Abstract
Background
Diagnostic imaging plays a critical role in the initial diagnosis and therapeutic monitoring of pancreatic adenocarcinoma. Over the past decade, the concept of ‘borderline resectable’ pancreatic cancer has emerged to describe a distinct subset of patients existing along the spectrum from resectable to locally advanced disease for whom a microscopically margin-positive (R1) resection is considered relatively more likely, primarily due to the relationship of the primary tumor with surrounding vasculature.
Materials and methods
This review traces the conceptual evolution of borderline resectability from a radiological perspective, including the debates over the key imaging criteria that define the thresholds between resectable, borderline resectable, and locally advanced or metastatic disease. This review also addresses the data supporting neoadjuvant therapy in this population and discusses current imaging practices before and during treatment.
Results
A growing body of evidence suggests that the borderline resectable group of patients may particularly benefit from neoadjuvant therapy to increase the likelihood of an ultimately margin-negative (R0) resection. Unfortunately, anatomic and imaging criteria to define borderline resectability are not yet universally agreed upon, with several classification systems proposed in the literature and considerable variance in institution-by-institution practice. As a result of this lack of consensus, as well as overall small patient numbers and lack of established clinical trials dedicated to borderline resectable patients, accurate evidence-based diagnostic categorization and treatment selection for this subset of patients remains a significant challenge.
Conclusions
Clinicians and radiologists alike should be cognizant of evolving imaging criteria for borderline resectability given their profound implications for treatment strategy, follow-up recommendations, and prognosis.
Keywords: pancreatic adenocarcinoma, borderline resectable, neoadjuvant therapy, staging criteria, CT
Introduction
Despite significant advances in the diagnosis and treatment of pancreatic adenocarcinoma, the prognosis for this disease remains relatively poor, representing the fourth most common overall cause of death due to cancer in the United States [1, 2]. Fewer than 25% of patients who are diagnosed with pancreatic cancer survive 1 year and no more than 5% survive 5 years. In the subset of patients with localized disease who undergo margin-negative resection, the survival rate at 5 years is substantially higher at 18%–24% [3–7]. However, fewer than 20% of patients present with disease localized to the pancreas, and about 70% of patients present with locally advanced or metastatic disease.
One promising development in recent years has been the description of a new subgroup of approximately 5%–10% of patients with borderline resectable disease who have disease that is too advanced achieve a negative margin with immediate surgery but potentially can reach a margin-negative resection after neoadjuvant therapy, although the true benefits of neoadjuvant therapy remain incompletely characterized [8]. There is disagreement about how this group should be defined, but its relationship to disease status can be conceptualized as a continuum of disease severity with two key thresholds (Figure 1). The first is between resectable and borderline resectable disease, beyond which there is a continually increasing risk of a margin-positive resection that potentially can be mitigated by neoadjuvant therapy. The second threshold is between borderline resectable and locally advanced disease, beyond which margin-positive resection risk is increasingly certain, and neoadjuvant therapy is theoretically less likely to be of any benefit. The patients who lie between these two thresholds are therefore in theory more likely to achieve the survival benefits of a margin-negative resection if they receive neoadjuvant chemotherapy. Similarly, patients above and below these thresholds may be more likely to experience the toxicities of treatment without improving their likelihood of margin-negative resection and thus overall prognosis [9, 10]. While there is currently insufficient evidence to conclusively demonstrate that neoadjuvant therapy achieves superior outcomes in borderline resectable patients, this model is useful as a general framework for understanding the rationale underlying the use of neoadjuvant therapy in this patient population.
Figure 1.
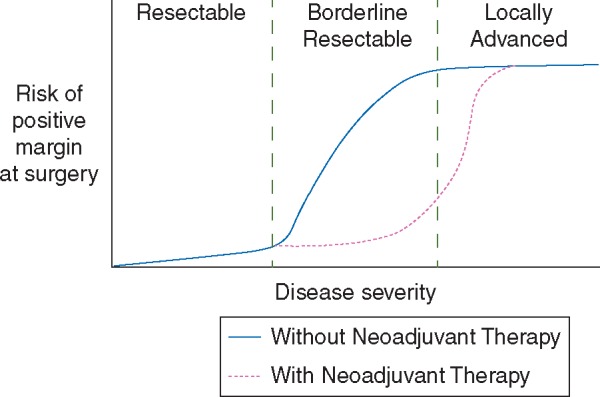
Conceptual representation of thresholds of disease severity versus risk of positive margin at surgery in pancreatic adenocarcinoma, with predicted effects of neoadjuvant therapy.
Encouragingly, the prognosis for the borderline resectable group of patients appears to be superior to that of patients with locally advanced and metastatic disease, although not as good as patients with immediately resectable disease [11, 12]. This subset of patients is also the focus of increasing research and clinical efforts (including a multi-institutional clinical trial organized by the Alliance for Clinical Trials in Oncology, a group of over 10 000 cancer specialists across the United States and Canada) in an attempt to provide a stronger evidence basis demonstrating the benefit of preoperative neoadjuvant therapy prior to resection. Here we trace the evolution of the borderline resectability concept from a radiological perspective and describe key imaging features that distinguish borderline resectable pancreatic cancer from locally advanced or metastatic disease.
