Abstract
Background
Localized early-stage extra-nodal marginal zone lymphoma (MZL) presents with heterogeneous organ involvement and is treated with various modalities, including resection, radiotherapy, and systemic therapy. We report the long-term outcome of a large cohort of extra-nodal MZL and assess the impact of patient and disease characteristics, organ site, and treatment strategy on disease control and survival.
Patients and methods
We identified 487 consecutive patients with stage IE or IIE MZL referred between 1992 and 2012 to Memorial Sloan Kettering Cancer Center. Pathology was reviewed by hematopathologists at our institution. Patient and disease factors as well as treatment types were analyzed for association with relapse-free survival, overall survival, and cumulative incidence of relapse.
Results
Median follow-up after treatment was 4.7 years. Five-year relapse-free survival and overall survival were 60% and 89%, respectively. Cumulative incidence of disease-specific death at 5 years was 1.3%. Radiotherapy alone was the initial treatment in 50% of patients, followed by surgical resection (30%), observation (8%), immunotherapy (4%), and chemotherapy (2%). Initial treatment type, primary disease site, and number of involved sites were significant factors in multivariable analysis of relapse (all P < 0.05). When compared with stomach, MZL originating in other disease sites (HR > 2.0, P ≤ 0.001), except for thyroid, had higher risk of relapse. Strategies such as antibiotics or topical therapies were associated with higher risk of relapse when compared with radiation therapy (P < 0.001). Crude rate of transformation to pathologically confirmed large-cell lymphoma was 2% (11 patients).
Conclusion
Overall and cause-specific survival are high in early-stage extra-nodal MZL. Curative-intent treatment led to fewer relapses and reduced the need for salvage. Stomach cases had lower risk of relapse than other anatomic primary sites. This study supports the use of local therapies to treat stage IE and IIE MZL.
Keywords: marginal zone lymphoma, outcomes, extranodal, early stage
Introduction
Extra-nodal mucosa-associated lymphoid tissue (MALT) lymphoma represents ∼70% of marginal zone B-cell lymphomas (MZL) while splenic and nodal sites make up the remaining 30% of cases [1]. Extra-nodal MZL presents in a variety of organs, such as the stomach, orbital adnexa, salivary glands, and lungs, among other sites. Extra-nodal MZL has been linked with two main bacterial infections: Helicobacter pylori infection in gastric MZL and Chlamydia psittaci in orbital tissues, with geographic variation in frequencies [1]. Treatment of H. pylori leads to regression of lymphoma in many gastric cases. In other sites, chronic autoimmune or inflammatory conditions are associated with development of MZL, such as Sjogren syndrome in salivary glands and Hashimoto thyroiditis in the thyroid.
Extra-nodal MZL is typically diagnosed at an early, localized stage in 60%–80% of cases [2, 3], making local treatments the preferred initial treatment approach. However, the treatment regimens used in practice are variable, with some patients receiving systemic therapy despite staging workup that confirms the localized nature of disease. Recent analysis of the National Cancer Database shows a survival detriment to omission of radiotherapy (RT) in early stage MZL [4]. A Surveillance, Epidemiology and End Results (SEER)-Medicare database analysis of stage IE gastric MALT lymphoma showed that RT was associated with lower risk of lymphoma-related death than chemotherapy [5]. The available studies on this disease have not consistently assessed the response to primary therapies, mostly due to small patient numbers, a lack of homogenous patient cohorts and variable follow-up strategy [3, 4, 6–12]. Furthermore, these studies find conflicting prognostic factors for disease relapse and survival.
In this study, we describe the response and long-term outcome of early-stage disease following various initial therapies. We also aim to find prognostic factors for relapse and survival.
Materials and methods
Patient selection
We carried out an IRB-approved retrospective analysis of the medical records of 487 consecutive patients treated at Memorial Sloan Kettering Cancer Center who had biopsy-proven stage I or II extra-nodal MZL diagnosed between January 1992 and September 2012. All patients had pathologic confirmation of MZL diagnosis by a MSKCC hematopathologist. We collected information on clinical features, stage, diagnostic studies, performance status, International Prognostic Index [13] score, treatments received, follow-up examinations, relapses or progression of disease (PD), and salvage therapies for recurrences.
