choose to use as a definition of clinical or partial success.
In recognition that AF ablation may not be curative, particularly for patients with persistent or long-standing persistent AF, the concepts of AF progression and regression, while unproven, could be of interest. Many patients might initially present with very infrequent episodes of PAF that could be quite manageable with minimal drug therapy. Ablation in this setting might help delay progression to higher burden paroxysmal or persistent AF, which could be associated with decreased functioning but also increased risks of stroke, HF, or death. On the other hand, patients with persistent AF who can be converted into infrequent, paroxysmal forms of AF (so-called AF regression) might experience not only QOL benefits but also a potential reduction in morbidity and mortality. In order for these endpoints to be widely implemented, thresholds of AF must be established under which patient QOL and risk of adverse outcomes are reliably improved, which has yet to be done. For example, one substudy of the STAR AF 1 trial showed that patients with very high-burden paroxysmal or persistent AF could continue to experience up to 2 or more hours of AF per month postablation and still report an improvement in QOL.1391 The patient-reported symptoms did not deteriorate until they experienced more than 27 hours of AF per month. This outcome remains an important focus for ongoing clinical investigation.
When an implanted device is not used, many trials have attempted to estimate changes in AF burden by using various methods. If careful recording of patient symptoms and clinically apparent recurrences is performed, including duration and frequency of episodes over a specific period of time, then these could be used to estimate AF burden pre- and postablation.1391 Total AF detected on intermittent continuous monitoring (like intermittent 7 day Holters) could be used, although the accuracy is somewhat limited depending on the duration and frequency of monitoring.378 Intermittent, but frequent, transtelephonic or other portable monitors can provide brief strips of rhythm status. Time in sinus rhythm could be estimated by the number of weeks (for example), with sinus transmissions divided by the total number of weeks of the monitoring period, akin to a time in therapeutic range for OAC with VKA.245 A combination of symptom reporting and ECG status at various time points can also be used to calculate estimated time in sinus rhythm, as was employed in a substudy of the Atrial Fibrillation and Congestive Heart Failure study.1392
Endpoint Differences for Paroxysmal vs Nonparoxysmal AF Ablation Studies
Important consideration should be given to differences in AF recurrence endpoint reporting in trials of paroxysmal versus persistent AF. For patients with PAF, the burden might not be well suited for determining the outcome of ablation. Because the preablation burden can be relatively low in the months preceding ablation, with a large range in the burden, it might be hard to realize a statistically significant change postablation or between treatment arms. This was demonstrated in the MANTRA-PAF trial, in which total AF burden (measured on 7-day Holters) did not differ between drug and ablation therapy, but the total number of patients free from any AF recurrence was significantly higher in the ablation arm.378 For these patients, a time to recurrence or proportion free from arrhythmia endpoint might be a better option. Other statistical concerns that need to be considered for AF burden as an outcome measure for ablation in PAF patients include regression to the mean and the clustered, nonrandom pattern of PAF episodes. For persistent AF, reduction in burden can be much more relevant because the preablation burden will be high (close to 100%), with little standard deviation, making a statistical reduction postablation easier to define. On the other hand, the use of freedom from 30-second endpoints could underestimate the true clinical effect of ablation in the persistent population. The consensus group still maintains that the 30-second endpoint should be reported, but secondary endpoints such as changes in AF burden and/or AF progression or regression should also be described. Both CABANA and EAST, with more extensive monitoring, should both shed additional light on these issues.
The writing group members encourage reporting of other secondary endpoints that might better represent clinically relevant outcomes of the ablation procedure. Improvements in patient QOL are very important to assessing the clinical success of AF ablation, but as with any intervention, the magnitude of the improvements might be confounded by expectancy bias (“placebo effect”). A detailed discussion of QOL measurements and potential benefits and limitations appears later in this section.
Symptomatic vs Asymptomatic Recurrence
Even in patients with highly symptomatic AF, as many as half of all episodes can occur without associated symptoms.56 The ratio of asymptomatic to symptomatic episodes increases up to 4-fold postablation, perhaps due to shorter durations, slower rates, or autonomic modulation after the procedure.58 In highly symptomatic AF patients, asymptomatic episodes often coexist with the symptomatic; thus, patient reporting of symptoms can still serve as a rough surrogate for procedural success. For clinical trial purposes, however, reporting of only symptomatic AF recurrences could overestimate procedural success by 20% or more by missing asymptomatic recurrences. The importance of asymptomatic AF detection depends in part on the purpose of the clinical trial. If patient QOL and symptom abatement is the primary goal of therapy in the study, then underdetection of asymptomatic AF could be of little relevance. However, if the study goal is to reduce the associated risks of AF (stroke, HF) and to change potential therapy, including OAC, then the detection of asymptomatic AF is much more critical. Typically, the detection of asymptomatic AF recurrence is accomplished by longer-term, frequent, or implantable monitoring approaches.
AF Monitoring Postablation
Arrhythmia monitoring can be performed with the use of noncontinuous or continuous ECG monitoring tools. The choice of either method depends on individual need and consequence of arrhythmia detection. Basically, more intensive monitoring is associated with a greater likelihood of detecting both symptomatic and asymptomatic AF.56,58,937 Identification of patients with AF and assessment of AF burden with intermittent monitoring have been shown to depend on a patient's actual AF burden, and improve with an increasing frequency or duration of intermittent monitoring. Conversely, the more complex and longer the method of monitoring that is used, the lower the patient compliance.
Available noncontinuous detection tools include scheduled or symptom-initiated standard ECGs, Holter (24 hours to 7 days), transtelephonic recordings, patient- and automatically activated devices, and external loop recorders (Table 6). Scheduled 7-day Holter ECG recordings or daily plus symptom-activated event recordings are estimated to document approximately 70% of AF recurrences, with an estimated negative predictive value for absence of AF between 25% and 40%.947,1393
Continuous ECG monitoring is permanent monitoring for a long time period (1, 2, or more years). Continuous ECG monitoring can be facilitated with the use of implantable devices. Implantable pacemakers or defibrillators with atrial leads allow the burden of AF to be assessed by tracking the number and duration of mode switch episodes, particularly when an arrhythmia duration of ≥5 minutes is used as the cutoff value.1394 More recently, a long-term subcutaneous implantable loop monitor has become available to facilitate continuous AF monitoring based on R-R interval analysis over a period of 2 years.58,952 These types of continuous ECG monitoring devices can be used to evaluate the results of AF ablation. Although implantable subcutaneous monitors hold promise for determination of AF burden long term, important limitations include less than 100% specificity due to myopotentials, atrial and ventricular premature beats, as well as limited memory resulting in electrograms not being retrievable to verify the correct rhythm diagnosis. Another major limitation for the performance of clinical trials is cost. If the consensus mandated ILR monitoring for all clinical trials, the cost of performing such trials would likely become prohibitive. There are also a number of patients who might refuse long-term devices.
Again, the purpose of the trial should be married to the type of monitoring performed. If the ultimate goal is to improve patients’ QOL, then excessive monitoring for asymptomatic AF might not be worth the effort. However, if the goal is to reduce AF burden, or change prognosis, particularly from a stroke point of view, then continuous monitoring should be required.
In the past, the consensus statement has provided minimum clinical requirements for postablation monitoring for clinical trials. Initially, these were quite stringent, and in the last consensus statement, the requirements were made more flexible. The current consensus recommends the following minimum monitoring requirements: For PAF, follow-up screening should include a minimum of three visits (e.g., at 3, 6, and 12 months), with a 12-lead ECG at each visit, a 24-hour Holter at the end of the follow-up period (e.g., 12 months), and more limited event recording from the end of the 3-month blanking period to the end of follow-up (e.g., 12 months), both at regular periods and with patient activated recordings obtained at the time of symptoms (or equivalent). Follow-up beyond 1 year is encouraged and might occur every 6 months with Holter and ECG (or equivalent). For persistent and long-standing persistent AF, follow-up screening should include a minimum of three visits (e.g., at 3, 6, and 12 months), with a 12-lead ECG at each visit, a 24-hour Holter every 6 months, and event recording from the end of the 3-month blanking period to the end of follow-up (e.g., 12 months), as well as at the time of symptoms (or equivalent). Follow-up beyond 1 year is encouraged and might occur every 6 months with Holter and ECG (or equivalent) (Table 10). In making these recommendations, it is important to recognize that the writing group views these as minimal monitoring recommendations. More intensive follow-up with more frequent Holters and/or extended ECG monitoring is encouraged. Similarly, follow-up beyond 1 year is encouraged and might occur every 6 months with Holter and ECG (or equivalent). It is acknowledged that this recommendation falls short of continuous monitoring and will largely detect symptomatic recurrences with only a limited ability to detect asymptomatic recurrences. However, this minimum standard will at least provide some consistency in trial reporting, and trials are encouraged to exceed this standard where possible. Details are specified in Table 10.
QOL Measurement
QOL should remain an important endpoint for AF ablation studies, but not necessarily the primary endpoint. QOL is limited by treatment expectancy bias. Although sham procedures have not been performed to assess the true magnitude of this bias, it is unlikely that such studies will be performed because they would be extremely challenging.
QOL can be measured both using well-established scales like the SF-36 and EQ5D, but also using more specific scales like the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT), University of Toronto Atrial Fibrillation Severity Scale, Mayo AF-Specific Symptom Inventory, or Symptom Severity Score. The advantages of the generalized scales is their wide usage in medicine, the ability to compare improvements in QOL with other medical interventions, and in the case of the EQ5D, converts QOL changes to cost-effectiveness measures through the use of QALYs; however, these scales can lack sensitivity to changes with reductions in AF burden. AF-specific scales, on the other hand, might improve sensitivity and discriminate more effectively between patients with successful and failed ablation. At present, the true value of AF-specific scales requires validation through randomized studies using standard of care therapy as a control arm, given the comorbidity associated with AF can impact the same symptoms that affect the EHRA score and the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Finally, we still need to know how these changes compare with other medical interventions and if the changes would result in substantial reductions in health care cost or patient morbidity.
The consensus group recommends that all clinical trials incorporate some measure of patient-reported outcomes and preferably measure them using both a general and an AF-specific measurement scale. A summary of QOL scales is provided in Table 11.
Other Endpoint Reporting
There are important subgroups of patients and clinical outcomes that need to be studied, but are unlikely to be addressed by any one study alone. To facilitate pooled data analysis, the consensus recommends routine reporting of additional subgroup analyses, particularly around modifiable lifestyle risk factors. BMI and OSA should be reported in the baseline characteristics and subgroup analysis, comparing high vs average BMI and those with and without sleep apnea, which should be ideally reported in recognition that modifiable risk factors are an important contributor to AF progression and ablation outcome.
The need for a better understanding of the most appropriate postablation anticoagulation strategy is particularly recognized by the consensus group. Due to the rarity of stroke, TIA, and peripheral thromboembolism, it is unlikely that sufficiently powered studies will ever be conducted to conclusively resolve this relevant aspect of clinical practice. In the absence of a clear strategy, it is possible that postablation patients are exposed to an excess stroke risk if untreated, or to an excess bleeding risk if treated with no real need. As a reasonable surrogate to an evidence-based demonstration, the consensus group recognizes the value of careful reporting of secondary outcomes in which individual data are made available for (1) baseline risk factors; (2) postablation anticoagulation strategy (e.g., if continued, and if so, which drug, or discontinued); and (3) postablation thromboembolic and/or bleeding events. An effort of this type would not only enhance the quality of the single studies, but it would also allow for pooled analyses in the future. Examples of specific secondary outcomes that could be reported are summarized in Tables 10 and 12.
Unanswered Questions in AF Ablation
There is still much to be learned about the mechanisms of AF, techniques of AF ablation, and long-term outcomes. The following are unanswered questions for future investigation:
AF ablation and modification of stroke risk and need for ongoing OAC: The CHA2DS2-VASc score was developed for patients with clinical AF. If a patient has received a successful ablation such that he/she no longer has clinical AF (subclinical, or no AF), then what is the need for ongoing OAC? Are there any patients in whom successful ablation could lead to discontinuation of OAC?
Substrate modification in catheter-based management of AF—particularly for persistent AF: What is the proper lesion set required beyond PVI? Do lines and CFAE have any remaining role? Are these approaches ill-advised or simply discouraged?
What is the role of targeting localized rotational activations? How do we ablate a localized rotational activation? How can scar be characterized and targeted for ablation? Do we need to replicate the MAZE procedure? Does the RA need to be targeted as well as the LA?
(3) Autonomic influence in AF: Is clinical AF really an autonomic mediated arrhythmia? Is elimination of GP required? Is there a role for autonomic modulation, for example, spinal cord or vagal stimulation?
(4) Contribution and modulation of risk factors on outcomes of AF ablation: Obesity reduction has been shown to reduce AF burden and recurrence in patients undergoing ablation. What is the role of bariatric surgery?
Does the modulation of other risk factors influence outcome such as hypertension, sleep apnea, and diabetes?
(5) Outcomes in ablation of high-risk populations: Do high-risk populations benefit from AF ablation?
Congestive HF has been assessed in smaller trials, but larger trials are required. Outcome data are needed in patients with very enlarged LAs, HCM, patients with renal failure on dialysis, and the very elderly.
(6) Surgical vs catheter-based vs hybrid ablation: There should be more comparative work between percutaneous and minimally invasive surgical approaches. Both report similar outcomes, but there is a dearth of comparative data. Is there any patient benefit to hybrid procedures?
(7) How do we characterize patients who are optimal candidates for ablation? Preablation LGE-MRI might identify patients with heavy burdens of scar who are unlikely to respond to ablation. These techniques must become reproducible and reliable and must be assessed in multicenter trials. Other markers need to be investigated, including genetic markers, biochemical markers, and clinical markers based on aggregated risk scores.
(8) The incremental role of new technologies: As newer and often more expensive technologies are produced for AF ablation, their definitive incremental value must be determined in order to justify change in practice or case cost. These technologies include global (basket) mapping techniques, newer ablation indices for assessing lesion durability, advanced imaging for viewing lesions in the myocardium, etc. New energy sources, including laser, low-intensity ultrasound, photonic particle therapy, external beam ablation, and MRI-guided ablation, must be assessed in comparative fashion.
(9) Outcomes of AF ablation: We need to better understand the clinical relevance of ablation outcomes. What is the significance of time to recurrence of 30 seconds of arrhythmia? How do we best quantify AF burden?
How do these outcomes relate to QOL and stroke risk?
(10) What is the role of surgical LA reduction? Does LAA occlusion or obliteration improve outcome of persistent AF ablation with an accompanying reduction in stroke? Does ablation work through atrial size reduction? What is the incidence of “stiff atrial” syndrome and does this mitigate the clinical impact of ablation?
(11) Working in teams: What is the role of the entire heart team in AF ablation? Does a team approach achieve better outcomes than a “silo” approach?
(12) Improving the safety of catheter ablation: As ablation extends to more operators and less experienced operators, the statistical occurrence of complications will increase. We need newer techniques to minimize complications and institute standards for operators to improve the reproducibility of ablation results and safety profiles at a variety of centers worldwide.
(13) How does catheter ablation affect mortality, stroke, and hospitalization in broad and selected patient populations receiving catheter ablation for AF?
(14) Management of patients who fail initial attempts at catheter ablation: Should there be specific criteria for repeat ablations (e.g., atrial size, BMI)? Should patients be referred for surgery for repeat ablation?
In order to address these and other important questions in the field of catheter and surgical AF ablation, we urge investigators to create and participate in multisite collaborations and electrophysiology research networks with involvement of senior and junior investigators on the steering committees to push forward the next phase of AF research. We also urge funding bodies to support these important initiatives.
Section 14: Conclusion
Catheter ablation of AF is a very commonly performed procedure in hospitals throughout the world. Surgical ablation of AF, although less widely available than catheter-based AF ablation, is also an important therapeutic option for patients with AF at many major medical centers. This document provides an up-to-date review of the indications, techniques, and outcomes of catheter and surgical ablation of AF. Areas for which a consensus can be reached concerning AF ablation are identified, and a series of consensus definitions have been developed for use in future clinical trials of AF ablation. Also included within this document are recommendations concerning indications for AF ablation, technical performance of this procedure, and training. It is our hope to improve patient care by providing a foundation for those involved with care of patients with AF as well as those who perform AF ablation. It is recognized that this field continues to evolve rapidly and that this document will need to be updated. Successful AF ablation programs optimally should consist of a cooperative team of cardiologists, electrophysiologists, and surgeons to ensure appropriate indications, procedure selection, and follow-up.
Acknowledgments
The authors acknowledge the support of Jun Dong, MD, PhD; Kan Fang, MD; and Mark Fellman at the Division of Cardiovascular Devices, Center for Devices and Radiological Health, U.S. Food and Drug Administration (FDA) during the preparation of this document. This document does not necessarily represent the opinions, policies, or recommendations of the FDA.
Appendix A.
Author disclosure table
| Writing group member | Institution | Consultant/Advisory board/Honoraria | Speakers’ bureau | Research grant | Fellowship support | Stock options/Partner | Board Mbs/Other |
|---|---|---|---|---|---|---|---|
| Hugh Calkins, MD (Chair) | Johns Hopkins Medical Institutions, Baltimore, MD | 1: Abbott Laboratories, 1: AtriCure, Inc., 1: Boston Scientific Corp., 1: Pfizer Inc., 1: St. Jude Medical, 1: Toray Industries Inc., 2: iRhythm, 3: Boehringer Ingelheim, 3: Medtronic, Inc. | None | 2: Medtronic, Inc., 2: Boston Scientific Corp. | None | None | None |
| Gerhard Hindricks, MD (Vice-Chair) | Heart Center Leipzig, Leipzig, Germany | None | None | 1: SIEMENS, 3: Biosense Webster, Inc., 3: Stereotaxis, Inc., 4: BIOTRONIK, 5: Boston Scientific Corp., 5: St. Jude Medical | None | None | None |
| Riccardo Cappato, MD (Vice-Chair) | Humanitas Research Hospital, Arrhythmias and Electrophysiology Research Center, Milan, Italy* | None | None | None | None | None | None |
| Young-Hoon Kim, MD, PhD (Vice-Chair) | Korea University, Seoul, South Korea | None | 1: St. Jude Medical | 2: St. Jude Medical | None | None | None |
| Eduardo B. Saad, MD, PhD (Vice-Chair) | Hospital Pro-Cardiaco and Hospital Samaritano, Botafogo, Rio de Janeiro, Brazil | None | None | None | None | None | None |
| Luis Aguinaga, MD, PhD | Centro Privado de Cardiología, Tucuman, Argentina | None | None | None | None | None | None |
| Joseph G. Akar, MD, PhD | Yale University School of Medicine, New Haven, CT | 1: Biosense Webster | None | None | None | None | None |
| Vinay Badhwar, MD | West Virginia University School of Medicine, Morgantown, WV | None | None | None | None | None | None |
| Josep Brugada, MD, PhD | Cardiovascular Institute, Hospital Clínic, University of Barcelona, Catalonia, Spain | None | None | None | None | None | None |
| John Camm, MD | St. George's University of London, London, United Kingdom | 1: Actelion Pharmaceuticals, 1: Daiichi-Sankyo, 1: Eli Lilly, 1: Gilead Sciences, Inc., 1: Heart Metabolics, 1: InCarda Therapeutics, 1: InfoBionic, 1: Johnson and Johnson, 1: Medtronic, Inc., 1: Milestone, 1: Pfizer, Inc., 2: Boehringer Ingelheim, 2: Boston Scientific Corp., 2: Novartis 3: Bayer HealthCare, LLC | 1: Daiichi-Sankyo, 1: Servier, 2: Bayer/Schering Pharma, 2: Boehringer Ingelheim | 3: Boehringer Ingelheim, 3: Daiichi-Sankyo, 3: Pfizer, Inc. | None | None | 0: European Heart Rhythm Association, 1: Oxford |
| Peng-Sheng Chen, MD | Indiana University School of Medicine, Indianapolis, IN | None | None | 5: National Institutes of Health | None | 5: Arrhythmotech | None |
| Shih-Ann Chen, MD | National Yang-Ming University, Taipei, Taiwan | 1: Bayer/Schering Pharma, 1: Biosense Webster, 1: Boehringer Ingelheim, 1: Boston Scientific Corp., 1: Daiichi-Sankyo, 1: Medtronic Inc., 1: Pfizer Inc., 1: St. Jude Medical | 1: St. Jude Medical | 2: Biosense Webster, 2: St. Jude Medical | None | None | None |
| Mina K. Chung, MD | Cleveland Clinic, Cleveland, OH | 0: Amarin, 0: BIOTRONIK, 0: Boston Scientific Corp., 0: Medtronic, Inc., 0: St. Jude Medical, 0: Zoll Medical Corporation | 1: American College of Cardiology | None | None | None | 1: Up to Date |
| Jens Cosedis Nielsen, DMSc, PhD | Aarhus University Hospital, Skejby, Denmark | None | None | 5. Novo Nordisk Foundation | None | None | None |
| Anne B. Curtis, MD | University at Buffalo, Buffalo, NY | 1: Daiichi-Sankyo, 1: Medtronic, Inc., 1: Projects in Knowledge, 2: St. Jude Medical | None | None | None | None | None |
| D. Wyn Davies, MD | Imperial College Healthcare NHS Trust, London, United Kingdom | 1: Boston Scientific Corp., 1: Janssen Pharmaceuticals, 1: Medtronic, Inc., 1: Rhythmia Medical | None | None | None | 3: Rhythmia Medical | None |
| John D. Day, MD | Intermountain Medical Center Heart Institute, Salt Lake City, UT | 1: BIOTRONIK, 1: Boston Scientific Corp., 3: St. Jude Medical | None | None | None | None | None |
| André d’Avila, MD, PhD | Hospital SOS Cardio, Florianopolis, SC, Brazil | None | 0: BIOTRONIK, 0: St. Jude Medical | 0: BIOTRONIK, 0: St. Jude Medical | None | None | None |
| N.M.S. (Natasja) de Groot, MD, PhD | Erasmus Medical Center, Rotterdam, the Netherlands | None | None | None | None | None | None |
| Luigi Di Biase, MD, PhD | Albert Einstein College of Medicine, Montefiore-Einstein Center for Heart & Vascular Care, Bronx, NY | 1: Atricure, 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: Boston Scientific Corp., 1: EpiEP, 1: Medtronic, Inc., 1: St. Jude Medical, 1: Stereotaxis, Inc. | None | None | None | None | None |
| Mattias Duytschaever, MD, PhD | Universitair Ziekenhuis Gent (Ghent University Hospital), Ghent, Belgium | None | None | None | None | None | None |
| James R. Edgerton, MD | The Heart Hospital, Baylor Plano, Plano, TX | 2: AtriCure, Inc. | 1: AtriCure, Inc. | 2: AtriCure, Inc. | None | None | None |
| Kenneth A. Ellenbogen, MD | Virginia Commonwealth University School of Medicine, Richmond, VA | 1: American Heart Association, 1: Heart Rhythm Society, 2: Boston Scientific Corp. | 1: AtriCure, Inc., 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: St. Jude Medical, 2: Boston Scientific Corp., 2: Medtronic, Inc. | 2: Biosense Webster, Inc., 2: Daiichi-Sankyo, 2: National Institutes of Health, 4: Boston Scientific Corp., 4: Medtronic, Inc. | None | None | 1: Elsevier, 1: Wiley-Blackwell |
| Patrick T. Ellinor, MD, PhD | Massachusetts General Hospital, Boston, MA | 1: Bayer HealthCare, LLC, 1: Quest Diagnostics | None | 1: Leducq Foundation, 3: American Heart Association, 3: National Institutes of Health, 5: Bayer HealthCare, LLC | None | None | None |
| Sabine Ernst, MD, PhD | Royal Brompton and Harefield NHS Foundation Trust, National Heart and Lung Institute, Imperial College London, London, United Kingdom | 2: Biosense Webster, Inc. | None | 4: Spectrum Dynamics | None | None | None |
| Guilherme Fenelon, MD, PhD | Albert Einstein Jewish Hospital, Federal University of São Paulo, São Paulo, Brazil | 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: St. Jude Medical | None | None | None | None | None |
| Edward P. Gerstenfeld, MS, MD | University of California, San Francisco, San Francisco, CA | 1: Boehringer Ingelheim, 1: Boston Scientific Corp., 1: Medtronic, Inc., 1: St. Jude Medical | None | 4: Biosense Webster, Inc., 4: St. Jude Medical | 2: Biosense Webster, Inc., 2: BIOTRONIK, 2: Boston Scientific Corp., 2: Medtronic, Inc. | 1: Rhythm Diagnostic Systems Inc. | None |
| David E. Haines, MD | Beaumont Health System, Royal Oak, MI | 1: Lake Region Medical, 1: Terumo Medical Corp | None | None | None | None | 1: Biosense Webster, Inc., 1: Boston Scientific Corp., 1: Medtronic, Inc., 1: St. Jude Medical |
| Michel Haissaguerre, MD | Hôpital Cardiologique du Haut-Lévêque, Pessac, France | None | None | None | None | None | None |
| Robert H. Helm, MD | Boston University Medical Center, Boston, MA | None | None | None | None | None | 1: Boston Scientific Corp. |
| Elaine Hylek, MD, MPH | Boston University School of Medicine, Boston, MA | 1: Bayer, 1: Boehringer Ingelheim, 1: Bristol-Myers Squibb, 1: Daiichi-Sankyo, 1: Medtronic, 1: Portola, 1: Pfizer | None | 2: Janssen Pharmaceuticals | None | None | None |
| Warren M. Jackman, MD | Heart Rhythm Institute, University of Oklahoma Health Sciences Center, Oklahoma City, OK | 1: ACT, 1: VytronUS, Inc., 2: Biosense Webster, Inc., 2: Boston Scientific Corp., 2: Spectrum Dynamics | 1: BIOTRONIK, 1: St. Jude Medical, 2: Biosense Webster, Inc., 2: Boston Scientific Corp. | None | None | None | None |
| Jose Jalife, MD | University of Michigan, Ann Arbor, MI, the National Center for Cardiovascular Research Carlos III (CNIC) and CIBERCV, Madrid, Spain | 1: Topera Medical | None | 1: Medtronic, Inc. | None | None | None |
| Jonathan M. Kalman, MBBS, PhD | Royal Melbourne Hospital and University of Melbourne, Melbourne, Australia | None | 1: Boston Scientific Corp., 1: Medtronic, Inc. | 4: Medtronic, Inc. | 3: St. Jude Medical, 4: Biosense Webster, Inc., 4: Medtronic, Inc. | None | 2: Biosense Webster, Inc., 4: Boston Scientific Corp. |
| Josef Kautzner, MD, PhD | Institute for Clinical and Experimental Medicine, Prague, Czech Republic | 1: Bayer/Schering Pharma, 1: Boehringer Ingelheim, 1: Boston Scientific Corp., 1: Daiichi-Sankyo, 1: Sorin Group, 1: St. Jude Medical, 1: Biosense Webster, Inc., 2: Medtronic, Inc. | 1: BIOTRONIK1: Medtronic, Inc. 1: St. Jude Medical | None | None | None | None |
| Hans Kottkamp, MD | Hirslanden Hospital, Dept. of Electrophysiology, Zurich, Switzerland | 1: Biosense Webster, Inc., 1: Kardium | None | None | None | 1: Kardium | None |
| Karl Heinz Kuck, MD, PhD | Asklepios Klinik St. Georg, Hamburg, Germany | 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: St. Jude Medical, 1: Stereotaxis, Inc. | None | 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: St. Jude Medical, 1: Stereotaxis, Inc. | None | 1: Endosense | None |
| Koichiro Kumagai, MD, PhD | Heart Rhythm Center, Fukuoka Sanno Hospital, Fukuoka, Japan | None | None | None | None | None | None |
| Richard Lee, MD, MBA | Saint Louis University Medical School, St. Louis, MO | None | None | None | None | None | None |
| Thorsten Lewalter, MD, PhD | Dept. of Cardiology and Intensive Care, Hospital Munich-Thalkirchen, Munich, Germany | 1: BIOTRONIK, 1: Medtronic, Inc., 1: St. Jude Medical | 1: Abbott Vascular, 1: BIOTRONIK, 1: Medtronic, Inc., 1: St. Jude Medical | None | None | None | None |
| Bruce D. Lindsay, MD | Cleveland Clinic, Cleveland, OH | 0: Medtronic, Inc., 1: Abbott Vascular, 1: Biosense Webster, Inc. | None | None | 3: Boston Scientific Corp., 3: Medtronic, Inc., 3: St. Jude Medical | None | None |
| Laurent Macle, MD | Montreal Heart Institute, Department of Medicine, Université de Montréal, Montréal, Canada | 1: Bayer HealthCare, LLC, 1: Biosense Webster, Inc., 1: Boehringer Ingelheim, 1: Bristol-Myers Squibb, 1: Medtronic, Inc., 1: Pfizer, Inc., 1: Servier, 1: St. Jude Medical | None | 4: Biosense Webster, Inc., 5: St. Jude Medical | None | None | None |
| Moussa Mansour, MD | Massachusetts General Hospital, Boston, MA | 1: Biosense Webster, Inc., 1: St. Jude Medical | None | 4: Biosense Webster, Inc., 4: St. Jude Medical, 5: Pfizer, 5: Boehringer Ingelheim | None | 4: NewPace Ltd. | None |
| Francis E. Marchlinski, MD | Hospital of the University of Pennsylvania, University of Pennsylvania School of Medicine, Philadelphia, PA | 1: Abbot Medical; 1: Biosense Webster, Inc., 2: BIOTRONIK, 1: Medtronic, Inc., 1: Boston Scientific Corp., 1: St. Jude Medical | None | 3: Medtronic, Inc., 4: Biosense Webster, Inc. | 1: BIOTRONIK, 3: Boston Scientific Corp., 3: Medtronic, Inc., 4: Biosense Webster, Inc., 5: St. Jude Medical | None | None |
| Gregory F. Michaud, MD | Brigham and Women's Hospital, Boston, MA | 1: Biosense Webster, Inc., 1: Boston Scientific Corp., 1: Medtronic, Inc., 1: St. Jude Medical | None | 4: Biosense Webster, Inc., 4: Boston Scientific Corp. | None | None | None |
| Hiroshi Nakagawa, MD, PhD | Heart Rhythm Institute, University of Oklahoma Health Sciences Center, Oklahoma City, OK | 2: Biosense Webster, Inc 1: Boston Scientific Corp., 2: Stereotaxis, Inc., 3: Japan Lifeline, 3: Fukuda Denshi | 1: Medtronic, Inc, 2: Boston Scientific Corp., 1: Spectrum Dynamics | 4: Biosense Webster, Inc., 2: Japan Lifeline, 2: Affera | None | None | None |
| Andrea Natale, MD | Texas Cardiac Arrhythmia Institute, St. David's Medical Center, Austin, TX | 1: Boston Scientific Corp., 1: Janssen Pharmaceuticals, 1: Medtronic, Inc., 1: St. Jude Medical, 2: Biosense Webster, Inc. | None | None | None | None | None |
| Stanley Nattel, MD | Montreal Heart Institute and Université de Montréal, Montreal, Canada, McGill University, Montreal, Canada, and University Duisburg-Essen, Essen, Germany | 1: Merck Pharmaceuticals, 1: Xention Discovery | None | 3: OMEICOS Therapeutics | None | None | 0: Montreal Heart Institute/Inventor Patents |
| Ken Okumura, MD, PhD | Division of Cardiology, Saiseikai Kumamoto Hospital, Kumamoto, Japan | 1: Biosense Webster, Inc., 1: Boehringer Ingelheim, 1: Bristol-Myers Squibb, 1: Medtronic, Inc., 2: Bayer/Schering Pharma, 3: Daiichi-Sankyo | None | 2: Biosense Webster, Inc., 2: Medtronic, Inc. | None | None | None |
| Douglas Packer, MD | Mayo Clinic, Rochester, MN | 0: Abbott Laboratories, 0: Abiomed, 0: Aperture Diagnostics, 0: Biosense Webster, Inc., 0: Boston Scientific Corp., 0: CardioFocus, Inc., 0: CardioInsight Technologies, 0: Johnson and Johnson, 0: Johnson and Johnson Healthcare Systems, 0: MediaSphere Medical, LLC, 0: Medtronic CryoCath, 0: SIEMENS, 0: St. Jude Medical | None | 0: American Heart Association, 0: Boston Scientific/EPT, 0: CardioInsight, 0: Endosense, 0: SIEMENS Acuson, 0: SIEMENS Acunav, 1: CardioFocus, 1: Hansen Medical, 1: Medtronic, Inc. 2: National Institutes of Health, 3: Thermedical (EP Limited), 5: Biosense Webster, 5: St. Jude Medical | None | None | 1: Medtronic, 1: Oxford Press (Royalty), 1: SIEMENS, 1: WebMD, 1: Wiley-Blackwell (Royalty), 2: Biosense Webster, 4: St. Jude Medical (Royalty) |
| Evgeny Pokushalov, MD, PhD | State Research Institute of Circulation Pathology, Novosibirsk, Russia | 1: Biosense Webster, Inc., 1: Boston Scientific Corp., 1: Medtronic, Inc. | None | None | None | None | None |
| Matthew R. Reynolds, MD, MSc | Lahey Hospital and Medical Center, Burlington, MA | 1: Biosense Webster, Inc., 1: Medtronic, Inc., 1: St. Jude Medical | None | None | None | None | None |
| Prashanthan Sanders, MBBS, PhD | Centre for Heart Rhythm Disorders, South Australian Health and Medical Research Institute, University of Adelaide and Royal Adelaide Hospital, Adelaide, Australia | 1: Biosense Webster, Inc., 1: Boston Scientific Corp., 1: CathRx, 1: Medtronic, Inc., 1: St. Jude Medical | 1: Biosense Webster, Inc., 1: Boston Scientific Corp., 1: Medtronic, Inc., 1: St. Jude Medical | 4: Sorin Group, 5: BIOTRONIK, 5: Boston Scientific Corp., 5: Medtronic, Inc., 5: St. Jude Medical | None | None | None |
| Mauricio Scanavacca, MD, PhD | Instituto do Coração (InCor), São Paulo, Brazil | 1: Biosense Webster, Inc., 1: St. Jude Medical | 1: Bayer/Schering Pharma, 1: Bristol-Myers Squibb, 1: Johnson and Johnson, 1: Daiichi-Sankyo | 2: Johnson and Johnson | 2: Johnson and Johnson | None | None |
| Richard Schilling, MD | Barts Heart Centre, London, United Kingdom | 1: Biosense Webster, Inc., 1: Boehringer Ingelheim, 1: Daiichi-Sankyo, 1: Hansen Medical, 1: Medtronic, Inc., 1: St. Jude Medical | None | 1: Boston Scientific Corp., 1: Hansen Medical, 1: Medtronic, Inc., 1: St. Jude Medical, 4: Boston Scientific Corp., 4: Medtronic, Inc., 4: St. Jude Medical | None | None | None |
| Claudio Tondo, MD, PhD | Cardiac Arrhythmia Research Center, Centro Cardiologico Monzino, IRCCS, Department of Cardiovascular Sciences, University of Milan, Milan, Italy | None | None | None | None | None | None |
| Hsuan-Ming Tsao, MD | National Yang-Ming University Hospital, Yilan City, Taiwan | None | None | None | None | None | None |
| Atul Verma, MD | Southlake Regional Health Centre, University of Toronto, Toronto, Canada | 1: Bayer HealthCare, LLC, 1: Boehringer Ingelheim | None | 5: Bayer HealthCare, LLC, 5: Biosense Webster, Inc., 5: BIOTRONIK, 5: Medtronic, Inc. | None | None | None |
| David J. Wilber, MD | Loyola University of Chicago, Chicago, IL | 1: Biosense Webster, Inc., 1: Janssen Pharmaceuticals, 1: Medtronic, Inc., 1: St. Jude Medical, 1: Thermedical | None | 1: Abbott Vascular, 1: Medtronic, Inc., 1: St. Jude Medical, 1: Thermedical, 3: Biosense Webster, Inc. | 3: Biosense Webster, Inc., 3: Medtronic, Inc., 3: St. Jude Medical | None | 1: Elsevier, 1: Wiley-Blackwell, 4: American College of Cardiology Foundation |
| Teiichi Yamane, MD, PhD | Jikei University School of Medicine, Tokyo, Japan | 1: Bayer HealthCare, 1: Medtronic, 2: Abott Japan, 2: Daiichi-Sankyo, 2: Boehringer Ingelheim, 2: Bristol-Myers Squibb | None | 1: Boehringer Ingelheim, 1: Bayer HealthCare | None | None | None |
Number Value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000.
