The effects of recurrent concussion on white matter integrity and functional neural recruitment are dependent on the playing position and football career duration of former collegiate and professional football players.
Abstract
Purpose
To better understand the relationship between exposure to concussive and subconcussive head impacts, white matter integrity, and functional task-related neural activity in former U.S. football athletes.
Materials and Methods
Between 2011 and 2013, 61 cognitively unimpaired former collegiate and professional football players (age range, 52–65 years) provided informed consent to participate in this cross-sectional study. Participants were stratified across three crossed factors: career duration, concussion history, and primary playing position. Fractional anisotropy (FA) and blood oxygen level–dependent (BOLD) percent signal change (PSC) were measured with diffusion-weighted and task-related functional magnetic resonance imaging, respectively. Analyses of variance of FA and BOLD PSC were used to determine main or interaction effects of the three factors.
Results
A significant interaction between career duration and concussion history was observed; former college players with more than three concussions had lower FA in a broadly distributed area of white matter compared with those with zero to one concussion (t29 = 2.774; adjusted P = .037), and the opposite was observed for former professional players (t29 = 3.883; adjusted P = .001). A separate interaction between concussion history and position was observed: Nonspeed players with more than three concussions had lower FA in frontal white matter compared with those with zero to one concussion (t25 = 3.861; adjusted P = .002). Analysis of working memory-task BOLD PSC revealed a similar interaction between concussion history and position (all adjusted P < .004). Overall, former players with lower FA tended to have lower BOLD PSC across three levels of a working memory task.
Conclusion
Career duration and primary playing position seem to modify the effects of concussion history on white matter structure and neural recruitment. The differences in brain structure and function were observed in the absence of clinical impairment, which suggested that multimodal imaging may provide early markers of onset of traumatic neurodegenerative disease.
© RSNA, 2017
See also the editorial by Maldjian in this issue.
Introduction
A remote history of recurrent concussion is associated with a wide range of detriments to psychologic (1–3), neurologic (1,4,5), and cognitive (2,6–8) health. Chronic traumatic encephalopathy has been identified in deceased collegiate (9) and professional football players (4); however, the contributing factors to the development of chronic traumatic encephalopathy are poorly understood. In addition to a high risk of concussion, football also involves exposure to many subconcussive impacts. The extent to which these subconcussive impacts affect neurologic health is unclear.
Large-volume exposure to concussive and subconcussive impacts over many years may be a risk factor for the development of neurodegenerative disease (2–8). The acute and chronic effects of subconcussive impact exposure on the brain is a nascent area of research. Neuroimaging has emerged as a powerful tool to study late-life effects of head trauma in vivo and may yield clinically relevant biomarkers of disease presence and progression. By using diffusion-tensor imaging, collegiate contact sport athletes showed greater changes in fractional anisotropy (FA) and mean diffusivity during a season compared with noncontact sport control participants (10). These changes were largely persistent after 6 months without contact. A resting-state functional magnetic resonance (MR) imaging study (11) of rugby players before and after a full contact game showed a differential effect of subconcussive impact exposure on connectivity within the default mode network between those with a history of concussion and those without. These findings suggest subconcussive impact exposure may affect both brain structure and function acutely after a relatively small amount and short duration of exposure.
Evidence of a persistent effect of large-volume subconcussive exposure on late-life neurologic health, however, is scant and equivocal. One study by Stamm et al (12) found that among retired professional football players, exposure to football at an early age was associated with deficits at neuropsychologic testing. Another study (13) that examined neuropsychologic test performance, outcomes from a detailed neurologic examination, and MR images in retired professional football players was unable to replicate the findings of Stamm et al. A recent article (14) observed that an index of cumulative exposure to football (accounting for seasons played, playing position, and level of play) was predictive of cognitive and behavioral symptoms in former high school and collegiate football players. There is also evidence of a difference in the incidence of neurodegenerative disease cause of death across playing positions, in which risk of dying from neurodegenerative disease is greater for former speed position players than for former nonspeed (ie, linemen) players (15). In light of these studies, the effects of the total volume and character of subconcussive impact exposure on later-life health remain unclear and more research is needed.
In our study of former collegiate and professional football players, we hypothesized that a longer playing career would result in cumulative white matter damage and would augment the effects of recurrent concussion on functional neural recruitment. Additionally, we expected former speed position players, with their greater exposure to large magnitude impacts, to show greater white matter damage across our cohort. The purpose of the current study was to better understand the relationship between exposure to concussive and subconcussive head impacts, white matter integrity, and functional task-related neural activity in former U.S. football athletes.
