Abstract
In acute myelogenous leukemia, the persistent detection of abnormal cytogenetics at complete remission (ACCR) is associated with inferior outcomes. However, the prognostic significance of ACCR in adult patients with acute lymphoblastic leukemia (ALL) is unknown. We evaluated 272 adult patients with ALL and abnormal cytogenetics at baseline who were treated with frontline induction chemotherapy, achieved complete remission (CR) and had cytogenetic analysis performed at the time of CR. ACCR was observed in 26 patients (9.6%). Median relapse-free survival was 22 months (95% CI, 12 months to not reached) for patients with ACCR vs. 48 months (range, 30–125 months) in patients with normal cytogenetics at CR (NCCR; P =0.31). Median overall survival also did not differ significantly between the ACCR (99 months [range, 17 months to not reached]) and NCCR groups (67 months [range, 47 months to not reached], P =0.86). The specificity of ACCR for minimal residual disease (MRD) positivity by multi-parameter flow cytometry (MFC) was 43%, and there was overall poor correlation between these two methods for the detection of residual disease. When patients were stratified by MRD status, the presence or absence of persistent cytogenetic abnormalities at CR did not add additional prognostic information. This study suggests that there is poor association between MRD assessment by MFC and the presence or absence of cytogenetic abnormalities at CR in adult patients with ALL. ACCR was not associated with adverse outcomes in ALL and did not add additional prognostic information when MRD status by MFC was known.
Introduction
While the vast majority of adult patients with acute lymphoblastic leukemia (ALL) achieve complete remission (CR) with induction chemotherapy, most patients relapse [1,2]. Despite recent advances in the treatment of ALL, the prognosis of patients with relapsed disease remains dismal [3]. Reliable identification of patients with high probability of relapse is imperative to improving the outcomes in ALL, as these patients may benefit from more intensive risk-adapted treatments. Minimal residual disease (MRD) status has emerged as a powerful prognostic tool in the risk-stratification of patients with ALL [4]. Post-therapy MRD by polymerase chain reaction (PCR) or multi-parameter flow cytometry (MFC) is strongly associated with higher rates of relapse and shorter overall survival (OS) in both Philadelphia chromosome-negative (Ph−) and Ph-positive (Ph+) ALL [5–8]. Identification of patients with MRD positivity after induction or consolidation may inform the decision to pursue more intensive postremission strategies, including stem cell transplantation (SCT) [6,9].
Cytogenetic abnormalities are identified at the time of diagnosis in approximately 80% of patients with ALL [10]. In theory, the persistence cytogenetic abnormalities at the time of remission could be used as a marker of MRD. Although cytogenetic analysis is expected to be less sensitive for the determination of MRD than more sophisticated methods such as MFC and PCR, it is unknown whether the persistence of these abnormalities at CR provides prognostic information in patients with ALL, especially if MRD status by other methods is known. In patients with acute myelogenous leukemia (AML), the persistence of abnormal cytogenetics at complete remission (ACCR) is strongly associated with shorter relapse-free survival (RFS) and OS [11,12]; these patients with ACCR may also benefit from SCT as consolidative therapy [11]. Given the prognostic significance of ACCR in patients with AML, we sought to investigate the prognostic value of ACCR in ALL and also to determine whether cytogenetic analysis at the time of CR in this population adds additional prognostic information when MRD status by MFC is also known.
Methods
Patients
Between April 2001 and March 2015, 571 adult patients with previously untreated ALL (including pre-B ALL, pre-T ALL, Burkitt, or Burkitt-like leukemia) received induction with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (hyperCVAD), or augmented Berlin-Frankfurt-Munster (AugBFM)-based chemotherapy. Five hundred forty-one patients (95%) achieved CR or CR with inadequate platelet recovery (CRp), 161 (30%) of whom had diploid cytogenetics at baseline, 52 (10%) had insufficient metaphases, and 7 (1%) did not have cytogenetics performed. Of the remaining 321 patients (59%) with abnormal cytogenetics at baseline, 272 had an adequate cytogenetic assessment performed at remission and were evaluable for this analysis. All patients signed an informed consent form for clinical trial participation, and all trials were approved by the institutional review board of The University of Texas MD Anderson Cancer Center.
