Abstract
Background
Aromatase inhibitors (AIs) have been associated with cardiovascular disease in adjuvant randomized controlled trials (RCTs) comparing these drugs to tamoxifen. However, it is unclear whether this risk is real or due to cardioprotective effects of tamoxifen. To address this question, we conducted a systematic review and meta-analysis of all RCTs of AIs and tamoxifen in adjuvant and extended adjuvant setting.
Patients and methods
We searched PubMed, Embase (OVID), Cochrane CENTRAL, WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov from inception to June 2016 for all RCTs comparing cardiovascular and cerebrovascular safety of AIs to tamoxifen, AIs to placebo or no-treatment, or tamoxifen to placebo or no-treatment in the adjuvant or extended adjuvant setting. Relative risks (RRs) were pooled using DerSimonian and Laird random-effects models with analyses stratified by RCT design.
Results
A total of 19 RCTs were included in the meta-analysis (n = 62 345). In the adjuvant setting, AIs were associated with a 19% (RR: 1.19, 95% confidence interval [CI]: 1.07–1.34) increased risk of cardiovascular events compared with tamoxifen. AIs were not associated with an increased risk compared with placebo in the extended-adjuvant setting (RR: 1.01, 95% CI: 0.85–1.20). In the adjuvant setting, tamoxifen was associated with a 33% (RR: 0.67, 95% CI: 0.45–0.98) decreased risk compared with placebo or no-treatment. The results from extended adjuvant RCTs comparing tamoxifen to placebo were inconclusive but suggestive of a small protective effect (RR: 0.91, 95% CI: 0.77–1.07).
Conclusions
The increased risk of cardiovascular events with AIs relative to tamoxifen is likely the result of cardioprotective effects of the latter. This new evidence should be considered when assessing the benefits and risks of AIs in the treatment of breast cancer.
Keywords: breast cancer, aromatase inhibitors, tamoxifen, anastrozole, letrozole, exemestane
Introduction
Third generation aromatase inhibitors (AIs) have replaced tamoxifen as the mainstay treatment of estrogen-receptor (ER) positive breast cancer in postmenopausal women [1]. According to a comprehensive individual patient data meta-analysis, AIs significantly reduce breast cancer recurrence and breast cancer-related mortality and increase overall survival in comparison with tamoxifen [2]. However, there have been concerns regarding the cardiovascular safety of AIs. Indeed, several adjuvant RCTs comparing AIs with tamoxifen have indicated that AIs increase the risk of cardiovascular disease [3–6] and as a result, current guidelines indicate that AIs are associated with increased ischemic heart disease [7].
To date, several systematic reviews and meta-analyses have compared the cardiovascular safety of AIs to tamoxifen [8–13], with several of these reporting increased risks with AIs [8–10]. However, previous clinical studies have demonstrated that tamoxifen may have favorable cardiovascular effects, including reducing total cholesterol levels and low-density lipoprotein cholesterol (LDL-C) levels, increasing high-density lipoprotein cholesterol levels, and reducing C-protein and fibrinogen levels [14–19]. Thus, the observed increased risk of cardiovascular events associated with AIs in RCTs comparing AIs to tamoxifen may be due to cardioprotective effects of the latter.
Given the known benefits of AI therapy [2], there is an urgent need to better understand the cardiovascular safety of these drugs. We therefore conducted a systematic review and meta-analysis of RCTs to determine whether AIs are associated with an increased risk of cardiovascular events and if present, whether this association is due to cardioprotective effects of tamoxifen.
Me thods
Search strategy
We systematic searched PubMed, Embase, Cochrane CENTRAL, WHO International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov from inception to March 2015 for all RCTs consisting of tamoxifen or AIs. The electronic search was updated in June 2016. Medical Subject Heading (MeSH) terms were used in PubMed and Cochrane CENTRAL, EMTREE terms in Embase, and keyword search terms for tamoxifen and AIs (including generic and brand names) in all databases. In PubMed and Embase, the BMJ RCT filter that optimizes sensitivity and specificity was applied to restrict inclusion to RCTs [20]. The search was also restricted to articles published in English. The detailed search strategy of each electronic database is shown in supplementary Tables 1–5 (available at Annals of Oncology online). Manual searches of the bibliographies of previous systematic reviews and relevant RCTs were conducted to retrieve additional RCTs that may not have been identified in our electronic search.
Study selection
The title and abstracts of identified publications were screened independently by two reviewers (FKK and SQ), with any publication deemed potentially relevant by either reviewer carried forward to full-text evaluation. Disagreements during full-text review were resolved by consensus or, when necessary, by a third independent reviewer (LA).
We restricted inclusion to phase III RCTs examining third generation AIs and tamoxifen among postmenopausal women with a diagnosis of breast cancer. These RCTs consisted of adjuvant phase III RCTs comparing AIs to tamoxifen, extended-adjuvant RCTs comparing AIs or tamoxifen to placebo or no-treatment, and adjuvant and extended adjuvant RCTs comparing tamoxifen to placebo or no-treatment. We only included studies if cardiovascular or cerebrovascular adverse events were reported.
We excluded phase I and II trials of AIs and tamoxifen, RCTs of first and second-generation AIs or raloxifene, RCTs comparing third generation AIs in combination with other adjuvant therapy including radiation therapy or chemotherapy, cancer prevention RCTs, and RCTs administered in premenopausal women (defined as any study where premenopausal population was greater than 50% of the study population). In addition, we also excluded RCTs that reported the combined results of RCTs, those that included non-cardiovascular events as part of their composite endpoints, and those published in a language other than English. Finally, we excluded RCTs where the primary indication for use of adjuvant hormonal therapy was not breast cancer (e.g. polycystic ovarian syndrome, ovulation induction, and uterine adenomyosis).