Materials and Methods
Study Design
The National Football League (NFL) Charities and NFL Players Association provided financial support for this research project. The authors had control of the data and information submitted for publication. From September 2011 to March 2013, we recruited a stratified sample of former football players. All participants gave written informed consent in accordance with the requirements of the institutional review board. Our three factors of interest had two levels each and were fully crossed, giving eight (2 × 2 × 2) distinct strata. Exposure to subconcussive impacts was represented by football career duration: retired after college football or continued on to professional football for 5 or more years after college. History of concussive injury was assessed by using self-report with a provided standard definition of concussion (16). With the inherent difficulty of accurately quantifying concussions on the basis of self-report because of recall bias and poor recognition of the injury in the past, we chose to dichotomize our strata for concussion history a priori as more than three concussions (high frequency) or zero to one concussion (low frequency). The cut-off of three for concussion history is based on previous studies in which players with three or more concussions had worse late-life outcomes (3,8). By excluding players with exactly two previous concussions, we are better able to detect differences between the high-frequency and low-frequency concussion groups. Playing position was operationalized according to Lehman et al (15) as nonspeed position (ie, offensive or defensive lineman) and speed position (ie, any other position, excluding punter and kickers). The number of participants in each stratum is shown in Table 1.
Table 1.
Demographics of Sample by Concussion History and Career Duration Stratification
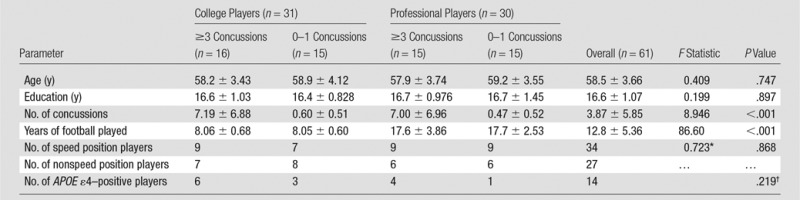
Note.—Unless otherwise indicated, data are mean ± standard deviation. APOE = apolipoprotein E.
*Reported as a χ2 test of independence; df = 3.
†Fisher exact test.
Recruitment of Participants
We recruited 32 former professional football player participants from our center’s data repository of 1330 former NFL players who completed general health surveys from our research center in 2001 and 2010. We excluded players who changed the number of concussions they reported between surveys or who reported two concussions (n = 784), played less than 5 years in the NFL (n = 202), or who were younger than 55 years or older than 65 years (n = 267). The remaining 77 potentially eligible participants were randomized and contacted by telephone until 32 participants were recruited into the study. Of the 63 former professional players who were screened, 16 players were not interested in the study and 15 players were excluded because they were a kicker or punter or had MR imaging contraindications, clinical cognitive impairments, or major neurologic disease. Thirty-two former college football athletes were similarly recruited from seven division-I football programs on the East Coast and were frequency matched according to the self-reported number of concussions and age at the time of the visit. The former college players must have played college football for at least 3 years. With 64 participants, the study had greater than 80% power to detect a main effect size of 1.0 between groups for continuous variables. MR imaging data from three participants were unusable because of excessive movement in the imager (>4 mm of movement during one or more functional MR examination) or cessation of the examination before completion because of claustrophobia; these participants were excluded from all analyses. The remaining participants completed every assessment (no missing data).
Neuropsychologic Testing
The Mini-Mental State Examination (known as the MMSE) (17), Repeatable Battery for the Assessment of Neuropsychological Status (known as the RBANS) (18), and Wechsler Adult Intelligence Scale–Third Edition (known as the WAIS-III) (19) were administered and scored by a licensed clinical neuropsychologist with 22 years of experience who was blinded to group assignment. Participants also completed self-report surveys of anxiety (Zung Anxiety Scale) (20) and depression (Beck Depression Inventory) (21). Separate one-way multivariate analyses of variance were used to assess for group differences in RBANS and WAIS-III domains across concussion-career duration and concussion-position stratifications. Separate univariate analyses of variance were used for Beck Depression Inventory, Zung Anxiety, and MMSE.
Structural MR Imaging
MR imaging data were obtained by using a 3-T imager (Trio; Siemens, Erlangen, Germany). T1-weighted images were obtained (repetition time msec/echo time msec, 1750/4.38; voxel size, 1 × 1 × 1 mm3; 160 sections). These structural images were used for registration of both functional and diffusion-weighted images.
Diffusion-weighted Imaging Acquisition and Processing
A spin-echo sequence was used to help acquire 54 diffusion-weighted directional images (b = 1000 sec/mm2) and nine unweighted images (b = 0) with an isotropic resolution of 2 × 2 × 2 mm3. Preprocessing of the raw diffusion images was performed by using FSL 5.0.9 (FMRIB Software Library; Oxford Center for Functional MR Imaging of the Brain, Oxford, England, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) (22) and included correction for eddy currents and head motion, and excluded nonbrain voxels through brain extraction (23). Tensor parameters were then estimated by using the FMRIB’s Diffusion Toolbox. FA maps of all participants were entered into the tract-based spatial statistics pipeline (24). First, each FA map was aligned into common space with FNIRT (FMRIB; Oxford Center for Functional MR Imaging of the Brain). Next, the group’s mean FA image was computed and thinned by using an FA threshold of 0.2, which created a mean skeleton map that represented the centers of all white matter tracts common to the group. Finally, the normalized FA image of each participant was projected onto the white matter skeleton and the resulting data were used to derive voxel-wise, cross-subject statistics. The FSL program Randomise was used for non-parametric, permutation-based statistical inference with threshold-free cluster enhancement (25,26). We used 10000 permutations, giving an approximate P value of .0500 ± .0044 (standard deviation). Family-wise error rate with a priori α level of .05 was used to correct for multiple comparisons.