Treatments
Of the 272 evaluable patients, 237 (87%) received a hyperCVAD-based regimen and 35 (13%) received AugBFM. The details of these backbone regimens are published elsewhere [13,14]. All patients with Ph+ ALL received a tyro-sine kinase inhibitor added to the chemotherapy regimen; patients with CD20-positive ALL also received an anti-CD20 antibody. The specific treatment regimens used in this study are summarized in Supporting Information Table 1.
Response and outcome definitions
CR was defined as the presence of <5% blasts in the bone marrow (BM) with >1 × 109/L neutrophils and >100 × 109/L platelets in the peripheral blood with no evidence of extramedullary disease. CRp was defined as meeting criteria for CR but with a platelet count ≤100 × 109/L. Relapse was defined by recurrence of ≥5% blasts in a BM aspirate or by the presence of extramedullary disease. RFS was calculated from the time of CR until relapse or death. OS was calculated from the time of treatment initiation until death.
Cytogenetic assessment
BM specimens for cytogenetic assessment were obtained prior to initiation of treatment and at the time of CR. Patients were risk-stratified by baseline cytogenetics in accordance with consensus guidelines [15]. The presence of Ph+, MLL rearrangement, hypodiploidy (<44 chromosomes) or complex cytogenetics (≥5 chromosomal abnormalities) were considered poor-risk. An adequate remission sample required either the identification of ≥10 normal metaphases or the presence of at least one abnormal metaphase that was also present in the pre-treatment specimen. ACCR was defined as the presence of an abnormal karyotype in ≥1 metaphase if the same abnormality was also identified in the pretreatment specimen.
MRD assessment
MRD by MFC was performed on BM specimens at the time of CR as previously described [5]. Initially, a 15-marker, 4-color panel was used; later, a 6-color panel was used. MRD positivity was defined as a cluster of at least 20 cells showing altered expression of ≥2 antigens. The sensitivity of this MRD assay was 0.01%.
Statistical methods
Patient characteristics were summarized using median (range) for continuous variables and frequencies (percentages) for categorical variables. Mean values were reported as mean ± standard error of the mean and were compared using the two-tailed Student’s t-test. Associations between categorical variables were assessed using Fisher exact tests. RFS and OS were calculated using Kaplan–Meier estimates, and survival estimates were compared using the log-rank test.
Results
ACCR frequency and predictors
The median duration of survivor follow-up was 43 months (range, 2–148 months). Best response was CR in 267 patients (98%) and CRp in five (2%). Median time to CR/CRp was 23 days (range, 14–84 days). Of the 272 patients evaluable for this analysis, 26 (9.6%) had ACCR and 246 (90.4%) had normal cytogenetics at CR (NCCR). Among the patients with ACCR, the median number of abnormal metaphases observed in the remission specimen was two (range, 1–18). Fifteen patients with ACCR (58%) had ≥2 abnormal metaphases at the time of CR, and 11 (42%) had only one abnormal metaphase identified. The most common chromosomal abnormality persisting at the time of CR was t(9;22), which was present in 14 (54%) out of the 26 patients with ACCR. Persistent deletion of chromosome Y and trisomy 21 (excluding patients with germline trisomy 21) were observed in two patients (8%) each. No other chromosomal abnormality was observed in more than one patient, and no patients had evidence of a new clonal chromosomal abnormality (i.e. ≥2 abnormal metaphases) in the remission sample that was not present in the pre-treatment sample. The number of metaphases examined in the NCCR and ACCR groups were similar, with ≥20 metaphases analyzed in 89% and 92% of patients, respectively (P =0.65).
