Abstract
Aims
This registry was created to describe the experience of 76 Italian centres with a large cohort of recipients of multipoint pacing (MPP) capable cardiac resynchronization therapy (CRT) devices.
Methods and results
A total of 507 patients in whom these devices had been successfully implanted were enrolled between August 2013 and May 2015. We analysed: (i) current clinical practices for the management of such patients, and (ii) the impact of MPP on heart failure clinical composite response and on the absolute change in ejection fraction (EF) at 6 months. Multipoint pacing was programmed to ‘ON’ in 46% of patients before discharge. Methods of optimizing MPP programming were most commonly based on either the greatest narrowing of the QRS complex (38%) or the electrical delays between the electrodes (34%). Clinical and echocardiographic follow-up data were evaluated in 232 patients. These patients were divided into two groups according to whether MPP was programmed to ‘ON’ (n = 94) or ‘OFF’ (n = 138) at the time of discharge. At 6 months, EF was significantly higher in the MPP group than in the biventricular-pacing group (39.1 ± 9.6 vs. 34.7 ± 7.6%; P < 0.001). Even after adjustments, early MPP activation remained an independent predictor of absolute increase in LVEF of ≥5% (odds ratio 2.5; P = 0.001). At 6 months, an improvement in clinical composite score was recorded in a greater proportion of patients with MPP-ON than in controls (56 vs. 38%; P = 0.009). On comparing optimal MPP and conventional vectors, QRS was also seen to have decreased significantly (P < 0.001).
Conclusion
This study provides information that is essential in order to deal with the expected increase in the number of patients receiving MPP devices in the coming years. The results revealed different practices among centres, and establishing the optimal programming that can maximize the benefit of MPP remains a challenging issue. Compared with conventional CRT, MPP improved clinical status and resulted in an additional increase in EF.
Clinical Trial Registration
http://www.clinicaltrial.gov/. Unique identifier: NCT02606071.
Keywords: Cardiac resynchronization therapy, Left ventricular pacing configurations, Heart failure, Multipoint pacing, MPP, Optimization
What's new?
Little is known about clinical outcomes and current clinical practices for the management of patients in whom MPP-endowed CRT devices are implanted. This is the first registry to describe a multicentre experience with a large cohort of recipients of MPP-capable CRT-D devices.
The results revealed different practices among centres, and establishing the optimal programming that can maximize the benefit of MPP remains a challenging issue.
After 6 months of MPP, clinical outcomes and LV function were seen to have improved in comparison with those of control subjects.
Introduction
Over the last decade, cardiac resynchronization therapy (CRT) has evolved considerably, becoming established as a treatment option in selected patients with heart failure (HF).1–4 However, the clinical response to CRT remains highly variable and a significant proportion of patients fail to respond positively (∼30% with current technology). Recent studies suggest that multipoint left-ventricular (LV) pacing [MultiPoint™ Pacing (MPP), St JudeMedical, Sylmar, CA] via a LV quadripolar pacing lead5–8 may be an alternative approach that can improve CRT response by simultaneously recruiting a larger volume of myocardium.9–13 However, almost all data in the literature refer to the acute phase after implantation while clinical follow-up data are limited to 44 patients enrolled in a single-centre study.14,15 With the current focus on improved quality and efficiency of healthcare delivery, additional data are of great importance in order to establish whether this benefit is sustained over the long-term.
This prospective, non-randomized, multi-centre, observational study was undertaken with a two-fold aim: (i) to identify current clinical practices in the management of CRT patients in whom MPP-enabled CRT devices are implanted; (ii) to verify the feasibility and the effects of CRT on the clinical status and echocardiographic parameters of recipients of devices with this capability.
Methods
The IRON-MPP registry: patient population and data collection
The IRON-MPP (ClinicalTrials.gov identifier: NCT02606071) is a prospective multicentre, observational registry designed to collect clinical and device data from a large cohort of HF patients treated in clinical practice with a CRT-D device endowed with the ability to deliver MPP. From August 2013 to May 2015, consecutive patients were prospectively enrolled at 76 Italian academic and private cardiology centres homogeneously distributed throughout Italy (see Supplementary material online, Appendix). Centres were asked to collect data before hospital discharge from consecutive patients who had undergone successful CRT-D implantation. Patients enrolled in any device study affecting programming or treatment were excluded. Eligibility for device implantation was based on international guidelines.16
Patients were included regardless of whether they were undergoing a first-device implantation procedure or whether the LV lead was placed as part of an upgrade procedure in patients who already had either a permanent pacemaker or an implantable cardioverter defibrillator. Baseline patient characteristics, procedural data, information on lead positions, electrical measurement values, and optimization methods used were requested in the survey questionnaire. Devices, LV vector combinations, and pacing delays for MPP were individually programmed as per the usual practice of each treating physician.
