Abstract
Background
Although 1% has been used as cut-off for estrogen receptor (ER) positivity, several studies have reported that tumors with ER < 1% have characteristics similar to those with 1% ≤ ER < 10%. We hypothesized that in patients with human epidermal growth factor 2 (HER2)-negative breast cancer, a cut-off of 10% is more useful than one of 1% in discriminating for both a better pathological complete response (pCR) rate to neoadjuvant chemotherapy and a better long-term outcome with adjuvant hormonal therapy. Our objectives were to identify a percentage of ER expression below which pCR was likely and to determine whether this cut-off value can identify patients who would benefit from adjuvant hormonal therapy.
Patients and methods
Patients with stage II or III HER2-negative primary breast cancer who received neoadjuvant chemotherapy followed by definitive surgery between June 1982 and June 2013 were included. Logistic regression models were used to assess the association between each variable and pCR. Cox models were used to analyze time to recurrence and overall survival. The recursive partitioning and regression trees method was used to calculate the cut-off value of ER expression.
Results
A total of 3055 patients were analyzed. Low percentage of ER was significantly associated with high pCR rate (OR = 0.99, 95% CI = 0.986–0.994, P < 0.001). The recommended cut-off of ER expression below which pCR was likely was 9.5%. Among patients with ER ≥ 10% tumors, but not those with 1%≤ER < 10% tumors, adjuvant hormonal therapy was significantly associated with long time to recurrence (HR = 0.24, 95% CI = 0.16–0.36, P < 0.001) and overall survival (HR = 0.32, 95% CI = 0.2–0.5, P < 0.001).
Conclusion
Stage II or III HER2-negative primary breast cancer with ER < 10% behaves clinically like triple-negative breast cancer in terms of pCR and survival outcomes and patients with such tumors may have a limited benefit from adjuvant hormonal therapy. It may be more clinically relevant to define triple-negative breast cancer as HER2-negative breast cancer with <10%, rather than <1%, of ER and/or progesterone receptor expression.
Keywords: breast cancer, ER positivity, triple-negative breast cancer, adjuvant hormonal therapy
Introduction
Approximately 12%–17% of breast cancers are triple-negative breast cancer (TNBC), which is defined as estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor 2 (HER2)-negative disease [1]. With contemporary treatments, patients with TNBC generally have a better overall response to chemotherapy but—particularly among those with chemotherapy-insensitive disease—a poorer prognosis than do patients with other breast cancer subtypes, such as breast cancers that are ER- and/or HER2-positive [1–6]. This suggests that TNBC is a heterogeneous disease and that patients with TNBC have chemotherapy responses and survival outcomes that differ from those of patients with other breast cancer subtypes. Compared with TNBC patients who do not achieve pathological complete response (pCR) to neoadjuvant chemotherapy (NACT), TNBC patients who do achieve pCR have a better prognosis; thus, pCR is a surrogate survival marker in this population [2, 6–10].
According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP), breast cancers with <1% of ER or PR expression should be considered hormone receptor–negative tumors [11]. However, we found in a previous retrospective study that breast cancers with 1%–9% of ER expression had gene expression profiles similar to those of breast cancers with <1% of ER expression [12]. Another retrospective study including both HER2-negative and HER2-positive cases showed that the recurrence-free survival (RFS) and overall survival (OS) rates of patients with 1%–9% of ER expression and those of patients with <1% of ER expression did not differ significantly (P = 0.96 and 0.10, respectively) and that both groups’ RFS and OS rates were significantly worse than those of patients with ≥10% of ER expression (P < 0.0001 for both) [13]. These results suggest that breast cancers with ≥1% but <10% of ER expression and those with ≤1% of ER expression have similar molecular features and clinical prognoses. Another retrospective study suggested that low percentage of ER expression as a continuous variable is associated with a high pCR rate [14]. However, those studies included both HER2-negative and HER2-positive diseases. In addition, no previous study has determined the optimal cut-off value of the ER expression level as a continuous variable in terms of the association with pCR rate or the difference in the survival benefit from adjuvant hormonal therapy based on the calculated cut-off value of ER. Some studies have reported that hormone receptor–positive tumors are less sensitive to systemic chemotherapies than hormone receptor–negative tumors are, which suggests that the ER expression level is associated with sensitivity to NACT [15–17]. More recently, the St. Gallen International Expert Consensus 2015 reported that ER expression values between 1% and 9% were considered equivocal and that endocrine therapy alone cannot be relied upon for patients with these values [18]. Thus, whether the percentage of ER expression as a continuous variable affects the pCR rate after NACT in patients with HER2-negative primary breast cancer is unknown and the exact clinical definition of TNBC with consideration of the survival benefit from adjuvant hormonal therapy has not been fully investigated.
We hypothesized that in patients with HER2-negative primary breast cancer, a cut-off ER expression level of 10% is more useful than one of 1% for discriminating patients who are likely to have a better pCR rate to NACT and those who are likely to have a better long-term outcome with adjuvant hormonal therapy. In this retrospective chart review study, our primary objective was to identify the percentage of ER expression below which pCR was likely in patients with newly diagnosed stage II or III HER2-negative primary invasive breast cancer treated with NACT. Our secondary objective was to determine whether this cut-off value can identify patients who would benefit from adjuvant hormonal therapy.