We used a general linear model in which FA was predicted by three factors (concussion history, career duration, and playing position) and their interactions; the grand mean was included in the model as a parameter of noninterest. F tests with separate contrast vectors were used to determine any main or interaction effects. To examine the direction of the observed interactions, significant voxels from the threshold-free cluster enhancement corrected statistical maps were masked and mean FA values from the voxels that composed the significant clusters were extracted for each participant. These mean FA values were viewed by using boxplots, and post hoc t tests were performed within and between groups with Tukey correction for multiple comparisons.
Assessment of Working Memory Functional MR Imaging Task
The in-imager working memory paradigm used in this study was an N-back task (27). The experimental task was a blocked design with three conditions defined by working memory load: 0-back (low), 1-back (moderate), and 2-back (high). The stimuli for this task included both upper- and lowercase letters that appeared on the screen, one at a time, for 3 seconds each. Stimuli were presented in six blocks, with 72 letters appearing in each block. Each condition was presented over two consecutive blocks with condition order randomized across participants. During the 0-back condition, participants were told to respond “yes” every time the letter “d” (or “D”) appeared on the screen, and “no” for every other letter. In the 1- and 2-back conditions, participants were told to respond “yes” any time the letter on the screen was the same as the previous letter or the letter that had appeared two positions before it, respectively, and “no” for nonmatches. Before the imaging sessions, participants were familiarized with the task, provided with verbal instructions, and completed practice sessions. Behavioral data from the task (reaction time and accuracy) were analyzed in separate mixed analyses of variance across concussion-career duration and concussion-position stratifications (between participants), and N-back level (within participants).
Functional MR Imaging Task Acquisition and Processing
Task-based functional MR imaging was performed by using a whole-brain, gradient-echo, echo-planar sequence, with a voxel size of 3 × 3 × 3 mm3 over 53 sections (3 sec/30; flip angle, 90°). Images were preprocessed and analyzed by using software (SPM12; Wellcome Department of Cognitive Neurology, London, England) implemented as a suite of commands in Matlab (MathWorks, Natick, Mass). Images were co-registered to the anatomic examination of each participant, section-time corrected, realigned, normalized to Montreal Neuroimaging Institute template, and smoothed by using a Gaussian 8-mm kernel.
Functional MR Imaging Task Blood Oxygen Level–Dependent Percent Signal Change Analyses
All functional MR imaging blood oxygen level–dependent (BOLD) percent signal changes (PSCs) were initially analyzed on the basis of prespecified univariate contrasts to capture differences in recruitment across working memory loads. The three contrasts were 1-back greater than 0-back, 2-back greater than 0-back, and 2-back greater than 1-back. Group-level analysis of these functional MR imaging differences followed the same general linear model framework as the diffusion-tensor imaging analysis. Only significant contrasts from the diffusion-tensor imaging analysis were examined, and these were the concussion history–career duration and concussion history–playing position interactions. This approach improves interpretability of any observed differences in a task-related activity. In the context of observable differences in white matter integrity, differences in neural activation are interpreted as either functional inefficiency as a result of white matter damage (relatively decreased activity in task-relevant regions) (28) or compensatory neural activity in response to white matter damage (relatively increased activity in task-unrelated regions, or regions adjacent to task-related regions) (29–31). An a priori P value less than .001 was corrected for multiple comparisons by using a cluster size correction of k greater than 10 contiguous voxels. For group contrasts with significant clusters, BOLD PSC β weights (PSC between conditions) of individual participants were extracted to probe directionality of the interaction.
Apolipoprotein Eε4 Carrier Status
Buccal DNA was collected with brush swabs and was used to genotype the APOE allele. Fourteen participants were carriers of the ε4 allele, a known risk factor for neurodegeneration, and two participants were homozygous (Table 1). We assessed whether carrier status was associated with concussion history or career duration in our sample by using a Fisher exact test and did not observe an association (P = .219). When neuropsychologic and imaging outcomes were analyzed, we restricted our analysis to noncarriers and observed the same significant interactions between concussion history, career duration, and playing position as in the blinded analysis (detailed in Results), with an overlapping distribution of significant voxel clusters. Furthermore, we observed no significant interaction between APOE ε4 carrier status and concussion history, career duration, or playing position for any outcome (all P > .05). In light of these findings, we included both carriers and noncarriers in the final analyses and excluded APOE ε4 carrier status from our linear models.
Results
Participants were male and included 31 former college football players and 30 former professional NFL players. A detailed description of the sample is provided in Table 1.
Concussion History: Career Duration Interaction
Neuropsychologic testing.—No group differences were observed across the four concussion history–career duration stratifications for any neuropsychologic test or behavioral symptom survey (all P > .05; Table 2). Group means, standard deviations, and analysis of variance statistics are provided in Table 2.
Table 2.
