Abstract
Aims
Cardiac resynchronization therapy (CRT) induces reverse cardiac remodelling in heart failure (HF), but many patients receiving CRT remain non-responders. This study assessed the role of amino-terminal-pro-B-type natriuretic peptide (NT-proBNP), mid-regional-pro-atrial natriuretic peptide (MR-proANP), and mid-regional-pro-adrenomedullin (MR-proADM) at the time of device implantation to predict favourable clinical course (CRT response and/or risk of MACE) in HF patients receiving CRT.
Methods and results
A total of 137 HF patients were prospectively included. Blood was drawn from the coronary sinus (CS) at CRT implantation, and from a peripheral vein (PV) simultaneously and after 6 months. Clinical CRT response at 6 months and major adverse cardiovascular events (MACE) at 2 years were assessed. Baseline PV-levels of MR-proANP (202 vs. 318 pmol/L, P = 0.009) and MR-proADM (843 vs. 1112 pmol/L, P = 0.02) were lower in CRT responders compared with non-responders. At 6 months, CRT responders showed a decrease in MR-proANP levels, compared with an increase in non-responders (−32 vs. +7 pmol/L, P = 0.02). During the same period, NT-proBNP decreased by a similar way in responders and non-responders, while MR-proADM was unchanged in both groups. High baseline MR-proANP, either in PV (OR 0.41, 95% CI 0.24–0.71, P = 0.002) or CS (OR 0.32, 95% CI 0.15–0.70, P = 0.005) was associated with reduced likelihood of CRT response. Furthermore, PV and CS levels of NT-proBNP, MR-proANP, and MR-proADM were all associated with increased risk of 2-year MACE (all P < 0.01).
Conclusion
Mid-regional-pro-atrial natriuretic peptide may assist prediction of clinical course in HF patients undergoing CRT implantation. Low circulating MR-proANP at the time of device implantation is associated with CRT response and more favourable outcome.
Keywords: Heart failure, Cardiac resynchronization therapy, Response, Biomarker, MR-proANP, MR-proADM, NT-proBNP
What's new?
Cardiovascular biomarker measurement at the time of device implantation may be helpful to predict favourable clinical course in heart failure (HF) patients receiving cardiac resynchronization therapy (CRT).
Baseline levels of mid-regional-pro-atrial natriuretic peptide (MR-proANP) was lower in CRT responders compared with non-responders (202 vs. 318 pmol/L, P = 0.009). At 6 months, CRT responders showed a decrease in MR-proANP levels, compared with an increase in non-responders (−32 vs. +7 pmol/L, P = 0.02).
High baseline MR-proANP was associated with reduced likelihood of CRT response (OR 0.41, 95% CI 0.24–0.71, P = 0.002) and increased risk of major adverse cardiovascular events at 2-year follow-up (HR 2.7, 95% CI 1.6–4.4, P < 0.001).
Mid-regional-pro-atrial natriuretic peptide may assist prediction of clinical course in HF patients receiving CRT.
Introduction
Heart failure (HF) is a major cause of death and unscheduled hospital admissions in industrialized countries. In eligible patients, cardiac resynchronization therapy (CRT) has been shown to reduce mortality and hospital readmissions and improve functional capacity of selected patients with chronic HF.1–3 Unfortunately, despite guideline-based patient selection and adjustment of device parameters, at least, one-third of patients receiving CRT remain non-responders.4,5 Hence, there is an unmet need for additional criteria to optimize patient selection. Despite intensive research, though high LA size before device implantation was shown to be associated with lower CRT response,6,7 the role of echocardiography-derived parameters for prediction of clinical course after CRT is still disappointing. In this regard, cardiovascular biomarkers might help clinicians in selecting those patients that would effectively benefit from CRT: biomarkers provide the potential advantage of providing a picture of active processes in HF that might influence CRT response, such as active myocardial remodelling. Cardiac resynchronization induces reverse remodelling of the failing heart with improvement of left-ventricular systolic and diastolic function with an improvement in left-sided filling pressures.8 Natriuretic peptides (NPs) are released by the failing heart in response to mechanical wall stretch and reflect severity of cardiac dysfunction. Among NPs, mid-regional-pro-atrial natriuretic peptide (MR-proANP) may correlate with left-sided filling pressures better than amino-terminal-pro-B-type natriuretic peptide (NT-proBNP) and be complementary to NT-proBNP for prognosis.9–12 Similarly, in HF patients, increased concentrations of the precursor fragment of adrenomedullin (MR-proADM), a peptide expressed in several tissues with vasodilatory, inotropic, and natriuretic properties, have also been shown to provide strong prognostic information with regard to mortality and hospital admission.10–14 However, data about the prognostic properties of MR-proADM in patients undergoing CRT device implantation are scarce.