Enrolled patients gave written informed consent and data were collected in accordance with institutional guidelines on ethics. Patient information was de-identified and all data were entered in a web-based database. The follow-up data presented in this paper were censored at 6 months; however, patients are still being followed-up in the ongoing registry.
Device characteristics and multipoint left-ventricular pacing
Commercially available MPP-capable CRT-D devices (Unify Quadra MP or Quadra Assura MP, St Jude Medical, Sylmar, CA) and leads were implanted in this study. Implantation was performed according to the standard practice of the individual centre. Connected to a quadripolar LV lead (Quartet™ LV lead, St Jude Medical, Sylmar, CA), these devices have the ability to deliver sequential pacing pulses from 2 LV sites (MPP), potentially capturing a larger area and engaging multiple zones in the long axis of the LV.
Two LV pacing vectors (LV1 and LV2) can be selected from the 10 vectors available with the quadripolar systems: six with both cathodes and anodes of the LV lead and the remaining four with an LV lead cathode and the right-ventricular coil anode. Interventricular (LV–RV) and intraventricular (LV–LV) delays are programmable in two different ranges (5–80 ms for delay 1; 5–50 ms for delay 2). Device programming and optimization were left to the discretion of the treating physician.
Follow-up
The variables captured at the 6-month follow-up examination included survival, hospitalization, NYHA functional class, electrocardiographic data, left-ventricular vector combinations and key echocardiographic data. Standard case-report forms were used for data collection. The timing of periodic follow-up examinations was left to the local investigators.
Two-dimensional echocardiography was performed at the baseline and at the 6-month follow-up examination, in order to assess changes in EF. Only patients (n = 232) in whom data were available both at the baseline and at the 6-month follow-up examination were included in the follow-up analysis. These 232 patients were divided into an ‘early MPP activation’ group, with MPP programmed to ‘ON’ at the time of post-implantation discharge (MPP-ON group), and a ‘non-MPP’ group, in whom MPP remained ‘OFF’ or was switched ‘ON’ after hospital discharge (MPP-OFF group). Left-ventricular EF was evaluated by means of Simpson's method and other conventional echocardiographic measurements in strict conformity with echocardiography guidelines.
As part of the protocol of the study, the electrical performance of the LV lead and the occurrence of phrenic nerve stimulation (PNS) were assessed during pacing from all 10 vectors. Programming adjustments, timing of periodic follow-up examinations, and pharmacological therapy were left to the local investigators.
Outcome measures
Response to CRT was evaluated through the HF clinical composite score (CCS) and the relative change in LV ejection fraction at 6 months. On the basis of the CCS, we classified patients into one of three response groups: worsened, improved, or unchanged according to a predefined scheme.17 Patients were classified as worsened if they died or were hospitalized for worsening HF or had an increase in NYHA functional class. Patients were classified as improved if they had not worsened and had a decrease in NYHA functional class. Patients who had neither worsened nor improved were classified as unchanged. An increase in LVEF of 5% or more was used in this study as an objective measure of cardiac structural improvement and response to CRT and has been used in previous trials.18–22
Hospitalization for HF was defined as admission to a healthcare facility for more than 24 h with symptoms of congestive HF and subsequent treatment for HF.
QRS analysis
Spontaneous and paced QRS duration were measured by means of resting 12-lead ECG and analysed by two independent investigators. Bundle-branch block was defined on the basis of the World Health Organization Task Force criteria.23 QRS duration was measured from the first deflection until return to baseline in any lead of the 12-lead surface ECG. The paced QRS duration was measured from the beginning of the ventricular pacing spike to the end of the QRS complex as the maximum paced QRS duration in any of the 12 ECG leads. In each patient, we selected the minimum QRS duration during MPP vs. conventional biventricular pacing.
Statistical analysis
Categorical variables describing the patient population are expressed as absolute numbers and percentages, while continuous variables are shown as means [with standard deviations (SD)] or medians (with quartiles) for continuous variables. Non-continuous variables expressed as proportions were compared by means of χ2 analysis with Yate correction or Fisher's exact test. Normally distributed, continuous variables were compared by means of the two-sample t-test for independent variables or paired t-test for paired data. Non-parametric Wilcoxon–Mann–Whitney and Wilcoxon signed rank (for paired data) tests were used for non-normally distributed variables.
Echocardiographic analysis was performed on the basis of the intention-to-treat principle, and only patients with 6 months follow-up data were included.
Multivariate logistic regression analysis was used to estimate the independent effect (Odds Ratio) of MPP activation on absolute increase in EF at 6 months, with selected confounder parameters (significant association in univariate analysis) and with their potential interactions. All P-values were two-sided, and a P-value of <0.05 was considered to indicate statistical significance.