Methods
Study population
This retrospective chart review study was approved by The University of Texas MD Anderson Cancer Center’s Institutional Review Board (protocol number: PA14-0046), and a waiver of informed consent was granted based on the study’s retrospective nature. We reviewed the Breast Medical Oncology management system database (protocol number: 2004-0541) to identify patients with newly diagnosed stage II or III HER2-negative primary invasive breast cancer who received NACT followed by definitive surgery between June 1982 and June 2013 at MD Anderson Cancer Center. We included only patients for whom the percentages of ER and PR expression levels were available.
Data collection
From the database, we extracted age, race, menopausal status, body mass index, clinical stage, percentage of ER, percentage of PR, histology, nuclear grade, NACT regimens [anthracycline alone (A), taxane alone (T), or anthracycline plus taxane (A + T)], treatment response (pCR or non-pCR), lymphovascular invasion (positive or negative), adjuvant hormonal therapy (yes or no), adjuvant chemotherapy (yes or no), and adjuvant radiation therapy (yes or no). Stage was assessed using American Joint Committee on Cancer (AJCC) classification. HER2 positivity was defined as a HER2/CEP17 fluorescence in situ hybridization (FISH) ratio of ≥2.0 and/or an immunohistochemical (IHC) staining score of 3+ [19]. pCR was defined as no invasive carcinoma in the breast or no tumor in the axilla at the time of surgery.
Data were initially extracted from electronic medical records and entered into a prospectively maintained database. The dataset was assessed and cleaned by TF and TK independently. Follow-up information for patients in the Breast Medical Oncology management system database is obtained every 2 years by direct review of the medical records and linkage to the MD Anderson Tumor Registry, which mails annual follow-up letters to each patient registered at MD Anderson to confirm that the patient is alive and free of cancer. The MD Anderson Tumor Registry also checks the Social Security Death Index and the Texas Bureau of Vital Statistics for the status of patients who do not respond to the letters.
Pathological evaluation
For IHC staining, the 1D5 antibody was used until 2000. Since then, the 6F11 antibody has been used for IHC staining of formalin-fixed paraffin-embedded (FFPE) tissue sections in our clinical IHC laboratory, which is certified under the provisions of the United States Clinical Laboratory Improvement Act (CLIA) and accredited by the CAP. The IHC protocol, whose use with 4-μm FFPE tissue sections, has been validated and includes a de-paraffinization step for 30 min at 72 °C and a rehydration with antigen retrieval carried out at 100 °C for 20 min with Citrate buffer. In the protocol, endogenous peroxidase is blocked with 3% peroxide for 5 min. The primary antibody is applied at a 1 : 35 dilution for 15 min. Post-primary antibody detection is carried out using a commercial polymer system (Bond Polymer Refine Detection, Leica), and stain development is achieved by incubation with DAB and DAB Enhancer (Leica). Hematoxylin is applied as a counterstain. A positive control (cervical tissue) is added to every slide. At MD Anderson, a multidisciplinary approach to grossing breast specimens, used for more than two decades, involves the use of whole and sectioned specimen radiography, the correlation of gross findings with imaging findings, and discussion among the pathologist, radiologist, and surgeon. For specimens obtained after neoadjuvant therapy, we follow protocol for the examination of specimens from patients with invasive carcinoma of the breast from College of American Pathologists (CAP) [20] and an international working group for standardization of specimen handing and reporting for breast specimens after neoadjuvant therapy [21].
Data were initially extracted from electronic medical records and entered into a prospectively maintained database. The dataset was assessed and cleaned by TF and TK independently. Follow-up information for patients in the Breast Medical Oncology management system database is obtained every 2 years by direct review of the medical records and linkage to the MD Anderson Tumor Registry, which mails annual follow-up letters to each patient registered at MD Anderson to confirm that the patient is alive and free of cancer. The MD Anderson Tumor Registry also checks the Social Security Death Index and the Texas Bureau of Vital Statistics for the status of patients who do not respond to the letters.
Statistical analysis
Standard descriptive statistics and frequency tabulation were used to summarize data. The chi-square test and Fisher’s exact test were used to evaluate the association between two categorical variables. The Kruskal–Wallis test was used to compare the distributions of continuous variables among different groups. Univariate and multivariate logistic regression models were used to investigate the association between each variable and pCR. The variables with P values of ≤0.2 in the univariate analysis were included in the selection of the full multivariate model. We obtained the reduced multivariate model using a backward selection approach, removing the least significant covariates from the full model one at a time, and P values of <0.05 were used as the limit for inclusion in this analysis. ER and PR were treated as continuous and categorical variables separately in the multivariate analyses.
Kaplan–Meier curves for patients sorted by prognostic factors of interest were produced, and the log-rank test was used to assess the difference between the prognostic factor groups. OS was defined as the time from surgery to death or last follow-up; patients who were alive at the end of the study period were censored at the date of last follow-up. Time to recurrence (TTR) was defined as the time from surgery to recurrence or breast cancer–specific death; patients whose disease had not recurred or who had not died from breast cancer were censored at the date of last follow-up. Univariate and multicovariate Cox proportional hazard models were used to determine the effects of prognostic factors on survival distributions. The recursive partitioning and regression trees method [22] was used to select a percentage of ER expression below which pCR was likely. The package was downloaded via the Comprehensive R ArchiveNetwork (CRAN, https://cran.r-project.org/web/packages/rpart/). All tests were two-sided. P values <0.05 were considered statistically significant. All analyses were conducted using SAS 9.3 (SAS, Cary, NC), S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA), and R 2.14.2.
