Abstract
Background and aim
High heart rate is an independent predictor of total cancer incidence and all-cause mortality in patients with cancer. We aimed to evaluate the impact of resting heart rate on the recurrence of colorectal polyp, using long-term surveillance follow-up data of colorectal cancer survivors.
Methods
Three hundred patients were selected from the colorectal cancer survivor cohort of Severance Hospital, Seoul, Korea. Resting heart rate, physical activity, and body composition analysis at the time of 5-year survival, and clinical data including colonoscopy surveillance results were collected for mean follow-up duration of 8 years.
Results
Patients with a high resting heart rate showed a significantly higher recurrence rate of advanced adenoma than those with a low resting heart rate (quartile 1, 45–66 beats per minute (b.p.m.); quartile 2, 67–73 b.p.m.; quartile 3, 74–80 b.p.m.; quartile 4, 81–120 b.p.m.; 3.8% vs. 7.9% vs. 10.0% vs. 14.7%, p for trend = 0.018). After adjustment for various risk factors, patients in the highest quartile of resting heart rate (≥ 81 b.p.m.) had a significantly higher risk of advanced adenoma recurrence (hazard ratio [HR]: 6.183, 95% confidence interval [CI]: 1.181–32.373, p = 0.031), compared to those in the lowest quartile (≤ 66 b.p.m.). In subgroup analysis, the association of resting heart rate with advanced adenoma recurrence appeared to be stronger among patients who had more than normal body fat mass or sedentary life style.
Conclusions
Elevated resting heart rate was independently associated with a higher rate of advanced adenoma recurrence in colorectal cancer survivors.
Introduction
Resting heart rate is a sensitive indicator of the autonomic nervous system. An increase in the resting heart rate is caused by activation of sympathetic activity more than parasympathetic activity and/or decreased vagal tone. Resting heart rate can be influenced by lifestyle factors, such as psychological stress and physical activity [1,2]. Knowing that psychological stress and physical activity are closely associated with prognosis of cancer, high resting heart rate may be used as a prognostic factor for cancer recurrence. In a recent meta-analysis, the authors found an increased risk of coronary heart disease, sudden cardiac death, heart failure, atrial fibrillation, stroke, cardiovascular disease, total cancer, and all-cause mortality in patients with a higher resting heart rate [3–5]. The relative risk (RR) of a 10-beats-per-minute (b.p.m.) increase in the resting heart rate for patients with cancer was 1.14 (95% confidence interval [CI]: 1.06–1.23, p < 0.0001), and the RR of a high versus low resting heart rate was 1.43 (95% CI: 1.12–1.82, p < 0.0001) [3].
Colorectal cancer (CRC) is highly prevalent, with its incidence increasing worldwide [6,7]. Several factors, such as obesity, physical inactivity, Western diet, smoking, alcohol use, and a personal history of CRC or polyps, have been linked to CRC development and recurrence [8,9]. Interestingly, in a previous study, a resting heart rate ≥ 75 b.p.m. was an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer (hazard ratio [HR]: 1.67, 95% CI: 1.01–2.78, p = 0.040) [10]. Another study showed a relationship between an elevated resting heart rate and an incident CRC risk in patients with manifest vascular disease (HR: 1.19, 95% CI: 1.00–1.42) [11].
The mechanisms underlying the relationship between resting heart rate and cancer are complex and not well understood. An increased heart rate impairs endothelial function in animal models and may contribute to reduced shear stress and vascular compliance [1,12]. Non-cardiac conditions, such as chronic inflammation associated with microvascular disease, might also be on the result of a high heart rate [13]. Moreover, increased sympathetic activation might also contribute to the initiation and progression of cancer, including inflammation, angiogenesis, tissue invasion, cellular immune response, and epithelial-mesenchymal transition [14].
To our knowledge, there has been no study that evaluated the association of heart rate with polyp recurrence in detail for a long-term surveillance period. In this study, we aimed to evaluate the impact of resting heart rate on the recurrence of colorectal polyp, using long-term surveillance follow-up data of CRC survivors.
Patients and methods
Patients
A total of 300 patients were randomly selected from the CRC survivor cohort of Severance Hospital, Seoul, Korea. These patients were followed-up for > 5 years after the diagnosis and treatment of CRC. The mean follow-up period from firstly diagnosed and treated was 8.0 years (median, 7 years; range, 5–21 years). After the curative resection of CRC, all patients underwent surveillance colonoscopy by experienced endoscopists. All patients underwent a baseline colonoscopy before colorectal resection or within 6 months after colorectal resection. In cases of incomplete colonoscopy before surgery, additional colonoscopic examinations were carried out within 6 months after the surgery, and the findings were included as part of the baseline colonoscopic findings. We excised all polyps detected during preoperative and postoperative colonoscopies, and all specimens were sent to the pathology department for histological evaluation. Follow-up colonoscopy was performed 1–4 times per patient, based on the guidelines [15]. Moreover, to overcome the interval diversities in surveillance colonoscopy, we investigated the total follow-up period and the timing of follow-up colonoscopy. The exclusion criteria were as follows: (i) incomplete medical records, (ii) a history of familial polyposis syndrome or Lynch syndrome, (iii) known inflammatory bowel disease (IBD), and (iv) incomplete baseline colonoscopy before colorectal resection or within 6 months after surgery. Cases of incomplete colonoscopy (nonvisualized cecum and/or inadequate bowel preparation) were also excluded.
Patients were identified using the electronic medical record system that includes all patients with cancer at Severance Hospital, Yonsei University College of Medicine. This study was approved by the institutional review board of Severance Hospital, Yonsei University (Seoul, Korea) and patients consents were waived.