Pretreatment characteristics of the cohort are summarized in Table I. The median age was 48 years (range, 16–85 years) and the median white blood cell count at presentation was 11.6 × 109/L (range, 0.4–629.4 × 109/L). Baseline characteristics associated with ACCR were Ph+ ALL (62% of ACCR group vs. 42% of NCCR group, P =0.06) and longer median time to CR (25.5 days for ACCR group vs. 23.0 days for NCCR group, P =0.07). There was also a trend towards more patients with ACCR undergoing SCT (31% vs. 17% in NCCR group, P =0.10). There was no association between the treatment regimen or cytogenetic risk-group and the presence or absence of cytogenetic abnormalities at the time of CR.
TABLE I.
Patient Characteristics and Association with Cytogenetic Assessment at Complete Remission
| Characteristica | NCCR (N =246) | ACCR (N =26) | P |
|---|---|---|---|
| Age (years) | 47 (16–80) | 54 (21–85) | 0.46 |
| WBC (109/L) | 11.7 (0.4–629.4) | 11.1 (2.0–31.5) | 0.43 |
| Hemoglobin (g/dL) | 9.5 (4.0–16.4) | 9.3 (5.0–11.5) | 0.48 |
| Platelets (109/L) | 40 (4–513) | 38.5 (10–253) | 0.96 |
| BM blasts (%) | 87 (0–100) | 83 (30–97) | 0.33 |
| LDH (U/L) | 1131 (172–36247) | 1334 (440–18406) | 0.54 |
| Time to CR (days) | 23.0 (14–82) | 25.5 (16–84) | 0.07 |
| Diagnosis | 1.0 | ||
| B-ALL | 221 (90) | 24 (92) | |
| T-ALL | 13 (5) | 1 (4) | |
| Burkitt leukemia | 12 (5) | 1 (4) | |
| Cytogenetics | 0.21 | ||
| Poor-risk | 143 (58) | 19 (73) | |
| Others | 103 (42) | 7 (27) | |
| Philadelphia chromosome (Ph) | 0.06 | ||
| Ph+ | 103 (42) | 16 (62) | |
| Ph− | 143 (58) | 10 (38) | |
| Prior chemotherapy or radiation | 0.25 | ||
| Yes | 21 (9) | 4 (15) | |
| No | 225 (91) | 22 (85) | |
| Chemotherapy | 0.22 | ||
| HyperCVAD | 212 (86) | 25 (96) | |
| AugBFM | 34 (14) | 1 (4) | |
| MRD at CR | 0.47 | ||
| Positive | 69 (34) | 9 (43) | |
| Negative | 137 (67) | 12 (57) | |
| Transplant | 0.10 | ||
| Yes | 41 (17) | 8 (31) | |
| No | 205 (83) | 18 (69) |
Continuous variables are listed as median (range) and categorical variables as N (%).
NCCR, normal cytogenetics at complete remission; ACCR, abnormal cytogenetics at complete remission; WBC, white blood cells; BM, bone marrow; LDH, lactate dehydrogenase; hyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone; AugBFM, augmented Berlin-Frankfurt-Munster; MRD, minimal residual disease; CR, complete remission.
Of the 119 patients with Ph+ ALL, 87 (73%) had additional chromosomal abnormalities (ACAs) identified prior to treatment. Patients with ACAs were less likely than those without ACAs to have ACCR (7% vs. 31%, respectively; P <0.001). Of the 6 Ph+ patients with ACAs and ACCR, 4 had persistence of t(9;22) and 2 had other cytogenetic abnormalities identified at the time of remission. Patients with ACAs had a lower mean percentage of Ph+ metaphases in the pre-treatment specimen compared to those without ACAs (56% ± 3% vs. 71% ± 6%, respectively; P =0.03), although the mean percentage of abnormal metaphases in these two groups were similar (65% ± 3% vs. 71% ± 6%, respectively; P =0.39).
