Abstract
Substantial progress has been made in the genetic basis of Parkinson’s disease (PD). In particular, by identifying genes that segregate with inherited PD or show robust association with sporadic disease, and by showing the same genes are found on both lists, we have generated an outline of the cause of this condition. Here, we will discuss what those genes tell us about the underlying biology of PD. We specifically discuss the relationships between protein products of PD genes and show that common links include regulation of the autophagy–lysosome system, an important way by which cells recycle proteins and organelles. We also discuss whether all PD genes should be considered to be in the same pathway and propose that in some cases the relationships are closer, whereas in other cases the interactions are more distant and might be considered separate.
Keywords: chaperone-mediated autophagy, genetics, lysosomes, mitophagy, Parkinson’s disease, protein complexes
Autophagy refers to a series of processes related to how cells can recycle specific components, the term being derived from the Greek for self-eating (Zhang and Baehrecke 2015). Autophagy in its various forms has been linked to many aspects of fundamental biology, but also to some specific disease states (Feng et al. 2015). In this review, we will discuss the various lines of evidence suggesting that dysregulated autophagy might play a causal role in the pathogenesis of Parkinson’s disease (PD), a common neu-rodegenerative condition.
The interested reader is directed toward many high-quality reviews on the basic biology of autophagy, but before discussing PD we will highlight a few essential concepts. Most importantly, ‘autophagy’ covers at least three distinct phenomena. Macroautophagy functions to degrade unnecessary or damaged proteins and organelles using a double membranous organelle, the autophagosome (Feng et al. 2014). In a series of regulated events, the autophagosome matures and fuses with lysosomes. In contrast, microautophagy involves direct invagination of lysosomal membranes to engulf cellular components (Mijaljica et al. 2011). Chaperone-mediated autophagy (CMA) is a distinct pathway dependent on recognition of proteins by a complex including the 70 kDa heat-shock cognate protein (Hsc70) that then is transferred across the lysosomal membrane (Cuervo and Wong 2014). In each form of autophagy, the net result is that the material to be degraded will be subject to hydrolysis by lysosomal enzymes and the final products are then available to the cell for re-use. Autophagy is therefore important in homeostasis, maintenance of cellular pools of nutrients such as amino acids, as well as in triggering some forms of cell death. Autophagy also plays a role in infectious diseases, by regulating the removal of bacterial or viral agents. Therefore, in broad concept there is no single ‘autophagy’ pathway but a series of inter-related events using overlapping sets of machinery that have multiple biological functions.
In recent years, it has been recognized that disruption of autophagy has specific effects in the central nervous system. For example, genetic ablation of key autophagy genes results in neurodegenerative phenotypes in mice (Komatsu et al. 2006). In parallel, the identification of many genes associated with PD and related conditions led to substantive understanding of how neurodegeneration can occur in this disease (Bonifati 2014). Interestingly, some of these genes appear to have specific roles in how autophagy is regulated. Here, we will critically discuss the evidence for regulation of autophagy in PD, focusing on gene products that appear to have related functions.
Mitophagy: Parkin, PINK1, and Fbxo7
Mutations in parkin were first reported in an early onset form of PD in 1998 and mutations in PTEN-induced novel kinase 1 (PINK1) were identified in several families with similar phenotypes a few years later (Kitada et al. 1998; Valente et al. 2004). Looking at the primary sequence of the encoded proteins, it was recognized that parkin is an E3 protein-ubiquitin ligase and PINK1 was a serine/threonine protein kinase with a mitochondrial targeting sequence. Mutations were associated with loss of protein function, in that several were large deletions or truncating mutations (Bonifati 2014). However, it was not immediately obvious what the function of these proteins was in cells and whether PINK1 and parkin had any relationship with each other.
Two sets of results in very different systems demonstrated that PINK1 and parkin were involved in related pathways. First, when PINK1 knockout flies were examined they were seen to have phenotypes that were related to the accumulation of damage to mitochondria in a variety of tissues (Clark et al. 2006; Park et al. 2006). This phenocopied a set of phenotypes previously reported in parkin knockout flies (Greene et al. 2003), and through a series of genetic experiments it was shown that PINK1 was genetically upstream of parkin function. Some of the key data include that double knockout PINK1/parkin flies had no worse phenotype than each knockout alone and that parkin could rescue PINK1 knockout but not vice-versa (Clark et al. 2006; Park et al. 2006).
Second, while parkin is present predominantly in the cytoplasm and nucleus (Cookson et al. 2003), it was shown first by the Youle group that parkin could be recruited to mitochondria under conditions that induce mitochondrial depolarization (Narendra et al. 2008). Subsequently, it was demonstrated that this was a PINK1-dependent phenomenon (Matsuda et al. 2010; Narendra et al. 2010; Vives-Bauza et al. 2010). PINK1 is normally subject to rapid processing by proteases in mitochondria that are themselves inactivated by loss of membrane polarization (Jin et al. 2010; Deas et al. 2011; Meissner et al. 2011; Sekine et al. 2012). Thus, when mitochondria are damaged, there is an accumulation of PINK1 on the surface of that subset of the organelle. More recently it was shown that the key enzymatic events were the phosphorylation of Ubiquitin by PINK1 (Kane et al. 2014; Koyano et al. 2014), which then acts as a receptor for parkin (Okatsu et al. 2015). Parkin is simultaneously activated, likely by one or more phosphorylation events (Ordureau et al. 2014), and attaches phosphorylated ubiquitin to a series of proteins on the outer mitochondrial membrane (Chan et al. 2011; Sarraf et al. 2013). The key steps of this pathway are shown in Fig. 1.
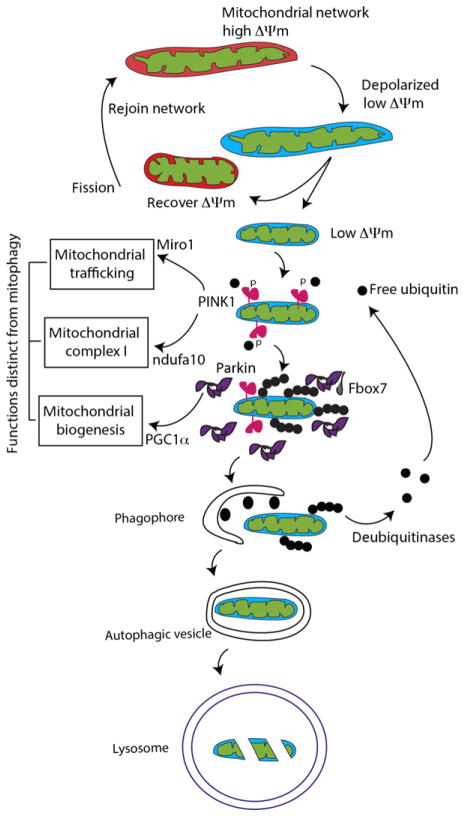
Fig. 1.
Mitophagy. Mitochondria normally exhibit polarization across their membranes as a result of an imbalance in H+ ion flow because of oxidative metabolism – this is known as mitochondrial membrane potential (ΔΨm). Mitochondria can be depolarized, in which case they can undergo fission and either recover membrane potential and rejoin the network by fusion or become isolated and be turned over via mitophagy. The mechanisms of mitophagy involve several PD-related genes. PINK1 (pink in the diagram) becomes stabilized on the mitochondrial surface, then phosphorylating ubiquitin (small black dots). Parkin is then recruited from the cytosol and activated, adding phosphorylated ubiquitin to multiple proteins on the outer mitochondrial membrane. Some data suggest that the adaptor protein Fbxo7 is required for this step. Once marked in this way, the damaged mitochondria are then engulfed by the nascent phagophore, a de novo lipid structure marked by the accumulation of LC3 (black ovals), a ubiquitin-like molecule that is lapidated once autophagy is initiated. The autophagic vesicle is completed and fuses with lysosomes, resulting in degradation of the damaged mitochondria.
After the ubiquitylation of mitochondrial membrane proteins by activated parkin, the marked mitochondria are degraded by a specialized form of macroautophagy called mitophagy (Narendra et al. 2008). It has been proposed that the turnover of mitochondria, specifically those that have lost mitochondrial membrane potential, that is, that are depolarized as above, is a way in which damaged mitochondria can be removed from the normal mitochondrial pool. It is likely that mitophagy is an important homeostatic mechanism that prevents accumulation of organelles that are diminished in their ability to make ATP, a critical function of mitochondria (Youle and Narendra 2011).
The PINK1/parkin pathway may also involve other genes related to inherited parkinsonism. For example, it has been shown that Fbxo7, a rare cause of recessive disease (Bonifati 2014) interacts physically with parkin and is required for mitophagy (Burchell et al. 2013). Interestingly, in Drosophila, Fbxo7 over-expression can rescue phenotypes linked to parkin deficiency, which suggests that that physical interaction with parkin is not required for at least some protective effects including in vivo.
In this way, PINK1, parkin, and Fbxo7 have been shown to be critically linked to mitophagy. While there are many details that still need to be mechanistically resolved, what is perhaps most interesting about these data are that they show how two different forms of protein degradation pathways, namely the ubiquitin–proteasome system and macroautophagy, co-operate to control a fundamental aspect of cellular homeostasis. Where there is less clarity is how disruption in these processes leads to a neurodegenerative phenotype.