Data collection
Resting heart rate and blood pressure were checked at the time of 5-year survival upon the outpatient visit, and were measured in a quiet place with the patient in a sitting position with the feet flat on the floor after resting for 5–10 minutes. Before the test, eating, drinking alcohol, smoking, exercising, and bathing were avoided for 30 minutes. Baseline heart rate and blood pressure were measured by resting the arm on a table so that the cuff was at the same level as the heart.
To evaluate the severity of obesity, we measured weight, height, body mass index (BMI), and waist circumference, and used InBody 720 (Biospace, Korea) for body composition analysis. InBody 720 can automatically measure various parameters, intracellular and extracellular water, body fat mass, protein, and body cell mass. [16]. The normal standard range of body fat mass for males is defined at 10–20%, and 18–28% for females by Inbody 720. We followed the World Health Organization (WHO) Asia-Pacific classification to define the categories of obesity as normal (BMI < 23.0), overweight (23 ≤ BMI < 25), and obese (BMI ≥ 25) [17].
Physical activity data were collected through the interviewer-administered Global Physical Activity Questionnaire (GPAQ). GPAQ is a survey developed by the WHO to evaluate physical activity, and includes 16 questions involving activity at work, travel to and from places, recreational activity, and sedentary behavior [18]. We estimated total metabolic equivalent (MET)-hours/week from walking and vigorous exercise by summing the MET-hours/week from walking (hours per week multiplied by 3.5) and the MET-hours/week from vigorous exercise (hours per week multiplied by 7.0) [19,20].
Concerning colorectal adenoma characteristics, we analyzed the number, size, location, and pathology of colorectal polyps [21]. Any colorectal adenoma detected during surveillance colonoscopy was defined as recurrent adenoma. We defined advanced adenoma as a lesion of ≥ 10 mm, and a histologic finding of a villous component, or high-grade dysplasia [22]. Also, we reviewed about type of surgery, colorectal cancer stage, and receiving subsequent adjuvant chemotherapy.
Statistical analyses
Means and standard deviations or medians and ranges were calculated for all continuous variables, as appropriate. Categorical variables were expressed as proportions (%) and statistical analyses were performed to compare the groups of variables. One-way analysis of variance (ANOVA) tests (or Kruskal-Wallis tests) were used to compare continuous variables, and chi-square tests (or Fisher’s exact test) were used for categorical variables, as appropriate. Kaplan-Meier analyses (log-rank tests) were carried out to compare the cumulative risk of advanced adenoma development across the quartiles of resting heart rates. Cox proportional hazards analyses were carried out to reveal the independent risk factors of the cumulative development of advanced adenoma with adjustment for various confounders, including age, sex, history of alcohol consumption, smoking, family history of CRC, DM, hypertension, skeletal muscle mass, body fat mass, physical activity, systolic blood pressure, diastolic blood pressure, resting heart rate, follow-up duration, time to surveillance colonoscopy, and number of surveillance colonoscopies. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS version 23.0; SPSS Inc., Armonk, NY, USA). A p-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 300 patients who were diagnosed with CRC and survived > 5 years after curative surgical resection were enrolled in this study. The CRC survivors were divided into 4 groups according to heart rate: quartile 1, 45–66 beat per minutes (b.p.m.); quartile 2, 67–73 b.p.m.; quartile 3, 74–90 b.p.m.; and quartile 4, 81–120 b.p.m.. There was no difference in education status, alcohol use, tobacco use, family history of CRC, height, weight, BMI, waist circumference, skeletal muscle mass, body cell mass, systolic blood pressure, diastolic blood pressure, tumor location, tumor stage, tumor grade, medical history, metabolic parameters, and medication use between the groups (Table 1). However, patients with low resting heart rate were older, had a higher percentage of men, undertook more physical activity, and had lower body fat mass, lower percentage body fat, and lower visceral fat area, although the difference in the average values was small.
Table 1. Baseline characteristics of colorectal cancer survivors according to resting heart rate.
| Variables | Quartile 1 45–66 b.p.m. (n = 79) |
Quartile 2 67–73 b.p.m. (n = 76) |
Quartile 3 74–80 b.p.m. (n = 70) |
Quartile 4 81–120 b.p.m. (n = 75) |
*p value |
|---|---|---|---|---|---|
| Age (years) | 62.4 ± 9.6 | 62.7 ± 10.2 | 59.9 ± 10.4 | 58.5 ± 12.0 | 0.041 |
| Males | 51 (64.6%) | 38 (50.0%) | 33 (47.1%) | 36 (48.0%) | 0.038 |
| Education | 0.477 | ||||