Prognostic significance of ACCR
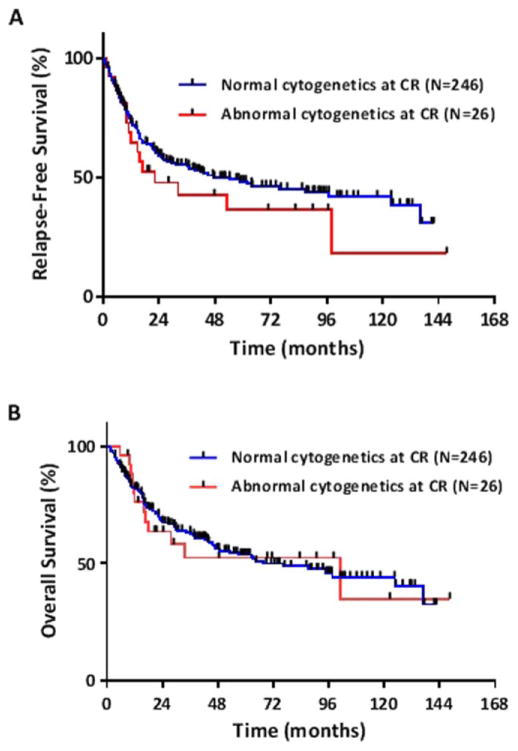
The median RFS was 22 months (95% CI, 12 months to not reached [NR]) in patients with ACCR and 48 months (95% CI, 30–125 months) in patients with NCCR (P =0.31; Fig. 1A). Median OS also did not differ between the ACCR (99 months [95% CI, 17 months to NR]) and NCCR groups (67 months [95% CI, 47 months to NR], P =0.86; Fig. 1B). Among those patients with ACCR, a significant difference in RFS or OS was not observed in patients with one abnormal metaphase vs. ≥2 abnormal metaphases at CR.
Figure 1.
(A) Relapse-free survival and (B) overall survival for patients with and without abnormal cytogenetics at complete remission. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary. com.]
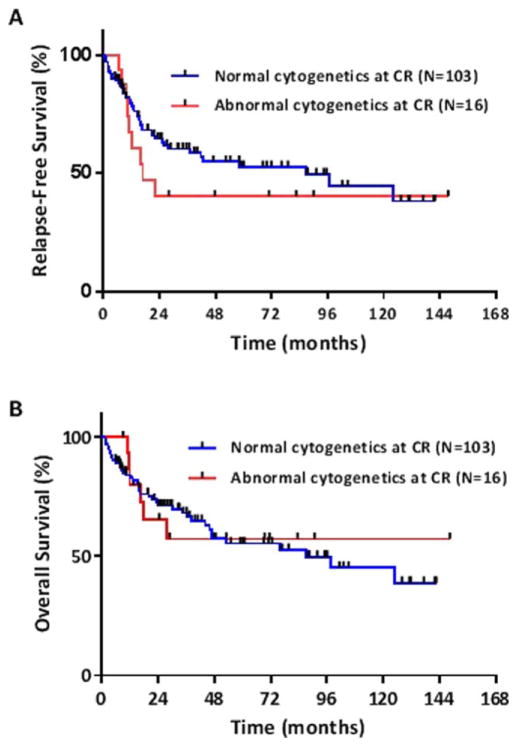
Of the 119 patients with Ph+ ALL, 16 (13%) had ACCR and 103 (87%) had NCCR. Among patients with Ph+ ALL, the median RFS for patients with ACCR was 17 months (95% CI, 10 months to NR) compared to 87 months (95% CI, 28 months to NR) for patients with NCCR (Fig. 2A). While there was a large numerical difference in RFS between the two groups, this did not reach statistical significance (P =0.35). Median OS did not differ between Ph+ patients with ACCR compared to those with NCCR (NR vs. 87 months [95% CI, 46 months to NR], respectively) (P =0.88; Fig. 2B).
Figure 2.