Key anatomic features
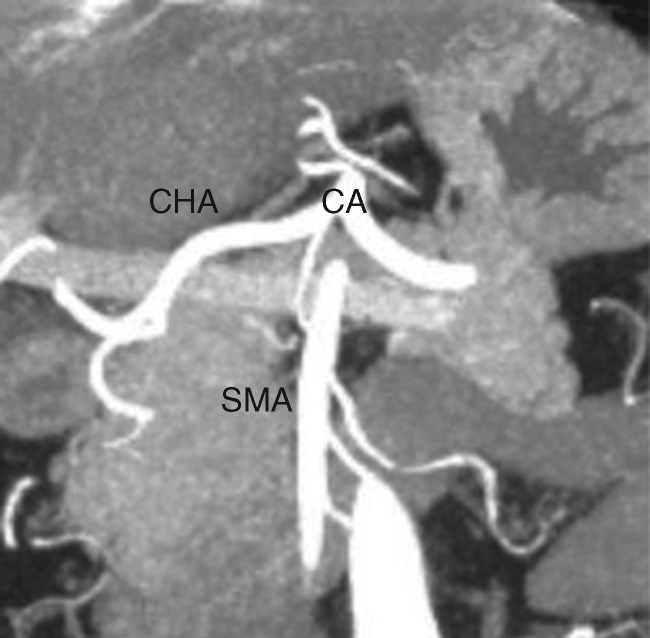
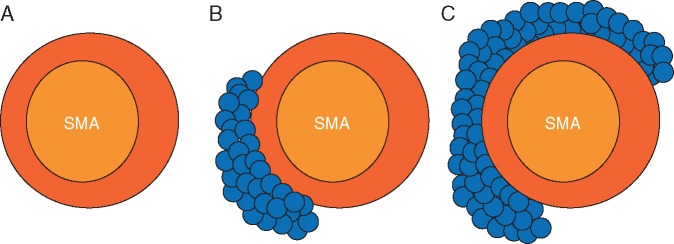
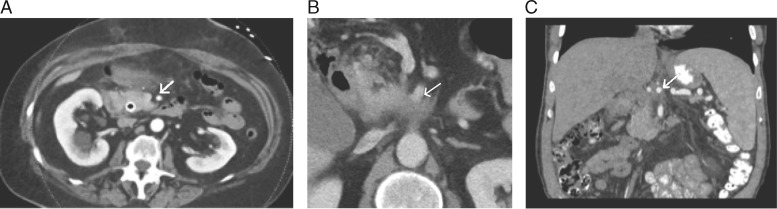
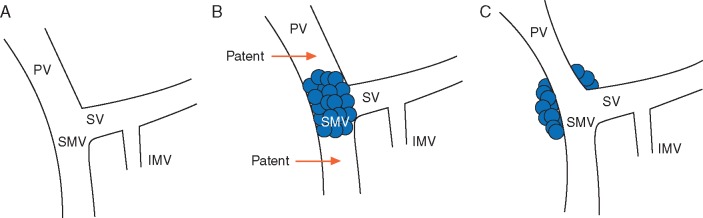
Aside from the presence of distant metastases, resectability is most clearly influenced by the degree of involvement of the primary tumor with critical adjacent vessels, chiefly the celiac artery, superior mesenteric artery (SMA), common hepatic artery (CHA), superior mesenteric vein (SMV), and portal vein (PV) (Figure 2). Accurate staging depends upon the ability to precisely delineate the degree of tumor–vessel contact. For arterial structures, taking the SMA in cross-section as a hypothetical example, a cancer may be deemed anywhere on the spectrum from resectable to unresectable depending on the degree of such contact (Figure 3A–C). This concept can be systematically applied in the interpretation of pancreatic protocol CT images to determine resectability status (Figure 4A–C). A similar approach can be applied in the evaluation of relevant venous anatomy. Taking a representative example of the hepatic portal venous system, depending on features such as tumor–vessel contact, venous occlusion, and contour irregularity, one can also systematically assess resectability status (Figure 5A–C). This concept, too, can be applied in interpreting CT images to arrive at an accurate image-based estimate of resectability status (Figure 6A and B).
Figure 2.
Coronal MIP reformatted image demonstrates normal anatomy of critical arterial vessels involved in pancreatic adenocarcinoma staging, including celiac artery (CA), superior mesenteric artery (SMA), and common hepatic artery (CHA) in relation to pancreas.
Figure 3.
(A) Cross-sectional representation of SMA uninvolved by tumor, compatible with resectable disease. (B) Less than 180° of tumor–vessel contact, compatible with borderline resectable disease. (C) Greater than 180° of tumor–vessel contact, representing locally advanced disease.
Figure 4.
(A) Axial CT image demonstrating SMA completely uninvolved by tumor with a clear surrounding fat plane, compatible with resectable disease (arrow). Anterior abdominal stranding was unrelated to the patient’s tumor. (B) Axial CT image demonstrating <180° of tumor–vessel contact of SMA (arrow), compatible with borderline resectable disease. (C) Coronal reformatted CT image demonstrating >180° of encasement of the celiac artery (arrow), compatible with locally advanced disease.
Figure 5.
(A) Schematic representation of hepatic portal venous system, uninvolved by tumor and compatible with resectable disease. (B) Short-segment occlusion of the SMV and PV. Note the patent vein above and below the level of the occlusion; if deemed safely surgically reconstructable, this would be considered borderline resectable disease. (C) Greater than 180° of tumor–vessel interface with the SMV/PV as well as contour irregularity, but no occlusion. This would be considered borderline resectable disease based on NCCN, AHPBA, and Alliance guidelines, but resectable based on MD Anderson criteria.
Figure 6.
(A) Sagittal reformatted CT image demonstrating distortion of the SMV with tumor–vessel contact of less than 180° (arrow), which would be considered borderline resectable disease based on NCCN and AHPBA guidelines, but resectable based on MD Anderson and Alliance criteria. (B) Coronal MIP image demonstrating short segment SMV occlusion (arrow); if deemed safely surgically reconstructable, this would be considered borderline resectable disease.