| Less than high school | 16 (20.3%) | 20 (26.3%) | 18 (25.7%) | 16 (21.3%) | |
| High school graduate | 48 (60.8%) | 41 (53.9%) | 35 (50.0%) | 39 (52.0%) | |
| College degree or higher | 15 (19.0%) | 15 (19.7%) | 17 (24.3%) | 20 (26.7%) | |
| Current alcohol | 47 (59.5%) | 37 (48.7%) | 31 (44.3%) | 38 (50.7%) | 0.224 |
| Current tobacco | 37 (46.9%) | 29 (38.1%) | 29 (41.4%) | 26 (34.6%) | 0.210 |
| Family history of CRC | 9 (11.4%) | 11 (14.5%) | 8 (11.4%) | 13 (17.3%) | 0.395 |
| Physical activity (MET-hours/wk) | 29.5 ± 34.2 | 27.7 ± 34.2 | 18.3 ± 34.0 | 18.3 ± 2.7 | 0.049 |
| Height (cm) | 162.2 ± 7.3 | 162.8 ± 7.9 | 160.9 ± 7.8 | 161.7 ± 9.2 | 0.504 |
| Weight (kg) | 63.2 ± 9.7 | 65.1 ± 8.7 | 63.9 ± 10.3 | 64.7 ± 9.9 | 0.635 |
| BMI (kg/m2) | 24.0 ± 2.7 | 24.6 ± 3.1 | 24.7 ± 3.3 | 24.7 ± 3.1 | 0.363 |
| Waist (cm) | 86.0 ± 7.8 | 87.8 ± 8.2 | 86.6 ± 9.7 | 87.4 ± 8.3 | 0.596 |
| Skeletal muscle mass (kg) | 25.4 ± 5.2 | 25.1 ± 4.8 | 24.3 ± 4.8 | 24.8 ± 5.1 | 0.545 |
| Body Fat mass (kg) | 16.1 ± 5.3 | 18.9 ± 6.9 | 19.7 ± 6.8 | 19.5 ± 6.7 | 0.003 |
| Percent body fat (%) | 26.3 ± 7.8 | 28.6 ± 9.8 | 30.1 ± 8.0 | 29.8 ± 8.3 | 0.029 |
| Visceral fat area (cm2) | 74.0 ± 28.5 | 91.5 ± 40.6 | 94.3 ± 4.1 | 93.2 ± 38.0 | 0.003 |
| Body cell mass (kg) | 30.2 ± 5.7 | 29.8 ± 6.0 | 28.7 ± 5.3 | 29.1 ± 5.4 | 0.480 |
| Systolic blood pressure (mmHg) | 124.4 ± 14.4 | 127.5 ± 15.0 | 125.6 ± 15.6 | 125.3 ± 15.2 | 0.643 |
| Diastolic blood pressure (mmHg) | 77.2 ± 11.2 | 79.5 ± 11.5 | 78.7 ± 10.9 | 79.1 ± 9.8 | 0.554 |
| Tumor location | 0.436 | ||||
| Colon | 46 (58.2%) | 38 (50.0%) | 37 (52.9%) | 48 (64.0%) | |
| Rectum | 33 (41.8%) | 38 (50.0%) | 33 (47.1%) | 27 (36.0%) | |
| Invasion through bowel wall | 0.056 | ||||
| T1-2 | 30 (38.0%) | 41 (53.9%) | 29 (41.4%) | 20 (26.7%) | |
| T3-4 | 49 (62.0%) | 35 (46.1%) | 41 (58.6%) | 55 (73.3%) | |
| No. of positive lymph nodes | 0.896 | ||||
| N1 | 71 (89.9%) | 71 (93.4%) | 65 (92.9%) | 68 (90.7%) | |
| N2 | 8 (10.1%) | 5 (6.6%) | 5 (7.1%) | 7 (9.3%) | |
| Stage of colorectal cancer | 0.087 | ||||
| Stage 1 | 28 (40.6%) | 36 (46.1%) | 27 (34.6%) | 20 (26.7%) | |
| Stage 2 | 33 (47.8%) | 35 (44.9%) | 37 (47.4%) | 48 (64.0%) | |
| Stage 3 | 8 (11.6%) | 7 (9.0%) | 14 (17.9%) | 7 (9.3%) | |
| Grade of differentiation | 0.345 | ||||
| Well | 17 (21.5%) | 26 (34.2%) | 16 (22.9%) | 18 (24.0%) | |
| Moderate | 59 (74.7%) | 48 (63.2%) | 52 (74.2%) | 51 (68.0%) | |
| Poor/undifferentiated | 3 (3.8%) | 2 (2.6%) | 2 (2.9%) | 6 (8.0%) | |
| Type of operation | 0.517 | ||||
| Low anterior resection | 31 (44.9%) | 44 (56.4%) | 40 (51.3%) | 34 (45.3%) | |
| Anterior resection | 14 (20.3%) | 15 (19.2%) | 19 (24.4%) | 22 (29.3%) | |
| Abdominopelvic resection | 0 (0.0%) | 3 (3.8%) | 4 (5.1%) | 0 (0.0%) | |
| Left hemicolectomy | 6 (8.7%) | 2 (2.6%) | 2 (2.6%) | 3 (4.0%) | |
| Right hemicolectomy | 16 (23.2%) | 12 (15.4%) | 13 (16.7%) | 16 (21.3%) | |
| Segmental resection | 2 (2.9%( | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | |
| Adjuvant chemotherapy | 7 (10.3%) | 7 (9.0%) | 13 (16.7%) | 6 (8.0%) | 0.954 |
| Medical history | |||||
| Hypertension | 23 (29.1%) | 30 (39.5%) | 25 (35.7%) | 21 (28.0%) | 0.784 |
| DM | 12 (15.2%) | 10 (13.2%) | 12 (17.1%) | 17 (22.7%) | 0.173 |
| Dyslipidemia | 15 (19.0%) | 15 (19.7%) | 12 (17.1%) | 20 (26.7%) | 0.322 |
| Coronary artery disease | 6 (7.6%) | 3 (3.9%) | 3 (4.3%) | 0 (0.0%) | 0.122 |
| Cerebrovascular disease | 0 (0.0%) | 1 (1.3%) | 4 (5.7%) | 5 (6.7%) | 0.058 |
| Peripheral arterial disease | 2 (2.5%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | 0.330 |
| Atrial fibrillation | 2 (2.5%) | 1 (1.3%) | 2 (2.9%) | 2 (2.7%) | 0.810 |
| Metabolic parameters | |||||
| Hemoglobin levels (g/dL) | 12 9 ± 1.8 | 13.3 ± 1.5 | 12.9 ± 1.8 | 12.9 ± 2.3 | 0.386 |
| Potassium (mmol/L) | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.4 | 0.386 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.7 | 0.9 ± 0.2 | 0.501 |
| eGFR (ml/min/1.73m2) | 83.6 ± 15.2 | 87.8 ± 18.1 | 89.0 ± 22.0 | 88.8 ± 16.7 | 0.206 |
| Serum glucose (mg/dL) | 114.2 ± 61.9 | 107.0 ± 20.2 | 102.9 ± 22.3 | 108.4 ± 34.3 | 0.360 |
| Total cholesterol (mg/dL) | 175.3 ± 38.7 | 176.6 ± 43.6 | 179.8 ± 33.8 | 180.5 ± 33.9 | 0.803 |
| Medication | |||||
| Beta blocker | 6 (7.6%) | 2 (2.6%) | 2 (2.9%) | 4 (5.3%) | 0.420 |
| Diuretics | 1 (1.3%) | 5 (6.6%) | 2 (2.9%) | 2 (2.7%) | 0.298 |
| ACE inhibitor/ARB | 8 (10.1%) | 9 (11.8%) | 4 (5.7%) | 8 (10.7%) | 0.778 |
| Calcium channel blocker | 7 (8.9%) | 5 (6.6%) | 3 (4.3%) | 4 (5.3%) | 0.687 |
| Alpha blocker | 1 (1.3%) | 1 (1.3%) | 0 (0.0%) | 1 (1.3%) | 0.820 |
| Lipid-lowering medication | 21 (26.6%) | 18 (23.7%) | 17 (24.3%) | 21 (28.0%) | 0.924 |
| Aspirin | 8 (10.1%) | 5 (6.6%) | 9 (12.9%) | 7 (9.3%) | 0.643 |
| Clopidogrel | 4 (5.1%) | 1 (1.3%) | 6 (8.6%) | 3 (4.0%) | 0.220 |
| Anticoagulant | 1 (1.3%) | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0.312 |
Variables are expressed as mean ± SD or n (%).