Results
Patients
A total of 3055 patients were included in the analysis (supplementary Figure S1, available at Annals of Oncology online). Among these 3055 patients, 1726 (56.5%) had stage II disease and 1329 (43.5%) had stage III disease; 932 (30.5%) had tumors with <1% of ER expression (ER < 1% tumors), 171 (5.6%) had tumors with ≥1% but <10% of ER expression (1%≤ER < 10% tumors), and 1952 (63.9%) had tumors with ≥10% of ER expression (ER ≥ 10% tumors). Most patients (2577; 84.4%) had ductal carcinoma, and most patients (2651; 86.8%) received A + T as NACT. Of the 171 patients with 1%≤ER < 10% tumors, 43 (25.1%) had adjuvant hormonal therapy, and of the 1952 patients with ER ≥ 10% tumors, 1906 (97.6%) had adjuvant hormonal therapy.
The patients’ demographic and clinicopathological characteristics by ER expression level are given in Table 1. Compared with patients with ER < 1% or 1 ≤ ER < 10% tumors, those with ER ≥ 10% tumors were more likely to be white (P < 0.001), have nuclear grade I or II (P < 0.001), have non-lobular disease (P < 0.001), have lymphovascular invasion (P = 0.005), and have received adjuvant radiation (P < 0.001).
Table 1.
Patient characteristics at diagnosis
| Variable | TOTAL (n = 3055) | ER < 1% (n = 932) | 1% ≤ ER < 10% (n = 171) | ER ≥ 10% (n = 1952) | P value |
|---|---|---|---|---|---|
| Age, median (range), years | 49 (19–83) | 49 (22–83) | 49 (19–77) | 49 (22–83) | 0.49 |
| BMI, median (range), kg/m2 | 27.7 (14.5–65.9) | 27.8 (14.5–56.4) | 29 (17.7–31.3) | 27.5 (15.5–66) | 0.03 |
| Race/ethnicity | |||||
| White | 1915 (62.7) | 565 (60.6) | 97 (56.7) | 1253 (64.2) | <0.001 |
| Black | 463 (15.2) | 197 (21.1) | 35 (20.5) | 231 (11.8) | |
| Hispanic | 483 (15.8) | 128 (13.7) | 25 (14.6) | 330 (16.9) | |
| Asian/others | 194 (6.4) | 42 (4.5) | 14 (8.2) | 138 (7.1) | |
| Menopausal status | |||||
| Premenopausal | 1461 (47.8) | 438 (47.2) | 84 (49.1) | 939 (48.4) | 0.82 |
| Postmenopausal | 1579 (51.7) | 489 (52.8) | 87 (50.9) | 1003 (51.6) | |
| Unknown | 15 (0.5) | 0 (0) | 0 (0) | 0 (0) | |
| Histology | |||||
| Ductal | 2577 (84.4) | 851 (91.3) | 156 (91.2) | 1570 (80.4) | <0.001 |
| Lobular | 231 (7.6) | 15 (1.6) | 2 (1.2) | 214 (11) | |
| Others | 215 (7.0) | 40 (4.3) | 7 (4.1) | 168 (8.6) | |
| Unknown | 32 (1.0) | 26 (2.8) | 6 (3.5) | 0 (0) | |
| Nuclear grade | |||||
| I/II | 1163 (38.1) | 91 (9.8) | 19 (11.1) | 1053 (53.9) | <0.001 |
| III | 1803 (59.0) | 813 (87.2) | 148 (86.5) | 842 (43.1) | |
| Unknown | 89 (2.9) | 28 (3) | 4 (2.3) | 57 (2.9) | |
| Clinical stage | |||||
| Stage II | 1726 (56.5) | 511 (54.8) | 99 (57.9) | 1116 (57.2) | 0.46 |
| Stage III | 1329 (43.5) | 421 (45.2) | 72 (42.1) | 836 (42.8) | |
| Neoadjuvant regimen | |||||
| A | 292 (95.6) | 70 (7.5) | 9 (5.3) | 213 (10.9) | <0.001 |
| T | 112 (36.7) | 36 (3.9) | 3 (1.8) | 73 (3.7) | |
| A+T | 2651 (86.8) | 826 (88.6) | 159 (93) | 1666 (85.3) | |
| ER, continuous, mean ± SD | 51.88 ± 43.17 | – | – | – | |
| PR, categorical | |||||
| PR < 1% | 1245 (40.8) | 818 (87.8) | 110 (64.3) | 317 (16.2) | <0.001 |
| 1% ≤ PR < 10% | 326 (10.7) | 61 (6.5) | 41 (24) | 224 (11.5) | |
| PR ≥ 10% | 1484 (48.6) | 53 (5.7) | 20 (11.7) | 1411 (72.3) | |
| PR, continuous, mean ± SD | 31.6 ± 38.24 | 2.4 ± 11.3 | 4.6 ± 12.5 | 47.9 ± 38.5 | <0.001 |
| LVI | |||||
| Negative | 2030 (66.4) | 631 (67.7) | 124 (72.5) | 1275 (65.3) | 0.005 |
| Positive | 915 (30) | 254 (27.3) | 36 (21.1) | 625 (32) | |
| Unknown | 110 (3.6) | 47 (5) | 11 (6.4) | 52 (2.7) | |
| Adjuvant chemotherapy | |||||
| No | 2594 (84.9) | 770 (82.6) | 155 (90.6) | 1669 (85.5) | 0.013 |
| Yes | 461 (15.1) | 162 (17.4) | 16 (9.4) | 283 (14.5) | |
| Adjuvant hormonal therapy | |||||
| No | 1020 (33.4) | 846 (90.8) | 128 (74.9) | 46 (2.4) | <0.001 |
| Yes | 2035 (66.6) | 86 (9.2) | 43 (25.1) | 1906 (97.6) | |
| Adjuvant radiation | |||||
| No | 636 (20.8) | 237 (25.4) | 50 (29.2) | 349 (17.9) | <0.001 |
| Yes | 2419 (79.2) | 659 (74.6) | 121 (70.8) | 1603 (82.1) | |
| pCR | |||||
| No | 2626 (86) | 687 (73.7) | 123 (71.9) | 1816 (93) | <0.001 |
| Yes | 429 (14) | 245 (26.3) | 48 (28.1) | 136 (7) |
All data are no. of patients (%) unless noted otherwise.