*Dr. Cappato is now with the Department of Biomedical Sciences, Humanitas University, Milan, Italy, and IRCCS, Humanitas Clinical and Research Center, Milan, Italy.
Appendix B.
Reviewer disclosure table
| Peer reviewer | Institution | Consultant/Advisory board/Honoraria | Speakers’ bureau | Research grant | Fellowship support | Stock options/ Partner | Board Mbs/Other |
|---|---|---|---|---|---|---|---|
| Carina Blomström-Lundqvist, MD, PhD | Department of Cardiology and Medical Science, Uppsala University, Uppsala, Sweden | 1: Bayer/Schering Pharma, 1: Boston Scientific Corp., 1: Medtronic, Inc., 1: Sanofi, 1: Pfizer, MSD, Bristol-Myers Squibb, Biosense Webster, Inc. | None | 1: Cardiome Pharma/ Astellas, 1: Medtronic, Inc. | None | None | None |
| Angelo A.V. De Paola, MD, PhD | Hospital São Paulo – Federal University of São Paulo, São Paulo, Brazil | None | None | None | None | None | None |
| Peter M. Kistler, MBBS, PhD | The Alfred Hospital Heart Centre, Melbourne, Australia | None | 1: St. Jude Medical | None | None | None | None |
| Gregory Y.H. Lip, MD | University of Birmingham, Birmingham, United Kingdom; Aalborg University, Aalborg, Denmark | 1: Medtronic, 3: Bayer/Janssen, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo | 3: Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo. No fees are received personally | None | None | None | None |
| Nicholas S. Peters, MD | St Mary's Hospital, Imperial College London, London, United Kingdom | 1: Boston Scientific Corp., 1: Cardialen, Inc., 1: Cardiologs, 1: Magnetecs, 1: Medtronic, Inc., 1: St. Jude Medical | None | None | None | None | None |
| Cristiano F. Pisani, MD | InCor, Heart Insitute, HCFMUSP, Arrhythmia Unit | None | None | None | None | None | None |
| Antonio Raviele, MD | ALFA-Alliance to Fight Atrial Fibrillation, Rimini, Italy | None | None | None | None | None | None |
| Eduardo B. Saad, MD, PhD | Hospital Pro-Cardiaco and Hospital Samaritano, Botafogo, Rio de Janeiro, Brazil | None | None | None | None | None | None |
| Kazuhiro Satomi, MD, PhD | Tokyo Medical University, Tokyo, Japan | 1: Bayer/Schering Pharma, 1: Boehringer Ingelheim, 1: Bristol-Myers Squibb, 1: Japan Lifeline, 1: Johnson and Johnson, 1: Medtronic, Inc., 1: Sankyo Pharmaceuticals, 1: St. Jude Medical | None | None | None | None | None |
| Martin K. Stiles, MB ChB, PhD | Waikato Hospital, Hamilton, New Zealand | 1: Boston Scientific Corp., 1: Biosense Webster, Inc., 1: BIOTRONIK, 1: Medtronic, Inc. | None | None | 1: Medtronic, Inc. | None | None |
| Stephan Willems, MD, PhD | University Medical Center Hamburg-Eppendorf, Hamburg, Germany | 1: Bayer HealthCare, LLC, 1: Biosense Webster, Inc., 1: Boehringer Ingelheim, 1: Bristol-Myers Squibb, 1: Sanofi, 1: St. Jude Medical, 1: Medtronic | None | None | None | None | None |
Number Value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000.
Contributor Information
Document Reviewers::
Carina Blomström-Lundqvist, Angelo A V De Paola, Peter M Kistler, Gregory Y H Lip, Nicholas S Peters, Cristiano F Pisani, Antonio Raviele, Eduardo B Saad, Kazuhiro Satomi, Martin K Stiles, and Stephan Willems
References
- 1. Calkins H, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2007;4(6):816–861. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 2012;9(4):632–696.e21. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs AK, Anderson JL, Halperin JL.. The evolution and future of ACC/AHA clinical practice guidelines: a 30-year journey: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64(13):1373–1384. [DOI] [PubMed] [Google Scholar]
- 4. Anderson JL. Evolution of the ACC/AHA clinical practice guidelines in perspective: guiding the guidelines. J Am Coll Cardiol 2015;65(25):2735–2738. [DOI] [PubMed] [Google Scholar]
- 5. January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016;50(5):e1–e88. [DOI] [PubMed] [Google Scholar]
- 7. Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12(10):1360–1420. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd-Jones DM, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110(9):1042–1046. [DOI] [PubMed] [Google Scholar]
- 9. Benjamin EJ, et al. Independent risk factors for atrial fibrillation in a population-based cohort: The Framingham Heart Study. JAMA 1994;271(11):840–844. [PubMed] [Google Scholar]
- 10. Miller JD, et al. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol 2015;66(25):2899–2906. [DOI] [PubMed] [Google Scholar]
- 11. EHRA Scientific Committee Task Force, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 2017;19(2):190–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allan V, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost 2017;117(5):837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Vos CB, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55(8):725–731. [DOI] [PubMed] [Google Scholar]
- 14. Wijffels MC, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92(7):1954–1968. [DOI] [PubMed] [Google Scholar]
- 15. Walters TE, et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: Implications for early ablative intervention. Heart Rhythm 2016;13(2):331–339. [DOI] [PubMed] [Google Scholar]
- 16. Wakili R, et al. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121(8):2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heijman J, et al. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114(9):1483–1499. [DOI] [PubMed] [Google Scholar]
- 18. Kopecky SL, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med 1987;317(11):669–674. [DOI] [PubMed] [Google Scholar]
- 19. Kochhauser S, et al. Predictors for progression of atrial fibrillation in patients awaiting atrial fibrillation ablation. Can J Cardiol 2016;32(11):1348–1354. [DOI] [PubMed] [Google Scholar]
- 20. Sugihara C, et al. The development of AF over time in patients with permanent pacemakers: objective assessment with pacemaker diagnostics demonstrates distinct patterns of AF. Europace 2015;17(6):864–870. [DOI] [PubMed] [Google Scholar]
- 21. Fox CS, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004;291(23):2851–2855. [DOI] [PubMed] [Google Scholar]
- 22. Arnar DO, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J 2006;27(6):708–712. [DOI] [PubMed] [Google Scholar]
- 23. Lubitz SA, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA 2010;304(20):2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christophersen IE, Ellinor PT.. Genetics of atrial fibrillation: from families to genomes. J Hum Genet 2016;61(1):61–70. [DOI] [PubMed] [Google Scholar]
- 25. Gudbjartsson DF, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448(7151):353–357. [DOI] [PubMed] [Google Scholar]
- 26. Benjamin EJ, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet 2009;41(8):879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gudbjartsson DF, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet 2009;41(8):876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellinor PT, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 2010;42(3):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellinor PT, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 2012;44(6):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinner MF, et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 2014;130(15):1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lubitz SA, et al. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol 2014;63(12):1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tada H, et al. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke 2014;45(10):2856–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Everett BM, et al. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J 2013;34(29):2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lubitz SA, et al. Genetic risk prediction of atrial fibrillation. Circulation 2017;135(14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hucker WJ, et al. Atrial fibrillation genetics: is there a practical clinical value now or in the future? Can J Cardiol 2016;32(11):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shoemaker MB, et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2015;8(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi EK, et al. Korean Atrial Fibrillation (AF) Network: genetic variants for AF do not predict ablation success. J Am Heart Assoc 2015;4(8):e002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chugh SS, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colilla S, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112(8):1142–1147. [DOI] [PubMed] [Google Scholar]
- 40. Kannel WB, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82(8A):2N–9N. [DOI] [PubMed] [Google Scholar]
- 41. Miller PS, Andersson FL, Kalra L.. Are cost benefits of anticoagulation for stroke prevention in atrial fibrillation underestimated? Stroke 2005;36(2):360–366. [DOI] [PubMed] [Google Scholar]
- 42. Miyasaka Y, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49(9):986–992. [DOI] [PubMed] [Google Scholar]
- 43. Chen LY, Benditt DG, Alonso A.. Atrial fibrillation and its association with sudden cardiac death. Circ J 2014;78(11):2588–2593. [DOI] [PubMed] [Google Scholar]
- 44. Lubitz SA, et al. Atrial fibrillation patterns and risks of subsequent stroke, HF, or death in the community. J Am Heart Assoc 2013;2(5):e000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang TJ, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107(23):2920–2925. [DOI] [PubMed] [Google Scholar]
- 46. Jacobs V, et al. Atrial fibrillation and dementia. Trends Cardiovasc Med 2015;25(1):44–51. [DOI] [PubMed] [Google Scholar]
- 47. Dorian P, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36(4):1303–1309. [DOI] [PubMed] [Google Scholar]
- 48. Boriani G, et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128(5):509–518.e2. [DOI] [PubMed] [Google Scholar]
- 49. Kim MH, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4(3):313–320. [DOI] [PubMed] [Google Scholar]
- 50. Go AS, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 51. Ganesan AN, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37(20):1591–1602. [DOI] [PubMed] [Google Scholar]
- 52. Van Gelder IC, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347(23):1834–1840. [DOI] [PubMed] [Google Scholar]
- 53. Wyse DG, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347(23):1825–1833. [DOI] [PubMed] [Google Scholar]
- 54. Carlsson J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003;41(10):1690–1696. [DOI] [PubMed] [Google Scholar]
- 55. Roy D, et al. Rhythm control versus rate control for atrial fibrillation and HF. N Engl J Med 2008;358(25):2667–2677. [DOI] [PubMed] [Google Scholar]
- 56. Hindricks G, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 2005;112(3):307–313. [DOI] [PubMed] [Google Scholar]
- 57. Vasamreddy CR, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2006;17(2):134–139. [DOI] [PubMed] [Google Scholar]
- 58. Verma A, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173(2):149–156. [DOI] [PubMed] [Google Scholar]
- 59. Rienstra M, et al. Asymptomatic persistent atrial fibrillation and outcome: results of the RACE study. Heart Rhythm 2014;11(6):939–945. [DOI] [PubMed] [Google Scholar]
- 60. Siontis KC, et al. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: Characteristics and prognostic implications. Heart Rhythm 2016;13(7):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reynolds MR, et al. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J 2006;152(6):1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pedrote A, et al. Paroxysmal atrial fibrillation burden before and after pulmonary veins isolation: an observational study through a subcutaneous leadless cardiac monitor. J Cardiovasc Electrophysiol 2013;24(10):1075–1082. [DOI] [PubMed] [Google Scholar]
- 63. Wokhlu A, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55(21):2308–2316. [DOI] [PubMed] [Google Scholar]
- 64. Ho SY, et al. Anatomy of the LA: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10(11):1525–1533. [DOI] [PubMed] [Google Scholar]
- 65. Ho SY, McCarthy KP.. Anatomy of the left atrium for interventional electrophysiologists. Pacing Clin Electrophysiol 2010;33(5):620–627. [DOI] [PubMed] [Google Scholar]
- 66. Ho SY, Cabrera JA, Sanchez-Quintana D.. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol 2012;5(1):220–228. [DOI] [PubMed] [Google Scholar]
- 67. Kato R, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation 2003;107(15):2004–2010. [DOI] [PubMed] [Google Scholar]
- 68. Jongbloed MR, et al. Noninvasive visualization of the cardiac venous system using multislice computed tomography. J Am Coll Cardiol 2005;45(5):749–753. [DOI] [PubMed] [Google Scholar]
- 69. Mlcochova H, et al. Magnetic resonance angiography of pulmonary veins: implications for catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2005;28(10):1073–1080. [DOI] [PubMed] [Google Scholar]
- 70. Nathan H, Eliakim M.. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation 1966;34(3):412–422. [DOI] [PubMed] [Google Scholar]
- 71. Weiss C, et al. Impact of the distribution and structure of myocardium in the pulmonary veins for radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol 2002;25(9):1352–1356. [DOI] [PubMed] [Google Scholar]
- 72. Wang K, et al. Architecture of atrial musculature in humans. Br Heart J 1995;73(6):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anne W, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res 2005;67(4):655–666. [DOI] [PubMed] [Google Scholar]
- 74. Goette A, Rocken C.. Atrial amyloidosis and atrial fibrillation: a gender-dependent “arrhythmogenic substrate”? Eur Heart J 2004;25(14):1185–1186. [DOI] [PubMed] [Google Scholar]
- 75. Pashakhanloo F, et al. Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging. Circ Arrhythm Electrophysiol 2016;9(4):e004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hansen BJ, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36(35):2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haissaguerre M, et al. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. J Physiol 2016;594(9):2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim DT, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol 2000;36(4):1324–1327. [DOI] [PubMed] [Google Scholar]
- 79. Han S, et al. Electrophysiological characteristics of the Marshall bundle in humans. Heart Rhythm 2010;7(6):786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cabrera JA, et al. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J 2008;29(3):356–362. [DOI] [PubMed] [Google Scholar]
- 81. Gittenberger-de Groot AC, et al. The role of neural crest and epicardium-derived cells in conduction system formation. Novartis Found Symp 2003;250:125–134. discussion 134-41, 276-9. [DOI] [PubMed] [Google Scholar]
- 82. Jongbloed MR, et al. Embryonic conduction tissue: a spatial correlation with adult arrhythmogenic areas. J Cardiovasc Electrophysiol 2004;15(3):349–355. [DOI] [PubMed] [Google Scholar]
- 83. Perez-Lugones A, et al. Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J Cardiovasc Electrophysiol 2003;14(8):803–809. [DOI] [PubMed] [Google Scholar]
- 84. Ehrlich JR, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 2003;551(Pt 3):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen YC, et al. Heterogeneous expression of potassium currents and pacemaker currents potentially regulates arrhythmogenesis of pulmonary vein cardiomyocytes. J Cardiovasc Electrophysiol 2009;20(9):1039–1045. [DOI] [PubMed] [Google Scholar]
- 86. Verheule S, et al. Tissue structure and connexin expression of canine pulmonary veins. Cardiovasc Res 2002;55(4):727–738. [DOI] [PubMed] [Google Scholar]
- 87. Honjo H, et al. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation 2003;107(14):1937–1943. [DOI] [PubMed] [Google Scholar]
- 88. Levin MD, et al. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J Clin Invest 2009;119(11):3420–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wongcharoen W, et al. Effects of a Na+/Ca2+ exchanger inhibitor on pulmonary vein electrical activity and ouabain-induced arrhythmogenicity. Cardiovasc Res 2006;70(3):497–508. [DOI] [PubMed] [Google Scholar]
- 90. Weerasooriya R, et al. Dissociated pulmonary vein arrhythmia: incidence and characteristics. J Cardiovasc Electrophysiol 2003;14(11):1173–1179. [DOI] [PubMed] [Google Scholar]
- 91. Hocini M, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002;105(20):2442–2448. [DOI] [PubMed] [Google Scholar]
- 92. Arora R, et al. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation 2003;107(13):1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ozgen N, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res 2007;75(4):758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kalifa J, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation 2003;108(6):668–671. [DOI] [PubMed] [Google Scholar]
- 95. Jais P, et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 2002;106(19):2479–2485. [DOI] [PubMed] [Google Scholar]
- 96. Chen SA, Tai CT.. Catheter ablation of atrial fibrillation originating from the non-pulmonary vein foci. J Cardiovasc Electrophysiol 2005;16(2):229–232. [DOI] [PubMed] [Google Scholar]
- 97. Kumagai K, et al. Electrophysiologic properties of pulmonary veins assessed using a multielectrode basket catheter. J Am Coll Cardiol 2004;43(12):2281–2289. [DOI] [PubMed] [Google Scholar]
- 98. Sanders P, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005;112(6):789–797. [DOI] [PubMed] [Google Scholar]
- 99. Atienza F, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 2006;114(23):2434–2442. [DOI] [PubMed] [Google Scholar]
- 100. Hou Y, Zhou Q, Po SS.. Neuromodulation for cardiac arrhythmia. Heart Rhythm 2016;13(2):584–592. [DOI] [PubMed] [Google Scholar]
- 101. Chiou CW, Eble JN, Zipes DP.. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation 1997;95(11):2573–2584. [DOI] [PubMed] [Google Scholar]
- 102. Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl) 2005;209(6):425–438. [DOI] [PubMed] [Google Scholar]
- 103. Armour JA, et al. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247(2):289–298. [DOI] [PubMed] [Google Scholar]
- 104. Pauza DH, Skripka V, Pauziene N.. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs 2002;172(4):297–320. [DOI] [PubMed] [Google Scholar]
- 105. Po SS, Nakagawa H, Jackman WM.. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2009;20(10):1186–1189. [DOI] [PubMed] [Google Scholar]
- 106. Hou Y, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol 2007;50(1):61–68. [DOI] [PubMed] [Google Scholar]
- 107. Chevalier P, et al. Quantitative study of nerves of the human LA. Heart Rhythm 2005;2(5):518–522. [DOI] [PubMed] [Google Scholar]
- 108. Tan AY, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol 2006;48(1):132–143. [DOI] [PubMed] [Google Scholar]
- 109. Chen PS, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114(9):1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Scherlag BJ, et al. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol 2005;45(11):1878–1886. [DOI] [PubMed] [Google Scholar]
- 111. Po SS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm 2006;3(2):201–208. [DOI] [PubMed] [Google Scholar]
- 112. Jayachandran JV, et al. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation 2000;101(10):1185–1191. [DOI] [PubMed] [Google Scholar]
- 113. Lu Z, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 2008;1(3):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Patterson E, et al. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2(6):624–631. [DOI] [PubMed] [Google Scholar]
- 115. Zhou J, et al. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol 2007;18(1):83–90. [DOI] [PubMed] [Google Scholar]
- 116. Choi EK, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 2010;121(24):2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lemery R, et al. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006;3(4):387–396. [DOI] [PubMed] [Google Scholar]
- 118. Scanavacca M, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation 2006;114(9):876–885. [DOI] [PubMed] [Google Scholar]
- 119. Katritsis D, et al. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol 2008;102(3):330–334. [DOI] [PubMed] [Google Scholar]
- 120. Pokushalov E, et al. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm 2009;6(9):1257–1264. [DOI] [PubMed] [Google Scholar]
- 121. Katritsis DG, et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 2011;8(5):672–678. [DOI] [PubMed] [Google Scholar]
- 122. Katritsis DG, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62(24):2318–2325. [DOI] [PubMed] [Google Scholar]
- 123. Driessen AH, et al. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J Am Coll Cardiol 2016;68(11):1155–1165. [DOI] [PubMed] [Google Scholar]
- 124. Nakagawa H, et al. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009;6(12 Suppl):S26–S34. [DOI] [PubMed] [Google Scholar]
- 125. Chen J, Wasmund SL, Hamdan MH.. Back to the future: the role of the autonomic nervous system in atrial fibrillation. Pacing Clin Electrophysiol 2006;29(4):413–421. [DOI] [PubMed] [Google Scholar]
- 126. Bauer A, et al. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm 2006;3(12):1428–1435. [DOI] [PubMed] [Google Scholar]
- 127. Pokushalov E, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60(13):1163–1170. [DOI] [PubMed] [Google Scholar]
- 128. Pokushalov E, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one-year follow-up of a randomized pilot study. Circ Arrhythm Electrophysiol 2015;8(6):1334–1341. [DOI] [PubMed] [Google Scholar]
- 129. Tsai WC, et al. Effects of renal sympathetic denervation on the stellate ganglion and brain stem in dogs. Heart Rhythm 2017;14(2):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mahnkopf C, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm 2010;7(10):1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol 2012;23(7):797–799. [DOI] [PubMed] [Google Scholar]
- 132. Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J 2013;34(35):2731–2738. [DOI] [PubMed] [Google Scholar]
- 133. Cochet H, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26(5):484–492. [DOI] [PubMed] [Google Scholar]
- 134. Chrispin J, et al. Clinical predictors of cardiac magnetic resonance late gadolinium enhancement in patients with atrial fibrillation. Europace 2017;19(3):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Konrad T, et al. Primary persistent atrial fibrillation: a distinct arrhythmia subentity of an ablation population. J Cardiovasc Electrophysiol 2015;26(12):1289–1294. [DOI] [PubMed] [Google Scholar]
- 136. Boldt A, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004;90(4):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. de Oliveira IM, et al. Fibrosis, myocardial crossings, disconnections, abrupt turns, and epicardial reflections: do they play an actual role in human permanent atrial fibrillation? A controlled necropsy study. Cardiovasc Pathol 2013;22(1):65–69. [DOI] [PubMed] [Google Scholar]
- 138. Smorodinova N, et al. Bioptic study of left and right atrial interstitium in cardiac patients with and without atrial fibrillation: interatrial but not rhythm-based differences. PLoS One 2015;10(6):e0129124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Platonov PG, et al. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58(21):2225–2232. [DOI] [PubMed] [Google Scholar]
- 140. Kottkamp H, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2016;27(1):22–30. [DOI] [PubMed] [Google Scholar]
- 141. Andrade J, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114(9):1453–1468. [DOI] [PubMed] [Google Scholar]
- 142. Andrade JG, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol 2014;7(1):69–75. [DOI] [PubMed] [Google Scholar]
- 143. Andrade J, et al. The time course of exit and entrance block during cryoballoon pulmonary vein isolation. Europace 2014;16(4):500–504. [DOI] [PubMed] [Google Scholar]
- 144. Swartz MF, et al. Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. J Am Coll Cardiol 2012;60(18):1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Akoum N, et al. Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J Cardiovasc Electrophysiol 2012;23(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kottkamp H, Schreiber D.. The Substrate in “Early Persistent” Atrial Fibrillation: Arrhythmia Induced, Risk Factor Induced, or From a Specific Fibrotic Atrial Cardiomyopathy? JACC: Clinical Electrophysiology 2016;2(2):140–142. [DOI] [PubMed] [Google Scholar]
- 147. Jalife J, et al. Mechanisms of atrial fibrillation: mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol 1998;9(8 Suppl):S2–S12. [PubMed] [Google Scholar]
- 148. Goette A, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18(10):1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nattel S, et al. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis 2005;48(1):9–28. [DOI] [PubMed] [Google Scholar]
- 150. Potpara TS, et al. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade Atrial Fibrillation study. Chest 2012;141(2):339–347. [DOI] [PubMed] [Google Scholar]
- 151. Holmqvist F, et al. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart 2015;101(11):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Veasey RA, et al. The natural history of atrial fibrillation in patients with permanent pacemakers: is atrial fibrillation a progressive disease? J Interv Card Electrophysiol 2015;44(1):23–30. [DOI] [PubMed] [Google Scholar]
- 153. Allessie M, Ausma J, Schotten U.. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54(2):230–246. [DOI] [PubMed] [Google Scholar]
- 154. Nattel S, Burstein B, Dobrev D.. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1(1):62–73. [DOI] [PubMed] [Google Scholar]
- 155. Filgueiras-Rama D, et al. Long-term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the LA. Circ Arrhythm Electrophysiol 2012;5(6):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pandit SV, Jalife J.. Rotors and the dynamics of cardiac fibrillation. Circ Res 2013;112(5):849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nattel S. Paroxysmal atrial fibrillation and pulmonary veins: relationships between clinical forms and automatic versus re-entrant mechanisms. Can J Cardiol 2013;29(10):1147–1149. [DOI] [PubMed] [Google Scholar]
- 158. Martins RP, et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129(14):1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wei SK, et al. Muscarinic modulation of the sodium-calcium exchanger in HF. Circulation 2007;115(10):1225–1233. [DOI] [PubMed] [Google Scholar]
- 160. Nattel S, Dobrev D.. The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J 2012;33(15):1870–1877. [DOI] [PubMed] [Google Scholar]
- 161. Corradi D. Atrial fibrillation from the pathologist's perspective. Cardiovasc Pathol 2014;23(2):71–84. [DOI] [PubMed] [Google Scholar]
- 162. Pinho-Gomes AC, et al. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Signal 2014;20(8):1268–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Deshmukh A, et al. Left atrial transcriptional changes associated with atrial fibrillation susceptibility and persistence. Circ Arrhythm Electrophysiol 2015;8(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Schiller M, Javelaud D, Mauviel A.. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 2004;35(2):83–92. [DOI] [PubMed] [Google Scholar]
- 165. He X, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res 2011;108(2):164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Nattel S, Harada M.. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63(22):2335–2345. [DOI] [PubMed] [Google Scholar]
- 167. Ben Amar M, Bianca C.. Towards a unified approach in the modeling of fibrosis: A review with research perspectives. Phys Life Rev 2016;17:61–85. [DOI] [PubMed] [Google Scholar]
- 168. Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol 2014;29(1):20–27. [DOI] [PubMed] [Google Scholar]
- 169. Jalife J, Kaur K.. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med 2015;25(6):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. de Bakker JM, et al. Slow conduction in the infarcted human heart ‘Zigzag’ course of activation. Circulation 1993;88(3):915–926. [DOI] [PubMed] [Google Scholar]
- 171. Bian W, Tung L.. Structure-related initiation of reentry by rapid pacing in monolayers of cardiac cells. Circ Res 2006;98(4):e29–e38. [DOI] [PubMed] [Google Scholar]
- 172. Spach MS, Boineau JP.. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol 1997;20(2 Pt 2):397–413. [DOI] [PubMed] [Google Scholar]
- 173. de Bakker JM, Stein M, van Rijen HV.. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm 2005;2(7):777–779. [DOI] [PubMed] [Google Scholar]
- 174. Valderrabano M, et al. Obstacle-induced transition from ventricular fibrillation to tachycardia in isolated swine right ventricles: insights into the transition dynamics and implications for the critical mass. J Am Coll Cardiol 2000;36(6):2000–2008. [DOI] [PubMed] [Google Scholar]
- 175. Basso C, Thiene G.. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy. Just a matter of fat? Cardiovasc Pathol 2005;14(1):37–41. [DOI] [PubMed] [Google Scholar]
- 176. Roberts-Thomson KC, Sanders P, Kalman JM.. Sinus node disease: an idiopathic right atrial myopathy. Trends Cardiovasc Med 2007;17(6):211–214. [DOI] [PubMed] [Google Scholar]
- 177. Haemers P, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J 2017;38(1):53–61. [DOI] [PubMed] [Google Scholar]
- 178. Pathak RK, et al. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol 2015;31(2):203–210. [DOI] [PubMed] [Google Scholar]
- 179. Nalliah CJ, et al. The role of obesity in atrial fibrillation. Eur Heart J 2016;37(20):1565–1572. [DOI] [PubMed] [Google Scholar]
- 180. Mahajan R, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66(1):1–11. [DOI] [PubMed] [Google Scholar]
- 181. Rocken C, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106(16):2091–2097. [DOI] [PubMed] [Google Scholar]
- 182. Podduturi V, et al. Isolated atrial amyloidosis and the importance of molecular classification. Proc (Bayl Univ Med Cent) 2013;26(4):387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hove-Madsen L, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 2004;110(11):1358–1363. [DOI] [PubMed] [Google Scholar]
- 184. Wang L, et al. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation. Circ Res 2014;114(9):1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Greiser M, Schotten U.. Dynamic remodeling of intracellular Ca(2)(+) signaling during atrial fibrillation. J Mol Cell Cardiol 2013;58:134–142. [DOI] [PubMed] [Google Scholar]
- 186. Voigt N, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125(17):2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chiang DY, et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol 2014;7(6):1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li N, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014;129(12):1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dong LW, et al. Impairment of the ryanodine-sensitive calcium release channels in the cardiac sarcoplasmic reticulum and its underlying mechanism during the hypodynamic phase of sepsis. Shock 2001;16(1):33–39. [DOI] [PubMed] [Google Scholar]
- 190. Caballero R, et al. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol 2010;55(21):2346–2354. [DOI] [PubMed] [Google Scholar]
- 191. Van Wagoner DR, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 1999;85(5):428–436. [DOI] [PubMed] [Google Scholar]
- 192. Voigt N, et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3(5):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Nattel S. Changes in the atrial transcriptome and atrial fibrillation: susceptibility, persistence, causes, and consequences. Circ Arrhythm Electrophysiol 2015;8(1):5–7. [DOI] [PubMed] [Google Scholar]
- 194. Jalife J, Berenfeld O, Mansour M.. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002;54(2):204–216. [DOI] [PubMed] [Google Scholar]
- 195. Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415(6868):219–226. [DOI] [PubMed] [Google Scholar]
- 196. Schotten U, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91(1):265–325. [DOI] [PubMed] [Google Scholar]
- 197. Haissaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339(10):659–666. [DOI] [PubMed] [Google Scholar]
- 198. Engelman TW. Ueber den Einfluss der Systole auf die motorische Leitung in der Herzkammer, mit Bemerkungen zur Theorie allorhythmischer Herzstörungen. Archiv für die gesamte Physiologie des Menschen und der Tiere 1896;62(12):543–566. [Google Scholar]
- 199. Moe GK, Rheinboldt WC, Abildskov JA.. A computer model of atrial fibrillation. Am Heart J 1964;67:200–220. [DOI] [PubMed] [Google Scholar]
- 200. Garrey WE. The nature of fibrillary contraction of the heart. Its relation to tissue mass and form. Annals of Noninvasive Electrocardiology 1998;3(2):163–180. [Google Scholar]
- 201. Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res 2011;89(4):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lewis T. Oliver-Sharpey Lectures on the nature of flutter and fibrillation of the auricle. Br Med J 1921;1(3146):551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. de Boer RA, et al. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009;11(9):811–817. [DOI] [PubMed] [Google Scholar]
- 204. Chen YJ, et al. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation 2001;104(23):2849–2854. [DOI] [PubMed] [Google Scholar]
- 205. Voigt N, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Christ T, et al. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc Natl Acad Sci U S A 2014;111(30):11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Greiser M, et al. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest 2014;124(11):4759–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Lee SH, et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol 2005;46(6):1054–1059. [DOI] [PubMed] [Google Scholar]
- 209. Allessie MA, Bonke FI, Schopman FJ.. Circus movement in rabbit atrial muscle as a mechanism of trachycardia. Circ Res 1973;33(1):54–62. [PubMed] [Google Scholar]
- 210. Mines GR. On circulating excitations in heart muscles and their possible relation to tachycardia and fibrillation; 1914.