Neuropsychologic Tests Performance and Neuropsychiatric Symptom Reporting by Concussion History and Career Duration Stratification
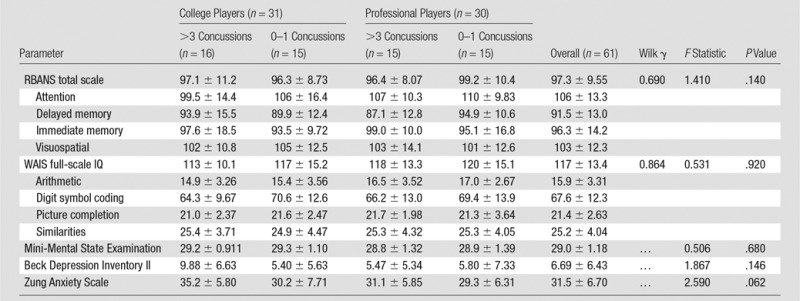
Note.—Unless otherwise indicated, data are mean ± standard deviation. IQ = intelligence quotient, RBANS = Repeatable Battery for the Assessment of Neuropsychological Status, WAIS = Wechsler Adult Intelligence Scale–Third Edition.
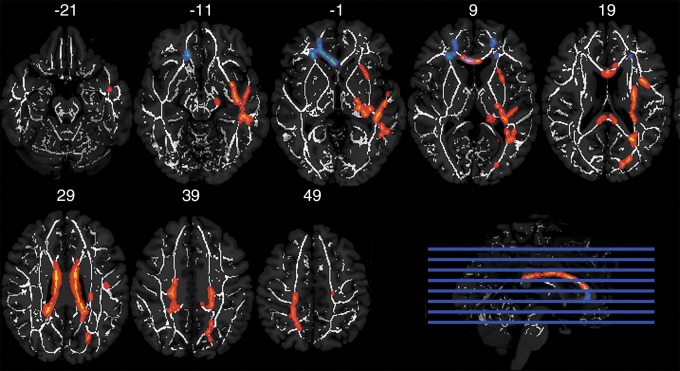
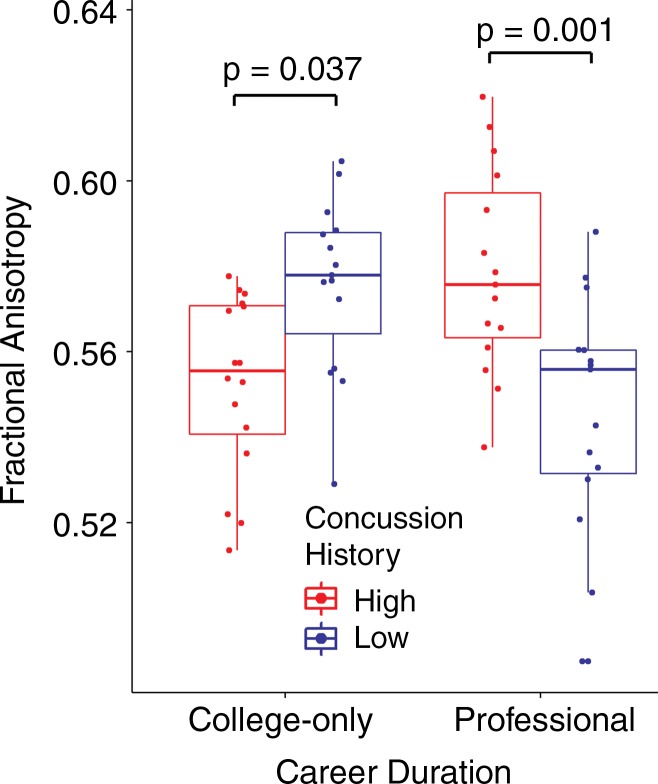
Diffusion-tensor imaging.—A significant cross-over interaction between concussion history and career duration was observed for 10 closely-grouped clusters that included the corpus callosum, superior longitudinal fasciculus, and other tracts (Fig 1, Table E1 [online]). The mean FA values across all voxels within the significant clusters are plotted with overlying boxplots in Figure 2. Post hoc t tests of the concussion history–career duration interaction revealed that within the college-only cohort, the high-frequency concussion group had a significantly lower mean FA than the low-frequency concussion group (t29 = 2.774; adjusted P = .037). The opposite was observed within the professional group: The low concussion group had a lower mean FA than the high concussion group (t29 = 3.883; adjusted P = .001).
Figure 1:
Axial MR images show results of tract-based spatial statistics analysis of white matter FA clusters of voxels with a significant crossover interaction (P < .05, corrected) between concussion history and career duration (red and orange) and between concussion history and playing position (blue). Clusters are overlaid on the mean white matter skeleton (white) in Montreal Neuroimaging Institute space (y coordinates are shown in millimeters). Voxels were filled out for viewing by using tbss_fill tool in FSL.
Figure 2:
Box-and-whisker plots show mean FA values from the significant clusters for the interaction of concussion history and career duration.