The manuscript was written by the principal investigator (G.B.F.), and the accuracy of the data reported was confirmed by all the authors who had full access to the data.
Results
The IRON-MPP registry enrolled 507 patients in 76 centres over a period of 22 months, when the database was frozen for interim analysis. Baseline characteristics of the entire study population are summarized in Table 1.
Table 1.
Baseline characteristics and procedural data
| Patients, n | 507 |
| Age, years | 70 ± 9.4 |
| Male sex, n (%) | 399 (79) |
| Left-ventricular ejection fraction (%) | 28.1 ± 5.8 |
| Coronary artery disease, n (%) | 228 (45) |
| History of AF | 118 (23) |
| Hypertension | 221 (44) |
| Diabetes Mellitus | 123 (24) |
| QRS duration, ms | 160 ± 25 |
| LBBB, n (%) | 380 (75) |
| RBBB, n (%) | 50 (10) |
| IVCD, n (%) | 77 (15) |
| NYHA Functional Class | |
| Class II, n (%) | 209 (41) |
| Class III, n (%) | 283 (56) |
| Class IV, n (%) | 15 (3) |
| Procedural Data | |
| Procedural Time, mina | 113 ± 43 |
| Fluoro Time, mina | 21 ± 14 |
| MPP LV pacing threshold, Va,b | 1.2 ± 0.8 |
| Final LV lead location (LAO 30° view) | |
| Anterior, n (%) | 15 (3) |
| Anterolateral, n (%) | 66 (13) |
| Lateral, n (%) | 259 (51) |
| Posterolateral, n (%) | 137 (27) |
| Posterior, n (%) | 30 (6) |
Values are mean ± SD unless otherwise specified.
IVCD, intraventricular conduction delay; LBBB, left bundle-branch block; LV, left ventricular; RBBB, right bundle-branch block; V, volts; MPP, multipoint pacing.
aOf successfully implanted patients.
bAverage of the two optimal pacing configurations.
The contribution of each centre was variable and enrolment ranged from 1 to 57 patients per centre (median 3, IQR 1.7–6). The cohort was 79% male, and age ranged from 32 to 89 years. The majority of left-ventricular leads were placed in a lateral (51%) or posterolateral (27%) position, whereas the right-ventricular lead was predominantly placed in the apex (52%).
Standards of care in multipoint left-ventricular pacing
The LV capture thresholds (CT) were measured in at least 2 of the 10 available configurations in all patients. Considering a CT < 5 V for at least two vectors without PNS at high pacing output, the MPP function was programmable in 97% of patients. At a lower CT cut-off (<3 V) for both vectors, MPP remained programmable in 87% of patients (Table 2). Phrenic nerve stimulation was reported in 93 patients in at least one configuration, but in only 17 patients (4%) was MPP not programmable owing to PNS.
Table 2.
MPP programmability based on pacing thresholds and PNS
| MPP Programmability | |
|---|---|
| CT in at least two MPP vectors <5 V | 98.6% |
| CT in at least two MPP vectors < 5 V without PNSa | 97.4% |
| CT in at least two MPP vectors < 3 V | 88.5% |
| CT in at least two MPP vectors < 3 V without PNSa | 87.5% |
aWithout PNS at high pacing output in these two vectors.
CT, capture threshold; MPP, multipoint pacing; PNS, phrenic nerve stimulation; V, volts.
Before discharge from hospital, MPP was programmed to ‘OFF’ in 273 patients (54%) and remained inactive for the entire follow-up except in two patients in whom the function was activated before the follow-up examination (in all analyses these patients were considered to have been in the ‘MPP-OFF’ group for the complete follow-up period). The mean LV capture threshold (at 0.5 ms) of the two lower MPP vectors was 0.9 ± 0.6 V and 1.5 ± 1.0 V for LV1 and LV2, respectively. By the 6-month follow-up evaluation, in 39 out of 232 patients (17%) initially programmed to conventional biventricular pacing, the MPP feature was switched to ‘ON’.
The choice and the timing of switching on MPP capabilities varied widely among centres. In the early post-implantation phase, MPP ‘OFF’ was regarded as the best option by 30% of the centres; 58% of the centres considered that MPP ‘ON’ should be preferred in the early post-implantation period while in the remaining centres (12%) both options were equally implemented.
In the MPP-ON group, information on acute MPP optimization after implantation was available in 199 out of 233 patients and device optimization was performed in 182 out of 199 patients (91%). In 38% of patients, MPP was programmed according to the configuration that resulted in the greatest narrowing of the QRS complex. In 34% the electrical delays between the electrodes were calculated through the automatic device algorithm (CRT Toolkit™ St Jude Medical, Sylmar, CA, USA) and the device was programmed accordingly. In 3% of patients optimal delays and configurations were based on echocardiographic evaluation, and few centres (10%) chose to use a pressure guidewire for haemodynamic measurements. Figure 1 shows the distribution of the different methods of MPP optimization prior to discharge. Additional data on final MPP pacing configurations and interventricular delays are reported in Supplementary material online, Appendix Tables A and B.