Initial workup
Staging workup followed institutional standards for the disease site as well as NCCN guidelines. Work-up included an appropriate imaging study [positron emission tomography (PET) scan, computed tomography (CT), or magnetic resonance imaging (MRI)] as well as complete blood count and basic metabolic panel. All patients with gastric MZL were diagnosed by esophagogastroduodenoscopy (EGD) inspection and biopsy. Bone-marrow biopsy was carried out at physician discretion.
Treatment
Extra-nodal MZLs were treated with a relatively homogenous therapeutic strategy. Treatment of early-stage extra-nodal MZL at our institution constitutes primarily either radiotherapy or surgical resection. A cohort of patients treated only with RT has been previously published [15]. Some patients are treated at physician discretion with systemic therapies including cytotoxic chemotherapy or targeted immunotherapies, in particular rituximab. Gastric MZL patients are referred for RT if they have H. pylori-independent disease (biopsy does not identify H. pylori) or fail antibiotic therapy for H. pylori eradication based on repeat gastric biopsy. Based on the clinical setting, such as skin MZL or patients who wish to delay treatment, a minority of patients are initially managed with antibiotics, topical steroids, or observation (active surveillance approach).
Follow-up
Patients were typically seen 1-4 months after completing treatment of initial response and toxicity assessment. Imaging studies were used to assess response for most sites, with the exception of skin and orbit sites, where principally clinical examination was used. For gastric MZL, EGD with biopsy was customarily carried out every 4-6 months for the first 2-3 years, after which annual EGD was carried out.
Radiographic response was determined according to the International Working Group response criteria [14] at time of first follow-up imaging study; in some cases, we retrospectively ascertained clinical or radiographic response. Responses were categorized as one of the following: complete response (CR), complete response uncertain (CRu), partial response (PR), stable disease (SD), and PD.
For all patients receiving treatment, a progression or relapse event during follow-up was classified as any measurable, biopsy-proven, or visible increase in existing disease; relapse in initial site after initial CR; or the development of an entirely new site of MZL. For gastric MZL, biopsy-proven recurrence was required to determine relapse. Disease that transformed to large cell lymphoma was also considered a progression event.
Treatment technique
Patients who received RT were treated with either involved field RT (IFRT) or involved site RT (ISRT) without intentional prophylactic treatment of regional nodes unless the nodal drainage fell within the involved field, as previously reported [10]. Surgical resection involved removal of all visible disease on imaging or clinical examination.
Statistical analysis
Endpoints of our study were overall survival (OS), relapse-free survival (RFS), disease-specific death rate, relapse rate, and in-field failure rate after RT. Follow-up began from treatment start date for all endpoints except in-field failure. For OS, patients were followed until date of death or last contact, if still alive; for disease-specific death, death due to causes unrelated to MZL was considered a competing risk event. For RFS, patients were followed until the date of progression, relapse in previous or new site, death, or last follow-up; for the relapse endpoint, death without relapse was a competing risk event. For in-field failure after RT, which included patients that received RT any time after diagnosis, patients were followed from end of RT until progression or recurrence within the irradiated site and death without in-field failure was a competing risk event. Median OS and RFS were estimated by Kaplan–Meier methods. Cumulative incidence rates of all other endpoints were estimated by competing risk methods.
We further estimated the cumulative incidence of first relapse at specific sites (local, regional, distant) after CR or CRu to initial treatment. Patients were followed from the date of CR/CRu determination until first relapse. Death without relapse was considered a competing risk event.
To identify prognostic factors associated with OS and relapse, we carried out multivariable analysis (MVA) using Cox regression and competing risk regression. For each endpoint, factors with a P < 0.2 by univariate analysis (UVA) were assessed by MVA and backwards selection was used to reduce the model and retain only factors with P < 0.05. To evaluate the association of relapse with OS, relapse was analyzed as a time-dependent variable to account for biases related to different lengths of waiting time between diagnosis and relapse. Age differences by disease site were assessed with analysis of variance. Competing risks analysis was carried out using package cmprsk in R version 2.11.1 (The R Foundation for Statistical Computing). All other statistical analyses were carried out in SAS version 9.2 (SAS Institute, Cary, NC).