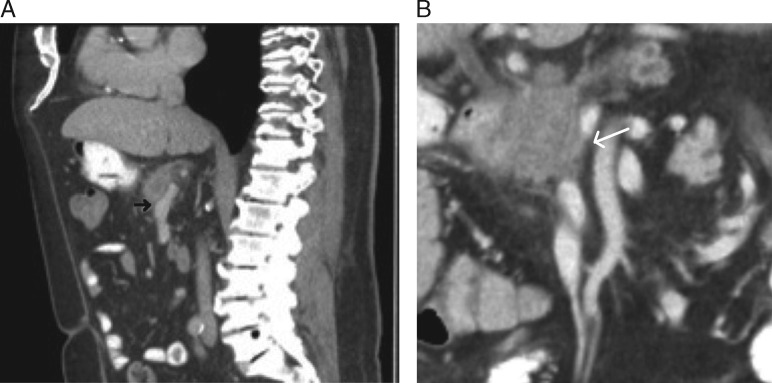
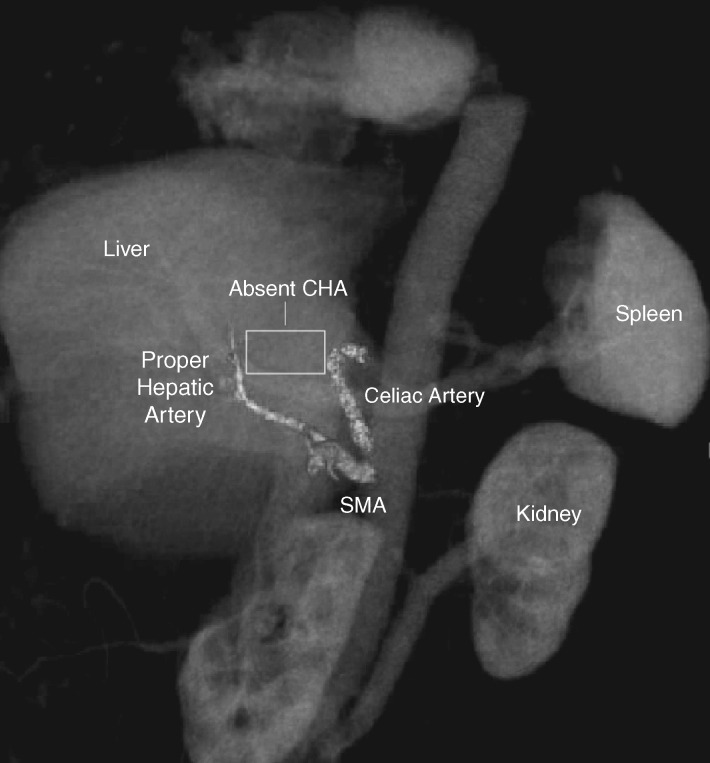
A detailed characterization of variant vascular anatomy is also crucial, as these anatomic variants can profoundly affect surgical planning and/or operative candidacy. The radiologist must accurately assess the arterial supply to the vital organs near the pancreas and report any variants that could compromise these organs if not recognized during surgery. In addition to well-known variants such as accessory hepatic arteries and replaced right hepatic artery (arising from the SMA), there are rarer variants such as an absent common hepatic artery with proper hepatic fed by the gastroduodenal artery, of which inadvertent ligation would almost certainly be fatal (Figure 7). This underscores the importance of the vigilance of the radiologist to identify and communicate such features to prevent a potentially catastrophic surgical outcome.
Figure 7.
Coronal 3D volume rendered image demonstrating variant arterial anatomy in which common hepatic artery is absent and does not arise from celiac axis (CA), and in which the proper hepatic artery (PHA) is instead fed by the gastroduodenal artery (GDA) arising from the superior mesenteric artery (SMA).
The origins and evolution of borderline resectability
While not formally proposed in the literature until 2006, the concept of borderline resectability had its roots in early studies from the 1990s and early 2000s demonstrating that patients with pancreatic cancer involving venous structures such as the SMV/PV could undergo vascular resection with outcomes comparable to patients with localized disease undergoing typical resections, and superior to patients with locally advanced disease being managed non-operatively [13, 14]. As it became increasingly apparent that resectability existed along a continuous spectrum rather than a series of discrete categories, and that accurate preoperative staging was imperative to guide appropriate treatment, nascent efforts at radiographic classification of pancreatic cancer incorporating an intermediate ‘borderline’ category began in earnest.
The term ‘borderline resectable’ was formally introduced by researchers at the MD Anderson Cancer Center in 2006 as part of a CT-based classification system, which was soon adopted by the National Comprehensive Cancer Network (NCCN) [8]. This defined borderline resectability as the subset of patients with any or all of the following: short segment abutment or encasement of the hepatic artery, amenable to surgical reconstruction; tumor abutment of the SMA involving <180° of the artery circumference; or short-segment occlusion of the SMV/PV amenable to surgical reconstruction (Table 1). The MD Anderson classification system was elaborated upon by Katz et al. in 2008 based on 160 patients with borderline resectable disease by the established criteria, and further introduced three specific subtypes of borderline resectable disease (Katz A, B, and C) [15]. In addition to the Katz A group based on the anatomic/imaging criteria above, borderline resectable B and C groups described populations of individuals with findings suggestive but not definitively diagnostic of metastasis and patients with poor functional status. Examples proposed for ‘type B’ borderline disease have included so-called ‘indeterminate’ hepatic lesions or lymph nodes, biopsy proof of involvement of a regional lymph node, or elevated tumor markers, all of which would theoretically convey an increased risk for micrometastatic disease below the threshold of imaging detection, with corresponding increased risk of early recurrence [10, 16, 17]. Of note, however, a recent retrospective analysis failed to validate the Katz B group, showing markedly worse outcomes in terms of overall survival and recurrence rate with respect to the more commonly utilized Katz A anatomic definition [18]. Additional work is warranted to further elucidate subsets of individuals for whom upfront resection is unlikely to be successful based on clinical or laboratory features beyond strict anatomic or imaging-based criteria, but is beyond the scope of this discussion.
Table 1.
