Abstract
Background
Combined cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) blockade induces high rates of immune-related adverse events (irAEs). The safety of resuming anti-PD-1 in patients who discontinue combination therapy due to irAEs is not known.
Patients and methods
We assessed patients who experienced clinically significant irAEs from combined CTLA-4 and PD-1 blockade leading to treatment discontinuation at four academic centers. We assessed the safety of resuming anti-PD-1 in terms of recurrent and distinct irAEs.
Results
Eighty patients discontinued combination therapy due to irAEs, including colitis (41%), hepatitis (36%), and pneumonitis (4%). Of these, 96% received corticosteroids and 21% received additional immunosuppression (e.g. infliximab). All were rechallenged with anti-PD-1, and 14 (18%) had recurrent irAEs at a median of 14 days after therapy resumption (six grade 1–2, seven grade 3–4, and one grade 5 Steven–Johnson Syndrome). Colitis was less likely to recur than other irAEs (6% versus 28%, P = 0.01). Clinically significant but distinct toxicities occurred in an additional 17 (21%) patients (11 grade 1–2 and 6 grade 3–4). Duration of steroid taper, severity of initial irAEs and use of additional immunosuppressants did not predict for toxicity on rechallenge, although patients remaining on steroid therapy at anti-PD-1 resumption had higher rates of toxicities (55% versus 31%, P = 0.03).
Conclusions
Patients who discontinued CTLA-4/PD-1 blockade for severe irAEs had relatively high rates of recurrent or distinct toxicities with anti-PD-1 resumption. However, many patients, particularly with combination-induced colitis, tolerated anti-PD-1 rechallenge well, and this approach can be considered in selected patients.
Keywords: nivolumab, ipilimumab, pembrolizumab, melanoma, colitis, immune-related adverse events
Introduction
Historically, the median survival of patients with metastatic melanoma was <1 year and long-term survivorship was rare [1]. However, with the advent of immune checkpoint inhibitors and effective targeted therapies, survival rates have dramatically increased and durable disease control is a real possibility [2, 3]. Immune checkpoint inhibitors targeting anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and anti-programmed death 1 (PD-1) block key immune suppressive molecules and thereby release cytotoxic T cells to target cancer cells. Along with their potent antitumor response, activated T-cells can recognize off-target epitopes in normal tissue and cause toxicity. The most common immune-related adverse events (irAEs) include colitis, hepatitis, pneumonitis, nephritis, and endocrinopathies [4, 5]. Although usually managed with corticosteroids, these irAEs can cause significant morbidity and, rarely, mortality [6, 7].
Combination immunotherapy with the PD-1 blocking antibody, nivolumab, and anti-CTLA-4, ipilimumab, has demonstrated higher response rates (RRs) than either therapy alone in metastatic melanoma. However, dual checkpoint blockade causes more frequent and severe irAEs, as 53% of patients experience grade 3–4 irAEs [8, 9]. During clinical trials, patients with high-grade irAEs requiring systemic corticosteroids were required to permanently discontinue both ipilimumab and nivolumab. Outside trials, given that most high-grade toxicities occur during the induction phase, clinicians frequently reinitiate nivolumab or pembrolizumab following the resolution of toxicities in the belief that further anti-PD-1 treatment is required to best achieve durable disease control. In part, this approach is based on the safety of using anti-PD-1 therapy in patients with major toxicities from ipilimumab [10]. Furthermore, given the distinct side-effect profiles of each agent, one could speculate that many combination-induced toxicities might not recur with anti-PD-1. The safety and efficacy of resuming anti-PD-1 monotherapy in patients with severe toxicity from combination therapy, however, is not known.
Given the benefits of anti-PD-1 therapy in patients with metastatic melanoma, counterbalanced by the potential risks of reactivating severe toxicities, characterizing the clinical utility of this approach is critical. Herein, we evaluate whether patients who experienced severe irAEs on combination PD-1 and CTLA-4 blockade benefit from resumption of anti-PD-1 monotherapy based on incidence of recurrent and distinct irAEs and clinical activity.