ER, estrogen receptor; BMI, body mass index; A, anthracycline; T, taxane; SD, standard deviation; PR, progesterone receptor; LVI, lymphovascular invasion; pCR, pathological complete response.
Univariate logistic regression analysis of pCR
In the univariate logistic regression analysis, high percentages of ER and PR expression as continuous variables were significantly associated with low pCR rates [ER: odds ratio (OR), 0.98; 95% confidence interval (CI), 0.978–0.983; P < 0.001; PR: OR, 0.976; 95% CI, 0.971–0.98; P < 0.001]. Consistent with this result, low ER and PR expression levels as categorical variables were significantly associated with high pCR rates. Ductal carcinoma, clinical stage II, high nuclear grade, and the A + T regimen were significantly associated with high pCR rates (supplementary Table S1, available at Annals of Oncology online).
Multivariate logistic regression analysis of pCR
The reduced multivariate logistic regression model using percentages of ER and PR expression as continuous variables is shown in Table 2. After adjustment for other covariates, low ER and PR expression levels remained significantly associated with high pCR rates (ER: OR, 0.99; 95% CI, 0.986–0.994; P < 0.001; PR: OR, 0.989; 95% CI, 0.984–0.995; P < 0.001). Ductal carcinoma, clinical stage II, high nuclear grade, and the A + T regimen also remained significantly associated with high pCR rates.
Table 2.
Multivariate logistic regression analysis by pathological complete response (pCR) status
| Variable | OR (95% CI) | P value |
|---|---|---|
| Clinical stage | ||
| Stage III | (reference) | |
| Stage II | 1.94 (1.53–2.45) | <0.001 |
| ER, continuous | 0.990 (0.986–0.994) | <0.001 |
| PR, continuous | 0.989 (0.984–0.995) | <0.001 |
| Race/ethnicity | ||
| White | (reference) | |
| Black | 1.18 (0.88–1.58) | 0.28 |
| Hispanic | 1.37 (1.00–1.85) | 0.04 |
| Asian/Others | 0.87 (0.52–1.45) | 0.58 |
| Histology | ||
| Ductal | (reference) | |
| Lobular | 0.36 (0.14–0.90) | 0.03 |
| Others | 0.510 (0.226–1.000) | 0.05 |
| Nuclear grade | ||
| III | (reference) | |
| I/II | 0.45 (0.32–0.63) | <0.001 |
| Neoadjuvant regimen | ||
| A+T | (reference) | |
| A | 0.30 (0.17–0.52) | <0.001 |
| T | 0.26 (0.10–0.66) | 0.004 |
OR, odds ratio; CI, confidential interval; ER, estrogen receptor; PR, progesterone receptor; A + T, anthracycline and taxane; A, anthracycline; T, taxane.
We also carried out multivariate logistic regression using ER expression levels as categorical variables. Compared with patients with ER ≥ 10% tumors, patients with ER < 1% or 1%≤ER < 10% tumors had a significantly higher probability of pCR (ER < 1%: OR, 2.15; 95% CI, 1.62–2.87; P < 0.001; 1%≤ER < 10%: OR, 2.27; 95% CI, 1.48–3.47; P < 0.001). There was no significant difference of pCR rates between ER < 1% and 1%≤ER < 10% groups in multivariate logistic regression (OR, 0.95; 95% CI, 0.64–1.40; P = 0.792).
