Abstract
Background
Based upon preclinical synergy in murine models, we carried out a phase I trial to determine the maximum tolerated dose (MTD), toxicities, pharmacokinetics, and biomarkers of response for the combination of BKM120, a PI3K inhibitor, and olaparib, a PARP inhibitor.
Patients and methods
Olaparib was administered twice daily (tablet formulation) and BKM120 daily on a 28-day cycle, both orally. A 3 + 3 dose-escalation design was employed with the primary objective of defining the combination MTD, and secondary objectives were to define toxicities, activity, and pharmacokinetic profiles. Eligibility included recurrent breast (BC) or ovarian cancer (OC); dose-expansion cohorts at the MTD were enrolled for each cancer.
Results
In total, 69 of 70 patients enrolled received study treatment; one patient never received study treatment because of ineligibility. Twenty-four patients had BC; 46 patients had OC. Thirty-five patients had a germline BRCA mutation (gBRCAm). Two DLTs (grade 3 transaminitis and hyperglycemia) were observed at DL0 (BKM120 60 mg/olaparib and 100 mg b.i.d.). The MTD was determined to be BKM120 50 mg q.d. and olaparib 300 mg b.i.d. (DL8). Additional DLTs included grade 3 depression and transaminitis, occurring early in cycle 2 (DL7). Anticancer activity was observed in BC and OC and in gBRCAm and gBRCA wild-type (gBRCAwt) patients.
Conclusions
BKM120 and olaparib can be co-administered, but the combination requires attenuation of the BKM120 dose. Clinical benefit was observed in both gBRCAm and gBRCAwt pts. Randomized phase II studies will be needed to further define the efficacy of PI3K/PARP-inhibitor combinations as compared with a PARP inhibitor alone.
Keywords: BKM120, olaparib, ovarian cancer, breast cancer, PARP-inhibitors
Introduction
The Cancer Genome Atlas has revealed shared genomic alterations of high-grade serous OC (HGSC) and triple negative BC (TNBC) including extensive copy number alterations, p53 mutations, PI3K pathway activation, and deficiencies in DNA damage repair and homologous recombination (HR) [1, 2] providing a rationale for examining similar treatment concepts in both diseases. Poly (ADP ribose) polymerase (PARP) inhibitors interfere with DNA damage repair and exhibit single agent activity in recurrent gBRCAm and gBRCAwt OC and BC [3–6]. However, despite the presence of PIK3CA amplification in HGSC and PIK3CA mutations in endometrial and breast cancer (BC), treatments with single agent PI3K inhibitors have had limited efficacy [7, 8]. Preclinical data supporting the combination and synergy of a PI3K and PARP inhibitor was derived from genetically engineered and patient-derived xenograft (PDX) mouse models and both demonstrated substantial improvement over single agent activity when the PI3K inhibitor BKM120 and PARP inhibitor olaparib were co-administered [9–11]. The underlying rationale is that PI3K inhibitors enhance the efficacy of PARP inhibitors through their antimetabolic activity [9, 10]. PI3K inhibition leads to decreased flux through the non-oxidative pentose phosphate pathway (PPP) that produces ribose-5-phosphate required for nucleoside synthesis [10]. Thus, PI3K inhibitors lower nucleotide pools required for DNA synthesis and S-phase progression, sensitizing BC cells to PARP inhibitor treatments [10]. Additionally, in gBRCAwt breast tumors, PI3K inhibition is thought to decrease BRCA1 expression through transcriptional regulation [11].
The pan-PI3K inhibitor BKM120 has been examined in phase I studies [8, 12–14] that reported disease stability in 30%–40% of patients as best responses, and a partial response (PR) was seen in one patient with TNBC [8]. Olaparib has single agent activity with response rates of 41% in gBRCAm and 24% in gBRCAwt OC; objective responses in BC were not seen in this study [6]. Notably, only one of 26 patients with platinum-resistant gBRCAwt OC (4%) had a response to olaparib [6]. In different series [4, 15], 13%–41% of BRCAm BC patients achieved objective responses.
The primary objective of this phase I dose escalation study was to determine the MTD and recommended phase 2 dose (RP2D) of the combination of BKM120 and olaparib. Secondary objectives were to define the safety and toxicity, anticancer activity and pharmacokinetic interactions of the combination.