- 211. Wiener N, Rosenblueth A.. The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex 1946;16(3):205–265. [PubMed] [Google Scholar]
- 212. Allessie MA, Bonke FI, Schopman FJ.. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res 1977;41(1):9–18. [DOI] [PubMed] [Google Scholar]
- 213. Winfree AT. Varieties of spiral wave behavior: An experimentalist's approach to the theory of excitable media. Chaos 1991;1(3):303–334. [DOI] [PubMed] [Google Scholar]
- 214. Rensma PL, et al. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res 1988;62(2):395–410. [DOI] [PubMed] [Google Scholar]
- 215. Winfree AT. Vortex action potentials in normal ventricular muscle. Ann N Y Acad Sci 1990;591:190–207. [DOI] [PubMed] [Google Scholar]
- 216. Davidenko JM, et al. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A 1990;87(22):8785–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Jalife J, Delmar M., Anumonwo J, Berenfeld O, Kalifa J.. Basic Cardiac Electrophysiology for the Clinician. Armonk, NY: Futura Pub; 1999. [Google Scholar]
- 218. Fast VG, Kleber AG.. Role of wavefront curvature in propagation of cardiac impulse. Cardiovasc Res 1997;33(2):258–271. [DOI] [PubMed] [Google Scholar]
- 219. Zhao J, et al. Integration of High-Resolution Optical Mapping and 3-Dimensional Micro-Computed Tomographic Imaging to Resolve the Structural Basis of Atrial Conduction in the Human Heart. Circ Arrhythm Electrophysiol 2015;8(6):1514–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kneller J, et al. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res 2005;96(5):e35–e47. [DOI] [PubMed] [Google Scholar]
- 221. Cuculich PS, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation 2010;122(14):1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Haissaguerre M, et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130(7):530–538. [DOI] [PubMed] [Google Scholar]
- 223. Narayan SM, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 2014;63(17):1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Lin YJ, et al. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6(5):851–858. [DOI] [PubMed] [Google Scholar]
- 225. Lin Y-J, et al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC: Clinical Electrophysiology 2016;2(6):667–678. [DOI] [PubMed] [Google Scholar]
- 226. Miller JM, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25(9):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Lee S, et al. Simultaneous biatrial high-density (510-512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation 2015;132(22):2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. van der Does LJ, et al. Dynamics of endo- and epicardial focal fibrillation waves at the right atrium in a patient with advanced atrial remodelling. Can J Cardiol 2016;32(10):1260.e19–1260.e21. [DOI] [PubMed] [Google Scholar]
- 229. de Groot N, et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol 2016;9(5). [DOI] [PubMed] [Google Scholar]
- 230. Perez FJ, et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol 2009;2(4):393–401. [DOI] [PubMed] [Google Scholar]
- 231. Pappone C, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003;42(2):185–197. [DOI] [PubMed] [Google Scholar]
- 232. Hsu LF, et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation 2004;109(7):828–832. [DOI] [PubMed] [Google Scholar]
- 233. Chen MS, et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 2004;43(6):1004–1009. [DOI] [PubMed] [Google Scholar]
- 234. Gentlesk PJ, et al. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18(1):9–14. [DOI] [PubMed] [Google Scholar]
- 235. Khan MN, et al. Pulmonary-vein isolation for atrial fibrillation in patients with HF. N Engl J Med 2008;359(17):1778–1785. [DOI] [PubMed] [Google Scholar]
- 236. MacDonald MR, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97(9):740–747. [DOI] [PubMed] [Google Scholar]
- 237. Hunter RJ, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7(1):31–38. [DOI] [PubMed] [Google Scholar]
- 238. Themistoclakis S, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010;55(8):735–743. [DOI] [PubMed] [Google Scholar]
- 239. Bunch TJ, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(8):839–845. [DOI] [PubMed] [Google Scholar]
- 240. Dagres N, et al. Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta-analysis. J Card Fail 2011;17(11):964–970. [DOI] [PubMed] [Google Scholar]
- 241. Lin YJ, et al. Successful catheter ablation reduces the risk of cardiovascular events in atrial fibrillation patients with CHA2DS2-VASc risk score of 1 and higher. Europace 2013;15(5):676–684. [DOI] [PubMed] [Google Scholar]
- 242. Ghanbari H, et al. Mortality and cerebrovascular events after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm 2014;11(9):1503–1511. [DOI] [PubMed] [Google Scholar]
- 243. Yaksh A, et al. Atrial fibrillation: to map or not to map? Neth Heart J 2014;22(6):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Pappone C, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation 2000;102(21):2619–2628. [DOI] [PubMed] [Google Scholar]
- 245. Verma A, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372(19):1812–1822. [DOI] [PubMed] [Google Scholar]
- 246. Kottkamp H, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol 2004;44(4):869–877. [DOI] [PubMed] [Google Scholar]
- 247. Pappone C, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109(3):327–334. [DOI] [PubMed] [Google Scholar]
- 248. Byrd GD, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation 2005;112(9 Suppl):I7–I13. [DOI] [PubMed] [Google Scholar]
- 249. Tan AY, et al. Electrical connections between left superior pulmonary vein, LA, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol 2006;290(1):H312–H322. [DOI] [PubMed] [Google Scholar]
- 250. Narayan SM, et al. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J Am Coll Cardiol 2013;62(2):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Gaita F, et al. Very long-term results of surgical and transcatheter ablation of long-standing persistent atrial fibrillation. Ann Thorac Surg 2013;96(4):1273–1278. [DOI] [PubMed] [Google Scholar]
- 252. Ganesan AN, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2(2):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Oral H, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002;40(1):100–104. [DOI] [PubMed] [Google Scholar]
- 254. Joshi S, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol 2009;20(10):1089–1094. [DOI] [PubMed] [Google Scholar]
- 255. Andrade JG, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol 2012;23(12):1295–1301. [DOI] [PubMed] [Google Scholar]
- 256. Grubman E, et al. Histopathologic effects of radiofrequency catheter ablation in previously infarcted human myocardium. J Cardiovasc Electrophysiol 1999;10(3):336–342. [DOI] [PubMed] [Google Scholar]
- 257. Hsieh MH, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation 1999;100(22):2237–2243. [DOI] [PubMed] [Google Scholar]
- 258. Fenelon G, Brugada P.. Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing Clin Electrophysiol 1996;19(4 Pt 1):484–489. [DOI] [PubMed] [Google Scholar]
- 259. Lodzinski P, et al. Does a blanking period after pulmonary vein isolation impact long-term results? Results after 55 months of follow-up. Cardiol J 2014;21(4):384–391. [DOI] [PubMed] [Google Scholar]
- 260. Liang JJ, et al. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: Timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm 2015;12(12):2461–2468. [DOI] [PubMed] [Google Scholar]
- 261. Jais P, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118(24):2498–2505. [DOI] [PubMed] [Google Scholar]
- 262. Calkins H, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2(4):349–361. [DOI] [PubMed] [Google Scholar]
- 263. Verma A, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation 2005;112(5):627–635. [DOI] [PubMed] [Google Scholar]
- 264. Eitel C, et al. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm 2010;7(2):157–164. [DOI] [PubMed] [Google Scholar]
- 265. Macle L, et al. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet 2015;386(9994):672–679. [DOI] [PubMed] [Google Scholar]
- 266. Ouyang F, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122(23):2368–2377. [DOI] [PubMed] [Google Scholar]
- 267. Weerasooriya R, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57(2):160–166. [DOI] [PubMed] [Google Scholar]
- 268. Wokhlu A, et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol 2010;21(10):1071–1078. [DOI] [PubMed] [Google Scholar]
- 269. Hsieh MH, et al. The different mechanisms between late and very late recurrences of atrial fibrillation in patients undergoing a repeated catheter ablation. J Cardiovasc Electrophysiol 2006;17(3):231–235. [DOI] [PubMed] [Google Scholar]
- 270. Sotomi Y, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013;111(4):552–556. [DOI] [PubMed] [Google Scholar]
- 271. Mainigi SK, et al. Incidence and predictors of very late recurrence of atrial fibrillation after ablation. J Cardiovasc Electrophysiol 2007;18(1):69–74. [DOI] [PubMed] [Google Scholar]
- 272. Gaztanaga L, et al. Time to recurrence of atrial fibrillation influences outcome following catheter ablation. Heart Rhythm 2012;10(1):2–9. [DOI] [PubMed] [Google Scholar]
- 273. January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 274. January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 275. EHRA Scientific Committee Task Force, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation. Endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Dublin S, et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med 2006;166(21):2322–2328. [DOI] [PubMed] [Google Scholar]
- 277. Gami AS, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49(5):565–571. [DOI] [PubMed] [Google Scholar]
- 278. Tedrow UB, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol 2010;55(21):2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Huxley RR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123(14):1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Munger TM, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol 2012;60(9):851–860. [DOI] [PubMed] [Google Scholar]
- 281. Abed HS, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10(1):90–100. [DOI] [PubMed] [Google Scholar]
- 282. Richter B, et al. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J 2006;27(21):2553–2559. [DOI] [PubMed] [Google Scholar]
- 283. Jongnarangsin K, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19(7):668–672. [DOI] [PubMed] [Google Scholar]
- 284. Shah AN, et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol 2008;19(7):661–667. [DOI] [PubMed] [Google Scholar]
- 285. Chang SL, et al. Comparison of outcome in catheter ablation of atrial fibrillation in patients with versus without the metabolic syndrome. Am J Cardiol 2009;103(1):67–72. [DOI] [PubMed] [Google Scholar]
- 286. Letsas KP, et al. Pre-ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation: the potential role of inflammation. Europace 2009;11(2):158–163. [DOI] [PubMed] [Google Scholar]
- 287. Tang RB, et al. Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circ J 2009;73(3):438–443. [DOI] [PubMed] [Google Scholar]
- 288. Jin Hwang H, et al. Atrial electroanatomical remodeling as a determinant of different outcomes between two current ablation strategies: circumferential pulmonary vein isolation vs pulmonary vein isolation. Clin Cardiol 2010;33(3):E69–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Patel D, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm 2010;7(2):167–172. [DOI] [PubMed] [Google Scholar]
- 290. Patel D, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010;3(5):445–451. [DOI] [PubMed] [Google Scholar]
- 291. Patel D, et al. The impact of statins and renin-angiotensin-aldosterone system blockers on pulmonary vein antrum isolation outcomes in post-menopausal females. Europace 2010;12(3):322–330. [DOI] [PubMed] [Google Scholar]
- 292. Chao TF, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J 2011;75(10):2326–2332. [DOI] [PubMed] [Google Scholar]
- 293. Winkle RA, et al. Relation of early termination of persistent atrial fibrillation by cardioversion or drugs to ablation outcomes. Am J Cardiol 2011;108(3):374–379. [DOI] [PubMed] [Google Scholar]
- 294. Wong CX, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 2011;57(17):1745–1751. [DOI] [PubMed] [Google Scholar]
- 295. Kang JH, et al. Prediction of long-term outcomes of catheter ablation of persistent atrial fibrillation by parameters of preablation DC cardioversion. J Cardiovasc Electrophysiol 2012;23(11):1165–1170. [DOI] [PubMed] [Google Scholar]
- 296. Mohanty S, et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J Am Coll Cardiol 2012;59(14):1295–1301. [DOI] [PubMed] [Google Scholar]
- 297. Ejima K, et al. Impact of diastolic dysfunction on the outcome of catheter ablation in patients with atrial fibrillation. Int J Cardiol 2013;164(1):88–93. [DOI] [PubMed] [Google Scholar]
- 298. Letsas KP, et al. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int J Cardiol 2013;164(1):94–98. [DOI] [PubMed] [Google Scholar]
- 299. Wong CX, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC: Clinical Electrophysiology 2015;1(3):139–152. [DOI] [PubMed] [Google Scholar]
- 300. Alonso A, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J 2015;170(4):770–777.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Pathak RK, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64(21):2222–2231. [DOI] [PubMed] [Google Scholar]
- 302. Bitter T, et al. Sleep-disordered breathing and cardiac arrhythmias. Can J Cardiol 2015;31(7):928–934. [DOI] [PubMed] [Google Scholar]
- 303. Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol 2000;119(2-3):189–197. [DOI] [PubMed] [Google Scholar]
- 304. Kraiczi H, et al. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol (1985) 2000;89(2):493–498. [DOI] [PubMed] [Google Scholar]
- 305. Ghias M, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol 2009;54(22):2075–2083. [DOI] [PubMed] [Google Scholar]
- 306. Linz D, et al. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011;8(9):1436–1443. [DOI] [PubMed] [Google Scholar]
- 307. Linz D, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension 2012;60(1):172–178. [DOI] [PubMed] [Google Scholar]
- 308. Linz D, et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension 2013;62(4):767–774. [DOI] [PubMed] [Google Scholar]
- 309. Iwasaki YK, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012;9(9):1409–1416.e1. [DOI] [PubMed] [Google Scholar]
- 310. Iwasaki YK, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64(19):2013–2023. [DOI] [PubMed] [Google Scholar]
- 311. Dimitri H, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012;9(3):321–327. [DOI] [PubMed] [Google Scholar]
- 312. Stevenson IH, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010;7(9):1263–1270. [DOI] [PubMed] [Google Scholar]
- 313. Holmqvist F, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2015;169(5):647–654.e2. [DOI] [PubMed] [Google Scholar]
- 314. Kwon Y, et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax 2015;70(9):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Kanagala R, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107(20):2589–2594. [DOI] [PubMed] [Google Scholar]
- 316. Fein AS, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62(4):300–305. [DOI] [PubMed] [Google Scholar]
- 317. Matiello M, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace 2010;12(8):1084–1089. [DOI] [PubMed] [Google Scholar]
- 318. Naruse Y, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10(3):331–337. [DOI] [PubMed] [Google Scholar]
- 319. Neilan TG, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2(6):e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Li L, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace 2014;16(9):1309–1314. [DOI] [PubMed] [Google Scholar]
- 321. Kannel WB, et al. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982;306(17):1018–1022. [DOI] [PubMed] [Google Scholar]
- 322. Krahn AD, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995;98(5):476–484. [DOI] [PubMed] [Google Scholar]
- 323. Thomas MC, et al. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 2008;21(10):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Tremblay-Gravel M, et al. Blood Pressure and Atrial Fibrillation: A Combined AF-CHF and AFFIRM Analysis. J Cardiovasc Electrophysiol 2015;26(5):509–514. [DOI] [PubMed] [Google Scholar]
- 325. Grundvold I, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension 2012;59(2):198–204. [DOI] [PubMed] [Google Scholar]
- 326. Conen D, et al. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009;119(16):2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Kistler PM, et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J 2006;27(24):3045–3056. [DOI] [PubMed] [Google Scholar]
- 328. Berruezo A, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007;28(7):836–841. [DOI] [PubMed] [Google Scholar]
- 329. Arya A, et al. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace 2010;12(2):173–180. [DOI] [PubMed] [Google Scholar]
- 330. Santoro F, et al. Impact of Uncontrolled Hypertension on Atrial Fibrillation Ablation Outcome. JACC: Clinical Electrophysiology 2015;1(3):164–173. [DOI] [PubMed] [Google Scholar]
- 331. Pokushalov E, et al. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: early experience. Heart Rhythm 2014;11(7):1131–1138. [DOI] [PubMed] [Google Scholar]
- 332. Healey JS, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol 2005;45(11):1832–1839. [DOI] [PubMed] [Google Scholar]
- 333. Al Chekakie MO, et al. The effects of statins and renin-angiotensin system blockers on atrial fibrillation recurrence following antral pulmonary vein isolation. J Cardiovasc Electrophysiol 2007;18(9):942–946. [DOI] [PubMed] [Google Scholar]
- 334. Tayebjee MH, et al. Impact of angiotensin-converting enzyme-inhibitors and angiotensin receptor blockers on long-term outcome of catheter ablation for atrial fibrillation. Europace 2010;12(11):1537–1542. [DOI] [PubMed] [Google Scholar]
- 335. Kato T, et al. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol 2006;17(8):890–894. [DOI] [PubMed] [Google Scholar]
- 336. Huxley RR, et al. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Huxley RR, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 2012;98(2):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lin KJ, et al. Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol 2014;39(3):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Ettinger PO, et al. Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J 1978;95(5):555–562. [DOI] [PubMed] [Google Scholar]
- 340. Patel N, et al. Does alcohol use increase the risk of atrial fibrillation recurrence after radiofrequency ablation? J Am Coll Cardiol 2013;61(10_S). [Google Scholar]
- 341. Mohanty S, et al. Impact of alcohol intake on thromboembolic events following catheter ablation for atrial fibrillation. J Am Coll Cardiol 2014;63(12_S). [Google Scholar]
- 342. Qiao Y, et al. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Wilber DJ. Fibroblasts, focal triggers, and persistent atrial fibrillation: is there a connection? Circ Arrhythm Electrophysiol 2012;5(2):249–251. [DOI] [PubMed] [Google Scholar]
- 344. Rohr S. Arrhythmogenic implications of fibroblast-myocyte interactions. Circ Arrhythm Electrophysiol 2012;5(2):442–452. [DOI] [PubMed] [Google Scholar]
- 345. Qureshi WT, et al. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) project. Circulation 2015;131(21):1827–1834. [DOI] [PubMed] [Google Scholar]
- 346. Malmo V, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation 2016;133(5):466–473. [DOI] [PubMed] [Google Scholar]
- 347. Abdulla J, Nielsen JR.. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace 2009;11(9):1156–1159. [DOI] [PubMed] [Google Scholar]
- 348. Aizer A, et al. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 2009;103(11):1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Mont L, Elosua R, Brugada J.. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace 2009;11(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hoogsteen J, et al. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace 2004;6(3):222–228. [DOI] [PubMed] [Google Scholar]
- 351. Pelliccia A, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol 2005;46(4):690–696. [DOI] [PubMed] [Google Scholar]
- 352. Swanson DR. Atrial fibrillation in athletes: implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med Hypotheses 2006;66(6):1085–1092. [DOI] [PubMed] [Google Scholar]
- 353. Heidbuchel H, et al. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol 2006;107(1):67–72. [DOI] [PubMed] [Google Scholar]
- 354. Andersen K, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 2013;34(47):3624–3631. [DOI] [PubMed] [Google Scholar]
- 355. Bettoni M, Zimmermann M.. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105(23):2753–2759. [DOI] [PubMed] [Google Scholar]
- 356. Pelliccia A, et al. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med 1999;130(1):23–31. [DOI] [PubMed] [Google Scholar]
- 357. Eijsvogels TM, Fernandez AB, Thompson PD.. Are There Deleterious Cardiac Effects of Acute and Chronic Endurance Exercise? Physiol Rev 2016;96(1):99–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Allison MA, et al. Sedentary behavior and adiposity-associated inflammation: the Multi-Ethnic Study of Atherosclerosis. Am J Prev Med 2012;42(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Kasapis C, Thompson PD.. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005;45(10):1563–1569. [DOI] [PubMed] [Google Scholar]
- 360. Engelmann MD, Svendsen JH.. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J 2005;26(20):2083–2092. [DOI] [PubMed] [Google Scholar]
- 361. Chung MK, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104(24):2886–2891. [DOI] [PubMed] [Google Scholar]
- 362. Korantzopoulos P, et al. Atrial fibrillation and electrical remodeling: the potential role of inflammation and oxidative stress. Med Sci Monit 2003;9(9):RA225–RA229. [PubMed] [Google Scholar]
- 363. Psychari SN, et al. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol 2005;95(6):764–767. [DOI] [PubMed] [Google Scholar]
- 364. Mohlenkamp S, et al. Running: the risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29(15):1903–1910. [DOI] [PubMed] [Google Scholar]
- 365. Marrouche NF, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311(5):498–506. [DOI] [PubMed] [Google Scholar]
- 366. Benito B, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123(1):13–22. [DOI] [PubMed] [Google Scholar]
- 367. Lindsay MM, Dunn FG.. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med 2007;41(7):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Gay-Jordi G, et al. Losartan prevents heart fibrosis induced by long-term intensive exercise in an animal model. PLoS One 2013;8(2):e55427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Sorokin AV, et al. Atrial fibrillation in endurance-trained athletes. Br J Sports Med 2011;45(3):185–188. [DOI] [PubMed] [Google Scholar]
- 370. Calvo N, et al. Efficacy of circumferential pulmonary vein ablation of atrial fibrillation in endurance athletes. Europace 2010;12(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Koopman P, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace 2011;13(10):1386–1393. [DOI] [PubMed] [Google Scholar]
- 372. Furlanello F, et al. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J Cardiovasc Electrophysiol 2008;19(5):457–462. [DOI] [PubMed] [Google Scholar]
- 373. Pathak RK, et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol 2015;66(9):985–996. [DOI] [PubMed] [Google Scholar]
- 374. Fjeldsoe B, et al. Systematic review of maintenance of behavior change following physical activity and dietary interventions. Health Psychol 2011;30(1):99–109. [DOI] [PubMed] [Google Scholar]
- 375. European Heart Rhythm A, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12(10):1360–1420. [DOI] [PubMed] [Google Scholar]
- 376. Camm AJ, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33(21):2719–2747. [DOI] [PubMed] [Google Scholar]
- 377. Wazni OM, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293(21):2634–2640. [DOI] [PubMed] [Google Scholar]
- 378. Cosedis Nielsen J, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367(17):1587–1595. [DOI] [PubMed] [Google Scholar]
- 379. Morillo CA, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311(7):692–700. [DOI] [PubMed] [Google Scholar]
- 380. Hakalahti A, et al. Radiofrequency ablation vs antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17(3):370–378. [DOI] [PubMed] [Google Scholar]
- 381. Hocini M, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 2003;108(10):1172–1175. [DOI] [PubMed] [Google Scholar]
- 382. Chen YW, et al. Pacing or ablation: which is better for paroxysmal atrial fibrillation-related tachycardia-bradycardia syndrome? Pacing Clin Electrophysiol 2014;37(4):403–411. [DOI] [PubMed] [Google Scholar]
- 383. Inada K, et al. The role of successful catheter ablation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses: outcome during a 5-year follow-up. Europace 2014;16(2):208–213. [DOI] [PubMed] [Google Scholar]
- 384. Tondo C, et al. Pulmonary vein vestibule ablation for the control of atrial fibrillation in patients with impaired left ventricular function. Pacing Clin Electrophysiol 2006;29(9):962–970. [DOI] [PubMed] [Google Scholar]
- 385. Bunch TJ, et al. Substrate and procedural predictors of outcomes after catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2008;19(10):1009–1014. [DOI] [PubMed] [Google Scholar]
- 386. Lutomsky BA, et al. Catheter ablation of paroxysmal atrial fibrillation improves cardiac function: a prospective study on the impact of atrial fibrillation ablation on left ventricular function assessed by magnetic resonance imaging. Europace 2008;10(5):593–599. [DOI] [PubMed] [Google Scholar]
- 387. Choi AD, et al. Ablation vs medical therapy in the setting of symptomatic atrial fibrillation and left ventricular dysfunction. Congest Heart Fail 2010;16(1):10–14. [DOI] [PubMed] [Google Scholar]
- 388. De Potter T, et al. Left ventricular systolic dysfunction by itself does not influence outcome of atrial fibrillation ablation. Europace 2010;12(1):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Cha YM, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol 2011;4(5):724–732. [DOI] [PubMed] [Google Scholar]
- 390. Jones DG, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in HF. J Am Coll Cardiol 2013;61(18):1894–1903. [DOI] [PubMed] [Google Scholar]
- 391. Machino-Ohtsuka T, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2013;62(20):1857–1865. [DOI] [PubMed] [Google Scholar]
- 392. Al Halabi S, et al. Catheter ablation for atrial fibrillation in heart failure patients: a meta-analysis of randomized controlled trials. JACC Clin Electrophysiol 2015;1(3):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Bunch TJ, et al. Five-year outcomes of catheter ablation in patients with atrial fibrillation and left ventricular systolic dysfunction. J Cardiovasc Electrophysiol 2015;26(4):363–370. [DOI] [PubMed] [Google Scholar]
- 394. Lobo TJ, et al. Atrial fibrillation ablation in systolic dysfunction: clinical and echocardiographic outcomes. Arq Bras Cardiol 2015;104(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Ling LH, et al. Sinus rhythm restores ventricular function in patients with cardiomyopathy and no late gadolinium enhancement on cardiac magnetic resonance imaging who undergo catheter ablation for atrial fibrillation. Heart Rhythm 2013;10(9):1334–1339. [DOI] [PubMed] [Google Scholar]
- 396. Spragg DD, et al. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol 2008;19(6):627–631. [DOI] [PubMed] [Google Scholar]
- 397. Kusumoto F, et al. Radiofrequency catheter ablation of atrial fibrillation in older patients: outcomes and complications. J Interv Card Electrophysiol 2009;25(1):31–35. [DOI] [PubMed] [Google Scholar]
- 398. Bunch TJ, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol 2010;33(2):146–152. [DOI] [PubMed] [Google Scholar]
- 399. Santangeli P, et al. Ablation for atrial fibrillation: termination of atrial fibrillation is not the end point. Card Electrophysiol Clin 2012;4(3):343–352. [DOI] [PubMed] [Google Scholar]
- 400. Santangeli P, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a meta-analysis. Circ Arrhythm Electrophysiol 2012;5(2):302–311. [DOI] [PubMed] [Google Scholar]
- 401. Santangeli P, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol 2012;23(7):687–693. [DOI] [PubMed] [Google Scholar]
- 402. Nademanee K, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm 2015;12(1):44–51. [DOI] [PubMed] [Google Scholar]
- 403. Bunch TJ, et al. The impact of age on 5-year outcomes after atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol 2016;27(2):141–146. [DOI] [PubMed] [Google Scholar]
- 404. Metzner I, et al. Ablation of atrial fibrillation in patients >/=75 years: long-term clinical outcome and safety. Europace 2016;18(4):543–549. [DOI] [PubMed] [Google Scholar]
- 405. Leong-Sit P, et al. Efficacy and risk of atrial fibrillation ablation before 45 years of age. Circ Arrhythm Electrophysiol 2010;3(5):452–457. [DOI] [PubMed] [Google Scholar]
- 406. Oral H, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006;114(8):759–765. [DOI] [PubMed] [Google Scholar]
- 407. Hussein AA, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4(3):271–278. [DOI] [PubMed] [Google Scholar]
- 408. Hunter RJ, et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 2012;98(1):48–53. [DOI] [PubMed] [Google Scholar]
- 409. Winkle RA, et al. Discontinuing anticoagulation following successful atrial fibrillation ablation in patients with prior strokes. J Interv Card Electrophysiol 2013;38(3):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Karasoy D, et al. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J 2015;36(5):307–314a. [DOI] [PubMed] [Google Scholar]
- 411. Schlingloff F, et al. Oral anticoagulation after successful atrial fibrillation ablation operations: is it necessary? Ann Thorac Surg 2016;101(4):1471–1476. [DOI] [PubMed] [Google Scholar]
- 412. Nuhrich JM, et al. Oral anticoagulation is frequently discontinued after ablation of paroxysmal atrial fibrillation despite previous stroke: data from the German Ablation Registry. Clin Res Cardiol 2015;104(6):463–470. [DOI] [PubMed] [Google Scholar]
- 413. Oral H, et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2004;15(8):920–924. [DOI] [PubMed] [Google Scholar]
- 414. Tondo C, et al. Rhythm-symptom correlation in patients on continuous monitoring after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(2):154–160. [DOI] [PubMed] [Google Scholar]
- 415. Kirchhof P, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 416. Forleo GB, et al. Clinical impact of catheter ablation in patients with asymptomatic atrial fibrillation: the IRON-AF (Italian registry on NavX atrial fibrillation ablation procedures) study. Int J Cardiol 2013;168(4):3968–3970. [DOI] [PubMed] [Google Scholar]
- 417. Mohanty S, et al. Catheter ablation of asymptomatic longstanding persistent atrial fibrillation: impact on quality of life, exercise performance, arrhythmia perception, and arrhythmia-free survival. J Cardiovasc Electrophysiol 2014;25(10):1057–1064. [DOI] [PubMed] [Google Scholar]
- 418. Wu L, et al. Comparison of radiofrequency catheter ablation between asymptomatic and symptomatic persistent atrial fibrillation: a propensity score matched analysis. J Cardiovasc Electrophysiol 2016;27(5):531–535. [DOI] [PubMed] [Google Scholar]
- 419. Cox JL, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101(3):406–426. [PubMed] [Google Scholar]
- 420. Sundt TM 3rd, Camillo CJ, Cox JL.. The maze procedure for cure of atrial fibrillation. Cardiol Clin 1997;15(4):739–748. [DOI] [PubMed] [Google Scholar]
- 421. Melo J, et al. Surgery for atrial fibrillation using radiofrequency catheter ablation: assessment of results at one year. Eur J Cardiothorac Surg 1999;15(6):851–854. discussion 855. [DOI] [PubMed] [Google Scholar]
- 422. Sueda T, et al. Efficacy of pulmonary vein isolation for the elimination of chronic atrial fibrillation in cardiac valvular surgery. Ann Thorac Surg 2001;71(4):1189–1193. [DOI] [PubMed] [Google Scholar]
- 423. Swartz JFP.G., Silvers J, Pattern L, Cervantez D.. A catheter-based curative approach to atrial fibrillation in humans. [abstract]. Circulation 1994;90(Suppl I):I–335. [Google Scholar]
- 424. Haissaguerre M, et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 1996;7(12):1132–1144. [DOI] [PubMed] [Google Scholar]
- 425. Gaita F, et al. Atrial mapping and radiofrequency catheter ablation in patients with idiopathic atrial fibrillation. Electrophysiological findings and ablation results. Circulation 1998;97(21):2136–2145. [DOI] [PubMed] [Google Scholar]
- 426. Jais P, et al. Long-term follow-up after right atrial radiofrequency catheter treatment of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1998;21(11 Pt 2):2533–2538. [DOI] [PubMed] [Google Scholar]
- 427. Calkins H, et al. A new system for catheter ablation of atrial fibrillation. Am J Cardiol 1999;83(5B):227D–236D. [DOI] [PubMed] [Google Scholar]
- 428. Natale A, et al. Catheter ablation approach on the right side only for paroxysmal atrial fibrillation therapy: long-term results. Pacing Clin Electrophysiol 2000;23(2):224–233. [DOI] [PubMed] [Google Scholar]
- 429. Kocheril AG, et al. Hybrid therapy with right atrial catheter ablation and previously ineffective antiarrhythmic drugs for the management of atrial fibrillation. J Interv Card Electrophysiol 2005;12(3):189–197. [DOI] [PubMed] [Google Scholar]
- 430. Haissaguerre M, et al. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J Cardiovasc Electrophysiol 1994;5(9):743–751. [DOI] [PubMed] [Google Scholar]
- 431. Jais P, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95(3):572–576. [DOI] [PubMed] [Google Scholar]
- 432. Pappone C, et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation 1999;100(11):1203–1208. [DOI] [PubMed] [Google Scholar]
- 433. Haissaguerre M, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 2000;101(12):1409–1417. [DOI] [PubMed] [Google Scholar]
- 434. Robbins IM, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998;98(17):1769–1775. [DOI] [PubMed] [Google Scholar]
- 435. Marrouche NF, et al. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation 2003;107(21):2710–2716. [DOI] [PubMed] [Google Scholar]
- 436. Oral H, et al. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights. Circulation 2002;106(10):1256–1262. [DOI] [PubMed] [Google Scholar]
- 437. Pappone C, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104(21):2539–2544. [DOI] [PubMed] [Google Scholar]
- 438. Ouyang F, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 2004;110(15):2090–2096. [DOI] [PubMed] [Google Scholar]
- 439. Takahashi A, et al. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation 2002;105(25):2998–3003. [DOI] [PubMed] [Google Scholar]
- 440. Callans DJ, et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(9):1050–1055. [DOI] [PubMed] [Google Scholar]