At this point, it is worth noting that the phenotype in people with disease related to parkin or PINK1 has some features that are distinct from ‘typical’ sporadic PD. Although the number of autopsied cases are rather few, neurodegeneration in parkin/PINK1 seems to be limited to loss of dopamine neurons in the substantia nigra pars compacta (Poulopoulos et al. 2012). While loss of nigral neurons is a major and important feature of sporadic PD, it has been known for some time that many other brain regions can also be involved (Langston 2006). In addition, sporadic PD tends to progress substantially over time, whereas PINK1 and parkin cases generally show slower progression and sustained response to low dose dopaminergic medication (Bonifati 2012). Thus, in some ways, these recessive diseases represent a very dopamine-focused form of PD. Mutations in Fbxo7 can be variable clinically and include features such as chorea (Gündüz et al. 2014).
The reason why the phenotype of PINK1/parkin/Fbox7 merits discussion is that there is no obvious link between the physiological functions of dopamine neurons and a specific need for mitophagy. Autophagy in general, and mitophagy specifically, are very general cell biological phenomenon conserved over a large evolutionary space from yeast through humans. So why, at least in humans, there might be any specific cells that are lost with aging after disruption of mitophagy is difficult to resolve.
Adding to this difficulty, there has been some controversy about the role of PINK1/parkin-dependent mitophagy in neurons. While some studies have reported that neurons are susceptible to depolarization-induced parkin recruitment (Cai et al. 2012; Joselin et al. 2012; Ashrafi et al. 2014; Ye et al. 2015), others have not (Van Laar et al. 2011, 2015). Some studies have suggested that excessive excitatory stimuli might be sufficient to trigger parkin recruitment in neurons (Van Laar et al. 2015), with a potential implication being that pathological rather than physiological situations might have a bigger impact on parkin function. One key idea is that the bioenergetics of neurons are distinct from other cell types. Our laboratory has shown that hexokinase, which converts glucose to glucose-6-phosphate and is the first committed step in glycolysis, is required for parkin recruitment at least in cultured cells (McCoy et al. 2014). While it is often stated that neurons use glycolysis only sparingly and instead predominantly rely on oxidative metabolism, recent measurements of ATP suggest that both major pathways can be used to support synaptic function in cultured hippocampal neurons (Rangaraju et al. 2014). To what extent dopamine neurons, which are reported to have unusual bioenergetic demands, might use different ways to generate ATP is not clear but worth pursuing in the future.
It is also important to note that mutations in PINK1 and parkin may impact mitochondrial regulation by mechanisms that are potentially separate from mitophagy. For example, loss of PINK1 is associated with lower mitochondrial complex I activity possibly because of direct phosphorylation of the inner mitochondrial membrane component of complex I ndufa10 (Morais et al. 2009, 2014). Work in Drosophila models has suggested that ndufa10 can rescue PINK1 deficiency independent of effects on mitophagy or parkin (Pogson et al. 2014). PINK1 and parkin can increase turnover of proteins of the respiratory chain in vivo (Vincow et al. 2013) and have been shown to influence the translation of mRNA species that are important for respiratory chain function, which may represent a mechanism of mitochondrial quality control again independent of mitophagy (Gehrke et al. 2015).
These results collectively suggest that while the regulation of mitophagy is likely to explain some aspects of the function of PINK1 and parkin, it is also possible that the overall pathway is more complex than a simple linear relationship, as others have discussed (Scarffe et al. 2014). Some of the additional functions assigned to either PINK1 or parkin include regulation of mitochondria along neuronal axons (Weihofen et al. 2009; Liu et al. 2012; Birsa et al. 2014) and control of mitochondrial biogenesis (Pacelli et al. 2011; Shin et al. 2011). There are also important data supporting the idea that parkin can play an important role in ER-mitochondrial cross talk (Van Laar et al. 2015), including control of the translocation of a subset of mitochondrial proteins to the ER during mitophagy (Saita et al. 2013), suggesting that parkin may impact multiple organelles in the neuron.
The considerations illustrate a central contention that we will expand upon later in this article; that although there are sometimes direct mechanistic links between proteins encoded by genes that cause PD, the pathways that link genes can be more complex than implied by a simple linear relationship. In a sense, this is an unsurprising thought as a given cell has many different processes to co-ordinate under variable physiological conditions. Thus, the genes involved in recessive parkinsonism may have functions outside of mitophagy that allow for the control of distinct cellular events that need to be tied together either spatially or temporally, as indicated by the boxes distinct from the mitophagy pathway in Fig. 1. Interestingly, Mendelian inheritance of mutations in some of the proposed effector genes for these functions (such as Miro1, ndufa10, or Pcg1α) is extremely rare, which might indicate that disruption of the central functions of mitochondria is poorly tolerated.
There is substantial evidence that mitochondrial turnover occurs by additional, PINK1/parkin-independent pathways. Mitophagy can be triggered by diverse stimuli including hypoxia (Liu et al. 2014), mitochondrial toxins such as rotenone (Dagda et al. 2013), or the mitochondrial lipid cardiolipin (Li et al. 2015), and it is not clear if PINK1/parkin is always required for these effects. For example, in cell culture, iron chelators (Allen et al. 2013) or the protein AMBRA1 (Strappazzon et al. 2015) can promote mitophagy in the absence of parkin. In vivo, parkin deficiency does not result in the accumulation of damaged mitochondria in a model of chronic mitochondrial complex I impairment in dopaminergic neurons, the mitopark mouse (Sterky et al. 2011). Similarly, there are circumstances where mitophagy can proceed in the absence of PINK1 (Dagda et al. 2009; East et al. 2014). In some tissues, the mitochondrial protein Drp1 acts in a parallel, parkin-independent pathway to control mitochondrial ubiquitylation and turnover (Kageyama et al. 2014), and Drp1 can rescue phenotypes resulting from the loss of function of PINK1 in vivo (Liu et al. 2011).
Collectively, these considerations indicate that PINK1 and parkin are regulatory proteins that are particularly important in mitophagy after exposure to some cellular signaling pathways but are unlikely to be central players in all forms of mitophagy. This point, that PD genes play modulatory rather than essential roles, will be returned to when discussing some of the dominant genes that appear to have roles in the regulation of other forms of autophagy.
Autophagy: α-synuclein, Vps35, LRRK2
α-Synuclein is one member of a group of small proteins predominantly found in the pre-synaptic terminals of neurons (Clayton and George 1999). In 1997, mutations in familial cases with Parkinson’s disease were discovered in the gene encoding α-synuclein, SNCA (Polymeropoulos et al. 1997). Subsequently, α-synuclein was found to be a major constituent of Lewy bodies and Lewy neurites, the pathological hallmarks of PD (Spillantini et al. 1997). In 2003 Singleton and colleagues reported a triplication of the SNCA locus in a large family with PD and dementia (Singleton et al. 2003). Subsequently, duplications and triplications of the α-synuclein locus were found in several families (Farrer et al. 2004; Ibáñez et al. 2004; Ikeuchi et al. 2008). The mRNA and protein levels of α-synuclein in triplication cases are twice the amount present in controls (Miller et al. 2004), showing that increased α-synuclein levels can cause Parkinson’s Disease. Remarkably, genome wide association studies (GWAS) identified the α-synuclein locus as contributing to lifetime risk of sporadic PD (International Parkinson Disease Genomics Consortium et al. 2011).
As increased α-synuclein levels are implicated in PD pathogenesis, a logical question is therefore to examine the pathway by which α-synuclein is degraded in cells. For example, Cuervo et al. (2004) found that α-synuclein is a substrate for the specialized form of autophagy, CMA. The amino acid sequence of α-synuclein contains a CMA-like recognition motif that is sufficient to allow translocation of α-synuclein through isolated intact lysosomes and further degradation in a CMA-dependent fashion. It was subsequently shown that CMA is a major pathway for degradation of α-synuclein in primary neuronal cultures and human neuronal cell lines (Vogiatzi et al. 2008). The siRNA-mediated knockdown of lysosome-associated membrane protein 2 (LAMP2a) resulted in slower turnover of wild-type α-synuclein, and α-synuclein mutant lacking the CMA motif exhibited slower degradation compared to wild type. In addition, inhibition of CMA can lead to the formation of insoluble, oligomeric α-synuclein species (Vogiatzi et al. 2008). Knockdown of LAMP2a in SH-SY5Y cells also results in increased half-life of α-synuclein (Alvarez-Erviti et al. 2010).
In addition to these effects of CMA on α-synuclein, mutant, or modified forms of the protein may inhibit CMA. Specifically, phosphorylated α-synuclein (Martinez-Vicente et al. 2008), A53T mutant (Cuervo et al. 2004; Xilouri et al. 2009), and dopamine-modified forms of the wild-type protein (Martinez-Vicente et al. 2008; Xilouri et al. 2009) lead to CMA dysfunction, and may induce compensatory macroautophagy (Xilouri et al. 2009). Indeed, pharmacological inhibition of macroautophagy resulted in α-synuclein accumulation in the lysosome (Webb et al. 2003; Vogiatzi et al. 2008) and activation of macroautophagy facilitated the degradation of both wild-type and mutant forms of α-synuclein (Webb et al. 2003; Spencer et al. 2009). A study in transgenic mice over-expressing α-synuclein under the regulatory control of the Thy-1 promoter provided evidence that CMA provides a major mechanism for α-synuclein degradation in vivo. Enhanced lysosomal content of α-synuclein, and up-regulation of CMA-related proteins (LAMP2a, Hsc70) were directly correlated with the level of α-synuclein over-expression throughout the brain (Mak et al. 2010). Impairment of the autophagy system in general, and CMA in particular, is therefore likely to increase the amount of α-synuclein in brain, thus contributing to PD development. There are additional genes that may have roles in macroautophagy and CMA that, therefore, may affect α-synuclein through overlapping mechanisms.