*p value for comparing quartile groups based on resting heart rate.
b.p.m., beat per minute; CRC, colorectal cancer; METS, metabolic equivalents; BMI, body mass index; DM, diabetes mellitus; GFR, glomerular filtration rate; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor antagonist; SD, standard deviation
Colonoscopic surveillance
The mean follow-up period was 8.0 years (median, 7 years; range, 5–21 years). The colonoscopy was completely inserted into the cecum in all 300 patients. The mean duration between CRC surgery and polyp recurrence was 45.2 months (median, 48 months; range, 6–204 months). Sixty-one (20.3%) patients underwent 1 follow-up colonoscopy, 136 (45.3%) underwent 2, 86 (28.7%) underwent 3, and 17 (5.7%) underwent 4 or more follow-up colonoscopies. The number of follow-up colonoscopies was not significantly different among the 4 groups (p = 0.414). There was also no significant difference in the time to the first follow-up colonoscopy (16.9 months vs. 15.6 months vs. 18.7 months vs. 17.6 months, p = 0.635; S1 Table).
Adenoma and advanced adenoma recurrence
A total of 162 (54.0%) patients showed 1 or more colorectal polyp. The total adenoma recurrence rate was 32.3%, and the total advanced adenoma recurrence rate was 9.0%. The tubular adenoma recurrence rate was 22.7% in the proximal colon and 14.3% in the distal colon and rectum. The advanced adenoma recurrence rate was 6.3% in the proximal colon and 3.3% in the distal colon and rectum.
Resting heart rate and adenoma recurrence
The group with a low baseline heart rate showed a significantly lower recurrence rate of advanced adenoma than those with a high baseline heart rate (3.8% vs. 7.9% vs. 10.0% vs. 14.7%, p = 0.018; Table 2). There was no significant difference in the total adenoma recurrence rate.
Table 2. Colorectal polyp recurrence rates according to resting heart rates.
| Resting heart rate (b.p.m.) | p for trend | ||||
|---|---|---|---|---|---|
| Quartile 1 45–66 (n = 79) |
Quartile 2 67–73 (n = 76) |
Quartile 3 74–80 (n = 70) |
Quartile 4 81–120 (n = 75) |
||
| Any polyp | |||||
| Event | 45 (57.0%) | 39 (51.3%) | 40 (57.1%) | 38 (50.7%) | 0.599 |
| Multivariable-adjusteda | Referent | 1.049 (0.648–1.697) |
1.337 (0.812–2.201) |
1.027 (0.625–1.688) |
|
| Hyperplastic polyp | |||||
| Event | 12 (15.2%) | 13 (17.1%) | 20 (28.6%) | 15 (20.0%) | 0.211 |
| Multivariable-adjusteda | Referent | 1.492 (0.614–3.625) |
2.712* (1.144–6.427) |
1.325 (0.541–3.236) |
|
| Tubular adenoma | |||||
| Event | 37 (46.8%) | 32 (42.1%) | 25 (35.7%) | 27 (36.0%) | 0.122 |
| Multivariable-adjusteda | Referent | 1.011 (0.564–1.810) |
0.643 (0.322–1.287) |
0.543 (0.274–1.077) |
|
| Advanced adenoma | |||||
| Event | 3 (3.8%) | 6 (7.9%) | 7 (10.0%) | 11 (14.7%) | 0.018 |
| Multivariable-adjusteda | Referent | 2.491 (0.431–14.394) |
5.623* (0.981–32.241) |
6.183** (1.181–32.373) |
|
Variables are expressed as n (%).
b.p.m., beat per minute
* p < 0.10
** p < 0.05
a multivariate Cox proportional hazards analysis, including the confounders, such as age, sex, history of alcohol consumption, smoking, family history of CRC, DM, hypertension, skeletal muscle mass, body fat mass, physical activity, colorectal cancer stage, type of surgery, adjuvant chemotherapy, use of aspirin, systolic blood pressure, diastolic blood pressure, resting heart rate, follow-up duration, time to surveillance colonoscopy, and number of surveillance colonoscopies
Physical activity, obesity, and resting heart rate
There was an association between vigorous exercise and heart rate (p for trend = 0.021); however, walking exercise and heart rate were not related (p for trend = 0.601; S2 Table). A correlation between total MET-hours/week and heart rate was also found (p for trend = 0.022), and the patient group with a low baseline heart rate showed a significantly higher proportion of active exercise (≥ 40 MET-hours/week) (27.8% vs. 30.3% vs. 11.4% vs. 13.3%, p for trend = 0.003). Furthermore, in the low heart rate group, more people undertook active exercise for more than 5 hours (44.3% vs. 41.3% vs. 28.6% vs. 29.3%, p for trend = 0.020). The mean heart rates of the active exercise group who had over 40 MET-hours/week and the sedentary group were 70.3 ± 10.5 and 75.0 ± 10.8 b.p.m. (p = 0.034), respectively.