- 441. Oral H, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003;108(19):2355–2360. [DOI] [PubMed] [Google Scholar]
- 442. Karch MR, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 2005;111(22):2875–2880. [DOI] [PubMed] [Google Scholar]
- 443. Arentz T, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation 2007;115(24):3057–3063. [DOI] [PubMed] [Google Scholar]
- 444. Yamada T, et al. Electrophysiological pulmonary vein antrum isolation with a multielectrode basket catheter is feasible and effective for curing paroxysmal atrial fibrillation: efficacy of minimally extensive pulmonary vein isolation. Heart Rhythm 2006;3(4):377–384. [DOI] [PubMed] [Google Scholar]
- 445. Gerstenfeld EP, et al. Utility of exit block for identifying electrical isolation of the pulmonary veins. J Cardiovasc Electrophysiol 2002;13(10):971–979. [DOI] [PubMed] [Google Scholar]
- 446. Nanthakumar K, et al. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation 2004;109(10):1226–1229. [DOI] [PubMed] [Google Scholar]
- 447. Ouyang F, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111(2):127–135. [DOI] [PubMed] [Google Scholar]
- 448. Cheema A, et al. Incidence and time course of early recovery of pulmonary vein conduction after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18(4):387–391. [DOI] [PubMed] [Google Scholar]
- 449. Pratola C, et al. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation 2008;117(2):136–143. [DOI] [PubMed] [Google Scholar]
- 450. Rajappan K, et al. Acute and chronic pulmonary vein reconnection after atrial fibrillation ablation: a prospective characterization of anatomical sites. Pacing Clin Electrophysiol 2008;31(12):1598–1605. [DOI] [PubMed] [Google Scholar]
- 451. Bansch D, et al. Circumferential pulmonary vein isolation: wait or stop early after initial successful pulmonary vein isolation? Europace 2013;15(2):183–188. [DOI] [PubMed] [Google Scholar]
- 452. Nakamura K, et al. Optimal observation time after completion of circumferential pulmonary vein isolation for atrial fibrillation to prevent chronic pulmonary vein reconnections. Int J Cardiol 2013;168(6):5300–5310. [DOI] [PubMed] [Google Scholar]
- 453. Neuzil P, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6(2):327–333. [DOI] [PubMed] [Google Scholar]
- 454. Jiang RH, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm 2014;11(6):969–976. [DOI] [PubMed] [Google Scholar]
- 455. Kim TH, et al. Pulmonary vein reconnection predicts good clinical outcome after second catheter ablation for atrial fibrillation. Europace 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 456. Kuck KH, et al. Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the Gap-Atrial Fibrillation-German Atrial Fibrillation Competence Network 1 Trial. Circ Arrhythm Electrophysiol 2016;9(1):e003337. [DOI] [PubMed] [Google Scholar]
- 457. Wang XH, et al. Early identification and treatment of PV re-connections: role of observation time and impact on clinical results of atrial fibrillation ablation. Europace 2007;9(7):481–486. [DOI] [PubMed] [Google Scholar]
- 458. Sauer WH, et al. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: response to ablation that incorporates slow-pathway modification. Circulation 2006;114(3):191–195. [DOI] [PubMed] [Google Scholar]
- 459. Ninomiya Y, et al. Usefulness of the adenosine triphosphate with a sufficient observation period for detecting reconduction after pulmonary vein isolation. Pacing Clin Electrophysiol 2009;32(10):1307–1312. [DOI] [PubMed] [Google Scholar]
- 460. Yamane T, et al. Repeated provocation of time- and ATP-induced early pulmonary vein reconnections after pulmonary vein isolation: eliminating paroxysmal atrial fibrillation in a single procedure. Circ Arrhythm Electrophysiol 2011;4(5):601–608. [DOI] [PubMed] [Google Scholar]
- 461. Kobori A, et al. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate (UNDER-ATP) trial. Eur Heart J 2015;36(46):3276–3287. [DOI] [PubMed] [Google Scholar]
- 462. Packer DL, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61(16):1713–1723. [DOI] [PubMed] [Google Scholar]
- 463. Arentz T, et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2004;15(9):1041–1047. [DOI] [PubMed] [Google Scholar]
- 464. Tritto M, et al. Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. Eur Heart J 2004;25(23):2155–2163. [DOI] [PubMed] [Google Scholar]
- 465. Datino T, et al. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation 2010;121(8):963–972. [DOI] [PubMed] [Google Scholar]
- 466. Dallaglio PD, et al. The role of adenosine in pulmonary vein isolation: a critical review. Cardiol Res Pract 2016;2016:8632509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Kapa S, et al. Dose-dependent pulmonary vein reconnection in response to adenosine: relevance of atrioventricular block during infusion. J Interv Card Electrophysiol 2016;47(1):117–123. [DOI] [PubMed] [Google Scholar]
- 468. Andrade JG, et al. Pulmonary vein isolation using “contact force” ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Heart Rhythm 2014;11(11):1919–1924. [DOI] [PubMed] [Google Scholar]
- 469. Miyazaki S, et al. Impact of adenosine-provoked acute dormant pulmonary vein conduction on recurrence of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23(3):256–260. [DOI] [PubMed] [Google Scholar]
- 470. Zhang J, et al. Origin and ablation of the adenosine triphosphate induced atrial fibrillation after circumferential pulmonary vein isolation: effects on procedural success rate. J Cardiovasc Electrophysiol 2014;25(4):364–370. [DOI] [PubMed] [Google Scholar]
- 471. Datino T, et al. Differential effectiveness of pharmacological strategies to reveal dormant pulmonary vein conduction: a clinical-experimental correlation. Heart Rhythm 2011;8(9):1426–1433. [DOI] [PubMed] [Google Scholar]
- 472. Steven D, et al. Loss of pace capture on the ablation line: a new marker for complete radiofrequency lesions to achieve pulmonary vein isolation. Heart Rhythm 2010;7(3):323–330. [DOI] [PubMed] [Google Scholar]
- 473. Andrade JG, et al. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Circ Arrhythm Electrophysiol 2013;6(6):1103–1108. [DOI] [PubMed] [Google Scholar]
- 474. Steven D, et al. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol 2013;62(1):44–50. [DOI] [PubMed] [Google Scholar]
- 475. Schaeffer B, et al. Loss of pace capture on the ablation line during pulmonary vein isolation versus “dormant conduction”: is adenosine expendable? J Cardiovasc Electrophysiol 2015;26(10):1075–1080. [DOI] [PubMed] [Google Scholar]
- 476. Weerasooriya R, et al. Cost analysis of catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2003;26(1 Pt 2):292–294. [DOI] [PubMed] [Google Scholar]
- 477. Vijayaraman P, et al. Assessment of exit block following pulmonary vein isolation: far-field capture masquerading as entrance without exit block. Heart Rhythm 2012;9(10):1653–1659. [DOI] [PubMed] [Google Scholar]
- 478. Ip JE, et al. Method for differentiating left superior pulmonary vein exit conduction from pseudo-exit conduction. Pacing Clin Electrophysiol 2013;36(3):299–308. [DOI] [PubMed] [Google Scholar]
- 479. Spector P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ Arrhythm Electrophysiol 2013;6(3):655–661. [DOI] [PubMed] [Google Scholar]
- 480. Chen S, et al. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol 2012;35(5):524–531. [DOI] [PubMed] [Google Scholar]
- 481. Kim JY, et al. Achievement of successful pulmonary vein isolation: methods of adenosine testing and incremental benefit of exit block. J Interv Card Electrophysiol 2016;46(3):315–324. [DOI] [PubMed] [Google Scholar]
- 482. Andrade JG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm 2011;8(9):1444–1451. [DOI] [PubMed] [Google Scholar]
- 483. Furnkranz A, et al. Characterization of conduction recovery after pulmonary vein isolation using the “single big cryoballoon” technique. Heart Rhythm 2010;7(2):184–190. [DOI] [PubMed] [Google Scholar]
- 484. Furnkranz A, et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol 2013;24(5):492–497. [DOI] [PubMed] [Google Scholar]
- 485. Ciconte G, et al. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J Cardiovasc Electrophysiol 2014;25(8):845–851. [DOI] [PubMed] [Google Scholar]
- 486. Metzner A, et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2014;7(2):288–292. [DOI] [PubMed] [Google Scholar]
- 487. Bordignon S, et al. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace 2015;17(5):725–731. [DOI] [PubMed] [Google Scholar]
- 488. Heeger CH, et al. Bonus-freeze: benefit or risk? Two-year outcome and procedural comparison of a “bonus-freeze” and “no bonus-freeze” protocol using the second-generation cryoballoon for pulmonary vein isolation. Clin Res Cardiol 2016;105(9):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Kuck KH, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374(23):2235–2245. [DOI] [PubMed] [Google Scholar]
- 490. Kuck KH, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37(38):2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Miyazaki S, et al. Durability of cryothermal pulmonary vein isolation— Creating contiguous lesions is necessary for persistent isolation. Int J Cardiol 2016;220:395–399. [DOI] [PubMed] [Google Scholar]
- 492. Straube F, et al. First-line catheter ablation of paroxysmal atrial fibrillation: outcome of radiofrequency vs. cryoballoon pulmonary vein isolation. Europace 2016;18(3):368–375. [DOI] [PubMed] [Google Scholar]
- 493. Straube F, et al. Outcome of paroxysmal atrial fibrillation ablation with the cryoballoon using two different application times: the 4- versus 3-min protocol. J Interv Card Electrophysiol 2016;45(2):169–177. [DOI] [PubMed] [Google Scholar]
- 494. Raatikainen MJ, et al. Radiofrequency catheter ablation maintains its efficacy better than antiarrhythmic medication in patients with paroxysmal atrial fibrillation: On-treatment analysis of the randomized controlled MANTRA-PAF trial. Int J Cardiol 2015;198:108–114. [DOI] [PubMed] [Google Scholar]
- 495. Kumar N, et al. Adenosine testing after second-generation cryoballoon ablation (ATSCA) study improves clinical success rate for atrial fibrillation. Europace 2015;17(6):871–876. [DOI] [PubMed] [Google Scholar]
- 496. Schmidt B, et al. Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol 2010;3(5):481–488. [DOI] [PubMed] [Google Scholar]
- 497. Dukkipati SR, et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: multicenter results of pulmonary vein remapping studies. Heart Rhythm 2012;9(6):919–925. [DOI] [PubMed] [Google Scholar]
- 498. Dukkipati SR, et al. Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience. Circ Arrhythm Electrophysiol 2013;6(3):467–472. [DOI] [PubMed] [Google Scholar]
- 499. Metzner A, et al. Acute and long-term clinical outcome after endoscopic pulmonary vein isolation: results from the first prospective, multicenter study. J Cardiovasc Electrophysiol 2013;24(1):7–13. [DOI] [PubMed] [Google Scholar]
- 500. Metzner A, et al. The influence of varying energy settings on efficacy and safety of endoscopic pulmonary vein isolation. Heart Rhythm 2012;9(9):1380–1385. [DOI] [PubMed] [Google Scholar]
- 501. Bordignon S, et al. Comparison of balloon catheter ablation technologies for pulmonary vein isolation: the laser versus cryo study. J Cardiovasc Electrophysiol 2013;24(9):987–994. [DOI] [PubMed] [Google Scholar]
- 502. Bordignon S, et al. Energy titration strategies with the endoscopic ablation system: lessons from the high-dose vs. low-dose laser ablation study. Europace 2013;15(5):685–689. [DOI] [PubMed] [Google Scholar]
- 503. Dukkipati SR, et al. Pulmonary vein isolation using the visually guided laser balloon: a prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol 2015;66(12):1350–1360. [DOI] [PubMed] [Google Scholar]
- 504. Patel NJ, et al. Contemporary utilization and safety outcomes of catheter ablation of atrial flutter in the United States: Analysis of 89,638 procedures. Heart Rhythm 2016;13(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 505. Ernst S, et al. Total pulmonary vein occlusion as a consequence of catheter ablation for atrial fibrillation mimicking primary lung disease. J Cardiovasc Electrophysiol 2003;14(4):366–370. [DOI] [PubMed] [Google Scholar]
- 506. Pappone C, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004;110(19):3036–3042. [DOI] [PubMed] [Google Scholar]
- 507. Sawhney N, et al. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3(3):243–248. [DOI] [PubMed] [Google Scholar]
- 508. Chae S, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 2007;50(18):1781–1787. [DOI] [PubMed] [Google Scholar]
- 509. Vogler J, et al. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol 2015;66(24):2743–2752. [DOI] [PubMed] [Google Scholar]
- 510. Ouyang F, et al. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation 2002;105(16):1934–1942. [DOI] [PubMed] [Google Scholar]
- 511. Wazni O, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation 2003;108(20):2479–2483. [DOI] [PubMed] [Google Scholar]
- 512. Matsuo S, et al. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm 2010;7(1):2–8. [DOI] [PubMed] [Google Scholar]
- 513. Tzeis S, et al. The modified anterior line: an alternative linear lesion in perimitral flutter. J Cardiovasc Electrophysiol 2010;21(6):665–670. [DOI] [PubMed] [Google Scholar]
- 514. Haissaguerre M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005;16(11):1138–1147. [DOI] [PubMed] [Google Scholar]
- 515. Scherr D, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8(1):18–24. [DOI] [PubMed] [Google Scholar]
- 516. Nademanee K, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43(11):2044–2053. [DOI] [PubMed] [Google Scholar]
- 517. O'Neill MD, et al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J 2009;30(9):1105–1112. [DOI] [PubMed] [Google Scholar]
- 518. Lo LW, et al. Predicting factors for atrial fibrillation acute termination during catheter ablation procedures: implications for catheter ablation strategy and long-term outcome. Heart Rhythm 2009;6(3):311–318. [DOI] [PubMed] [Google Scholar]
- 519. Zhang Z, et al. Linear ablation following pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol 2016;39(6):623–630. [DOI] [PubMed] [Google Scholar]
- 520. Kim TH, et al. Linear ablation in addition to circumferential pulmonary vein isolation (Dallas lesion set) does not improve clinical outcome in patients with paroxysmal atrial fibrillation: a prospective randomized study. Europace 2015;17(3):388–395. [DOI] [PubMed] [Google Scholar]
- 521. Wynn GJ, et al. Biatrial linear ablation in sustained nonpermanent AF: results of the substrate modification with ablation and antiarrhythmic drugs in nonpermanent atrial fibrillation (SMAN-PAF) trial. Heart Rhythm 2016;13(2):399–406. [DOI] [PubMed] [Google Scholar]
- 522. Kumagai K, et al. A new approach for complete isolation of the posterior left atrium including pulmonary veins for atrial fibrillation. J Cardiovasc Electrophysiol 2007;18(10):1047–1052. [DOI] [PubMed] [Google Scholar]
- 523. Yamaguchi Y, et al. Long-term effects of box isolation on sympathovagal balance in atrial fibrillation. Circ J 2010;74(6):1096–1103. [DOI] [PubMed] [Google Scholar]
- 524. Kumagai K. Catheter ablation of atrial fibrillation. State of the Art. Circ J 2011;75(10):2305–2311. [DOI] [PubMed] [Google Scholar]
- 525. Kim JS, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? A prospective randomized clinical trial. Int J Cardiol 2015;181:277–283. [DOI] [PubMed] [Google Scholar]
- 526. He X, et al. Left atrial posterior wall isolation reduces the recurrence of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol 2016;46(3):267–274. [DOI] [PubMed] [Google Scholar]
- 527. Tamborero D, et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol 2009;2(1):35–40. [DOI] [PubMed] [Google Scholar]
- 528. Di Biase L, et al. Left atrial appendage isolation in patients with long-standing persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol 2016;68(18):1929–1940. [DOI] [PubMed] [Google Scholar]
- 529. Di Biase L, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133(17):1637–1644. [DOI] [PubMed] [Google Scholar]
- 530. Shah D, et al. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol 2003;26(7 Pt 2):1631–1635. [DOI] [PubMed] [Google Scholar]
- 531. Lin D, et al. Provocability of atrial fibrillation triggers during pulmonary vein isolation in patients with infrequent AF [abstract]. Heart Rhythm 2004;1(Suppl):S231. [Google Scholar]
- 532. Di Biase L, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010;121(23):2550–2556. [DOI] [PubMed] [Google Scholar]
- 533. Di Biase L, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122(2):109–118. [DOI] [PubMed] [Google Scholar]
- 534. Santangeli P, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm 2016;13(2):374–382. [DOI] [PubMed] [Google Scholar]
- 535. Lin WS, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003;107(25):3176–3183. [DOI] [PubMed] [Google Scholar]
- 536. Lakkireddy D, et al. Effect of atrial fibrillation ablation on gastric motility: the atrial fibrillation gut study. Circ Arrhythm Electrophysiol 2015;8(3):531–536. [DOI] [PubMed] [Google Scholar]
- 537. Lee RJ, et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J 2015;170(6):1184–1194. [DOI] [PubMed] [Google Scholar]
- 538. Zhao Y, et al. Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm 2016;13(1):141–149. [DOI] [PubMed] [Google Scholar]
- 539. Dixit S, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol 2012;5(2):287–294. [DOI] [PubMed] [Google Scholar]
- 540. Dagres N, et al. Current ablation techniques for persistent atrial fibrillation: results of the European Heart Rhythm Association Survey. Europace 2015;17(10):1596–1600. [DOI] [PubMed] [Google Scholar]
- 541. Hocini M, et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm 2011;8(12):1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Panikker S, et al. Left atrial appendage electrical isolation and concomitant device occlusion: A safety and feasibility study with histologic characterization. Heart Rhythm 2015;12(1):202–210. [DOI] [PubMed] [Google Scholar]
- 543. Panikker S, et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol 2016;9(7). [DOI] [PubMed] [Google Scholar]
- 544. Rillig A, et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol 2016;9(5):e003461. [DOI] [PubMed] [Google Scholar]
- 545. Nademanee K, et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J Am Coll Cardiol 2008;51(8):843–849. [DOI] [PubMed] [Google Scholar]
- 546. Haissaguerre M, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 2006;113(5):616–625. [DOI] [PubMed] [Google Scholar]
- 547. Haissaguerre M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol 2005;16(11):1125–1137. [DOI] [PubMed] [Google Scholar]
- 548. Takahashi Y, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol 2008;51(10):1003–1010. [DOI] [PubMed] [Google Scholar]
- 549. Singh SM, et al. Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms: preliminary assessment of a modified step-wise approach to ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2010;21(6):608–616. [DOI] [PubMed] [Google Scholar]
- 550. Narayan SM, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm 2011;8(2):244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Verma A, et al. Selective CFAE targeting for atrial fibrillation study (SELECT AF): clinical rationale, design, and implementation. J Cardiovasc Electrophysiol 2011;22(5):541–547. [DOI] [PubMed] [Google Scholar]
- 552. Quintanilla JG, et al. Mechanistic approaches to detect, target, and ablate the drivers of atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9(1):e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Kottkamp H, Bender R, Berg J.. Catheter ablation of atrial fibrillation: how to modify the substrate? J Am Coll Cardiol 2015;65(2):196–206. [DOI] [PubMed] [Google Scholar]
- 554. Rolf S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7(5):825–833. [DOI] [PubMed] [Google Scholar]
- 555. Bai R, et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm 2016;13(1):132–140. [DOI] [PubMed] [Google Scholar]
- 556. Cutler MJ, et al. Impact of voltage mapping to guide whether to perform ablation of the posterior wall in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 2016;27(1):13–21. [DOI] [PubMed] [Google Scholar]
- 557. Yang G, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2016;9(2):e003382. [DOI] [PubMed] [Google Scholar]
- 558. Verma A, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 2005;45(2):285–292. [DOI] [PubMed] [Google Scholar]
- 559. Kapa S, et al. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(10):1044–1052. [DOI] [PubMed] [Google Scholar]
- 560. Oakes RS, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119(13):1758–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. McGann C, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Lin YJ, et al. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation 2005;112(12):1692–1700. [DOI] [PubMed] [Google Scholar]
- 563. Narayan SM, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60(7):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Rappel WJ, Narayan SM.. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos 2013;23(2):023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565. Gianni C, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016;13(4):830–835. [DOI] [PubMed] [Google Scholar]
- 566. Narayan SM, Zaman JA.. Mechanistically based mapping of human cardiac fibrillation. J Physiol 2016;594(9):2399–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567. Sommer P, et al. Successful repeat catheter ablation of recurrent long-standing persistent atrial fibrillation with rotor elimination as the procedural endpoint: a case series. J Cardiovasc Electrophysiol 2016;27(3):274–280. [DOI] [PubMed] [Google Scholar]
- 568. Buch E, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm 2016;13(3):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569. Benharash P, et al. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8(3):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Ramanathan C, et al. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 2004;10(4):422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Lim HS, et al. Noninvasive mapping to guide atrial fibrillation ablation. Card Electrophysiol Clin 2015;7(1):89–98. [DOI] [PubMed] [Google Scholar]
- 572. Yamashita S, et al. Body surface mapping to guide atrial fibrillation ablation. Arrhythm Electrophysiol Rev 2015;4(3):172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Guillem MS, et al. Noninvasive mapping of human atrial fibrillation. J Cardiovasc Electrophysiol 2009;20(5):507–513. [DOI] [PubMed] [Google Scholar]
- 574. Guillem MS, et al. Noninvasive localization of maximal frequency sites of atrial fibrillation by body surface potential mapping. Circ Arrhythm Electrophysiol 2013;6(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Rodrigo M, et al. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: a clinical-computational study. Heart Rhythm 2014;11(9):1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576. Pauza DH, et al. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000;259(4):353–382. [DOI] [PubMed] [Google Scholar]
- 577. Scherlag BJ, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol 2005;13(Suppl 1):37–42. [DOI] [PubMed] [Google Scholar]
- 578. Patterson E, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol 2006;47(6):1196–1206. [DOI] [PubMed] [Google Scholar]
- 579. Lemola K, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation 2008;117(4):470–477. [DOI] [PubMed] [Google Scholar]
- 580. Nishida K, et al. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc Res 2011;89(4):825–833. [DOI] [PubMed] [Google Scholar]
- 581. Nishida K, et al. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol 2014;64(8):823–831. [DOI] [PubMed] [Google Scholar]
- 582. Stavrakis S, et al. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol 2015;1(1-2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Pokushalov E, et al. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2010;33(10):1231–1238. [DOI] [PubMed] [Google Scholar]
- 584. Pokushalov E, et al. Ganglionated plexi ablation for long-standing persistent atrial fibrillation. Europace 2010;12(3):342–346. [DOI] [PubMed] [Google Scholar]
- 585. Pokushalov E, et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol 2013;24(12):1338–1343. [DOI] [PubMed] [Google Scholar]
- 586. Pokushalov E, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm 2013;10(9):1280–1286. [DOI] [PubMed] [Google Scholar]
- 587. Morillo CA, et al. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation 1995;91(5):1588–1595. [DOI] [PubMed] [Google Scholar]
- 588. Harada A, et al. Atrial activation during chronic atrial fibrillation in patients with isolated mitral valve disease. Ann Thorac Surg 1996;61(1):104–111. discussion 111-2. [DOI] [PubMed] [Google Scholar]
- 589. Gray RA, Pertsov AM, Jalife J.. Spatial and temporal organization during cardiac fibrillation. Nature 1998;392(6671):75–78. [DOI] [PubMed] [Google Scholar]
- 590. Berenfeld O, et al. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the Langendorff-perfused sheep heart. J Cardiovasc Electrophysiol 2000;11(8):869–879. [DOI] [PubMed] [Google Scholar]
- 591. Mansour M, et al. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation 2001;103(21):2631–2636. [DOI] [PubMed] [Google Scholar]
- 592. Lazar S, et al. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation 2004;110(20):3181–3186. [DOI] [PubMed] [Google Scholar]
- 593. Atienza F, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Atienza F, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol 2014;64(23):2455–2467. [DOI] [PubMed] [Google Scholar]
- 595. Zhao Q, et al. Effect of renal sympathetic denervation on the inducibility of atrial fibrillation during rapid atrial pacing. J Interv Card Electrophysiol 2012;35(2):119–125. [DOI] [PubMed] [Google Scholar]
- 596. Wang X, et al. Effect of renal sympathetic denervation on the progression of paroxysmal atrial fibrillation in canines with long-term intermittent atrial pacing. Europace 2015;17(4):647–654. [DOI] [PubMed] [Google Scholar]
- 597. Zhou Q, et al. Renal sympathetic denervation suppresses atrial fibrillation induced by acute atrial ischemia/infarction through inhibition of cardiac sympathetic activity. Int J Cardiol 2016;203:187–195. [DOI] [PubMed] [Google Scholar]
- 598. Vollmann D, et al. Renal artery ablation instead of pulmonary vein ablation in a hypertensive patient with symptomatic, drug-resistant, persistent atrial fibrillation. Clin Res Cardiol 2013;102(4):315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599. Schlaich MP, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 2009;20(5):933–939. [DOI] [PubMed] [Google Scholar]
- 600. Bakris GL, et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J Am Coll Cardiol 2015;65(13):1314–1321. [DOI] [PubMed] [Google Scholar]
- 601. Boersma LV, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125(1):23–30. [DOI] [PubMed] [Google Scholar]
- 602. Wang S, Liu L, Zou C.. Comparative study of video-assisted thoracoscopic surgery ablation and radiofrequency catheter ablation on treating paroxysmal atrial fibrillation: a randomized, controlled short-term trial. Chin Med J (Engl) 2014;127(14):2567–2570. [PubMed] [Google Scholar]
- 603. Phan K, et al. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardiothorac Surg 2016;49(4):1044–1051. [DOI] [PubMed] [Google Scholar]
- 604. Krul SP, et al. Epicardial and endocardial electrophysiological guided thoracoscopic surgery for atrial fibrillation: a multidisciplinary approach of atrial fibrillation ablation in challenging patients. Int J Cardiol 2014;173(2):229–235. [DOI] [PubMed] [Google Scholar]
- 605. Probst J, et al. Thoracoscopic epicardial left atrial ablation in symptomatic patients with atrial fibrillation. Europace 2016;18(10):1538–1544. [DOI] [PubMed] [Google Scholar]
- 606. Mahapatra S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91(6):1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607. Gersak B, et al. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23(10):1059–1066. [DOI] [PubMed] [Google Scholar]
- 608. Pison L, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60(1):54–61. [DOI] [PubMed] [Google Scholar]
- 609. Zembala M, et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol 2012;70(8):819–828. [PubMed] [Google Scholar]
- 610. Bisleri G, et al. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg 2013;44(5):919–923. [DOI] [PubMed] [Google Scholar]
- 611. Gehi AK, et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10(1):22–28. [DOI] [PubMed] [Google Scholar]
- 612. La Meir M, et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol 2013;167(4):1469–1475. [DOI] [PubMed] [Google Scholar]
- 613. Gersak B, et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg 2014;147(4):1411–1416. [DOI] [PubMed] [Google Scholar]
- 614. Kurfirst V, et al. Two-staged hybrid treatment of persistent atrial fibrillation: short-term single-centre results. Interact Cardiovasc Thorac Surg 2014;18(4):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Pison L, et al. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg 2014;3(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Bulava A, et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4(3):e001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Raitt MH, et al. Reversal of electrical remodeling after cardioversion of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(5):507–512. [DOI] [PubMed] [Google Scholar]
- 618. Chalfoun N, et al. Reverse electrical remodeling of the atria post cardioversion in patients who remain in sinus rhythm assessed by signal averaging of the P-wave. Pacing Clin Electrophysiol 2007;30(4):502–509. [DOI] [PubMed] [Google Scholar]
- 619. Igarashi M, et al. Effect of restoration of sinus rhythm by extensive antiarrhythmic drugs in predicting results of catheter ablation of persistent atrial fibrillation. Am J Cardiol 2010;106(1):62–68. [DOI] [PubMed] [Google Scholar]
- 620. Rivard L, et al. Improved outcome following restoration of sinus rhythm prior to catheter ablation of persistent atrial fibrillation: a comparative multicenter study. Heart Rhythm 2012;9(7):1025–1030. [DOI] [PubMed] [Google Scholar]
- 621. Mohanty S, et al. Effect of periprocedural amiodarone on procedure outcome in patients with long-standing persistent atrial fibrillation undergoing extended pulmonary vein antrum isolation: results from a randomized study (SPECULATE). Heart Rhythm 2015;12(3):477–483. [DOI] [PubMed] [Google Scholar]
- 622. Gerstenfeld EP, et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 2004;110(11):1351–1357. [DOI] [PubMed] [Google Scholar]
- 623. Chugh A, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol 2005;46(1):83–91. [DOI] [PubMed] [Google Scholar]
- 624. Chugh A, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2005;2(5):464–471. [DOI] [PubMed] [Google Scholar]
- 625. Deisenhofer I, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 2006;8(8):573–582. [DOI] [PubMed] [Google Scholar]
- 626. Mesas CE, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J Am Coll Cardiol 2004;44(5):1071–1079. [DOI] [PubMed] [Google Scholar]
- 627. Anter E, et al. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm 2016;13(10):2048–2055. [DOI] [PubMed] [Google Scholar]
- 628. Shah D, et al. Narrow, slow-conducting isthmus dependent left atrial reentry developing after ablation for atrial fibrillation: ECG characterization and elimination by focal RF ablation. J Cardiovasc Electrophysiol 2006;17(5):508–515. [DOI] [PubMed] [Google Scholar]
- 629. Chugh A, et al. Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation 2006;113(5):609–615. [DOI] [PubMed] [Google Scholar]
- 630. Gerstenfeld EP, et al. Surface electrocardiogram characteristics of atrial tachycardias occurring after pulmonary vein isolation. Heart Rhythm 2007;4(9):1136–1143. [DOI] [PubMed] [Google Scholar]
- 631. Steven D, et al. Mapping of atrial tachycardias after catheter ablation for atrial fibrillation: use of bi-atrial activation patterns to facilitate recognition of origin. Heart Rhythm 2010;7(5):664–672. [DOI] [PubMed] [Google Scholar]
- 632. Anter E, et al. Pulmonary vein isolation using the Rhythmia mapping system: Verification of intracardiac signals using the Orion mini-basket catheter. Heart Rhythm 2015;12(9):1927–1934. [DOI] [PubMed] [Google Scholar]
- 633. Di Biase L, et al. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm 2011;8(3):368–372. [DOI] [PubMed] [Google Scholar]
- 634. Hutchinson MD, et al. Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm 2013;10(3):347–353. [DOI] [PubMed] [Google Scholar]
- 635. Nair KK, et al. The prevalence and risk factors for atrioesophageal fistula after percutaneous radiofrequency catheter ablation for atrial fibrillation: the Canadian experience. J Interv Card Electrophysiol 2014;39(2):139–144. [DOI] [PubMed] [Google Scholar]
- 636. Di Biase L, et al. Relationship between catheter forces, lesion characteristics, “popping,” and char formation: experience with robotic navigation system. J Cardiovasc Electrophysiol 2009;20(4):436–440. [DOI] [PubMed] [Google Scholar]
- 637. Di Biase L, et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol 2009;2(2):108–112. [DOI] [PubMed] [Google Scholar]
- 638. Martinek M, et al. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol 2009;20(7):726–733. [DOI] [PubMed] [Google Scholar]
- 639. Martinek M, et al. Acute development of gastroesophageal reflux after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm 2009;6(10):1457–1462. [DOI] [PubMed] [Google Scholar]
- 640. Sadek MM, et al. Recurrent atrial arrhythmias in the setting of chronic pulmonary vein isolation. Heart Rhythm 2016;13(11):2174–2180. [DOI] [PubMed] [Google Scholar]
- 641. Elayi CS, et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm 2010;7(9):1216–1223. [DOI] [PubMed] [Google Scholar]
- 642. Lim HS, et al. Is ablation to termination the best strategy for ablation of persistent atrial fibrillation? Persistent atrial fibrillation is best ablated by a strategy that terminates the arrhythmia: procedural termination is associated with improved long-term outcomes. Circ Arrhythm Electrophysiol 2015;8(4):963–971. [DOI] [PubMed] [Google Scholar]
- 643. Kochhauser S, et al. Impact of acute atrial fibrillation termination and prolongation of atrial fibrillation cycle length on the outcome of ablation of persistent atrial fibrillation: a substudy of the STAR AF II trial. Heart Rhythm 2017;14(4):476–483. [DOI] [PubMed] [Google Scholar]
- 644. Scott PA, Silberbauer J, Murgatroyd FD.. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace 2016;18(3):359–367. [DOI] [PubMed] [Google Scholar]
- 645. Zaman JA, Peters NS, Narayan SM.. Rotor mapping and ablation to treat atrial fibrillation. Curr Opin Cardiol 2015;30(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646. Bai R, et al. Ablation of perimitral flutter following catheter ablation of atrial fibrillation: impact on outcomes from a randomized study (PROPOSE). J Cardiovasc Electrophysiol 2012;23(2):137–144. [DOI] [PubMed] [Google Scholar]