The Biomarkers to Predict CRT Response in Patients with HF (BIOCRT) study was designed to investigate the role of cardiovascular biomarkers to predict favourable clinical course of HF patients undergoing CRT implantation.15 The aim of the present study was to assess the role of NPs (NT-proBNP and MR-proANP) and MR-proADM at the time of device implantation to predict favourable clinical course (CRT response and/or risk of MACE) in HF patients receiving CRT. We hypothesized MR-proANP and MR-proADM may provide unique information regarding CRT response and prognosis in this patient population.
Methods
Study population
A total of 137 patients with chronic HF undergoing CRT device implantation at Massachusetts General Hospital (Boston, MA, USA) were prospectively included in the observational BIOCRT study (ClinicalTrials.gov number: NCT01949246) between December 2007 and July 2012. The protocol has been previously described in detail, inclusion and exclusion criteria are detailed in Supplementary material online, Table S1.15 At the time of device implantation, coronary sinus (CS) blood was drawn through the guiding catheter before delivering the CRT lead. Simultaneously, at 6-month follow-up peripheral venous (PV), blood was drawn from one of the upper extremity veins. All patients underwent CRT implantation and optimization of the CRT device at 1 month. Transthoracic echocardiography was performed at baseline and at 6-month follow-up.
Blood samples storage and analysis
All samples were immediately centrifuged and stored in microcentrifuge tubes at −80°C until assayed by a standardized way. De-identified specimens were sent to independent core laboratories for analysis. Measurements of concentrations of NT-proBNP were performed using a 1-step sandwich chemiluminescent immunoassay (Dimension Vista Flex, Siemens, Newark, DE, USA) at Siemens Healthcare Diagnostics, Inc., Newark, DE, USA. Concentrations of MR-proANP and MR-proADM were determined using an automated immunoassay technique (B.R.A.H.M.S, Hennigsdorf, Germany) at Lariboisière University Hospital, Paris, France.
Outcome analysis
Patients returned for regular clinic visits at 1, 3, and 6 months after device implantation and were followed for events during up to 2 years. CRT response was assessed according to the HF Clinical Composite Score.16 Patients who experienced a favourable change in NYHA functional class or in the global assessment or both, but did not experience any major adverse cardiovascular event (MACE) were defined as responders. Major adverse cardiovascular event was defined as the composite endpoint of death, cardiac transplant, left-ventricular assist device (LVAD) implantation, and HF hospitalization at 2 years. An outcome panel consisting of two cardiologists, blinded to the biomarker results, determined the clinical response of each subject based on review of the medical record, with disagreement resolved by consensus with a third cardiologist.
Statistical analysis
Continuous variables are expressed, after testing for normality using the Shapiro–Wilk test, as mean ± standard deviation or median [interquartile range], as appropriate. Nominal variables are expressed as frequency (percentages). Differences between two independent groups were assessed with a t-test, Wilcoxon rank sum test or Fisher's exact test, as appropriate. Differences between Wilcoxon-signed rank tests were used to examine the differences between baseline and 6-month biomarker values.
Multivariable regression was used to evaluate the association of biomarkers (logarithmic scale) and CRT response. Unadjusted and adjusted Cox proportional hazards models were used to evaluate the association of biomarkers (logarithmic scale) and MACE. Adjustment was performed for a priori selected covariates with strong evidence on CRT response (age, sex, left-ventricular ejection fraction, estimated glomerular filtration rate, left-atrial diameter, right-ventricular systolic pressure, left bundle-branch block, duration of QRS complex) and MACE (age, sex, left-ventricular ejection fraction, estimated glomerular filtration rate). The performances of NT-proBNP, MR-proANP, and MR-proADM for predicting CRT response and MACE were assessed with calculating the area under the curve (AUC) of receiver-operating characteristic (ROC) curves. Net reclassification indices (NTI) were calculated to assess the added predictive performance of MR-proANP or MR-proADM in addition to NT-proBNP.