Data extraction
Data extraction was conducted independently by two reviewers (FKK and SQ) using a standardized, pilot-tested data extraction form. Disagreements between reviewers were resolved by consensus or by a third reviewer (LA). For each RCT, the following data were extracted: year of publication, total number of randomized patients, number of patients included in analysis, dosage, and duration of follow-up time. We also extracted the following baseline demographic and clinical characteristics: mean age, proportion of postmenopausal women, proportion of node-positive patients, proportion of ER/progesterone-receptor positive patients, tumor size, and previous breast cancer therapy (chemotherapy, radiation therapy, and mastectomy). Count data for all cardiovascular and cerebrovascular events were extracted from included RCTs. When multiple follow-up periods were reported for a given RCT, we selected the trial with the most comprehensive reporting of cardiovascular and cerebrovascular events and/or the longest follow-up reported.
Quality assessment
The quality of each included RCT was assessed using the Cochrane Collaboration’s tool for assessing risk of bias [21]. Each RCT was evaluated for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Potential conflicts of interest were determined by considering funding sources. Each domain was assigned a “high,” “low,” or “unclear” risk of bias independently by two reviewers (FKK and SQ), with disagreements adjudicated by a third reviewer (LA).
Statistical analysis
The cardiovascular endpoints reported in RCTs of AIs and tamoxifen are presented in supplementary Table 6 (available at Annals of Oncology online) and the definition of composite endpoints and corresponding counts that we used in the quantitative analysis are reported in supplementary Table 7 ((available at Annals of Oncology online). Where possible composite endpoints of cardiovascular disease reported in RCTs were used as the definition of cardiovascular events in the quantitative analysis. For trials for which composite endpoints were not reported, cardiovascular events were combined (excluding hypertension, hypercholesterolemia, and thromboembolism). Cardiovascular death was not pooled with ischemic heart disease when these endpoints were reported separately as more than half of cardiovascular deaths are attributed to ischemic heart disease, and thus these events are not mutually-exclusive [22]. Cardiovascular death was used to define cardiovascular events when only this outcome was reported. Similarly, cerebrovascular death was not combined with stroke or transient ischemic attack when reported separately. We conducted secondary analysis using the outcome of ischemic heart disease. Similar to cardiovascular events, in the absence of a composite endpoint of ischemic heart disease, we combined myocardial infarction and angina (for RCTs that reported both events separately). Finally, we conducted sensitivity analyses for this endpoint using myocardial infarctions only for such trials since the occurrences of these two events are not mutually exclusive.
Data were meta-analyzed across RCTs to obtained pooled relative risks (RRs) and corresponding 95% confidence intervals (CIs) using DerSimonian and Laird random-effects models with inverse variance weighting [23]. All analyses were stratified by RCT design: (i) adjuvant RCTs of upfront AIs in comparison to upfront tamoxifen, (ii) sequential treatment with tamoxifen and AIs (or vice versa) in comparison to tamoxifen, (iii) sequential treatment with AIs and tamoxifen (or vice versa) in comparison to AIs, (iv) extended adjuvant RCTs comparing AIs to placebo, (v) adjuvant RCTs comparing tamoxifen to placebo or no treatment, and (vi) extended adjuvant RCTs comparing tamoxifen to placebo. The amount of heterogeneity across the RCTs was estimated using the I2 statistic [24]. To examine the impact of our choice of meta-analytic model, we conducted sensitivity analyses using the fixed-effects models with inverse variance weighting. A continuity correction was applied in RCTs with zero events [25]. All statistical analyses were conducted using R metafor package [26].
Results
Search results
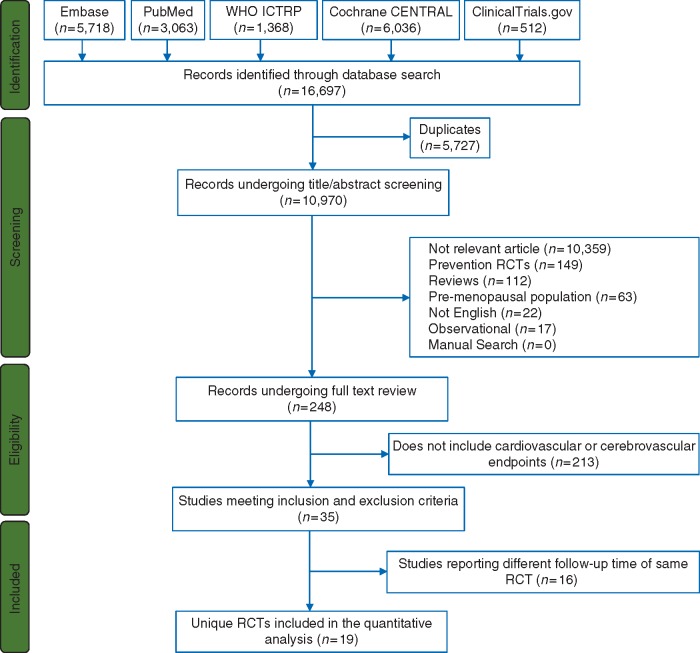
The flow diagram for our electronic search strategy and study selection is shown in Figure 1. Our electronic search identified 16 697 potentially relevant publications. After removing duplicates and screening titles, abstracts, and full-texts, we identified 35 publications corresponding to 19 different RCTs that met our inclusion criteria and were included in our systematic review and meta-analysis.
Figure 1.
PRISMA flow diagram describing systematic search for RCTs of aromatase inhibitors and tamoxifen. RCTs, randomized controlled trials.
Study and patient characteristics
The study design and population characteristics of included RCTs are shown in Table 1. The mean age ranged across RCTs from 55 to 71 years. Most of the RCTs were completely restricted to postmenopausal women (n = 12, 63%). RCTs that did not have this restriction (n = 7, 37%) predominantly randomized postmenopausal women, including Breast International Group 1-98 (BIG 1-98) (98%) [27], SITAM-01 (94%) [28], Adjuvant Tamoxifen, Longer Against Shorter (90%) [29], Scottish (82%) [30], NSABP-B14 (73%) [31], NSABP-B14 phase I trial (70%) [31], and UK Over 50s (52%) [32]. In addition, the majority of RCTs were restricted to patients with hormone-receptor (estrogen or progesterone receptor) positive breast cancer. The tamoxifen dose was 20 mg/day in the majority of the RCTs (n = 15, 88%). In terms of individual AIs, the dose was consistent across RCTs using anastrozole (1 mg/day), letrozole (2.5 mg/day), and exemestane (25 mg/day).