(A) Relapse-free survival and (B) overall survival for patients with Philadelphia chromosome-positive ALL with and without abnormal cytogenetics at complete remission. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Association of ACCR with MRD by MFC
MRD by MFC was performed in 229 patients (84% of the evaluable population); two of these patients had indeterminate MRD assessment and were excluded from the analysis. Among the 227 patients evaluable for MRD by MFC, MRD positivity at CR was observed in 78 (34%); 149 (66%) were MRD-negative. The median RFS for patients positive for MRD was 22 months (95% CI, 17 to 48 months) vs. 99 months (95% CI, 45 months vs. NR) for patients who were MRD-negative (P <0.01). Median OS for patients who were MRD-positive at CR was 33 months (95% CI, 23–76 months) compared to 137 months (95% CI, 87 months to NR) for those who were MRD-negative (P <0.01).
Of the 21 patients with ACCR and who had an evaluable MRD assessment, nine (43%) were positive for MRD and 12 (57%) were negative for MRD, yielding a poor correlation between these two methods. Among the 12 patients with ACCR who were negative for MRD, six were Ph+ and six were Ph−. There was no association between MRD status and the number of abnormal metaphases at CR, with only one abnormal metaphase observed in five of the MRD-positive patients (56%) and six of the MRD-negative patients (50%; P =0.80). Of the 64 patients who were MRD positive and had a quantified MRD result, the mean percent of aberrant leukemic cells identified by MFC was 2.3% ± 1.5% in the ACCR group (n =7) vs. 0.9% ± 0.2% (n =57) in the NCCR group (P =0.09).
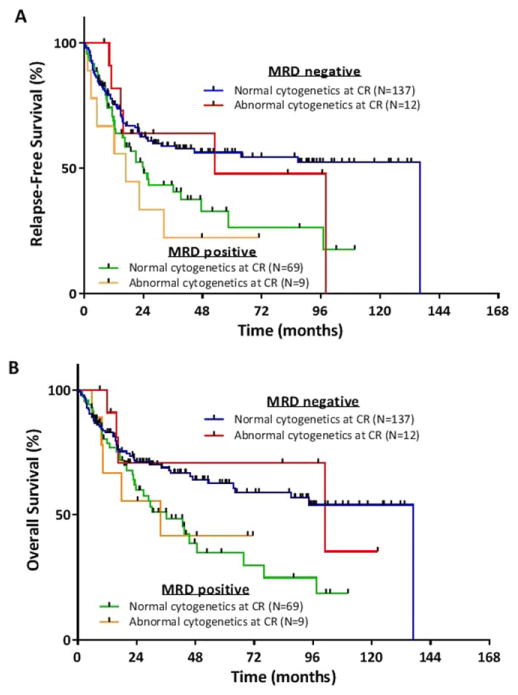
When patients were stratified by MRD status as assessed by MFC, the presence or absence of persistent cytogenetic abnormalities at CR did not add additional prognostic information (Fig. 3A, B). Median RFS for MRD-negative patients with ACCR was 53 months (95% CI, 16 to NR) vs. 137 months (95% CI, 37 months to NR) for those with NCCR (P =0.74). Among MRD-positive patients, median RFS was 17 months (95% CI, 5 to NR) for those with ACCR vs. 24 months (95% CI, 17 to 59 months) for those with NCCR (P =0.42). Median OS for MRD-negative patients with ACCR was 99 months (95% CI, 16 months to NR) vs. 137 months (95% CI, 64 months to NR) for those with NCCR (P =0.87). Among MRD-positive patients, median OS was 33 months (95% CI, 10 months to NR) for those with ACCR vs. 36 months (95% CI, 23–76 months) for those with NCCR (P =0.90).
Figure 3.
(A) Relapse-free survival and (B) overall survival for patients with and without abnormal cytogenetics at complete remission (CR), stratified by minimal residual disease (MRD) assessment. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
Post-treatment MRD status by PCR or MFC has been well-established as a powerful prognostic factor in patients with ALL and can help to inform the decision to pursue SCT [5–9]. This analysis was designed to address whether ACCR might also serve as a form of MRD in this population and be similarly prognostic for long-term outcomes; to our knowledge, this study is the first to address this question in ALL. In this large retrospective study of adults patients with previously untreated ALL receiving induction chemotherapy, ACCR was not found to be prognostic for RFS or OS and did not offer additional prognostic information when MRD status by MFC was known.