Body fat mass and visceral fat area were also related to a higher heart rate (p for trend = 0.041 and 0.015, respectively); however, BMI and heart rate were not significantly associated (p for trend = 0.571; S3 Table). The mean heart rates of patients with a lower than average body fat mass (n = 10), with an average body fat mass (n = 104), and with a higher than average body fat mass (n = 186) were 65.4 ± 9.9, 73.4 ± 10.5, and 74.7 ± 10.9 b.p.m. (p = 0.024).
Resting heart rate, physical activity, obesity, and adenoma recurrence
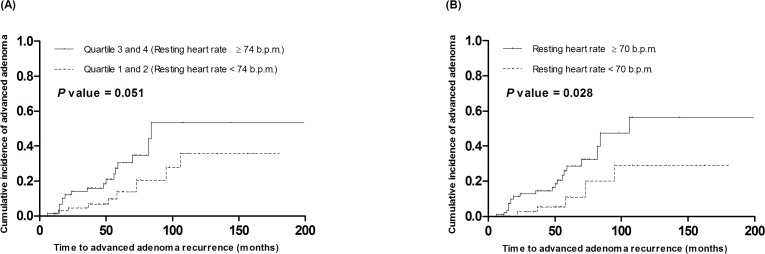
We performed Kaplan-Meier analysis (log-rank tests) to compare the cumulative rate of the development of advanced adenoma between the low resting heart rate groups (quartile 1 and 2, < 74 b.p.m.) and high resting heart rate groups (quartile 3 and 4, ≥ 74 b.p.m.; Fig 1A). An increased resting heart rate was positively associated with the development of advanced adenoma, although it was not statistically significant (p = 0.051). However, a significant difference was found in the cumulative rate of advanced adenoma development between patients with a resting heart rate ≥ 70 b.p.m. and those with a rate < 70 b.p.m. (p = 0.028; Fig 1B). Subsequently, multivariate Cox proportional hazards analysis, including the confounders, such as age, sex, history of alcohol consumption, smoking, family history of CRC, DM, hypertension, skeletal muscle mass, body fat mass, physical activity, colorectal cancer stage, type of surgery, adjuvant chemotherapy, use of aspirin, systolic blood pressure, diastolic blood pressure, resting heart rate, follow-up duration, time to surveillance colonoscopy, and number of surveillance colonoscopies, showed that patients in the highest quartile of resting heart rate (≥ 81 b.p.m.) had a significantly higher risk of advanced adenoma recurrence (hazard ratio [HR]: 6.183, 95% confidence interval [CI]: 1.181–32.373, p = 0.031), compared to those in the lowest quartile (≤ 66 b.p.m.) (Table 2). Higher than normal body fat mass (hazard ratio [HR]: 10.743, 95% CI: 2.212–52.178, p = 0.003) and progressive colorectal cancer stage (stage 2 vs. stage 1; HR: 5.067, 95% CI: 1.446–17.756, p = 0.011) were also positively associated with increased risk of advanced adenoma recurrence (Table 3).
Fig 1.
Kaplan-Meier analysis (log-rank tests) to compare the cumulative rate of development of advanced adenoma between low resting heart rate group (quartile 1 and 2, < 74 b.p.m.) and high resting heart rate group (quartile 3 and 4, ≥ 74 b.p.m.) (A), and between high resting heart rate group (≥ 70 b.p.m.) and low resting heart rate group (< 70 b.p.m.) (B).
Table 3. Cox proportional hazards analysis for advanced adenoma recurrence.