Table 1.
Patient characteristics at baseline in randomized controlled trials of AIs and tamoxifen included in the study
| Trial | Trial Funding | Trial arm (randomized) | Age (mean) | PM (%) | Node positive (%) | HR-positive (%) | Tumor size >2 cm (%) | Primary treatment |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Mastectomy (%) | Radiotherapy (%) | Chemotherapy (%) | ||||||||
| ATAC [53] | Industry | A-3125 | A-64 | A-100 | A-35 | A-84 | A-35 | A-48 | A-63 | A-22 |
| T-3116 | T-64 | T-100 | T-34 | T-83 | T-36 | T-47 | T-63 | T-21 | ||
| BIG 1-98 [27] | Industry & Nonprofit | L-4003 | L-61a | L-99 | L-42 | L-∼100 | L-37 | L-44 | L-72 | L-25 |
| T-4007 | T-61a | T-98 | T-41 | T-∼100 | T-38 | T-42 | T-72 | T-25 | ||
| Abo-Touk N et al. [54] | NA | L-60 | L-NA | L-100 | L-48 | L-100 | L-87 | L-70 | L-95 | L-NA |
| T-60 | T-NA | T-100 | T-35 | T-100 | T-83 | T-68 | T-93 | T-NA | ||
| N-SAS BC03 [55] | Industry & Nonprofit | A-347 | A-60 | A-100 | A-41 | A-100 | A-21c | A-48 | A-NA | A-54 |
| T-349 | T-60 | T-100 | T-40 | T-100 | T-21c | T-48 | T-NA | T-53 | ||
| ITA [56] | Industry | A-223 | A-63a | A-100 | A-100 | A-91 | A-24 | A-52 | A-54 | A-67 |
| T-225 | T-63a | T-100 | T-100 | T-86 | T-19 | T-55 | T-49 | T-67 | ||
| ARNO95 [57] | Industry | A-489 | A-61 | A-100 | A-26 | A-97 | A-36 | A-33 | A-67 | A-NA |
| T-490 | T-61 | T-100 | T-27 | T-96 | T-37 | T-30 | T-68 | T-NA | ||
| IES [58] | Industry & Nonprofit | E-2352 | E-64 | E-100 | E-44 | E-81b | E-NA | E-52 | E-NA | E-32 |
| T -2372 | T-64 | T-100 | T-44 | T-81b | T-NA | T-52 | T-NA | T-32 | ||
| Paridaens RJ et al. [59] | Industry & Nonprofit | E-190 | E-63a | E-100 | E-NA | E-92 | E-NA | E-NA | E-41 | E-30 |
| T-192 | T-62a | T-100 | T-NA | T-94 | T-NA | T-NA | T-42 | T-33 | ||
| TEAM [6] | Industry | E-4904 | E-64a | E-100 | E-47 | E-100 | E-42 | E-44 | E-69 | E-36 |
| T→E-4875 | T→E-64a | T→E-100 | T→E-47 | T→E-100 | T→E-41 | T→E-45 | T→E-68 | T→E-36 | ||
| N-SAS BC04 [60] | Industry & Nonprofit | A-55 | A-63 | A-100 | A-66 | A-95b | A-NA | A-33 | A-62 | A-38 |
| E-55 | E-63 | E-100 | E-62 | E-96b | E-NA | E-27 | E-64 | E-38 | ||
| T→E-56 | T→E-63 | T→E-100 | T→E-66 | T→E-96b | T→E-NA | T→E-32 | T→E-64 | T→E-41 | ||
| MA.17 [33] | Industry & Nonprofit | L-2593 | L-62a | L-100 | L-46 | L-98 | L-NA | L-50 | L-60 | L-46 |
| P-2594 | P-62a | P-100 | P-46 | P-98 | P-NA | P-50 | P-59 | P-46 | ||
| MA.17R [35] | Industry & Non-profit | L-959 | L-66a | L-100 | L-51 | L-99 | L-9 | L-48 | L-NA | L-59 |
| P-959 | P-65a | P-100 | P-52 | P-99 | P-8 | P-49 | P-NA | P-58 | ||
| ATLAS [29] | Industry & Nonprofit | T-6454 | T-NA | T-90 | T-41 | T-53b | T-52 | T-72 | T-NA | T-NA |
| NT-6440 | NT-NA | NT-90 | NT-40 | NT-53b | NT-52 | NT-71 | NT-NA | NT-NA | ||
| SITAM-01 [28] | Industry | T-943 | T-61 | T-94 | T-44 | T-59b | T-45 | T-63 | T-40 | T-11 |
| NT-958 | NT-61 | NT-95 | NT-43 | NT-61b | NT-43 | NT-64 | NT-39 | NT-9 | ||
| NSABP B-14 [31] | Nonprofit | T-583 | T-56 | T-73 | T-0 | T-100b | T-32 | T-56 | T-NA | T-NA |
| P-570 | P-56 | P-74 | P-0 | P-100b | P-35 | P-56 | P-NA | P-NA | ||
| UK Over 50s [61, 62] | Nonprofit | T-1725 | T-62a | T-53 | T-25 | T-NA | T-NA | T-38 | T-62 | T-NA |
| NT-1724 | NT-62a | NT-52 | P-26 | NT-NA | NT-NA | NT-37 | NT-62 | NT-NA | ||
| Scottish [63] | Industry & Nonprofit | T-661 | T-59 | T- 82 | T -32 | T-41b | T-68 | T-100 | T-32 | T-NA |
| NT-651 | NT-59 | NT-82 | NT-33 | NT-39b | NT-71 | NT-100 | NT-31 | NT-NA | ||
| NSABP-B14 phase I [31] | Nonprofit | T-1404 | T-55 | T-71 | T-0 | T-100b | T-43 | T-62 | T-NA | T-NA |
| P-1414 | P-55 | P-68 | P-0 | P-100b | P-41 | P-62 | P-NA | P-NA | ||
| Cummings et al. [64] | Nonprofit | T-85 | T-71a | T-100 | T-100 | T-86b | T-33c | T-100 | T-NA | T-NA |
| P-83 | P-70a | P-100 | P-100 | P-84b | P-28c | P-100 | P-NA | P-NA | ||
Proportions do not include patients with unknown, uncertain, or other status, arrow indicates switch between endocrine therapy.