Methods
Study design and treatment
The phase I study used a 3 + 3 design, dose escalating if 0/3 or 1/6 participants experienced a DLT during the first cycle of therapy (first 28 days) (see supplementary data, available at Annals of Oncology online). Once the MTD and RP2D were determined, up to 12 patients were entered into two separate expansion cohorts, one for OC and another for BC. BKM120 and olaparib were administered p.o., daily (q.d.) and continuously; BKM120 was given q.d. and olaparib b.i.d. Tumor assessment by RECIST 1.1 occurred every two cycles. PK blood samples were collected for BKM120 and olaparib on Day 1, time 0 and then days 8 and 15 at time 0, 1, 2, 4, and 8 h of cycle 1.
Eligibility
Patients were eligible if they had a confirmed diagnosis of either recurrent ovarian, fallopian tube, or primary peritoneal cancer (collectively referred to as ‘OC’), HGSC or TNBC histology; diagnosis of BC or ovarian other than HGSC or TNBC but with known gBRCAm; RECIST 1.1 measurable or evaluable (BC only) disease, normal organ function, and ability to provide informed consent. Prior PARP inhibitor use was allowed for patients in dose escalation but not in the dose expansion cohort; no prior PI3K inhibitor use was allowed. Only patients with a known deleterious BRCA mutation were classified as BRCAm positive.
Results
Seventy patients were enrolled into the study between October 2012 and November 2014. During the dose-escalation, 47 patients were enrolled: 35 patients with OC (26 ovarian, six fallopian tube, three primary peritoneal) and 12 patients with BC. Twenty-three patients were enrolled in the dose-expansion cohorts: 11 patients with OC and 12 patients with BC. One patient was deemed ineligible after signing the informed consent and never received study drug. The cutoff date for analysis was 5 April 2015 when 60 of 69 patients (78%) had stopped study treatment.
Patient characteristics are summarized in Table 1. Of the 46 women with OC, 89% had HGSC, and 70% were gBRCAm positive, 20% were gBRCAwt, and 10% were unknown/not tested. Of the BC patients, 54% had a diagnosis of TNBC, and 46% were hormone receptor positive (either ER+ and/or PR+); 63% were gBRCAm, 29% gBRCAwt, and 8% unknown/not tested. Median ages of the OC and BC patients were 60 and 48 years of age, respectively. In those patients who were negative for a known deleterious germline mutation in BRCA1 or BRCA2, no somatic mutations or mutations of unknown significance were found in patients whose DNA was available for WES or MSKImpact [17].
Table 1.
Patient and tumor characteristics
| Characteristic | Ovarian cancer | Breast cancer |
|---|---|---|
| Number of patients | 46 | 24 |
| Age, years | ||
| Median (range) | 60 (34–78) | 48 (27–70) |
| Histology | ||
| High-grade serous | 41 (89%) | – |
| High-grade endometrioid | 1 (2%) | – |
| Carcinosarcoma | 2 (4%) | – |
| Poorly differentiated carcinoma | 2 (4%) | – |
| Platinum status | ||
| Platinum resistant | 26 (57%) | – |
| Platinum sensitive | 20 (43%) | – |
| Breast cancer subtypea | ||
| Triple negative | – | 13 (54%) |
| Hormone receptor positive | – | 11 (46%) |
| ECOG performance status | ||
| 0 | 32 (70%) | 17 (71%) |
| 1 | 14 (30%) | 7 (29%) |
| Germline BRCA status | ||
| gBRCAm | 32 (70%) | 15 (63%) |
| wt BRCA | 9 (20%) | 7 (29%) |
| Unknown | 5 (10%) | 2 (8%) |
All breast cancer patients tested Her2-negative by FISH or IHC standard criteria.
Ten dose combinations were evaluated (supplementary Table S1, available at Annals of Oncology online). The starting dose, DL0, was BKM120 60 mg q.d. and olaparib 100 mg b.i.d. Two DLTs occurred during DL0, one patient with grade 3 hyperglycemia and another with grade 3 transaminitis who was found to have liver metastases after undergoing repeat diagnostic imaging after cycle 1. The doses were then decreased to BKM120 40 mg q.d. and olaparib 50 mg b.i.d. (DL-1) which were deemed safe. Doses were then escalated (supplementary Table S1, available at Annals of Oncology online) to BKM120 60 mg q.d. and olaparib 300 mg b.i.d. (DL7). At DL7, there were no DLT’s during cycle 1, but grade 4 transaminase elevation occurred in one patient on cycle 2, day 8 as well as grade 3 depression in another patient on cycle 2, day 10, both episodes resulting in hospitalization and both considered dose-limiting; doses were then de-escalated to BKM120 50 mg q.d. and olaparib 300 mg b.i.d. (DL8) which were subsequently deemed safe and selected as the MTD. Patients on the expansion cohort were treated at this dose level.