Patients and methods
Patients
Eligible patients were 18 years of age or older, had a confirmed diagnosis of advanced melanoma, and had received at least one cycle of combination anti-PD-1 + ipilimumab followed by at least one dose of anti-PD-1 (nivolumab or pembrolizumab) between 8 January 2013 and 11 January 2016. Patients were treated at four academic centers (Melanoma Institute Australia, Memorial Sloan Kettering Cancer Center, Moffitt Cancer Center, and Vanderbilt Ingram Cancer Center). In addition, patients must have experienced at least one clinically significant irAE resulting in treatment discontinuation of combination anti-PD-1 and anti-CTLA-4 treatment, with later resumption of anti-PD-1 monotherapy. Patients who experienced grade 3–4 irAEs following the fourth dose of combination therapy (but before start of anti-PD-1 monotherapy) were included. The decision to discontinue combination therapy due to an irAE was determined by the treating oncologist. All irAE adverse event grades were recorded retrospectively. Duration of corticosteroids was collected and patients were considered “off steroids” when on hormone replacement doses for hypopituitarism.
Study design
This was a multicenter, retrospective analysis of patients treated with anti-PD-1 + anti-CTLA-4 who experienced toxicities leading to treatment discontinuation and resumed single-agent anti-PD-1 thereafter. We collected patient demographics, frequency, timing, and spectrum of irAEs as well as management of these events. We collected efficacy data consisting of RR, progression-free survival (PFS), and overall survival (OS).
Outcomes
Safety end points were irAEs as defined in the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.03). We specifically evaluated recurrent or new irAEs with anti-PD-1 therapy after treatment discontinuation of anti-PD-1 + anti-CTLA-4. Efficacy outcomes were secondary and included RR per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [11], PFS, and OS.
Statistical analysis
Baseline demographics and treatment characteristics were analyzed using descriptive statistics, listed with frequencies and percentages for categorical variables and medians and ranges for continuous variables. PFS was defined as time from first dose of anti-PD-1 + anti-CTLA-4 therapy to disease progression as documented by serial cross-sectional imaging or initiation of a new local or systemic therapy. OS was defined as the time from first dose of therapy to death from any cause. Characteristics of patients who had toxicities were compared with those who did not using chi-square (nominal variables), Mann–Whitney U (continuous variables), or logrank test (time-dependent variables). P < 0.05 was considered statistically significant. All surviving patients were censored at the time of last follow-up. Survival distributions were estimated using the Kaplan–Meier method.
Results
Patients
A total of 80 patients were identified who experienced severe irAEs while being treated with anti-CTLA-4 + anti-PD-1 (hereafter referred to as ‘combination therapy’). The median age was 56 years (range 25–89 years); 55% were male and 68% had stage IV M1c disease (Table 1). Of these, 13 (11%) patients had received prior therapies for metastatic disease (largely BRAF ± MEK inhibitors). The median number of cycles of combination therapy received was 2; 26% received 1 dose, 33% received 2 doses, and 41% received 3–4 doses before treatment discontinuation. Median follow-up time was 14.3 months.
Table 1.
Demographics
| Characteristic | N (%) |
|---|---|
| Median age, years (range) | 56 (25–89) |
| Male | 44 (55) |
| Stage | |
| IIIc | 9 (11) |
| IV M1a/b | 17 (21) |
| IV M1c | 54 (68) |
| ≥1 prior therapy | 9 (11) |
| Anti-CTLA-4/PD-1 regimen | |
| Ipilimumab 3 mg/kg + Nivolumab 1 mg/kg | 76 (95) |
| Pembrolizumab 2 mg/kg + Ipilimumab 1 mg/kg | 4 (5) |
| Number of aCTLA4 + aPD1 doses | |
| 1 | 21 (26) |
| 2 | 26 (33) |
| 3 | 21 (26) |
| 4 | 12 (15) |
| Length of steroid taper, days (median, range) | 35 (5–240) |
| Duration between last aCTLA4 + aPD1 dose and aPD1 resumption, days (median, range) | 58 (14–395) |
CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death 1.
Adverse events
All patients discontinued combination therapy for irAEs (Table 2); the worst grade of irAE experienced with combination therapy was grade 2 in 25 (31%) patients, grade 3 in 49 (61%), and grade 4 in 6 (8%) patients. Events leading to treatment discontinuation included colitis/diarrhea in 33 (41%) patients, hepatitis in 29 (36%), symptomatic hypophysitis in 5 (6%), rash in 5 (6%), and pneumonitis in 3 (4%). Neurologic complications (acute inflammatory demyelinating polyneuropathy (AIDP) and myasthenia gravis), pancreatitis, nephritis, uveitis, mucositis, and idiopathic thrombocytopenic purpura (ITP) occurred in 1–2 cases each. Other AEs leading to treatment discontinuation included wheezing, arthralgias, severe fatigue, and hyperkalemia/hyponatremia. Ten (13%) patients had >1 concurrent toxicity that led to treatment discontinuation.