Median age.
Estrogen-receptor positive.
≥3 cm.
A, Anastrozole; E, Exemestane; HR, Hormone-receptor (estrogen or progesterone) positive; L, Letrozole; NT, No treatment; P, Placebo; PM, postmenopausal; T, Tamoxifen; ARNO 95, German Adjuvant Breast Cancer Group/Arimidex-Novaldex 95; ATAC, Anastrozole, Tamoxifen, Alone or in Combination; ATLAS, Adjuvant Tamoxifen, Longer Against Shorter trial; BIG 1-98, Breast International Group 1-98; IES, Intergroup Exemestane Study; ITA, Italian Tamoxifen Anastrozole trial; NSABP-B14, National Surgical Adjuvant Breast and Bowel Project B14; N-SAS BC03, National Surgical Adjuvant Study Breast Cancer 03 trial; N-SAS BC04, National Surgical Adjuvant Study Breast Cancer 04 trial; SITAM-01, Italian Study of Adjuvant Treatment in Breast Cancer-01; TEAM, Tamoxifen Exemestane Adjuvant Multinational trial; AI, Aromatase Inhibitors; CI, Confidence Interval; NT, No treatment; RR, Relative risk. Arrow indicates switch between endocrine therapy.
Quality assessment
The majority of RCTs were of low risk of bias in different domains of Cochrane Collaboration’s tool (Table 1 and supplementary Table 8, available at Annals of Oncology online) and were funded by industry. In the BIG 1-98 trial, 25% of patients selectively crossed over from the tamoxifen arm to the letrozole arm in 2005 after it was demonstrated that letrozole significantly reduces distance recurrences and improves disease-free progression in comparison to tamoxifen [4]. However, all adverse events were reported within 30 days of selective crossover from tamoxifen to letrozole, and thus the crossover did not bias the results reported [4]. Similarly, in MA.17 trial, participants were unblinded at a median 2.4 years of follow-up [33]. However, the cardiovascular and cerebrovascular events were reported at 30 months of follow-up. Finally, in the Scottish trial, 330 patients out of 656 patients randomized to the control arm received tamoxifen during the study period due to relapse or suspicion of relapse [34]. The cardiovascular and cerebrovascular events were reported by treatment allocation at randomization and patients were censored at the date of systemic relapse [30].
Cardiovascular disease
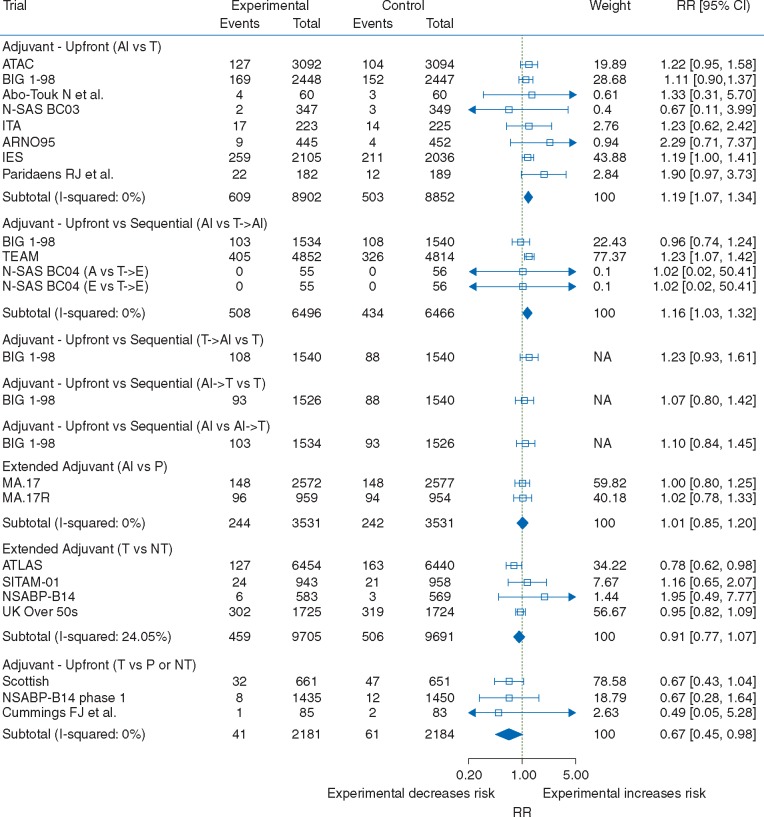
The counts for cardiovascular events for all RCTs meeting the inclusion and exclusion and included in the quantitative analysis are reported in supplementary Tables 6 and 7 (available at Annals of Oncology online). Pooled analysis of eight RCTs comparing upfront adjuvant AIs to tamoxifen showed a 19% increased risk of cardiovascular events (RR: 1.19, 95% CI: 1.07–1.34; Figure 2). Similar results were obtained among RCTs comparing AIs to tamoxifen (RR: 1.19, 95% CI: 1.02-1.39; supplementary Figure 1, available at Annals of Oncology online) and among RCTs comparing these two drugs after initial adjuvant treatment with tamoxifen (RR: 1.20, 95% CI: 1.02–1.41; supplementary Figure 1, available at Annals of Oncology online). These results are also consistent when examining different AIs independently (supplementary Figure 2, available at Annals of Oncology online). Similar results were also observed when comparing sequential treatment with tamoxifen and AIs to upfront treatment with tamoxifen alone (RR: 1.23, 95% CI: 0.93–1.61; Figure 2), although the 95% CIs were wide and included unity. Pooled analyses of RCTs comparing AIs to sequential treatment with tamoxifen and AIs (RR: 1.16, 95% CI: 1.03–1.32; Figure 2) also demonstrate an increased risk of cardiovascular events associated with AIs. RCTs comparing sequential treatment with AIs and tamoxifen to upfront treatment with tamoxifen (RR: 1.07, 95% CI: 0.80-1.42; Figure 2), and RCTs of upfront treatment with AIs to sequential treatment with AIs and tamoxifen (RR: 1.10, 95% CI: 0.84–1.45; Figure 2) were all inconclusive due to wide 95% CIs. In the MA.17 trial, patients initially treated for a median 5 years with tamoxifen were