Functional MR imaging task.—There was a significant main effect of N-back level on both accuracy (F2,56 = 73.131; P < .001) and reaction time (F2,56 = 200.778; P < .001); participants were faster and more accurate on the 0-back than on the 1-back (accuracy: t60 = 4.183, P < .001; reaction time: t60 = 9.220, P < .001) and 2-back (accuracy: t60 = 10.782; reaction time: t60 = 19.816, P < .001), and on the 1-back than on the 2-back (accuracy: t60 = 7.434, P < .001; reaction time: t60 = 10.895, P < .001). There was no interaction between concussion history and career duration for accuracy (F2,56 = 0.040, P = .843) or reaction time (F2,56 = 1.569, P = .215), and there was no significant three-way interaction between these two factors and N-back level for accuracy (F2,56 = 1.313, P = .273) or reaction time (F2,56 = 1.452, P = .233).
BOLD functional MR imaging.—There were no significant interactions observed between concussion history and career duration in the functional MR imaging data for any condition contrast.
Playing Position Interaction and Concussion History
Neuropsychologic testing.—No group differences were observed across the four concussion history–playing position stratifications for any neuropsychologic test or behavioral symptom survey (all P > .05; Table 3). Group means, standard deviations, and analysis of variance statistics are given in Table 3.
Table 3.
Neuropsychologic Tests Performance and Neuropsychiatric Symptom Reporting by Concussion and Position Stratification
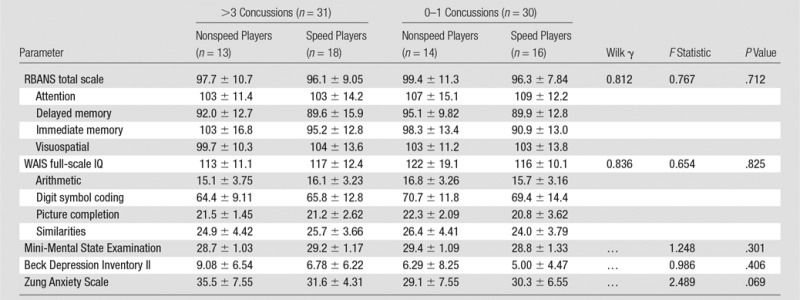
Note.—Unless otherwise indicated, data are mean ± standard deviation. IQ = intelligence quotient, RBANS = Repeatable Battery for the Assessment of Neuropsychological Status, WAIS = Wechsler Adult Intelligence Scale–Third Edition.
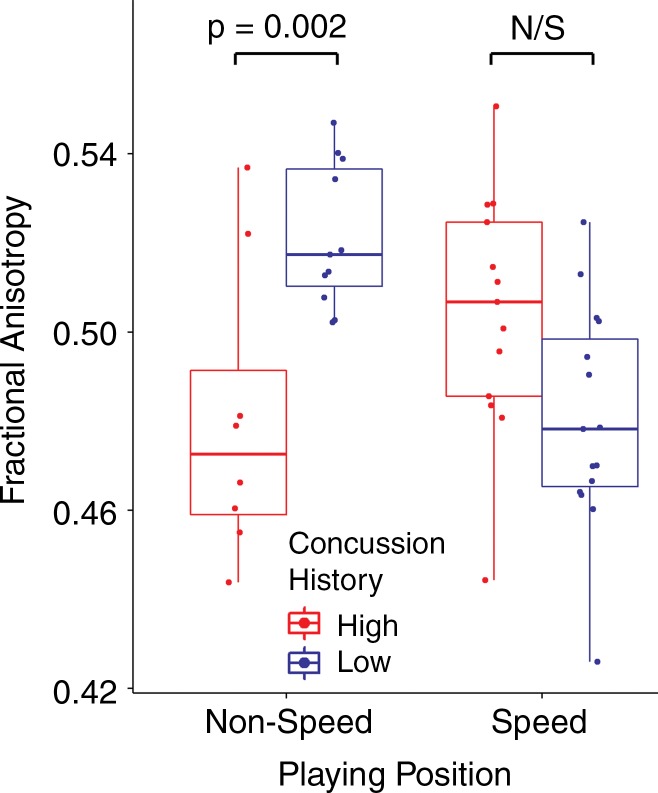
Diffusion-tensor imaging.—Four significant clusters were observed in the frontal white matter, specifically within the forceps minor, for the interaction of concussion history and playing position (Fig 1, Table E2 [online]). The mean FA values across all voxels within the significant clusters are plotted with overlaid boxplots in Figure 3. Post hoc t tests of the concussion history–playing position interaction revealed that within the nonspeed players, the high concussion group had a significantly lower mean FA than did the low concussion group (t25 = 3.861; adjusted P = .002). No significant difference was observed between the high and low concussion groups within the speed position players (t32 = 2.342; adjusted P = .100).
Figure 3:
Box-and-whisker plots show mean FA values from the significant clusters for the interaction of concussion history and playing position.
Functional MR imaging task.—There was no concussion history–playing position interaction for accuracy (F2,56 = 1.171; P = .284) or reaction time (F2,56 = 3.071; P = .085) and no significant three-way interaction between these two factors and N-back level for accuracy (F2,56 = 0.500; P = .608) or reaction time (F2,56 = 1.386; P = .254).