Comparison of CT-based criteria distinguishing resectable, borderline resectable, and locally advanced disease
| MD Anderson [8, 15] | AHPBA/SSAT/SSO [19] | NCCN (Version 2.2016) [22] | Alliance [23] | ||
|---|---|---|---|---|---|
| Celiac | Resectable | No involvement | No involvement | No arterial tumor contact | No involvement |
| Borderline | No involvement | No involvement | Solid tumorb contact ≤180°, or >180° without involvement of the aorta and with intact and uninvolved gastroduodenal arterya (body/tail only) | Tumor-vessel interface <180° | |
| Locally Advanced | Any involvement | Any involvement | >180° solid tumor contact (any portion of pancreas), or any degree of solid tumor contact with aortic involvement (body/tail only) | Tumor-vessel interface ≥180° | |
| SMA | Resectable | No involvement | No involvement | No arterial tumor contact | No involvement |
| Borderline | Abutment ≤180° | Abutment ≤180° | Solid tumor contact ≤180° | Tumor-vessel interface <180° | |
| Locally Advanced | >180° involvement | >180° involvement | >180° solid tumor contact (any portion of pancreas), or any solid tumor contact with the first jejunal branch off SMA (head/uncinate only) | Tumor-vessel interface ≥180° | |
| CHA | Resectable | No involvement | No involvement | No arterial tumor contact | No involvement |
| Borderline | Short segment abutment <180° or encasement ≥180° amenable to reconstruction | Short segment abutment <180° or encasement ≥180° amenable to reconstruction | Solid tumor contact without extension to celiac axis or hepatic bifurcation, allowing for safe/complete reconstruction | Any degree of reconstructible involvement | |
| Locally Advanced | Involvement not amenable to reconstruction | Involvement not amenable to reconstruction | Any solid tumor contact with extension to celiac axis or hepatic bifurcation | Nonreconstructible involvement | |
| SMV/PV | Resectable | Any involvement without occlusion | No involvement | No tumor contact with the SMV/PV or ≤180° contact without vein contour irregularity | Tumor-vessel interface <180°, no occlusion |
| Borderline | Short segment occlusion only, with patent vein above and below the occlusion amenable to surgical reconstruction | Abutment, encasement, and/or occlusion amenable to surgical reconstruction (any involvement) | Solid tumor contact with the SMV/PV of > 180°, contact of ≤ 180° with contour irregularity of the vein, or thrombosis of the vein but with suitable vessel proximal and distal to the site allowing for safe and complete reconstruction | Tumor-vessel interface ≥180° and/or occlusion amenable to surgical reconstruction | |
| Locally Advanced | Non-reconstructible occlusion | Any non-reconstructible involvement or major venous thrombosis extending for several cm | Unreconstructible SMV/PV due to tumor involvement or occlusion (tumor or bland thrombus) | Any non-reconstructible involvement | |
| Head/uncinate only: Contact with most proximal draining jejunal branch into SMV | |||||
This is a point of some controversy and would be considered unresectable according to certain NCCN 2016 panel members as noted in the most recent guidelines.
‘Solid tumor contact’ can also be considered hazy density or stranding of the fat surrounding relevant peripancreatic vessels, reported on staging and follow-up imaging, with resectability decisions made through consensus at multidisciplinary meetings per most recent NCCN guidelines [22].
The next major effort to standardize the classification system for borderline resectable disease was undertaken by the American Hepato-Pancreato-Biliary Association/Society for Surgery of the Alimentary Tract/Society of Surgical Oncology (AHPBA/SSAO/SSO) in 2009. At a consensus conference, experts voted to expand the acceptable criteria for borderline resectable disease to include tumor abutment and encasement, in addition to short-segment occlusion, of the SMV and/or PV (Table 1) [19, 20]. This addition was later incorporated into the NCCN guidelines, which have been revised several times, most recently in 2016 [18, 21, 22].
A third radiographic classification system was proposed by the Alliance for Clinical Trials in Oncology group, in conjunction with multiple additional oncology groups (Southwest, Eastern, Radiation Therapy) and as part of an ongoing multi-institutional trial for patients with borderline resectable cancer (Alliance A021101, and the recently activated A021501 randomized phase II trial), which incorporates neoadjuvant as well adjuvant therapy in addition to surgery [23, 24]. This classification system arose out of a desire for a more simplified and standardized nomenclature in describing borderline resectable disease based on CT imaging criteria. Accordingly, the Alliance system strictly refers to circumferential tumor–vessel interface strictly in terms of degrees of overall contact. The chief difference of the Alliance system is again regarding the allowable extent of SMV/PV involvement, intermediate between the prior definitions, defining only a tumor–vessel interface of greater than or equal to 180° (and/or reconstructable short segment occlusion) as borderline resectable (Table 1). A tumor–vessel interface of less than 180° with the celiac artery and/or SMA is also considered borderline resectable in this definition.
Realistically, resectability status exists along a continuous spectrum, with an increasing incidence of margin-positive resection as involvement of the key vascular structures described above increases [25]. The 2014 update to the NCCN guidelines acknowledged this reality with conclusion that ‘no perfect definition of borderline resectable disease is currently possible because of insufficient data’. The recent update in 2016 still retains an accommodative definition for general practice, in which solid tumor contact with the SMV/PV of >180°, or contact of ≤180° with contour irregularity of the vein, or thrombosis of the vein but with suitable vessel proximal and distal to the site allowing for safe and complete reconstruction, are all considered borderline resectable (Table 1). This stems primarily from concern that too many patients would erroneously be deemed to have resectable disease, not be offered neoadjuvant therapy, and found to be unresectable or have margin-positive resections at surgery [18, 22]. Of note, however, the NCCN panel has previously endorsed the use of the more restrictive but standardized Alliance definition for use in clinical trials, primarily in the interest of uniformity for research purposes.
In summary, there are areas of agreement but also areas of profound inconsistency among the various consensus guidelines released. This creates a diagnostic dilemma for radiologists attempting to provide accurate and reliable CT-based assessments of resectability status. For example, while all guidelines concur that lack of involvement of relevant arterial structures (Celiac/SMA/CHA) is necessary in order to deem a pancreatic adenocarcinoma ‘resectable’, there is significant disparity as to what constitutes the thresholds between resectable/borderline and borderline/locally advanced disease. While any involvement of the celiac artery is sufficient to deem a mass ‘unresectable’ based on MD Anderson/AHPBA criteria, the Alliance definition allows tumor–vessel interface less than 180° in the ‘borderline’ category. NCCN goes even further, allowing any tumor contact 180° or less, but also contact greater than 180° without involvement of the aorta and with intact and uninvolved gastroduodenal artery (Table 1). Complicating matters further, there is even internal conflict within the individual consensus statements. For example, it is noted in the 2016 NCCN guidelines that there is disagreement among various panelists regarding the second celiac criterion (>180° contact without involvement of the aorta and with intact and uninvolved gastroduodenal artery), which some would deem unresectable [22].