Mutations in leucine-rich repeat kinase 2 (LRRK2) (Paisán-Ruíz et al. 2004; Zimprich et al. 2004) are a relatively common genetic cause of late-onset PD, accounting for up to 40% of familial cases in some populations (Bras et al. 2005; Deng et al. 2006; Ozelius et al. 2006; International Parkinson Disease Genomics Consortium, Nalls M. A., Plagnol V., Hernandez D. G., Sharma M., Sheerin U.-M., Saad M., et al. 2011). Similar to α-synuclein, GWAS have also identified variants at the LRRK2 locus as having increased risk for sporadic PD (International Parkinson Disease Genomics Consortium, Nalls M. A., Plagnol V., Hernandez D. G., Sharma M., Sheerin U.-M., Saad M., et al. 2011). LRRK2 encodes a large (286 kDa) protein with multiple protein–protein interaction domains, namely ankyrin repeats, leucine-rich repeats, C-terminal WD40 domain, and two enzymatic regions, the ROC-COR bidomain that acts as a GTPase and kinase. Although many LRRK2 mutations have already been described, only a few have been proven to cause PD: G2019S, R1441C/G/H, Y1699C, I2020T (Cookson 2010). Interestingly, pathogenic mutations tend to cluster within the three domains that form the enzymatic core of LRRK2, namely the ROC-COR and kinase domain.
LRRK2 has been implicated in multiple functions, including cytoskeletal organization, endosomal vesicle trafficking, translational control, and miRNA processing (Cookson 2010), but here we will discuss the connections to autophagy. An important set of observations linking LRRK2 with macroautophagy came from cellular localization investigations. A study of HEK293 cells expressing low levels of LRRK2 localized the protein to several structures associated with autophagy, including microvilli/filipodia, neck of cave-olae, multivesicular bodies, and autophagosomes (Alegre-Abarrategui et al. 2009). In human brains, LRRK2 is present in cytoplasmic punctae corresponding to multivesicular bodies and autophagic vacuoles (Biskup et al. 2006). LRRK2 can be co-localized with the late endosomal markers Rab7, and less frequently with the lysosomal marker LAMP2 (Higashi et al. 2009). LRRK2 is also found in autophagic vesicles when expressed in cells or mouse models (Biskup et al. 2006; Plowey et al. 2008; Alegre-Abarrategui et al. 2009; Ramonet et al. 2011).
Protein interactions of LRRK2 may also provide clues as to its function. It has been shown to interact with early endosomal marker Rab5b (Shin et al. 2008; Heo et al. 2010). More recent data suggest that LRRK2 might phosphorylate Rab5b and activate its GTPase activity, negatively regulating its signaling (Yun et al. 2015). A Drosophila melanogaster homolog, lrrk, physically interacts with the late endosomal GTPase rab7 and to negatively regulate rab7-dependent perinuclear localization of lysosomes (Dodson et al. 2012). Gómez-Suaga and colleagues reported that pathogenic LRRK2 mutations reduce Rab7 activity in mammalian cells, causing a delay in trafficking of Rab7 out of late endosomes (Gómez-Suaga et al. 2014).
Physical interaction with another homolog of Rab7, Rab7L1 has been reported by our lab and by others (MacLeod et al. 2013; Beilina et al. 2014). In our hands, we found that LRRK2 forms a complex with Rab7L1, Hsc70, chaperone regulator BCL2-associated athanogene 5 (Bag5), and cyclin G-associated kinase (GAK) to promote the removal of Golgi derived vesicles by autophagy-dependent mechanisms (Beilina et al. 2014), as shown in Fig. 2. Interestingly, GAK and Rab7L1 genes have been nominated as risk factors for sporadic PD by GWAS (Simón-Sánchez et al. 2009; International Parkinson Disease Genomics Consortium, Nalls M. A., Plagnol V., Hernandez D. G., Sharma M., Sheerin U.-M., Saad M., et al. 2011; Sharma et al. 2012). Previous functional studies demonstrated that GAK is recruited to the trans-golgi network (TGN) (Zhao et al. 2001; Kametaka et al. 2007) and promotes the uncoating of endocytosed clathrin-coated vesicles (Greener et al. 2000; Lee et al. 2006). Experimental evidence demonstrated that depletion of GAK in cells impaired the sorting of cathepsin D to lysosomes (Kametaka et al. 2007).
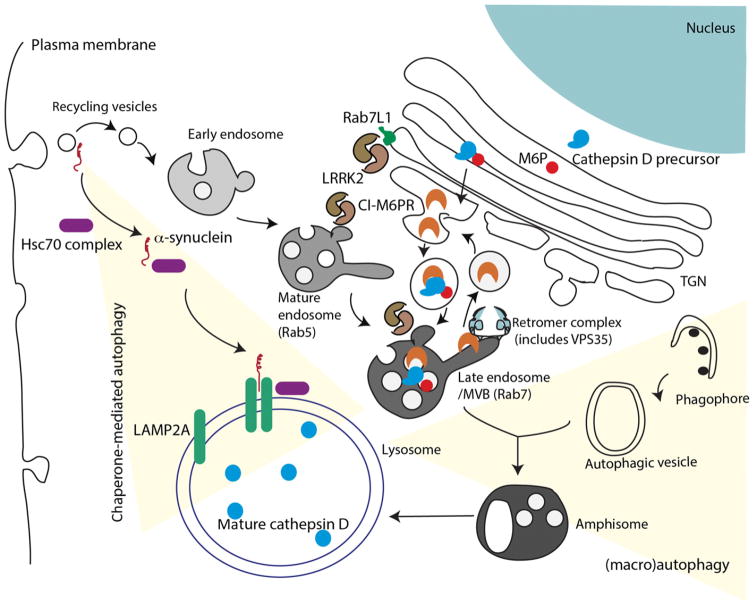
Fig. 2.
Chaperone-mediated autophagy (CMA) and macroautophagy. At least two PD genes are thought to play roles in CMA and macroautophagy, shown here integrated into some of the other major vesicular transport systems in cells. α-Synuclein (red) is associated with several lipid structures, but particularly in vesicles coming from the plasma membrane. When bound to a complex of chaperones (mainly Hsc70) and co-chaperones, α-synuclein can be transported to the lysosome and translocated through a pore formed by dimerized LAMP2A and degraded. Recycling vesicles can fuse with endosomes that then mature, a process that includes switches of small GTPases from Rab5 to Rab7. Late endosomes fuse with autophagic vesicles to generate amphisomes, which then also target their contents to the lysosome. The endosomal system is also important in the sorting of proteins to the lysosome; here in blue the protease cathepsin D is shown maturing through the ER and Golgi via binding to mannose-6-phosphate that is recognized by the cation-independent mannose-6-phosphate receptor (CI-M6PR). A portion of CI-M6PR is recycled back to the trans-Golgi network (TGN) via the retromer complex (light blue). Two genes for PD, LRRK2 (shown as a dimeric protein in brown) and VPS35 are involved in different aspects of these mechanisms. LRRK2 can be found in several different vesicular structures from the TGN where it interacts with the PD risk factor protein Rab7L1, through several autophagic vesicles. Current data support the hypothesis that LRRK2 has a general inhibitory effect on autophagy. VPS35 is a direct component of the retromer and is reported to change interactions within the recycling aspect of this pathway. Although the diagram here presents the CMA and macroautophagy pathways as somewhat distinct, in practice they are likely to influence each other. For example, there is some evidence that mutant forms of α-synuclein and LRRK2 both inhibit CMA.
Collectively, the observations of LRRK2 interaction with one or more small GTPases in the Rab family support that LRRK2 plays a critical role in sorting of a variety of vesicles in the cell. However, this does not indicate a direct role of LRRK2 in autophagy specifically. Strong evidence supporting the role of LRRK2 in autophagy comes from studies of LRRK2 knockout mice (Herzig et al. 2011; Tong et al. 2012). These mice appeared to have kidney and lung pathology with lipofuscin accumulation and biphasic alteration in the autophagy–lysosomal markers. Interestingly, striking accumulation and aggregation of α-synuclein was observed in kidney of LRRK2 −/− mice at 20 months of age (Tong et al. 2012).
Studies using iPSc-derived dopaminergic neurons bearing the G2019S mutation in LRRK2 showed a basal increase in autophagy LC3- and p62-positive puncta and increase in LC3II protein levels compared to control cells (Sánchez-Danés et al. 2012). In addition, inhibition of autophagy in these dopaminergic cells resulted in mutant-specific reduction in autophagic flux measured by LC3II immunoblotting, suggesting problems in clearance of autophagosomes (Sánchez-Danés et al. 2012). Increased levels of α-synuclein were observed in iPSc-derived G2019S mDA neurons and these increases are not associated with increased mRNA levels of α-synuclein, suggesting impaired degradation of α-synuclein (Sánchez-Danés et al. 2012; Reinhardt et al. 2013). As discussed above, because α-synuclein is degraded in part by CMA, a reasonable interpretation is that inhibition of general macroautophagy by LRRK2-G2019S would secondarily result in excessive stability of α-synuclein because of the disruption of the more specialized form of autophagy, CMA. It has also been reported that LRRK2 may inhibit CMA directly, perhaps by a similar mechanism to that reported for α-synuclein (Orenstein et al. 2013), suggesting that LRRK2 mutations may have effects on both bulk macroautophagy and CMA. Whether LRRK2 mutations also affect other specialized forms of autophagy, and whether the mechanisms by which they do are shared with a more general inhibition of macroautophagy, is not resolved at this time.