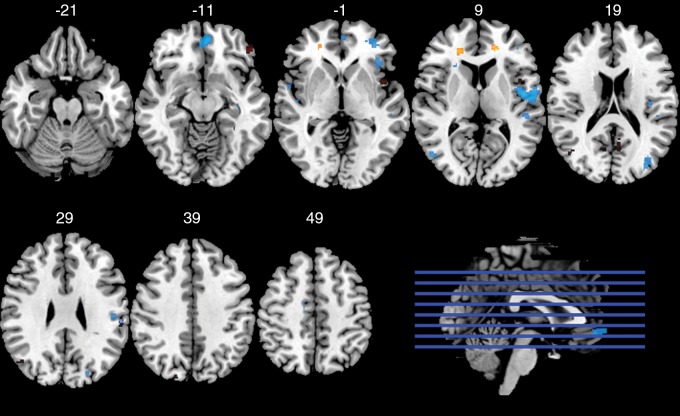
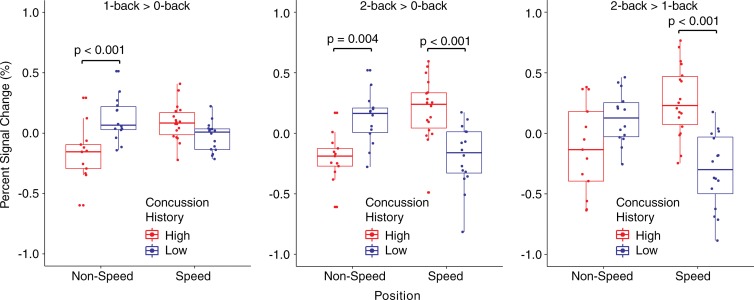
BOLD functional MR imaging.—There was a significant interaction between concussion history and playing position at each task condition contrast (Fig 4, 5). Further analysis of regions shown to exhibit BOLD PSC modulations as a function of concussion history and playing position revealed diffuse patterns of differences (Table E3 [online]). Among nonspeed position players, the low concussion group showed greater BOLD PSC in the 1-back greater than 0-back contrast than in the high concussion group (t25 = 4.378; adjusted P < .001). However, at the 2-back greater than 1-back contrast, speed position athletes in the high concussion group had greater BOLD PSC than the speed position athletes in the low concussion group (t32 = 5.539; adj. P < .001). In the 2-back greater than 0-back contrast, however, both of the effects noted above were observed (Fig 5). Low concussion, nonspeed position athletes had greater BOLD PSC than high concussion, nonspeed position athletes (t25 = 3.537; adjusted P = .004), whereas high concussion, speed position athletes had greater BOLD PSC over low concussion speed position athletes (t25 = 4.791; adjusted P < .001).
Figure 4:
Axial functional MR images show analysis of the interaction between concussion history and playing position for the task-related functional MR imaging BOLD response during a working memory N-back task. Each task-level contrast was modeled separately: 1-back greater than 0-back (orange), 2-back greater than 1-back (red), 2-back greater than 0-back (blue). Clusters with a significant interaction (P < .001) between concussion history and playing position are overlaid on the Montreal Neuroimaging Institute template brain. Numbers are the y coordinate of the Montreal Neuroimaging Institute space (in millimeters).
Figure 5:
Box-and-whisker plots show mean PSC from the significant clusters for the interaction of concussion history and playing position for each comparison of the functional MR imaging task.
Discussion
In our cohort of 61 former football athletes without cognitive or mood impairments, we found significant subgroup differences in white matter integrity (FA) and working memory-related functional neural activation patterns (BOLD PSC). Our findings indicate the effects of recurrent concussion on white matter integrity and functional neural recruitment in former football players depend on the impact exposure history of the athlete, including their career duration and playing position. Regarding the structural and functional imaging outcomes, both career duration and playing position were associated with concussion history. We will discuss the nature of these relationships.
Within our college-only cohort, those with more than three concussions had lower FA than those with zero to one concussions; these differences were broadly distributed. This result is consistent with a similar cross-sectional imaging study of former collegiate contact sport athletes (32). Similar to our study, Tremblay et al (32) used tract-based spatial statistics and none of the participants were impaired in cognition or mood; they found diffusely lower FA in former university-level athletes with a history of concussions compared with those without previous concussion. Similarly, we observed lower FA throughout the corpus callosum and extending into the superior longitudinal fasciculus in those who reported more than three concussions (32).
We observed an unexpected result in our professional football cohort: Those who reported multiple concussions had greater mean FA than those with zero to one concussion. Interestingly, the professional football cohort with more than three concussions was not significantly different (in FA, BOLD PSC, or clinical measures) than our lowest exposed group, the college-only, 0–1 concussion players. We do not have data to explain this result, but speculate that this lack of difference may reflect a selection bias in favor of former professional players with a relatively high amount of brain reserve. In other words, those players with a long career duration, exposure to recurrent concussive events, and who are cognitively normal in their late 50s may not be reflective of the highly exposed former professional football player population as a whole. These individuals may constitute a relatively more resilient subset of former players who were well enough to participate in the study. Although this hypothesis is speculative, in general, our diffusion-tensor imaging findings in the professional players are consistent with previous reports (2,33,34). Three previous studies found no differences in group mean FA when retired NFL players with a history of concussions were compared with age-matched, nonathlete control participants. Hart et al (2) reported no white matter FA differences between unimpaired players (without deficits of cognition or mood) and control participants. Strain et al (33) found no differences in FA between nondepressed retired NFL players with significant concussion history and matched control participants. In Goswami et al (34), the authors restricted their tract-based spatial statistics analysis of white matter FA to the uncinate fasciculus and found no differences between former NFL players and control participants.