randomized to additional 5 years of additional treatment with either letrozole or placebo [33]. In the MA.17R trial, patients initially treated with 4.5–6 years of adjuvant treatment with any AI (preceded in most patients with tamoxifen treatment) were randomized to letrozole of placebo for an additional five years [35]. In this extended adjuvant setting, AIs were not associated with cardiovascular events (RR: 1.01, 95% CI: 0.85–1.20; Figure 2) when pooling data across these RCTs or when considering each RCT independently. Consistent with these results, pooled estimate showed a 33% decreased risk when comparing upfront tamoxifen to placebo or no treatment in the adjuvant setting (RR: 0.67, 95% CI: 0.45–0.98; Figure 2). In Scottish trial, approximately 50% of participants in the control arm received the treatment, which could lead to a dilution of the effect. However, similar results were obtained in a sensitivity analysis that excluded this study (RR: 0.65, 95% CI: 0.28–1.49), though estimates were less precise (supplementary Figure 3, available at Annals of Oncology online). The cardiovascular effects of tamoxifen in RCTs comparing tamoxifen to placebo or no treatment in the extended adjuvant setting is inconclusive due to lack of precision (RR: 0.91, 95% CI: 0.77–1.07). Similar results were obtained for all the above contrasts using fixed-effects analyses (supplementary Figure 4, available at Annals of Oncology online).
Figure 2.
Forest plot of relative risks of cardiovascular events with AIs and tamoxifen by trial design. Pooled relative risks and confidence intervals were obtained using DerSimonian and Laird random-effects models. AIs, aromatase inhibitors.
Ischemic heart disease
Restricting the definition of cardiovascular events to ischemic heart disease yielded similar results (supplementary Figures 5 and 6, available at Annals of Oncology online). In RCTs comparing upfront AIs to tamoxifen in the adjuvant setting, there was a 30% increased risk of ischemic heart disease when comparing AIs to tamoxifen (RR: 1.30, 95% CI: 1.11–1.53). In the extended adjuvant setting, AIs were not associated with an increased risk of ischemic heart disease in comparison to placebo (RR: 0.82, 95% CI: 0.60–1.13). Pooled analysis showed a significant 34% decreased risk when comparing upfront tamoxifen to placebo or no-treatment in the adjuvant setting (RR: 0.66, 95% CI: 0.45–0.98). The association between tamoxifen and cardiovascular ischemic events in the extended adjuvant setting remained inconclusive due to low precision (RR: 1.21, 95% CI: 0.58–2.53) and high degree of heterogeneity (I2 statistic: 60.6%). Similar results were also obtained when restricting the definition of ischemic heart disease to myocardial infarction in RCTs reporting myocardial infarction and angina (rather than a composite endpoint of ischemic heart disease). However, in this analysis, there was loss of precision in the pooled estimate when comparing tamoxifen to placebo in the adjuvant setting and tamoxifen to placebo or no-treatment in the extended adjuvant setting (supplementary Figures 7 and 8, available at Annals of Oncology online).
Cerebrovascular disease
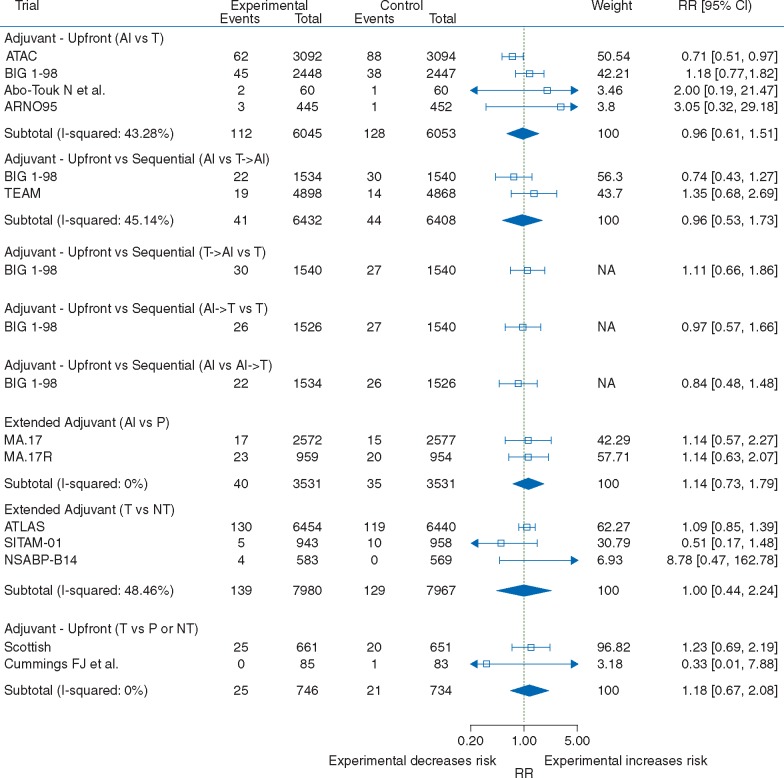
Cerebrovascular endpoints were reported inconsistently across RCTs, leading to low events rates. When data were pooled across trials, no evidence of a difference was observed (Figure 3). However, these analyses were inconclusive due to wide 95% CIs (upfront treatment with AIs versus tamoxifen: RR: 0.96, 95% CI: 0.61–1.51; AIs versus sequential treatment with tamoxifen and AIs: RR: 0.96, 95% CI: 0.53–1.73; tamoxifen versus no treatment in the extended adjuvant setting: RR: 1.00, 95% CI: 0.44–2.24; and tamoxifen versus placebo or no treatment in the adjuvant setting: RR: 1.18, 95% CI: 0.67–2.08). Similar results were obtained using fixed-effects analysis (supplementary Figure 9, available at Annals of Oncology online).