Results
Patient characteristics
Median follow-up was 4.7 years (range 0.01-21.3 years) overall and 4.6 years among 415 survivors (range 0.01-21 years). Median age at diagnosis was 60 years (range 9–92). A 57% of patients were female (Table 1). Most patients (89%) had Ann Arbor stage IE disease. Primary site of disease was the stomach in 32%, orbit in 14%, lung in 12%, skin in 13%, parotid in 5%, thyroid in 2%, and other in 22% (Table 1; supplementary Figure S1, available at Annals of Oncology online). IPI score was zero or one (low risk) in 95% and two (low-intermediate risk) in 5%; 4% of patients had B-symptoms at diagnosis. Age differed significantly between the major categories of primary disease site (supplementary Table S1, available at Annals of Oncology online), with parotid and skin MZL presenting at younger ages.
Table 1.
Characteristics of early stage patients
| Characteristic | N = 487 | |
|---|---|---|
| Age | Median (range) | 60 (9–92) |
| Gender | ||
| Female | 277 (57%) | |
| Male | 210 (43%) | |
| Stage | ||
| I | 434 (89%) | |
| II | 53 (11%) | |
| Primary site at diagnosis | ||
| Stomach | 155 (32%) | |
| Orbit | 68 (14%) | |
| Lung | 60 (12%) | |
| Skin | 61 (13%) | |
| Head and neck | 26 (5%) | |
| Thyroid | 9 (2%) | |
| Other | 108 (22%) | |
| IPI risk group | ||
| Unknown | 2 (0%) | |
| Low risk (0–1 points) | 461 (95%) | |
| Low-intermediate risk (2 points) | 24 (5%) | |
| B-symptoms at diagnosis | ||
| Not present | 463 (95%) | |
| Present | 18 (4%) | |
| N/A | 6 (1%) | |
| Initial treatment type | ||
| Surgery | 144 (30%) | |
| Chemotherapy | 9 (2%) | |
| Radiation therapy | 244 (50%) | |
| Chemotherapy and RTa | 2 (0%) | |
| Immunotherapy | 19 (4%) | |
| Observation | 39 (8%) | |
| Other | 30 (6%) | |
| Sites at diagnosis | ||
| >1 site | 37 (8%) | |
| 1 site | 450 (92%) | |
| Bone marrow biopsy | ||
| Not done or N/A | 222 (46%) | |
| Negative | 265 (54%) | |
| Among patients receiving RT as initial treatment (N = 246)b | ||
| RT dose | ||
| <3000 | 42 (17%) | |
| 3000 | 157 (64%) | |
| >3000 | 37 (15%) | |
| Unknown dose | 10 (4%) | |
Percentages may not add up to 100 due to rounding.
Grouped with RT for analysis.
Includes ChemoRT as initial treatment.
IPI, International Prognostic Index; RT, radiotherapy.
For staging workup, 58% of patients had PET scan, 87% had CT scan, and 49% had both PET and CT. EGD was carried out in 41% of patients and MRI was done in 18%. Bone-marrow biopsy was carried out and was negative in 54% of patients; 45% of patients did not undergo bone marrow sampling.
Forty-five patients (9%) had a prior autoimmune disorder diagnosis, including Sjogren’s disease in 20 patients, Hashimoto’s thyroiditis in nine, rheumatoid arthritis in 11, systemic lupus erythematosus in three, and inflammatory bowel disease in two patients. Helicobacter pylori infection was diagnosed in 44 patients with gastric MZL. The majority of H. pylori-positive gastric patients (84%) received antibiotic therapy. Non-gastric patients were not treated with antibiotics against H. pylori. Patients were referred for radiotherapy if found to have persistent gastric MZL after completion of antibiotics and repeat endoscopy with biopsy.
Initial treatments
Treatments started shortly after diagnosis with a median interval of 1.5 months. Radiotherapy was the most common initial treatment, given in 50% of patients (Tables 1 and 2). Surgical resection was the second most common therapy (30%), followed by observation (8%), other treatments (6%: includes oral antibiotics in 19 patients and topical corticosteroids in five), immunotherapy (4%), and systemic chemotherapy (2%). Observation was the initial strategy in 39 patients representing various disease sites, with the most common site being skin (44%) followed by orbit (18%). Immunotherapy consisted of rituximab in 17 of 19 patients. Patients who received non-local therapies were not more likely to have B-symptoms, nor did they have higher IPI score. See supplementary results, available at Annals of Oncology online for details about systemic therapy and RT, including toxicity.