Our analysis of playing position found that among nonspeed players, those with three or more concussions had significantly lower FA than those with zero to one concussion. These differences were localized to the frontal white matter, particularly the forceps minor. We also observed several frontoparietal regions with lower BOLD PSC in the nonspeed high concussion group relative to the nonspeed low concussion group, particularly when the task conditions (1- and 2-back) was compared with the baseline condition (0-back). This corroboration of functional MR imaging and diffusion-tensor imaging effects suggests that compromised white matter integrity in frontal white matter tracts reduces functional neural efficiency in these athletes during the working memory task, with corresponding under-recruitment of task-relevant regions. Athletes with clusters of lower FA tended to have a lower and more negative BOLD PSC in task-relevant areas across all task contrasts, which suggested that this compromised structural integrity resulted in relatively reduced activity in task-relevant regions (28).
The interactions we observed between concussion history and playing position suggest that there may be important differences in the mechanisms of injury between speed and nonspeed players. The magnitude, location, and frequency of head impacts in football differ by position (35,36). For example, offensive backs are 1.24 and 1.52 times more likely to experience an impact exceeding 80 g of peak linear acceleration than offensive or defensive linemen, respectively (37). Linemen, however, tend to experience a greater overall frequency of impacts than speed positions and have the greatest proportion of impacts to the front of the helmet (36,38). The high proportion of frontal impacts experienced by nonspeed players may result in more localized damage to frontal white matter tracts compared with the more variable impact locations experienced by speed position players. It is important to note that the frequency, magnitude, and location of both concussive and subconcussive impacts may differ between speed and nonspeed players. Because of this, it is unknown whether the significant interactions we observed between concussion history and playing position are a result of differences in subconcussive or concussive impacts, or a combination of the two.
Both functional MR imaging contrasts comparing high working memory load (2-back) with lower working memory load (1- and 0-back) revealed a significant difference between speed position players on the basis of concussion history. Specifically, high-concussion speed athletes showed greater recruitment of the task-relevant regions, along with other adjacent regions, relative to low-concussion speed athletes. This finding was unexpected and mirrors the FA pattern observed in the speed group, in which speed players with more than three concussions tended to have greater mean FA in the forceps minor region. These results highlight preliminary associations between changes in structural networks and functional neural recruitment, which may help us further understand the neurodegenerative nature of repeated traumatic head injuries.
One limitation of our study is that our recruitment strategy purposely selected unimpaired former players. We chose this strategy because we were primarily interested in the early changes in brain structure and function preceding clinical impairment secondary to traumatic neurodegeneration. In applying this selection criterion, we may have biased our sample, particularly among professional players with a history of recurrent concussion. This group has been shown to have a greater risk of mild cognitive impairment, depression, and pathologic analysis–confirmed chronic traumatic encephalopathy compared with the general population. Our sample may have a higher than normal amount of brain reserve, as reflected in higher FA in the absence of clinical impairments. We are also limited by our sample size. A priori power calculations were performed in regard to observing main effects, not interactions. Thus, our inability to observe a significant interaction may be the result of inadequate power.
The effects of recurrent concussion on white matter integrity and functional neural recruitment are dependent on the playing position and football career duration of former collegiate and professional football players. A longer football career does not appear to augment the effects of recurrent concussion, as we expected it would. We observed that playing a nonspeed position (ie, offensive or defensive linemen) resulted in lower white matter integrity and more inefficient neural recruitment for those with a history of recurrent concussion. This finding was observed in both collegiate and professional players, which suggests that the primary playing position of a football athlete is a modifier of the effects of recurrent concussion, and may be a risk factor for the development of traumatic neurodegenerative disease. Of note, we observed no group differences in neuropsychologic test results, psychiatric symptom surveys, or functional MR imaging task performance across our concussive and subconcussive exposure strata, as expected given our recruitment strategy. Our data suggest that measureable changes to white matter integrity and neural recruitment may precede cognitive and behavioral impairment as measured by standard clinical neuropsychologic tasks. Multimodal imaging may provide early markers of traumatic neurodegenerative disease onset and progression.
Advances in Knowledge
■ We observed a significant interaction between concussion history and career duration for white matter integrity (P < .05 with family-wise error rate correction), and between concussion history and playing position for both white matter integrity and functional neural recruitment (P < .05 with family-wise error rate correction) during a working memory task.
■ Former football athletes who played a nonspeed position (ie, offensive or defensive linemen) and reported three or more concussions had lower white matter fractional anisotropy (t25 = 3.861; adjusted P = .002) and lower blood oxygen level–dependent percent signal change during a working memory task (t25 = 3.537; adjusted P = .004) than did those with zero to one concussion.