There is also some evidence that this observation with G2019S might generalize to other mutations in LRRK2. In response to starvation, fibroblasts from patients with different LRRK2 mutations across multiple functional domains showed lower levels of lipidation of LC3 compared to wild-type controls (Manzoni et al. 2013b). Conversely, inhibition of kinase activity of LRRK2 enhanced LC3 lipidation and the effects of kinase inhibitors required the presence of LRRK2 (Manzoni et al. 2013a). Consistent with the reports in LRRK2 knockout mice, these results suggest that LRRK2 normally acts to suppress autophagy, as measured by LC3-II formation. How these results relate to the observations of LRRK2 on various vesicular structures marked by Rab GTPases remains to be clarified. That LRRK2 can be found in several different compartments, including the TGN, endosomes, and autophagic vesicles, is consistent with earlier localization data (Alegre-Abarrategui et al. 2009).
Despite this substantial body of literature suggesting that LRRK2 plays a regulatory role in macroautophagy, there are some unresolved questions about the mechanisms and direction of effect. For example, expression of G2019S LRRK2 increased the number of LC3-positive punctae, presumably representing autophagic vacuoles, in SH-SY5Y cells (Plowey et al. 2008), whereas G2019S expressed at endogenous levels in human fibroblasts has been reported to decrease LC3-II levels under starvation conditions (Manzoni et al. 2013b). While some of the discrepancies could be because of technical differences between studies, such as the use of slightly different measures in different cell lines, it is also possible that some important biology could be discerned by investigating the mechanism(s) underlying how LRRK2 impacts macroautophagy. For example, the observation that LRRK2 knockout results in autophagy markers can be both increased or decreased in vivo depending on the age of the animals (Tong et al. 2012) shows that some autophagy markers are potentially regulated at stages downstream of LRRK2 itself. In other words, measurement of any given marker of macroautophagy could reflect both primary consequences of LRRK2 alterations and compensatory changes that follow.
Two independent exome sequencing studies of Swiss (Vilariño-Güell et al. 2011) and Australian families (Zimprich et al. 2011) with autosomal dominant Parkinson’s disease identified a D620N mutation in the vacuolar protein sorting 35 (VPS35) gene. Other variants – P316S and L774M – have been identified for this gene, however, their pathogenicity is less clear as only D620N shows clear evidence of segregation in families (Vilariño-Güell et al. 2011; Zimprich et al. 2011; Ando et al. 2012).
VPS35 is a part of the retromer complex involved in endosomal–lysosomal retrograde transport of vesicles to trans-Golgi network (Seaman et al. 1997). The retromer has two subcomplexes: the cargo selection trimer consisting of Vps26, Vps29, and Vps35 and the membrane association sorting nexin dimer (Hierro et al. 2007). One of the well-studied types of cargo for retromer is a cation-independent mannose-6-phospate receptor (CI-MPR) that binds to the newly made lysosomal enzymes in the TGN and delivers them to the lysosome (Seaman 2004). After delivering lysosomal enzymes to lysosomes, the retromer returns CI-MPR back to the TGN.
Cathepsin D is a lysosomal enzyme that is modified by the mannose-6-phospate at the TGN (Fig. 2). This modification of cathepsin D is recognized by CI-MPR, which enables the delivery of the protease to lysosomes. Cathepsin D matures during the delivery and becomes active in lysosomes. Interestingly, cathepsin D is one of the main lysosomal endopeptidases responsible for the degradation of α-synuclein (Sevlever et al. 2008; Cullen et al. 2009). Moreover, marked increase in aggregated levels of α-synuclein have been observed in cathepsin D knockout mice (Qiao et al. 2008). In studies with transgenic flies expressing human wild-type α-synuclein, RNAi-mediated silencing of VPS35 resulted in the aberrant maturation of cathepsin D and the absence of cathepsin D led to the accumulation of α-synuclein in late endosomal and lysosomal compartments, accompanied by locomotor abnormalities in flies and mild eye disorganization (Miura et al. 2014). These data suggest that retromer may play an important role in the control of the degradation of α-synuclein and that depletion of VPS35 function may result in an increased load of synuclein.
Retromer containing the D620N VPS35 mutation incorrectly traffics CI-M6PR, resulting in improper processing, and increased secretion, of unprocessed cathepsin D (Follett et al. 2014). The impairment of cathepsin D processing was also confirmed in fibroblasts of patients carrying the same mutation (Follett et al. 2014). It is worth noting, that another study on pathogenicity of D620N in the rodent primary neurons and patient-derived human fibroblasts did not find any changes in localization of the retromer complex between endosomal and lysosomal vesicles, or the vesicular sorting of the retromer cargo, CI-M6PR (Tsika et al. 2014).
The D620N mutation does not disrupt interaction of VPS35 with any of the other retromer subunits (Follett et al. 2014; Tsika et al. 2014; Zavodszky et al. 2014) but selectively impairs the interaction between retromer and the WASH complex (McGough et al. 2014; Zavodszky et al. 2014), which functions to promote F-actin nucleation on endosomes and proper sorting of multiple cargo proteins (Derivery et al. 2009; Gomez and Billadeau 2009). Consequentially, the disrupted WASH-retromer interaction results in perturbed trafficking of the autophagy-related protein 9A (ATG9A), which is necessary for proper autophagy induction. ATG9A is retained more at TGN-golgi areas, and was not able to traffic to autophagic structures, leading to impairment in autophagy measured by decrease in LC3II levels resulted in increased levels of α-synuclein (Zavodszky et al. 2014). There is additional experimental evidence showing that retromer plays specific roles in autophagy. The autophagosome protein Atg9 cycles between the Golgi and endosomes under normal conditions but upon induction of autophagy Atg9 moves toward endosomes and then autophagosomes (Young et al. 2006). These data suggest that there are multiple links between retromer function and autophagy and, as discussed above, from autophagy to LRRK2.
Supporting this, MacLeod and colleagues have linked LRRK2 to retromer function in cells by showing that VPS35 can physically interact with LRRK2 (MacLeod et al. 2013). In fly models, over-expression of wild-type VPS35 rescued DA neuronal loss and shorter life span caused by expression of G2019S LRRK2 (MacLeod et al. 2013). A functional interaction between LRRK2 and VPS35 has been independently confirmed in lines expressing pathogenic mutations I2020T, Y1699C, and I1122V (Linhart et al. 2014). Again, locomotor deficits, shortened life span and abnormal eye phenotype were rescued by expression of two retromer subunits VPS35 or VPS26 (Linhart et al. 2014).
The cumulative evidence discussed here suggests that three genes, LRRK2, VPS35, and α-synuclein, along with risk factor genes such as Rab7L1 and GAK, have functional and/or physical links to each other. In our opinion, a likely nexus for these events is at the TGN, which a key vesicular environment that mediates overlapping function between the retromer and autophagy control. It is likely that the function of this set of genes is to regulate autophagy, suggesting that the pathway that is perturbed in these forms of Parkinson’s disease is macroautophagy. A likely outcome, at least in neurons, is that there would be accumulation of α-synuclein as a downstream event, although there are other models that might also be reasonable.
Lysosomal genes: GBA and ATP13A2
There are many other genes and risk factor loci for PD, and it is not our intention here to try to link all of them to autophagy-related functions. However, there are two genes in particular that bear some further examination as they are linked to autophagy–lysosome function, which in turn is required for the execution of degradation in autophagy. However, the two examples discussed here, PD is only part of the clinical picture and we will emphasize that these are in some ways distinct from the autophagy genes discussed above.
Mutations in the gene encoding glucocerebrosidase (GBA) cause Gaucher’s disease, a condition that is characterized by enlargement of multiple organs and, in a subset of cases, neurological problems. Gaucher’s disease shows autosomal recessive inheritance and is related to the loss of normal function of GBA, which is to degrade glycosylceramide, an important lipid for cell membranes and as a source for ceramide for cell signaling. In patients, usually children, lack of GBA activity causes the accumulation of glycosylceramide in lysosomes and, as such, Gaucher’s is the most common lysosomal storage disease (Baris et al. 2014).
Several years ago, it was noted clinically that grandparents of Gaucher’s children had PD more often than would be expected by chance alone. Sequencing was used to identify heterozygous variants in the GBA gene in PD patients (Lwin et al. 2004). A multicenter analysis confirmed that these results generalize across cases, and that possession of a single copy of the defective GBA allele confers an ~5fold increase in risk of PD over lifetime (Sidransky et al. 2009). Similar estimates of risk have been seen in other studies (Lesage et al. 2011) and there is signal around the GBA gene in recent GWAS (Nalls et al. 2014).