The null hypothesis was rejected with an adjusted two-sided P-value < 0.05. Analyses were performed with the use of SAS, Version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Ethical considerations
The study was performed in observation of national laws and in accordance with the ethical standards of the Declaration of Helsinki, and was approved by local Ethical Committees. All patients provided written informed consent.
Results
Patient characteristics and biomarker levels at the time of CRT implantation
Baseline characteristics of the 137 included patients are summarized in Table 1. The mean left-ventricular ejection fraction was 26 ± 7% and 91% of patients were in NYHA class III or IV. Most patients were in sinus rhythm with a median QRS duration of 160 [142, 178] ms.
Table 1.
Baseline characteristics of study participants
| Overall n = 137 | CRT responders n = 82 | CRT non-responders n = 55 | P-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | 66 [57, 77] | 65 [52, 77] | 71 [60, 78] | 0.21 |
| Male | 77% | 73% | 84% | 0.21 |
| BMI, kg/m2 | 27.6 [24.9, 31.9] | 28.0 [24.8, 32.6] | 26.4 [25.1, 30.3] | 0.17 |
| Diabetes | 34% | 28% | 42% | 0.10 |
| Hypertension | 66% | 62% | 71% | 0.36 |
| Hyperlipidaemia | 64% | 59% | 73% | 0.10 |
| History of smoking | 54% | 48% | 64% | 0.08 |
| Ischemic cardiomyopathy | 46% | 40% | 55% | 0.12 |
| NYHA class | 0.34 | |||
| II | 9% | 6% | 13% | |
| III | 85% | 89% | 80% | |
| IV | 6% | 5% | 7% | |
| Medications | ||||
| ACEi/ARB | 80% | 82% | 78% | 0.66 |
| β-Blockers | 85% | 84% | 87% | 0.81 |
| Aldosterone antagonists | 31% | 26% | 38% | 0.13 |
| Diuretics | 78% | 77% | 80% | 0.83 |
| Laboratory | ||||
| Creatinine, mg/dL | 1.22 [0.98, 1.55] | 1.14 [0.95, 1.45] | 1.27 [1.03, 1.81] | 0.02 |
| eGFR | 61 ± 21 | 64 ± 21 | 56 ± 21 | 0.03 |
| ECG parameters | ||||
| Sinus rhythm | 69% | 72% | 65% | 0.45 |
| Heart rate, bpm | 72 ± 12 | 72 ± 13 | 72 ± 10 | 0.95 |
| QRS duration, ms | 160 [142, 178] | 163 [146, 178] | 158 [140, 176] | 0.46 |
| LBBB | 53% | 62% | 38% | 0.009 |
| Paced | 21% | 21% | 22% | 0.83 |
| Echocardiographic parameters | ||||
| LVEF, % | 26 ± 7 | 26 ± 7 | 24 ± 8 | 0.12 |
| LV dimensions, mm | ||||
| End-diastole (EDD) | 62 [57, 67] | 61 [56, 67] | 63 [58, 68] | 0.31 |
| End-systole (ESD) | 54 [48, 58] | 53 [47, 58] | 54 [50, 59] | 0.36 |
| LV volumes, mL | ||||
| End-diastole (EDV) | 217 [178, 280] | 208 [167, 272] | 225 [193, 301] | 0.14 |
| End-systole (ESV) | 157 [128, 205] | 152 [118, 202] | 164 [134, 232] | 0.08 |
| Left-atrial diameter, mm | 45 [41, 49] | 44 [40, 49] | 47 [43, 51] | 0.02 |
| Mitral regurgitation | 0.36 | |||
| None/trace | 22% | 24% | 18% | |
| Mild | 34% | 36% | 32% | |
| Moderate | 33% | 32% | 34% | |
| Severe | 11% | 8% | 16% | |
| RV systolic pressure, mmHg | 42 [35, 51] | 40 [35, 48] | 46 [39, 57] | 0.02 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; LBBB, left bundle-branch block; LV, left ventricular; LVEF, left-ventricular ejection fraction; NYHA, New York Heart Association; RV right ventricular.