Table 2.
Clinically significant toxicities with combination PD-1 and CTLA-4 blockade and anti-PD-1 resumption
| irAEs with CTLA-4 + PD-1 blockade |
irAEs with anti-PD-1 resumption (recurrent or de novo) |
|||
|---|---|---|---|---|
| irAE | All grade irAE, n (%) | Grade 3/4 irAE, n (%) | All grade irAE, n (%) | Grade 3/4 irAE, n (%) |
| Colitis | 33 (41) | 20 (25) | 6 (8) | 2 (3) |
| Hepatitis | 29 (36) | 19 (24) | 8 (10) | 5 (7) |
| Hypophysitis | 5 (6) | 2 (3) | 2 (3) | 1 (1) |
| Dermatitis/rash | 5 (6) | 3 (4) | 6 (8) | 2 (3)a |
| Pneumonitis | 3 (4) | 1 (1) | 4 (5) | – |
| Elevated lipase | 4 (5)b | 4 (5) | 3 (5) | 2 (3)b |
| Nephritis | 2 (3) | 1 (1) | 1 (1) | – |
| Neurologic | 2 (3) | 1 (1) | – | – |
| ITP | 1 (1) | 1 (1) | – | – |
| Other | 7 (9) | 2 (3) | 8 (10) | 2 (3)c |
Includes one patient with grade 5 Stevens–Johnson Syndrome.
Two patients with clinical pancreatitis.
Grade 3 type 1 diabetes and grade 3 arthralgias.
CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death 1; irAE, immune-related adverse event.
Among all patients, 77 (96%) received corticosteroid treatment. The remaining 3 (4%) patients who did not receive steroids had grade 3 lipase elevation, grade 2 hepatitis, and hyperkalemia with hyponatremia, and were managed by holding therapy. Of the 77 patients who received steroids, 63 (82%) received a dose approximately equivalent to prednisone 1 mg/kg or greater, whereas 14 (18%) received prednisone 0.5 mg/kg or less. The median duration of corticosteroid administration was 35 days (range 5–240 days). Other immunosuppressants were also administered in a subset of patients (n = 17), including infliximab in 12 (15%: all colitis), mycophenolate mofetil in 4 (5%: 3 with hepatitis and 1 with infliximab-refractory colitis), and intravenous immunoglobulin in 2 (3%: ITP and AIDP).
Outcomes with anti-PD-1
All patients resumed single-agent anti-PD-1 therapy. The median duration between the last dose of combination therapy to the first dose of anti-PD-1 was 58 days (range 14–395 days). Thirty-one (39%) patients were still on immunosuppression when they resumed anti-PD-1; all but 6 patients were receiving less than or equal to prednisone 10 mg daily dose or equivalent. Seventeen (21%) patients had persistent irAEs (symptoms or laboratory value abnormalities) at anti-PD-1 resumption, but all were improved and grade 1–2. Thirteen (16%) patients resumed anti-PD-1 for disease progression, and 65 (81%) resumed as continued/maintenance therapy for incomplete responses (2 unknown).
Among all patients, 40 (50%) experienced any grade of irAEs with anti-PD-1 resumption (Table 2). Of these, 26 (33%) had grade 1–2 events and 14 (18%) had grade 3–5 toxicities; 24 (30%) patients discontinued anti-PD-1 due to these events. To provide further insights, we divided these events into whether the same irAE that led to combination therapy discontinuation recurred (recurrent) or whether new irAEs occurred (distinct).
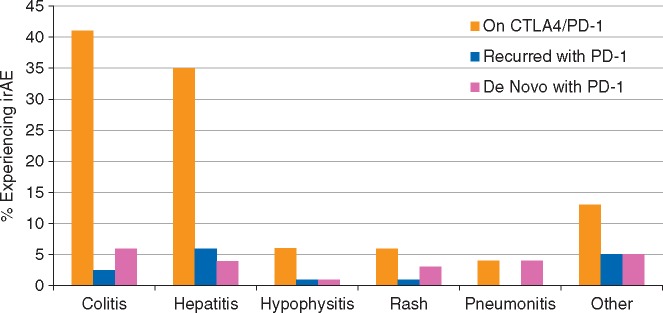
We first assessed whether particular toxicities had a tendency to recur with anti-PD-1 resumption. Colitis seemed especially unlikely to recur, with only 2 of 33 (6%) patients experiencing recurrent colitis or diarrhea with anti-PD-1 resumption (Figure 1). Patients with neurologic toxicity (n = 2), uveitis (n = 2), and ITP (n = 1) did not experience recurrences. By contrast, hepatitis (5 recurrences of 29 patients, 17%), pancreatitis (2 of 2, 100%), pneumonitis (1 of 3, 33%), and nephritis (1 of 2, 50%) appeared to recur more often. Symptomatic hypophysitis (e.g. severe headache in 1 of 8; 13%) and rash (1 of 4; 25%) also appeared to have an intermediate likelihood of recurrence. Overall, patients with colitis were less likely to have recurrent toxicity compared with other patients (6% versus 28%, P = 0.01).