Related non-hematologic and hematologic toxicities occurring in ≥10% of all treated patients (n = 69) are listed in Table 2. There were no unexpected toxicities observed based on the known toxicities of olaparib and BKM120. Nausea and fatigue were the most common toxicities occurring in 78% and 65% of patients respectively, mostly grades 1 and 2. Hyperglycemia, an expected toxicity of PI3K-inhibitors, was seen in 39% of patients, mostly grades 1 and 2. Transaminase elevations, a known toxicity of PI3K inhibitors, were also observed in 20% of patients and were responsible for two DLT’s as described above. Depression and anxiety, which were observed in 36% and 28% of patients, respectively are known toxicities of BKM120; these toxicities were reversible with either dose reductions or cessation of BKM120 based on the toxicity grade. Anemia, an expected toxicity of olaparib, occurred at an incidence of 23%, mostly grades 1 and 2 (Table 2). Neutropenia was observed in 12% of patients, and thrombocytopenia and lymphopenia were each observed in 10% of patients.
Table 2.
Study treatment-related toxicities occurring in ≥10% of patients
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Non-hematologic toxicities | |||||
| Nausea | 42 (61%) | 10 (14%) | 2 (3%) | 0 | 54 (78%) |
| Fatigue | 30 (43%) | 13 (19%) | 2 (3%) | 0 | 45 (65%) |
| Hyperglycemia | 18 (26%) | 8 (12%) | 1 (1%) | 0 | 27 (39%) |
| Anorexia | 17 (25%) | 8 (12%) | 1 (1%) | 0 | 26 (38%) |
| Depression | 15 (22%) | 6 (9%) | 3 (4%) | 1 (1%) | 25 (36%) |
| Diarrhea | 21 (30%) | 3 (4%) | 0 | 0 | 24 (35%) |
| Anxiety | 13 (19%) | 5 (7%) | 1 (1%) | 0 | 19 (28%) |
| Mucositis | 12 (17%) | 4 (6%) | 0 | 0 | 16 (23%) |
| Vomiting | 13 (19%) | 1 (1%) | 1 (1%) | 0 | 15 (22%) |
| ↑ALT | 7 (10%) | 3 (4%) | 3 (4%) | 1 (1%) | 14 (20%) |
| ↑AST | 8 (12%) | 2 (3%) | 4 (6%) | 0 | 14 (20%) |
| Dysgeusia | 9 (13%) | 3 (4%) | 0 | 0 | 12 (17%) |
| Dyspepsia | 5 (7%) | 4 (6%) | 0 | 0 | 9 (13%) |
| Constipation | 7 (10%) | 0 | 0 | 0 | 7 (10%) |
| ↑Creatinine | 7 (10%) | 0 | 0 | 0 | 7 (10%) |
| Dizziness | 6 (9%) | 0 | 1 (1%) | 0 | 7 (10%) |
| Hematologic toxicities | |||||
| Anemia | 10 (14%) | 3 (4%) | 2 (3%) | 1 (1%) | 16 (23%) |
| Neutropenia | 0 | 6 (9%) | 1 (1%) | 1 (1%) | 8 (12%) |
| Thrombocytopenia | 6 (9%) | 1 (1%) | 0 | 0 | 7 (10%) |
| Lymphopenia | 2 (3%) | 5 (7%) | 0 | 0 | 7 (10%) |
Best overall response using RECIST 1.1 criteria was assessable in 59 of 69 treated patients (86%) (Table 3). All patients had measurable disease at baseline. Tumor measurements at restaging were not available for seven non-responders who came off study before first restaging. Nine of 17 PRs were confirmed, confirmation of response was not required. Activity of the combination was similar for both cancer types, with a 29% response rate for OC (90%CI: 18%–43%) irrespective of platinum-sensitivity status and a 28% response rate for BC (90%CI: 12%–50%); all PRs. Slightly less than half of patients with both cancers had stable disease (SD) as best overall response. The median duration of SD for assessable patients without progressive disease as best overall response (n = 45) was 6.9 months (90%CI: 5.5–7.5 months). Among all treated patients, 16 of 45 OC patients (36%) and 8 of 24 BC patients (33%) achieved disease stability for >6 months.