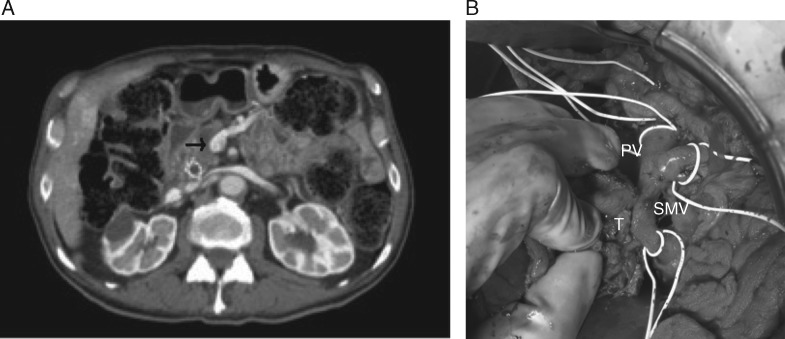
Similar diagnostic dilemmas are present with evaluation of venous involvement. Take the aforementioned example of a mass in which there is greater than 180° of tumor–vessel interface with the SMV/PV as well as contour irregularity, but no occlusion. Because there is greater than 180° of tumor–vessel contact, this would be considered borderline resectable disease according to NCCN criteria, whereas MD Anderson criteria would consider the mass resectable regardless of degree of tumor–vessel contact (provided there is no occlusion) (Table 1). Similarly, if there were less than 180° of contact, NCCN would still consider the mass borderline resectable (due to the contour irregularity); however, now both the Alliance and MD Anderson criteria would consider the mass resectable in the absence of occlusion. Conversely, the AHPBA criteria would deem any degree of involvement sufficient to call the mass borderline resectable. Additionally, if this lesion happened to contact the most proximal draining jejunal branch into the SMV, it would automatically be deemed unresectable according to NCCN criteria, whereas the other consensus statements provide no such caveats (Table 1). This ambiguity in venous involvement definitions can lead to unanticipated margin-positive resections (Figure 8A and B).
Figure 8.
(A) Axial CT image demonstrating less than 180° of tumor–vessel interface with SMV without evidence of distortion, which would be considered resectable disease based on NCCN guidelines. (B) Intraoperative photograph from the same patient demonstrating gross adherence of tumor (T) to SMV near confluence with portal vein (PV). The resection margin was positive.
Clearly, there are numerous unresolved details in the CT determination of resectability status between these various consensus guidelines, highlighting the need for more standardized terminology and validated classification criteria going forward.
Role of neoadjuvant therapy
Neoadjuvant therapy in borderline resectable pancreatic cancer offers several theoretical benefits, including sterilization of margins preoperatively to maximize the chance of R0 resection, as well as treatment of metastases too small to be detected by preoperative imaging [26]. Given that these surgeries are very complex, a non-trivial percentage of patients with borderline resectable disease suffer postoperative complications (the overall morbidity rate among all patients undergoing pancreatic surgery remains high, estimated between 30 and 60%) that delay adjuvant therapy or are otherwise unable to endure additional treatment; thus, the neoadjuvant approach also ensures that patients receive the benefits of multimodality therapy [26, 27].
Several studies utilizing neoadjuvant therapy have shown high R0 resection rates (often ∼90% or higher) (supplementary Table S1, available at Annals of Oncology online) [10, 28–37]. Unfortunately, results are somewhat confounded by low patient numbers and lack of consensus regarding what precisely constitutes borderline resectable disease. As early as 2001, Mehta et al. described 15 patients with ‘marginal’ disease defined by any degree of PV, SMV, and/or SMA involvement, and all nine of whom underwent resection had negative margins [38]. A 2011 study by Stokes et al. included 40 patients with borderline resectable disease (based on MD Anderson criteria), of whom 34 completed neoadjuvant therapy and 16 completed pancreatic resection; in addition, two sets of eight patients received 50 Gy radiation in 20 or 28 fractions over four or six weeks in addition to capecitabine. R0 resection was achieved in 75% (12/16) of patients, and borderline patients completing surgery had similar survival to standard resectable patients undergoing surgery, with improved survival in those who received chemoradiation [39]. These data were corroborated by Katz et al. of the MD Anderson group, who also lamented the relative lack of evidence-based data from prospective trials since the early closure of the Eastern Cooperative Oncology Group Trial 1200 in 2005 (the first trial devoted to studying borderline resectable pancreatic adenocarcinoma) [40, 41]. A major challenge in these studies is the non-standardized nature of the selection process for borderline resectable disease and neoadjuvant therapy due to site bias in treatment regimens. This challenge underscores the need for dedicated multi-institutional trials to aid in development of uniform evidence-based management guidelines.
More recent data remain optimistic. Multiple meta-analyses suggest that up to one third of initially borderline resectable tumors were able to achieve successful margin negative resection following neoadjuvant therapy [42, 43]. A recent review by Winner et al. similarly concluded that, although there is no high-level evidence to universally recommend neoadjuvant therapy, wider use of neoadjuvant therapy in non-metastatic (locally advanced and borderline resectable) disease is likely beneficial [44]. Finally, a recent study evaluated long-term outcomes for both induction chemotherapy and neoadjuvant stereotactic body radiotherapy for 110 borderline resectable and 49 locally advanced pancreatic cancer patients, and found a relatively high R0 resection rate (96%) among the 51% of borderline resectable patients who underwent subsequent surgery with a trend toward improved survival and relatively low rate of grade 3+ radiation-related toxicity among those who underwent neoadjuvant therapy (7%) [45].
While the use of neoadjuvant therapy is the initial treatment approach of choice for borderline resectable disease in many centers [46, 47], the optimal treatment strategy remains an unsettled issue, in part due to uncertainty over inconsistent definitions of the borderline resectable cohort across published trials. However, a majority of NCCN expert panelists in the 2014 guidelines felt that a neoadjuvant approach based on a more inclusive definition of borderline resectable disease was superior. Upfront resection was accordingly downgraded from a category 2A to a 2B recommendation for borderline resectable patients in 2014, with neoadjuvant therapy remaining at category 2A, meaning that a majority of the panel believed the neoadjuvant approach was acceptable in this population of patients. Further research is needed in this area to guide evidence-based treatment regimens, which will be aided by the widespread adoption of more standardized, consistent, and objective Alliance classification criteria for borderline disease, thus facilitating comparison of results between studies.