Recommended cut-off value for ER positivity
Recursive partitioning and regression trees method without boundary values including all 3055 patients was used to find an optimal cut-off point for the continuous ER with respect to pCR. In this method, the best threshold to split the observations into two separated subgroups was investigated. The recursive partitioning and regression trees method revealed that the recommended cut-off of ER expression below which pCR was likely was 9.5%. Multivariate analysis revealed that the pCR probability of patients with ER < 10% tumors (26.6%) was significantly higher than that of patients with ER ≥ 10% tumors (7.0%; OR, 2.17; 95% CI, 1.64–2.87; P < 0.0001). Using the same methods, we found that the recommended cut-off of ER expression with respect to TTR was also 9.5%.
Survival analysis
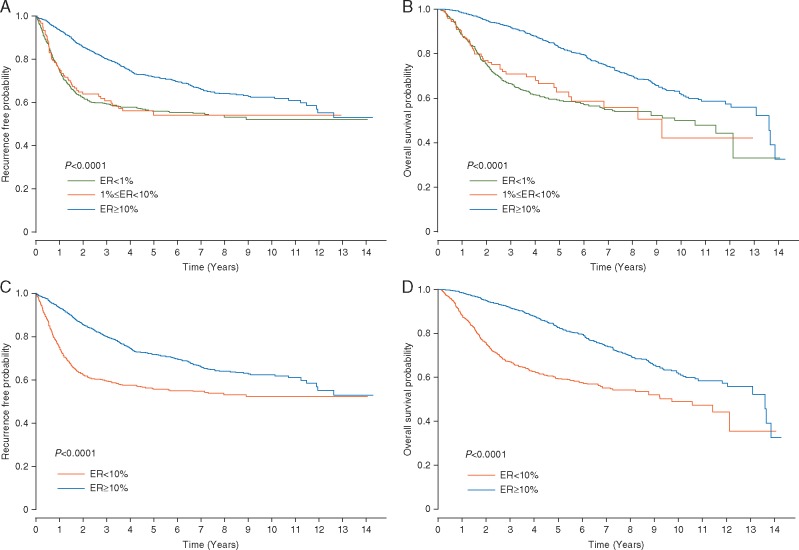
The median follow-up time was 3.9 years for OS. The TTR and OS curves of the ER < 1% and 1%≤ER < 10% groups were overlapping. The TTR and OS rates of the patients with ER ≥ 10% tumors were significantly higher than those of the patients with ER < 1% or 1%≤ER < 10% tumors (log-rank P < 0.0001 for both TTR and OS) (Figure 1A and B). When 10% was used as the cut-off to categorize ER expression level, the TTR and OS of the patients with ER < 10% tumors were significantly lower than those of the patients with ER ≥ 10% tumors (log-rank P < 0.0001 for both TTR and OS) (Figure 1C and D).
Figure 1.
Time to recurrence (TTR) and overall survival (OS) analysis. (A) TTR and (B) OS curves for patients with tumors with <1% of ER expression (ER < 1%), patients with tumors with ≥1% but <10% of ER expression (1% ≤ ER < 10%), and patients with tumors with ≥10% of ER expression (ER ≥ 10%). (C) TTR and (D) OS curves for patients with tumors with <10% of ER expression (ER < 10%) and patients with tumors with ≥10% of ER expression (ER ≥ 10%).
The univariate Cox proportional hazard models revealed that compared with ER < 1% or 1%≤ER < 10% tumors, ER ≥ 10% tumors were significantly associated with better TTR and OS (supplementary Table S2, available at Annals of Oncology online). Patients with ER < 1% or 1%≤ER < 10% tumors did not have significantly different TTR (HR, 1.033; 95% CI, 0.78–1.37: P < 0.82) or OS (HR, 1.086; 95% CI, 0.8–1.48: P < 0.6). After adjustment for covariates including pCR status and adjuvant hormonal therapy, the percentage of ER expression was not significantly associated with TTR or OS (supplementary Table S3, available at Annals of Oncology online). Compared with PR ≥ 10% tumors, PR < 1% tumors were significantly associated with worse TTP and OS, and 1%≤PR < 10% tumors were not a significant factor for TTP or OS (supplementary Table S3, available at Annals of Oncology online).
Next, to explore the effect of the percentage of ER expression on survival outcomes, we removed adjuvant hormonal therapy from the multivariate Cox regression model. After the removal of adjuvant hormonal therapy and adjustment for other covariates, the multivariate Cox regression model revealed that compared with patients with ER ≥ 10% tumors, those with ER < 1% or 1%≤ER < 10% tumors had worse TTR (ER < 1%: HR, 1.64; 95% CI, 1.34–1.99; P < 0.001; 1%≤ER < 10%: HR, 1.92; 95% CI, 1.4–2.62; P < 0.001) and OS (ER < 1%: HR, 2.07; 95% CI, 1.67–2.58; P < 0.001; 1%≤ER < 10%: HR, 2.35; 95% CI, 1.66–3.32; P < 0.001).
Difference of survival benefit from adjuvant hormonal therapy
To explore the difference of survival benefit of adjuvant hormonal therapy between patients with 1%≤ER < 10% tumors and those with ER ≥ 10% tumors, we included an interaction term between adjuvant hormonal therapy and ER category (1% ≤ ER < 10% versus ER ≥ 10%), which was statistically significant in the multivariate models (P < 0.001 for TTR and P = 0.048 for OS). We carried out additional subgroup analyses by these two ER categories. Multivariate analysis revealed that among the 171 patients with 1% ≤ ER < 10% tumors, adjuvant hormonal therapy was not significantly associated with either TTR (HR, 0.88; 95% CI, 0.48–1.6; P = 0.67) or OS (HR, 0.65; 95% CI, 0.32–1.36; P = 0.25) (Table 3). However, among the 1952 patients with ER ≥ 10% tumors, adjuvant hormonal therapy was significantly associated with better TTR (HR, 0.24; 95% CI, 0.16–0.36; P < 0.001) and OS (HR, 0.32; 95% CI, 0.2–0.5; P < 0.001) (Table 4).