Cytogenetic responses have been shown to be highly prognostic in a variety of leukemias. In patients with chronic myelogenous leukemia, complete cytogenetic response has been well-established as predictive of superior survival, regardless of the treatment used [16–19]. Cytogenetic response is also predictive for better long-term outcomes in patients with myelodysplastic syndrome treated with hypomethylating agents [20], and in AML, abnormal cytogenetics at various time points after induction and/or consolidative chemotherapy has consistently been shown to confer an adverse prognosis [11,12,21–23]. Given the prognostic significance of a lack of complete cytogenetic response in these other diseases, it is notable that a similarly heightened risk for relapse and death was not observed in the present study.
One potential explanation why ACCR did not appear to have prognostic significance in this analysis may be that, in some patients, the abnormal karyotype observed at the time of CR does not represent a persistent malignant clone of lymphoblasts but rather chromosomal abnormalities in nonmalignant lymphoid, myeloid, or erythroid cells. This phenomenon has been described previously in patients with Ph+ ALL [24] and has also been suggested in a study of MRD in Ph+ ALL that observed conflicting results between MRD assessment of BCR-ABL transcripts by PCR and MRD measurement by MFC [5]. A subset of these patients had high transcript levels of BCR-ABL but MRD negativity by MFC, suggesting that the BCR-ABL transcripts were present in the non-ALL compartment.
This explanation could also account for the poor correlation between ACCR and MRD positivity by MFC observed in this study, at least in the subset of Ph+ patients in whom this phenomenon has been previously reported. While the detection of persistent cytogenetic abnormalities would be expected to be less sensitive than more sophisticated methods of MRD assessment such as PCR and MFC, if the cytogenetic abnormalities observed at CR represented a persistent malignant clone of lymphoblasts, one would expect that ACCR would be highly specific for MRD-positive disease as assessed by MFC. However, in the present study, only 43% of patients with ACCR were also positive for MRD by MFC.
One surprising finding of the present study was that this discordance between cytogenetic abnormalities at CR and assessment of MRD by MFC was seen in both Ph+ and Ph− patients. In fact, of the 12 patients with ACCR who were MRD-negative, half had Ph− ALL. While previous reports have suggested that persistent chromosomal abnormalities in nonlymphoblasts may be observed in Ph+ ALL, this phenomenon has not been described in Ph− disease. Our observation of poor correlation between ACCR and MRD assessment by MFC in both Ph+ and Ph− ALL is hypothesis generating; however, the small size of these subgroups and the lack of cytogenetic analysis of individual cellular lineages limit our ability to reach any definitive conclusions about the reason for this discordance.
Another unexpected finding was that among Ph+ patients, patients harboring ACAs were significantly less likely to develop ACCR than were those without ACAs. The cause of the lower ACCR rate in patients with ACAs is unclear, although it is notable that the ACA cohort had a lower density of Ph+ metaphases identified in the pretreatment sample than did the non-ACA group (although both groups had similar overall densities of abnormal metaphases). In our study, patients with Ph+ ALL had an approximately two-fold higher rate of ACCR than those with Ph− disease (13% vs. 7%, respectively), suggesting that t(9;22) is more likely to persist after induction chemotherapy than are other cytogenetic abnormalities. Thus, the lower relative density of Ph+ metaphases in patients with ACAs may have contributed to their lower rate of ACCR.
It should be noted that although these data were derived from a relatively large database of 571 patients, only 26 patients (9.6% of the evaluable patients) were identified as having ACCR. The median RFS was numerically shorter in the ACCR group compared to the NCCR group when the entire cohort was analyzed (22 vs. 48 months, respectively) as well as when the analysis was limited to Ph+ patients (17 vs. 87 months, respectively), MRD-positive patients (17 vs. 24 months, respectively), and MRD-negative patients (53 vs. 137 months), although none of these comparisons reached statistical significance. It remains possible that a larger study might identify a statistically significant relationship between cytogenetic abnormalities at CR in ALL; however, such an endeavor would likely require pooling data across multiple institutions.