Table 2.
Initial treatment types for each major MZL disease site
| Initial treatment | Stomach | Orbit | Lung | Skin | Parotid | Thyroid | Other |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Radiation therapy | 122 (79) | 44 (65) | 1 (2) | 19 (31) | 7 (27) | 5 (56) | 48 (44) |
| Surgery | 4 (3) | 10 (15) | 54 (90) | 18 (30) | 16 (62) | 4 (44) | 38 (35) |
| Chemotherapy | 3 (2) | 0 | 1 (2) | 1 (2) | 0 | 0 | 4 (4) |
| Immunotherapy | 6 (4) | 3 (4) | 2 (3) | 1 (2) | 1 (4) | 0 | 6 (6) |
| Observation | 2 (1) | 7 (10) | 2 (3) | 17 (28) | 1 (4) | 0 | 10 (9) |
| Other | 18 (12) | 4 (6) | 0 | 5 (8) | 1 (4) | 0 | 2 (2) |
Percentages may not add up to 100 due to rounding.
Response rates
All 144 patients treated with surgery had complete resections and therefore achieved CR. Among the 274 total patients receiving curative initial treatments (RT, chemotherapy, or immunotherapy), 82% achieved CR, 4% achieved CRu, 6% achieved PR, 4% had stable disease, and 1% experienced PD during treatment. Response was not recorded in 2% of patients. Among the RT-only group, 89% achieved CR, 5% had CRu, 3% had PR, 1% had SD, 1 patient had PD, and 2% had unknown response.
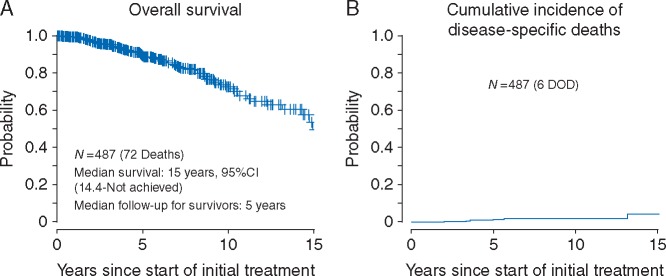
Overall survival
Seventy-two patients died during the follow-up period. Median OS was 15 years (95% CI 14.4–not reached). Five-year OS was 89% (95% CI 85%–92%), and 10-year OS was 73% (95% CI 65%–79%) (Figure 1A). Cause of death was MZL in six patients, other cause in 44 patients, and uncertain in 22 patients.
Figure 1.
(A) Overall survival. (B) Cumulative incidence of disease-specific deaths.
The 5- and 10-year cumulative incidences of disease-specific death are 1.3% (95% CI 0.02%––2.6%) and 1.8% (95% CI 0.2%––3.3%), respectively (Figure 1B). Non-lymphoma causes of death included second primary solid tumor malignancies (not associated with treatment) in 22 patients, second primary hematologic malignancies in 6 patients, medical causes including pneumonia in 6 patients, and car accident in one. Eight of the 22 patients with uncertain cause of death had experienced relapse at some time before death, including two with transformed disease; all eight were treated for their relapse. The median age at death for these patients was 73 years.
Relapses and progression
One-hundred ninety-five patients experienced relapse or death during the follow-up period, of which 151 patients relapsed (28 of whom died subsequently), and 44 patients died without a relapse. Median RFS was 8.1 years (95% CI 6.3–9.7 years). Five-year RFS was 60% (95% CI 55%––65%), and 10-year RFS was 42% (95% CI 35%––49%) (Figure 2A). For details of relapse locations and salvage therapies, refer to supplementary results and Tables S3–S5, and Figure S2, available at Annals of Oncology online.
Figure 2.
(A) Relapse-free survival. (B) Cumulative incidence of relapse for all patients. (C) Cumulative incidence of relapse by primary disease site.