Table 3.
Response to treatment
| RECIST 1.1 Response | Ovarian cancer | Breast cancer | Total |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Number treateda | 45 | 24 | 69 |
| Not assessable | 4 (9%) | 6 (25%) | 10 (14%) |
| Non-measurable disease | 3 (7%) | 6 (25%) | 9 (13%) |
| Discontinued cycle 1 without PD | 1 (2%) | 0 | 1 (1%) |
| Number assessable | 41 | 18 | 59 |
| Complete response | 0 | 0 | 0 |
| Partial response | 12 (29%) | 5 (28%) | 17 (29%) |
| Stable disease | 20 (49%) | 8 (44%) | 28 (47%) |
| Progressive disease | 9 (22%) | 5 (28%) | 14 (24%) |
| Response by BRCA status | |||
| gBRCAm | 28 | 12 | 40 |
| Partial response | 8 (29%) | 4 (33%) | 12 (30%) |
| Stable disease | 13 (46%) | 5 (42%) | 18 (45%) |
| Progressive disease | 7 (25%) | 3 (25%) | 10 (25%) |
| gBRCAwt | 8 | 5 | 13 |
| Partial response | 1 (12%) | 1 (20%) | 2 (15%) |
| Stable disease | 5 (62%) | 2 (40%) | 7 (54%) |
| Progressive disease | 2 (25%) | 2 (40%) | 4 (31%) |
| Unknown | 5 | 1 | 6 |
| Partial response | 3 (60%) | 0 | 3 (50%) |
| Stable disease | 2 (40%) | 1 (100%) | 3 (50%) |
| Progressive disease | 0 | 0 | 0 |
| Response by platinum status | |||
| Platinum resistant | 22 | – | – |
| Partial response | 6 (27%) | – | – |
| Stable disease | 11 (50%) | – | – |
| Progressive disease | 5 (23%) | – | – |
| Platinum sensitive | 19 | – | – |
| Partial response | 6 (32%) | – | – |
| Stable disease | 9 (47%) | – | – |
| Progressive disease | 4 (21%) | – | – |
Response to treatment of all patients based on RECIST 1.1 and BRCA status. One patient was deemed ineligible before initiating treatment.
Of the originally consented 46 patients, one withdrew consent before treatment.
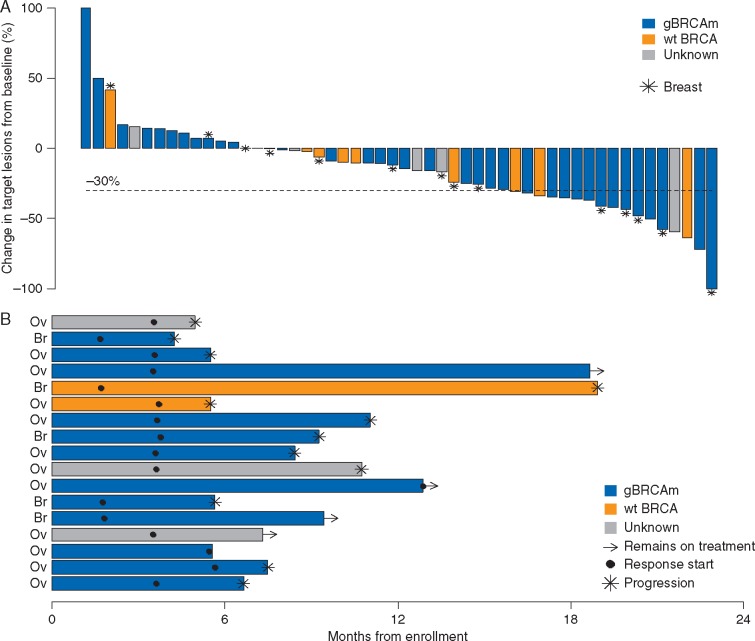
Thirty-seven of 52 assessable patients with restaging radiographic imaging showed tumor shrinkage from baseline (Figure 1A). Among the 17 patients with PR’s, 12 progressed while on treatment, one discontinued treatment of intolerability, and four remain on study treatment, with a median duration of response of 4.8 months by Kaplan–Meier estimation (Figure 1B). In the OC cohort, all of the patients with a PR had HGSC histology, and 8 of the 12 patients who achieved a PR were known gBRCAm carriers; we were not able to obtain sufficient quality DNA in those four ovarian cancers (OCs) that achieved partial remissions and were gBRCAwt. In the patients with BC who exhibited a tumor response, four out of the five patients with a PR had a gBRCAm; one patient who had a PR had TNBC and was gBRCAwt. Supplementary Figure S1, available at Annals of Oncology online, describes a patient with a gBRCA2m and ER+/PR+ HER2 negative BC who achieved a PR.