No comparative randomized clinical trials have been performed between populations who received up front surgery versus neoadjuvant therapy, but several phase I/II trials are underway and will hopefully yield additional insight into the benefits of the neoadjuvant approach. The recently published Alliance A021101 prospective pilot trial demonstrated that among 22 patients with borderline resectable disease, 68% underwent pancreatectomy with a median overall survival of 22 months, demonstrating the feasibility of multimodality therapy in a multi-institutional setting. The recently activated A021501 randomized phase II trial will hopefully help further define preoperative treatment regimens with the goal of future evaluation of the superior arm in future phase III trials [24]. In addition to the ongoing Alliance trial, there is another trial currently underway in the Netherlands by the Dutch Pancreatic Group, entitled the Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC) trial. This randomized, controlled, multicenter trial aims to determine whether preoperative therapy impacts overall survival as measured by increased R0 resection rate as its endpoint [48].
Current practice of preoperative imaging for borderline resectable disease
Accurate staging of pancreatic adenocarcinoma draws upon a large body of information ranging from clinical and laboratory data including tumor markers to tissue sampling and laparoscopy. However, the mainstay remains multi-detector computed tomography (MDCT) due to its wide availability and relative ease of use; it is also overwhelmingly the most well-studied imaging modality in pancreatic cancer. The reported sensitivity for detecting small pancreatic adenocarcinomas with MDCT is as high as 89%–97% [49, 50]. Preoperative imaging is typically performed using a ‘pancreatic protocol’ involving multi-phase acquisitions (including approximately 45 s pancreatic parenchymal and 70 s portal venous phases) with multiplanar reformats and thin-section (<3 mm) reconstructions. Some centers also perform a 25 s early arterial phase CT angiogram for surgical planning purposes, although this is not uniformly accepted [51]. The pancreatic parenchymal phase at 45 s is chosen to maximize the distinction between normally enhancing pancreatic parenchyma and the characteristically hypoenhancing adenocarcinoma. Neutral oral contrast agents such as water are used to distend the stomach and proximal duodenum, and thereby potentially increase the visibility of pancreatic lesions, without the artifacts of standard positive contrast that could potentially obscure subtle lesions [52]. Incorporation of post-processed presentations such as maximum intensity projections (MIPs) into study protocols has also been shown to improve identification of subtle areas of vascular involvement and ability to predict margin-negative/R0 resection given the superior visualization of the surrounding mesenteric vasculature [53, 54]. This is of particular interest in the borderline resectable cancer population given the level of specificity based on CT imaging criteria required to accurately stage these patients. Several studies have also demonstrated the utility of curved multiplanar reformations for cancer detection and evaluation of ductal anatomy and vasculature over axial images alone [55, 56].
While CT is the mainstay for preoperative staging of pancreatic cancer, recent studies have suggested that a dedicated MRI protocol may (due to its superior contrast resolution) have particular value for detection of small (<2 cm) and/or isoattenuating tumors that do not alter contours of adjacent structures, as well as signs of vascular encasement, detection of lymph nodes, small peritoneal metastases, and liver metastases [49, 54, 57–59]. MRI is also of utility in patients with an allergy to standard iodinated CT contrast agents, and for general troubleshooting when an ambiguous or otherwise equivocal finding as identified on CT [49]. However, MRI is uniquely sensitive to several artifacts such as respiratory motion and magnetic susceptibility exacerbated by commonly administered contrast agents (such as gadotexate disodium) that can severely limit accurate detection and staging [49, 60–62]. While the NCCN had previously recommended either pancreatic protocol CT or MRI in initial workup strategy as late as 2014, the latter was subsequently removed and is absent from the most recent 2016 guidelines, confirming the widespread adoption of MDCT as the modality of choice for initial staging [21, 49, 63].
Current practice of imaging during treatment
Radiologic evaluation should occur both prior to and at the conclusion of neoadjuvant therapy [8, 10, 26, 64–66]. Following neoadjuvant therapy, the radiologist should be primarily focused on evaluation for worsening of disease that would preclude resection, such as the development of new metastases or worsening vascular involvement. While infrequent, distinct tumor regression away from vessels on post-neoadjuvant imaging has been associated with a very high rate of R0 resection, irrespective of decrease in tumor size or residual vascular involvement [67]. However, there is growing awareness that clinical response to neoadjuvant therapy may not reliably result in visible regression of the extensive fibrous tumor stroma of pancreatic adenocarcinomas [68].
In a study by Katz et al. of 122 borderline resectable patients treated with various neoadjuvant strategies, 15 patients showed partial response, 84 showed stable disease, and 23 showed progressive disease using standard Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Only one patient had disease downstaged to resectable status by imaging criteria, but 81 of 85 who underwent pancreatectomy after neoadjuvant therapy achieved R0 resection status [69]. In another study by Ferrone et al., 40 patients with locally advanced or borderline cancer received FOLFIRINOX and 87 received no neoadjuvant therapy. Despite imaging after FOLFIRNOX still classifying 19 patients as locally advanced and 9 as borderline, ultimately 92% had a R0 resection [70]. Cassinotto et al. also found that CT after neoadjuvant therapy overestimated tumor size and degree of vascular involvement, reducing the accuracy of predicting R0 resection status [71]. Some authors have indeed concluded that surgery with intent for primary resection and cure should be considered in all operable candidates without evidence of disease progression or metastasis after neoadjuvant therapy [10].
Current research is ongoing to utilize other imaging modalities that may more readily differentiate desmoplastic reaction or tumor fibrosis from true neoplastic tissue. For example, recent studies utilizing quantitative dynamic contrast-enhanced MR imaging to measure fibrotic and vascular density has shown promise in reliably differentiating tumor from non-tumoral tissue [72, 73]. As our understanding of these advanced applications and functional imaging improve, MRI and other modalities may eventually be incorporated into what is now a strictly CT-based set of borderline pancreatic cancer imaging classification criteria. For the time being, however, CT remains the mainstay of imaging.