In conclusion, this study found a poor association between MRD assessment by MFC and the presence or absence of cytogenetic abnormalities at CR in adult patients with ALL. ACCR was not associated with adverse outcomes and therefore should not be used to guide prognostication or therapeutic decisions. Further studies may elucidate why some patients have persistently abnormal cytogenetics but no evidence of MRD by MFC.
Supplementary Material
Acknowledgments
Contract grant sponsor: MD Anderson Cancer Center Support Grant; Contract grant number: CA016672.
Footnotes
Conflict of interest: Nothing to report
Additional Supporting Information may be found in the online version of this article.
References
- 1.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): Results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157:463–471. doi: 10.1111/j.1365-2141.2012.09095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding AK, Rowe JM, Buck G, et al. UKALL-XII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120:4470–4481. doi: 10.1182/blood-2012-06-379040. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32:1595–1604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- 7.Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148:80–89. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagafuji K, Miyamoto T, Eto T, et al. Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with Ph-negative ALL: results of a prospective study (ALL MRD2002 Study) J Hematol Oncol. 2013;6:14. doi: 10.1186/1756-8722-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhedin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125:2486–2496. doi: 10.1182/blood-2014-09-599894. quiz 2586. [DOI] [PubMed] [Google Scholar]
- 10.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Cortes J, Estrov Z, et al. Persistence of cytogenetic abnormalities at complete remission after induction in patients with acute myeloid leukemia: prognostic significance and the potential role of allogeneic stem-cell transplantation. J Clin Oncol. 2011;29:2507–2513. doi: 10.1200/JCO.2010.34.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcucci G, Mrozek K, Ruppert AS, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22:2410–2418. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 14.Nachman J, Sather HN, Gaynon PS, et al. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. Acute Lymphoblastic Leukemia (Version 1.2015) [Google Scholar]
- 16.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118:4541–4546. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabbour E, Kantarjian H, Ghanem H, et al. The achievement of a 3-month complete cytogenetic response to second-generation tyrosine kinase inhibitors predicts survival in patients with chronic phase chronic myeloid leukemia after imatinib failure. Clin Lymphoma Myeloma Leuk. 2013;13:302–306. doi: 10.1016/j.clml.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarjian HM, O’Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 19.Milojkovic D, Nicholson E, Apperley JF, et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2010;95:224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubbert M, Wijermans P, Kunzmann R, et al. Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2′-deoxycytidine. Br J Haematol. 2001;114:349–357. doi: 10.1046/j.1365-2141.2001.02933.x. [DOI] [PubMed] [Google Scholar]
- 21.Freireich EJ, Cork A, Stass SA, et al. Cytogenetics for detection of minimal residual disease in acute myeloblastic leukemia. Leukemia. 1992;6:500–506. [PubMed] [Google Scholar]
- 22.Grimwade D, Walker H, Oliver F, et al. What happens subsequently in AML when cytogenetic abnormalities persist at bone marrow harvest? Results of the 10th UK MRC AML trial. Bone Marrow Transpl. 1997;19:1117–1123. doi: 10.1038/sj.bmt.1700804. [DOI] [PubMed] [Google Scholar]
- 23.Balleisen S, Kuendgen A, Hildebrandt B, et al. Prognostic relevance of achieving cytogenetic remission in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome following induction chemotherapy. Leuk Res. 2009;33:1189–1193. doi: 10.1016/j.leukres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Schenk TM, Keyhani A, Bottcher S, et al. Multilineage involvement of Philadelphia chromosome positive acute lymphoblastic leukemia. Leukemia. 1998;12:666–674. doi: 10.1038/sj.leu.2400986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.