Baseline PV and CS levels of NT-proBNP, MR-proANP, and MR-proADM are reported in Supplementary material online, Table S2. Notably, the median transcardiac gradient (difference between CS and PV) was +237 pg/mL for NT-proBNP, +71 pmol/L for MR-proANP, and −19 pmol/L for MR-proADM.
Clinical characteristics of cardiac resynchronization therapy responders
A total of 82 patients (60%) were considered CRT responders 6 months after device implantation.
As reported in Table 1, CRT responders had similar characteristics as CRT non-responders, at the time of CRT implantation, except for slightly lower creatinine levels, smaller left atria and lower right-ventricular systolic pressures. A CRT device with combined cardioverter-defibrillator (CRT-D) was implanted in 95% of CRT responders and 96% of CRT non-responders, P = 1.0.
At 6-month follow-up, in addition to clinical improvement, CRT responders showed significantly smaller left-ventricular end-diastolic volume (184 [138, 231] vs. 217 [164, 271] mL, P = 0.03), smaller left-ventricular end-systolic volume (117 [77, 154] vs. 154 [107, 203] mL, P = 0.003), smaller left-atrial diameter (43 ± 8 vs. 47 ± 7, P = 0.001) and better systolic function (left-ventricular ejection fraction 37 ± 10 vs. 28 ± 9%, P < 0.001) compared with CRT non-responders.
Biomarker levels of cardiac resynchronization therapy responders
Concerning circulating cardiovascular biomarkers (Supplementary material online, Table S3), at the time of CRT implantation, PV concentrations of MR-proANP, and MR-proADM were lower in CRT responders compared with CRT non-responders (202 [112, 348] vs. 318 [238, 506] pmol/L, P = 0.009 and 843 [657, 1129] vs. 1112 [776, 1449] pmol/L, P = 0.02, respectively). Although not reaching statistical significance, a similar trend was observed also for NT-proBNP (1019 [444, 2507] vs. 2227 [736, 3309] pg/mL, P = 0.06).
At 6 months after device implantation, CRT responders showed a decrease in MR-proANP levels (−32 [−89, +5] pmol/L) compared with baseline, while MR-proANP increased (+7 [−49, +123] pmol/L, P = 0.02) in CRT non-responders (Figure 1). During the same time period, NT-proBNP decreased by a similar way in CRT responders and CRT non-responders. Concentrations of MR-proADM were unchanged in CRT responders and CRT non-responders (Figure 1).
Figure 1.
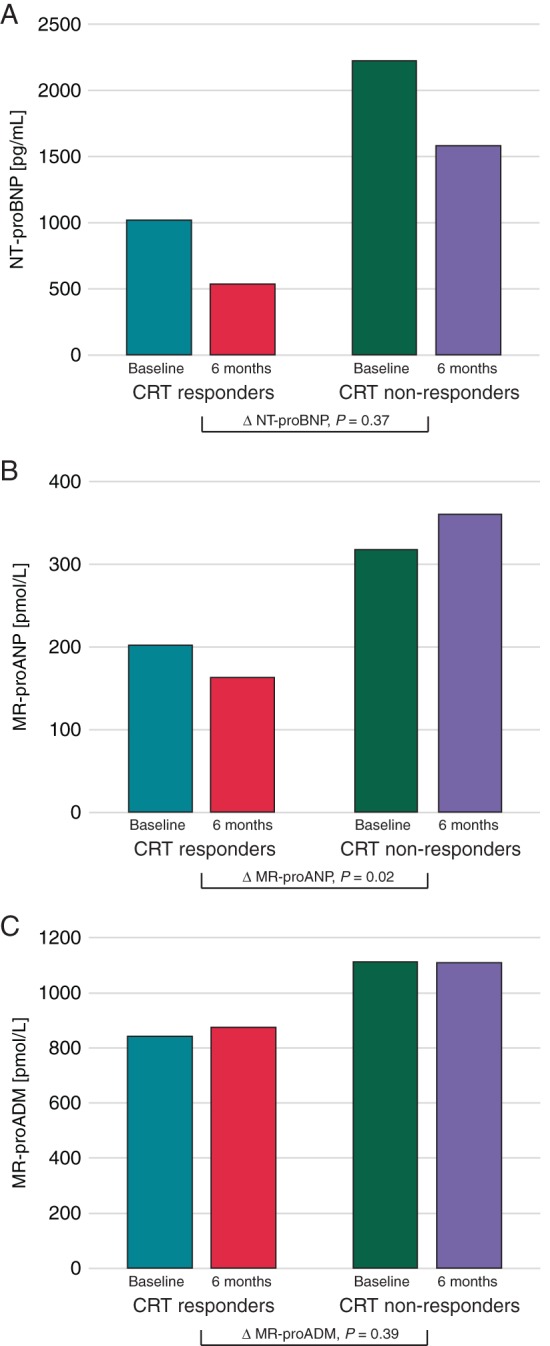
Peripheral venous NT-proBNP (A), MR-proANP (B), and MR-proADM (C) values at baseline and at 6 months in CRT responders and CRT non-responders. Median plasma levels of NT-proBNP, MR-proANP, and MR-proADM at baseline and 6-month follow-up in CRT responders and CRT non-responders.