- 647. Bai R, et al. Worldwide experience with the robotic navigation system in catheter ablation of atrial fibrillation: methodology, efficacy and safety. J Cardiovasc Electrophysiol 2012;23(8):820–826. [DOI] [PubMed] [Google Scholar]
- 648. Kobza R, et al. Late recurrent arrhythmias after ablation of atrial fibrillation: incidence, mechanisms, and treatment. Heart Rhythm 2004;1(6):676–683. [DOI] [PubMed] [Google Scholar]
- 649. Ouyang F, et al. Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation 2005;112(20):3038–3048. [DOI] [PubMed] [Google Scholar]
- 650. Boersma LV, et al. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm 2008;5(12):1635–1642. [DOI] [PubMed] [Google Scholar]
- 651. Jais P, et al. Successful irrigated-tip catheter ablation of atrial flutter resistant to conventional radiofrequency ablation. Circulation 1998;98(9):835–838. [DOI] [PubMed] [Google Scholar]
- 652. Haines DE. The biophysics and pathophysiology of lesion formation during radiofrequency catheter ablation In: Zipes D JJ, editor. Cardiac Electrophysiology: From Cell to Bedside. 4th ed New York: WB Saunders; 2006. p. 1018–1027. [Google Scholar]
- 653. Haines DE, et al. The biophysics of passive convective cooling during catheter ablation with gold versus platinum electrodes and multielectrode phased radiofrequency energy delivery. J Cardiovasc Electrophysiol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 654. Nakagawa H, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation 1995;91(8):2264–2273. [DOI] [PubMed] [Google Scholar]
- 655. Reddy VY, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) study. Circulation 2015;132(10):907–915. [DOI] [PubMed] [Google Scholar]
- 656. Finta B, Haines DE.. Catheter ablation therapy for atrial fibrillation. Cardiol Clin 2004;22(1):127–145. ix. [DOI] [PubMed] [Google Scholar]
- 657. Thomas SP, et al. A comparison of open irrigated and non-irrigated tip catheter ablation for pulmonary vein isolation. Europace 2004;6(4):330–335. [DOI] [PubMed] [Google Scholar]
- 658. Bruce GK, et al. Discrepancies between catheter tip and tissue temperature in cooled-tip ablation: relevance to guiding left atrial ablation. Circulation 2005;112(7):954–960. [DOI] [PubMed] [Google Scholar]
- 659. Oh S, et al. Avoiding microbubbles formation during radiofrequency left atrial ablation versus continuous microbubbles formation and standard radiofrequency ablation protocols: comparison of energy profiles and chronic lesion characteristics. J Cardiovasc Electrophysiol 2006;17(1):72–77. [DOI] [PubMed] [Google Scholar]
- 660. Tanno K, et al. Histopathology of canine hearts subjected to catheter ablation using radiofrequency energy. Jpn Circ J 1994;58(2):123–135. [DOI] [PubMed] [Google Scholar]
- 661. Nath S, et al. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation 1993;88(4 Pt 1):1826–1831. [DOI] [PubMed] [Google Scholar]
- 662. Everett THt, et al. Role of calcium in acute hyperthermic myocardial injury. J Cardiovasc Electrophysiol 2001;12(5):563–569. [DOI] [PubMed] [Google Scholar]
- 663. Saad EB, et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation 2003;108(25):3102–3107. [DOI] [PubMed] [Google Scholar]
- 664. Bunch TJ, et al. Analysis of catheter-tip (8-mm) and actual tissue temperatures achieved during radiofrequency ablation at the orifice of the pulmonary vein. Circulation 2004;110(19):2988–2995. [DOI] [PubMed] [Google Scholar]
- 665. Haines DE. Determinants of lesion size during radiofrequency catheter ablation: the role of electrode-tissue contact pressure and duration of energy delivery. Journal of Cardiovascular Electrophysiology 1991;2(6):509–515. [Google Scholar]
- 666. Strickberger SA, et al. Relation between impedance and endocardial contact during radiofrequency catheter ablation. Am Heart J 1994;128(2):226–229. [DOI] [PubMed] [Google Scholar]
- 667. Avitall B, et al. The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol 1997;20(12 Pt 1):2899–2910. [DOI] [PubMed] [Google Scholar]
- 668. Yokoyama K, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008;1(5):354–362. [DOI] [PubMed] [Google Scholar]
- 669. Kuck KH, et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 2012;9(1):18–23. [DOI] [PubMed] [Google Scholar]
- 670. Ikeda A, et al. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode-tissue contact force and lesion size. Circ Arrhythm Electrophysiol 2014;7(6):1174–1180. [DOI] [PubMed] [Google Scholar]
- 671. Nakagawa H, et al. Locations of high contact force during left atrial mapping in atrial fibrillation patients: electrogram amplitude and impedance are poor predictors of electrode-tissue contact force for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6(4):746–753. [DOI] [PubMed] [Google Scholar]
- 672. Nakagawa H, et al. Prospective study to test the ability to create RF lesions at predicted depth and diameter using a new formula incorporating contact force, radiofrequency power and application time (force-power-time index) in the beating heart [abstract]. Heart Rhythm 2014;11(Suppl):S548. [Google Scholar]
- 673. Natale A, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64(7):647–656. [DOI] [PubMed] [Google Scholar]
- 674. Kumar S, et al. Predictive value of impedance changes for real-time contact force measurements during catheter ablation of atrial arrhythmias in humans. Heart Rhythm 2013;10(7):962–969. [DOI] [PubMed] [Google Scholar]
- 675. Kumar S, et al. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol 2012;5(6):1124–1129. [DOI] [PubMed] [Google Scholar]
- 676. Reddy VY, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9(11):1789–1795. [DOI] [PubMed] [Google Scholar]
- 677. Haldar S, et al. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int J Cardiol 2013;168(2):1160–1166. [DOI] [PubMed] [Google Scholar]
- 678. Perna F, et al. Assessment of catheter tip contact force resulting in cardiac perforation in swine atria using force sensing technology. Circ Arrhythm Electrophysiol 2011;4(2):218–224. [DOI] [PubMed] [Google Scholar]
- 679. Kimura M, et al. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm 2014;11(6):984–991. [DOI] [PubMed] [Google Scholar]
- 680. Sohns C, et al. Quantitative magnetic resonance imaging analysis of the relationship between contact force and left atrial scar formation after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(2):138–145. [DOI] [PubMed] [Google Scholar]
- 681. Martinek M, et al. Clinical impact of an open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. Pacing Clin Electrophysiol 2012;35(11):1312–1318. [DOI] [PubMed] [Google Scholar]
- 682. Marijon E, et al. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J Cardiovasc Electrophysiol 2014;25(2):130–137. [DOI] [PubMed] [Google Scholar]
- 683. Sigmund E, et al. Optimizing radiofrequency ablation of paroxysmal and persistent atrial fibrillation by direct catheter force measurement-a case-matched comparison in 198 patients. Pacing Clin Electrophysiol 2015;38(2):201–208. [DOI] [PubMed] [Google Scholar]
- 684. Wilber DJ, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303(4):333–340. [DOI] [PubMed] [Google Scholar]
- 685. Ullah W, et al. Randomized trial comparing pulmonary vein isolation using the SmartTouch catheter with or without real-time contact force data. Heart Rhythm 2016;13(9):1761–1767. [DOI] [PubMed] [Google Scholar]
- 686. Wakili R, et al. Impact of real-time contact force and impedance measurement in pulmonary vein isolation procedures for treatment of atrial fibrillation. Clin Res Cardiol 2014;103(2):97–106. [DOI] [PubMed] [Google Scholar]
- 687. Van Belle Y, et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J 2007;28(18):2231–2237. [DOI] [PubMed] [Google Scholar]
- 688. Neumann T, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol 2008;52(4):273–278. [DOI] [PubMed] [Google Scholar]
- 689. Reddy VY, et al. Durability of pulmonary vein isolation with cryoballoon ablation: results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) study. J Cardiovasc Electrophysiol 2015;26(5):493–500. [DOI] [PubMed] [Google Scholar]
- 690. Coulombe N, Paulin J, Su W.. Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J Cardiovasc Electrophysiol 2013;24(8):919–925. [DOI] [PubMed] [Google Scholar]
- 691. Straube F, et al. Comparison of the first and second cryoballoon: high-volume single-center safety and efficacy analysis. Circ Arrhythm Electrophysiol 2014;7(2):293–299. [DOI] [PubMed] [Google Scholar]
- 692. Straube F, et al. Differences of two cryoballoon generations: insights from the prospective multicentre, multinational FREEZE Cohort Substudy. Europace 2014;16(10):1434–1442. [DOI] [PubMed] [Google Scholar]
- 693. Schmidt M, et al. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: results from the German Ablation Registry. J Cardiovasc Electrophysiol 2014;25(1):1–7. [DOI] [PubMed] [Google Scholar]
- 694. Linhart M, et al. Comparison of cryoballoon and radiofrequency ablation of pulmonary veins in 40 patients with paroxysmal atrial fibrillation: a case-control study. J Cardiovasc Electrophysiol 2009;20(12):1343–1348. [DOI] [PubMed] [Google Scholar]
- 695. Aryana A, et al. Acute and long-term outcomes of catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: a multicenter experience. J Cardiovasc Electrophysiol 2015;26(8):832–839. [DOI] [PubMed] [Google Scholar]
- 696. Hunter RJ, et al. Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: a randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (The Cryo Versus RF Trial). J Cardiovasc Electrophysiol 2015;26(12):1307–1314. [DOI] [PubMed] [Google Scholar]
- 697. Furnkranz A, et al. Rationale and Design of FIRE AND ICE: A multicenter randomized trial comparing efficacy and safety of pulmonary vein isolation using a cryoballoon versus radiofrequency ablation with 3D-reconstruction. J Cardiovasc Electrophysiol 2014;25(12):1314–1320. [DOI] [PubMed] [Google Scholar]
- 698. Namdar M, et al. Isolating the pulmonary veins as first-line therapy in patients with lone paroxysmal atrial fibrillation using the cryoballoon. Europace 2012;14(2):197–203. [DOI] [PubMed] [Google Scholar]
- 699. Dukkipati SR, et al. Visual balloon-guided point-by-point ablation: reliable, reproducible, and persistent pulmonary vein isolation. Circ Arrhythm Electrophysiol 2010;3(3):266–273. [DOI] [PubMed] [Google Scholar]
- 700. Metzner A, et al. Esophageal temperature change and esophageal thermal lesions after pulmonary vein isolation using the novel endoscopic ablation system. Heart Rhythm 2011;8(6):815–820. [DOI] [PubMed] [Google Scholar]
- 701. Metzner A, et al. One-year clinical outcome after pulmonary vein isolation using the novel endoscopic ablation system in patients with paroxysmal atrial fibrillation. Heart Rhythm 2011;8(7):988–993. [DOI] [PubMed] [Google Scholar]
- 702. Koruth JS, et al. Pre-clinical investigation of a low-intensity collimated ultrasound system for pulmonary vein isolation in a porcine model. JACC: Clinical Electrophysiology 2015;1(4):306–314. [DOI] [PubMed] [Google Scholar]
- 703. Meininger GR, et al. Initial experience with a novel focused ultrasound ablation system for ring ablation outside the pulmonary vein. J Interv Card Electrophysiol 2003;8(2):141–148. [DOI] [PubMed] [Google Scholar]
- 704. Metzner A, et al. Long-term clinical outcome following pulmonary vein isolation with high-intensity focused ultrasound balloon catheters in patients with paroxysmal atrial fibrillation. Europace 2010;12(2):188–193. [DOI] [PubMed] [Google Scholar]
- 705. Neven K, et al. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circ Arrhythm Electrophysiol 2010;3(3):260–265. [DOI] [PubMed] [Google Scholar]
- 706. Sohara H, et al. Feasibility of the radiofrequency hot balloon catheter for isolation of the posterior left atrium and pulmonary veins for the treatment of atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2(3):225–232. [DOI] [PubMed] [Google Scholar]
- 707. Evonich RF, Nori DM, Haines DE.. Efficacy of pulmonary vein isolation with a novel hot balloon ablation catheter. J Interv Card Electrophysiol 2012;34(1):29–36. [DOI] [PubMed] [Google Scholar]
- 708. Yamaguchi Y, et al. Long-term results of radiofrequency hot balloon ablation in patients with paroxysmal atrial fibrillation: safety and rhythm outcomes. J Cardiovasc Electrophysiol 2015;26(12):1298–1306. [DOI] [PubMed] [Google Scholar]
- 709. Sohara H, et al. HotBalloon ablation of the pulmonary veins for paroxysmal AF: a multicenter randomized trial in Japan. J Am Coll Cardiol 2016;68(25):2747–2757. [DOI] [PubMed] [Google Scholar]
- 710. Mansour M, et al. Initial experience with the Mesh catheter for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Heart Rhythm 2008;5(11):1510–1516. [DOI] [PubMed] [Google Scholar]
- 711. Steinwender C, et al. Acute results of pulmonary vein isolation in patients with paroxysmal atrial fibrillation using a single mesh catheter. J Cardiovasc Electrophysiol 2009;20(2):147–152. [DOI] [PubMed] [Google Scholar]
- 712. Hofmann R, et al. Pulmonary vein isolation with Mesh Ablator versus cryoballoon catheters: 6-month outcomes. J Interv Card Electrophysiol 2010;29(3):179–185. [DOI] [PubMed] [Google Scholar]
- 713. Steinwender C, et al. One-year follow-up after pulmonary vein isolation using a single mesh catheter in patients with paroxysmal atrial fibrillation. Heart Rhythm 2010;7(3):333–339. [DOI] [PubMed] [Google Scholar]
- 714. Koch L, et al. Mesh ablator vs. cryoballoon pulmonary vein ablation of symptomatic paroxysmal atrial fibrillation: results of the MACPAF study. Europace 2012;14(10):1441–1449. [DOI] [PubMed] [Google Scholar]
- 715. Shin DI, et al. Initial results of using a novel irrigated multielectrode mapping and ablation catheter for pulmonary vein isolation. Heart Rhythm 2014;11(3):375–383. [DOI] [PubMed] [Google Scholar]
- 716. Zellerhoff S, et al. Pulmonary vein isolation using a circular, open irrigated mapping and ablation catheter (nMARQ): a report on feasibility and efficacy. Europace 2014;16(9):1296–1303. [DOI] [PubMed] [Google Scholar]
- 717. Rodriguez-Entem F, et al. Initial experience and treatment of atrial fibrillation using a novel irrigated multielectrode catheter: Results from a prospective two-center study. J Arrhythm 2016;32(2):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718. Stabile G, et al. Safety and efficacy of pulmonary vein isolation using a circular, open-irrigated mapping and ablation catheter: A multicenter registry. Heart Rhythm 2015;12(8):1782–1788. [DOI] [PubMed] [Google Scholar]
- 719. Deneke T, et al. Acute safety and efficacy of a novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. J Cardiovasc Electrophysiol 2014;25(4):339–345. [DOI] [PubMed] [Google Scholar]
- 720. Vurma M, et al. Safety and efficacy of the nMARQ catheter for paroxysmal and persistent atrial fibrillation. Europace 2016;18(8):1164–1169. [DOI] [PubMed] [Google Scholar]
- 721. Rosso R, et al. Radiofrequency ablation of paroxysmal atrial fibrillation with the new irrigated multipolar nMARQ ablation catheter: verification of intracardiac signals with a second circular mapping catheter. Heart Rhythm 2014;11(4):559–565. [DOI] [PubMed] [Google Scholar]
- 722. Kiss A, et al. Cerebral microembolization during atrial fibrillation ablation: comparison of different single-shot ablation techniques. Int J Cardiol 2014;174(2):276–281. [DOI] [PubMed] [Google Scholar]
- 723. Gaita F, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J Cardiovasc Electrophysiol 2011;22(9):961–968. [DOI] [PubMed] [Google Scholar]
- 724. Herrera Siklody C, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58(7):681–688. [DOI] [PubMed] [Google Scholar]
- 725. De Greef Y, et al. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation: comparative 3-year data. Europace 2014;16(6):820–825. [DOI] [PubMed] [Google Scholar]
- 726. McCready J, et al. Safety and efficacy of multipolar pulmonary vein ablation catheter vs. irrigated radiofrequency ablation for paroxysmal atrial fibrillation: a randomized multicentre trial. Europace 2014;16(8):1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727. Wasmer K, et al. Safety profile of multielectrode-phased radiofrequency pulmonary vein ablation catheter and irrigated radiofrequency catheter. Europace 2016;18(1):78–84. [DOI] [PubMed] [Google Scholar]
- 728. Verma A, et al. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol 2013;6(5):835–842. [DOI] [PubMed] [Google Scholar]
- 729. Nagy-Balo E, et al. Predictors of cerebral microembolization during phased radiofrequency ablation of atrial fibrillation: analysis of biophysical parameters from the ablation generator. Heart Rhythm 2014;11(6):977–983. [DOI] [PubMed] [Google Scholar]
- 730. Zellerhoff S, et al. Modified phased radiofrequency ablation of atrial fibrillation reduces the number of cerebral microembolic signals. Europace 2014;16(3):341–346. [DOI] [PubMed] [Google Scholar]
- 731. De Greef Y, et al. Low rate of asymptomatic cerebral embolism and improved procedural efficiency with the novel pulmonary vein ablation catheter GOLD: results of the PRECISION GOLD trial. Europace 2016;18(5):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732. Leitz P, et al. Data on procedural handling and complications of pulmonary vein isolation using the pulmonary vein ablation catheter GOLD(R). Europace 2016;18(5):696–701. [DOI] [PubMed] [Google Scholar]
- 733. Hummel J, et al. Phased RF ablation in persistent atrial fibrillation. Heart Rhythm 2014;11(2):202–209. [DOI] [PubMed] [Google Scholar]
- 734. Boersma LV, et al. Multielectrode pulmonary vein isolation versus single tip wide area catheter ablation for paroxysmal atrial fibrillation: a multinational multicenter randomized clinical trial. Circ Arrhythm Electrophysiol 2016;9(4):e003151. [DOI] [PubMed] [Google Scholar]
- 735. Bourier F, et al. Sensor-Based Electromagnetic Navigation (Mediguide(R)): How Accurate Is It? A Phantom Model Study. J Cardiovasc Electrophysiol 2015;26(10):1140–1145. [DOI] [PubMed] [Google Scholar]
- 736. Nakagawa H, et al. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circ Arrhythm Electrophysiol 2012;5(2):417–424. [DOI] [PubMed] [Google Scholar]
- 737. Dong J, et al. Integrated electroanatomic mapping with three-dimensional computed tomographic images for real-time guided ablations. Circulation 2006;113(2):186–194. [DOI] [PubMed] [Google Scholar]
- 738. Kistler PM, et al. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006;17(10):1093–1101. [DOI] [PubMed] [Google Scholar]
- 739. Ector J, et al. Biplane three-dimensional augmented fluoroscopy as single navigation tool for ablation of atrial fibrillation: accuracy and clinical value. Heart Rhythm 2008;5(7):957–964. [DOI] [PubMed] [Google Scholar]
- 740. Sporton SC, et al. Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. J Cardiovasc Electrophysiol 2004;15(3):310–315. [DOI] [PubMed] [Google Scholar]
- 741. Estner HL, et al. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX). Europace 2006;8(8):583–587. [DOI] [PubMed] [Google Scholar]
- 742. Scaglione M, et al. Visualization of multiple catheters with electroanatomical mapping reduces X-ray exposure during atrial fibrillation ablation. Europace 2011;13(7):955–962. [DOI] [PubMed] [Google Scholar]
- 743. Martinek M, et al. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol 2007;30(10):1215–1223. [DOI] [PubMed] [Google Scholar]
- 744. Kistler PM, et al. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. Eur Heart J 2008;29(24):3029–3036. [DOI] [PubMed] [Google Scholar]
- 745. Bertaglia E, et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 2009;11(8):1004–1010. [DOI] [PubMed] [Google Scholar]
- 746. Della Bella P, et al. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J Cardiovasc Electrophysiol 2009;20(3):258–265. [DOI] [PubMed] [Google Scholar]
- 747. Caponi D, et al. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome? A randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 2010;12(8):1098–1104. [DOI] [PubMed] [Google Scholar]
- 748. Filgueiras-Rama D, et al. Remote magnetic navigation for accurate, real-time catheter positioning and ablation in cardiac electrophysiology procedures. J Vis Exp 2013(74). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749. Proietti R, et al. Remote magnetic with open-irrigated catheter vs. manual navigation for ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2013;15(9):1241–1248. [DOI] [PubMed] [Google Scholar]
- 750. Wutzler A, et al. Robotic ablation of atrial fibrillation with a new remote catheter system. J Interv Card Electrophysiol 2014;40(3):215–219. [DOI] [PubMed] [Google Scholar]
- 751. Dello Russo A, et al. Analysis of catheter contact force during atrial fibrillation ablation using the robotic navigation system: results from a randomized study. J Interv Card Electrophysiol 2016;46(2):97–103. [DOI] [PubMed] [Google Scholar]
- 752. Faddis MN, et al. Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 2002;106(23):2980–2985. [DOI] [PubMed] [Google Scholar]
- 753. Greenberg S, et al. Remote controlled magnetically guided pulmonary vein isolation in canines. Heart Rhythm 2006;3(1):71–76. [DOI] [PubMed] [Google Scholar]
- 754. Pappone C, et al. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol 2006;47(7):1390–1400. [DOI] [PubMed] [Google Scholar]
- 755. Saliba W, et al. Atrial fibrillation ablation using a robotic catheter remote control system: initial human experience and long-term follow-up results. J Am Coll Cardiol 2008;51(25):2407–2411. [DOI] [PubMed] [Google Scholar]
- 756. Wazni OM, et al. Experience with the hansen robotic system for atrial fibrillation ablation–lessons learned and techniques modified: Hansen in the real world. J Cardiovasc Electrophysiol 2009;20(11):1193–1196. [DOI] [PubMed] [Google Scholar]
- 757. Bradfield J, et al. Catheter ablation utilizing remote magnetic navigation: a review of applications and outcomes. Pacing Clin Electrophysiol 2012;35(8):1021–1034. [DOI] [PubMed] [Google Scholar]
- 758. Hlivak P, et al. Robotic navigation in catheter ablation for paroxysmal atrial fibrillation: midterm efficacy and predictors of postablation arrhythmia recurrences. J Cardiovasc Electrophysiol 2011;22(5):534–540. [DOI] [PubMed] [Google Scholar]
- 759. Willems S, et al. Persistence of pulmonary vein isolation after robotic remote-navigated ablation for atrial fibrillation and its relation to clinical outcome. J Cardiovasc Electrophysiol 2010;21(10):1079–1084. [DOI] [PubMed] [Google Scholar]
- 760. Troianos CA, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011;24(12):1291–1318. [DOI] [PubMed] [Google Scholar]
- 761. Wynn GJ, et al. Improving safety in catheter ablation for atrial fibrillation: a prospective study of the use of ultrasound to guide vascular access. J Cardiovasc Electrophysiol 2014;25(7):680–685. [DOI] [PubMed] [Google Scholar]
- 762. Saliba W, Thomas J.. Intracardiac echocardiography during catheter ablation of atrial fibrillation. Europace 2008;10(Suppl 3) iii42–47. [DOI] [PubMed] [Google Scholar]
- 763. Ferguson JD, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol 2009;2(6):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764. Kim SS, et al. The use of intracardiac echocardiography and other intracardiac imaging tools to guide noncoronary cardiac interventions. J Am Coll Cardiol 2009;53(23):2117–2128. [DOI] [PubMed] [Google Scholar]
- 765. Schmidt M, et al. Intracardiac echocardiography improves procedural efficiency during cryoballoon ablation for atrial fibrillation: a pilot study. J Cardiovasc Electrophysiol 2010;21(11):1202–1207. [DOI] [PubMed] [Google Scholar]
- 766. Packer DL, et al. New generation of electro-anatomic mapping: full intracardiac ultrasound image integration. Europace 2008;10(Suppl 3):iii35–iii41. [DOI] [PubMed] [Google Scholar]
- 767. Rostock T, et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol 2008;51(22):2153–2160. [DOI] [PubMed] [Google Scholar]
- 768. Ren JF, Marchlinski FE, Callans DJ.. Left atrial thrombus associated with ablation for atrial fibrillation: identification with intracardiac echocardiography. J Am Coll Cardiol 2004;43(10):1861–1867. [DOI] [PubMed] [Google Scholar]
- 769. Vasamreddy CR, et al. Technique and results of pulmonary vein angiography in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(1):21–26. [DOI] [PubMed] [Google Scholar]
- 770. Strohmer B, Schernthaner C, Pichler M.. Simultaneous angiographic imaging of ipsilateral pulmonary veins for catheter ablation of atrial fibrillation. Clin Res Cardiol 2006;95(11):591–599. [DOI] [PubMed] [Google Scholar]
- 771. Fynn SP, Kalman JM.. Pulmonary veins: anatomy, electrophysiology, tachycardia, and fibrillation. Pacing Clin Electrophysiol 2004;27(11):1547–1559. [DOI] [PubMed] [Google Scholar]
- 772. Walters TE, Ellims AH, Kalman JM.. The role of left atrial imaging in the management of atrial fibrillation. Prog Cardiovasc Dis 2015;58(2):136–151. [DOI] [PubMed] [Google Scholar]
- 773. Lin WS, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation 2000;101(11):1274–1281. [DOI] [PubMed] [Google Scholar]
- 774. Tsao HM, et al. Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2001;12(12):1353–1357. [DOI] [PubMed] [Google Scholar]
- 775. Perez-Lugones A, et al. Three-dimensional reconstruction of pulmonary veins in patients with atrial fibrillation and controls: morphological characteristics of different veins. Pacing Clin Electrophysiol 2003;26(1 Pt 1):8–15. [DOI] [PubMed] [Google Scholar]
- 776. Schwartzman D, Lacomis J, Wigginton WG.. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol 2003;41(8):1349–1357. [DOI] [PubMed] [Google Scholar]
- 777. Lickfett L, et al. Characterization of a new pulmonary vein variant using magnetic resonance angiography: incidence, imaging, and interventional implications of the “right top pulmonary vein”. J Cardiovasc Electrophysiol 2004;15(5):538–543. [DOI] [PubMed] [Google Scholar]
- 778. Mansour M, et al. Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(4):387–393. [DOI] [PubMed] [Google Scholar]
- 779. Chun KR, et al. The ′single big cryoballoon′ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J 2009;30(6):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780. Donal E, et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging 2016;17(4):355–383. [DOI] [PubMed] [Google Scholar]
- 781. Hadid C, et al. Value of intraprocedural radiologic rotational angiography in atrial fibrillation ablation. Comparison with other imaging techniques. Rev Esp Cardiol (Engl Ed) 2012;65(6):574–575. [DOI] [PubMed] [Google Scholar]
- 782. Lehar F, et al. Comparison of clinical outcomes and safety of catheter ablation for atrial fibrillation supported by data from CT scan or three-dimensional rotational angiogram of left atrium and pulmonary veins. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159(4):622–628. [DOI] [PubMed] [Google Scholar]
- 783. Hauser TH, et al. Prognostic value of pulmonary vein size in prediction of atrial fibrillation recurrence after pulmonary vein isolation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2015;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784. Dewire J, et al. The association of pre-existing left atrial fibrosis with clinical variables in patients referred for catheter ablation of atrial fibrillation. Clin Med Insights Cardiol 2014;8(Suppl 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785. Kim RJ, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100(19):1992–2002. [DOI] [PubMed] [Google Scholar]
- 786. Kim RJ, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343(20):1445–1453. [DOI] [PubMed] [Google Scholar]
- 787. Karim R, et al. Evaluation of current algorithms for segmentation of scar tissue from late gadolinium enhancement cardiovascular magnetic resonance of the LA: an open-access grand challenge. J Cardiovasc Magn Reson 2013;15:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788. Khurram IM, et al. MRI evaluation of radiofrequency, cryothermal, and laser left atrial lesion formation in patients with atrial fibrillation. Pacing Clin Electrophysiol 2015;38(11):1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789. Khurram IM, et al. Left atrial LGE and arrhythmia recurrence following pulmonary vein isolation for paroxysmal and persistent AF. JACC Cardiovasc Imaging 2016;9(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790. Sramko M, et al. Clinical value of assessment of left atrial late gadolinium enhancement in patients undergoing ablation of atrial fibrillation. Int J Cardiol 2015;179:351–357. [DOI] [PubMed] [Google Scholar]
- 791. Spragg DD, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm 2012;9(12):2003–2009. [DOI] [PubMed] [Google Scholar]
- 792. Grothoff M, et al. MR imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: initial results. Radiology 2014;271(3):695–702. [DOI] [PubMed] [Google Scholar]
- 793. Nazarian S, et al. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation 2008;118(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794. Halperin HR, Kolandaivelu A.. MRI-Guided Electrophysiology Intervention. Rambam Maimonides Med J 2010;1(2):e0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795. Vergara GR, et al. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm 2011;8(2):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796. Scherr D, et al. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009;20(4):379–384. [DOI] [PubMed] [Google Scholar]
- 797. Vazquez SR, Johnson SA, Rondina MT.. Peri-procedural anticoagulation in patients undergoing ablation for atrial fibrillation. Thromb Res 2010;126(2):e69–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798. Liu Y, et al. Incidence and outcomes of cerebrovascular events complicating catheter ablation for atrial fibrillation. Europace 2016;18(9):1357–1365. [DOI] [PubMed] [Google Scholar]
- 799. Noseworthy PA, et al. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity-matched study of 24,244 patients. Heart Rhythm 2015;12(6):1154–1161. [DOI] [PubMed] [Google Scholar]
- 800. Deneke T, et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol 2015;26(4):455–463. [DOI] [PubMed] [Google Scholar]
- 801. Dorwarth U, et al. Radiofrequency catheter ablation: different cooled and noncooled electrode systems induce specific lesion geometries and adverse effects profiles. Pacing Clin Electrophysiol 2003;26(7 Pt 1):1438–1445. [DOI] [PubMed] [Google Scholar]
- 802. Maleki K, et al. Intracardiac ultrasound detection of thrombus on transseptal sheath: incidence, treatment, and prevention. J Cardiovasc Electrophysiol 2005;16(6):561–565. [DOI] [PubMed] [Google Scholar]
- 803. Wazni OM, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005;16(6):576–581. [DOI] [PubMed] [Google Scholar]
- 804. Shah D. Filamentous thrombi during left-sided sheath-assisted catheter ablations. Europace 2010;12(12):1657–1658. [DOI] [PubMed] [Google Scholar]
- 805. Sparks PB, et al. Left atrial “stunning” following radiofrequency catheter ablation of chronic atrial flutter. J Am Coll Cardiol 1998;32(2):468–475. [DOI] [PubMed] [Google Scholar]
- 806. Cappato R, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111(9):1100–1105. [DOI] [PubMed] [Google Scholar]
- 807. Abhishek F, et al. Effectiveness of a strategy to reduce major vascular complications from catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2011;30(3):211–215. [DOI] [PubMed] [Google Scholar]
- 808. Hoyt H, et al. Complications arising from catheter ablation of atrial fibrillation: temporal trends and predictors. Heart Rhythm 2011;8(12):1869–1874. [DOI] [PubMed] [Google Scholar]
- 809. Chilukuri K, et al. Transoesophageal echocardiography predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. Europace 2010;12(11):1543–1549. [DOI] [PubMed] [Google Scholar]
- 810. Schernthaner C, et al. High incidence of echocardiographic abnormalities of the interatrial septum in patients undergoing ablation for atrial fibrillation. Echocardiography 2013;30(4):402–406. [DOI] [PubMed] [Google Scholar]
- 811. Yamamoto M, et al. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ Cardiovasc Imaging 2014;7(2):337–343. [DOI] [PubMed] [Google Scholar]
- 812. Puwanant S, et al. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol 2009;54(22):2032–2039. [DOI] [PubMed] [Google Scholar]
- 813. McCready JW, et al. Incidence of left atrial thrombus prior to atrial fibrillation ablation: is pre-procedural transoesophageal echocardiography mandatory? Europace 2010;12(7):927–932. [DOI] [PubMed] [Google Scholar]
- 814. Gula LJ, et al. Impact of routine transoesophageal echocardiography on safety, outcomes, and cost of pulmonary vein ablation: inferences drawn from a decision analysis model. Europace 2010;12(11):1550–1557. [DOI] [PubMed] [Google Scholar]
- 815. Calkins H, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376(17):1627–1636. [DOI] [PubMed] [Google Scholar]
- 816. Kapa S, et al. ECG-gated dual-source CT for detection of left atrial appendage thrombus in patients undergoing catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2010;29(2):75–81. [DOI] [PubMed] [Google Scholar]
- 817. Romero J, et al. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6(2):185–194. [DOI] [PubMed] [Google Scholar]
- 818. Gottlieb I, et al. Diagnostic accuracy of arterial phase 64-slice multidetector CT angiography for left atrial appendage thrombus in patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol 2008;19(3):247–251. [DOI] [PubMed] [Google Scholar]
- 819. Lazoura O, et al. A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging 2016;32(2):347–354. [DOI] [PubMed] [Google Scholar]
- 820. Saksena S, et al. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the intracardiac echocardiography guided cardioversion helps interventional procedures study. Circ Arrhythm Electrophysiol 2010;3(6):571–577. [DOI] [PubMed] [Google Scholar]
- 821. Baran J, et al. Intracardiac echocardiography for detection of thrombus in the left atrial appendage: comparison with transesophageal echocardiography in patients undergoing ablation for atrial fibrillation: the Action-Ice I Study. Circ Arrhythm Electrophysiol 2013;6(6):1074–1081. [DOI] [PubMed] [Google Scholar]
- 822. Ren JF, et al. Intracardiac echocardiographic diagnosis of thrombus formation in the left atrial appendage: a complementary role to transesophageal echocardiography. Echocardiography 2013;30(1):72–80. [DOI] [PubMed] [Google Scholar]
- 823. Anter E, et al. Comparison of intracardiac echocardiography and transesophageal echocardiography for imaging of the right and left atrial appendages. Heart Rhythm 2014;11(11):1890–1897. [DOI] [PubMed] [Google Scholar]
- 824. Sriram CS, et al. Detection of left atrial thrombus by intracardiac echocardiography in patients undergoing ablation of atrial fibrillation. J Interv Card Electrophysiol 2015;43(3):227–236. [DOI] [PubMed] [Google Scholar]