Table 3.
Subgroup analysis of patients with 1%≤ER < 10% expression (n = 171)
| TTR |
OS |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Clinical stage | ||||
| Stage III | (reference) | (reference) | ||
| Stage II | 0.38 (0.21–0.67) | <0.001 | 0.22 (0.11–0.45) | <0.001 |
| Pathological response | ||||
| pCR | (reference) | (reference) | ||
| Non-pCR | 3.79 (1.47–9.74) | 0.006 | 4.55 (1.37–15.08) | 0.01 |
| LVI | ||||
| Negative | (reference) | (reference) | ||
| Positive | 2.22 (1.24–3.97) | 0.007 | 1.93 (1.00–3.68) | 0.048 |
| Adjuvant hormonal therapy | ||||
| No | (reference) | (reference) | ||
| Yes | 0.88 (0.48–1.60) | 0.67 | 0.65 (0.32–1.36) | 0.25 |
Multivariate Cox regression analysis of time to recurrence (TTR) and overall survival (OS).
HR, hazard ratio; CI, confidential interval; pCR, pathological complete response; LVI, lymphovascular invasion.
Table 4.
Subgroup analysis of patients with ER ≥ 10% expression (n = 1952)
| TTR |
OS |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 0.974 (0.965–0.983) | <0.001 | – | – |
| BMI | – | – | 1.02 (1.01–1.04) | 0.01 |
| Race | ||||
| White | (reference) | (reference) | ||
| Black | 1.00 (0.75–1.34) | 0.98 | – | – |
| Hispanic | 0.74 (0.56–0.98) | 0.03 | – | – |
| Asian/Others | 1.03 (0.70–1.52) | 0.87 | – | – |
| Nuclear grade | – | – | (reference) | |
| III | – | – | 0.77 (0.6–0.98) | 0.03 |
| I/II | ||||
| Histology | ||||
| Ductal | – | – | (reference) | |
| Lobular | – | – | 0.78 (0.52–1.16) | 0.22 |
| Others | – | – | 0.57 (0.37–0.89) | 0.01 |
| Clinical stage | ||||
| Stage III | (reference) | (reference) | ||
| Stage II | 0.51 (0.42–0.62) | <0.001 | 0.51 (0.4–0.65) | <0.001 |
| PR, categorical | ||||
| PR ≥ 10% | (reference) | (reference) | ||
| PR < 1% | 1.77 (1.39–2.24) | <0.001 | 1.80 (1.36–2.36) | <0.001 |
| 1% ≤ PR < 10% | 1.34 (1.01–1.76) | 0.04 | 1.44 (1.03–2.00) | 0.03 |
| Pathological response | ||||
| pCR | (reference) | (reference) | ||
| Non-pCR | 3.31 (1.75–6.25) | <0.001 | 5.61 (2.07–15.16) | <0.001 |
| LVI | ||||
| Negative | (reference) | (reference) | ||
| Positive | 1.72 (1.42–2.10) | <0.001 | 1.68 (1.32–2.13) | <0.001 |
| Neoadjuvant regimens | ||||
| A+T | (reference) | – | – | |
| A | 1.17 (0.90–1.51) | 0.24 | – | – |
| T | 1.93 (1.27–2.93) | 0.002 | – | – |
| Adjuvant hormonal therapy | ||||
| No | (reference) | (reference) | ||
| Yes | 0.24 (0.16–0.36) | <0.001 | 0.32 (0.20–0.50) | <0.001 |
Multivariate Cox regression analysis of time to recurrence (TTR) and overall survival (OS).
HR, hazard ratio; CI, confidential interval; BMI, body mass index; PR, progesterone receptor; pCR, pathological complete response; LVI, lymphovascular invasion; A + T, anthracycline and taxane; A, anthracycline; T, taxane.
Discussion
We demonstrated that for patients with newly diagnosed stage II or III HER2-negative primary invasive breast cancer treated with NACT, a low percentage of ER expression was significantly associated with a high pCR rate. We also demonstrated that the percentage of ER expression below which pCR was likely was 10%, which is the cut-off value previously recommended by the ASCO/CAP. In addition, we found adjuvant hormonal therapy to have a significant benefit in terms of TTR and OS only in patients with ER ≥ 10% tumors. Although patients with 1% ≤ ER < 10% tumors are currently recommended to receive adjuvant hormonal therapy, such patients in the present study did not receive a significant survival benefit from adjuvant hormonal therapy. At MD Anderson Cancer Center, treatment decisions regarding both neoadjuvant and adjuvant systemic therapies follow Breast Medical Oncology department consensus based on National Comprehensive Cancer Network (NCCN) clinical practice guidelines. The consensus meeting is a monthly meeting, and the influence of the preference of the treating physician is minimal.