The 5-year cumulative incidence of relapse for all patients was 33% (95% CI 28%–38%), with a 10-year incidence of 42% (95% CI 36%–48%) (Figure 2B). Patients who experienced progression or relapse during the follow-up period had higher risk of death that was borderline significant (HR 1.60, P = 0.06).
In both UVA and MVA for relapse, primary disease site, initial treatment type, and number of sites at diagnosis were significantly associated with relapse (Table 3; Figure 2C). Among treatment types, only “other” treatments, such as topical therapies or antibiotics, were significantly associated with a greater risk of relapse than RT (HR 8.37, P < 0.001).
Table 3.
Competing risk regression analysis for association of factors with relapse/progression (N = 483)a
| Characteristic | Strata | Univariate | Multivariable | ||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Age (per 10 years) | 1.01 (0 .89, 1.15) | 0 .87 | |||
| Gender | Female | 1.0 | 0 .76 | ||
| Male | 1.05 (0 .76, 1.45) | ||||
| Stage | I | 1.0 | 0 .06 | ||
| II | 1.5 (0 .99, 2.29) | ||||
| Primary site at diagnosis | Stomach | 1.0 | <0 .001 | 1.0 | < .001 |
| Orbit | 1.9 (1.13, 3.2) | 2.01 (1.07, 3.78) | |||
| Lung | 1.81 (1.03, 3.17) | 3.16 (1.5, 6.66) | |||
| Skin | 2.73 (1.6, 4.65) | 2.99 (1.67, 5.35) | |||
| H&N | 2.6 (1.34, 5.05) | 3.8 (1.92, 7.54) | |||
| Thyroid | 0.8 (0 .22, 2.93) | 1.23 (0 .34, 4.41) | |||
| Other | 1.91 (1.2, 3.06) | 2.5 (1.51, 4.14) | |||
| IPI risk group | Low (0–1 points) | 1.0 | 0 .26 | ||
| Low-intermediate risk (2 points) | 1.53 (0 .73, 3.18) | ||||
| B-symptomsa | Not present at diagnosis | 1.0 | 0 .88 | ||
| Present | 1.08 (0 .4, 2.91) | ||||
| Initial treatment | RT or ChemoRT | 1.0 | <0 .001 | 1.0 | < .001 |
| Surgery | 1.22 (0 .83, 1.80) | 0 .69 (0 .42, 1.14) | |||
| Chemo or immunotherapy | 2.27 (1.19, 4.31) | 1.5 (0 .73, 3.09) | |||
| Observation | 2.03 (1.19, 3.49) | 1.24 (0 .69, 2.24) | |||
| Other | 8.27 (4.07, 17) | 8.37 (4.39, 15.94) | |||
| No. sites at diagnosis | >1 site | 1.0 | 0 .007 | 1.0 | 0 .04 |
| 1 site | 0.54 (0 .34, 0 .84) | 0.58 (0 .35, 0 .97) | |||
| Bone marrow biopsy carried out | No | 1.0 | 0 .09 | ||
| Yes | 0.76 (0 .55, 1.04) | ||||
Variables with P-value <0 .2 were selected for multivariable analysis and only variables with P-value < 0 .05 were retained in the final model.
Unknown relapse status not included.
HR, hazard ratio; IPI, International Prognostic Index, RT, radiotherapy.
Among the 294 patients treated with RT either upfront or for a relapse, 11 experienced failures within the radiation field. The 5- and 10-year cumulative incidences of in-field failure after RT were 2.4% (95% CI 0.5%–4%) and 4.7% (95% CI 0.8%–8.7%), respectively.
Eleven patients (2%) experienced transformation of MZL to pathologically confirmed large-cell lymphoma. Initial sites of disease were stomach (n = 5), orbit (3), parotid (2), and breast (1). Of these, two died of disease. Transformation occurred at the primary disease site in three patients, and in a regional or distant site in eight patients.
Discussion
We present, to our knowledge, the largest report of early-stage extra-nodal marginal zone lymphoma treatment and outcomes in the literature. With a median follow-up of almost 5 years, our study presents a comprehensive picture of the disease course of extra-nodal MZL. Our study confirms that extra-nodal MZL disease control is excellent following local or systemic therapies and disease-specific death is rare. For patients who achieve CR to treatment, local relapse is uncommon; when relapse does occur, as is the case in one-third of patients, the relapsed disease is almost always a non-transformed MZL emerging in an extra-nodal site and can be treated successfully in the relapsed setting.