Figure 3.
Forest plot of relative risks of cerebrovascular events with AIs and tamoxifen by trial design. Pooled relative risks and confidence intervals were obtained using DerSimonian and Laird random-effects models. AIs: aromatase inhibitors.
Discussion
The risk of cardiovascular disease increases with age and is considerably higher in postmenopausal women in comparison to premenopausal women [36]. Thus, in postmenopausal women, excess risk of cardiovascular disease from breast cancer treatment is a major health concern. Similar to previous studies, we found that adjuvant treatment with AIs increases the risk of cardiovascular events in comparison to tamoxifen in postmenopausal women with breast cancer. However, we also found that tamoxifen is associated with 33% reduction in risk of cardiovascular events in RCTs comparing tamoxifen to placebo or no treatment. Thus, the cardioprotective effects of tamoxifen can completely account for the increase risk in cardiovascular events observed in the RCTs comparing AIs to tamoxifen. This conclusion is further supported by the MA.17 and MA.17R RCTs, where there was no association between AIs and cardiovascular event or ischemic heart disease in the extended adjuvant setting [35, 37]. The cardiovascular safety of AIs may also be compared with placebo in the MAP.3 breast cancer prevention RCT [38]. In this setting, there was also no increased risk of cardiovascular events when comparing exemestane to placebo in postmenopausal women at moderate risk of breast cancer at 35 months of follow-up [38].
Tamoxifen has been shown to decrease cardiovascular disease in previous studies. A meta-analysis of all RCTs comparing tamoxifen to placebo or no-treatment (in the presence of co-interventions) demonstrated that tamoxifen decreases the risk of myocardial infarction by 26% (RR: 0.74, 95% CI: 0.47–1.16) and the risk of myocardial infarction-associated mortality by 45% (RR: 0.55, 95% CI: 0.36–0.87) in breast cancer treatment RCTs [39]. In the Swedish Breast Cancer Group RCT, postmenopausal women with early stage breast cancer were randomized to either 5 or 2 years of treatment with tamoxifen [40, 41]. In this setting, treatment with tamoxifen for 5 years led to lower incidence of coronary heart disease and coronary heart disease-associated mortality in comparison to 2 years of treatment with tamoxifen during the treatment period [40]. There remained a decrease in the risk of coronary heart disease (HR: 0.83, 95% CI: 0.70–1.00) and coronary heart disease mortality (HR: 0.72, 95% CI: 0.53–0.97) at a median 12 years of follow-up [40]. Finally, in IBIS-I tamoxifen breast-cancer prevention RCT, there remained a non-significant decrease in the incidence of myocardial infarction when comparing tamoxifen to placebo at 16 years of median follow-up in healthy women at risk of breast cancer (OR: 0.76, CI: 0.34–1.67) [42].
A major mechanism proposed for the cardioprotective effects of tamoxifen is alterations in serum lipid levels. In RCTs comparing tamoxifen to placebo, tamoxifen decreases serum total and LDL cholesterol, while increasing apoliprotein A-I levels in postmenopausal women with breast cancer [16, 19] and in healthy postmenopausal women [18]. Tamoxifen may lower LDL and total cholesterol by inhibiting enzymes involve in cholesterol metabolism pathway including sterol-Δ8,7 isomerase and Acetyl-Coenzyme A acetyltransferase [17]. In contrast, evidence from RCTs that suggests AIs do not significantly alter plasma lipoproteins. In ATENA and a MA.17L substudy, there were no differences in plasma lipoprotein between patients who received AIs and those who received placebo or no treatment [43–45]. Consistent with these results, it has been demonstrated that AIs do not systematically alter plasma lipoproteins from baseline to follow-up assessments [12]. Tamoxifen also has anti-inflammatory effects and lowers C-reactive protein and fibrinogen levels, both of which are strong predictors of cardiovascular disease [19, 46–49]. The anti-inflammatory effects of tamoxifen may also be mediated through cytokine TGF-β, which maintains vessel wall structure during atherogenesis [17, 50]. Finally, tamoxifen has been shown to have antioxidant properties which protect LDL cholesterol from harmful oxidation [51, 52].
Previous systematic reviews and meta-analyses have compared the cardiovascular effects of AIs to tamoxifen with discordant results [8–13]. The discordance between these studies may be due to consideration of trials comparing AIs to tamoxifen in adjuvant setting only [8], absence of a systematic search or limited search of electronic databases [9, 10, 13], and qualitative assessment of evidence [12]. This is the first study to date to additionally include adjuvant and extended-adjuvant RCTs comparing the cardiovascular effects of tamoxifen to placebo or no treatment. We have also included up-to-date results from adjuvant RCTs comparing AIs to tamoxifen and the extended-adjuvant RCTs comparing AIs to placebo. Thus, the major strength of this study is the consideration of the totality of evidence from all RCTs of AIs and tamoxifen in the adjuvant and extended-adjuvant setting.
There are also some limitations to this study. First, there was heterogeneity in reporting of cardiovascular and cerebrovascular endpoints between studies. However, we additionally used ischemic heart disease as definition of cardiovascular event in our secondary analysis and found similar results in comparison to using composite endpoint of cardiovascular events. Second, there was some heterogeneity present among RCTs with respect to duration of follow-up, patient recruitment periods, and patient characteristics. Nevertheless, results were consistent when analysis was conducted across trial subtypes. We used random-effects models to account for between-study heterogeneity and found results were consistent with fixed-effects analysis. Efficacy was the primary endpoint of RCTs of AIs and tamoxifen included in this study and thus publication bias in regards to cardiotoxicity of these drugs is not anticipated. In addition, there was not sufficient information to assess risk of cardiovascular events by patients’ baseline cardiovascular disease risk. We were also not able to conduct analysis for cardiovascular mortality as this endpoint was reported inconsistently across trials. Pharmacoepidemiologic studies will need to address whether AIs increase the risk of cardiovascular-associated mortality in comparison to tamoxifen.