■ Measurable changes to white matter integrity and neural recruitment in former football athletes (P < .05 with family-wise error rate correction) may develop in the absence of cognitive and behavioral impairments measured by standard clinical neuropsychologic tests.
SUPPLEMENTAL TABLES
Received March 6, 2017; revision requested May 5; revision received May 26; accepted June 4; final version accepted July 28.
From the 2015 RSNA Annual Meeting.
Supported by the National Football League Charities and National Football League Players’ Association. M.D.C. supported by F30NS090816 from the National Institute of Neurologic Disorders and Stroke.
M.D.C. supported by National Institute of Neurologic Disorders and Stroke (F30NS090816). Study supported by the National Football League Charities and the National Football League Players’ Association.
M.D.C. and E.M.L.V. contributed equally to this work.
Disclosures of Conflicts of Interest: M.D.C. disclosed no relevant relationships. E.M.L.V. disclosed no relevant relationships. A.A.C. disclosed no relevant relationships. K.S.G. disclosed no relevant relationships. F.S. disclosed no relevant relationships. Z.Y.K. disclosed no relevant relationships. J.K.S. disclosed no relevant relationships. K.M.G. disclosed no relevant relationships.
Abbreviations:
- BOLD
- blood oxygen level–dependent
- FA
- fractional anisotropy
- NFL
- National Football League
- PSC
- percent signal change
References
- 1.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30(1):179–188, xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart J, Jr, Kraut MA, Womack KB, et al. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol 2013;70(3):326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 2007;39(6):903–909. [DOI] [PubMed] [Google Scholar]
- 4.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68(7):709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005;57(1):128–134; discussion 128–134. [DOI] [PubMed] [Google Scholar]
- 6.De Beaumont L, Théoret H, Mongeon D, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009;132(Pt 3):695–708. [DOI] [PubMed] [Google Scholar]
- 7.Ford JH, Giovanello KS, Guskiewicz KM. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J Neurotrauma 2013;30(20):1683–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005;57(4):719–726; discussion 719–726. [DOI] [PubMed] [Google Scholar]
- 9.Mez J, Solomon TM, Daneshvar DH, Stein TD, McKee AC. Pathologically confirmed chronic traumatic encephalopathy in a 25-year-old former college football player. JAMA Neurol 2016;73(3):353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazarian JJ, Zhu T, Zhong J, et al. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One 2014;9(4):e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson B, Neuberger T, Gay M, Hallett M, Slobounov S. Effects of subconcussive head trauma on the default mode network of the brain. J Neurotrauma 2014;31(23):1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamm JM, Bourlas AP, Baugh CM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 2015;84(11):1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon GS, Kuhn AW, Zuckerman SL, et al. Participation in pre-high school football and neurological, neuroradiological, and neuropsychological findings in later life: a study of 45 retired National Football League players. Am J Sports Med 2016;44(5):1106–1115. [DOI] [PubMed] [Google Scholar]
- 14.Montenigro PH, Alosco ML, Martin BM, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 2017;34(2):328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012;79(19):1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47(5):250–258. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 18.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Technical manual for the Wechsler Adult Intelligence Test. 3rd ed. San Antonio, Tex: Psychological Corporation, 1997. [Google Scholar]
- 20.Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1971;12(6):371–379. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 1988;8(1):77–100. [Google Scholar]
- 22.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 25.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 27.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol 1958;55(4):352–358. [DOI] [PubMed] [Google Scholar]
- 28.O’Conaill CR, Malisza KL, Buss JL, et al. Visual search for feature conjunctions: an fMRI study comparing alcohol-related neurodevelopmental disorder (ARND) to ADHD. J Neurodev Disord 2015;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakun JG, Zhu Z, Brown CA, Johnson NF, Gold BT. Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI-DTI study. Neuropsychologia 2015;71:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002;17(3):1394–1402. [DOI] [PubMed] [Google Scholar]
- 31.Persson J, Nyberg L, Lind J, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex 2006;16(7):907–915. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay S, Henry LC, Bedetti C, et al. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain 2014;137(Pt 11):2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strain J, Didehbani N, Cullum CM, et al. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 2013;81(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goswami R, Dufort P, Tartaglia MC, et al. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct 2016;221(4):1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr ZY, Littleton AC, Cox LM, et al. Estimating contact exposure in football using the Head Impact Exposure Estimate (HIEE). J Neurotrauma 2015;32(14):1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crisco JJ, Fiore R, Beckwith JG, et al. Frequency and location of head impact exposures in individual collegiate football players. J Athl Train 2010;45(6):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihalik JP, Bell DR, Marshall SW, Guskiewicz KM. Measurement of head impacts in collegiate football players: an investigation of positional and event-type differences. Neurosurgery 2007;61(6):1229–1235; discussion 1235. [DOI] [PubMed] [Google Scholar]
- 38.Schnebel B, Gwin JT, Anderson S, Gatlin R. In vivo study of head impacts in football: a comparison of National Collegiate Athletic Association Division I versus high school impacts. Neurosurgery 2007;60(3):490–495; discussion 495–496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.