An important finding of our data is that, despite the ability of systemic chemotherapy or rituximab to treat disseminated microscopic disease, patients treated with these modalities were not less likely to relapse. Given these results, we prefer to avoid the toxicities of systemic therapy, and instead offer all patients local therapy for this disease. We reserve systemic therapy for the few patients who relapse and require a systemic approach. The low radiation doses required for CR are associated with few serious complications [15].
We identify several factors associated with risk of relapse in early-stage extra-nodal MZL, including primary disease site and initial treatment type. Compared with gastric primaries, patients with orbit, lung, skin, parotid, and other disease sites are significantly more likely to relapse or progress. These sites tend to represent (i) paired organs such as orbit or parotid, and (ii) organs where only the involved site of disease, and not the whole organ, is locally treated—such as the lung and skin. In contrast, RT for stomach MZL involves treatment of the entire organ. This variation in treatment extent for different disease sites may explain higher relapse rates between stomach and other sites. Our finding confirms similar results from previous smaller studies [4, 12, 16–18]. The association of certain organ sites with higher risk of relapse may support closer follow-up for those patients, with special attention to the remainder of the involved organ or to its contralateral, paired structure.
We find that, compared with RT, patients receiving “other” treatments, including antibiotics and corticosteroids, were significantly more likely to experience relapse while patients undergoing surgery were not. Gastric, skin, or orbit sites are more likely to receive “other” therapies. Because these treatments do not address the entire organ, patients will more likely have partial response and eventual relapse. Patients who were observed were highly selected, and likely had minimal residual disease burden after biopsy before embarking on “observation only” approach.
Recent analyses of population databases have identified improved outcomes in patients receiving RT as initial treatment of an early stage extra-nodal MZL. Ling et al. identified over 22 000 patients in the NCDB with stage I-II extra-nodal and nodal MZL from 1998 to 2012 [4]. They found that RT utilization had decreased over time with a corresponding increase in systemic therapy. Propensity-score adjusted survival analysis found that RT was independently associated with improved OS (Hazard ratio 0.75). An analysis of 1134 cases of stage IE gastric MZL from the SEER-Medicare database found that RT was associated with a better cause-specific survival compared with those treated with chemotherapy [5].
Other groups have reported excellent outcomes of early-stage extra-nodal MZL that are further supported by our study. A multicenter report [19] from the International Extra-nodal Lymphoma Study Group (IELSG) reported on 102 patients receiving RT for gastric MZL. Ten-year freedom from treatment failure and overall survival were 88% and 70%, respectively. Princess Margaret Hospital investigators identified 192 patients with stage IE-IIE MZL treated with RT, 23 of whom also received chemotherapy [6, 11]. Ten-year RFS was 68% and 10-year cause-specific survival was 98%. Thyroid and stomach sites had >90% 10-year recurrence-free rates. A prospective phase II trial of RT in stage IEA MZL showed 3-year OS of 100% and local control of 97% [7]. Lastly, a study of stage IE orbital MZL show >95% local control following RT [20]; 6 patients had a contralateral orbital relapse and were salvaged with RT.
The limitation of our study is its retrospective nature. The nature of treatments at a tertiary referral center such as ours does raise the possibility of referral biases, which may explain the high number of patients who received radiotherapy as primary treatment of gastric MZL. Similarly, very few patients with gastric disease were treated with surgery. Given the lack of a prospective study, our retrospective study presents a clear picture of the natural history of various treatment strategies for early-stage extra-nodal MZL, one that can inform treatment paradigms for future patients.
Supplementary Material
Acknowledgement
Dr Sewit Teckie was a Dr Mortimer J. Lacher, MD, Lymphoma Fellow at Memorial Sloan Kettering Cancer Center. The authors thank Karen Chau, BA for editorial assistance in preparing the manuscript.
Key Message
Early-stage, extra-nodal marginal zone lymphoma can present in various anatomic sites. Curative-intent local therapies, such as low-dose radiotherapy and surgical excision, are effective and safe. While relapse is common, most occur in distant sites and are salvaged with additional therapy. Overall and cause-specific survival are excellent.