Conclusion
Borderline resectable pancreatic cancer remains a concept in continued evolution. Accurate imaging-based identification of the subset of patients who would benefit most from neoadjuvant therapy is imperative to improve treatment outcomes and overall survival. Critically, this includes an understanding of the key evolving imaging thresholds of disease severity, both between resectable/borderline and borderline/locally advanced disease, as they specifically relate to increasing risk of positive margin at surgery. Due to the potential for overestimating the extent of vascular involvement following neoadjuvant therapy, radiologists should focus on excluding disease progression rather than expecting tumor regression in response to therapy. The armamentarium of tools at the radiologist’s disposal includes traditional CT with multiplanar reformations, 3D imaging such as MIPs for elucidation of subtle vascular involvement, with PET/CT and MRI performing important and evolving complementary roles. Ongoing trials using more standardized, reproducible Alliance criteria for borderline resectability based on degree of tumor–vessel contact will hopefully provide the foundation for further evidence-based staging and treatment recommendations in this area.
Supplementary Material
Acknowledgements
Support from Hale Center for Pancreatic Cancer Research, NIH/NCI U01 CA210171, Department of Defense CA130288, Lustgarten Foundation, Pancreatic Cancer Action Network, Noble Effort Fund, Peter R. Leavitt Family Fund, Wexler Family Fund, and Promises for Purple to B.M. Wolpin.
Funding
Support from Hale Center for Pancreatic Cancer Research, NIH/NCI U01 CA210171, Department of Defense CA130288, Lustgarten Foundation, Pancreatic Cancer Action Network, Noble Effort Fund, Peter R. Leavitt Family Fund, Wexler Family Fund, and Promises for Purple to B.M. Wolpin.
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Hariharan D, Saied A, Kocher HM.. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008; 10(1): 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel R, Ma J, Zou Z, Jemal A.. Cancer statistics, 2014. CA 2014; 64(1): 9–29. [DOI] [PubMed] [Google Scholar]
- 3. Yeo CJ, Cameron JL, Sohn TA. et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226(3): 248–257; discussion 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo CJ, Cameron JL, Lillemoe KD. et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221(6): 721–731. discussion 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeo CJ, Abrams RA, Grochow LB. et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997; 225(5): 621–633; discussion 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cameron JL, Crist DW, Sitzmann JV. et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 1991; 161(1): 120–124; discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 7. Puleo F, Marechal R, Demetter P. et al. New challenges in perioperative management of pancreatic cancer. World J Gastroenterol 2015; 21(8): 2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varadhachary GR, Tamm EP, Abbruzzese JL. et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006; 13(8): 1035–1046. [DOI] [PubMed] [Google Scholar]
- 9. Sohn TA, Yeo CJ, Cameron JL. et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4(6): 567–579. [DOI] [PubMed] [Google Scholar]
- 10. Schwarz L, Katz MH.. Diagnosis and management of borderline resectable pancreatic adenocarcinoma. Hematol Oncol Clin North Am 2015; 29(4): 727–740. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi S, Kinoshita T, Konishi M. et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepato-Biliary-Pancreatic Sci 2011; 18(4): 567–574. [DOI] [PubMed] [Google Scholar]
- 12. Tamburrino D, Partelli S, Crippa S. et al. Selection criteria in resectable pancreatic cancer: a biological and morphological approach. WJG. 2014; 20(32): 11210–11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allema JH, Reinders ME, van Gulik TM. et al. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. Br J Surg 1994; 81(11): 1642–1646. [DOI] [PubMed] [Google Scholar]
- 14. Leach SD, Lee JE, Charnsangavej C. et al. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg 1998; 85(5): 611–617. [DOI] [PubMed] [Google Scholar]
- 15. Katz MH, Pisters PW, Evans DB. et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008; 206(5): 833–846; discussion 46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. La Torre M, Nigri G, Lo Conte A. et al. Is a preoperative assessment of the early recurrence of pancreatic cancer possible after complete surgical resection? Gut Liver 2014; 8(1): 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarz L, Lupinacci RM, Svrcek M. et al. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg 2014; 101(5): 530–538. [DOI] [PubMed] [Google Scholar]
- 18. Tempero MA, Malafa MP, Behrman SW. et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compreh Cancer Netw 2014; 12(8): 1083–1093. [DOI] [PubMed] [Google Scholar]
- 19. Callery MP, Chang KJ, Fishman EK. et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009; 16(7): 1727–1733. [DOI] [PubMed] [Google Scholar]
- 20. Vauthey JN, Dixon E.. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol 2009; 16(7): 1725–1726. [DOI] [PubMed] [Google Scholar]
- 21. Tempero MA, Arnoletti JP, Behrman SW. et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compreh Cancer Netw 2012; 10(6): 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NCCN. Pancreatic Adenocarcinoma (Version 2.2016) 2016. Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (12 January 2016, date last accessed).