Value of biomarker levels at the time of device implantation for predicting cardiac resynchronization therapy response
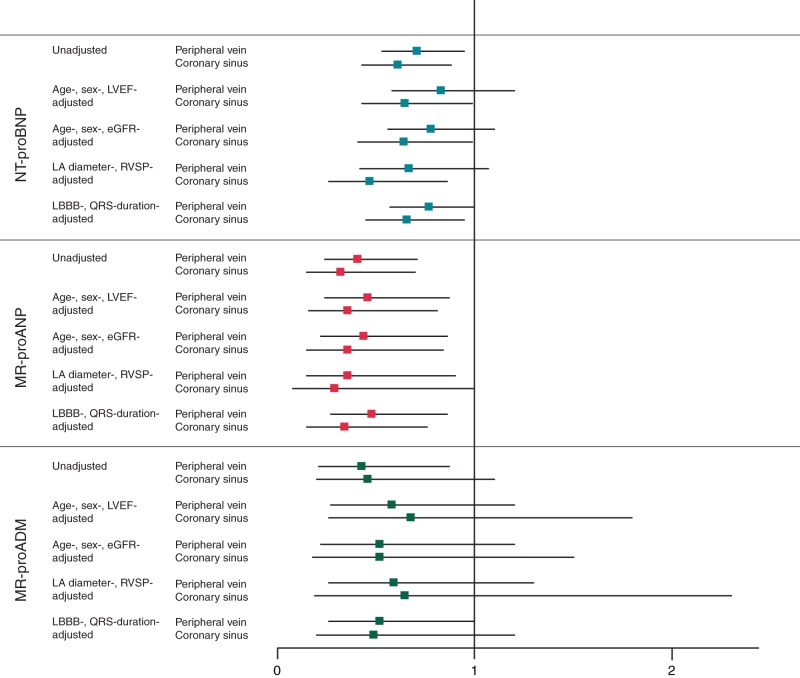
The association between biomarker levels at the time of device implantation and CRT response at 6 months is summarized in Figure 2. High baseline CS levels of NT-proBNP were associated with reduced likelihood of CRT response in the univariate analysis [odds ratio (OR) 0.61, 95% confidence interval (95% CI) 0.43–0.88, P = 0.009] and after several adjustments for clinical confounders. Peripheral vein levels of NT proBNP were associated with reduced likelihood of CRT response in the univariate analysis (OR 0.71, 95% CI 0.53–0.95, P = 0.02) but lost statistical significance after adjustments for clinical confounders.
Figure 2.
Prognostic value of plasma NT-proBNP, MR-proANP, and MR-proADM for CRT response at 6 months. Odds ratio with 95% confidence interval of log-transformed PV and CS levels of NT-proBNP, MR-proANP, and MR-proADM. eGFR, estimated glomerular filtration rate; LAD, left-atrial diameter; LBBB, left bundle-branch block; LVEF, left-ventricular ejection fraction; RVSP, right-ventricular systolic pressure.
In contrast, high concentrations of MR-proANP, either in PV (OR 0.41, 95% CI 0.24–0.71, P = 0.002) or in CS (OR 0.32, 95% CI 0.15–0.70, P = 0.005) were consistently associated with reduced likelihood of CRT response in the univariate analysis and after adjustment for clinical confounders.
Higher PV concentrations of MR-proADM were also associated with reduced likelihood of CRT response in the univariate analysis but lost statistical significance after adjustment for clinical confounders.