- 825. Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 826. Granger CB, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 827. Patel MR, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365(10):883–891. [DOI] [PubMed] [Google Scholar]
- 828. Giugliano RP, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369(22):2093–2104. [DOI] [PubMed] [Google Scholar]
- 829. Wazni OM, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: comparison of strategies of anticoagulation management in the periprocedural period. Circulation 2007;116(22):2531–2534. [DOI] [PubMed] [Google Scholar]
- 830. Schmidt M, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratios. Pacing Clin Electrophysiol 2009;32(8):995–999. [DOI] [PubMed] [Google Scholar]
- 831. Kwak JJ, et al. Safety and convenience of continuous warfarin strategy during the periprocedural period in patients who underwent catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2010;21(6):620–625. [DOI] [PubMed] [Google Scholar]
- 832. Gopinath D, et al. Pulmonary vein antrum isolation for atrial fibrillation on therapeutic coumadin: special considerations. J Cardiovasc Electrophysiol 2011;22(2):236–239. [DOI] [PubMed] [Google Scholar]
- 833. Hakalahti A, et al. Catheter ablation of atrial fibrillation in patients with therapeutic oral anticoagulation treatment. Europace 2011;13(5):640–645. [DOI] [PubMed] [Google Scholar]
- 834. Di Biase L, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129(25):2638–2644. [DOI] [PubMed] [Google Scholar]
- 835. Bassiouny M, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6(3):460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836. Bin Abdulhak AA, et al. Safety and efficacy of interrupted dabigatran for peri-procedural anticoagulation in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2013;15(10):1412–1420. [DOI] [PubMed] [Google Scholar]
- 837. Hohnloser SH, Camm AJ.. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace 2013;15(10):1407–1411. [DOI] [PubMed] [Google Scholar]
- 838. Providencia R, et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014;16(8):1137–1144. [DOI] [PubMed] [Google Scholar]
- 839. Winkle RA, et al. Peri-procedural interrupted oral anticoagulation for atrial fibrillation ablation: comparison of aspirin, warfarin, dabigatran, and rivaroxaban. Europace 2014;16(10):1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840. Armbruster HL, et al. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother 2015;49(3):278–284. [DOI] [PubMed] [Google Scholar]
- 841. Calkins H, et al. RE-CIRCUIT study-randomized evaluation of Dabigatran etexilate compared to warfarin in pulmonary vein ablation: assessment of an uninterrupted periprocedural anticoagulation strategy. Am J Cardiol 2015;115(1):154–155. [DOI] [PubMed] [Google Scholar]
- 842. Cappato R, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36(28):1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843. Phan K, et al. Rivaroxaban versus warfarin or dabigatran in patients undergoing catheter ablation for atrial fibrillation: A meta-analysis. Int J Cardiol 2015;185:209–213. [DOI] [PubMed] [Google Scholar]
- 844. Pollack CV, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med 2015;373(6):511–520. [DOI] [PubMed] [Google Scholar]
- 845. Siegal DM, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 2015;373(25):2413–2424. [DOI] [PubMed] [Google Scholar]
- 846. Ren JF, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast. J Cardiovasc Electrophysiol 2005;16(5):474–477. [DOI] [PubMed] [Google Scholar]
- 847. Bruce CJ, et al. Early heparinization decreases the incidence of left atrial thrombi detected by intracardiac echocardiography during radiofrequency ablation for atrial fibrillation. J Interv Card Electrophysiol 2008;22(3):211–219. [DOI] [PubMed] [Google Scholar]
- 848. Asbach S, et al. Early heparin administration reduces risk for left atrial thrombus formation during atrial fibrillation ablation procedures. Cardiol Res Pract 2011;2011:615087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849. Briceno DF, et al. Clinical impact of heparin kinetics during catheter ablation of atrial fibrillation: meta-analysis and meta-regression. J Cardiovasc Electrophysiol 2016;27(6):683–693. [DOI] [PubMed] [Google Scholar]
- 850. Lakshmanadoss U,, Kutinsky I, Williamson B, Wong W, Haines DE. . Figure-of-eight suture for vascular hemostasis in fully anticoagulated patients after atrial fibrillation catheter ablation [abstract]. Europace 2015;15(Suppl 3):iii136–iii159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851. Chilukuri K, et al. Incidence and outcomes of protamine reactions in patients undergoing catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2009;25(3):175–181. [DOI] [PubMed] [Google Scholar]
- 852. Majeed A, et al. Thromboembolic safety and efficacy of prothrombin complex concentrates in the emergency reversal of warfarin coagulopathy. Thromb Res 2012;129(2):146–151. [DOI] [PubMed] [Google Scholar]
- 853. Connolly SJ, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364(9):806–817. [DOI] [PubMed] [Google Scholar]
- 854. Mega JL, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366(1):9–19. [DOI] [PubMed] [Google Scholar]
- 855. Daoud EG, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8(9):1416–1423. [DOI] [PubMed] [Google Scholar]
- 856. Riley MP, et al. Risk of stroke or transient ischemic attack after atrial fibrillation ablation with oral anticoagulant use guided by ECG monitoring and pulse assessment. J Cardiovasc Electrophysiol 2014;25(6):591–596. [DOI] [PubMed] [Google Scholar]
- 857. Saad EB, et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score </=3: a long-term outcome study. Circ Arrhythm Electrophysiol 2011;4(5):615–621. [DOI] [PubMed] [Google Scholar]
- 858. Yagishita A, et al. Incidence of late thromboembolic events after catheter ablation of atrial fibrillation. Circ J 2011;75(10):2343–2349. [DOI] [PubMed] [Google Scholar]
- 859. Zuern CS, et al. Anticoagulation after catheter ablation of atrial fibrillation guided by implantable cardiac monitors. Pacing Clin Electrophysiol 2015;38(6):688–693. [DOI] [PubMed] [Google Scholar]
- 860. Tran VN, et al. Thromboembolic events 7-11 years after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2015;38(4):499–506. [DOI] [PubMed] [Google Scholar]
- 861. Piccini JP, et al. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol 2009;2(6):626–633. [DOI] [PubMed] [Google Scholar]
- 862. Noseworthy PA, et al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863. Sjalander S, et al. Assessment of use vs discontinuation of oral anticoagulation after pulmonary vein isolation in patients with atrial fibrillation. JAMA Cardiol 2017;2(2):146–152. [DOI] [PubMed] [Google Scholar]
- 864. Elkassabany N, et al. Anesthetic management of patients undergoing pulmonary vein isolation for treatment of atrial fibrillation using high-frequency jet ventilation. J Cardiothorac Vasc Anesth 2012;26(3):433–438. [DOI] [PubMed] [Google Scholar]
- 865. Santangeli P, Lin D.. Catheter Ablation of Paroxysmal Atrial Fibrillation: Have We Achieved Cure with Pulmonary Vein Isolation? Methodist Debakey Cardiovasc J 2015;11(2):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866. Pappone C, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004;109(22):2724–2726. [DOI] [PubMed] [Google Scholar]
- 867. Cummings JE, et al. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med 2006;144(8):572–574. [DOI] [PubMed] [Google Scholar]
- 868. Shah D, et al. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol 2005;46(2):327–330. [DOI] [PubMed] [Google Scholar]
- 869. Lemola K, et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation 2004;110(24):3655–3660. [DOI] [PubMed] [Google Scholar]
- 870. Cummings JE, et al. Left atrial flutter following pulmonary vein antrum isolation with radiofrequency energy: linear lesions or repeat isolation. J Cardiovasc Electrophysiol 2005;16(3):293–297. [DOI] [PubMed] [Google Scholar]
- 871. Cummings JE, et al. Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the LA. Circulation 2005;112(4):459–464. [DOI] [PubMed] [Google Scholar]
- 872. Good E, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol 2005;46(11):2107–2110. [DOI] [PubMed] [Google Scholar]
- 873. Kottkamp H, et al. Topographic variability of the esophageal left atrial relation influencing ablation lines in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2005;16(2):146–150. [DOI] [PubMed] [Google Scholar]
- 874. Redfearn DP, et al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2005;16(6):589–593. [DOI] [PubMed] [Google Scholar]
- 875. Ren JF, Marchlinski FE, Callans DJ.. Real-time intracardiac echocardiographic imaging of the posterior left atrial wall contiguous to anterior wall of the esophagus. J Am Coll Cardiol 2006;48(3):594. author reply 594–595. [DOI] [PubMed] [Google Scholar]
- 876. Ruby RS, et al. Prevalence of fever in patients undergoing left atrial ablation of atrial fibrillation guided by barium esophagraphy. J Cardiovasc Electrophysiol 2009;20(8):883–887. [DOI] [PubMed] [Google Scholar]
- 877. Ripley KL, et al. Time course of esophageal lesions after catheter ablation with cryothermal and radiofrequency ablation: implication for atrio-esophageal fistula formation after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2007;18(6):642–646. [DOI] [PubMed] [Google Scholar]
- 878. Ahmed H, et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm 2009;6(7):962–969. [DOI] [PubMed] [Google Scholar]
- 879. Kawasaki R, et al. Atrioesophageal fistula complicating cryoballoon pulmonary vein isolation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(7):787–792. [DOI] [PubMed] [Google Scholar]
- 880. Lim HW, et al. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(2):208–213. [DOI] [PubMed] [Google Scholar]
- 881. Yokoyama K, et al. Canine model of esophageal injury and atrial-esophageal fistula after applications of forward-firing high-intensity focused ultrasound and side-firing unfocused ultrasound in the left atrium and inside the pulmonary vein. Circ Arrhythm Electrophysiol 2009;2(1):41–49. [DOI] [PubMed] [Google Scholar]
- 882. Singh SM, et al. Esophageal injury and temperature monitoring during atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2008;1(3):162–168. [DOI] [PubMed] [Google Scholar]
- 883. Kuwahara T, et al. Safe and effective ablation of atrial fibrillation: importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol 2009;20(1):1–6. [DOI] [PubMed] [Google Scholar]
- 884. Contreras-Valdes FM, et al. Severity of esophageal injury predicts time to healing after radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm 2011;8(12):1862–1868. [DOI] [PubMed] [Google Scholar]
- 885. Leite LR, et al. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol 2011;4(2):149–156. [DOI] [PubMed] [Google Scholar]
- 886. Tschabrunn CM, et al. Comparison between single- and multi-sensor oesophageal temperature probes during atrial fibrillation ablation: thermodynamic characteristics. Europace 2015;17(6):891–897. [DOI] [PubMed] [Google Scholar]
- 887. Deneke T, et al. Utility of esophageal temperature monitoring during pulmonary vein isolation for atrial fibrillation using duty-cycled phased radiofrequency ablation. J Cardiovasc Electrophysiol 2011;22(3):255–261. [DOI] [PubMed] [Google Scholar]
- 888. Carroll BJ, et al. Multi-sensor esophageal temperature probe used during radiofrequency ablation for atrial fibrillation is associated with increased intraluminal temperature detection and increased risk of esophageal injury compared to single-sensor probe. J Cardiovasc Electrophysiol 2013;24(9):958–964. [DOI] [PubMed] [Google Scholar]
- 889. Muller P, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm 2015;12(7):1464–1469. [DOI] [PubMed] [Google Scholar]
- 890. Tsuchiya T, et al. Atrial fibrillation ablation with esophageal cooling with a cooled water-irrigated intraesophageal balloon: a pilot study. J Cardiovasc Electrophysiol 2007;18(2):145–150. [DOI] [PubMed] [Google Scholar]
- 891. Arruda MS, et al. Feasibility and safety of using an esophageal protective system to eliminate esophageal thermal injury: implications on atrial-esophageal fistula following AF ablation. J Cardiovasc Electrophysiol 2009;20(11):1272–1278. [DOI] [PubMed] [Google Scholar]
- 892. Kuwahara T, et al. Oesophageal cooling with ice water does not reduce the incidence of oesophageal lesions complicating catheter ablation of atrial fibrillation: randomized controlled study. Europace 2014;16(6):834–839. [DOI] [PubMed] [Google Scholar]
- 893. Kuwahara T, et al. Incidences of esophageal injury during esophageal temperature monitoring: a comparative study of a multi-thermocouple temperature probe and a deflectable temperature probe in atrial fibrillation ablation. J Interv Card Electrophysiol 2014;39(3):251–257. [DOI] [PubMed] [Google Scholar]
- 894. Chugh A, et al. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm 2009;6(3):319–322. [DOI] [PubMed] [Google Scholar]
- 895. Koruth JS, et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2012;23(2):147–154. [DOI] [PubMed] [Google Scholar]
- 896. Zellerhoff S, Lenze F, Eckardt L.. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace 2011;13(9):1219–1221. [DOI] [PubMed] [Google Scholar]
- 897. Zellerhoff S, et al. Fatal course of esophageal stenting of an atrioesophageal fistula after atrial fibrillation ablation. Heart Rhythm 2011;8(4):624–626. [DOI] [PubMed] [Google Scholar]
- 898. Khan M, et al. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;(2):CD003244. [DOI] [PubMed] [Google Scholar]
- 899. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med 2008;359(16):1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900. Martinek M, et al. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm 2010;7(9):1224–1230. [DOI] [PubMed] [Google Scholar]
- 901. Shaheen NJ, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005;41(3):588–594. [DOI] [PubMed] [Google Scholar]
- 902. Halm U, et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol 2010;105(3):551–556. [DOI] [PubMed] [Google Scholar]
- 903. Knopp H, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm 2014;11(4):574–578. [DOI] [PubMed] [Google Scholar]
- 904. Dagres N, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006;17(11):1213–1215. [DOI] [PubMed] [Google Scholar]
- 905. Eitel C, et al. Successful nonsurgical treatment of esophagopericardial fistulas after atrial fibrillation catheter ablation: a case series. Circ Arrhythm Electrophysiol 2013;6(4):675–681. [DOI] [PubMed] [Google Scholar]
- 906. Singh SM, et al. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10(11):1591–1597. [DOI] [PubMed] [Google Scholar]
- 907. Bunch TJ, et al. Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol 2006;17(4):435–439. [DOI] [PubMed] [Google Scholar]
- 908. Cappato R, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009;53(19):1798–1803. [DOI] [PubMed] [Google Scholar]
- 909. Tan C, Coffey A.. Atrioesophageal fistula after surgical unipolar radiofrequency atrial ablation for atrial fibrillation. Ann Thorac Surg 2013;95(3):e61–e62. [DOI] [PubMed] [Google Scholar]
- 910. Mohanty S. Outcomes of atrio-esophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol 2014;25(9):E6. [DOI] [PubMed] [Google Scholar]
- 911. Mohanty S, et al. Outcomes of atrioesophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol 2014;25(6):579–584. [DOI] [PubMed] [Google Scholar]
- 912. Hazell W, et al. Atrio-oesophageal fistula: an emergent complication of radiofrequency ablation. Emerg Med Australas 2009;21(4):329–332. [DOI] [PubMed] [Google Scholar]
- 913. Cazavet A, et al. Successful surgery for atrioesophageal fistula caused by transcatheter ablation of atrial fibrillation. J Thorac Cardiovasc Surg 2010;140(3):e43–e45. [DOI] [PubMed] [Google Scholar]
- 914. Podgaetz E, Deschamps C.. Esophageal complications of catheter ablation for atrial fibrillation: a case report. J Thorac Cardiovasc Surg 2013;145(1):e9–13. [DOI] [PubMed] [Google Scholar]
- 915. Queneherve L, et al. Endoscopic management of an esophagopericardial fistula after radiofrequency ablation for atrial fibrillation. World J Gastroenterol 2013;19(21):3352–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916. Gunes MF, et al. Ablating the posterior heart: cardioesophageal fistula complicating radiofrequency ablation in the coronary sinus. J Cardiovasc Electrophysiol 2015;26(12):1376–1378. [DOI] [PubMed] [Google Scholar]
- 917. Qadeer MA, et al. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc 2007;66(3):605–611. [DOI] [PubMed] [Google Scholar]
- 918. Ellis CR, et al. Successful treatment of esophageal perforation following atrial fibrillation ablation with a fully covered esophageal stent: prevention of atrial-esophageal fistula. Journal of Innovations in Cardiac Rhythm Management 2012;3:874–878. [Google Scholar]
- 919. Markar SR, et al. Novel multimodality endoscopic closure of postoperative esophageal fistula. Int J Surg Case Rep 2012;3(11):577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920. Cappato R, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3(1):32–38. [DOI] [PubMed] [Google Scholar]
- 921. Deshmukh A, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128(19):2104–2112. [DOI] [PubMed] [Google Scholar]
- 922. Gupta A, et al. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 2013;6(6):1082–1088. [DOI] [PubMed] [Google Scholar]
- 923. Takahashi Y, et al. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol 2005;16(10):1104–1107. [DOI] [PubMed] [Google Scholar]
- 924. Chilukuri K, et al. Association of transseptal punctures with isolated migraine aura in patients undergoing catheter ablation of cardiac arrhythmias. J Cardiovasc Electrophysiol 2009;20(11):1227–1230. [DOI] [PubMed] [Google Scholar]
- 925. Noheria A, et al. Migraine headaches following catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2011;30(3):227–232. [DOI] [PubMed] [Google Scholar]
- 926. Barbhaiya CR, et al. Global survey of esophageal and gastric injury in atrial fibrillation ablation: incidence, time to presentation, and outcomes. J Am Coll Cardiol 2015;65(13):1377–1378. [DOI] [PubMed] [Google Scholar]
- 927. Holmes DR Jr., Monahan KH, Packer D.. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv 2009;2(4):267–276. [DOI] [PubMed] [Google Scholar]
- 928. Fender EA, Packer DL, Holmes DR. Jr.. Pulmonary vein stenosis after atrial fibrillation ablation. EuroIntervention 2016;12(Suppl X):X31–X34. [DOI] [PubMed] [Google Scholar]
- 929. Kirchhof P, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14(1):8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930. Oral H, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006;113(15):1824–1831. [DOI] [PubMed] [Google Scholar]
- 931. Vasamreddy CR, et al. Predictors of recurrence following catheter ablation of atrial fibrillation using an irrigated-tip ablation catheter. J Cardiovasc Electrophysiol 2004;15(6):692–697. [DOI] [PubMed] [Google Scholar]
- 932. Bertaglia E, et al. Predictive value of early atrial tachyarrhythmias recurrence after circumferential anatomical pulmonary vein ablation. Pacing Clin Electrophysiol 2005;28(5):366–371. [DOI] [PubMed] [Google Scholar]
- 933. Das M, et al. Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhythm Electrophysiol 2015;8(4):846–852. [DOI] [PubMed] [Google Scholar]
- 934. Kaitani K, et al. Efficacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation (EAST-AF) trial. Eur Heart J 2016;37(7):610–618. [DOI] [PubMed] [Google Scholar]
- 935. Willems S, et al. Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation: insights from the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) trial. Circ Arrhythm Electrophysiol 2016;9(8). [DOI] [PubMed] [Google Scholar]
- 936. Klemm HU, et al. Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. J Cardiovasc Electrophysiol 2006;17(2):146–150. [DOI] [PubMed] [Google Scholar]
- 937. Senatore G, et al. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol 2005;45(6):873–876. [DOI] [PubMed] [Google Scholar]
- 938. Pokushalov E, et al. Ablation of paroxysmal and persistent atrial fibrillation: 1-year follow-up through continuous subcutaneous monitoring. J Cardiovasc Electrophysiol 2011;22(4):369–375. [DOI] [PubMed] [Google Scholar]
- 939. Dagres N, et al. Influence of the duration of Holter monitoring on the detection of arrhythmia recurrences after catheter ablation of atrial fibrillation: implications for patient follow-up. Int J Cardiol 2010;139(3):305–306. [DOI] [PubMed] [Google Scholar]
- 940. Winkle RA, et al. Atrial arrhythmia burden on long-term monitoring in asymptomatic patients late after atrial fibrillation ablation. Am J Cardiol 2012;110(6):840–844. [DOI] [PubMed] [Google Scholar]
- 941. Kapa S, et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J Cardiovasc Electrophysiol 2013;24(8):875–881. [DOI] [PubMed] [Google Scholar]
- 942. Pecha S, et al. Concomitant surgical atrial fibrillation ablation and event recorder implantation: better monitoring, better outcome? Interact Cardiovasc Thorac Surg 2013;16(4):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943. Takagi T, et al. Role of extended external auto-triggered loop recorder monitoring for atrial fibrillation. Circ J 2014;78(11):2637–2642. [DOI] [PubMed] [Google Scholar]
- 944. Damiano RJ, Jr, et al. Detection of atrial fibrillation after surgical ablation: conventional versus continuous monitoring. Ann Thorac Surg 2016;101(1):42–47; discussion 47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945. Piorkowski C, et al. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2005;16(12):1286–1292. [DOI] [PubMed] [Google Scholar]
- 946. Janse PA, et al. Symptoms versus objective rhythm monitoring in patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Eur J Cardiovasc Nurs 2008;7(2):147–151. [DOI] [PubMed] [Google Scholar]
- 947. Ziegler PD, Koehler JL, Mehra R.. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006;3(12):1445–1452. [DOI] [PubMed] [Google Scholar]
- 948. Edgerton JR, et al. Long-term monitoring after surgical ablation for atrial fibrillation: how much is enough? J Thorac Cardiovasc Surg 2011;142(1):162–165. [DOI] [PubMed] [Google Scholar]
- 949. Mulder AA, et al. Arrhythmia detection after atrial fibrillation ablation: value of incremental monitoring time. Pacing Clin Electrophysiol 2012;35(2):164–169. [DOI] [PubMed] [Google Scholar]
- 950. Gussak I, et al. Wireless remote monitoring of reconstructed 12-lead ECGs after ablation for atrial fibrillation using a hand-held device. J Electrocardiol 2012;45(2):129–135. [DOI] [PubMed] [Google Scholar]
- 951. Tarakji KG, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015;12(3):554–559. [DOI] [PubMed] [Google Scholar]
- 952. Hindricks G, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3(2):141–147. [DOI] [PubMed] [Google Scholar]
- 953. Bogachev-Prokophiev A, et al. Assessment of concomitant paroxysmal atrial fibrillation ablation in mitral valve surgery patients based on continuous monitoring: does a different lesion set matter?. Interact Cardiovasc Thorac Surg 2014;18(2):177–181. discussion 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954. Ad N, et al. The Cox-Maze III procedure success rate: comparison by electrocardiogram, 24-hour holter monitoring and long-term monitoring. Ann Thorac Surg 2009;88(1):101–105. [DOI] [PubMed] [Google Scholar]
- 955. Hanke T, et al. Twenty-four-hour holter monitor follow-up does not provide accurate heart rhythm status after surgical atrial fibrillation ablation therapy: up to 12 months experience with a novel permanently implantable heart rhythm monitor device. Circulation 2009;120(11 Suppl):S177–S184. [DOI] [PubMed] [Google Scholar]
- 956. Liu J, et al. The value of transtelephonic electrocardiogram monitoring system during the “Blanking Period” after ablation of atrial fibrillation. J Electrocardiol 2010;43(6):667–672. [DOI] [PubMed] [Google Scholar]
- 957. Bogachev-Prokophiev A, et al. Ablation for atrial fibrillation during mitral valve surgery: 1-year results through continuous subcutaneous monitoring. Interact Cardiovasc Thorac Surg 2012;15(1):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958. Pokushalov E, et al. Does atrial fibrillation burden measured by continuous monitoring during the blanking period predict the response to ablation at 12-month follow-up? Heart Rhythm 2012;9(9):1375–1379. [DOI] [PubMed] [Google Scholar]
- 959. Perez-Castellano N, et al. The COR trial: a randomized study with continuous rhythm monitoring to compare the efficacy of cryoenergy and radiofrequency for pulmonary vein isolation. Heart Rhythm 2014;11(1):8–14. [DOI] [PubMed] [Google Scholar]
- 960. Charitos EI, et al. Long-term outcomes after surgical ablation for atrial fibrillation in patients with continuous heart rhythm monitoring devices. Interact Cardiovasc Thorac Surg 2015;21(6):712–721. [DOI] [PubMed] [Google Scholar]
- 961. Pecha S, et al. Implantable loop recorder monitoring after concomitant surgical ablation for atrial fibrillation (AF): insights from more than 200 continuously monitored patients. Heart Vessels 2016;31(8):1347–1353. [DOI] [PubMed] [Google Scholar]
- 962. Eitel C, et al. Performance of an implantable automatic atrial fibrillation detection device: impact of software adjustments and relevance of manual episode analysis. Europace 2011;13(4):480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963. Seidl K, et al. Is the atrial high rate episode diagnostic feature reliable in detecting paroxysmal episodes of atrial tachyarrhythmias? Pacing Clin Electrophysiol 1998;21(4 Pt 1):694–700. [DOI] [PubMed] [Google Scholar]
- 964. Purerfellner H, et al. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol 2004;27(7):983–992. [DOI] [PubMed] [Google Scholar]
- 965. Martinek M, et al. New insights into long-term follow-up of atrial fibrillation ablation: full disclosure by an implantable pacemaker device. J Cardiovasc Electrophysiol 2007;18(8):818–823. [DOI] [PubMed] [Google Scholar]
- 966. Steven D, et al. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur Heart J 2008;29(8):1037–1042. [DOI] [PubMed] [Google Scholar]
- 967. Puskas JD, et al. “Spot” ECGs underestimate atrial fibrillation recurrence after surgical ablation. Innovations (Phila) 2008;3(1):7–11. [DOI] [PubMed] [Google Scholar]
- 968. Kaufman ES, et al. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm 2012;9(8):1241–1246. [DOI] [PubMed] [Google Scholar]
- 969. Arbelo E, et al. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J 2014;35(22):1466–1478. [DOI] [PubMed] [Google Scholar]
- 970. O'Donnell D, et al. Delayed cure despite early recurrence after pulmonary vein isolation for atrial fibrillation. Am J Cardiol 2003;91(1):83–85. [DOI] [PubMed] [Google Scholar]
- 971. Jiang H, et al. Predictors of early recurrence and delayed cure after segmental pulmonary vein isolation for paroxysmal atrial fibrillation without structural heart disease. J Interv Card Electrophysiol 2006;15(3):157–163. [DOI] [PubMed] [Google Scholar]
- 972. Richter B, et al. Frequency of recurrence of atrial fibrillation within 48 hours after ablation and its impact on long-term outcome. Am J Cardiol 2008;101(6):843–847. [DOI] [PubMed] [Google Scholar]
- 973. Reddy VY, et al. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm 2008;5(3):353–360. [DOI] [PubMed] [Google Scholar]
- 974. Kirchhof P, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J 2007;28(22):2803–2817. [DOI] [PubMed] [Google Scholar]
- 975. Issac TT, Dokainish H, Lakkis NM.. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol 2007;50(21):2021–2028. [DOI] [PubMed] [Google Scholar]
- 976. Li XP, et al. Predictive value of early recurrence and delayed cure after catheter ablation for patients with chronic atrial fibrillation. Circ J 2008;72(7):1125–1129. [DOI] [PubMed] [Google Scholar]
- 977. Andrade JG, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2012;35(1):106–116. [DOI] [PubMed] [Google Scholar]
- 978. Mugnai G, et al. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: Predictive role of atrial arrhythmias occurring in the blanking period on the incidence of late recurrences. Heart Rhythm 2016;13(4):845–851. [DOI] [PubMed] [Google Scholar]
- 979. Roux JF, et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation 2009;120(12):1036–1040. [DOI] [PubMed] [Google Scholar]
- 980. Leong-Sit P, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011;4(1):11–14. [DOI] [PubMed] [Google Scholar]
- 981. Koyama T, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010;56(18):1463–1472. [DOI] [PubMed] [Google Scholar]
- 982. Kim YR, et al. Effect of short-term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8(6):1366–1372. [DOI] [PubMed] [Google Scholar]
- 983. Abed HS, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310(19):2050–2060. [DOI] [PubMed] [Google Scholar]
- 984. Imazio M, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011;124(21):2290–2295. [DOI] [PubMed] [Google Scholar]
- 985. Deftereos S, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol 2012;60(18):1790–1796. [DOI] [PubMed] [Google Scholar]
- 986. Deftereos S, et al. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm 2014;11(4):620–628. [DOI] [PubMed] [Google Scholar]
- 987. Chilukuri K, et al. Outcomes in patients requiring cardioversion following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2010;21(1):27–32. [DOI] [PubMed] [Google Scholar]
- 988. Baman TS, et al. Time to cardioversion of recurrent atrial arrhythmias after catheter ablation of atrial fibrillation and long-term clinical outcome. J Cardiovasc Electrophysiol 2009;20(12):1321–1325. [DOI] [PubMed] [Google Scholar]
- 989. Malasana G, et al. A strategy of rapid cardioversion minimizes the significance of early recurrent atrial tachyarrhythmias after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(7):761–766. [DOI] [PubMed] [Google Scholar]
- 990. Sponga S, et al. Role of an aggressive rhythm control strategy on sinus rhythm maintenance following intra-operative radiofrequency ablation of atrial fibrillation in patients undergoing surgical correction of valvular disease. J Cardiol 2012;60(4):316–320. [DOI] [PubMed] [Google Scholar]
- 991. Sairaku A, et al. Learning curve for ablation of atrial fibrillation in medium-volume centers. J Cardiol 2011;57(3):263–268. [DOI] [PubMed] [Google Scholar]
- 992. Sairaku A, et al. How many electrical cardioversions should be applied for repetitive recurrences of atrial arrhythmias following ablation of persistent atrial fibrillation? Europace 2011;13(12):1703–1708. [DOI] [PubMed] [Google Scholar]
- 993. Lellouche N, et al. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J Cardiovasc Electrophysiol 2008;19(6):599–605. [DOI] [PubMed] [Google Scholar]
- 994. Lee SH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2004;10(3):221–226. [DOI] [PubMed] [Google Scholar]
- 995. Thomas SP, Wallace EM, Ross DL.. The effect of a residual isthmus of surviving tissue on conduction after linear ablation in atrial myocardium. J Interv Card Electrophysiol 2000;4(1):273–281. [DOI] [PubMed] [Google Scholar]
- 996. Oral H, Knight BP, Morady F.. Left atrial flutter after segmental ostial radiofrequency catheter ablation for pulmonary vein isolation. Pacing Clin Electrophysiol 2003;26(6):1417–1419. [DOI] [PubMed] [Google Scholar]
- 997. Villacastin J, et al. Left atrial flutter after radiofrequency catheter ablation of focal atrial fibrillation. J Cardiovasc Electrophysiol 2003;14(4):417–421. [DOI] [PubMed] [Google Scholar]
- 998. Gerstenfeld EP, et al. Reentrant and nonreentrant focal left atrial tachycardias occur after pulmonary vein isolation. Heart Rhythm 2005;2(11):1195–1202. [DOI] [PubMed] [Google Scholar]
- 999. Gerstenfeld EP, Marchlinski FE.. Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm 2007;4(3 Suppl):S65–S72. [DOI] [PubMed] [Google Scholar]
- 1000. Lim TW, et al. Atrial arrhythmias after single-ring isolation of the posterior left atrium and pulmonary veins for atrial fibrillation: mechanisms and management. Circ Arrhythm Electrophysiol 2008;1(2):120–126. [DOI] [PubMed] [Google Scholar]
- 1001. Anousheh R, et al. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol 2010;33(4):460–468. [DOI] [PubMed] [Google Scholar]
- 1002. Brooks AG, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7(6):835–846. [DOI] [PubMed] [Google Scholar]
- 1003. Themistoclakis S, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008;5(5):679–685. [DOI] [PubMed] [Google Scholar]
- 1004. Nalliah CJ, et al. Clinical significance of early atrial arrhythmia type and timing after single ring isolation of the pulmonary veins. Europace 2015;17(7):1038–1044. [DOI] [PubMed] [Google Scholar]
- 1005. Satomi K. Electrophysiological characteristics of atrial tachycardia after pulmonary vein isolation of atrial fibrillation. Circ J 2010;74(6):1051–1058. [DOI] [PubMed] [Google Scholar]
- 1006. Jais P, et al. Flutter localized to the anterior left atrium after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006;17(3):279–285. [DOI] [PubMed] [Google Scholar]
- 1007. Patel AM, et al. Atrial tachycardia after ablation of persistent atrial fibrillation: identification of the critical isthmus with a combination of multielectrode activation mapping and targeted entrainment mapping. Circ Arrhythm Electrophysiol 2008;1(1):14–22. [DOI] [PubMed] [Google Scholar]
- 1008. Jais P, et al. A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J Cardiovasc Electrophysiol 2009;20(5):480–491. [DOI] [PubMed] [Google Scholar]
- 1009. Sauer WH, et al. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm 2006;3(9):1024–1028. [DOI] [PubMed] [Google Scholar]
- 1010. Marchlinski FE, et al. Efficacy and safety of targeted focal ablation versus PV isolation assisted by magnetic electroanatomic mapping. J Cardiovasc Electrophysiol 2003;14(4):358–365. [DOI] [PubMed] [Google Scholar]
- 1011. Choi JI, et al. Clinical significance of early recurrences of atrial tachycardia after atrial fibrillation ablation. J Cardiovasc Electrophysiol 2010;21(12):1331–1337. [DOI] [PubMed] [Google Scholar]
- 1012. Al-Hijji MA, et al. Trends and predictors of repeat catheter ablation for atrial fibrillation. Am Heart J 2016;171(1):48–55. [DOI] [PubMed] [Google Scholar]
- 1013. Gerstenfeld EP, et al. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol 2003;14(7):685–690. [DOI] [PubMed] [Google Scholar]
- 1014. Wasmer K, et al. Pulmonary vein reconnection and arrhythmia progression after antral linear catheter ablation of paroxysmal and persistent atrial fibrillation. Clin Res Cardiol 2016;105(9):738–743. [DOI] [PubMed] [Google Scholar]
- 1015. Bassiouny M, et al. Randomized study of persistent atrial fibrillation ablation: ablate in sinus rhythm versus ablate complex-fractionated atrial electrograms in atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9(2):e003596. [DOI] [PubMed] [Google Scholar]
- 1016. Rolf S, Dagres N, Hindricks G.. Voltage-based ablation: the growing evidence for the role of individually tailored substrate modification for atrial fibrillation. J Cardiovasc Electrophysiol 2016;27(1):31–33. [DOI] [PubMed] [Google Scholar]