- 23. Katz MH, Marsh R, Herman JM. et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013; 20(8): 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz MH, Shi Q, Ahmad SA. et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg 2016; 151(8): e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez NE, Prendergast C, Lowy AM.. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol 2014; 20(31): 10740–10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varadhachary GR. Preoperative therapies for resectable and borderline resectable pancreatic cancer. J Gastrointest Oncol 2011; 2(3): 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho CK, Kleeff J, Friess H, Buchler MW.. Complications of pancreatic surgery. HPB (Oxford). 2005; 7(2): 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang K, Lu W, Qin W, Wu Y.. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. Pancreatology 2016; 16(1): 28–37. [DOI] [PubMed] [Google Scholar]
- 29. Rose JB, Rocha FG, Alseidi A. et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol 2014; 21(5): 1530–1537. [DOI] [PubMed] [Google Scholar]
- 30. Motoi F, Unno M, Takahashi H. et al. Influence of preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: project study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2014; 21(2): 148–158. [DOI] [PubMed] [Google Scholar]
- 31. Tzeng CW, Balachandran A, Ahmad M. et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB 2014; 16(5): 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi H, Ohigashi H, Gotoh K. et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 2013; 258(6): 1040–1050. [DOI] [PubMed] [Google Scholar]
- 33. Chuong MD, Springett GM, Freilich JM. et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013; 86(3): 516–522. [DOI] [PubMed] [Google Scholar]
- 34. Kang CM, Chung YE, Park JY. et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg 2012; 16(3): 509–517. [DOI] [PubMed] [Google Scholar]
- 35. Chun YS, Cooper HS, Cohen SJ. et al. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann Surg Oncol 2011; 18(13): 3601–3607. [DOI] [PubMed] [Google Scholar]
- 36. Brown KM, Siripurapu V, Davidson M. et al. Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. Am J Surg 2008; 195(3): 318–321. [DOI] [PubMed] [Google Scholar]
- 37. Turrini O, Viret F, Moureau-Zabotto L. et al. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology 2009; 76(6): 413–419. [DOI] [PubMed] [Google Scholar]
- 38. Mehta VK, Fisher G, Ford JA. et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001; 5(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 39. Stokes JB, Nolan NJ, Stelow EB. et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011; 18(3): 619–627. [DOI] [PubMed] [Google Scholar]
- 40. Katz MH, Pisters PW, Lee JE, Fleming JB.. Borderline resectable pancreatic cancer: what have we learned and where do we go from here?. Ann Surg Oncol 2011; 18(3): 608–610. [DOI] [PubMed] [Google Scholar]
- 41. Landry J, Catalano PJ, Staley C. et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol 2010; 101(7): 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Assifi MM, Lu X, Eibl G. et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 2011; 150(3): 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gillen S, Schuster T, Meyer Zum Buschenfelde C. et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010; 7(4): e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winner M, Goff SL, Chabot JA.. Neoadjuvant therapy for non-metastatic pancreatic ductal adenocarcinoma. Semin Oncol 2015; 42(1): 86–97 [DOI] [PubMed] [Google Scholar]
- 45. Mellon EA, Hoffe SE, Springett GM. et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015; 54(7): 979–985. [DOI] [PubMed] [Google Scholar]
- 46. Abrams RA, Lowy AM, O'Reilly EM. et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009; 16(7): 1751–1756. [DOI] [PubMed] [Google Scholar]
- 47. Evans DB, Erickson BA, Ritch P.. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol 2010; 17(11): 2803–2805. [DOI] [PubMed] [Google Scholar]
- 48. Versteijne E, van Eijck CH, Punt CJ. et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016; 17(1): 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pietryga JA, Morgan DE.. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol 2015; 6(4): 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wong JC, Lu DS.. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol 2008; 6(12): 1301–1308. [DOI] [PubMed] [Google Scholar]
- 51. Fletcher JG, Wiersema MJ, Farrell MA. et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi–detector row CT. Radiology 2003; 229(1): 81–90. [DOI] [PubMed] [Google Scholar]
- 52. Horton KM, Fishman EK.. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am 2002; 40(6): 1263–1272. [DOI] [PubMed] [Google Scholar]
- 53. House MG, Yeo CJ, Cameron JL. et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J of Gastrointest Surg 2004; 8(3): 280–288. [DOI] [PubMed] [Google Scholar]
- 54. Raman SP, Chen Y, Fishman EK.. Cross-sectional imaging and the role of positron emission tomography in pancreatic cancer evaluation. Semin Oncol 2015; 42(1): 40–58. [DOI] [PubMed] [Google Scholar]
- 55. Fukushima H, Itoh S, Takada A. et al. Diagnostic value of curved multiplanar reformatted images in multislice CT for the detection of resectable pancreatic ductal adenocarcinoma. Eur Radiol 2006; 16(8): 1709–1718. [DOI] [PubMed] [Google Scholar]
- 56. Kakihara D, Yoshimitsu K, Irie H. et al. Usefulness of the long-axis and short-axis reformatted images of multidetector-row CT in evaluating T-factor of the surgically resected pancreaticobiliary malignancies. Eur J Radiol 2007; 63(1): 96–104. [DOI] [PubMed] [Google Scholar]
- 57. Vachiranubhap B, Kim YH, Balci NC, Semelka RC.. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging 2009; 20(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 58. Tamm EP, Bhosale PR, Vikram R. et al. Imaging of pancreatic ductal adenocarcinoma: State of the art. WJR. 2013; 5(3): 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim JH, Park SH, Yu ES. et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010; 257(1): 87–96. [DOI] [PubMed] [Google Scholar]
- 60. Davenport MS, Viglianti BL, Al-Hawary MM. et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 2013; 266(2): 452–461. [DOI] [PubMed] [Google Scholar]
- 61. Pietryga JA, Burke LM, Marin D. et al. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 2014; 271(2): 426–434. [DOI] [PubMed] [Google Scholar]
- 62. Bashir MR, Castelli P, Davenport MS. et al. Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology 2015; 274(1): 141–8. [DOI] [PubMed] [Google Scholar]
- 63. Bockhorn M, Uzunoglu FG, Adham M. et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014; 155(6): 977–988. [DOI] [PubMed] [Google Scholar]
- 64. Evans DB, Varadhachary GR, Crane CH. et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. JCO 2008; 26(21): 3496–3502. [DOI] [PubMed] [Google Scholar]
- 65. Varadhachary GR, Evans DB.. Rational study endpoint(s) for preoperative trials in pancreatic cancer: pathologic response rate, margin negative resection, overall survival or ′all of the above′? Ann Surg Oncol 2013; 20(12): 3712–3714. [DOI] [PubMed] [Google Scholar]
- 66. Varadhachary GR, Wolff RA, Crane CH. et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. JCO 2008; 26(21): 3487–3495. [DOI] [PubMed] [Google Scholar]
- 67. Cassinotto C, Mouries A, Lafourcade JP. et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 2014; 273(1): 108–116. [DOI] [PubMed] [Google Scholar]
- 68. Wagner M, Antunes C, Pietrasz D. et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 2016; doi: 10.1007/s00330-016-4632-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69. Katz MH, Fleming JB, Bhosale P. et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012; 118(23): 5749–5756. [DOI] [PubMed] [Google Scholar]
- 70. Ferrone CR, Marchegiani G, Hong TS. et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015; 261(1): 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cassinotto C, Cortade J, Belleannee G. et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol 2013; 82(4): 589–593. [DOI] [PubMed] [Google Scholar]
- 72. Bali MA, Metens T, Denolin V. et al. Pancreatic perfusion: noninvasive quantitative assessment with dynamic contrast-enhanced MR imaging without and with secretin stimulation in healthy volunteers–initial results. Radiology 2008; 247(1): 115–121. [DOI] [PubMed] [Google Scholar]
- 73. Bali MA, Metens T, Denolin V. et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology 2011; 261(2): 456–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.