The areas under the ROC curve of PV levels of NT-proBNP, MR-proANP, and MR-proADM for predicting CRT response were 0.58, 0.67, and 0.65, respectively. Mid-regional-pro-atrial natriuretic peptide was superior to NT-proBNP in this regard (P = 0.01). The combination of MR-proANP and NT-proBNP was superior to NT-proBNP alone for prediction of CRT response [Net reclassification index (NRI) 0.56, 95% CI 0.2–0.9, P = 0.003], while the combination of MR-proADM and NT-proBNP was similar to NT-proBNP alone (NRI 0.24, 95% CI −0.1 to 0.6, P = 0.19).
Notably, the transcardiac gradient of any of the three biomarkers did not provide any prognostic information with regard to CRT response (Supplementary material online, Table S4).
Value of biomarker levels at the time of device implantation for predicting 2-year major adverse cardiovascular event
During a median follow-up of 2 years, there were 35 (25%) patients with MACE, including 14 deaths, 32 HF hospitalizations, 2 LVAD implantations, and 3 heart transplants. Baseline characteristics of patients with and without MACE have been published elsewhere (Arrigo et al., in revision).
As reported in Supplementary material online, Table S5, higher concentrations of NT-proBNP, MR-proANP, and MR-proADM in PV or CS were all associated with increased risk of 2-year MACE. Indeed, PV levels of NT-proBNP [hazard ratio (HR) 1.7, 95% CI 1.3–2.4, P < 0.001], MR-proANP (HR 2.7, 95% CI 1.6–4.4, P < 0.001), and MR-proADM (HR 4.7, 95% CI 2.4–9.5, P < 0.001) were all associated with increased risk of 2-year MACE in the univariate analysis and after adjustment for clinical confounders. Accordingly, the areas under the ROC curve of PV levels of NT-proBNP, MR-proANP, and MR-proADM for predicting MACE were similar (0.68, 0.70, and 0.63, respectively, all P > 0.05). The combination of MR-proANP and NT-proBNP or MR-proADM and NT-proBNP was similar to NT-proBNP alone for prediction of MACE (NRI 0.02, 95% CI −0.4 to 0.5, P = 0.94 and NRI −0.09, 95% CI −0.5 to 0.3, P = 0.67, respectively).
Notably, transcardiac gradient of MR-proANP and MR-proADM also provided prognostic information (Supplementary material online, Table S5).
Baseline mid-regional-pro-atrial natriuretic peptide and favourable long-term clinical course
Receiver-operating characteristic analysis indicated that optimal cut-off level of baseline PV MR-proANP for its association with both outcomes (CRT response and MACE) was 250 pmol/L. As depicted in Figure 3, CRT response in patients with MR-proANP levels <250 pmol/L was 74% compared with 46% in patients with higher concentrations (P = 0.003) and MACE was only 10% compared with 39% with MR-proANP higher than 250 pmol/L (P < 0.001).
Figure 3.
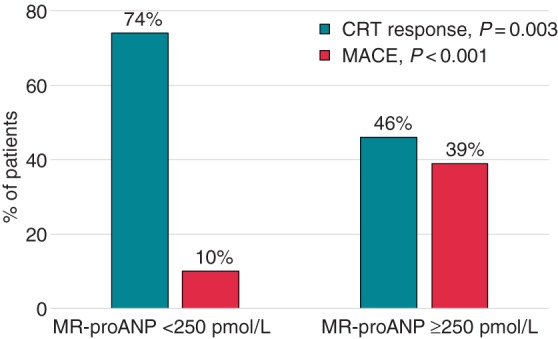
Cardiac resynchronization therapy response at 6 months and MACE at 2 years according to baseline values of PV MR-proANP levels. CRT response at 6 months (blue) and MACE at 2 years (yellow) in patients with MR-proANP levels <250 and ≥250 pmol/L.
Discussion
The current study showed that MR-proANP may assist prediction of clinical course in severe HF patients undergoing CRT implantation. In particular, low circulating MR-proANP at the time of device implantation is associated with high likelihood of CRT response and low risk of MACE.