Conclusions
RCTs directly comparing AIs to tamoxifen suggest that AIs are associated with an increased risk of cardiovascular events. As a result, current clinical practice guidelines indicate that ischemic heart disease is a major adverse event associated with AIs [7]. However, the results from this study demonstrate that the cardiovascular events associated with AIs in RCTs directly comparing AIs to tamoxifen may be accounted for by the cardioprotective effects of tamoxifen. Concordant with these results, AIs are not associated with cardiovascular events when compared with placebo in the extended-adjuvant setting. The results from this study are consistent with the putative mechanisms for cardioprotective actions of tamoxifen in previous studies. The findings of this systematic review and meta-analysis should be considered when assessing the benefits and risks of AIs in treatment of breast cancer in postmenopausal women.
Supplementary Material
Acknowledgements
KBF holds a Canadian Institutes of Health Research New Investigator Award. SS is the recipient of the James McGill Chair. LA is the recipient of a Chercheur-Boursier career award from the Fonds de la recherche en santé du Québec.
Funding
This study was supported by a Foundation Grant from the Canadian Institutes of Health Research (FDN-333744). The funding source has no role design and conduct of the study, collection, management, analysis, writing, review, or approval of the manuscript.
Disclosure
NBF served as a consultant from Amgen, Novartis, Amgen, and Roche. SS has received research funding, participated in advisory board meetings or as a speaker for AstraZeneca, Boehringer-Ingelheim, Novartis, Pfizer and Merck. All other authors have no conflicts of interest to declare.
References
- 1. Kelly E, Lu CY, Albertini S, Vitry A.. Longitudinal trends in utilization of endocrine therapies for breast cancer: an international comparison. J Clin Pharm Ther 2015; 40: 76–82. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists' Collaborative G, Dowsett M, Forbes JF. et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 3. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006; 7: 633–643. [DOI] [PubMed] [Google Scholar]
- 4. Colleoni M, Giobbie-Hurder A, Regan MM. et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol 2011; 29: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bliss JM, Kilburn LS, Coleman RE. et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol 2012; 30: 709–717. [DOI] [PubMed] [Google Scholar]
- 6. Van De Velde CJ, Rea D, Seynaeve C. et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 2011; 377: 321–331. [DOI] [PubMed] [Google Scholar]
- 7. Burstein HJ, Temin S, Anderson H. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol 2014; 32: 2255–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amir E, Seruga B, Niraula S. et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011; 103: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 9. Aydiner A. Meta-analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal women. Breast 2013; 22: 121–129. [DOI] [PubMed] [Google Scholar]
- 10. Cuppone F, Bria E, Verma S. et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer 2008; 112: 260–267. [DOI] [PubMed] [Google Scholar]
- 11. Josefsson ML, Leinster SJ.. Aromatase inhibitors versus tamoxifen as adjuvant hormonal therapy for oestrogen sensitive early breast cancer in post-menopausal women: meta-analyses of monotherapy, sequenced therapy and extended therapy. Breast 2010; 19: 76–83. [DOI] [PubMed] [Google Scholar]
- 12. Younus M, Kissner M, Reich L, Wallis N.. Putting the cardiovascular safety of aromatase inhibitors in patients with early breast cancer into perspective: a systematic review of the literature. Drug Safety 2011; 34: 1125–1149. [DOI] [PubMed] [Google Scholar]
- 13. Ryden L, Heibert Arnlind M, Vitols S. et al. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo—meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast 2016; 26: 106–114. [DOI] [PubMed] [Google Scholar]
- 14. Bruning PF, Bonfrer JM, Hart AA. et al. Tamoxifen, serum lipoproteins and cardiovascular risk. Br J Cancer 1988; 58: 497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuzick J, Allen D, Baum M. et al. Long term effects of tamoxifen. Biological effects of Tamoxifen Working Party. Eur J Cancer 1992; 29A: 15–21. [DOI] [PubMed] [Google Scholar]
- 16. Dewar JA, Horobin JM, Preece PE. et al. Long term effects of tamoxifen on blood lipid values in breast cancer. BMJ 1992; 305: 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grainger DJ, Schofield PM.. Tamoxifen for the prevention of myocardial infarction in humans: preclinical and early clinical evidence. Circulation 2005; 112: 3018–3024. [DOI] [PubMed] [Google Scholar]
- 18. Guetta V, Lush RM, Figg WD. et al. Effects of the antiestrogen tamoxifen on low-density lipoprotein concentrations and oxidation in postmenopausal women. Am J Cardiol 1995; 76: 1072–1073. [DOI] [PubMed] [Google Scholar]
- 19. Love RR, Wiebe DA, Feyzi JM. et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst 1994; 86: 1534–1539. [DOI] [PubMed] [Google Scholar]
- 20. British Medical Journal Evidence Center Information Specialists. Study Design Search Filters. BMJ Clinical Evidence. http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665076.html. [Google Scholar]
- 21. Higgins JP, Altman DG, Gotzsche PC. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Center for Disease Control and Prevention. Heart Disease Facts 2015. https://www.cdc.gov/heartdisease/facts.htm.