These genetic results suggest that while full loss of GBA function is associated with a multisystem lysosomal storage disease, single mutant alleles are tolerated during development but increase the risk of a late-onset neurodegenerative disease. There has therefore been a great deal of interest in examining the links between GBA and other PD genes, particularly α-synuclein. An important study from Mazzulli et al. (2011) suggested not only that GBA dysfunction would impact α-synuclein accumulation via altered lysosomal processing, but also that the presence of a α-synuclein in an oligomeric form would limit the maturation of GCase, which similar to cathepsin D discussed above, requires the enzyme to transit from the ER to the TGN. Supporting this idea, iPSC lines from PD patients who have mutant GBA alleles have higher synuclein levels compared to controls (Schöndorf et al. 2014), as do cell lines edited to have diminished GCase activity (Bae et al. 2015).
There is an ongoing discussion as to whether the risk of PD is associated with lower GCase activity or whether gain of function mechanisms might contribute to disease risk. For example, increasing the expression of mutant forms of GBA was associated with increased amounts of α-synuclein, irrespective of the effects of the mutations on enzyme activity (Cullen et al. 2011). A similar dissociation between enzyme activity and levels of α-synuclein was noted in vivo by comparing mutant knockin and knockout GBA alleles in mice (Cullen et al. 2011). While these studies indicate that there are still important mechanistic aspects of the role of GBA in PD that need to be resolved, they also show that risk factor genes are difficult to place easily into loss of gain of function categories.
Along the same lines, there have been several attempts to link the function of the lysosomal enzyme ATP13A2 with α-synuclein. Mutations in ATP13A2 were first reported in a recessively inherited disease, Kufor–Rakeb syndrome (Ramirez et al. 2006). The phenotype of Kufor–Rakeb syndrome is complex, with multiple brain regions being involved leading to immobility, mutism, and dementia. However, mutations in ATP13A2 are also found in early onset parkinsonism cases without these additional features (Di et al. 2007), suggesting that the protein has one or more functions relevant to PD even if the overall clinical picture can be broader.
The ATP13A2 gene encodes a P-type ATPase normally initially localized to lysosomes, with some mutations preventing maturation through the ER-TGN and promoting degradation of the protein product (Ramirez et al. 2006). ATPases are known to transport metal cations, and so several studies have focused on the role of metal ions in ATP13A2-dependent phenotypes. In a range of organisms from yeast to mammals, it has been shown that loss of ATP13A2 sensitizes cells to excess divalent metal cations of various types (Gitler et al. 2009; Chesi et al. 2012; Podhajska et al. 2012; Ramonet et al. 2012; Kong et al. 2014; Park et al. 2014). Interestingly, some data suggest that ATP13A2 is expressed in components of the autophagy–lysosomal system other than the lysosomes themselves (Kong et al. 2014).
A picture therefore emerges that ATP13A2 mutations lead to an inability to regulate divalent metal cations in the lumen of various vesicular structures, including but not limited to lysosomes (Lopes da Fonseca and Outeiro 2014). Given that GBA is also involved in lysosomal function, it becomes reasonable to think that both GBA and ATP13A2 have common effects (Fig. 3). This would put both genes in a pathway that indirectly regulates α-synuclein clearance via the autophagy–lysosome system. Supporting this contention, patient fibroblasts carrying ATP13A2 mutations have been shown to have higher levels of α-synuclein (Tsunemi and Krainc 2014) and accumulation of α-synuclein may occur in one ATP13A2 knockout mouse model (Schultheis et al. 2013). It has further been suggested that part of the effects of ATP13A2 mutations on α-synuclein may be mediated via exocytosis (Kong et al. 2014).
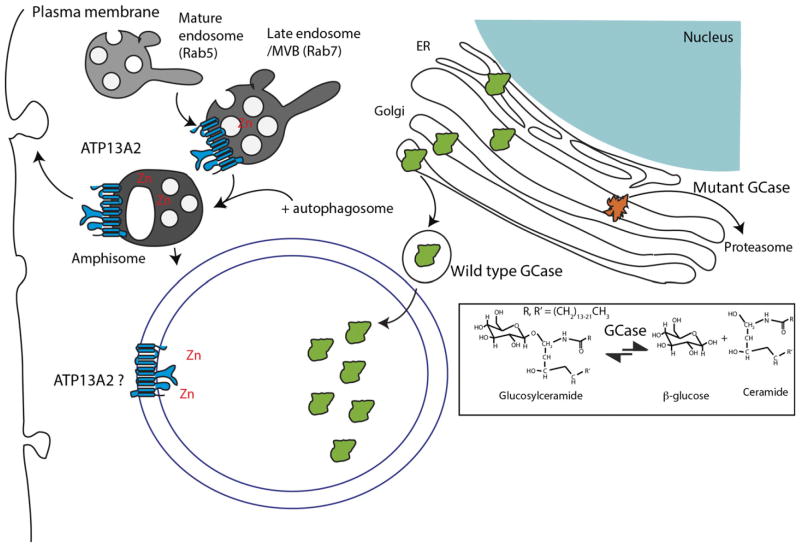
Fig. 3.
Lysosomal proteins. Similar to figure 2, two additional genes for PD are shown in the context of the regulation of some of the broader aspects of function in the autophagy–lysosomal system. Glucocere-brosidase (GCase) is, like cathepsin D in figure 2, trafficked from the ER through the Golgi to the lysosome where it catalyzes the degradation of glycosylceramide to glucose and ceramides (in the reaction outlined in the box). Mutant forms of GCase are often improperly folded and degraded by the proteasome, leading to accumulation of glucosylceramides. ATP13A2 is reported to be present on a number of regulatory vesicles in the autophagy–lysosome system, where it allows for the transport of Zn and potentially other divalent metal cations (not shown) into the lumen of those vesicles. There is some evidence that amphisomes containing ATP13A2 can fuse with the plasma membrane, thus influencing exocytosis.
Although elegant, this proposal is not fully supported by all the available data. For example, genome editing using zinc finger nucleases to remove ATP13A2 does not result in either lysosomal dysfunction or α-synuclein accumulation (Bae et al. 2014), despite the observation that manipulation of GBA does have the expected effects in the same cell line (Bae et al. 2015). In an ATP13A2 knockout mouse where lysosomal and protein trafficking deficiencies were noted, locomotor phenotypes were found to be independent of α-synuclein (Kett et al. 2015). In fact, in this in vivo model, which does show motor dysfunction and a variety of neuropathological phenotypes, no accumulation of α-synuclein was observed. A similar lack of dependency of ATP13A2 mutant induced neuropathology was noted in a rat model (Daniel et al. 2014). How these different observations will be resolved is not yet clear and will likely require confirmation or refutation of the key data.
Further complicating where to place ATP13A2, mitophagy can be impaired cells where ATP13A2 is knocked down with siRNA (Gusdon et al. 2012) or mutated at the endogenous level in patient fibroblasts (Grünewald et al. 2012). These studies might imply that ATP13A2 should be considered to be part of the PINK1/parkin/Fbxo7 pathway alluded to above. However, the available data suggest that the mitophagy phenotype in ATP13A2-deficient cells is secondary to an autophagy defect, presumably related to lysosomal dysfunction as, for example, the same effects can be achieved by siRNA against Atg7 (Gusdon et al. 2012).
Collectively, these observations suggest that while a substantial loss of lysosomal function is associated with lysosomal storage disorders, in some cases, parkinsonism can be part of the phenotype, but the phenotype tends to be broader than PD alone. The affected pathways at a cellular level are also broader for lysosomal enzyme deficiency than for recessive parkinsonism, but include defects in mitophagy as a consequence of the primary defects in the autophagy–lysosome system.
Sporadic PD
Predating the identification of genes, it was known that sporadic PD cases have pathologic features that might indicate dysregulation of autophagy. For example, Anglade and colleagues performed ultrastructural analysis of dopaminergic neurons in the substantia nigra of patients with PD, and found autophagic degeneration, accumulation of high density lysosome-like vacuoles, and presence of lipofuscin granules (Anglade et al. 1997). Similar results were seen by other groups (Chu et al. 2009; Alvarez-Erviti et al. 2010).
Postmortem studies in the substantia nigra pars compacta and amygdala of PD brains revealed significant reductions in expression levels of autophagy proteins LAMP2a and Hsc70 compared to age-matched controls or Alzheimer’s disease samples (Chu et al. 2009; Alvarez-Erviti et al. 2010, 2013; Wu et al. 2011b; Murphy et al. 2015). Neuropathological examination of human brains derived from PD patients found alterations in the main CMA components, Hsc70 and LAMP2A using immunohistochemistry (Chu et al. 2009). In addition, this study found changes in other auto-lysosomal components, suggesting more generalized autophagy impairment, not specific to CMA (Chu et al. 2009). Similarly, using immunoblotting, Alvarez-Erviti et al. (2010) showed significant decreases in both Hsc70 and LAMP2A in the nigra and amygdala of PD brains compared to controls. An additional study correlated the loss of LAMP2 and Hsc70 proteins with the increased levels of α-synuclein seen in PD brains (Murphy et al. 2015). Mechanistically, several miRNAs that down-regulate CMA are significantly increased in substantia nigra compacta and amygdala PD brains compared to both age-matched controls and brain samples with AD (Alvarez-Erviti et al. 2013).
There is some evidence that these diminished autophagy functions may be a systemic effect rather than brain specific. For example, in one study, reduced LAMP2A and Hsc70 expression, along with impairment in fusion of autophago-some and lysosome has been noted in sporadic PD patients (Wu et al. 2011b). Others have confirmed the diminished Hsc70 expression but did not note changes in LAMP2A (Sala et al. 2014). Examination of cerebrospinal fluid (CSF) from PD patients showed significant decreased activity of multiple lysosomal hydrolases compared to age-matched controls (Balducci et al. 2007). Another study in the peripheral leukocytes of sporadic PD patients found significant reduction in activity of alpha-galactosidase A (GLA), one of the enzyme active in lysosomes (Wu et al. 2011a).