| Variables | Multivariate analysis | ||||
|---|---|---|---|---|---|
| HR | 95% CI | *p value | |||
| Age (years) | 0.997 | 0.935–1.056 | 0.836 | ||
| Male sex | 1.736 | 0.340–8.874 | 0.508 | ||
| History of alcohol | 1.119 | 0.203–6.177 | 0.897 | ||
| History of smoking | 0.419 | 0.105–1.664 | 0.216 | ||
| Family history of colorectal cancer | 1.016 | 0.199–5.190 | 0.985 | ||
| Hypertension | 2.460 | 0.679–8.908 | 0.170 | ||
| DM | 0.656 | 0.148–2.895 | 0.577 | ||
| Skeletal muscle mass (kg) | 0.361 | 0.038–3.386 | 0.372 | ||
| Body Fat mass (kg) | 10.743 | 2.212–52.178 | 0.003 | ||
| Exercise amount (METs) | 1.006 | 0.988–1.025 | 0.492 | ||
| Colorectal cancer stage | |||||
| Stage 1 | 1.000 | (reference) | |||
| Stage 2 | 5.067 | 1.446–17.756 | 0.011 | ||
| Stage 3 | 0.139 | 0.000–152.492 | 0.581 | ||
| Type of surgery | 0.865 | 0.538–1.391 | 0.550 | ||
| Adjuvant chemotherapy | 2.669 | 0.002–2895.610 | 0.783 | ||
| Use of aspirin | 0.553 | 0.080–3.835 | 0.549 | ||
| Systolic blood pressure (mmHg) | 0.988 | 0.939–1.040 | 0.651 | ||
| Diastolic blood pressure (mmHg) | 1.026 | 0.956–1.102 | 0.469 | ||
| Heart rates | |||||
| Qualtile1 | 1.000 | (reference) | |||
| Qualtile2 | 2.491 | 0.431–14.394 | 0.308 | ||
| Qualtile3 | 5.623 | 0.981–32.241 | 0.053 | ||
| Qualtile4 | 6.183 | 1.181–32.373 | 0.031 | ||
| Follow-up duration (years) | 0.891 | 0.732–1.085 | 0.252 | ||
| Time to first surveillence colonoscopy (months) | 0.975 | 0.947–1.004 | 0.094 | ||
| Number of surveillence colonoscopy | 1.094 | 0.400–2.993 | 0.864 | ||
HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus
*p value for comparing advanced adenoma group and non-advanced adenoma group
In addition, we performed subgroup analysis according to the amount of exercise performed and body fat mass. In the higher than normal body fat mass group, 2 (4.5%), 6 (13.6%), 7 (14.3%), and 10 (20.4%) advanced adenomas were found in 44 patients with a resting heart rate of 45–66 b.p.m., in 44 patients with a resting heart rate of 67–73 b.p.m., in 49 patients with a resting heart rate of 74–80 b.p.m., and in 49 patients with a resting heart rate of 81–120 b.p.m., respectively (p = 0.032; Table 4). Furthermore, in the sedentary exercise group (< 40 MET-hours/week), 2 (3.5%), 4 (7.5%), 7 (11.3%), and 11 (16.9%) advanced adenomas were found in 57 patients with a resting heart rate of 45–66 b.p.m., in 53 patients with a resting heart rate of 67–73 b.p.m., in 62 patients with a resting heart rate of 74–80 b.p.m., and in 65 patients with a resting heart rate of 81–120 b.p.m., respectively (p = 0.011; Table 4). However, in patients with normal or less body fat mass or active exercise, resting heart rate was not a statistically significant independent factor for advanced adenoma recurrence. Therefore, the association of resting heart rate with advanced adenoma recurrence appeared to be stronger among patients who had more than normal body fat mass and sedentary life style.
Table 4. Subgroup analyses of multivariable-adjusted hazard ratios of advanced adenoma recurrence associated with heart rate.
| Resting heart rate (b.p.m.) | p for trend | ||||
|---|---|---|---|---|---|
| Quartile 1 45–66 (n = 79) |
Quartile 2 67–73 (n = 76) |
Quartile 3 74–80 (n = 70) |
Quartile 4 81–120 (n = 75) |
||
| Body fat mass | |||||
| Normal or less body fat mass (n = 114) | |||||
| Event | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 0.835 |
| Multivariable-adjusteda | Referent | NS | NS | NS | |
| More than normal body fat mass (n = 186) | |||||
| Event | 2 (4.5%) | 6 (13.6%) | 7 (14.3%) | 10 (20.4%) | 0.032 |
| Multivariable-adjusteda | Referent | 2.895 (0.461–18.174) |
7.117** (1.142–44.351) |
7.114** (1.133–44.686) |
|
| Physical activity | |||||
| Active exercise (≥ 40 MET-hours/wk) (n = 63) | |||||
| Event | 1 (4.5%) | 2 (8.7%) | 0 (0.0%) | 0 (0.0%) | 0.472 |
| Multivariable-adjusteda | Referent | NS | NS | NS | |
| Sedentary (< 40 MET-hours/wk) (n = 237) | |||||
| Event | 2 (3.5%) | 4 (7.5%) | 7 (11.3%) | 11 (16.9%) | 0.011 |
| Multivariable-adjusteda | Referent | 1.679 (0.267–10.563) |
4.266* (0.820–22.204) |
4.681* (0.923–23.725) |
|
Variables are expressed as n (%).
b.p.m., beat per minute
aMultivariable model adjusted for the same covariates used in Table 2.
* p < 0.10
** p < 0.05
Discussion
A history of CRC is a very strong risk factor for the recurrence of advanced adenoma, and many studies have investigated the risk factors for polyp recurrence in patients with CRC [21,23]. Age, sex, a family history of CRC, and obesity were described as risk factors for colorectal neoplasia recurrence [24]. The study by Anker et al. reported that a high heart rate was an independent predictor of survival in cancer patients [10]. The study group was heterogeneous because it included various cancer types, such as colorectal (n = 36), pancreatic (n = 72), and non-small cell lung cancer (n = 37) (HR: 1.67, 95% CI: 1.01–2.78, p = 0.040). Van Kruiksdijk et al. showed that an elevated resting heart rate was related to the incident CRC (n = 67) risk in patients with manifest vascular disease (n = 6007) (HR: 1.19, 95% CI: 1.00–1.42) [11]. However, because of the high risk of cardiovascular mortality from vascular disease, the patients might not live long enough to develop cancer. With regard to colon polyps, there have been no data evaluated the association between resting heart rate and polyp recurrence.
In our study, surveillance colonoscopy after treatment of CRC revealed an adenoma recurrence rate of 32.3% and an advanced adenoma recurrence rate of 9.0%, which are similar to the results of previous studies showing an adenoma recurrence rate of 34.4–41.4% and an advanced adenoma recurrence rate of 4.4–6.5% [25–27]. With regard to the heart rate, multivariate analysis showed that an increased resting heart rate was associated with a significantly greater risk of advanced adenoma recurrence, but not with tubular adenoma and overall polyps. Our study showed a relationship between resting heart rate and advanced adenoma recurrence in the Cox proportional hazards analysis, with an HR of 11.550 (HR: 1.218–109.525, p = 0.033) for the highest quartile of resting heart rate compared with the lowest stratum. Furthermore, in subgroup analyses based on body fat mass and amount of exercise, our data showed that obese patients with a low resting heart rate had a lower risk of advanced adenoma than obese patients with a high resting heart rate. In addition, sedentary patients with a low resting heart rate had a valid lower risk of advanced adenoma than sedentary patients with a high resting heart rate. However, for patients with normal or less body fat mass or active exercise did not show any correlation between resting heart rate and advanced adenoma recurrence.