Cardiac resynchronization therapy improves survival and functional capacity through reverse remodelling of the failing heart via reduction of left-ventricular dimensions and improved left-ventricular ejection fraction.17 However, despite guideline-based patient selection, rates of CRT non-response remain high. In our study, 40% of patients were considered clinical non-responders at 6 months, which is in line with rates reported in the literature.4,5 Mechanisms of CRT non-response are unknown, but a lack in reverse cardiac remodelling is likely to play a central role. Supporting this assumption, our study confirmed the close relationship between clinical and echocardiographic course after CRT. Indeed, 6 months after device implantation, CRT responders showed smaller left-ventricular volumes and higher left-ventricular ejection fraction compared with CRT non-responders, in accordance with previous studies.5
Our study suggests that CRT response may be inversely related to the severity of myocardial dysfunction at the time of CRT implantation. Indeed, our present study showed that, at the time of device implantation, CRT responders had lower concentrations of NPs (in particular MR-proANP) compared with CRT non-responders. In stable HF patients, circulating NPs reflects the severity of myocardial stretch due to ventricular dysfunction.9 By showing positive transcardiac gradients, our study further supported the cardiac source of NPs. In addition, we showed that CRT responders, despite similar left-ventricular volumes and ejection fraction, had smaller left atria compared with CRT non-responders. The association between smaller atria and CRT response is in line with results of previous studies.6,7,18,19 Hence, lower NP levels and smaller atria strongly suggest less severe myocardial dysfunction at the time of device implantation in our CRT responders than in CRT non-responders. According to this statement, we also observed no differences in baseline levels of MR-proADM, according to CRT response. Indeed, MR-proADM has a systemic—rather than cardiac—origin, as suggested by the negative transcardiac gradient seen in our study, and was described as a marker of increased plasma volume and activated sympathetic nervous system in HF.20 In summary, our study suggests the severity of myocardial dysfunction as an important mechanism of CRT response.
The present study suggests a closer relationship of circulating MR-proANP with clinical course after CRT implantation when compared with NT-proBNP albeit with similar trends in concentration of the latter marker in this small cohort. Indeed, PV levels of MR-proANP decreased in CRT responders and increased in CRT non-responders during the 6 initial months after device implantation, while NT-proBNP decreased in both subgroups. Our results suggest that MR-proANP may better reflect reverse remodelling and improvement in LV systolic and diastolic functions induced by CRT than NT-proBNP.8 This is in line with the recent study showing that MR-proANP correlates better with left-ventricular filling pressures than NT-proBNP.9
Finally, the present study showed that baseline plasma levels of NT-proBNP, MR-proANP, and MR-proADM were all associated with 2-year MACE: the higher the plasma levels, the higher the risk of MACE. Those data are in line with studies showing NPs and MR-proADM as prognosticators of death in HF patients.10–14 Our data extend these properties for the assessment of MACE in HF patients with CRT.
Clinical implications
Our study first shows that measurement of MR-proANP may improve selection of CRT patients. Indeed, in patients with criteria of CRT implantation, whether this might be used as a criterion to assist in decision-making for implantation is speculative and more data are needed in this regard. In the light of our data, it is premature to discourage CRT implantation in patients with highly elevated MR-proANP, but clinicians should be aware of increased risk of adverse clinical course in those patients. In addition, MR-proANP measured during the follow-up objectifies CRT response; in those with non-response by clinical or biochemical criteria, interventions to improve response (such as adjustment in device parameters and/or lead placement) might be undertaken.
Limitations
The study has some notable limitations. First, this is a small cohort of selected patients from a single academic tertiary care centre with experienced care and follow-up of CRT patients. This may represent selection bias and limit its generalizability. Due to small number of included patients and events, the ability to perform multivariable adjustments is limited. However, this is the largest cohort of CRT patients for whom CS and PV blood samples for biomarker analysis are available. Larger prospective studies are needed to confirm our results.
Conclusion
Mid-regional-pro-atrial natriuretic peptide may assist prediction of clinical course in severe HF patients undergoing CRT implantation. In particular, low circulating MR-proANP at the time of device implantation is associated with the favourable outcome.
Supplementary material
Funding
M.A. is recipient of a fellowship of the Collège de Médecine des Hôpitaux de Paris. Q.A.T. was supported by NIH/NHLBI K23HL098370 and L30HL093896. J.S. was supported by NIH/NHLBI K23HL098370.