Funding
This work was supported in part by donations from the Lymphoma Foundation; the Connecticut Sports Foundation; and the National Cancer Institute at the National Institutes of Health Cancer Center Support Grant [P 30 CA008748]. MSKCC was supported by the NIH/NCI Cancer Center Support Grant [P 30 CA008748].
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Swerdlow S, Campo E, Harris N. et al. WHO classification of tumours of haematopoietic and lymphoid tissues In Bosman F, Jaffe E, Lakhani S, Ohgaki H (eds): World Health Organization classification of tumours 4th Edition Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2. Armitage JO, Weisenburger DD.. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998; 16: 2780–2795. [DOI] [PubMed] [Google Scholar]
- 3. Thieblemont C, Berger F, Dumontet C. et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000; 95: 802–806. [PubMed] [Google Scholar]
- 4. Ling DC, Vargo JA, Balasubramani GK. et al. Underutilization of radiation therapy in early-stage marginal zone lymphoma negatively impacts overall survival. Pract Radiat Oncol 2015; 6: 97–105. [DOI] [PubMed] [Google Scholar]
- 5. Olszewski AJ, Castillo JJ.. Comparative outcomes of oncologic therapy in gastric extranodal marginal zone (MALT) lymphoma: analysis of the SEER-Medicare database. Ann Oncol 2013; 24: 1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goda JS, Gospodarowicz M, Pintilie M. et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010; 116: 3815–3824. [DOI] [PubMed] [Google Scholar]
- 7. Isobe K, Kagami Y, Higuchi K. et al. A multicenter phase II study of local radiation therapy for stage IEA mucosa-associated lymphoid tissue lymphomas: a preliminary report from the Japan Radiation Oncology Group (JAROG). Int J Radiat Oncol Biol Phys 2007; 69: 1181–1186. [DOI] [PubMed] [Google Scholar]
- 8. Kalpadakis C, Pangalis GA, Vassilakopoulos TP. et al. Non-gastric extra-nodal marginal zone lymphomas–a single centre experience on 76 patients. Leuk Lymphoma 2008; 49: 2308–2315. [DOI] [PubMed] [Google Scholar]
- 9. Mazloom A, Medeiros LJ, McLaughlin PW. et al. Marginal zone lymphomas: factors that affect the final outcome. Cancer 2010; 116: 4291–4298. [DOI] [PubMed] [Google Scholar]
- 10. Schechter NR, Portlock CS, Yahalom J.. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16: 1916–1921. [DOI] [PubMed] [Google Scholar]
- 11. Tsang RW, Gospodarowicz MK, Pintilie M. et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21: 4157–4164. [DOI] [PubMed] [Google Scholar]
- 12. Zucca E, Conconi A, Pedrinis E. et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003; 101: 2489–2495. [DOI] [PubMed] [Google Scholar]
- 13. Project TIN-HsLPF. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993; 329: 987–994. [DOI] [PubMed] [Google Scholar]
- 14. Cheson BD, Pfistner B, Juweid ME. et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 15. Teckie S, Qi S, Lovie S. et al. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys 2015; 92: 130–137. [DOI] [PubMed] [Google Scholar]
- 16. Raderer M, Streubel B, Woehrer S. et al. High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res 2005; 11: 3349–3352. [DOI] [PubMed] [Google Scholar]
- 17. Zinzani PL, Magagnoli M, Galieni P. et al. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol 1999; 17: 1254.. [DOI] [PubMed] [Google Scholar]
- 18. Ueda K, Terui Y, Yokoyama M. et al. Non-gastric advanced mucosa-associated lymphoid tissue (MALT) lymphoma has worse prognosis than gastric MALT lymphoma even when treated with rituximab-containing chemotherapy. Leuk Lymphoma 2013; 54: 1928–1933. [DOI] [PubMed] [Google Scholar]
- 19. Wirth A, Gospodarowicz M, Aleman BM. et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol 2013; 24: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 20. Harada K, Murakami N, Kitaguchi M. et al. Localized ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiation therapy: a long-term outcome in 86 patients with 104 treated eyes. Int J Radiat Oncol Biol Phys 2014; 88: 650–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.