With regard to physical activity, our study demonstrated that a low heart rate was positively associated with exercise for more than 5 hours or over 40 MET-hours/week. If aerobic exercise is performed for a long time, it affects the parasympathetic nerve, thus increasing the stroke volume and lowering the resting heart rate [28]. It is well known that bradycardia syndrome (< 60 b.p.m.) occurs in athletes, especially in those who undertake marathon running [29]. In terms of obesity, our data showed that a low heart rate was negatively associated with body fat mass and the visceral fat area, but not with BMI. We already showed usefulness of body composition analysis and its relationship with colorectal polyp recurrence [30]. BMI may be an inaccurate measure of percentage body fat for an individual [31]. For example, greater loss of muscle mass with age exacerbates the prevalence of false-negative BMIs. Meanwhile, our results also showed that low body fat mass and more exercise could contribute to lowering the rate of advanced adenoma recurrence associated with resting heart rate.
As mentioned above, active exercise was directly related to low body fat mass and low resting heart rate. However, in multivariate analysis and Cox proportional hazards analysis after adjustment for related factors, including these 3 factors, resting heart rate was still remained as a significant independent factor associated with a lower rate of advanced adenoma recurrence. Therefore, we need to understand the mechanism of the tumor preventive effect of a lower resting heart rate, which is not related to the direct effects of active exercise. In this regard, the molecular biologic mechanism of the relationship between heart rate and tumor development has not yet been clearly demonstrated. In a previous study, the catecholamine stress hormone norepinephrine was shown to influence tumor progression by modulating the expression of factors implicated in angiogenesis and metastasis [32]. Masur et al. reported that norepinephrine-induced locomotion of SW 480 colon carcinoma cells is mediated by the β2-adrenoceptor and inhibited by beta blockers [33]. Yang et al. suggested that norepinephrine could stimulate the aggressive potential of melanoma cancer cells by inducing the production of vascular endothelial growth factor (VEGF), interleukin (IL)-8, and IL-6 [34]. In addition, an epidemiological meta-analysis demonstrated that beta blocker use is associated with improved overall survival (HR: 0.79, 95% CI: 0.67–0.93, p = 0.004) and disease free survival (HR: 0.69, 95% CI: 0.53–0.91, p = 0.009) in cancer patients [35]. Recently, Choi et al. reported that perioperative propranolol was effective in reducing the ovarian cancer burden measured using CA 125, suggesting its potential benefits in decreasing perioperative tumor growth (p = 0.044) [36]. Therefore, an intervention targeting components of the activated sympathetic-adrenal medullary axis or the utilization of beta blockers might represent new strategies for slowing the progression of malignant disease and improving cancer patients’ quality of life. Furthermore, this new strategy might be applicable to cancer prevention in the future.
This study has the innate limitations of a retrospective cross-sectional case-control study performed in a single-tertiary university hospital. Another weak point was that only a single measurement of physical activity, body composition data, and resting heart rate at least 5 years after the diagnosis and treatment of CRC was available. The serial measurements during 5 years would provide more useful information. However, our study has the strong points of including a patient group with a history of CRC that is a high risk group of colorectal neoplasia, having detailed long-term follow-up surveillance data, and analyzing detailed exercise amount and body composition, which are related with resting heart rate. In addition, because 5-year survivors of CRC were included, and physical activity, body composition, and resting heart rate were measured after a minimum period of 5 years from the initial diagnosis and treatment of CRC, these data may be reflective of a stabilized status without variance from the impact of cancer treatment or cancer-related stress. Last weak point was that we had relative small sample size and few recurrence events. Further studies with larger sample size, longer follow-up and more events will be needed.
In conclusion, we found that a high resting heart rate were meaningful independent risk factors of advanced adenoma recurrence in CRC survivors.
Supporting information
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1631020).