Figure 1.
Percentage of patients with clinically significant toxicities with cytotoxic T-lymphocyte antigen 4 (CTLA-4)/programmed death 1 (PD-1) blockade (gold), recurrent toxicities with PD-1 blockade (blue), and distinct de novo toxicities with PD-1 blockade (red).
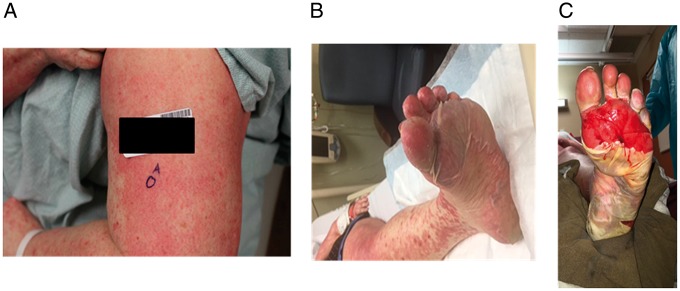
In total, the same irAE that caused combination therapy discontinuation recurred in 14 (18%) patients at a median of 14 days following therapy resumption (range 7–167 days). Of these, 6 were grade 1–2 irAEs and 7 were grade 3–4 events. There was one grade 5 event: a 50-year-old woman initially had a grade 2 rash with ipilimumab and nivolumab which improved to grade 1 with low-dose corticosteroids (methylprednisolone dose pack). After a single dose of anti-PD-1 therapy, she developed grade 3 rash which initially improved with prednisone 1 mg/kg. However, while still on steroids, she developed worsening rash and blistering; biopsy showed Steven–Johnson Syndrome/toxic epidermal necrolysis that ultimately involved 90% body surface areas including oral and genital surfaces (Figure 2). Despite high-dose steroid administration, IVIG, infliximab, and admission to a burn unit, the patient died approximately 50 days after restarting anti-PD-1. Ten of the 14 patients (71%) discontinued anti-PD-1 therapy due to these recurrent irAEs, but no other fatal events occurred.
Figure 2.
Initial grade 2 rash (not shown) with combination therapy progressing to grade 3 rash with anti-programmed death 1 (PD-1) rechallenge (A) followed by desquamation and fatal Stevens–Johnson Syndrome (B and C).
To further characterize safety, we then assessed whether patients who discontinued combination therapy for toxicity experienced ‘distinct’ irAEs upon anti-PD-1 resumption. Nine (11%) patients experienced distinct low-grade events not requiring therapy interruption or systemic steroids, specifically low-grade hypothyroidism (n = 2), rash (n = 5), asymptomatic lipase elevation (n = 1), and myalgias (n = 1). Clinically significant irAEs requiring therapy discontinuation or systemic steroids occurred in 17 (21%) patients (Figure 1). Of these, the worst grade was 1–2 in 11 patients, grade 3 in 5, and grade 4 in 1 patient (hepatitis); 5 patients had more than one irAE during anti-PD-1 treatment. These clinically significant irAEs included colitis/diarrhea (n = 5), hepatitis (n = 3), pneumonitis (n = 3), rash (n = 1), hypophysitis (n = 1), type I diabetes (n = 1), severe myalgias (n = 1), and arthralgias (n = 2). Seven patients discontinued therapy due to these distinct irAEs, 16 received systemic steroids (9 received prednisone 0.5 mg/kg or less and 7 received approximately 1 mg/kg or more), and 1 patient received infliximab for colitis. No patients died from these distinct anti-PD-1–induced irAEs. Ultimately, in total, 39% (n = 31) of patients experienced clinically significant recurrent or distinct toxicities.