Figure 1.
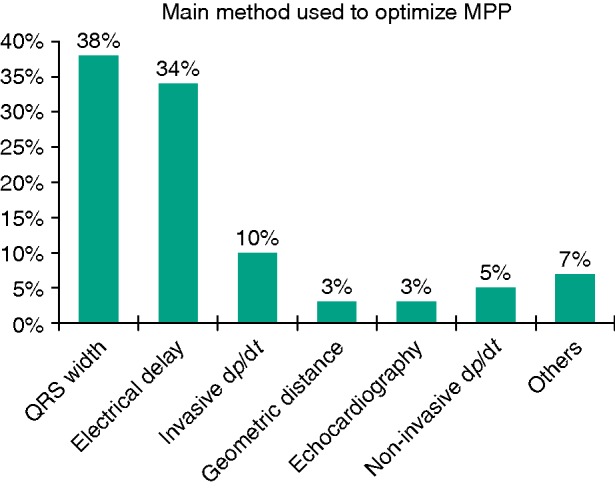
Distribution of the different methods of MPP optimization prior to discharge.
Influence of the pacing mode on QRS duration
With regard to the best QRS shortening, we analysed the adjunctive value of MPP compared with the best conventional biventricular configuration. In the 108 patients in whom both MPP and conventional biventricular configurations were evaluated, MPP significantly reduced QRS duration in comparison with conventional biventricular pacing (134.8 ± 26 ms vs. 141.3 ± 25 ms; P < 0.001).
Six-month follow-up
At the 6-month follow-up examination, both clinical and echocardiographic data were available in 232 patients (46%); these patients (median three patients per centre, IQR 2–8) are described in the follow-up analysis of this manuscript. Of these 232 patients, 94 were enrolled in the MPP-ON group and 138 were enrolled in the MPP-OFF group. The characteristics of patients without follow-up data were similar to those of the remaining population.
Patients in the MPP-ON and MPP-OFF groups did not differ significantly in terms of baseline clinical and echocardiographic parameters and aetiology of their cardiomyopathy (Table 3). However, MPP was more likely to be activated in patients with wider QRS at baseline.
Table 3.
Baseline characteristics and outcomes of patients with follow-up data
| MPP-OFF (n = 138) | MPP-ON (n = 94) | P-value | |
|---|---|---|---|
| Age, years | 71 ± 10.0 | 69 ± 11.0 | 0.10 |
| Male sex, n (%) | 109 (79) | 76 (81) | 0.40 |
| Left-ventricular EF (%) | 28.1 ± 6.0 | 28.2 ± 5.9 | 0.9 |
| QRS duration, ms | 157 ± 23 | 164 ± 3 | 0.03 |
| LBBB, n (%) | 108 (78) | 67 (71) | 0.27 |
| NYHA Class (II/III/IV), (%) | 45/53/2 | 30/68/2 | 0.10 |
| Coronary artery disease, n (%) | 65 (47) | 39 (41) | 0.2 |
| 6-month follow-up | |||
| Δ Left-ventricular EF, (%)a | 6.5 ± 8.2 | 10.7 ± 10.2 | <0.001 |
| Ejection fraction (%) | 34.7 ± 7.6 | 39.0 ± 9.6 | 0.001 |
| Increase in EF ≥5% (%)a | 58 | 74 | 0.012 |
| CCS score (improved; unchanged; worsened), (%) | 38/47/15 | 56/35/9 | 0.02 |
Values are mean ± SD unless otherwise specified.
LBBB, left bundle branch block; EF, ejection fraction; V, volts; MPP, multipoint pacing.
a6-month vs. baseline.
During a median of 6 months' follow-up, the MPP feature was switched ‘ON’ in 17% of the patients in the ‘MPP-OFF’ group, whereas nine patients in the MPP-ON group were reprogrammed to ‘MPP-OFF’. At 6 months, the average percentage of ventricular pacing was 96.7% in the MPP-OFF group and 96.4% in the MPP-ON group (P = 0.85).