References
- 1.Bohm M, Reil JC, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. 2015;128: 219–228. doi: 10.1016/j.amjmed.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 2.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm. 2009;6: S68–75. doi: 10.1016/j.hrthm.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Sen A, o'Hartaigh B, Janszky I, Romundstad PR, Tonstad S, et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017;27: 504–517. doi: 10.1016/j.numecd.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Park S, Lim SM, Lee MK, Giovannucci EL, Kim JH, et al. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res Treat. 2016;159: 375–384. doi: 10.1007/s10549-016-3938-1 [DOI] [PubMed] [Google Scholar]
- 5.Yang HI, Kim HC, Jeon JY. The association of resting heart rate with diabetes, hypertension, and metabolic syndrome in the Korean adult population: The fifth Korea National Health and Nutrition Examination Survey. Clin Chim Acta. 2016;455: 195–200. doi: 10.1016/j.cca.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Yoon M, Kim N, Nam B, Joo J, Ki M. Changing trends in colorectal cancer in the Republic of Korea: contrast with Japan. Epidemiol Health. 2015;37: e2015038 doi: 10.4178/epih/e2015038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res Treat. 2012;44: 219–226. doi: 10.4143/crt.2012.44.4.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle T, Fritschi L, Tabatabaei SM, Ringwald K, Heyworth JS. Smoking, alcohol, diabetes, obesity, socioeconomic status, and the risk of colorectal cancer in a population-based case-control study. Cancer Causes Control. 2014;25: 1659–1668. doi: 10.1007/s10552-014-0470-7 [DOI] [PubMed] [Google Scholar]
- 9.Vallance JK, Boyle T, Courneya KS, Lynch BM. Accelerometer-assessed physical activity and sedentary time among colon cancer survivors: associations with psychological health outcomes. J Cancer Surviv. 2015;9: 404–411. doi: 10.1007/s11764-014-0409-8 [DOI] [PubMed] [Google Scholar]
- 10.Anker MS, Ebner N, Hildebrandt B, Springer J, Sinn M, Riess H, et al. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study. Eur J Heart Fail. 2016;18: 1524–1534. doi: 10.1002/ejhf.670 [DOI] [PubMed] [Google Scholar]
- 11.van Kruijsdijk RC, van der Graaf Y, Bemelmans RH, Nathoe HM, Peeters PH, Visseren FL, et al. The relation between resting heart rate and cancer incidence, cancer mortality and all-cause mortality in patients with manifest vascular disease. Cancer Epidemiol. 2014;38: 715–721. doi: 10.1016/j.canep.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56: 1973–1983. doi: 10.1016/j.jacc.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Bohm M. Heart rate: from heart failure to chronic diseases and cancer. Is there a role for supportive care by heart rate reduction? Eur J Heart Fail. 2017;19: 250–252. doi: 10.1002/ejhf.689 [DOI] [PubMed] [Google Scholar]
- 14.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18: 1201–1206. doi: 10.1158/1078-0432.CCR-11-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130: 1865–1871. doi: 10.1053/j.gastro.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 16.Gibson AL, Holmes JC, Desautels RL, Edmonds LB, Nuudi L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in Hispanic, black, and white adults. Am J Clin Nutr. 2008;87: 332–338. [DOI] [PubMed] [Google Scholar]
- 17.Anuurad E, Shiwaku K, Nogi A, Kitajima K, Enkhmaa B, Shimono K, et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health. 2003;45: 335–343. [DOI] [PubMed] [Google Scholar]
- 18.Bauman AE, Nelson DE, Pratt M, Matsudo V, Schoeppe S. Dissemination of physical activity evidence, programs, policies, and surveillance in the international public health arena. Am J Prev Med. 2006;31: S57–65. doi: 10.1016/j.amepre.2006.06.026 [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25: 71–80. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg L, Boggs D, Wise LA, Palmer JR, Roltsch MH, Makambi KH, et al. A follow-up study of physical activity and incidence of colorectal polyps in African-American women. Cancer Epidemiol Biomarkers Prev. 2006;15: 1438–1442. doi: 10.1158/1055-9965.EPI-06-0079 [DOI] [PubMed] [Google Scholar]
- 21.Martinez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120: 1077–1083. doi: 10.1053/gast.2001.0050101083 [DOI] [PubMed] [Google Scholar]
- 22.Francois F, Park J, Bini EJ. Colon pathology detected after a positive screening flexible sigmoidoscopy: a prospective study in an ethnically diverse cohort. Am J Gastroenterol. 2006;101: 823–830. doi: 10.1111/j.1572-0241.2006.00433.x [DOI] [PubMed] [Google Scholar]
- 23.Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc. 2000;51: 433–437. [DOI] [PubMed] [Google Scholar]
- 24.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24: 3535–3541. doi: 10.1200/JCO.2006.06.0863 [DOI] [PubMed] [Google Scholar]
- 25.Paik JH, Jung EJ, Ryu CG, Hwang DY. Detection of Polyps After Resection of Colorectal Cancer. Ann Coloproctol. 2015;31: 182–186. doi: 10.3393/ac.2015.31.5.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan C, Gaglia P, Zullo A, Scaccianoce G, Piglionica D, Rossini FP, et al. Endoscopic follow-up after colorectal cancer resection: an Italian multicentre study. Dig Liver Dis. 2006;38: 45–50. doi: 10.1016/j.dld.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Kim BC, Han KS, Hong CW, Sohn DK, Park SC, et al. Incidence and risk factors of metachronous colorectal neoplasm after curative resection of colorectal cancer in Korean patients. J Dig Dis. 2014;15: 367–376. doi: 10.1111/1751-2980.12154 [DOI] [PubMed] [Google Scholar]
- 28.Oh DJ, Hong HO, Lee BA. The effects of strenuous exercises on resting heart rate, blood pressure, and maximal oxygen uptake. J Exerc Rehabil. 2016;12: 42–46. doi: 10.12965/jer.150258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114: 1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562 [DOI] [PubMed] [Google Scholar]
- 30.Park J, Kim JH, Lee HJ, Park SJ, Hong SP, Cheon JH, et al. The Effects of Physical Activity and Body Fat Mass on Colorectal Polyp Recurrence in Patients with Previous Colorectal Cancer. Cancer Prev Res (Phila). 2017;10: 478–484. [DOI] [PubMed] [Google Scholar]
- 31.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89: 500–508. doi: 10.3945/ajcn.2008.26847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1: 628–638. doi: 10.18632/oncotarget.101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61: 2866–2869. [PubMed] [Google Scholar]
- 34.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23: 267–275. doi: 10.1016/j.bbi.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi CH, Song T, Kim TH, Choi JK, Park JY, Yoon A, et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol. 2014;140: 1179–1188. doi: 10.1007/s00432-014-1658-7 [DOI] [PubMed] [Google Scholar]
- 36.Jang HI, Lim SH, Lee YY, Kim TJ, Choi CH, Lee JW, et al. Perioperative administration of propranolol to women undergoing ovarian cancer surgery: A pilot study. Obstet Gynecol Sci. 2017;60: 170–177. doi: 10.5468/ogs.2017.60.2.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.