We found that 5.4% of the HER2-negative breast cancers in the present study had ER expression rates of 1%–9%. This is in line with previous studies that reported that ∼5% of all breast cancers have ER expression rates of 1%–9%. Because of the low frequency of this category, conducting prospective trials targeting this cohort is not feasible. Until recently, only retrospective studies and post hoc reviews have informed the discussion of stratifying patients with breast cancer according to their likelihood of benefiting from hormonal therapy. However, our findings shed some light on whether patients with HER2-negative breast cancer can be grouped in this manner. St. Gallen International Expert Consensus 2005 recommended three categories of endocrine responsiveness: endocrine responsive, endocrine response uncertain, and endocrine non-responsive [23]. Following this statement, St. Gallen International Expert Consensus 2009 recommended adjuvant endocrine therapy for almost all patients whose tumors show evidence of endocrine responsiveness, which is now defined as the presence of any detectable ER expression [24]. Then, St. Gallen International Expert Consensus 2015 reported that ER values between 1% and 9% were considered equivocal and that endocrine therapy alone cannot be relied upon for patients with these values [18]. However, these categories have not been clearly defined.
One of the novelties of the current study is that we evaluated ER expression as a continuous variable. Also, we derived the cut-off value of ER expression to identify patients who would be more or less likely to achieve pCR after NACT and investigated the benefit from adjuvant hormonal therapy in terms of TTR and OS.
The current study had several potential limitations. First, this was a retrospective study and treatment was not randomly assigned. However, conducting a randomized controlled trial that includes patients with 1%≤ER < 10% tumors would be extremely difficult, given the small number of patients with such tumors; moreover, the current recommendations for defining ER and PR positivity are based on retrospective analyses. Second, there were fewer patients with 1%≤ER < 10% tumors than patients with ER ≥ 10% tumors. This sample size of patients with 1%≤ER < 10% tumors might have been too small to detect statistically significant differences. Despite the small sample size, our multivariate analysis revealed that the interaction term between adjuvant hormonal therapy and ER category (1% ≤ ER < 10% versus ER ≥ 10%) was statistically significant for both TTR and OS, which supported this significant result. Third, some patients’ diagnostic biopsies were carried out at outside centers, which could have contributed to low reproducibility of biomarker evaluation in our study sample. However, at MD Anderson Cancer Center, only dedicated breast pathologists review and evaluate all slides, including slides from outside centers. Also, cases are reviewed as part of MD Anderson’s internal quality control program and its concordance rate in biomarker evaluation is >95%. Thus, the reproducibility problem of ER and PR expression in the current study must be small. Fourth, data on the biological features of tumors, such as PAM50 results, were not included in the study. ASCO guidelines state that clinicians may use the PAM50 risk of recurrence score to inform their decision to use adjuvant systemic therapy in patients with ER/PR-positive, HER2-negative, node-negative breast cancer [25]. Fifth, owing to its retrospective nature, our analysis did not account for Ki-67 expression status, which may be associated with pCR and survival outcomes. However, the interlaboratory reproducibility of current methods of Ki-67 assessment is inadequate, and the role of Ki-67 in patients treated with NACT without endocrine therapy is still unclear [26, 27]. In addition, the NCCN Guidelines do not recommend routine testing of Ki-67. Given the lack of reproducibility and the recommendation of the NCCN, Ki67 staining is not routinely carried out as standard practice, and many patients in our study did not have Ki-67 data. Lastly, there is a heterogeneity of systemic treatments after definitive surgery because of the nature of retrospective study. This might affect the analysis of benefit from adjuvant hormonal therapy on the survival outcomes.
One factor that may need to be taken into consideration when interpreting the findings of the present study is BRCA1 somatic mutation status and its association with poly ADP ribose polymerase (PARP) inhibitors and platinum agents. Approximately 60% or more of breast cancer patients with BRCA1 mutations have TNBC and ∼35% of TNBC patients have BRCA1 mutation [28, 29]. Tumors with BRCA mutation tend to respond to PARP inhibitors and/or platinum agents [29–32]. In the era of developing platinum and PARP inhibitors for BRCA-mutated breast tumors, the comprehensive analysis with IHC and genomic analysis of breast tumors, especially low ER expression HER2-negative breast tumors, would become more important. Something else that should be considered in the interpretation of the present study’s findings is the fact that two different antibodies were used during the study period. Both the 1D5 and 6F11 antibodies have been demonstrated to produce results that correlate with clinical outcomes. However, the quality of antibody as well as the quality of IHC staining methods have improved. Stricter guidelines for the documentation of pre-analytical factors such as fixation time have been implemented, and more robust detection systems are available for archival tissues.
Current ASCO/CAP guidelines recommend that, regardless of HER2 status, breast cancers with <1% of ER expression should be considered ER-negative. However, we found that for patients with newly diagnosed stage II or III HER2-negative primary invasive breast cancer, a low percentage of ER expression was associated with a high pCR rate and that the cut-off percentage of ER expression below which pCR was likely was 10%. In addition, only patients with ER ≥ 10% tumors had better survival with adjuvant hormonal therapy. Also, the cut-off of 1% is more reproducible than that of 10% in IHC staining, and the cut-off of 10% tends to be more affected by the number of cells on the slide and the quality of the slides. The quality of pathological assessment was maintained in the current study because only dedicated pathologists evaluated all slides.