Figure 1.
Response to study treatment. (A) Summary of the maximum reduction in target lesion size in n = 52 patients with measurable disease and restaging scans. (B) Summary of the duration of response for the 17 patients who achieved a partial response by RECIST 1.1 criteria labeled by cancer type. The interval from date of enrollment to date of either progression or date of last disease assessment is displayed along with the first disease assessment to achieve partial response. Median duration of response was 4.8 months (maximum 17.2 months).
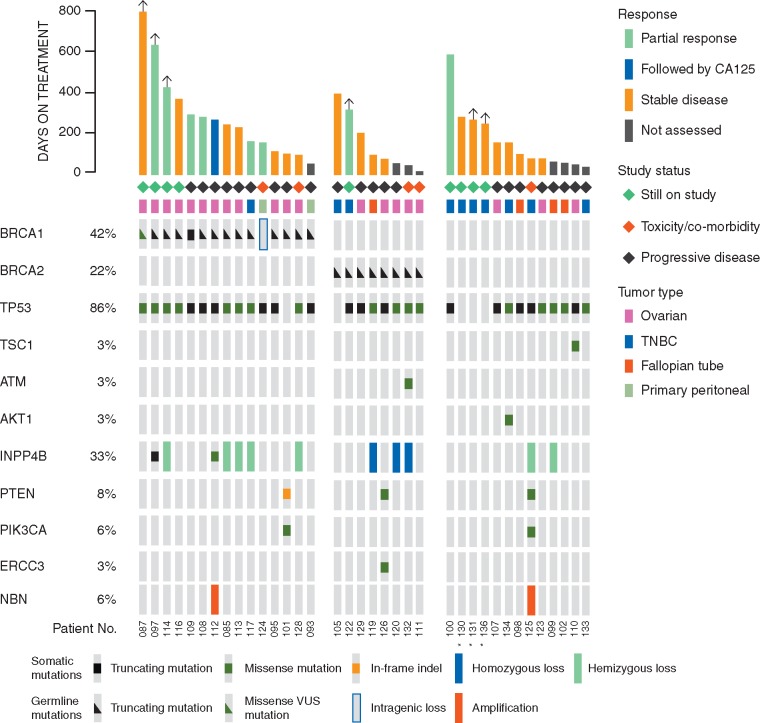
To identify potentially predictive markers for this combination, we examined archival tumor tissue from 40 patients enrolled in this study by Next Generation Sequencing (NGS, MSKImpact panel [17]); of these 36 were also subjected to Whole Exome Sequencing (WES) (supplementary Figure S2, available at Annals of Oncology online). Sixty-four percent were germline mutation carriers for BRCA1 (42%) or BRCA2 (22%) (Figure 1B). Forty-four percent of patients had a genetic alteration that could activate the PI3K pathway, but there was no apparent relationship between the presence of these and response to the treatment combination (Figure 2).
Figure 2.
Genomic aberrations in the PI3K pathway or in DNA damage repair in patients with metastatic breast or ovarian cancer on this study. Data are shown for the 36 patients who could be evaluated for a response and in whom sufficient material was available as described in supplementary Figure S1, available at Annals of Oncology online. Mutations and were identified through next-generation sequencing (MSK-IMPACT panel). Not shown are FANCA, CHEK2, PALB2, HDAC2, RAD51, MLH3, and MRE11 (no mutations), ERCC3 (mutated in one patient) and NBN (amplified in two patients). An asterisk marks cases with low DNA purity, and low frequency mutations may be present.