As discussed above, diminished function in the autophagy–lysosome system may contribute to the accumulation of α-synuclein. Interestingly, Gegg et al. (2012) found reduced glucocerebrosidase protein levels and enzyme activity in a range of affected brain regions in patients with sporadic PD. Supporting this observation GBA protein levels and enzyme activity were selectively reduced in brain regions that accumulate abnormal α-synuclein (Murphy et al. 2014).
Collectively, these observations suggest that, as well as being involved in familial PD, there may be some contribution of autophagy–lysosomal pathways to the more common sporadic form of the same disease. Whether the observed responses in sporadic disease indicate that autophagy is causal or a consequence of the disease process is less clear. However, it is reasonable to ask to what extent autophagy-related pathways contribute to the pathogenic mechanism relevant to the different forms of PD.
Linking the pathways: could we and should we?
An ongoing debate in the PD field revolves around the extent to which we can integrate what we have found about genetic forms of the disease. There are really parts to this question: are all genetic forms of parkinsonism related to each other and what do they tell us, if anything about sporadic disease?
There are good reasons to distinguish between the different genes for parkinsonism. It has been argued persuasively, for example, that α-synuclein positive Lewy bodies are important for assignment of a disease to PD (Hardy and Lees 2005) but this position is complicated by the fact that not all LRRK2 cases have Lewy bodies despite being clinically homogenous (Cookson et al. 2008). Clinical information is also useful, and would suggest distinction between recessive and dominant genes, but as discussed for ATP13A2 and Fbxo7, there can be a wide range of symptoms for mutations in a single gene (Ramirez et al. 2006; Gündüz et al. 2014). In addition, because the number of cases, especially those with autopsy information, is small for the rarer inherited conditions, then defining pathways by patient information is a fraught process.
However, there is good evidence to think that at least some of the genetic forms of disease have relevance to sporadic PD. Strongest among these ideas is the observation that some of the known genes for inherited PD are in loci that are nominated by GWAS for risk of sporadic PD. This leads to the concept of pleomorphic risk loci, regions of the genome that contain multiple variants, some of which cause disease by changing amino acids whereas others affect disease risk because of differences in gene expression levels (Singleton and Hardy 2011). PD has multiple such loci, including α-synuclein and LRRK2 but to date none of the recessive genes has been shown to act as strong risk factors for sporadic PD. Therefore, for a subset of PD genes, but not all of them, it is reasonable to assume that there is a relationship with sporadic disease.
The functional data discussed above relating PD genes and proteins to forms of autophagy may be helpful in resolving these issues. It is clear that PINK1 and parkin are in a tightly related pathway linked to mitophagy and it is likely that this can be extended to Fbxo7 (Burchell et al. 2013). Therefore, for this form of parkinsonism there is reasonable evidence to support the idea that one form of autophagy is important in the disease process. Importantly, however, because mitophagy is a specialized form of autophagy it is not reasonable to infer that the disease in this case is a generalized defect in autophagy–lysosomal system. In addition, because some of the protein products of genes in the PINK1/parkin/Fbxo7 pathway have functions outside of mitophagy, it is possible that diminished mitophagy is not sufficient for parkinsonism.
It is also reasonably well established that LRRK2 is that it has physical and/or genetic partners that link it in to other forms of PD. Interactions with Rab7L1 and GAK (MacLeod et al. 2013; Beilina et al. 2014) suggest relevance to sporadic disease, as these are candidates from GWAS, and physical interaction with VPS35 (MacLeod et al. 2013) would provide a link to inherited PD. Furthermore, it has been shown that lack of LRRK2 limits the toxic effects of inflammation or α-synuclein over-expression in cells and animals (Lin et al. 2009; Daher et al. 2014; Skibinski et al. 2014). Therefore, LRRK2 appears to be a highly connected hub in the network of PD genes. Given the multiple links, it has been suggested previously that LRRK2 and α-synuclein each contribute to the toxicity seen in dopamine neurons in the disease (Taymans and Cookson 2010).
The crucially difficult question that next arises is whether the mitophagy defect in PINK1/parkin/Fbxo7 disease is functionally related to the regulation of autophagy that occurs as a result of mutations in LRRK2 and its interaction partners. To some extent this depends upon what the meaning of the word ‘is’ is. If it is the case that LRRK2 exerts an upstream effect on general autophagy, as might be inferred from the effects of mutations on autophagosome formation (Manzoni et al. 2013a,b), then it would be reasonable to infer that one could see defects in mitophagy as a secondary consequence. There is some evidence of such defects in cells expressing mutant LRRK2 (Su et al. 2015), although such results need confirmation and mechanistic development to be certain of their applicability to different models. At this time, we think that the distinction between PINK1/parkin and LRRK2 is sufficient to consider them separately but with the reasonable possibility that those pathways may intersect at the level of autophagic regulation of mitochondria. This will certainly be a hot topic area for the PD field in the next few years.
Along the same lines, the relationship between each of these genes and GBA and ATP13A2 also requires clarification. A reasonable interpretation of the data available to date is that loss of function of either of these genes results in a lysosomal storage disease that, as part of the spectrum, can include symptoms of PD. Again, the real question here is to what extent do we consider the effects of LRRK2 and interaction partners on autophagy regulation to be ‘the same’ pathway as lysosomal function. On the one hand, it is certainly reasonable to infer that a block in lysosomal function would result in limited ability to turn over clients by all forms of autophagy. On the other hand, the examples of parkin and LRRK2 suggest that in those causes of disease, the proteins are regulatory for forms of autophagy rather than essential to lysosomal function, which is an important distinction at least at the mechanistic level.
There are data that would be consistent with the concept that even though there are groupings of genes as outlined above, there are effects that cross any boundaries placed between them. For example, PINK1 and parkin are reported to influence diverse autophagic pathways including starvation responses (Parganlija et al. 2014), recognition of intracellular pathogens (Manzanillo et al. 2013) and perhaps most relevant to PD, α-synuclein turnover (Lonskaya et al. 2013). Conversely, expression of α-synuclein can influence mitochondrial morphology in the mouse brain (Chinta et al. 2010; Chen et al. 2015), as can expression of LRRK2 in cell culture models (Cherra et al. 2013; Su et al. 2015).
Our view at this time is that there are three broad groupings of PD genes; the mitophagy regulators, the effectors of autophagy–endosomal recycling and the lysosomal proteins. There are likely to be important higher level relationships between these groups and those are worth pursuing further experimentally in the future. Nonetheless, the discreteness of subsets of genes supports the idea that there are distinctions in disease mechanisms at the level of the individual causative mutations.
Future directions
There are some key areas that must be explored in the near future. Most narrowly, additional mechanistic data are required. To give one example, exactly how LRRK2 influences autophagy is not well understood at this time. By analogy to parkin, it is possible that LRRK2 is not active basally but requires activation steps, which remain to be fully elucidated. Understanding mechanisms related to control of these genes are likely to provide important ways in to understanding pathways in more depth.
Mechanistic data are also important to clarify some of the genetic data that are currently available. Although most GWAS studies list genes that are near to the peak of statistical association, this gives a false sense of precision as any gene that is within a region of linkage disequilibrium remains a valid candidate until it can be disproved. Thus, while at some loci such as that around SNCA we can be reasonably certain of the best candidate, in other regions there are not such obvious ‘smoking guns’. We have claimed recently that interactions between candidates, especially those that come from unbiased approaches, might be one way to rationally promote one gene over all others (Beilina et al. 2014), although whether that generalizes to all loci remains to be proven.
Importantly, the role of α-synuclein in different forms of PD needs to be clarified. The issue of Lewy bodies remains difficult to understand, but the available data still support α-synuclein as being required for neuronal damage (Lin et al. 2009; Daher et al. 2014; Skibinski et al. 2014). Perhaps, a better way to think about the role of α-synuclein is to consider Lewy bodies and contribution to cell death as separate hypotheses. If so, then testing whether α-synuclein is required for neurodegeneration in the context of other genes, such as ATP13A2 or VPS35 would be instructive.
A much more difficult question alluded to above is why changes in autophagy or its regulation would result in a neurodegenerative condition, much less one that affects the motor system prominently. Some of the genes nominated for risk for PD, specifically α-synuclein and tau, encode proteins with relatively restricted neuronal expression, which may contribute to expression of a brain disease. However, some proteins are also expressed in other cell types – LRRK2 is expressed in macrophages and microglia, for example (Moehle et al. 2012), leaving open the possibility that some of the neurodegenerative process in PD is non-cell autonomous. We therefore need consider that proteins expressed outside of neurons may also contribute to disease.
It should also be noted that while it is possible, with nuanced views, multiple genes to a series of related pathways, it is not yet clear if all PD genes can be placed in one of these categories. A full discussion of this complex problem is probably outside the scope of this review, but as an example we might consider the MAPT gene encoding tau that is a nominated gene for PD risk (Simón-Sánchez et al. 2009). Tau is a microtubule binding protein and could therefore be linked to autophagy either because vesicular transport depends on cytoskeletal transport (Yan 2014) and/or because some forms of tau are substrates for degradation by the autophagy–lysosomal system (Chesser et al. 2013). However, additional mechanism-based studies are required to distinguish whether risk factor variants in MAPT or other risk genes require alterations in autophagy for their actions relevant to PD pathogenesis.