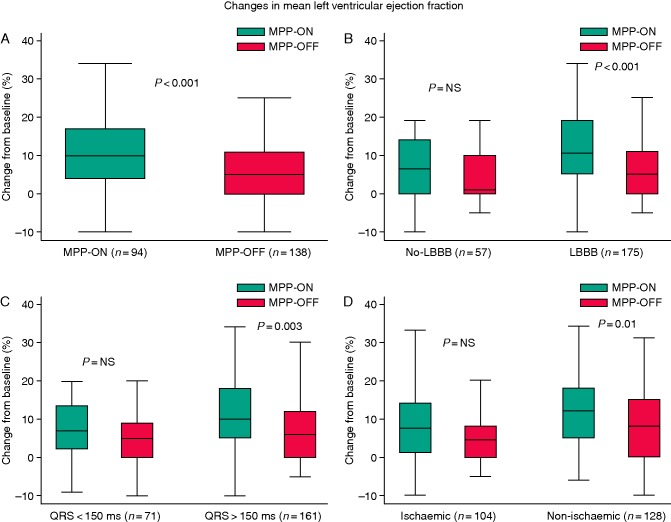
Between study enrolment and the 6-month follow-up visit, LVEF increased from 28.2% ± 5.9 to 39.0% ± 9.6% in the MPP-ON group (P < 0.001 vs. baseline) and from 28.1% ± 6.0 to 34.7% ± 7.6% in the MPP-OFF group (P < 0.001 vs. baseline). At 6 months, the MPP-ON group had a significantly higher mean left-ventricular EF than the biventricular-pacing group (Figure 2), with an absolute difference of 4.2% between the groups in the change from baseline (relative difference from baseline of 15% (Table 3).
Figure 2.
Six-month echocardiographic follow-up. Changes in mean left ventricular ejection fraction between baseline and 6-month follow-up examination according to MPP ‘OFF’ vs. ‘ON’(A); QRS morphology (B); QRS duration (C); and aetiology (D). P-values are for comparison between groups; I bars indicate 95% confidence intervals.
Using logistic regression, univariate predictors of absolute increase in LVEF of ≥5% at 6 months (Table 4) were analysed. Even after adjustments, early MPP activation remained independent predictor of absolute increase in LVEF of ≥5% with odds ratio of 2.5 [(95% CI 1.3–4.9); P = 0.001]. Significant factors included also left-ventricular bundle block, baseline EF, and coronary artery disease (Table 5).
Table 4.
Baseline characteristics of patients with or without 6-month absolute increase in left ventricular ejection fraction of ≥5%a
| EF < 5% (n = 82) | EF ≥ 5% (n = 150) | P-value | |
|---|---|---|---|
| Age, years | 70.4 ± 9.2 | 69.5 ± 10.7 | 0.5 |
| Male sex, n (%) | 72 (87.8) | 113 (75.3) | 0.03 |
| Left-ventricular EF (%) | 31.21 ± 4.4 | 26.6 ± 6.0 | 0.001 |
| QRS duration, ms | 155 ± 29 | 162 ± 25 | 0.05 |
| LBBB, n (%) | 54 (65) | 121 (81) | 0.01 |
| Coronary artery disease, n (%) | 47 (58) | 57 (38) | 0.004 |
| NYHA Class (II; III; IV), (%) | 1/38/58/2 | 0/38/59/2 | 0.7 |
| Early MPP-ON activation | 24 (30) | 70 (47) | 0.012 |
Table 5.
Multivariate predictors of 6-month absolute increase in left-ventricular ejection fraction of ≥5%a
| OR | (95% CI) | P-value | |
|---|---|---|---|
| LBBB | 1.005 | 1.001–1.010 | 0.017 |
| Left-ventricular EF | 0.851 | 0.797–0.909 | 0.001 |
| Coronary artery disease | 0.464 | 0.247–0.874 | 0.017 |
| Early MPP-ON activationb | 2.506 | 1.278–4.914 | 0.008 |
A 6-month improvement in HF clinical composite score was observed in 104 patients (45%). Twenty-eight patients (12%) were judged to have worsened, and the remaining 100 were classified as unchanged (43%). An improvement in clinical composite score at 6 months was seen in a greater proportion of patients with MPP-ON (56 vs. 38%; P = 0.009) than in controls. In the MPP group, 9% of patients were worsened vs. 15% in the CRT group. The response to CRT evaluated at the 6-month follow-up examination, measured according to the aforementioned clinical composite score, is reported in Figure 3 for the two study groups.
Figure 3.
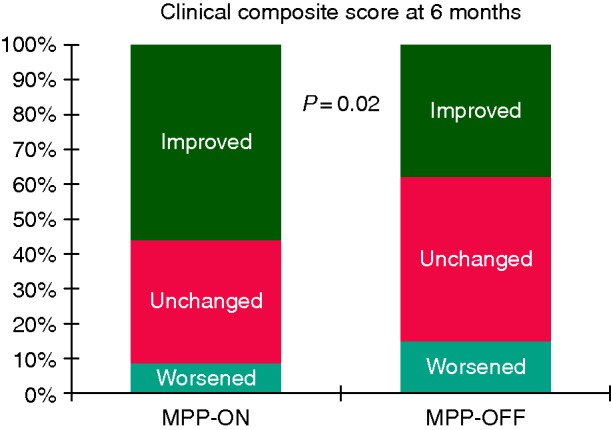
Distribution of ‘improved’, ‘unchanged’, and ‘worsened’ patients as defined on the basis of Packer's clinical composite score, between baseline and 6-month follow-up according to MPP ‘OFF’ vs. ‘ON’.