In conclusion, stage II or III HER2-negative primary invasive breast cancer with <10% of ER expression behaves clinically like TNBC in terms of pCR and survival outcomes and patients with such tumors may have a limited benefit from adjuvant hormonal therapy. It may be more clinically relevant to define TNBC as HER2-negative breast cancer with <10%, rather than <1%, of ER and/or PR expression. Studies with other datasets to confirm our findings are warranted.
Supplementary Material
Acknowledgements
Joseph A. Munch of the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center provided scientific editing services.
Funding
Morgan Welch Inflammatory Breast Cancer Research Program; a grant from the State of Texas Rare and Aggressive Breast Cancer Research Program; MD Anderson’s Cancer Center Support Grant from the National Cancer Institute (CA016672), which supports the Biostatistics Shared Resource; and a grant from the National Cancer Institute (CA079466).
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Foulkes WD, Smith IE, Reis-Filho JS.. Triple-negative breast cancer. N Engl J Med 2010; 363: 1938–1948. [DOI] [PubMed] [Google Scholar]
- 2. Liedtke C, Mazouni C, Hess KR. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 3. De Laurentiis M, Cianniello D, Caputo R. et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer Treat Rev 2010; 36(Suppl 3): S80–S86. [DOI] [PubMed] [Google Scholar]
- 4. Santana-Davila R, Perez EA.. Treatment options for patients with triple-negative breast cancer. J Hematol Oncol 2010; 3: 42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carey LA, Dees EC, Sawyer L. et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13: 2329–2334. [DOI] [PubMed] [Google Scholar]
- 6. Lin NU, Vanderplas A, Hughes ME. et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012; 118: 5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Minckwitz G, Untch M, Blohmer JU. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 8. Esserman LJ, Berry DA, DeMichele A. et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012; 30: 3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mieog JS, van der Hage JA, van de Velde CJ.. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007; CD005002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kong X, Moran MS, Zhang N. et al. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 2011; 47: 2084–2090. [DOI] [PubMed] [Google Scholar]
- 11. Hammond ME, Hayes DF, Dowsett M. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28: 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamoto T, Booser D, Valero V. et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol 2012; 30: 729–734. [DOI] [PubMed] [Google Scholar]
- 13. Yi M, Huo L, Koenig KB. et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol 2014; 25: 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colleoni M, Bagnardi V, Rotmensz N. et al. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer 2010; 46: 2216–2224. [DOI] [PubMed] [Google Scholar]
- 15. Sanchez-Munoz A, Plata-Fernandez Y, Fernandez M. et al. Tumor histological subtyping determined by hormone receptors and HER2 status defines different pathological complete response and outcome to dose-dense neoadjuvant chemotherapy in breast cancer patients. Clin Transl Oncol 2014; 16: 548–554. [DOI] [PubMed] [Google Scholar]
- 16. Gianni L, Eiermann W, Semiglazov V. et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010; 375: 377–384. [DOI] [PubMed] [Google Scholar]
- 17. Gianni L, Pienkowski T, Im Y-H. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 25–32. [DOI] [PubMed] [Google Scholar]
- 18. Coates AS, Winer EP, Goldhirsch A. et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26: 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolff AC, Hammond ME, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 20. Lester SC, Bose S, Chen Y-Y. et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. (http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-breast-invasive-13protocol-3200.pdf): In. College of American Pathologists (http://www,cap.org): 2013 (30 July 2017, date last accessed).
- 21. Provenzano E, Bossuyt V, Viale G. et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol 2015; 28: 1185–1201. [DOI] [PubMed] [Google Scholar]
- 22. Therneau TA, B, Ripley B, Package ‘rpart’: recursive partitioning and regression trees. http://rileylab.org/wp-content/uploads/2016/08/rpart.pdf. 2014. (30 July 2017, date last accessed).
- 23. Goldhirsch A, Glick JH, Gelber RD. et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16: 1569–1583. [DOI] [PubMed] [Google Scholar]
- 24. Goldhirsch A, Ingle JN, Gelber RD. et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009; 20: 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris LN, Ismaila N, McShane LM. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016; 34: 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polley MY, Leung SC, McShane LM. et al. An international Ki67 reproducibility study. J Natl Cancer Inst 2013; 105: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dowsett M, Nielsen TO, A’Hern R. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011; 103: 1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner NC, Tutt AN.. Platinum chemotherapy for BRCA1-related breast cancer: do we need more evidence? Breast Cancer Res 2012; 14: 115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peshkin BN, Alabek ML, Isaacs C.. BRCA1/2 mutations and triple negative breast cancers. Breast Dis 2010; 32: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karginova O, Siegel MB, Van Swearingen AE. et al. Efficacy of carboplatin alone and in combination with ABT888 in intracranial murine models of BRCA-mutated and BRCA-wild-type triple-negative breast cancer. Mol Cancer Ther 2015; 14: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tutt A, Robson M, Garber JE. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376: 235–244. [DOI] [PubMed] [Google Scholar]
- 32. Livraghi L, Garber JE.. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med 2015; 13: 188.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.