At the end, of all these considerations comes the acknowledgement that despite the advances in understanding causes of disease there has been relatively little published on curing PD. While wanting to contribute to lessening symptoms or progression for PD patients is aspirational, an additional appeal of mechanism-based therapeutics is that they may further resolve some of the questions raised above. As a thought experiment, would a parkin-based drug work on LRRK2 disease? If so, then this would suggest that the different pathways alluded to above are in fact closely related.
Conclusions
Many significant discoveries about the causation of PD have occurred in the past two decades by studying the different genetic contributions to disease risk. More recently, it has become clear that at least some of the nominated genes are functionally related. Our working hypothesis is that there are strong relationships between subsets of these proteins but that there are distinct themes that relate to the regulation of autophagy. Further testing this hypothesis is critical for moving these observations toward clinical application.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH (Z01-AG000948), National Institute on Aging.
Abbreviations used
- ATG
autophagy gene
- BAG
bcl2-associated athanogene
- CI-MPR
cation independent mannose 6 phosphate receptor
- CMA
chaperone-mediated autophagy
- COR
C-terminal of ROC
- GAK
cyclin-G-associated kinase
- GBA
Glucocerebrosidase
- GWAS
genome-wide association study
- Hsc70
heat-shock cognate protein, 70 kDa
- IPSC
induced pluripotent stem cells
- LC3
microtubule-associated protein light chain 2
- LRRK2
leucine-rich repeat kinase 2
- PD
Parkinson’s disease
- PINK1
PTEN-induced novel kinase 1
- ROC
ras of complex proteins
- TGN
trans-Golgi network
- VPS
vesicular protein sorting
Footnotes
Conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Alegre-Abarrategui J, Christian H, Lufino MMP, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GFG, Toth R, James J, Ganley IG. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013;14:1127–1135. doi: 10.1038/embor.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AHV. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM. Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson’s disease. Cell Death Dis. 2013;4:e545. doi: 10.1038/cddis.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M, Funayama M, Li Y, et al. VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov Disord. 2012;27:1413–1417. doi: 10.1002/mds.25145. [DOI] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Lee C, Lee HJ, Kim S, Lee SJ. ATP13A2/PARK9 Deficiency Neither Cause Lysosomal Impairment Nor Alter α-Synuclein Metabolism in SH-SY5Y Cells. Exp Neurobiol. 2014;23:365–371. doi: 10.5607/en.2014.23.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Yang NY, Lee C, Lee HJ, Kim S, Sardi SP, Lee SJ. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp Mol Med. 2015;47:e153. doi: 10.1038/emm.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Pierguidi L, Persichetti E, Parnetti L, Sbaragli M, Tassi C, Orlacchio A, Calabresi P, Beccari T, Rossi A. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov Disord. 2007;22:1481–1484. doi: 10.1002/mds.21399. [DOI] [PubMed] [Google Scholar]
- Baris HN, Cohen IJ, Mistry PK. Gaucher disease: the metabolic defect, pathophysiology, phenotypes and natural history. Pediatr Endocrinol Rev. 2014;12(Suppl 1):72–81. [PMC free article] [PubMed] [Google Scholar]
- Beilina A, Rudenko IN, Kaganovich A, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci USA. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsa N, Norkett R, Wauer T, et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem. 2014;289:14569–14582. doi: 10.1074/jbc.M114.563031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Suppl 1):S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- Bonifati V. Genetics of Parkinson’s disease–state of the art, 2013. Parkinsonism Relat Disord. 2014;20(Suppl 1):S23–S28. doi: 10.1016/S1353-8020(13)70009-9. [DOI] [PubMed] [Google Scholar]
- Bras JM, Guerreiro RJ, Ribeiro MH, Januario C, Morgadinho A, Oliveira CR, Cunha L, Hardy J, Singleton A. G2019S dardarin substitution is a common cause of Parkinson’s disease in a Portuguese cohort. Mov Disord. 2005;20:1653–1655. doi: 10.1002/mds.20682. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Nelson DE, Sanchez-Martinez A, et al. The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci. 2013;16:1257–1265. doi: 10.1038/nn.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xie Z, Turkson S, Zhuang X. A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J Neurosci. 2015;35:890–905. doi: 10.1523/JNEUROSCI.0089-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra SJ, Steer E, Gusdon AM, Kiselyov K, Chu CT. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol. 2013;182:474–484. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesi A, Kilaru A, Fang X, Cooper AA, Gitler AD. The role of the Parkinson’s disease gene PARK9 in essential cellular pathways and the manganese homeostasis network in yeast. PLoS ONE. 2012;7:e34178. doi: 10.1371/journal.pone.0034178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser AS, Pritchard SM, Johnson GVW. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O’Farrell C, Schlossmacher M, Farrer MJ. RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet. 2003;12:2957–2965. doi: 10.1093/hmg/ddg328. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson’s disease. Int J Clin Exp Pathol. 2008;1:217–231. [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Cullen V, Lindfors M, Ng J, et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. 2009;2:5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen V, Sardi SP, Ng J, et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Banerjee T, Das Janda E. How Parkinsonian toxins dysregulate the autophagy machinery. Int J Mol Sci. 2013;14:22163–22189. doi: 10.3390/ijms141122163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JPL, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci USA. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G, Musso A, Tsika E, Fiser A, Glauser L, Pletnikova O, Schneider BL, Moore DJ. α-Synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson’s disease occurs independent of ATP13A2 (PARK9) Neurobiol Dis. 2014;73C:229–243. doi: 10.1016/j.nbd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Deas E, Plun-Favreau H, Gandhi S, et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet. 2011;20:867–879. doi: 10.1093/hmg/ddq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Le W, Guo Y, Hunter CB, Xie W, Huang M, Jankovic J. Genetic analysis of LRRK2 mutations in patients with Parkinson disease. J Neurol Sci. 2006;251:102–106. doi: 10.1016/j.jns.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East DA, Fagiani F, Crosby J, Georgakopoulos ND, Bertrand H, Schaap M, Fowkes A, Wells G, Campanella M. PMI: a ΔΨm independent pharmacological regulator of mitophagy. Chem Biol. 2014;21:1585–1596. doi: 10.1016/j.chembiol.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25:354–363. doi: 10.1016/j.tcb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett J, Norwood SJ, Hamilton NA, et al. The Vps35 D620N mutation linked to Parkinson’s Disease disrupts the cargo sorting function of retromer. Traffic. 2014;15:230–244. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW, Schapira AHV. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke S, Wu Z, Klinkenberg M, Sun Y, Auburger G, Guo S, Lu B. PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab. 2015;21:95–108. doi: 10.1016/j.cmet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P, Rivero-Ríos P, Fdez E, Blanca Ramírez M, Ferrer I, Aiastui A, López De Munain A, Hilfiker S. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet. 2014;23:6779–6796. doi: 10.1093/hmg/ddu395. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem. 2000;275:1365–1370. doi: 10.1074/jbc.275.2.1365. [DOI] [PubMed] [Google Scholar]
- Grünewald A, Arns B, Seibler P, Rakovic A, Münchau A, Ramirez A, Sue CM, Klein C. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging. 2012;33(1843):e1–e7. doi: 10.1016/j.neurobiolaging.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Gündüz A, Eken AG, Bilgiç B, Hanagasi HA, Bilgüvar K, Günel M, Başak AN, Ertan S. FBXO7-R498X mutation: phenotypic variability from chorea to early onset parkinsonism within a family. Parkinsonism Relat Disord. 2014;20:1253–1256. doi: 10.1016/j.parkreldis.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Gusdon AM, Zhu J, Houten B, Van Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Lees AJ. Parkinson’s disease: a broken nosology. Mov Disord. 2005;20(Suppl 12):S2–S4. doi: 10.1002/mds.20532. [DOI] [PubMed] [Google Scholar]
- Heo HY, Kim KS, Seol W. Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5. Exp Neurobiol. 2010;19:97–105. doi: 10.5607/en.2010.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig MC, Kolly C, Persohn E, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, Moore DJ, Yamamoto R, Minegishi M, Sato K, Togo T, Katsuse O, et al. Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J Neuropathol Exp Neurol. 2009;68:994–1005. doi: 10.1097/NEN.0b013e3181b44ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñnez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Kakita A, Shiga A, et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol. 2008;65:514–519. doi: 10.1001/archneur.65.4.514. [DOI] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium. Nalls MA, Plagnol V, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW, Shen J, Slack RS, Park DS. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum Mol Genet. 2012;21:4888–4903. doi: 10.1093/hmg/dds325. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Hoshijima M, Seo K, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametaka S, Moriyama K, Burgos PV, Eisenberg E, Greene LE, Mattera R, Bonifacino JS. Canonical interaction of cyclin G associated kinase with adaptor protein 1 regulates lysosomal enzyme sorting. Mol Biol Cell. 2007;18:2991–3001. doi: 10.1091/mbc.E06-12-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett LR, Stiller B, Bernath MM, et al. α-Synuclein-independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2. J Neurosci. 2015;35:5724–5742. doi: 10.1523/JNEUROSCI.0632-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kong SMY, Chan BKK, Park JS, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- Koyano F, Okatsu K, Kosako H, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Lee DW, Wu X, Eisenberg E, Greene LE. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci. 2006;119:3502–3512. doi: 10.1242/jcs.03092. [DOI] [PubMed] [Google Scholar]
- Lesage S, Anheim M, Condroyer C, et al. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet. 2011;20:202–210. doi: 10.1093/hmg/ddq454. [DOI] [PubMed] [Google Scholar]