Predisposing factors for recurrent or distinct irAEs
We then assessed for whether the length and type of immunosuppressive treatment, severity of toxicity, presence of ongoing steroids or symptoms at anti-PD-1 resumption, or delay in resuming anti-PD-1 influenced whether patients experienced clinically significant irAEs on anti-PD-1. For this analysis, we included both patients who had recurrence of the same irAE (n = 14) or clinically significant, distinct irAEs (n = 17) as described above. The duration of steroid taper was not significantly different in patients who had toxicities on anti-PD-1 compared with those not experiencing toxicities (median 43 versus 32 days, P = 0.5). Similarly, patients who required additional immunosuppression (e.g. infliximab, mycophenolate mofetil) with combination therapy were no more likely to experience severe toxicities with anti-PD-1 than those who received only corticosteroids (16% versus 22%; P = 0.5). Furthermore, the presence of grade 3–4 toxicities versus grade 1–2 toxicities did not predict recurrent/distinct toxicities (P = 0.9). The duration between final dose of CTLA-4 and PD-1 blockade to resumption of anti-PD-1 was slightly higher in patients without toxicities versus those who experienced toxicities (median 62 versus 56 days, P = 0.03). Patients who remained on steroids at anti-PD-1 resumption appeared to have higher rates of toxicity than patients who had discontinued steroids (55% versus 31%, P = 0.03), as did those whose symptoms had not resolved at resumption (30% versus 17%, P = 0.2) although not statistically significant.
Efficacy
Among these 80 patients, 56 (70%) experienced partial or complete response. An additional 15 patients (19%) had stable disease, while the remaining 9 (11%) patients had progressive disease as best response. Neither median PFS nor OS were reached (supplementary Figure S1, available at Annals of Oncology online). Only 4 (5%) patients experienced initial response followed by progression during study follow-up. Thirteen (16%) patients received anti-PD-1 therapy for disease progression after stopping combination therapy. Of these, 4 (31%) had partial responses, 3 (23%) had stable disease, and 6 (46%) had progressive disease.
Discussion
Combination immune checkpoint blockade with ipilimumab and nivolumab induces high RRs but frequent irAEs [8, 9]. In this study, we specifically focused on patients who experienced clinically significant irAEs while on combination therapy, and were rechallenged with anti-PD-1 monotherapy. This study is the first to evaluate the safety and efficacy of this increasingly common practice.
Herein, we found that almost 40% of patients who discontinued combination therapy for toxicities experienced recurrent or clinically significant distinct toxicities with anti-PD-1 monotherapy resumption. Importantly, one patient who had a grade 2 rash with combination therapy subsequently experienced fulminant and fatal Steven–Johnson Syndrome upon anti-PD-1 rechallenge. Thus, severe toxicities can occur with anti-PD-1 resumption, and clinical vigilance is required.
We sought to determine clinical features that would predict recurrent or novel severe toxicities. Although the severity of initial toxicity or duration/type of immunosuppression was not associated with subsequent irAEs, the absence of steroids at rechallenge and the interval before rechallenge appeared to have a weak correlation. By contrast, the type of toxicity appeared to be more informative. Very few patients with ‘ipilimumab-like’ toxicities, including colitis and hypophysitis, experienced recurrences with anti-PD-1. This is consistent with prior studies that have shown that ipilimumab-induced irAEs rarely recur with anti-PD-1 [10, 12]. By contrast, ‘anti-PD-1-like’ toxicities such as hepatitis, nephritis, pancreatitis, and pneumonitis appeared to have some risk of recurrence; although the small number of patients with individual toxicities limits definitive conclusions. Together, these data suggest that even with dual immune therapies, either ipilimumab or nivolumab may be the primary ‘culprit’ in driving specific toxicities. We suggest that patients with colitis or hypophysitis can safely resume anti-PD-1, but caution should be maintained with most other toxicities.
We also noted a relatively high rate (21%) of clinically significant but distinct irAEs upon anti-PD-1 rechallenge (e.g. patients with colitis that later experienced hepatitis). This incidence appears somewhat higher than the rate of severe irAEs with single-agent anti-PD-1 [9, 13], suggesting that immune priming by combination therapy may predispose to other subsequent toxicities or that combination toxicities may present in a delayed fashion. One could also postulate that patients who experienced irAEs with combination therapy have an intrinsic genetic tendency for toxicities with other immune therapies.