- 1017. Dumonceau JM, et al. Acute delayed gastric emptying after ablation of atrial fibrillation: treatment with botulinum toxin injection. Endoscopy 2006;38(5):543. [DOI] [PubMed] [Google Scholar]
- 1018. Bunch TJ, et al. Vagus nerve injury after posterior atrial radiofrequency ablation. Heart Rhythm 2008;5(9):1327–1330. [DOI] [PubMed] [Google Scholar]
- 1019. Kuwahara T, et al. Clinical characteristics and management of periesophageal vagal nerve injury complicating left atrial ablation of atrial fibrillation: lessons from eleven cases. J Cardiovasc Electrophysiol 2013;24(8):847–851. [DOI] [PubMed] [Google Scholar]
- 1020. Miyazaki S, et al. Factors associated with periesophageal vagal nerve injury after pulmonary vein antrum isolation. J Am Heart Assoc 2014;3(5):e001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021. Chopra N, Shadchehr A.. Achalasia cardia as a unique complication of pulmonary vein isolation. Heart Rhythm 2014;11(12):2297–2299. [DOI] [PubMed] [Google Scholar]
- 1022. Bertaglia E, et al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace 2010;12(2):181–187. [DOI] [PubMed] [Google Scholar]
- 1023. Sotomi Y, et al. Incidence and risk factors for very late recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace 2013;15(11):1581–1586. [DOI] [PubMed] [Google Scholar]
- 1024. Steinberg JS, et al. Very long-term outcome after initially successful catheter ablation of atrial fibrillation. Heart Rhythm 2014;11(5):771–776. [DOI] [PubMed] [Google Scholar]
- 1025. Krittayaphong R, et al. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai 2003;86(Suppl 1):S8–S16. [PubMed] [Google Scholar]
- 1026. Oral H, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006;354(9):934–941. [DOI] [PubMed] [Google Scholar]
- 1027. Pappone C, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48(11):2340–2347. [DOI] [PubMed] [Google Scholar]
- 1028. Stabile G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J 2006;27(2):216–221. [DOI] [PubMed] [Google Scholar]
- 1029. Forleo GB, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol 2009;20(1):22–28. [DOI] [PubMed] [Google Scholar]
- 1030. Mont L, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35(8):501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031. Skelly A, et al. Catheter ablation for treatment of atrial fibrillation. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 1032. Siontis KC, et al. Radiofrequency ablation versus antiarrhythmic drug therapy for atrial fibrillation: meta-analysis of quality of life, morbidity, and mortality. JACC: Clinical Electrophysiology 2016;2(2):170–180. [DOI] [PubMed] [Google Scholar]
- 1033. Khaykin Y, et al. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J Cardiovasc Electrophysiol 2009;20(1):7–12. [DOI] [PubMed] [Google Scholar]
- 1034. Cappato R, et al. Delayed cardiac tamponade after radiofrequency catheter ablation of atrial fibrillation: a worldwide report. J Am Coll Cardiol 2011;58(25):2696–2697. [DOI] [PubMed] [Google Scholar]
- 1035. Themistoclakis S, et al. Prospective European survey on atrial fibrillation ablation: clinical characteristics of patients and ablation strategies used in different countries. J Cardiovasc Electrophysiol 2014;25(10):1074–1081. [DOI] [PubMed] [Google Scholar]
- 1036. Zado E, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2008;19(6):621–626. [DOI] [PubMed] [Google Scholar]
- 1037. Corrado A, et al. Efficacy, safety, and outcome of atrial fibrillation ablation in septuagenarians. J Cardiovasc Electrophysiol 2008;19(8):807–811. [DOI] [PubMed] [Google Scholar]
- 1038. Tan HW, et al. Efficacy, safety and outcome of catheter ablation for atrial fibrillation in octogenarians. Int J Cardiol 2010;145(1):147–148. [DOI] [PubMed] [Google Scholar]
- 1039. Piccini JP, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation 2012;126(18):2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040. Hao SC, et al. Acute safety outcomes in younger and older patients with atrial fibrillation treated with catheter ablation. J Interv Card Electrophysiol 2012;35(2):173–182. [DOI] [PubMed] [Google Scholar]
- 1041. Guiot A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012;23(1):36–43. [DOI] [PubMed] [Google Scholar]
- 1042. Hsu LF, et al. Catheter ablation for atrial fibrillation in congestive HF. N Engl J Med 2004;351(23):2373–2383. [DOI] [PubMed] [Google Scholar]
- 1043. Olivotto I, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104(21):2517–2524. [DOI] [PubMed] [Google Scholar]
- 1044. Providencia R, et al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Heart 2016;102(19):1533–1543. [DOI] [PubMed] [Google Scholar]
- 1045. Chun KR, et al. Catheter ablation of atrial fibrillation in the young: insights from the German Ablation Registry. Clin Res Cardiol 2013;102(6):459–468. [DOI] [PubMed] [Google Scholar]
- 1046. Humphries KH, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation 2001;103(19):2365–2370. [DOI] [PubMed] [Google Scholar]
- 1047. Ong L, et al. Gender differences and quality of life in atrial fibrillation: the mediating role of depression. J Psychosom Res 2006;61(6):769–774. [DOI] [PubMed] [Google Scholar]
- 1048. Dagres N, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol 2007;49(5):572–577. [DOI] [PubMed] [Google Scholar]
- 1049. Volgman AS, et al. Women with atrial fibrillation: Greater risk, less attention. Gend Med 2009;6(3):419–432. [DOI] [PubMed] [Google Scholar]
- 1050. Balk EM, et al. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol 2010;21(11):1208–1216. [DOI] [PubMed] [Google Scholar]
- 1051. Heist EK, et al. Predictors of atrial fibrillation termination and clinical success of catheter ablation of persistent atrial fibrillation. Am J Cardiol 2012;110(4):545–551. [DOI] [PubMed] [Google Scholar]
- 1052. Zhang XD, et al. Efficacy and safety of catheter ablation for long-standing persistent atrial fibrillation in women. Pacing Clin Electrophysiol 2013;36(10):1236–1244. [DOI] [PubMed] [Google Scholar]
- 1053. Forleo GB, et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace 2007;9(8):613–620. [DOI] [PubMed] [Google Scholar]
- 1054. Takigawa M, et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int J Cardiol 2013;168(3):1984–1991. [DOI] [PubMed] [Google Scholar]
- 1055. Beck H, Curtis AB.. Sex differences in outcomes of ablation of atrial fibrillation. J Atr Fibrillation 2014;6(6):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056. Baman TS, et al. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(6):626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057. Hoyt H, et al. Demographic profile of patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2011;22(9):994–998. [DOI] [PubMed] [Google Scholar]
- 1058. Shah RU, et al. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2012;59(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059. Bertaglia E, et al. Updated national multicenter registry on procedural safety of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2013;24(10):1069–1074. [DOI] [PubMed] [Google Scholar]
- 1060. Su W, et al. Best practice guide for cryoballoon ablation in atrial fibrillation: The compilation experience of more than 3000 procedures. Heart Rhythm 2015;12(7):1658–1666. [DOI] [PubMed] [Google Scholar]
- 1061. Takami M, et al. Impact of freezing time and balloon size on the thermodynamics and isolation efficacy during pulmonary vein isolation using the second generation cryoballoon. Circ Arrhythm Electrophysiol 2015;8(4):836–845. [DOI] [PubMed] [Google Scholar]
- 1062. Furnkranz A, et al. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2014;25(8):840–844. [DOI] [PubMed] [Google Scholar]
- 1063. Di Giovanni G, et al. One-year follow-up after single procedure Cryoballoon ablation: a comparison between the first and second generation balloon. J Cardiovasc Electrophysiol 2014;25(8):834–839. [DOI] [PubMed] [Google Scholar]
- 1064. Liu J, et al. Second generation of cryoballoons can improve efficiency of cryoablation for atrial fibrillation. Pacing Clin Electrophysiol 2015;38(1):129–135. [DOI] [PubMed] [Google Scholar]
- 1065. Khoueiry Z, et al. Outcomes after cryoablation vs. radiofrequency in patients with paroxysmal atrial fibrillation: impact of pulmonary veins anatomy. Europace 2016;18(9):1343–1351. [DOI] [PubMed] [Google Scholar]
- 1066. Mugnai G, et al. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am J Cardiol 2014;113(9):1509–1513. [DOI] [PubMed] [Google Scholar]
- 1067. Cheng X, et al. The long-term efficacy of cryoballoon vs irrigated radiofrequency ablation for the treatment of atrial fibrillation: a meta-analysis. Int J Cardiol 2015;181:297–302. [DOI] [PubMed] [Google Scholar]
- 1068. Jourda F, et al. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace 2015;17(2):225–231. [DOI] [PubMed] [Google Scholar]
- 1069. Luik A, et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority FreezeAF study. Circulation 2015;132(14):1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070. Squara F, et al. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace 2015;17(5):718–724. [DOI] [PubMed] [Google Scholar]
- 1071. Wasserlauf J, et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2015;38(4):483–489. [DOI] [PubMed] [Google Scholar]
- 1072. Schmidt M, et al. German ablation registry: Cryoballoon vs. radiofrequency ablation in paroxysmal atrial fibrillation–One-year outcome data. Heart Rhythm 2016;13(4):836–844. [DOI] [PubMed] [Google Scholar]
- 1073. Ciconte G, et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: One-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 2015;12(1):60–66. [DOI] [PubMed] [Google Scholar]
- 1074. Koektuerk B, et al. Cryoballoon ablation for pulmonary vein isolation in patients with persistent atrial fibrillation: one-year outcome using second generation cryoballoon. Circ Arrhythm Electrophysiol 2015;8(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 1075. Guhl EN, et al. Efficacy of cryoballoon pulmonary vein isolation in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 2016;27(4):423–427. [DOI] [PubMed] [Google Scholar]
- 1076. Straube F, et al. Cryoballoon ablation for persistent atrial fibrillation—Large single-center experience. J Cardiol 2016;68(6):492–497. [DOI] [PubMed] [Google Scholar]
- 1077. Su WW, et al. Novel usage of the cryoballoon catheter to achieve large area atrial substrate modification in persistent and long-standing persistent atrial fibrillation. J Interv Card Electrophysiol 2016;46(3):275–285. [DOI] [PubMed] [Google Scholar]
- 1078. Ciconte G, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 2015;17(4):559–565. [DOI] [PubMed] [Google Scholar]
- 1079. Spitzer SG, et al. Treatment of Recurrent Nonparoxysmal Atrial Fibrillation Using Focal Impulse and Rotor Mapping (FIRM)-Guided Rotor Ablation: Early Recurrence and Long-Term Outcomes. J Cardiovasc Electrophysiol 2017;28(1):31–38. [DOI] [PubMed] [Google Scholar]
- 1080. Berntsen RF, et al. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: A within-patient controlled study with implanted cardiac monitoring. Heart Rhythm 2016;13(9):1768–1774. [DOI] [PubMed] [Google Scholar]
- 1081. Lo LW, et al. Pearls and Pitfalls in Catheter Ablation of Persistent Atrial Fibrillation. Circ J 2016;80(2):306–313. [DOI] [PubMed] [Google Scholar]
- 1082. Perrotta L, et al. How to learn pulmonary vein isolation with a novel ablation device: learning curve effects using the endoscopic ablation system. J Cardiovasc Electrophysiol 2014;25(12):1293–1298. [DOI] [PubMed] [Google Scholar]
- 1083. Sediva L, et al. Visually guided laser ablation: a single-centre long-term experience. Europace 2014;16(12):1746–1751. [DOI] [PubMed] [Google Scholar]
- 1084. Gal P, et al. First Dutch experience with the endoscopic laser balloon ablation system for the treatment of atrial fibrillation. Neth Heart J 2015;23(2):96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1085. Osca J, et al. Electrical Isolation of Pulmonary Veins Using Laser Catheter in the Treatment of Paroxysmal and Persistent Atrial Fibrillation. One-year Results. Rev Esp Cardiol (Engl Ed) 2016;69(5):488–493. [DOI] [PubMed] [Google Scholar]
- 1086. Sawhney N, et al. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am J Cardiol 2009;104(3):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087. Medi C, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011;22(2):137–141. [DOI] [PubMed] [Google Scholar]
- 1088. Reynolds MR, Ellis E, Zimetbaum P.. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol 2008;19(7):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1089. Ware JE, et al. SF-36 health survey: manual and interpretation guide. The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 1090. Bubien RS, et al. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation 1996;94(7):1585–1591. [DOI] [PubMed] [Google Scholar]
- 1091. Walfridsson H, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health-related quality of life and symptom burden. The MANTRA-PAF trial. Europace 2015;17(2):215–221. [DOI] [PubMed] [Google Scholar]
- 1092. Thrall G, et al. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119(5). 448 e1–448 e19. [DOI] [PubMed] [Google Scholar]
- 1093. Berkowitsch A, et al. Comparison of generic health survey SF-36 and arrhythmia related symptom severity check list in relation to post-therapy AF recurrence. Europace 2003;5(4):351–355. [DOI] [PubMed] [Google Scholar]
- 1094. Reynolds MR, et al. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes 2010;3(6):615–623. [DOI] [PubMed] [Google Scholar]
- 1095. Spertus J, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4(1):15–25. [DOI] [PubMed] [Google Scholar]
- 1096. Walfridsson U, Arestedt K, Stromberg A.. Development and validation of a new Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA) with focus on symptom burden. Health Qual Life Outcomes 2012;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097. Fichtner S, et al. Prospective assessment of short- and long-term quality of life after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2012;23(2):121–127. [DOI] [PubMed] [Google Scholar]
- 1098. Kistler PM, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 2004;44(1):109–116. [DOI] [PubMed] [Google Scholar]
- 1099. Wijffels MC, et al. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 1997;96(10):3710–3720. [DOI] [PubMed] [Google Scholar]
- 1100. Scharf C, et al. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol 2003;14(2):150–155. [DOI] [PubMed] [Google Scholar]
- 1101. Jayam VK, et al. Atrial volume reduction following catheter ablation of atrial fibrillation and relation to reduction in pulmonary vein size: an evaluation using magnetic resonance angiography. J Interv Card Electrophysiol 2005;13(2):107–114. [DOI] [PubMed] [Google Scholar]
- 1102. Tsao HM, et al. Morphologic remodeling of pulmonary veins and left atrium after catheter ablation of atrial fibrillation: insight from long-term follow-up of three-dimensional magnetic resonance imaging. J Cardiovasc Electrophysiol 2005;16(1):7–12. [DOI] [PubMed] [Google Scholar]
- 1103. Canpolat U, et al. The impact of cryoballoon-based catheter ablation on left atrial structural and potential electrical remodeling in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2015;44(2):131–139. [DOI] [PubMed] [Google Scholar]
- 1104. Lemola K, et al. Effect of left atrial circumferential ablation for atrial fibrillation on left atrial transport function. Heart Rhythm 2005;2(9):923–928. [DOI] [PubMed] [Google Scholar]
- 1105. Verma A, et al. Extensive ablation during pulmonary vein antrum isolation has no adverse impact on left atrial function: an echocardiography and cine computed tomography analysis. J Cardiovasc Electrophysiol 2006;17(7):741–746. [DOI] [PubMed] [Google Scholar]
- 1106. Jeevanantham V, et al. Meta-analysis of the effect of radiofrequency catheter ablation on left atrial size, volumes and function in patients with atrial fibrillation. Am J Cardiol 2010;105(9):1317–1326. [DOI] [PubMed] [Google Scholar]
- 1107. Machino-Ohtsuka T, et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ J 2013;77(7):1695–1704. [DOI] [PubMed] [Google Scholar]
- 1108. Muellerleile K, et al. Cardiovascular magnetic resonance demonstrates reversible atrial dysfunction after catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2013;24(7):762–767. [DOI] [PubMed] [Google Scholar]
- 1109. Hanazawa K, et al. Effect of radiofrequency catheter ablation of persistent atrial fibrillation on the left atrial function: assessment by 320-row multislice computed tomography. Int J Cardiol 2015;179:449–454. [DOI] [PubMed] [Google Scholar]
- 1110. Cochet H, et al. Atrial structure and function 5 years after successful ablation for persistent atrial fibrillation: an MRI study. J Cardiovasc Electrophysiol 2014;25(7):671–679. [DOI] [PubMed] [Google Scholar]
- 1111. Gibson DN, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8(9):1364–1371. [DOI] [PubMed] [Google Scholar]
- 1112. Reynolds MR, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes 2012;5(2):171–181. [DOI] [PubMed] [Google Scholar]
- 1113. Chang CH, et al. Effect of radiofrequency catheter ablation for atrial fibrillation on morbidity and mortality: a nationwide cohort study and propensity score analysis. Circ Arrhythm Electrophysiol 2014;7(1):76–82. [DOI] [PubMed] [Google Scholar]
- 1114. Friberg L, Tabrizi F, Englund A.. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37(31):2478–2487. [DOI] [PubMed] [Google Scholar]
- 1115. Abecasis J, et al. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 2009;11(10):1289–1294. [DOI] [PubMed] [Google Scholar]
- 1116. McCready JW, et al. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace 2011;13(3):355–361. [DOI] [PubMed] [Google Scholar]
- 1117. D'Ascenzo F, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation? A meta-analysis. Int J Cardiol 2013;167(5):1984–1989. [DOI] [PubMed] [Google Scholar]
- 1118. Chan P, et al. Cost-effectiveness of left atrial catheter ablation, antiarrhythmic therapy, and rate control therapy for paroxysmal and chronic atrial fibrillation [abstract]. Circulation 2005:e318.16286593 [Google Scholar]
- 1119. Eckard N, et al. Cost-effectiveness of catheter ablation for patients with symptomatic atrial fibrillation. J Atr Fibrillation 2009;2(2):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1120. Reynolds MR, et al. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2(4):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1121. Martin-Doyle W, Reynolds MR.. Is AF ablation cost effective? J Atr Fibrillation 2010;2(1):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1122. Assasi N, et al. Ablation procedures for rhythm control in patients with atrial fibrillation: clinical and cost-effectiveness analyses. CADTH Technol Overv 2012;2(1):e2101. [PMC free article] [PubMed] [Google Scholar]
- 1123. Neyt M, Van Brabandt H, Devos C.. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124. Reynolds MR, et al. Cost-effectiveness of cryoballoon ablation for the management of paroxysmal atrial fibrillation. Europace 2014;16(5):652–659. [DOI] [PubMed] [Google Scholar]
- 1125. Aronsson M, et al. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace 2015;17(1):48–55. [DOI] [PubMed] [Google Scholar]
- 1126. Winkle RA, et al. Physician-controlled costs: the choice of equipment used for atrial fibrillation ablation. J Interv Card Electrophysiol 2013;36(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127. Ladapo JA, et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol 2012;23(1):1–8. [DOI] [PubMed] [Google Scholar]
- 1128. McKenna C, et al. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart 2009;95(7):542–549. [DOI] [PubMed] [Google Scholar]
- 1129. Adragao PP, et al. Safety and long-term outcomes of catheter ablation of atrial fibrillation using magnetic navigation versus manual conventional ablation: a propensity-score analysis. J Cardiovasc Electrophysiol 2016;27(Suppl 1):S11–S16. [DOI] [PubMed] [Google Scholar]
- 1130. Anderson JL, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(21):2304–2322. [DOI] [PubMed] [Google Scholar]
- 1131. Eick OJ, Gerritse B, Schumacher B.. Popping phenomena in temperature-controlled radiofrequency ablation: when and why do they occur? Pacing Clin Electrophysiol 2000;23(2):253–258. [DOI] [PubMed] [Google Scholar]
- 1132. Fisher JD, et al. Internal transcardiac pericardiocentesis for acute tamponade. Am J Cardiol 2000;86(12):1388–1389, A6. [DOI] [PubMed] [Google Scholar]
- 1133. Hsu LF, et al. Transcardiac pericardiocentesis: an emergency life-saving technique for cardiac tamponade. J Cardiovasc Electrophysiol 2003;14(9):1001–1003. [DOI] [PubMed] [Google Scholar]
- 1134. Bunch TJ, et al. Outcomes after cardiac perforation during radiofrequency ablation of the atrium. J Cardiovasc Electrophysiol 2005;16(11):1172–1179. [DOI] [PubMed] [Google Scholar]
- 1135. Hsu LF, et al. Incidence and prevention of cardiac tamponade complicating ablation for atrial fibrillation. Pacing Clin Electrophysiol 2005;28(Suppl 1):S106–S109. [DOI] [PubMed] [Google Scholar]
- 1136. Nairooz R, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol 2015;187:426–429. [DOI] [PubMed] [Google Scholar]
- 1137. Latchamsetty R, et al. Management and outcomes of cardiac tamponade during atrial fibrillation ablation in the presence of therapeutic anticoagulation with warfarin. Heart Rhythm 2011;8(6):805–808. [DOI] [PubMed] [Google Scholar]
- 1138. Wu S, et al. Meta-analysis of efficacy and safety of new oral anticoagulants compared with uninterrupted vitamin K antagonists in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol 2016;117(6):926–934. [DOI] [PubMed] [Google Scholar]
- 1139. Michowitz Y, et al. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol 2014;7(2):274–280. [DOI] [PubMed] [Google Scholar]
- 1140. Tsang TS, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 2002;77(5):429–436. [DOI] [PubMed] [Google Scholar]
- 1141. O'Neill MD, et al. Two techniques to avoid surgery for cardiac tamponade occurring during catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19(3):323–325. [DOI] [PubMed] [Google Scholar]
- 1142. Katz ES, et al. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36(2):468–471. [DOI] [PubMed] [Google Scholar]
- 1143. Di Biase L, et al. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: clinical implications after long-term follow-up. J Am Coll Cardiol 2006;48(12):2493–2499. [DOI] [PubMed] [Google Scholar]
- 1144. Prieto LR, et al. Comparison of stent versus balloon angioplasty for pulmonary vein stenosis complicating pulmonary vein isolation. J Cardiovasc Electrophysiol 2008;19(7):673–678. [DOI] [PubMed] [Google Scholar]
- 1145. Packer DL, et al. Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation. Circulation 2005;111(5):546–554. [DOI] [PubMed] [Google Scholar]
- 1146. Taylor GW, et al. Pathological effects of extensive radiofrequency energy applications in the pulmonary veins in dogs. Circulation 2000;101(14):1736–1742. [DOI] [PubMed] [Google Scholar]
- 1147. Arentz T, et al. Incidence of pulmonary vein stenosis 2 years after radiofrequency catheter ablation of refractory atrial fibrillation. Eur Heart J 2003;24(10):963–969. [DOI] [PubMed] [Google Scholar]
- 1148. Tse HF, et al. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol 2003;42(4):752–758. [DOI] [PubMed] [Google Scholar]
- 1149. Kasper L, et al. Hemoptysis and lung disease as a manifestation of pulmonary vein stenosis after cryoballoon catheter ablation for atrial fibrillation. Pol Arch Med Wewn 2016;126(1-2):94–96. [PubMed] [Google Scholar]
- 1150. Dong J, et al. Incidence and predictors of pulmonary vein stenosis following catheter ablation of atrial fibrillation using the anatomic pulmonary vein ablation approach: results from paired magnetic resonance imaging. J Cardiovasc Electrophysiol 2005;16(8):845–852. [DOI] [PubMed] [Google Scholar]
- 1151. Hoyt RH, et al. Transvenous catheter cryoablation for treatment of atrial fibrillation: results of a feasibility study. Pacing Clin Electrophysiol 2005;28(Suppl 1):S78–S82. [DOI] [PubMed] [Google Scholar]
- 1152. Saad EB, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med 2003;138(8):634–638. [DOI] [PubMed] [Google Scholar]
- 1153. Hilbert S, et al. Pulmonary vein collateral formation as a long-term result of post-interventional pulmonary vein stenosis. Eur Heart J 2016;37(31):2474. [DOI] [PubMed] [Google Scholar]
- 1154. Hilbert S, Sommer P, Bollmann A.. Pulmonary vein dilatation in a case of total pulmonary vein occlusion: contemporary approach using a combination of 3D-mapping system and image integration. Catheter Cardiovasc Interv 2016;88(7):E227–E232. [DOI] [PubMed] [Google Scholar]
- 1155. Fender EA, et al. Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: presentation, management, and clinical outcomes. Circulation 2016;134(23):1812–1821. [DOI] [PubMed] [Google Scholar]
- 1156. De Potter TJ, et al. Drug-eluting stents for the treatment of pulmonary vein stenosis after atrial fibrillation ablation. Europace 2011;13(1):57–61. [DOI] [PubMed] [Google Scholar]
- 1157. Kanter KR, Kirshbom PM, Kogon BE.. Surgical repair of pulmonary venous stenosis: a word of caution. Ann Thorac Surg 2014;98(5):1687–1691. discussion 1691–1692. [DOI] [PubMed] [Google Scholar]
- 1158. Patel NS, et al. Successful surgical repair of iatrogenic pulmonary vein stenosis. J Cardiovasc Electrophysiol 2012;23(6):656–658. [DOI] [PubMed] [Google Scholar]
- 1159. Bharat A, et al. Lung transplant is a viable treatment option for patients with congenital and acquired pulmonary vein stenosis. J Heart Lung Transplant 2013;32(6):621–625. [DOI] [PubMed] [Google Scholar]
- 1160. Ponamgi SP, et al. Catheter-based intervention for pulmonary vein stenosis due to fibrosing mediastinitis: the Mayo Clinic experience. Int J Cardiol Heart Vasc 2015;8:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161. Kumar S, et al. Esophageal hematoma after atrial fibrillation ablation: incidence, clinical features, and sequelae of esophageal injury of a different sort. Circ Arrhythm Electrophysiol 2012;5(4):701–705. [DOI] [PubMed] [Google Scholar]
- 1162. Gillinov AM, Pettersson G, Rice TW.. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2001;122(6):1239–1240. [DOI] [PubMed] [Google Scholar]
- 1163. Mohr FW, et al. Curative treatment of atrial fibrillation with intraoperative radiofrequency ablation: short-term and midterm results. J Thorac Cardiovasc Surg 2002;123(5):919–927. [DOI] [PubMed] [Google Scholar]
- 1164. Doll N, et al. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg 2003;125(4):836–842. [DOI] [PubMed] [Google Scholar]
- 1165. Sonmez B, et al. A fatal complication due to radiofrequency ablation for atrial fibrillation: atrio-esophageal fistula. Ann Thorac Surg 2003;76(1):281–283. [DOI] [PubMed] [Google Scholar]
- 1166. Scanavacca MI, et al. Left atrial-esophageal fistula following radiofrequency catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(8):960–962. [DOI] [PubMed] [Google Scholar]
- 1167. Borchert B, et al. Lethal atrioesophageal fistula after pulmonary vein isolation using high-intensity focused ultrasound (HIFU). Heart Rhythm 2008;5(1):145–148. [DOI] [PubMed] [Google Scholar]
- 1168. Ghia KK, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2009;24(1):33–36. [DOI] [PubMed] [Google Scholar]
- 1169. Gilcrease GW, Stein JB.. A delayed case of fatal atrioesophageal fistula following radiofrequency ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2010;21(6):708–711. [DOI] [PubMed] [Google Scholar]
- 1170. Stockigt F, et al. Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol 2012;23(11):1254–1257. [DOI] [PubMed] [Google Scholar]
- 1171. Yousuf O, Calkins H.. Sounding the warning on the potential for oesophageal injury resulting from use of the nMARQ for ablation of atrial fibrillation. Europace 2015;17(3):343–344. [DOI] [PubMed] [Google Scholar]
- 1172. Jackson, et al. The vagus plays a role in the anti-reflux barrier by controlling both the lower esophageal sphincter pressure and crural diaphragm activity. J Am Coll Surg 2005;201(3)(Suppl):S11. [Google Scholar]
- 1173. Nolker G, et al. Esophageal acid levels after pulmonary vein isolation for atrial fibrillation. Pacing Clin Electrophysiol 2009;32(Suppl 1):S228–S230. [DOI] [PubMed] [Google Scholar]
- 1174. Medeiros De, Vasconcelos JT, et al. Atrial-oesophageal fistula following percutaneous radiofrequency catheter ablation of atrial fibrillation: the risk still persists. Europace 2017;19(2):250–258. [DOI] [PubMed] [Google Scholar]
- 1175. Rillig A, et al. Modified energy settings are mandatory to minimize oesophageal injury using the novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. Europace 2015;17(3):396–402. [DOI] [PubMed] [Google Scholar]
- 1176. Chavez P, et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart 2015;2(1):e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177. Mateos JC, et al. Simplified method for esophagus protection during radiofrequency catheter ablation of atrial fibrillation–prospective study of 704 cases. Rev Bras Cir Cardiovasc 2015;30(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178. Shim HB, et al. Successful management of atrio-esophageal fistula after cardiac radiofrequency catheter ablation. Korean J Thorac Cardiovasc Surg 2013;46(2):142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1179. Schwartz TW, et al. Pancreatic-polypeptide response to food in duodenal-ulcer patients before and after vagotomy. Lancet 1976;1(7969):1102–1105. [DOI] [PubMed] [Google Scholar]
- 1180. Ajaj W, et al. Real time high resolution magnetic resonance imaging for the assessment of gastric motility disorders. Gut 2004;53(9):1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181. Pisani CF, et al. Gastric hypomotility following epicardial vagal denervation ablation to treat atrial fibrillation. J Cardiovasc Electrophysiol 2008;19(2):211–213. [DOI] [PubMed] [Google Scholar]
- 1182. Kanaeda T, et al. Evaluation of periesophageal nerve injury after pulmonary vein isolation using the (13)C-acetate breath test. J Arrhythm 2015;31(6):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1183. Lo LW, et al. A novel finding—impairment of gastric myoelectricity after catheter ablation of atrial fibrillation. Circ J 2013;77(8):2014–2023. [DOI] [PubMed] [Google Scholar]
- 1184. Janssens J, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 1990;322(15):1028–1031. [DOI] [PubMed] [Google Scholar]
- 1185. Jones MP, Maganti K.. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol 2003;98(10):2122–2129. [DOI] [PubMed] [Google Scholar]
- 1186. Earley MJ, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J 2006;27(10):1223–1229. [DOI] [PubMed] [Google Scholar]
- 1187. Sarabanda AV, et al. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol 2005;46(10):1902–1912. [DOI] [PubMed] [Google Scholar]
- 1188. Guiot A, et al. Collateral nervous damages after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol 2012;23(4):346–351. [DOI] [PubMed] [Google Scholar]
- 1189. Metzner A, et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 2014;25(5):466–470. [DOI] [PubMed] [Google Scholar]
- 1190. Okumura Y, et al. Distortion of right superior pulmonary vein anatomy by balloon catheters as a contributor to phrenic nerve injury. J Cardiovasc Electrophysiol 2009;20(10):1151–1157. [DOI] [PubMed] [Google Scholar]
- 1191. Arruda M, et al. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol 2007;18(12):1261–1266. [DOI] [PubMed] [Google Scholar]
- 1192. Miyazaki S, et al. Prevalence and clinical outcome of phrenic nerve injury during superior vena cava isolation and circumferential pulmonary vein antrum isolation using radiofrequency energy. Am Heart J 2014;168(6):846–853. [DOI] [PubMed] [Google Scholar]
- 1193. Wissner E, et al. Catheter ablation of atrial fibrillation in patients with persistent left superior vena cava is associated with major intraprocedural complications. Heart Rhythm 2010;7(12):1755–1760. [DOI] [PubMed] [Google Scholar]
- 1194. Elayi CS, et al. Left superior vena cava isolation in patients undergoing pulmonary vein antrum isolation: impact on atrial fibrillation recurrence. Heart Rhythm 2006;3(9):1019–1023. [DOI] [PubMed] [Google Scholar]
- 1195. Liu H, et al. Electrogram-guided isolation of the left superior vena cava for treatment of atrial fibrillation. Europace 2007;9(9):775–780. [DOI] [PubMed] [Google Scholar]
- 1196. Yong Ji S, et al. Phrenic nerve injury: An underrecognized and potentially preventable complication of pulmonary vein isolation using a wide-area circumferential ablation approach. J Cardiovasc Electrophysiol 2013;24(10):1086–1091. [DOI] [PubMed] [Google Scholar]
- 1197. Franceschi F, et al. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm 2011;8(7):1068–1071. [DOI] [PubMed] [Google Scholar]
- 1198. Miyazaki S, et al. Prospective evaluation of bilateral diaphragmatic electromyograms during cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2015;26(6):622–628. [DOI] [PubMed] [Google Scholar]
- 1199. Mondesert B, et al. Clinical experience with a novel electromyographic approach to preventing phrenic nerve injury during cryoballoon ablation in atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7(4):605–611. [DOI] [PubMed] [Google Scholar]
- 1200. Sacher F, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol 2006;47(12):2498–2503. [DOI] [PubMed] [Google Scholar]
- 1201. Andrade JG, et al. Histopathology of cryoballoon ablation-induced phrenic nerve injury. J Cardiovasc Electrophysiol 2014;25(2):187–194. [DOI] [PubMed] [Google Scholar]
- 1202. Patel D, et al. Long-term functional and neurocognitive recovery in patients who had an acute cerebrovascular event secondary to catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2010;21(4):412–417. [DOI] [PubMed] [Google Scholar]
- 1203. Kochhauser S, et al. Comparison of outcomes after cardioversion or atrial fibrillation ablation in patients with differing periprocedural anticoagulation regimens. Can J Cardiol 2014;30(12):1541–1546. [DOI] [PubMed] [Google Scholar]
- 1204. Kosiuk J, et al. Early cerebral thromboembolic complications after radiofrequency catheter ablation of atrial fibrillation: incidence, characteristics, and risk factors. Heart Rhythm 2014;11(11):1934–1940. [DOI] [PubMed] [Google Scholar]
- 1205. Merchant FM, Delurgio DB.. Catheter ablation of atrial fibrillation and risk of asymptomatic cerebral embolism. Pacing Clin Electrophysiol 2014;37(3):389–397. [DOI] [PubMed] [Google Scholar]
- 1206. Lickfett L, et al. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation. J Cardiovasc Electrophysiol 2006;17(1):1–7. [DOI] [PubMed] [Google Scholar]
- 1207. Gaita F, et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 2010;122(17):1667–1673. [DOI] [PubMed] [Google Scholar]
- 1208. Schrickel JW, et al. Incidence and predictors of silent cerebral embolism during pulmonary vein catheter ablation for atrial fibrillation. Europace 2010;12(1):52–57. [DOI] [PubMed] [Google Scholar]