Discussion
Early experiences strongly support the finding that MPP improves acute haemodynamic response in comparison with conventional biventricular pacing.24 However, the clinical value of MPP has yet to be tested in larger prospective studies. Furthermore, no information is available on current practices for the management of patients who receive MPP-capable devices. This prospective, multicentre clinical experience is the first to provide a unique snapshot of the characteristics and management of patients in whom MPP devices are implanted in cardiology practices across Italy. The main results of this study can be summarized as follows: (i) MPP reduced the degree of ventricular dyssynchrony (as evidenced by shortening of the QRS interval), and this effect was accompanied by an increase in left-ventricular ejection fraction; (ii) a QRS-based strategy was the option adopted by most cardiologists to optimize MPP programming, whereas few chose echocardiographic or haemodynamic optimization; (iii) in 46% of patients the MPP function was activated early (i.e. before hospital discharge) and this percentage increased to 53% at 6 months.
Multipoint pacing optimization: keep it simple?
The beneficial effect of MPP is thought to depend on appropriate LV pacing vectors and on inter-and intra-ventricular timing delays. Owing to the relatively short clinical experience with MPP-capable devices and considering the high number of settings available, there is, as yet, no agreement on the optimal setting of sequence and delays in LV multipoint stimulations. In the present study, MPP programming was left to the choice of the investigators; we therefore provide the first picture of how these devices are optimized in clinical practice.
In this series, investigators used different strategies for guiding MPP optimization. No echocardiographic-based optimization was performed in the vast majority of patients. This is not surprising; although echocardiography is widely used to optimize CRT programming, it is time-consuming, especially in view of the high number of MPP configurations. Some investigators (34%) focused on the latest LV pacing site activated, which is potentially associated with improved CRT response;25 the integrated electrical delay measurement software (CRT Toolkit™ St Jude Medical, Sylmar, CA, USA) might facilitate measurement of the electrical activation of the four LV electrodes, thereby allowing the earliest and latest LV pole to be identified. Others used a more anatomic approach in which MPP vectors were empirically chosen to pace both with the distal and the most proximal LV electrode. Thibault et al.9 were the first to show that this approach is frequently associated with the best CRT response. Similarly, on using a pressure–volume loop to optimize MPP, Pappone et al.10 found that the use of the widest anatomically separated vectors is associated with improved CRT response. In the present series, the most common method of MPP optimization was based on the configuration that resulted in the greatest narrowing of the QRS complex. QRS morphology and the reduction of its width has been, until now, one of the main goals of CRT optimization. However, in MPP patients the effects of such optimization on chronic remodelling remain unknown. Notably, few centres chose an approach guided by invasive or non-invasive haemodynamic measurements. This is an important finding since MPP-related improved clinical and echocardiographic response has to date been observed mainly after invasive haemodynamic optimization. Novel sophisticated techniques seem promising means of optimizing CRT response but need adequate training and refinement; it is likely that investigators preferred simpler and less time-consuming strategies for guiding MPP optimization.26
The wide range of vectors and interval combinations does not permit univocal programming of an MPP device and it is likely that the final choice was also based on LV pacing thresholds and on the optimal safety ratio with regard to PNS. Interestingly, despite the theoretical CT and PNS issues, MPP was programmable in at least 90% of patients. In order to simplify the utilization of this tool, it is important to establish clinical, anatomical, and electrical parameters for the optimization of device programming and to find reasonable empirical MPP settings. It is reasonable to suppose that any improvement in this field will translate into improved CRT response, and therefore into improved cost-effectiveness.
Multipoint pacing: treat or wait?
The choice and the timing of switching on the MPP function varied widely among centres. Turning MPP ‘ON’ in the early post-implantation phase was regarded as the best option by 58% of centres, while 30% of centres left the function ‘OFF’ in the majority of patients during the 6 months of follow-up. The reasons behind this choice may be different. A possible explanation might be that physicians are concerned about premature battery depletion, though the real impact of MPP on battery longevity remains to be determined. Thus, some clinicians might prefer a watch and wait approach activating the MPP only in non-responders to conventional CRT rather than turning it on soon after implantation. This strategy is currently under investigation (MOre REsponse on Cardiac Resynchronization Therapy with MultiPoint Pacing—the MORE-CRT MPP study, ClinicalTrials.gov identifier: NCT02006069). Differences in the characteristics of patients, physicians, and hospitals or economic limitations may also account for these disparities.