- 23. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedrich JO, Adhikari NK, Beyene J.. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 2007; 7: 5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viechtbauer W. Conducting meta-analysis in R with metafor Package. J Stat Soft 2010; 36: 1–48. [Google Scholar]
- 27. Thurlimann B, Keshaviah A, Coates AS. et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 353: 2747–2757. [DOI] [PubMed] [Google Scholar]
- 28. Sacco M, Valentini M, Belfiglio M. et al. Randomized trial of 2 versus 5 years of adjuvant tamoxifen for women aged 50 years or older with early breast cancer: Italian Interdisciplinary Group for Cancer Evaluation Study of Adjuvant Treatment in Breast Cancer 01. J Clin Oncol 2003; 21: 2276–2281. [DOI] [PubMed] [Google Scholar]
- 29. Davies C, Pan H, Godwin J. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonald CC, Alexander FE, Whyte BW. et al. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomised trial. The Scottish Cancer Trials Breast Group. BMJ 1995; 311: 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fisher B, Dignam J, Bryant J. et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996; 88: 1529–1542. [DOI] [PubMed] [Google Scholar]
- 32. Hackshaw A, Roughton M, Forsyth S. et al. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol 2011; 29: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 33. Goss PE, Ingle JN, Martino S. et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003; 349: 1793–1802. [DOI] [PubMed] [Google Scholar]
- 34. Stewart HJ, Prescott RJ, Forrest AP.. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst 2001; 93: 456–462. [DOI] [PubMed] [Google Scholar]
- 35. Goss PE, Ingle JN, Pritchard KI. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016; 375: 209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosano GM, Vitale C, Marazzi G, Volterrani M.. Menopause and cardiovascular disease: the evidence. Climacteric 2007; 10(Suppl 1): 19–24. [DOI] [PubMed] [Google Scholar]
- 37. Goss PE, Ingle JN, Martino S. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 38. Goss PE, Ingle JN, Ales-Martinez JE. et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 39. Braithwaite RS, Chlebowski RT, Lau J. et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med 2003; 18: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosell J, Nordenskjold B, Bengtsson NO. et al. Effects of adjuvant tamoxifen therapy on cardiac disease: results from a randomized trial with long-term follow-up. Breast Cancer Res Treat 2013; 138: 467–473. [DOI] [PubMed] [Google Scholar]
- 41.Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst 1996; 88: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 42. Cuzick J, Sestak I, Cawthorn S. et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Markopoulos C, Chrissochou M, Michailidou A. et al. Effect of exemestane on the lipidemic profile of post-menopausal operable breast cancer patients following 5-7 years of adjuvant tamoxifen: Preliminary results of the ATENA substudy. Anti-Cancer Drugs 2005; 16: 879–883. [DOI] [PubMed] [Google Scholar]
- 44. Markopoulos C, Dafni U, Misitzis J. et al. Extended adjuvant hormonal therapy with exemestane has no detrimental effect on the lipid profile of postmenopausal breast cancer patients: final results of the ATENA lipid substudy. Breast Cancer Res 2009; 11: R35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wasan KM, Goss PE, Pritchard PH. et al. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol 2005; 16: 707–715. [DOI] [PubMed] [Google Scholar]
- 46. Bonanni B, Johansson H, Gandini S. et al. Effect of tamoxifen at low doses on ultrasensitive C-reactive protein in healthy women. J Thromb Haemost 2003; 1: 2149–2152. [DOI] [PubMed] [Google Scholar]
- 47. Cushman M, Costantino JP, Tracy RP. et al. Tamoxifen and cardiac risk factors in healthy women: suggestion of an anti-inflammatory effect. Arterioscler Thromb Vasc Biol 2001; 21: 255–261. [DOI] [PubMed] [Google Scholar]
- 48. Stec JJ, Silbershatz H, Tofler GH. et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham offspring population. Circulation 2000; 102: 1634–1638. [DOI] [PubMed] [Google Scholar]
- 49. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E. et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012; 367: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grainger DJ, Witchell CM, Metcalfe JC.. Tamoxifen elevates transforming growth factor-beta and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med 1995; 1: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 51. Wiseman H. Tamoxifen as an antioxidant and cardioprotectant. Biochem Soc Symp 1995; 61: 209–219. [DOI] [PubMed] [Google Scholar]
- 52. Wiseman H, Laughton MJ, Arnstein HR. et al. The antioxidant action of tamoxifen and its metabolites. Inhibition of lipid peroxidation. FEBS Lett 1990; 263: 192–194. [DOI] [PubMed] [Google Scholar]
- 53. Baum M, Buzdar AU, Cuzick J. et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002; 359: 2131–2139. [DOI] [PubMed] [Google Scholar]
- 54. Abo-Touk NA, Sakr HA, Abd El-Lattef A.. Switching to letrozole versus continued tamoxifen therapy in treatment of postmenopausal women with early breast cancer. J Egypt Natl Canc Inst 2010; 22: 79–85. [PubMed] [Google Scholar]
- 55. Aihara T, Takatsuka Y, Ohsumi S. et al. Phase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 study. Breast Cancer Res Treat 2010; 121: 379–387. [DOI] [PubMed] [Google Scholar]
- 56. Boccardo F, Rubagotti A, Puntoni M. et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole trial. J Clin Oncol 2005; 23: 5138–5147. [DOI] [PubMed] [Google Scholar]
- 57. Kaufmann M, Jonat W, Hilfrich J. et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 study. J Clin Oncol 2007; 25: 2664–2670. [DOI] [PubMed] [Google Scholar]
- 58. Coombes RC, Hall E, Gibson LJ. et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081–1092. [DOI] [PubMed] [Google Scholar]
- 59. Paridaens RJ, Dirix LY, Beex LV. et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European organisation for research and treatment of cancer breast cancer cooperative group. J Clin Oncol 2008; 26: 4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takei H, Ohsumi S, Shimozuma K. et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04). Breast Cancer Res Treatment 2012; 133: 227–236. [DOI] [PubMed] [Google Scholar]
- 61. Current Trials working Party of the Cancer Research Campaign Breast Cancer Trials Group. Preliminary results from the cancer research campaign trial evaluating tamoxifen duration in women aged fifty years or older with breast cancer. J Natl Cancer Inst 1996; 88: 1834–1839. [DOI] [PubMed] [Google Scholar]
- 62. Hackshaw A, Roughton M, Forsyth S. et al. Long-term benefits of 5 years of tamoxifen: 10-Year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol 2011; 29: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 63.Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh (Lancet 1987; 171–175). [PubMed]
- 64. Cummings FJ, Gray R, Tormey DC. et al. Adjuvant tamoxifen versus placebo in elderly women with node-positive breast cancer: long-term follow-up and causes of death. J Clin Oncol 1993; 11: 29–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.