- 1209. Deneke T, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm 2011;8(11):1705–1711. [DOI] [PubMed] [Google Scholar]
- 1210. Sauren LD, et al. Transcranial measurement of cerebral microembolic signals during endocardial pulmonary vein isolation: comparison of three different ablation techniques. J Cardiovasc Electrophysiol 2009;20(10):1102–1107. [DOI] [PubMed] [Google Scholar]
- 1211. Wieczorek M, et al. Investigation into causes of abnormal cerebral MRI findings following PVAC duty-cycled, phased RF ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2013;24(2):121–128. [DOI] [PubMed] [Google Scholar]
- 1212. Bendszus M, Stoll G.. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol 2006;5(4):364–372. [DOI] [PubMed] [Google Scholar]
- 1213. Kruis RW, Vlasveld FA, Van Dijk D.. The (un)importance of cerebral microemboli. Semin Cardiothorac Vasc Anesth 2010;14(2):111–118. [DOI] [PubMed] [Google Scholar]
- 1214. Medi C, et al. Subtle post-procedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol 2013;62(6):531–539. [DOI] [PubMed] [Google Scholar]
- 1215. Ichiki H, et al. The incidence of asymptomatic cerebral microthromboembolism after atrial fibrillation ablation: comparison of warfarin and dabigatran. Pacing Clin Electrophysiol 2013;36(11):1328–1335. [DOI] [PubMed] [Google Scholar]
- 1216. Nagy-Balo E, et al. Transcranial measurement of cerebral microembolic signals during pulmonary vein isolation: a comparison of two ablation techniques. Circ Arrhythm Electrophysiol 2013;6(3):473–480. [DOI] [PubMed] [Google Scholar]
- 1217. Vermeer SE, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348(13):1215–1222. [DOI] [PubMed] [Google Scholar]
- 1218. Helps SC, et al. The effect of gas emboli on rabbit cerebral blood flow. Stroke 1990;21(1):94–99. [DOI] [PubMed] [Google Scholar]
- 1219. Krivonyak GS, Warren SG.. Cerebral arterial air embolism treated by a vertical head-down maneuver. Catheter Cardiovasc Interv 2000;49(2):185–187. [DOI] [PubMed] [Google Scholar]
- 1220. Cauchemez B, et al. High-flow perfusion of sheaths for prevention of thromboembolic complications during complex catheter ablation in the LA. J Cardiovasc Electrophysiol 2004;15(3):276–283. [DOI] [PubMed] [Google Scholar]
- 1221. Kuwahara T, et al. Clinical characteristics of massive air embolism complicating left atrial ablation of atrial fibrillation: lessons from five cases. Europace 2012;14(2):204–208. [DOI] [PubMed] [Google Scholar]
- 1222. Franzen OW, et al. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter Cardiovasc Interv 2008;71(4):553–558. [DOI] [PubMed] [Google Scholar]
- 1223. Ryu KH, et al. Heparin reduces neurological impairment after cerebral arterial air embolism in the rabbit. Stroke 1996;27(2):303–309. discussion 310. [DOI] [PubMed] [Google Scholar]
- 1224. Aldhoon B, et al. Complications of catheter ablation for atrial fibrillation in a high-volume centre with the use of intracardiac echocardiography. Europace 2013;15(1):24–32. [DOI] [PubMed] [Google Scholar]
- 1225. Mugnai G, et al. Complications in the setting of percutaneous atrial fibrillation ablation using radiofrequency and cryoballoon techniques: A single-center study in a large cohort of patients. Int J Cardiol 2015;196:42–49. [DOI] [PubMed] [Google Scholar]
- 1226. Murakawa Y, et al. Nationwide survey of catheter ablation for atrial fibrillation: The Japanese catheter ablation registry of atrial fibrillation (J-CARAF)-A report on periprocedural oral anticoagulants. J Arrhythm 2015;31(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227. Palaniswamy C, et al. Catheter ablation of postinfarction ventricular tachycardia: ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014;11(11):2056–2063. [DOI] [PubMed] [Google Scholar]
- 1228. Peichl P, et al. Complications of catheter ablation of ventricular tachycardia: a single-center experience. Circ Arrhythm Electrophysiol 2014;7(4):684–690. [DOI] [PubMed] [Google Scholar]
- 1229. Waigand J, et al. Percutaneous treatment of pseudoaneurysms and arteriovenous fistulas after invasive vascular procedures. Catheter Cardiovasc Interv 1999;47(2):157–164. [DOI] [PubMed] [Google Scholar]
- 1230. Lakkireddy D, et al. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol 2014;63(10):982–988. [DOI] [PubMed] [Google Scholar]
- 1231. Tanaka-Esposito CC, et al. Real-time ultrasound guidance reduces total and major vascular complications in patients undergoing pulmonary vein antral isolation on therapeutic warfarin. J Interv Card Electrophysiol 2013;37(2):163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232. Errahmouni A, et al. Ultrasound-guided venous puncture in electrophysiological procedures: a safe method, rapidly learned. Pacing Clin Electrophysiol 2014;37(8):1023–1028. [DOI] [PubMed] [Google Scholar]
- 1233. Chugh A, et al. Manifestations of coronary arterial injury during catheter ablation of atrial fibrillation and related arrhythmias. Heart Rhythm 2013;10(11):1638–1645. [DOI] [PubMed] [Google Scholar]
- 1234. Makimoto H, et al. Aborted sudden cardiac death due to radiofrequency ablation within the coronary sinus and subsequent total occlusion of the circumflex artery. J Cardiovasc Electrophysiol 2013;24(8):929–932. [DOI] [PubMed] [Google Scholar]
- 1235. Kitamura T, et al. Transient sinus node dysfunction following sinus node artery occlusion due to radiofrequency catheter ablation of the septal superior vena cava-right atrium junction. J Electrocardiol 2016;49(1):18–22. [DOI] [PubMed] [Google Scholar]
- 1236. Ouali S, et al. Acute coronary occlusion during radiofrequency catheter ablation of typical atrial flutter. J Cardiovasc Electrophysiol 2002;13(10):1047–1049. [DOI] [PubMed] [Google Scholar]
- 1237. Mykytsey A, et al. Right coronary artery occlusion during RF ablation of typical atrial flutter. J Cardiovasc Electrophysiol 2010;21(7):818–821. [DOI] [PubMed] [Google Scholar]
- 1238. Sticherling C, Pfister O, Osswald S.. Thrombo-embolic occlusion of the left anterior descending coronary artery complicating left atrial radiofrequency ablation. Europace 2009; 11(1):117–118. [DOI] [PubMed] [Google Scholar]
- 1239. Wong KC, et al. High incidence of acute sub-clinical circumflex artery ‘injury' following mitral isthmus ablation. Eur Heart J 2011;32(15):1881–1890. [DOI] [PubMed] [Google Scholar]
- 1240. Fayad G, et al. Circumflex artery stenosis induced by intraoperative radiofrequency ablation. Ann Thorac Surg 2003;76(4):1291–1293. [DOI] [PubMed] [Google Scholar]
- 1241. Calkins H, et al. Radiation exposure during radiofrequency catheter ablation of accessory atrioventricular connections. Circulation 1991;84(6):2376–2382. [DOI] [PubMed] [Google Scholar]
- 1242. Lindsay BD, et al. Radiation exposure to patients and medical personnel during radiofrequency catheter ablation for supraventricular tachycardia. Am J Cardiol 1992;70(2):218–223. [DOI] [PubMed] [Google Scholar]
- 1243. Kovoor P, et al. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation 1998;98(15):1534–1540. [DOI] [PubMed] [Google Scholar]
- 1244. Macle L, et al. Radiation exposure during radiofrequency catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2003;26(1 Pt 2):288–291. [DOI] [PubMed] [Google Scholar]
- 1245. Lickfett L, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation 2004;110(19):3003–3010. [DOI] [PubMed] [Google Scholar]
- 1246. Ector J, et al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol 2007;50(3):234–242. [DOI] [PubMed] [Google Scholar]
- 1247. Chen J, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J Am Coll Cardiol 2010;56(9):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1248. Khaykin Y, et al. CARTO-guided vs. NavX-guided pulmonary vein antrum isolation and pulmonary vein antrum isolation performed without 3-D mapping: effect of the 3-D mapping system on procedure duration and fluoroscopy time. J Interv Card Electrophysiol 2011;30(3):233–240. [DOI] [PubMed] [Google Scholar]
- 1249. Stabile G, et al. Reduced fluoroscopy exposure during ablation of atrial fibrillation using a novel electroanatomical navigation system: a multicentre experience. Europace 2012;14(1):60–65. [DOI] [PubMed] [Google Scholar]
- 1250. De Ponti R, et al. Simulator training reduces radiation exposure and improves trainees' performance in placing electrophysiologic catheters during patient-based procedures. Heart Rhythm 2012;9(8):1280–1285. [DOI] [PubMed] [Google Scholar]
- 1251. Kleemann T, et al. Development of radiation exposure in patients undergoing pulmonary vein isolation in Germany between 2007 and 2014: great potential to minimize radiation dosage. Clin Res Cardiol 2016;105(10):858–864. [DOI] [PubMed] [Google Scholar]
- 1252. Steven D, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol 2010;21(1):6–12. [DOI] [PubMed] [Google Scholar]
- 1253. Weiss JP, et al. A comparison of remote magnetic irrigated tip ablation versus manual catheter irrigated tip catheter ablation with and without force sensing feedback. J Cardiovasc Electrophysiol 2016;27(Suppl 1):S5–S10. [DOI] [PubMed] [Google Scholar]
- 1254. Dragusin O, et al. Evaluation of a radiation protection cabin for invasive electrophysiological procedures. Eur Heart J 2007;28(2):183–189. [DOI] [PubMed] [Google Scholar]
- 1255. Reddy VY, et al. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm 2010;7(11):1644–1653. [DOI] [PubMed] [Google Scholar]
- 1256. Bulava A, Hanis J, Eisenberger M.. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol 2015;38(7):797–806. [DOI] [PubMed] [Google Scholar]
- 1257. Stabile G, et al. Low incidence of permanent complications during catheter ablation for atrial fibrillation using open-irrigated catheters: a multicentre registry. Europace 2014;16(8):1154–1159. [DOI] [PubMed] [Google Scholar]
- 1258. Luckie M, et al. Dressler's syndrome following pulmonary vein isolation for atrial fibrillation. Acute Card Care 2008;10(4):234–235. [DOI] [PubMed] [Google Scholar]
- 1259. Lambert T, et al. Cardiac tamponade following pericarditis 18 days after catheter ablation of atrial fibrillation. Clin Res Cardiol 2010;99(9):595–597. [DOI] [PubMed] [Google Scholar]
- 1260. Torihashi S, et al. Two cases of delayed cardiac tamponade due to pericarditis after pulmonary vein (PV) isolation for atrial fibrillation. Intern Med 2015;54(7):791–796. [DOI] [PubMed] [Google Scholar]
- 1261. Kim DR, et al. Comparison of two different doses of single bolus steroid injection to prevent atrial fibrillation recurrence after radiofrequency catheter ablation. Yonsei Med J 2015;56(2):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262. Kesek M, et al. Entrapment of circular mapping catheter in the mitral valve. Heart Rhythm 2007;4(1):17–19. [DOI] [PubMed] [Google Scholar]
- 1263. Tavernier R, Duytschaever M, Taeymans Y.. Fracture of a circular mapping catheter after entrapment in the mitral valve apparatus during segmental pulmonary vein isolation. Pacing Clin Electrophysiol 2003;26(8):1774–1775. [DOI] [PubMed] [Google Scholar]
- 1264. Grove R, et al. Demand for open heart surgery due to entrapment of a circular mapping catheter in the mitral valve in a patient undergoing atrial fibrillation ablation. Clin Res Cardiol 2008;97(9):628–629. [DOI] [PubMed] [Google Scholar]
- 1265. Lakkireddy D, et al. Radiofrequency ablation of atrial fibrillation in patients with mitral or aortic mechanical prosthetic valves: a feasibility, safety, and efficacy study. Heart Rhythm 2011;8(7):975–980. [DOI] [PubMed] [Google Scholar]
- 1266. Zeljko HM, et al. Entrapment of the circular mapping catheter in the mitral valve in two patients undergoing atrial fibrillation ablation. Europace 2011;13(1):132–133. [DOI] [PubMed] [Google Scholar]
- 1267. Desimone CV, et al. Catheter ablation related mitral valve injury: the importance of early recognition and rescue mitral valve repair. J Cardiovasc Electrophysiol 2014;25(9):971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1268. Gurbuz O, et al. Case report: paravalvular leak as a complication of percutaneous catheter ablation for atrial fibrillation. J Cardiothorac Surg 2014;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1269. Mansour M, et al. Successful release of entrapped circumferential mapping catheters in patients undergoing pulmonary vein isolation for atrial fibrillation. Heart Rhythm 2004;1(5):558–561. [DOI] [PubMed] [Google Scholar]
- 1270. Shoemaker MB, et al. Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J Am Coll Cardiol 2011;57(19):1918–1919. [DOI] [PubMed] [Google Scholar]
- 1271. Welch TD, et al. Symptomatic pulmonary hypertension with giant left atrial v waves after surgical maze procedures: evaluation by comprehensive hemodynamic catheterization. Heart Rhythm 2013;10(12):1839–1842. [DOI] [PubMed] [Google Scholar]
- 1272. Witt C, et al. Recurrent dyspnea following multiple ablations for atrial fibrillation explained by the “stiff left atrial syndrome”. Catheter Cardiovasc Interv 2013;82(5):E747–E749. [DOI] [PubMed] [Google Scholar]
- 1273. Pilote L, et al. Stiff left atrial syndrome. Can J Cardiol 1988;4(6):255–257. [PubMed] [Google Scholar]
- 1274. Khurram IM, et al. Association between left atrial stiffness index and atrial fibrillation recurrence in patients undergoing left atrial ablation. Circ Arrhythm Electrophysiol 2016;9(3). [DOI] [PubMed] [Google Scholar]
- 1275. Kosiuk J, et al. Prevalence and predictors of worsened left ventricular diastolic dysfunction after catheter ablation of atrial fibrillation. Int J Cardiol 2013;168(4):3613–3615. [DOI] [PubMed] [Google Scholar]
- 1276. Wong GR, et al. Novel use of sildenafil in the management of pulmonary hypertension due to post-catheter ablation ‘stiff left atrial syndrome’. Int J Cardiol 2015;181:55–56. [DOI] [PubMed] [Google Scholar]
- 1277. Aryana A, et al. Impact of Pulmonary Vein Cryoballoon Ablation on Bronchial Injury. J Cardiovasc Electrophysiol 2016;27(7):861–867. [DOI] [PubMed] [Google Scholar]
- 1278. Verma N, et al. Ice formation in the left mainstem bronchus during cryoballoon ablation for the treatment of atrial fibrillation. Heart Rhythm 2016;13(3):814–815. [DOI] [PubMed] [Google Scholar]
- 1279. Kang KW, et al. Long-term changes in heart rate variability after radiofrequency catheter ablation for atrial fibrillation: 1-year follow-up study with irrigation tip catheter. J Cardiovasc Electrophysiol 2014;25(7):693–700. [DOI] [PubMed] [Google Scholar]
- 1280. Bunch TJ, et al. Mechanisms of phrenic nerve injury during radiofrequency ablation at the pulmonary vein orifice. J Cardiovasc Electrophysiol 2005;16(12):1318–1325. [DOI] [PubMed] [Google Scholar]
- 1281. Bai R, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol 2006;17(9):944–948. [DOI] [PubMed] [Google Scholar]
- 1282. De Ponti R, et al. Superiority of simulator-based training compared with conventional training methodologies in the performance of transseptal catheterization. J Am Coll Cardiol 2011;58(4):359–363. [DOI] [PubMed] [Google Scholar]
- 1283. Talbot H, et al. Interactive training system for interventional electrocardiology procedures. Med Image Anal 2016;35:225–237. [DOI] [PubMed] [Google Scholar]
- 1284. Zipes DP, et al. 2015 ACC/AHA/HRS advanced training statement on clinical cardiac electrophysiology (a revision of the ACC/AHA 2006 update of the clinical competence statement on invasive electrophysiology studies, catheter ablation, and cardioversion). J Am Coll Cardiol 2015;66(24):2767–2802. [DOI] [PubMed] [Google Scholar]
- 1285. Wojcik M, et al. Learning curve in cryoballoon ablation of atrial fibrillation: eight-year experience. Circ J 2014;78(7):1612–1618. [DOI] [PubMed] [Google Scholar]
- 1286. Martirosyan M, et al. Learning curve in circular multipolar phased radiofrequency ablation of atrial fibrillation. Cardiol J 2015;22(3):260–266. [DOI] [PubMed] [Google Scholar]
- 1287. Cox JL, Ad N, Palazzo T.. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118(5):833–840. [DOI] [PubMed] [Google Scholar]
- 1288. Feinberg MS, et al. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation 1994;90(5 Pt 2):II285–II292. [PubMed] [Google Scholar]
- 1289. Prasad SM, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126(6):1822–1828. [DOI] [PubMed] [Google Scholar]
- 1290. U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data: AtriCure Synergy Ablation System, PMA P100046. 2011.
- 1291. Demaria RG, et al. Surgical radiofrequency ablation induces coronary endothelial dysfunction in porcine coronary arteries. Eur J Cardiothorac Surg 2003;23(3):277–282. [DOI] [PubMed] [Google Scholar]
- 1292. Laczkovics A, Khargi K, Deneke T.. Esophageal perforation during left atrial radiofrequency ablation. J Thorac Cardiovasc Surg 2003;126(6):2119–2120. author reply 2120. [DOI] [PubMed] [Google Scholar]
- 1293. Doll N, et al. Epicardial treatment of atrial fibrillation using cryoablation in an acute off-pump sheep model. Thorac Cardiovasc Surg 2003;51(5):267–273. [DOI] [PubMed] [Google Scholar]
- 1294. Santiago T, et al. Epicardial radiofrequency applications: in vitro and in vivo studies on human atrial myocardium. Eur J Cardiothorac Surg 2003;24(4):481–486. discussion 486. [DOI] [PubMed] [Google Scholar]
- 1295. Thomas SP, et al. Comparison of epicardial and endocardial linear ablation using handheld probes. Ann Thorac Surg 2003;75(2):543–548. [DOI] [PubMed] [Google Scholar]
- 1296. van Brakel TJ, et al. Evaluation of epicardial microwave ablation lesions: histology versus electrophysiology. Ann Thorac Surg 2004;78(4):1397–1402. discussion 1397–1402. [DOI] [PubMed] [Google Scholar]
- 1297. Deneke T, et al. Histopathology of intraoperatively induced linear radiofrequency ablation lesions in patients with chronic atrial fibrillation. Eur Heart J 2005;26(17):1797–1803. [DOI] [PubMed] [Google Scholar]
- 1298. Schuessler RB, et al. Animal studies of epicardial atrial ablation. Heart Rhythm 2009;6(12 Suppl):S41–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1299. Melby SJ, et al. Epicardial microwave ablation on the beating heart for atrial fibrillation: the dependency of lesion depth on cardiac output. J Thorac Cardiovasc Surg 2006;132(2):355–360. [DOI] [PubMed] [Google Scholar]
- 1300. Gammie JS, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2008;85(3):909–914. [DOI] [PubMed] [Google Scholar]
- 1301. Damiano RJ, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141(1):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1302. Ad N, et al. Impact of follow-up on the success rate of the cryosurgical maze procedure in patients with rheumatic heart disease and enlarged atria. J Thorac Cardiovasc Surg 2006;131(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 1303. Gammie JS, et al. A multi-institutional experience with the CryoMaze procedure. Ann Thorac Surg 2005;80(3):876–880. discussion 880. [DOI] [PubMed] [Google Scholar]
- 1304. Gaynor SL, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 2004;128(4):535–542. [DOI] [PubMed] [Google Scholar]
- 1305. Weimar T, et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2011;31(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1306. Henn MC, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2015;150(5):1168–1176. 1178 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1307. Lall SC, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg 2007;133(2):389–396. [DOI] [PubMed] [Google Scholar]
- 1308. Voeller RK, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg 2008;135(4):870–877. [DOI] [PubMed] [Google Scholar]
- 1309. Blackshear JL, Odell JA.. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61(2):755–759. [DOI] [PubMed] [Google Scholar]
- 1310. McCarthy PM, et al. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg 2000;12(1):25–29. [DOI] [PubMed] [Google Scholar]
- 1311. Stulak JM, et al. Ten-year experience with the Cox-maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg 2007;83(4):1319–1324. [DOI] [PubMed] [Google Scholar]
- 1312. Buber J, et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol 2011;58(15):1614–1621. [DOI] [PubMed] [Google Scholar]
- 1313. Holmes DR, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374(9689):534–542. [DOI] [PubMed] [Google Scholar]
- 1314. Holmes DR, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64(1):1–12. [DOI] [PubMed] [Google Scholar]
- 1315. Gillinov AM, Pettersson G, Cosgrove DM.. Stapled excision of the left atrial appendage. J Thorac Cardiovasc Surg 2005;129(3):679–680. [DOI] [PubMed] [Google Scholar]
- 1316. Healey JS, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150(2):288–293. [DOI] [PubMed] [Google Scholar]
- 1317. Romanov A, et al. Effect of left atrial appendage excision on procedure outcome in patients with persistent atrial fibrillation undergoing surgical ablation. Heart Rhythm 2016;13(9):1803–1809. [DOI] [PubMed] [Google Scholar]
- 1318. Kanderian AS, et al. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52(11):924–929. [DOI] [PubMed] [Google Scholar]
- 1319. Lee R, et al. A randomized, prospective pilot comparison of 3 atrial appendage elimination techniques: internal ligation, stapled excision, and surgical excision. J Thorac Cardiovasc Surg 2016;152(4):1075–1080. [DOI] [PubMed] [Google Scholar]
- 1320. Bartus K, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62(2):108–118. [DOI] [PubMed] [Google Scholar]
- 1321. Price MJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol 2014;64(6):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1322. Pillarisetti J, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: Understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm 2015;12(7):1501–1507. [DOI] [PubMed] [Google Scholar]
- 1323. Ailawadi G, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011;142(5):1002–1009, 1009.e1. [DOI] [PubMed] [Google Scholar]
- 1324. Emmert MY, et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45(1):126–131. [DOI] [PubMed] [Google Scholar]
- 1325. Lee R, et al. Late neurologic events after surgery for atrial fibrillation: rare but relevant. Ann Thorac Surg 2013;95(1):126–131; discussion 131–132. [DOI] [PubMed] [Google Scholar]
- 1326. Badhwar V, et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: a report of the Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg 2016;101(6):2265–2271. [DOI] [PubMed] [Google Scholar]
- 1327. Abreu Filho CA, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation 2005;112(9 Suppl):I20–I25. [DOI] [PubMed] [Google Scholar]
- 1328. Doukas G, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA 2005;294(18):2323–2329. [DOI] [PubMed] [Google Scholar]
- 1329. Blomstrom-Lundqvist C, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J 2007;28(23):2902–2908. [DOI] [PubMed] [Google Scholar]
- 1330. Chevalier P, et al. Left atrial radiofrequency ablation during mitral valve surgery: a prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis 2009;102(11):769–775. [DOI] [PubMed] [Google Scholar]
- 1331. Cheng DC, et al. Surgical ablation for atrial fibrillation in cardiac surgery: a meta-analysis and systematic review. Innovations (Phila) 2010;5(2):84–96. [DOI] [PubMed] [Google Scholar]
- 1332. Budera P, et al. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicentre study. Eur Heart J 2012;33(21):2644–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333. Phan K, et al. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomised controlled trials. Heart 2014;100(9):722–730. [DOI] [PubMed] [Google Scholar]
- 1334. Gillinov AM, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372(15):1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335. Rankin JS, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg 2013;95(4):1484–1490. [DOI] [PubMed] [Google Scholar]
- 1336. Louagie Y, et al. Improved patient survival with concomitant Cox Maze III procedure compared with heart surgery alone. Ann Thorac Surg 2009;87(2):440–446. [DOI] [PubMed] [Google Scholar]
- 1337. Chiappini B, Di Bartolomeo R, Marinelli G.. Radiofrequency ablation for atrial fibrillation: different approaches. Asian Cardiovasc Thorac Ann 2004;12(3):272–277. [DOI] [PubMed] [Google Scholar]
- 1338. Barnett SD, Ad N.. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg 2006;131(5):1029–1035. [DOI] [PubMed] [Google Scholar]
- 1339. Edgerton JR, Jackman WM, Mack MJ.. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88(5):1655–1657. [DOI] [PubMed] [Google Scholar]
- 1340. Edgerton JR, et al. Totally thorascopic surgical ablation of persistent AF and long-standing persistent atrial fibrillation using the “Dallas” lesion set. Heart Rhythm 2009;6(12 Suppl):S64–S70. [DOI] [PubMed] [Google Scholar]
- 1341. Lockwood D, et al. Linear left atrial lesions in minimally invasive surgical ablation of persistent atrial fibrillation: techniques for assessing conduction block across surgical lesions. Heart Rhythm 2009;6(12 Suppl):S50–S63. [DOI] [PubMed] [Google Scholar]
- 1342. Malaisrie SC, et al. Atrial fibrillation ablation in patients undergoing aortic valve replacement. J Heart Valve Dis 2012;21(3):350–357. [PubMed] [Google Scholar]
- 1343. Cherniavsky A, et al. Assessment of results of surgical treatment for persistent atrial fibrillation during coronary artery bypass grafting using implantable loop recorders. Interact Cardiovasc Thorac Surg 2014;18(6):727–731. [DOI] [PubMed] [Google Scholar]
- 1344. Yoo JS, et al. Impact of concomitant surgical atrial fibrillation ablation in patients undergoing aortic valve replacement. Circ J 2014;78(6):1364–1371. [DOI] [PubMed] [Google Scholar]
- 1345. Damiano RJ, Jr, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg 2003;126(6):2016–2021. [DOI] [PubMed] [Google Scholar]
- 1346. Akpinar B, et al. Combined off-pump coronary artery bypass grafting surgery and ablative therapy for atrial fibrillation: early and mid-term results. Ann Thorac Surg 2006;81(4):1332–1337. [DOI] [PubMed] [Google Scholar]
- 1347. Geidel S, et al. Permanent atrial fibrillation ablation surgery in CABG and aortic valve patients is at least as effective as in mitral valve disease. Thorac Cardiovasc Surg 2006;54(2):91–95. [DOI] [PubMed] [Google Scholar]
- 1348. Geidel S, et al. Persistent atrial fibrillation ablation concomitant to coronary surgery. Thorac Cardiovasc Surg 2011;59(4):207–212. [DOI] [PubMed] [Google Scholar]
- 1349. Krul SP, et al. Navigating the mini-maze: systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int J Cardiol 2013;166(1):132–140. [DOI] [PubMed] [Google Scholar]
- 1350. Kainuma S, et al. Dilated left atrium as a predictor of late outcome after pulmonary vein isolation concomitant with aortic valve replacement and/or coronary artery bypass grafting. Eur J Cardiothorac Surg 2015;48(5):765–777; discussion 777. [DOI] [PubMed] [Google Scholar]
- 1351. Cox JL, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101(4):569–583. [PubMed] [Google Scholar]
- 1352. Rodriguez E, et al. Minimally invasive bi-atrial CryoMaze operation for atrial fibrillation. Oper Tech Thorac Cardiovasc Surg 2009;14(3):208–223. [Google Scholar]
- 1353. Wolf RK, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130(3):797–802. [DOI] [PubMed] [Google Scholar]
- 1354. Edgerton JR, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg 2008;86(1):35–38; discussion 39. [DOI] [PubMed] [Google Scholar]
- 1355. Edgerton JR, et al. Minimally invasive surgical ablation of atrial fibrillation: six-month results. J Thorac Cardiovasc Surg 2009;138(1):109–113; discussion 114. [DOI] [PubMed] [Google Scholar]
- 1356. Beyer E, Lee R, Lam BK.. Point: Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J Thorac Cardiovasc Surg 2009;137(3):521–526. [DOI] [PubMed] [Google Scholar]
- 1357. Kearney K, et al. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann Cardiothorac Surg 2014;3(1):15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1358. Ad N, et al. Surgical ablation of atrial fibrillation trends and outcomes in North America. J Thorac Cardiovasc Surg 2012;144(5):1051–1060. [DOI] [PubMed] [Google Scholar]
- 1359. Driessen AH, et al. Electrophysiologically guided thoracoscopic surgery for advanced atrial fibrillation: 5-year follow-up. J Am Coll Cardiol 2017;69(13):1753–1754. [DOI] [PubMed] [Google Scholar]
- 1360. Khargi K, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg 2005;27(2):258–265. [DOI] [PubMed] [Google Scholar]
- 1361. Wazni OM, et al. Atrial arrhythmias after surgical maze: findings during catheter ablation. J Am Coll Cardiol 2006;48(7):1405–1409. [DOI] [PubMed] [Google Scholar]
- 1362. Magnano AR, et al. Mechanisms of atrial tachyarrhythmias following surgical atrial fibrillation ablation. J Cardiovasc Electrophysiol 2006;17(4):366–373. [DOI] [PubMed] [Google Scholar]
- 1363. McElderry HT, et al. Proarrhythmic aspects of atrial fibrillation surgery: mechanisms of postoperative macroreentrant tachycardias. Circulation 2008;117(2):155–162. [DOI] [PubMed] [Google Scholar]
- 1364. McCarthy PM, et al. Where does atrial fibrillation surgery fail? Implications for increasing effectiveness of ablation. J Thorac Cardiovasc Surg 2010;139(4):860–867. [DOI] [PubMed] [Google Scholar]
- 1365. Zeng Y, et al. Recurrent atrial arrhythmia after minimally invasive pulmonary vein isolation for atrial fibrillation. Ann Thorac Surg 2010;90(2):510–515. [DOI] [PubMed] [Google Scholar]
- 1366. Lee R, et al. Surgical treatment for isolated atrial fibrillation: minimally invasive vs. classic cut and sew maze. Innovations (Phila) 2011;6(6):373–377. [DOI] [PubMed] [Google Scholar]
- 1367. Edgerton JR, et al. Totally thoracoscopic surgical ablation or catheter ablation of atrial fibrillation: a systematic review and preliminary meta-analysis. Card Electrophysiol Clin 2012;4(3):413–423. [DOI] [PubMed] [Google Scholar]
- 1368. Edgerton Z, et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of long-standing persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol 2016;27(5):524–530. [DOI] [PubMed] [Google Scholar]
- 1369. Wright M, et al. State of the art: catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19(6):583–592. [DOI] [PubMed] [Google Scholar]
- 1370. Sueda T, et al. Long-term results after the box pulmonary vein isolation procedure for chronic atrial fibrillation in mitral valve surgery. Ann Thorac Cardiovasc Surg 2012;18(2):101–108. [DOI] [PubMed] [Google Scholar]
- 1371. Maagh P, et al. Pulmonary vein isolation in 2012: is it necessary to perform a time consuming electrophysical mapping or should we focus on rapid and safe therapies? A retrospective analysis of different ablation tools. Int J Med Sci 2013;10(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1372. Bisleri G, et al. The need of a hybrid approach for the treatment of atrial fibrillation. Heart Surg Forum 2005;8(5):E326–E330. [DOI] [PubMed] [Google Scholar]
- 1373. Nitta T. Surgery for atrial fibrillation: a worldwide review. Semin Thorac Cardiovasc Surg 2007;19(1):3–8. [DOI] [PubMed] [Google Scholar]
- 1374. Byrne JG, et al. Hybrid cardiovascular procedures. JACC Cardiovasc Interv 2008;1(5):459–468. [DOI] [PubMed] [Google Scholar]
- 1375. La Meir M, et al. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg 2012;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1376. Prasad SM, et al. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg 2002;124(4):708–713. [DOI] [PubMed] [Google Scholar]
- 1377. Kiser AC, et al. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum 2010;13(5):E317–E321. [DOI] [PubMed] [Google Scholar]
- 1378. Kiser AC, et al. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations (Phila) 2011;6(4):243–247. [DOI] [PubMed] [Google Scholar]
- 1379. Natale A, et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of long-standing persistent atrial fibrillation: results from a single center [abstract]. Heart Rhythm 2011;8(Suppl 5):S79. [DOI] [PubMed] [Google Scholar]
- 1380. Belden W, Moraca R.. The convergent procedure for AF: experience at Allegheny General Hospital. EP Lab Digest 2013;13(5). [Google Scholar]
- 1381. Civello KC, Smith CA, Boedefeld W. . Combined endocardial and epicardial ablation for symptomatic atrial fibrillation: single center experience in 100+ consecutive patients. Journal of Innovations in Cardiac Rhythm Management 2013:1–7. [Google Scholar]
- 1382. Robinson M, et al. Maximizing ablation, limiting invasiveness, and being realistic about atrial fibrillation: the convergent hybrid ablation procedure for advanced AF. EP Lab Digest 2013;13(6). [Google Scholar]
- 1383. Thosani AJ, et al. Closed chest convergent epicardial-endocardial ablation of non-paroxysmal atrial fibrillation—a case series and literature review. Arrhythm Electrophysiol Rev 2013;2(1):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1384. Je HG, Shuman DJ, Ad N.. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating-heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg 2015;48(4):531–540; discussion 540–541. [DOI] [PubMed] [Google Scholar]
- 1385. Farina E, et al. Neuropsychological deficits in asymptomatic atrial fibrillation. Acta Neurol Scand 1997;96(5):310–316. [DOI] [PubMed] [Google Scholar]
- 1386. De Groot JC, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52(3):335–341. [DOI] [PubMed] [Google Scholar]
- 1387. Sacco RL, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1388. Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1389. Wang X, et al. Early recurrences after paroxysmal atrial fibrillation ablation: when is the proper timing for reablation? Pacing Clin Electrophysiol 2011;34(6):709–716. [DOI] [PubMed] [Google Scholar]
- 1390. Healey JS, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366(2):120–129. [DOI] [PubMed] [Google Scholar]
- 1391. Mantovan R, et al. Relationship of quality of life with procedural success of atrial fibrillation (AF) ablation and postablation AF burden: substudy of the STAR AF randomized trial. Can J Cardiol 2013;29(10):1211–1217. [DOI] [PubMed] [Google Scholar]
- 1392. Suman-Horduna I, et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive HF. J Am Coll Cardiol 2013;61(4):455–460. [DOI] [PubMed] [Google Scholar]
- 1393. Arya A, et al. Clinical implications of various follow up strategies after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2007;30(4):458–462. [DOI] [PubMed] [Google Scholar]
- 1394. Glotzer TV, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107(12):1614–1619. [DOI] [PubMed] [Google Scholar]
- 1395. Di Biase L, et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm 2015;12(6):1162–1168. [DOI] [PubMed] [Google Scholar]
- 1396. Wu RC, et al. Circular mapping catheter entrapment in the mitral valve apparatus: a previously unrecognized complication of focal atrial fibrillation ablation. J Cardiovasc Electrophysiol 2002;13(8):819–821. [DOI] [PubMed] [Google Scholar]
- 1397. Natale A, et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol 2000;35(7):1898–1904. [DOI] [PubMed] [Google Scholar]
- 1398. Black-Maier E, et al. Risk of atrioesophageal fistula formation with contact-force sensing catheters. Heart Rhythm 2017. pii: S1547-5271(17) 30452–30456. [DOI] [PubMed] [Google Scholar]
- 1399. Thygesen K, et al. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50(22):2173–2195. [DOI] [PubMed] [Google Scholar]