The European CRT survey demonstrated considerable ‘off-label’ use of CRT devices in patients with a non-LBBB pattern or a narrow QRS.27 There is a growing body of evidence that MPP might be most beneficial in patients who are unlikely to benefit from standard CRT, including those with myocardial scarring, a type I LV activation pattern, and less marked QRS prolongation.28,29 Conversely, we found that 6-month echocardiographic outcomes were statistically improved in LBBB patients, in patients with non-ischaemic cardiomyopathy, and in patients with QRS ≥ 150 ms. A trend towards improved outcomes was also seen in their counterparts but it did not reach statistical significance. It is likely that our study lacked the power to reveal differences, as a result of the relatively small number of patients with follow-up data. Further studies are needed to address this issue.
With regard to CRT response, in a preliminary single-centre experience, Pappone et al.14,15 found that MPP improved echocardiographic and clinical response to CRT. Extending the observations by Pappone et al. to a large multi-centre experience, we observed that MPP pacing resulted in a significant 6-month improvement in absolute LVEF compared with conventional biventricular pacing. If these observations are confirmed in larger series, we feel that early MPP activation should be undertaken in order to improve the prognosis of CRT patients.
Limitations
This study has potential limitations: (i) although our data were collected in a single European country, they were nevertheless derived from a multi-centre experience that brought together a high number of centres with different workloads and reflecting different behaviours. Thus, the mix of 76 participating centres might provide a sufficiently representative sample of current clinical practice in Italy. (ii) The registry was entirely voluntary and did not employ a quality-control system for data collection nor an independent system of monitoring. (iii) Clinical and echocardiographic outcome analysis is inherently limited by the non-randomized design of the study, and is therefore prone to the effects of confounding variables even though no differences were detected between the groups in terms of clinical characteristics. (iv) The implantation volume varied considerably among the participating centres, and 6-month follow-up data were available for a limited number of patients.
Although the existing registry data have inherent limitations, this study provides the most detailed clinical record available and yields a sufficiently representative sample of current clinical practice in Italy.
Conclusions
This study provides information that is essential in order to face the expected increase in the number of patients treated with MPP in the coming years. The results revealed different practices among centres, and establishing the optimal programming in order to maximize the benefit of MPP remains a challenging issue.
Compared with conventional CRT, MPP reduced QRS duration. Furthermore, MPP resulted in an additional increase in ejection fraction and clinical composite response. Further investigation is required in order to better determine which patient populations benefit most from these devices and to assess whether the implantation of MPP-enabled CRT devices is superior and should become the standard of care.
Supplementary material
Funding
This was an independent study. No external funding was achieved for this project. Funding to pay the Open Access publication charges for this article was provided by St. Jude Medical Italia.
Conflict of interest: G.F. received speaker's fee from St Jude Medical. A.C. and F.Z. received fee from Medtronic, St Jude Medical, Boston Scientific, Biotronik, and Sorin. L.S. received speaker's fee from St Jude Medical, Boston Scientific, and Biotronik. The remaining authors have no disclosures.
Supplementary Material
Acknowledgements
The contribution of the individual centres to the registry is gratefully acknowledged. We also wish to express our gratitude to Eliana Cipolla and Enrico Romano for their assistance in data analysis, the interpretation of results, and the editing of tables/figures.
References
- 1. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T et al. . Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L et al. . Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 3. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C et al. . Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873–80. [DOI] [PubMed] [Google Scholar]
- 4. Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B et al. . Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003;289:2685–94. [DOI] [PubMed] [Google Scholar]
- 5. Forleo GB, Della Rocca DG, Papavasileiou LP, Molfetta AD, Santini L, Romeo F. Left ventricular pacing with a new quadripolar transvenous lead for CRT: early results of a prospective comparison with conventional implant outcomes. Heart Rhythm 2011;8:31–7. [DOI] [PubMed] [Google Scholar]
- 6. Sperzel J, Dänschel W, Gutleben KJ, Kranig W, Mortensen P, Connelly D et al. . First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace 2012;14:365–72. [DOI] [PubMed] [Google Scholar]