PK results for BKM120 and olaparib are located in supplementary Tables S2 and S3, available at Annals of Oncology online. Steady state Cmax values determined on day 8, 2 h post dosing for both olaparib and BKM120 appear comparable to values when testing both of these agents as single agents in the phase I setting, i.e. drug exposures increased appropriately with increasing dose [3, 14]. BKM120 Cmax results appeared unaffected by olaparib dosing, and olaparib Cmax results appeared unaffected by BKM120 dosing. However, at the higher doses, PKs varied as much as fivefold for each drug (supplementary Tables S2 and S3, available at Annals of Oncology online). None of the patients with DLT toxicities had abnormally elevated steady state Cmax levels of either olaparib or BKM120.
Despite the molecular similarities of TNBC and HGSOC [1, 2], there are notable differences, namely the greater genomic instability of OC and the predominance of PIK3CA amplifications rather than activating mutations. We carried out preclinical experiments with OC PDX to examine if the PI3K-inhibitor enhanced the efficacy of the PARP inhibitor olaparib similar to what was found in BC [9, 11, 16]. We used PDX derived from 10 different OC patients, none of whom were enrolled on this study) (supplementary files ‘Murine ovarian cancer PDX models’ and Figures S3 and S4, available at Annals of Oncology online). In 7/10 PDX-models, the combination of BKM120 with olaparib showed better outcomes than the PARP inhibitor alone (supplementary Figure S4, available at Annals of Oncology online). There was, however, no obvious correlation between baseline status of PIK3CA or DNA damage repair parameters and response to the BKM120 and olaparib combination in these PDX models.
Discussion
The rationale for this phase I study of the oral PI3K inhibitor BKM120 and the oral PARP inhibitor olaparib was based on preclinical work showing in vivo synergy of this combination [9, 11]. Both agents were able to be combined in this study with RECIST 1.1 responses occurring in both recurrent ovarian and BC patients; the MTD was BKM120 50 mg q.d. and olaparib 300 mg b.i.d. Overall, the combination was well tolerated, but toxicities prevented further escalation of BKM120 which included CNS toxicity, specifically grade 3 depression and grade 3 transaminase elevation, both known toxicities of BKM120. In addition, no predictive genotypic markers using NGS were identified in this study for the combination.
Though the PK’s of both olaparib and BKM120 did not show any drug drug interactions (DDI), we were not able to dose escalate BKM120 beyond 50 mg; the single agent MTD of BKM120 is 100 mg. Therefore, there are likely DDI between BKM120 and olaparib possibly leading to cumulative BKM120 drug levels given the delayed DLT toxicities observed early in cycle 2 in two patients (grade 3 transaminase elevations and grade 3 depression), providing rationale for extended PK testing beyond completion of cycle 1. An amendment to this phase I study adding dose escalation testing for the alpha specific PI3K inhibitor BYL719 combined with olaparib is now underway given this PI3K inhibitor’s selectivity on the PI3K pathway and absence of CNS toxicities.
Our group has previously established and characterized a group of OC PDX models that faithfully model the clinical spectrum and responsiveness to standard-of-care chemotherapy [18]. In this mouse model system of HGSC, the addition of BKM120 improved responses over olaparib monotherapy in both BRCA-related and unrelated OC (supplementary Figure S4, available at Annals of Oncology online), similar to what was seen previously in BC [9, 11]. Our approach, using human OC PDX models rather than select cell lines, models the inter-individual variability of patients tumors, and as expected, responses were also highly variable (supplementary Figure S4, available at Annals of Oncology online). However, we did not identify any biomarkers that predicted response, thus underscoring the complexity of the biology underlying responses to this regimen even in a mouse model; this raises the possibility that a constellation of genomic or proteomic markers might be predictive.