- Li XX, Tsoi B, Li YF, Kurihara H, He RR. Cardiolipin and its different properties in mitophagy and apoptosis. J Histochem Cytochem. 2015;63:301–311. doi: 10.1369/0022155415574818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart R, Wong SA, Cao J, et al. Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-rich repeat kinase 2 (LRRK2) Mol Neurodegener. 2014;9:23. doi: 10.1186/1750-1326-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Acín-Peréz R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci USA. 2011;108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, et al. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Sakakibara K, Chen Q, Okamoto K. Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res. 2014;24:787–795. doi: 10.1038/cr.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonskaya I, Desforges NM, Hebron ML, Moussa CE-H. Ubiquitination increases parkin activity to promote autophagic α-synuclein clearance. PLoS ONE. 2013;8:e83914. doi: 10.1371/journal.pone.0083914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lopes da Fonseca T, Outeiro TF. ATP13A2 and Alpha-synuclein:a Metal Taste in Autophagy. Exp Neurobiol. 2014;23:314–323. doi: 10.5607/en.2014.23.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- MacLeod DA, Rhinn H, Kuwahara T, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal Degradation of a-Synuclein in Vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni C, Mamais A, Dihanich S, Abeti R, Soutar MPM, Plun-Favreau H, Giunti P, Tooze SA, Bandopadhyay R, Lewis PA. Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim Biophys Acta. 2013a;1833:2900–2910. doi: 10.1016/j.bbamcr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni C, Mamais A, Dihanich S, et al. Pathogenic Parkinson’s disease mutations across the functional domains of LRRK2 alter the autophagic/lysosomal response to starvation. Biochem Biophys Res Commun. 2013b;441:862–866. doi: 10.1016/j.bbrc.2013.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, et al. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Kaganovich A, Rudenko IN, Ding J, Cookson MR. Hexokinase activity is required for recruitment of parkin to depolarized mitochondria. Hum Mol Genet. 2014;23:145–156. doi: 10.1093/hmg/ddt407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough IJ, Steinberg F, Jia D, et al. Retromer Binding to FAM21 and the WASH Complex Is Perturbed by the Parkinson Disease-Linked VPS35(D620N) Mutation. Curr Biol. 2014;24:1670–1676. doi: 10.1016/j.cub.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem. 2011;117:856–867. doi: 10.1111/j.1471-4159.2011.07253.x. [DOI] [PubMed] [Google Scholar]
- Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–1838. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- Miura E, Hasegawa T, Konno M, et al. VPS35 dysfunction impairs lysosomal degradation of α-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol Dis. 2014;71:1–13. doi: 10.1016/j.nbd.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, et al. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Haddad D, Craessaerts K, et al. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T, Halliday GM. Lysosomal-associated membrane protein 2 Isoforms Are Differentially Affected in Early Parkinson’s Disease. Mov Disord. 2015 doi: 10.1002/mds.26141. In press. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatsu K, Koyano F, Kimura M. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J Cell Biol. 2015;209:111–128. doi: 10.1083/jcb.201410050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau A, Sarraf SA, Duda DM, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56:360–375. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein SJ, Kuo SH, Tasset I, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Rasmo D, De Signorile A, et al. Mitochondrial defect and PGC-1α dysfunction in parkin-associated familial Parkinson’s disease. Biochim Biophys Acta. 2011;1812:1041–1053. doi: 10.1016/j.bbadis.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Paisán-Ruíz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Parganlija D, Klinkenberg M, Domínguez-Bautista J, et al. Loss of PINK1 impairs stress-induced autophagy and cell survival. PLoS ONE. 2014;9:e95288. doi: 10.1371/journal.pone.0095288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet. 2014;23:2802–2815. doi: 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowey ED, Cherra SJ, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhajska A, Musso A, Trancikova A, Stafa K, Moser R, Sonnay S, Glauser L, Moore DJ. Common pathogenic effects of missense mutations in the P-type ATPase ATP13A2 (PARK9) associated with early-onset parkinsonism. PLoS ONE. 2012;7:e39942. doi: 10.1371/journal.pone.0039942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson JH, Ivatt RM, Sanchez-Martinez A, Tufi R, Wilson E, Mortiboys H, Whitworth AJ. The complex I subunit NDUFA10 selectively rescues Drosophila pink1 mutants through a mechanism independent of mitophagy. PLoS Genet. 2014;10:e1004815. doi: 10.1371/journal.pgen.1004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson’s disease. Mov Disord. 2012;27:831–842. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Hamamichi S, Caldwell KA, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ramonet D, Daher JPL, Lin BM, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS ONE. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet. 2012;21:1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P, Schmid B, Burbulla LF, et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12:354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun. 2013;4:1410. doi: 10.1038/ncomms2400. [DOI] [PubMed] [Google Scholar]
- Sala G, Stefanoni G, Arosio A, Riva C, Melchionda L, Saracchi E, Fermi S, Brighina L, Ferrarese C. Reduced expression of the chaperone-mediated autophagy carrier hsc70 protein in lymphomonocytes of patients with Parkinson’s disease. Brain Res. 2014;1546:46–52. doi: 10.1016/j.brainres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37:315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöndorf DC, Aureli M, McAllister FE, et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Fleming SM, Clippinger AK, et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet. 2013;22:2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S, Kanamaru Y, Koike M, et al. Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J Biol Chem. 2012;287:34635–34645. doi: 10.1074/jbc.M112.357509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever D, Jiang P, Yen SHC. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Ioannidis JPA, Aasly JO, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology. 2012;79:659–667. doi: 10.1212/WNL.0b013e318264e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N, Jeong H, Kwon J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Sánchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A, Hardy J. A generalizable hypothesis for the genetic architecture of disease: pleomorphic risk loci. Hum Mol Genet. 2011;20:R158–R162. doi: 10.1093/hmg/ddr358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Nakamura K, Cookson MR, Finkbeiner S. Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies. J Neurosci. 2014;34:418–433. doi: 10.1523/JNEUROSCI.2712-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci USA. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strappazzon F, Nazio F, Corrado M, et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015;22:419–432. doi: 10.1038/cdd.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YC, Guo X, Qi X. Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim Biophys Acta. 2015;1852:12–21. doi: 10.1016/j.bbadis.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Cookson MR. Mechanisms in dominant parkinsonism: the toxic triangle of LRRK2, alpha-synuclein, and tau. BioEssays. 2010;32:227–235. doi: 10.1002/bies.200900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Giaime E, Yamaguchi H, Ichimura T, Liu Y, Si H, Cai H, Bonventre JV, Shen J. Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener. 2012;7:2. doi: 10.1186/1750-1326-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsika E, Glauser L, Moser R, et al. Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum Mol Genet. 2014;23:4621–4638. doi: 10.1093/hmg/ddu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Krainc D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet. 2014;23:2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet. 2011;20:927–940. doi: 10.1093/hmg/ddq531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, Holbein CD, Berman SB. Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and in the presence of N-acetyl cysteine, mitophagy. Neurobiol Dis. 2015;74:180–193. doi: 10.1016/j.nbd.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C, Wider C, Ross OA, et al. VPS35 Mutations in Parkinson Disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci USA. 2013;110:6400–6405. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang J, Feng X, et al. Decreased expression of lysosomal alpha-galactosiase A gene in sporadic Parkinson’s disease. Neurochem Res. 2011a;36:1939–1944. doi: 10.1007/s11064-011-0516-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Wang X, Feng X, et al. Altered expression of autophagic genes in the peripheral leukocytes of patients with sporadic Parkinson’s disease. Brain Res. 2011b;1394:105–111. doi: 10.1016/j.brainres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant α-Synuclein Confers Toxicity to Neurons in Part through Inhibition of Chaperone-Mediated Autophagy. PLoS ONE. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. Interplay between HDAC6 and its interacting partners: essential roles in the aggresome-autophagy pathway and neurodegenerative diseases. DNA Cell Biol. 2014;33:567–580. doi: 10.1089/dna.2013.2300. [DOI] [PubMed] [Google Scholar]
- Ye X, Sun X, Starovoytov V, Cai Q. Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet. 2015;24:2938–2951. doi: 10.1093/hmg/ddv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ARJ, Chan EYW, Hu XW, Köchl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- Yun HJ, Kim H, Ga I, Oh H, Ho DH, Kim J, Seo H, Son I, Seol W. An early endosome regulator, Rab5b, is an LRRK2 kinase substrate. J Biochem. 2015;157:485–495. doi: 10.1093/jb/mvv005. [DOI] [PubMed] [Google Scholar]
- Zavodszky E, Seaman MNJ, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014;5:3828. doi: 10.1038/ncomms4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Baehrecke EH. Eaten alive: novel insights into autophagy from multicellular model systems. Trends Cell Biol. 2015;7:376–387. doi: 10.1016/j.tcb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Greener T, Al-Hasani H, Cushman SW, Eisenberg E, Greene LE. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J Cell Sci. 2001;114:353–365. doi: 10.1242/jcs.114.2.353. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagés A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]