- 7. Forleo GB, Di Biase L, Bharmi R, Dalal N, Panattoni G, Pollastrelli A et al. . Hospitalization rates and associated cost analysis of cardiac resynchronization therapy with an implantable defibrillator and quadripolar vs. bipolar left ventricular leads: a comparative effectiveness study. Europace 2015;17:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomassoni G, Baker J, Corbisiero R, Love C, Martin D, Niazi I et al. . Postoperative performance of the Quartet® left ventricular heart lead. J Cardiovasc Electrophysiol 2013;24:449–56. [DOI] [PubMed] [Google Scholar]
- 9. Thibault B, Dubuc M, Khairy P, Guerra PG, Macle L, Rivard L et al. . Acute haemodynamic comparison of multisite and biventricular pacing with a quadripolar left ventricular lead. Europace 2013;15:984–91. [DOI] [PubMed] [Google Scholar]
- 10. Pappone C, Ćalović Z, Vicedomini G, Cuko A, McSpadden LC, Ryu K et al. . Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure-volume loops in cardiac resynchronization therapy patients. Heart Rhythm 2014;11:394–401. [DOI] [PubMed] [Google Scholar]
- 11. Zanon F, Baracca E, Pastore G, Fraccaro C, Roncon L, Aggio S et al. . Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Heart Rhythm 2015;12:975–98. [DOI] [PubMed] [Google Scholar]
- 12. Rinaldi CA, Leclercq C, Kranig W, Kacet S, Betts T, Bordachar P et al. . Improvement in acute contractility and hemodynamics with multipoint pacing via a left ventricular quadripolar pacing lead. J Interv Card Electrophysiol 2014;40:75–80. [DOI] [PubMed] [Google Scholar]
- 13. Osca J, Alonso P, Cano O, Miro V, Tello MJ, Olagüe J et al. . The use of multisite left ventricular pacing via quadripolar lead improves acute haemodynamics and mechanical dyssynchrony assessed by radial strain speckle tracking: initial results. Europace 2015;18:560–7. [DOI] [PubMed] [Google Scholar]
- 14. Pappone C, Ćalović Ž, Vicedomini G, Cuko A, McSpadden LC, Ryu K et al. . Multipoint left ventricular pacing in a single coronary sinus branch improves mid-term echocardiographic and clinical response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2015;26:58–63. [DOI] [PubMed] [Google Scholar]
- 15. Pappone C, Ćalović Ž, Vicedomini G, Cuko A, McSpadden LC, Ryu K et al. . Improving cardiac resynchronization therapy response with multipoint left ventricular pacing: twelve-month follow-up study. Heart Rhythm 2015;12:1250–8. [DOI] [PubMed] [Google Scholar]
- 16. Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H et al. . Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace 2007;9:959–98. [DOI] [PubMed] [Google Scholar]
- 17. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–82. [DOI] [PubMed] [Google Scholar]
- 18. Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D et al. . Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med 2002;346:1357–65. [DOI] [PubMed] [Google Scholar]
- 19. Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E et al. . Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol 2003;92:1238–40. [DOI] [PubMed] [Google Scholar]
- 20. Bleeker GB, Bax JJ, Fung JWH, van der Wall EE, Zhang Q, Schalij MJ et al. . Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol 2006;97:260–3. [DOI] [PubMed] [Google Scholar]
- 21. Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail 2009;2:189–96. [DOI] [PubMed] [Google Scholar]
- 22. Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD et al. . Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation 2010;121:1985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willems J, Robles de Medina E, Bernard R, Coumel P, Fisch C, Krikler D et al. . Criteria for intraventricular conduction disturbances and pre-excitation: World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol 1985;5:1261–75. [DOI] [PubMed] [Google Scholar]
- 24. Rinaldi CA, Burri H, Thibault B, Curnis A, Rao A, Gras D et al. . Review of multisite pacing to achieve cardiac resynchronization therapy. Europace 2015;17:7–17. [DOI] [PubMed] [Google Scholar]
- 25. Zanon F, Baracca E, Pastore G, Fraccaro C, Roncon L, Aggio S et al. . Determination of the longest intrapatient left ventricular electrical delay may predict acute hemodynamic improvement in patients after cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2014;7:377–83. [DOI] [PubMed] [Google Scholar]
- 26. Gras D, Gupta MS, Boulogne E, Guzzo L, Abraham WT. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol 2009;32 (Suppl. 1):S236–9. [DOI] [PubMed] [Google Scholar]
- 27. Sciaraffia E, Dagres N, Hernandez-Madrid A, Proclemer A, Todd D, Blomström-Lundqvist C. Do cardiologists follow the European guidelines for cardiac pacing and resynchronization therapy? Results of the European Heart Rhythm Association survey. Europace 2015;17:148–51. [DOI] [PubMed] [Google Scholar]
- 28. Sohal M, Shetty A, Niederer S, Lee A, Chen Z, Jackson T et al. . Mechanistic insights into the benefits of multisite pacing in cardiac resynchronization therapy: The importance of electrical substrate and rate of left ventricular activation. Heart Rhythm 2015;12:2449–57. [DOI] [PubMed] [Google Scholar]
- 29. Ginks MR, Shetty AK, Lambiase PD, Duckett SG, Bostock J, Peacock JL et al. . Benefits of endocardial and multisite pacing are dependent on the type of left ventricular electric activation pattern and presence of ischemic heart disease: insights from electroanatomic mapping. Circ Arrhythm Electrophysiol 2012;5:889–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.