Our study demonstrated a response rate of 29% in advanced OC and 28% in BC patients with measurable disease. Of the women with OC, all of the patients who responded had HGSC histology, and the majority (8 out of 12 responders) had a known gBRCAm. There was no difference in response to the BKM120 + olaparib regimen between OC patients with platinum-sensitive versus platinum-resistant disease. In previous studies overall response rates to olaparib in gBRCAmt OC was 28% [5], and response rates of olaparib in platinum-resistant patients is usually lower than observed with platinum-sensitive patients [5, 6]. Within the group of BC patients with gBRCAm, 4 out of 12 patients had a PR and 5 out of 12 patients had SD, which translates into a clinical benefit for the majority of these patients; even more remarkably, one out of five patients with gBRCAwt had a prolonged PR and two out of five BC patients with gBRCAwt had SD and continued on study at the time of the data lock. These observations in a small cohort of patients do raise the possibility that the PI3K and PARP inhibitor combination might improve outcomes over olaparib alone for BC patients. For OC patients who are gBRCAm, it is impossible to determine the benefit of the combination of olaparib and BKM120, given the known activity of olaparib in this patient population; in fact, the U.S. Food and Drug Administration (FDA) granted accelerated approval to olaparib in December 2014 for patients with gBRCAm recurrent OC. Additional biomarker strategies besides presence of a gBRCAm or a somatic BRCAm will be needed to select patients who could benefit from the combination versus single agent olaparib given the activity of olaparib in both gBRCAm and gBRCAwt ovarian and BC.
Funding
Ursula Matulonis: Ovarian Cancer Research Foundation, Breast Cancer Research Foundation. William Barry: CJL Foundation. Gordon Mills: Adelson Medical Research Foundation and 5 P50CA083639-13. Gerburg Wulf: Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209), by the Breast Cancer Research Foundation, Mary Kay Ash Foundation, by the Men’s Initiative of the Dana-Farber Harvard Cancer Center and the Breast Cancer Alliance. Lewis Cantley: R01-GM041890. Shannon Westin: National Institutes of Health K12CA088084 K12 Calabresi Scholar Award, National Institutes of Health 2P50CA083639 SPORE in Ovarian Cancer. Joyce Liu: Ovarian Cancer Research Foundation.
Disclosure
UAM: consultant for Astrazeneca, Tesaro, Genentech, Pfizer, and Immunogen. GBM: consultant for Astrazeneca, receives sponsored research support from Astrazeneca and holds a patent related to this manuscript. KMB-M: consultant for Astrazeneca, Clovis, travel funding by Abbvie to attend FDA and EMA meetings (last in May 2014). EW: unpaid consultant to Astrazeneca and Novartis. LC: consultant for Novartis, Genentech and Pfizer. SNW: consultant for AstraZeneca, Medivation, Genentech, sponsored research support from AstraZeneca and Novartis. JL: consultant for Astrazeneca. CW: consultant for Astrazeneca. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fong PC, Boss DS, Yap TA. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009; 361: 123–134. [DOI] [PubMed] [Google Scholar]
- 4. Tutt A, Robson M, Garber JE. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376: 235–244. [DOI] [PubMed] [Google Scholar]
- 5. Matulonis UA, Penson RT, Domchek SM. et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol 2016; 27: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 6. Gelmon KA, Tischkowitz M, Mackay H. et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011; 12: 852–861. [DOI] [PubMed] [Google Scholar]
- 7. Matulonis U, Vergote I, Backes F. et al. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol Oncol 2015; 136: 246–253. [DOI] [PubMed] [Google Scholar]
- 8. Rodon J, Brana I, Siu LL. et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs 2014; 32: 670–681. [DOI] [PubMed] [Google Scholar]
- 9. Juvekar A, Burga LN, Hu H. et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov 2012; 2: 1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu H, Juvekar A, Lyssiotis CA. et al. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 2016; 164: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibrahim YH, Garcia-Garcia C, Serra V. et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012; 2: 1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu YL, Zhang LI, Trandafir L. et al. Phase I study of the pan-PI3K inhibitor buparlisib in adult Chinese patients with advanced solid tumors. Anticancer Res 2016; 36: 6185–6194. [DOI] [PubMed] [Google Scholar]
- 13. Ando Y, Inada-Inoue M, Mitsuma A. et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci 2014; 105: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bendell JC, Rodon J, Burris HA. et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012; 30: 282–290. [DOI] [PubMed] [Google Scholar]
- 15. Kaufman B, Shapira-Frommer R, Schmutzler RK. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JF, Palakurthi S, Zeng Q. et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for pre-clinical evaluation of novel therapeutics. Clin Cancer Res 2016. doi: 10.1158/1078-0432.CCR-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng DT, Mitchell TN, Zehir A. et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT). J Mol Diagn 2015; 17: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.