This and other recent studies question the risk–benefit ratio of resuming anti-PD-1 following severe combination toxicities. Randomized studies have recently shown that patients with these irAEs have high RRs and excellent clinical outcomes with observation alone [8, 9]. Thus, many patients with ongoing stable or responding disease may not need to resume anti-PD-1 as maintenance therapy (as most patients in our study did), although long-term data are needed to truly examine this issue. Patients who progress after combination therapy may benefit from anti-PD-1 resumption: 4 of 13 (31%) patients responded to anti-PD-1 in this clinical setting.
This study has several limitations. First, it only evaluates patients who did reinitiate anti-PD-1 following combination therapy cessation. This eliminates patients without clinical indications to resume therapy (e.g. dramatic progressive disease or rapid clinical response) and may have influenced the high RR observed. One study suggests that a similar proportion of patients never reinitiated monotherapy [14]. Second, this study was unable to evaluate the safety of resuming anti-PD-1 in patients where toxicities were deemed too serious to resume any immune therapy (e.g. severe pneumonitis, neurologic toxicities). Thus, clinicians should use critical judgment and extreme caution in resuming anti-PD-1 in patients with life-threatening irAEs (particularly non-colitis events). Third, metrics for discontinuing either combination therapy were physician-specific, and the decision to recommence anti-PD-1 similarly so. While there are some clear indications to permanently discontinue anti-PD-1 (e.g. grade 3–4 pneumonitis), many more subjective areas exist, such as bothersome and persistent grade II events. Fourth, this study primarily included patients who received ipilimumab 3 mg/kg and nivolumab 1 mg/kg. Studies evaluating different doses could demonstrate distinct toxicity rates upon rechallenge. Anti-PD-1 resumption was permitted following combination therapy discontinuation in the Keynote-029 study (testing pembrolizumab 2 mg/kg + ipilimumab 1 mg/kg), although safety in these patients was not reported [15].
In conclusion, this report provides the first assessment of safety and efficacy of resuming anti-PD-1 agents following toxicity with dual PD-1 and CTLA-4 blockade. Approximately 40% of patients who resumed anti-PD-1 developed clinically significant recurrent or distinct irAEs. These toxicities often occur early, and certain toxicities are more likely to recur. While these events are generally low-grade and manageable with standard treatment algorithms, they can occasionally be life-threatening. Thus, anti-PD-1 resumption may be considered for selected patients with appropriate monitoring and standard treatment algorithms for toxicities.
Funding
National Cancer Institute (K23 CA204726) and the James C. Bradford Jr. Melanoma Fund to DBJ (no grant number applies); Cancer Institute New South Wales Fellowship to AMM (no grant number applies); National Health and Medical Research Council Practitioner Fellowship to GVL (no grant number applies).
Disclosure
DBJ has served as an advisory board member for Bristol-Myers Squibb, Genoptix, Incyte, and Merck. ANS has served as an advisory board member for Vaccinex, Castle Biosciences, and has received travel support from Bristol-Myers Squibb. GVL has served as an advisory board member for Amgen, Array, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, and Roche, and has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche. AMM has served as an advisory board member for Chugai, Merck Sharp & Dohme, Novartis, and Pierre Fabre, and has received honoraria from Bristol-Myers Squibb and Roche. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Korn EL, Liu PY, Lee SJ. et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008; 26: 527–534. [DOI] [PubMed] [Google Scholar]
- 2. Long GV, Weber JS, Infante JR. et al. Overall survival and durable responses in patients with braf v600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 2016; 34: 871–878. [DOI] [PubMed] [Google Scholar]
- 3. Topalian SL, Sznol M, McDermott DF. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32(10): 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber JS, Hodi FS, Wolchok JD. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017; 35(7): 785–792. [DOI] [PubMed] [Google Scholar]
- 5. Weber JS, Yang JC, Atkins MB, Disis ML.. Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015; 33(18): 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson DB, Balko JM, Compton ML. et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375(18): 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eggermont AM, Chiarion-Sileni V, Grob JJ. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16(5): 522–530. [DOI] [PubMed] [Google Scholar]
- 8. Postow MA, Chesney J, Pavlick AC. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372(21): 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menzies AM, Johnson DB, Ramanujam S. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017; 28(2): 368–376. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 12. Weber JS, Kudchadkar RR, Gibney GT. et al. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. J Clin Oncol 2013; 31: 9011. [Google Scholar]
- 13. Robert C, Schachter J, Long GV. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372(26): 2521–2532. [DOI] [PubMed] [Google Scholar]
- 14. Shoushtari AN, Friedman CF, Navid-Azarbaijani P. et al. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long GV, Atkinson V, Cebon JS. et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 2017; 18(9): 1202–1210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.