Conflict of interest: Q.A.T. received grant support from St Jude Medical and ACRIN. J.L.J. has received grant support from Roche Diagnostics, Siemens Diagnostics, Singulex, and Prevencio; J.L.J. has received consulting income from Roche Diagnostics, Critical Diagnostics, Sphingotec, Philips, Cardiorentis, Novartis, Amgen, and Boeringer-Ingelheim. J.P.S. has received grant support from St Jude Medical, Medtronic, BostonScientific, SorinGroup, Biotronik, BG Medicine, and Siemens. A.M. received speaker's honoraria from Abbott, Novartis, Orion, Roche and Servier and fee as member of advisory board and/or steering committee from Cardiorentis, Adrenomed, MyCartis, ZS Pharma and Critical Diagnostics.
Supplementary Material
References
- 1. Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 2. Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 3. Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405. [DOI] [PubMed] [Google Scholar]
- 4. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 5. Ypenburg C, van Bommel RJ, Borleffs CJW, Bleeker GB, Boersma E, Schalij MJ et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol 2009;53:483–90. [DOI] [PubMed] [Google Scholar]
- 6. Rossi L, Malagoli A, Piepoli M, Franchi F, Malavasi V, Casali E et al. Indexed maximal left atrial volume predicts response to cardiac resynchronization therapy. Int J Cardiol 2013;168:3629–33. [DOI] [PubMed] [Google Scholar]
- 7. Kuperstein R, Goldenberg I, Moss AJ, Solomon S, Bourgoun M, Shah A et al. Left atrial volume and the benefit of cardiac resynchronization therapy in the MADIT-CRT trial. Circ Heart Fail 2014;7:154–60. [DOI] [PubMed] [Google Scholar]
- 8. Doltra A, Bijnens B, Tolosana JM, Gabrielli L, Castel MÁ, Berruezo A et al. Effect of cardiac resynchronization therapy on left ventricular diastolic function: implications for clinical outcome. J Card Fail 2013;19:795–801. [DOI] [PubMed] [Google Scholar]
- 9. Andersen MJ, Ersbøll M, Bro-Jeppesen J, Møller JE, Hassager C, Køber L et al. Relationships between biomarkers and left ventricular filling pressures at rest and during exercise in patients after myocardial infarction. J Card Fail 2014;20:959–67. [DOI] [PubMed] [Google Scholar]
- 10. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010;55:2062–76. [DOI] [PubMed] [Google Scholar]
- 11. Masson S, Latini R, Carbonieri E, Moretti L, Rossi MG, Ciricugno S et al. The predictive value of stable precursor fragments of vasoactive peptides in patients with chronic heart failure: data from the GISSI-heart failure (GISSI-HF) trial. Eur J Heart Fail 2010;12:338–47. [DOI] [PubMed] [Google Scholar]
- 12. Shah RV, Truong QA, Gaggin HK, Pfannkuche J, Hartmann O, Januzzi JL. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur Heart J 2012;33:2197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adlbrecht C, Hülsmann M, Strunk G, Berger R, Mörtl D, Struck J et al. Prognostic value of plasma midregional pro-adrenomedullin and C-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail 2009;11:361–6. [DOI] [PubMed] [Google Scholar]
- 14. Haehling von S, Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, Doehner W et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail 2010;12:484–91. [DOI] [PubMed] [Google Scholar]
- 15. Truong QA, Januzzi JL, Szymonifka J, Thai W-E, Wai B, Lavender Z et al. Coronary sinus biomarker sampling compared to peripheral venous blood for predicting outcomes in patients with severe heart failure undergoing cardiac resynchronization therapy: the BIOCRT study. Heart Rhythm 2014;11:2167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–82. [DOI] [PubMed] [Google Scholar]
- 17. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53. [DOI] [PubMed] [Google Scholar]
- 18. Feneon D, Behaghel A, Bernard A, Fournet M, Mabo P, Daubert J-C et al. Left atrial function, a new predictor of response to cardiac resynchronization therapy? Heart Rhythm 2015;12:1800–6. [DOI] [PubMed] [Google Scholar]
- 19. Kloosterman M, Rienstra M, Mulder BA, Van Gelder IC, Maass AH. Atrial reverse remodelling is associated with outcome of cardiac resynchronization therapy. Europace 2016;18:1211–9. [DOI] [PubMed] [Google Scholar]
- 20. Nishikimi T, Saito Y, Kitamura K, Ishimitsu T, Eto T, Kangawa K et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol 1995;26:1424–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.